 Open Access Article
Open Access ArticleAdvancing ovarian cancer care: recent innovations and challenges in the use of MXenes and their composites for diagnostic and therapeutic applications
Neda
Farzizadeh
a,
Atefeh
Zarepour
bc,
Arezoo
Khosravi
de,
Siavash
Iravani
 *f and
Ali
Zarrabi
*f and
Ali
Zarrabi
 *g
*g
aDepartment of Midwifery, School of Nursing and Midwifery, Ardabil University of Medical Sciences, Ardabil, Iran
bDepartment of Biology, Faculty of Arts and Sciences, Kocaeli University, İzmit, Kocaeli 41001, Türkiye
cDepartment of Research Analytics, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai 600 077, India
dDepartment of Genetics and Bioengineering, Faculty of Engineering and Natural Sciences, Istanbul Okan University, Istanbul 34959, Türkiye
eGraduate School of Biotechnology and Bioengineering, Yuan Ze University, Taoyuan 320315, Taiwan
fIndependent Researcher, W Nazar ST, Boostan Ave, Isfahan, Iran. E-mail: siavashira@gmail.com
gDepartment of Biomedical Engineering, Faculty of Engineering and Natural Sciences, Istinye University, Istanbul 34396, Türkiye. E-mail: alizarrabi@gmail.com
First published on 12th July 2025
Abstract
Ovarian cancer remains the deadliest form of gynecologic malignancy, largely owing to the absence of reliable early diagnostic tools and the limited effectiveness of current therapeutic strategies. Recent advances in nanotechnology—particularly the emergence of two-dimensional materials known as MXenes—offer promising avenues to address these challenges. This review highlights the emerging role of MXenes and their composites in the management of ovarian cancer, focusing on their potential in biomarker detection and targeted treatment strategies. We provide a comprehensive analysis of the latest studies examining the physicochemical features of MXenes, their synthesis and surface functionalization approaches, and their application in ovarian cancer, including biosensing, drug delivery, and combinatorial therapeutic systems. MXene-based biosensors have shown remarkable detection limits in detecting ovarian cancer biomarkers, such as cancer antigen 125 (CA125), human epididymis protein 4 (HE4), lipolysis-stimulated lipoprotein receptor (LSR), and carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5). However, several challenges remain, including issues of biocompatibility, structural stability, and clinical scalability. Continued interdisciplinary research is essential to address these limitations, optimize MXene functionalization, and translate their laboratory success into clinical settings. With appropriate advancements, MXenes hold significant promise for enabling more precise, efficient, and patient-specific approaches to ovarian cancer diagnosis and therapy.
1. Introduction
Among the three major gynecologic malignancies, ovarian cancer is the most lethal, despite being the third most commonly diagnosed.1 According to the World Health Organization (WHO), over 324![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 new cases and more than 206
000 new cases and more than 206![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths were reported globally in 2022,2 with mortality projected to exceed 313
000 deaths were reported globally in 2022,2 with mortality projected to exceed 313![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 by 2040.3,4 The main causes of ovarian cancer's elevated mortality rate are its gradual progression, delayed onset of symptoms, and ineffective screening techniques, which frequently lead to an advanced stage diagnosis.3 Owing to its position within the abdominal cavity, the initial manifestations of ovarian cancer are subtle, resulting in a diagnosis at an advanced stage in roughly 75% of patients that were characterized by extensive metastases, rendering simple surgical intervention insufficient for tumor eradication.5 The WHO has categorized ovarian cancer into distinct types: epithelial tumors, mesenchymal tumors, mixed epithelial and mesenchymal tumors, sex-cord stromal tumors, germ cell tumors, monodermal teratoma and somatic-type tumors originating in dermoid cysts, various tumors, mesothelial neoplasms, tissue neoplasms, tumor-like lesions, lymphoid/myeloid neoplasms, and metastatic tumors.6
000 by 2040.3,4 The main causes of ovarian cancer's elevated mortality rate are its gradual progression, delayed onset of symptoms, and ineffective screening techniques, which frequently lead to an advanced stage diagnosis.3 Owing to its position within the abdominal cavity, the initial manifestations of ovarian cancer are subtle, resulting in a diagnosis at an advanced stage in roughly 75% of patients that were characterized by extensive metastases, rendering simple surgical intervention insufficient for tumor eradication.5 The WHO has categorized ovarian cancer into distinct types: epithelial tumors, mesenchymal tumors, mixed epithelial and mesenchymal tumors, sex-cord stromal tumors, germ cell tumors, monodermal teratoma and somatic-type tumors originating in dermoid cysts, various tumors, mesothelial neoplasms, tissue neoplasms, tumor-like lesions, lymphoid/myeloid neoplasms, and metastatic tumors.6
In patients exhibiting indicative symptoms, the diagnostic evaluation comprises a physical examination and radiographic imaging, including transvaginal ultrasonography (TVUS). A common screening strategy for the early detection of ovarian cancer is not yet available for asymptomatic women. At present, the Cancer Antigen 125 (CA125) blood test and TVUS are the most promising screening modalities for ovarian cancer detection.7 More than 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 women globally undergo exploratory surgery for suspicious masses, resulting in nearly 300
000 women globally undergo exploratory surgery for suspicious masses, resulting in nearly 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 new diagnoses of ovarian cancer; this highlights the critical need for improved non-invasive diagnostic tools to reduce unnecessary surgical interventions, mitigate patient risk, and alleviate healthcare burdens, particularly given that the majority of these procedures reveal benign conditions.8,9 Ultrasound-based models can differentiate ovarian cancer from benign mimickers; however, preoperative diagnosis remains incomplete. Moreover, ovarian cancer manifests 5.1 years prior to the onset of clinical symptoms but may progress rapidly from early to late stages in less than a year. This creates a narrow but crucial window for early detection, which remains difficult to capture with current techniques.9
000 new diagnoses of ovarian cancer; this highlights the critical need for improved non-invasive diagnostic tools to reduce unnecessary surgical interventions, mitigate patient risk, and alleviate healthcare burdens, particularly given that the majority of these procedures reveal benign conditions.8,9 Ultrasound-based models can differentiate ovarian cancer from benign mimickers; however, preoperative diagnosis remains incomplete. Moreover, ovarian cancer manifests 5.1 years prior to the onset of clinical symptoms but may progress rapidly from early to late stages in less than a year. This creates a narrow but crucial window for early detection, which remains difficult to capture with current techniques.9
Despite these promising developments, ovarian cancer treatment still faces significant hurdles, including chemoresistance and metastasis, which are the leading causes of mortality. Current clinical strategies integrate debulking surgery, maximal cytoreductive surgery, platinum- and taxane-based chemotherapy, and targeted therapies like PARP inhibitors and antibody–drug conjugates, yet resistance often limits long-term success.6 Debulking surgery removes as much of the tumor as possible, sometimes including the uterus and both ovaries, especially in advanced stages.10 Maximal cytoreductive surgery removes all remaining disease, which greatly improves survival rates in patients in stages 3 and 4, and is frequently carried out after initial chemotherapy.11 Chemotherapy mainly uses platinum-based agents like carboplatin or cisplatin in combination with taxanes like paclitaxel, which are administered intraperitoneally or intravenously to target cancer cells. Neoadjuvant chemotherapy, given before surgery, can also help shrink tumors and improve surgical outcomes.12
The current methods for diagnosis and treatment lack sufficient sensitivity and efficiency to detect ovarian cancer in its early stages. Furthermore, the elevated expenses and unspecified detection points resulted in delayed diagnosis.13 Also, systemic therapies like chemotherapy and radiation therapy are associated with numerous adverse effects, including harm to healthy tissue, alopecia, nausea, and gastrointestinal complications. Researchers have increasingly turned to nanotechnology to develop more selective and efficient drug delivery systems.14 By working at the nanoscale, it becomes possible to design materials with properties that significantly differ from their bulk counterparts, offering new opportunities for precise diagnosis and therapy.15 Emerging technologies such as nanocarriers (nano-gels, aptamers, peptide-mediated formulations) and novel drug conjugates aim to enhance drug delivery specificity, reduce side effects, and improve pharmacokinetics by enabling sustained release near tumor sites. Clinical trials are actively investigating new agents targeting molecular subtypes of ovarian cancer, including CDK2 inhibitors and immunotherapy combinations, reflecting a shift towards personalized medicine. Parallel to these material innovations, advances in nanoparticle (NP)-packed hydrogels targeting female-specific biology offer new avenues for treating platinum-resistant ovarian cancer with fewer systemic toxicities, such as ocular side effects seen in current antibody–drug conjugates.16
Recent advancements in ovarian cancer care have focused on innovative targeted therapies and nanotechnology-based drug delivery systems to improve treatment efficacy and reduce side effects. Nanoparticles (NPs)—such as gold (Au), silver (Ag), carbon nanotubes, graphene oxide, quantum dots (QDs), and MXenes—have emerged as promising candidates in the fight against cancer due to their unique optical, electrical, and structural characteristics.17–19 MXenes are a class of two-dimensional (2D) materials characterized by M representing an early transition metal, X denoting carbon or nitrogen, and -ene analogous to graphene, the first identified 2D substance.14 MXenes possess an extensive surface area, high conductivity, outstanding photothermal conversion efficiency (PTCE), and strong near-infrared (NIR) absorption15 that appropriate them for variety of biomedical applications, encompassing tumor identification as contrast agents, drug administration, biomedicine, cancer therapy, and diagnostics.14,20–25 These 2D structures enable synergistic photothermal and chemotherapeutic approaches, targeted drug delivery, and enhanced bioimaging and biosensing capabilities. Researchers are exploring MXene-based systems for noninvasive, efficient cancer therapy, including photothermal therapy and controlled drug release. However, challenges remain regarding their stability in physiological environments, biodegradability, and the need for sustained and controlled drug delivery to maximize therapeutic outcomes.26–29 Future perspectives emphasize overcoming biological barriers, improving MXene stability and biodegradability, and integrating multi-modal therapies to enhance efficacy and patient quality of life.
The aim of this review is to explore recent advancements in the use of MXenes for the diagnosis and treatment of ovarian cancer. We highlight progress in synthesis and functionalization strategies aimed at improving their biocompatibility and therapeutic targeting, with a focus on the development of MXene-based biosensors and drug delivery platforms.
2. Overview of MXenes
2.1. Definition and structure of MXenes
MXenes are a family of 2D transition metal carbides, nitrides, or carbonitrides, first discovered by Naguib et al. in 2011 through the selective etching of MAX phases, layered ternary carbides or nitrides. The general formula for MAX phases is Mn+1AXn (n = 1, 2, or 3), where M is an early transition metal, A is a group 13 or 14 element (typically aluminum), and X is carbon and/or nitrogen. The resulting MXene, typically denoted as Mn+1XnTx, retains a layered structure after removal of the A layer, where Tx represents surface terminations such as –OH, –O, or –F introduced during synthesis.30–32Structurally, MXenes feature strong M–X bonds (a mix of ionic and covalent interactions) and exhibit metallic conductivity due to their layered architecture.33 The etching process commonly involves hydrofluoric acid (HF) or a combination of fluoride salts and hydrochloric acid (HCl) to selectively remove the A layer from MAX phases (e.g., Ti3AlC2), resulting in the formation of Ti3C2Tx, the most extensively studied MXene.34
To date, more than 50 MXene compositions have been experimentally synthesized by varying M and X elements, offering a broad platform for tailoring their chemical, electrical, and physical properties. Unlike other 2D materials such as graphene or transition metal dichalcogenides (TMDs), MAX phases exhibit strong interlayer bonding and cannot be exfoliated mechanically. Instead, MXenes are typically obtained via chemical exfoliation, producing nanosheets with a high degree of functional tunability.30,35 All four of MXene's dimensions, 0D, 1D, 2D, and 3D, have been used in recent studies to examine its properties.36 They exhibit several desirable properties for biomedical and technological applications, including hydrophilicity, paramagnetism, a large surface area, and a high atomic number due to the transition metal content. They also show exceptional electrical conductivity, corrosion resistance, and mechanical strength. The 2D morphology of MXenes offers a superior surface-to-volume ratio, enabling efficient interaction with biomolecules, critical for sensing, drug delivery, and biosensor platforms.13,37,38 Therefore, the unique structural features and customizable surface chemistry of MXenes distinguish them from conventional 2D materials, making them highly attractive for diverse applications, including those in the biomedical field.
2.2. Synthesis and functionalization of MXenes
Two main strategies have been established for the synthesis of MXene compounds: top-down method and bottom-up method.39 Each of these techniques has shown success in producing single-layer, few-layer, and multilayer MXene structures, so offering different advantages for fine-tuning material properties to fit intended uses.35,40Among these, HF etching is the most widely used due to its ability to effectively remove the A element and yield thin MXene flakes with functional surface groups such as hydrogen oxide, fluorine, and oxygen.43 These intrinsic groups act as anchoring sites for additional functionalization, like covalent grafting, through diazonium salt reactions introduces organic groups (such as amines) to improve dispersibility,44 or polymer wrapping, with poly(ethylene glycol) (PEG) that improves biocompatibility.45,46 However, the use of HF raises safety concerns because of its corrosive and toxic nature, and the associated organic delaminating agents may introduce additional biocompatibility risks.47 Although the human body contains trace fluoride ions, exposure to elevated levels from these procedures has been linked to oxidative stress, cellular damage, and organ toxicity.48–50
Ying et al. studied the removal of hexavalent chromium [Cr(VI)] using Ti3C2 nanosheets produced via HF etching and ultrasonic delamination. Their findings showed that the delaminated MXene could adsorb and reduce Cr(VI), achieving a final concentration well below WHO standards.51 Mashtalir et al. examined exfoliation kinetics of Ti3AlC2 and found temperature, time, and particle size significantly influenced sheet formation and resistivity profiles. Electron microscopy confirmed structural exfoliation, while elemental analysis showed the presence of titanium, carbon, oxygen, and fluorine in the resulting materials.52
To improve safety and flake quality, researchers have turned to in situ HF generation by combining fluoride salts like lithium fluoride (LiF) with HCl, reducing the handling of HF directly.53–55 This technique produces MXenes with similar surface terminations, but the presence of interlayer water molecules in the resulting materials can prolong drying time and reduce stability. Furthermore, surface terminations influence interlayer spacing; for example, hydrophobic fluorine groups reduce spacing and water retention.55
Another etching technique known as alkaline treatment etches the A layer using strong bases such as sodium hydroxide (NaOH) or potassium hydroxide (KOH). Though slower and less effective than HF techniques, it is less toxic and environmentally benign.56 In this method, an HF-free approach for synthesis MXenes with fluorine-free terminations has evolved as etching MAX phases using concentrated alkali solutions. Reacting MAX phases such as Ti3AlC2 with concentrated NaOH at elevated temperatures (270 °C) removes aluminum atoms and produces hydrophilic hydrogen oxide surface groups.57 Improved hydrophilicity allows this method to achieve 214% higher mass capacitance than HF-etched MXenes.57 But handling concentrated alkaline solutions and high-temperature reactions creates safety issues that complicate scaling for industrial manufacture.57,58 Usually displaying multilamellar “accordion-like” structures, the resulting MXenes,57,59 need further processing steps including chemical interaction with agents such as tetrabutylammonium hydroxide (TBAOH) and dimethyls sulfoxide (DMSO) to increase interlayer spacing and mechanical delamination through sonication to isolate single-layer nanosheets.59 These post-processing needs complicate matters more than other synthesis methods like molten-salt etching.60
Molten salt etching is another emerging approach, leveraging low-melting-point salts such as chlorides and carbonates to promote uniform diffusion and dissolution of reactants. This technique allows for lower reaction temperatures and high product purity.61,62 This method might lessen the requirement for pre-treatment with ball milling. Metal chlorides, carbonates, nitrates, and fluorides are among the frequently utilized molten salts.63 Using mild solutions like sodium chloride (NaCl) or HCl, electrochemical etching uses a three-electrode system including a MAX phase material as the working electrode. Although this approach avoids dangerous chemicals and is safer, it takes more processing time.64,65 Salt etchants involve the use of molten salts, for example potassium fluoride (KF) or LiF at high temperatures to etch the A layer. It is effective for nitride-based MXenes but results in smaller flake sizes and lower crystallinity.66,67
Although this strategy reduces environmental hazards and facilitates recycling, challenges such as carbide-derived carbon (CDC) formation, intercalator toxicity, and poor scalability still hinder industrial application.68 Optimization of parameters like voltage, time, and electrolyte concentration is ongoing. Thermo-assisted etching may offer better control, but reproducibility remains an issue. Guan et al. introduced a microwave-assisted molten salt method to synthesize Ti3AlC2 powders. This process, using a mixture of titanium hybride (TiH2), aluminum, titanium carbide (TiC), and NaCl/KCl, achieved up to 98.5% purity at lower temperatures with shorter reaction times, demonstrating the synergy between microwave heating and molten salts.61
Using gaseous precursors, CVD is a high-precision technique that creates MXenes by depositing layers on substrates such as silicon or copper.70,71 Although it has a limited yield and is expensive, it produces high-quality, crystalline MXene films.42 Using transition metal oxide (TMO) nanosheets as templates, the template method transforms them into MXenes via ammoniation or carbonization.42 Although it takes longer to process, this method is more economical and produces higher yields than CVD.72 Wang et al. established a direct synthesis methodology for the large-scale and cost-efficient manufacturing of MXenes by interaction between metal halides/metals with methane, nitrogen, or graphite. This method enables the CVD fabrication of MXenes and spherulite structures.73
Chuan Xu et al. fabricated ultrathin molybdenum carbide (Mo2C) crystals using CVD, enabling low-temperature synthesis of superconducting 2D materials.74 In contrast, Zhang et al. used PEPLD to produce uniform face-centered cubic (FCC)-Mo2C films with tunable thickness by adjusting laser pulse frequencies and methane plasma intensity.75 While PEPLD allows fine control over film morphology, the crystallinity and throughput are typically lower than CVD.
Table 1 provides a comparative summary of the major synthesis techniques under the three main categories including top-down, bottom-up, and green synthesis.
| Synthesis type | Method | Key reagents/conditions | Advantages | Limitations |
|---|---|---|---|---|
| Top-down | HF etching | HF | High yield; functional surfaces (hydrogen oxide, fluorine, and oxygen) | Highly toxic; environmental and health concerns |
| LiF–HCl in situ HF | LiF + HCl | Safer than direct HF; similar surface terminations | Interlayer water retention; longer drying times | |
| Alkaline treatment | NaOH or KOH at high temperature | Fluorine-free surfaces; eco-friendlier | Requires high temp; lower yield; multiple post-processing steps | |
| Molten salt etching | Metal chlorides/carbonates | High purity; low-temp synthesis | Limited scalability; low flake size | |
| Electrochemical etching | Salt solution + 3-electrode system | Safer; HF-free | Longer processing time; scalability challenges | |
| Bottom-up | CVD | Gaseous precursors on substrates | High-quality, crystalline MXenes | Expensive; low yield |
| Template method | TMO nanosheets + ammoniation | Higher yield than CVD; cost-effective | Complex setup; limited flake control | |
| PEPLD | Plasma & pulsed laser deposition | Tunable film thickness | Low throughput; lower crystallinity | |
| Green | Green etchants (e.g., NaBF4, ZnCl2) | Eco-friendly salts | Reduced toxicity; improved biocompatibility | Limited industrial maturity; integration challenges |
| Electrochemical & thermal etching | Voltage + heat + benign electrolytes | No HF; safer disposal | Low reaction rate; reproducibility issues | |
• Functionalization via termination engineering
Surface terminations on MXene have a major impact on the material's stability, electronic structure, and other chemical and physical characteristics. Obtaining MXene materials with distinct structural features and properties effectively requires the controllable regulation of MXene surface terminations.90 The physical characteristics of MXenes, such as the types and concentrations of electrochemical active sites and electronic structures, can be altered by altering their surface by regulating the T groups.91 Oxygen and fluorine surface terminations are readily accomplished using standard etching methods like HF or fluoride-containing salts which are mentioned before. By varying the etching time and etchant concentration, as well as by employing different post-treatments like hydrazine and annealing in different atmospheres, the concentrations and kinds of surface terminations can be tuned.52,92 For example, Halim et al. reported that NaOH treatment enabled low-temperature vacuum annealing (550 °C) to produce predominantly oxygen-terminated Ti3C2 MXene by facilitating fluorine removal, altering lattice parameters and titanium oxidation states.93 Also, density functional theory calculations revealed that surface terminations critically influence the Fermi level density of states, with reduced fluorine concentrations during annealing (300 to 775 °C) significantly enhancing conductivity.94 Furthermore, hydrogen annealing transformed surface groups (e.g., carbon–titanium–hydroxyl [C–Ti–OH] to oxygen–titanium–oxygen/carbon–titanium–oxygen [O–Ti–O and C–Ti–O]) and generated titanium–carbon (Ti–C) vacancies, which drastically increased saturation magnetization in otherwise nonmagnetic Ti3C2.95
• Functionalization via small molecules
The outer surface of MXenes has a range of surface terminations that can serve as molecular anchoring points through covalent/noncovalent functionalization.96 Small molecule functionalization significantly enhances MXene properties and applications; for instance, introducing the non-ionic surfactant hexaethylene glycol monododecyl ether (C12E6) onto Ti3C2 improved molecular interactions via hydrogen bonding between MXene surface groups and C12E6's hydrogen oxide, leading to enhanced packing symmetry.97 Solvothermal treatment of niobium carbide (Nb2C) with ethanol increased surface functional groups and interlayer spacing, which promoted multiple reflections and provided polarization sites, resulting in superior electromagnetic wave (EMW) absorption performance.98 Diazonium-based surface chemistry enabled large-scale MXene delamination by weakening M-X bonds and expanding interlayer spacing through aryl-surface linkages.44
• Functionalization via polymerization
Surface groups of MXenes enable polymer integration via ex situ blending—offering defined structures and tunable compositions facilitated by hydrogen bonding/electrostatic interactions—or in situ polymerization.99,100 Specifically, Sun et al. produced highly conductive nanocomposite materials composed of MXenes embedded within a polystyrene matrix (MXene@polystyrene nanocomposites) via electrostatic assembly, observing that electrostatic forces dominated assembly at lower MXene contents while van der Waals forces and hydrogen bonding prevailed at higher contents.101 Ling et al. fabricated Ti3C2/polyvinyl alcohol (Ti3C2/PVA) composites using hydrogen bonding and Ti3C2/polydiallyldimethylammonium (Ti3C2/PDDA) composites via electrostatic interactions, demonstrating intercalated and orderly stacked structures respectively.102 For in situ approaches, Boota et al. polymerized pyrrole between Ti3C2 layers, forming a periodic pattern with hydrogen bonds between MXene surface groups and the polymer,103 while Lin et al. functionalized Ti3C2 with soybean phospholipid to improve physiological stability and tumor targeting.104 Furthermore, amino-silane modification of Ti3C2, inspired by Shamsabadi et al.'s work on titanium dioxide (TiO2), creates covalent bonds and free amine groups enabling diverse applications.105 Research demonstrated self-initiated photo-grafting and photopolymerization (SIPGP) can be readily performed at room temperature under UV radiation and has been successfully applied to diverse substrates including graphene, carbon nanotubes, silicon-based materials, and diamond.106–108 Furthermore, it is indicated PEGylation via electrostatic adsorption is an economical and effective strategy to improve MXene's water dispersibility.109 Additionally, a covalent modification strategy was developed employing (3-aminopropyl) triethoxysilane (APTES) as a bridge to conjugate PEG onto MXene; this approach provided dual benefits: APTES bonding to MXene hydroxyl groups enables subsequent binding to amino groups, while the APTES amino group itself reacts with PEG to form PEGylated MXene for biomedical applications.45
2.3. Types of MXenes
Many MXene variants including those derived from titanium (Ti–MXenes),46,110 vanadium (V–MXenes),111,112 molybdenum (Mo–MXenes),113,114 tantalum (Ta–MXene),115 and niobium (Nb–MXene)116,117 have been investigated over the past few years; each of them shows unique properties and possible uses in different fields of science.118 The mostly studies MXene is Ti3C2 because of its electrical conductivity, chemical stability, and mechanical strength.119Ti3C2 is typically created by selectively etching the A element from MAX phases like Ti3AlC2. This leads to delaminated layers with adjustable surface chemistry and flexible electronic characteristics.120 Ti3C2 has shown great promise in the field of biomedical sciences for antibacterial applications,121 photothermal treatment (PTT),122,123 and biosensor development.124,125 Its ultrathin nanosheets exhibit a natural PTCE of 48.6%, enabling effective photothermal hyperthermia for the treatment of cancer.126
Vanadium-based MXenes are a different subclass of MXenes where vanadium serves as the transition metal. These nanomaterials, which belong to the larger family of 2D transition metal carbides, adhere to the general structural formula Vn+1XnTx, where the functional properties depend much on the surface terminations (Tx). They are generated by selectively etching MAX phases, such vanadium aluminum carbide (V2AlC), so allowing their use in several advanced uses.118 Algae-based extraction has shown success in intercalating and delaminating V2AlC, producing high volume vanadium carbide (V2C) nanosheets best fit for PTT. These V2C nanosheets show remarkable NIR absorption and great structural integrity by achieving PTCE higher than that of other MXenes. Particularly studies using PTT have shown promising results in vitro and in vivo as well as their effectiveness in eradicating cancer cells.127,128
Molybdenum-based MXenes, represented by the general formula Mn+1AXn, include molybdenum titanium carbide MXene (Mo2TiC2), which is derived from the MAX phase molybdenum titanium carbide molybdenum titanium aluminum carbide (Mo2TiAlC2).118 Recently, these molybdenum-based MXenes, Mo2CTx in particular, have shown great promise in PTT.129,130 Apart from their great absorption in the near-infrared spectrum, molybdenum-based nanomaterials (Mo-NMs) have interesting properties including high light absorption, biodegradability, low cytotoxicity, biocompatibility, and a large surface area.129,131 These features make molybdenum-based nanomaterials strong contenders for cancer theranoscopic applications.130,132 Moreover, advanced molybdenum-based nanocomposites were synthesized to improve the functionality of Mo-NMs, facilitating synergistic combination therapies.131,132
Tantalum-based MXenes display unique features resulted from their adjustable electronic and mechanical features, making them highly promising for biomedical applications like drug delivery and imaging.133,134 Furthermore, because of their high PTCE, ultrathin 2D Ti3C2 MXenes show great potential in tumor ablation, so supporting efficient cancer treatment.14
First presented in 2013 by selectively etching MAX phases, niobium-based MXene, especially Nb2C, led to the synthesis of layered materials with various surface functional groups.118,135 In the NIR-I and NIR-II bio-windows, respectively, these niobium-based MXenes show amazing PTCEs of 36.4% and 45.65% respectively together with proving photothermal stability and biocompatibility.23
In drug delivery systems, Nb2C nanocomposites have demonstrated high loading capacity (32.57%) and efficient cancer cell inhibition. For example, they achieved a 92.37% reduction in U87 glioblastoma cells through photothermal hyperthermia, underscoring their therapeutic potential.136
3. Role of MXenes in ovarian cancer diagnosis and treatment
3.1. MXenes in CA125 detection
CA125 is a type of mucin-like glycoprotein with molecular weight of about 200 kDa, regarded as the most reliable or in other words the gold standard biomarker for ovarian cancer detection.137 It was first identified in 1981 as an antigen of ovarian carcinoma epithelial cells138 and is observable in the blood of ovarian cancer patients. CA125 is present in more than 90% of patients with advanced epithelial ovarian cancer (stages II, III, and IV) and in 50% of individuals with early-stage cancer.139,140 Thus, the identification of CA125 is crucial for the early diagnosis of ovarian cancer.141As a diagnostic biomarker, a blood CA125 level beyond 35 U mL−1 is generally correlated with a heightened risk of ovarian cancer, while in healthy person, this level is below 35 U mL−1.137 Various techniques have been developed for the detection of CA125, including radioimmunoassays, colorimetric assays, electrochemical analysis, surface plasmon resonance, quartz crystal microbalance, chemiluminescence, and enzyme-linked immunosorbent assays (ELISA).137,142 Due to the disadvantages in conventional approaches relative to contemporary alternatives, like false positive results, diminished sensitivity, and inability in trace detection in ELISAs143 or costly apparatus in chemiluminescence,144 novel approaches like biosensors have emerged as the superior alternative to conventional detection methods in clinical and point-of-care diagnostics in recent decades.145–147
Biosensors offer superior selectivity and sensitivity, enhanced mobility, and reduced sample preparation compared to conventional diagnostic methods.148 Nanomaterials enhance the sensitivity, specialization, and rapid responsiveness of biosensors for distinct tasks.149–151 Numerous nanomaterial and biomaterial biosensors employing various sensing modalities have been developed to detect and monitor cancer biomarkers in recent years.152
MXenes has changed to effective options in biomarker detection because of their high surface area and superior electrical conductivity, and also adjustable characteristics.153,154 Due to these features, MXene-based electrochemical biosensors can detect cancer biomarkers with high sensitivity and selectivity, reaching detection limits as low as femtomolar concentrations.154,155 The studies investigating MXene-based structures in CA125 detection in ovarian cancer are discussed in the following.
An electrochemical immunosensor was designed to detect the ovarian cancer using an immunosensor composed of a glassy carbon electrode (GCE) functionalized with MXene, graphene QD (GQD), and Au NPs in real samples.137 Adding Au NPs improved the absorption and increased the effective surface area performance of GCE (Fig. 1A). When the GCE was modified by MXene, GQD, and AuNPs, the currents of cyclic voltammetry (CV) peaks were increased and the electron transfer resistance was reduced in comparison to GCE alone (Fig. 1B). The bare electrode had a charge transfer resistance (Rct) of 5665.97 Ω, while the modified electrode depicted Rct = 322.15 Ω due to its increased conductivity. This multilayer MXene provided a wide specific surface area for a lot of Au NP attachments. The concentration of MXene-GQDs nanocomposites on the surface of GCE was optimized by various volumes of MXene-GQD suspension which 4 μL was the optimal volume. Ten calibration plots of the improved system were made in the range of −0.2 to 0.6 V and at various concentrations of CA125 antigen. High sensitivity for CA125 detection was provided by increasing the concentration of CA125, which decreased square wave voltammetry (SWV) voltammograms. For the developed immunosensor, the limit of detection (LOD) of the CA125 antigen was 0.075 nU mL−1 and the results demonstrated a suitable linear connection between the SWV response and the quantities of CA125 (linear range 0.1–1 nU mL−1) (Fig. 1C). Analytical techniques confirmed excellent performance, stability over four days, and reproducibility (relative standard deviation [RSD] = 2.04%). The biosensor exhibited minimal interference from other biomarkers (e.g., prostate-specific antigen [PSA], cancer antigen 153 [CA153]). The immunosensor was highly stable with no obvious signal decay over several days, only showing 2% reduction in the electrochemical signals after 4 days at 8 °C and about 15% after storage for 120 hours at 4 °C. Importantly, detection of CA125 in both diluted healthy serum and serum samples from ovarian cancer patients, with recovery rates ranging from 98.1 to 105%, indicated that the sensor is applicable for complex biological matrices with a certain degree of biosafety for short-term diagnostic applications. However, further evaluation of long-term toxicity and immune response is needed.137
 | ||
| Fig. 1 (A) Schematic image of fabrication process of MXene-GQD/AuNPs immunosensor. (B) Results of sensing kinetic of fabricated sensor in the presence of different scan rates (10–500 mV s−1). (C) Detection of different concentrations of CA125 in real sample. Reprinted with permission from ref. 137. Copyright 2024, The Author(s). | ||
The strong interlayer van der Waals forces in 2D MXenes lead to significant stacking and aggregation, which drastically lowers their specific surface area and restricts their practical performance.159,160 A viable approach is incorporating the conductive materials between the layers of MXene that acted as spacers161 among them is one-dimensional carbon nanotubes (CNTs) that have high electrical conductivity and excellent chemical stability.162,163 Unfortunately, random physical mixing of MXene with CNTs does not effectively resolve the stacking issue, as both MXene and CNTs carry a negative charge. In contrast, amino-functionalized CNTs (NH2–CNT), with positively charged, could address the stacking problem and enhance the electrochemical sensing performance of MXene.164 Chitosan (CS), known for its excellent film-forming feature, could be used as a functionalizing agent in the structure of biosensors.165
A disposable ultrasensitive immunosensor was constructed based on Ti3C2TxMXene/NH2–CNT modified screen-printed carbon electrode (SPCE) for the detection of the CA125 in ovarian cancer (Fig. 2A).166 The redox peak current of Ti3C2Tx/NH2–CNT modified SPCE was significantly increased compared to bare SPCE due to its superior electronic conductivity and large active area. However, the immobilization of antibody 1 (Ab1) decreased the redox peak current due to the non-conductive biomacromolecule. After blocking non-specific active sites with bovine serum albumin (BSA), the CA125 antigen and antibody 2 (Ab2) were modified on the electrode, decreasing the redox peak current stepwise. This successful construction of a CA125 electrochemical immunosensor was confirmed by comparing the CV plots of the modified and control groups. The current signal showed significant change after adding CA125, demonstrating the immunosensor's specificity. The study demonstrated the successful construction of a CA125 electrochemical immunosensor by modifying the electrode with Ti3C2Tx/NH2–CNT, which significantly reduced the Rct of the bare SPCE (Fig. 2B and C). However, the Rct was then raised when Ab1 was immobilized on the electrode surface. The Rct was then raised gradually while the BSA, CA125, and Ab2 were changed one after the other on SPCE. This was explained by the fact that the electron transport between the electrode and hexacyanoferrate(III/II) (Fe[CN]6)3−/4 was impeded by these non-conductive proteins. The use of 0.25 wt% CS solution as a dispersant enhanced the stability of the composites on the working electrode. CV tests showed that the electrode material of Ti3C2Tx/NH2–CNT(H2O)/SPCE behaved stably, confirming that CS could improve electrode stability (Fig. 2D). The use of CS in the experimental group greatly improved the current signal and reduced background, attributed to the formation of a homogeneous conductive network and adsorption on the electrode surface. The fabricated immunosensor displayed a wide linear detection range (1 mU mL−1 to 500 U mL−1) and low LOD (1 mU mL−1). It demonstrated good selectivity and reproducibility (RSD ∼ 1.04%). Clinical validation showed satisfactory alignment with chemiluminescence methods used in hospitals, with relative deviations within ±8.55%. The sensor showed good selectivity for CA125, with minimal interference from other common proteins like PSA, cystatin C (CysC), and pro-gastrin releasing peptide (ProGRP). The test was conducted using 100 U mL−1 of CA125 and 1000 U mL−1 of the four interferents to bolster the results. The CA125 current signal was substantially altered in comparison to the interferents' signals, suggesting that the developed immunosensor has good selectivity for CA125 detection (Fig. 2E). Although the present study primarily focused on the analytical performance of the Ti3C2Tx/NH2–CNT modified with CS electrochemical immunosensor, the experimental results also offered indirect insights into its biocompatibility and clinical applicability. The differential pulse voltammetry (DPV) response of the constructed immunosensor was observed on days 1, 3, 7, 14, and 21, respectively, after it was stored at 4 °C. On day 21, the sensor's activity stayed at 99.3% of its starting level indicating chemical robustness suitable for practical applications. Moreover, successful detection of CA125 in both spiked healthy serum samples and clinical serum from ovarian cancer patients—without significant deviation from the chemiluminescence method used in hospitals—suggested strong compatibility with biological fluids. The high recovery rates (85–110%) and low RSD values (1.71–7.1%) observed in serum testing further reflected its reliability in complex physiological matrices. These findings, together with the sensor's resistance to signal interference and high reproducibility, implied a promising level of biosafety and short-term operational safety. Nevertheless, further dedicated studies—including cytotoxicity assessments, immune response evaluations, and long-term in vivo toxicity tests—are required to comprehensively determine the biocompatibility of Ti3C2Tx/NH2–CNT composites for clinical or implantable applications.166
 | ||
| Fig. 2 (A) Schematic image of fabrication of electrochemical immunosensor used for the detection of CA125. Effect of utilizing different modifications ((a) Bare SPCE, (b) Ti3C2Tx/NH2–CNT/SPCE, (c) Ab1/Ti3C2Tx/NH2–CNT/SPCE, (d) BSA/Ab1/Ti3C2Tx/NH2–CNT/SPCE, (e) CA125/BSA/Ab1/Ti3C2Tx/NH2–CNT/SPCE, and (f) Ab2/CA125/BSA/Ab1/Ti3C2Tx/NH2–CNT/SPCE) on sensing performance (B) and interfacial features (C) of the electrode. (D) Effect of different scan rate (20–200 mV s−1) on redox peak current of Ti3C2Tx/NH2–CNT/SPCE. (E) Assessment of the selectivity feature of the sensor in the presence of different sample. Reprinted with permission from ref. 166. Copyright 2025, Published by Elsevier. | ||
An innovative electrochemical immunosensor was constructed using a dual MOF sandwich strategy for the detection of the CA125.142 They fabricated immunosensor via combining MXene (Ti3C2), amino-functionalized MIL-101 MOF with iron (MIL-101(Fe)-NH2), and zirconium-based MOF composed of zirconium oxide/hydroxide cluster coordinated by six 1,4-benzenedicarboxylate linkers (Zr6O4[OH]4[BDC]6 [UiO66]) loaded with methylene blue (MB). The DPV current response of composite material of MXene sheets coated with CS polymer, further integrated with MIL-101 MOF particles (MXene@CS@MIL101) was 30.63 ± 0.59 μA significantly bigger than the DPV current response of composite material of MXene sheets coated with CS polymer (MXene@CS), indicating that the MXene@CS@MIL101 composites as the base layer could amplify the electrochemical signal to improve the immunosensor performance significantly. Additionally, it showed that the MXene@CS@MIL101 composites work in concert, which could be a major factor in this immunosensor's high sensitivity. MIL-101(Fe)-NH2 enhanced the primary antibody loading capacity, while composite of UiO66 MOF loaded or functionalized with MB (UiO66@MB) served as a signal probe for redox processes. This sensor achieved high sensitivity with LOD of 0.006 U mL−1 in detecting CA125. The sensitivity for the CA125 detection was calculated to be 83.26 μA mL U−1 cm−2. The immunosensor showed potential for clinical applications, with recovery rates of 99.94–100.1% in spiked serum samples. The study focused on the detection of a single antigen (CA125) and the potential for simultaneous detection of multiple tumor markers was suggested but not explored. The results also suggested the biocompatibility and clinical feasibility of the MXene-based electrochemical immunosensor for CA125 detection. The immunosensor showed good specificity to CA125, high reproducibility (RSD < 5%) and signal stability with no significant degradation or instability over a period of 72 hours storage at 4 °C, and good accuracy for detecting CA125 in patient serum samples and recovery experiments, with strong agreement compared to standard chemiluminescence assays (relative error [Er] = 0.50–5.21%). These results suggested that the MXene-based electrochemical immunosensor functioned well as a matrix of the nanocomposite, and can be used to detect CA125.142
The combination of amino-functionalized UiO66 MOF (UiO66-NH2 MOF) and Ti3C2 MXenes was used in another research for CA125 detection.167 It was a sandwich-like electrochemical immunosensor (STEM) using nanoribbon-like Ti3C2 MXenes (Ti3C2TxNR) as a carrier for the primary antibody (PAb) and UiO-66-NH2 MOFs coupled with toluidine blue (Tb) as a signal amplifier. Results of this study demonstrated a wide linear range (0.2–150.0 U mL−1) and low LOD; the results showed that the Tb current peaks increased linearly between 0.2 and 150.0 U mL−1, as did the increase in CA125 concentration. The linear equation was y = 0.308 + 0.103x (R2 = 0.9983), where x was the concentration (ng mL−1) of CA125 and y was the current response (μA) of Tb. The LOD value derived from S/N = 3 was 0.05 U mL−1. The current peaks' corresponding RSD was 3.22%. In the meantime, the assembled Tb-MOFs/SAb and PAb/Ti3C2TxNR were stored at 4 °C until they were no longer useful for assessing the stability of the planned STEM test. After 35 days of storage, the trials showed that the present peak reaction shows virtually no decrease (∼9.25%) with a good reproducibility (RSD = 3.22%) suggesting physicochemical robustness and low degradation in aqueous environments. Next, a 100-fold excess quantity of additional interfering chemicals, was used to examine the specificity of the STEM assay. The computed results showed that the value of ki,g for human serum albumin (HAS), vascular endothelial growth factor (VEGF), carcinoembryonic antigen (CEA), CA153, cancer antigen 199 (CA199), and lysozyme (Lys) were −3.25, −2.78, −3.26, −2.53, and −2.68, respectively. This indicated the superior specificity of the STEM platform for CA125. The designed STEM assay was tested in real human serum samples, with results showing adaptability in real applications. The assay measured CA125 in a local hospital sample, with recoveries ranging from 94.35–99.66%. Concurrent ELISA technology confirmed the assay's precision, indicating the compatibility of the nanomaterial interfaces in biological matrices and suitability for real sample measurement. These results indicated that this Ti3C2TxNR and MOF-based composite platform was not likely to release toxic byproducts or cause matrix interference during short-term assays. However, as new materials for bioanalytical platforms, further investigation of their toxicity and matrix interference potential is required.167 Additionally, self-assembled Prussian blue (PB) NPs were decorated on MXene QDs supported by electrodeposited Au NPs modified glassy carbon electrode (AuNP/PB/MXene QD/GCE) (Fig. 3A). The measurement of CA125 using DPV yielded an impressive sensitivity, with a linear range of 1 pU mL−1 to 0.12 nU mL−1 (R2 = 0.9824) and a LOD of 0.57 pU mL−1. It was in consistent with their previous findings which reported an enhanced conductivity and promoted the attachment of anti-CA125 onto the modified GCE, consequently improving sensitivity using an electrochemical immunosensor to detect the ovarian cancer using an immunosensor composed of a GCE functionalized with MXene, GQD, and AuNPs in real samples, however, this time they had added phosphate-buffered saline (PBS) because of its remarkable qualities, which include strong redox reaction activity, magnetic qualities, electrochemical traits, and photophysical effects.137,168
3.2. MXenes and innovative biomarkers in ovarian cancer detection
Early detection of ovarian cancer depends on the identification of strong biomarkers devoid of invasive treatments.171 Functionalized MXenes show the capacity to detect cancer-related proteins or nucleic acids exactly. Target biomolecules binding to the MXene surface causes observable changes in electrical signals, exactly proportional to the concentration of biomarkers. This allows for the quantification of biomarker levels, providing critical insights into cancer staging and progression.172 Beside the MXene-based biosensors engineered to detect CA125 in ovarian cancer patients, other biomarkers are utilized to provide MXene-based biosensors for cancer detection which are discussed in the following.3.3. Optical biosensors
According to earlier reports, endometrial, bladder, and colon cancers exhibit increased expression of the lipolysis-stimulated LSR.173–175 Among several whey acidic protein (WAP)-domain coding genes in that area, the WAP four-disulfide core domain protein 2 (WFDC2) gene on chromosome 20q12-13 codes human epididymis protein 4 (HE4). HE4 is overexpressed in various forms of ovarian cancer and ovarian cancer cell lines. The role of HE4 in early detection of ovarian cancer has been previously studied.176–180A pressure-colorimetric biosensor was developed for ovarian cancer diagnosis by detecting HE4 (Fig. 4A). Through the use of Nb2C MXene and Ag–polysulfide hybrids (Ag–Sx) hybrids, the biosensor converted photothermal effects into visual color changes and pressure elevation, enabling highly sensitive detection through a multi-signal readout system. The modification of Nb2C MXene with Ag–Sx improved the photothermal property of the MXene (from 27.8 °C to 66.7 °C in the absence and presence of Ag–Sx) (Fig. 4B). Moreover, it could affect the UV-visible spectra of Nb2C MXene via changing the light absorption from 900 nm to about 808 nm (Fig. 4C). Superior sensitivity over current methods was provided by the LOD, which was 3.01 × 10−7 ng mL−1 for pressure analysis and 4.86 × 10−6 ng mL−1 for colorimetric analysis. The rolling amplification mechanism improved signal conversion under NIR light, allowing for precise and quick detection. Indeed, the Nb2C MXene absorbed the NIR light and led to increase the temperature on the surface of multi-functional signal conversion paper (MSCP). This led to converting the Ag–glutathione (Ag-GSH) compounds into the Ag–Sx on the MSCP, and their attachment to the MXene. As more Ag–Sx was formed, the heating effect increases in a cycle, enhancing the system's overall performance (Fig. 4D). Moreover, the concentration of Nb2C MXene had also affect changing the temperature so that higher concentration of MXene led to greater temperature increased and a visible color change from pale yellow to dark brown (Fig. 4E). Besides the high photothermal conversion efficiency and catalase-mimicking activity of the Nb2C MXene/Ag–Sx hybrid, biocompatibility and long-term safety are also important considerations for advancing toward clinical or point-of-care applications. The in situ synthesis of Ag–Sx on Nb2C MXene through mild NIR-induced decomposition of Ag–GSH avoided strong chemical reagents that may leave cytotoxic residues during the fabrication process and enhance the biocompatibility. In addition, the self-limiting rolling deposition mechanism provided localized signal amplification without the need for continuous external chemical inputs to minimize systemic exposure and improve in vivo adaptability. The stable pressure and colorimetric outputs over several cycles with low RSD (4.5% for colorimetric and 3.76% for pressure analysis) indicated that the material was stable under repetitive thermal and catalytic stress. Importantly, negligible interferences from other species were observed.181
 | ||
| Fig. 4 (A) Schematic image related to the fabrication and biosensing application of multimodal photothermal platform, (B) photothermal profiles of a thermally sensitive Ag-GSH complex, Nb2C MXene, and combination of Nb2C MXene and Ag-GSH irradiated with NIR light (1.8 W cm−2), (C) UV-visible spectra of Nb2C MXene (a) and Nb2C MXene/Ag–Sx (b), (D) schematic image of the effect of laser irradiation on enhancing photothermal effect of Nb2C MXene/Ag–Sx, (E) effect of laser irradiation on increasing temperature of different concentrations of Nb2C MXene (a) and MSCP (b). Reprinted with permission from ref. 181. Copyright 2023, Published by Elsevier. | ||
A highly sensitive fluorescence quenching-based immunoassay was developed for the detection of HE4 using a modified fluorescent quencher. The system employed a carboxymethyl cellulose (CMC)–functionalized Nb2C MXene nanocomposite (CMC@MXene) to effectively quench the fluorescence of Tb-norfloxacin coordination polymer NPs (Tb-NFX CPNPs). In the assay, CMC@MXene was functionalized with HE4 antigen (Ag) and immobilized with antibody (Ab) in a 96-well plate, enabling an indirect competitive format. The addition of Tb-NFX CPNPs facilitated the transduction of biomolecular interactions into fluorescence signals. Upon NIR exposure, localized heating from the Ag–CMC@MXene composite further suppressed the emission intensity. This dual-mode quenching effect enabled highly selective and sensitive detection of HE4, with a broad linear range (10−5 to 10 ng mL−1), low detection limit of 3.3 fg mL−1, and recovery rate between 98.4–101.5%. The assay leveraged the aggregation-induced emission (AIE) characteristics of Tb-NFX and the dual role of CMC@MXene, as both a quencher and a protein carrier. This well-engineered platform demonstrated strong performance in real sample analysis and offers a promising approach for extending fluorescent biosensing strategies to other biomarkers.182
3.4. Electrochemical biosensors
The excretion of hydrogen peroxide (H2O2) was elevated in cancer cells, as previously observed. The release of H2O2 has been documented in the triggering of cellular apoptosis. Cancer cells typically do not secrete H2O2; but, following the introduction of a stimulant, they can leak H2O2 due to altered intracellular redox homeostasis.183,184 Nagarajan et al. conducted a study to develop a biocompatible MXene-based biosensor for H2O2 detection in ovarian cancer diagnosis. They utilized a novel electrode, where flavin adenine dinucleotide (FAD) was immobilized on 2D MXene (Ti3C2), improving electron transfer and catalytic properties. The biosensor exhibited LOD of about 0.7 nM and a broad linear range of 5 nM to 2 μM for H2O2. The biosensor operated by electrocatalytically reduction of H2O2via the FAD/Ti3C2-modified electrode at −0.47 V, a significant improvement over bare electrode. The hydrophilic surface terminations on Ti3C2 enhanced the adsorption of FAD and stably immobilized it without any other binders, and the FAD/Ti3C2Tx-modified GCEs maintained a high electrocatalytic activity toward H2O2 reduction with little current loss (<2%) after 50 potential cycles and maintained 87% repeatability after three days in physiological buffers, suggesting strong structural and functional stability. In addition, the fabricated biosensor exhibited high selectivity (>98%) in the presence of common biomolecular interferents and demonstrated a capacity for H2O2 detection in ovarian cancer cell lines with good recovery (92–97.7% after spiking), demonstrating both diagnostic potential and biointerface stability. However, these results also confirmed that Ti3C2-based MXene was biocompatible and functionally robust under biological conditions, and the long-term in vivo fate, immunogenicity, and degradability of this material remain to be elucidated.185A sensitive detection method was developed for epithelial ovarian cancer by targeting the carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5) tumor marker using a DNA nanobiosensor. They utilized an electrochemical biosensor based on a nanocomposite of MXene multi walled CNTs polypyrrole (MXene/MWCNTs/PPY), which displayed high sensitivity (93.3%) and specificity in detecting CEACAM5 in 26 clinical samples. The biosensor had a wide linear range (5 × 10−11 to 5 × 10−7 M), low LOD (0.12 pM), and stability for up to 20 days. The biosensor functioned through the immobilization of single-stranded DNA probes on a modified electrode, facilitating hybridization with target DNA and electrochemical detection using methylene blue as an indicator. MXenes enhanced the biosensor's performance by increasing conductivity and surface area, enabling efficient electron transfer. This approach demonstrated potential as a low-cost, rapid, and non-invasive method for early epithelial ovarian cancer diagnosis. The stability and real sample analysis indirectly assessed the biocompatibility and safety profile of the fabricated MXene/MWCNTs/PPY-based electrochemical DNA biosensor but no direct cytotoxicity assays were reported. The stable electrochemical performance over 25 days and reliable detection of target DNA in complex biological matrices suggested that the nanocomposite did not undergo rapid degradation or induce interfering side effects in these settings, while the successful detection of the target with high recovery rates (87–111%) and low RSDs (<2%) in serum indicated a low level of nonspecific interactions and minimal matrix toxicity, which implied acceptable biocompatibility for diagnostic applications. However, no direct in vitro or in vivo toxicity studies were conducted.186
3.5. Multimodal biosensors
In a recent study, a compact, multimodal sensor was developed via integrating optical, electrical, and visual detection components through assembling three sub-sensors capable of independently responding to NIR light. The system utilized photothermal properties of V2C MXene QDs (MQDs) combined with polyaniline and NiFe2O4 to modulate luminescence, color, and resistance outputs within a multilayer chip. This chip comprised thermochromic paper and a thermoelectric module layered beneath a transparent indium tin oxide (ITO) electrode functionalized with a ECL probe. The V2C MQDs contributed to strong luminescence, while NiFe2O4 enhanced electrocatalytic activity, enabling sensitive ECL signal generation. Upon NIR irradiation, the probe converted light to heat, triggering ECL enhancement, visible color change in CoCl2·6H2O-based thermochromic paper, and precised resistance variation via the thermoelectric layer. This light-responsive, multi-signal platform enabled accurate detection of lipolysis-induced lipoprotein receptors across a wide linear range (10−6 to 10 ng mL−1), showing high selectivity with LOD of about 3.3 × 10−7 ng mL−1, stability (about 90.3% of its beginning values after 15 days), and reproducibility, which were comparable with other researches. In the case of real sample, the fabricated sensor showed high ECL recovery of about 99.4–101.2%. The integration of intermediate thermal modulation and signal separation provided a novel strategy for advancing multifunctional sensing technologies.187An electrochemiluminescence–photothermal (ECL–photothermal) bimodal immunosensor was developed by Huang et al. to identify LSR as an ovarian cancer biomarker. V2C/Ag nanocomposites, which were created by self-reducing silver ammonia on MXene nanosheets, were used in the sensor. By utilizing the complementary properties of MXene and Ag NPs, the sensor integrated ECL and photothermal detection techniques. The photothermal detection showed a linear correlation between temperature increase and LSR concentration, with a LOD of 1.53 × 10−6 ng mL−1, whereas the ECL detection showed a wide linear range from 10−5 to 102 ng mL−1 with a LOD of 1.34 × 10−6 ng mL−1. With recovery rates ranging from 97.8% to 107.6%, real sample analysis in human serum validated the sensor's usefulness. The PTCE was improved by the MXene/Ag nanocomposite's increased light absorption due to surface plasmon resonance (SPR) effects. In particular, V2C/Ag had a PTCE of 27.6%, which was greater than V2C MXene's PTCE of 26.8%. Furthermore, the biosensor's ECL response was greatly improved by the MXene/Ag nanocomposites' unexpected oxygen reduction reaction (ORR) catalytic activity. The biosensor worked by using antibodies to capture the target biomarker on a modified electrode, which allowed for dual-mode signal output when exposed to laser light. Non-specific proteins such as thyroglobulin (Tg), Interleukin-6 (IL-6), and HE4 were used to test the biosensor's selectivity. Since the ECL intensity and temperature increase (ΔT) were nearly the same as those of the blank sample, the non-specific protein was unable to impede the signal responses and the LSR assay's high specificity. Consistent photothermal behavior during multiple on/off laser cycles, reproducible ECL signals (RSD = 1.3%) and thermal readings (RSD = 2.43%), high specificity against interfering biomolecules (e.g., HE4, IL-6, Tg), and high recovery rates (97.8–107.6%) in serum samples without signal suppression indicated minimal degradation or NP leaching of the composite in aqueous conditions, as well as its compatibility with complex biological matrices. Besides showing good electrochemical and photothermal properties of the V2C/Ag-based dual-mode immunosensor, the material design was also suitable for biocompatibility and long-term safety considerations necessary for clinical translation. The mild conditions used in the in situ reduction of Ag ions by V2C MXene minimized residual oxidizing agents to facilitate cleaner synthesis with fewer toxic byproducts.188
Table 2 summarizes the LODs and linear range of various designed MXene-based structures in diagnosis of cancer.
| Target biomarker | Type of MXene | LOD | Linear detection range | Ref. |
|---|---|---|---|---|
| CA125 | GCE modified with MXene, GQDs, and AuNPs | 0.075 nU mL−1 | 0.1–1 nU mL−1 | 137 |
| Ti3C2Tx–MXene/NH2–CNT composites with CS | 1 mU mL−1 | 1 mU mL−1–500 U mL−1 | 166 | |
| MXene (Ti3C2), MIL-101(Fe)-NH2, UiO66@MB | 0.006 U mL−1 | Not specified | 142 | |
| Ti3C2NR-based sandwich immunosensor with UiO-66-NH2 MOF and TB | 0.05 U mL−1 | 0.2–150.0 U mL−1 | 167 | |
| LSR | V2C/Ag nanocomposites | 1.34 × 10−6 ng mL−1 (ECL) 1.53 × 10−6 ng mL−1 (photothermal) | Wide detection range | 188 |
| HE4 | Nb2C MXene, Ag–Sx hybrids | 3.01 × 10−7 ng mL−1 (pressure) 4.86 × 10−6 ng mL−1 (colorimetric) | Not specified | 181 |
| H2O2 | Ti3C2 MXene modified with FAD | 0.7 nM | 5 nM–2 μM | 185 |
| CEACAM5 | MXene/MWCNTs/PPY nanocomposite | 0.12 pM | 5 × 10−11 to 5 × 10−7 M | 186 |
| Exosomes | Iron(III) ferrocyanide [Fe4(Fe[CN]6)3]/Ti3C2 MXene | 229 particles μL−1 | 5 × 102 particles μL−1 to 5 × 105 particles μL−1 | 189 |
| Exosomes | Cyanine 3-labeled aptamer specific for CD63 protein (Cy3-CD63 aptamer)/Ti3C2 MXenes nanocomplex | 1.4 × 103 particles mL−1 | Not reported | 190 |
| H2O2 | 3D electrode composed of reduced graphene oxide, Ti3C2 MXene, and MWCNTs (3D rGO–Ti3C2–MWCNTs electrode) | 0.3 μM | 1–60 μM and 60 μM–9.77 mM | 191 |
| Cytokeratin 19 fragment 21-1 (CYFRA 21-1) | L-Cysteine functionalized magnetic NPs (L-Cyst@MNPs)/Ti3C2–MXene | 0.023 ng mL−1 | 0.5–30 ng mL−1 | 192 |
| Carcinoembryonic antigen | Functionalized Ti3C2 MXene nanosheets (f-Ti3C2–MXene) | 0.000018 ng mL−1 | 0.0001–2000 ng mL−1 | 45 |
| miRNA-135b | Molybdenum disulfide (MoS2) QDs-MXene heterostructure and Au NPs coated with a biomimetic (AuNPs@biomimetic) lipid layer | 10 fM | 30 fM to 20 nM | 193 |
| miRNA-182 | Molybdenum disulfide nanosheets decorated with Au (MoS2@Au) NPs | 6.61 am | 10 am to 1 nm | 194 |
| miRNA-122 | Au hollow flower-like nanostructures combined with poly(n-butyl acrylate (AuHFGNs/PnBA)-MXene | 0.0035 aM | 0.01 aM to 10 nM | 195 |
3.6. Therapeutic applications of MXenes in ovarian cancer
The unique integration of electrical conductivity, optical responsiveness, magnetic behavior, and structural versatility makes MXenes a compelling platform for biomedical innovation.196 So far, various MXene platforms have been successfully synthesized to combat malignancies.23 Experts in biology are encouraged to contribute to this new field in order to produce more useful MXenes for a variety of uses. Among their many outstanding characteristics, they are unique and guarantee their use in biological applications in two ways: first, they have several functional groups, such as hydroxyl and oxygen, which allow them to carry a variety of drugs. Second, they are cytocompatible, which guarantees less toxicity and focused administration.197 Because MXenes have unmatched PTCE, biocompatibility, and multifunctional engineering capabilities, they have completely changed cancer treatment. Numerous treatment techniques, such as photothermal therapy (PTT), chemodynamic therapy (CDT), photodynamic therapy (PDT), and sonodynamic therapy (SDT), are enhanced by these materials, which frequently combine several strategies to get around the drawbacks of traditional treatments.198–210It was demonstrated that the engineered NP, curcumin-loaded porous MXene-derived carbon nanocarrier (PMCS) functionalized with a tumor-targeting peptide (CBP-sip65) and enhanced with the hyaluronidase enzyme PH20 (PH20/CCM@PMCS@CBP-sip65), constructed from PH20-overexpressing cancer-associated fibroblast (CAF)-cancer cell hybrid membranes and poly(dimethyl diallyl ammonium chloride) (PDDA)-modified MXene (a 2D transition metal carbide/nitride) loaded with carboplatin (CBP) and small interfering RNA targeting p65 (sip65), exhibited dual targeting of tumor cells and CAFs (Fig. 5A). Key quantitative results included 82.98% entrapment efficiency and 71.34% drug loading for CBP, and 71.79% entrapment efficiency and 1.75% loading for small interfering RNA (siRNA). NPs showed pH-dependent drug release (∼70% CBP and ∼50% siRNA released at pH 5.0). In vitro, curcumin-loaded porous carbon-based delivery system, enhanced with PH20 enzyme (PH20/CCM@PMCS) reduced cancer cell proliferation by inducing apoptosis (significant increase in Annexin V+ cells), generated reactive oxygen species (ROS), and triggered immunogenic cell death (ICD) via calreticulin (CRT) exposure and adenosine triphosphate (ATP)/high mobility group box 1 (HMGB1) release (2–3-fold increases in ATP and HMGB1 levels) (Fig. 5B and C). In CAFs, the NPs reduced pro-angiogenic cytokines (VEGF and angiogenin by ∼50%) and increased M1 macrophage polarization (CD86+ macrophages: 14.2% → 19.3% in human, 15.4% → 55.2% in mouse models). In vivo, PH20/CCM@PMCS reduced tumor volume by 85.30% (vs. 63.37% for non-PH20-coated CCM@PMCS), decreased angiogenesis (cluster of differentiation 31 [CD31] by ∼75%), and increased cytotoxic CD8+ T-cell infiltration (∼50% higher activation). The NPs showed low systemic toxicity (hemolysis <5% at 10 μg mL−1) and synergized with programmed death-ligand 1 (anti-PD-L1), enhancing tumor inhibition. These results highlight the platform's ability to quantitatively target multiple tumor microenvironment (TME) components, overcoming drug resistance and immune suppression in ovarian cancer (Fig. 5D). So, PH20/CCM@PMCS NPs had good biocompatibility and low toxicity both in vitro and in vivo. Cell viability assays demonstrated that PH20/CCM, PMXene, and PH20/CCM@PMXene had no cytotoxic effects on cancer cells, CAFs, and normal cell lines, suggesting the inherent safety of these coatings on cell membranes and MXene nanocarriers. Repeated intravenous injections in mice did not alter body weight or induce histopathological damage to major organs, indicating low toxicity of PH20/CCM@PMCS NPs. Hemolysis assays showed no significant red blood cell damage at working concentrations, suggesting good blood compatibility. Moreover, NP levels in plasma decreased rapidly to undetectable levels within 48 hours post-injection, while excretion analysis indicated efficient clearance by urine and feces with low NP accumulation in major organs.211
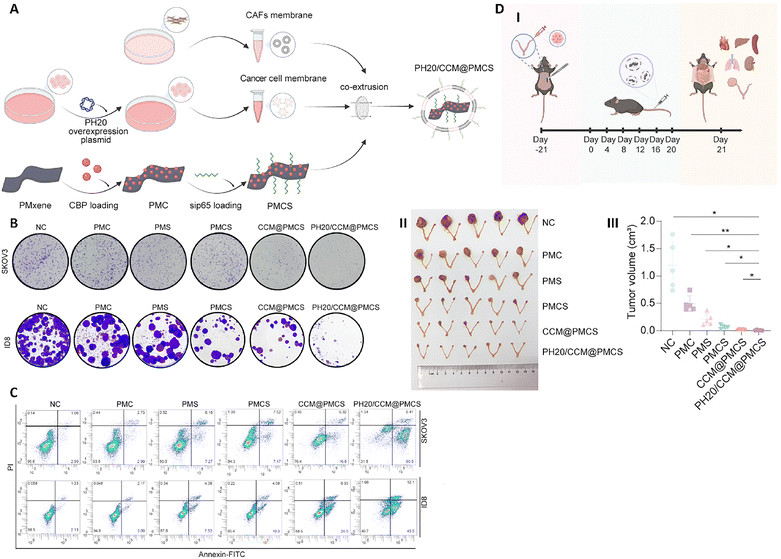 | ||
| Fig. 5 (A) The production procedure of PH20/CCM@PMCS. Results of colony formation (B) and apoptotic assay (C) of different treatments (including NC, PMC, PMS, PMCS, CCM@PMCS, and PH20/CCM@PMCS on SKOV3 and ID8 cells (D) schematic illustration of in vivo assessment of PH20/CCM@PMCS therapeutic effect (I). Effect of different treatments on size (II) and volume (III) of tumor. Reprinted with permission from ref. 211. Copyright 2025, Published by Springer Nature. | ||
Yang et al., conducted a study using a mouse model to investigate whether Ti3C2 nanosheets can translocate to the ovaries and cause ovarian damage, potentially impairing ovarian function. Results showed that intravenously injected Ti3C2 nanosheets were internalized by ovarian granulosa cells, reducing the number of primary, secondary, and antral follicles. This was associated with increased levels of follicle stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), and progesterone (P4), along with decreased testosterone (T) levels. Mechanistically, Ti3C2 nanosheets activated autophagy via the phosphoinositide 3-kinase/protein kinase B/mechanistic target of rapamycin (PI3K/AKT/mTOR) pathway, with oxidative stress playing a key role. However, autophagic flux was impaired, as indicated by increased Beclin1, autophagy-related 5 (ATG5), microtubule-associated protein 1A/1B-light chain 3 (LC3II/I) ratio, and SQSTM1 (P62) accumulation. In vitro experiments using KGN cells showed that inhibiting autophagy initiation with 3-methyladenine (3-MA) partially reduced estradiol and progesterone secretion, while blocking autophagic flux with Rapamycin (RAPA) exacerbated the disruption of hormone secretion. While MXene nanosheets exhibited ovarian toxicity in this context, their ability to modulate autophagy and hormonal secretion suggested potential applications in ovarian cancer treatment, particularly in leveraging autophagy-related mechanisms for targeted therapies.210
A study was conducted with ovarian cancer cell lines (ID8 and SKOV3) and C57BL/6 mice tumor models, aiming to develop an ultrasound-responsive bismuth molybdate (Bi2MoO6) MXene (BMO-MXene) heterojunction as a ferroptosis inducer for stimulating ICD against ovarian cancer (Fig. 6A). MXene was utilized in combination with Bi2MoO6 to form a Schottky heterojunction, synthesized through a hydrothermal method followed by mixing MXene and BMO at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio via electrostatic adsorption under ultrasonication. The BMO-MXene, upon ultrasound stimulation, inhibited ovarian cancer proliferation by over 90% (Fig. 6B), induced ferroptosis through lipid peroxidation, mitochondrial membrane potential reduction, and inactivation of glutathione peroxidase (GPX4) and cystathionine transporter protein (SLC7A11), and activated ICD, enhancing dendritic cell maturation and antitumor immunity. The study found that BMO-MXene showed high ROS production and strong cytotoxicity against ovarian cancer cells in vitro, while in vivo experiments (Fig. 6C) demonstrated significant tumor volume reduction, increased ICD markers (CRT exposure, ATP secretion, HMGB1 release), and greater infiltration of immune cells such as CD8+ T cells and mature dendritic cells. It was concluded that BMO-MXene effectively induced ferroptosis and boosted antitumor immunity through ferroptosis-ICD pathway. Therefore, BMO-MXene presents a promising noninvasive, tumor-targeted ferroptosis induction strategy with immune-stimulatory effects, potentially overcoming chemotherapy resistance and enhancing ovarian cancer treatment.209
1 ratio via electrostatic adsorption under ultrasonication. The BMO-MXene, upon ultrasound stimulation, inhibited ovarian cancer proliferation by over 90% (Fig. 6B), induced ferroptosis through lipid peroxidation, mitochondrial membrane potential reduction, and inactivation of glutathione peroxidase (GPX4) and cystathionine transporter protein (SLC7A11), and activated ICD, enhancing dendritic cell maturation and antitumor immunity. The study found that BMO-MXene showed high ROS production and strong cytotoxicity against ovarian cancer cells in vitro, while in vivo experiments (Fig. 6C) demonstrated significant tumor volume reduction, increased ICD markers (CRT exposure, ATP secretion, HMGB1 release), and greater infiltration of immune cells such as CD8+ T cells and mature dendritic cells. It was concluded that BMO-MXene effectively induced ferroptosis and boosted antitumor immunity through ferroptosis-ICD pathway. Therefore, BMO-MXene presents a promising noninvasive, tumor-targeted ferroptosis induction strategy with immune-stimulatory effects, potentially overcoming chemotherapy resistance and enhancing ovarian cancer treatment.209
 | ||
| Fig. 6 (A) Schematic image related to the fabrication and anticancer application of BMO-MXene. (B) Fluorescence images of ovarian cancer cells exposed with different treatments (control, BMO-MXene, ultrasound, and the combination of BMO-MXene and ultrasonic therapy) (scar bar = 100 μm). (C) Schematic image related to in vivo process (I). Effect of different treatments on body weight (II), size (III), and volume (IV) of tumors exposed with different treatments. Reprinted from ref. 209 under the terms of the Creative Commons CC BY license. Copyright 2024, The Author(s). | ||
4. Challenges and future perspectives
Nowadays, physical examination, TVUS imaging, and serum level tests for CA125 are used to diagnose ovarian cancer.212 Findings from the prostate, lung, colorectal and ovarian (PLCO) screening trial demonstrated that testing with CA125 showed a positive predictive value of only 4%, that could be improved to 26.5% in combination with TVUS, but this combination has still not been shown to provide a noticeable improvement in survival outcomes for patients after a 15-year follow-up.213,214 Thus, more efficient diagnostic techniques are required since CA125 screening lacks clinical sensitivity in the early stages, resulting in late-stage diagnoses and decreased survival prospects.212Biomarkers are detected using conventional methods such as radioimmunoassay,215 immune polymerase chain reaction (PCR) assay,216 electrophoretic immunoassay,217 mass spectrometric immunoassay,218 and ELISA.219 The ELISA is mostly used for the detection and determination of CA125.220 The development of low-cost, simple, sensitive, and rapid detection techniques for point-of-care diagnosis is urgently needed due to the drawbacks of the other immunoassays, such as lengthy analysis, high cost, complex instrumentation, and the need for professional personnel, as well as the time-consuming and labor-intensive process of ELISA.221 In recent decades, biosensors have become an important state of the art technology in laboratory medicine, notably in point-of-care diagnostics and their applications in this sector are increasing very quickly.222
Recent studies have demonstrated the versatility of MXene-based platforms, achieving remarkable LODs, wide linear ranges, and excellent reproducibility.137,142,167,183 However, the severe stacking issue that 2D MXene faces as a result of the strong interlayer van der Waals force significantly lowers its specific surface area and restricts its useful performance.159,160 The integration of MXenes with other types of nanomaterials, such as CNTs, metal NPs, MOFs, etc., has significantly enhanced biosensor performance. As research progresses, MXene-based biosensors are poised to revolutionize cancer diagnostics, offering new avenues for early detection, disease monitoring, and improved patient outcomes on a global scale. Adding certain conductive elements into the MXene interlayers is a workable approach to achieve this.161 The potential of MXene-based proposed immunosensors like what engineered by Hosseinchi Ghareaghaji et al.137 in which AuNPs were added to the biosensors and showed a noticeably improved LOD and linear detection range in comparison to previous findings;223–228 however, this study was evaluated on a small number of real samples and there is a need for more focus on developing new portable tools with the advantages of their platform that will bring benefits for point-of-care diagnostics in clinical settings. Furthermore, PB NPs, one of the many electrochemical indicators, has drawn a lot of interest because of its remarkable qualities, which include strong redox reaction activity, magnetic properties, electrochemical features, and photo-physical effects. The self-assembly method of preparing PB is simple and repeatable, which makes it appropriate for commercialization and can shorten the preparation time needed to produce immunosensors. However, PB frequently has poor conductivity, which can seriously impair the created immunosensor's functionality.229 Some researchers have regularly used conducting AuNPs230,231 to create PB-conducting nanocomposites in an effort to overcome these limitations and improve PB conductivity. In the field of ovarian cancer, Hosseini Ghareaghaji et al. reported an improved conductivity using the combination of PB and AuNP in detecting CA125.232 Additionally, a MOF-based system was designed which successfully detected one antigen CA125. However, given its outstanding performance in this investigation, it should be able to identify numerous antigens at once. In order to enable precise and timely diagnosis of various cancers, researchers will concentrate their future efforts on developing electrochemical sensors that can concurrently detect several tumor markers in sera samples.142 One of the limitations previously reported in the immobilization of antibodies in the immunosensor was the decreased redox peak current due to the non-conductive biomacromolecule166 which should be considered in the future studies to overcome this hindering by antibodies for an improved conductivity in the biosensors.
Many factors influence the biocompatibility profile of MXenes, such as size, morphology, exposure duration, dosage, environmental conditions like temperature, and experimental conditions like PH. For example, in the study by Qu et al. pH, PBS concentration, and MB concentration all had a significant impact on an immunosensor's performance; acidic or alkaline PBS reduced the electrical signal because of the activity of antigenic and antibody proteins; neutral conditions were better for methylene blue redox reactions; the ideal pH was 7.0; PBS concentration improved peak current but decreased as concentration changed; MB concentration also had an impact, increasing proportionately with concentration; the ideal concentration was 15.0 mmol l−1; and incubation time also had an impact on the sensor's performance, with peak current increasing as the incubation period was prolonged.142 The need for systematic, standardized evaluations to check variations in safety assessments is highlighted by the fact that some studies report low toxicity under controlled parameters, such as cell line compatibility or specific incubation periods.233,234
One of the other challenges facing with MXene-based products is high amounts of HF or fluoride-based chemicals that are frequently used in nanosheet production techniques;137,142,181,188 nevertheless, their toxicity restricts their effectiveness and safety precautions. This creates practical obstacles to scaling and makes large-scale production more difficult. In order to solve these problems and adhere to sustainable chemistry principles, scientists are creating green synthesis protocols that completely do away with the use of fluorine.80,235 Optimizing these procedures is the major goal in order to guarantee scalability while preserving good production yields. The biodegradation kinetics and long-term metabolic interactions of MXenes are still poorly understood, despite their encouraging biocompatibility. Systematic investigations into their breakdown mechanisms under in vivo circumstances are lacking in current studies.235 Moreover, suboptimal drug-loading efficiency and limited spatiotemporal control limit the therapeutic potential of MXene-based nanosheets, resulting in nonspecific biodistribution and compromised therapeutic precision; it is difficult to predict systemic biodistribution patterns because unintended dispersion can cause irreversible physiological disruptions; to mitigate off-target cytotoxicity, precise dosage control is necessary to address these biosafety concerns.236
In the field of treatment, because of their stability, biocompatibility, and targeting properties, MXene NPs have demonstrated promise in drug delivery and imaging applications. Stability, biocompatibility, and targeting capabilities can all be enhanced via surface modification. Green precursors, surface modifications using natural components, material size reduction, the use of natural ligands/linkers, and green solvents are some strategies. MXenes are useful in customized therapy because targeted delivery techniques can increase tumor tissue accumulation, decrease off-target effects, and improve drug delivery specificity.237–239
Converting MXenes' in vitro results into clinical applications requires in vivo studies, which assess pharmacokinetics, biodistribution, stability, and toxicity to determine safety and efficacy; balancing stability and biodegradability; assessing the environmental impact of large-scale production and disposal; and comprehending the long-term effects on human health and the environment.238,240,241 Currently, different cell lines, different dosage schedules, and diverse experimental models all contribute to methodological inconsistencies in current research. Dual evaluation in biological contexts relevant to both health and disease is necessary for accurate safety profiling, and systematic investigations are required to establish correlations.242
Artificial intelligence (AI) has the potential to revolutionize cancer treatment, especially for complex malignancies like ovarian cancer. AI-driven algorithms can analyze large datasets to optimize MXene synthesis, predict biocompatibility, and customize surface modifications for targeted drug delivery. Machine learning models can help design MXene nanostructures with enhanced stability, drug loading efficiency, and controlled release profiles, improving therapeutic outcomes. For instance, Marchwiany et al.243 used four ML algorithms: logistic regression, random forest, support vector machine, and extreme random tree. The results showed that only two MXenes with surface functional groups like lithium atoms and metal oxides were hazardous, indicating that the MXenes are not harmful. Additionally, by evaluating patient-specific information, such as genetic markers and tumor microenvironment profiles, AI can be used to tailor MXene-based therapies and inform the development of intelligent MXene platforms for precision medicine. AI makes it easier to understand MXene-enhanced diagnostic pictures in imaging-guided therapy, allowing for precise tumor localization and real-time therapeutic response monitoring.244
5. Conclusion
MXenes are a revolutionary class of 2D nanomaterials that hold great promise for the diagnosis and treatment of ovarian cancer. High surface area, conductivity, biocompatibility, and programmable surface chemistry are just a few of their remarkable physicochemical characteristics that have made them attractive options for targeted drug administration, biosensing, and imaging. By detecting well-known ovarian cancer biomarkers like CA125, HE4, LSR, and CEACAM5, advances in MXene-based biosensors have shown previously unheard-of sensitivity, opening the door to early, non-invasive diagnoses. Furthermore, there is considerable promise for improving therapy efficacy while reducing systemic toxicity by their incorporation into photothermal, photodynamic, and chemodynamic therapies.Even with these developments, a number of obstacles still exist. Additional explorations are needed to determine the long-term toxicity and biocompatibility of MXenes, especially with regard to their stability in physiological settings and any off-target effects. For repeatability and clinical translation to be guaranteed, standardization in synthesis techniques and functionalization strategies is essential. Furthermore, further clinical trials and thorough in vivo research are necessary to confirm the safety and therapeutic efficacy of MXene-based theranostic techniques, even though preclinical studies have shown their efficiency. MXenes tend to undergo oxidation and degradation in biological environments, which compromises their functional integrity and therapeutic efficacy. Controlled and sustained drug release from MXene-based carriers remains difficult to achieve, limiting precise dosing and treatment duration. Moreover, comprehensive in vivo biosafety assessments and long-term toxicity studies are still lacking, raising concerns about potential side effects and biodegradability. Large-scale, reproducible synthesis of MXenes with consistent quality and functionalization is another significant barrier, as variability can affect clinical outcomes. Additionally, the absence of standardized regulatory guidelines and safety standards for MXene-based nanomaterials complicates their approval process for clinical use.
Future perspectives for MXenes in ovarian cancer focus on enhancing their biocompatibility and targeting capabilities through advanced surface engineering and functionalization with bioactive molecules. This could improve their selective uptake by ovarian tumor cells and reduce off-target effects. Integration with emerging technologies such as microfluidic platforms for early diagnosis and real-time monitoring could further boost their clinical utility. Moreover, combining MXene-based photothermal and photodynamic therapies with epigenetic targeting strategies, such as those addressing oncogenes like MECOM implicated in ovarian cancer chemoresistance, may provide synergistic therapeutic benefits. Integration of MXene platforms with emerging ovarian cancer biomarkers and theranostic targets, such as MUC16 and MSLN, could improve diagnostic accuracy and personalized treatment strategies. Furthermore, clinical translation will benefit from designing specialized clinical trials and regulatory frameworks tailored to the dual diagnostic and therapeutic nature of MXene-based agents. Addressing tumor heterogeneity and resistance mechanisms in ovarian cancer through MXene-enabled combination therapies, including photothermal and immunotherapies, represents a promising avenue for improving patient outcomes.
Author contributions
Neda Farzizadeh: writing – review & editing; Atefeh Zarepour: writing – review & editing; Arezoo Khosravi: visualization, graphical abstract, writing – review & editing; Siavash Iravani: supervision, conceptualization, writing – review & editing; Ali Zarrabi: supervision, writing – review & editing.Conflicts of interest
Author(s) declare no conflict of interest.Data availability
No data was used for the research described in the article.References
- X. Fu, Q. Zhang, Z. Wang, Y. Xu and Q. Dong, Cell Death Dis., 2024, 15, 21 CrossRef CAS PubMed.
- E. M. J. Ferlay, F. Lam, M. Laversanne, M. Colombet, L. Mery, M. Piñeros, A. Znaor, I. Soerjomataram and F. Bray, Cancer Today, International Agency for Research on Cancer, Lyon, France, 2022, https://gco.iarc.who.int/media/globocan/factsheets/cancers/25-ovary-fact-sheet.pdf.
- Z. Momenimovahed, A. Tiznobaik, S. Taheri and H. Salehiniya, Int. J. Women's Health, 2019, 287–299 CrossRef PubMed.
- L. M. J. Ferlay, M. Ervik, F. Lam, M. Colombet, L. Mery, M. Piñeros, A. Znaor, I. Soerjomataram and F. Bray, Global Cancer Observatory: Cancer Tomorrow (version 1.1), International Agency for Research on Cancer, Lyon, France, 2022, https://gco.iarc.fr/tomorrow/en/about#references Search PubMed.
- S. Morand, M. Devanaboyina, H. Staats, L. Stanbery and J. Nemunaitis, Int. J. Mol. Sci., 2021, 22, 6532 CrossRef CAS PubMed.
- U. M. Zamwar and A. P. Anjankar, Cureus, 2022, 14, e30561 Search PubMed.
- Y. Xiao, M. Bi, H. Guo and M. Li, EBioMedicine, 2022, 79, 104001 CrossRef CAS PubMed.
- J. R. van Nagell Jr and R. W. Miller, Obstet. Gynecol., 2016, 127, 848–858 CrossRef PubMed.
- Y. Gao, S. Zeng, X. Xu, H. Li, S. Yao, K. Song, X. Li, L. Chen, J. Tang and H. Xing, Lancet Digital Health, 2022, 4, e179–e187 CrossRef CAS PubMed.
- G. Moreno–Bueno, C. Gamallo, L. Pérez–Gallego, J. C. de Mora, A. Suárez and J. Palacios, Diagn. Mol. Pathol., 2001, 10, 116–122 CrossRef PubMed.
- R. E. Bristow, R. S. Tomacruz, D. K. Armstrong, E. L. Trimble and F. Montz, J. Clin. Oncol., 2002, 20, 1248–1259 CrossRef PubMed.
- J. B. Trimbos, I. Vergote, G. Bolis, J. B. Vermorken, C. Mangioni, C. Madronal, M. Franchi, S. Tateo, G. Zanetta and G. Scarfone, J. Natl. Cancer Inst., 2003, 95, 113–125 CrossRef CAS PubMed.
- M. Barani, M. Bilal, F. Sabir, A. Rahdar and G. Z. Kyzas, Life Sci., 2021, 266, 118914 CrossRef CAS PubMed.
- S. Shurbaji, N. P. A. Manaph, S. M. Ltaief, A. R. Al-Shammari, A. Elzatahry and H. C. Yalcin, Front. Nanotechnol., 2021, 3, 689718 CrossRef.
- S. Ranjbari, M. Darroudi, B. Hatamluyi, R. Arefinia, S. H. Aghaee-Bakhtiari, M. Rezayi and M. Khazaei, Front. Bioeng. Biotechnol., 2022, 10, 984336 CrossRef PubMed.
- Q. Lin, J. Li, Z. Abudousalamu, Y. Sun, M. Xue, L. Yao and M. Chen, Int. J. Nanomed., 2024, 19, 9351–9370 CrossRef PubMed.
- H. Wang, J. Sun, L. Lu, X. Yang, J. Xia, F. Zhang and Z. Wang, Anal. Chim. Acta, 2020, 1094, 18–25 CrossRef CAS PubMed.
- M. Darroudi, K. Ghasemi, M. Rezayi and M. Khazaei, Mini-Rev. Med. Chem., 2023, 23, 1033–1049 CrossRef CAS PubMed.
- Y. Ma, K. Jiang, H. Chen, Q. Shi, H. Liu, X. Zhong, H. Qian, X. Chen, L. Cheng and X. Wang, Acta Biomater., 2022, 149, 359–372 CrossRef CAS PubMed.
- X. Han, J. Huang, H. Lin, Z. Wang, P. Li and Y. Chen, Adv. Healthcare Mater., 2018, 7, 1701394 CrossRef PubMed.
- P. Zhang, X.-J. Yang, P. Li, Y. Zhao and Q. J. Niu, Soft Matter, 2020, 16, 162–169 RSC.
- B. Zhu, J. Shi, C. Liu, J. Li and S. Cao, Ceram. Int., 2021, 47, 24252–24261 CrossRef CAS.
- H. Lin, S. Gao, C. Dai, Y. Chen and J. Shi, J. Am. Chem. Soc., 2017, 139, 16235–16247 CrossRef CAS PubMed.
- X. Yu, X. Cai, H. Cui, S.-W. Lee, X.-F. Yu and B. Liu, Nanoscale, 2017, 9, 17859–17864 RSC.
- H. Lin, Y. Chen and J. Shi, Adv. Sci., 2018, 5, 1800518 CrossRef PubMed.
- S. Iravani and R. S. Varma, ACS Biomater. Sci. Eng., 2021, 7, 1900–1913 CrossRef CAS PubMed.
- S. Iravani and R. S. Varma, Nanomaterials, 2022, 12, 3360 CrossRef CAS PubMed.
- G. Jamalipour Soufi, P. Iravani, A. Hekmatnia, E. Mostafavi, M. Khatami and S. Iravani, Comments Inorg. Chem., 2022, 42, 174–207 CrossRef CAS.
- F. Mohajer, G. Mohammadi Ziarani, A. Badiei, S. Iravani and R. S. Varma, Micromachines, 2022, 13, 1773 CrossRef PubMed.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, MXenes, Jenny Stanford Publishing, 2023, pp. 15–29 Search PubMed.
- T. Qin, Z. Wang, Y. Wang, F. Besenbacher, M. Otyepka and M. Dong, Nano-Micro Lett., 2021, 13, 183 CrossRef CAS PubMed.
- T. Dutta, P. Alam and S. K. Mishra, J. Mater. Chem. B, 2025, 13, 4279–4312 RSC.
- N. Hemanth and B. Kandasubramanian, Chem. Eng. J., 2020, 392, 123678 CrossRef CAS.
- A. Zarepour, Ç. Karasu, Y. Mir, M. H. Nematollahi, S. Iravani and A. Zarrabi, Biomater. Sci., 2023, 11, 6687–6710 RSC.
- S. Wang, H. Ma, S. Ge, M. Rezakazemi and J. Han, Mater. Sci. Eng., R, 2025, 163, 100925 CrossRef.
- N. Xu, H. Li, Y. Gan, H. Chen, W. Li, F. Zhang, X. Jiang, Y. Shi, J. Liu and Q. Wen, Adv. Sci., 2020, 7, 2002209 CrossRef CAS PubMed.
- R. M. Ronchi, J. T. Arantes and S. F. Santos, Ceram. Int., 2019, 45, 18167–18188 CrossRef CAS.
- Z. Xiang, Y. Shi, X. Zhu, L. Cai and W. Lu, Nano-Micro Lett., 2021, 13, 150 CrossRef CAS PubMed.
- M. Malaki, A. Maleki and R. S. Varma, J. Mater. Chem. A, 2019, 7, 10843–10857 RSC.
- M. Sun, W. Ye, J. Zhang and K. Zheng, Inorganics, 2024, 12, 112 CrossRef CAS.
- Y. Li, H. Shao, Z. Lin, J. Lu, L. Liu, B. Duployer, P. O. Persson, P. Eklund, L. Hultman and M. Li, Nat. Mater., 2020, 19, 894–899 CrossRef CAS PubMed.
- A. J. Borah, V. Natu, A. Biswas and A. Srivastava, Oxford Open Mater. Sci., 2025, 5, itae017 CrossRef.
- M. Naguib, O. Mashtalir, J. Carle, V. Presser, J. Lu, L. Hultman, Y. Gogotsi and M. W. Barsoum, ACS Nano, 2012, 6, 1322–1331 CrossRef CAS PubMed.
- H. Wang, J. Zhang, Y. Wu, H. Huang, G. Li, X. Zhang and Z. Wang, Appl. Surf. Sci., 2016, 384, 287–293 CrossRef CAS.
- S. Kumar, Y. Lei, N. H. Alshareef, M. Quevedo-Lopez and K. N. Salama, Biosens. Bioelectron., 2018, 121, 243–249 CrossRef CAS PubMed.
- K. Khan, A. K. Tareen, W. Ahmad, I. Hussain, M. U. Chaudhry, A. Mahmood, M. F. Khan, H. Zhang and Z. Xie, Adv. Sci., 2024, 11, 2303998 CrossRef CAS PubMed.
- S. Zhang, L. Meng, Y. Hu, Z. Yuan, J. Li and H. Liu, Small, 2024, 20, 2308600 CrossRef CAS PubMed.
- P. Kuang, H. Deng, H. Cui, L. Chen, J. Fang, Z. Zuo, J. Deng, X. Wang and L. Zhao, Oncotarget, 2016, 8, 4703 CrossRef PubMed.
- Q. Luo, H. Cui, H. Deng, P. Kuang, H. Liu, Y. Lu, J. Fang, Z. Zuo, J. Deng and Y. Li, Oncotarget, 2017, 8, 50430 CrossRef PubMed.
- H. Guo, P. Kuang, Q. Luo, H. Cui, H. Deng, H. Liu, Y. Lu, J. Fang, Z. Zuo and J. Deng, Oncotarget, 2017, 8, 85504 CrossRef PubMed.
- Y. Ying, Y. Liu, X. Wang, Y. Mao, W. Cao, P. Hu and X. Peng, ACS Appl. Mater. Interfaces, 2015, 7, 1795–1803 CrossRef CAS PubMed.
- O. Mashtalir, M. Naguib, B. Dyatkin, Y. Gogotsi and M. W. Barsoum, Mater. Chem. Phys., 2013, 139, 147–152 CrossRef CAS.
- X. Wang, C. Garnero, G. Rochard, D. Magne, S. Morisset, S. Hurand, P. Chartier, J. Rousseau, T. Cabioc'h and C. Coutanceau, J. Mater. Chem. A, 2017, 5, 22012–22023 RSC.
- Z. Lin, D. Barbara, P.-L. Taberna, K. L. Van Aken, B. Anasori, Y. Gogotsi and P. Simon, J. Power Sources, 2016, 326, 575–579 CrossRef CAS.
- Y. Wei, P. Zhang, R. A. Soomro, Q. Zhu and B. Xu, Adv. Mater., 2021, 33, 2103148 CrossRef CAS PubMed.
- J. Xuan, Z. Wang, Y. Chen, D. Liang, L. Cheng, X. Yang, Z. Liu, R. Ma, T. Sasaki and F. Geng, Angew. Chem., 2016, 128, 14789–14794 CrossRef.
- S. Bai, M. Yang, J. Jiang, X. He, J. Zou, Z. Xiong, G. Liao and S. Liu, npj 2D Mater. Appl., 2021, 5, 78 CrossRef CAS.
- J. Wu, Y. Yu and G. Su, Nanomaterials, 2022, 12, 828 CrossRef CAS PubMed.
- K. Arole, S. E. Pas, R. M. Thakur, L. A. Amiouny, M. H. Kabir, M. Dujovic, M. Radovic, J. L. Lutkenhaus, M. J. Green and H. Liang, ACS Appl. Mater. Interfaces, 2024, 16, 64156–64165 CrossRef CAS PubMed.
- M. Mim, K. Habib, S. N. Farabi, S. A. Ali, M. A. Zaed, M. Younas and S. Rahman, ACS Omega, 2024, 9, 32350–32393 CAS.
- K. Guan, W. Lei, H. Wang, X. Liu, J. Luo, Q. Jia, H. Zhang and S. Zhang, Ceram. Int., 2022, 48, 16357–16363 CrossRef CAS.
- T. Galvin, N. Hyatt, W. Rainforth, I. Reaney and D. Shepherd, J. Eur. Ceram. Soc., 2018, 38, 4585–4589 CrossRef CAS.
- H. Liu, Y. Wang, L. Yang, R. Liu and C. Zeng, J. Mater. Sci. Technol., 2020, 37, 77–84 CrossRef CAS.
- W. Sun, S. Shah, Y. Chen, Z. Tan, H. Gao, T. Habib, M. Radovic and M. Green, J. Mater. Chem. A, 2017, 5, 21663–21668 RSC.
- C. Zhou, X. Zhao, Y. Xiong, Y. Tang, X. Ma, Q. Tao, C. Sun and W. Xu, Eur. Polym. J., 2022, 167, 111063 CrossRef CAS.
- P. Urbankowski, B. Anasori, T. Makaryan, D. Er, S. Kota, P. L. Walsh, M. Zhao, V. B. Shenoy, M. W. Barsoum and Y. Gogotsi, Nanoscale, 2016, 8, 11385–11391 RSC.
- H. Yu, Y. Wang, Y. Jing, J. Ma, C. F. Du and Q. Yan, Small, 2019, 15, 1901503 CrossRef PubMed.
- U. U. Rahman, M. Humayun, U. Ghani, M. Usman, H. Ullah, A. Khan, N. M. El-Metwaly and A. Khan, Molecules, 2022, 27, 4909 CrossRef CAS PubMed.
- A. Parihar, A. Singhal, N. Kumar, R. Khan, M. A. Khan and A. K. Srivastava, Nano-Micro Lett., 2022, 14, 100 CrossRef CAS PubMed.
- A. K. Patel, A. J. Borah and A. Srivastava, Oxford Open Mater. Sci., 2023, 3, itad020 CrossRef CAS.
- R. Dutt, R. R. Srivastava, H. Mishra and A. Srivastava, Opt. Mater., 2024, 149, 115050 CrossRef CAS.
- Y. Xie, D. Kocaefe, C. Chen and Y. Kocaefe, J. Nanomater., 2016, 2016, 2302595 Search PubMed.
- D. Wang, C. Zhou, A. S. Filatov, W. Cho, F. Lagunas, M. Wang, S. Vaikuntanathan, C. Liu, R. F. Klie and D. V. Talapin, Science, 2023, 379, 1242–1247 CrossRef CAS PubMed.
- C. Xu, L. Wang, Z. Liu, L. Chen, J. Guo, N. Kang, X.-L. Ma, H.-M. Cheng and W. Ren, Nat. Mater., 2015, 14, 1135–1141 CrossRef CAS PubMed.
- F. Zhang, Z. Zhang, H. Wang, C. H. Chan, N. Y. Chan, X. X. Chen and J.-Y. Dai, Phys. Rev. Mater., 2017, 1, 034002 CrossRef.
- I. Ijaz, E. Gilani, A. Nazir and A. Bukhari, Green Chem. Lett. Rev., 2020, 13, 223–245 CrossRef CAS.
- M. Huston, M. DeBella, M. DiBella and A. Gupta, Nanomaterials, 2021, 11, 2130 CrossRef CAS PubMed.
- A. Gour and N. K. Jain, Artif. Cells, Nanomed., Biotechnol., 2019, 47, 844–851 CrossRef CAS PubMed.
- S. Jadoun, R. Arif, N. K. Jangid and R. K. Meena, Environ. Chem. Lett., 2021, 19, 355–374 CrossRef CAS.
- T. Amrillah, Cryst. Growth Des., 2022, 22, 4640–4660 CrossRef CAS.
- C. Peng, P. Wei, X. Chen, Y. Zhang, F. Zhu, Y. Cao, H. Wang, H. Yu and F. Peng, Ceram. Int., 2018, 44, 18886–18893 CrossRef CAS.
- Y. Guo, S. Jin, L. Wang, P. He, Q. Hu, L.-Z. Fan and A. Zhou, Ceram. Int., 2020, 46, 19550–19556 CrossRef CAS.
- S.-Y. Pang, Y.-T. Wong, S. Yuan, Y. Liu, M.-K. Tsang, Z. Yang, H. Huang, W.-T. Wong and J. Hao, J. Am. Chem. Soc., 2019, 141, 9610–9616 CrossRef CAS PubMed.
- S. Jolly, M. P. Paranthaman and M. Naguib, Mater. Today Adv., 2021, 10, 100139 CrossRef CAS.
- A. Rafieerad, W. Yan, A. Amiri and S. Dhingra, Mater. Des., 2020, 196, 109091 CrossRef CAS.
- S. Sezen, A. Zarepour, A. Zarrabi and S. Iravani, Microchem. J., 2023, 193, 109258 CrossRef CAS.
- B. Xu, C. Zhi and P. Shi, J. Phys. Mater., 2020, 3, 031001 CrossRef CAS.
- M. Mozafari and M. Soroush, Mater. Adv., 2021, 2, 7277–7307 RSC.
- J. Chen, Q. Huang, H. Huang, L. Mao, M. Liu, X. Zhang and Y. Wei, Nanoscale, 2020, 12, 3574–3592 RSC.
- M. Tang, J. Li, Y. Wang, W. Han, S. Xu, M. Lu, W. Zhang and H. Li, Symmetry, 2022, 14, 2232 CrossRef CAS.
- C. Wang, S. Chen and L. Song, Adv. Funct. Mater., 2020, 30, 2000869 CrossRef CAS.
- R. Thakur, A. VahidMohammadi, J. Moncada, W. R. Adams, M. Chi, B. Tatarchuk, M. Beidaghi and C. A. Carrero, Nanoscale, 2019, 11, 10716–10726 RSC.
- J. Halim, I. Persson, P. Eklund, P. O. Persson and J. Rosen, RSC Adv., 2018, 8, 36785–36790 RSC.
- J. L. Hart, K. Hantanasirisakul, A. C. Lang, B. Anasori, D. Pinto, Y. Pivak, J. T. van Omme, S. J. May, Y. Gogotsi and M. L. Taheri, Nat. Commun., 2019, 10, 522 CrossRef CAS PubMed.
- D. Zhang, S. Wang, R. Hu, J. Gu, Y. Cui, B. Li, W. Chen, C. Liu, J. Shang and S. Yang, Adv. Funct. Mater., 2020, 30, 2002471 CrossRef CAS.
- N. Parra-Muñoz, M. Soler and A. Rosenkranz, Adv. Colloid Interface Sci., 2022, 309, 102792 CrossRef PubMed.
- Y. Xia, T. S. Mathis, M.-Q. Zhao, B. Anasori, A. Dang, Z. Zhou, H. Cho, Y. Gogotsi and S. Yang, Nature, 2018, 557, 409–412 CrossRef CAS PubMed.
- Z. Jin, Y. Fang, X. Wang, G. Xu, S. Wei, C. Zhou, Y. Zhang and Y. Xu, J. Colloid Interface Sci., 2019, 537, 306–315 CrossRef CAS PubMed.
- Q. Gao, X. Wang, D. W. Schubert and X. Liu, Adv. Nanocompos., 2024, 1, 52–76 CrossRef.
- L. Qin, Q. Tao, X. Liu, M. Fahlman, J. Halim, P. O. Persson, J. Rosen and F. Zhang, Nano Energy, 2019, 60, 734–742 CrossRef CAS.
- R. Sun, H. B. Zhang, J. Liu, X. Xie, R. Yang, Y. Li, S. Hong and Z. Z. Yu, Adv. Funct. Mater., 2017, 27, 1702807 CrossRef.
- Z. Ling, C. E. Ren, M. Q. Zhao, J. Yang, J. M. Giammarco, J. Qiu, M. W. Barsoum and Y. Gogotsi, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 16676–16681 CrossRef CAS PubMed.
- M. Boota, B. Anasori, C. Voigt, M.-Q. Zhao, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2016, 28, 1517–1522 CrossRef CAS PubMed.
- H. Lin, X. Wang, L. Yu, Y. Chen and J. Shi, Nano Lett., 2017, 17, 384–391 CrossRef CAS PubMed.
- A. A. Shamsabadi, F. Seidi, E. Salehi, M. Nozari, A. Rahimpour and M. Soroush, J. Mater. Chem. A, 2017, 5, 4011–4025 RSC.
- M. Seifert, A. H. Koch, F. Deubel, T. Simmet, L. H. Hess, M. Stutzmann, R. Jordan, J. A. Garrido and I. D. Sharp, Chem. Mater., 2013, 25, 466–470 CrossRef CAS.
- P. Xiao, C. Wan, J. Gu, Z. Liu, Y. Men, Y. Huang, J. Zhang, L. Zhu and T. Chen, Adv. Funct. Mater., 2015, 25, 2428–2435 CrossRef CAS.
- N. A. Hutter, A. Reitinger, N. Zhang, M. Steenackers, O. A. Williams, J. A. Garrido and R. Jordan, Phys. Chem. Chem. Phys., 2010, 12, 4360–4366 RSC.
- Z. Li, H. Zhang, J. Han, Y. Chen, H. Lin and T. Yang, Adv. Mater., 2018, 30, 1706981 CrossRef PubMed.
- S. Shah, I. Mubeen, E. Pervaiz, H. Nasir and S. Ahsan, Chem. Pap., 2023, 77, 5601–5621 CrossRef CAS.
- Z.-L. Tan, J.-X. Wei, Y. Liu, F. U. Zaman, W. Rehman, L.-R. Hou and C.-Z. Yuan, Rare Met., 2022, 41, 775–797 CrossRef CAS.
- P. Asen, J. Energy Storage, 2025, 105, 114601 CrossRef.
- L. Zhao and B. Li, Tungsten, 2020, 2, 176–193 CrossRef.
- J. Wang, Q. Qin, F. Li, Y. Anjarsari, W. Sun, R. Azzahiidah, J. Zou, K. Xiang, H. Ma and J. Jiang, Carbon Lett., 2023, 33, 1381–1394 CrossRef CAS.
- Y. S. Worku, L. L. Sikeyi, A. S. Olaleru, T. H. Dolla, N. Palaniyandy and M. M. Mathe, ChemistrySelect, 2025, 10, e202405649 CrossRef CAS.
- P. A. Rasheed, R. P. Pandey, F. Banat and S. W. Hasan, Matter, 2022, 5, 546–572 CrossRef CAS.
- A. Arjun, M. Ankitha, N. Shabana, P. Vaishag, F. Shamsheera, M. Mufeeda and P. A. Rasheed, FlatChem, 2023, 41, 100538 CrossRef CAS.
- D. L. Pawara, R. S. Tade, S. N. Nangare, P. O. Patil, P. K. Deshmukh, B. A. Vyas, S. B. Bari and M. P. More, J. Ind. Eng. Chem., 2024, 145, 1–19 Search PubMed.
- X. Cheng, L. Zu, Y. Jiang, D. Shi, X. Cai, Y. Ni, S. Lin and Y. Qin, Chem. Commun., 2018, 54, 11622–11625 RSC.
- M. Anayee, C. E. Shuck, M. Shekhirev, A. Goad, R. Wang and Y. Gogotsi, Chem. Mater., 2022, 34, 9589–9600 CrossRef CAS.
- H. Chen, Y. Wang, X. Chen, Z. Wang, Y. Wu, Q. Dai, W. Zhao, T. Wei, Q. Yang and B. Huang, Molecules, 2024, 29, 2902 CrossRef CAS PubMed.
- G. Perini, A. Rosenkranz, G. Friggeri, D. Zambrano, E. Rosa, A. Augello, V. Palmieri, M. De Spirito and M. Papi, Biomed. Pharmacother., 2022, 153, 113496 CrossRef CAS PubMed.
- C. Yu, S. Sui, X. Yu, W. Huang, Y. Wu, X. Zeng, Q. Chen, J. Wang and Q. Peng, Colloids Surf., B, 2022, 217, 112663 CrossRef CAS PubMed.
- R. Kumar and L. Singh, Adv. Mater. Technol., 2022, 7, 2200151 CrossRef CAS.
- B. Ran, C. Chen, B. Liu, M. Lan, H. Chen and Y. Zhu, Electrophoresis, 2022, 43, 2033–2043 CrossRef CAS PubMed.
- Z. Liu, M. Zhao, H. Lin, C. Dai, C. Ren, S. Zhang, W. Peng and Y. Chen, J. Mater. Chem. B, 2018, 6, 3541–3548 RSC.
- S. Zada, W. Dai, Z. Kai, H. Lu, X. Meng, Y. Zhang, Y. Cheng, F. Yan, P. Fu and X. Zhang, Angew. Chem., Int. Ed., 2020, 59, 6601–6606 CrossRef CAS PubMed.
- J. Wu, H. Yin, X. Song, M. Deng, B. Wang, X. Fei, J. Zhu, Z. Guo, H. Zhang and W. Chen, Adv. Funct. Mater., 2024, 34, 2313997 CrossRef CAS.
- N. Li, Y. Wang, Y. Li, C. Zhang and G. Fang, Small, 2024, 20, 2305645 CrossRef CAS PubMed.
- Z. Huang, X. Cui, S. Li, J. Wei, P. Li, Y. Wang and C.-S. Lee, Nanophotonics, 2020, 9, 2233–2249 CrossRef CAS.
- E. A. Hussein, M. M. Zagho, B. R. Rizeq, N. N. Younes, G. Pintus, K. A. Mahmoud, G. K. Nasrallah and A. A. Elzatahry, Int. J. Nanomed., 2019, 14, 4529–4539 CrossRef CAS PubMed.
- Z. Zhou, X. Li, T. Hu, B. Xue, H. Chen, L. Ma, R. Liang and C. Tan, Adv. NanoBiomed Res., 2022, 2, 2200065 CrossRef CAS.
- P.-S. Ganesh and S.-Y. Kim, J. Ind. Eng. Chem., 2022, 109, 52–67 CrossRef CAS.
- H. Lin, Y. Wang, S. Gao, Y. Chen and J. Shi, Adv. Mater., 2018, 30, 1703284 CrossRef PubMed.
- M. Naguib, J. Halim, J. Lu, K. M. Cook, L. Hultman, Y. Gogotsi and M. W. Barsoum, J. Am. Chem. Soc., 2013, 135, 15966–15969 CrossRef CAS PubMed.
- X. Han, X. Jing, D. Yang, H. Lin, Z. Wang, H. Ran, P. Li and Y. Chen, Theranostics, 2018, 8, 4491 CrossRef CAS PubMed.
- Z. H. Gharehaghaji, B. Khalilzadeh, H. Yousefi and R. Mohammad-Rezaei, Microchim. Acta, 2024, 191, 418 CrossRef CAS PubMed.
- R. C. Bast Jr, T. L. Klug, E. S. John, E. Jenison, J. M. Niloff, H. Lazarus, R. S. Berkowitz, T. Leavitt, C. T. Griffiths and L. Parker, N. Engl. J. Med., 1983, 309, 883–887 CrossRef CAS PubMed.
- I. Jacobs and R. C. Bast Jr, Hum. Reprod., 1989, 4, 1–12 CrossRef CAS PubMed.
- D. Gupta and C. G. Lis, J. Ovarian Res., 2009, 2, 1–20 CrossRef PubMed.
- P. S. Pakchin, M. Fathi, H. Ghanbari, R. Saber and Y. Omidi, Biosens. Bioelectron., 2020, 153, 112029 CrossRef PubMed.
- L. Qu, M. Wu, L. Zhao, J. Li and H. Pan, Microchim. Acta, 2023, 190, 147 CrossRef CAS PubMed.
- Q. Zhao, D. Lu, G. Zhang, D. Zhang and X. Shi, Talanta, 2021, 223, 121722 CrossRef CAS PubMed.
- P. D. Mehta, B. A. Patrick, D. L. Miller, P. K. Coyle and T. Wisniewski, J. Alzheimer's Dis., 2020, 78, 1237–1244 CAS.
- X. Huang, Y. Zhu and E. Kianfar, J. Mater. Res. Technol., 2021, 12, 1649–1672 CrossRef CAS.
- V. S. Sivasankarapillai, A. K. Somakumar, J. Joseph, S. Nikazar, A. Rahdar and G. Z. Kyzas, Nano-Struct., Nano-Objects, 2020, 22, 100457 CrossRef CAS.
- H. Li, 3rd International Academic Exchange Conference on Science and Technology Innovation (IAECST), 2021, pp. 1515–1519.
- V. Naresh and N. Lee, Sensors, 2021, 21, 1109 CrossRef CAS PubMed.
- M. H. Hooshiar, M. A. Moghaddam, M. Kiarashi, A. Y. Al-Hijazi, A. F. Hussein, H. A. Alrikabi, S. Salari, S. Esmaelian, H. Mesgari and S. Yasamineh, J. Biol. Eng., 2024, 18, 28 CrossRef PubMed.
- M. Li, R. Singh, Y. Wang, C. Marques, B. Zhang and S. Kumar, Biosensors, 2022, 12, 843 CrossRef CAS PubMed.
- P. Malik, R. Gupta, V. Malik and R. K. Ameta, Measurement: Sens., 2021, 16, 100050 Search PubMed.
- M. Pourmadadi, A. Moammeri, A. Shamsabadipour, Y. F. Moghaddam, A. Rahdar and S. Pandey, Biosensors, 2023, 13, 99 CrossRef CAS PubMed.
- R. S. Chouhan, M. Shah, D. Prakashan, R. PR, P. Kolhe and S. Gandhi, Diagnostics, 2023, 13(4), 697 CrossRef CAS PubMed.
- A. Parihar, A. Singhal, N. Kumar, R. Khan, M. A. Khan and A. K. Srivastava, Nanomicro Lett., 2022, 14, 100 CAS.
- J. Sengupta and C. M. Hussain, Biosensors, 2025, 15, 127 CrossRef CAS PubMed.
- F. Mollarasouli, S. Kurbanoglu and S. A. Ozkan, Biosensors, 2019, 9, 86 CrossRef CAS PubMed.
- M. Yang, H. Lu and S. Liu, Appl. Sci., 2022, 12, 5630 CrossRef CAS.
- E. P. Nguyen, C. d C. C. Silva and A. Merkoçi, Nanoscale, 2020, 12, 19043–19067 RSC.
- Z. Wu, T. Shang, Y. Deng, Y. Tao and Q. H. Yang, Adv. Sci., 2020, 7, 1903077 CrossRef CAS PubMed.
- J. Wu, Z. Wang, S. Zhang, Q. Yang, Z. Li, X. Zang, X. Zhao, N. Shang, N. Khaorapapong and X. Xu, Small, 2024, 20, 2305730 CrossRef CAS PubMed.
- Y. Cai, X. Chen, Y. Xu, Y. Zhang, H. Liu, H. Zhang and J. Tang, Carbon Energy, 2024, 6, e501 CrossRef CAS.
- J. Chen, Y. Chen, S. Li, J. Yang, J. Dong and X. Lu, Carbon, 2022, 199, 110–118 CrossRef CAS.
- S. Chen, J. Xu, M. Shi, Y. Yu, Q. Xu, X. Duan, Y. Gao and L. Lu, Appl. Surf. Sci., 2021, 570, 151149 CrossRef CAS.
- X. Ma, X. Tu, F. Gao, Y. Xie, X. Huang, C. Fernandez, F. Qu, G. Liu, L. Lu and Y. Yu, Sens. Actuators, B, 2020, 309, 127815 CrossRef CAS.
- Q. Wang, X. Xiao, X. Hu, L. Huang, T. Li and M. Yang, Mater. Lett., 2021, 285, 129158 CrossRef CAS.
- M. Yang, L. Wang, C. Xie, H. Lu, J. Wang, Y. Li, H. Li, J. Yang, T. Zhang and S. Liu, Talanta, 2025, 281, 126893 CrossRef CAS PubMed.
- X. Mei, Z. Zeng, W. Xu, H. Yang, Y. Zheng, H. Gao, C. Wu, Y. Zheng, Q. Xu and G. Wang, Anal. Sci., 2024, 40, 1081–1087 CrossRef CAS PubMed.
- Z. H. Gharehaghaji, R. Mohammad-Rezaei and B. Khalilzadeh, Microchem. J., 2024, 206, 111633 CrossRef.
- Y. Xu, Y. S. Ang, L. Wu and L. K. Ang, Nanomaterials, 2019, 9, 165 CrossRef CAS PubMed.
- Z. Zou, Y. Chen, R. Sun, B. Shi, L. Jiang and F. Huang, Microchim. Acta, 2025, 192, 168 CrossRef CAS PubMed.
- K. Maeda, H. Sasaki, S. Ueda, S. Miyamoto, S. Terada, H. Konishi, Y. Kogata, K. Ashihara, S. Fujiwara and Y. Tanaka, J. Ovarian Res., 2020, 13, 1–9 CrossRef PubMed.
- G. Jamalipour Soufi, P. Iravani, A. Hekmatnia, E. Mostafavi, M. Khatami and S. Iravani, Comments Inorg. Chem., 2022, 42, 174–207 CrossRef CAS.
- J. M. Garcia, C. Peña, V. García, G. Domínguez, C. N. Muñoz, J. Silva, I. Millán, R. Diaz, Y. Lorenzo and R. Rodriguez, Clin. Cancer Res., 2007, 13, 6351–6358 CrossRef CAS PubMed.
- M. Herbsleb, K. Birkenkamp-Demtroder, T. Thykjaer, C. Wiuf, A.-M. K. Hein, T. F. Ørntoft and L. Dyrskjøt, BMC Med. Genomics, 2008, 1, 1–17 CrossRef PubMed.
- H. Shimada, S. Satohisa, T. Kohno, S. Takahashi, T. Hatakeyama, T. Konno, M. Tsujiwaki, T. Saito and T. Kojima, Oncotarget, 2016, 7, 27735 CrossRef PubMed.
- J. Li, S. Dowdy, T. Tipton, K. Podratz, W.-G. Lu, X. Xie and S.-W. Jiang, Expert Rev. Mol. Diagn., 2009, 9, 555–566 CrossRef CAS PubMed.
- K. Huhtinen, P. Suvitie, J. Hiissa, J. Junnila, J. Huvila, H. Kujari, M. Setälä, P. Härkki, J. Jalkanen and J. Fraser, Br. J. Cancer, 2009, 100, 1315–1319 CrossRef CAS PubMed.
- J. M. Escudero, J. M. Auge, X. Filella, A. Torne, J. Pahisa and R. Molina, Clin. Chem., 2011, 57, 1534–1544 CrossRef CAS PubMed.
- R. G. Moore, A. K. Brown, M. C. Miller, S. Skates, W. J. Allard, T. Verch, M. Steinhoff, G. Messerlian, P. DiSilvestro and C. Granai, Gynecol. Oncol., 2008, 108, 402–408 CrossRef CAS PubMed.
- D. Trudel, B. Têtu, J. Grégoire, M. Plante, M.-C. Renaud, D. Bachvarov, P. Douville and I. Bairati, Gynecol. Oncol., 2012, 127, 511–515 CrossRef CAS PubMed.
- Y. Chen, Y. Huang, S. Chen, L. Gao, S. Zhang, H. Dai and B. Zeng, Talanta, 2023, 265, 124876 CrossRef CAS PubMed.
- L. Du, Y. Chen, Y. Huang, S. Yan, S. Zhang and H. Dai, Microchim. Acta, 2023, 190, 108 CrossRef CAS PubMed.
- N. Yang, W. Xiao, X. Song, W. Wang and X. Dong, Nano-Micro Lett., 2020, 12, 1–27 CrossRef PubMed.
- S. Ramaraj, M. Sakthivel, S.-M. Chen, B.-S. Lou and K.-C. Ho, ACS Appl. Mater. Interfaces, 2019, 11, 7862–7871 CrossRef CAS PubMed.
- R. D. Nagarajan, P. Murugan, K. Palaniyandi, R. Atchudan and A. K. Sundramoorthy, Micromachines, 2021, 12, 862 CrossRef PubMed.
- S. Rajaie, M. Nasiri, A. Pasdar, M. Rezayi and M. Khazaei, Microchem. J., 2023, 193, 109016 CrossRef CAS.
- S. Zhang, Y. Huang, L. Gao, Y. Chen and H. Dai, Biosens. Bioelectron., 2025, 277, 117224 CrossRef CAS PubMed.
- Y. Huang, S. Chen, S. Zhang, L. Gao, F. Lin and H. Dai, J. Colloid Interface Sci., 2024, 661, 793–801 CrossRef CAS PubMed.
- H. Zhang, Z. Wang, F. Wang, Y. Zhang, H. Wang and Y. Liu, Talanta, 2021, 224, 121879 CrossRef CAS PubMed.
- Q. Zhang, F. Wang, H. Zhang, Y. Zhang, M. Liu and Y. Liu, Anal. Chem., 2018, 90, 12737–12744 CrossRef CAS PubMed.
- S.-Q. Yu, P. Li, H.-J. Li, L.-J. Shang, R. Guo, X.-M. Sun and Q.-Q. Ren, Biosensors, 2024, 14, 261 CrossRef CAS PubMed.
- M. Choramle, D. Verma, A. Kalkal, R. Pradhan, A. K. Rai and G. Packirisamy, Anal. Methods, 2024, 16, 4938–4950 RSC.
- Y. Guo, Y. Nie, P. Wang, Z. Li and Q. Ma, Talanta, 2023, 259, 124559 CrossRef CAS PubMed.
- L. Liu, C. Shangguan, J. Guo, K. Ma, S. Jiao, Y. Yao and J. Wang, Adv. Opt. Mater., 2020, 8, 2001214 CrossRef CAS.
- S. Ranjbari, M. Rezayi, R. Arefinia, S. H. Aghaee-Bakhtiari, B. Hatamluyi and A. Pasdar, Talanta, 2023, 255, 124247 CrossRef CAS PubMed.
- Y. Gogotsi and B. Anasori, MXenes, Jenny Stanford Publishing, 2023, pp. 3–11 Search PubMed.
- Y. Chen, L. Wang and J. Shi, Nano Today, 2016, 11, 292–308 CrossRef CAS.
- W. Feng, R. Wang, Y. Zhou, L. Ding, X. Gao, B. Zhou, P. Hu and Y. Chen, Adv. Funct. Mater., 2019, 29, 1901942 CrossRef.
- Y. Dong, L. Guo, L. Song, T. Liu, G. Zheng, M. Zheng and B. Li, Int. J. Nanomed., 2025, 1983–1998 CrossRef PubMed.
- T. Liao, Z. Chen, Y. Kuang, Z. Ren, W. Yu, W. Rao, L. Li, Y. Liu, Z. Xu and B. Jiang, Acta Biomater., 2023, 159, 312–323 CrossRef CAS PubMed.
- D. An, X. Wu, Y. Gong, W. Li, G. Dai, X. Lu, L. Yu, W. X. Ren, M. Qiu and J. Shu, Nanophotonics, 2022, 11, 5177–5188 CrossRef CAS PubMed.
- P. Xiong, X. Wei, L. Zhou, W. Zhou, M. Li, Y. Ge, J. Zou, S. Peng, L. Jiang and L. Tian, Adv. Funct. Mater., 2024, 34, 2405124 CrossRef CAS.
- A. Gazzi, L. Fusco, A. Khan, D. Bedognetti, B. Zavan, F. Vitale, A. Yilmazer and L. G. Delogu, Front. Bioeng. Biotechnol., 2019, 7, 295 CrossRef PubMed.
- Q. Zhang, W. Huang, C. Yang, F. Wang, C. Song, Y. Gao, Y. Qiu, M. Yan, B. Yang and C. Guo, Biomater. Sci., 2019, 7, 2729–2739 RSC.
- X. Wang, X. Wang, Q. Yue, H. Xu, X. Zhong, L. Sun, G. Li, Y. Gong, N. Yang and Z. Wang, Nano Today, 2021, 39, 101170 CrossRef CAS.
- X. Jiang, H. Wang, Y. Shen, N. Hu and W. Shi, Sens. Actuators, B, 2022, 350, 130891 CrossRef CAS.
- G. Liu, J. Zhu, H. Guo, A. Sun, P. Chen, L. Xi, W. Huang, X. Song and X. Dong, Angew. Chem., Int. Ed., 2019, 58, 18641–18646 CrossRef CAS PubMed.
- B. E. Scheibe, J. K. Wychowaniec, M. Scheibe, B. Peplińska, M. Jarek, G. Nowaczyk and Ł. Przysiecka, ACS Biomater. Sci. Eng., 2019, 5, 6557–6569 CrossRef CAS PubMed.
- S. Cheng, T. Zhou, Y. Luo, J. Zhang, K. Dong, Q. Zhang, W. Shu, T. Zhang, Q. Zhang and R. Shi, J. Nanobiotechnol., 2024, 22, 408 CrossRef CAS PubMed.
- L. Yang, Z. He, L. Hu, H. Tang, Y. Geng, Q. Tan, Y. Zhang, Y. Wen, W. Wu and H. Gu, J. Nanobiotechnol., 2024, 22, 242 CrossRef CAS PubMed.
- Y. Yao, J. Zhang, K. Huang, Y. Peng, S. Cheng, S. Liu, T. Zhou, J. Chen, H. Li and Y. Zhao, J. Nanobiotechnol., 2025, 23, 83 CrossRef CAS PubMed.
- J. M. Liberto, S.-Y. Chen, I.-M. Shih, T.-H. Wang, T.-L. Wang and T. R. Pisanic, Cancers, 2022, 14, 2885 CrossRef CAS PubMed.
- S. S. Buys, E. Partridge, M. H. Greene, P. C. Prorok, D. Reding, T. L. Riley, P. Hartge, R. M. Fagerstrom, L. R. Ragard and D. Chia, Am. J. Obstet. Gynecol., 2005, 193, 1630–1639 CrossRef PubMed.
- P. F. Pinsky, K. Yu, B. S. Kramer, A. Black, S. S. Buys, E. Partridge, J. Gohagan, C. D. Berg and P. C. Prorok, Gynecol. Oncol., 2016, 143, 270–275 CrossRef PubMed.
- S. J. Goldsmith, Semin. Nucl. Med., 1975, 5(2), 125–152 CrossRef CAS PubMed.
- K. Saito, D. Kobayashi, M. Sasaki, H. Araake, T. Kida, A. Yagihashi, T. Yajima, H. Kameshima and N. Watanabe, Clin. Chem., 1999, 45, 665–669 CrossRef CAS.
- A. C. Moser and D. S. Hage, Electrophoresis, 2008, 29, 3279–3295 CrossRef CAS PubMed.
- S. Hu, S. Zhang, Z. Hu, Z. Xing and X. Zhang, Anal. Chem., 2007, 79, 923–929 CrossRef CAS PubMed.
- R. Aebersold and M. Mann, Nature, 2003, 422, 198–207 CrossRef CAS PubMed.
- A. M. Yates, S. J. Elvin and D. E. Williamson, J. Immunoassay, 1999, 20, 31–44 CrossRef CAS PubMed.
- N. Scholler, M. Crawford, A. Sato, C. W. Drescher, K. C. O'Briant, N. Kiviat, G. L. Anderson and N. Urban, Clin. Cancer Res., 2006, 12, 2117–2124 CrossRef CAS PubMed.
- N. Razmi and M. Hasanzadeh, TrAC, Trends Anal. Chem., 2018, 108, 1–12 CrossRef CAS.
- M. Li, M. Zhang, S. Ge, M. Yan, J. Yu, J. Huang and S. Liu, Sens. Actuators, B, 2013, 181, 50–56 CrossRef CAS.
- W. Liu, C. Ma, H. Yang, Y. Zhang, M. Yan, S. Ge, J. Yu and X. Song, Microchim. Acta, 2014, 181, 1415–1422 CrossRef CAS.
- L. Wu, Y. Sha, W. Li, S. Wang, Z. Guo, J. Zhou, X. Su and X. Jiang, Sens. Actuators, B, 2016, 226, 62–68 CrossRef CAS.
- W. Mu, C. Wu, F. Wu, H. Gao, X. Ren, J. Feng, M. Miao, H. Zhang, D. Chang and H. Pan, J. Pharm. Biomed. Anal., 2024, 243, 116080 CrossRef CAS PubMed.
- Z. Chen, B. Li, J. Liu, H. Li, C. Li, X. Xuan and M. Li, Microchim. Acta, 2022, 189, 257 CrossRef CAS PubMed.
- A. Sangili, T. Kalyani, S.-M. Chen, A. Nanda and S. K. Jana, ACS Appl. Bio Mater., 2020, 3, 7620–7630 CrossRef CAS PubMed.
- Z. Wang, H. Yang, B. Gao, Y. Tong, X. Zhang and L. Su, Analyst, 2014, 139, 1127–1133 RSC.
- G. Wang, G. Zhang, H. Huang and L. Wang, Anal. Methods, 2011, 3, 2082–2087 RSC.
- T. Yang, Y. Gao, Z. Liu, J. Xu, L. Lu and Y. Yu, Sens. Actuators, B, 2017, 239, 76–84 CrossRef CAS.
- Z. Gharehaghaji, R. Mohammad-Rezaei and B. Khalilzadeh, Microchem. J., 2024, 206, 111633 CrossRef.
- V. Bhardwaj, A. Kaushik, Z. M. Khatib, M. Nair and A. J. McGoron, Front. Pharmacol., 2019, 10, 1369 CrossRef PubMed.
- A. Szuplewska, D. Kulpińska, M. Jakubczak, A. Dybko, M. Chudy, A. Olszyna, Z. Brzózka and A. M. Jastrzębska, Adv. Drug Delivery Rev., 2022, 182, 114099 CrossRef CAS PubMed.
- S. Nandi, D. Karati and S. Mukherjee, Inorg. Chem. Commun., 2024, 170, 113214 CrossRef CAS.
- N. Gao, J. Zhao, X. Zhu, J. Xu, G. Ling and P. Zhang, Acta Biomater., 2022, 154, 1–22 CrossRef CAS PubMed.
- S. Iravani and R. S. Varma, Nanomaterials, 2022, 12, 3360 CrossRef CAS PubMed.
- B. F. Far, N. Rabiee and S. Iravani, RSC Adv., 2023, 13, 34562–34575 RSC.
- N. Rabiee and S. Iravani, Mater. Chem. Horiz., 2023, 2, 171–184 Search PubMed.
- J. Huang, Z. Li, Y. Mao and Z. Li, Nano Select., 2021, 2, 1480–1508 CrossRef CAS.
- M. Liao, Q. Cui, Y. Hu, J. Xing, D. Wu, S. Zheng, Y. Zhao, Y. Yu, J. Sun and R. Chai, Neural Regener. Res., 2024, 19, 258–263 CrossRef CAS PubMed.
- A. Maleki, M. Ghomi, N. Nikfarjam, M. Akbari, E. Sharifi, M. A. Shahbazi, M. Kermanian, M. Seyedhamzeh, E. Nazarzadeh Zare and M. Mehrali, Adv. Funct. Mater., 2022, 32, 2203430 CrossRef CAS.
- M. E. Marchwiany, M. Birowska, M. Popielski, J. A. Majewski and A. M. Jastrzębska, Materials, 2020, 13, 3083 CrossRef CAS PubMed.
- S. Iravani, A. Khosravi, E. Nazarzadeh Zare, R. S. Varma, A. Zarrabi and P. Makvandi, RSC Adv., 2024, 14, 36835–36851 RSC.
| This journal is © The Royal Society of Chemistry 2025 |

