 Open Access Article
Open Access ArticleAn outstanding, efficient visible-light-driven BiOI/LaCoO3 Z-scheme system toward cefixime degradation†
Razieh
Ahesteh
a,
Alireza
Nezamzadeh-Ejhieh
 *a and
Seyed Nezamoddin
Mirsattari
b
*a and
Seyed Nezamoddin
Mirsattari
b
aDepartment of Chemistry, Shah. C., Islamic Azad University, Shahreza, P.O. Box 311-86145, Islamic Republic of Iran. E-mail: r.ahesteh@iau.ir; arnezamzadeh@iau.ac.ir; arnezamzadeh@iaush.ac.ir; arnezam1346@gmail.com; Alireza_nezamzadeh@yahoo.com
bDepartment of Chemistry, Institute of Agriculture, Water, Food, and Nutraceuticals, Isf. C., Islamic Azad University, Isfahan, Islamic Republic of Iran. E-mail: n.mirsattari@iau.ac.ir
First published on 16th May 2025
Abstract
Multiple pollutants, especially antibiotics, are polluting water systems, prompting the development of novel photocatalysts with synergistic activity for mineralizing these pollutants. In this study, we synthesized three-dimensional BiOI microspheres, supported by noble metal-free LaCoO3 co-catalysts, to construct an enhanced hybrid photocatalyst featuring superior charge separation properties. Various techniques were used to characterize the samples, including FTIR, SEM-EDX, XRD, and UV-Vis DRS (diffuse reflectance spectroscopy). Based on the Williamson–Hall equation, the BiOI/LaCoO3 sample had an average crystallite size of 50.70 nm. BiOI, LaCoO3, and BiOI/LaCoO3 have band gaps of 1.80, 1.56, and 1.57 eV, relating to absorption edge wavelengths of 684, 790, and 789, respectively. For BiOI, LaCoO3, and BiOI/LaCoO3, the pHpzc (point of zero charge pH) values were 5.8, 10.5, and 8.9, respectively. In this coupled system, the moles of LaCoO3 oxide are four times greater than those of another component, resulting in boosted activity. According to the response surface methodology (RSM) study, the suggested model shows a F-value of 50.26 > F0.05,14,15 in the model, as well as a LOF F-value of 3.44 < F0.05,10,5 and high R2-values (R2 = 0.9908, pred-R2 = 0.9989, and adj-R2 = 0.9996). The proposed binary catalyst of BiOI/LaCoO3, the direct Z-scheme, is the preferred method of illustrating Cefixime photodegradation. Using chemical oxygen demand (COD) data, we could derive the rate constants of 0.053 min−1 and 0.061 min−1 for photodegrading solutions. During photodegradation, CEF molecules degrade at a t1/2 of 13.07 and mineralize at a t1/2 of 11.36.
1. Introduction
Among the most important categories of pharmaceutical compounds are antibiotics, used in animal and plant breeding and human health care.1–3 However, these compounds are increasingly overused and can be found in the environment, particularly in aquatic environments.4 Among the most commonly used antibiotics is Cefixime (CEF). Following its FDA approval in 1980, it has become one of the most widely used antibiotics on the planet. CEF is an antibiotic classified as a cephalosporin of the third generation and is widely used. There are many antibiotics containing active pharmaceutical ingredients (API). These antibiotics are intended to treat various human and animal illnesses. Still, due to their extensive use and persistence in water sources, soils, and sediments, they have a negative environmental impact. Despite their non-biodegradability, many antibiotics make conventional chemical and biological treatments ineffective, resulting in increased levels of the original drugs and their intermediates on both land and water. Thus, methods for decomposing and removing antibiotic compounds from water sources (surface water, groundwater, or wastewater) are essential. AOPs (advanced oxidation processes, including photochemical, electrochemical, and catalytic oxidation, ozonation, Fenton and photo-Fenton, and Sonolysis) are among the various wastewater treatment methods that have attracted increasing attention to remove organic pollutants in wastewater.5–7 Most of these methods employ in situ formation of highly reactive species (e.g., hydroxyl radicals) that degrade and oxidize many organic pollutants and/or increase their biodegradability. The AOP systems still face several difficulties, such as inefficient energy use, reliance on specific materials, and/or introduction of additional chemicals.8 In water and wastewater systems, heterogeneous photocatalysis is widely used to eliminate pharmaceutical and organic contaminants.9–11This technique involves irradiating semiconductors with enough energy through photons (in the UV-Vis region).12,13 With the photoinduced electron–hole (e−/h+) pairs, superoxide radicals and hydroxyl radicals are immediately produced in the presence of dissolved oxygen and water/hydroxyl.14,15 In this process, these strong oxidants can break organic pollutants into smaller fragments, eventually breaking into H2O and CO2, and other inorganic ions depending on the structural heteroatoms.16–18 The e−/h+ recombination, the critical heterogeneous photocatalysis’ drawback, causes a significant reduction in overall process activity.19–22 The number of techniques for decreasing e−/h+ recombination has increased over the past few decades. Semiconductor coupling creates a novel heterostructure to transport the photogenerated electrons from one semiconductor's negative CB to another's more positive CB.23–28 There is an opposite trend between the holes between VB positions. Transitions of this kind reduce e−/h+ recombination while decreasing photodegradation.26,29–33 In general, heterogeneous photocatalysis has a wide application range.34–41
BiOX is a VVI-VII ternary oxide semiconductor with an individual structure and a low band gap energy (Eg: 1.77–1.92 eV), capable of absorbing most visible light.42,43 Although it has been regarded for sensitizing broadband semiconductors, it is highly recombinant in single semiconductors, resulting in poor activity since it cannot produce sufficient ˙O2− and ˙OH, which are conducive to photocatalysis. Thus, many researchers have attempted to combine BiO-halides with other semiconductors to increase their separation efficiency.44–47 It has been widely reported that bismuth oxide (BiOI) has a very narrow Eg of about 1.8 eV.48,49
It has been discovered that lanthanum cobaltite (LaCoO3) is a highly efficient and reliable co-catalyst that is noble metal-free. LaCoO3 is an essential member of the perovskite family of cobalt oxides, a vital semiconductor compound. Its excellent characteristics (excellent visible light absorption capacity and electronic conductivity, high charge carrier mobility, and low-cost, nontoxic compound) make it an excellent choice for CO2 reduction and organic pollutant degradation.42,44,50 The photocatalytic activity of a similar compound, LaFeO3, has been well-reviewed.51
During the development of this study, nano-dimensioned BiOI and LaCoO3 were synthesized, and their binary BiOI/LaCoO3 catalyst was mechanically prepared. After confirming the synergistic effect of the binary system concerning the individual system in the photodegradation of cefixime (CEF), we investigated the impact of influencing variables on the degradation process by designing the experiment via an RSM approach. Finally, by performing the photodegradation tests against the scavengers (chloride, nitrate, ascorbic acid, and isopropanol act as scavengers of hydroxyl, electron, superoxide, and hole radicals, respectively), the relative roles of the reactive species in the overall photocatalytic activity were estimated.
2. Experimental
2.1. Materials and preparations
Cobalt nitrate hexahydrate (99.99%), lanthanum nitrate hexahydrate (99.99%), citric acid (99%), urea (99%), KI (AR), HNO3 (AR), Bi(NO3)3·5H2O (AR), and other chemicals were purchased from Sigma/Aldrich Co., Ltd, and used as received. All experiments were conducted with deionized water.By modifying a published procedure, BiOI microspheres were produced by a facile solvothermal method.52 An amount of 1.9400 g (4 mmol) Bi(NO3)3·5H2O and 0.6640 g (4 mmol) KI is typically dissolved over 10 minutes in 40 mL ethylene glycol under critical stirring. The Bi(NO3)3·5H2O solution was then added dropwise to the KI solution while vigorously stirring it. Vigorous stirring of the mixture was then performed for another hour at room temperature. A 100 mL Teflon-lined stainless-steel autoclave was used to heat the mixture at 160 °C for 12 hours after mixing. Centrifugation was used to collect the product after cooling naturally to room temperature, several washes with distilled water and ethanol, followed by 24 hours of drying at 80 °C.
Using a modified procedure, LaCoO3 nanoparticles were prepared using sol–gel and citrate complexation. A 30 mL La (NO3)3·6H2O solution (2.1650 g, 5 mmol) was added dropwise to a 30 mL aqueous solution containing 1.4552 g (5 mmol) of Co(NO3)2·6H2O and 2.1014 g (10 mmol) of citric acid under stirring, and vigorous stirring continued at room temperature for 5 hours. The solution was then heated to 80 °C for 2 hours, and a xerogel-like precursor was formed. Then, it was dried in an oven at 100 °C for 12 hours. The precursor was powdered over the milling in an agate mortar, transferred to an aluminum crucible, and air calcined at 700 °C for four hours (rising temperature rate: 20 °C min−1). After careful washing with water and ethanol, the black powder was dried at 80 °C for 12 h.53
To fabricate the binary system (BiOI/LaCoO3), depending on the BiOI:LaCoO3 molar ratio requested, the adequate weight of BiOI and LaCoO3 NPs was added to an agate mortar and completely hand-mixed for 30 min.
Cefixime solution: a Cefixime tablet (400 mg) was weighed. To obtain an analytical sample, 3 cefixime tablets were completely turned into powder in an agate mortar, and 6.25 mg of the powder was completely dissolved in water and filtered in a 250 mL volumetric flask, reaching the mark to achieve a 100 mg L−1 stock CEF solution. This stock solution was used to prepare diluter solutions via the serial dilution pathway.
2.2. Characterization techniques
In this study, different analyses were conducted on the synthesized nanoparticles. Analyzing the samples' powder X-ray diffraction patterns (XRD) identified their crystal structure (PW1730, Philips Company, Netherlands). A Fourier Transform Infrared (FTIR) spectrophotometer (Agilent PerkinElmer Spectrum 65) was used to identify functional groups in the samples. Nanoparticle size and morphological and structural information were achieved with a field emission scanning electron microscope (FESEM), coated with gold (Tescan Company, Czech Republic). An elemental composition of photocatalysts was determined using energy-dispersive X-ray spectroscopy (EDX) (Quantum 200, USA). A UV-Vis diffuse reflection spectrum (DRS: Biomate5, Thermo Company USA) was used to obtain the optical features of catalysts. A UV-Vis spectrophotometer (PG-Instrument T80, Australia) was used to record absorption spectra of cefixime solutions before and after the photodegradation process. The NPs were separated using a centrifuge instrument (Sigma).2.3. Photodegradation tests
During the preliminary photodegradation tests of CEF, 3 mg of the alone and binary systems (BiOI or LaCoO3 or BiOI/LaCoO3) was added to 10 mL 10 mg L−1 CEF solution and homogenized in the dark for 10 min under magnetic stirring. Under this circumstance, an equilibrated surface adsorption/desorption was achieved, and the catalyst species were well-separated and dispersed. We irradiated the suspensions with three 40 W W-lamps for a specific time, centrifuged the suspension (at rpm ∼13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), and acquired the absorption spectrum of the supernatant (A). All experiments were done at room temperature. The reaction efficiency was compared with the absorbance of a blank CEF solution (A0) to calculate the photocatalytic degradation efficiency. The formula was applied to calculate the CEF degradation percentage, in which C0 is the initial CEF concentration and A is the CEF concentration at time t after photodegradation, according to Beer–Lamberts’ law.
000), and acquired the absorption spectrum of the supernatant (A). All experiments were done at room temperature. The reaction efficiency was compared with the absorbance of a blank CEF solution (A0) to calculate the photocatalytic degradation efficiency. The formula was applied to calculate the CEF degradation percentage, in which C0 is the initial CEF concentration and A is the CEF concentration at time t after photodegradation, according to Beer–Lamberts’ law.| CEF Deg. (%) = [(A0 − A)/A0] × 100 = [(C0 − C)/C0] × 100 | (1) |
The effect of the direct photolysis in the absence of the catalysts and the effect of the surface adsorption on the CEF removal by the individual and binary catalysts (under the dark condition) were also studied under the same conditions mentioned above.
3. Results and discussion
3.1. Characterization studies
 | ||
| Fig. 1 XRD patterns (A), W–H plot (for the binary catalyst) (B), and FTIR spectra (B) of BiOI and LaCoO3 NPs and the related binary BiOI/LaCoO3 compound. | ||
A striking aspect of the findings was the similarity in XRD patterns between the obtained LaCoO3/BiOI hybrids and pure BiOI. Furthermore, in the XRD patterns of the LaCoO3/BiOI hybrid, a visible peak showing LaCoO3 (2θ = 32.88°) content emerged as the content of LaCoO3 increased, providing evidence that LaCoO3/BiOI composites were successfully synthesized. Furthermore, no additional peaks associated with possible impurities were detected, indicating that the samples were of high purity as prepared. The average size of the sample crystallites was calculated using the Debye–Scherrer formula, eqn (2),58 and the Williamson–Hall (W–H) eqn (3).59 The W–H model shows how the size (the Scherrer component) and the strain (ε, at ε = 0, W–H gives the Scherrer equation) simultaneously affect the peak broadening (D is the average size, D, β is the FWHM, the full width at half maximum, θ is the Bragg angle, k is the Scherrer constant (0.9), and λ is the X-ray source wavelength (Cu-Ka: 0.154 nm).60
D = (Kλ)/(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) θ) | (2) |
β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos(θ) = (0.9λ/d) + (ε cos(θ) = (0.9λ/d) + (ε![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) sin(θ)) sin(θ)) | (3) |
Averagely, the sizes of 23.06 nm, 31.87 nm, and 27.11 nm were respectively obtained for crystallites of BiOI, LaCoO3, and the coupled photocatalyst, while the values for these crystallites were obtained by the W–H plot (see the ESI,†Fig. 1A and Fig. S1) were 195.41, 31.08, and 50.70 nm, respectively. A complete set of data, information, and results is also summarized in Tables S1–S3 (ESI†).
 | ||
| Fig. 2 (A) UV-Vis DRS absorption spectra of BiOI, LaCoO3, and LaCoO3/BiOI samples. (B) and (C) Tauc plots for Eg estimation for n = 2 and n = 1/12. | ||
In addition, the band gap energy (Eg) of a typical semiconductor could be determined by a Kubelka–Munk equation64–66 by using a typical Tauc formula of αhv = A(hv − Eg)n/2, where A, α, v, and h are respectively the proportionality constant, absorption coefficient, frequency, and Planck constant. Typical semiconductor's optical transitions are indicated by n (n = 1 for direct transition, and n = 4 for indirect transition).53,64,67 Various Tauc formula formats for different electronic transitions have been well illustrated and compared in the literature.68
Various Tauc formats have been used in the literature, all summarized in Table 1.69 Another Tauc formula format is written as follows:
| (αhv)n = k(hv − Eg) | (4) |
| Band gap energies | ||||||
|---|---|---|---|---|---|---|
| Catalysts | Tauc plots (eV) | Absorption edge | ||||
| 1/2 | 2 | 3/2 | 3 | λ (nm) | E g (eV) | |
| BiOI | 1.95 | 2.11 | 2.22 | 2.23 | 684 | 1.81 |
| LaCoO3 | 1.67 | 1.91 | 1.95 | 2.00 | — | — |
| BiOI/LaCoO3 | 1.90 | 2.00 | 2.05 | 2.10 | 789 | 1.57 |
| The elemental electron affinity and ionization potentials | |||
|---|---|---|---|
| Element | E a (eV) | E i (eV) | 1/2(Ea + Ei) (eV) |
| Bi | 0.94 | 7.24 | 4.09 |
| I | 3.04 | 10.45 | 6.75 |
| La | −0.46 | 5.57 | 2.55 |
| Co | −0.66 | 7.78 | 3.56 |
| O | −1.46 | 13.62 | 6.08 |
| Potential positions and Mulliken's electronegativities | ||||
|---|---|---|---|---|
| Catalyst | χ (eV) | E g (eV) | E VB (eV) | E CB (eV) |
| BiOI | 5.92 | 2.11 | +2.47 | +0.36 |
| LaCoO3 | 4.62 | 1.91 | +1.07 | −0.84 |
The exponent n is subjected to different values depending on the electronic transition type.78–81 Typical Tauc plots for n = 2 and n = 1/2 can be seen in Fig. 2B and C (others are summarized in the ESI,† Fig. S2). Calculating Eg requires extrapolating the rising slope of curves toward the x-axis or photon energy. As a result of crossing the x-axis, the Eg is calculated for the definite electronic transition considered. A summary of all the values obtained is presented in Table 1.
The composite showed a considerable red shift concerning the bismuth oxycarbonate, indicating its ability to transfer electrons more efficiently. Using the following formulas (5) and (6) we calculated the valence (VB) and conduction band potentials (EVB and ECB) of BiOI and LaCoO3 based on the Eg values for n = 2, the electron free energy (Ee; 4.5 eV versus SHE), and the electronegativity of semiconductors (χ).82
| EVB = χ − Ee + 0.5Eg | (5) |
| ECB = EVB − Eg | (6) |
The χ value is the geometric mean of the semiconductor's atoms’ electronegativity. In addition, it is possible to calculate the atom's electronegativity from its first ionization and electron affinity (Ei and Ea), both of which can be calculated using the formula presented in Table 1.
Furthermore, energy dispersive X-ray spectrum analysis (EDS) and corresponding elemental mapping were performed to confirm the elemental composition and distribution in the hybrid LaCoO3/BiOI. As confirmed by a homogeneous distribution of La, Co, O, Bi, and I elements in the hybrid, LaCoO3 NPs were successfully immobilized on BiOI plate-like surfaces. It can be rationally inferred, based on the XRD, FTIR, SEM, EDS, and elemental mapping results described above, that the LaCoO3/BiOI hybrid was not only well-fabricated but also that a uniform 0D/3D heterojunction exists between LaCoO3 and BiOI that exhibits a strong interfacial interaction. There was a significant amount of contact area between LaCoO3 NPs and BiOI nanosheets in this heterojunction, which increased the rate of interfacial charge transfer, ultimately allowing for increased photocatalytic activity.
3.2. Photodegradation experiments
Fig. 5B shows the effects of the change in the components’ moles in the coupled catalyst on the photocatalytic activity, and the highest activity was reached when the moles of BiOI were two times greater than that of another component. In this optimized value, the largest e/h pairs with the highest isolation extent were reached, which was used in the following steps (see Table S4, ESI†).
 | (7) |
| Y = +78.28 − 3.64A + 7.01B − 10.06C + 1.98D − 0.5425AB + 2.45AC − 9.49AD + 6.39BC − 0.2800BD + 4.89CD − 1.08A2 − 0.9167B2 − 1.66C2 − 1.61D2 | (8) |
| Chosen variables | |||||
|---|---|---|---|---|---|
| Code | Unite | −α | −1 level | +1 level | +α |
| A: pH | — | 4 | 10 | 1 | 13 |
| B: catal. dose | g L−1 | 0.6 | 1 | 0.4 | 1.2 |
| C: CCEF | mg L−1 | 6 | 16 | 1 | 21 |
| D: time | min | 30 | 80 | 5 | 105 |
| ANOVA results | |||||
|---|---|---|---|---|---|
| Source | Sum squares | df | Mean sq. | F value | p-values |
| Model | 6741.9 | 14 | 481.6 | 5026.3 | <0.0001 |
| A: pH | 317.6 | 1 | 317.6 | 3314.5 | <0.0001 |
| B: catal. dose | 1180 | 1 | 1180 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 315.5 315.5 |
0.0005 |
| C: | 2425 | 1 | 2425 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 326.5 326.5 |
<0.0001 |
| D: time | 95 | 1 | 95 | 987 | <0.0001 |
| AB | 4.7 | 1 | 4.7 | 49.2 | <0.0001 |
| AC | 96.2 | 1 | 96.2 | 1004.5 | <0.0001 |
| AD | 1440 | 1 | 1440 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 028.2 028.2 |
<0.0001 |
| BC | 652.6 | 1 | 652.6 | 6811 | <0.0001 |
| BD | 1.3 | 1 | 1.3 | 13.1 | 0.0025 |
| CD | 382 | 1 | 382 | 3989.2 | <0.0001 |
| A 2 | 32 | 1 | 32 | 331.9 | <0.0001 |
| B 2 | 23 | 1 | 23 | 240.6 | <0.0001 |
| C 2 | 76 | 1 | 76 | 790.5 | 0.0001 |
| D 2 | 71.1 | 1 | 71.1 | 790.5 | <0.0001 |
| Residual | 1.44 | 15 | 75.7 | ||
| Lack of fit | 1.25 | 10 | 0.0958 | 3.44 | 0.0925 |
| Pure error | 0.1823 | 5 | 0.1255 | ||
| Cor total | 6743.3 | 29 | |||
| Adj. R2: 0.9989 | Pred. R2: 0.9996 | R 2: 0.9998 | |||
The results were statistically analyzed (ANOVA) at a 95% confidence level to validate the model's goodness. According to Table 2, the model is significant at a 95% confidence interval for the data processing compared to its F-value of 5026 with F(0.05,14,15) = 2.29. To provide further evidence of the soundness of the model, we also analyze the non-significant lake of fit (LOF) term based on an F-value of 3.44 < F0.05,10,5 = 4.74.87
The regression coefficient (R2) value of 0.9998 confirms the model's total efficiency, indicating less than 1% of the total variations cannot be handled. There is a good degree of agreement in light of the close relationship between the experimental and predicted data. All variables selected significantly affected the response, as evidenced by the near value of R2 and adjusted R2 (0.9996). The predicted R2 considers the model's ability to recognize new responses, which was achieved at a value close to the model's merit (0.9998) and predicted R2 (0.9989). Furthermore, the closely predicted R2 and the adjusted R2 also agreed that additional variables had no effect on the response variable. A precision of greater than 4 (288.143) was also achieved, measuring the predicted response versus its standard deviation. The Pareto formula was used to predict the relative importance of each model's term. For the quadratic equation discussed above, βi is the coefficient. A summary of the results is shown in Fig. S4 (ESI†).
 | (9) |
A high catalytic amount, about 1 g L−1, combined with pH 4 resulted in the greatest amount of degradation of cefixime in the study of the simultaneous effects of catalytic amount and pH on cefixime photodegradation (Fig. 6B). The binary catalyst has a pHpzc about 8.9. At pH 4, its surface is expected to be positive. For CEF molecules, two carboxylic acid functional groups have pKa values of 2.1 and 3.9, and most of the second carboxylic acid group will be in carboxylate form. Furthermore, its structure has two sulfur and five nitrogen heteroatoms and many oxygen atoms. Thus, the presence of anionic carboxylate and free electron pairs of these hetero atoms attract the CEF molecules by the catalyst's surface at pH 4, resulting in higher degradation efficiency. It would be expected that CEF anionic species at higher alkaline pHs critically repelled because of the repulsive force between both carboxylic functional groups (are in carboxylate anions) and high amounts of free electron pairs of the structural heteroatoms and the negatively charged catalyst surface. Thus, the lower degradation efficiency at pH 10 relatively compensated by increasing the catalyst dose that available more active sites for the degradation process.
Fig. 6C shows that the adverse effects of alkaline pH cannot be compensated at longer irradiation times. The results generally show lower CEF degradation at alkaline pH values, showing high stability of the anionic CEF because of the probable involvement of the free electrons in resonance effects. Higher CEF degradation efficiency at pH 4 and longer illumination times confirm the formation of relatively stable degradation intermediates that can be degraded at longer times.
Fig. 6D shows how pH and pollutant concentration affect the degradation, with a pH of about 4 and pollutant concentration of 6 mg L−1 giving the best degradation. At the mentioned pH, the catalyst is positively charged and protonated. Consequently, a repulsive force can cause degradation to decrease. These protonated groups may, on the other hand, result in significant destabilization of the cefixime molecules, which allows for maximum degradation. In another way, in moderate concentrations, the collision probability between CEF molecules and the catalyst surface is high enough to reach the highest degradation efficiency. At higher CEF concentrations, H-bonding formation between CEF molecules may be high, resulting in lower interaction of CEF molecules with the catalyst surface. In the high CEF concentration, absorption of a significant part of the arrived photons by CEF molecules may result in the catalyst's lower photo-excitation and lower production of the reactive species.
According to the reasons mentioned above for the effects of the catalyst dose and CEF concentration, higher CEF efficiency can be reached at a lower CEF concentration of about 6 ppm and a higher catalyst dose of about 1 g L−1, as shown in Fig. 6E. Finally, Fig. 6E shows the CEF molecules can be photodegraded at shorter times at a CEF concentration of 6 ppm. From the results, it can be concluded that some intermediate degradation radicles may react at longer times, forming larger species with harder degradation probabilities.
| Aλ = I0 − I | (10) |
I = I0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−εbC) exp(−εbC) | (11) |
It has been proven by Grotthus–Draper's law that absorbed photons have a significant effect on photodegradation rates. Using eqn (12), you can calculate the rate of time-dependent photodegradation under constant irradiance (k0 is a constant). By using a polychromatic beam, the equation above becomes eqn (13)
| −dC/dt = k0·I0(1 − exp(−bC)) | (12) |
| −dC/dt = k0·I0(1 − exp(−bC)) | (13) |
A solution based on eqn (14) can be used when photodegradation intermediates cannot absorb the arrived photons. Based on these formulas, we know that k1 and ε2 are the reactants' reaction rate constants and absorption coefficients, respectively.
| dC/dt = k1(1 − exp(2C)) | (14) |
Throughout the photodegradation process, photons are absorbed by degradation products, decreasing the number of photons absorbed. Thus, the solution absorbed a large amount of light related to the reactant. You can calculate degradation rates using eqn (15) (ε3 is the degradation fragments’ absorption coefficient).
| −dC/dt = k1[1 − exp(−(2C + 3(C0 − C)))](2C)/[(2C) + (C0 − C)] | (15) |
According to the formula below (k1: the pseudo-rate constant), photodegradation can be predicted if the degradation fragments and reactants have similar absorption spectra (or ε2 = ε3).
| −dC/dt = k1[1 − exp(−2C0)][(C)/(C0)] | (16) |
A fourth type of photodegradation process involves high concentrations of reactants, in which the exponential term (exp(−(ε2C + ε3(C0 − C))) in eqn (16) reaches zero (e.g., a zero-order rate process).
| −dC/dt = K1 | (17) |
In terms of reaction kinetics, we can obtain a pseudo-first-order equation by using eqn (17) as an integral format of eqn (18). In consequence, this holds for most photocatalytic degradations including photon-absorbing reactants and intermediates.88–94
| ln(C/C0) = −Kt(or Ct = C0e−kt) | (18) |
A series of photodegradation runs were done at various times to examine the kinetics of the CEF photodegradation using the LaCoO3/BiOI catalyst.
Over time, the final CEF solution's absorbance was decreased, as shown in Fig. 7A. The C/C0 value is calculated according to the maximum wavelength absorbance. Fig. 7B shows the results with the slope (0.053 min−1) representing the CEF photodegradation rate constant as calculated using the Hinshelwood equation Y = −0.0530X − 0.3590 (r2 = 0.9743). The 0.693/k equation can calculate the k-value for a t1/2 time of 13.07 min. A photodegradation reaction takes about 13 min to degrade half of the CEF molecules under the applied conditions.
 | ||
| Fig. 7 (A) and (B) CEF photodegradation results versus time, and (C) COD results of the solutions obtained in case A (inset: the corresponding Hinshelwood plot). | ||
Chemical oxygen demand (COD) experiments were used to measure the mineralization values of CEF molecules during photocatalytic degradation. COD is the extent of the amount of oxygen needed to convert organic substances to inorganic substances, such as H2O and CO2. Other inorganic species can be formed by pollutant molecules depending on the nature of the heteroatom. This means that water or wastewater with a high COD content has a high pollution level. An extensive mineralization extent indicates that a more significant initial pollutant and its degradation fragments will be mineralized during photodegradation. One would expect a low COD value if a COD run was was carried out with such a solution. The results in Fig. 7D illustrate that parent CEF and its degradation fragments were mineralized, resulting in a lower oxygen consumption over the COD process.
As a result of the elongated photodegradation of CEF, the COD remaining in the solution decreased. Based on the COD data, Hinshelwood plots were created. A Hinshelwood equation is derived using Fig. 7C (inset), Y = −0.0610X + 0.1660 (r2 = 0.9957) and a k-value of 0.061 min−1 (t1/2 = 11.36). During photodegradation, k-values were used to determine how fast the mineralized CEF molecules degraded. Based on the k-values, CEF photodegradation and CEF mineralization rates are comparable, and CEF degradation intermediates can be well mineralized during the photocatalytic process.
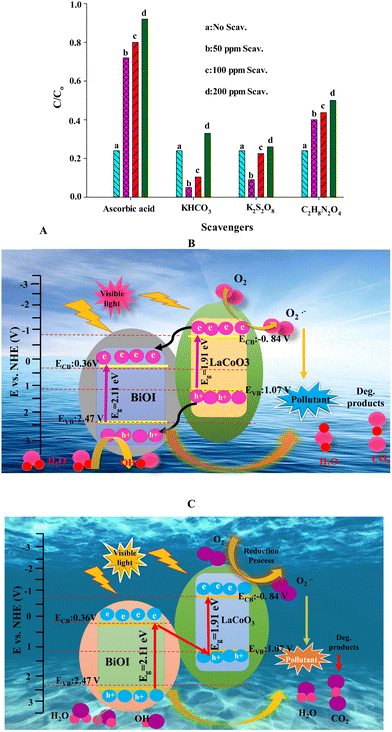 | ||
| Fig. 8 (A) Results of scavenging agents in CEF photodegradation; (B) and (C) suggest mechanisms based on the results in case A. | ||
In heterogeneous photocatalysis, peroxydisulfate (PDS), peromonosulfate, and hydrogen peroxide are electron scavengers. As a result of this scavenging process, PDS is produced as sulfate anion radicals (SO4˙−). There have been reported values of rate constants for PDS and sulfate radical reactions in the pH range of 3–8 involving acidic radicals.96
| S2O82− + heat → 2SO4˙− (k1: 5.7 × 10−5 s−1) |
| SO4˙− + S2O82− → S2O8˙− + SO42− (k2: 6.1 × 10−5 M−1 s−1) |
| SO4˙− + H2O → ˙OH + H+ + SO42− (k3 [H2O] < 2 × 10−3 M−1 s−1) |
| SO4˙− + SO4˙− → S2O82− (k4: 5 × 108 M−1 s−1) |
It is possible to produce powerful ˙OH for photochemical decontamination of freshwater by exposing it to nitrates, nitrites, and dissolved organic matter (DOM).
Some solutes can also scavenge the radicals, including DOM itself, carbonate, and bicarbonate salts derived from inorganic carbon. Regarding kinetics, the scavenging reaction between ˙OH and carbonate or bicarbonate follows a second-order model (k: 3.9 × 108 M−1 s−1 and 8.5 × 106 M−1 s−1, respectively). There is less reactivity in CO3˙− produced compared to ˙OH.97 As a hole scavenger, C2H8N2O4 has excellent performance.98 Through the one or two electron steps redox reactions, ascorbic acid forms semi-dehydroascorbic acid and dehydroascorbic acid (DHA +2H+ + 2e− → ASC + H2O, E° (pH 7) = 60 mV).98
Ascorbic acid can scavenge superoxide radicals. In the presence of acetaldehyde and xanthine oxidase (as the superoxide radical sources), scavenging of superoxide by AA has been reported to have a second-order rate constant of 8.2 × 107 M−1 s−1. Scavenging reactions of this type have also been reported to have a rate constant of 2.7 × 105 M−1 s−1 (in the presence of the xanthine oxidase system).99Fig. 8A shows the inhibition trend as ascorbic acid > ammonium oxalate > KHCO3 ≈ K2S2O8, demonstrating the critical role played by the photogenerated ˙O2− followed by the ˙OH in the photodegradation, based on the following trend.
| Superoxide radicals > photogenerated h+ > photogenerated e− ∼ hydroxyl radicals |
This study shows that superoxide radicals and photoinduced holes play a relatively higher role in CEF photodegradation. The following section will discuss a photodegradation mechanism based on the results obtained here and the VB/CB positions obtained in the DRS section.
Several mechanisms have been described that illustrate photodegradation pathways (the charge carriers transfer) in heterogeneous photocatalysis up to this point. In addition, these mechanisms use direct Z-scheme photocatalysts,100 traditional Z-scheme photocatalysts, S-scheme,101–104 and Z-scheme types II heterojunctions, among others.105,106 These mechanisms have been well-reviewed in the literature.107–112 Usually, type-II photocatalytic mechanisms are used in heterojunction systems. When the semiconductors are considered to be successive binary heterojunctions, this mechanism is illustrated in Fig. 8B. In the fabricated binary catalyst, LaCoO3 and BiOI also exhibit narrow band gaps, so they are appropriate for Vis light applications due to their ability to produce photo-induced e−/h+ pairs after illumination.
For the type-II heterojunction mechanism, photoinduced electrons must be transferred from the LaCoO3-CB position (−0.84 V) to the BiOI-CB position (36 V). Alternatively, photo-induced holes should simultaneously migrate from BiOI-VB (2.47 V) to LaCoO3-VB (1.07 V). Consequently, photo-induced electrons accumulate in the BiOI-CB position while the holes are in the LaCoO3-VB position. The potential positions in this pathway show that the accumulated electrons in the BiOI-CB position, cannot reduce dissolved oxygen to superoxide species (E° = −0.28 V).
Fig. 8C shows a schematic depiction of the direct Z-Scheme mechanism. In this mechanism, BiOI-CB has a more negative potential (0.36 V) than LaCoO3-VB (1.07 V). Thus, photoinduced electrons in BiOI-CB can be transferred to the LaCoO3-VB position after being photoinduced. These electrons' photoexcitation finally results in e-accumulation in the LaCoO3-CB position, as more potent than those accumulated in the BiOI-CB position (see the type II heterojunction mechanism). In other trends, hole-accumulation happened in the BiOI-VB position, as more potent than those accumulated in the LaCoO3-VB (see the type II heterojunction mechanism). This description states that the photo-accumulated electrons in the LaCoO3-CB position can only produce enough superoxide radicals (not by the photoinduced electrons in the BiOI-CB position). In another trend, the accumulated holes in the BiOI-VB position are strong enough to oxidize CEF molecules concerning the accumulated holes in the LaCoO3-VB. Thus, the direct Z-scheme mechanism can describe the CEF photodegradation by the constructed binary catalyst.
Conclusions
This study reports the successful synthesis of a binary LaCoO3/BiOI composite photocatalyst using a facile solvothermal approach. LaCoO3 nanoparticles were immobilized onto the surface of flower-like BiOI species, resulting in a composite with better interface contact. As confirmed by the relative change in band gap energies of 2.32, 2.21, and 2.30 eV for BiOI, LaCoO3, and BiOI/LaCoO3 catalysts, the coupled catalyst experienced a small redshift relative to BiOI alone, but decreased the e/h recombination via the charge carrier transfer between two components. pHpzc values of 5.8 and 10.5 were found for the BiOI and LaCoO3 samples and 10.5 for the coupling sample, confirming the relative difference in native accumulated charges of the catalysts. The relative differences in average crystallite sizes obtained by the Scherrer and W–H formulas confirm the relative role of the strain effect in peak broadening. The LaCoO3/BiOI composite exhibited significantly increased photocatalytic activity to degrade cefixime (CEF) under visible light illumination compared to single BiOI and LaCoO3. A significant part of the improved activity can be attributed to the efficient charge transfer across the heterojunction interface created by the largely conductive LaCoO3 co-catalyst as the acceptor of the photo-excited electrons in the CB-BiOI. Under visible light illumination, the reactive species scavenging runs indicated that superoxide radicals acted as the primary reactive agent for CEF degradation. Over time, the decreased COD impact of the photodegraded CEF solutions confirmed that the CEF degradation intermediates have relatively mineralized into water, carbon dioxide, etc.Data availability
All data used and required are summarized in the ESI.†Conflicts of interest
There are no conflicts to declare.References
- C. H.-F. Lau, K. van Engelen, S. Gordon, J. Renaud and E. Topp, Novel antibiotic resistance determinants from agricultural soil exposed to antibiotics widely used in human medicine and animal farming, Appl. Environ. Microbiol., 2017, 83, e00989–00917 CrossRef CAS PubMed.
- X. Dong, Y. Li, D. Li, D. Liao, T. Qin, O. Prakash, A. Kumar and J. Liu, A new 3D 8-connected Cd(II) MOF as a potent photocatalyst for oxytetracycline antibiotic degradation, CrystEngComm, 2022, 24, 6933–6943 RSC.
- M. Zheng, J. Chen, L. Zhang, Y. Cheng, C. Lu, Y. Liu, A. Singh, M. Trivedi, A. Kumar and J. Liu, Metal organic frameworks as efficient adsorbents for drugs from wastewater, Mater. Today Commun., 2022, 31, 103514 CrossRef CAS.
- B. Van den Bergh, J. E. Michiels, T. Wenseleers, E. M. Windels, P. V. Boer, D. Kestemont, L. De Meester, K. J. Verstrepen, N. Verstraeten and M. Fauvart, Frequency of antibiotic application drives rapid evolutionary adaptation of Escherichia coli persistence, Nat. Microbiol., 2016, 1, 1–7 CrossRef PubMed.
- D. B. Miklos, C. Remy, M. Jekel, K. G. Linden, J. E. Drewes and U. Hübner, Evaluation of advanced oxidation processes for water and wastewater treatment–A critical review, Water Res., 2018, 139, 118–131 CrossRef CAS.
- J. Wang and S. Wang, Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism, Chem. Eng. J., 2020, 401, 126158 CrossRef CAS.
- M. Liu, Y. Ye, J. Ye, T. Gao, D. Wang, G. Chen and Z. Song, Recent Advances of Magnetite (Fe3O4)-Based Magnetic Materials in Catalytic Applications, Magnetochemistry, 2023, 9, 100 CrossRef.
- M. A. Oturan and J.-J. Aaron, Advanced oxidation processes in water/wastewater treatment: principles and applications. A review, Crit. Rev. Environ. Sci. Technol., 2014, 44, 2577–2641 CrossRef CAS.
- M. Cai, Y. Liu, K. Dong, X. Chen and S. Li, Floatable S-scheme Bi2WO6/C3N4/carbon fiber cloth composite photocatalyst for efficient water decontamination, Chin. J. Catal., 2023, 52, 239–251 CrossRef CAS.
- D. Behera, P. Priyadarshini and K. Parida, ZIF-8 metal–organic frameworks and their hybrid materials: emerging photocatalysts for energy and environmental applications, Dalton Trans., 2025, 54, 2681–2708 RSC.
- M. Liu, C. Shan, H. Chang, Z. Zhang, R. Huang, D. W. Lee, W. Qi, Z. He and R. Su, Nano-engineered natural sponge as a recyclable and deformable reactor for ultrafast conversion of pollutants from water, Chem. Eng. Sci., 2022, 247, 117049 CrossRef CAS.
- Y. Wu, X. He, X. Wang, J. Xv, M. Muddassir, I. A. Ansari and A. Zhong, Synergistic efficacy unleashed: Co/Ni-based catalysts as a versatile powerhouse for photocatalytic degradation of ornidazole, Inorg. Chim. Acta, 2024, 568, 122115 CrossRef CAS.
- J. Wang, C. Rao, L. Lu, S. Zhang, M. Muddassir and J. Liu, Efficient photocatalytic degradation of methyl violet using two new 3D MOFs directed by different carboxylate spacers, CrystEngComm, 2021, 23, 741–747 RSC.
- P. Priyadarshini, A. Mishra, S. Nayak and K. Parida, NH2-MIL-125(Ti) and its functional nanomaterials – a versatile platform in the photocatalytic arena, Nanoscale, 2025, 17, 4906–4957 RSC.
- S. Subudhi, S. P. Tripathy and K. Parida, Metal oxide integrated metal organic frameworks (MO@MOF): rational design, fabrication strategy, characterization and emerging photocatalytic applications, Inorg. Chem. Front., 2021, 8, 1619–1636 RSC.
- A. Buthiyappan, A. R. Abdul Aziz and W. M. A. Wan Daud, Recent advances and prospects of catalytic advanced oxidation process in treating textile effluents, Rev. Chem. Eng., 2016, 32, 1–47 CAS.
- M. Ahmaruzzaman, Biochar based nanocomposites for photocatalytic degradation of emerging organic pollutants from water and wastewater, Mater. Res. Bull., 2021, 140, 111262 CrossRef CAS.
- K. A. Isai and V. S. Shrivatava, Photocatalytic degradation of methyl orange using ZnO and Fe doped ZnO: A comparative study, Iran. J. Catal., 2024, 9(3), 259–268 Search PubMed.
- A. Rahmani-Aliabadi and A. Nezamzadeh-Ejhieh, A visible light FeS/Fe2S3/zeolite photocatalyst towards photodegradation of ciprofloxacin, J. Photochem. Photobiol., A, 2018, 357, 1–10 CrossRef CAS.
- C. Shen, X. Li, B. Xue, D. Feng, Y. Liu, F. Yang, M. Zhang and S. Li, Surface plasmon effect combined with S-scheme charge migration in flower-like Ag/Ag6Si2O7/Bi12O17Cl2 enables efficient photocatalytic antibiotic degradation, Appl. Surf. Sci., 2025, 679, 161303 CrossRef CAS.
- A. Singh, A. K. Singh, J. Liu and A. Kumar, Syntheses, design strategies, and photocatalytic charge dynamics of metal–organic frameworks (MOFs): a catalyzed photo-degradation approach towards organic dyes, Catal. Sci. Technol., 2021, 11, 3946–3989 RSC.
- C. El Bekkali, H. Bouyarmane, S. Laasri, A. Laghzizil and A. Saoiabi, Effects of metal oxide catalysts on the photodegradation of antibiotics effluent, Iran. J. Catal., 2024, 8(4), 241–247 Search PubMed.
- C. Rao, L. Zhou, Y. Pan, C. Lu, X. Qin, H. Sakiyama, M. Muddassir and J. Liu, The extra-large calixarene-based MOFs-derived hierarchical composites for photocatalysis of dye: Facile syntheses and contribution of carbon species, J. Alloys Compd., 2022, 897, 163178 CrossRef CAS.
- R.-Q. Yan, G.-H. Liu, Q.-F. Wang, W. Liu and C.-L. Song, Fast Photodegradation of Malachite Green using Nano-ZnO on Ceramic MgAl Carbonate Layered Double Hydroxides Support, Chin. J. Chem. Phys., 2016, 29, 241–244 CrossRef CAS.
- M. Liu, G. Chen, Z. Song, Z. He, A. Zhong and M. Cui, Catalytic Dechlorination of Three Organochlorides by Recyclable Nano-Palladium-Engineered Natural Sponge with Formic Acid, Catalysts, 2024, 14(7), 424 CrossRef CAS.
- J. Zhao, Z. Dang, M. Muddassir, S. Raza, A. Zhong, X. Wang and J. Jin, A New Cd(II)-Based Coordination Polymer for Efficient Photocatalytic Removal of Organic Dyes, Molecules, 2023, 28(19), 6848 CrossRef CAS.
- M. Rezaei, A. A. Ensafi and E. Heydari-Bafrooei, MoS2/SrTiO3−x perovskite heterostructure: Fabrication, characterization, and comprehensive photodegradation study towards Rhodamine B, Colloids Surf., A, 2025, 708, 135993 CrossRef CAS.
- A. Rostami-Vartooni, A. Moradi-Saadatmand, M. Bagherzadeh and M. Mahdavi, Green synthesis of Ag/Fe3O4/ZrO2 nanocomposite using aqueous Centaurea cyanus flower extract and its catalytic application for reduction of organic pollutants, Iran. J. Catal., 2024, 9(1), 27–35 Search PubMed.
- Y. Ao, J. Bao, P. Wang, C. Wang and J. Hou, Bismuth oxychloride modified titanium phosphate nanoplates: a new pn type heterostructured photocatalyst with high activity for the degradation of different kinds of organic pollutants, J. Colloid Interface Sci., 2016, 476, 71–78 CrossRef CAS.
- D. Zhou, B. Yu, Q. Chen, H. Shi, Y. Zhang, D. Li, X. Yang, W. Zhao, C. Liu and G. Wei, Improved visible light photocatalytic activity on Z-scheme g-C3N4 decorated TiO2 nanotube arrays by a simple impregnation method, Mater. Res. Bull., 2020, 124, 110757 CrossRef CAS.
- L. Jing, Y. Xu, C. Qin, J. Liu, S. Huang, M. He, H. Xu and H. Li, Visible-light-driven ZnFe2O4/Ag/Ag3VO4 photocatalysts with enhanced photocatalytic activity under visible light irradiation, Mater. Res. Bull., 2017, 95, 607–615 CrossRef CAS.
- A. A. Santiago, E. M. Macedo, F. K. Oliveira, R. L. Tranquilin, M. D. Teodoro, E. Longo, F. V. Motta and M. R. Bomio, Enhanced photocatalytic activity of CaMoO4/g-C3N4 composites obtained via sonochemistry synthesis, Mater. Res. Bull., 2022, 146, 111621 CrossRef CAS.
- M. Rezaei and A. A. Ensafi, TiO2−x-MoS2 heterostructure: A study of kinetic, thermodynamic, and scavenging agents’ effects in photodegradation, Mater. Sci. Semicond. Process., 2025, 188, 109162 CrossRef CAS.
- S. Li, C. Wang, K. Dong, P. Zhang, X. Chen and X. Li, MIL-101(Fe)/BiOBr S-scheme photocatalyst for promoting photocatalytic abatement of Cr(VI) and enrofloxacin antibiotic: Performance and mechanism, Chin. J. Catal., 2023, 51, 101–112 CrossRef CAS.
- C. Wang, K. Rong, Y. Liu, F. Yang and S. Li, Carbon quantum dots-modified tetra (4-carboxyphenyl) porphyrin/BiOBr S-scheme heterojunction for efficient photocatalytic antibiotic degradation, Sci. China Mater., 2024, 67, 562–572 CrossRef CAS.
- M. Liu, Y. Wan, C. Zhu, G. Chen and X. Li, FeNi bimetallic modified pg-C3N4−x and its photoelectrocatalytic hydrogen production coupling anodic oxidation, Sep. Purif. Technol., 2025, 357, 130142 CrossRef CAS.
- W. Chen, R.-Q. Yan, G.-H. Chen, M.-Y. Chen, G.-B. Huang and X.-H. Liu, Hydrothermal route to synthesize helical CdS@ZnIn2S4 core-shell heterostructures with enhanced photocatalytic hydrogeneration activity, Ceram. Int., 2019, 45, 1803–1811 CrossRef CAS.
- Z. Pan, S. Wang, R. Yan, C. Song, Y. Jin, G. Huang and J. Huang, Enhanced photocatalytic properties of Mn doped CdS catalysts by decomposition of complex precursors, Opt. Mater., 2020, 109, 110324 CrossRef CAS.
- R. Xiang, C. Zhou, Y. Liu, T. Qin, D. Li, X. Dong, M. Muddassir and A. Zhong, A new type Co(II)-based photocatalyst for the nitrofurantoin antibiotic degradation, J. Mol. Struct., 2024, 1312, 138501 CrossRef CAS.
- V. Soni, A. Khosla, P. Singh, V.-H. Nguyen, Q. V. Le, R. Selvasembian, C. M. Hussain, S. Thakur and P. Raizada, Current perspective in metal oxide based photocatalysts for virus disinfection: A review, J. Environ. Manage., 2022, 308, 114617 CrossRef CAS PubMed.
- M. Rezaei, A. A. Ensafi and E. Heydari-Bafrooei, Oxygen vacancy mediated TiO2−x-MoS2/FTO heterostructure as an efficient photoanode for photoelectrochemical water splitting, J. Ind. Eng. Chem., 2025, 146, 589–602 CrossRef CAS.
- H. Sun, Z. Tian, G. Zhou, J. Zhang and P. Li, Exploring the effects of crystal facet in Bi2WO6/BiOCl heterostructures on photocatalytic properties: a first-principles theoretical study, Appl. Surf. Sci., 2019, 469, 125–134 CrossRef CAS.
- C. You, C. Wang, M. Cai, Y. Liu, B. Zhu and S. Li, Improved Photo-Carrier Transfer by an Internal Electric Field in BiOBr/Nrich C3N5 3D/2D S-Scheme Heterojunction for Efficiently Photocatalytic Micropollutant Removal, Acta Phys.-Chim. Sin., 2024, 40, 2407014 CrossRef.
- T. B. Li, G. Chen, C. Zhou, Z. Y. Shen, R. C. Jin and J. X. Sun, New photocatalyst BiOCl/BiOI composites with highly enhanced visible light photocatalytic performances, Dalton Trans., 2011, 40, 6751–6758 RSC.
- S. Li, C. You, K. Rong, C. Zhuang, X. Chen and B. Zhang, Chemically bonded Mn0.5Cd0.5S/BiOBr S-scheme photocatalyst with rich oxygen vacancies for improved photocatalytic decontamination performance, Adv. Powder Mater., 2024, 3, 100183 CrossRef.
- S. Li, K. Dong, M. Cai, X. Li and X. Chen, A plasmonic S-scheme Au/MIL-101(Fe)/BiOBr photocatalyst for efficient synchronous decontamination of Cr(VI) and norfloxacin antibiotic, eScience, 2024, 4, 100208 CrossRef.
- D. Kandi, A. Behera, S. Sahoo and K. Parida, CdS QDs modified BiOI/Bi2MoO6 nanocomposite for degradation of quinolone and tetracycline types of antibiotics towards environmental remediation, Sep. Purif. Technol., 2020, 253, 117523 CrossRef CAS.
- Y. Wang, K. Deng and L. Zhang, Visible light photocatalysis of BiOI and its photocatalytic activity enhancement by in situ ionic liquid modification, J. Phys. Chem. C, 2011, 115, 14300–14308 CrossRef CAS.
- T.-H. Chen, M. Yoshida, S. Tsunekawa, J.-H. Wu, K.-Y. A. Lin and C. Hu, Development of BiOI as an effective photocatalyst for oxygen evolution reaction under simulated solar irradiation, Catal. Sci. Technol., 2020, 10, 3223–3231 RSC.
- J. Cao, B. Xu, B. Luo, H. Lin and S. Chen, Novel BiOI/BiOBr heterojunction photocatalysts with enhanced visible light photocatalytic properties, Catal. Commun., 2011, 13, 63–68 CrossRef CAS.
- A. Mishra, N. Priyadarshini, S. Mansingh and K. Parida, Recent advancement in LaFeO3-mediated systems towards photocatalytic and photoelectrocatalytic hydrogen evolution reaction: A comprehensive review, Adv. Colloid Interface Sci., 2024, 333, 103300 CrossRef CAS PubMed.
- L. Wang, D. Yan, L. Lyu, C. Hu, N. Jiang and L. Zhang, Notable light-free catalytic activity for pollutant destruction over flower-like BiOI microspheres by a dual-reaction-center Fenton-like process, J. Colloid Interface Sci., 2018, 527, 251–259 CrossRef CAS PubMed.
- J. Luo, R. Li, Y. Chen, X. Zhou, X. Ning, L. Zhan, L. Ma, X. Xu, L. Xu and L. Zhang, Rational design of Z-scheme LaFeO3/SnS2 hybrid with boosted visible light photocatalytic activity towards tetracycline degradation, Sep. Purif. Technol., 2019, 210, 417–430 CrossRef CAS.
- H. Aghaei and M. Ghiaci, Use of H3PO4/ZrO2–TiO2–surfactant mixed oxide for catalytic vapor-phase dehydration of 1-octanol, Reaction Kinetics, Mech. Catal., 2020, 131, 233–246 CAS.
- X. Xiao and W.-D. Zhang, Facile synthesis of nanostructured BiOI microspheres with high visible light-induced photocatalytic activity, J. Mater. Chem., 2010, 20, 5866–5870 RSC.
- J. Qin, L. Lin and X. Wang, A perovskite oxide LaCoO 3 cocatalyst for efficient photocatalytic reduction of CO 2 with visible light, Chem. Commun., 2018, 54, 2272–2275 RSC.
- J. Guo, Y.-z Dai, X.-j Chen, L.-l Zhou and T.-h Liu, Synthesis and characterization of Ag3PO4/LaCoO3 nanocomposite with superior mineralization potential for bisphenol A degradation under visible light, J. Alloys Compd., 2017, 696, 226–233 CrossRef CAS.
- D. Kumar, G. Agarwal, B. Tripathi, D. Vyas and V. Kulshrestha, Characterization of PbS nanoparticles synthesized by chemical bath deposition, J. Alloys Compd., 2009, 484, 463–466 CrossRef CAS.
- A. K. Zak, W. A. Majid, M. E. Abrishami and R. Yousefi, X-ray analysis of ZnO nanoparticles by Williamson–Hall and size–strain plot methods, Solid State Sciences, 2011, 13, 251–256 CrossRef.
- N. Pourshirband, A. Nezamzadeh-Ejhieh and S. N. Mirsattari, The CdS/g-C3N4 nano-photocatalyst: brief characterization and kinetic study of photodegradation and mineralization of methyl orange, Spectrochim. Acta, Part A, 2021, 248, 119110 CrossRef CAS PubMed.
- M. Sakthivel, S. Ramaraj, S.-M. Chen and B. Dinesh, Synthesis of rose like structured LaCoO3 assisted functionalized carbon nanofiber nanocomposite for efficient electrochemical detection of anti-inflammatory drug 4-aminoantipyrine, Electrochimica Acta, 2018, 260, 571–581 CrossRef CAS.
- S. Qu, Y. Xiong and J. Zhang, Fabrication of GO/CDots/BiOI nanocomposites with enhanced photocatalytic 4-chlorophenol degradation and mechanism insight, Sep. Purif. Technol., 2019, 210, 382–389 CrossRef CAS.
- Z. Jiang, W. Wan, H. Li, S. Yuan, H. Zhao and P. K. Wong, A hierarchical Z-scheme α-Fe2O3/g-C3N4 hybrid for enhanced photocatalytic CO2 reduction, Adv. Mater., 2018, 30, 1706108 CrossRef PubMed.
- C. Li, A. Zhang, L. Zhang, J. Song, S. Su, Z. Sun and J. Xiang, Enhanced photocatalytic activity and characterization of magnetic Ag/BiOI/ZnFe2O4 composites for Hg0 removal under fluorescent light irradiation, Appl. Surf. Sci., 2018, 433, 914–926 CrossRef CAS.
- L. Yosefi and M. Haghighi, Fabrication of nanostructured flowerlike p-BiOI/p-NiO heterostructure and its efficient photocatalytic performance in water treatment under visible-light irradiation, Appl. Catal., B, 2018, 220, 367–378 CrossRef CAS.
- A. Sobhani-Nasab, M. Eghbali-Arani, S. M. Hosseinpour-Mashkani, F. Ahmadi and M. Rahimi-Nasrabadi, Eco-friendly preparation and characterization of CuMn2O4 nanoparticles with the green capping agent and their photocatalytic and photovoltaic applications, Iran. J. Catal., 2024, 10(2), 91–99 Search PubMed.
- H. R. Pouretedal, M. Fallahgar, F. Sotoudeh Pourhasan and M. Nasiri, Taguchi optimization of photodegradation of yellow water of trinitrotoluene production catalyzed by nanoparticles TiO2/N under visible light, Iran. J. Catal., 2024, 7(4), 317–326 Search PubMed.
- N. Omrani and A. Nezamzadeh-Ejhieh, A quadripartite Cu2O-CdS-BiVO4-WO3 visible-light driven photocatalyst contained three cascade Z-scheme systems: Focus on conditions’ optimization, scavenging agents and the mechanism pathway towards sulfasalazine, Iran. J. Catal., 2025 DOI:10.57647/j.ijc.2025.1502.15.
- N. Mehrabanpour, A. Nezamzadeh-Ejhieh, S. Ghattavi and A. Ershadi, A magnetically separable clinoptilolite supported CdS-PbS photocatalyst: Characterization and photocatalytic activity toward cefotaxime, Appl. Surf. Sci., 2023, 614, 156252 CrossRef CAS.
- S. K. Suram, P. F. Newhouse and J. M. Gregoire, High Throughput Light Absorber Discovery, Part 1: An Algorithm for Automated Tauc Analysis, ACS Comb. Sci., 2016, 18, 673–681 CrossRef CAS PubMed.
- P. Norouzzadeh, K. Mabhouti, M. Golzan and R. Naderali, Investigation of structural, morphological and optical characteristics of Mn substituted Al-doped ZnO NPs: a Urbach energy and Kramers-Kronig study, Optik, 2020, 204, 164227 CrossRef CAS.
- P. Makuła, M. Pacia and W. Macyk, How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra, J. Phys. Chem. Lett., 2018, 9, 6814–6817 CrossRef PubMed.
- J. Tauc, Optical Properties of Solids, ed. F. Abeles, North-Holland Publishing Company, Amsterdam, 1972, ch. 5 Search PubMed.
- J. B. Coulter and D. P. Birnie III, Assessing Tauc Plot Slope Quantification: ZnO Thin Films as a Model System, Phys. Status Solidi B, 2018, 255, 1700393 CrossRef.
- R. Köferstein, L. Jäger and S. G. Ebbinghaus, Magnetic and optical investigations on LaFeO3 powders with different particle sizes and corresponding ceramics, Solid State Ionics, 2013, 249–250, 1–5 CrossRef.
- R. Köferstein and S. G. Ebbinghaus, Investigations of BaFe0.5Nb0.5O3 nano powders prepared by a low temperature aqueous synthesis and resulting ceramics, J. Eur. Ceram. Soc., 2017, 37, 1509–1516 CrossRef.
- K.-i Katsumata, R. Motoyoshi, N. Matsushita and K. Okada, Preparation of graphitic carbon nitride (g-C3N4)/WO3 composites and enhanced visible-light-driven photodegradation of acetaldehyde gas, J. Hazard. Mater., 2013, 260, 475–482 CrossRef CAS PubMed.
- P. Kubelka, Ein beitrag zur optik der farbanstriche, Z. tech. Phys, 1931, 12, 593–601 Search PubMed.
- J. Tauc, R. Grigorovici and A. Vancu, Optical properties and electronic structure of amorphous germanium, Phys. Status Solidi B, 1966, 15, 627–637 CrossRef CAS.
- S. Shanthi, S. Poovaragan, M. Arularasu, S. Nithya, R. Sundaram, C. M. Magdalane, K. Kaviyarasu and M. Maaza, Optical, magnetic and photocatalytic activity studies of Li, Mg and Sr doped and undoped zinc oxide nanoparticles, J. Nanosci. Nanotechnol., 2018, 18, 5441–5447 CrossRef CAS PubMed.
- B. Manikandan and R. John, Properties of sol-gel synthesized multiphase TiO2 (AB)-ZnO (ZW) semiconductor nanostructure: An effective catalyst for methylene blue dye degradation, Iran. J. Catal., 2024, 10(1), 1–16 Search PubMed.
- Y. Nosaka and A. Y. Nosaka, Generation and detection of reactive oxygen species in photocatalysis, Chem. Rev., 2017, 117, 11302–11336 CrossRef CAS PubMed.
- S. Dianat, Visible light induced photocatalytic degradation of direct red 23 and direct brown 166 by InVO4-TiO2 nanocomposite, Iran. J. Catal., 2024, 8(2), 121–132 Search PubMed.
- A. Eslami, A. Oghazyan and M. Sarafraz, Magnetically separable MgFe2O4 nanoparticle for efficient catalytic ozonation of organic pollutants, Iran. J. Catal., 2024, 8(2), 95–102 Search PubMed.
- N. Elmi Fard and R. Fazaeli, Experimental design study of RB 255 photocatalytic degradation under visible light using synthetic Ag/TiO2 nanoparticles: Optimization of experimental conditions, Iran. J. Catal., 2024, 8(2), 133–141 Search PubMed.
- N. Parsafard, Optimization of main parameters affecting activity and octane number produced from catalytic isomerization of n-heptane using response surface methodology, Iran. J. Catal., 2023, 13, 125–133 CAS.
- S. A. Hosseini and R. Saeedi, Photocatalytic degradation of rhodamine B by nano bismuth oxide: Process modeling by response surface methodology (RSM), ( 2017).
- A. Nezamzadeh-Ejhieh and H. Zabihi-Mobarakeh, Heterogeneous photodecolorization of mixture of methylene blue and bromophenol blue using CuO-nano-clinoptilolite, J. Ind. Eng. Chem., 2014, 20, 1421–1431 CrossRef CAS.
- A. Yousefi and A. Nezamzadeh-Ejhieh, Preparation and characterization of SnO2-BiVO4-CuO catalyst and kinetics of phenazopyridine photodegradation, Iran. J. Catal., 2021, 11, 247–259 CAS.
- R. J. Manikandan Balakrishnan, Properties of sol-gel synthesized multiphase TiO2 (AB)-ZnO (ZW) semiconductor nanostructure: an effective catalyst for methylene blue dye degradation, Iran. J. Catal., 2020, 10, 1–16 Search PubMed.
- R. Sheikhsamany, A. Nezamzadeh-Ejhieh, R. Ensandoost and B. Kakavandi, BaTi0. 85Zr0. 15O3/MOF-5 nanocomposite: Synthesis, characterization and photocatalytic activity toward tetracycline, J. Mol. Liq., 2024, 403, 124850 CrossRef CAS.
- R. Sheikhsamany, H. Faghihian and M. Shirani, The MIL100 (Fe)/BaTi0.85Zr0.15O3 nanocomposite with the photocatalytic capability for study of tetracycline photodegradation kinetics, Spectrochim. Acta, Part A, 2023, 291, 122323 CrossRef CAS PubMed.
- B. Divband, A. Jodaie and M. Khatmian, Enhancement of photocatalytic degradation of 4-nitrophenol by integrating Ag nanoparticles with ZnO/HZSM-5 nanocomposite, Iran. J. Catal., 2024, 9(1), 63–70 Search PubMed.
- S. D. Khairnar, M. R. Patil and V. S. Shrivastava, Hydrothermally synthesized nanocrystalline Nb2O5 and its visible-light photocatalytic activity for the degradation of congo red and methylene blue, Iran. J. Catal., 2024, 8(2), 143–150 Search PubMed.
- M. Zebardast, A. Fallah Shojaei and K. Tabatabaeian, Enhanced removal of methylene blue dye by bimetallic nano-sized MOF-5s, Iran. J. Catal., 2024, 8(4), 297–309 Search PubMed.
- K. Govindan, H. T. Chandran, M. Raja, S. U. Maheswari and M. Rangarajan, Electron scavenger-assisted photocatalytic degradation of amido black 10B dye with Mn3O4 nanotubes: a response surface methodology study with central composite design, J. Photochem. Photobiol., A, 2017, 341, 146–156 CrossRef CAS.
- D. Vione, S. Khanra, S. C. Man, P. R. Maddigapu, R. Das, C. Arsene, R.-I. Olariu, V. Maurino and C. Minero, Inhibition vs. enhancement of the nitrate-induced phototransformation of organic substrates by the OH scavengers bicarbonate and carbonate, Water Res., 2009, 43, 4718–4728 CrossRef CAS PubMed.
- T. Tan, D. Beydoun and R. Amal, Effects of organic hole scavengers on the photocatalytic reduction of selenium anions, J. Photochem. Photobiol., A, 2003, 159, 273–280 CrossRef CAS.
- A. Nandi and I. Chatterjee, Scavenging of superoxide radical by ascorbic acid, J. Biosci., 1987, 11, 435–441 CrossRef CAS.
- S. Sultana, S. Mansingh and K. M. Parida, Facile Synthesis of CeO2 Nanosheets Decorated upon BiOI Microplate: A Surface Oxygen Vacancy Promoted Z-Scheme-Based 2D-2D Nanocomposite Photocatalyst with Enhanced Photocatalytic Activity, J. Phys. Chem. C, 2018, 122, 808–819 CrossRef CAS.
- J. Zhang, G. Yu, C. Yang and S. Li, Recent progress on S-scheme heterojunction strategy enabling polymer carbon nitrides C3N4 and C3N5 enhanced photocatalysis in energy conversion and environmental remediation, Curr. Opin. Chem. Eng., 2024, 45, 101040 CrossRef.
- S. Li, K. Rong, X. Wang, C. Shen, F. Yang and Q. Zhang, Design of Carbon Quantum Dots/CdS/Ta3N5 S-Scheme Heterojunction Nanofibers for Efficient Photocatalytic Antibiotic Removal, Acta Physico-Chimica Sinica, 2024, 40, 2403005 CrossRef.
- Z.-C. Zhao, K. Wang, L. Chang, R.-Q. Yan, J. Zhang, M. Zhang, L. Wang, W. Chen and G.-B. Huang, Construction of S-scheme MIL-101(Fe)/Bi2MoO6 heterostructures for enhanced catalytic activities towards tetracycline hydrochloride photodegradation and nitrogen photofixation, Solar Energy, 2023, 264, 112042 CrossRef CAS.
- H. Yang, Z.-C. Zhao, Y.-P. Yang, Z. Zhang, W. Chen, R.-Q. Yan, Y. Jin and J. Zhang, Defective WO3 nanoplates controllably decorated with MIL-101(Fe) nanoparticles to efficiently remove tetracycline hydrochloride by S-scheme mechanism, Sep. Purif. Technol., 2022, 300, 121846 CrossRef CAS.
- Q. Xu, L. Zhang, J. Yu, S. Wageh, A. A. Al-Ghamdi and M. Jaroniec, Direct Z-scheme photocatalysts: Principles, synthesis, and applications, Mater. Today, 2018, 21, 1042–1063 CrossRef CAS.
- P. Priyadarshini, A. Mishra, A. Majhi, K. Parida and K. Parida, Engineering rGO–Driven Z-Scheme Charge Dynamics in NH2-MIL-125(Ti)/ZIF-67 for Superior Green Energy Applications, ACS Appl. Energy Mater., 2025, 8(8), 5067–5081 CrossRef CAS.
- K. Sharma, V. Hasija, M. Malhotra, P. K. Verma, A. A. Parwaz Khan, S. Thakur, Q. Van Le, H. H. Phan Quang, V.-H. Nguyen, P. Singh and P. Raizada, A review of CdS-based S-scheme for photocatalytic water splitting: Synthetic strategy and identification techniques, Int. J. Hydrogen Energy, 2024, 52, 804–818 CrossRef CAS.
- Y. Kumar, A. Sudhaik, K. Sharma, Sonu, P. Raizada, A. Aslam Parwaz Khan, V.-H. Nguyen, T. Ahamad, P. Singh and A. M. Asiri, Construction of magnetically separable novel arrow down dual S-scheme ZnIn2S4/BiOCl/FeVO4 heterojunction for improved photocatalytic activity, J. Photochem. Photobiol., A, 2023, 435, 114326 CrossRef CAS.
- M. Malhotra, K. Poonia, P. Singh, A. A. P. Khan, P. Thakur, Q. Van Le, E. T. Helmy, T. Ahamad, V.-H. Nguyen, S. Thakur and P. Raizada, An overview of improving photocatalytic activity of MnO2 via the Z-scheme approach for environmental and energy applications, J. Taiwan Inst. Chem. Eng., 2024, 158, 104945 CrossRef CAS.
- A. Kumar, P. Singh, V.-H. Nguyen, Q. Van Le, T. Ahamad, S. Thakur, L. Huong Nguyen and P. Raizada, Rationally constructed synergy between dual-vacancies and Z-scheme heterostructured MoS2-x/g-C3N4/Ca-α-Fe2O3 for high-performance photodegradation of sulfamethoxazole antibiotic from aqueous solution, Chem. Eng. J., 2023, 474, 145720 CrossRef CAS.
- K. Sharma, A. Sudhaik, P. Raizada, P. Thakur, X. M. Pham, Q. Van Le, V.-H. Nguyen, T. Ahamad, S. Thakur and P. Singh, Constructing α-Fe2O3/g-C3N4/SiO2 S-scheme-based heterostructure for photo-Fenton like degradation of rhodamine B dye in aqueous solution, Environ. Sci. Pollut. Res., 2023, 30, 124902 CrossRef CAS PubMed.
- V. Dutta, S. Sonu, P. Raizada, V. K. Thakur, T. Ahamad, S. Thakur, P. Kumar Verma, H. H. P. Quang, V.-H. Nguyen and P. Singh, Prism-like integrated Bi2WO6 with Ag-CuBi2O4 on carbon nanotubes (CNTs) as an efficient and robust S-scheme interfacial charge transfer photocatalyst for the removal of organic pollutants from wastewater, Environ. Sci. Pollut. Res., 2023, 30, 124530 CrossRef CAS PubMed.
- M. Sheydaei, H. R. K. Shiadeh, B. Ayoubi-Feiz and R. Ezzati, Preparation of nano N-TiO2/graphene oxide/titan grid sheets for visible light assisted photocatalytic ozonation of cefixime, Chem. Eng. J., 2018, 353, 138–146 CrossRef CAS.
- M. Sheydaei, P. Moharramkhani, B. Ayoubi-Feiz and F. Khodabandeloo, Experimental and computational investigation of cold atmospheric plasma/visible-light/N-TiO2 in treatment of synthetic and real wastewaters, Water Resour. Ind., 2025, 33, 100276 CrossRef CAS.
- S. O. Babalola, P. A. Steenkamp, M. O. Daramola and S. A. Iwarere, Mechanistic study of cefixime degradation with an atmospheric air dielectric barrier discharge – Influence of radical scavengers and metal ion catalyst, Sep. Purif. Technol., 2025, 353, 128376 CrossRef CAS.
- F. Mahdavian, A. Dargahi, M. Vosoughi, A. Mokhtari, H. Sadeghi and Y. Rashtbari, Enhanced removal of cefixime from aqueous solutions using Fe3O4@GO nanocomposite with ultrasonic: isotherm and kinetics study, Desalin. Water Treat., 2022, 280, 224–239 CrossRef CAS.
- S. Aber, L. Ghalamchi, H. Hosseinzadeh, K. Nofouzi and A. Naseri, Biological treatment of ranitidine and cefixime trihydrate mixed solution by activated sludge in sequencing batch reactor, Desalin. Water Treat., 2021, 225, 94–103 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ma00279f |
| This journal is © The Royal Society of Chemistry 2025 |





