 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Polybutene, polyisobutylene, and beyond: a comprehensive review of synthesis to sustainability
J. I.
Mnyango
 *ab,
B.
Nyoni
ab,
N.
Mama
b,
B. G.
Fouda-Mbanga
*ab,
B.
Nyoni
ab,
N.
Mama
b,
B. G.
Fouda-Mbanga
 b,
Z.
Tywabi-Ngeva
b and
S. P.
Hlangothi
*ab
b,
Z.
Tywabi-Ngeva
b and
S. P.
Hlangothi
*ab
aCentre for Rubber Science and Technology, Department of Chemistry, Nelson Mandela University, P.O. BOX 77000, Gqeberha, 6031, South Africa. E-mail: mnyangojabulani@gmail.com
bDepartment of Chemistry, Nelson Mandela University, Gqeberha, 6031, South Africa. E-mail: percy.hlangothi@mandela.ac.za
First published on 8th July 2025
Abstract
Polymers derived from butene and isobutylene are highly versatile and exhibit unique properties. These include polybutene, polyisobutylene, butyl rubber, polybutylene terephthalate, polybutylene succinate, and poly(styrene-b-isobutylene-b-styrene), each of which has a specific use in sectors such as packaging, automotive, and healthcare. However, despite their potential, the body of research on butene- and isobutylene-based polymers is less extensive than that of more significantly researched and used polymers like polyethylene or polystyrene. This disparity underscores the need for further comparative research to evaluate their performance, sustainability, and commercial viability relative to these traditional polymers. By highlighting new trends and finding knowledge gaps, this study presents a thorough analysis of progress in the synthesis, properties, processability, and applications of butene- and isobutylene-based polymers. The development of eco-friendly synthesis processes and the growing interest in high-performance applications—especially in the packaging, automotive, biomedical, and electronics industries—are some of the key findings. Particularly, polybutylene succinate and poly(butylene adipate-co-terephthalate) demonstrate how combining eco-conscious polymerization processes with biodegradability can result in the manufacture of more sustainable materials, with definite benefits when evaluated via life cycle assessments. Furthermore, concerns about the degradation capacities of biodegradable butene- and isobutylene-based polymers were noted in the review. Combining all the findings provides insights into future research directions, highlighting the necessity of more investigation into the economical manufacturing, green chemistry solutions, and innovative applications of these polymers. The inferences of this work suggest that these polymers have considerable potential for utilization not just in domestic and high-performance industrial applications but also as sustainable materials.
1. Introduction
Butene- and isobutylene-based polymers have emerged as a key component of contemporary polymer science and engineering due to their versatility, potential for sustainability, and various applications across several industries. These butene- or isobutylene-derived polymers comprise a wide range of materials, each with unique properties that make them valuable in different domains. A broad spectrum of polymer families, including polybutene (PB), polyisobutylene (PIB), butyl rubber (IIR), polybutylene terephthalate (PBT), polybutylene succinate (PBS), and poly(styrene-b-isobutylene-b-styrene) (SIBS rubber), has drawn a lot of interest from academic, domestic, and industrial circles because of their performance-based properties, biodegradability, and potential economic benefits in various sectors, which include packaging, automotive, healthcare, and electronics.One of the earliest innovations in the history of isobutylene-based polymers was the development of IIR in the early 20th century.1 When this polyolefin-like material was initially introduced in the 1940s, its remarkable gas impermeability and excellent resilience made it indispensable for applications like tyre production.1,2 Thenceforth, polyolefins (PB and PIB) have established themselves in specific markets, especially those requiring high flexibility and resistance to chemicals.3 Such homopolymers, because of their completely saturated backbones and low glass transition temperature (Tg) values, present superior flexibility and long-term chemical stability, which underpin their widespread utilization in various applications, such as seals, adhesives, and flexible materials.3 Research into the synthesis, processability, and applications of materials with enhanced mechanical properties and biodegradability was further fueled by the development of polyesters (PBT and PBS) in subsequent decades.4,5 Moreover, due to its superior elastomeric properties, processability, and thermal stability, as well as the evolving need for high-performance block copolymers, SIBS, which emerged in the late 20th century, has commercially matured into a versatile block copolymer that finds usage in adhesives, coatings, and medical devices.6,7
The synthesis of butene- and isobutylene-based polymers has undergone significant changes over the years, with improvements in polymerization pathways enabling precise control over characteristics such as molecular weight (Mw) and branching as well as processes like copolymerization. For example, Ziegler–Natta or metallocene catalyst systems, which offer a high Mw and a controlled polymer structure, are employed to synthesize PB, with the former being used commercially for isotactic PB-1 synthesis.8–10 Similarly, cationic polymerization, which can also be precisely controlled to obtain the required molecular characteristics, is primarily used to synthesize PIB.11,12 The ability to tailor these polymers for specific end-use applications, from flexible packaging items to high-strength fibers utilized in engineering plastics, has improved with the advent of innovative catalysts and reaction conditions. Nevertheless, despite these developments, much remains unknown about the long-term stability and degradation mechanisms of these materials in practical environments.
Generally, butene- and isobutylene-based polymers are not as widely studied as traditional polymers like polyethylene (PE) and polystyrene (PS). The main differences between representative butene- and isobutylene-based polymers (PB, PIB, PBT, and PBS) and PE/PS in a few selected properties are outlined in Table 1, with an emphasis on mechanical and thermal behavior, as well as processability. Several factors contribute to this limited scope of research. The commercial adoption of some butene- and isobutylene-based polymers (e.g., PIB and PBS) is restricted by their comparatively complex and expensive synthesis pathways.5,12 In comparison, because of their wide range of applications, inexpensive cost of production, and well-established processing infrastructure, the market continues to demand well-known polymers like PE and PS.2,3 Also, the specialized uses of some butene- and isobutylene-based materials, like PBS in biodegradable packaging13,14 and PIB in sealants,11,12 have not yet reached the volume or consistency required to support in-depth scholarly or commercial research.
| Property | Polymer | |||||
|---|---|---|---|---|---|---|
| PB8 | PIB12 | PBT4 | PBS13 | PE3 | PS2 | |
| Ultimate tensile strength, MPa | 20–30 | 0.5–1.0 | 50–60 | 30–40 | 10–30 | 30–50 |
| Elongation at break, % | 300–600 | ≥800 | 50–200 | 100–300 | 100–500 | 1–2 |
| Thermal behavior, °C | ca. 120–150 | ca. 100–120 | 220–250 | ca. 90–100 | 120–135 | ca. 80–100 |
| T g, °C | −25 to −40 | −65 to −70 | ca. 50 | ca. −30 | −100 to −125 | ca. 100 |
| Processing ease | Moderate | Complex | Complex | Moderate | Easy | Easy |
As a biodegradable substitute for traditional plastics, PBS has gained significant attention from the perspective of environmental sustainability. This polyester material shares mechanical properties with polymers that are based on petrochemicals (e.g., PE and polypropylene (PP)) and is made from plant-based feedstocks and renewable resources like succinic acid.13–15 Durmaz et al.16 have shown that solvent casting and melt mixing methods can be used to synthesize PBS-based films reinforced with alkaline-treated halloysite nanotubes. According to the literature, these films can greatly increase the shelf life of fruits by scavenging ethylene gas and controlling gas transport. This special feature demonstrates the potential of PBS as an active food packaging material. Notwithstanding these promising applications, comprehensive studies on maximizing the properties of this polymer for widespread commercial use—particularly concerning its mechanical strength and long-term durability—are still lacking.
Furthermore, PBT has earned prominence for its superior mechanical properties, high thermal stability, and chemical resistance, which make it an ideal material for use in fibers, electronics, as well as automotive exterior and interior components.17,18 The main advantages of PBT lie in its high-performance qualities and durability, as opposed to PBS's emphasis on biodegradability, similar to poly(butylene adipate-co-terephthalate) (PBAT, also commonly known as Ecoflex). In engineering plastics, where strength and resistance to heat are of utmost importance, PBT is typically utilized.17,19,20 However, even though it has been extensively studied for various applications, concerns with processing methods and cost-effectiveness still prevent it from being widely adopted in some sectors.21,22 Therefore, the applications of PBT in a range of high-performance industries may grow as a result of further studies into developing more efficient processing methods and further enhancement of its properties.
PIB is another isobutylene-based polymer that is essential to various applications, including adhesives, sealants, and coatings.11,12,23,24 Excellent flexibility, low-temperature performance, and exceptional chemical resistance are all made possible by the distinct chemical structure of PIB, even though the extent of these properties can depend on factors such as Mw, crosslinking, and the presence of additives.23–26 The potential of blending this polymer with more biodegradable polymers to get hybrid materials with enhanced mechanical and environmental performance is one field that has not been thoroughly investigated. Exploring the compatibility and interactions of PIB with polymers like PBS or PBAT may result in new materials that combine high performance with biodegradability.
Ultimately, even though each of the butene- and isobutylene-based polymers investigated in this review has achieved notable commercial success in a variety of applications, there is still a lack of studies into their long-term effects on the environment and their recyclability, as well as the development of more sustainable raw materials and alternative butene- and isobutylene-based polymers. For example, whilst IIR is widely recognized for its effectiveness in tyre applications, details are scarce on its capacity to be recycled and its potential for integration into models of the circular economy. Similarly, the challenges in enhancing the mechanical properties and degradation rates of biodegradable butene- and isobutylene-based polymers remain important ongoing research topics.
This review aims to present a comprehensive exploration of PB, PIB, IIR, PBT, PBS, and SIBS polymers, emphasizing their synthesis, processability, properties, and applications. Special attention is given to these saturated polymers. Unsaturated polymers, such as polybutadiene and polyisoprene, along with their derivatives, are excluded from the present discussion due to their distinct chemical behavior, synthesis methods, and application profiles, which warrant a separate and focused review. In addition to surveying established knowledge, this review highlights areas that are still under-explored, including scalability, environmental impact, and recyclability of the butene- and isobutylene-based materials, while offering some suggestions for future research directions to address these gaps. Accordingly, this review promotes a better understanding of these polymers and their potential for domestic and industrial applications by consolidating the existing body of knowledge and identifying opportunities that necessitate more research.
2. Synthesis of butene- and isobutylene-based polymers: key steps, conditions, and challenges
This section covers the different types of butene- and isobutylene-based polymers, focusing on their classification and synthesis processes. These polymers can be generally divided into four primary groups, polyolefins, polyolefin-like materials, polyesters, and block copolymers, based on their backbone structure, the monomers involved, polymerization methods employed, as well as resultant material properties and applications (Fig. 1).3,14,27,28 A variety of industrial applications can benefit from the unique characteristics of each group, which each represents a different category of materials. An extensive understanding of synthesis processes is essential for the design and development of innovative materials with desired functionalities and applications.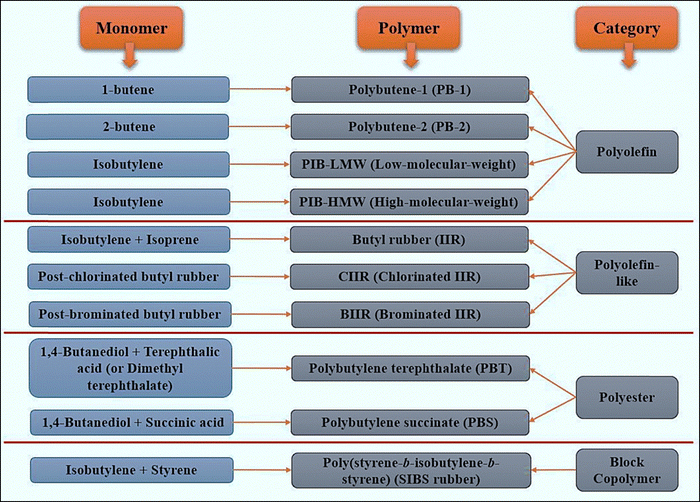 | ||
| Fig. 1 Classification of butene- and isobutylene-based polymers by monomer composition, polymer type, and category.3,14,27,28 | ||
Since the polyolefins are synthesized from butene and isobutylene (commonly known as isobutene in industrial contexts) through homopolymerization, respectively, PB and PIB are included in the polyolefin group. Catalytic methods such as Ziegler–Natta or metallocene-based systems (for PB)8–10,29 and cationic polymerization (for PIB)11,12,30–33 are used to synthesize these materials. Although polyolefin-like materials (IIRs), which are typically synthesized via solution and cationic polymerization, exhibit a structure that is relatively similar to polyolefins as a result of isobutylene monomer copolymerized with 1 to 4 wt% of isoprene,34 their synthesis methods and the resultant properties differ, making them a separate category. Furthermore, PBT and PBS, two isobutylene-based polymers that are polyesters, are synthesized via polycondensation reactions between diols and dicarboxylic acids.35–41 PBS is made from succinic acid and butylene glycol, whereas PBT is made by reacting butylene glycol with terephthalic acid. Despite being a block copolymer (another category), SIBS is considered an isobutylene-based polymer due to the significant presence of an isobutylene-originating central block polymer, PIB, which generally makes up 40–70 wt% of SIBS, where the styrene blocks occupy the remaining portion.6,42,43 Even though fewer studies are devoted to the core synthesis of SIBS itself, cationic polymerization has been used to synthesize this SIBS.
2.1. Synthesis of polyolefins
Among the primary advantages of the radical polymerization process are its versatility as well as simplicity as it does not require highly specialized equipment and can be initiated with easily accessible initiators (therefore, it is economical).54,55 It can also polymerize a wide variety of monomers.54 This makes it well-suited for use in bulk, solution, or suspension conditions as well as for the large-scale industrial manufacture of polyolefins with different Mws.54–56 However, radical polymerization can also produce homopolymers with a rather broad distribution of molecular chain lengths due to the random nature of the process, especially the initiation and termination stages.57,58 The resultant broad MWD is detrimental as it causes processing challenges, e.g., issues in controlling melt flow during injection molding or extrusion, as well as complex polymer blending.58 It also lacks precise control over the polymer stereochemistry or block structure, i.e., results in irregular arrangement of the side groups along the polymer backbone.58,59 Accordingly, the radical polymerization method, especially if the reaction conditions are not carefully controlled, leads to the polymer's inconsistent behavior, which can be a result of the formation of increased branched structures, cross-linking, and gelation (a network that prohibits polymerization from occurring), all of which decrease performance consistency in numerous applications.60
The advantage of using cationic polymerization to synthesize polyolefins is that it offers more control over the resultant polymer's molecular structure, weight, and stereochemistry.62,67 This level of control, however, is typically accompanied by elevated operational costs, especially as a result of the need for stringent purity, dry conditions, and the employment of moisture-sensitive and often expensive catalysts. In contrast to the radical-based process, cationic polymerization produces a polymer with (i) a narrower MWD (i.e., lower Đ), indicating greater uniformity of chain lengths, and (ii) chains that have constant tacticity—the arrangement of side groups along the backbone chain.61–66 In addition, the cationic polymerization method is selective, i.e., not every butene isomer may be suitable for it, and is more effective when 1-butene and isobutylene monomers are used, which generally counts as its limitation.67 Oxygen, moisture, or other contaminants can also deactivate the cationic initiators and impede the polymerization process, making cationic polymerization susceptible to these effects.63,66 However, to overcome some of the challenges, the cationic polymerization of PIB has been advanced to produce a highly reactive version of PIB (viz.: HR PIB), which has been thoroughly discussed by Karthikeyan et al.31 Because of its reactive nature, HR PIB is the most vital industrial polymeric material used as an additive for lubricants, fuels, and various polymers.31,52,65,68 The authors31 described a variety of synthetic methods for HR PIB, such as the living carbocationic approach, organometallic catalyst systems, and the extensively studied carbocationic polymerization that involves the chain transfer promoting catalysts based on Lewis acids. An actual example of this is a study by Li et al.,30 who used the cationic polymerization method to synthesize HR PIB. They used a water/titanium tetrachloride (H2O/TiCl4) initiating system in the 1-butyl-3-methylimidazolium hexafluorophosphate ([Bmim][PF6]) ionic liquid (IL) at −10 °C to make the HR PIB (Fig. 2), which had a high exo-olefin end group content (>80%) and a relatively narrow MWD (Mw/Mn ≤ 2.7). According to the authors, the main characteristic of isobutylene polymerization in ionic liquids (ILs) is that it occurs heterogeneously at the interface of the isobutylene droplets and the IL particles. The [PF6]− anion served as both a deactivator, similar to a nucleophile or electron donor for the carbenium ion, and an activating initiator in conjunction with a Lewis acid.30 The narrow MWD and functionality selectivity of PIB were enhanced by the conditions of a mixture.30 ILs thus exhibit significant benefits and promise for HR PIB synthesis.30
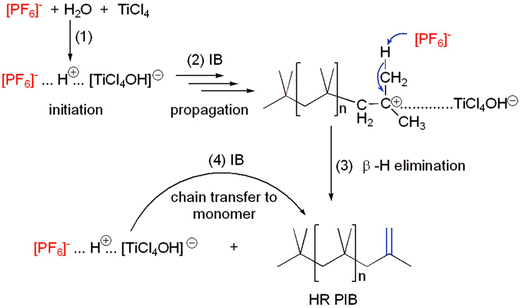 | ||
| Fig. 2 Feasible mechanism of H2O/TiCl4-initiated cationic polymerization of isobutylene in [Bmim][PF6] ionic liquid at −10 °C. Reproduced from ref. 30 with permission from Royal Society of Chemistry, copyright 2019. | ||
Additionally, Yang et al.69 sought to develop novel PIB telechelic prepolymers with a range of epoxide functionalities, such as cycloaliphatic epoxide, exo-olefin epoxide, aliphatic glycidyl ether, and phenyl glycidyl ether. A complete process is schematized in Fig. 3, where the method employed involved combining living carbocationic polymerization with post-polymerization modifications, including reaction with epichlorohydrin, epoxidation with meta-chloroperoxybenzoic acid, and nucleophilic substitution to introduce the desired end-group functionalities. The findings revealed that all PIB prepolymers exhibited a functionality of 2.0 and achieved the targeted Mw with narrow distributions. The study also showed how versatile the synthesis approach employed is, enabling the production of prepolymers with a variety of additional functional groups. The authors suggested that the synthesized PIB prepolymers have the potential to be applied in coatings, adhesives, sealants, and other industrial products through thermal curing or photoinitiated cationic ring-opening polymerization.
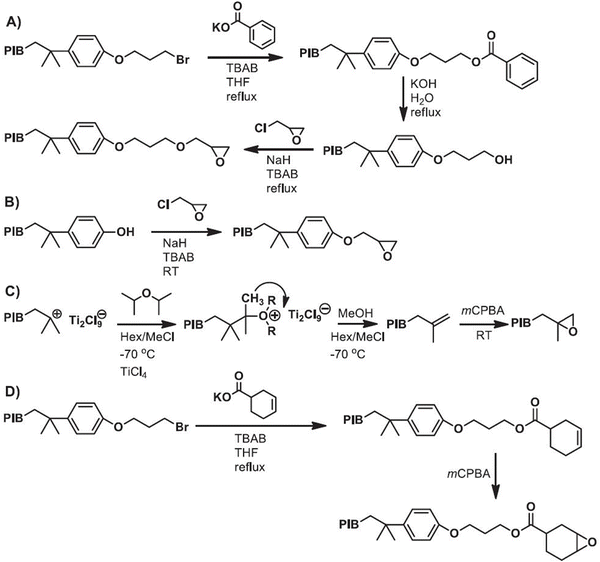 | ||
| Fig. 3 Synthesis of PIB telechelic prepolymers with different epoxide functionalities: (A) PIB with aliphatic glycidyl ether, (B) PIB with phenyl glycidyl ether, (C) PIB with exo-olefin epoxide, and (D) PIB with cyclohexene epoxide. Reproduced from ref. 69 with permission from Elsevier, copyright 2020. | ||
On the other hand, Hulnik et al.12 demonstrated the advantage of inducing the cationic polymerization of isobutylene (and β-pinene) using visible light. Their study aimed to investigate a visible-light-induced cationic polymerization system for synthesizing PIB-LMW (and poly(β-pinene)) (Fig. 4). The method involved using a photo-initiating system comprising dimanganese decacarbonyl (Mn2(CO)10) and diphenyl iodonium hexafluorophosphate ([Ph2I]+[PF6]−) in a dichloromethane (CH2Cl2)/n-hexane mixture at −30 °C. The polymerization reaction was initiated by the formation of chloromethylisobutyl carbocations, generated by oxidizing the chloromethylisobutyl radicals using [Ph2I]+[PF6]−. These radicals were generated through chlorine abstraction from the CH2Cl2 by MnCO5˙ radicals, which were formed upon photo-induced decomposition of Mn2(CO)10. The findings revealed that the studied photo-initiating system allowed the controlled synthesis of PIB with relatively low Mw (2000 to 3000 g mol−1) and a Đ of less than 1.7. Notably, the synthesized PIB exhibited a high content of exo-olefin end groups (up to 90%), and the Mw of the polymer could be easily tuned in the range of 2000 to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 by adjusting the diphenyl iodonium salt concentration. This study highlighted the significant advantage of using visible light as an initiation method for cationic polymerization, offering greater control over polymerization conditions and properties compared to traditional methods that do not employ light.
000 g mol−1 by adjusting the diphenyl iodonium salt concentration. This study highlighted the significant advantage of using visible light as an initiation method for cationic polymerization, offering greater control over polymerization conditions and properties compared to traditional methods that do not employ light.
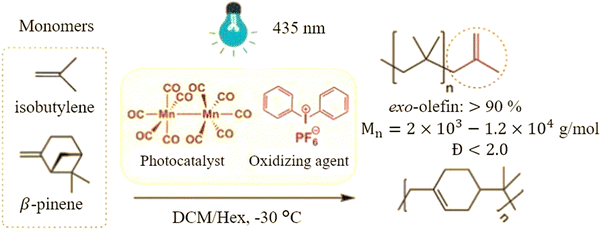 | ||
| Fig. 4 Cationic polymerization of isobutylene and β-pinene via a photo-initiating system. Reproduced from ref. 12 with permission from American Chemical Society (ACS), copyright 2023. | ||
PB synthesized via the coordination polymerization method often has a narrower MWD, ensuring consistent properties and performance.9,70–75 This is especially the case if metallocene catalysts are used, whereas the Ziegler–Natta catalysts generally make the method stereoselective, meaning that it often produces a polymer with specific tacticity such as isotactic, syndiotactic, or atactic configuration.70–73 As a result, while both systems use transition metals to facilitate coordination polymerization, they differ in terms of control over stereochemistry and final polymer characteristics. They are also susceptible to moisture and impurities, which typically deactivate their ability to perform optimally and reduce polymerization efficiency, all of which disadvantage the coordination synthesis method.70,72 Additionally, the Ziegler–Natta and metallocene catalysts are expensive due to the necessity for their precise manufacturing and activation as well as the need to be handled cautiously, which increases the overall cost of the synthesis process and commercialization.9,70–76 The complexity and challenges associated with scaling up, due to the need for precise control over reaction conditions, are additional disadvantages of the coordination polymerization method compared to other options.77,78 Even so, Huang et al.10 used a catalyst precursor, viz.: η5-pentamethyl cyclopentadienyl titanium trinzyloxide (Cp*Ti(OBz)3), which was novel when reported in 2001, in combination with methylaluminoxane (MAO) to synthesize a stereoregular PB-1. The primary focus of the study was to find out how the conditions of polymerization affected the catalyst's activity as well as the Mw and stereoregularity of the final polymer. Variables like the polymerization temperature, Al/Ti ratio, and concentration of Ti were investigated. According to the results, the catalyst reached its maximum activity of 91.2 kgPB mol Ti−1 h−1 at 30 °C and an Al/Ti ratio of 200. Furthermore, changes in the polymerization conditions, specifically the temperature and the Al/Ti ratio, had an impact on both Mw and catalytic activity. As the temperature decreased, the Mw of the PB-1 samples increased, and the microstructure of the polymer varied: the ether-soluble portion was atactic, whereas the heptane-extracted fraction was stereoregular. It was found that the PB-1 had a narrow MWD (Mw/Mn = 1.1 to 1.2) and a Mw greater than 1 × 106 g mol−1. These results provided important insights into the synthesis of highly stereoregular PB-1, although further recent research may build on this work using more refined catalysts or different polymerization conditions.
Similarly, Huang et al.62 explored the synthesis and application of a catalyst precursor, viz.: the monotitanocene (η5-pentamethylcyclopentadienyl) titanium tricinnamyloxide [Cp*Ti(OCH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC6H5)3], for the polymerization of 1-butene in the presence of MAO. The study aimed to investigate how various coordination polymerization conditions impacted the catalytic activity, Mw, stereoregularity, and regioregularity of the resultant PB-1. The findings demonstrated that the monotitanocene catalyst was effective in producing atactic PB-1 with good yields under typical polymerization conditions. The polymer exhibited high Mw (5.3 to 9.6 × 105 g mol−1), stereoirregularity, and a Bernoullian factor (B) of 0.95, which suggested that chain-end control was a predominant mechanism in the polymerization process. This study also provided important insights into the synthesis of PB-1 with specific properties, further advancing the understanding of catalyst behavior in coordination polymerization pathways. In contrast, Resconi et al.79 explored the performance of C2- and C1-symmetric zirconocenes in the polymerization of 1-butene, focusing on the synthesis of isotactic PB-1 with variable isotacticity and melting points. Their results revealed that the C1-symmetric zirconocenes, especially those with heterocyclic ligands, created PB-1 with high Mw (up to 4.0 × 105) and fully regioregular structure. The degree of isotacticity could be tuned by altering the substitution pattern of the indenyl ligand, achieving isotactic triad contents ranging from 86 to ca. 100%. Some C2-symmetric zirconocenes exhibited even higher isoselectivity, though they were less regioselective, with a small % of 4,1 units from secondary 1-butene insertion. The study also illustrated that the melting point and mechanical properties of the PBs correlated with their stereoregularity, with higher isotacticity resulting in higher melting points and stiffness. Notably, catalysts revealed high productivity and activity even at lower AlMAO/Zr ratios, suggesting their potential for industrial application. These findings highlighted the versatility of zirconocene catalysts in producing a broad range of PBs and copolymers, with Fig. 5 illustrating the proposed elementary steps following a secondary 1-butene insertion for the synthesis of isotactic PB.
CHC6H5)3], for the polymerization of 1-butene in the presence of MAO. The study aimed to investigate how various coordination polymerization conditions impacted the catalytic activity, Mw, stereoregularity, and regioregularity of the resultant PB-1. The findings demonstrated that the monotitanocene catalyst was effective in producing atactic PB-1 with good yields under typical polymerization conditions. The polymer exhibited high Mw (5.3 to 9.6 × 105 g mol−1), stereoirregularity, and a Bernoullian factor (B) of 0.95, which suggested that chain-end control was a predominant mechanism in the polymerization process. This study also provided important insights into the synthesis of PB-1 with specific properties, further advancing the understanding of catalyst behavior in coordination polymerization pathways. In contrast, Resconi et al.79 explored the performance of C2- and C1-symmetric zirconocenes in the polymerization of 1-butene, focusing on the synthesis of isotactic PB-1 with variable isotacticity and melting points. Their results revealed that the C1-symmetric zirconocenes, especially those with heterocyclic ligands, created PB-1 with high Mw (up to 4.0 × 105) and fully regioregular structure. The degree of isotacticity could be tuned by altering the substitution pattern of the indenyl ligand, achieving isotactic triad contents ranging from 86 to ca. 100%. Some C2-symmetric zirconocenes exhibited even higher isoselectivity, though they were less regioselective, with a small % of 4,1 units from secondary 1-butene insertion. The study also illustrated that the melting point and mechanical properties of the PBs correlated with their stereoregularity, with higher isotacticity resulting in higher melting points and stiffness. Notably, catalysts revealed high productivity and activity even at lower AlMAO/Zr ratios, suggesting their potential for industrial application. These findings highlighted the versatility of zirconocene catalysts in producing a broad range of PBs and copolymers, with Fig. 5 illustrating the proposed elementary steps following a secondary 1-butene insertion for the synthesis of isotactic PB.
 | ||
| Fig. 5 Key steps involved in secondary 1-butene insertion for the synthesis of isotactic PB-1. Reproduced from ref. 79 with permission from Wiley, copyright 2006. | ||
Recently, Zheng et al.80 studied the impacts of alkylaluminium compounds, including TEA, triisobutylaluminium (TIBA), and diethylaluminium chloride (DEAC), as co-catalysts in 1-butene polymerization with MgCl2-supported TiCl4 Ziegler–Natta catalysts. The study aimed to evaluate the influences of these co-catalysts on catalyst activity, isotacticity, and the Mw of PB-1. The results demonstrated that TEA exhibited the highest catalytic activity, but produced PB with the lowest Mw compared to TIBA and DEAC. TIBA led to more high-isotactic PB-1 fractions with higher Mw. The solvent sequential extraction fractionation process successfully separated PB into atactic PB-1, medium-high isotactic PB-1, and high-isotactic PB-1. The incorporation of TIBA or DEAC with TEA enhanced the content of high-isotactic PB-1 fractions, with the results supporting the hypothesis that multiple active species resulted during polymerization, influencing the properties of the final polymer. Similar work has been undertaken and reported by Hakim et al.,70 who synthesized the isotactic PB-1 in n-hexane by employing excess 1-butene monomer and an industrial Ziegler–Natta catalyst with TEA as a co-catalyst. Different properties of PB-1 were observed by varying the 1-butene pressure and the amount of hydrogen. The study demonstrated that PB-1 may be produced with a narrow MWD and enhanced mechanical properties, allowing a range of products to be commercialized under expanded operational conditions.70 On the other hand, D'Anania et al.81 conducted a comprehensive mechanistic study of 1-butene polymerization promoted by a C2-symmetric ansa-metallocene prototype complex using density functional theory (DFT) calculations. The results showed that chain termination from a secondary growing chain is favored over propagation, contributing to the lower Mw observed in PB synthesized by ansa-metallocene systems. The study also identified a stepwise mechanism for the isomerization of 2,1 units into 4,1 units, with DFT findings supporting this mechanism as more feasible than a concerted one. These insights can be applied to other analogous systems, and the results lay the foundation for future work aimed at enhancing the Mw of PB. This research helps address the key limitations of PB and provides a pathway for expanding polymer molecular structures, as shown in Fig. 6, which schematizes the mechanisms for the isomerization of 2,1-last inserted butene units leading to 4,1 units.
 | ||
| Fig. 6 Two different mechanisms for the isomerization of 2,1-last inserted butene units to 4,1 units. Reproduced from ref. 81 with permission from Frontiers Media S.A., copyright 2024. | ||
2.2. Synthesis of polyolefin-like polymer
IIR is mostly synthesized by copolymerizing isobutylene (98%) with isoprene (2%), making the process more complex compared to that of PIB synthesis. Cationic polymerization is a preferred method for IIR synthesis because it properly addresses the unique challenges presented by the monomers and ensures the generation of a high-performance material with tailored characteristics and properties for a range of applications.1,82 The reaction during IIR synthesis typically takes place in a low-temperature environment, between −105 °C and −90 °C, to control the highly reactive carbocation intermediates.82 A strong Lewis acid—AlCl3, BF3, tin(IV) chloride (SnCl4), or TiCl4—is utilized as the catalyst in the presence of a co-catalyst, commonly H2O or alcohol, which creates the initiating proton source.1,83,84 To ensure the solubility as well as adequate dispersion of the monomers and catalysts, the polymerization is conducted in an inert solvent such as hexane or chloromethane.1,82,84 Controlled unsaturation sites are introduced by adding isoprene (ca. 1 to 3%), which has a more reactive conjugated diene architecture, to the predominantly isobutylene backbone, enhancing the vulcanization characteristics of the polymer.1,82–84 This special structural characteristic makes IIR different from other cationically polymerized polymers. The end product is a rubbery material with final properties that are dependent on the isobutylene-to-isoprene ratio, which is generally versatile in that it can be adjusted thereby enabling these properties to be tuned and making the IIR suitable for a variety of industrial applications. Furthermore, because of its lower polarity and minimal reactivity, in contrast to isoprene, which reacts more readily with the growing molecular chain, isobutylene requires precise reaction conditions in order to achieve controlled Mw, tacticity, composition, and topology.1,82–85 This emphasizes how important the interplay between reaction kinetics and thermodynamics is in the manufacture of high-performance IIR.A detailed overview of developments in IIR synthesis through cationic polymerization, with a highlight on enhancing the process's efficiency and sustainability, has recently been presented by Sharma et al.1 The objective of the exploration was to provide a description of the advancements in the cationic copolymerization of isobutylene and isoprene, emphasizing how different initiating systems affect the microstructure and Mw of the polymer as well as the unsaturation content. The authors presented details regarding the chemistry and performance of initiating systems based on rare earth metals, metallocenes, boron, aluminum, aluminoxane, and zinc. They also discussed the drawbacks of commercial polymerization practices, such as the requirement for cryogenic settings and the effects of chlorinated solvents on the environment, as well as a slurry procedure (Table 2) and the solution process. Methodologically, the study looked at the cationic polymerization reaction mechanisms; Fig. 7 partly schematizes the synthesis process. Here, Lewis acid-based or organometallic initiating systems were used to first generate the highly reactive carbocation. The polymer chain is propagated by the sequential addition of monomers, whereas it is terminated by chain transfer processes, chain terminating agents, or ion pair collapse. The results demonstrated important developments in the field, especially the creation of catalysts based on metallocene and containing weakly coordinating anions, which allow for high-molecular-weight IIR synthesis at elevated temperatures. The environmental issues and energy consumption of traditional processes are addressed by these advances.
| Step | Description of a process |
|---|---|
| 1 | Mixing of chloromethane-dissolved isobutylene and isoprene with chloromethane-dissolved AlCl3 |
| 2 | Polymerization in a reactor kept between −105 to −85 °C |
| 3 | Formation of IIR slurry in chloromethane |
| 4 | Removal of chloromethane and volatiles in a flash tank, followed by mixing with hot H2O |
| 5 | Incorporation of anti-agglomerates and stabilizers, followed by drying to remove H2O and other impurities |
| 6 | Attainment of the final product, the IIR bales |
 | ||
| Fig. 7 Initiation (A) and propagation (B) for the synthesis of IIR via cationic polymerization. Reproduced from ref. 1 with permission from John Wiley & Sons, Ltd., copyright 2021. | ||
2.3. Synthesis of polyester polymer
A polycondensation reaction, which is a type of step-growth polymerization process, is used to synthesize an engineering thermoplastic or polyester copolymer known as PBT.22,35,86–93 As illustrated in Table 3 and Fig. 8, this synthesis usually commences with either dimethyl terephthalate (DMT) or terephthalic acid (TPA) as the acid material, which reacts with a butene derivative (viz.: 1,4-butanediol or butane-1,4-diol (BDO)) that provides the diol group (HO–CH2–CH2–CH2–CH2–OH) under carefully monitored conditions.87,90,93 The usage of DMT results in the production of bis(hydroxybutyl) terephthalate or bis(4-hydroxybutyl) terephthalate (C16H22O6) through the process of transesterification, in which DMT reacts with BDO in the presence of a catalyst, i.e., Ti-based compounds or zinc acetate (Zn(OAc)2), of which its function is to improve the polymerization rate and control the polymer's Mw and yield.35,86,88,93 A small molecule like methanol (CH3OH) is produced as a by-product during this stage and is continually eliminated to drive the reaction forward (i.e., towards high Mw polymer formation).22,35,87,93 On the other hand, TPA provides the dicarboxylic acid group (–COOH) (Fig. 9) and produces H2O rather than CH3OH when it interacts directly with BDO to form the same intermediate.88,93 Specifically, the hydroxyl group (–OH) of BDO reacts with the –COOH of TPA to initiate the esterification reaction. Economic and environmental considerations frequently influence a selection between DMT and TPA, with the latter being utilized on more occasions because it is less expensive and does not produce a relatively toxic by-product (CH3OH).86–93| Stage | Process | Condition | Catalyst | By-product | Ref. |
|---|---|---|---|---|---|
| Transesterification | DMT + BDO → C16H22O6 + CH3OH | 150 to 220 °C, atmospheric pressure | Ti-based compound or Zn(OAc)2 | CH3OH | 86 |
| Esterification | TPA + BDO → C16H22O6 + H2O | 200 to 280 °C, atmospheric pressure | Ti-based compound or Zn(OAc)2 | H2O | 88 |
| Polycondensation | C16H22O6 → PBT + excess BDO + CH3OH/H2O | 250 to 300 °C, decreased pressure or inert gas | Sb2O3 or GeO2 | CH3OH/H2O | 93 |
 | ||
| Fig. 9 Synthetic pathway for poly(butylene terephthalate-co-tetramethylene ether glycol) copolymer. Reproduced from ref. 94 with permission from MDPI, copyright 2020. | ||
An intermediate material (C16H22O6) is polycondensed in the second stage to generate long PBT chains with high Mw.90,92,93 To avoid oxidation and make it easier to remove by-products and excess BDO, this stage is normally carried out at high temperatures (250 to 280 °C) while under vacuum or inert gas flow.89,92,93 Antimony trioxide (Sb2O3) and germanium dioxide (GeO2) are two examples of catalysts that are regularly used to increase the rate of polycondensation reaction.22,91 To limit adverse reactions like crosslinking or heat degradation and to attain the required degree of polymerization, the process is meticulously regulated. Purity is another important factor, and the employment of an inert environment, vacuum, or low-pressure system typically ensures the effective elimination of volatile by-products. The resultant polymer melt is extruded, cooled, and pelletized for use or additional processing.22,91,93 The step-growth polymerization process guarantees the effective synthesis of high-purity PBT suitable for use in various applications.22,35,86–93
Ittobane et al.90 synthesized the partially renewable PBT copolyesters containing alditol units in the presence of titanium(IV) butoxide (Ti(OBu)4) catalyst. The goal was to attach alditol-derived units—more precisely, 2,3-di-O-benzyl-L-threitol—to the PBT backbone in order to explore the impact on the resultant polymer structure and characteristics. The synthesis was carried out via solution-phase polycondensation, by means of mixtures of BDO and 2,3-di-O-benzyl-L-threitol with terephthaloyl chloride in 1,2-dichlorobenzene as the solvent. The chemical reaction yielded PBT copolyesters with weight-average Mws ranging from 4.0 × 103 to 1.2 × 104 g mol−1 and Đ of ca. 1.5. The results revealed that the copolyesters had high Mws, a random microstructure, and were thermally stable at temperatures >300 °C. Copolyesters containing up to 30% alditol units retained a semi-crystalline topology, with the same crystal form as parent homopolyester PBT. However, increasing the alditol content led to a significant decline in melting temperature (Tm) and crystallinity, but the Tg increased. On the other hand, Mao et al.94 synthesized the poly(butylene terephthalate-co-tetramethylene ether glycol) (PBT-co-PTMEG) copolymers through melt polymerization, where polycondensation reactions were carried out between DMT, BDO, and PTMEG with contents of the latter ranging from 0 to 40 wt%. As schematized in Fig. 9, the synthesis route highlights the reaction scheme for incorporating PTMEG into the PBT backbone during melt polymerization. The findings demonstrated that all synthesized PBT-co-PTMEG copolymers exhibited excellent thermal behavior with a 5% decomposition temperature of ca. 370 °C. However, the incorporation of PTMEG significantly influenced the crystallization behavior of the copolymers. For instance, the crystallization temperature reduced from 182.3 to 135.1 °C, and the enthalpy of crystallization decreased from 47.0 to 22.1 J g−1 as PTMEG content increased from 0 to 40 wt%. This suggested a decrease in crystallinity due to the disruption of the ordered PBT chains by PTMEG. The crystal structure of PBT remained in α-form, but the X-ray diffraction's intensity of the characteristic peaks weakened with increasing PTMEG content, supporting the differential scanning calorimetry's crystallinity results.
2.4. Synthesis of block copolymer
The most often used approach for synthesizing the SIBS copolymer is the living cationic polymerization through the block copolymerization process.6,43,95–98 Synthesis of well-defined block copolymers with narrow MWD and precise control over the block structure is made possible by this method's capacity to regulate the process. Vinylic monomer (styrene) and isobutylene are typically polymerized using a cationic initiator, such as AlCl3 or TiCl4, in the presence of a suitable solvent, such as toluene or hexane.6,95,98 Isobutylene's weaker reactivity than styrene has been reported to make polymerization challenging, especially in cationic conditions, necessitating careful reaction condition control.6,97,98 Even so, a triblock structure (i.e., styrene–isobutylene–styrene) is still formed by the sequential addition of styrene blocks to the isobutylene chain due to the living nature of the cationic polymerization, including the choice of a solvent and Lewis acid catalyst, which help facilitate controlled polymerization and thereby leading to the formation of thermoplastic elastomer with well-defined block structures.95,96 Although fewer studies are devoted to the core synthesis of SIBS itself, a sizable body of literature concentrates, instead of the initial polymerization strategies, on the modification and processing of SIBS for specific purposes, such as improving its chemical properties or strengthening its functionality for specific end applications.6,45,94,98 Nevertheless, the synthesis of SIBS for biocompatible materials, specifically for leaflet heart valve prostheses, was investigated by Rezvova et al.99 The study produced SIBS with a narrow MWD (Mw/Mn = 1.3) via controlled cationic polymerization and demonstrated the resultant material's mechanical properties. This material showed greater elongation and Young's modulus (elastic modulus), but a lower strength when compared to xenopericard and expanded polytetrafluoroethylene (ePTFE). An investigation also illustrated that altering the core polyisobutylene block and varying the Mw could maximize the biocompatibility of SIBS, and accordingly, SIBS has promise for usage in biomedical applications, especially in prosthetic heart valves.99 Makarevich et al.95 also utilized the cationic polymerization method to synthesize SIBS, with a DiCumCl/TiCl4/2,6-lutidine initiating system under open conditions. The work aimed to create a streamlined synthesis methodology for clearly defined SIBS without the need for the glove box method, which is commonly employed in cationic polymerization. The authors found that they could control the resultant copolymer's Mw and Đ by adding a proton trap. The ultimate tensile strength (UTS) of this SIBS was comparable to that of SIBS made in glove box conditions, demonstrating that SIBS copolymers with varying lengths of polyisobutylene and polystyrene blocks may be synthesized with minimal Đ. According to the results by both Makarevich et al.95 and Rezvova et al.,99 living cationic polymerization provides a practical way to create well-defined SIBS with favorable mechanical characteristics, which makes it ideal for a range of applications, in the biomedical sector and other industries.2.5. Recent advances in synthesis
Synthesis of butene- and isobutylene-based polymers has advanced significantly as a result of the growing need for high-performance, environmentally friendly, and sustainable polymers. Exploring novel approaches and catalysts that can improve polymerization operations' efficiency while reducing their negative effects on the environment is, therefore, becoming more and more important. As noted previously, conventional catalysts, which may have limits in terms of selectivity, activity, sustainability, and economic aspects, are frequently used in traditional techniques of synthesizing certain butene- and isobutylene-based polymers. These methods also face numerous processing-related difficulties, such as high viscosity of reaction mixtures, limited control over molecular structure, high energy demands for thermal control, and the instability of catalysts under industrial-scale temperature and pressure conditions. In addition, scaling up from lab-scale to continuous industrial manufacturing typically presents challenges with heat monitoring, mixing efficiency, and real-time process management. To overcome these restrictions, current innovations like metallocene-based single-site catalysts (SSCs), continuous flow polymerization systems, green solvent utilization (such as scCO2, ILs), and real-time reaction regulation strategies have been proposed (Table 4). These advances aim to enhance yield, control, and sustainability across the synthesis pipeline while addressing economic and environmental limitations.| Issue | Example | Suggested solution | Ref. |
|---|---|---|---|
| High reaction medium viscosity | PIB and IIR polymerization | Utilizing diluent systems or ILs to decrease viscosity and improve mixing | 30 |
| High cost and toxicity of catalysts | AlCl3 and TiCl4 in conventional systems | Greener alternatives, including bio-based catalysts, earth-abundant metals, and SSCs | 83 |
| Inadequate thermal regulation at an industrial scale | Continuous synthesis of PIB | Heat exchangers-integrated continuous flow reactors | 12 |
| Poor control of MWD | PB and PIB polymerization | Utilization of metallocene SSCs, RAFT/ATRP methods for accuracy | 46 |
| Toxicity of solvents and environmental impact | Chlorinated hydrocarbons | Employing scCO2 and ILs as green solvents | 82 |
| Deactivation of catalysts at elevated pressures | PIB synthesis through cationic polymerization | Modifications of a reactor; immobilized or supported catalysts | 74 |
| Upscaling batch processes | All common thermal polymerization processes | Transition to continuous flow synthesis, process intensification | 75 |
Additionally promising for sustainable polymerization is the development of bimetallic catalysts, which combine two metals to increase catalytic activity, enhance the efficiency and selectivity of reactions, and reduce energy consumption.113,117,118 These catalysts have the ability to improve selectivity towards desired polymer structures, raise Mw control, and increase the rate of polymerization.113,119–121 Moreover, to increase the stability, reactivity, and sustainability of the catalytic system, hybrid catalysts—combinations of metal catalysts with organic ligands—have been used.121–123 For instance, in the synthesis of high-density PB, bimetallic catalysts have demonstrated enhanced polymerization efficiency with superior control over stereoregularity and polymer architecture.124 Also, high specificity and control over polymerization are provided by SSCs, which can create polymers with narrow MWD and controlled stereochemistry—two characteristics that are particularly important for the synthesis of isotactic PB or PB copolymers.46,81,125,126 Metallocene catalysts are well-known SSCs. Recent developments have concentrated on adjusting their functionality and structure to increase selectivity, sustainability, and efficiency.127–131 Metallocene catalysts have demonstrated exceptional control over stereoregularity and MWD in the polymerization of butene-based monomers in some recent studies. For example, the use of ansa-zirconocene dichlorides bearing electron-rich ligands can improve isotactic selectivity in PB-1 synthesis while preserving narrow MWD.132,133 Similarly, in the cationic copolymerization of Raffinate-1 monomers, increased catalytic activity and better tacticity have been observed when employing hafnocene-based systems.9,134 These developments highlight metallocenes’ ongoing significance as precise instruments for tailoring polymer structure, especially for high-performance applications where syndiotactic or isotactic structures are preferred. Furthermore, three key polymerization pathways—Ziegler–Natta, metallocene, and cationic polymerization—are shown in comparison in Fig. 10, with an emphasis on differences in active sites, control over polymer structure, and suitability for butene- and isobutylene-based polymers.
 | ||
| Fig. 10 Comparative pathways of Ziegler–Natta, metallocene, and cationic polymerization systems for butene- and isobutylene-based monomers.135–139 | ||
Metallocene catalysts provide single-site precision, generating polymers with distinct microstructures and narrow MWD, in contrast to the heterogeneous nature of Ziegler–Natta catalysts, which often produce broader MWDs and mixed tacticity.136,137 On the other hand, cationic polymerization offers extremely rapid initiation, especially for isobutylene-derived systems like PIB; however, it usually lacks stereoregulation, though new hybrid and IL-supported systems are increasing its selectivity as well as sustainability.138,139
 | ||
| Fig. 11 Chemical recycling of PBAT via enzymatic hydrolysis to break it down into its monomer components, followed by their separation, recycling, and possible upcycling. Reproduced from ref. 166 with permission from Royal Society of Chemistry, copyright 2024. | ||
2.6. Environmental footprint and life cycle analysis
The sustainable development of polymer products requires careful consideration of the environmental impact of polymer synthesis. A common approach for assessing the environmental effects of various polymerization methods is life cycle assessment, or life cycle analysis (LCA).167,168 From the extraction of raw materials to the disposal or recycling of products, LCA offers a methodical way to evaluate the overall environmental impact, considering factors like energy consumption, toxicity, waste production, and carbon emissions. Promoting more eco-conscious and sustainable production techniques for butene- and isobutylene-based polymers require an awareness of the effects that their polymerization processes have on the environment.As summarized in Table 5, the comparative evaluation of different polymerization systems, such as the well-known Ziegler–Natta and metallocene-based catalysts, is made possible by LCA. Overall energy efficiency, environmental footprint, and sustainability of these two systems vary significantly. When compared to the Ziegler–Natta counterpart, the metallocene-based polymerization pathway often exhibits better environmental performance and sustainability because it is highly selective and controlled, generates fewer by-products and pollutants (greenhouse gas (GHG) emissions), and consumes less energy because it operates under milder conditions throughout the polymer synthesis process.74,75,79,127,129,130,169 As highlighted before, the metallocene-based systems are those like MAO-activated metallocenes that convert butene into ethylene–butene copolymers, propylene-butene copolymers, etc. The more complex Ziegler–Natta systems have a higher carbon footprint because of both their production of higher amounts of waste and their higher reactor energy consumption resulting from the higher temperature and pressure requirements.170–174 Also, in addition to improving product quality, the decreased polymer structural variation offered by metallocene catalysts reduces the necessity for excess reagents and solvents, which are frequently required in less efficient methods like Ziegler–Natta polymerization.172 The selectivity of the metallocene catalyst also makes this system a more sustainable choice for large-scale polymer manufacturing.129,130,169 When taking into account the broader life cycle of butene- and isobutylene-based polymers, the disparity in environmental impact between the two systems becomes particularly evident. For example, because of their superior material properties, such as greater UTS and improved chemical resistance, metallocene-catalyzed polymers typically have a longer service life.175–177 This extended lifespan of products created from these polymers means that their contribution to environmental waste is prolonged, further lessening their overall environmental footprint.
| Aspect | Ziegler–Natta polymerization pathway | Metallocene polymerization pathway | Ref. |
|---|---|---|---|
| (1) Preparation of raw materials | Feedstock: butane derivative | Feedstock: butane derivative | 74 and 80 |
| (2) Preparation of a catalyst | Preparing Ziegler–Natta catalysts necessitates higher temperatures (70–90 °C) and pressures (10–30 bar) | Lower temperatures (40–60 °C) and pressures (2–10 bar) are employed in the manufacture of metallocene catalysts, meaning the process is less complex, more controlled, and inexpensive | 75 and 100 |
| (3) Polymerization reaction as well as by-products and waste production | Synthesis of broader MWD polymer | A narrower MWD polymer is produced through more selective polymerization | 130 and 179 |
| More by-products and waste, such as solvent waste and excess reagents, are produced because of less precise control of the process | Fewer by-products and less waste are generated because of a more controlled process, meaning more efficient use of resources | ||
| (4) Energy consumption and gas emissions | Higher GHG emissions from solvents and excess reagents because of high temperatures | Lesser energy consumption and GHG emissions because of meticulous catalyst selection, precise control, and milder reaction conditions | 74 and 100 |
| Significant energy input because of complex steps that necessitate high temperatures and pressures | |||
| (5) Polymer product | Broader MWD results in less material recyclability | Higher recyclability as well as better UTS and other material properties | 75 and 130 |
| (6) Sustainability (generally) | Sustainability is reduced by higher energy consumption, pollutants, and waste production | Sustainability is strengthened by small energy requirements, lesser pollutant emissions, and lower waste production | 130 and 179 |
Furthermore, metallocene-based polymerization processes relatively allow for the recycling of butene- and isobutylene-based polymers at the end of their life cycle.172,178 Because of their controlled molecular architecture and consistent properties, the polymers generated by the metallocene catalysts tend to be simpler to recycle. For instance, it has been shown that ethylene–butene copolymers synthesized with metallocene catalysts are more recyclable than common ethylene–butene copolymers, which are made via Ziegler–Natta systems.179–183 This improved recyclability is essential for encouraging a circular economy and lowering the demand for the manufacturing of virgin polymers, which further lessens the impact on the environment. The greater complexity and lesser selectivity of Ziegler–Natta catalysts would then be the primary reasons for this system to produce butene- and isobutylene-based polymers that are more challenging to recycle.10,70,80,100,179,183 Impurities and structural variations in polymers often lead to lower-quality recyclates, which may need to be blended with virgin material or undergo further treatment to meet product specifications. This additional processing can decrease the overall life cycle's efficiency and increase the environmental effect of recycling.
Nonetheless, Ziegler–Natta systems are still often utilized in the polymerization of butene- and isobutylene-based polymers because of their well-established industrial infrastructure and lower costs,70,80,100 even though metallocene catalysts have substantial environmental advantages. Therefore, future widespread use or adoption of these more environmentally friendly technologies is anticipated to be driven by continued developments in catalyst design, metallocene system process optimization, and sustainable polymer synthesis. The efficiency of metallocene catalysts is still superior, but recent research on the development of SSCs to produce polymers has shown a decrease in the energy consumption and waste generation of Ziegler–Natta systems.184 Ultimately, the overall decrease in carbon footprint and energy consumption is essential for both the sustainability of polymer synthesis and the mitigation of the environmental effects of the polymer's use in consumer goods. For industries looking into lessening their environmental impact while satisfying the increasing demand for butene- and isobutylene-based polymers in a variety of applications, energy-efficient and cleaner processes of metallocene catalysts are essential. Accordingly, one important step in the direction of more environmentally friendly synthesis methods in the butene- and isobutylene-based polymer sector could be the shift to metallocene-based polymerization technology. However, whilst metallocene-based polymerization systems have several advantages, the impact that the final product has on the environment is just as important, especially when considering biodegradability.
Biodegradable butene- and isobutylene-based polymers seem promising for lessening the impact of plastic waste on ecosystems when taking into account the entire life cycle, including end-of-life degradation. For example, as compared to traditional petroleum-based plastics, PBS, a biodegradable polyester synthesized similarly to PBT using BDO and succinic acid (instead of DMT or TPA), provides notable environmental advantages. It is much less harmful when it decomposes in natural settings because it produces non-toxic metabolites that lessen its long-term persistence in landfills and waterways.13–16,185,186 Compared to conventional techniques like Ziegler–Natta, the manufacture of PBS utilizing metallocene catalysts also uses less energy and produces fewer by-products, making it a more environmentally and industrially sustainable option.38,39,187 The growing need for eco-friendly materials is effectively served by this combination of energy-efficient manufacture and biodegradability. Similarly, PBAT is another isobutylene-based copolymer that combines biodegradability with advantageous life cycle characteristics. Because it decomposes naturally in industrial composting environments, it is a more environmentally friendly option for packaging applications than traditional plastics.16,38,39,187 The PBAT synthesis, when carried out using metallocene-based systems, also benefits from a more precisely controlled route that uses less energy and produces fewer emissions. These characteristics highlight the potential of metallocene-based catalysts to generate materials that help lessen environmental plastic pollution in addition to high-performance butene- and isobutylene-based polymers. Accordingly, PBS and PBAT both exemplify how combining biodegradability with advanced polymerization methods can result in the manufacture of more sustainable materials, with definite benefits when assessed via life cycle analyses.
3. Processing characteristics of butene- and isobutylene-based polymers
Key processing characteristics of butene- and isobutylene-based polymers are essential for efficiently transforming them into final, high-quality products. These, which primarily include ease of processing, flow behavior, and formability, depend on several factors such as molecular structure, viscosity, as well as thermal and crystallization behaviors.24,188,189 Additionally, the processing characteristics also influence the final cost and performance of the material in its intended application. Therefore, it is important to understand and control the factors they depend on to optimize processing methods—i.e., extrusion, injection, and blow molding, as well as film casting—and ensure that the finished product meets desired application specifications but is also cost-effective. This section examines these characteristics, along with how each characteristic influences the processing efficiency and final performance of the material.3.1. Ease of processing, flow behavior, and formability
The ease of processing refers to the simplicity of transforming polymers into their respective, final forms using traditional processing methods.103 A viscosity, which varies with temperature, is directly correlated with how well a polymer flows. Generally, better flow from a decreased viscosity makes it easier for a polymer to fill extruders or molds.190 Because higher Mws typically results in higher viscosities, which slow down the flow and necessitate higher temperatures or pressures to facilitate processing, Mw and shear sensitivity also have a substantial impact on flow behavior. Accordingly, because they require less energy to flow and mold, polymers with lower Mws, lower crystallinity, and moderate viscosity are generally easier to process.190,191 Conversely, because of their greater flow resistance, the high Mw and crystalline polymers are typically more challenging to process.190,191 For instance, the moderate Mw and semi-crystallinity of PB (particularly isotactic PB-1) necessitate rather high processing temperatures (usually 180–200 °C) for better flow behavior and extrusion or molding.29,192 In addition, because of its rigid and regular chain structure, both of which cause it to flow less easily, PB-1 may be more challenging to process, especially at lower temperatures, than more amorphous butene- and isobutylene-based polymers.193–196 When cooled too rapidly, for example, PB tends to show slower flow rates, which can lead to defects in the finished product, particularly in applications like pipelines where dimensional stability—a material's ability to retain its size and form under varying environmental factors including temperature, mechanical stress, or chemical exposure—and uniformity are crucial.193–199 Nonetheless, in the study by Li et al.,200 effective melting and extrusion of PB-1 pellets into filaments was made possible by the melt-spinning system, which included a spin pack and a single-screw extruder (Fig. 12(a)). According to the authors, processing was made easier by the comparatively mild extrusion temperatures (i.e., 110–260 °C), which promoted favorable flow characteristics and reduced thermal degradation. Similarly, uniform melting and smooth film formation were guaranteed by the hot-pressing method (Fig. 12(b)) employed to make PB-1 films at 180 °C. These processes worked well for processing PB-1, providing good control over material properties and characteristics, including mechanical performance and crystallinity. They also made it possible to produce high-quality filaments and films that could be used for intended testing and applications.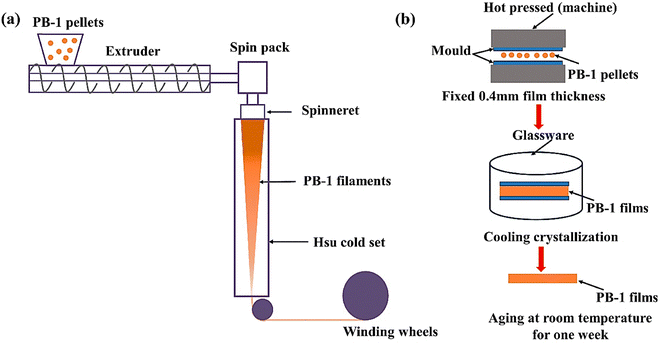 | ||
| Fig. 12 Schematic of the melt-spinning (a) and hot-pressing (b) processes for the production of polybutene-1 filaments and films. Reproduced from ref. 200 with permission from MDPI, copyright 2023. | ||
In addition to its greater amorphousness, PIB, on the other hand, is generally easier to process at lower temperatures (150–170 °C) due to its lower Mw.24,52,201,202 It is especially well-suited for the extrusion of flexible products like adhesives, sealants, medical tubing, coatings, and packing films because of its low Tg, which permits it to flow more readily at lower processing temperatures.201–209 Moreover, PIB exhibits shear-thinning behavior, meaning that as shear forces rise, its viscosity drops.23,202,203,206,209 This makes it easier to process under high shear conditions, which makes it easier to fabricate thin, flexible products. However, PIB is typically not applicable for applications needing high-dimensional stability because it lacks the structural rigidity of PB.210
Furthermore, specialized machinery, like extruders and Banbury mixers, is typically used for IIR processing under high-shear conditions to overcome the material's intrinsic flow resistance despite its amorphousness.211–213 Because of its cross-linkable structure, it is less flowable than thermoplastic polymers like PB and PIB under typical molding or extrusion conditions, necessitating the use of more precise control over the processing conditions.211,213 This structure is a result of a highly reactive monomer (isoprene) that adds double bonds to the polymer chain, enabling crosslinking—a crucial process for the material's ultimate functionality. IIR's elastic properties, in spite of its poor flowability, are made better by the presence of isoprene in the polymer structure, which makes it ideal for uses requiring great strength and flexibility under stress, where stretchability and durability are crucial.214 Also, in contrast to materials based only on isobutylene, the addition of isoprene makes the IIR have a higher viscosity, which has an impact on the melt flow characteristics of the polymer.26 Because IIR may need higher temperatures, between 140 and 180 °C for Banbury mixing, ca. 160–200 °C for extrusion, and ca. 180–220 °C for injection molding, to obtain significant flow during processing, this increase in viscosity might make processing more challenging.211,212 However, after being crosslinked, the isoprene units contribute to the formation of a network structure that gives the finished product enhanced wear resistance, dimensional stability, and chemical resistance.214,215 It is important to strike a balance between the amounts of isoprene and isobutylene since too much of the former can diminish the Mw and processability, while too little can limit the crosslinking capability and jeopardize the final mechanical properties.
Some isobutylene-based copolymers alter the material's properties and processability by combining isobutylene with styrene. One important aspect of this alteration is the impact of styrene on isobutylene's processability, particularly when it comes to SIBS block copolymers. The addition of styrene to isobutylene improves the polymer's melt strength and viscosity, among other processability characteristics.95,97 Higher melt strength enables the polymer to retain its structure and shape throughout fabrication, which is especially advantageous in techniques like extrusion, as well as injection and blow molding.95,216,217 Better phase separation between the hard styrene blocks and the soft isobutylene segments is made possible by styrene's compatibilizing role in the polymer blend.43,95,217 The isobutylene segments retain flexibility and rubberiness, but the stiff, crystalline styrene blocks give the polymer structural stability, creating a material that is easy to process while balancing rigidity with elasticity and maintaining the necessary mechanical properties for demanding applications like coatings and films. Adding styrene, however, also presents certain challenges. Because styrene is stiffer than isobutylene, it can elevate the melting point and viscosity of the polymer. To handle the changed material efficiently, greater processing temperatures and specialized equipment may be needed. Additionally, depending on the polymerization conditions, block copolymerization of styrene and isobutylene could result in branched architectures, which can affect the process consistency as well as the mechanical performance of the final material.97,216 The amount of styrene must also be carefully regulated because too much of it might cause the finished product to become brittle, which will lessen its flexibility and resistance to impact. Similar to the case with IIR, to guarantee that the material works as well as it can for a given application, styrene must be balanced even if it enhances the processability and mechanical properties of isobutylene-based copolymers. PBS is another type of copolymer, but different from isobutylene-based copolymers, where Ewurum et al.218 have recently studied its different processing techniques, including extrusion and molding, with the former making it possible to incorporate lignin into the PBS matrix effectively (Fig. 13). According to the authors, the PBS-lignin materials were processed smoothly and with good flow characteristics and uniform lignin dispersion throughout the polymer thanks to the use of a single-screw extruder and a hot press at relatively moderate temperatures (140 °C for extrusion and 120 °C for molding). By altering PBS's structure and enhancing its compatibility with lignin, the processing techniques—specifically, reaction extrusion with DCP and molding—further improved the polymer's processability. These methods demonstrated the viability and simplicity of working with lignin-filled PBS systems for the production of copolymers with different lignin contents.
 | ||
| Fig. 13 Schematic illustration of the processing of biodegradable PBS-lignin copolymers via extrusion and molding. Reproduced from ref. 218 with permission from MDPI, copyright 2025. | ||
The ease of processing of a butene- and isobutylene-based polymer can also be achieved by blending it with another polymer. The properties of two polymers together can reveal important information about how the materials can be treated in different applications. By optimizing the blend composition to meet desired processing characteristics, this approach can assist in addressing processing-related issues like temperature sensitivity in addition to viscosity and flow behavior. For example, PB-1 can be made more processable by mixing it with a more flexible polymer. This will make it easier to handle during extrusion, injection molding, and other fabrication processes. The material's overall flow characteristics and melt viscosity can all be improved by the interaction between the two polymers. The resultant mixture may exhibit enhanced flexibility, toughness, or thermal stability, depending on the polymer selected for blending. This will facilitate processing into finished products such as films, fibers, or molded components. The study by Palai et al.219 offers a pertinent example to support the notion that polymer blending improves ease of processability and material properties. In their study, they prepared PLA/poly(butylene succinate-co-adipate) (PBSA) blend-based films by mixing these two polymers with a fixed wt% of epoxy-functionalized styrene acrylate (ESA), using a single-step blending process followed by a blown film extrusion process (Fig. 14). The material's integrity was maintained by the effective blending of PLA, PBSA, and ESA during the dry blending process, which was conducted at 50 °C and 80 rpm without causing undue shear. The melt blending procedure, carried out in a co-rotating twin-screw extruder with regulated screw speeds (i.e., 80–100 rpm) and temperature profiles (from 155–175 °C), guaranteed a consistent dispersion of ESA throughout the polymer blend matrix, thereby enhancing PLA and PBSA compatibility. Additionally, by precisely controlling the residence duration (2–4 minutes), this approach ensured that the polymers were processed under optimal conditions and optimized the flow behavior, allowing the components to blend efficiently without degrading. Stable PLA/PBSA/ESA blend-based film manufacturing with strong optical qualities and effective control over material thickness and blow-up ratio was made possible by the blown film extrusion method, which used a conventional mixing screw and controlled temperatures (160–175 °C) to preserve favorable flow behavior. This is similar to a recent study by Zhang et al.,220 who created PLA/PBAT blend- and PLA/PBS blend-based films through melt blending and extrusion cast processing, and Choi et al.,221 who created PLA/PBS blend-based fibers by blending PLA and PBS using a melt-spinning technique, from twin screw extrusion to fiber spinning process.
 | ||
| Fig. 14 Schematic of the processing steps for PLA/PBSA/ESA blend-based films: dry blending and blown film extrusion. Reproduced from ref. 219 with permission from Elsevier, copyright 2020. | ||
The polymer's ability to be shaped into the desired finished product—a sheet, film, fiber, or three-dimensional (3D) object—without failure is referred to as formability.192,222 This characteristic is also influenced by the molecular structure.222 For instance, since they soften more readily when heated and may be molded into a variety of shapes without cracking or breaking, isobutylene-based polymers like PIB with low crystallinity (low Tg) and high amorphous nature (flexible molecular chains) are often more formable.223,224 Thus, formability is somewhat more challenging for PB, which normally leads to issues like warping during rapid cooling.192,225,226 However, when processed under optimized conditions, PB also shows good formability.192,226 For instance, the crystalline nature of PB means that higher temperatures and pressures, as well as longer processing durations, are required to ensure that it flows into molds sufficiently and maintains its shape while cooling, thereby achieving optimal formability, especially during the manufacture of PB pipes.192,198,199,226 As expected, IIR is less formable than PB and PIB because of its cross-linkable architecture, which limits its melt-processability.227 However, just like PB, IIR can be processed into molds or shapes via specialized molding and vulcanization methods, although moldability and formability are limited in conventional thermoplastic processes. On the other hand, because additional monomers often enhance the balance of flexibility and stiffness, isobutylene-based copolymers normally show higher formability when compared to pure PIB.42,64,228 When PIB is blended with a polymer like PE, the resultant PIB/PE blend also provides a more highly formable material than pure PIB and PE because of the synergistic effects of both polymers.206,229,230 Because of their better formability, such blends can be processed into films, coatings, and other products that need both flexibility and strength. Table 6 provides a summary of the butene- and isobutylene-based polymers' processing characteristics while also contrasting them with those of common polymers.
| Polymer | Processing characteristic | Suitable processing method | Ref. | ||
|---|---|---|---|---|---|
| Ease of processing | Flow behavior | Formability | |||
| PB-1 | Moderate | Moderate at high temperatures | Ideal for rigid products | Extrusion, as well as injection and blow molding | 192 and 199 |
| PIB | High | Excellent at low temperatures | Excellent for films | Extrusion and film casting | 224 and 225 |
| IIR | Moderate to low | Restricted due to crosslinking | Limited | Molding, hot-press vulcanization, and extrusion | 208 |
| PE | High | Good at high temperatures | Moderate | Extrusion, injection molding, and blown film | 230 |
| PP | High | Moderate to good | Good | Extrusion, injection molding, and thermoforming | 231 |
4. Properties of butene- and isobutylene-based polymers
Butene- and isobutylene-based polymers have drawn a lot of interest recently because of their unique set of properties and competitive costs.22,38,52,95,227,232 These desirable properties make them useful in a variety of applications, from packaging materials to electronics. Moreover, the growing focus on sustainability has driven the development of biodegradable and recyclable butene- and isobutylene-based polymers, positioning them as a viable alternative to conventional plastics. This section offers an overview of the key properties of butene- and isobutylene-based polymers, building upon their section on synthesis, and setting the context for their section on applications. By examining these properties, we hope to highlight the potential of butene- and isobutylene-based polymers in addressing both functional and environmental challenges in contemporary material science.4.1. Mechanical properties
Butene- and isobutylene-based polymers exhibit good mechanical properties that can be harnessed for specific applications. For instance, IIR, CIIR, and BIIR have been demonstrated to have excellent mechanical properties because of the polymer's isobutylene backbone, which offers inherent flexibility, low gas permeability, and high resilience, as well as the halogenation in CIIR and BIIR (chlorine and bromine), which improves their strength, wear resistance, and overall mechanical performance.214,233–236 Also, PBT exhibits good mechanical properties, including high strength, rigidity, good impact resistance, dimensional stability, low creep, and excellent wear resistance, making it suitable for applications requiring both mechanical strength and heat resistance.17,237–239 On the other hand, because of aromatic units found in the molecule chains, PBAT exhibits good mechanical properties such as elastic modulus, UTS, and flexibility.240–242 Furthermore, PBAT is biodegradable; however, this characteristic greatly depends on the process variables chosen during synthesis.240,242 PBS polymers have been shown to exhibit a good compromise of mechanical endurance, ductility, toughness, and impact resistance. Studies have shown that the mechanical properties of PBS are comparable to those of PE and PP.13,38,243 The mechanical behavior of PB and PIB has been studied extensively, and compared to traditional polyolefins, these butene- and isobutylene-based polyolefins possess unique chain and helical structures that result in excellent mechanical properties.199,207,208,244–246 Furthermore, functionalized PB and PIB exhibit beneficial properties concerning toughness.45,208,247–249 Block copolymers like SIBS exhibit exceptional UTS and elongation properties because of the phase separation between the hard styrene blocks and the soft isoprene/butylene blocks, as well as their characteristic Mw.95,99,250,251 A comparison of tensile properties of different types of butene- and isobutylene-based polymers is presented in Table 7. It is evident that the structure and composition of butene- and isobutylene-based polymers greatly affect their mechanical characteristics. PBT has a higher elastic modulus and is considerably stiffer than polymers like PB-1 and PBS, which show high elongation, indicating good elasticity or flexibility. PIB exhibits high elongation and low stiffness, indicating exceptional flexibility. When compared to unmodified PBS, modified PBS (e.g., PBS-0.03M and PBS-0.2TMP, where M and TMP are malic acid and tri-methyl propane, respectively), exhibit better strength, underscoring the advantages of surface modification. Depending on their structure, block copolymers such as poly(styrene–isobutylene–styrene) (PS–PIB–PS) exhibit different properties; the star block version offers good strength, while linear versions offer both superior strength and high elongation. The trade-offs between strength, flexibility, and how changes affect these polymers' performance are demonstrated by this comparison.| Polymer | Composition/trade name | Young modulusa (MPa) | Tensile strengtha (MPa) | Elongation at breaka (%) | Study |
|---|---|---|---|---|---|
| a Approximate values, —: not reported. | |||||
| PB-1 | Toppyl PB 8340M | 270 | 30 | 300 | 252 |
| PIB | — | 1.6 | 14 | 650 | 253 |
| CIIR | — | 2 | 2 | 400 | 254 |
| BIIR | — | — | 15 | 566 | 213 |
| PBT | — | 925 | 47 | — | 255 |
| PBAT | — | 126 | 21 | 670 | 240 |
| Starch/PBAT | KINGFA | — | 20 | 287 | |
| PLA/PBAT | KINGFA | — | 22 | 258 | |
| PBS | — | — | 19 | 375 | 13 |
| PBS | Bionelle 1020 MD | 707 | 34 | — | 38 |
| PBS-0.03 M | 0.03 mol% branching ratio by M | 160 | 23 | 100 | 256 |
| PBS-0.2TMP | 0.2 mol% branching ratio by TMP | 200 | 37 | 130 | |
| PBS/15%SRF | 15%SRF | — | 15 | 48 | 257 |
| PS–PIB–PS | Linear block copolymer | 1 | 24 | 906 | 258 |
| PS–PIB–PS | Linear block copolymer | 19 | 13 | 460 | |
| PS–PIB–PS | Star block copolymer | 52 | 10 | 339 | |
Furthermore, crosslinking, which involves chemically connecting polymer molecules by primary valence bonds, introduces stability to a polymer because of the imposed restrictions on molecular mobility. The rigidity of a polymer is increased by crosslinking, depending on the degree of crosslinking. The length of the crosslink and the distance between crosslinks vary. The shorter the crosslinks, or the smaller the separation between them, the stronger and more rigid the polymer becomes. While more significantly crosslinked materials are rigid and may be brittle, the minimally crosslinked polymers are typically rubbery. Dziemidkiewicz et al.268 have examined the influence of different curing agents for the BIIR crosslinking process on the mechanical behavior of the vulcanizates, which were cured with metal acetylacetonates and showed good mechanical properties with UTS in the range of 9–14 MPa and better damping properties. The chemicals and materials, known as additives and fillers, that are typically added into polymer formulations to obtain the properties required for desired end applications, also affect the mechanical properties of the resultant polymer product.13,14,28,269,270 Adding additives like fillers to polymers can significantly affect their strength, often enhancing it by increasing stiffness, improving impact resistance, or creating a more durable internal structure, depending on the amount and whether it is a reinforcing filler or not. However, some additives can weaken a polymer depending on their interaction with the polymer chains, like plasticizers, as low-vapor liquids that lower the Tg of polymers, thus increasing flexibility at the cost of UTS and elastic modulus.237,271,272 This decrease in UTS and modulus is because the introduction of plasticizers results in polymer chains in the amorphous regions being forced apart, thereby decreasing the intermolecular forces between chains and allowing them to slip more readily over one another.264,273 Nonetheless, reinforcing butene- and isobutylene-based polymers with fillers like synthetic materials (e.g., glass fibers, carbon black (CB), and carbon nanotubes (CNTs)) and natural materials (e.g., clay, coffee husk, and natural fibers) can significantly increase their mechanical properties, such as UTS, modulus, hardness, and impact resistance.18,20,23,232,233,271
4.2. Thermal stability
PBS has been reported to have good thermal stability in addition to excellent mechanical properties, and due to these properties, injection or blow molded and extruded items have been effectively made from PBS.38,274–276 Furthermore, blending a butene- or isobutylene-based polymer with another butene- or isobutylene-based polymer or a different type of polymer can induce structural changes in the resulting polymer, such as differences in sequence distribution and phase morphology, which significantly impact its thermal properties.277,278 For example, blending PBS with PBT38,279,280 or polycarbonate (PC)281,282 can enhance the thermal stability of PBS, as both PBT and PC have higher Tg and melting temperatures or points (Tms) than PBS, which contributes to improved thermal properties. Additionally, PBAT has been reported to have good crystallization and thermal stability. As a result, it has good processing stability to be used alone or blended with other materials through conventional manufacturing processes like extrusion, injection molding, and blowing film.240,283 It has been reported that the melting point of PBAT is ca. 120 °C and has a crystallization point of 60 °C, a 5% weight loss temperature of 350 °C, and a heat distortion temperature of 55 °C.240 Hence, although PBAT may not function well in elevated-temperature applications due to its relatively low melting and heat distortion temperatures, its high 5% weight loss temperature indicates good thermal stability and resistance to degradation at moderate temperatures. On the other hand, IIR and its halogen-functionalized counterparts are unique in that they have a high percentage of methyl groups (–CH3) attached to the polymer chain. This structure makes the polymer chains more rigid and contributes to their excellent resistance to thermal and UV oxidation as well as environmental aging.284–287 As for SIBS, it exhibits high thermal and oxidative stability due to its saturated polyisobutylene mid-block, performing well in applications with temperatures up to ca. 200 °C; however, it is not suitable for high-temperature environments above this threshold.6,95,288 A comparison of thermal properties of different types of butene- and isobutylene-based polymers is presented in Table 8. Similar to tensile properties, this comparison demonstrates how blends, additives, as well as polymer structure and composition, affect the thermal behavior of butene- and isobutylene-based polymers. Good thermal stability is indicated by the comparatively high melting and crystallization temperatures of polymers like PB-1 and polyethylene terephthalate (PET)/PBT (PET/PBT) blends. In contrast, PIB exhibits a high heat distortion temperature and a very low Tg, indicating superior flexibility at low temperatures and resistance to high heat. Their melting and crystallization temperatures are higher when PBS is modified (for example, by adding M or TMP as a branching agent) to enhance its thermal properties. PBS/15%SRF (i.e., PBS with 15% sugarcane rind fiber) exhibits Tm similar to that of PB-1, probably due to the influence of SRF on the crystallization behavior and polymer chain interactions since pure PBS and PBS with lower SRF contents had lower Tm values in the study. Moreover, linear block copolymers maintain high heat distortion temperatures while displaying low glass transition temperatures (Tgs), which suggests flexibility at low temperatures.| Polymer or blend | Composition/trade name | Melting temp.a (°C) | Crystallization temp.a (°C) | Glass transition temp.a (°C) | Heat distortion temp.a (°C) | Study |
|---|---|---|---|---|---|---|
| a Approximate values. b A thermoplastic polyurethane (TPU) composed of PIB diols (hydroxyallyl telechelic PIBs) and poly(tetramethylene oxide) (PTMO), where PTMO serves as a compatibilizer. | ||||||
| PB-1 | Toppyl PB 8340M | 97 | 111 | −21 | — | 29 |
| PIB | — | — | — | −63.2 | 260 | 253 |
| PIB-PTMO-TPUb | 80% PIB diol | — | — | −25 | — | 289 |
| BIIR | — | — | — | −55 | — | 290 |
| PBT | — | 224 | 198 | 51 | — | 291 |
| PET/PBT | 80/20 | 238 | 188 | 63 | — | |
| PET/PBT | 60/40 | 211 | 165 | 58 | — | |
| PET/PBT | 80/20 | 254 | 216 | — | — | 292 |
| PBAT | — | 115 | 60 | — | 55 | 240 |
| PBS | Bionelle 1020 MD | 115 | — | −32 | — | 38 |
| PBS | — | — | 19 | — | 375 | 13 |
| PBS-0.03 M | 0.03 mol% branching ratio by M | 160 | 23 | — | 100 | 256 |
| PBS-0.2TMP | 0.2 mol% branching ratio by TMP | 200 | 37 | — | 130 | |
| PBS/15%SRF | 15% SRF | 97 | 57 | — | — | 257 |
| PS-PIB-PS | Linear block copolymer | 1 | 24 | −61 | 906 | 258 |
| PS-PIB-PS | Linear block copolymer | 19 | 13 | −61 | 460 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1.293,294 This is because the polymer chains get sufficiently large that further molecular weight increases do not substantially alter how the chains move.293,294 As a result, not much more heat is needed to make the transition from a rigid, glassy state to a more flexible, rubbery state. The Fox–Flory equation is as follows:293,294
000 g mol−1.293,294 This is because the polymer chains get sufficiently large that further molecular weight increases do not substantially alter how the chains move.293,294 As a result, not much more heat is needed to make the transition from a rigid, glassy state to a more flexible, rubbery state. The Fox–Flory equation is as follows:293,294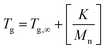 | (1) |
| K = 2VcρNA/α | (2) |
Similar to mechanical behavior, the thermal properties of a polymer can also be influenced by the secondary bonds, where the strong intermolecular forces in polymer units result in higher Tgs because they have the potential to limit polymer chain mobility but increase stiffness.2,295Tgs for halogenated IIRs, such as CIIR and BIIR, have been found to typically be higher than those of non-halogenated butyl rubber.284,296,297 This is mostly because of the halogen atoms, which, because of the polarity of the C–Cl and C–Br bonds, can produce stronger intermolecular interactions, like dipole–dipole forces. The Tg rises as a result of these interactions, which limit the mobility of polymer chains. Also, the Tg rises due to the decreased flexibility of the chains caused by the larger size of the halogen atoms and possible steric hindrance.298 Therefore, in addition to any dipole–dipole forces, the higher Tg of halogenated IIRs is primarily caused by a combination of increased intermolecular forces and steric effects. However, the addition of plasticizers results in polymer chains in the amorphous regions being forced apart, thereby decreasing the intermolecular forces between chains and allowing them to slip more readily over one another.264,275,299–302 As a result, the Tg is lowered. One of the common plasticizers that have been used with butene- and isobutylene-based polymers, particularly PBS and PIB, is oligomeric poly(ethylene glycol) (PEG Mw = 4000).56,301 Furthermore, it has been shown that a certain degree of chain stiffness affects the degradation of polymers, i.e., the degradability of the polymer decreases with an increase in the thermal stability, which explains the non-degradability of high thermally stable butene- and isobutylene-based polymers like pure PB, PIB, IIRs, PBT, and SIBS. Nonetheless, the rigidity of a polymer is also increased by crosslinking, depending on the crosslinking system used and thereby the degree of crosslinking; e.g., higher crosslinked polymers will exhibit a higher Tg than those with lower crosslinked molecules. Because they form dense network structures, epoxy crosslinking, radiation crosslinking, and isocyanate crosslinking are the systems that typically result in the highest degree of crosslinking and higher Tg.303,304 On the other hand, the thermal stability of IIR vulcanizates is not significantly affected by crosslinking agents like metal acetylacetonates.268 Moreover, blending with PBT could improve the thermal stability of PBS, as the former contributes to a more rigid and thermally stable structure because it exhibits a higher Tg and Tm than the latter. This can be shown by comparing the Tms of the reactants (i.e., PBT and PBS) to that of the resultant product (i.e., a biodegradable plastic called poly(butylene succinate-co-butylene terephthalate) (PBST)). For example, when compared to PBS alone, which has Tm generally ranging from ca. 115–125 °C, the higher Tm (ca. 220–250 °C) of PBST is comparable to that of PBT alone (ca. 225–250 °C), depending on the specific formulation, composition, molecular weight, crystallinity, and processing conditions.305–307 Reinforcing butene- and isobutylene-based polymers with reinforcing fillers like glass fibers, CB, silica, or talc, could also significantly improve their thermal stability.
4.3. Chemical stability
The chemical stability of a polymer directly impacts the material's performance, durability, and suitability for specific applications. For example, polymers used for medical devices must be able to resist body fluids and the various sterilization processes. Polymers used for food packaging must not react with food or leach chemicals. In aerospace and automotive industries, polymer materials used must be able to endure fuels, oils, and extreme temperatures. PIB-based elastomers provide superior oxidative and chemical stability.208,308 For specific applications, PIB-based tri-blocks are expected to yield barrier and damping properties that cannot be obtained with diene-based materials.258 These unique properties make PIB-based materials ideal for use in industries where chemical resistance and durability are critical. On the other hand, although IIR exhibits excellent resistance to a wide range of chemicals, including aliphatic hydrocarbons, oxygenated solvents (e.g., alcohols and ketones), as well as oils, it remains susceptible to degradation when exposed to environmental conditions that involve aromatic hydrocarbons, chlorinated solvents, and strong acids (e.g., sulphuric and nitric acids).213,233,269,309 Consequently, unmodified IIR is typically not suitable for applications in environments with prolonged exposure to these substances, such as in automotive fuel systems, chemical processing, and highly acidic environments, where chemical resistance is critical.213,269,309 As for other butene- and isobutylene-based polymers, PB is susceptible to aromatic hydrocarbons, chlorinated solvents, and oxidizing agents.9,73,310 PBT is highly resistant to acids and alkalies; however, it has limitations with oxidizers and high pH.18,311 The modified versions (i.e., PBAT and PBST) perform well in specific applications but are limited by chlorinated solvents and strong acids.312,313 PBS is suitable for environments without exposure to aromatic solvents and strong acids,14,314 and SIBS is resistant to aliphatic hydrocarbons but not suitable for specific aromatic solvents or strong acids.315Furthermore, the chemical composition of butene- and isobutylene-based polymers play a key role in determining their resistance to chemical degradation. PB and PIB have saturated hydrocarbon chains, which provide inherent resistance to different chemicals (acids, bases, and water).9,52,64,73 Because these polymers do not contain reactive functional groups like amines or esters, they are less likely to oxidize or hydrolyze. However, the presence of unsaturation in PIB makes it more susceptible to oxidative degradation. IIR is also characterized by a combination of saturated (isobutylene) and unsaturated (isoprene) units. Though susceptible to aromatic hydrocarbons, etc., the unsaturation in the isoprene segment enables crosslinking, and the resulting crosslinked network enhances the chemical resistance of the polymer by reducing chain mobility, which makes it less vulnerable to chemical attack.285,316 However, extreme conditions like exposure to strong oxidizing agents have been found to still compromise the chemical resistance of IIR, especially when non-halogenated.284 Similarly, although the presence of ester functional groups in the polymer backbone contributes to its resistance to a variety of acids, bases, and oils, PBT, much like PET, is more prone to hydrolysis when exposed to strong acids and bases, leading to possible degradation over time.317,318 PBS, another ester group-containing polymer, is susceptible to hydrolysis, mainly in the presence of moisture or acids, which then limits its chemical resistance, particularly in environments exposed to oils or solvents.319,320 On the other hand, SIBS benefits from the presence of styrene blocks in the copolymer, which enhances its chemical resistance properties. However, as stated previously, SIBS may be more vulnerable to oxidizing agents and aromatic solvents due to the phenyl groups in the styrene units.315 Overall, the ability of these butene- and isobutylene-based polymers to withstand chemical degradation under various environmental conditions is largely determined by their chemical makeup, which includes the type of bonding (saturation vs. unsaturation), functional groups, as well as crosslinking or halogenation.
Nonetheless, a variety of additives can be incorporated into butene- and isobutylene-based polymers to enhance their chemical properties. For PB and PIB, antioxidants, such as phenolic antioxidants and phosphites, are often utilized to improve their oxidative stability.321,322 Plasticizers, such as esters and paraffinic oils, are commonly added to enhance flexibility in these materials.323 For IIR, crosslinking agents (sulphur or peroxides) are used to introduce crosslinks in the polymer chain and resistance to chemicals. Halogenation agents like chlorine or bromine are also introduced to get CIIR or BIIR, respectively, with modified chemical resistance properties. Antioxidants, such as butylated hydroxytoluene (BHT), phosphites, and aminic antioxidants, can also be utilized to increase the chemical durability of IIR under harsh conditions. In PBT, specific additives, including dioctyl phthalate, epoxidized soybean oil, di(2-ethylhexyl) adipate, and trioctyl trimellitate, can be included in their formulations to improve resistance to chemical degradation from oils, solvents, and strong acids. For PBS, plasticizers and stabilizers (especially antioxidants and biostabilizers) are employed to enhance its thermal stability, particularly for biodegradation formulations.324,325 SIBS benefits from the incorporation of antioxidants, especially non-staining and non-discoloring ones, like BHT and many phenolic types to enhance its chemical attack, especially oils, aromatic solvents or acids, and oxidizing agents.6,270,288
5. Applications of butene- and isobutylene-based polymers
Due to their unique, exceptional properties, butene- and isobutylene-based polymers are finding use across diverse industries. Along with a comprehensive overview that follows, Table 9 presents a summary of the applications of butene- and isobutylene-based polymers studied, highlighting the specific properties that render them suitable for these applications. These unique, properties-driven applications highlight the significant versatility of butene- and isobutylene-based polymers. They make them highly adaptable for use in various sectors, from automotive to medical, packaging, and even emerging technologies. The ability of each polymer to meet the specific demands of its application, whether it be low gas permeability in automotive tyres or biocompatibility in medical devices, guarantees that these materials are performing optimally in diverse environments and under different conditions. Moreover, the importance of this versatility cannot be overstated. In addition to the continual emergence of challenges in the field, having a polymer base that can be tailored for specific applications becomes crucial as industries evolve, including the need for sustainable materials, enhanced performance in challenging environments, or the development of advanced medical treatments. The ability to modify and optimize properties and biodegradability allows for continuous innovation. Because of this adaptability, butene- and isobutylene-based polymers are anticipated to remain essential components in advancing various industries and enabling better, more effective products that satisfy both functional and environmental requirements.| Polymer | Properties-based application | Study |
|---|---|---|
| PB | Especially PB-1, used as lubricants, adhesives, sealants, coatings, tubing, flexible films, and appliance parts because of its high tackiness; low density (0.91 g mL−1 at 25 °C); good dimensional stability; low dielectric constant (2.2–2.5 at 25 °C); high transparency (generally translucent to opaque); low gas permeability (5–20 cc per (m2 day atm) for oxygen); and good adhesion that is due to its low surface energy | 29,192,199 and 225 |
| PIB | Because its dielectric constant (2.2–2.3 at 25 °C) and gas permeability (10−12–10−9 cm3 (cm2 s cm Hg)−1 for oxygen) are low, and it offers excellent anti-aging, creep resistance, and adhesive properties as well as high tackiness, it can be used in lubricant formulations, automotive parts, tubing, and as an insulation and encapsulant material in photovoltaic modules and other electronic devices. Especially PIB-HMW, it can be used in stretch films and eyewear or optical devices because of its high strength (15 to 30 MPa); excellent transparency; low moisture diffusivity (10−13–10−12 cm2 s−1); high flexibility (εb up to 600%); and low Tg (−70 to −60 °C). It is also used in coatings, linings, as well as pressure-sensitive adhesives (PSAs) and sealants, due to its high adhesive strength, non-reactivity under ambient conditions, and solubility in hydrocarbons, along with its flexibility and easy removability | 23,24,43,202,206,207 and 229 |
| IIR | Its excellent air retention properties; good resistance to both heat (up to 150, 160, and 170 °C for IIR, CIIR, and BIIR, respectively) and chemicals; low gas permeability; good flexibility; good damping properties; and excellent sealing performance explain its typical use in automotive tyres (inner liners of tubeless tyres and inner tubes), vibration dampers, gaskets, ball bladders, footwear, protective wear, and other domestic and industrial applications. Particularly, a low molecular weight polymer, chlorinated butyl, is used as a sealant and adhesive | 2,28,215,227,254 and 269 |
| PBT | Its applications in engineering, biomedicine, conductive microfibers, electrical connectors, and appliance parts are made possible by its excellent moldability (250–280 °C molding temp.) and properties like high strength (50–80 MPa) and modulus (2.5–4 GPa); good dimensional stability; and good electrical resistance or insulation capability (i.e., high dielectric strength, 15–20 kV mm−1). When blended with PC, the resultant thermoplastic blend is used in the automotive (e.g., bumpers, relays, sockets, switches), aerospace, electronics, consumer goods (like packaging), etc. because of the blend's enhanced notched Izod impact strength (4–8 kJ m−2 of pure PBT vs. 15–40 kJ m−2 at 25 °C of PBT/PC blend); dimensional stability (due to the reduced moisture absorption and higher thermal resistance from PC); and resistance to stress cracking, chemicals, fuel, heat, and weather. Its outdoor electrical insulation is also possible when copolymerized with PTMEG | 18,19,27,94,239,255 and 291 |
| PBAT | Used in cable insulation because of its flexibility; good chemical resistance; good UTS (15–35 MPa); high εb (from 200–600%); low Tg (−20 to −10 °C); and good electrical properties. Its biodegradability (occurring both in aerobic and anaerobic environments), along with compostable capabilities and good barrier properties against moisture, makes it ideal for food containers and disposable cutlery. When blended with PLA, the resultant PBAT/PLA blend is used in packaging films because of enhanced tensile properties (UTS (higher at higher PLA content), elastic modulus (higher at higher PLA content), and εb (higher at higher PBAT content)) | 153,186,240,242,283 and 312 |
| PBS | It is ideal for packaging, agricultural films, and biodegradable plastics because of its environmental impact reduction combined with flexibility, high UTS (40–50 MPa), flexural modulus (ca. 100–300 MPa), and εb (up to 500%) | 5,14,37,154,219,301 and 324 |
| SIBS | It finds use in medical applications, such as drug-eluting coronary stents, ophthalmic implants, artificial heart valves, tissue scaffolds, wound dressings, hydrophobic mats, etc. due to its hemocompatibility, hydrophobicity, biocompatibility, high flexibility, ability to support cell growth for medical use, and suitable UTS (3–25 MPa), tear strength (15–50 kN m−1), εb (up to 700%), elastic modulus (0.5–10 MPa), and strain at yield (5–15%) | 6,99,217,250 and 315 |
5.1. Packaging
Approximately 146 million tonnes, or ca. 36% of the world's total plastic production, are used in packaging.326,327 The most common polymers employed for packaging include polyvinyl chloride (PVC), which is found in cling films, bottles, and non-food packaging; low-density PE, which is used for grocery bags, food wraps, and squeezable bottles; PP, which is found in yogurt containers, straws, and bottle caps; PET, which is found in beverage bottles and food containers; high-density PE, which is used in milk jugs, detergent bottles, and grocery bags; and polystyrene, which is used in disposable cutlery, plastic cups, and food containers.328 These polymers contribute significantly to plastic pollution because they are not naturally degradable and can persist in the environment for hundreds of years.328 Additionally, only ca. 9% of plastic waste is recycled worldwide. This striking disparity draws attention to the difficulties in managing plastic waste and the pressing need for initiatives to address the growing environmental concerns associated with packaging waste. Accordingly, biodegradable plastics are gaining popularity in packaging applications. Because of their biodegradability and distinct physical properties, PBAT and PBS are increasingly being utilized in the packaging industry to address the growing concerns regarding plastic waste.186,301 PBS has gained significant traction for compostable packaging like food containers, films, and wraps.154,301 Its capacity to break down organically in the environment without leaving harmful residues is in line with the increasing demand for compostable packaging. Although it shows great promise for single-use applications, the low melting point and relatively low stiffness limit the use of PBS in more demanding packaging. Therefore, to more effectively compete with conventional plastic alternatives, recent developments have concentrated on enhancing the ease of processing, mechanical properties (e.g., increased stiffness), and heat resistance of PBS by blending it or forming composites with other biodegradable polymers for more specialized packaging applications.301,324 Additionally, because it provides a suitable strength, flexibility, and reduction of plastic waste environmental footprint, PBAT has emerged as the material of choice for compostable plastic bags and single-use packaging like agricultural films.186,242 While PBS and PBAT are the most prominent options, PBT is also making strides in the packaging sector, especially for rigid, lightweight packaging applications like bottle caps and food containers where durability and performance under high heat or chemical exposure (e.g., cosmetic packaging) conditions are essential.19,945.2. Automotive
In the automotive industry, IIR remains a staple due to its unique properties like low permeability, heat resistance, and chemical durability. Specifically, it plays a crucial role in inner tubes and tyre linings, where it retains air pressure, resists damage from environmental factors, and provides durability throughout the long service life of a tyre.28,215,254,269 IIR is resilient enough to be also used in vehicle seals to protect against dust, water, and specific chemicals. Its use in more durable forms, like CIIR and BIIR, which have enhanced chemical resistance, has recently gained popularity. Additionally, PIB, with its strong adhesive properties, continues to be used as a base polymer in PSAs.206,207,329 However, there has been significant attention focused on more recyclable rubber and plastic materials and greener alternatives to conventional IIRs and PIB in some automotive parts due to the rising demand for electric vehicles (EVs). The automotive industry's transition to sustainable rubber and plastic materials in an attempt to meet environmental goals has been covered in detail by Vieyra et al.330 Their analysis highlights how important it is to replace plastics made from fossil fuels with those derived from renewable resources in order to reduce plastic waste, lighten automobiles, and facilitate the switch to electric and driverless vehicles. They also discussed the challenges and opportunities of using recycled and biodegradable plastics, emphasizing the significance of addressing material selection and guaranteeing durability throughout the lifecycle of a vehicle. Furthermore, materials like IIRs and PIB exhibit more complex chemical structures that make them generally not considered widely recyclable in most standard recycling streams, which generally focus on more commonly recycled materials like PE, PP, and PET.331,332 SIBS, with its excellent properties combined with high biostability and biocompatibility, is increasingly used for medicinal devices as well as automotive sealants and adhesives, responding to the industries’ demands for more versatile and high-performance but also sustainable materials that can withstand various harsh environmental conditions.6,217 Meanwhile, PBT is making a significant mark in automotive bumpers, engine components, and interior parts like electronics (e.g., switches and connectors) because of its excellent dimensional stability and resistance to mechanical wear and tear as well as to elevated temperatures (up to 140 °C).94,239,333–335 Also, by incorporating IL moieties, nanofillers, or improving the material's chemical resistance through particular functional groups, the surface-modified PBT has improved performance in automotive sensors and control units, guaranteeing increased durability and resistance to environmental challenges.335–3375.3. Healthcare
Similar to SIBS, PIB (particularly, the crosslinked form) has long been used in the medical field because of its suitable properties combined with biostability and biocompatibility.43,224,338 Recent trends in medical adhesives, surgical drapes, drug delivery systems, implantable devices, and wound care focus on PB (particularly, in its hydrogenated form) and PIB, as they remain favored materials where their bio-adhesive strength, inertness, and low toxicity ensure safe and effective contact with human tissues.224,339 The suitability for drug delivery systems is further increased by the ability of the particularly biocompatible butene- and isobutylene-based polymers to be functionalized with different molecules.340,341 In response to the growing demand for biodegradable medical materials with favorable mechanical properties, PBS has been explored for tissue engineering and biomaterial scaffolds, with research emphasizing its degradation rate and mechanical performance to match the specific needs of biological tissues.13,14,38,342 Similarly, because of its biocompatibility and chemical resistance, PBT is being used in medical applications, especially for injection molded connectors in health-related devices like tips for electrosurgical instruments, pulse oximeters, and clips for breathing masks.343,344 Another biocompatible polymer used in medicinal applications, particularly in biodegradable drug-eluting stents, drug delivery systems, and different temporary implants that naturally degrade in the body thereby decreasing the need for surgical removal, is SIBS.270,345–3475.4. Other
As research and development efforts continue, the applications of butene- and isobutylene-based polymers are expected to be diversified, driven by advancements in polymer science and engineering as well as the need for innovative materials in various industries. For instance, PIB, like other addition polymers, is a material that can undergo depolymerization under controlled conditions to yield original isobutylene monomers, making it possible to produce new and recycled thermoplastic elastomers for use in domestic and industrial products, such as the automotive, electronics, and specific consumer goods.348 Moreover, the versatility of PIB and PBT in next-generation energy technologies is demonstrated by their development for use in energy storage systems, including as possible components of polymer electrolytes.349 Research into functionalizing these polymers with ILs and other materials shows promise for enhancing the performance of energy storage systems, even though these materials are not typically the main electrolytes used in supercapacitors and batteries.350 These advancements aim to improve characteristics like stability, ionic conductivity, and flexibility, which may make them useful for future energy storage applications.350 Stretchability and resilience are crucial for next-generation electronic applications, and polymers like SIBS are gaining prominence for flexible electronics, smart textiles, and wearable technology because of their exceptional balance of flexibility, mechanical strength, and durability.6,7,351New avenues for advanced applications have been made possible by recent advancements in hybrid materials based on polymers derived from butene and isobutylene. For example, because of the synergetic combination of PIB's inherent flexibility and graphene's electrical conductivity and thermal stability, polymer–graphene nanocomposites have shown exceptional electromagnetic interference (EMI) shielding capabilities. These hybrids show promise as lightweight shielding materials for aerospace and flexible electronics applications.352 Similar to this, PBS reinforced with nanocellulose has demonstrated noticeably better mechanical strength and oxygen barrier performance, making it appropriate for environmentally friendly food packaging films. In keeping with the objectives of green packaging, these PBS–nanocellulose composites also maintain their biodegradability.353 Despite these benefits, there are still issues, especially with dispersion and compatibility between polymers and fillers. To increase interfacial adhesion, compatibilizers or surface functionalization of nanofillers are frequently required. For these hybrids, the most common processing techniques are still the melt blending and solvent casting, with the former being preferred due to its scalability and industrial viability. These hybrid systems are anticipated to be crucial in packaging, electronics, and biomedical applications as the need for sustainable, multifunctional materials grows.
5.5. Emerging applications
Butene- and isobutylene-based polymers are being investigated more and more for many cutting-edge and novel applications in the high-tech and sustainable innovation fields, in addition to their well-established uses. The functional potential of these polymers is being expanded by recent studies that show their increasing relevance in domains like energy storage, additive manufacturing, and soft electronics.Energy storage systems: isobutylene-based polymers such as PBS, PBAT, and PBT are gaining significant interest as structural or membrane components in biodegradable batteries and polymer electrolytes as a result of their processability, compatibility with ILs, and chemical inertness. Particularly, PBT functionalized with IL moieties has been studied to enhance ionic conductivity and mechanical integrity in flexible energy storage devices.354,355
3D printing and additive manufacturing: biodegradable polymers like PBS and PBAT are emerging as customizable filaments for fused deposition modeling 3D printing, owing to their thermal stability, low warpage, and biodegradability.356 They offer an important potential for manufacturing eco-friendly parts for prototyping, medical models, and lightweight consumer goods.357
Flexible and wearable electronics: elastomeric isobutylene-based copolymers such as SIBS and modified PIB have been suggested as substrates or dielectric layers in stretchable electronics and soft robotics.358 Their flexibility, chemical resistance, and ability to retain properties under cyclic strain make them feasible for utilization in wearable sensors, actuators, and biomedical electronics.359
6. Narrowing the focus to biodegradability and recyclability
6.1. Biodegradability
As partly highlighted before, researchers are becoming increasingly concerned about the environmental effects of bio-based plastics, as well as their capacity to degrade. While both biodegradable and non-biodegradable bio-based plastics are predicted to minimize environmental plastic pollution, neither is genuinely sustainable.360 Bio-based plastics like bio-PE, bio-PP, and bio-PET are considered not biodegradable, have extended disposal timeframes, and can be detrimental to the environment in a way that compares to their fossil-fuel counterparts.361 They break down into microplastics, which are consumed by aquatic and terrestrial species and contain hazardous chemicals and microbes unless properly disposed of in a designated composting facility. Chemicals added to increase the material's functioning cause nonspecific toxicity, and their migratory processes are poorly understood. A recent study discovered that bioplastics and plant-based polymers were just as harmful to the environment and individuals as traditional plastics.362 On the other hand, researchers have been working on producing biodegradable, non-volatile, and nontoxic additives to significantly increase the mechanical, thermal, and physical characteristics of bioplastics, as well as their biodegradability, with minimum concomitant leaching.363,364 As evidenced by the papers retrieved, incorporating polysaccharide or lipid-based plasticizers into conventional plastics such as PVC has become more appealing than traditional phthalate plasticizers, demonstrating competitive performance with potential in industrial applications.365,366 Combining such studies on the chemical modification of bio-based polymers with studies on the potential risks of additives will aid the design of a greener product with better performance while minimizing potential damage both to the end users and the environment.Furthermore, bioplastics' biodegradability is determined more by their chemical structure rather than their biobased origin. In contrast to other biodegradable polymers such as PLA, PBS, and polyhydroxyoctanoate, biodegradability studies using polyhydroxyalkanoates and their blends have shown success in aqueous and soil testing.367,368 Nevertheless, most degradation experiments are performed on a laboratory scale, and inconsistencies may occur owing to suboptimal circumstances in the natural environment.369–371 Moreover, PBAT and PBST, while being biodegradable and more easily dispersed in the environment, their natural degradation capacities are highly modest. As a result of their increasing usage, significant amounts of waste have accumulated, potentially causing a significant environmental burden. Thus, establishing strategies to minimize and recycle PBAT and PBST waste is vital for their long-term use.372,373 This demands considerable studies into environmental parameters that influence bioplastic degradation, such as pH, salinity, nutrient content, and microbial population, in order to better understand degradation pathways and processes. Blending certain bio-based plastics with other biodegradable or non-biodegradable polymers can have both antagonistic and synergistic effects on biodegradability. Recent research has identified marine biodegradable bio-based polymers with potential uses in a variety of fields. However, merely creating biodegradable materials may not be sufficient; manufacturing should include a practical strategy for recovery and treatment using the current infrastructure. Also, genetically engineered organisms, persistent bio-accumulative and toxic compounds, and petroleum-based co-polymers utilized in some bio-based plastics are causing environmental concerns, especially in terms of pollution and long-term ecological effects. Accordingly, although bio-based plastics provide several environmental benefits, there is rising worry regarding their inputs, GHG emissions, and end-of-life management. Concerns about chemical stability have an impact on the lifecycle and performance of butene- and isobutylene-based polymers in addition to biodegradability. For instance, it has been demonstrated that PBS degrades hydrolytically in humid environments, losing over 10% of its mass after 30 days at 60 °C and 90% relative humidity.374 In contrast, PIB is vulnerable to oxidative degradation when exposed to UV light; without stabilizers, molecular weight reductions of up to 40% have been documented following several hours of exposure at specific nm.375 To improve environmental resistance while maintaining functionality, these vulnerabilities can be lessened by adding UV stabilizers (such as hindered amine light stabilizers), incorporating inorganic nanoparticles like titanium dioxide (TiO2) or zinc oxide (ZnO), or copolymerizing PIB with PBAT or other biodegradable segments.376 These consequences are quantified through life cycle evaluations (as previously discussed), which can also include biological carbon content analysis and carbon isotope assays. Ultimately, while bio-based plastics benefit the environment, production and end-of-life choices should be improved. Switching to renewable energy and innovative feedstocks can result in higher emission reductions and cheaper prices.
The overall environmental performance of butene- and isobutylene-based polymers is largely determined by sustainable supply chain factors. These materials can be derived from renewable feedstocks like 1,4-butanediol or succinic acid derived from plants, or they can be made from fossil-based monomers (like butenes in petrochemical streams). The feedstock selection has a direct impact on these polymers' cost structure, long-term viability, and carbon footprint. Although bio-based sources typically emit lower GHGs, their sustainability can vary greatly based on factors like land-use change, water needs, and agricultural inputs. Additionally, LCA tools like SimaPro and GaBi should be used to assess the energy intensity of polymer synthesis routes, including the use of high-pressure reactors, specialized catalysts, and purification steps. Researchers can use these tools to measure effects at every stage of the supply chain, from the procurement of raw materials to recycling or end-of-life disposal. Closing the loop and lowering reliance on virgin resources in this situation requires the use of circular economy models, especially those that incorporate mechanical recycling, chemical depolymerization, and renewable energy inputs. Researchers and industry stakeholders can more effectively optimize the economic and environmental trade-offs of these polymer systems by implementing such multi-metric LCA approaches.
6.2. Recycling and reuse
Polymer recycling involves a sequence of well-defined steps designed to recover and repurpose end-of-life plastic products. The process typically includes the collection and sorting of waste, followed by identification using spectroscopic or density-based techniques, thorough washing to remove contaminants, and subsequent size reduction through shredding. The shredded material is then pelletized to produce uniform granulates, which can be reprocessed into new polymeric materials.51 Among recycling methods, mechanical recycling remains the most widely implemented due to its relative simplicity and cost-effectiveness. It relies mainly on the effective separation of single-polymer streams and requires clean, uncontaminated feedstock to maintain polymer properties after reprocessing. Chemical recycling, on the other hand, offers an alternative route by breaking down polymers into their monomeric units or other value-added chemicals using heat, solvents, catalysts, and/or pressure.377 This approach is particularly beneficial for dealing with complex or contaminated plastics that are unsuitable for mechanical recycling. Technologies under chemical recycling include solvent-based dissolution processes, which preserve polymer chains, and depolymerization approaches that cleave the covalent bonds within polymer backbones—an especially relevant technique for butene- and isobutylene-based elastomers. Both mechanical and chemical recycling methods can be adapted to recover and reuse butene- and isobutylene-derived polymers (Fig. 15), such as polyisobutylene and its derivatives. | ||
| Fig. 15 Overview of mechanical and chemical recycling pathways for butene- and isobutylene-based polymer materials.166,348,377–381 | ||
Presently, most polymer waste is recycled mechanically, which is only successful for uniform, contaminant-free plastic waste that is challenging to collect. However, using chemical recycling techniques to complete the mechanical recycling of polymers can considerably increase recycling rates and assist in revitalizing the whole petrochemical industry. Furthermore, depolymerization is a process that is commonly used to recycle synthetic polymers, such as addition and condensation polymers. This process not only enhances sustainability but also supports the circular economy by enabling the recycling and reuse of butene- and isobutylene-based polymers. Heat is frequently used to depolymerize addition polymers like PB, PIB, IIR, and SIBS, but hydrolysis is required for condensation polymers like PBT, PBAT, and PBS.51 Pyrolysis, a process in which waste polymers are heated and pressed in an oxygen-free environment, is one example of thermochemical recycling of polymer waste. It may be modified to produce considerable volumes of monomeric compounds for reuse from a specific polymer waste stream. However, pyrolysis is not without its significant drawbacks, which include environmental concerns, high energy requirements, and potential challenges in waste management. Therefore, other recycling processes with less environmental impact have been developed. For instance, Watson et al.348 carried out the depolymerization of PIB at room temperature in the presence of trifluoromethanesulfonic acid and an arene solvent such as benzene. This enthalpically driven process formed tert-butyl carbocations, which were stabilized by the solvent, resulting in stable products like tert-butylbenzene. The depolymerization occurred in minutes, with the extent dependent on the end groups of the PIB and the solvent utilized. This method lowered the ceiling temperature of the polymer, offering a new approach to polymer recycling. On the other hand, PBT has been successfully depolymerized in supercritical methanol378 and hot compressed water-containing reactors,379,380 as well as via enzymatic pathways.381 By utilizing and/or advancing these approaches, the butene- and isobutylene-based polymer wastes could be effectively addressed, lowering their contribution to the overall polymer waste concerns.
The widespread adoption of efficient recycling techniques for butene- and isobutylene-based polymers is hampered by a number of challenges, despite encouraging developments. The requirement for pure, single-polymer streams, which is rarely met because of contamination and multilayer packaging, limits mechanical recycling. After multiple reprocessing cycles, polymer degradation also results in a loss of thermal stability and mechanical integrity, rendering materials unsuitable for high-performance reuse. Regarding chemical recycling, the recovery and purity of monomers are still hampered by the separation of copolymer components, additives, and catalysts. Moreover, several chemical depolymerization methods are still energy-intensive and reliant on harmful solvents or high pressures, which makes them less sustainable. These limitations are being addressed by recent innovations. For example, under mild conditions, selective depolymerization techniques show tailored breakdown of polymer chains, such as acid-mediated room-temperature degradation of PIB348 or supercritical and enzymatic depolymerization of PBT and PBAT.378–381 Compared to conventional pyrolysis, these techniques increase selectivity and have a lower environmental impact. Additionally, separation-enhanced procedures, such as membrane filtration and tandem enzymatic depolymerization, demonstrate how biocatalytic and hybrid systems can increase recycling yield and material purity, particularly for condensation polymers like PBAT.166
6.3. Environmental impact and recyclability
Understanding the environmental fate of butene- and isobutylene-based polymers is crucial, especially when it comes to their performance in different environmental conditions, hydrolysis behavior, and degradation kinetics. Although biodegradable polymers like PBS and PBAT have potential benefits, environmental factors like temperature, microbial diversity, and moisture content have a significant impact on how quickly they degrade. PBS films, for instance, degraded up to 60% in 180 days under industrial composting conditions, according to Borelbach et al.,382 while only 90 days of controlled composting conditions showed 60.7%, as Shin et al.383 stated. Similarly, PBS showed hydrolytic degradation rates exceeding 50% weight loss in controlled marine mesocosms within 90 days, according to Suzuki et al.,384 indicating partial marine degradability. However, broad environmental integration is still hampered by chemical stability under particular conditions. Despite being biodegradable under ideal conditions, PBT and PBAT exhibit strong resistance in landfills and freshwater systems.283,385 High temperatures and steady enzymatic activity are necessary for their degradation, which is not always feasible in open environments.386,387 Also, chemical additives like plasticizers and UV stabilizers that are frequently used in commercial formulations may disrupt the kinetics of degradation and present a risk of leaching during breakdown, which could result in ecological toxicity.388,389 Regarding recyclability, the environmental trade-offs of mechanical and chemical methods must be taken into account. Despite its effectiveness in recovering monomers, pyrolysis has significant energy requirements and emissions. However, new solvent-based and enzymatic depolymerization processes present viable, low-impact substitutes. For example, a recent study by Yang et al.390 reports on the enzymatic depolymerization of PBAT film using engineered cutinases, and reveals 55% gel formation after UV exposure for 24 h and immersing in a solvent (trichloromethane) for 48 h. However, the majority of solvents used are toxic as well. These findings underscore the need for a multifaceted approach when assessing the life cycle of butene- and isobutylene-based polymers. To promote the sustainable use of these polymers, more case-specific environmental impact analyses and scalable recycling techniques are essential. Ultimately, directing policy and design decisions for more environmentally friendly polymer solutions will depend on combining LCA data with actual degradation behavior.7. Future directions and research opportunities
Despite recent significant advancements, there are still several promising research prospects in the field of butene- and isobutylene-based polymers discussed in this review. More research into cutting-edge synthesis methods, novel copolymers, and the sustainability of these materials will ensure their widespread adoption as they evolve. Creating new butene- and isobutylene-based copolymers that combine the desirable qualities of different monomers to achieve distinctive performance characteristics is a primary avenue for future studies. For instance, butylene has been incorporated into copolymers like PBS, PBAT, and PBST to produce polymers that combine material biodegradability and enhanced mechanical properties. Future research can concentrate on broadening the variety of renewable resource-based monomers added to butene- and isobutylene-based copolymers, which may produce more biodegradable polymers with unique properties for a wider range of applications.Specific butene- and isobutylene-based copolymer systems are beginning to show promise in meeting application-specific requirements, in keeping with current trends in material innovation (Table 10). PBS and polycaprolactone copolymerization (PBS–PCL), for instance, may provide improved elasticity and delayed degradation, making it ideal for medical applications like drug delivery scaffolds and bioresorbable implants.391 Similar to this, combining PIB and PBAT (PIB–PBAT) may result in flexible, semi-biodegradable films with increased toughness, making them desirable options for agricultural and flexible food packaging applications.392 Such studies highlight how crucial strategic monomer selection is for tuning mechanical and environmental performance. Future research should build upon these by integrating copolymer architectures for advanced and sustainable applications with functionality, responsiveness, and compatibility.
| Copolymer systems | Monomer combination | Target property/benefit | Potential application | Ref. |
|---|---|---|---|---|
| PBS–PCL | Butylene succinate + ε-caprolactone | Increased biocompatibility, reduced rate of biodegradation, and improved elasticity | Medical devices like scaffolds, sutures, and implants | 391 |
| PIB–PBAT | Isobutylene + butylene adipate/terephthalate | Improved toughness, flexibility, and partial biodegradability | Flexible packaging and agricultural films | 392 |
| PB–PLA | Butene + lactic acid | Balance of flexibility, rigidity, and biodegradability | Compostable containers and semi-rigid packaging | 393 |
| PBS–starch | 1,4-Butanediol/succinic acid + glucose | Increased biodegradability and reduced cost | Disposable food packaging and mulch films | 394 |
| PBAT–PEG | Butylene adipate/terephthalate + ethylene glycol | Stimuli responsiveness and water uptake control | Smart materials and controlled drug release | 395 |
The characteristics and performance of butene- and isobutylene-based polymers could be greatly enhanced by advanced processing methods. For example, to get more control over the MWD and polymer architecture, the application of metallocene-based catalysts in the synthesis of PB can be investigated further. New methods for adjusting the structure and characteristics of butene- and isobutylene-based polymers are provided by recent advancements in the use of supercritical fluids in polymer processing. PBS and PBAT, in particular, have been made highly porous by the use of scCO2, which may make it appropriate for use in drug delivery systems or as a bio-based substitute for synthetic polymers in packaging applications.144,145,232,297 In addition, investigating the use of other innovative polymerization processes like plasma and microwave-assisted polymerization could open the door to more effective, scalable, and ecologically friendly ways to synthesize these polymers.396,397
Another crucial area for further study is the role of butene- and isobutylene-based polymers in the context of the circular economy. In addition to the biodegradability, the recyclability of these polymers will become a crucial characteristic to investigate as the need for sustainable materials grows. Creating methods to enhance the mechanical and chemical recycling of these materials is a promising direction of study. PBS, PBAT, and PBST are biodegradable, but further studies are still needed to determine how quickly they break down in different environmental settings. This might be the main strategy in which their roles in the circular economy can be more clearly defined. Moreover, PB, PBT, and IIR recycling procedures are currently not maximized. Future studies could concentrate on developing more effective depolymerization and re-polymerization processes to make these materials viable contenders for circular product life cycles. Biodegradable butene- and isobutylene-based polymers should also have their LCA extended to incorporate a wider range of environmental metrics, such as carbon sequestration, water use, and land use. These comprehensive assessments will shed more light on the true environmental advantages of these polymers (in contrast to traditional plastics) and direct their continued use across a range of sectors.
Further studies could also explore the copolymerization, functionalization, and nanoparticle reinforcement of butene- and isobutylene-based polymers to fabricate smart materials that react differently to environmental stimuli. For example, to make PBAT well-suited for high-tech uses like environmental sensors and self-healing materials, it might be developed to have improved features like stimuli-responsiveness (for example, in response to temperature, light, or pH) (Fig. 16).398,399 A glimpse of these smart functionalities can be found in recent case studies. For instance, because of their low Tg and intrinsic segmental mobility, self-healing coatings derived from PIB-based elastomers have shown exceptional mechanical integrity recovery following minor damage.24,400 In aerospace and marine applications, where microcracks often form during cyclic stress, these materials could be ideal for long-term protective coatings. In addition, controlled shape recovery and responsiveness to external stimuli like heat and moisture have been demonstrated for PBS-based nanocomposites embedded with thermo-responsive fillers like graphene oxide or poly(N-isopropylacrylamide).401 These systems are becoming sustainable alternatives in high-tech automotive, electronics systems, and temperature-sensitive sensors, as well as in the medical field (e.g., biomedical scaffolds and current use of SIBS in drug delivery systems) and smart packaging (e.g., packaging that effectively responds to environmental factors like temperature and humidity).348,391
 | ||
| Fig. 16 Conceptual representation of stimuli-responsive behavior in smart butene- and isobutylene-based polymer systems.398–401 | ||
The investigation of butene- and isobutylene-based polymers for high-performance applications in sectors like aerospace is another important area of research. For such a demanding sector in terms of components’ properties, where some require high strength, others need high rigidity, and some demand both and more, great effort is necessary to enhance the processing capabilities, mechanical behavior, dimensional stability, and heat resistance of butene- and isobutylene-based polymers. Future studies could concentrate on the creation of hybrid materials, which combine the qualities of butene- and isobutylene-based polymers with inorganic fibers or fillers, like graphene or CNTs, to fabricate high-strength and ultra-lightweight materials that can be used in next-generation electronics, vehicles, or systems. This is particularly because butene- and isobutylene-based polymers have demonstrated a basic solution for the automotive industry's increasing need for lightweight, durable, and environmentally friendly materials in areas like external components, interior panels, and under-the-hood applications.265,316,330,333
Another relevant field for research is the incorporation of renewable feedstocks into the production of butene- and isobutylene-based polymers. Currently, some of these polymers, i.e., PBS, PBAT, and PBST, are made from plant-based feedstocks and renewable resources. The carbon footprint of these polymers may be further reduced with additional studies into alternate biomass sources like algae or lignocellulosic materials for their synthesis. Optimizing the synthesis process will require an understanding of how different feedstocks affect the finished polymer's performance. Therefore, to ensure that the environmental advantages of butene- and isobutylene-based polymers are optimized, researchers might have to look into the sustainability of the whole supply chain, from the sourcing of feedstock to polymerization and end-of-life management.
Developing advanced blends and composites comprising butene- and isobutylene-based polymers and other biodegradable materials is a notable research opportunity. Materials with improved mechanical properties and increased biodegradability may result from further blending PBS with materials like starch and PLA, opening up a wider range of packaging industry applications.176,402,403 Similarly, the creation of composite materials made up of PBS, PBAT, or PBST and natural fibers may provide a more environmentally friendly substitute for traditional plastics in automotive and construction applications. This means that researchers can reduce reliance on fossil-based plastics by developing novel materials that meet new market demands by combining the special properties of butene- and isobutylene-based polymers with other biopolymers.
As PBS, PBAT, and PBST, in particular, gain traction in the market, there is an increasing demand to make their synthesis methods more scalable. Optimizing the methods for these polymers is crucial to satisfy the growing demand for sustainable polymer products. For instance, although PBAT is made from a variety of renewable feedstocks, its complex synthesis pathway makes it costly to produce on a commercial scale.403 Future studies could concentrate on increasing the synthesis process's efficiency for PBAT as well as other isobutylene-based copolymers and polymers. Improvements in reactor technology, reaction kinetics, and catalyst design may make it possible to produce biodegradable plastics on a larger scale and at a lower cost.403,404 To summarize and promote further research on overcoming challenges related to processing, Fig. 17 illustrates a flow diagram outlining current processing restrictions and emerging strategic solutions for enhancing the efficiency, scalability, and sustainability of butene- and isobutylene-based polymer synthesis and applications.
 | ||
| Fig. 17 General flow diagram of processing challenges and proposed strategic solutions for butene- and isobutylene-based polymers.241,274,398,406 | ||
Researchers should explore the role of butene- and isobutylene-based polymers in energy-efficient applications in keeping with sustainability. PBT, for example, has good electrical and thermal properties, making it an ideal option for energy-efficient electronics.405 The use of this material in energy harvesting, storage, and even as part of photovoltaics or energy-efficient windows may be the subject of future research. In line with the expanding trend of sustainability in electronics, PBS, PBAT, and PBST may also be viable materials for use in biodegradable energy storage devices due to their biodegradability and mechanical strengths. In general, the ongoing development of the applicability and sustainability of butene- and isobutylene-based polymers will be essential in widening their usage while tackling environmental issues and furthering the green chemistry agenda as awareness of plastic pollution and its effects grows worldwide.
7.1. Proposed experimental methodologies and evaluation frameworks
This subsection describes particular experimental techniques and evaluation instruments to direct the real-world application of the research directions suggested in this review. For butene- and isobutylene-based polymers, these approaches facilitate the implementation of innovative processing methods, environmental evaluations, and application-focused advancements.• For renewable feedstocks:
o Explore bio-based butene synthesis from glucose by Clostridium acetobutylicum fermentation.
o Use gas chromatography-mass spectrometry and nuclear magnetic resonance (NMR, 1H/13C) to characterize fermentation outputs.
o Use high-performance liquid chromatography, elemental analysis, and Fourier transform infrared spectroscopy (FT-IR) to assess purity and conversion.
• For scalable synthesis:
o Assess continuous flow reactors at different temperatures (e.g., 50–120 °C) and contact times.
o Use size-exclusion chromatography, thermogravimetric analysis, and in-line FT-IR to monitor polymer conversion.
o Assess throughput and stability by optimizing catalyst loading and flow rates.
• For circular economy integration and recyclability:
o Employ supercritical methanol or enzymatic digestion to perform chemical depolymerization.
o Use techniques like liquid chromatography-mass spectrometry, NMR, and respirometry to characterize degradation products.
o Employ differential scanning calorimetry (DSC), dynamic mechanical analysis, and other mechanical testing to assess recyclability after multiple thermal cycles.
• For the development of smart material:
o Use nanoparticles, including ZnO, CNTs, and graphene, to functionalize polymers through melt blending or in situ polymerization.
o Employ UV-Vis spectroscopy, DSC, and dynamic mechanical analysis to test stimuli-responsiveness under pH, light, and thermal stimuli.
o Use electrochemical impedance spectroscopy and gas permeability testing to assess conductivity or barrier properties, more especially for electronics and packaging.
• For environmental impact and degradation evaluation:
o Use tools like SimaPro or GaBi to conduct LCA, covering cradle-to-grave metrics, which typically include GHG emissions, water consumption, and land-use change.
o Employ respirometry, mass loss tracking, and scanning electron microscopy imaging to assess polymer degradation in marine, composting, and soil conditions.
o Utilize carbon isotope ratio analysis to measure renewable content.
8. Conclusion
Significant progress has been achieved in the synthesis of butene- and isobutylene-based polymers, with several approaches improving polymerization efficiency, processability, material properties, and applicability. With precise control over molecular weight distribution and polymer design, metallocene-based catalyst systems have demonstrated notable efficacy in the synthesis of high-quality PBT, PBAT, and PBS. In addition to producing materials with enhanced mechanical and thermal properties, these synthesis process advancements have also lessened the environmental impact of polymerization by using less energy and generating fewer by-products than traditional Ziegler–Natta catalyst systems, which are typically used to synthesize PB, PIB, and IIR. On the other hand, the core synthesis of SIBS itself has received less attention.Properties of butene- and isobutylene-based polymers have been crucial to their expanding use in various industries. For example, PB and PIB find use in adhesive applications because of their high bonding strength. On the other hand, as an alternative to conventional, non-biodegradable polymers, PBAT and PBS, are notable for their biodegradability and have demonstrated significant potential in eco-friendly packaging solutions, agricultural films, and medicinal uses. Like PBS, the applications of SIBS extend to the healthcare sector, where its biocompatibility makes it an ideal fit for controlled-release drug delivery systems. Meanwhile, because of its good resistance to both heat and chemicals, low gas permeability, and excellent sealing performance, IIR finds use in protective wear, automotive tyres, seals, and gaskets. Ultimately, the growth of these polymers in various industries is anticipated to accelerate as the need for high-performance and ecologically friendly materials keeps increasing and research advances.
Benefits for the environment from isobutylene-based polymers, especially PBS, PBAT, and PBST, are essential for addressing the rising issues of plastic waste and pollution. These biodegradable polymers contribute significantly to the transition to more sustainable materials as they present a viable biodegradable substitute for conventional plastics derived from petroleum-based resources. Notwithstanding these promising characteristics, the synthesis costs of the overall butene- and isobutylene-based polymers are high. Additionally, even though the synthesis process optimization has advanced, more may be done to lessen the environmental impact of polymer synthesis, particularly concerning energy use and GHG emissions. Future studies should, therefore, concentrate on investigating different, more sustainable catalytic methods and enhancing the scalability of metallocene-based systems. There are also still concerns with the full biodegradability of PBS and PBAT and their ability to be completely integrated into the circular economy. On the other hand, PB, PIB, PBT, IIR, and SIBS recycling procedures are currently underexplored. This can be addressed by improving the degradation rates of PBS and PBAT under various environmental conditions and enhancing the recyclability of the other butene- and isobutylene-based polymers, both of which will maximize the polymers’ environmental benefits and thereby their adoption across various domestic and industrial sectors.
Author contributions
J. I. Mnyango: conceptualization, methodology, literature investigation, writing – original draft, writing – review & editing, visualization, validation; B. Nyoni: writing – original draft; N. Mama: writing – review & editing; B. G. Fouda-Mbanga: writing – original draft; Z. Tywabi-Ngeva: writing – review & editing; S. P. Hlangothi: conceptualization, supervision, writing – review & editing, validation.Conflicts of interest
The authors declared no potential conflicts of interest concerning the research, authorship, and/or publication of this article.Data availability
As this paper is a literature review, no original research data or datasets were generated or analyzed during the study. All data and information presented in this paper are derived from publicly available scientific literature, research articles, and academic sources. References to the relevant studies are provided throughout the paper. Any additional data, if required, would have to be accessed by consulting the cited sources in the reference list.Acknowledgements
The National Research Foundation (NRF) [PSTD240422215462] is acknowledged for financial support.References
- R. K. Sharma, S. Mohanty and V. Gupta, Advances in butyl rubber synthesis via cationic polymerization: an overview, Polym. Int., 2021, 70(9), 1165–1175, DOI:10.1002/pi.6180.
- J. G. Speight, Monomers, polymers, and plastics, in Handbook of Industrial Hydrocarbon Processes, ed. Speight, J. G., Gulf Professional Publishing, Houston, US, 2nd edn, 2020, ch. 14, pp. 597–649 DOI:10.1016/B978-0-12-809923-0.00014-X.
- M. Gahleitner and C. Paulik, Polypropylene and other polyolefins, in Brydson's Plastics Materials, ed. Gilbert, M., Butterworth-Heinemann, Oxford, UK, 8th edn, 2017, ch. 11, pp. 279–309 DOI:10.1016/B978-0-323-35824-8.00011-6.
- S. R. Turner and Y. Liu, Chemistry and technology of step-growth polyesters, in Polymer Science: A Comprehensive Reference, ed. Matyjaszewski, K. and Möller, M., Elsevier, Amsterdam, Netherlands, 2012, ch. 5.14, pp. 311–331 DOI:10.1016/B978-0-444-53349-4.00143-6.
- M. Barletta, A. Genovesi, M. P. Desole and A. Gisario, Melt processing of biodegradable poly(butylene succinate) (PBS)—a critical review, Clean Technol. Environ. Policy, 2025, 27, 683–725, DOI:10.1007/s10098-024-03005-8.
- L. Pinchuk, G. J. Wilson, J. J. Barry, R. T. Schoephoerster, J.-M. Parel and J. P. Kennedy, Medical applications of poly(styrene-block-isobutylene-block-styrene) (“SIBS”), Biomaterials, 2008, 29(4), 448–460, DOI:10.1016/j.biomaterials.2007.09.041.
- P. Maji and K. Naskar, Styrenic block copolymer-based thermoplastic elastomers in smart applications: Advances in synthesis, microstructure, and structure–property relationships—A review, J. Appl. Polym. Sci., 2022, 139(39), e52942, DOI:10.1002/app.52942.
- A. He, W. Zheng, Y. Shi, G. Liu, W. Yao and B. Huang, In situ synthesis of polybutene-1/polypropylene spherical alloys within a reactor with an MgCl2-supported Ziegler–Natta catalyst, Polym. Int., 2012, 61(10), 1575–1581, DOI:10.1002/pi.4250.
- A. Ghavampoor, N. Bahri-Laleh, S. Sadjadi, M. Nekoomanesh, A. Vahid, J. Duran, M. Spiegel and A. Poater, A green approach to synthesize polybutene lubricants from mixed C4 monomers using supported dendritic ionic liquids, J. Mol. Liq., 2024, 412, 125814, DOI:10.1016/j.molliq.2024.125814.
- Q. Huang, F. Zhu, Q. Wu and S. Lin, The synthesis of high molecular weight polybutene-1 catalyzed by Cp·Ti(OBz)3/MAO, Polym. Int., 2001, 50(1), 45–48, DOI:10.1002/1097-0126(200101)50:1<45::AID-PI531>3.0.CO;2-S.
- L. Cai, B. Liu, X. Liu and D. Cui, Synthesis of high molecular weight polyisobutylene with Al(C6F5)3-based initiating systems under mild conditions, Polymer, 2022, 241, 124539, DOI:10.1016/j.polymer.2022.124539.
- M. Hulnik, D. Trofimuk, P. A. Nikishau, H. C. Kiliclar, B. Kiskan and S. V. Kostjuk, Visible-light-induced cationic polymerization of isobutylene: A route toward the synthesis of end-functional polyisobutylene, ACS Macro Lett., 2023, 12(8), 1125–1131, DOI:10.1021/acsmacrolett.3c00384.
- M. Barletta, C. Aversa, M. Ayyoob, A. Gisario, K. Hamad, M. Mehrpouya and H. Vahabi, Poly(butylene succinate) (PBS): Materials, processing, and industrial applications, Prog. Polym. Sci., 2022, 132, 101579, DOI:10.1016/j.progpolymsci.2022.101579.
- L. Aliotta, M. Seggiani, A. Lazzeri, V. Gigante and P. Cinelli, A brief review of poly (butylene succinate) (PBS) and its main copolymers: Synthesis, blends, composites, biodegradability, and applications, Polymers, 2022, 14, 844, DOI:10.3390/polym14040844.
- S. Thakur, J. Chaudhary, P. Singh, W. F. Alsanie, S. A. Grammatikos and V. K. Thakur, Synthesis of Bio-based monomers and polymers using microbes for a sustainable bioeconomy, Bioresour. Technol., 2022, 344, 126156, DOI:10.1016/j.biortech.2021.126156.
- B. U. Durmaz, F. U. Nigiz and A. Aytac, Active packaging films based on poly(butylene succinate) films reinforced with alkaline halloysite nanotubes: Production, properties, and fruit packaging applications, Appl. Clay Sci., 2024, 259, 107517, DOI:10.1016/j.clay.2024.107517.
- X. Liu, Y. Ge, S. Liu, Y. Wang, B. Guo and X. Wang, Simultaneous enhancement of strength and toughness of polybutylene terephthalate composites via oriented shish-kebab crystals induced by oriented polytetrafluoroethylene nanofibers, Compos. Commun., 2025, 55, 102325, DOI:10.1016/j.coco.2025.102325.
- W. Tabaka and B. Schartel, Less is more: Optimised fire performance in glass fibre-reinforced polybutylene terephthalate laminates with concentrated flame retardant top layer, Compos., Part C: Open Access, 2025, 16, 100577, DOI:10.1016/j.jcomc.2025.100577.
- D. C. Souza, J. H. S. Carvalho, R. C. Freitas, I. C. Bellettini and B. C. Janegitz, Production of conductive microfibers based on Polybutylene adipate terephthalate and graphite using a 3D printed electrospinning system and their application as electrochemical dopamine sensor, Microchem. J., 2024, 206, 111520, DOI:10.1016/j.microc.2024.111520.
- H. Uematsu, S. Yorikane, A. Yamaguchi, S. Sugihara, F. Nishimura, M. Yamane and S. Tanoue, Role of glass fiber surface treatment on hydrothermal aging at the interface between polybutylene terephthalate and glass fiber, Composites, Part A, 2025, 192, 108811, DOI:10.1016/j.compositesa.2025.108811.
- R. G. Kleijnen, M. Schmid and K. Wegener, Production and processing of a spherical polybutylene terephthalate powder for laser sintering, Appl. Sci., 2019, 9(7), 1308, DOI:10.3390/app9071308.
- B. K. Ratshoshi, S. Farzad and J. F. Görgens, A techno-economic study of polybutylene adipate terephthalate (PBAT) production from molasses in an integrated sugarcane biorefinery, Food Bioprod. Process., 2024, 145, 11–20, DOI:10.1016/j.fbp.2024.01.011.
- Y. Kang, Y. Zhao, X. Feng, J. Fang, L. Shui, M. Xin and C. Hao, Adhesive and rheological properties of Polyisobutylene-based adhesives with different white carbon black fillers, Int. J. Adhes. Adhes., 2024, 133, 103755, DOI:10.1016/j.ijadhadh.2024.103755.
- U. G. Centa, A. Oseli, M. Mihelčič, A. Kralj, M. Žnidaršič, M. Halilovič and L. S. Perše, Long-term viscoelastic behavior of polyisobutylene sealants before and after thermal stabilization, Polymers, 2024, 16(1), 22, DOI:10.3390/polym16010022.
- A. Prudnikau, D. I. Shiman, E. Ksendzov, J. Harwell, E. A. Bolotina, P. A. Nikishau, S. V. Kostjuk, I. D. W. Samuel and V. Lesnyak, Design of cross-linked polyisobutylene matrix for efficient encapsulation of quantum dots, Nanoscale Adv., 2021, 3, 1443–1454, 10.1039/D0NA01012J.
- A. V. Pocius, Adhesives and sealants, in Polymer Science: A Comprehensive Reference, ed. Matyjaszewski, K. and Möller, M., Elsevier, Amsterdam, Netherlands, 2012, ch. 8.12, pp. 305–324 DOI:10.1016/B978-0-444-53349-4.00210-7.
- V. Soni, V. Pandey, S. Asati, V. Gour and R. K. Tekade, Biodegradable block copolymers and their applications for drug delivery, in Advances in Pharmaceutical Product Development and Research, Basic Fundamentals of Drug Delivery, ed. Tekade, R. K., Academic Press, Cambridge, US, 2019, ch. 11, pp. 401–447 DOI:10.1016/B978-0-12-817909-3.00011-X.
- J. J. Higgins, F. C. Jagisch and N. E. Stucker, Butyl rubber and polyisobutylene, in Handbook of Adhesives, ed. I. Skeist, Springer, Boston, US, 1990, pp. 185–186 DOI:10.1007/978-1-4613-0671-9_10.
- S. Sängerlaub, K. Reichert, J. Sterr, N. Rodler, D. von der Haar, I. Schreib, C. Stramm, A. Gruner, J. Voigt, H. Raddatz and M. Jesdinszki, Identification of polybutene-1 (PB-1) in easy peel polymer structures, Polym. Test., 2018, 65, 142–149, DOI:10.1016/j.polymertesting.2017.11.007.
- X. Li, Y. Wu, J. Zhang, S. Li, M. Zhang, D. Yang, H. Wang, Y. Shang, W. Guo and P. Yan, Synthesis of highly reactive polyisobutylenes via cationic polymerization in ionic liquids: characteristics and mechanism, Polym. Chem., 2019, 10, 201–208, 10.1039/C8PY01141A.
- S. Karthikeyan and V. K. Gupta, Highly reactive polyisobutylene through cationic polymerization of isobutylene, J. Polym. Res., 2023, 30(337), 1–18, DOI:10.1007/s10965-023-03706-6.
- I. V. Vasilenko, A. N. Frolov and S. V. Kostjuk, Cationic polymerization of isobutylene using AlCl3OBu2 as a Coinitiator: Synthesis of highly reactive polyisobutylene, Macromolecules, 2010, 43(13), 5503–5507, DOI:10.1021/ma1009275.
- Z. Yu, X. Feng, C. Zhao, J. Li, R. Liu, Y. Jin and Y. Wu, Synthesis of linear and star-shaped telechelic polyisobutylene by cationic polymerization, RSC Adv., 2022, 12, 27380–27388, 10.1039/D2RA04504D.
- S. T. Semegen, ( 2003), Rubber, synthetic, in Encyclopedia of Physical Science and Technology, ed. Meyers, R. A., Academic Press, Cambridge, US, 3rd edn, pp. 395–405 DOI:10.1016/B0-12-227410-5/00671-2.
- J.-G. Rosenboom, L. De Lorenzi, G. Storti and M. Morbidelli, Reaction kinetics and simulations of ring-opening polymerization for the synthesis of polybutylene terephthalate, Polymer, 2018, 146, 120–132, DOI:10.1016/j.polymer.2018.05.029.
- A. Díaz, R. Katsarava and J. Puiggalí, Synthesis, properties and applications of biodegradable polymers derived from diols and dicarboxylic acids: From polyesters to poly(ester amide)s, Int. J. Mol. Sci., 2014, 15(5), 7064–7123, DOI:10.3390/ijms15057064.
- S. Kato, T. Ueda, T. Aoshima, N. Kosaka and S. Nitta, BioPBS™ (Polybutylene Succinate), in Synthetic Biodegradable and Biobased Polymers, Advances in Polymer Science, ed. Künkel, A., Battagliarin, G., Winnacker, M., Rieger, B., Coates, G., Springer, Cham, Switzerland, 2023, vol. 293, pp. 269–304 DOI:10.1007/12_2023_159.
- S. A. Rafiqah, A. Khalina, A. S. Harmaen, I. A. Tawakkal, K. Zaman, M. Asim, M. N. Nurrazi and C. H. Lee, A Review on properties and application of bio-based poly(butylene succinate, Polymers, 2021, 13(9), 1436, DOI:10.3390/polym13091436.
- A. Gomoll, J. Hallstein, A. Lieske, E. Metzsch-Zilligen, R. Pfaendner and D. Zehm, Unraveling the cause for the unusual processing behavior of poly(butylene succinate), Part 2: The effect of synthesis on the processing stability of poly(butylene succinate), J. Appl. Polym. Sci., 2025, 1–17, DOI:10.1002/app.56897.
- J. Devroede, R. Duchateau, C. E. Koning and J. Meuldijk, The synthesis of poly(butylene terephthalate) from terephthalic acid, part I: The influence of terephthalic acid on the tetrahydrofuran formation, J. Appl. Polym. Sci., 2009, 114(4), 2435–2444, DOI:10.1002/app.30782.
- C. Zhu, Z. Zhang, Q. Liu, Z. Wang and J. Jin, Synthesis and biodegradation of aliphatic polyesters from dicarboxylic acids and diols, J. Polym. Sci., 2003, 90(4), 982–990, DOI:10.1002/app.12722.
- J. Kántor, G. Fekete and A. L. Gergely, Oil sorption properties of centrifugally spun polyisobutylene-based thermoplastic elastomer microfibers, Polymers, 2024, 16(18), 2624, DOI:10.3390/polym16182624.
- L. Pinchuk, The use of polyisobutylene-based polymers in ophthalmology, Bioact. Mater., 2022, 10, 185–194, DOI:10.1016/j.bioactmat.2021.09.005.
- V. Volkis, H. Mei, R. K. Shoemaker and J. Michl, LiCB11(CH3)12-catalyzed radical polymerization of isobutylene: highly branched polyisobutylene and an isobutylene−ethyl acrylate copolymer, J. Am. Chem. Soc., 2009, 131(9), 3132–3133, DOI:10.1021/ja807297g.
- S. C. Hong, T. Pakula and K. Matyjaszewski, Preparation of polyisobutene-graft-poly(methyl methacrylate) and polyisobutene-graft-polystyrene with different compositions and side chain architectures through atom transfer radical polymerization (ATRP), Macromol. Chem. Phys., 2001, 202(17), 3392–3402, DOI:10.1002/1521-3935(20011101)202:17<3392::AID-MACP3392>3.0.CO;2-4.
- Y.-H. Fu, S. T. Madrahimov and D. E. Bergbreiter, Block copolymers derived from polyisobutylene oligomers, J. Polym. Sci., Part A:Polym. Chem., 2018, 56(16), 1860–1867, DOI:10.1002/pola.29069.
- J. Iyer, I. Saraf, A. Ray, M. Brunsteiner and A. Paudel, Assessment of diverse solid-state accelerated autoxidation methods for droperidol, Pharmaceutics, 2022, 14(6), 1114, DOI:10.3390/pharmaceutics14061114.
- Y. Shen, W. Zhu, M. Papadaki, M. S. Mannan, C. V. Mashuga and Z. Cheng, Thermal decomposition of solid benzoyl peroxide using advanced reactive system screening tool: Effect of concentration, confinement and selected acids and bases, J. Loss Prev. Process Ind., 2019, 60, 28–34, DOI:10.1016/j.jlp.2019.04.001.
- K. Sun, Y. Zhang, D. Zhu, X. Peng, J. Zhang, T. Gong, M. Ma and P. Xiao, Visible-light photopolymerization activated by nanocarbon materials as photocatalysts, J. Photochem. Photobiol., C, 2023, 57, 100637, DOI:10.1016/j.jphotochemrev.2023.100637.
- L. A. Camacho-Cruz, M. A. Velazco-Medel and E. Bucio, Aqueous polymerizations, inGreen Sustainable Process for Chemical and Environmental Engineering and Science, ed. Inamuddin, Boddula, R. and Asiri, A. M., Elsevier, Amsterdam, Netherlands, 2020, ch. 9, pp. 275–318 DOI:10.1016/B978-0-12-819542-0.00009-9.
- G. R. Jones, H. S. Wang, K. Parkatzidis, R. Whitfield, N. P. Truong and A. Anastasaki, Reversed controlled polymerization (RCP): Depolymerization from well-defined polymers to monomers, J. Am. Chem. Soc., 2023, 145(18), 9898–9915, DOI:10.1021/jacs.3c00589.
- J. B. Alves, M. K. Vasconcelos, L. H. R. Mangia, M. Tatagiba, J. Fidalgo, D. Campos, P. L. Invernici, M. V. Rebouças, M. H. S. Andrade and J. C. Pinto, A bibliometric survey on polyisobutylene manufacture, Processes, 2021, 9(8), 1315, DOI:10.3390/pr9081315.
- P. De and R. Faust, Relative reactivity of C4 olefins toward the polyisobutylene cation, Macromolecules, 2006, 39(20), 6861–6870, DOI:10.1021/ma0611725.
- B. E. Obi, Polymer chemistry and synthesis, in Polymeric Foams Structure-Property-Performance, ed. Obi, B. E., William Andrew Publishing, Norwich, US, 2018, ch. 2, pp. 17–40 DOI:10.1016/B978-1-4557-7755-6.00002-1.
- I. Capek, Solution radical polymerization, in Nanocomposite Structures and Dispersions, ed. Capek, I., Elsevier, Amsterdam, Netherlands, 2nd edn, 2019, ch. 2, pp. 95–174 DOI:10.1016/B978-0-444-63748-2.00002-X.
- D. Hero and G. Kali, Free- and reversible deactivation radical (co)polymerization of isobutylene in water under environmentally benign conditions, Eur. Polym. J., 2021, 147, 110336, DOI:10.1016/j.eurpolymj.2021.110336.
- J. Qiu, B. Charleux and K. Matyjaszewski, Controlled/living radical polymerization in aqueous media: homogeneous and heterogeneous systems, Prog. Polym. Sci., 2001, 26(10), 2083–2134, DOI:10.1016/S0079-6700(01)00033-8.
- Y.-N. Zhou, J.-J. Li, T.-T. Wang, Y.-Y. Wu and Z.-H. Luo, Precision polymer synthesis by controlled radical polymerization: Fusing the progress from polymer chemistry and reaction engineering, Prog. Polym. Sci., 2022, 130, 101555, DOI:10.1016/j.progpolymsci.2022.101555.
- M. Chen, M. Zhong and J. A. Johnson, Light-controlled radical polymerization: Mechanisms, methods, and applications, Chem. Rev., 2016, 116(17), 10167–10211, DOI:10.1021/acs.chemrev.5b00671.
- H. Nakatani, T. Ichizyu, H. Miura and M. Terano, Preparation of modified polybutene-1 by oxidation and limonene radical grafting using an Nd2O3-assisted radical initiator system and its characterization, Polym. Int., 2010, 59(12), 1673–1682, DOI:10.1002/pi.2902.
- Y. He and Y. Lu, Living cationic polymerization of isobutylene in seconds based on microflow system, Eur. Polym. J., 2022, 174, 111335, DOI:10.1016/j.eurpolymj.2022.111335.
- Q. Huang, P. He, J. Wang and Y. Wu, Synthesis of high molecular weight polyisobutylene via cationic polymerization at elevated temperatures, Chin. J. Polym. Sci., 2013, 31(8), 1139–1147, DOI:10.1007/s10118-013-1304-x.
- C. Zhang, Y. Wu, X. Meng, Q. Huang, G. Wu and R. Xu, Synthesis of polyisobutylene with sec-arylamino terminal group by combination of cationic polymerization with alkylation, Chin. J. Polym. Sci., 2009, 27(4), 551–559, DOI:10.1142/S0256767909004230.
- I. E. Nifant’ev, S. A. Korchagina, M. S. Chinova and A. N. Tavtorkin, Polyisobutylenes with controlled molecular weight and chain-end structure: Synthesis and actual applications, Polymers, 2023, 15, 3415, DOI:10.3390/polym15163415.
- T. Rajasekhar, G. Singh, G. S. Kapur and S. S. V. Ramakumar, Recent advances in catalytic chain transfer polymerization of isobutylene: a review, RSC Adv., 2020, 10, 18180–18191, 10.1039/D0RA01945C.
- Y. Zhu, Z. Yu, R. Liu, Y. Jin, Q. Shi and Y. Wu, Recent advances in green cationic polymerization, J. Polym. Sci., 2024, 62(11), 2549–2573, DOI:10.1002/pol.20230971.
- P.-F. Yan, A.-R. Guo, Q. Liu and Y.-X. Wu, Living cationic polymerization of isobutylene coinitiated by FeCl3 in the presence of isopropanol, J. Polym. Sci. Part A: Polymer Chemistry, 2012, 50(16), 3383–3392, DOI:10.1002/pola.26126.
- S. V. Kostjuk, H. Y. Yeong and B. Voit, Cationic polymerization of isobutylene at room temperature, J. Polym. Sci., Part A:Polym. Chem., 2013, 51(3), 471–486, DOI:10.1002/pola.26423.
- B. Yang and R. F. Storey, Synthesis and characterization of polyisobutylene telechelic prepolymers with epoxide functionality, React. Funct. Polym., 2020, 150, 104563, DOI:10.1016/j.reactfunctpolym.2020.104563.
- S. Hakim, M. Nekomanesh and H. Honarkar, Synthesis of isotactic polybutene-1 with Ziegler-Natta catalyst, Iran. J. Polym. Sci. Technol., 2022, 35(2), 163–174, DOI:10.22063/jipst.2022.3152.2150.
- B. Jiang, H. Shao, H. Nie and A. He, Sequential two-stage polymerization to synthesis isotactic polypropylene/isotactic polybutene-1 alloys: compositions, morphologies and granule growing mechanism, Polym. Chem., 2015, 6, 3315–3323, 10.1039/C5PY00205B.
- C. Liu, F. Wang, Y. Kang, X. Mao, L. Pan, Z. Ma and Y. Li, Preparation of polybutene-based thermoplastic elastomers through the copolymerization of 1-butene with higher α-olefins, Polym. Chem., 2024, 15(13), 1331–1338, 10.1039/d4py00062e.
- Z. I. Kahkeshi, M. N. Haghighi, N. Bahri-Laleh and S. Sadjadi, Effect of support type on the characteristics of polybutene polymers from C4 monomers employing supported ionic liquid/AlCl3 initiating systems, J. Mol. Struct., 2024, 1308, 138111, DOI:10.1016/j.molstruc.2024.138111.
- Q. G. Huang, Y. P. Sheng, K. X. Deng, L. F. Ma, Y. X. Wu and W. T. Yang, The effect of polymerization conditions on crystalline of polybutene-1 catalyzed by metallocene catalyst, Chin. Chem. Lett., 2007, 18(2), 217–220, DOI:10.1016/j.cclet.2006.12.030.
- J. L. D. de Tuesta, E. Casas, J. Moreno, P. P. Nebreda, J. M. Escola and R. Van Grieken, Synthesis and characterization of low molecular weight poly(1-butene) macromolecules prepared using metallocene catalysts, Appl. Catal., A, 2013, 460–461, 70–77, DOI:10.1016/j.apcata.2013.04.020.
- A. Klaue, M. Kruck, N. Friederichs, F. Bertola, H. Wu and M. Morbidelli, Insight into the synthesis process of an industrial Ziegler−Natta catalyst, Ind. Eng. Chem. Res., 2019, 58(2), 886–896, DOI:10.1021/acs.iecr.8b05296.
- E. R. Engel and J. L. Scott, Advances in the green chemistry of coordination polymer materials, Green Chem., 2020, 22, 3693–3715, 10.1039/D0GC01074J.
- K. Vellingiri, P. Kumar and K. H. Kim, Coordination polymers: Challenges and future scenarios for capture and degradation of volatile organic compounds, Nano Res., 2016, 9, 3181–3208, DOI:10.1007/s12274-016-1230-7.
- L. Resconi, I. Camurati and F. Malizia, Metallocene catalysts for 1-butene polymerization, Macromol. Chem. Phys., 2006, 207(24), 2257–2279, DOI:10.1002/macp.200600307.
- W. Zheng, A. He, C. Liu, H. Shao and R. Wang, The influences of alkylaluminium as cocatalyst on butene-1 polymerization with MgCl2-supported TiCl4 Ziegler-Natta catalysts, Polymer, 2020, 210, 122998, DOI:10.1016/j.polymer.2020.122998.
- O. D’Anania, C. De Rosa and G. Talarico, Mechanistic insights on 1-butene polymerization catalyzed by homogeneous single-site catalysts: a DFT computational study, Front. Chem., 2024, 12, 1377740, DOI:10.3389/fchem.2024.1377740.
- J.-F. Chen, H. Gao, Y.-X. Wu, H.-K. Zou, G.-W. Chu and L. Zhang, ( 2009), Method for synthesis of butyl rubber, US20090286948A1, [online] Available at: https://patents.google.com/patent/US20090286948A1/en, [Accessed: 15 March 2025].
- H. T. Kim, S. Lee, W.-Y. Jeon, M.-J. Jang, S.-H. Seo, H.-J. Lee, J. Jeong, J. Park, H. Park, B. D. Pardhe, K. T. Heo, Y. S. Kim, Y. Jeong, S. J. Yum, Y.-H. Yang, H. Lee, J.-J. Yoon, J. Park, S.-H. Choi and J. Ahn, Sustainable butyl rubber production from microbial isobutanol-derived isobutylene, ACS Sustainable Chem. Eng., 2025, 13(6), 2275–2282, DOI:10.1021/acssuschemeng.4c05992.
- X. Yan, M. Du, J. Li, Y. Xue, Y. Wu, H. Zhang, X. Wang and D. Xu, A theoretical study of the mechanism of cationic polymerization of isobutylene catalysed by EtAlCl2/t-BuCl with bis(2-chloroethyl)ether in hexanes, Phys. Chem. Chem. Phys., 2024, 26, 6763–6773, 10.1039/D3CP05337G.
- K. Cao, S. Elliott, S. A. Sirohey, N. Durrell and G. Davidson, Fast, efficient, catalyst-free epoxidation of butyl rubber using oxone/acetone for improved filler dispersion, ACS Omega, 2024, 9, 19601–19612 CrossRef CAS PubMed.
- F. E. Guckert, C. Sayer, D. de Oliveira, P. H. H. de Araújo and B. F. Oechsler, Synthesis of polybutylene succinate via lipase-catalyzed transesterification: Enzyme stability, reuse and PBS properties in bulk polycondensations, Eur. Polym. J., 2022, 179, 111573, DOI:10.1016/j.eurpolymj.2022.111573.
- A. Abdullin, T. Magsumov, A. Kusova, A. Sokolov, T. Mukhametzyanov and I. Sedov, Influence of cross-link density on the non-isothermal crystallization kinetics of polybutylene terephthalate, Thermochimica Acta, 2024, 732, 179672, DOI:10.1016/j.tca.2024.179672.
- J. Mi, Y. Li, W. Wang, Z. Li, T. Liu, G. Nie and Z. Zhang, Synthesis and characterization of erythritol-modified poly(butylene adipate-co-terephthalate), Mater. Today Commun., 2025, 42, 111318, DOI:10.1016/j.mtcomm.2024.111318.
- T. Zhou, G. Liu, M. Wang, Y. Liu and L. Zheng, Synthesis and properties of polybutylene (succinate)-b-poly(dimethylsiloxane) with unprecedented combined performance and functions, Biomacromolecules, 2024, 24(12), 5951–5963, DOI:10.1021/acs.biomac.3c00956.
- N. Ittobane, A. M. de Ilarduya, A. Alla and S. Muñoz-Guerra, Synthesis and characterization of poly(butylene terephthalate) copolyesters derived from threitol, Polym. Polym. Compos., 2021, 29(9), S817–S825, DOI:10.1177/09673911211023298.
- P. J. Darda, J. A. Hogendoorn, G. F. Versteeg and F. Souren, Reaction kinetics of polybutylene terephthalate polycondensation reaction, AIChE J., 2005, 51(2), 622–630, DOI:10.1002/aic.10360.
- P. J. Darda, K. Hogendoorn, T. Molenkamp and G. Versteeg, Determination of polybutylene terephthalate polycondensation equilibrium constant using a batch reactor, Macromol. Symp., 2004, 206(1), 275–290, DOI:10.1002/masy.200450222.
- V. V. Antić and M. V. Pergal, Poly(butylene terephthalate)—synthesis, properties, application, in Handbook of Engineering and Speciality Thermoplastics: Polyethers and Polyesters, ed. Thomas, S. and Visakh P. M., Scrivener Publishing, Beverly, US, 2011, ch. 5, pp. 127–180 DOI:10.1002/9781118104729.ch5.
- H.-I. Mao, C.-W. Chen and S.-P. Rwei, Synthesis and nonisothermal crystallization kinetics of poly(butylene terephthalate-co-tetramethylene ether glycol) copolyesters, Polymers, 2020, 12, 1897, DOI:10.3390/polym12091897.
- M. I. Makarevich, P. A. Nikishau, I. A. Berezianko, T. V. Glushkova, M. A. Rezvova, E. A. Ovcharenko, G. E. Bekmukhamedov, D. G. Yakhvarov and S. V. Kostjuk, Aspects of the synthesis of poly(styrene-block-isobutylene-block-styrene) by TiCl4-Co-initiated cationic polymerization in open conditions, Macromol, 2021, 1, 243–255, DOI:10.3390/macromol1040017.
- B. D. Ratner, Polymeric implants, in Polymer Science: A Comprehensive Reference, ed. Matyjaszewski, K. and Möller, M., Elsevier, Amsterdam, Netherlands, 2012, ch. 9.21, pp. 397–411 DOI:10.1016/B978-0-444-53349-4.00230-2.
- R. F. Storey and B. J. Chisholm, Aspects of the synthesis of poly(styrene-b-isobutylene-b-styrene) block copolymers using living carbocationic polymerization, Macromolecules, 1993, 26(25), 6727–6733, DOI:10.1021/ma00077a006.
- H. T. Zhang, Z.-T. Wei, F. Zhang, T. Yang and Y.-X. Wu, Nanocrystallization-locked network of poly(styrene-b-isobutylene-b-styrene)-g-polytetrahydrofuran block graft copolymer, Chin. J. Polym. Sci., 2021, 39, 874–886, DOI:10.1007/s10118-021-2536-9.
- M. A. Rezvova, E. A. Ovcharenko, P. A. Nikishev, S. V. Kostyuk, T. V. Glushkova, D. V. Trebushat, V. S. Chernonosova, G. Y. Shevelev, K. Y. Klyshnikov, Y. A. Kudryavtseva and L. S. Barabash, Prospects for using styrene-isobutylene-styrene (SIBS) triblock copolymer as a cusp material for leaflet heart valve prostheses: Evaluation of physicochemical and mechanical properties, Russ. J. Appl. Chem., 2019, 92, 9–19, DOI:10.1134/S1070427219010026.
- N. Bahri-Laleh, Interaction of different poisons with MgCl2/TiCl4 based Ziegler-Natta catalysts, Appl. Surf. Sci., 2016, 379, 395–401, DOI:10.1016/j.apsusc.2016.04.034.
- A. Vaughan, D. A. Davis and J. R. Hagadorn, Industrial Catalysts for alkene polymerization, in Polymer Science: A Comprehensive Reference, ed. Matyjaszewski, K. and Möller, M., Elsevier, Amsterdam, Netherlands, ch. 3.20, 2012, pp. 657–672 DOI:10.1016/B978-0-444-53349-4.00080-7.
- Z. Behrooznia and J. Nourmohammadi, Polysaccharide-based materials as an eco-friendly alternative in biomedical, environmental, and food packaging, Giant, 2024, 19, 100301, DOI:10.1016/j.giant.2024.100301.
- A. Samir, F. H. Ashour, A. A. A. Hakim and M. Bassyouni, Recent advances in biodegradable polymers for sustainable applications, npj Mater. Degrad., 2022, 6, 68, DOI:10.1038/s41529-022-00277-7.
- A. Das, T. Ringu, S. Ghosh and N. Pramanik, A comprehensive review on recent advances in preparation, physicochemical characterization, and bioengineering applications of biopolymers, Polym. Bull., 2023, 80, 7247–7312, DOI:10.1007/s00289-022-04443-4.
- N. Patra, P. Ramesh, V. Donthu and A. Ahmad, Biopolymer-based composites for sustainable energy storage: recent developments and future outlook, J. Mater. Sci.: Mater. Eng., 2024, 19, 34, DOI:10.1186/s40712-024-00181-9.
- A. Gamage, P. Thiviya, A. Liyanapathiranage, M. L. D. Wasana, Y. Jayakodi, A. Bandara, A. Manamperi, R. S. Dassanayake, P. Evon, O. Merah and T. Madhujith, Polysaccharide-based bioplastics: Eco-friendly and sustainable solutions for packaging, J. Compos. Sci., 2024, 8, 413, DOI:10.3390/jcs8100413.
- J. Baranwal, B. Barse, A. Fais, G. L. Delogu and A. Kumar, Biopolymer: A sustainable material for food and medical applications, Polymers, 2022, 14(5), 983, DOI:10.3390/polym14050983.
- S. Sudheer, S. Bandyopadhyay and R. Bhat, Sustainable polysaccharide and protein hydrogel-based packaging materials for food products: A review, Int. J. Biol. Macromol., 2023, 248, 125845, DOI:10.1016/j.ijbiomac.2023.125845.
- P. Gururani, P. Bhatnagar, P. Dogra, H. C. Joshi, P. K. Chauhan, M. S. Vlaskin, N. C. Joshi, A. Kurbatova, A. Irina and V. Kumar, Bio-based food packaging materials: A sustainable and Holistic approach for cleaner environment- a review, Curr. Res. Green Sustainable Chem., 2023, 7, 100384, DOI:10.1016/j.crgsc.2023.100384.
- G. H. Matar and M. Andac, Recent advances in sustainable biopolymer films incorporating vanillin for enhanced food preservation and packaging, Polym. Bull., 2025, 82, 2751–2777, DOI:10.1007/s00289-025-05661-2.
- N. R. Chodankar, S. V. Karekar, M. Safarkhani, A. M. Patil, P. A. Shinde, R. B. Ambade, J.-K. Kim, Y.-K. Han, Y.-S. Huh, A. Al Ghaferi and E. Alhajri, Revolutionizing implantable technology: Biocompatible supercapacitors as the future of power sources, Adv. Funct. Mater., 2024, 34(42), 2406819, DOI:10.1002/adfm.202406819.
- Y. Bao, L. Shao, G. Xing and C. Qi, Cobalt, nickel and iron embedded chitosan microparticles as efficient and reusable catalysts for Heck cross-coupling reactions, Int. J. Biol. Macromol., 2019, 130, 203–212, DOI:10.1016/j.ijbiomac.2019.02.143.
- M. S. Akhtar, M. T. Naseem, S. Ali and W. Zaman, Metal-based catalysts in biomass transformation: From plant feedstocks to renewable fuels and chemicals, Catalysts, 2025, 15(1), 40, DOI:10.3390/catal15010040.
- N. A. Kamaruzaman, W. M. K. W. M. Zin, K. H. Kamarudin, N. Md Saleh and F. Yusoff, Recent advances in transition metals- based materials as electrocatalysts for water splitting, Int. J. Electrochem. Sci., 2023, 18(7), 100187, DOI:10.1016/j.ijoes.2023.100187.
- N. Kaplaneris and L. Ackermann, Earth-abundant 3d transition metals on the rise in catalysis, Beilstein J. Org. Chem., 2022, 18, 86–88, DOI:10.3762/bjoc.18.8.
- Z. I. Kahkeshi, N. Bahri-Laleh, S. Sadjadi and M. N. Haghighi, An environmentally benign approach for the synthesis of low molar mass polybutenes from mixed C4 monomers using AlCl3/ionic-liquid initiating systems, Mol. Catal., 2023, 547, 113332, DOI:10.1016/j.mcat.2023.113332.
- X. Zhang, M. Zhang, S. Liu and Z. Li, Synthesis of amido-quinoline Hafnium and Zirconium complexes with improved performances toward ethylene/1-octene copolymerization, Organometallics, 2024, 43(20), 2472–2479, DOI:10.1021/acs.organomet.4c00037.
- R. N. Ewuzie, J. R. Genza and A. Z. Abdullah, Review of the application of bimetallic catalysts coupled with internal hydrogen donor for catalytic hydrogenolysis of lignin to produce phenolic fine chemicals, Int. J. Biol. Macromol., 2024, 265(2), 131084, DOI:10.1016/j.ijbiomac.2024.131084.
- Q. Zhang, C. Hu and X. Pang, Multinuclear catalyst: An efficient tool for the synthesis of polyesters and polycarbonates by ring-opening polymerization, Fundam. Res., 2023 DOI:10.1016/j.fmre.2023.06.016.
- M. R. Radlauer and T. Agapie, Bimetallic Zirconium amine bis(phenolate) polymerization catalysts: Enhanced activity and tacticity control for polyolefin synthesis, Organometallics, 2014, 33(13), 3247–3250, DOI:10.1021/om500608j.
- S. Xie, S. Qian, K. Zhu, L. Sun, W. Chen and S. Chen, Comparison of eco-friendly Ti–M bimetallic coordination catalysts and commercial monometallic Sb- or Ti-based catalysts for the synthesis of poly(ethylene-co-isosorbide terephthalate), ACS Omega, 2023, 8(22), 19237–19248, DOI:10.1021/acsomega.2c07831.
- V. Pascanu, G. G. Miera, A. K. Inge and B. Martín-Matute, Metal–organic frameworks as catalysts for organic synthesis: A Critical Perspective, J. Am. Chem. Soc., 2019, 141(18), 7223–7234, DOI:10.1021/jacs.9b00733.
- J. García-Serna, R. Piñero-Hernanz and D. Durán-Martín, Inspirational perspectives and principles on the use of catalysts to create sustainability, Catal. Today, 2022, 387, 237–243, DOI:10.1016/j.cattod.2021.11.021.
- Q. Yang, R. Hou and K. Sun, Tuning butene selectivities by Cu modification on Pd-based catalyst for the selective hydrogenation of 1,3-butadiene, J. Catal., 2019, 374, 12–23, DOI:10.1016/j.jcat.2019.04.018.
- P. Fu, J. Huo, J. Li, C. De Rosa and S. Jiang, Form II to form I transition in solution-crystallized isotactic polybutene-1, Soft Matter, 2024, 20, 3191–3202, 10.1039/D4SM00152D.
- B. Heurtefeu, C. Bouilhac, E. Cloutet, D. Taton, A. Deffieux and H. Cramail, Polymer support of “single-site” catalysts for heterogeneous olefin polymerization, Prog. Polym. Sci., 2011, 36(1), 89–126, DOI:10.1016/j.progpolymsci.2010.09.002.
- H. Mazhar, F. Shehzad, S.-G. Hong and M. A. Al-Harthi, Enhancing metallocene catalyst activity: Utilizing layered double hydroxide for ethylene–propylene copolymerization, Macromol. Mater. Eng., 2024, 309(1), 2300245, DOI:10.1002/mame.202300245.
- A. Ali, M. Nadeem, A. Naveed, J. M. Moradian, S. N. Haider, S. Khan, A. M. Bhayo, J. Lu, R. N. Ali, N. Ahmad, F. Zhiqiang and L. Guo, Copolymerization of ethylene and isoprene initiated by metallocene catalyst, Arabian J. Chem., 2024, 17(11), 105989, DOI:10.1016/j.arabjc.2024.105989.
- A. Ali, A. Naveed, A. Maroń, M. A. Younis, J. M. Moradian, B. Yousaf, T. Aziz, R. N. Ali, N. Ahmad, S. Y. Alomar, F. Zheqiang and L. Guo, Copolymerization of ethylene and isoprene via silicon bridge metallocene [rac-Me2Si(2-Me-4-Ph-Ind)2ZrCl2] catalyst: A new way to control the composition and microstructure of copolymers, Chemosphere, 2024, 347, 140700, DOI:10.1016/j.chemosphere.2023.140700.
- Y. Wang, Y. Qin and J.-Y. Dong, Trouble-free combination of ω-alkenylmethyldichlorosilane copolymerization-hydrolysis chemistry and metallocene catalyst system for highly effective and efficient direct synthesis of long-chain-branched polypropylene, Polymer, 2022, 259, 125327, DOI:10.1016/j.polymer.2022.125327.
- C. Ehm, A. Mingione, A. Vittoria, F. Zaccaria, R. Cipullo and V. Busico, High-throughput experimentation in olefin polymerization catalysis: Facing the challenges of miniaturization, Ind. Eng. Chem. Res., 2020, 59(31), 13940–13947, DOI:10.1021/acs.iecr.0c02549.
- P. V. Kovyazin, L. M. Khalilov and L. V. Parfenova, Enantiomerically pure ansa-η5-complexes of transition metals as an effective tool for chirality transfer, Molecules, 2025, 30(12), 2511, DOI:10.3390/molecules30122511.
- L. V. Parfenova, P. V. Kovyazin, A. K. Bikmeeva, E. R. Palatov, P. V. Ivchenko, I. E. Nifant’ev and L. M. Khalilov, Catalytic Properties of Zirconocene-Based Systems in 1-Hexene Oligomerization and Structure of Metal Hydride Reaction Centers, Molecules, 2023, 28(6), 2420, DOI:10.3390/molecules28062420.
- X. Duan, P. Yu, Y. Yu, L. Ma, Y. Xia, A. Moreno, L. Chen, R. Cong, C. Liu and Z. Zhou, Rapid and quantitative 1D 13C NMR analysis of polypropylene tacticity with relaxation agent and proton polarization transfer, Magn. Reson. Lett., 2025, 200198, DOI:10.1016/j.mrl.2025.200198.
- W. Posch, Polyolefins: Plastics Engineering Encyclopedia—Elsevier, in Plastics Design Library, Applied Plastics Engineering Handbook, ed. Myer Kutz, William Andrew Publishing, Norwich, US, 3rd edn, 2024, ch. 2, pp. 9–56 DOI:10.1016/B978-0-323-88667-3.00012-6.
- M. Matsko and V. Zakharov, Heterogeneity of active sites in the polymer chain transfer reactions at olefin polymerization over multisite supported Ziegler-Natta catalysts, Polymers, 2023, 15(21), 4316, DOI:10.3390/polym15214316.
- A. Ali, J. M. Moradian, A. Naveed, S. Najeeb-Uz-Zaman Haider, W. A. Qureshi, S. Khan, H. Naz, Y. Guo, J. Lu, M. Ahmed, F. hiqiang and L. Guo, To overcome the unconventional polymerization behavior of single-site symmetrical metallocene catalyst in the presence of borate, Appl. Catal., A, 2024, 683, 119830, DOI:10.1016/j.apcata.2024.119830.
- Y. Feng, P. Guo, W. Ding, Y. Jin and Y. Wu, Cationic copolymerization of isobutylene and bio-renewable β-myrcene towards sustainable elastomers: synthesis and mechanism, J. Polym. Res., 2025, 32, 80, DOI:10.1007/s10965-025-04306-2.
- F. Zaccaria, A. Vittoria, G. Antinucci, R. Cipullo and V. Busico, α-Olefin oligomerization mediated by group 4 metallocene catalysts: An extreme manifestation of the multisite nature of methylaluminoxane, Polymers, 2025, 17(1), 46, DOI:10.3390/polym17010046.
- N. Kasmi, L. Papadopoulos, Y. Chebbi, G. Z. Papageorgiou and D. N. Bikiaris, Effective and facile solvent-free synthesis route to novel biobased monomers from vanillic acid: Structure–thermal property relationships of sustainable polyesters, Polym. Degrad. Stab., 2020, 181, 109315, DOI:10.1016/j.polymdegradstab.2020.109315.
- U. A. Weerasinghe, T. Wu, P. L. Chee, P. Y. M. Yew, H. K. Lee, X. J. Loh and K. Dan, Deep eutectic solvents towards green polymeric materials, Green Chem., 2024, 26, 8497–8527, 10.1039/D4GC00532E.
- B. Joseph, S. Krishnan, S. V. Kavil, A. R. Pai, J. James, N. Kalarikkal and S. Thomas, Green chemistry approach for fabrication of polymer composites, Sustainable Chem., 2021, 2, 254–270, DOI:10.3390/suschem2020015.
- L. Niedner and G. Kali, Green engineered polymers: Solvent free, room-temperature polymerization of monomer from a renewable resource, without utilizing initiator, ChemistrySelect, 2019, 4(12), 3495–3499, DOI:10.1002/slct.201900930.
- I. Zaborniak, M. Klamut, C. M. Warne, K. Kisiel, M. Niemiec, P. Błoniarz, A. Pellis, K. Matyjaszewski and P. Chmielarz, Controlled polymer synthesis toward green chemistry: deep insights into atom transfer radical polymerization in biobased substitutes for polar aprotic solvents, ACS Sustainable Chem. Eng., 2024, 12(12), 4933–4945, DOI:10.1021/acssuschemeng.3c07993.
- W. Li, Z. Gong, K. Wu, L. Zhao and D. Hu, Effect of crystalline transformation on supercritical CO2 foaming and cell morphology of isotactic polybutene-1, J. CO2 Util., 2023, 74, 102546, DOI:10.1016/j.jcou.2023.102546.
- L. Li, T. Liu, L. Zhao and W. Yuan, CO2-induced phase transition from form II to I in isotactic poly-1-butene, Macromolecules, 2009, 42(6), 2286–2290, DOI:10.1021/ma8025496.
- L. Li, T. Liu, L. Zhao and W. Yuan, CO2-induced phase transition of isotactic poly-1-butene with form III upon heating, Macromolecules, 2011, 44(12), 4836–4844, DOI:10.1021/ma200988y.
- L. Li, T. Liu and L. Zhao, Direct fabrication of porous isotactic poly-1-butene with form I from the melt using CO2, Macromol. Rapid Commun., 2011, 32, 1834–1838, DOI:10.1002/marc.201100462.
- D. Hu, W. Li, K. Wu, L. Cui, Z. Xu and L. Zhao, Utilization of supercritical CO2 for controlling the crystal phase transition and cell morphology of isotactic polybutene-1 foams, J. CO2 Util., 2022, 66, 102265, DOI:10.1016/j.jcou.2022.102265.
- J. Shi, P. Wu, L. Li, T. Liu and L. Zhao, Crystalline transformation of isotactic polybutene-1 in supercritical CO2 studied by in-situ Fourier transform infrared spectroscopy, Polymer, 2009, 50(23), 5598–5604, DOI:10.1016/j.polymer.2009.09.078.
- L. Li, T. Liu and L. Zhao, Direct melt-crystallization of isotactic poly-1-butene with form I′ using high-pressure CO2, Polymer, 2011, 52(24), 5659–5668, DOI:10.1016/j.polymer.2011.10.011.
- D. Hu, K. Xue, Z. Liu, Z. Xu and L. Zhao, The essential role of PBS on PBAT foaming under supercritical CO2 toward green engineering, J. CO2 Util., 2022, 60, 101965, DOI:10.1016/j.jcou.2022.101965.
- B. Li, G. Zhao, G. Wang, L. Zhang, J. Gong and Z. Shi, Biodegradable PLA/PBS open-cell foam fabricated by supercritical CO2 foaming for selective oil-adsorption, Sep. Purif. Technol., 2021, 257, 117949, DOI:10.1016/j.seppur.2020.117949.
- M. H. Reis, F. A. Leibfarth and L. M. Pitet, Polymerizations in continuous flow: Recent advances in the synthesis of diverse polymeric materials, ACS Macro Lett., 2020, 9(1), 123–133, DOI:10.1021/acsmacrolett.9b00933.
- N. Zaquen, M. Rubens, N. Corrigan, J. Xu, P. B. Zetterlund, C. Boyer and T. Junkers, Polymer synthesis in continuous flow reactors, Prog. Polym. Sci., 2020, 107, 101256, DOI:10.1016/j.progpolymsci.2020.101256.
- J. H. Vrijsen, C. O. Medeiros, J. Gruber and T. Junkers, Continuous flow synthesis of core cross-linked star polymers via photo-induced copper mediated polymerization, Polym. Chem., 2019, 10, 1591–1598, 10.1039/C9PY00134D.
- S. Zhu, Y. Lu, K. Wang and G. Luo, Flow synthesis of medium molecular weight polyisobutylene coinitiated by AlCl3, Eur. Polym. J., 2016, 80, 219–226, DOI:10.1016/j.eurpolymj.2016.01.034.
- D. Rackl, P. Kreitmeier and O. Reiser, Synthesis of a polyisobutylene-tagged fac-Ir(ppy)3 complex and its application as recyclable visible-light photocatalyst in a continuous flow process, Green Chem., 2016, 18, 214–219, 10.1039/C5GC01792K.
- N. P. Truong, G. R. Jones, K. G. E. Bradford, D. Konkolewicz and A. Anastasaki, A comparison of RAFT and ATRP methods for controlled radical polymerization, Nat. Rev. Chem., 2021, 5, 859–869, DOI:10.1038/s41570-021-00328-8.
- D. Sun, Y. Li, C. Yang, Y. Su, Y. Yamada and S. Sato, Production of 1,3-butadiene from biomass-derived C4 alcohols, Fuel Process. Technol., 2020, 197, 106193, DOI:10.1016/j.fuproc.2019.106193.
- M. Martín and I. E. Grossmann, Optimal simultaneous production of i-butene and ethanol from switchgrass, Biomass Bioenergy, 2014, 61, 93–103, DOI:10.1016/j.biombioe.2013.11.022.
- T. A. Ewing, N. Nouse, M. van Lint, J. van Haveren, J. Hugenholtz and D. S. van Es, Fermentation for the production of biobased chemicals in a circular economy: a perspective for the period 2022–2050, Green Chem., 2022, 24, 6373–6405, 10.1039/D1GC04758B.
- B. Choe, S. Lee and W. Won, Coproduction of butene oligomers and adipic acid from lignocellulosic biomass: Process design and evaluation, Energy, 2021, 235, 121278, DOI:10.1016/j.energy.2021.121278.
- P. Mandree, G. A. Thopil and S. Ramchuran, Potential opportunities to convert waste to bio-based chemicals at an industrial scale in South Africa, Fermentation, 2023, 9(10), 908, DOI:10.3390/fermentation9100908.
- R. El-Araby, Biofuel production: exploring renewable energy solutions for a greener future, Biotechnol. Biofuels Bioprod., 2024, 17, 129, DOI:10.1186/s13068-024-02571-9.
- M. Ismail, A. Abouhmad, N. Warlin, S.-H. Pyo, O. E. Örn, B. Al-Rudainy, C. Tullberg, B. Zhang and R. Hatti-Kaul, Closing the loop for poly(butylene-adipate-co-terephthalate) recycling: depolymerization, monomers separation, and upcycling, Green Chem., 2024, 26, 3863–3873, 10.1039/D3GC04728H.
- A. A. Rashid, S. A. Khan and M. Koç, Life cycle assessment on fabrication and characterization techniques for additively manufactured polymers and polymer composites, Cleaner Environ. Syst., 2024, 12, 100159, DOI:10.1016/j.cesys.2023.100159.
- P. Ramesh and S. Vinodh, State of art review on Life Cycle Assessment of polymers, Int. J. Sustain. Eng., 2020, 13(6), 411–422, DOI:10.1080/19397038.2020.1802623.
- W. Kaminsky, Metallocene based polyolefin nanocomposites, Materials, 2014, 7, 1995–2013, DOI:10.3390/ma7031995.
- K. Schröder, K. Matyjaszewski, K. J. T. Noonan and R. T. Mathers, Towards sustainable polymer chemistry with homogeneous metal-based catalysts, Green Chem., 2014, 16, 1673–1686, 10.1039/C3GC42159G.
- W. L. da Silva, M. A. Lansarin, F. C. Stedile and J. H. Z. dos Santos, The potential of chemical industrial and academic wastes as a source of supported photocatalysts, J. Mol. Catal. A:Chem., 2014, 393, 125–133, DOI:10.1016/j.molcata.2014.05.040.
- A. Shamiri, M. H. Chakrabarti, S. Jahan, M. A. Hussain, W. Kaminsky, P. V. Aravind and W. A. Yehye, The influence of Ziegler-Natta and metallocene catalysts on polyolefin structure, properties, and processing ability, Materials, 2014, 7, 5069–5108, DOI:10.3390/ma7075069.
- H.-F. Joaquin and L. Juan, Quantification of poisons for Ziegler Natta catalysts and effects on the production of polypropylene by gas chromatographic with simultaneous detection: Pulsed discharge helium ionization, mass spectrometry and flame ionization, J. Chromatogr. A, 2020, 1614, 460736, DOI:10.1016/j.chroma.2019.460736.
- D. C. Pernusch, G. Spiegel, C. Paulik and W. Hofer, Influence of poisons originating from chemically recycled plastic waste on the performance of Ziegler–Natta catalysts, Macromol. React. Eng., 2022, 16(1), 2100020, DOI:10.1002/mren.202100020.
- C. Chen, M. I. Shekh, S. Cui and F. J. Stadler, Rheological behavior of blends of metallocene catalyzed long-chain branched polyethylenes. Part I: Shear rheological and thermorheological behavior, Polymers, 2021, 13(3), 328, DOI:10.3390/polym13030328.
- M. Ansari, S. G. Hatzikiriakos, A. M. Sukhadia and D. C. Rohlfing, Rheology of Ziegler–Natta and metallocene high-density polyethylenes: broad molecular weight distribution effects, Rheologica Acta, 2011, 50, 17–27, DOI:10.1007/s00397-010-0503-4.
- E. Kokko, A. Malmberg, P. Lehmus, B. Löfgren and J. V. Seppälä, Influence of the catalyst and polymerization conditions on the long-chain branching of metallocene-catalyzed polyethenes, J. Polym. Sci., Part A:Polym. Chem., 2000, 38(2), 376–388, DOI:10.1002/(SICI)1099-0518(20000115)38:2<376::AID-POLA12>3.0.CO;2-5.
- W. Kaminsky, Production of polyolefins by metallocene catalysts and their recycling by pyrolysis, Macromol. Symp., 2016, 360(1), 10–22, DOI:10.1002/masy.201500127.
- O. R. de Ballesteros, F. Auriemma, R. Di Girolamo, A. Malafronte, M. Scoti and C. De Rosa, Mechanical properties of isotactic 1-butene-ethylene copolymers from Ziegler-Natta catalyst, Polymer, 2021, 216, 123408, DOI:10.1016/j.polymer.2021.123408.
- Z. Liu, S. Chen and J. Zhang, Preparation and characterization of ethylene–butene copolymer (EBC)/mica composites, J. Polym. Res., 2011, 18, 2403–2413, DOI:10.1007/s10965-011-9654-y.
- J. C. Falk and R. J. Schlott, Synthesis and properties of ethylene–butene-1 block copolymers, Macromolecules, 1971, 4(2), 152–154, DOI:10.1021/ma60020a004.
- R. Li, G. Yang and L. Liu, Characterization of ethylene–butene polyethylene copolymers by DSC and SSA, IOP Conf. Ser.:Mater. Sci. Eng., 2020, 774, 012026, DOI:10.1088/1757-899X/774/1/012026.
- M. Rahaman, M. A. Parvez, J. B. P. Soares and I. A. Hussein, Effect of polymerization conditions on thermal and mechanical properties of ethylene/1-butene copolymer made with Ziegler-Natta catalysts, Int. J. Polym. Sci., 2014, 654260, 1–10, DOI:10.1155/2014/654260.
- V. K. Soni, R. Rani and G. Singh, Advancing Ziegler-Natta catalysis with internal donors, Eur. Polym. J., 2025, 226, 113741, DOI:10.1016/j.eurpolymj.2025.113741.
- D. Liu, Z. Qi, Y. Zhang, J. Xu and B. Guo, Poly(butylene succinate) (PBS)/ionic liquid plasticized starch blends: Preparation, characterization, and properties, Starch/Staerke, 2015, 67(9–10), 802–809, DOI:10.1002/star.201500060.
- S. Roy, T. Ghosh, W. Zhang and J.-W. Rhim, Recent progress in PBAT-based films and food packaging applications: A mini-review, Food Chem., 2024, 437(1), 137822, DOI:10.1016/j.foodchem.2023.137822.
- Y. K. Han, S. R. Kim and J. Kim, Preparation and characterization of high molecular weight poly(butylene succinate), Macromol. Res., 2002, 10, 108–114, DOI:10.1007/BF03218299.
- S. Şen, M. I. Cengiz and E. Tekay, Thermal, mechanical, electrical properties of poystyrene/poly (styrene-b-isobutylene-b-styrene/carbon nanotube nanocomposites, J. Elastomers Plast., 2024, 56(4), 311–329, DOI:10.1177/00952443241236915.
- E. Manaila, A. Airinei, M. D. Stelescu, M. Sonmez, L. Alexandrescu, G. Craciun, D. Pamfil, N. Fifere, C.-D. Varganici, F. Doroftei and A. Bele, Radiation processing and characterization of some ethylene-propylene-diene terpolymer/butyl (halobutyl) rubber/nanosilica composites, Polymers, 2020, 12, 2431, DOI:10.3390/polym12102431.
- Z. Ahmad, M. K. Abdullah, M. Z. Ali and M. A. Md Zawawi, Electronic packaging and thermal management, in Polymers in Electronics, ed. Ahmad, Z., Abdullah, M. K., Ali, M. Z. and Md Zawawi, M. A., Elsevier, Amsterdam, Netherlands, 2023, ch. 6, pp. 389–427 DOI:10.1016/B978-0-323-98382-2.00002-8.
- C. Abeykoon, P. Pérez and A. L. Kelly, The effect of materials’ rheology on process energy consumption and melt thermal quality in polymer extrusion, Polym. Eng. Sci., 2020, 60(6), 1244–1265, DOI:10.1002/pen.25377.
- L. Doyle, Extrusion foaming behavior of polybutene-1. Toward single-material multifunctional sandwich structures, J. Appl. Polym. Sci., 2022, 139(12), 51816, DOI:10.1002/app.51816.
- J. Ping, G. Ma and Z. Ma, Crystallization behavior of isotactic polybutene blended with polyethylene, Molecules, 2022, 27, 2448, DOI:10.3390/molecules27082448.
- Y. Liu, Y. Wang, Q. Yu, L. Ren, Y. Wu, Z. Wang, Q. Li and B. S. Hsiao, Crystal structural evolution of polybutene-1 in solid state upon deformation and stress relaxation, Polymer, 2021, 226, 123833, DOI:10.1016/j.polymer.2021.123833.
- W. Wang, B. Wang, A. Tercjak, A. J. Müller, Z. Ma and D. Cavallo, Origin of transcrystallinity and nucleation kinetics in polybutene-1/fiber composites, Macromolecules, 2020, 53(20), 8940–8950, DOI:10.1021/acs.macromol.0c02038.
- M. Kaszonyiova, F. Rybnikar and P. H. Geil, Crystallization and transformation of polybutene-1, J. Macromol. Sci., Part B:Phys., 2004, 43(5), 1095–1114, DOI:10.1081/MB-200033322.
- L. Andena, M. Rink, R. Frassine and R. Corrieri, A fracture mechanics approach for the prediction of the failure time of polybutene pipes, Eng. Fract. Mech., 2009, 76(18), 2666–2677, DOI:10.1016/j.engfracmech.2009.10.002.
- Y. Zhao, C. Liu, H. Shao and A. He, High-temperature creep behaviors of polybutene-1 with different chain microstructure and molecular weight, Polym. Test., 2023, 124, 108084, DOI:10.1016/j.polymertesting.2023.108084.
- X. Zhang, K. Zhu, J. Li, Q. Ding and S. Jiang, Tensile yield behavior of isotactic polybutene-1, Mater. Today Commun., 2024, 39, 109024, DOI:10.1016/j.mtcomm.2024.109024.
- J. Li, Y. Qiao, H. Zhang, Y. Zheng, Z. Tang, Z. Zeng, P. Yao, F. Bao, H. Liu, J. Yu, C. Zhu and J. Xu, Microstructure and tensile properties of melt-spun filaments of polybutene-1 and butene-1/ethylene copolymer, Polymers, 2023, 15, 3729, DOI:10.3390/polym15183729.
- Q. Liu, Y.-X. Wu, Y. Zhang, P.-F. Yan and R.-W. Xu, A cost-effective process for highly reactive polyisobutylenes via cationic polymerization coinitiated by AlCl3, Polymer, 2010, 51(25), 5960–5969, DOI:10.1016/j.polymer.2010.10.012.
- H. Zheng, Y. Zhu, B. Zhao, Y. Lin, W. Lan, W. Shi and B. Wei, Temperature endurable face-sealing polyisobutylene film with contactable liquid desiccant for the encapsulation of OLEDs, Mater. Today Commun., 2024, 41, 110345, DOI:10.1016/j.mtcomm.2024.110345.
- J. Heine, U. Rodehorst, J. P. Badillo, M. Winter and P. Bieker, Chemical stability investigations of polyisobutylene as new binder for application in lithium air-batteries, Electrochim. Acta, 2015, 155, 110–115, DOI:10.1016/j.electacta.2015.01.001.
- S. K. Schickhardt, G. Łabuz, D. J. Munro, I. Lieberwirth, L. Zhang, H. Fang and G. U. Auffarth, In-vitro assessment of a novel intraocular lens made of crosslinked polyisobutylene, J. Mech. Behav. Biomed. Mater., 2024, 152, 106368, DOI:10.1016/j.jmbbm.2023.106368.
- P. Cadieux, J. D. Watterson, J. Denstedt, R. R. Harbottle, J. Puskas, J. Howard, B. S. Gan and G. Reid, Potential application of polyisobutylene-polystyrene and a Lactobacillus protein to reduce the risk of device-associated urinary tract infections, Colloids Surf., B, 2003, 28(2–3), 95–105, DOI:10.1016/S0927-7765(02)00147-9.
- A. V. Kostyuk, N. M. Smirnova and S. O. Ilyin, Two-functional phase-change pressure-sensitive adhesives based on polyisobutylene matrix filled with paraffin wax, J. Energy Storage, 2022, 52, 104797, DOI:10.1016/j.est.2022.104797.
- D. Ding, C. Liu, Y. Zhang, W. Xu, Y. Cai, T. Zhong and L. Fang, Mechanistic insights of different release behaviors dominated by drug physicochemical properties in polyisobutylene pressure sensitive adhesive, Int. J. Pharm., 2023, 630, 122416, DOI:10.1016/j.ijpharm.2022.122416.
- J. E. Puskas, E. Krisch and K. Molnar, Thermoplastic elastomers based on polyisobutylene, in Advances in Thermoplastic Elastomers, ed. Singha, N. K. and Jana, S. C., Elsevier, Amsterdam, Netherlands, 2024, ch. 9, pp. 243–274 DOI:10.1016/B978-0-323-91758-2.00015-5.
- P. P. Patil, Y. Gori, A. Kumar and M. R. Tyagi, Experimental analysis of tribological properties of polyisobutylene thickened oil in lubricated contacts, Tribol. Int., 2021, 159, 106983, DOI:10.1016/j.triboint.2021.106983.
- C. M. Small, G. M. McNally, A. Marks and W. R. Murphy, The effect of extrusion processing conditions and polyisobutylene concentration on the properties of polyethylene for stretch and cling film applications, J. Plast. Film Sheeting, 2002, 18(4), 245–258, DOI:10.1177/8756087902034639.
- W. Wang, H. Zou, G. Chu, Y. Xiang, H. Peng and J. Chen, Effects of assistant solvents and mixing intensity on the bromination process of butyl rubber, Chin. J. Chem. Eng., 2014, 22(4), 398–404, DOI:10.1016/S1004-9541(14)60052-5.
- K. K. Kar, N. L. Ravikumar, P. B. Tailor, J. Ramkumar and D. Sathiyamoorthy, Performance evaluation and rheological characterization of newly developed butyl rubber based media for abrasive flow machining process, J. Mater. Process. Technol., 2009, 209(4), 2212–2221, DOI:10.1016/j.jmatprotec.2008.05.012.
- S. Liu, X. Mao, Q. Qu, F. Li, J. Mao, J. Chen, H. Ma and H. Wang, Effect of antioxidant structure on bromobutyl rubber composites' processability and age resistance, React. Funct. Polym., 2024, 205, 106082, DOI:10.1016/j.reactfunctpolym.2024.106082.
- S. Yang, H. Wu, Y. Xiong and S. Guo, In-situ constructing hybrid cross-linked networks in brominated butyl rubber via amphiphilic graphene oxide cross-linkers: Retaining excellent gas barrier and mechanical properties after fatigue, Composites, Part B, 2024, 272, 111224, DOI:10.1016/j.compositesb.2024.111224.
- E. Su, G. Bayazit, S. Ide and O. Okay, Butyl rubber-based interpenetrating polymer networks with side chain crystallinity: Self-healing and shape-memory polymers with tunable thermal and mechanical properties, Eur. Polym. J., 2022, 168, 111098, DOI:10.1016/j.eurpolymj.2022.111098.
- M. Fittipaldi, L. A. Rodriguez, A. Damley-Strnad and L. R. Grace, Improving tensile strength of an injection-molded biocompatible thermoplastic elastomer, Mater. Des., 2015, 86, 6–13, DOI:10.1016/j.matdes.2015.07.070.
- N. Shen, S. Liu, P. Kasbe, F. Khabaz, J. P. Kennedy and W. Xu, Macromolecular engineering and additive manufacturing of poly(styrene-b-isobutylene-b-styrene), ACS Appl. Polym. Mater., 2021, 3(9), 4554–4562, DOI:10.1021/acsapm.1c00616.
- N. Ewurum and A. G. McDonald, Lignin reinforcement in polybutylene succinate copolymers, Polymers, 2025, 17, 194, DOI:10.3390/polym17020194.
- B. Palai, S. Mohanty and S. K. Nayak, Synergistic effect of polylactic acid (PLA) and Poly(butylene succinate-co-adipate) (PBSA) based sustainable, reactive, super toughened eco-composite blown films for flexible packaging applications, Polym. Test., 2020, 83, 106130, DOI:10.1016/j.polymertesting.2019.106130.
- Y. Zhang, M. Zhu, Z. Huang, F. Yang, Y. Weng and C. Zhang, The effect of polylactic acid-based blend films modified with various biodegradable polymers on the preservation of strawberries, Food Packag. Shelf Life, 2024, 45, 101333, DOI:10.1016/j.fpsl.2024.101333.
- I. S. Choi, Y. K. Kim, S. H. Hong, H.-J. Seo, S.-H. Hwang, J. Kim and S. K. Lim, Effects of polybutylene succinate content on the rheological properties of polylactic acid/polybutylene succinate blends and the characteristics of their fibers, Materials, 2024, 17, 662, DOI:10.3390/ma17030662.
- G. Hussain, M. Hassan, H. Wei, J. Buhl, M. Xiao, A. Iqbal, H. Qayyum, A. A. Riaz, R. Muhammad and K. Ostrikov, Advances on incremental forming of composite materials, Alexandria Eng. J., 2023, 79, 308–336, DOI:10.1016/j.aej.2023.07.045.
- P. C. Thapliyal, Utilization of chemical additives to enhance biodegradability of plastics, in Biodegradability of Conventional Plastics, ed. Sarkar, A., Sharma, B. and Shekhar, S., Elsevier, Amsterdam, Netherlands, 2023, ch. 13, pp. 259–281 DOI:10.1016/B978-0-323-89858-4.00006-3.
- D. Barczikai, J. Domokos, D. Szabó, K. Molnar, D. Juriga, E. Krisch, K. S. Nagy, L. Kohidai, C. A. Helfer, A. Jedlovszky-Hajdu and J. E. Puskas, Polyisobutylene—new opportunities for medical applications, Molecules, 2021, 26, 5207, DOI:10.3390/molecules26175207.
- M. Kashif, H. Li, J. Liu, S. Rasul, Q. Ullah and Y. Liu, Competitive behavior of isotactic polybutene-1 polymorphs in electrospun membranes and solution cast films via cold crystallization, J. Macromol. Sci., Part B:Phys., 2022, 61(7–8), 897–913, DOI:10.1080/00222348.2022.2116920.
- B. Wang, F.-h Lin, Y.-y Zhao, X.-y Li, Y.-c Liu, J.-b Li, X.-J. Han, S.-x Liu, X.-r Ji, J. Luo and Y.-h Wei, Isotactic polybutene-1/bamboo powder composites with excellent properties at initial stage of molding, Polymers, 2019, 11, 1981, DOI:10.3390/polym11121981.
- A. Smejda-Krzewicka, E. Irzmańska, K. Mrozowski, A. Adamus-Włodarczyk, N. Litwicka, K. Strzelec and M. I. Szynkowska-Jóźwik, The new elastomeric compounds made of butyl rubber filled with phyllosilicates, characterized by increased barrier properties and hydrophobicity and reduced chemical degradation, Molecules, 2024, 29, 1306, DOI:10.3390/molecules29061306.
- A. Lisovskii, E. Nelkenbaum, V. Volkis, R. Semiat and M. S. Eisen, Polymerization of isobutylene and copolymerization of isobutylene with isoprene promoted by methylalumoxane, Inorg. Chim. Acta, 2002, 334, 243–252, DOI:10.1016/S0020-1693(02)00799-5.
- C. M. Small, G. M. Mcnally, W. R. Murphy and A. Marks, The manufacture and performance of polyethylene-polyisobutylene films for cling applications, Dev. Chem. Eng. Miner. Process., 2008, 11(1–2), 169–184, DOI:10.1002/apj.5500110217.
- R. Krishnamoorti, W. W. Graessley, L. J. Fetters, R. T. Garner and D. J. Lohse, Anomalous mixing behavior of polyisobutylene with other polyolefins, Macromolecules, 1995, 28(4), 1252–1259, DOI:10.1021/ma00108a064.
- P. Szabó, E. Epacher, E. Földes and B. Pukánszky, Miscibility, structure and properties of PP/PIB blends, Mater. Sci. Eng. A, 2004, 383(2), 307–315, DOI:10.1016/j.msea.2004.04.035.
- Z. C. Lule and J. Kim, Properties of economical and eco-friendly polybutylene adipate terephthalate composites loaded with surface treated coffee husk, Composites, Part A, 2021, 140, 106154, DOI:10.1016/j.compositesa.2020.106154.
- G. Zhang, J. Peng, H. Wang, Y. Lu and Y. Zhang, Curing and reinforcement effect of recovered carbon black from waste tires on brominated butyl rubber, Compos. Sci. Technol., 2024, 258, 110879, DOI:10.1016/j.compscitech.2024.110879.
- L. Li, J. Zhang, J. O. Jo, S. Datta and J. K. Kim, Effects of variation of oil and zinc oxide type on the gas barrier and mechanical properties of chlorobutyl rubber/epoxidised natural rubber blends, Mater. Des., 2013, 49, 922–928, DOI:10.1016/j.matdes.2013.02.057.
- T. Lei, Y.-W. Zhang, D.-L. Kuang and Y.-R. Yang, Preparation and properties of rubber blends for high-damping-isolation bearings, Polymers, 2019, 11, 1374, DOI:10.3390/polym11081374.
- S. Gopisathi, C. Park, Y. I. Huh, J. Jeon, C. H. Yun, J. Won, K.-U. Jeong and C. Nah, Enhancing the reversion resistance, crosslinking density and thermo-mechanical properties of accelerated sulfur cured chlorobutyl rubber using 4,4′-bis (maleimido) diphenyl methane, Rubber Chem. Technol., 2019, 92(1), 110–128, DOI:10.5254/rct.18.82605.
- N. T.-H. Pham and V.-T. Nguyen, Morphological and mechanical properties of poly (butylene terephthalate)/high-density polyethylene blends, Adv. Mater. Sci. Eng., 2020, 8890551, DOI:10.1155/2020/8890551.
- J. X. Chan, J. F. Wong, A. Hassan, N. Othman, J. A. Razak, U. Nirmal, S. Hashim, Y. C. Ching, M. Z. Yunos and T. M. S. U. Gunathilake, Enhanced tribological and mechanical properties of polybutylene terephthalate nanocomposites reinforced with synthetic wollastonite nanofibers/graphene oxide hybrid nanofillers, Diamond Relat. Mater., 2023, 135, 109835, DOI:10.1016/j.diamond.2023.109835.
- J. X. Chan, J. F. Wong, A. Hassan, N. Othman, J. A. Razak, U. Nirmal, S. Hashim, Y. C. Ching, M. Z. Yunos, R. Yahaya and T. M. S. U. Gunathilake, Synthetic wollastonite nanofiber for polybutylene terephthalate nanocomposite: Mechanical, thermal, tribological and flammability properties, Polymer, 2022, 256, 125259, DOI:10.1016/j.polymer.2022.125259.
- J. Jian, Z. Xiangbin and H. Xianbo, An overview on synthesis, properties and applications of poly(butylene-adipate-co-terephthalate)–PBAT, Adv. Ind. Eng. Polym. Res., 2020, 3(1), 19–26, DOI:10.1016/j.aiepr.2020.01.001.
- J. Zhang, D. Hu, S. Wei, Z. Xi, W. Zhen and L. Zhao, Effects of chain composition of PBAT on the supercritical CO2 foaming and degradation behavior, J. CO2 Util., 2023, 72, 102500, DOI:10.1016/j.jcou.2023.102500.
- T. Burford, W. Rieg and S. Madbouly, Biodegradable poly(butylene adipate-co-terephthalate) (PBAT, Phys. Sci. Rev., 2023, 8(8), 1127–1156, DOI:10.1515/psr-2020-0078.
- C. Scolaro, S. Brahimi, A. Falcone, V. Beghetto and A. Visco, Mechanical and physical changes in bio-polybutylene-succinate induced by UVC ray photodegradation, Polymers, 2024, 16, 1288, DOI:10.3390/polym16091288.
- R. D. K. Misra, H. Nathani, A. Dasari, S. D. Wanjale and J. P. Jog, The determining role of clay particles on mechanically induced surface damage and associated stress whitening in polybutene–clay nanocomposites, Mater. Sci. Eng., A, 2004, 386(1–2), 175–185, DOI:10.1016/j.msea.2004.07.010.
- J. E. Puskas, P. Antony, M. E. Fray and V. Altstädt, The effect of hard and soft segment composition and molecular architecture on the morphology and mechanical properties of polystyrene–polyisobutylene thermoplastic elastomeric block copolymers, Eur. Polym. J., 2003, 39(10), 2041–2049, DOI:10.1016/S0014-3057(03)00130-7.
- S. Bagrodia, M. R. Tant, G. L. Wilkes and J. P. Kennedy, Sulphonated polyisobutylene telechelic ionomers: 12. Solid-state mechanical properties, Polymer, 1987, 28(13), 2207–2226, DOI:10.1016/0032-3861(87)90377-6.
- Y. Du, C. Li, J. Jin, C. Li and W. Jiang, Surface modification of polyisobutylene via grafting amino acid-based poly (acryloyl-6-aminocaproic acid) as multifunctional material, Colloids Surf., B, 2018, 161, 73–82, DOI:10.1016/j.colsurfb.2017.10.035.
- Y.-B. Wu, K. Li, D. Xiang, M. Zhang, D. Yang, J.-H. Zhang, J. Mao, H. Wang and W.-L. Guo, Surface immobilization of heparin on functional polyisobutylene-based thermoplastic elastomer as a potential artificial vascular graft, Appl. Surf. Sci., 2018, 445, 8–15, DOI:10.1016/j.apsusc.2018.03.048.
- M. L. Di Lorenzo, R. Androsch and I. Stolte, Tailoring the rigid amorphous fraction of isotactic polybutene-1 by ethylene chain defects, Polymer, 2014, 55(23), 6132–6139, DOI:10.1016/j.polymer.2014.09.040.
- D. Suleiman, A. M. Padovani, A. A. Negrón, J. M. Sloan, E. Napadensky and D. M. Crawford, Mechanical and chemical properties of poly(styrene-isobutylene-styrene) block copolymers: Effect of sulfonation and counter ion substitution, J. Appl. Polym. Sci., 2014, 40344, 1–8, DOI:10.1002/app.40344.
- T. Kwee, S. J. Taylor, K. A. Mauritz and R. F. Storey, Morphology and mechanical and dynamic mechanical properties of linear and star poly(styrene-b-isobutylene-b-styrene) block copolymers, Polymer, 2005, 46(12), 4480–4491, DOI:10.1016/j.polymer.2005.02.031.
- L. W. McKeen, Polyolefins, in In Plastics Design Library, Film Properties of Plastics and Elastomers, ed. McKeen, L. W., William Andrew Publishing, Norwich, US, 3rd edn, 2012, ch. 9, pp. 189–218 DOI:10.1016/B978-1-4557-2551-9.00009-8.
- M. F. Ashby, Material profiles, in Materials and the Environment, ed. Ashby, M. F., Butterworth-Heinemann, Oxford, UK, 2nd edn, 2013, ch. 15, pp. 459–595 DOI:10.1016/B978-0-12-385971-6.00015-4.
- Z. Sheng, S. Yang, J. Wang, Y. Lu, K. Tang and S. Song, Preparation and properties analysis of chlorinated butyl rubber (CIIR)/organic diatomite damping composites, Materials, 2018, 11, 2172, DOI:10.3390/ma11112172.
- M. A. A. Saidi, F. S. Mazlan, A. Hassan, R. Abd Rashid and A. R. Rahmat, Flammability, thermal and mechanical properties of polybutylene terephthalate/dolomite composites, J. Phys. Sci., 2019, 30(3), 175–189, DOI:10.21315/jps2019.30.3.11.
- M. Vandesteene, N. Jacquel, R. Saint-Loup, N. Boucard, C. Carrot, A. Rousseau and F. Fenouillot, Synthesis of branched poly(butylene succinate): Structure properties relationship, Chin. J. Polym. Sci., 2016, 34, 873–888, DOI:10.1007/s10118-016-1805-5.
- Z. Huang, L. Qian, Q. Yin, N. Yu, T. Liu and D. Tian, Biodegradability studies of poly(butylene succinate) composites filled with sugarcane rind fiber, Polym. Test., 2018, 66, 319–326, DOI:10.1016/j.polymertesting.2018.02.003.
- R. F. Storey, B. J. Chisholm and M. A. Masse, Morphology and physical properties of poly(styrene-b-isobutylene-b-styrene) block copolymers, Polymer, 1996, 37(14), 2925–2938, DOI:10.1016/0032-3861(96)89388-8.
- S. Coiai, M. L. Di Lorenzo, P. Cinelli, M. C. Righetti and E. Passaglia, Binary green blends of poly(lactic acid) with poly(butylene adipate-co-butylene terephthalate) and poly(butylene succinate-co-butylene adipate) and their nanocomposites, Polymers, 2021, 13(15), 2489, DOI:10.3390/polym13152489.
- R. Herrera, L. Franco, A. Rodríguez-Galán and J. Puiggalí, Characterization and degradation behavior of poly(butylene adipate-co-terephthalate)s, J. Polym. Sci., Part A:Polym. Chem., 2002, 40(23), 4141–4157, DOI:10.1002/pola.10501.
- K. Buaksuntear, P. Limarun, S. Suethao and W. Smitthipong, Non-covalent interaction on the self-healing of mechanical properties in supramolecular polymers, Int. J. Mol. Sci., 2022, 23, 6902, DOI:10.3390/ijms23136902.
- M. Wohlert, T. Benselfelt, L. Wågberg, I. Furó, L. A. Berglund and J. Wohlert, Cellulose and the role of hydrogen bonds: not in charge of everything, Cellulose, 2022, 29, 1–23, DOI:10.1007/s10570-021-04325-4.
- B. Gayretli, R. Shanthar, T. T. Öpöz and C. Abeykoon, Mechanical properties of LDPE and PS polymer matrix composites reinforced with GNP and CF—A critical review, Int. J. Lightweight Mater. Manuf., 2024, 7(4), 572–596, DOI:10.1016/j.ijlmm.2024.03.005.
- Z. Eslami, S. Elkoun, M. Robert and K. Adjallé, A review of the effect of plasticizers on the physical and mechanical properties of alginate-based films, Molecules, 2023, 28, 6637, DOI:10.3390/molecules28186637.
- Q. Dong, N. Li, X. Wang, Z. Fu, J. Xu and Z. Fan, Regulating the structure of ethylene-propylene copolymer for polyolefin in-reactor alloy with improved properties, in Studies in Surface Science and Catalysis, ed. Shiono, T., Nomura, K. and Terano, M., Elsevier, Amsterdam, Netherlands, 2006, ch. 5, vol. 161, pp. 25–30 DOI:10.1016/S0167-2991(06)80429-5.
- Y. Yu, L. Sang, Z. Wei, X. Leng and Y. Li, Unique isodimorphism and isomorphism behaviors of even-odd poly(hexamethylene dicarboxylate) aliphatic copolyesters, Polymer, 2017, 115, 106–117, DOI:10.1016/j.polymer.2017.03.034.
- H. Feng, X. Lu, W. Wang, N.-G. Kang and J. W. Mays, Block copolymers: Synthesis, self-assembly, and applications, Polymers, 2017, 9, 494, DOI:10.3390/polym9100494.
- A. Dziemidkiewicz, M. Maciejewska and M. Pingot, Thermal analysis of halogenated rubber cured with a new cross-linking system, J. Therm. Anal. Calorim., 2019, 138, 4395–4405, DOI:10.1007/s10973-019-08881-7.
- M. Shiva, M. K. Dallakeh, M. Ahmadi and M. Lakhi, Effects of silicon carbide as a heat conductive filler in butyl rubber for bladder tire curing applications, Mater. Today Commun., 2021, 29, 102773, DOI:10.1016/j.mtcomm.2021.102773.
- M. A. Rezvova, A. E. Yuzhalin, T. V. Glushkova, M. I. Makarevich, P. A. Nikishau, S. V. Kostjuk, K. Y. Klyshnikov, V. G. Matveeva, M. Y. Khanova and E. A. Ovcharenko, Biocompatible nanocomposites based on poly(styrene-block-isobutylene-block-styrene) and carbon nanotubes for biomedical application, Polymers, 2020, 12, 2158, DOI:10.3390/polym12092158.
- W. Phetwarotai, M. Zawong, N. Phusunti and D. Aht-Ong, Toughening and thermal characteristics of plasticized polylactide and poly(butylene adipate-co-terephthalate) blend films: Influence of compatibilization, Int. J. Biol. Macromol., 2021, 183, 346–357, DOI:10.1016/j.ijbiomac.2021.04.172.
- R. Li, X. Zhang, Y. Hou, R. Wang and J. Han, A type of low-temperature resistant material based on ethylene propylene monomer/polypropylene thermoplastic vulcanizates compatibilized by polyisobutylene, J. Appl. Polym. Sci., 2025, 142(15), e56734, DOI:10.1002/app.56734.
- J. Y. Boey, C. K. Lee and G. S. Tay, Factors affecting mechanical properties of reinforced bioplastics: A review, Polymers, 2022, 14, 3737, DOI:10.3390/polym14183737.
- J. P. Greene, Bio-based and biodegradable plastics, in In Plastics Design Library, Automotive Plastics and Composites, ed. Greene, J. P., William Andrew Publishing, Norwich, US, 2021, ch. 10, pp. 149–174 DOI:10.1016/B978-0-12-818008-2.00020-9.
- E. Rudnik, Compostable polymer properties and packaging applications, in In Plastics Design Library, Plastic Films in Food Packaging, ed. Ebnesajjad, S., William Andrew Publishing, Norwich, US, 2013, ch. 13, pp. 217–248 DOI:10.1016/B978-1-4557-3112-1.00013-2.
- L. Jiang and J. Zhang, Biodegradable and biobased polymers, in In Plastics Design Library, Applied Plastics Engineering Handbook, ed. Kutz, M., William Andrew Publishing, Norwich, US, 2nd edn, 2017, ch. 7, pp. 127–143 DOI:10.1016/B978-0-323-39040-8.00007-9.
- M. Ramesh and M. Muthukrishnan, Biodegradable polymer blends and composites for food-packaging applications, in Woodhead Publishing Series in Composites Science and Engineering, Biodegradable Polymers, Blends and Composites, ed. Rangappa, S. M., Parameswaranpillai, J., Siengchin, S. and Ramesh, M., Woodhead Publishing, Sawston, UK, 2022, ch. 25, pp. 693–716 DOI:10.1016/B978-0-12-823791-5.00004-1.
- V. R. Sastri, Materials used in medical devices, in In Plastics Design Library, Plastics in Medical Devices, ed. Sastri, V. R., William Andrew Publishing, Norwich, US, 2010, ch. 3, pp. 21–32 DOI:10.1016/B978-0-8155-2027-6.10003-0.
- N. Kantor-Malujdy, S. Skowron, B. Michalkiewicz and M. E. Fray, Poly(butylene-succinate)-based blends with enhanced oxygen permeability, Mater. Today Commun., 2022, 33, 104306, DOI:10.1016/j.mtcomm.2022.104306.
- Y. Tokiwa, B. P. Calabia, C. U. Ugwu and S. Aiba, Biodegradability of plastics, Int. J. Mol. Sci., 2009, 10, 3722–3742, DOI:10.3390/ijms10093722.
- T. P. Gumede, K. Shingange, P. Mbule and B. Motloung, Miscibility effect of biodegradable aliphatic poly(butylene succinate)/aromatic polycarbonate blends, Polym. Renewable Resour., 2022, 13(1–2), 28–43, DOI:10.1177/20412479221109912.
- Z. C. Lule, E. Wondu and J. Kim, Compatibilized bio-based polybutylene-succinate blended composites filled with surface modified Si3N4 for improved rigidity and thermal performance, Mater. Today Commun., 2023, 35, 106033, DOI:10.1016/j.mtcomm.2023.106033.
- B. E. Itabana, A. K. Mohanty, P. Dick, M. Sain, A. Bali, M. Tiessen, L.-T. Lim and M. Misra, Poly (butylene adipate-co-terephthalate) (PBAT)–based biocomposites: A comprehensive review, Macromol. Mater. Eng., 2024, 309(12), 2400179, DOI:10.1002/mame.202400179.
- K. V. Sukhareva, N. R. Sukharev, I. I. Levina, P. O. Offor and A. A. Popov, Solvent swelling-induced halogenation of butyl rubber using polychlorinated N-alkanes: Structure and properties, Polymers, 2023, 15, 4137, DOI:10.3390/polym15204137.
- N. Mandlekar, M. Joshi and B. S. Butola, A review on specialty elastomers based potential inflatable structures and applications, Adv. Ind. Eng. Polym. Res., 2022, 5(1), 33–45, DOI:10.1016/j.aiepr.2021.05.004.277.
- S. Ebnesajjad, Surface preparation of thermoplastics, thermosets, and elastomers, in Surface Treatment of Materials for Adhesive Bonding, ed. Ebnesajjad, S., William Andrew Publishing, Norwich, US, 2nd edn, 2014, ch. 8, pp. 185–226 DOI:10.1016/B978-0-323-26435-8.00008-3.
- J. S. Parent, D. J. Thom, G. White, R. A. Whitney and W. Hopkins, Thermal stability of brominated poly(isobutylene-co-isoprene), J. Polym. Sci., Part A:Polym. Chem., 2001, 39(12), 2019–2026, DOI:10.1002/pola.1177.
- M. A. Rezvova, P. A. Nikishau, M. I. Makarevich, T. V. Glushkova, K. Y. Klyshnikov, T. N. Akentieva, O. S. Efimova, A. P. Nikitin, V. Y. Malysheva, V. G. Matveeva, E. A. Senokosova, M. Y. Khanova, V. V. Danilov, D. M. Russakov, Z. R. Ismagilov, S. V. Kostjuk and E. A. Ovcharenko, Biomaterials based on carbon nanotube nanocomposites of poly(styrene-b-isobutylene-b-styrene): The effect of nanotube content on the mechanical properties, biocompatibility and hemocompatibility, Nanomaterials, 2022, 12(5), 733, DOI:10.3390/nano12050733.
- P. Kulkarni, U. Ojha, X. Wei, N. Gurung, K. Seethamraju and R. Faust, Thermal and mechanical properties of polyisobutylene-based thermoplastic polyurethanes, J. Appl. Polym. Sci., 2013, 130(2), 891–897, DOI:10.1002/app.39236.
- W. Zhang, Y. Zang, Y. Lu, W. Lin, S. Zhao and J. Xiong, Thermal decomposition of brominated butyl rubber, Materials, 2021, 14, 6767, DOI:10.3390/ma14226767.
- M. A. A. Saidi, A. Hassan, M. U. Wahit and L. J. Choy ( 2018) Mechanical and thermal properties of polyethylene terephthalate/polybutylene terephthalate blends. 7th International Graduate Conference On Engineering, Science and Humanities. At: Faculty of Built Environment and Surveying, Universiti Teknologi Malaysia, Johor Bahru, Malaysia. pp. 1–7.
- M. Szostak, Mechanical and thermal properties of PET/PBT blends, Mol. Cryst. Liq. Cryst., 2004, 416(1), 209–215, DOI:10.1080/15421400490481377.
- R. P. D’Amelia and B. Khanyan, An experimental review: Evaluation of the Flory-Fox equation for the relationship of glass transition temperature (Tg) vs molar mass of polystyrene using differential scanning calorimetry (DSC), J. Polym. Biopolym. Phys. Chem., 2022, 10(1), 10–17, DOI:10.12691/jpbpc-10-1-2.
- S. Pásztor, B. Becsei, G. Szarka, Y. Thomann, R. Thomann, R. Mühlhaupt and B. Iván, The scissors effect in action: The Fox-Flory relationship between the glass transition temperature of crosslinked poly(methyl methacrylate) and Mc in nanophase separated poly(methyl methacrylate)-l-polyisobutylene conetworks, Materials, 2020, 13(21), 4822, DOI:10.3390/ma13214822.
- P. G. Cortes, R. Araya-Hermosilla, K. Wrighton-Araneda, D. Cortés-Arriagada, F. Picchioni, F. Yan, P. Rudolf, R. K. Bose and F. Quero, Effect of intermolecular interactions on the glass transition temperature of chemically modified alternating polyketones, Mater. Today Chem., 2023, 34, 101771, DOI:10.1016/j.mtchem.2023.101771.
- C. Chandrasekaran, Rubbers mostly used in process equipment lining, in In Plastics Design Library, Anticorrosive Rubber Lining, ed. Chandrasekaran, C., William Andrew Publishing, Norwich, US, 2017, ch. 12, pp. 87–101 DOI:10.1016/B978-0-323-44371-5.00012-8.
- J. Charles and S. Muthusamy, Comparative study of butyl rubber (IIR) and bromobutyl rubber (BIIR) based on FTIR, dielectric and thermal studies, J. Appl. Sci. Eng. Methodol., 2016, 2(1), 206–211 Search PubMed.
- T. Qu, G. Nan, Y. Ouyang, B. Bieketuerxun, X. Yan, Y. Qi and Y. Zhang, Structure–property relationship, glass transition, and crystallization behaviors of conjugated polymers, Polymers, 2023, 15(21), 4268, DOI:10.3390/polym15214268.
- J. N. Fowler, B. R. Chapman and D. L. Green, Impact of plasticizers and tackifiers on the crystallization of isotactic poly(1-butene, Eur. Polym. J., 2010, 46(3), 568–577, DOI:10.1016/j.eurpolymj.2009.11.013.
- R. K. Raman, S. A. G. Thangavelu, S. Venkataraj and A. Krishnamoorthy, Materials, methods and strategies for encapsulation of perovskite solar cells: From past to present, Renewable Sustainable Energy Rev., 2021, 151, 111608, DOI:10.1016/j.rser.2021.111608.
- S. Su, R. Kopitzky, S. Tolga and S. Kabasci, Polylactide (PLA) and its blends with poly(butylene succinate) (PBS): A brief review, Polymers, 2019, 11(7), 1193, DOI:10.3390/polym11071193.
- Y. Y. Jiang, L. Ren, G. H. Wu, W. Guo, X. F. Guan, M. Y. Zhang and H. X. Zhang, An environmentally sustainable isosorbide-based plasticizer for biodegradable poly(butylene succinate), J. Polym. Eng., 2022, 42(4), 331–342, DOI:10.1515/polyeng-2021-0232.
- S. Mashhadikhan, R. Ahmadi, A. E. Amooghin, H. Sanaeepur, T. M. Aminabhavi and M. Rezakazemi, Breaking temperature barrier: Highly thermally heat resistant polymeric membranes for sustainable water and wastewater treatment, Renewable Sustainable Energy Rev., 2024, 189, 113902, DOI:10.1016/j.rser.2023.113902.
- C. W. H. Rajawasam, O. J. Dodo, M. A. S. N. Weerasinghe, I. O. Raji, S. V. Wanasinghe, D. Konkolewicz and N. D. A. Watuthanthrige, Educational series: characterizing crosslinked polymer networks, Polym. Chem., 2024, 15, 219–247, 10.1039/D3PY00914A.
- C. Zheng, G. Zhu, Y. Shi, L.-Z. Liu, M. Ren, W. Zhang and L. Han, Crystallization, structures and properties of biodegradable poly (butylene succinate-co-butylene terephthalate) with a symmetric composition, Mater. Chem. Phys., 2021, 260, 124183, DOI:10.1016/j.matchemphys.2020.124183.
- S. Wei, J. Xie, J. Zhang, L. Zhao and D. Hu, Green preparation of poly (butylene succinate-co-butylene terephthalate) foam with tunable degradability and mechanical properties by supercritical CO2, Polym. Degrad. Stab., 2024, 223, 110732, DOI:10.1016/j.polymdegradstab.2024.110732.
- G. Fredi and A. Dorigato, Recycling of bioplastic waste: A review, Adv. Ind. Eng. Polym. Res., 2021, 4(3), 159–177, DOI:10.1016/j.aiepr.2021.06.006.
- K. Wu, Y. Wu, S. Huang, Z. Chen, H. Wanga, Y. Shang and S. Li, Synthesis and characterization of hydroxyl-terminated butadiene-end-capped polyisobutylene and its use as a diol for polyurethane preparation, RSC Adv., 2020, 10, 9601–9609, 10.1039/D0RA00132E.
- G. Luo, B. Pang, X. Luo, X. Zeng, Y. Wang and L. Zhao, Self-healing and enhanced anticorrosion coatings based on graphene-reinforced brominated butyl rubber ionomer, Prog. Org. Coat., 2023, 174, 107245, DOI:10.1016/j.porgcoat.2022.107245.
- X. Meng, H. Ma, Y. Ma, C. Liu and A. He, Shape memory polybutene-1 alloy with superior high-temperature resistances to creep and raw petroleum, Polymer, 2025, 324, 128266, DOI:10.1016/j.polymer.2025.128266.
- J. Andrzejewski, A. Danielak, A. Piasecki, A. Islam and M. Szostak, Biocarbon-based sustainable reinforcing system for technical polymers. The structure-properties correlation between polycarbonate (PC) and polybutylene terephthalate (PBT)-based blends containing acrylonitrile-butadiene-styrene (ABS), Sustainable Mater. Technol., 2023, 36, e00612, DOI:10.1016/j.susmat.2023.e00612.
- F. V. Ferreira, L. S. Cividanes, R. F. Gouveia and L. M. F. Lona, An overview on properties and applications of poly(butylene adipate-co-terephthalate)-PBAT based composites, Polym. Eng. Sci., 2019, 59(2), E7–E15, DOI:10.1002/pen.24770.
- H. Shen, Y. Yuan, C. Liu, M. Yang and J. Xing, Rapid degradation of poly(butylene succinate-co-butylene terephthalate)s by microbial communities at high-temperature, Biochem. Eng. J., 2024, 204, 109230, DOI:10.1016/j.bej.2024.109230.
- P. Nuamduang, R. Auras, C. Winotapun, B. Hararak, W. Wanmolee and P. Leelaphiwat, Enhanced antifungal properties of poly(butylene succinate) film with lignin nanoparticles and trans-cinnamaldehyde for mango packaging, Int. J. Biol. Macromol., 2024, 267, 131185, DOI:10.1016/j.ijbiomac.2024.131185.
- K. Barrios-Tarazona and D. Suleiman, Chemical and morphological effects of blended sulfonated poly(styrene-isobutylene-styrene) and isopentylamine for direct methanol fuel cell applications, J. Appl. Polym. Sci., 2021, 138(11), 50034, DOI:10.1002/app.50034.
- J. P. Greene, Elastomers and rubbers, in In Plastics Design Library, Automotive Plastics and Composites, ed. Greene, J. P., William Andrew Publishing, Norwich, US, 2021, ch. 9, pp. 127–147 DOI:10.1016/B978-0-12-818008-2.00016-7.
- C. Loyer, G. Régnier, V. Duval, Y. Ould and E. Richaud, PBT plasticity loss induced by oxidative and hydrolysis ageing, Polym. Degrad. Stab., 2020, 181, 109368, DOI:10.1016/j.polymdegradstab.2020.109368.
- T. Sang, C. J. Wallis, G. Hill and G. J. P. Britovsek, Polyethylene terephthalate degradation under natural and accelerated weathering conditions, Eur. Polym. J., 2020, 136, 109873, DOI:10.1016/j.eurpolymj.2020.109873.
- F. Wu, M. Misra and A. K. Mohanty, Challenges and new opportunities on barrier performance of biodegradable polymers for sustainable packaging, Prog. Polym. Sci., 2021, 117, 101395, DOI:10.1016/j.progpolymsci.2021.101395.
- C. Kanemura, S. Nakashima and A. Hotta, Mechanical properties and chemical structures of biodegradable poly(butylene-succinate) for material reprocessing, Polym. Degrad. Stab., 2012, 97(6), 972–980, DOI:10.1016/j.polymdegradstab.2012.03.015.
- C. M. Parada, G. L. Parker and R. F. Storey, Polyisobutylene containing covalently bound antioxidant moieties, J. Polym. Sci., Part A:Polym. Chem., 2019, 57(17), 1836–1846, DOI:10.1002/pola.29457.
- M. Lundbäck, J. Hassinen, U. Andersson, T. Fujiwara and U. W. Gedde, Polybutene-1 pipes exposed to pressurized chlorinated water: Lifetime and antioxidant consumption, Polym. Degrad. Stab., 2006, 91(4), 842–847, DOI:10.1016/j.polymdegradstab.2005.06.015.
- G. Wypych, Plasticizers use and selection for specific polymers, in Handbook of Plasticizers, ed. Wypych, G., ChemTec Publishing, Toronto, Canada, 3rd edn, 2017, ch. 11, pp. 333–483 DOI:10.1016/B978-1-895198-97-3.50013-5.
- B. T. Hiller, J. L. Azzi and M. Rennert, Improvement of the thermo-oxidative stability of biobased poly(butylene succinate) (PBS) using biogenic wine by-products as sustainable functional fillers, Polymers, 2023, 15(11), 2533, DOI:10.3390/polym15112533.
- I.-N. Georgousopoulou, S. Vouyiouka, P. Dole and C. D. Papaspyrides, Thermo-mechanical degradation and stabilization of poly(butylene succinate), Polym. Degrad. Stab., 2016, 128, 182–192, DOI:10.1016/j.polymdegradstab.2016.03.012.
- H. Ritchie ( 2018) FAQs on plastics. [online] Available at: https://ourworldindata.org/faq-on-plastics#article-citation [Accessed: 19 March 2025].
- N. Harrington and H. Dena ( 2023) 30 Plastic Facts. [online] Available at: https://www.greenpeace.org/africa/en/blogs/54418/30-plastic-facts/#:~:text=In%202021%2C%20Coca%2DCola%20produced%2013%20billion%20more,purposes%2C%20including%20disposable%20food%20and%20beverage%20containers. [Accessed: 19 March 2025].
- M. T. Siraj, S. N. S. Jamil, A. M. Arka, S. Tasnim, M. Ghosh, M. R. B. Shahadat and M. Z. Rahman, Eco-friendly food packaging innovations: A review of recent progress on recyclable polymers, in Comprehensive Materials Processing, ed. Hashmi, S., Elsevier, Amsterdam, Netherlands, 2nd edn, 2024, ch. 12.47, pp. 693–709 DOI:10.1016/B978-0-323-96020-5.00077-7.
- V. Y. Melekhina, A. V. Kostyuk, N. M. Smirnova and S. O. Ilyin, Asphaltene-stabilized polyisobutylene pressure-sensitive adhesives for ultraviolet protection and surface bonding, Materials, 2023, 16, 1209, DOI:10.3390/ma16031209.
- H. Vieyra, J. M. Molina-Romero, J. D. D. Calderón-Nájera and A. Santana-Díaz, Engineering, recyclable, and biodegradable plastics in the automotive industry: A review, Polymers, 2022, 14(16), 3412, DOI:10.3390/polym14163412.
- M. Alaghemandi, Sustainable solutions through innovative plastic waste recycling technologies, Sustainability, 2024, 16(23), 10401, DOI:10.3390/su162310401.
- K. Ragaert, L. Delva and K. Van Geem, Mechanical and chemical recycling of solid plastic waste, Waste Manage., 2017, 69, 24–58, DOI:10.1016/j.wasman.2017.07.044.
- D. Kim, J. Lim, D. Jung, W. Oh, J. Kyeong, S. H. Kwon and S. G. Lee, Thermal and mechanical properties of polymeric materials for automotive applications using molecular dynamics simulation, Mater. Today Commun., 2023, 36, 106529, DOI:10.1016/j.mtcomm.2023.106529.
- B. H. Soudmand and K. Shelesh-Nezhad, Failure and wear analysis of poly(butylene terephthalate) nanocomposite spur gears, Tribol. Int., 2020, 151, 106439, DOI:10.1016/j.triboint.2020.106439.
- S. A. Vyavahare, B. M. Kharat and A. P. More, Polybutylene terephthalate (PBT) blends and composites: A review, Vietnam J. Chem., 2024, 62(5), 579–589, DOI:10.1002/vjch.202300177.
- C. Salvagnini, A. Roback, M. Momtaz, V. Pourcelle and J. Marchand-Brynaert, Surface functionalization of a poly(butylene terephthalate) (PBT) melt-blown filtration membrane by wet chemistry and photo-grafting, J. Biomater. Sci., Polym. Ed., 2007, 18(12), 1491–1516, DOI:10.1163/156856207794761934.
- P. Qian, Y. Zhang, H. Mao, H. Wang and H. Shi, Nucleation and mechanical enhancements in poly(butylene terephthalate) nanocomposites influenced by functionalized graphene oxide, SN Appl. Sci., 2019, 1, 443, DOI:10.1007/s42452-019-0466-8.
- J. E. Puskas, Y. Chen, Y. Dahman and D. Padavan, Polyisobutylene-based biomaterials, J. Polym. Sci., Part A:Polym. Chem., 2004, 42(13), 3091–3109, DOI:10.1002/pola.20114.
- H. S. Tan and W. R. Pfister, Pressure-sensitive adhesives for transdermal drug delivery systems, Pharm. Sci. Technol. Today, 1999, 2(2), 60–69, DOI:10.1016/S1461-5347(99)00119-4.
- T. Zahra, U. Javeria, H. Jamal, M. M. Baig, F. Akhtar and U. Kamran, A review of biocompatible polymer-functionalized two-dimensional materials: Emerging contenders for biosensors and bioelectronics applications, Anal. Chim. Acta, 2024, 1316, 342880, DOI:10.1016/j.aca.2024.342880.
- B. Kost, M. Brzeziński, M. Socka, M. Baśko and T. Biela, Biocompatible polymers combined with cyclodextrins: Fascinating materials for drug delivery applications, Molecules., 2020, 25(15), 3404, DOI:10.3390/molecules25153404.
- Z. U. Arif, The role of polysaccharide-based biodegradable soft polymers in the healthcare sector, Adv. Ind. Eng. Polym. Res., 2025, 8(1), 132–156, DOI:10.1016/j.aiepr.2024.05.001.
- N. Maheshwari, M. Tekade, Y. Chourasiya, M. C. Sharma, P. K. Deb and R. K. Tekade, Nanotechnology in tissue engineering, in In Advances in Pharmaceutical Product Development and Research, Biomaterials and Bionanotechnology, ed. Tekade, R. K., Academic Press, Cambridge, US, 2019, ch. 7, pp. 225–261 DOI:10.1016/B978-0-12-814427-5.00007-X.
- E. Waris, N. Ashammakhi, M. Lehtimäki, R.-M. Tulamo, P. Törmälä, M. Kellomäki and Y. T. Konttinen, Long-term bone tissue reaction to polyethylene oxide/polybutylene terephthalate copolymer (Polyactive®) in metacarpophalangeal joint reconstruction, Biomaterials, 2008, 29(16), 2509–2515, DOI:10.1016/j.biomaterials.2008.02.013.
- M. N. Helmus, D. F. Gibbons and D. Cebon, Biocompatibility: Meeting a key functional requirement of next-generation medical devices, Toxicol. Pathol., 2008, 36(1), 70–80, DOI:10.1177/0192623307310949.
- M. F. Maitz, Applications of synthetic polymers in clinical medicine, Biosurface Biotribol., 2015, 1(3), 161–176, DOI:10.1016/j.bsbt.2015.08.002.
- L. Pinchuk, M. Boden and D. Bluestein, The use of poly(styrene-block-isobutylene-block-styrene) and analogs for long-term implant applications, in Macromolecular Engineering, ed. Lubnin, A. and Erdodi, G., Elsevier, Amsterdam, Netherlands, 2021, ch. 11, pp. 211–235 DOI:10.1016/B978-0-12-821998-0.00011-9.
- C. B. Watson, D. Tan and D. E. Bergbreite, Enthalpy-driven polyisobutylene depolymerization, Macromolecules, 2019, 52(8), 3042–3048, DOI:10.1021/acs.macromol.9b00313.
- J. Chattopadhyay, T. S. Pathak and D. M. F. Santos, Applications of polymer electrolytes in lithium-ion batteries: A review, Polymers, 2023, 15(19), 3907, DOI:10.3390/polym15193907.
- X. Lv, C. Liu, D. Wu, S. Song, Z. Shao and S. Sun, Epoxy-based ionic liquid towards multi-walled carbon nanotubes/polybutylene terephthalate composite with excellent dispersion and conductivity behaviors, J. Polym. Res., 2020, 27, 237, DOI:10.1007/s10965-020-02224-z.
- S. E. Takalloo, A. Fannir, G. T. M. Nguyen, C. Plesse, F. Vidal and J. D. W. Madden, Impermeable and compliant: SIBS as a promising encapsulant for ionically electroactive devices, Robotics, 2019, 8(3), 60, DOI:10.3390/robotics8030060.
- J. T. Orasugh, C. Pal, M. S. Ali and D. Chattopadhyay, Electromagnetic interference shielding property of polymer-graphene composites, in Series in Composites Science and Engineering, Polymer Nanocomposites Containing Graphene, ed. Mostafizur R., Lalatendu N., Ibnelwaleed A. H., Narayan C. D., Woodhead Publishing, Sawston, UK, 2022, ch. 8, pp. 211–243 DOI:10.1016/B978-0-12-821639-2.00006-9.
- J. Sun, X. Yang, Y. Bai, Z. Fang, S. Zhang, X. Wang, Y. Yang and Y. Guo, Recent advances in cellulose nanofiber modification and characterization and cellulose nanofiber-based films for eco-friendly active food packaging, Foods, 2024, 13(24), 3999, DOI:10.3390/foods13243999.
- M. S. M. Misenan, R. Hempelmann, M. Gallei and T. Eren, Phosphonium-based polyelectrolytes: Preparation, properties, and usage in lithium-ion batteries, Polymers, 2023, 15(13), 2920, DOI:10.3390/polym15132920.
- U. C. Obi, E. O. Onche, O. F. Ngasoh, F. Barzegar, B. N. Ekwe, A. P. Onwualu and A. Bello, Polymer electrolytes for enhanced mechanical integrity in lithium-ion batteries: a review of recent progress and future directions, J. Polym. Eng., 2025, 45(5), 375–387, DOI:10.1515/polyeng-2024-0200.
- J. Hong, J. Lee, S. K. Kim, D. Son, D. Kang and J. K. Shim, Enhancing thermal insulation property and flexibility of starch/poly(butylene adipate terephthalate) (PBAT) blend foam by improving rheological properties, Polymers, 2025, 17(2), 138, DOI:10.3390/polym17020138.
- M. A. S. Siddiqui, M. S. Rabbi, R. U. Ahmed and M. M. Billah, Biodegradable natural polymers and fibers for 3D printing: A holistic perspective on processing, characterization, and advanced applications, Cleaner Mater., 2024, 14, 100275, DOI:10.1016/j.clema.2024.100275.
- Y. Shao, J. Yan, Y. Zhi, C. Li, Q. Li, K. Wang, R. Xia, X. Xiang, L. Liu, G. Chen, H. Zhang, D. Cai, H. Wang, X. Cheng, C. Yang, F. Ren and Y. Yu, A universal packaging substrate for mechanically stable assembly of stretchable electronics, Nat. Commun., 2024, 15, 6106, DOI:10.1038/s41467-024-50494-8.
- N. Shen, J. Bu, M. E. Prévôt, T. Hegmann, J. P. Kennedy and W. Xu, Macromolecular engineering and additive manufacturing of polyisobutylene-based thermoplastic elastomers. II. The poly(styrene-b-isobutylene-b-styrene)/poly(phenylene oxide) system, Macromol. Rapid Commun., 2023, 44(1), 2200109, DOI:10.1002/marc.202200109.
- C. R. Álvarez-Chávez, S. Edwards, R. Moure-Eraso and K. Geiser, Sustainability of bio-based plastics: general comparative analysis and recommendations for improvement, J. Cleaner Prod., 2012, 23(1), 47–56, DOI:10.1016/j.jclepro.2011.10.003.
- L. S. Dilkes-Hoffman, P. A. Lant, B. Laycock and S. Pratt, The rate of biodegradation of PHA bioplastics in the marine environment: A meta-study, Mar. Pollut. Bull., 2019, 142, 15–24, DOI:10.1016/j.marpolbul.2019.03.020.
- L. Zimmermann, A. Dombrowski, C. Völker and M. Wagner, Are bioplastics and plant-based materials safer than conventional plastics? In vitro toxicity and chemical composition, Environ. Int., 2020, 145, 106066, DOI:10.1016/j.envint.2020.106066.
- J. Han, S.-H. Shin, K.-M. Park and K. M. Kim, Characterization of physical, mechanical, and antioxidant properties of soy protein-based bioplastic films containing carboxymethylcellulose and catechin, Food Sci. Biotechnol., 2015, 24, 939–945, DOI:10.1007/s10068-015-0121-0.
- T. Tamara, S. Sumari, N. Nazriati and S. Arni, Properties of cassava starch-based bioplastics and CMC with sorbitol as A plasticizer, IOP Conf. Ser.: Earth Environ. Sci., 2020, 456, 012077, DOI:10.1088/1755-1315/456/1/012077.
- G. Feng, L. Hu, Y. Ma, P. Jia, Y. Hu, M. Zhang, C. Liu and Y. Zhou, An efficient bio-based plasticizer for poly (vinyl chloride) from waste cooking oil and citric acid: Synthesis and evaluation in PVC films, J. Cleaner Prod., 2018, 189, 334–343, DOI:10.1016/j.jclepro.2018.04.085.
- H. B. Pyeon, J. E. Park and D. H. Suh, Non-phthalate plasticizer from camphor for flexible PVC with a wide range of available temperature, Polym. Test., 2017, 63, 375–381, DOI:10.1016/j.polymertesting.2017.08.029.
- S. Muniyasamy, O. Ofosu, M. J. John and R. D. Anandjiwala, Mineralization of poly(lactic acid) (PLA), poly(3-hydroxybutyrate-co-valerate) (PHBV) and PLA/PHBV blend in compost and soil environments, J. Renewable Mater., 2016, 4(2), 133–145, DOI:10.7569/JRM.2016.634104.
- T. Narancic, S. Verstichel, S. R. Chaganti, L. Morales-Gamez, S. T. Kenny, B. De Wilde, R. B. Padamati and K. E. O’Connor, Biodegradable plastic blends create new possibilities for end-of-life management of plastics but they are not a panacea for plastic pollution, Environ. Sci. Technol., 2018, 52(18), 10441–10452, DOI:10.1021/acs.est.8b02963.
- A. Pischedda, M. Tosin and F. Degli-Innocenti, Biodegradation of plastics in soil: The effect of temperature, Polym. Degrad. Stab., 2019, 170, 109017, DOI:10.1016/j.polymdegradstab.2019.109017.
- A. N. Boyandin, S. V. Prudnikova, V. A. Karpov, V. N. Ivonin, N. L. Do, T. H. Nguyen, T. M. H. Le, N. L. Filichev, A. L. Levin, M. L. Filipenko, T. G. Volova and I. I. Gitelson, Microbial degradation of polyhydroxyalkanoates in tropical soils, Int. Biodeterior. Biodegrad., 2013, 83, 77–84, DOI:10.1016/j.ibiod.2013.04.014.
- D. Adhikari, M. Mukai, K. Kubota, T. Kai, N. Kaneko, K. S. Araki and M. Kubo, Degradation of bioplastics in soil and their degradation effects on environmental microorganisms, J. Agric. Chem. Environ., 2016, 5(1), 23–34, DOI:10.4236/jacen.2016.51003.
- C. C. Chen, L. Dai, L. Ma and R.-T. Guo, Enzymatic degradation of plant biomass and synthetic polymers, Nat. Rev. Chem., 2020, 4, 114–126, DOI:10.1038/s41570-020-0163-6.
- F. Kawai, Emerging strategies in polyethylene terephthalate hydrolase research for biorecycling, ChemSusChem., 2021, 14(19), 4115–4122, DOI:10.1002/cssc.202100740.
- R. Muthuraj, M. Misra and A. K. Mohanty, Hydrolytic degradation of biodegradable polyesters under simulated environmental conditions, J. Appl. Polym. Sci., 2015, 132(27) DOI:10.1002/app.42189.
- J. G. Drobny, Electron beam processing of commercial polymers, monomers, and oligomers, in In Plastics Design Library, Ionizing Radiation and Polymers, ed. J. George Drobny, William Andrew Publishing, Norwich, US, 2013, ch. 5, pp. 101–147 DOI:10.1016/B978-1-4557-7881-2.00005-5.
- W. Brostow, X. Lu, O. Gencel and A. T. Osmanson, Effects of UV stabilizers on polypropylene outdoors, Materials, 2020, 13(7), 1626, DOI:10.3390/ma13071626.
- A. Rahimi and J. García, Chemical recycling of waste plastics for new materials production, Nat. Rev. Chem., 2017, 1, 0046, DOI:10.1038/s41570-017-0046.
- Z. Jiang, F. Hou, J. Chen, B. Wang, S. Song, J. Li, L. Huang, C. Wang and H. Wang, Synthesis and properties of biodegradable PBAT prepared from PBT chemically recycled resources, Polymer, 2024, 307, 127326, DOI:10.1016/j.polymer.2024.127326.
- A. Parodi, V. Arpaia, C. Samori, L. Mazzocchetti and P. Galletti, Novel strategies for recycling poly(butylene adipate-co-terephthalate)-starch-based plastics: Selective solubilization and depolymerization–repolymerization processes, ACS Sustainable Chem. Eng., 2023, 11(39), 14518–14527, DOI:10.1021/acssuschemeng.3c03588.
- R. Yang, G. Xu, B. Dong, X. Guo and Q. Wang, Selective, sequential, and “One-Pot” depolymerization strategies for chemical recycling of commercial plastics and mixed plastics, ACS Sustainable Chem. Eng., 2022, 10(30), 9860–9871, DOI:10.1021/acssuschemeng.2c01708.
- S. Kaabel, J. P. D. Therien, C. E. Deschênes, D. Duncan, T. Friščić and K. Auclair, Enzymatic depolymerization of highly crystalline polyethylene terephthalate enabled in moist-solid reaction mixtures, Proc. Natl. Acad. Sci. U. S. A., 2021, 118(29), e2026452118, DOI:10.1073/pnas.2026452118.
- P. Borelbach, R. Kopitzky, J. Dahringer and P. Gutmann, Degradation behavior of biodegradable man-made fibers in natural soil and in compost, Polymers, 2023, 15(13), 2959, DOI:10.3390/polym15132959.
- N. Shin, S. H. Kim, J. Oh, S. Kim, Y. Lee, Y. Shin, S. Choi, S. K. Bhatia, Y.-G. Kim and Y.-H. Yang, Reproducible polybutylene succinate (PBS)-degrading artificial consortia by introducing the least type of PBS-degrading strains, Polymers, 2024, 16(5), 651, DOI:10.3390/polym16050651.
- M. Suzuki, S. Ishii, K. Gonda, H. Kashima, S. Suzuki, K. Uematsu, T. Arai, Y. Tachibana, T. Iwata and K. Kasuya, Marine biodegradation mechanism of biodegradable plastics revealed by plastisphere analysis, Res. Square, 2022 DOI:10.21203/rs.3.rs-2014166/v1.
- J. Li, Biodegradable PBAT Plastics and Composites, Springer, Gateway East, Singapore, 2025 DOI:10.1007/978-981-96-2057-9_1.
- J. Xu, K. Feng, Y. Li, J. Xie, Y. Wang, Z. Zhang and Q. Hu, Enhanced biodegradation rate of poly(butylene adipate-co-terephthalate) composites using reed fiber, Polymers, 2024, 16(3), 411, DOI:10.3390/polym16030411.
- W.-Z. Zheng, X. Li, J. Xie, Z. Y. Zhang, P.-L. Wang, D. Huang, Z.-L. Ren, J.-H. Ji and G.-X. Wang, Closed-loop recycling of biodegradable poly(butylene adipate-co-terephthalate) based on hydrolysis and repolymerization strategy, J. Environ. Chem. Eng., 2024, 12(6), 114354, DOI:10.1016/j.jece.2024.114354.
- N. R. Maddela, D. Kakarla, K. Venkateswarlu and M. Megharaj, Additives of plastics: Entry into the environment and potential risks to human and ecological health, J. Environ. Manage., 2023, 348, 119364, DOI:10.1016/j.jenvman.2023.119364.
- K. C. Omidoyin and E. H. Jho, Environmental occurrence and ecotoxicological risks of plastic leachates in aquatic and terrestrial environments, Sci. Total Environ., 2024, 954, 176728, DOI:10.1016/j.scitotenv.2024.176728.
- Y. Yang, J. Min, T. Xue, R. Peng, J.-W. Huang, Y. Qu, X. Li, N. Ma, F.-C. Tsai, L. Dai, Y. Liu, C.-C. Chen and R.-T. Guo, Complete bio-degradation of poly(butylene adipate-co-terephthalate) via engineered cutinases, Nat. Commun., 2023, 14, 1645, DOI:10.1038/s41467-023-37374-3.
- M. Safari, R. A. Pérez-Camargo, L. Ballester-Bayarri, G. Liu, A. Mugica, M. Zubitur, D. Wang and A. J. Müller, Biodegradable binary blends of poly (butylene succinate) or poly (ε-caprolactone) with poly (butylene succinate-ran-ε-caprolactone)copolymers: Crystallization behavior, Polymer, 2022, 256, 125206, DOI:10.1016/j.polymer.2022.125206.
- W. Chen, C. Qi, Y. Li and H. Tao, The degradation investigation of biodegradable PLA/PBAT blend: Thermal stability, mechanical properties and PALS analysis, Radiat. Phys. Chem., 2021, 180, 109239, DOI:10.1016/j.radphyschem.2020.109239.
- L. W. McKeen, Introduction to use of plastics in food packaging, in Plastics Design Library, Plastic Films in Food Packaging, ed. S. Ebnesajjad, William Andrew Publishing, Norwich, US, 2013, ch. 1, pp. 1–15 DOI:10.1016/B978-1-4557-3112-1.00001-6.
- Y. K. A. A. Iasya, R. C. Nissa, S. S. Kusumah, R. U. Annifah, Ismadi and Y. Nurhamiyah, Comparative study on the blends of PBS with thermoplastic starch and agar on their potential as renewable source of bioplastic, Polym. Bull., 2025 DOI:10.1007/s00289-025-05796-2.
- B. M. Tselana, S. Muniyasamy, V. O. Ojijo and W. Mhike, Melt processible biodegradable blends of polyethylene glycol plasticized cellulose diacetate with polylactic acid and polybutylene adipate-co-terephthalate, J. Polym. Environ., 2023, 31, 4891–4908, DOI:10.1007/s10924-023-02925-8.
- T. Dufour, From basics to frontiers: A comprehensive review of plasma-modified and plasma-synthesized polymer films, Polymers, 2023, 15(17), 3607, DOI:10.3390/polym15173607.
- E. Kabir, Application of microwave heating in polymer synthesis: A review, Results Chem., 2023, 6, 101178, DOI:10.1016/j.rechem.2023.101178.
- G. Wu, P. Bian, R. Xu, T. Wang, Z. Li, H. Mao, Y. Tai, C. Wang, Z. Ma, X. Hou, N. Carpentier, S. Dutta, S. Wuttke, M. Panahi-Sarmad, S. Van Vlierberghe and X. Xiao, Electro-thermal responses polymer systems with continuous shape memory alloys: Merging rapid shape memory and color transitions, Chem. Eng. J., 2025, 503, 158264, DOI:10.1016/j.cej.2024.158264.
- Y. Cheng and Y. Lu, Physical stimuli-responsive polymeric patches for healthcare, Bioact. Mater., 2025, 43, 342–375, DOI:10.1016/j.bioactmat.2024.08.025.
- M. Zhu, J. Liu, L. Gan and M. Long, Research progress in bio-based self-healing materials, Eur. Polym. J., 2020, 129, 109651, DOI:10.1016/j.eurpolymj.2020.109651.
- G. Ciarleglio, E. Toto and M. G. Santonicola, Conductive and thermo-responsive composite hydrogels with poly(N-isopropylacrylamide) and carbon nanotubes fabricated by two-step photopolymerization, Polymers, 2023, 15(4), 1022, DOI:10.3390/polym15041022.
- Z. Niu, F. Chen, H. Zhang and C. Liu, High content of thermoplastic starch, poly(butylenes adipate-co-terephthalate) and poly(butylene succinate) ternary blends with a good balance in strength and toughness, Polymers, 2023, 15(9), 2040, DOI:10.3390/polym15092040.
- M. Soccio, F. Dominici, S. Quattrosoldi, F. Luzi, A. Munari, L. Torre, N. Lotti and D. Puglia, PBS-based green copolymer as an efficient compatibilizer in thermoplastic inedible wheat flour/poly(butylene succinate) blends, Biomacromolecules, 2020, 21(8), 3254–3269, DOI:10.1021/acs.biomac.0c00701.
- S. S. Ali, E. A. Abdelkarim, T. Elsamahy, R. Al-Tohamy, F. Li, M. Kornaros, A. Zuorro, D. Zhu and J. Sun, Bioplastic production in terms of life cycle assessment: A state-of-the-art review, Environ. Sci. Ecotechnol., 2023, 15, 100254, DOI:10.1016/j.ese.2023.100254.
- D. Negrete-Bolagay and V. H. Guerrero, Opportunities and challenges in the application of bioplastics: Perspectives from formulation, processing, and performance, Polymers, 2024, 16(18), 2561, DOI:10.3390/polym16182561.
- F. Sandrolini, A. Motori and A. Saccani, Electrical properties of poly(butylene terephthalate, J. Appl. Polym. Sci., 1992, 44(5), 765–771, DOI:10.1002/app.1992.070440503.
| This journal is © The Royal Society of Chemistry 2025 |







