Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Natural dielectrics for organic field effect transistors: a study on resins derived from larch, spruce and Atlas cedar Pinaceae trees
Corina
Schimanofsky
 a,
Andreas
Petritz
a,
Andreas
Petritz
 b,
Boyuan
Ban
b,
Boyuan
Ban
 cd,
Cristian Vlad
Irimia
cd,
Cristian Vlad
Irimia
 ab,
Rosarita
D’Orsi
ab,
Rosarita
D’Orsi
 e,
Cigdem
Yumusak
e,
Cigdem
Yumusak
 a,
Felix
Mayr
a,
Felix
Mayr
 a,
Yasin
Kanbur
a,
Yasin
Kanbur
 af,
Sunwoo
Kim
ag,
Alessandra
Operamolla
af,
Sunwoo
Kim
ag,
Alessandra
Operamolla
 e,
Klara
Saller
e,
Klara
Saller
 h,
Manuela
Schiek
h,
Manuela
Schiek
 ai,
Yolanda
Salinas
ai,
Yolanda
Salinas
 jk,
Oliver
Brüggemann
jk,
Oliver
Brüggemann
 j,
Christian
Teichert
j,
Christian
Teichert
 c,
Chunlin
Xu
c,
Chunlin
Xu
 l,
Bong Sup
Shim
l,
Bong Sup
Shim
 g,
Clemens
Schwarzinger
g,
Clemens
Schwarzinger
 h,
Barbara
Stadlober
h,
Barbara
Stadlober
 b,
Niyazi Serdar
Sariciftci
b,
Niyazi Serdar
Sariciftci
 a and
Mihai
Irimia-Vladu
a and
Mihai
Irimia-Vladu
 *ab
*ab
aJohannes Kepler University Linz, Institute of Physical Chemistry, Linz Institute for Organic Solar Cells (LIOS), Altenberger Str. 69, Linz, 4040, Austria. E-mail: mihai.irimia-vladu@jku.at
bJoanneum Research Materials, Institute for Surface Technologies and Photonics, Franz-Pichler Str. 30, Weiz, 8169, Austria
cChair of Physics, Department of Physics, Mechanics, and Electrical Engineering, Montanuniversität Leoben, Franz Josef Str. 18, 8700 Leoben, Austria
dInstitute of Solid-State Physics, Hefei Institute of Physical Science, Key Lab of Photovoltaic and Energy Conservation Materials, Hefei, China
eDepartment of Chemistry and Industrial Chemistry, University of Pisa, via Moruzzi 13, 56124 Pisa, Italy
fDepartment of Chemistry, Karabük University, Baliklarkayasi Mevkii, Karabük 78050, Turkey
gDepartment of Chemical Engineering, Inha University, 100 Inha-ro, Michuhol-gu, Incheon 22212, Republic of Korea
hInstitute for Chemical Technology of Organic Materials, Johannes Kepler University Linz, Altenberger Str. 69, Linz, 4040, Austria
iJohannes Kepler University Linz, Center for Surface and Nanoanalytics (ZONA), Altenberger 69, 4040 Linz, Austria
jInstitute of Polymer Chemistry, Johannes Kepler University Linz, Altenberger 69, 4040 Linz, Austria
kIMC Krems University of Applied Sciences, Institute of Applied Chemistry, Piaristengasse 1, 3500 Krems, Austria
lLaboratory of Natural Materials Technology/Wood and Paper Chemistry, Åbo Akademi University, Porthansgatan 3-5, 20500 Åbo, Turku, Finland
First published on 18th June 2025
Abstract
Three Pinaceae resins originating from trees of high industrial significance—European larch, European spruce, and Atlas cedar—were examined in this work. These resins exhibited ease of processing using ethyl alcohol solutions, exceptional film formation, and great dielectric qualities with measured breakdown fields in the range of 5–7.3 MV cm−1. Because their film surface was essentially trap-free, it was possible to fabricate organic field effect transistors that are hysteresis-free and have outstanding stability under 12-hour bias stress at working voltages below 10 V, with current retention approaching 90% of the original value and transfer curve recovery occurring within 90 minutes. These environmentally friendly materials, which are freely available, are a great option for applications aiming to produce sustainable electronics.
1. Introduction
Despite the industrial and commercial potential of organic materials in applications such as organic and perovskite photovoltaics, with reports of efficiencies exceeding 20%, or even light emitting diodes, the development of organic field effect transistors lags behind that of the two counterparts mentioned above. This is mainly due to the low field effect mobility of the employed organic semiconductors and their limited stability and performance when operated in the radio frequency (RF) regime.1–11 Although the field of synthetic chemistry has contributed to the tremendous advancement in the performance of organic semiconductors in the recent past,3,12–14 many other challenges in terms of materials and their performance remain to be addressed. In this respect, the development of novel substrate and packaging materials, dielectrics and processing conditions are topics of utmost interest for materials scientists.15–20 Because of their inherent softness and flexibility, which match the elastic modulus of living cells, organic electronic component materials seem to be perfectly suited for applications interfacing electronics and sensors with living systems and developing disposable diagnostic and drug-delivery technologies.21–40 In these applications, fast recording and data transmission speeds are not essential requirements. In order to lessen the environmental impact of e-waste, there is a significant need for sustainable waste management solutions as well as green and biodegradable materials owing to the rapidly expanding application of electronic and sensor devices.41–45 In the recent studies on flexible, conformable, and even imperceptible substrates, organic field-effect transistors (OFETs) and organic electrochemical transistors (OECTs) have been explored as viable electrical devices for a variety of applications.46–50 In general, OFETs consist of an organic semiconducting and organic dielectric layer sandwiched between three electrodes (gate, source and drain).51 Traditional FETs usually employ inorganic materials such as silicon dioxide (SiO2),52 aluminum oxide (Al2O3)53,54 and other metal oxides (such as titanium, hafnium, tungsten, and tantalum oxides) as the insulating layer.55 Despite the inherently higher performance of inorganic dielectric layers, mostly due to the higher purity and order provided by the strong covalent bonds between their component atoms,56 the scientific community is becoming increasingly interested in creating OFETs using organic materials that are environmentally benign (even biodegradable), non-toxic, renewable and inexpensive and enable easy processing at low temperatures.57–61 Thus, in this work, we complement the efforts by the scientific community in the direction of sustainable electronics production by demonstrating that three natural resins stemming from the widely exploited, industrially relevant Pinaceae trees, i.e., larch, Atlas cedar and spruce, are excellent dielectrics to consider for the fabrication of environmentally friendly electronics.The Atlas cedar (Cedrus atlantica) resin reported herein was collected from a living tree growing in the city of Graz, Austria at an altitude of circa 350 m. The original resin deposit was captured in the photograph in Fig. 1. The tree from which the resin was collected was an ornamental cultivar. Cedrus atlantica is considered to have its origins from the Atlas Mountains in Northern Africa (Morocco) and is widespread around the Mediterranean basin, being closely related to the Lebanese cedar (Cedrus libani).62 However, Quai et al.63 argued based on the Phylogenies dataset analysis of Cedrus constructed from cpDNA, mtDNA and the combined cp- and mt-DNA that Cedrus atlantica could have originated in the high altitudes of Eurasia, and that Cedrus migrated into North Africa in the very late Tertiary Period. Many plantations of Atlas cedar are intentionally developed in Europe for timber production.62,64 Due to its majestic-round crown, the Atlas cedar is also intentionally planted in many city parks and residential areas for esthetic reasons.
 | ||
| Fig. 1 Photograph of the original deposit of the resins analyzed in this study: (a) larch; (b) Atlas cedar; and (c) spruce. | ||
The European larch (Larix decidua) resin reported herein was collected from a cultivar tree growing in the forest around Joanneum Research Materials, Weiz, Austria, at an altitude of circa 570 m, with the original resin deposit being captured in the photograph in Fig. 1. European larch is a fast-growing deciduous coniferous tree, which is mainly found in the southern, eastern and central mountains of Europe.65 It can be divided into three groups according to its geographic distribution including Alpine larch (Larix decidua var. decidua), Carpathian larch (Larix decidua var. carpatica), and Poland larch (Larix decidua var. polonica). Larch trees can grow up to approximately 50 meters with a lifespan of up to a couple of thousand years, being in fact the tallest of all Pinaceae trees.66,67 Owing to their strong adaptability, avoiding foliage desiccation by losing their needles in the winter in the harsh cold environment, larch trees have also been planted in other areas, such as north-western Europe, Siberia, Japan, North America, and New Zealand. Considering that larch wood has high durability, it is often used as timber and pulp fibers for manufacturing furniture and paper, respectively. Moreover, attempts have been made to valorize other components in addition to fibers. For example, as side products to pulp and paper, tannins can be extracted from bark and resins from wood. Distillation of larch resin produces turpentine, which is used as traditional medicine for treating colds.68
The European spruce (Picea abies) resin reported herein was collected from a cultivar tree growing in the forest around the Mariatrost neighborhood in the city of Graz, Austria, at an altitude of circa 405 m. The original resin deposit was captured in the photograph in Fig. 1. The Latin name abies means “fir-like” in the sense that it refers to it being tall., i.e., fir-like tall. The European spruce is widely planted for wood and represents the most prevalent tree in Eurasia, filling more than 30 million hectares of forest land, or more than 55% of the entire forest area of Eurasia.69 The reason for this widespread distribution is the re-plantation of the European forests over the past centuries. Because of this re-plantation, nowadays spruce occupies more than 20% of the land outside its native range, primarily on the land of broadleaved forest sites at low altitudes.70Picea abies is also used as the main Christmas tree in several countries around the world. Importantly, spruce also represents the first gymnosperm to have its genome sequenced.71
2. Experimental
The resins employed in this work were collected from living trees in Austria, Europe. The resins were solubilized in pure ethanol (99.9%) in 0.1 g mL−1 concentration by heating the solution while stirring for 30 min at 50 °C. The solution was filtered through a Chromafil, 0.2 μm pore size hydrophilic filter paper, and then diluted to the concentration employed for depositing thin films in this work, i.e., 25 mg mL−1 or 20 mg mL−1, respectively. All the resin thin films investigated in this work were spin coated at a rotation speed of 2500 rpm, and then dried on the top of a hot plate in air for 1 h at a temperature not exceeding 80 °C.A TGA/PerkinElmer Q5000 instrument at the Institute of Polymer Chemistry, Johannes Kepler University Linz, Austria was used to perform thermogravimetric tests utilizing platinum pans and scanning from 70 °C to 900 °C at a heating rate of 10 °C min−1 in a nitrogen environment (25 mL min−1). Using the same experimental heating setup and protocol, all four pine resin samples (in amounts ranging from 5 to 15 mg) were examined. The content of each resin material was taken from its corresponding pellet (nugget).
We performed gas chromatography measurements for the three resins in this study by dissolving each resin in 2 mL ethanol and used the solutions for the respective study (usually containing around 200 mg dry resin per sample; however, some solutions contained around 40–60 mg of dry resin). From each solution, 100 μL was withdrawn and transferred into a tared 1.5-mL glass vial with the aid of a microbalance. The solvent was evaporated to dryness using a stream of nitrogen, and then the vials were kept in a vacuum oven (at 40 °C) for ca. 30 min. After cooling to room temperature, the vials were weighed for the determination of the dry extract weight. A volume of 1.0 or 1.5 mL of acetone was added, and the vials were kept for circa 1 min in an ultrasonic bath. A volume corresponding to around 0.6 mg of each dry resin was withdrawn and transferred to a 6 mL test tube. Then, 2 mL of a solution containing 40 μg each of four internal standards (ISs) was added, and the solvent was evaporated to dryness using a stream of nitrogen in a 40 °C water bath. Subsequently, silylation reagents were added including pyridine-BSTFA-TMCS 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 μL, and the tubes were kept in an oven at 70 °C for 30 min, where BSTFA-TMSC stands for N,O-bis-(trimethylsilyl)-trifluoroacetamide with trimethylchlorosilane. After this, the solutions were transferred to 1.5-mL GC vials using Pasteur pipettes with a glass insert. Then approximately 1 μL of each sample was injected to the GC-MS. Identification was performed by comparing the mass spectra with that in spectra databases including NIST12/Wiley 11th and our own database (i.e., ABÖ Akademi, Finland). The peak areas were integrated and the concentration of each compound was calculated by dividing the peak area with that of the IS (heneicosanoic acid for all compounds was eluted before cholestadiene, and cholesterol or betulinol for cholestadiene and lignans). Then, the result was multiplied by the added amount of IS, and the result was divided by the amount of dry resin taken for the analysis.
20 μL, and the tubes were kept in an oven at 70 °C for 30 min, where BSTFA-TMSC stands for N,O-bis-(trimethylsilyl)-trifluoroacetamide with trimethylchlorosilane. After this, the solutions were transferred to 1.5-mL GC vials using Pasteur pipettes with a glass insert. Then approximately 1 μL of each sample was injected to the GC-MS. Identification was performed by comparing the mass spectra with that in spectra databases including NIST12/Wiley 11th and our own database (i.e., ABÖ Akademi, Finland). The peak areas were integrated and the concentration of each compound was calculated by dividing the peak area with that of the IS (heneicosanoic acid for all compounds was eluted before cholestadiene, and cholesterol or betulinol for cholestadiene and lignans). Then, the result was multiplied by the added amount of IS, and the result was divided by the amount of dry resin taken for the analysis.
To gain a better understanding of the three resin compositions, in addition to gas chromatography, we also performed HPLC high-resolution MS analyses (HPLC-MS) of all the samples and pyrolysis-gas chromatography-mass spectrometry (pyrolysis-GC-MS) analysis, given that this technique does not discriminate the insoluble fractions of the resins. The molar mass distribution of the resins was obtained by size exclusion chromatography. In the case of HPLC-MS, roughly 10 mg of the sample was mixed with 1 mL of acetonitrile and sonicated for 5 min, centrifuged and the soluble fraction analyzed. The characterization of resin compounds was performed using a Thermo Scientific Surveyor HPLC system coupled to a LTQ Orbitrap Velos mass spectrometer, available at the Institute for Chemical Technology of Organic Materials, Johannes Kepler University Linz, Austria. The compounds were separated on a Thermo Scientific Accucore C18 column (150 mm × 3.0 mm, 2.6 μm particle size) using a gradient with mobile phase A containing 0.1% formic acid in water and mobile phase B containing 0.1% formic acid in acetonitrile, at a flow rate of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mL min−1. The elution gradient starting conditions were 95% A and 5% B. After 2
mL min−1. The elution gradient starting conditions were 95% A and 5% B. After 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min of equilibration time, the proportion of B was increased to 20% at 8
min of equilibration time, the proportion of B was increased to 20% at 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min, to 40% at 12
min, to 40% at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min, to 60% at 15
min, to 60% at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min and to 95% at 19
min and to 95% at 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min, and held constant for another 4 min. UV detection was done by a photodiode array detector and mass spectra were recorded with an atmospheric pressure chemical ionization interface in FT mode with a resolution of 30
min, and held constant for another 4 min. UV detection was done by a photodiode array detector and mass spectra were recorded with an atmospheric pressure chemical ionization interface in FT mode with a resolution of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. In pyrolysis-GC-MS, to achieve a better performance in the analysis of natural products, pyrolysis in the presence of tetramethylammonium hydroxide (also known as thermally assisted hydrolysis and methylation) was performed. Experiments were carried out with a CDS Pyroprobe 5250 pyrolyzer (CDS Analytical Inc.) coupled to a Trace GC Ultra (Thermo Electron Corp.) equipped with a Restek RTX35 (30 m × 0.32 mm × 0.25 μm) capillary column, and an MD 800 quadrupole mass spectrometer (Fisons Instruments), available at the Institute for Chemical Technology of Organic Materials, Johannes Kepler University Linz, Austria. Briefly, 5 μL of a saturated aqueous tetramethylammonium hydroxide (TMAH, Fluka) solution was added to about 100 μg of sample and pyrolysis was performed at 550 °C for 10 s. The pyrolizer interface was set at 300 °C and the injector at 280 °C. The GC column temperature conditions were as follows: initial temperature 50 °C, hold for 2 min, increase at 20 °C min−1 to 300 °C, and hold this temperature for 10 min. The helium gas flow was set to 0.8 mL min−1 and the split flow was 14 mL min−1. Mass spectra were recorded under electron impact ionization at 70 eV electron energy in the m/z range of 15–400. Identification of the compounds was done by comparison of their mass spectra with the NIST 2011 electronic library and literature. In size exclusion chromatography, the samples were mixed with tetrahydrofuran (THF) and the insoluble part filtered off. Separation was carried out using three Phenomenex Phenogel columns (300 × 4.6 mm) with pore sizes of 50, 500 and 1000 Å at a temperature of 40 °C under a THF flow of 0.35 mL min−1. Detection was done using a refractive index and a UV detector, and for calibration, a set of polystyrene standards was used.
000. In pyrolysis-GC-MS, to achieve a better performance in the analysis of natural products, pyrolysis in the presence of tetramethylammonium hydroxide (also known as thermally assisted hydrolysis and methylation) was performed. Experiments were carried out with a CDS Pyroprobe 5250 pyrolyzer (CDS Analytical Inc.) coupled to a Trace GC Ultra (Thermo Electron Corp.) equipped with a Restek RTX35 (30 m × 0.32 mm × 0.25 μm) capillary column, and an MD 800 quadrupole mass spectrometer (Fisons Instruments), available at the Institute for Chemical Technology of Organic Materials, Johannes Kepler University Linz, Austria. Briefly, 5 μL of a saturated aqueous tetramethylammonium hydroxide (TMAH, Fluka) solution was added to about 100 μg of sample and pyrolysis was performed at 550 °C for 10 s. The pyrolizer interface was set at 300 °C and the injector at 280 °C. The GC column temperature conditions were as follows: initial temperature 50 °C, hold for 2 min, increase at 20 °C min−1 to 300 °C, and hold this temperature for 10 min. The helium gas flow was set to 0.8 mL min−1 and the split flow was 14 mL min−1. Mass spectra were recorded under electron impact ionization at 70 eV electron energy in the m/z range of 15–400. Identification of the compounds was done by comparison of their mass spectra with the NIST 2011 electronic library and literature. In size exclusion chromatography, the samples were mixed with tetrahydrofuran (THF) and the insoluble part filtered off. Separation was carried out using three Phenomenex Phenogel columns (300 × 4.6 mm) with pore sizes of 50, 500 and 1000 Å at a temperature of 40 °C under a THF flow of 0.35 mL min−1. Detection was done using a refractive index and a UV detector, and for calibration, a set of polystyrene standards was used.
Phosphitylation of samples (i.e., 31P-NMR spectra) was performed adapting the method described elsewhere.72 The samples were dried overnight in an oven set at 40 °C, and then transferred to a desiccator until they reached room temperature. A mixture of pyridine and deuterated chloroform (CDCl3) with the ratio 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v
1 v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v was prepared and dried over molecular sieves. Using this mixture, a 0.1 M solution of the relaxation reagent, chromium(III) acetylacetonate (5 mg mL−1), and the internal standard, cholesterol (40 mg mL−1), was prepared. All the solutions were stored in the dark. About 40 mg of sample was dissolved in 0.5 mL of solvent solution in a vial equipped with a stirring bar. Then, 0.1 mL of the internal standard and relaxation solution was added, and the solution was stirred for ∼4 h to achieve solubilization of the sample. 0.1 mL of 2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane (TMDP) was added to the clear solution and it was kept under vigorous magnetic stirring for 30 min. The resulting solution was transferred into a NMR tube. 31P-NMR spectra were recorded on a JEOL YH spectrometer with a probe operating at 202.468 MHz at 25 °C, available at the Department of Chemistry and Industrial Chemistry of University of Pisa, Italy. Chemical shifts were calibrated using the 31P-NMR signal at 132.2 ppm arising from the reaction product between residual water and TMDP. The spectra were quantitative and proton broadband decoupling was applied during the acquisition time. Cholesterol was used as an internal standard. Spectra were acquired with a 100 ppm spectral width, 32
v was prepared and dried over molecular sieves. Using this mixture, a 0.1 M solution of the relaxation reagent, chromium(III) acetylacetonate (5 mg mL−1), and the internal standard, cholesterol (40 mg mL−1), was prepared. All the solutions were stored in the dark. About 40 mg of sample was dissolved in 0.5 mL of solvent solution in a vial equipped with a stirring bar. Then, 0.1 mL of the internal standard and relaxation solution was added, and the solution was stirred for ∼4 h to achieve solubilization of the sample. 0.1 mL of 2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane (TMDP) was added to the clear solution and it was kept under vigorous magnetic stirring for 30 min. The resulting solution was transferred into a NMR tube. 31P-NMR spectra were recorded on a JEOL YH spectrometer with a probe operating at 202.468 MHz at 25 °C, available at the Department of Chemistry and Industrial Chemistry of University of Pisa, Italy. Chemical shifts were calibrated using the 31P-NMR signal at 132.2 ppm arising from the reaction product between residual water and TMDP. The spectra were quantitative and proton broadband decoupling was applied during the acquisition time. Cholesterol was used as an internal standard. Spectra were acquired with a 100 ppm spectral width, 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 data points, 11 s relaxation delay, and 256 scans. The spectra were analysed using the JEOL Delta software.
000 data points, 11 s relaxation delay, and 256 scans. The spectra were analysed using the JEOL Delta software.
Attenuated total reflection Fourier-transform infrared (ATR-FTIR) spectra were measured on a Bruker Vertex 80 FTIR spectrometer equipped with a Bruker Platinum ATR unit and a liquid N2-cooled mercury cadmium telluride (MCT) detector (at the Institute of Physical Chemistry, Linz Institute for Organic Solar Cells, of Johannes Kepler University Linz, Austria). Spectra were recorded with a resolution of 1 cm−1 and averaging of 200 scans. The solid resin material for ATR-FTIR measurements was obtained from ethanolic solutions of the resins by depositing the resin on glass substrates via drop-casting, drying the resulting film at 80 °C and carefully scraping off the resin material from the substrate.
Atomic force microscopy (AFM) and Kelvin probe force microscopy (KPFM) measurements were performed using an Asylum Research MFP-3DAFM system available at the Department of Physics of Montan University Leoben, Austria. ASYELEC-01-R2 probes were used (Ti/Ir coating on both the reflective and tip sides of the cantilever, spring constant of ∼2.8 N m−1, resonant frequency of ∼75 kHz, and tip radius of 25 ± 10 nm). For both types of experiments, the resin was deposited by spin coating from an ethanol solution on a gold-coated glass slide, and during the measurement, the gold back electrode of the resin films was grounded. AM-KPFM measurements providing contact potential differences (CPD)73 were carried out in a two-pass mode, with the probe lifted by 10 nm in the second pass. The root mean square (RMS) data was proven for both the topography roughness and the CPD fluctuations as the average with the standard deviation considering five arbitrarily chosen 20 × 20 μm2 areas of each resin sample. Topography and CPD images were processed in the open-source software Gwyddion v2.62. In the case of the topography images, first-order line filtering was applied and leveling of the base plane. In the CPD images, only zero-order line filtering was applied.
Contact angle measurements conducted at Joanneum Research Materials, on a KRÜSS DSA 100 contact angle measuring system, were performed to determine the surface energy. The liquids used were ultrapure water (a very polar liquid with a surface tension γ = 72.8 mN m−1 separable in a polar component γP = 51 mN m−1 and a dispersive component γD = 21.8 mN m−1), and diiodomethane (a very nonpolar liquid with γ = 50.8 mN m−1, γP = 0 mN m−1 and γD = 50.8 mN m−1). The surface energy was calculated from five droplets of water and diiodomethane, respectively, for each of the resins, and the mean values from three droplets with their standard derivations are reported herein.
In the dielectric strength measurement (breakdown field), the resin was measured in the metal–insulator–metal (MIM) configuration. Therefore, the resin was spin-coated on top of a 1 mm wide and 80 nm thick aluminum electrode. The resulting film was dried in ambient air at 80 °C before another 1 mm wide and 80 nm thick aluminum electrode was deposited on top in a cross configuration. The processing of all the resin thin films deposited in this study (i.e. deposition and drying) was done in ambient air. The MIM device was connected via alligator clips to a Novocontrol impedance analyzer with a DC booster instrument working up to 500 V DC voltage, available at the Institute of Physical Chemistry of Johannes Kepler University Linz, Austria. To establish the breakdown field, the DC voltage scan was performed at an increment of 2 V and 2.5-s time at each applied voltage starting from 0 V. As the breakdown voltage, the voltage considered was the one where a sudden loss of several orders of magnitude in capacitance occurred. The break down field was calculated by dividing the breakdown voltage to the measured thickness of the film via profilometry in the immediate vicinity of the measured MIM structure. For the determination of the dielectric constant, we performed impedance spectroscopy between 10 kHz and 1 mHz and used it to calculate the capacitance value at 1 kHz. For that, we employed the spin-coated resins in the MIM structure described above.
In the ellipsometry measurement, resin layers were prepared on fused silica substrates. Therefore, the resin was spin-coated, and subsequently dried at 80 °C. Standard ellipsometry scans to obtain reflection and transmission intensity data were recorded using a J. A. Woollam M 2000 DI ellipsometer in the spectral range of 195 nm to 1685 nm, available at the Center for Surface and Nanoanalytics (ZONA), Johannes Kepler University Linz, Austria. However, the transmission measurements were limited by the fused silica substrate given that it is only sufficiently transparent down to 230 nm. Data were analyzed with Complete EASE starting with a Cauchy layer in the transparent range to fit the layer thickness. Conversion to a transparent B-spline (Kramers-Kronig consistent mode on, 0.2 eV spectral resolution) with automated wavelength expansion fit gave the complex refractive index of the resin layers. Ellipsometric and transmission data were analyzed jointly, and backside reflection was included in the fitting.74
The aluminum gate electrode itself has a thickness of 80 nm and was obtained by fast evaporation (rate of 4–5 nm s−1) of high purity aluminum wires (99.999%, ChemPUR GmbH). The resulting film was anodized at a voltage of 10 V, while maintaining a steady current of 15 mA. After the 10 V compliance was reached, the sample was allowed to continue anodization until a final current of ∼4.5 μA. The typical thickness of the anodized layer is given by the following equation:
| d = α(V − Vox), |
3. Results and discussion
3.1 Materials analysis
The photographs of the original resin materials are presented in Fig. 1. A particular case was represented by spruce resin, for which we complemented the OFET study with one more resin sample collected from a tree in the vicinity of the city of Linz (altitude of ca. 570 m), Austria. For this particular spruce resin, only a size exclusion chromatography experiment was run for comparison with the original resin collected from the forest surrounding the city of Graz, Austria. The rationale for this decision will be discussed in the OFET section herein.The ATR-FTIR spectra of the investigated resins are shown in Fig. 2a–c. In case of the larch resin (Fig. 2a), the broad absorption band in the ATR-FTIR spectrum, which can be seen at 3385 cm−1, is characteristic of the O–H vibration of intermolecularly hydrogen-bonded hydroxyl groups in alcohol or phenol groups. The higher alcoholic group content in larch resin was indeed confirmed by 31P NMR spectroscopy, and this aspect will also be discussed in the following section. At lower wavenumbers, other absorption bands are visible, including the C–H stretching vibration modes of aromatic or alkene C–H groups (3070 cm−1), as well as the C–H in methylene and methyl groups (2935–2846 cm−1).75 Additionally, the spectrum exhibits a very broad absorption band in the region of approximately 3600–2400 cm−1, which can be assigned to the O–H stretching vibration of hydrogen-bonded carboxylic acid groups. This band appears with a very low relative intensity, and a more accurate evaluation of its intensity is difficult due to its partial overlap with the alcohol or phenol O–H vibration bands. However, considering the low content of free carboxyl groups according to NMR (will be discussed in the following section), the shape of the spectrum looks consistent with the data. The comparatively broad band of medium intensity at 1711 cm−1 can be attributed to the carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching vibration and indicates the presence of ester and/or carboxylic acid groups.75 The sharp and intense bands at 1604 cm−1 and 1515 cm−1 arise from the aromatic ring C
O) stretching vibration and indicates the presence of ester and/or carboxylic acid groups.75 The sharp and intense bands at 1604 cm−1 and 1515 cm−1 arise from the aromatic ring C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations and are typical for substituted phenolic groups such as guaiacyl units (4-hydroxy-3-methoxyphenyl group)76 or 4-hydroxyphenyl substituent groups.77,78 The spectrum of Atlas cedar (Fig. 2b) shows a broad O–H stretching vibration band (3600–2500 cm−1), a sharp C
C stretching vibrations and are typical for substituted phenolic groups such as guaiacyl units (4-hydroxy-3-methoxyphenyl group)76 or 4-hydroxyphenyl substituent groups.77,78 The spectrum of Atlas cedar (Fig. 2b) shows a broad O–H stretching vibration band (3600–2500 cm−1), a sharp C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration (1692 cm−1), and overtone vibrations (2657 and 2536 cm−1), which can be assigned to the carboxylic acid group of terpenoid acids. However, in the spectrum no characteristic aromatic C
O vibration (1692 cm−1), and overtone vibrations (2657 and 2536 cm−1), which can be assigned to the carboxylic acid group of terpenoid acids. However, in the spectrum no characteristic aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of phenol derivatives can be found (typically near 1604 and 1515 cm−1). This is consistent with the low content of phenols or substituted phenol in the composition determined by GC-MS (to be discussed in the following). The FTIR spectrum of spruce resin (Fig. 2c) is similar to that of larch resin. The spectrum shows a broad band centered at 3347 cm−1, which arises from the O–H stretching vibration occurring in alcohols and phenols. Additionally, the presence of a significant content of carboxylic acid groups in the resin is shown by the very broad hydrogen-bonded carboxylic acid O–H stretching vibration in the wavenumber range of 3600–2400 cm−1, the carbonyl C
C stretching of phenol derivatives can be found (typically near 1604 and 1515 cm−1). This is consistent with the low content of phenols or substituted phenol in the composition determined by GC-MS (to be discussed in the following). The FTIR spectrum of spruce resin (Fig. 2c) is similar to that of larch resin. The spectrum shows a broad band centered at 3347 cm−1, which arises from the O–H stretching vibration occurring in alcohols and phenols. Additionally, the presence of a significant content of carboxylic acid groups in the resin is shown by the very broad hydrogen-bonded carboxylic acid O–H stretching vibration in the wavenumber range of 3600–2400 cm−1, the carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration band at 1690 cm−1 and the weak overtone vibration bands at 2658 cm−1 and 2533 cm−1, confirming the higher concentration of free COOH groups found in these two samples by 31P NMR spectroscopy. The strong characteristic C
O stretching vibration band at 1690 cm−1 and the weak overtone vibration bands at 2658 cm−1 and 2533 cm−1, confirming the higher concentration of free COOH groups found in these two samples by 31P NMR spectroscopy. The strong characteristic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching bands at 1604 cm−1 and 1514 cm−1 indicate the presence of compounds with phenolic groups in the resin.76–78 The coalescing bands at 832, 821 and 798 cm−1 can be assigned to the out-of-plane C–H bending of the different aromatic constituents. The observed bands corroborate the results of GC-MS analysis of the investigated spruce resin and can be related to the occurrence of both phenolic group-containing aromatic compounds (e.g., 4-hydroxycinnamic acid and pinoresinol) and terpenoid acids as the major constituents of the resins.
C stretching bands at 1604 cm−1 and 1514 cm−1 indicate the presence of compounds with phenolic groups in the resin.76–78 The coalescing bands at 832, 821 and 798 cm−1 can be assigned to the out-of-plane C–H bending of the different aromatic constituents. The observed bands corroborate the results of GC-MS analysis of the investigated spruce resin and can be related to the occurrence of both phenolic group-containing aromatic compounds (e.g., 4-hydroxycinnamic acid and pinoresinol) and terpenoid acids as the major constituents of the resins.
 | ||
| Fig. 2 ATR-FTIR spectra of (a) larch, (b) Atlas cedar, and (c) spruce resins. The absorption bands are marked on each graph. | ||
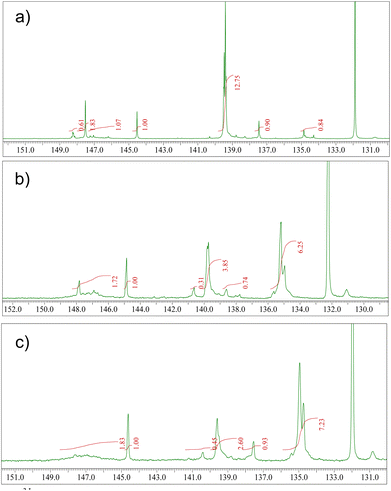
31P-NMR spectra were recorded after derivatization of the resins with 2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane (TMDP). The spectra were analyzed in the spectral range of 130 to 150 ppm (Fig. 3). The calculated hydroxyl content, obtained after standardization with a known quantity of cholesterol, is reported as millimole per gram of sample in Table 1. The respective table shows that the spruce and Atlas cedar resins possess a high content of carboxylic groups, in good agreement with the results from mass spectrometry determination of the molecular composition of the three resins (will be presented in the following section). Among the resins, Atlas cedar possessed the greatest content of aromatic compounds, where the major content corresponds to the substituted compound (see Table 1). Larch resin seems to be different from the others because it has more defined peaks in the aliphatic hydroxylic group region, with particular attention to the peak at 147.5 ppm, which can be ascribed to 2-substituted ethanolic groups. Larch has a higher content of aromatic groups, in particular substituted ones, and different from the others it presents a very low content of carboxylic acids. The high content of lignans found in larch resin well explains the high content of substituted aromatic groups revealed by NMR (Fig. 3).
| Resin | Aliphatic OHa | Phenolic OHb | Substituted phenolic OHc | Carboxylic acidd |
|---|---|---|---|---|
| a 149.0–145.0 ppm. b Phenolic hydroxyl content, 138.8–137.4 ppm. c Content of phenolic hydroxyls with substitution on C5 carbon of the aromatic ring, 144.0–139 ppm. d 136–133.6 ppm. Integration is referred to as cholesterol used as an internal standard. | ||||
| Larch | 0.73 | 0.19 | 2.66 | 0.18 |
| Atlas cedar | 0.35 | 0.15 | 0.86 | 1.29 |
| Spruce | 0.39 | 0.20 | 0.65 | 1.55 |
The composition of the individual resins was determined through their MS spectra. The species detected in the measurement are listed in Table 2. In our collected Atlas cedar resin, we observed that resin acids were the dominant compound group (24%), with isopimaric acid dominating (8%), followed by dehydroabietic acid (7%) and abietic acid (5%). Overall, diterpenoids accounted for 32% of the resin weight; however, a significant amount (9.0%) of the GC eluted compounds (42% in total) were unidentified. Holmbom et al.79 characterized callus resin (three approximately one-year-old, separate samples) and found that lariciresinol-9-acetate (up to 19.5%), lariciresinol (up to 12.2%), caffeic acid (up to 7.5%), lariciresinol-coumarate (up to 6.2%), abietol (up to 7.6%), abieta-7,13-diene (up to 4.8%), and lignan esters (up to 3.9%) were the major components among the GC-identified compounds. Unidentified peaks accounted for 16%, while compounds not eluted by GC accounted for 36% of the resin weight. In comparison, the major constituents of larch resin analyzed by us via the GC technique are presented as lariciresinol-9-acetate, a derivative of the lignan lariciresinol, which was the dominant component of our larch resin sample, accounting for 18.2% of the resin weight, followed by the underivatised lariciresinol, which accounted for 10.3%. The other lignans present were isolariciresinol (0.26%), which is easily formed from lariciresinol, and pinoresinol (0.77%). This is well in accordance with the result obtained by Holmbom et al.79 Altogether, the lignans accounted for 29% of the resin weight, and formed the major components of this larch resin sample. In fact, lariciresinol received its name from the Larix decidua species. The other major components were caffeic acid (5.8%), also well in accordance with a previous study (Holmbom et al.79), and the diterpenoids manool and larixol, both accounting for 2.2% of the resin weight. However, the diterpenoid composition was different than that previously reported for larch callus resin (Holmbom et al.79). The unidentified GC-eluted compounds accounted for only 2.4% of the resin weight, and compounds not eluted on GC for 48%. This means that the identity of almost half of the material is still unknown. Zoubi et al.80 analyzed the content of Atlas cedar resin and found ∼1.1% content of essential oils. The major components identified in the GC experiment run in this study were α-, β-, and γ-himachalene, which accounted for ∼60% of the resin composition, as well as resin alcohols such as cedrol and isocedranol in the proportion of ∼10% and 5%, respectively. In our collected Atlas cedar resin, we observed that resin acids were the dominant compound group (24%), with isopimaric acid dominating (8%), followed by dehydroabietic acid (7%) and abietic acid (5%). The other detected notable diterpenoids were neoabietic and palustric acid (1.02% and 0.32%, respectively), and isopimarol, manool, isopimaral, and pimarol (2.93%, 1.45%, 0.49%, and 0.12%, respectively). Overall, diterpenoids accounted for 32% of the resin weight; however, a significant amount (9.0%) of the GC-eluted compounds (altogether 42%) were unidentified. The HPLC-HRMS and SEC investigations presented in the following show the full composition of Atlas cedar resin employed in this work, and a list of its constituents is presented in Tables 3 and 4. Similar to the case of Atlas cedar, for the European spruce, also the resin acids were the dominant compound group (15%), with dehydroabietic acid dominating (5.8%), followed by palustric and isopimaric acid (around 2% each). The other noticeable detected diterpenoids were the resin acids abietic, sandaracopimaric, neoabietic, and pimaric acid (1.59%, 1.26%, 1.01%, and 0.49%, respectively) and pimarol (0.14%). However, in European spruce, lignans were also a significant compound group (17%), dominated by pinoresinol (4.4%) and lariciresinols (3.1%). The other detected noticeable lignans were secoisolarici-, matai-, and isolarici-resinol (0.39%, 0.098, and 0.059%, respectively). Among the resins in this study, only larch contained more lignans that spruce. The other important detected aromatic compounds were 4-hydroxycinnamic acid (concentration 8.19%), caffeic acid, ferulic acid, and isoferulic acid (0.26%, 0.18%, and 0.010%, respectively). Cholestadiene accounted for 0.048% of the resin weight. In the case of larch and Atlas cedar, only around 40% of the material was eluted from the GC column in our study. The composition of European spruce resin was analyzed by Holmbom et al.,79 who obtained very different results for European spruce callus resins (four different resins analyzed in their study), where pinoresinol and p-coumaric acid were the dominant compounds with a much higher content (up to 19% and 15%, respectively). Dehydroabietic acid was detected only in minor amounts, and the content of resin acids was overall much lower than in the resin used in the present study, which has more similarities to oleoresin of European spruce, although it contains lignans (Holmbom et al.79). However, the analysis results are not directly comparable, given that different quantification methods were used in the study presented in ref. 79 compared to the present study.
| Component | Larch (mg g−1) | Atlas cedar (mg g−1) | Spruce (mg g−1) |
|---|---|---|---|
| Mono- and sesquiterpenoids | |||
| β-Phellandrene | nd | nd | nd |
| Longifolene | nd | nd | nd |
| Cadinenes (β, δ) | nd | nd | 1.18 |
| 11-Hydroxy-eremophil-1(10)-ene | nd | nd | nd |
| α-Terpineol | nd | nd | 0.721 |
| α-Terpinolene | nd | nd | 0.125 |
| β-Cubebene | nd | nd | 0.105 |
| Germacrene D | nd | nd | 0.091 |
| Sum | 0 | 0 | 2.22 |
| Diterpenoids | |||
| Secodehydroabietic acids | nd | 11.9 | nd |
| Pimaric acid | nd | nd | 4.93 |
| Sandaracopimaric acid | 0.506 | 7.63 | 12.6 |
| Isopimaric acid | nd | 81.0 | 20.4 |
| Abietatetraenoic acid(s) | nd | 1.01 | 0.688 |
| Palustric acid | 0.391 | 3.25 | 23.9 |
| Dehydroabietic acid (DeAb) | 2.71 | 69.0 | 57.9 |
| Methyl dehydroabietate | nd | nd | nd |
| Abietic acid (Ab) | nd | 53.4 | 15.9 |
| Abietapentaenoic acid | nd | nd | nd |
| Neoabietic acid | nd | 10.2 | 10.1 |
| 8,15-Pimaradien-18-oic acid | nd | nd | nd |
| Sum | 3.61 | 237 | 146 |
| Oxidised compounds | |||
| Hydroxy-DeAbs | nd | 5.28 | 9.26 |
| Hydroxy-Ab(s) | nd | 13.0 | 5.79 |
| Hydroxy-RA | nd | 1.92 | 0.523 |
| Dihydroxy-DeAb(s) | nd | 0.385 | 0.825 |
| Hydroxy-7-oxo-DeAb | nd | nd | 0.507 |
| Sum | 0 | 20.6 | 16.9 |
| Other diterpenoids | |||
| Thunbergene(s) | nd | nd | 0.683 |
| Thunbergol | nd | nd | nd |
| Cis-abienol | nd | nd | 3.61 |
| Isopimaradiene | nd | 1.09 | nd |
| 19-Norabieta-8,11,13-triene | nd | 0.275 | nd |
| Abieta-7,13-diene | 0.381 | 0.678 | nd |
| Manool | 21.9 | 14.5 | nd |
| Manool oxide | nd | nd | 0.363 |
| Pimaral | nd | nd | nd |
| Isopimaral | nd | 4.86 | nd |
| Palustral | nd | nd | nd |
| Dehydroabietal | nd | 2.49 | nd |
| Pimarol | nd | 1.20 | 1.37 |
| Isopimarol | 10.6 | 29.3 | nd |
| Palustrol | nd | nd | 0.949 |
| Abietal | 2.40 | 3.24 | nd |
| Dehydroabietol | 0.795 | 7.11 | 0.788 |
| Neoabietol | nd | 0.661 | 0.523 |
| Larixol | 21.7 | nd | nd |
| Sum | 57.8 | 65.4 | 8.29 |
| Small-molecular aliphatic acids | |||
| Lactic acid | 4.56 | 3.03 | 1.72 |
| Glycolic acid | nd | 1.02 | nd |
| 3-Hydroxypropanoic acid | nd | 0.521 | nd |
| Succinic acid | nd | 0.422 | 0.492 |
| Levulinic acid | nd | nd | nd |
| 2-Methyl-4-oxopentanoic acid | nd | nd | nd |
| Methylsuccinic acid | nd | nd | nd |
| Sum | 4.56 | 4.99 | 2.21 |
| Fatty acids and alcohols | |||
| n-Octanoic acid | nd | nd | nd |
| n-Nonanoic acid | nd | 0.207 | nd |
| n-Hexadecanoic acid | 0.896 | 1.14 | 0.589 |
| n-Heptadecanoic acid | nd | nd | nd |
| n-Octadecanoic acid | nd | nd | nd |
| n-Hexadecanol | 0.258 | nd | 0.892 |
| n-Octadecanol | 0.265 | nd | 0.712 |
| Sum | 1.42 | 1.35 | 2.19 |
| Aromatic compounds | |||
| Resorcinol | nd | nd | 0.284 |
| Vanillin | 3.50 | nd | nd |
| Cinnamic acids | 0.318 | nd | nd |
| p-Coumaric acid | nd | nd | nd |
| 4-Hydroxy-benzaldehyde | nd | nd | 0.512 |
| 3,4-Dihydroxybenz-aldehyde | 0.978 | nd | nd |
| 3-Hydroxy-4-methoxybenzaldehyde | nd | nd | nd |
| Vanillic acid | 0.323 | nd | nd |
| 3-Hydroxybenzoic acid | nd | nd | 0.176 |
| 3,4-Dihydroxybenzoic acid | 0.183 | nd | nd |
| Vanillic acid | nd | nd | 0.417 |
| Ferulic acids | 2.27 | nd | 1.77 |
| Isoferulic acid | nd | nd | 0.103 |
| 4-Hydroxycinnamic acid | 3.82 | nd | 81.9 |
| 3,4-Dihydroxycinnamic acid | 57.6 | nd | 2.61 |
| 1-Guaiacylglycerols | nd | nd | nd |
| Monomethyl pinosylvin | nd | nd | nd |
| Isolariciresinol | 2.60 | nd | 0.593 |
| Secoisolariciresinol | nd | nd | 3.93 |
| Lariciresinol | 103 | nd | 13.3 |
| Matairesinol | nd | nd | 0.983 |
| Lariciresinol-9-acetate | 182 | nd | nd |
| Pinoresinol | 7.66 | nd | 43.8 |
| 7′-Oxolariciresinol (?) | nd | nd | 1.04 |
| Lariciresinol coumarates | nd | nd | 18.4 |
| Sum | 364 | 0 | 170 |
| Miscellaneous | |||
| Carbonic acid | nd | nd | nd |
| Glycerol | 1.47 | 0.541 | 0.630 |
| Cholestadiene | 0.734 | nd | 0.476 |
| Diacetone alcohol | 6.02 | 0.303 | 1.14 |
| N,N-Diethylcarbamic acid | 0.327 | 0.419 | nd |
| Ethylene glycol | 1.50 | nd | 0.865 |
| Pentitol | nd | nd | 0.268 |
| Hexitol | 0.407 | nd | nd |
| Monopalmitin | 0.402 | nd | nd |
| Decalin derivative | 11.1 | nd | nd |
| Sum | 21.9 | 1.26 | 3.38 |
| Identified, sum | 453 | 331 | 351 |
| Unidentified peaks | 24 | 90 | 41 |
| % GC eluted | 47.7% | 42.1% | 39.2% |
| t R (UV)/min | MH+ | Name/sum formula | Atlas cedar | Larch | Spruce |
|---|---|---|---|---|---|
| % area (UV) | |||||
| 6.15 | 181.0493 | C9H8O4 | 0.51 | 1.89 | — |
| 8.17 | 165.0544 | Hydroxycinnamic acid | — | 1.24 | 13.37 |
| 9.24 | 195.065 | C10H10O4 | — | — | 0.28 |
| 9.93 | 377.1593 | C20H24O7 | — | 0.5 | — |
| 11.47 | 375.1438 | C20H22O7 | — | 0.51 | 0.31 |
| 11.58 | 327.1588 | C20H22O4 | — | 0.58 | 0.24 |
| 11.92 | 219.1014 | C13H14O3 | 2.28 | 15.68 | 1.77 |
| 12.39 | 359.1486 | C20H22O6 | — | 1.93 | — |
| 13.1 | 341.1382 | C20H20O5 | 0.57 | 5.39 | 7.97 |
| 13.52 | 417.1542 | C22H24O8 | — | 2.38 | — |
| 14.32 | 343.1536 | C20H22O5 | — | 20.79 | — |
| 14.62 | 523.196 | C29H30O9 | 1.35 | 2.79 | — |
| 15.39 | 507.2011 | C29H30O8 | 2.36 | 27.65 | 9.85 |
| 16.54 | 299.2004 | C20H26O2 | — | — | 1.12 |
| 17.18 | 319.2265 | C20H30O3 | 3.81 | — | 1.55 |
| 17.75 | 301.2159 | C20H28O2 | 1.6 | — | 0.9 |
| 18.18 | 315.1952 | C20H26O3 | 0.47 | — | 0.59 |
| 18.41 | 289.252 | C20H32O2 | — | 0.35 | — |
| 19.74 | 301.2159 | Dehydroabietic acid | 1.93 | 0.54 | 1.62 |
| 20.62 | No mass signal | Overlapping with isopimaric acid | 21.19 | — | — |
| 20.69 | 303.2314 | Isopimaric acid | 35.51 | 6.06 | 43.76 |
| 21.29 | 271.2418 | C20H30 | 2.17 | — | — |
| 21.35 | 289.2522 | C20H32O | 5.91 | — | — |
| 21.44 | 273.2573 | C20H32 | — | — | 4.42 |
| 21.77 | 273.2574 | C20H32 | — | 0.49 | — |
| 22.23 | 287.2367 | C20H30O | 11.64 | 0.52 | 0.55 |
| Total | 91.3 | 89.29 | 88.3 | ||
| Resin | M n (g mol−1) | M w (g mol−1) | Đ |
|---|---|---|---|
| Atlas cedar | 301 | 533 | 1.77 |
| Larch | 316 | 526 | 1.66 |
| Spruce (Graz) | 318 | 596 | 1.87 |
| Spruce (Linz) | 307 | 582 | 1.89 |
For all the resins investigated in this study (Atlas cedar, larch, and spruce), we investigated the dielectric film surface via contact angle measurement, AFM, and KPFM.
Fig. 4a presents the typical surface morphology observed for the larch samples. The RMS roughness of the spin-coated larch surface was determined to be essentially flat, with a height roughness of 0.71 ± 0.3 nm. The corresponding surface potential map is shown in Fig. 4b. The main features observed in the KPFM image of larch resin films correlated to higher and lower regions of the resin layer, most likely originating simply from the probe being closer and further from the grounded electrode underneath the resin layer, respectively. The CPD RMS roughness was found to be 6.49 ± 1.52 mV. Considering the smaller scale images and focusing on the flat surface yields, the CPD variation was within the error bar of the system. We also performed surface investigation of Atlas cedar and spruce resins via contact angle measurement, AFM, and KPFM. The surface roughness of the spin-coated Atlas cedar resins was found to be comparable to that of polished Si wafers (i.e., ∼0.3 nm, see Fig. 4c), whereas the surface potential of the Atlas cedar resin film was similar to the other analyzed resins, i.e., 2.98 ± 0.41 mV. In fact, the spatial variations of the CPD were found to be almost negligible on the scale of 20 μm. The surfaces of the spruce resin films were atomically smooth and comparable to that of SiO2 formed via the wet thermal oxidation of Si chips, with a root mean square height of 1.23 ± 0.25 nm. The spatial variations of the CPD values of spruce films were found to be very similar to most of the other resins investigated in this study, i.e., 4.55 ± 1.94 mV. A compilation of the results from the AFM and KPFM investigations for the analyzed resins is presented in Fig. 5. Also, the results are compared with two well-known inorganic dielectrics, silicon dioxide and aluminum oxide, as well as the surface of plain gold (i.e., a film of Cr/Au), respectively.
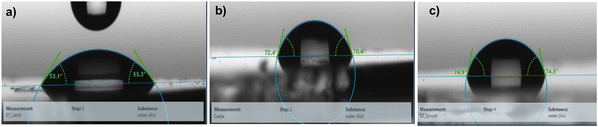
The water droplet contact angle measurement for larch, Atlas cedar and spruce revealed that they possessed hydrophilic surfaces (see Fig. 6). The water droplet contact angle for the larch resin, as displayed in Fig. 6a, was 53.1° ± 2.4°. The contact angle of the same resin with diiodomethane (not shown in the figure) was 43.1° ± 3°. The deviation in both quantities shows the spread of the measurement values of at least three droplets (left and right). The total surface energy, 56.5 mN m−1, was divided into a disperse component of 38 mN m−1 and a polar component of 18.5 mN m−1. The water droplet contact angle of the Atlas cedar resin was 70.4° ± 3.4°, as displayed in Fig. 6b. The contact angle of the same resin with diiodomethane (not shown in the figure) was 35.5° ± 2.5°. The deviation in both quantities shows the spread of the measurement values of at least three droplets (left and right). The total surface energy, 47.5 mN m−1, was divided into a disperse component of 41.8 mN m−1 and a polar component of 5.7 mN m−1. The water droplet contact angle of the spruce resin, as displayed in Fig. 6c, was 74.5° ± 2.1°. The contact angle of the same resin with diiodomethane (not shown in the figure) was 37.2° ± 1.2°. The deviation in both quantities shows the spread of the measurement values of at least three droplets (left and right). The total surface energy, 46.3 mN m−1, was divided into a disperse component of 41 mN m−1 and a polar component of 5.3 mN m−1.
The larch resin sample was analyzed by TGA under a nitrogen atmosphere (see Fig. 7a), and the first weight loss was detected below 120 °C (0.51%), which is attributed to possible traces of adsorbed water. The second main weight loss was centered at 330 °C; however, decomposition was also detected at the temperature of 243 °C. Here, the sample lost approximately 83% of its weight up to 450 °C. Another decomposition peak was detected at a temperature of 397 °C. From 450 °C to 650 °C, 4.32% weight loss was observed; similarly, up to 900 °C, ∼4.7% weight loss was also detected. A residual weight of 7.7% was detected, which is attributed to the incomplete decomposition of the sample.
Atlas cedar resin was analyzed by TGA (see Fig. 7b). The first transition below 120 °C showed a weight loss of 1.22%, which is attributed to the loss of water adsorbed from the environment, possibly occurring on the surface of the sample. The second step and more pronounced weight loss was centered at 270 °C (carrying approximately 91% weight loss). In this range (120–450 °C), two decomposition temperatures (at 149 °C and 362 °C) were detected. Moreover, the sample lost ca. 3% weight after 450 °C, with the respective peak centered at approximately 568 °C. Up to 900 °C, the very small weight loss of approximately 0.015% was detected, leaving a residual unburned weight of approximately 5%, which is attributed to the incomplete decomposition of the sample. In the case of spruce resin (see Fig. 7c), the adsorbed water was evaporated and the first weight loss was detected below 120 °C (0.94%). The second and more pronounced weight loss was centered at 251 °C, where the sample lost approximately 85% of its weight between 120 °C and 450 °C. Another decomposition peak was observed at a temperature of 418 °C, followed by a third considerable weight loss up to 650 °C of approximately 8.3%. The final step between 650 °C and 900 °C showed a minor loss of approximately 0.08%, leaving approximately 6% of residual weight.
For the entire set of dielectric measurements (impedance spectroscopy and breakdown field), a metal–insulator–metal (MIM) configuration was used. In this investigation, each resin was spin-coated onto a metal bottom electrode, and subsequently covered with a metal top electrode. We fabricated 4 slides, consisting of 4 MIM structures on each slide for each of the resins studied herein. Subsequently, we provide the results of one example of the MIM for each resin. The resulting films were measured by profilometry in the vicinity of the MIM cross and the resulting thicknesses were 105 nm for Atlas cedar, 110 nm for larch, and 465 nm for spruce. In all three materials (see Fig. 8), we observed a very uniform capacitance across the measured frequency range, and the loss angle (tangent delta) exhibited no relaxation behavior within this range. Both observations indicate the very high purity of the dielectric film with excellent dielectric performance. This is counterintuitive, considering that the resins were simply collected from the host trees, solubilized in pure ethanol and simply filtered through a hydrophilic filter.
In the case of each material, to establish its dielectric constant, we considered its capacitance at 1 kHz. The calculated dielectric constants were 5.4 for larch, 5.0 for Atlas cedar and 4.3 for spruce. The standard deviation of the dielectric constant for the 16 MIM samples analyzed was ±0.4 for larch and Atlas cedar, and ±0.3 for spruce. In addition, the breakdown voltage of the materials was determined by coupling the impedance analyzer to a DC voltage booster with the possible voltage window of ±500 V, and the results of this study for one particular MIM structure for each resin are presented in Fig. 9. The thickness, dielectric constant, breakdown voltage, and breakdown field of the investigated materials are summarized in Table 5.
| Material | Thickness/nm | Dielectric constant | Breakdown voltage/V | Breakdown field/MV cm−1 |
|---|---|---|---|---|
| Larch | 110 | 5.4 ± 0.4 | 25 (1st stage) | 2.3 |
| 80 (2nd stage) | 7.3 | |||
| Atlas cedar | 105 | 5.0 ± 0.4 | 52 | 4.95 |
| Spruce | 465 | 4.3 ± 0.3 | 296 | 6.35 |
Given that the 16 MIM structures under analysis were all made of resin films that were cast from the same solution and rotated at the same speed, there was very little variation in the film thickness. To generate a reliable Weibull distribution of the breakdown field values, one should follow a traditional breakdown field analysis that accounts for the fluctuation in the dielectric thickness. Nevertheless, this topic will be part of our future investigation on the breakdown field of plant resins. The spruce and Atlas cedar resins broke suddenly (the measured capacitance dropped abruptly to a negative value) when the appropriate voltage was reached (see Fig. 9a). However, the larch film broke in two stages, and thus Fig. 9b presents two separate breakdown field values. Considering that the breakdown field of anodized aluminum (i.e., Al2O3) is likewise not greater than 5 MV cm−1, the dielectric strength values approaching or exceeding 5 MV cm−1 for all the examined resins are notable81 (see Fig. 9 and Table 5). These values are consistent with the trend observed for other investigated tree or animal resins reported previously by our group.82–84 In comparison, synthetic polymer resins such as benzocyclobutene (BCB) have a breakdown field or dielectric strength of no more than 4.5 MV cm−1, while the stated breakdown field for typical low-k polymer dielectrics is between 1 and 2 MV cm−1. Generally, it is known that the breakdown voltage is dependent on the film thickness, as shown by the equation V = At2/3, where t is the film thickness and A is a material constant. Consequently, the dielectric strength decreases as the dielectric thickness increases. Accordingly, as shown elsewhere,85 the breakdown field values reported in this work may need to be reexamined in a more systematic investigation because they represent only a specific example, and are particular for the thickness of the dielectric films used.
For the determination of the complex refractive index, n and k, were obtained by spectroscopic ellipsometry in the spectral range of 195 nm to 1685 nm. The resulting graphs can be seen in Fig. 10. All the investigated resins show multiple optical transitions in the UV range. However, they are basically transparent within the visible and near IR range. The tail absorptions extend into the visible range, giving the resins a slightly tainted appearance in thick layers. The resins are isotropic, and their real refractive indices are in the range of 1.5 to 1.6, which are similar to that of float glass.
3.2 Transistor measurements
All three resins investigated in this study were tested as a capping layer of the aluminum oxide dielectric in OFETs. Two different semiconductors (pentacene and C60) were selected to obtain p- and n-type operating field effect transistors, respectively (see Fig. 11). For each resin used in this investigation, two batches of transistors were made, one with pentacene and the other with C60. Given that our fabrication mask has six glass slide positions, similar to that schematically shown in ref. 84, and that each slide contains four transistors, as shown in Fig. 11, a total of 48 OFETs was made for each resin and pentacene and likewise 48 OFETs for each resin and C60. In addition to the classic transfer and output characteristics recordings, bias stress measurements and stability measurements of the fabricated transistors were performed. Fig. 12 depicts the AFM images of the three resins with either pentacene or C60 grown on top. Fig. 12a and b present the topography of the semiconductors (pentacene and C60) grown on the films of larch. Both the pentacene and C60 grains are uniform in size; however, pentacene grew in aggregates with a typical size of 400 to 650 nm, whereas the grains of C60 are very small (in the range of 10 nm). In the AFM images of Atlas cedar (pentacene in Fig. 12c and C60 in the panel of Fig. 12d), it can be seen that the two morphologies also significantly differ. Pentacene grew in a uniform size of 300 to 500 nm, while C60 grew in rather small grains (10 to 30 nm). On spruce (pentacene in the panel of Fig. 12e and C60 in the panel of Fig. 12f), the grains of both semiconductors are uniform in size. However, pentacene grew in much larger grains than on larch and Atlas cedar (1.5 to 3 μm dendritically shaped aggregates), whereas the grains of C60 again have a uniform size of 10 nm.The transistors produced with larch resin as the capping layer on 10 V anodized Al2O3 (i.e., ∼16–17 nm thick aluminum oxide) showed hysteresis-free behavior both in transfer and output characteristics (with both semiconductors, see Fig. 13, panels a–d). The dielectric behavior was characterized by low leakage in the range of 100 pA through the whole measurement range (i.e., −2 to 5 V for the fullerene semiconductor and 2 to −7 V for pentacene as the semiconductor). In the case of both semiconductors, the mobility was calculated to be in the range of 0.05 cm2 V−1 s−1. The subthreshold swing of the two semiconductors was 0.8 V dec−1 for pentacene and 1.9 V dec−1 for the fullerene film. In the case of the normalized subthreshold swing values, we obtained 41.42 V nF cm−2 dec−1 for pentacene and 50.47 V nF cm−2 dec−1 for C60. In general, the normalized subthreshold swing is the more convenient method to compare the performance of organic semiconductors in OFET devices when deposited on various dielectrics or on dielectrics of different thicknesses.11,86 For both semiconductors, the subthreshold swing and the normalized subthreshold swing were in the same range, despite the slight difference in the thickness of the larch dielectric, which is visible in the small discrepancy of the normalized capacitances, C0d, listed as insets in the transfer characteristic graphs. A characteristic of the larch resin was that it induced a relatively high OFF level in the organic transistor characteristics, possibly because of the tendency of the dielectric material to charge the semiconductor in the OFF state. As shown in Fig. 13a and b, the OFF level of both p- and n-type devices was greater than 1 nA. Nevertheless, despite this high OFF level, the transistors showed virtually hysteresis-free characteristics. In addition to the classic transistor characterization measurements, bias stress investigations of the fabricated OFET with pentacene as semiconductor were performed, as presented in Fig. 14. We stressed the device at the maximum voltage (−7 V), while the voltage for both source and drain were kept constant for 12 h stress time (Fig. 14a). The transfer characteristics were measured at the beginning of the experiment as well as immediately after releasing the electrical stress. The larch-based OFET device retained ∼87.7% of its initial Ids after the stress time of 12 h, which is a remarkable value that ranks high among the best reported combinations of organic dielectrics and semiconductors.2,10,87,88 We continued to measure the recovery curve of the devices with 15 min increment between measurements (Fig. 14b), but for simplicity and to avoid cluttering the graph, we only show the transfer curve, where full or nearly full recovery was measured. In the case of larch, the ON level of the drain current, as well as the threshold voltage recovered after 90 min relaxation after the bias stress time of 12 h.
The transistor, which was fabricated with a capping layer of Atlas cedar resin on aluminum oxide dielectric, also exhibited hysteresis-free behavior in both its transfer and output characteristics (Fig. 15, panels a–d). Similar to the case to larch resin, the Atlas cedar resin-based OFETs displayed a high OFF level for both the p- and n-type devices, this time in the range of 10−8 A or higher. In the case of the field effect mobilities, values of 0.1 cm2 V−1 s−1 for pentacene and 0.37 cm2 V−1 s−1 for C60 were calculated, with both OFET devices working at an applied gate voltage of ±8 V. For the subthreshold swing, we obtained a value of 2.4 V dec−1 for pentacene and 2.2 V dec−1 for fullerene, which are basically in the same range, a fact not surprising given the similarity of the two transfer curves. The normalized subthreshold swing was around the same value too, i.e., 23.5 V nF cm−2 dec−1 for the pentacene-based device and 21.8 V nF cm−2 dec−1 for the device with fullerene, given that the two devices also have a similar specific capacitance (displayed as the inset of the two transfer characteristics curves). The higher mobility values of the Atlas cedar resin-based OFETs compared to their larch resin-based counterparts is a direct consequence of the inherently high OFF level for the Atlas cedar-based transistors, which generates a high value for the slope (Δ) in the mobility formula. If hypothetically the OFF level current would have been in the range of microamps, the recorded field effect mobilities would have surely surpassed the value of 1 cm2 V−1 s−1, as shown before.89
We performed the bias stress investigations with the transistor consisting of an Al2O3 dielectric, an Atlas cedar resin capping layer, and pentacene as the semiconductor. The transistor device was stressed for 12 h at the maximum allowed voltage before breaking would occur (−8 V). The device showed very good bias stress resistance, with ∼89% of the drain current retained after 12 h of stress (Fig. 16a), which similar to the larch-based OFET presented above, represents a remarkable result. The transfer characteristic was fully recovered after 4 h relaxation after the stress (see Fig. 16b).
Similar to the other two resins investigated in this study, spruce resin was also implemented as a capping layer interface to aluminum oxide, with pentacene and C60 semiconductors. When interfaced to pentacene semiconductor, the OFET displayed a very high OFF level in the transfer characteristics, in the range of 10−7 A (see Fig. 17). In contrast, when interfaced with C60, the OFF level was one order of magnitude lower, i.e., 2 × 10−8 A, resulting in a low ON–OFF ratio OFET (data not shown). Because of the very high OFF level of both the n-type and p-type devices, we were not able to record the bias stress for spruce. In the case of bias stress for the aluminum oxide-spruce-pentacene OFET, the transfer characteristic turned into a perfect horizontal line at the end of the 12 h bias stress, and this horizontal Idsvs. Vgs line never recovered during the next four days when the system was allowed to relax. In this respect, thus far, spruce remained the only resin in the entire set of Pinaceae resins for which this type of test could not be completed.83,84 To account for the stability of the spruce-based OFET device, we instead measured the device with the pentacene semiconductor in a glove box and compared it with the measurement obtained by employing a probe station in ambient air. The cables used in the experiment were identical to remove any possible discrepancy in the obtained results due to the difference in impedance of the measurement cables. The respective comparison is presented in Fig. 17, proving the relatively good stability of the interface between the spruce resin and the pentacene semiconductor, with the threshold field values differing by only 0.1 V. In addition, we remeasured the transfer curve presented in Fig. 17a after one year storage in a glove box, and the results are displayed in Fig. 17c and d, proving the good stability of the spruce resin interface with pentacene. In this respect, a drift in the threshold voltage of ∼0.7 V was recorded.
The bias stress event of spruce prompted us to investigate one more spruce resin for the fabrication of OFETs. The new resin was collected from a tree in the vicinity of the city of Linz, Austria, which was only characterized via a size exclusion chromatography experiment, as presented in Table 4. Although the number average molecular weight (Mn) and weight average (Mw) values differ slightly, as presented in Table 4, the new resin also displayed hysteresis-free behavior in both the transfer and output characteristics of OFETs when interfaced with pentacene, and small hysteresis in the respective characteristics when interfaced with C60 (see Fig. 18). Basically, these results are identical with that of the original spruce resin sample collected from a tree growing in the vicinity of the city of Graz, Austria. Nevertheless, the OFF level of the new resin had lower values by one to two orders of magnitude, as clearly shown in Fig. 18a and b. Interestingly, in the case of the field effect mobilities, we obtained relatively similar values for both semiconductors following the identical trend as the respective values obtained for the original resin, i.e., in the range of 4.3 × 10−2 cm2 V−1 s−1 for the pentacene- and 2.6 × 10−2 cm2 V−1 s−1 for the C60-based devices. In the case of the original resin (its transfer curves with pentacene are shown in Fig. 17), these values were 3.9 × 10−2 cm2 V−1 s−1 for the pentacene- and 4.6 × 10−2 cm2 V−1 s−1 for the C60-based devices, respectively. A subthreshold swing of 0.85 V dec−1 for pentacene and 1.7 V dec−1 for C60 were recorded for the fabricated OFETs with the new resin. The calculated values of the normalized subthreshold swing were 22.1 V nF cm−2 dec−1 for the pentacene-based device and 44.1 V nF cm−2 dec−1 for fullerene, respectively. The minimum drain–source (Vds) voltage that could run the spruce resin-based OFET was 150 mV, as shown in Fig. 18a, given that at an applied drain–source voltage of 100 mV, the curve still pitched down in full saturation.
The entire set of OFET parameters for the three analyzed resins in this study is summarized in Table 6. Inspecting Table 6, one can observe that the three resins investigated showed a relatively uniform performance in OFETs, with the values of the critical parameters that characterize the respective devices being in the same range. Thus, it is very difficult to clearly state which one is more or less efficient than other when employed as the capping layer for the aluminum oxide dielectric. Even when collected from trees that are situated several hundred kilometers away, the resins showed similar trends and performances, as demonstrated by the spruce resin collected from two distant trees, which had almost identical polydispersity index values, Đ (i.e., 1.89 vs. 1.87). Obviously, the number average molecular weight (Mn) and weight average (Mw) values may differ even when comparing resin samples collected from the same tree, given the unknown age of the respective material or the position on the tree stem (i.e., in direct sun exposure or on the north side, where the UV-exposure is negligible). In this case, we performed size exclusion chromatography measurements on a third spruce sample, which was collected from a different deposit situated on the same tree in Linz for which the resin was discussed above as part of the OFET devices in Fig. 18. The obtained values for the Mn, Mw, and Đ parameters were 311, 565, and 1.81 respectively, for the third resin, which are not significantly different than the values of the two other spruce resins investigated herein. Interestingly, also, all the resins examined in this study generated high OFF level values in the transfer characteristics of OFETs, and hysteresis-free behavior especially when interfaced with pentacene. Thus, these resins seem to be an excellent capping layer for inorganic dielectrics such as aluminum oxide, which show great reactivity especially towards electron transport.90,91
| Resin | OFET parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| With pentacene | With C60 | |||||||||
| V th (V) | C 0d (nF cm−2) | I ON/IOFF | μ h (cm2 V−1 s−1) | S SW (V dec−1) | V th (V) | C 0d (nF cm−2) | I ON/IOFF | μ e (cm2 V−1 s−1) | S SW (V dec−1) | |
| Larch | −1.4 | 33.54 | 1966 | 5 × 10−2 | 0.8 | 2 | 26.57 | 176 | 4.5 × 10−2 | 1.9 |
| Atlas cedar | −1.8 | 29.9 | 250 | 0.1 | 2.4 | 4.5 | 31.02 | 97 | 0.37 | 2.2 |
| Spruce | −0.4 | 26.02 | 1.5 × 104 | 4.3 × 10−2 | 0.85 | 5 | 20.37 | 1 × 104 | 2.6 × 10−2 | 1.7 |
Also, the nearly hysteresis-free electric performance of all the materials under investigation in this study is noteworthy and impressive, particularly when they were interfaced with the p-type semiconductor pentacene. This was also noted for fir and pine Pinaceae resins, as recently published by us.83,84 Compared to other traditional dielectrics used as a capping layer for aluminum oxide, such as parylene-C, divinyltetramethyldisiloxane-bis(benzocyclobutene) (BCB), low density polyethylene, adenine, shellac, lignin and cellulose in combination with aluminum oxide,54,82,92–97 the examined Pinaceae resins reported herein perform exceptionally well.
4. Conclusion
In this study, we investigated three resins originating from widespread Pinaceae trees and applied them as dielectrics in organic field effect transistors. If a single advantage of these resins has to be chosen, it is their abundant availability at virtually no cost. Spruce is the most widespread tree in the Eurasian forests, and has extensive industrial applications in the production of paper and nanocellulose fibers. Alternatively, larch is not as abundant; nevertheless, it is the material of choice for the production of floor planks, especially in Europe. Atlas cedar is a majestic tree of optically pleasant crown appearance, which is used extensively as an ornamental tree in many parks and residential areas in nearly all cities in Europe. In this work, we analyzed the resins collected from the above-mentioned living trees via a large set of experimental techniques to understand their composition, processing and film-forming characteristics, film surface performance in terms of rms roughness and contact potential difference fluctuations, optical behavior, and finally dielectric and electronic properties when employed as a thin capping layer for aluminum oxide in organic field effect transistors. We demonstrated that their performance is arguably similar, and the obtained results are not very different. Considering that (i) these materials are free of charge, representing non-commercialized residues of the industrial trees processed for a plethora of applications, (ii) they can be easily processed using non-toxic solvents such as ethanol and potentially many other alcohols and do not require any purification step except filtration, and (iii) they are completely non-toxic and even have medicinal properties, these resins may represent materials of choice for the future development of “green” and sustainable electronic applications. Although intense efforts have been devoted to the synthetic field of research to provide materials that are stable during operation, future consumable electronics may definitely require abundant and sustainable biomaterials with minimal cost, such as those exemplified in this study, that do not pose a threat to the environment.Conflicts of interest
The authors do not declare any conflict of interest.Data availability
Data are available from the authors upon request.Acknowledgements
This work is supported by Johannes Kepler University Open Access Publishing Fund. Cristian Vlad Irimia gratefully acknowledges the support from the Austrian Research Promotion Agency (FFG) (“DeftFAB”, project no. 867266, “ORGELE”, project no. 879855, and “AQDerivaten22”, project no. 895505). Corina Schimanofsky, Cigdem Yumusak, and Mihai Irimia-Vladu gratefully acknowledge the financial support from the project ‘‘EINSTEIN’’(HORIZON-WIDERA-2023-ACCESS-03), project no. 101136377. Andreas Petritz and Barbara Stadlober thank the financial support of the Austrian Science Fund (FWF), (project no. J4145-N30, project no. I5967-N and Grant-DOI: https://doi.org/10.55776/PIN1377723). Boyuan Ban acknowledges the financial support by the Chinese Scholarship Council (CSC) for his research studies at Montan Universität Leoben, Austria. Manuela Schiek thanks the Linz Institute of Technology (LIT-2023-12-SEE-114 SLIME) for funding. Felix Mayr gratefully acknowledges the financial support from the Austrian Research Promotion Agency (FFG) in the framework of the project “PlasIon-PhotoKat” (project no. FO999888408).References
- H. Sirringhaus, P. J. Brown, R. H. Friend, M. M. Nielsen, K. Bechgaard, B. M. W. Langeveld-Voss, A. J. H. Spiering, R. A. J. Janssen, E. W. Meijer, P. Herwig and D. M. de Leeuw, Two-dimensional charge transport in self-organized, high-mobility conjugated polymers, Nature, 1999, 401(6754), 685–688 CrossRef CAS.
- S. Bisoyi, U. Zschieschang, M. J. Kang, K. Takimiya, H. Klauk and S. P. Tiwari, Bias-stress stability of low-voltage p-channel and n-channel organic thin-film transistors on flexible plastic substrates, Org. Electron., 2014, 15, 3173–3182 CrossRef CAS.
- H. E. Katz, Z. Bao and S. L. Gilat, Synthetic chemistry for ultrapure, processable, and high-mobility organic transistor semiconductors, Acc. Chem. Res., 2001, 34(5), 359–369 CrossRef CAS.
- T. Sekitani, T. Yokota, U. Zschieschang, H. Klauk, S. Bauer, K. Takeuchi, M. Takamiya, T. Sakurai and T. Someya, Organic nonvolatile memory transistors for flexible sensor arrays, Science, 2009, 326, 1516–1519 CrossRef CAS.
- J. Zaumseil and H. Sirringhaus, Electron and ambipolar transport in organic field-effect transistors, Chem. Rev., 2007, 107(4), 1296–1323 CrossRef CAS.
- F. Ante, D. Kälblein, T. Zaki, U. Zschieschang, K. Takimiya, M. Ikeda, T. Sekitani, T. Someya, J. N. Burghartz, K. Kern and H. Klauk, Contact resistance and megahertz operation of aggressively scaled organic transistors, Small, 2012, 8(1), 73–79 CrossRef CAS.
- H. Sirringhaus, Reliability of organic field-effect transistors, Adv. Mater., 2009, 21, 3859–3873 CrossRef CAS.
- M. Irimia-Vladu, Y. Kanbur, F. Camaioni, C. Yumusak, A. A. Vlad, C. V. Irimia, A. Operamolla, G. Farinola, G. Romanazzi, G. P. Suranna, N. Gonzalez, M. C. Molina, L. F. Bautista, H. Langhals, E. D. Glowacki and N. S. Sariciftci, Stability of selected hydrogen-bonded semiconductors in organic electronic devices, Chem. Mater., 2019, 31(17), 6315–6346 CrossRef CAS.
- Y. Kanbur, H. Coskun, E. D. Glowacki, M. Irimia-Vladu, N. S. Sariciftci and C. Yumusak, High temperature stability of organic thin-film transistors based on quinacridone pigments, Org. Electron., 2019, 66, 53–57 CrossRef CAS.
- H. Klauk, Will we see gigahertz organic transistors?, Adv. Electron. Mater., 2018, 4, 1700474 CrossRef.
- C. Yumusak, N. S. Sariciftci and M. Irimia-Vladu, Purity of organic semiconductors as a key factor for the performance of organic electronic devices, Mater. Chem. Front., 2020, 4, 3678–3689 RSC.
- J. Mei, Y. Diao, A. L. Appleton, L. Fang and Z. Bao, Integrated materials design of organic semiconductors for field-effect transistors, J. Am. Chem. Soc., 2013, 135(18), 6724–6746 CrossRef CAS.
- S. Inal, J. Rivnay, A. O. Suiu, G. G. Malliaras and I. McCulloch, Conjugated polymers in bioelectronics, Acc. Chem. Res., 2018, 51(6), 1368–1376 CrossRef CAS.
- P. Hu, X. He and H. Jiang, Greater than 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm2 V−1s−1: a breakthrough of organic semiconductors for field-effect transistors, InfoMat, 2021, 3, 613–630 CrossRef CAS.
cm2 V−1s−1: a breakthrough of organic semiconductors for field-effect transistors, InfoMat, 2021, 3, 613–630 CrossRef CAS. - S. Inal, G. G. Malliaras and J. Rivnay, Benchmarking organic mixed conductors for transistors, Nat. Commun., 2017, 8(1), 1767 CrossRef.
- M. L. Chabinyc, L. H. Jimison, J. Rivnay and A. Salleo, Connecting electrical and molecular properties of semiconducting polymers for thin-film transistors, MRS Bull., 2008, 33, 683–689 CrossRef CAS.
- A. J. Morfa, T. Rödlmeier, N. Jürgensen, S. Stolz and G. Hernandez-Sosa, Comparison of biodegradable substrates for printed organic electronic devices, Cellulose, 2016, 23, 3809–3817 CrossRef CAS.
- X. Cheng, M. Caironi, Y. Y. Noh, J. Wang, C. Newman, H. Yan, A. Facchetti and H. Sirringhaus, Air stable cross-linked cytop ultrathin gate dielectric for high yield low-voltage top-gate organic field-effect transistors, Chem. Mater., 2010, 22(4), 1559–1566 CrossRef CAS.
- W. Lee, J. J. Seong, B. Ozlu, B. S. Shim, A. Marakhimov and S. Lee, Biosignal sensors and deep learning-based speech recognition: a review, Sensors, 2021, 21(4), 1399 CrossRef.
- D. Hughes, A. H. Mousa, C. Musumeci, M. Larsson, M. A. Shameem, U. Aydemir, L. Schmiderer, J. Larsson, M. Berggren, F. Ek, R. Olsson and M. Hjort, Lithography-Free Water Stable Conductive Polymer Nanowires, Nano Lett., 2025, 25(8), 3059–3065 CrossRef CAS.
- H. Karimi-Maleh, Y. Orooji, F. Karimi, M. Alizadeh, M. Baghayeri, J. Rouhi, S. Tajik, H. Beitollahi, S. Agarwal, V. Gupta, S. Rajendran, A. Ayati, L. Fu, A. Sanati, B. Tanhaei, F. Sen, M. Shabani-nooshabadi, N. Asrami and A. Al-Othman, A critical review on the use of potentiometric based biosensors for biomarkers detection, Biosens. Bioelectron., 2021, 184, 113252 CrossRef CAS.
- T. Someya, Z. Bao and G. G. Malliaras, The rise of plastic bioelectronics, Nature, 2016, 540(7633), 379–385 CrossRef CAS.
- S. R. McCuskey, J. Chatsirisupachai, E. Zeglio, O. Parlak, P. Panoy, A. Herland, G. C. Bazan and T.-Q. Nguyen, Current progress of interfacing organic semiconducting materials with bacteria, Chem. Rev., 2021, 122(4), 4791–4825 CrossRef.
- M. Irimia-Vladu, “Green” electronics: biodegradable and biocompatible materials and devices for sustainable future, Chem. Soc. Rev., 2014, 43, 588–610 RSC.
- A. Cetkovic, A. Bellapianta, M. Irimia-Vladu, J. Hofinger, C. Yumusak, A. Corna, M. C. Scharber, G. Zeck, N. S. Sariciftci, M. Bolz and A. Salti, In vitro cytotoxicity of D18 and Y6 as potential organic photovoltaic materials for retinal prostheses, Int. J. Mol. Sci., 2022, 23(15), 8666 CrossRef CAS.
- J. Y. Oh, S. Rondeau-Gagné, Y. C. Chiu, A. Chortos, F. Lissel, G. J. N. Wang, B. C. Schroeder, T. Kurosawa, J. Lopez, T. Katsumata, J. Xu, C. Zhu, X. Gu, W.-G. Bae, Y. Kim, L. Jin, J. W. Chung, J. B.-H. Tok and Z. Bao, Intrinsically stretchable and healable semiconducting polymer for organic transistors, Nature, 2016, 539(7629), 411–415 CrossRef CAS.
- M. L. Hammock, A. Chortos, B. C. K. Tee, J. B. H. Tok and Z. Bao, 25th anniversary article: the evolution of electronic skin (e-skin): a brief history, design considerations, and recent progress, Adv. Mater., 2013, 25(42), 5997–6038 CrossRef CAS.
- K. Feron, R. Lim, C. Sherwood, A. Keynes, A. Brichta and P. C. Dastoor, Organic bioelectronics: materials and biocompatibility, Int. J. Mol. Sci., 2018, 19(8), 2382 CrossRef.
- K. L. Peterson, R. P. Shukla and M. A. Daniele, Percutaneous wearable biosensors: a brief history and systems perspective, Adv. Sens. Res., 2024, 3(12), 2400068 CrossRef CAS.
- A. H. Alami, K. Aokal, D. Zhang, A. Taieb, M. Faraj, A. Alhammadi, B. Soudan, J. El-Hajjar and M. Irimia-Vladu, Low-cost dye-sensitized solar cells using ball-milled tellurium-doped graphene for counter electrode and a natural sensitizer dye, Int. J. Energy Res., 2019, 43(11), 5824–5833 CrossRef CAS.
- J. Park, Y. Lee, T. Y. Kim, S. Hwang and J. Seo, Functional bioelectronic materials for long-term biocompatibility and functionality, ACS Appl. Electron. Mater., 2022, 4(4), 1449–1468 CrossRef CAS.
- M. D. Angione, R. Pilolli, D. Cotrone, M. Magliulo, A. Mallardi, G. Palazzo, L. Sabbatini, D. Fine, A. Dodabalapur, N. Cioffi and L. Torsi, Carbon based materials for electronic bio-sensing, Mater. Today, 2011, 14, 424–433 CrossRef CAS.
- Y. van De Burgt, A. Melianas, S. T. Keene, G. Malliaras and A. Salleo, Organic electronics for neuromorphic computing, Nat. Electron., 2018, 1(7), 386–397 CrossRef.
- M. Muskovich and C. J. Bettinger, Biomaterials-based electronics: polymers and interfaces for biology and medicine, Adv. Healthcare Mater., 2012, 1, 248–266 CrossRef CAS.
- K. Kuribara, H. Wang, N. Uchiyama, K. Fukuda, T. Yokota, U. Zschieschang, C. Jaye, D. Fischer, H. Klauk, T. Yamamoto, K. Takimiya, M. Ikeda, H. Kuwabara, T. Sekitani, Y.-L. Loo and T. Someya, Organic transistors with high thermal stability for medical applications, Nat. Commun., 2012, 3, 723 CrossRef.
- B. Stadlober, M. Zirkl and M. Irimia-Vladu, The route towards sustainable smart sensors: ferroelectric PVDF-based materials and their integration into flexible electronics, Chem. Soc. Rev., 2019, 48, 1787–1825 RSC.
- J. Rivnay, P. Leleux, M. Ferro, M. Sessolo, A. Williamson, D. A. Koutsouras, D. Khodagholy, M. Ramuz, X. Strakosas, R. M. Owens, C. Benar, J.-M. Badier, C. Bernard and G. G. Malliaras, High-performance transistors for bioelectronics through tuning of channel thickness, Sci. Adv., 2015, 1(4), e1400251 CrossRef.
- J. Rivnay, S. Inal, A. Salleo, R. M. Owens, M. Berggren and G. G. Malliaras, Organic electrochemical transistors, Nat. Rev. Mater., 2018, 3(2), 1–14 Search PubMed.
- J. Wünssche, Y. Deng, P. Kumar, E. Di Mauro, E. Josberger, J. Sayago, A. Pezzella, F. Soavi, F. Cicoira, M. Rolandi and C. Santato, Protonic and electronic transport in hydrated thin films of the pigment eumelanin, Chem. Mater., 2015, 27(2), 436–442 CrossRef.
- Y. van de Burgt, A. Melianas, S. T. Keene, G. Malliaras and A. Salleo, Organic electronics for neuromorphic computing, Nat. Electron., 2018, 1(7), 386–397 CrossRef.
- Y. H. Jung, T. H. Chang, H. Zhang, C. Yao, Q. Zheng, V. W. Yang, H. Mi, M. Kim, S. J. Cho, D. W. Park, H. Jiang, J. Lee, Y. Qiu, W. Zhou, Z. Cai, S. Gong and Z. Ma, High-performance green flexible electronics based on biodegradable cellulose nanofibril paper, Nat. Commun., 2015, 6, 1–11 Search PubMed.
- J. Reynolds, M. Wilkins, D. Martin, M. Taggart, K. R. Rivera, M. Tunc-Ozdemir, T. Rufty, E. Lobaton, A. Bozkurt and M. A. Daniele, Evaluating bacterial nanocellulose interfaces for recording surface biopotentials from plants, Sensors, 2024, 24(7), 2335 CrossRef CAS.
- R. Geyer, J. R. Jambeck and K. L. Law, Production, use, and fate of all plastics ever made, Sci. Adv., 2017, 3(7), e1700782 CrossRef.
- S. M. F. Cruz, L. A. Rocha and J. C. Viana, Printing technologies on flexible substrates for printed electronics, Intech Open, 2018, 2, 64 Search PubMed.
- A. Camus, S. Choe, C. Bour-Cardinal, J. Isasmendi, Y. Cho, Y. Kim, C. V. Irimia, C. Yumusak, M. Irimia-Vladu, D. Rho, J. Myung and C. Santato, Electrical response and biodegradation of sepia melanin-shellac films printed on paper, Commun. Mater., 2024, 5(1–13), 173 CrossRef CAS.
- S. Bauer, S. Bauer-Gogonea, I. Graz, M. Kaltenbrunner, C. Keplinger and R. Schwödiauer, 25th Anniversary article: a soft future: from robots and sensor skin to energy harvesters, Adv. Mater., 2014, 26(1), 149–162 CrossRef CAS.
- B. Crone, A. Dodabalapur, Y. Y. Lin, R. W. Filas, Z. Bao, A. LaDuca, R. Sarpeshkar, H. E. Katz and W. Li, Large-scale complementary integrated circuits based on organic transistors, Nature, 2000, 403(6769), 521–523 CrossRef CAS.
- L. Migliaccio, M. G. Say, G. Pathak, I. Gablech, J. Brodský, M. J. Donahue and E. D. Głowacki, Ultrathin Indium Tin Oxide Accumulation Mode Electrolyte-Gated Transistors for Bioelectronics, Adv. Mater. Technol., 2025, 10(4), 2302219 CrossRef CAS.
- M. Kaltenbrunner, T. Sekitani, J. Reeder, T. Yokota, K. Kuribara, T. Tokuhara, M. Drack, R. Schwödiauer, I. Graz, S. Bauer-Gogonea, S. Bauer and T. Someya, An ultra-lightweight design for imperceptible plastic electronics, Nature, 2013, 499(7459), 458–463 CrossRef CAS.
- L. Fabbri, L. Migliaccio, A. Širvinskytė, G. Rizzi, L. Bondi, C. Tamarozzi, S. A. L. Weber, B. Fraboni, E. D. Glowacki and T. Cramer, How to achieve high spatial resolution in organic optobioelectronic devices?, Adv. Mater. Interface, 2024, 6(26), 2400822 Search PubMed.
- S. M. Sze, Physics of semiconductor devices, Wiley, New York, 3rd edn, with Kwok K. Ng, 2007 Search PubMed.
- H. Sirringhaus, 25th anniversary article: organic field-effect transistors: the path beyond amorphous silicon, Adv. Mater., 2014, 26(9), 1319–1335 CrossRef CAS.
- D. Saadi, F. Mayr, C. Yumusak, D. Wielend, B. Kahraman, C. V. Irimia, Y. Kanbur, M. Bednorz, K. Kotwica, A. ben Fredj, S. Romdhane, M. C. Scharber, N. S. Sariciftci and M. Irimia-Vladu, N,N′-substituted quinacridones for organic field effect transistors, Mater. Adv., 2023, 4, 2214–2225 RSC.
- R. D’Orsi, C. V. Irimia, J. J. Lucejko, B. Kahraman, Y. Kanbur, C. Yumusak, F. Babudri, M. Irimia Vladu and A. Operamolla, Kraft lignin: from pulping waste to bio-based dielectric polymer for organic field-effect transistors, Adv. Sustainable Syst., 2022, 6(12), 2200285 CrossRef.
- C. M. Siket, N. Tillner, A. I. Mardare, A. Reuveny, C. D. Grill, F. Hartmann, G. Kettlgruber, R. Moser, J. P. Kollender, T. Someya, A. W. Hassel, M. Kaltenbrunner and S. Bauer, Direct writing of anodic oxides for plastic electronics, npj flex. Electron., 2018, 2(1), 23 CrossRef.
- G. A. Torres Sevilla and M. M. Hussain, Printed organic and inorganic electronics: devices to systems, IEEE J. Emerg. Selected Top. Circuits Syst., 2017, 7(1), 147–160 Search PubMed.
- M. Baumgartner, M. E. Coppola, N. S. Sariciftci, E. D. Glowacki, S. Bauer and M. Irimia-Vladu, Emerging “green” materials and technologies for electronics, in Green materials for electronics, ed. M. Irimia-Vladu, E. D. Glowacki, N. S. Sariciftci, S. Bauer, Wiley-VCH, Weinheim, 2017, pp. 1–52 Search PubMed.
- F. Torricelli, I. Alessandri, E. Macchia, I. Vassalini, M. Maddaloni and L. Torsi, Green materials and technologies for sustainable organic transistors, Adv. Mater. Technol., 2022, 7(2), 2100445 CrossRef CAS.
- L. Wang, K. Wang, Z. Lou, K. Jiang and G. Shen, Plant-based modular building blocks for “green” electronic skins, Adv. Funct. Mater., 2018, 28, 1804510 CrossRef.
- X. Ren, Z. Lu, X. Zhang, S. Grigorian, W. Deng and J. Jie, Low-voltage organic field-effect transistors: challenges, progress, and prospects, ACS Mater. Lett., 2022, 4(8), 1531–1546 CrossRef CAS.
- L. Wang, D. Chen, K. Jiang and G. Shen, New insights and perspectives into biological materials for flexible electronics, Chem. Soc. Rev., 2017, 46, 6764–6815 RSC.
- J. E. Eckenwalder, Conifers of the world: the complete reference, 2009, Timber Press, ISBN 0-88192-974-3.
- C.-Y. Qiao, J.-H. Ran, Y. Li and X.-Q. Wang, Phylogeny and biogeography of cedrus (pinaceae) inferred from sequences of seven paternal chloroplast and maternal mitochondrial DNA regions, Ann. Bot., 2007, 100(3), 573–580 CrossRef CAS.
- W. M. Walters, European garden flora, 1986, vol 1 Search PubMed.
- F. Da Ronch, G. Caudullo, W. Tinner and D. de Rigo, Larix decidua and other larches in Europe: distribution, habitat, usage and threats, in European Atlas of Forest Tree Species: Tree species, ed. S.-J. Miguel-Ayanz, D. de Rigo, G. Caudullo, T. Houston Durrant and A. Mauri, European Atlas of Forest Tree Species, Publication Office of the European Union, Luxembourg, 2016 Search PubMed.
- P. Bannister and G. Neuner, Frost resistance and the distribution of conifers, in Conifer cold hardiness, ed. F. J. Bigras and S. J. Colombo, Kluwer Academic Publishers, Dordrecht, 2001, pp. 3–22 Search PubMed.
- J. E. Eckenwalder, Conifers of the world: the complete reference, Timber Press, Portland, OR, 2009. ISBN 978-0-88192-974-4 Search PubMed.
- A. Tschirch and C. Weigel, Arbeiten aus dem pharmaseutischen Institute der Universitilt Bern. Untersuchungen Uber die Sekrete. Ueber den Harzbalsam von Larix decidua. (Lärchen terpentin), 1900, pp. 387–400 Search PubMed.
- E. Klimo, H. Hager and J. Kulhavý, Spruce monocultures in Central Europe-problems and prospects, EFI Proc., 2000, 33, 5–9 Search PubMed.
- S. Jansen, H. Konrad and T. Geburek, The extent of historic translocation of Norway spruce forest reproductive material in Europe, Ann. For. Sci., 2017, 74, 56 CrossRef.
- B. Nystedt, N. Street, A. Wetterbom, A. Zuccolo and Y. C. Lin, et al., The Norway spruce genome sequence and conifer genome evolution, Nature, 2013, 497, 579–584 CrossRef CAS.
- D. S. Argyropoulos, N. Pajer and C. Crestini, Quantitative 31P NMR analysis of lignins and tannins, J. Visualized Exp., 2021, 174, e62696 Search PubMed.
- Y. Udum, P. Denk, G. A. Workneh, D. H. Apaydin, A. Nevosad, C. Teichert, M. S. White, N. S. Sariciftci and M. C. Scharber, Inverted bulk-heterojunction solar cell with cross-linked hole-blocking layer, Org. Electron., 2014, 15, 997–1001 CrossRef CAS.
- A. Kamptner, M. C. Scharber and M. Schiek, Accurate determination of the uniaxial complex refractive index and the optical band gap of polymer thin films to correlate their absorption strength and onset of absorption, ChemPhysChem, 2024, 25, e202400233 CrossRef CAS.
- R. M. Silverstein, F. X. Webster and D. J. Kiemle, Spectrometric identification of organic compounds, John Wiley & Sons, Hoboken (NJ), 7 edn, 2005, pp. 72–126 Search PubMed.
- H. L. Hergert, Infrared spectra of lignin and related compounds. II. Conifer lignin and model compounds, J. Org. Chem., 1960, 25, 405–413 CrossRef CAS.
- R. Świsłocka, M. Kowczyk-Sadowy, M. Kalinowska and W. Lewandowski, Spectroscopic (FT-IR, FT-Raman, 1H and 13C NMR) and theoretical studies of p-coumaric acid and alkali metal p-coumarates, Spectroscopy, 2012, 27, 35–48 CrossRef.
- R. J. Jakobsen, The vibrational spectra of p-cresol, Spectrochim. Acta, 1965, 21, 433–442 CrossRef CAS.
- T. Holmbom, M. Reunanen and P. Fardim, Composition of callus resin of Norway spruce, Scots pine, European larch and Douglas fir, Holzforschung, 2008, 62, 417–422 CrossRef CAS.
- Y. Ez Zoubi, F. El-AkhAL, A. Farah, K. Taghzouti and A. El Ouali Lalami, Chemical composition and larvicidal activity of Moroccan Atlas Cedar (Cedrus atlantica Manetti) against Culex pipiens (Diptera: Culicidae), J. Appl. Pharm. Sci., 2017, 7(07), 030–034 CAS.
- B. Wang, W. Huang, L. Chi, M. Al-Hashimi, T. J. Marks and A. Facchetti, Chem. Soc. Rev., 2018, 118, 5690 CrossRef CAS.
- M. Irimia-Vladu, E. D. Głowacki, G. Schwabegger, L. Leonat, H. Z. Akpinar, H. Sitter, S. Bauer and N. S. Sariciftci, Natural resin shellac as substrate and dielectric layer for organic field-effect transistors, Green Chem., 2013, 15, 1473–1476 RSC.
- M. E. Coppola, A. Petritz, C. V. Irimia, C. Yumusak, F. Mayr, M. Bednorz, A. Matkovic, M. A. Aslam, K. Saller, C. Schwarzinger, M. D. Ionita, M. Schiek, A. I. Smeds, Y. Salinas, O. Brüggemann, R. D’Orsi, M. Mattonai, E. Ribechini, A. Operamolla, C. Teichert, C. Xu, B. Stadlober, N. S. Sariciftci and M. Irimia-Vladu, Pinaceae pine resins (black pine, shore pine, rosin and Baltic amber) as natural dielectrics for low operating voltage, hysteresis-free, organic field effect transistors, Global Challenges, 2023, 7, 2300062 CrossRef.
- J. Ivić, A. Petritz, C. V. Irimia, B. Kahraman, Y. Kanbur, M. Bednorz, C. Yumusak, M. A. Aslam, A. Matković, K. Saller, C. Schwarzinger, W. Schühly, A. I. Smeds, Y. Salinas, M. Schiek, F. Mayr, C. Xu, C. Teichert, M. Osiac, N. S. Sariciftci, B. Stadlober and M. Irimia-Vladu, Pinaceae fir resins as natural dielectrics for low voltage operating, hysteresis-free organic field effect transistors, Adv. Sustainable Syst., 2022, 6(10), 2200234 CrossRef.
- L. Zha and C. L. Liu, Review and mechanism of the thickness effect of solid dielectrics, Nanomaterials, 2020, 10, 2473 CrossRef.
- C. R. Newman, C. D. Frisbie, D. A. da Silva Filho, J. L. Bredas, P. C. Ewbank and K. R. Mann, Introduction to organic thin film transistors and design of n-channel organic semiconductors, Chem. Mater., 2004, 16, 4436 CrossRef CAS.
- U. Zschieschang, R. T. Weitz, K. Kern and H. Klauk, Bias stress effect in low-voltage organic thin-film transistors, Appl. Phys. A: Mater. Sci. Process., 2009, 95, 139–145 CrossRef CAS.
- J. H. Park, Y. T. Lee, H. S. Lee, J. Y. Lee, K. Lee, G. B. Lee, J. Han, T. W. Kim and S. Im, Origin of bias-stress induced instability in organic thin-film transistors with semiconducting small-molecule/insulating polymer bend channel, Appl. Mater. Interface, 2013, 5, 1625–1629 CrossRef CAS.
- Z. Chen, M. J. Lee, R. S. Ashraf, Y. Gu, S. Albert-Seifried, M. M. Nielsen, B. Schroeder, T. D. Anthopoulos, M. Heeney, I. McCulloch and H. Sirringhaus, High-performance ambipolar diketopyrrolopyrrolethieno[3,2-b]thiophene copolymer field-effect transistors with balanced hole and electron mobilities, Adv. Mater., 2012, 24, 647–652 CrossRef CAS.
- C. Yumusak, F. Mayr, D. Wielend, B. Kahraman, Y. Kanbur, H. Langhals and M. Irimia-Vladu, 1,7-diazaperylene in organic field effect transistors, Isr. J. Chem., 2022, e202100126 CrossRef CAS.
- C. V. Irimia, C. Yumusak, B. Ban, E. Leeb, F. Mayr, C. Schimanofsky, A. I. Mardare, A. M. Molnar, C. Teichert, N. S. Sariciftci and M. Irimia-Vladu, Natural alkaloids (caffeine, theobromine and theophylline) as gate dielectrics on gold and aluminum gate electrodes in low operating voltage organic field-effect transistors, IEEE J. Flex. Electron., 2025 DOI:10.1109/JFLEX.2025.3539612.
- S. Kim, C. Yumusak, C. V. Irimia, M. Bednorz, E. Yenel, M. Kus, N. S. Sariciftci, B. S. Shim and M. Irimia-Vladu, Amplifying the dielectric constant of shellac by incorporating natural clays for organic field effect transistors (OFETs), Turkish J. Chem., 2023, 47, 1169–1182 CrossRef.
- G. Schwabegger, M. Ullah, M. Irimia-Vladu, M. Reisinger, Y. Kanbur, R. Ahmed, P. Stadler, S. Bauer, N. S. Sariciftci and H. Sitter, high mobility, low voltage operating C60 based n-type organic field effect transistors, Synth. Met., 2011, 161, 2058–2062 CrossRef CAS.
- Y. Kanbur, H. Coskun, E. D. Głowacki, M. Irimia-Vladu, N. S. Sariciftci and C. Yumusak, High temperature stability of organic thin-film transistors based on quinacridone pigments, Org. Electron., 2019, 66, 53–57 CrossRef CAS.
- Y. Kanbur, M. Irimia-Vladu, E. D. Glowacki, M. Baumgartner, G. Schwabegger, L. N. Leonat, M. Ullah, H. Sitter, R. Schwödiauer, Z. Kücükyavuz, S. Bauer and N. S. Sariciftci, Vacuum processed polyethylene as a dielectric for low voltage operating organic field effect transistors, Org. Electron., 2012, 13, 919–924 CrossRef CAS.
- A. Petritz, A. Wolfberger, A. Fian, T. Griesser, M. Irimia-Vladu and B. Stadlober, Cellulose derivative-based gate dielectric for high performance organic complementary inverters, Adv. Mater., 2015, 27, 7645–7656 CrossRef CAS.
- A. Petritz, A. Wolfberger, A. Fian, A. Haase, M. Irimia-Vladu, H. Gold, T. Rothländer, T. Griesser and B. Stadlober, Cellulose as biodegradable high-k dielectric layer in organic complementary inverters, Appl. Phys. Lett., 2013, 103, 153303 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |