Open Access Article
Open Access ArticleAn efficient strategy for simultaneous gold deposition and obtention of hierarchical Au/TS-1 applied to liquid-phase propylene epoxidation†
Ignacio
Centeno-Vega
 *a,
Lorenzo José
González-Rubio
b,
Cristina
Megías-Sayago
a and
Svetlana
Ivanova
b
*a,
Lorenzo José
González-Rubio
b,
Cristina
Megías-Sayago
a and
Svetlana
Ivanova
b
aDepartamento de Química Inorgánica, Instituto de Investigaciones Químicas and Centro de Innovación en Química Avanzada (ORFEO-CINQA), Centro mixto CSIC-Universidad de Sevilla, 41092 Sevilla, Spain. E-mail: icenteno@us.es
bDepartamento de Química Inorgánica e Instituto de Ciencia de Materiales de Sevilla, Centro Mixto CSIC-Universidad de Sevilla, 41092 Sevilla, Spain
First published on 6th June 2025
Abstract
A novel and straightforward one-step method has been developed for the controlled deposition of gold nanoparticles (AuNPs) with uniform diameters onto the titanosilicate (TS-1) zeolitic surface via a direct anionic exchange (DAE) approach. This innovative process simultaneously introduces auxiliary mesoporosity into the zeolite framework, overcoming critical limitations associated with traditional microporous catalysts, including diffusion constraints and rapid deactivation. The resultant hierarchical Au/TS-1 catalyst demonstrates remarkable enhancements in catalytic performance for the liquid-phase propylene epoxidation with H2O2 coupled with outstanding stability, a challenge that has long hindered the application of such materials. With its exceptional catalytic properties and simplified preparation procedure, this system represents a significant advancement in catalyst design. The developed material shows great potential for industrial applications and paves the way for the creation of next-generation catalysts essential for sustainable development.
1. Introduction
Plastic materials have undergone exponential growth in production during the last decades due to their ease and low cost of manufacturing in addition to their versatility. In 2019, it was estimated that worldwide industrial production of plastics reached a value of 460 million tons,1 with the majority (around 99%) coming from fossil-fuel-based resources.2 In our current society these materials are truly essential and it would be almost impossible to imagine a world without their presence so, evidently, this industry will keep growing every year, with the annual demand of these materials being expected to triple by 2060 to keep up with population increase.3 Of the total current synthetic plastic production, the packaging sector represents the largest plastic market, accounting for 40% of all the polymers generated by this industry.2,4 This industry is currently designed on the basis of a linear economy, founded on the single-usage of plastics which are readily discarded afterwards, producing an excessive amount of waste that has not been designed to be easily recyclable. Therefore, the industry needs to shift to a circular economy and use the mentioned materials, designing and producing them from renewable feedstocks while maintaining their properties for the corresponding purpose, resulting in the reduction of the associated carbon footprint, which was estimated to be 860 million tons in 2019.4 In this sector, polyethylene terephthalate (commonly referred to as PET) is of great relevance for the bottling of various beverages, accounting for 7% of global plastic production.2,5,6 Although it is a recyclable material, its production involves non-renewable fossil feedstocks, in addition to being a material that does not decompose easily when disposed of in the environment, thus contributing to soil and water pollution.A sustainable alternative to petroleum-derived polymers is polypropylene furanoate (PPF), which stands out as a competitive and bio-renewable option. PPF is a new generation plastic with matching properties to PET (even outperforming it in certain aspects such as improved barrier properties for O2 and CO2),7–10 which can be obtained through the polymerization of 2,5-furandicarboxylic acid (an oxidation product of the platform chemical HMF derived from biomass) and propylene glycol. A sustainable approach for the production of glycol is to use plastic waste (such as polyethylene and polypropylene) as starting feedstock, an inexpensive source of hydrocarbon chains which has proven to be exceptionally promising for the selective obtention of propylene through cutting-edge metathesis processes.11–14 Subsequently through its corresponding epoxidation and posterior ring-opening hydration, the respective diol could be obtained starting from propylene. This way a truly circular process could be developed, reducing the amount of fossil-derived plastic waste found in the environment by replacing it with its bio-based counterpart PPF.
The traditional industrial routes for propylene oxide (PO) synthesis, namely chlorohydrin and hydroperoxide processes, are efficient but present significant environmental challenges. In the past years, an environmentally benign process known as the HPPO (hydrogen peroxide propylene oxide) process has risen as the most promising alternative due to the obtention of water as the sole by-product and the use of hydrogen peroxide as a green oxidant. However, methanol is also employed in the process to increase propylene solubility and because of the mixture of solvents employed, the resulting epoxide is susceptible to suffer from ring-opening reactions due to the nucleophilic attack of the solvent, whether methanol or water. This way, the corresponding glycol can be obtained through the hydrolysis of the epoxide, while other products can also be found such as monomethyl ethers of propylene glycol, 1-methoxypropan-2-ol (1M2P) and 2-methoxypropan-1-ol (2M1P), highlighting the need for the development of a highly selective catalyst in this transformation (Fig. 1). In this process, titanium silicalite-1 (TS-1), a microporous zeolite with an MFI-type structure, is used as a catalyst due to the presence of tetrahedrally coordinated Ti(IV) sites within the TS-1 framework which are capable of activating H2O2. Despite its remarkable activity in the oxidation of organic substrates, TS-1 suffers from limitations in liquid-phase reactions because of its purely microporous structure, restricting the diffusion of larger molecules thus hindering its catalytic efficiency, in addition to poor stability and rapid deactivation due to pore blockage and carbon deposition. To address these limitations, zeolites can be endowed with a hierarchical structure through the creation of mesopores alongside the intrinsic micropores, allowing better access to active sites and avoiding their deactivation by pore-blockage or carbon deposition.
 | ||
| Fig. 1 Obtention of propylene glycol through HPPO route and posterior polymerization with FDCA to produce PEF. | ||
In this study, several TS-1 catalysts with different Si/Ti ratios have been synthesized and subsequently used as support for the deposition of gold nanoparticles through a direct anionic exchange (DAE) procedure which simultaneously introduces auxiliary mesoporosity in the zeolitic framework and increases the number of catalytically active tetrahedral Ti sites in the silicalite surface. The resulting TS-1 and Au/TS-1 catalysts’ performance has been studied in the H2O2-assisted liquid phase epoxidation of propylene and in the case of the gold-containing catalysts they were compared to their conventional counterparts prepared by incipient wetness impregnation and colloidal deposition. Key parameters influencing catalytic activity and selectivity were systematically analyzed, such as gold loading, Si/Ti molar ratio and presence of auxiliary mesoporosity. Additionally, catalyst stability was assessed over multiple consecutive runs.
2. Experimental section
2.1. Materials
For the synthesis of TS-1 zeolites with different Si/Ti molar ratios the following precursors were employed: tetraethyl orthosilicate (TEOS, ≥98%, Sigma-Aldrich) as a Si source, tetraethyl orthotitanate (TEOT, ≥97%, Sigma-Aldrich) as a Ti source and tetrapropylammonium hydroxide (TPAOH, 1 M aqueous solution, Sigma-Aldrich) as a structure directing agent. In the posterior deposition of gold nanoparticles HAuCl4 (Johnson Matthey) was employed as an Au precursor and NH3 (Sigma-Aldrich) was used for the DAE procedure. For the preparation of the Au/TS-1 catalyst through colloidal deposition polyvinyl alcohol (PVA, Sigma Aldrich) and NaBH4 (Alfa-Aesar) were required.For the liquid-phase catalytic epoxidation tests the following reagents were used: hydrogen peroxide (H2O2 30% wt Sigma-Aldrich), methanol (≥99.9%, Merck Millipore) and propylene (99.95%, Linde). Additionally, for the residual hydrogen peroxide concentration titration KMnO4 (≥99%, Panreac) and Na2C2O4 (≥99.5%, Sigma-Aldrich) were employed.
2.2. Preparation of TS-1 catalysts
TS-1 zeolites employed in this research have been synthesized following the two-step hydrolysis procedure described by Deng et al.15 Initially, a mixture of the entirety of TEOT (0.375–1.5 mmol) to be employed in the reaction and a third of the total amount of TEOS (25 mmol) is prepared. This mixture is added dropwise into a 1 M TPAOH solution under constant stirring at room temperature. The resulting gel is heated at 323 K for 30 min in a hydrolysis step and subsequently temperature is increased to 353 K for another 30 minutes in order to promote alcohol removal by evaporation, obtaining a sol with molar composition: 1.0SiO2:(0.015–0.06)TiO2:0.7TPAOH:38H2O.After cooling down, the remaining TEOS (50 mmol) is added to the previously prepared sol dropwise under continuous stirring. Moreover, 20 mL of distilled water is poured into the vial to favor hydrolysis and replenish the lost volume in the system. The heating steps for hydrolysis and alcohol removal are repeated under the same conditions as the previous step. The resulting mixture is transferred to a stainless-steel autoclave where its crystallization will take place for 48 hours at 443 K. The obtained solid is recovered and washed several times through centrifugation. Ultimately, said whitish dust is dried overnight at 373 K and calcined at 873 K for 6 hours to remove the remaining organic species present in the framework's pores. The resulting zeolites are designated as “TS_X” being X the molar ratio Si/Ti for each sample.
2.3. Au nanoparticle deposition over TS-1
For the deposition of gold nanoparticles over the surface of previously prepared titanium silicalite a direct anionic exchange (DAE) procedure assisted by NH3 was followed.16,17 Deposition of gold (1% wt) in the several zeolitic supports was achieved through their addition to a HAuCl4 aqueous solution (10−4 M) at 343 K under constant stirring. Once mixed, the solution was aged for 20 minutes before the addition of 20 mL NH3. The resulting slurry was finally filtered, washed with water and dried at 373 K overnight. Subsequently, samples are reduced at 423 K for 1 hour under a 4% H2 flow and ultimately calcined at 573 K for 1 hour in an 8% O2 flow (both balances performed with N2 as inert gas). The resulting zeolitic catalysts are labeled “Au/TS_X” being “X” once again the molar Si/Ti ratio of each sample.Moreover, conventional Au/TS-1 catalysts were prepared by incipient wetness impregnation and colloidal deposition for comparison. For the colloidal method, a 5 × 10−4 M solution of HAuCl4 was employed upon which was added a PVA solution (1% wt aqueous solution) in order to reach a PVA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au weight ratio of 0.85. After 20 minutes of stirring, the required amount of a freshly prepared 0.1 M NaBH4 solution was poured until achieving a NaBH4
Au weight ratio of 0.85. After 20 minutes of stirring, the required amount of a freshly prepared 0.1 M NaBH4 solution was poured until achieving a NaBH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au molar ratio equal to 10. Upon additional 20 minutes of stirring, the resulting colloid was put in contact with the adequate amount of TS-1 in order to have a nominal gold loading of 1 wt%. The obtained mixture was centrifuged at 15
Au molar ratio equal to 10. Upon additional 20 minutes of stirring, the resulting colloid was put in contact with the adequate amount of TS-1 in order to have a nominal gold loading of 1 wt%. The obtained mixture was centrifuged at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm with the aim to successfully anchor the entirety of the colloidal gold into the support. Subsequently, the sample was filtered, dried overnight at 100 °C and finally calcined at 300 °C for 2 h in static air, obtaining the catalyst labeled as Au/TS_30-C (C stands for “colloidal”).18 The sample prepared by incipient wetness impregnation was named Au/TS_30-I (I stands for “impregnation”).
000 rpm with the aim to successfully anchor the entirety of the colloidal gold into the support. Subsequently, the sample was filtered, dried overnight at 100 °C and finally calcined at 300 °C for 2 h in static air, obtaining the catalyst labeled as Au/TS_30-C (C stands for “colloidal”).18 The sample prepared by incipient wetness impregnation was named Au/TS_30-I (I stands for “impregnation”).
2.4. Catalytic tests
Catalytic activity of the previously synthesized TS_X zeolites and their gold-modified analogues (Au/TS_X) was analyzed in the liquid phase epoxidation of propylene. These trials were carried out in a 25 mL Parr autoclave reactor, equipped with mechanical stirring in addition to a precise temperature and precision control.In a typical run 150 mg catalyst, 10 mL methanol and 2.58 mL H2O2 (30 mmol, 35% wt) were fed into the reactor. Once the reactor has been sealed and checked for leaks with N2, it is purged twice with propylene to replace internal air. Reaction was performed at 323 K for 2 hours with constant stirring under a propylene pressure of 0.4 MPa. After this period, the reactor is cooled down with an ice bath and a reaction sample is taken through microfiltration for its posterior product identification by gas chromatography in a Shimadzu GC-2010 chromatograph equipped with an Sh-rxi-5ms column coupled to a Shimadzu GCMS-TQ8040 mass spectrometer. Product quantification was performed in a Shimadzu GC-2010 Pro chromatograph equipped with the same column and a flame ionization detector (FID). In order to be able to carry out the quantification, the response factors of each compound had been previously determined using acetonitrile as an internal standard. Residual H2O2 concentration in the liquid phase is determined by indirect permanganometric titration, through its previous standardization with sodium oxalate. The catalytic performance was evaluated using the following expressions (eqn (1)–(4)):
 | (1) |
 | (2) |
 | (3) |
 | (4) |
 and nH2O2 represent the initial and final molar amount of hydrogen peroxide, respectively.
and nH2O2 represent the initial and final molar amount of hydrogen peroxide, respectively.
2.5. Characterization
XRD measurements were performed in an X’Pert Pro PANalytical diffractometer equipped with a Cu anode and working at 45 kV/40 mA. Diffractograms were registered in the 5 to 90° 2θ range with a 0.05° step and 300 seconds of acquisition time. Structure and phase determination were conducted in comparison to the database PDF2 ICDD2000 (Powder Diffraction File 2 International Center for Diffraction Data).Catalysts’ textural properties were determined through N2 physisorption at 77 K in a Micromeritics Tristar II equipment. Previous to the adsorption, samples were degassed at 573 K for 4 hours in a Micromeritics 061 VacPrep vacuum system. BET method was employed to determine specific surface area through the adsorption isotherms and pore size distribution was calculated according to the BJH method.
Inductively couple plasma optical emission spectroscopy (ICP-OES) was employed in order to perform the elemental and quantitative analysis of the synthesized catalysts. Si, Ti and Au content was determined using an iCAP 7200 ICP-OES Duo (ThermoFisher Scientific) spectrometer. To carry out the measurements samples were previously digested in an acid media employing a microwave digestion system ETHOS EASY (Milestone).
TS-1 zeolite particles were visualized through scanning electron microscopy (SEM) in an S4800 Hitachi equipment with a 200 kV acceleration potential. After gold nanoparticles’ deposition in the zeolitic surface, samples were analyzed in a high-resolution transmission electron microscope (HR-TEM) JEOL 2100Plus (200 kV) equipped with a LaB6 filament. Digital images were taken with a CCD camera (Gatan) with the samples being deposited over a copper grid.
X-Ray photoelectron spectroscopy (XPS) experiments were performed in a PHOIBOS-100 spectrometer with a nonmonochromatic Al-Kα (1486.6 eV). Low resolution survey spectra were obtained with a pass energy = 50 eV, while high energy resolution spectra of detected elements (i.e., Au4f, Ti2p, C1s, O1p, and Si2p) were obtained with a pass energy = 35 eV. The analytical chamber operates at ultra-high vacuum at around 10−9 torr pressure. The XPS spectra are recorded at room temperature and referenced to the C1s, adjusted to 284.6 eV and fitted using CasaXPS software with Gaussian–Lorentzian peak shapes and Shirley baselines.
3. Results and discussion
3.1. Characterization of TS-1 and Au/TS-1 catalysts
Tables 1 and 2 display the textural properties of the several catalysts studied in this research. In general terms, it can be affirmed that as the Ti content is increased the BET surface area is also enhanced due to silicalite unit cell expansion because of the introduction of titanium species in the MFI-type structure, as previously reported in the literature.24,26,27 All TS_X samples exhibit similar pore volume; however, it can be seen how for the ones with the highest Ti content the mesopore proportion is increased to the detriment of micropores (Table 1, Samples TS_20 and TS_30). After gold deposition, all catalysts exhibit increased surface area, pore volume and pore size in comparison to their pure titanosilicate counterparts. During the deposition process, the change in the pH values to which the zeolite is subjected due to the presence of NH3 may cause the titanosilicate to undergo ammonia-assisted surface etching, further enhancing its surface area due to the formation of additional porosity. Aforementioned auxiliary porosity introduced in the samples during the gold deposition procedure is made up solely of mesopores, since it can be seen how equivalent samples show the same (or even lower, due to micropore blockage by the gold nanoparticles) values of microporosity while its mesoporosity is greatly enhanced after the DAE procedure (e.g.Table 1, entry TS_20 vs.Table 2, entry Au/TS_20), as illustrated in Fig. 4 displaying the change in pore size distribution after the deposition of Au nanoparticles. This way, we can confirm the successful controlled introduction of uniform intracrystalline mesopores of ca. 4 nm width in the TS-1 samples, achieving an enhanced mesoporosity at the expense of microporosity.
| Sample | Surface area (m2 g−1) | Pore volume (cm3 g−1) | Pore size (Å) | ||||
|---|---|---|---|---|---|---|---|
| S BET | S micro | S BJH | V T | V micro | V meso | ||
| TS_20 | 389 | 110 | 279 | 0.21 | 0.05 | 0.16 | 21.68 |
| TS_30 | 417 | 142 | 275 | 0.23 | 0.06 | 0.17 | 21.85 |
| TS_80 | 350 | 259 | 91 | 0.21 | 0.12 | 0.09 | 23.70 |
| TS_100 | 351 | 250 | 101 | 0.22 | 0.12 | 0.10 | 24.90 |
| Sample | Surface area (m2 g−1) | Pore volume (cm3 g−1) | Pore size (Å) | ||||
|---|---|---|---|---|---|---|---|
| S BET | S micro | S BJH | V T | V micro | V meso | ||
| Au/TS_20 | 429 | 130 | 299 | 0.34 | 0.05 | 0.29 | 31.48 |
| Au/TS_30 | 449 | 127 | 322 | 0.34 | 0.05 | 0.29 | 30.71 |
| Au/TS_80 | 434 | 186 | 248 | 0.41 | 0.09 | 0.32 | 38.14 |
| Au/TS_100 | 444 | 204 | 240 | 0.42 | 0.10 | 0.32 | 38.04 |
 | ||
| Fig. 5 SEM images of synthesized catalyst (a) TS_20 and (b) after its corresponding gold deposition; (c) synthesized TS_80 and (d) after its corresponding gold deposition. | ||
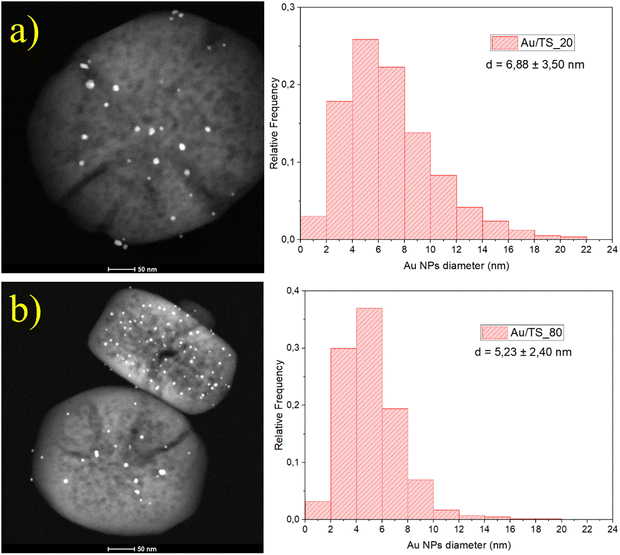
In Fig. 6 it can be observed a representative TEM image taken with a HAADF detector of both Au/TS_X catalysts previously analyzed by SEM along with their respective size distribution histogram for the Au nanoparticles. This way it is evidenced once again by the additional mesoporous structure that has been induced during the deposition procedure and etching treatment, resulting in wider pores that penetrate deep into the particle structure. Regarding the gold nanoparticles, it can be stated that they have been introduced in a controlled way, obtaining highly dispersed particles throughout the surface with a narrow size range that obeys a normal distribution.
| Catalyst | Si/Ti (ICP) | % Au wt (ICP) | Au NPs diameter (nm) |
|---|---|---|---|
| Au/TS_20 | 23.8 | 1.13 | 6.88 ± 3.50 |
| Au/TS_30 | 25.4 | 0.92 | 5.72 ± 3.01 |
| Au/TS_80 | 81.4 | 0.96 | 5.23 ± 2.40 |
| Au/TS_100 | 108.7 | 1.07 | 5.16 ± 2.06 |
| Au/TS_120 | 115.4 | 0.84 | 3.66 ± 1.50 |
| Au/TS_150 | 149.1 | 0.55 | 5.67 ± 2.60 |
A detailed analysis of the Ti 2p high resolution spectra (Fig. 7) reveals two doublets, the one at 458.2 ± 0.2 eV assigned to octahedral Ti like the one present in titania and the second at 460.2 ± 0.2 eV assigned to tetrahedral Ti incorporated to the zeolite framework.42–44
While the tetrahedral coordination refers to titanium accommodated into the silica framework the octahedral coordination derives either from the conversion of tetracoordinated titanium located at the surface to octahedrally coordinated through reaction with water or to the segregation of TiO2 during TS-1 synthesis. Considering the lower Si/Ti ratio of this sample one can presume that an important part of Ti is segregated as octahedral Ti. The fresh TS-30 sample shows a TiOh/TiTd ratio of 2 which diminishes after the gold deposition (Table S1, ESI†) due to the decreased TiO2 contribution after ammonia treatment. On the other hand, the surface Si/Ti ratio is close to the measured by ICP and increases after gold deposition due to the decrease of the Ti surface composition owing to the ammonia-assisted etching, being the TiOh loss much more important than that of TiTd, resulting in an increase in the number of catalytically active sites relative to silicalite weight.
As for gold, the surface composition of the Au/TS_30 sample indicates a very good dispersion and pure metallic state (Fig. S4, ESI†).
3.2. Propylene epoxidation
For the zeolites with the lower Ti content the overall propylene conversion and PO yield are still rather low even though they still show a remarkable selectivity. As the Si/Ti ratio is decreased, the PO yield is greatly enhanced at the cost of sacrificing its initial selectivity. Catalyst Au/TS_30 achieves a PO yield close to 50% while still maintaining its selectivity value proximate to 90%, reaching a compromise between both parameters and resulting in the most promising catalyst for this transformation due to its minority production of secondary compounds. Increasing the Ti content even further results in a slight improvement of PO yield, displaying an accurate exponential relationship between the obtained PO yield and the Si/Ti ratio of the studied catalysts, as shown in Fig. S5 (ESI†). This increase in activity noticeably enhances MP production as well, as a result of using methanol as a solvent in the process.
Usage of water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol mixtures could shift the catalyst selectivity towards the production of PG, leading to an increased yield of this compound and resulting in a promising and greener process for the in situ production of bio-based polymers such as PPF. Besides the drop in selectivity with this catalyst we can highlight its outstanding performance in the activation of propylene, being the catalyst that provides the highest conversion values.
methanol mixtures could shift the catalyst selectivity towards the production of PG, leading to an increased yield of this compound and resulting in a promising and greener process for the in situ production of bio-based polymers such as PPF. Besides the drop in selectivity with this catalyst we can highlight its outstanding performance in the activation of propylene, being the catalyst that provides the highest conversion values.
As for the use of hydrogen peroxide as an oxidant, it is evidenced that the presence of gold nanoparticles in the catalyst enhances both the conversion and efficiency in its usage by promoting H2O2 activation. This way, the synergistic interaction between the gold nanoparticles and the Ti active sites can be confirmed, resulting in higher yields for PO formation in this transformation.45
Catalysts prepared with the DAE procedure and their counterparts obtained by impregnation and colloidal deposition are compared in Table S4 (ESI†) where their catalytic performances are summarized and it can be observed that the DAE procedure stands out with the highest PO yield. Gold deposition through other methods not only does not achieve the same values as DAE but also worsens the catalytic activity of bare TS_30, promoting the formation of side-products, mainly MP, at the expense of reduced PO yields. Gold nanoparticle size can be ruled out as the possible explanation for this loss of selectivity, since colloidal deposition (Au/TS_30-C) achieves small particles of controlled-size that are outperformed by its DAE equivalent. Despite the lower selectivities, gold presence improves propylene conversion in every case through enhanced H2O2 activation. Therefore, it can be stated with certainty that the gold deposition method plays a crucial role in enhancing this transformation, not only by facilitating propylene conversion and increasing oxidant utilization efficiency, but also by tailoring TS-1 inherent textural properties in a simple deposition procedure. In this way, enlarged pore channels are obtained, favoring mass transfer and diffusion processes, which in addition to the improved hydrophobicity of the resulting surface, reduced acid strength and the exposure of a larger number of active sites (tetrahedral Ti species, as evidenced by XPS analysis) translates into an enhanced propylene conversion and PO yield.29,31,32,39,40,46,47
Activity studies were also carried out over different reaction times in order to gather a better insight into Au/TS_30 catalyst's performance, as shown in Fig. 9. It can be observed how for an initial reaction time of 30 minutes PO is exclusively formed, highlighting the outstanding selectivity achieved with this catalyst when propylene is the only compound present in the media. As the reaction time is increased to 2 hours and PO starts accumulating in the reactor, the extent to which ring-opening reactions take place is enhanced, detecting noticeable quantities of PG and MP, thus resulting in a decline of PO selectivity. Further extending the reaction time to 4 hours does not lead to the conversion of any additional propylene, suggesting that at this point only the previously formed PO is the reactive species and exclusively ring-opening reactions are taking place, diminishing the resulting PO yield. This fact can be attributed to pore-blocking deactivation by PO physisorption as a plausible mechanism, in addition to the selective chemical deactivation of Ti active sites through PO chemisorption forming a bidentate ether species among Ti–OH and adjacent Si–OH groups.48,49
4. Conclusions
In summary, a simple and straightforward one-step process has been developed for the controlled deposition of gold nanoparticles of uniform diameter in a titanosilicate zeolitic surface through a direct anionic exchange (DAE) procedure, simultaneously introducing auxiliary mesoporosity in the framework and removing detrimental octahedral Ti species, exposing a larger amount of catalytically active tetrahedral Ti sites. The resulting hierarchic system addresses the main drawbacks encountered with these catalysts, such as diffusion issues and rapid deactivation, associated with their microporous nature. This way, the obtained Au_TS-1 catalyst displays an improved performance in the propylene epoxidation reaction along with outstanding stability typically challenging to achieve with these catalysts. Therefore, due to the exceptional performance of the developed catalyst and its extraordinarily simple preparation procedure without the need for a separate hierarchization step, we do believe that this material can have great relevance in industrial applications upon further optimization, paving the way for the design of new-generation materials that will be truly essential for the sustainable development of our society.Author contributions
Ignacio Centeno-Vega: conceptualization, data curation, writing – original draft, writing – review & editing. Cristina Megías-Sayago: conceptualization, methodology, supervision, validation, writing – review & editing. Svetlana Ivanova: conceptualization, methodology, supervision, validation, writing – review & editing. Lorenzo José González Rubio: investigation, data curation, writing – original draft.Data availability
Data for this article, including textural properties of the synthesized catalysts, calibration curves for the epoxidation products and catalytic activities are available at SienceDB at https://www.scidb.cn/en/s/vQrM3m.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
I. Centeno-Vega would like to thank the Spanish Catalysis Society (SECAT) for the funding as part of the program of scholarships for the completion of a master's thesis in catalysis, as well as the Spanish Ministry of Science, Innovation and Universities for their support through an FPU grant (Ref. FPU23/00446). C. Megías-Sayago acknowledges the European Union's Horizon 2020 research and innovation programme for the funding received for this work under the Marie Skłodowska-Curie grant agreement no. 101022598.References
- Organisation for Economic Cooperation and Development (OECD), Global Plastics Outlook: Economic drivers, environmental impacts and policy options, 10.1787/de747aef-en, (accessed 17 June 2024).
- M. Rabnawaz, I. Wyman, R. Auras and S. Cheng, Green Chem., 2017, 19, 4737–4753 RSC.
- Organisation for Economic Cooperation and Development (OECD), Global Plastics Outlook: Policy Scenarios to 2060, 10.1787/aa1edf33-en, (accessed 17 June 2024).
- P. Sahu, A. Thorbole and R. K. Gupta, ACS Sustainable Chem. Eng., 2024, 12(18), 6811–6826, DOI:10.1021/acssuschemeng.4c01123.
- G. Lopez, M. Artetxe, M. Amutio, J. Bilbao and M. Olazar, Renewable Sustainable Energy Rev. 73, 2017, 346–368 DOI:10.1016/j.rser.2017.01.142.
- A. Carniel, N. Ferreira dos Santos, F. S. Buarque, J. V. Mendes Resende, B. D. Ribeiro, I. M. Marrucho, M. A. Z. Coelho and A. M. Castro, Green Chem., 2024, 26, 5708–5743 10.1039/d4gc00528g.
- Z. Terzopoulou, L. Papadopoulos, A. Zamboulis, D. G. Papageorgiou, G. Z. Papageorgiou and D. N. Bikiaris, Polymers, 2020, 12(6), 1209, DOI:10.3390/POLYM12061209.
- K. Loos, R. Zhang, I. Pereira, B. Agostinho, H. Hu, D. Maniar, N. Sbirrazzuoli, A. J. D. Silvestre, N. Guigo and A. F. Sousa, Front. Chem., 2020, 8, 585, DOI:10.3389/fchem.2020.00585.
- H. Zhang, G. Zhou, M. Jiang, H. Zhang, H. Wang, Y. Wu and R. Wang, Macromolecules, 2020, 53, 5475–5486 CrossRef CAS.
- S. K. Burgess, R. M. Kriegel and W. J. Koros, Macromolecules, 2015, 48, 2184–2193 CrossRef CAS.
- N. M. Wang, G. Strong, V. Dasilva, L. Gao, R. Huacuja, I. A. Konstantinov, M. S. Rosen, A. J. Nett, S. Ewart, R. Geyer, S. L. Scott and D. Guironnet, J. Am. Chem. Soc., 2022, 144, 18526–18531 CrossRef CAS PubMed.
- R. J. Conk, S. Hanna, J. X. Shi, J. Yang, N. R. Ciccia, L. Qi, B. J. Bloomer, S. Heuvel, T. Wills, J. Su, A. T. Bell and J. F. Hartwig, Science, 2022, 377, 1561–1566 CrossRef CAS PubMed.
- V. Farkas, P. Albrecht, Á. Erdélyi, M. Nagyházi, B. Csutorás, G. Turczel, N. Miskolczi, J. Bobek-Nagy, O. Osterthun, J. Klankermayer and R. Tuba, Green Chem., 2024, 26, 10225–10231 RSC.
- R. J. Conk, J. F. Stahler, J. X. Shi, J. Yang, N. G. Lefton, J. N. Brunn, A. T. Bell and J. F. Hartwig, Science, 2024, 385, 1322–1327 CrossRef CAS PubMed.
- X. Deng, Y. Wang, L. Shen, H. Wu, Y. Liu and M. He, Ind. Eng. Chem. Res., 2013, 52, 1190–1196 CrossRef CAS.
- S. Ivanova, C. Petit and V. Pitchon, Appl. Catal., A, 2004, 267, 191–201 CrossRef CAS.
- C. Megías-Sayago, A. Lolli, S. Ivanova, S. Albonetti, F. Cavani and J. A. Odriozola, Catal. Today, 2019, 333, 169–175 CrossRef.
- C. Megías-Sayago, J. L. Santos, F. Ammari, M. Chenouf, S. Ivanova, M. A. Centeno and J. A. Odriozola, Catal. Today, 2018, 306, 183–190 CrossRef.
- M. Liu, Z. Huang, W. Wei, X. Wang and Y. Wen, Front. Chem., 2021, 9, 682404 CrossRef CAS PubMed.
- X. Feng, N. Sheng, Y. Liu, X. Chen, D. Chen, C. Yang and X. Zhou, ACS Catal., 2017, 7, 2668–2675 CrossRef CAS.
- H. Huang, J. Zheng, K. Wu, J. Lin, X. Lin, Q. Yao, Q. Fan, D. Wang, Y. Huang, J. Jiang and Z. Zheng, J. Anal. Appl. Pyrolysis, 2024, 182, 106698 CrossRef CAS.
- Y. Zhu, J. Chen, W. Li, D. Wang, S. Li and Z. Zheng, J. Anal. Appl. Pyrolysis, 2020, 151, 104906 CrossRef CAS.
- H. Ahmad Alyosef, H. Roggendorf, D. Schneider, A. Inayat, J. Welscher, W. Schwieger, T. Münster, G. Kloess, S. Ibrahim and D. Enke, J. Porous Mater., 2016, 23, 1609–1618 CrossRef CAS.
- A. Thangaraj, R. Kumar, S. P. Mirajkar and P. Ratnasamy, Catalytic Properties of Crystalline Titanium Silicalites I. Synthesis and Characterization of Titanium-Rich Zeolites with MFI Structure, 1991, vol. 130 Search PubMed.
- S. Krishnamurthy, A. Esterle, N. C. Sharma and S. V. Sahi, Nanoscale Res. Lett., 2014, 9, 627, DOI:10.1186/1556-276X-9-627.
- S. P. D. Ormond, M. Ratova, P. Kelly, M. Edge, B. Mihailova and L. Tosheva, J. Porous Mater., 2016, 23, 1421–1429 CrossRef CAS.
- W. S. Lee, M. Cem Akatay, E. A. Stach, F. H. Ribeiro and W. Nicholas Delgass, J. Catal., 2012, 287, 178–189 CrossRef CAS.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Pure Appl. Chem., 2015, 87, 1051–1069 CrossRef CAS.
- C. Du, N. Cui, L. Li, Z. Hua and J. Shi, RSC Adv., 2019, 9, 9694–9699 RSC.
- G. Xiong, Q. Jia, Y. Cao, L. Liu and Z. Guo, RSC Adv., 2017, 7, 24046–24054 RSC.
- Z. Jie, T. Yang, X. Wang, Y. Ji, Y. Li, B. Meng, X. Tan and S. Liu, Surf. Interfaces, 2022, 29, 101741 CrossRef CAS.
- S. Karimi Haghighi and A. Nemati Kharat, Inorg. Chem. Commun., 2021, 125, 108413 CrossRef CAS.
- N. Sheng, D. Lin, W. Liang, C. Zhao, Y. Liu, Y. Zhu, Z. Song, J. Jiang, W. Xu, Z. Yang, B. Sun, X. Feng and C. Yang, Chem. Eng. Sci., 2024, 300, 120538 CrossRef CAS.
- T. Zhang, X. Chen, G. Chen, M. Chen, R. Bai, M. Jia and J. Yu, J. Mater. Chem. A, 2018, 6, 9473–9479 RSC.
- Y. Liu, C. Zhao, B. Sun, H. Zhu and W. Xu, Appl. Catal., A, 2021, 624, 118329, DOI:10.1016/j.apcata.2021.118329.
- I. I. Ivanova and E. E. Knyazeva, Chem. Soc. Rev., 2013, 42, 3671–3688 RSC.
- K. Zhu and X. Zhou, Curr. Opin. Chem. Eng., 9, 2015, 42–48 DOI:10.1016/j.coche.2015.07.009.
- J. Yang, S. Liu, Y. Liu, L. Zhou, H. Wen, H. Wei, R. Shen, X. Wu, J. Jiang and B. Li, iScience, 2024, 27(3), 109064, DOI:10.1016/j.isci.2024.109064.
- S. Shi, Y. Jing, Z. Zhang, W. Du, Y. Cao, X. Duan and X. Zhou, Chem. Eng. J., 2024, 491, 151732 CrossRef CAS.
- M. Lin, Z. Su and W. Ma, Chem. Phys., 2024, 581, 112259 CrossRef CAS.
- S. Ivanova, V. Pitchon and C. Petit, J. Mol. Catal. A: Chem., 2006, 256, 278–283 CrossRef CAS.
- G. Moretti, A. M. Salvi, M. R. Guascito and F. Langerame, Surf. Interface Anal., 2004, 36, 1402–1412 CrossRef CAS.
- D. Trong, L. Bonneviot, A. Bittar, A. Sayari and S. Kaliaguine, Titanium sites in titanium silicalites: an XPS, XANES and EXAFS study, 1992, vol. 74 Search PubMed.
- A. C. Alba-Rubio, J. L. G. Fierro, L. León-Reina, R. Mariscal, J. A. Dumesic and M. López Granados, Appl. Catal., B, 2017, 202, 269–280 CrossRef CAS.
- J. Huang, C. Liu, D. Sun, Y. Hong, M. Du, T. Odoom-Wubah, W. Fang and Q. Li, Chem. Eng. J., 2014, 235, 215–223 CrossRef CAS.
- M. Lin, C. Xia, B. Zhu, H. Li and X. Shu, Chem. Eng. J., 2016, 295, 370–375 CrossRef CAS.
- Y. Li, Y. Yi, X. Wang, B. Gao, Y. Guo, G. Wu, Z. Feng and H. Guo, Ind. Eng. Chem. Res., 2023, 62, 12152–12173 CrossRef CAS.
- D. Liu, R. Wang, Y. Yu, Z. Chen, N. Fang, Y. Liu and M. He, Catal. Sci. Technol., 2023, 13, 1437–1447 RSC.
- J. W. Harris, J. Arvay, G. Mitchell, W. N. Delgass and F. H. Ribeiro, J. Catal., 2018, 365, 105–114 CrossRef CAS.
- X. Feng, X. Duan, G. Qian, X. Zhou, D. Chen and W. Yuan, Appl. Catal., B, 2014, 150–151, 396–401 CrossRef CAS.
- Z. Lu, J. Kunisch, Z. Gan, M. Bunian, T. Wu and Y. Lei, ChemCatChem, 2020, 12, 5993–5999 CrossRef CAS.
- J. Huang, T. Takei, T. Akita, H. Ohashi and M. Haruta, Appl. Catal., B, 2010, 95, 430–438 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ma00203f |
| This journal is © The Royal Society of Chemistry 2025 |