 Open Access Article
Open Access ArticleDFT insights into the gas sensing performance of M2X (M = Zr, Hf; X = C, N) MXenes and their Janus derivatives for toxic gases
Muhitul
Islam
a,
Siraj Ud Daula
Shamim
 *a,
Bivas Kumar
Dash
a,
Aditi Ahmed
Ananna
a,
Mohammad Sadiqur
Rahman
*a,
Bivas Kumar
Dash
a,
Aditi Ahmed
Ananna
a,
Mohammad Sadiqur
Rahman
 b,
Tanu
Arefin
b,
Tanu
Arefin
 c and
Afiya Akter
Piya
c and
Afiya Akter
Piya
 a
a
aDepartment of Physics, Mawlana Bhashani Science and Technology University, Tangial-1902, Dhaka, Bangladesh. E-mail: sdshamim@mbstu.ac.bd
bDhaka University of Engineering & Technology, Gazipur-1707, Bangladesh
cDepartment of Physics, Bangladesh University of Textiles, Dhaka-1208, Bangladesh
First published on 28th October 2025
Abstract
Widespread air pollution caused by toxic, flammable, and hazardous gases has been linked to numerous health issues. Gas sensors play a crucial role in the detection and monitoring of these harmful substances. In this study, Zr and Hf-based carbide and nitride MXenes (Zr2C, Zr2N, Hf2C, and Hf2N) and their Janus structures (ZrHfC and ZrHfN) were investigated for their sensing performance towards the toxic CO, NO, NO2, and SO2 gas molecules using density functional theory (DFT). The adsorption behavior in terms of adsorption energy, charge transfer, recovery time, electronic properties in terms of band structures, density of states (DOS), and work function were examined to understand the sensing behavior of the nanosheets. Chemisorption interactions were found for all of the complexes except CO and SO2 adsorption on ZrHfC. Favorable adsorption characteristics were observed for the CO and NO gases on all the nanosheets. The adsorption energies ranged from −1.65 to −3.37 eV for CO and −4.42 to −5.32 eV for NO, with corresponding Mulliken charge transfers of −0.152 to −1.00e and −0.172 to −1.038e, respectively. But in the case of NO2 and SO2, very high adsorption characteristics with high deformation of the gases in the complexes were found, which leads to unfavorable adsorption performances. Furthermore, the work functions of the nanosheets varied during the adsorption of gas molecules. Thus, all calculations indicate that all six nanosheets can be useful for monitoring CO and NO.
1. Introduction
The existence of hazardous gas molecules in the environment is increasing rapidly due to rapid industrialization, which is alarming for the environment. Hazardous, poisonous, combustible, and explosive gases such as ethanol (C2H6O), formaldehyde (HCHO), nitrogen oxides (NOx), acetone (C3H6O), ammonia (NH3), sulfur oxides (SOx), and carbon monoxide (CO) pose serious threats to human health, environmental safety, and urban air quality and contribute significantly to carbon emissions and other global concerns. Toxic gases are the prime cause of cardiovascular diseases, cardiac dysfunction, nausea, acute headaches, respiratory issues, asthma, neural damage, and so on.1,2 CO is a poisonous gas without smell, color, or taste. It forms when fuels such as wood, coal, or gas don’t burn completely or when gas doesn’t get enough fresh air. When inhaled, it binds to blood cells faster than oxygen, developing a lack of oxygen in the body and leading to tissue hypoxia, brain injury, or even death.3 SO2 is a colorless, corrosive, and highly irritant gas that can cause significant health effects. It causes bronchoconstriction in asthmatic subjects, exacerbates asthma, chronic bronchitis, and chronic obstructive pulmonary disease, and contributes to acid rain.4 Nitric oxide (NO) and nitrogen dioxide (NO2) are major air pollutants that harm both health and the environment by contributing to acid rain, ozone depletion, and the greenhouse effect.5 Air contamination disturbs the natural atmospheric conditions and creates an ecological imbalance, resulting in 9 out of 10 people inhaling highly contaminated air, according to WHO.6Researchers have concentrated on developing complex gas sensors that offer high accuracy and speed in detecting the concentrations of gases to enable the rapid identification of these gas molecules. These sensors have several applications, such as the detection of explosives, medical diagnostics, food and beverage quality evaluation, and air quality monitoring. Many two-dimensional (2D) nanomaterials, including phosphorene, graphene, related 2D crystals, and even MXenes, have been utilized as gas-sensing materials over the past few decades due to their ultrahigh surface area, strong exterior actions, high mobility, outstanding thermal and electrical conductivity values, and high chemical and thermodynamic durability.7–9 A new class of two-dimensional materials called MXenes was discovered in 2011. It comprises nitrides, carbides, oxycarbides, and nitrocarbides of transition metals. These substances have generated considerable attention due to their various applications in energy storage, hydrogen generation, gas sensing, biological sensing catalysis, and water purification.10,11 The structures are composed of 2–5 sections of initial metals that transition (M), linked with 1–4 stages of X (C or N) that can be prepared by eliminating “A” sections (including Al or Si) via MAX indicators and by employing non-MAX ones.12 By taking out the “A” element from the bulk MAX phases, 2D MXene systems as suitable models were built.13,14 For simplicity, the focus was placed on M2X-type MXenes, the thinnest members of the MXene family, where M represents transition metals such as Sc, Ti, Zr, Hf, V, Nb, Ta, and Cr, and X stands for either carbon (C) or nitrogen (N). Finally, the overall findings were discussed on thicker MXene systems.15–18 It is anticipated that the produced metal surfaces will undergo chemical termination following the removal of the “A” element.13,19 When hydrofluoric acid (HF) acid solutions chemically exfoliate the MXenes, it has been observed experimentally that the exterior layers dissolve via F and/or OH groups.20,21 Additionally, O-terminated surfaces could be created chemically or by post-processing OH-terminated systems, for example, by thermal treatment or an ultraviolet-ozone cleaning method.19,22,23
From Table 1, it is clear that most previous DFT investigation focused on single-component MXenes that were utilized for detecting various toxic gases. These studies reported good charge transfer and sensitivity. However, they did not investigate Janus or heterostructure MXenes and were mainly restricted to conventional MXenes. Furthermore, common defects in MXenes, such as vacancies or missing atoms, can significantly affect the gas sensing properties. They often create additional active sites, enhancing adsorption and charge transfer with gas molecules, which can improve sensitivity and selectivity. However, a high concentration of defects may reduce structural stability. In recent years, numerous theoretical investigations have reported promising gas sensing properties of Janus material transition metal dichalcogenides beyond MXenes. Therefore, in this work, we focused on Janus MXenes. For instance, H. Dou et al.32 and B. Paul et al. explored Janus WSTe and WSeTe monolayers to determine toxic gases including CO, NO, and O2, while L. Ju et al. demonstrated that Se-vacancies in Janus WSSe enhance sensitivity toward NO2.32–34 The selective adsorption of SO2 and NO2 on Janus MoSSe was also reported by B. Babariya et al.35 Our exploration of Janus MXenes as novel gas sensing materials is further motivated by these works, which demonstrate how the intrinsic asymmetry of Janus structures can greatly enhance the gas sensing performance. Therefore, the current work depends on DFT to methodically investigate Hf2C, Hf2N, Zr2C, Zr2N, and their Janus heterostructures (ZrHfC and ZrHfN).
| Author | Sensing materials | Targeted gases | Methods | Key findings | Ref. |
|---|---|---|---|---|---|
| J. Liang et al. | MXene-based biosensor | Biomolecules | DFT | Good biocompatibility; potential piezoresistive biosensor. | 24 |
| Q. Zhou et al. | Ni-doped Zr2CO2/MoS2 | HCN | DFT | Enhanced adsorption/sensing via O-vacancies and doping. | 25 |
| R. K. Choudhury | W2CT2 (T = O, F) | NH3 | DFT | Strong physisorption, charge transfer, and conductivity. | 26 |
| Z. Li et al. | Ti3C2O2 | NH3, H2S, CO2 | DFT | Strong adsorption; excellent sensitivity to toxic gases. | 27 |
| R. P. Reji et al. | Sc2CO2 | VOCS (ethanol, acetonitrile, 2-propanol) | DFT | Dual-mode sensing; high sensitivity, fast recovery. | 28 |
| J. Su et al. | Zn–Co doped Ti3C2O2 | NO | DFT | Enhanced NO adsorption; improved sensitivity. | 29 |
| K. Boonpalit et al. | Sc@Zr3C2O2, Y@Zr3C2O2 | CO | DFT | Good sensitivity, stability, and fast recovery. | 30 |
| Xi. Li et al. | Zr3C2O2 | Multiple gases | DFT | Gas sensing tunable via surface groups and strain. | 31 |
2. Computational details
Using the DMol3 component in the Materials Studio software, DFT calculations were performed to investigate the interactions among six nanosheets and unique gas molecules, such as CO, NO, SO2, and NO2.36,37 The exchange–correlation functions were analyzed using the GGA (generalized gradient approximation) with the Perdew–Burke–Ernzerhof (PBE) approach.36 To adjust for relativistic impacts in the core region, a double numerical basis set with polarization (DNP) and the DFT semi-core pseudopotential (DSPP) were utilized.38,39 All the calculations were completed with Grimme dispersion correction (DFT-D2) for van der Waals forces and large interactions.40 We used the spin-unrestricted method, which employed separate orbitals for spin-up and spin-down electrons, to acquire a more accurate representation of uncoupled electron systems. Furthermore, the Tkatchenko–Scheffler (TS) dispersion correction was utilized to account for interactions produced by van der Waals forces.41–43 For this study, we used a 3 × 3 × 1 supercell with a 20 Å vacuum layer, containing 34 atoms. Earlier studies confirm that a 3 × 3 supercell works well for gas adsorption, and a 20 Å vacuum space prevents interactions between neighboring units.44,45 All calculations were performed using an established k-point grid of 4 × 4 × 1 and an entire cut-off radius of 5.4 Å. In an optimization process, the maximum force, displacement convergence, and energy tolerance are 0.002 Ha Å−1, 0.005 Å, and 1 × 10−5 Ha, respectively.46,47The cohesive energy of our proposed nanosheets was calculated using the following equation to investigate their structural stability.46
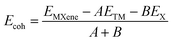 | (1) |
The adsorption energy was obtained to explore the ability of gas molecules and nanosheets to adsorb,48
| Eads = Enanosheet+gas − Enanosheet − Egas | (2) |
Through Mulliken and Hirshfeld's charge evaluation, the total charge transfer (Qt) between the gas molecules and the nanosheets was studied. Qt was determined by,49
| Qt = Qads-gas − Qiso-gas | (3) |
| Δρ = ρnanosheet+gas − ρnanosheet − ρgas | (4) |
3. Results and discussion
3.1 Geometry and structural stability
In this investigation, four 3 × 3 × 1 supercells of carbide and nitride MXenes (Hf2C, Hf2N, Zr2C, and Zr2N) and their Janus MXenes (ZrHfC and ZrHfN) were chosen. The Janus MXenes were created by replacing one Zr/Hf layer of any of the initial four nanosheets (Hf2C/N or Zr2C/N) with Hf/Zr, forming ZrHfC and ZrHfN. Each of these nanosheets has 27 atoms. Hf2N, Zr2N, and ZrHfN contain 9 N atoms each, and the same goes for Hf2C, Zr2C, and ZrHfC, but here they have 9 C atoms each. In both the Hf2N and Hf2C structures, 18 Hf atoms are present, and 18 Zr atoms are present in both the Zr2N and Zr2C structures. Additionally, 9 N or C atoms, along with 9 Hf and Zr atoms, respectively, are present in the ZrHfN and ZrHfC structures. The N–Hf bond length and bond angle of Hf–N–Hf in the optimized Hf2N structure are determined to be 2.204 Å and 87.150°, respectively, consistent with previous findings.50 For Hf2C, the C–Hf bond length and bond angle of Hf–C–Hf are determined to be 2.204 Å and 87.150°, respectively, and are comparable to the prior value.51 Additionally, the obtained value is the same in the instances of Zr2N and Zr2C. The ZrHfC monolayer, as seen in Fig. 1, is made up of three distinct atom layers: Hf, Zr, and N. The C layer is located in the center of the Hf and Zr layers. In the same way, the ZrHfN monolayer is composed of three layers, with the N layer remaining in the midst of the Hf and Zr layers. The ZrHfC and ZrHfN heterolayer structures show the maximum stability and lowest formation energies of all the mixed phases. The Hf–C and Zr–C bond lengths in the ZrHfC structure are optimized to be 2.192 Å and 2.218 Å, respectively, and the Hf–N–Zr bond angle is calculated to be 92.909°. For ZrHfN, the optimized Hf–N–Hf bond angle is 46.128°, and the Hf–N bond length is 2.192 Å, both in useful agreement with previously revealed findings.50 It is shown in Fig. 2–5 that no structural deformation occurs in these nanosheets after optimization. Therefore, the stability of the structure remains unchanged in this gas sensor. In this investigation, we also relaxed four hazardous gases, namely CO, NO, NO2, and SO2, in the ground state. The optimized structures of these gas molecules are shown in Fig. 1. In CO and NO, the C–O and N–O bonds have lengths of 1.145 Å and 1.166 Å, respectively. The N–O bond length in the NO2 molecule is 1.213 Å, while the S–O bond length in the SO2 molecule is 1.464 Å. NO2 and SO2 have V-shaped structures, with calculated O–N–O and O–S–O bond angles of 133.271° and 119.712°, respectively.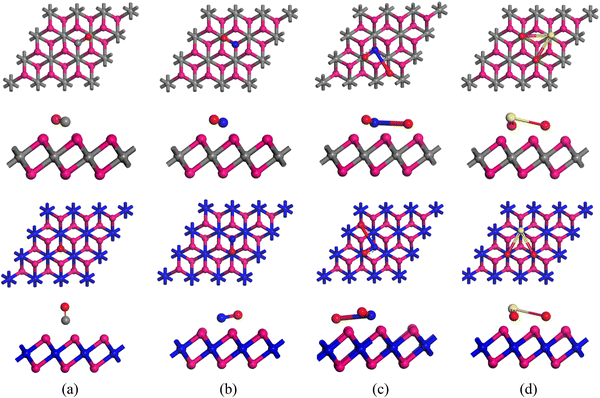 | ||
| Fig. 2 Top and side views of (a) CO, (b) NO, (c) NO2, and (d) SO2 adsorbed on the Hf2C (top two rows) and Hf2N (bottom two rows) nanosheets. | ||
We calculated the cohesive energy using eqn (1) to understand the bond strength in the crystal structures—the more negative the cohesive energy, the more stable the structure. The cohesive energies are −6.96, −6.59, −6.97, −6.61, −6.98, and −6.62 eV for Hf2C, Hf2N, Zr2C, Zr2N, ZrHfC, and ZrHfN, respectively, which is consistent with previous findings.50 In our findings, the cohesive energy is larger (more negative) in the carbide MXene than that of nitride ones, which was also found by Shein et al.52 The cohesive energies increase in this order: Hf2N < Zr2N < ZrHfN < Hf2C < Zr2C < ZrHfC, which shows that ZrHfC is the most stable among the nanosheets.
3.2 Adsorption behavior of Hf2C and Hf2N towards the CO, NO, NO2, and SO2
The adsorption properties of CO, NO, NO2, and SO2 on the Hf2C and Hf2N nanosheets were evaluated by analyzing the adsorption energy (Eads), minimum adsorption distance (dmin), and charge transfer (Qt). The calculated values are summarized in Table 2, and the corresponding optimized structures are illustrated in Fig. 2. When the adsorption process is positive, it indicates an endothermic reaction; conversely, the negative adsorption value indicates an exothermic reaction and also illustrates the attractive connection between gas molecules and nanosheets.53 Here, the significant values of Eads are −2.32, −4.42, −7.63, and −9.61 eV for CO, NO, NO2, and SO2 gas molecule adsorption on the Hf2C nanosheet at a distance of about 2.155, 2.102, 2.085, and 2.048 Å, respectively. Similarly, in the case of the Hf2N nanosheet, the values of Eads are −1.65, −4.82, −7.38, and −11.52 eV for CO, NO, NO2, and SO2 gas molecules at a distance of 2.354, 2.054, 2.040, and 2.034 Å, respectively. Based on the study findings by Yong et al., the highest Eads values for CO, NO, NO2, and SO2 on the C2N monolayer are −0.239 eV, −0.471 eV, −1.665 eV, and −1.304 eV, respectively.54 Aghaei et al. observed that the adsorption energies of toxic gas molecules (NO, CO, and NO2) on graphene-like boron carbide (BC3) were −1.11 eV, −1.34 eV, and −1.13 eV, respectively.55 Q. Hu et al. demonstrated Hf3C2O2 MXene's gas sensing potential through its moderate adsorption energies of −0.197 eV (CO) and −0.174 eV (NO).56 According to Salih et al., gold (Au) and silver (Ag) co-doped molybdenum disulfide (Au–Ag–MoS2) demonstrated promising NO and NO2 gas sensing capabilities, with adsorption energies of −0.553 eV and −2.603 eV, respectively.56 Based on the current comprehensive study, the Eads energy for the Hf2C and Hf2N nanosheets is better than the previous ones, confirming the superior sensing potential of Hf-based MXenes compared with many other 2D materials.For both Hf2C and Hf2N nanosheets, the Eads increases in the order CO < NO < NO2 < SO2, while dmin follows the reverse trend. Furthermore, all of the Eads values are observed to be negative, indicating an exothermic reaction. NO2 and SO2 molecules show a stronger interaction with Hf2C and Hf2N nanosheets than CO and NO molecules, which are also adsorbed on the surfaces of Hf2C and Hf2N in chemisorption. However, when the adsorption energy is too high, the desorption process slows, i.e., the recovery time increases, which is unsuitable for a good conductor. This indicates that these two nanosheets show better performance as a gas sensor material for detecting CO and NO rather than NO2 and SO2.
Mulliken and Hirshfeld charge analysis also indicates that Hf2C and gas molecules interact strongly by sharing a vast amount of charge (Mulliken) −0.200e (CO), −0.432e (NO), −1.906e (NO2) and −1.964e (SO2), where Hf2C plays the role of electron donor and toxic gas molecules are used as electron acceptors. Charge transfer analysis reveals that the Hf2C nanosheets exhibit a slightly greater charge transfer with gas molecules than Hf2N. However, a significant amount of electron transfer occurs in Hf2N. The Hirshfeld charge of 0.214e and Mulliken charge of 0.359e, 0.522e, and 0.327e are transferred to the gas molecules CO, NO, NO2, and SO2, respectively. The Qt values for CO, NO, NO2, and SO2 adsorption indicate that Hf2N serves as an electron donor. Thus, for Hf2C, the charge transfer follows the order: CO < NO < NO2 < SO2, while for Hf2N, the trend is CO < SO2 < NO < NO2. Previously, Yong et al. reported charge transfers of 0.031e, 0.294e, −0.017e, and −0.088e between C2N and CO, NO, NO2, and SO2 gas molecules, respectively.54 According to S. M. Aghaei et al., charge transfers occur between BC3 and gas molecules as follows: 0.09e from BC3 to CO, 0.65e from NO to BC3, and 0.48e from NO2 to BC3.55 E. Salih et al. demonstrated significant charge transfer from NO (0.220e) and NO2 (0.448e) to the Au–Ag–MoS2.57 Our results, in particular, exhibit higher charge transfer values, proving that the material according to this study interacts with these gas molecules more strongly, which implies it has promising potential for gas-sensing purposes.
3.3 Adsorption behavior of Zr2C and Zr2N towards the CO, NO, NO2, and SO2
To measure the adsorption capacity of the aforementioned gas molecules on other nanosheets, specifically Zr2C and Zr2N, we calculated the adsorption energy with adsorption distance and net charge transfer using eqn (2) and (3), which are listed in Table 1, and Fig. 3 depict the top and side views of the following gas molecule adsorption on the Zr2N and Zr2C nanosheets. The various adsorption sites on the nanosheets and gas molecules' orientations were investigated to find the most stable complex. The significant values of Eads are −2.84, −4.97, −9.27, and −10.60 eV, respectively, for CO, NO, NO2, and SO2 gas molecule adsorption on Zr2C nanosheets from distances of 2.176, 2.136, 2.108, and 2.063 Å, respectively. Again, the values of Eads are −3.37, −5.13, −9.87, and −6.56 eV for CO, NO, NO2, and SO2 gas molecule adsorption on Zr2N nanosheets at a distance of 2.139, 2.154, 2.165, and 2.099 Å, respectively. According to H. Y. Ammar et al., the Eads values of CO and NO are significantly enhanced when the B12N12 nanocage is doped with transition metals (Mn and Fe). Specifically, MnB11N12 exhibits adsorption energies of −0.510 eV (CO) and −0.740 eV (NO), while FeB11N12 shows higher adsorption energies of −0.760 eV (CO) and −1.013 eV (NO).58 Y. Wu et al. calculated adsorption energies on B-doped graphdiyne (B-GDY), showing weak CO interaction (−0.068 eV), moderate SO2 adsorption (−0.173 eV), and stronger NOx capture (−0.458 eV for NO, −0.541 eV for NO2).59 J. Ren et al. demonstrated that pristine hexagonal boron arsenide (BAs) exhibits distinct gas adsorption capabilities, with calculated adsorption energies of −0.27 eV for CO, −0.18 eV for NO, −0.43 eV for NO2, and a significantly stronger −0.92 eV for SO2.60 Our study shows that Zr2C and Zr2N nanosheets have stronger adsorption energy than the previous findings. Numerically, the adsorption energy trend upon adsorption on Zr2C is CO < NO < NO2 < SO2, while for Zr2N it follows CO < NO < SO2 < NO2. From the above observation, we have seen that the maximum adsorption occurs for SO2 in the case of Zr2C nanosheets, and for Zr2N nanosheets, it occurs for NO2 gas molecules. Also, the adsorption energy for CO and NO for both of these nanosheets is in the chemisorption range. | ||
| Fig. 3 Top and side views of (a) CO, (b) NO, (c) NO2, and (d) SO2 adsorbed on the Zr2C (top two rows) and Zr2N (bottom two rows) nanosheets. | ||
Mulliken charge analysis also shows that a considerable amount of charge is transferred to the nanosheets, indicating a strong interaction between the gas molecules and these two nanosheets. In terms of Zr2C, the charge transfer increases in the order: CO (−0.913e) < NO (−1.030e) < NO2 (−1.871e) < SO2 (−1.959e), while for Zr2N the trend follows CO (−0.152e) < SO2 (−0.329e) < NO2 (−0.440e) < NO (−0.477e). H. Y. Ammar et al. found that CO transferred 0.222e to MnB11N12 and 0.269e to FeB11N12, while NO received 0.045e from MnB11N12 and transferred 0.053e to FeB11N12.58 Y. Wu et al. reported charge sharing between the B-GDY supercell and gas molecules, with values of −0.008e (CO), 0.083e (NO), −0.563e (NO2), and −0.071e (SO2).59 J. Ren et al. reported minimal charge transfer between monolayer BAs and CO/NO molecules, while stronger interactions were observed with NO2 (−0.231e) and SO2 (−0.179e).60
From this table (Table 2), the atomic-level characteristics of the gases and the MXene surfaces can be used to explain the adsorption energies and charge transfer trends seen for distinct gas molecules on different MXenes. Because of their high electronegativity and polarity, which enable them to draw more electrons from the MXene surface, SO2 and NO2 exhibit the strongest adsorption and largest charge transfer. CO, on the other hand, has the smallest charge transfer and the weakest adsorption because of its smaller size and lower electronegativity. Furthermore, compared to carbides, nitride-based MXenes (Hf2N, Zr2N) frequently exhibit slightly greater adsorption because nitrogen atoms offer more active sites for electron transfer, improving the interaction with polar gas molecules.
3.4 Adsorption behavior of ZrHfC and ZrHfN towards the CO, NO, NO2, and SO2
To get more insight into the adsorption phenomena of Janus carbide and nitride MXene, we similarly observed the adsorption behavior of ZrHfC and ZrHfN towards the gas molecules. Fig. 4 and 5 show the top and side views after gas molecule adsorption on the ZrHfC and ZrHfN nanosheets. To better investigate the adsorption behavior, gas molecules are adsorbed on the Zr and Hf sites of the nanosheets. The significant values of Eads at the Zr sites are −2.77, −4.98, −9.17, and −0.40 eV, respectively, for CO, NO, NO2, and SO2 gas molecule adsorption on the ZrHfC nanosheets at a distance of 2.455, 2.097, 2.092, and 2.046 Å, respectively (Table 3). The significant values of Eads at the Hf sites are −0.29, −4.57, −9.50, and −10.68 eV, respectively, for CO, NO, NO2, and SO2 gas molecule adsorption on the ZrHfC nanosheets at a distance of 2.134, 2.183, 2.135, and 2.046 Å, respectively. Numerically, the absolute values of adsorption energy for the Zr sites are SO2 < CO < NO < NO2, and for the Hf sites are CO < NO < NO2 < SO2. In these nanosheets, the Zr site for CO and the Hf site for SO2 are substantially more reactive. Comparing the above two adsorption sites, we observe that the maximum adsorption occurs at NO2 gas molecules, which is −9.17 eV for Zr sites, and for the Hf sites, it occurs for SO2 (−10.68 eV) gas molecules. The most favorable adsorption sites are found at distances of 2.115 Å, 2.051 Å, 2.039 Å, and 1.098 Å on the Zr site of ZrHfN, with adsorption energies of approximately −3.30 eV, −4.78 eV, −10.13 eV, and −6.54 eV for CO, NO, NO2, and SO2, respectively. | ||
| Fig. 4 Top and side views of (a) CO, (b) NO, (c) NO2, and (d) SO2 adsorbed on the ZrHfC, where the top two rows are Hf sites and the bottom two rows are Zr sites of the nanosheets. | ||
 | ||
| Fig. 5 Top and side views of (a) CO, (b) NO, (c) NO2, and (d) SO2 adsorbed on the ZrHfN, where the top two rows are Hf sites and the bottom two rows are Zr sites of the nanosheets. | ||
| Nanosheets | Gas molecules | E ads (eV) | d min (Å) | Q t (e) (Mulliken) | Q t (e) (Hirshfeld) |
|---|---|---|---|---|---|
| Hf2C | CO | −2.32 | 2.155 | −0.200 | −0.162 |
| NO | −4.42 | 2.102 | −0.432 | −0.288 | |
| NO2 | −7.63 | 2.085 | −1.906 | −0.673 | |
| SO2 | −9.61 | 2.048 | −1.964 | −0.822 | |
| Hf2N | CO | −1.65 | 2.354 | −0.109 | −0.214 |
| NO | −4.82 | 2.054 | −0.359 | −0.276 | |
| NO2 | −7.38 | 2.04 | −0.522 | −0.437 | |
| SO2 | −11.52 | 2.034 | −0.327 | −0.228 | |
| Zr2C | CO | −2.84 | 2.176 | −0.913 | −0.333 |
| NO | −4.97 | 2.136 | −1.030 | −0.349 | |
| NO2 | −9.27 | 2.108 | −1.871 | −0.657 | |
| SO2 | −10.60 | 2.063 | −1.959 | −0.812 | |
| Zr2N | CO | −3.37 | 2.139 | −0.152 | −0.107 |
| NO | −5.13 | 2.154 | −0.477 | −0.29 | |
| NO2 | −9.87 | 2.165 | −0.44 | −0.331 | |
| SO2 | −6.56 | 2.099 | −0.329 | −0.276 | |
According to Table 3, SO2 and NO2 show the greatest amount of adsorption and strongest charge transfer on both ZrHfC and ZrHfN due to their high electronegativity and polarity, while CO exhibits the weakest because of its tiny dimensions and low electronegativity. Since nitrogen provides more active sites for electron transfer, nitride-based MXenes (ZrHfN) generally adsorb gases more strongly than carbides (ZrHfC).
| Nanosheets | Gas molecules | Sites | E ads (eV) | d min (Å) | Q t (e) (Mulliken) | Q t (e) (Hirshfeld) |
|---|---|---|---|---|---|---|
| ZrHfC | CO | Zr | −2.77 | 2.455 | −0.915 | −0.364 |
| Hf | −0.29 | 2.134 | −0.027 | −0.038 | ||
| NO | Zr | −4.98 | 2.097 | −0.172 | −0.124 | |
| Hf | −4.57 | 2.183 | −1.033 | −0.367 | ||
| NO2 | Zr | −9.17 | 2.092 | −1.88 | −0.656 | |
| Hf | −9.50 | 2.135 | −1.887 | −0.677 | ||
| SO2 | Zr | −0.40 | 2.046 | −0.245 | −0.252 | |
| Hf | −10.68 | 2.046 | −1.945 | −0.830 | ||
| ZrHfN | CO | Zr | −3.30 | 2.115 | −1.00 | −0.384 |
| Hf | −2.76 | 2.167 | −0.955 | −0.340 | ||
| NO | Zr | −4.78 | 2.051 | −0.701 | −0.349 | |
| Hf | −5.32 | 2.146 | −1.038 | −0.358 | ||
| NO2 | Zr | −10.13 | 2.039 | −1.908 | −0.667 | |
| Hf | −10.14 | 2.039 | −2.585 | −1.018 | ||
| SO2 | Zr | −6.54 | 1.098 | −0.053 | −0.504 | |
| Hf | −11.55 | 2.038 | −1.923 | −0.805 | ||
Similarly, for the Hf site, CO, NO, NO2, and SO2 molecules adsorb on ZrHfN at minimum distances of 2.167 Å, 2.146 Å, 2.039 Å, and 2.026 Å, with corresponding adsorption energies of about −2.76 eV, −5.32 eV, −10.14 eV, and −11.52 eV, respectively. The adsorption energies on the Zr site follow the trend: CO < NO < SO2 < NO2, and on the Hf site, that is, CO < NO < NO2 < SO2. The adsorption energies indicate that all four gases interact with both ZrHfC and ZrHfN through chemisorption, with substantially larger values for NO2 and SO2 gas molecules. Previous study by Kharb et al. demonstrates that O-functionalized TiVC MXene exhibits exceptional gas adsorption capabilities, with energies of −0.88 eV (CO), −0.72 eV (NO), −1.06 eV (NO2), and −0.28 eV (SO2).61 The highest adsorption energy was found on Pt-decorated Hf2CO2, with values of −3.45 eV for CO as reported by Boonpalit et al.30 Hu et al. reported adsorption energies of 2.07 eV for CO, 3.05 eV for NO, and 2.53 eV for NO2 on Cr–C9N4, while for Fe2–C9N4, the Eads values were 2.60 eV for CO, 3.07 eV for NO, and 2.45 eV for NO2.62 Compared to these earlier findings, our nanosheets demonstrate stronger adsorption.
We also noticed that the electron transfer between the gases and the heterostructures is significantly larger on both sites of the nanosheets when considering Mulliken and Hirshfeld charge analysis. The Mulliken charge transfer from ZrHfC to gas molecules is significant, with approximately 0.915e for CO, 0.172e for NO, 1.88e for NO2, and 0.245e for SO2 at the Zr site during the interaction between the nanosheets and gas molecules. The charge transfers of 0.027e, 1.033e, 1.887e, and 1.945e at the Hf site from ZrHfC to CO, NO, NO2, and SO2 gas molecules, respectively. For ZrHfN, a notable amount of charge is transferred from the nanosheets to the toxic gases. At the Zr site, Mulliken charges of 1.00e, 0.701e, 1.908e, and 0.504e (Hirshfeld) are transferred to CO, NO, NO2, and SO2, respectively. Meanwhile, at the Hf site, the Mulliken charge transferred to CO, NO, NO2, and SO2 has values of 0.955e, 1.038e, 2.585e, and 1.93e, respectively. K. Ma et al. reported charge transfers of −0.98e (CO–Zr–stanene), −1.293e (NO–Nb–stanene), −0.63e (NO2–Zr–stanene), and −0.67e (SO2–Nb–stanene).63 Z. Wu et al. found charge transfers of 0.108e (CO), 0.306e (NO), 0.513e (NO2), and 0.589e (SO2) for adsorption on Al-doped Ti2CO2 nanosheets.64 Mushtaq et al. observed the highest charge transfers: −2.52e (NO on a hollow site) and −3.59e (NO2 on a Si-atom site), with negative values indicating electron donation from the CFMS (001) surface to NOx.65 Compared to previous studies, our proposed heterostructure demonstrates significantly higher charge transfer values.
3.5 Electronic properties
To better understand the performance of these MXene nanosheets, the electronic characteristics of all six nanosheets have been examined. We checked their band structures, density of states (DOS) spectra, and charge density difference (CDD) maps. The electronic band structures were computed along a high-symmetry path through the Brillouin zone: starting at Γ (0,0,0), then to F (0,0.5,0), next to K (−0.333,0.667,0), and back to Γ (0,0,0) (Fig. 6). In all cases, the results clearly indicate that several bands cross the Fermi level, confirming the metallic character of the system, which matches with previous findings.50 The absence of a band gap ensures a continuous distribution of electronic states at the Fermi level, which is favorable for fast electron transport. In addition, the presence of steeply dispersive bands near the Fermi level suggests low effective mass and high carrier mobility. The DOS plots represent the distribution of electronic states at different energy levels. To investigate how gas adsorption affects the electronic properties of the nanosheets, we analyzed both the total density of states (TDOS) and partial density of states (PDOS), as illustrated in Fig. 7. After adsorption of the CO and NO gases on the nanosheets, no significant DOS peak was found at the Fermi level, but minor changes were found near the Fermi level after the adsorption of NO2 and SO2 gas molecules. From the DOS spectra, it also confirmed that all the optimized complexes exhibited metallic behavior due to the non-zero DOS at the Fermi level (EF). From the plots, it is evident that there is significant overlap between the orbitals of the gas molecules and the MXene surface atoms, particularly near the Fermi level. This orbital hybridization indicates strong electronic interactions between the gas molecules and MXenes, confirming the chemisorption mechanism. The intensity and position of the peaks also suggest that Hf- and Zr-based MXenes interact more strongly with NO and NO2 compared to CO and SO2, consistent with the adsorption energy and charge transfer results. | ||
| Fig. 6 The band structures of the nanosheets (a) Hf2C, (b) Hf2N, (c) Zr2C, (d) Zr2N, (e) ZrHfC, and (f) ZrHfN. | ||
 | ||
| Fig. 7 The total and partial DOS for (a) Hf2C, (b) Hf2N, (c) Zr2C, (d) Zr2N, (e) ZrHfC, and (f) ZrHfN nanosheets before and after adsorption of CO, NO, NO2, and SO2 gas molecules. | ||
We also studied the charge density difference (CDD) maps to examine charge redistribution, bonding interactions, and electric charge transfer behavior. The CDD maps in Fig. 8 illustrate the charge transfer process, where cyan and yellow colors indicate regions of charge loss and gain, respectively. For clearer visualization, the iso-surface value was set to 0.03e Å−3 to effectively represent charge density distributions. The CDD map clearly shows electron redistribution throughout the system by highlighting these regions. From Fig. 8, the transfer of a significant amount of charges between the gas molecules (CO) and the nanosheets is clearly observed. From the Mulliken charge analysis, we observe electron transfers of 0.200e (for CO) to Hf2C, 0.109e to Hf2N, 0.913e to Zr2C, 0.152e to Zr2N, 0.915e to ZrHfC, and 1.000e to ZrHfN. Charge transfer took place between the gas molecules and the adjacent atoms on the nanosheet surface.
3.6 Recovery time
In nanosheets, the recovery time (τ) is the time needed for gas molecules to desorb from the surface. Higher performance and accurate repeated use require faster sensor renewal, which is indicated by a shorter recovery time. An increase in adsorption energy leads to the recovery time τ being boosted exponentially, and standard transition state theory may clarify this fact as:66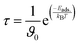 | (5) |
3.7 Sensitivity
It's crucial to examine a material's work function (φ) before using it as a sensing device. Because the work function of material surfaces influences the adsorption energy and charge transfer process, it is important for gas sensing. We studied how gas molecules change the work function of the nanosheets. To account for the interaction of dipoles, particular attention has been paid to the dipole slab correction in the work function calculation.55 In φ-based sensors, adsorbed gas molecules modify the work function, altering the material's electron emission. This work function shift changes the gate voltage, creating a detectable electrical signal for gas sensing.67,68 The variation in work function due to gas adsorption was computed by,47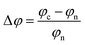 | (6) |
After the adsorption of CO, NO2, and SO2 on Hf2C, the φ values decreased, with the most significant negative change in φ observed for NO2 (about 3.11%). For Hf2N, the greatest positive change in φ was observed for SO2 (approximately 4.11%) due to a significant Δq of around 0.327e from the Hf2N to SO2. For Zr2C, φ experiences minor changes while adsorbing CO, NO, and NO2 gas molecules, but it significantly rises with SO2 adsorption (around 3.82%) because of high Δq values of about 1.959e from Zr2C to SO2. For Zr2N, the only positive variation in the work function is found for the adsorption of NO2 of about 4% because there is a large charge transfer of 0.329e to SO2, and for other gas adsorption, it is reduced minimally. For the heterostructures, φ varies significantly for the adsorption of SO2 at about 3.85% and 2.12% for ZrHfC and ZrHfN, respectively, due to the significant charge transfer of about 1.945 and 1.93, respectively.
4 Conclusions
Many harmful gases are currently present in our environment, which affect not only plants and animals but also human health and our daily lives. These harmful gases must be eliminated or filtered to ensure a healthy existence. MXenes have attracted a great deal of attention for their gas-sensing applications. The adsorption characteristics of Hf2C, Hf2N, Zr2C, Zr2N, ZrHfC, and ZrHfN nanosheets for CO, NO, NO2, and SO2 gas molecule adsorption have been examined in this study by employing the DFT approach. Our four MXenes and their Janus MXenes show stable thermodynamic stability, which was confirmed by the negative values of cohesive energies. All nanosheets show metallic behavior. To comprehend the adsorption behavior, the adsorption energy, charge transfer, work function, and electronic characteristics were investigated. The chemisorption of CO and NO on all nanosheets demonstrated better results, according to the adsorption energy, charge transfer, and electric characteristics. However, the adsorption behavior of NO2 and SO2 gas molecules was higher, but the recovery time was comparatively much larger for these two gases. The adsorption energies demonstrate strong CO and NO affinity across all six nanosheets, with values of −2.32 eV (CO) and −4.42 eV (NO) for Hf2C, −1.65 eV (CO) and −4.82 eV (NO) for Hf2N, −2.84 eV (CO) and −4.97 eV (NO) for Zr2C, −3.37 eV (CO) and −5.13 eV (NO) for Zr2N, −2.77 eV (CO) and −4.98 eV (NO) for ZrHfC at the Zr site, and −3.30 eV (CO) and −4.78 eV (NO) for ZrHfN at the Zr site. A significant amount of charge is shared between the nanosheets and the gas molecules, which are also visualized in the CDD maps. The DOS spectra demonstrate that no additional electronic states are created at the Fermi level for CO and NO adsorption on the nanosheets, but a minor contribution has been found near to the Fermi level in the case of NO2 and SO2. Moreover, there is a notable variation in the work functions of the nanosheets when gas molecules are adsorbed. Consequently, every computation suggests that every one of the six nanosheets could be helpful in tracking CO and NO toxic gas molecules.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included in the manuscript.Acknowledgements
We thankfully acknowledge the Bangladesh Research and Education Network (BdREN) for their computational access.References
- D. E. Schraufnagel, J. R. Balmes, C. T. Cowl, S. De Matteis, S.-H. Jung, K. Mortimer, R. Perez-Padilla, M. B. Rice, H. Riojas-Rodriguez and A. Sood, Chest, 2019, 155, 417–426 CrossRef PubMed.
- M. S. Mian, M. S. Rahman, J. Islam, K. N. Sakib, M. M. Tasnim and S. Yeasmin, J. Sci. Res., 2019, 11, 263–272 CrossRef CAS.
- L. D. Rosenthal, Am. J. Nurs., 2006, 106, 40–46 CrossRef.
- K. Ueda, Overcoming Environmental Risks to Achieve Sustainable Development Goals: Lessons from the Japanese Experience, Springer, 2021, pp. 39–46 Search PubMed.
- O. Badr and S. D. Probert, Appl. Energy, 1993, 46, 1–67 CrossRef CAS.
- L.-T. Le, K.-B. V. Quang, T.-V. Vo, T.-M. T. Nguyen, T.-V.-H. Dao and X.-T. Bui, Vietnam J. Sci. Technol. Eng., 2024, 66, 120–128 CrossRef.
- N. Baig, Composites, Part A, 2023, 165, 107362 CrossRef CAS.
- M. Kiani, M. U. Rehman, X. Tian and B. Yakobson, Adv. Mater. Technol., 2022, 7, 2101252 CrossRef CAS.
- X. Liu, T. Ma, N. Pinna and J. Zhang, Adv. Funct. Mater., 2017, 27, 1702168 CrossRef.
- A. VahidMohammadi, J. Rosen and Y. Gogotsi, Science, 2021, 372, eabf1581 CrossRef CAS.
- S. Alwarappan, N. Nesakumar, D. Sun, T. Y. Hu and C.-Z. Li, Biosens. Bioelectron., 2022, 205, 113943 CrossRef CAS PubMed.
- M. Ozkan, MRS Energy Sustain., 2024, 11, 181–190 CrossRef.
- M. Naguib, V. N. Mochalin, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2014, 26, 992–1005 CrossRef CAS PubMed.
- J.-F. Zhang, H.-Y. Cao and H.-B. Wang, J. Inorg. Mater., 2017, 32, 561–570 CrossRef.
- S. Gasso, R. Saini, R. Kaur, N. Joshi, S. Sharma and P. Devi, Mxene-Based Hybrid Nano-Architectures for Environmental Remediation and Sensor Applications, Elsevier, 2024, pp. 305–326 Search PubMed.
- M. Malaki, X. Jiang, H. Wang, R. Podila, H. Zhang, P. Samori and R. S. Varma, Chem. Eng. J., 2023, 463, 142351 CrossRef CAS.
- J. Zhu, E. Ha, G. Zhao, Y. Zhou, D. Huang, G. Yue, L. Hu, N. Sun, Y. Wang and L. Y. S. Lee, Coord. Chem. Rev., 2017, 352, 306–327 CrossRef CAS.
- N. García-Romeral, Á. Morales-García, F. Viñes, I. de, P. R. Moreira and F. Illas, Phys. Chem. Chem. Phys., 2023, 25, 31153–31164 RSC.
- S. Kumar, N. Kumari and Y. Seo, J. Energy Chem., 2024, 90, 253–293 CrossRef CAS.
- J. Bjork and J. Rosen, Chem. Mater., 2021, 33, 9108–9118 CrossRef.
- P. Srivastava, A. Mishra, H. Mizuseki, K.-R. Lee and A. K. Singh, ACS Appl. Mater. Interfaces, 2016, 8, 24256–24264 CrossRef CAS PubMed.
- M. Tang, J. Li, Y. Wang, W. Han, S. Xu, M. Lu, W. Zhang and H. Li, Symmetry, 2022, 14, 2232 CrossRef CAS.
- G. Li, S. Lian, J. Wang, G. Xie, N. Zhang and X. Xie, J. Mater., 2023, 9, 1160–1184 Search PubMed.
- J.-G. Liang and L. Pan, Materials Nanoarchitectonics, Elsevier, 2024, pp. 281–353 Search PubMed.
- Q. Zhou, L. Wang, W. Ju, Y. Yong, S. Wu, Y. Wang and H. Miao, Colloids Surf., A, 2023, 673, 131870 CrossRef CAS.
- R. K. Choudhury, B. R. Bhagat, K. H. Mali, R. Pokar and A. Dashora, Appl. Surf. Sci., 2022, 603, 154426 CrossRef.
- Z. Li, X. Cui, L. Jia, W. Zeng and Q. Zhou, Diamond Relat. Mater., 2023, 139, 110375 CrossRef CAS.
- R. P. Reji, S. K. C. Balaji, Y. Sivalingam, Y. Kawazoe and S. Velappa Jayaraman, ACS Appl. Nano Mater., 2023, 6, 5345–5356 CrossRef CAS.
- J. Su, X. Liu, H. Zhang, B. Zhao and T. Shen, Micro Nanostruct., 2023, 183, 207658 CrossRef CAS.
- K. Boonpalit, J. Kinchagawat, C. Prommin, S. Nutanong and S. Namuangruk, Phys. Chem. Chem. Phys., 2023, 25, 28657–28668 RSC.
- X. Li, H. Wang, H. Li, Y. Chen, Y. Ni and Y. Xia, Appl. Surf. Sci., 2023, 624, 157125 CrossRef CAS.
- H. Dou, B. Yang, X. Hu, C. Huo, X. Wang and C. Shi, Comput. Theor. Chem., 2021, 1195, 113089 CrossRef CAS.
- B. Paul, R. Babarao and S. Kanungo, Surf. Interfaces, 2025, 106573 CrossRef CAS.
- L. Ju, X. Tang, X. Li, B. Liu, X. Qiao, Z. Wang and H. Yin, Molecules, 2023, 28, 1644 CrossRef PubMed.
- B. Babariya, D. Raval, S. K. Gupta and P. N. Gajjar, Phys. Chem. Chem. Phys., 2022, 24, 15292–15304 RSC.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef.
- B. Delley, J. Chem. Phys., 1990, 92, 508–517 CrossRef.
- J. Wang, G. Wang and J. Zhao, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 35418 CrossRef.
- A. A. Piya, T. Ahmed, M. A. Khaleque, K. Ahmed and S. U. D. Shamim, Comput. Theor. Chem., 2022, 1217, 113902 CrossRef PubMed.
- V. A. Basiuk and L. V. Henao-Holguín, J. Comput. Theor. Nanosci., 2014, 11, 1609–1615 CrossRef.
- R. Pollet and H. Amara, J. Chem. Theory Comput., 2009, 5, 1719–1722 CrossRef PubMed.
- S. Grimme, J. Comput. Chem., 2004, 25, 1463–1473 Search PubMed.
- J. Hu, Q. Zhang, Q. Zhang and H. Cui, J. Mater. Res. Technol., 2022, 20, 763–771 CrossRef.
- T. Liu, Z. Cui, X. Li, H. Cui and Y. Liu, ACS Omega, 2020, 6, 988–995 CrossRef PubMed.
- H. Cui, X. Zhang, J. Zhang and Y. Zhang, High Voltage, 2019, 4, 242–258 CrossRef.
- S. U. D. Shamim, T. Akter, A. A. Ananna, B. K. Dash and A. A. Piya, RSC Adv., 2025, 15, 20712–20722 RSC.
- S. U. D. Shamim, D. Roy, S. Alam, A. A. Piya, M. S. Rahman, M. K. Hossain and F. Ahmed, Appl. Surf. Sci., 2022, 596, 153603 CrossRef.
- M. Amin, M. M. Rahman, M. K. Rokunuzzaman, M. K. Hossain and F. Ahmed, Comput. Theor. Chem., 2024, 1242, 114954 CrossRef.
- K. N. Munny, T. Ahmed, A. A. Piya and S. U. D. Shamim, Struct. Chem., 2023, 34, 2089–2105 CrossRef.
- T. Ahmed, A. A. Piya and S. U. D. Shamim, Nanoscale Adv, 2024, 6, 3441–3449 RSC.
- M.-Z. Liu, X.-H. Li, X.-H. Cui, H.-T. Yan, R.-Z. Zhang and H.-L. Cui, Appl. Surf. Sci., 2022, 605, 154830 CrossRef.
- I. R. Shein and A. L. Ivanovskii, Comput. Mater. Sci., 2012, 65, 104–114 CrossRef.
- M. H. Opi, T. Ahmed, M. R. Swarna, A. A. Piya and S. U. D. Shamim, Nanoscale Adv., 2024, 6, 5042–5054 RSC.
- Y. Yong, H. Cui, Q. Zhou, X. Su, Y. Kuang and X. Li, Appl. Surf. Sci., 2019, 487, 488–495 CrossRef.
- S. M. Aghaei, M. M. Monshi, I. Torres, S. M. J. Zeidi and I. Calizo, Appl. Surf. Sci., 2018, 427, 326–333 CrossRef.
- Q. Hu, W. Liu, D. Li, Q. Wu, Y. Chang, J. Wang, Q. Xia, L. Wang and A. Zhou, Mater. Today Commun., 2024, 40, 109467 CrossRef.
- E. Salih and A. I. Ayesh, Phys. E, 2021, 131, 114736 CrossRef.
- H. Y. Ammar, H. M. Badran and K. M. Eid, Mater. Today Commun., 2020, 25, 101681 CrossRef.
- Y. Wu, X. Chen, K. Weng, J. Arramel, W. Jiang, P. Ong, X. Zhang, N. Zhao and N. Li, Adv. Electron. Mater., 2021, 7, 2001244 CrossRef.
- J. Ren, W. Kong and J. Ni, Nanoscale Res. Lett., 2019, 14, 133 CrossRef.
- A. S. Kharb, K. Kumar, A. K. Chawla and A. K. Mishra, Phys. Chem. Chem. Phys., 2025, 27, 9041–9055 RSC.
- Y. Hu, L. Liu, S. Zhang and X. Chen, Colloids Surf., A, 2024, 689, 133716 CrossRef.
- K. Ma, J. Chen, X. Dai, J. Xiao, L. Wang, L. Xu and Z. Wang, Results Phys., 2021, 28, 104617 CrossRef.
- Z. Wu, J. Zhou, D. Li, Z. Ao, T. An and G. Wang, Sustain. Mater. Technol., 2021, 29, e00294 Search PubMed.
- M. Mushtaq, N. Algethami, M. Abdul Rauf Khan, A. I. Ayesh, M. Mateen, A. Laref, S. A. M. Abdelmohsen and M. K. Hossain, ACS Omega, 2023, 8, 14005–14012 CrossRef.
- N. L. Hadipour, A. Ahmadi Peyghan and H. Soleymanabadi, J. Phys. Chem. C, 2015, 119, 6398–6404 CrossRef.
- A. A. Kistanov, Y. Cai, K. Zhou, S. V. Dmitriev and Y.-W. Zhang, 2D Mater., 2016, 4, 15010 CrossRef.
- R. Pohle, A. Tawil, P. Davydovskaya and M. Fleischer, Proc. Eng., 2011, 25, 108–111 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |



