Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Gallium-in-glycerol phase change material emulsions (PCMEs) with superior latent heat capacity and thermal conductivity†
Chae Young
Park‡
a,
Suji
Kim‡
a,
Chimin
Song
a,
Jieun
Kim
b and
Joohyung
Lee
 *a
*a
aDepartment of Chemical Engineering, Myongji University, 116 Myongji-ro, Cheoin-gu, Yongin, Gyeonggi-do 17058, Korea. E-mail: ljbro@mju.ac.kr
bHyundai Kia Motors R&D Center, Hyundai Motor Company, 150, Hyundaiyeonguso-ro, Namyang-eup, Hwaseong, Gyeonggi-do 18280, Korea
First published on 14th May 2025
Abstract
In this study, we demonstrate a phase change material emulsion (PCME) with high thermal conductivity and latent heat capacity, produced by emulsifying Ga in glycerol as a flowable, electrically insulating liquid matrix. Polyvinylpyrrolidone (PVP) was employed as an emulsifier to achieve high Ga loadings (50–80 vol%) with stable dispersion, leveraging the strong binding affinity of PVP to Ga and the increased matrix viscosity. The Ga-in-glycerol emulsions retained solid–liquid phase transition temperatures near those of bulk Ga, melting at approximately 30 °C and crystallizing between −30 °C and −40 °C, unlike previous nano-sized dispersions that exhibited substantial depression of the phase transition temperatures. These PCMEs displayed significantly enhanced thermal properties compared to conventional emulsions with organic PCMs, with thermal conductivities reaching up to 4.85 W m−1 K−1 and latent heat capacities up to 241.52–262.91 J cm−3 at 80 vol% Ga loading. Despite the high Ga loading, the emulsions maintained electrical insulation. Additionally, these emulsions exhibited viscoelasticity, which confers them with high sedimentation stability and structure integrity while enabling their fluidic processing under shear. The unique combination of high thermal conductivity, substantial latent heat capacity, electrical insulation, and excellent rheological processability of these Ga-in-glycerol emulsions demonstrates their potential for advanced thermal management and energy storage applications, including electronics cooling and renewable energy systems.
1. Introduction
A phase change material (PCM) is a substance capable of absorbing or releasing a substantial amount of thermal energy in the form of latent heat through its phase transitions at specific temperatures.1 This property makes PCMs promising materials for effective thermal management or thermal energy utilization across various fields, including energy storage systems,2,3 electronics devices,4 housing,5 and textiles.6One method of utilizing PCMs is to disperse them within a carrier fluid to create a two-phase fluid system.7 In this configuration, the dispersed PCM particles perform the function of absorbing and releasing latent heat, while the carrier fluid facilitates heat transfer and provides fluidity to the dispersed PCM particles, allowing them to be applied to target systems with complex structures through various fluidic processes. Such two-phase fluids are commonly referred to as phase change emulsions (PCEs) or phase change material emulsions (PCMEs). They can be circulated using pumps if their viscosities are low, while those with higher viscosity or viscoelastic properties can be applied as pastes for coating applications.
Typical PCMs dispersed in carrier fluids of PCMEs have very low thermal conductivities.8 For example, paraffin-based PCMs, which are among the most widely used, have a thermal conductivity of about 0.2 W m−1 K−1, even lower than that of commonly used carrier fluids like water, which has a thermal conductivity of approximately 0.6 W m−1 K−1. Other PCMs also exhibit low thermal conductivity, including hexadecanol (0.141 W m−1 K−1), dodecanol (0.22 W m−1 K−1), stearic acid (0.24 W m−1 K−1), polyethylene glycol (0.24 W m−1 K−1), tetradecanol (0.481 W m−1 K−1), xylitol (0.41 W m−1 K−1), calcium chloride (0.5 W m−1 K−1), magnesium chloride (0.694 W m−1 K−1), and erythritol (0.77 W m−1 K−1). As a result, the PCMEs formed by dispersing these PCMs in carrier fluids inevitably exhibit low thermal conductivity as well.7
Recently, low-melting-point metals and alloys with melting points below room temperature up to approximately 250 °C have gained attention as promising PCMs due to their high latent heat capacity from solid–liquid phase transitions and high thermal conductivity.9,10 Among these, Gallium (Ga; melting point 30 °C) and certain Ga-based alloys, such as eutectic gallium-indium (EGaIn; melting point 16 °C) and eutectic gallium-indium-tin (Galinstan; melting point 13 °C), are particularly notable.11 These materials exhibit excellent thermal properties, with high thermal conductivities and high latent heat, in addition to being malleable near room temperatures and non-toxic, which greatly enhances their potential applicability.
However, directly applying these low-melting-point metals for thermal applications is often limited due to their low wettability on most surfaces, corrosiveness to metal surfaces, and poor electrical insulation.12 To overcome these issues, they can be dispersed as micro- or nano-sized particles within an organic, electrically insulating matrix. The organic matrix is typically a thermosetting material, such as silicone13 or epoxy,14 which hardens in appropriate conditions to form a solid composite material with dispersed metal particles exhibiting no fluidity.
Relatively few studies have discussed colloidal dispersions of low-melting-point metals in non-curing, flowable carrier fluids with long pot life as high-thermal-conductivity fluids. For example, a low-viscosity and a high-viscosity fluid, both containing 50 vol% EGaIn dispersed in liquid paraffin doped with an ionic surfactant, showed thermal conductivities of 0.7 W m−1 K−1 and 0.9 W m−1 K−1, respectively.15 Colloidal dispersions of EGaIn in pure liquid paraffin at 55 vol%, produced using ultrasonication, achieved a thermal conductivity of 1.1 W m−1 K−1, which could be further enhanced by adding high-thermal-conductivity ceramic fillers.16 In another study, silicone oil-based dispersions of Ga particles at 50 vol% demonstrated thermal conductivities of approximately 1.3–2 W m−1 K−1–these conductivities could be significantly increased by applying pressure to disrupt the insulating oxide layer on the Ga surface17 or by adding other metallic fillers with even higher thermal conductivity that could alloy with Ga.18 Finally, a viscoelastic paste of EGaIn emulsified at 50 vol% in ethanol with high concentrations of polyvinylpyrrolidone (PVP) as an emulsifier has shown a thermal conductivity of 3.1 W m−1 K−1.19
These studies have focused on the enhanced thermal conductivity of the resulting colloidal suspensions, with little attention given to the phase change behavior of the dispersed low-melting-point metal particles. In rare instances, a few studies have examined the phase transition behavior of micronized low-melting-point metal particles. For example, Mingear and collaborators systematically observed the solid–liquid phase transition behavior of various Ga–In mixtures in ethanol, including pure Ga, In, and their eutectic compositions in nano-particle form.20 In this study, dispersed metal particles were below 100 nanometers in size and exhibited melting points significantly lower than their bulk counterparts, e.g., reaching below −20 °C for Ga whose melting point in bulk is 30 °C, due to increased surface energy effects. Similarly, nano-sized Ga particles dispersed in isopropanol21 and EGaIn nanoparticles dispersed in polydimethylsiloxane13 showed melting points of approximately −14 °C and −25 °C, respectively, both much lower than their bulk melting points. These sub-zero melting characteristics indicate that these dispersed particles can remain in a liquid state over a broad temperature range, suggesting potential utility for low-temperature thermal energy applications. However, to date, there has been little evidence that low-melting-point metals dispersed in flowable carrier fluids can retain phase transition behaviors similar to their bulk solid–liquid phase transitions.
In this study, we demonstrate a PCME consisting of Ga particles with several tens of micrometers in size dispersed in glycerol as a carrier fluid with low volatility and excellent thermal stability suitable for thermal applications. This PCME absorbs or releases a substantial amount of latent heat through solid–liquid phase transitions of the dispersed Ga particles at temperatures similar to those of bulk Ga, i.e., at about 30 °C during melting and at about −30 to −40 °C during crystallization, different from previously reported PCMEs containing nano-sized Ga or Ga-based low-melting-point metal alloys which showed significant depressions in phase transition temperatures. Despite the relatively large particle size compared to previously reported nano-sized colloidal Ga or Ga-based particles, with the higher density of Ga relative to the carrier fluid, the resulting emulsion with Ga volume loadings above 70 vol% exhibited high sedimentation stability owing to the internal structuring of the dispersed Ga particles. As Ga possess both high latent heat capacity and high thermal conductivity, these critical thermal properties of the resulting PCME improve simultaneously, without trade-offs, as the loading of Ga particles in the carrier fluid increases. These Ga-dispersed PCMEs with various Ga loadings display considerably higher latent heat capacities and thermal conductivities compared to conventional PCMEs containing organic PCMs, such as paraffins and fatty acids, while maintaining beneficial electrical insulation properties, setting them apart from the electrically conductive bulk Ga. This unique combination of properties makes these PCMEs promising candidates for diverse thermal management and thermal energy utilization applications.
2. Materials and methods
2.1. Materials
Ga was purchased from Suzhou Chuanmao Metal Materials Co., Ltd (China). Glycerol (99.0%) was obtained from Samchun Chemical Co., Ltd (Korea). PVP K30 with a molecular weight of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 was supplied by Sigma-Aldrich (USA).
000 g mol−1 was supplied by Sigma-Aldrich (USA).
2.2. Sample preparation
To prepare Ga-in-glycerol emulsions, melted Ga and glycerol solutions with varying PVP concentrations (0–20 wt%, with respect to the glycerol solution as the continuous phase) were shear-mixed using a mortar and pestle for approximately 10 min. The volume ratios of Ga to glycerol solution were adjusted to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 6
5, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 7
4, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 8
3, and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
2.3. Characterization
To observe the morphology of emulsified Ga microdroplets, the sample emulsions were deposited onto glass slides, rinsed with ethanol using a squeeze bottle, and left under ambient conditions over several days to allow the ethanol to evaporate completely. The dried samples were then observed using an Eclipse Ts2-FL optical microscope (Nikon, Japan). The compositions and thermal stability of the emulsions were investigated through thermogravimetric analysis (TGA) in the temperature range of 30–800°, with a heating rate of 10 °C min−1 under a nitrogen environment, using TGA 2 apparatus (Mettler Toledo, USA). To characterize the rheological properties of Ga-in-glycerol emulsions, a Haake MARS-40 rheometer (Thermo Fisher Scientific, USA) equipped with parallel plates of 35 mm diameter and a gap distance of 1 mm was used. Oscillatory frequency sweep tests were conducted on emulsions with Ga contents of 50, 60, 70, and 80 vol% under a constant shear stress of 1 Pa over a frequency range of 0.01–100 Hz. Stress sweep tests were performed on emulsions with Ga contents of 70 and 80 vol% under a constant oscillatory frequency of 1 Hz, across a shear stress range of 0.01–1000 Pa. Steady-state viscosity measurements for glycerol solutions containing 0, 10, and 20 wt% PVP were also conducted using the same rheometer at shear rates ranging from 10 to 100 s−1. Latent heat storage and release properties of the emulsions were analyzed using a Discovery DSC25 (TA Instruments, USA) differential scanning calorimetry (DSC) apparatus in the temperature range between −70 and 70 °C, with a scanning rate of 5 °C min−1 under a nitrogen environment. Thermal conductivities of the emulsions were measured using the Hot Disk method with a TPS 2500 (Hot Disk AB, Sweden) apparatus.2.4. Heating of PCMEs
To study the temperature evolution of the PCMEs during heating, PCMEs with Ga contents of 50, 60, 70, and 80 vol%, as well as a glycerol solution with 20 wt% dissolved PVPs, were charged in square-shaped silicone molds with dimensions of 1 cm (W) × 1 cm (L) × 1 cm (H), which were frozen at −85 °C for two days. The sample-filled molds were then placed on an isothermal hot plate maintained at 100 °C, and the time-dependent temperature variations of the samples were monitored using an Optris Xi 400 infrared camera (Optris Infrared Sensing, LLC, USA).3. Results and discussion
Two-phase PCMEs consisting of Ga particles (dispersed phase) suspended in glycerol (continuous phase) at high volume loadings (50–80 vol%) were prepared. Ga, one of the most popular low-melting-point metals,11 has high latent heat (460 J cm−3) for their solid–liquid transition and high thermal conductivity (40 W m−1 K−1). Glycerol, the carrier fluid, has high thermal stability and low volatility, which make it suitable for various thermal applications. Since Ga has unusually high interfacial tensions with most common organic and inorganic liquids, a direct emulsification of Ga and the pure carrier fluid at high Ga loadings is challenging.19To enhance the emulsification efficiency of Ga with glycerol, polyvinylpyrrolidone (PVP) was used as an emulsifier. When dissolved in glycerol at high concentrations, PVPs significantly increase the viscosity of the continuous phase (Fig. S1, ESI†), which can help partly offset the high interfacial tension acting between the dispersed and continuous phase during emulsification.22 Additionally, PVPs can bind to the surface of Ga particles through coordination bonds mediated by electron pairs of nitrogen (N) atoms, which not only prevents the coalescence of dispersed Ga into larger droplets but also mitigates surface oxidation on the bound particles.19,23
Fig. 1 shows the visual appearance of samples in which an equal volume mixture of Ga and glycerol solution, with pre-dissolved PVPs at 0, 10, and 20 wt% (with respect to the glycerol as the continuous phase), was emulsified using a mortar and pestle. As shown in Fig. 1a, when pure glycerol without PVP was used, emulsification was ineffective, and the non-emulsified, shiny bulk Ga was visible. In Fig. 1b, where glycerol contained 10 wt% PVP, a moderate level of emulsification occurred, leading to a significant reduction in the size of the dispersed Ga. However, the boundaries between the dispersed and continuous phases remained visible to the naked eye. In contrast, as shown in Fig. 1c, with glycerol containing 20 wt% PVP, complete emulsification was achieved resulting in a uniformly opaque gray suspension wherein shiny Ga was no longer visible. Fig. 2a shows the optical microscopy image of Ga particles dispersed in the sample prepared using the glycerol solution with 20 wt% PVPs, confirming that the bulk Ga was emulsified as fine particles with sizes in the order of 10 μm.
 | ||
| Fig. 2 Optical microscopy images of dispersed Ga particles in Ga-in-glycerol emulsions with Ga contents of (a) 50 vol%, (b) 60 vol%, (c) 70 vol%, and (d) 80 vol%. The scale bars indicate 20 μm. | ||
Using a glycerol solution with 20 wt% PVP, Ga-in-glycerol emulsions with higher Ga volume loadings (60–80 vol%) were also readily prepared. Fig. 1d–f and 2b–d present the visual appearances of the samples and the optical microscopy images of the dispersed Ga particles, respectively. All the samples exhibited uniformly opaque gray suspensions retaining the fluidity. The sizes of dispersed Ga particles slightly increased with higher Ga loadings but remained within a similar range (10–30 μm). It is notable that, unlike the protocols reported in the literature for producing highly low-melting-point metal-loaded emulsions or composites, the Ga particles in our study were not pre-produced and incrementally added to the continuous phase. Instead, substantial amounts of Ga were directly emulsified with the carrier fluid within minutes, yielding uniformly dispersed, fluidic colloidal suspensions.
Fig. 3 presents the thermogravimetric analysis (TGA) results of the produced PCMEs. As a control, the glycerol solution containing 20 wt% PVP without Ga exhibited significant mass loss in the temperature range of 200–300 °C due to the decomposition of glycerol. The remaining PVP, accounting for 20 wt%, began to decompose at 400 °C and was fully decomposed by 450 °C. For the PCMEs, slight inflection points were observed at approximately 200 °C and 400 °C, corresponding to the decomposition of glycerol and PVP, respectively. However, due to the high-volume fraction of thermally stable, high-density Ga in the emulsions, the residual mass remained remarkably high up to 800 °C. At 800 °C, the residual masses of PCMEs with 50, 60, 70, and 80 vol% Ga were 84.1%, 88.2%, 92.5%, and 95.6%, respectively. These values closely aligned with the fractions corresponding to the actually loaded Ga mass, indicating minimal component loss during the emulsification process and confirming the high efficiency of the employed emulsification method.
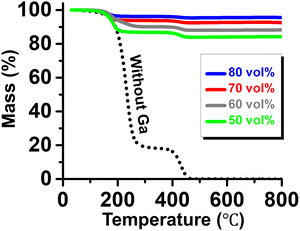 | ||
| Fig. 3 TGA curves of the PCMEs with various Ga contents. The dashed line represents the data for the glycerol solution without Ga. | ||
This high emulsification efficiency is likely due to the synergetic effect of the grinding-based mixing method–proven effective in inducing mechanochemistry24 at low-melting-point metal interfaces–and high binding affinity of the emulsifier PVPs to the Ga surface through coordination bonding.19 It was found that implementing common preparation methods for colloidal suspensions, such as vortexing, rotor-stator homogenization, and sonication, did not lead to a successful emulsification of the same formulation within the same mixing time (Fig. S2, ESI†). As another control, when ethanol, which has lower viscosity than glycerol, was used as the carrier fluid, emulsification was not achieved at the same PVP concentration (20 wt% with respect to the continuous phase) due to the insufficiently high viscosity of the continuous phase (Fig. S3, ESI†). This result aligns with our previous observation with EGaIn.19 At a much higher PVP concentration (40 wt% with respect to the continuous phase), it has been shown that EGaIn, which is similar to Ga, can be emulsified in ethanol. However, when the EGaIn volume loading exceeded 60 vol%, the EGaIn particles burst and coalesced, forming a continuous metallic phase that facilitated electron transport. Consequently, the resulting emulsion lost its electrical insulation, rendering it unsuitable for practical thermal applications. In this study, using glycerol as the carrier fluid, which possess stronger intermolecular forces and higher viscosity due to multiple hydrogen bonds compared to ethanol, a large quantity of Ga was successfully dispersed within the continuous phase without particle rupture, achieving a volume fraction of up to 80 vol% with a moderate amount of PVP. As a result, all Ga-in-glycerol emulsions produced under these conditions maintained electrical insulation (see Fig. S4 for experimental confirmation, ESI†).
Fig. 4 presents results of the oscillatory viscoelasticity measurements conducted on Ga-in-glycerol emulsions with various Ga loadings (50–80 vol%), performed under a constant shear stress of 1 Pa and over a frequency range of 0.01–100 Hz. At a Ga content of 50 vol%, the viscous modulus (G′′) exceeds the elastic modulus (G′) across all frequencies (Fig. 4a), which explains the relatively higher fluidity of this formulation compared to others, as observed in Fig. 1c. With a Ga content of 60 vol%, both G′ and G′′ values increase, and the two curves nearly overlap (Fig. 4b). For Ga contents above 60 vol%, G′ surpasses G′′, indicating the development of solid-like characteristics (Fig. 4c and d). The increasing solid-like properties with higher Ga contents above 60 vol% are also evident from their visual appearances (Fig. 1d–f), imparting high sedimentation stability and shape retention to the samples (Fig. 1g).16 At high Ga volume fractions, the Ga particles become densely packed and form a three-dimensional network structure, which reduces the free volume available for the continuous phase fluid. As a result, the emulsion flows less easily and begins to exhibit solid-like characteristics.
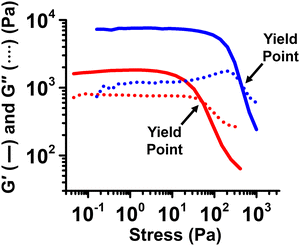
Fig. 5 shows the results of stress-sweep oscillatory viscoelasticity measurements performed at a constant frequency of 1 Hz and over a shear stress range of 0.01–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Pa for samples with Ga content of 70 vol% and 80 vol%, where G′ > G′′ was especially pronounced in the former frequency-sweep measurements. These samples exhibit a linear viscoelastic region at low shear stress, characterized by G′ > G′′, reflecting their solid-like characteristics. This region is followed by a sharp decrease in both G′ and G′′ due to the breakdown of the internal structure, eventually leading to a crossover point beyond a certain stress threshold–defined as the yield stress. The yield stress values, determined at the crossover points of the curves, were 49 and 425 Pa for the samples with Ga loadings of 70 and 80 vol%, respectively, These results indicate that, beyond these stress levels, the samples can also flow as the internal structure disintegrates. The diverse viscoelastic behaviors observed across varying Ga contents and processing parameters suggest the potential for Ga-in-glycerol emulsions in various rheological applications. For example, in applications where the PCME must maintain its shape without leakage—such as when applied at the interface of electronic components—solid-like formulations with Ga loadings above 60 vol% would be advantageous. In contrast, in situations where the PCME needs to be transported or circulated via pumping, formulations with Ga loadings below 60 vol% would be more suitable.
000 Pa for samples with Ga content of 70 vol% and 80 vol%, where G′ > G′′ was especially pronounced in the former frequency-sweep measurements. These samples exhibit a linear viscoelastic region at low shear stress, characterized by G′ > G′′, reflecting their solid-like characteristics. This region is followed by a sharp decrease in both G′ and G′′ due to the breakdown of the internal structure, eventually leading to a crossover point beyond a certain stress threshold–defined as the yield stress. The yield stress values, determined at the crossover points of the curves, were 49 and 425 Pa for the samples with Ga loadings of 70 and 80 vol%, respectively, These results indicate that, beyond these stress levels, the samples can also flow as the internal structure disintegrates. The diverse viscoelastic behaviors observed across varying Ga contents and processing parameters suggest the potential for Ga-in-glycerol emulsions in various rheological applications. For example, in applications where the PCME must maintain its shape without leakage—such as when applied at the interface of electronic components—solid-like formulations with Ga loadings above 60 vol% would be advantageous. In contrast, in situations where the PCME needs to be transported or circulated via pumping, formulations with Ga loadings below 60 vol% would be more suitable.
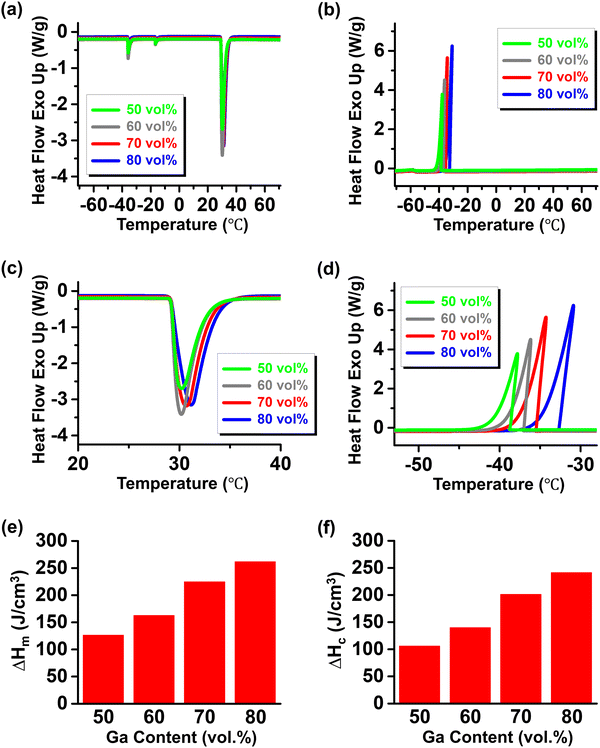
Fig. 6 shows the differential scanning calorimetry (DSC) curves for Ga-in-glycerol emulsions with Ga contents of 50–80 vol%. Upon heating (Fig. 6a), a main endothermic peak due to the solid-to-liquid phase transition appears around 30 °C, which is similar to the melting behavior observed in bulk Ga (Fig. S5, ESI†).21 This melting point above room temperature contrasts with previously reported melting points of approximately −20 °C for Ga particles dispersed at nanoscale dimensions (tens of nanometers).20,21 The difference is attributed to the relatively large size (10–30 μm) of dispersed Ga particles in the Ga-in-glycerol emulsions presented in this study, which fall outside the size range where melting point depression due to the enhanced surface effect occurs.20 The gradual shift of these melting peaks to higher temperatures with increasing Ga content (Fig. 6c) is probably due to a slight increase in Ga particle size. The latent heat absorption properties at temperatures above room temperature could be advantageous for various thermal management and energy applications, such as in battery and electronic device thermal regulation and solar thermal energy harvesting. Minor endothermic peaks observed at sub-zero temperatures during heating are likely due to the presence of a small fraction of nanoscale Ga particles, which may have formed during the emulsification process.
Upon cooling (Fig. 6b), the primary exothermic peak corresponding to the liquid-to-solid phase transition occurs at sub-zero temperatures (approximately −30 to −40 °C), significantly lower than the melting points, indicating a supercooling effect similar to that observed in bulk Ga (Fig. S5, ESI†).21 Similar to the phase transition behavior upon heating, the phase transition of Ga particles dispersed in Ga-in-glycerol emulsions in this study occurs at a much higher temperature than the freezing point of approximately −130 °C reported for Ga particles dispersed at nanoscale sizes (tens of nanometers) in literature,20,21 owing to the relatively large size of dispersed Ga particles. The crystallization peaks during cooling gradually shifted to higher temperatures with increasing Ga content (Fig. 6d), likely due to the increase in Ga particle size.
As shown in Fig. 6e and f, the latent heat absorption and release capacity of Ga-in-glycerol emulsions due to the solid–liquid or liquid–solid phase transition of Ga particles increases almost linearly with the Ga loading in the emulsion (with linear fits of the measured latent heat capacities provided in Fig. S6 (ESI†) to facilitate interpolation within the investigated range of Ga concentrations). Due to Ga's high latent heat capacity (460 J cm−3), Ga-in-glycerol emulsions with high Ga volume fractions exhibit substantial latent heat capacities. For instance, at 80 vol% Ga content, the latent heat absorption and release capacities are 261.91 and 241.52 J cm−3, respectively, far exceeding the 100–120 J cm−3 range of commercial PCM capsules. In conclusion, the emulsified Ga particles with PVPs shown here are large enough to exhibit phase transition behaviors similar to those of bulk Ga, yet they remain stably dispersed in a fluid medium with both flowability and electrical insulation. This enables the resulting PCMEs with high Ga loadings to absorb and release substantial amounts of latent heat, above room temperature and in sub-zero temperatures, respectively, while maintaining the high sedimentation stability and electrical insulation, suggesting their high potential for unique applications in thermal management and thermal energy storage.
The thermal conductivities for Ga-in-glycerol emulsions with Ga content ranging from 50 to 80 vol% are shown in Fig. 7. Given the high thermal conductivity (40 W m−1 K−1) of Ga, it is not surprising that the resulting emulsions with high Ga loadings exhibit significantly higher thermal conductivities than those of pure glycerol and a glycerol solution containing 20 wt% of dissolved PVP (approximately 0.3 W m−1 K−1) as the continuous phase. The thermal conductivity measured for the emulsion with the lowest Ga content, at 50 vol%, was 1.70 W m−1 K−1, which is higher than any values previously reported for conventional PCMEs, typically prepared with PCMs like paraffin wax.7 As the Ga content increased to 60, 70, and 80 vol%, the thermal conductivity of the resulting PCMEs further increased to 2.17, 3.21, and 4.85 W m−1 K−1, respectively. The measured thermal conductivities were well fitted using the Agari model,25 which is widely used for analyzing the thermal conductivity of polymer composites:
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kc = ϕfC2 kc = ϕfC2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kf + ϕm kf + ϕm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(C1km). log(C1km). |
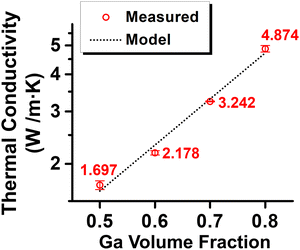 | ||
| Fig. 7 Thermal conductivities of Ga-in-glycerol emulsions with various Ga contents. (Circled data points: measured values; dotted line: predicted by the Agari model). | ||
Fig. 8 shows the temperature evolution of Ga-in-glycerol PCMEs with varying Ga contents, filled into silicone molds (1 mL) and placed on an isothermal hot plate maintained at 100 °C. As a control, a glycerol solution with 20 wt% PVP dissolved and no dispersed Ga showed a continuous and uninterrupted temperature rise. As the Ga content increased, the rate of temperature rise in the PCMEs gradually increased, which can be attributed to the enhanced thermal conductivity. For all the PCMEs, the initial temperature rise was followed by plateau regions within the temperature range of 30–35 °C, consistent with the Ga melting range observed in the DSC analysis. These delays in temperature increase are attributed to the latent heat storage associated with the solid–liquid phase transition of the dispersed Ga particles. As the Ga content increased, the plateau region extended from approximately 7 min for the 50 vol% Ga PCME to 12 min for the 80 vol% Ga PCME. This extension corresponds to the increased latent heat storage capacity, as indicated by the DSC analysis, which shows that the 80 vol% Ga PCME can store approximately 107% more latent heat compared to the 50 vol% Ga PCME at the same volume. Interestingly, after the plateau region, the temperature rose more steeply with increasing Ga content, with all samples reaching a final temperature of approximately 62 °C in nearly the same amount of time (30 min). Again, this behavior is attributed to the enhanced thermal conductivity of PCMEs with higher Ga content. These experiments highlight the dual thermal functions of the Ga particles suspended in the PCMEs, which simultaneously enhance thermodynamic latent heat storage at their melting points and dynamic heat transfer both below and above their melting points.
4. Conclusion
In this study, we demonstrated PCMEs with highly loaded Ga particles in glycerol as a stable, electrically insulating carrier fluid. By employing PVP as an emulsifier, we achieved efficient emulsification of Ga within glycerol at Ga loadings up to 80 vol%, forming stable colloidal suspensions with high thermal conductivity and latent heat capacities suitable for advanced thermal applications. Unlike prior studies on nano-sized Ga particles, the relatively large Ga particles (10–30 μm) used in this work retained solid–liquid phase transition temperatures near those of bulk Ga, i.e., melting near 30 °C and crystallizing between −30 °C and −40 °C, thus offering robust thermal properties without significant depression of the phase transition temperatures typically seen in nanoscale particles. The thermal conductivity and latent heat capacities of Ga-in-glycerol PCMEs were shown to increase nearly linearly with Ga content, with values far surpassing those of conventional organic-based PCMEs, such as paraffin or fatty acids. This unique combination of high thermal conductivity and substantial latent heat at high Ga loadings, along with the electrical insulation preserved by the glycerol matrix, represents a significant advance in PCME technology, potentially useful in various thermal applications including next-generation cooling systems, electronics thermal regulation, and renewable energy storage solutions.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the 2024 Hyundai Motor Group Future Technology Research Project, funded by Hyundai NGV.References
- B. Zalba, J. M. Marin, L. F. Cabeza and H. Mehling, Review on Thermal Energy Storage with Phase Change: Materials, Heat Transfer Analysis and Applications, Appl. Therm. Eng., 2003, 23, 251–283 CrossRef CAS.
- J. Zhang, D. Shao, L. Jiang, G. Zhang, H. Wu, R. Day and W. Jiang, Advanced Thermal Management System Driven by Phase Change Materials for Power Lithium-ion Batteries: A Review, Renewable Sustainable Energy Rev., 2022, 159, 112207 CrossRef CAS.
- R. Gad, H. Mahmoud, S. Ookawara and H. Hassan, Evaluation of Thermal Management of Photovoltaic Solar Cell via Hybrid Cooling System of Phase Change Material Inclusion Hybrid Nanoparticles Coupled with Flat Heat Pipe, J. Energy Storage, 2023, 57, 106185 CrossRef.
- V. Bianco, M. D. Rosa and K. Vafai, Phase-Change Materials for Thermal Management of Electronic Devices, Appl. Therm. Eng., 2022, 214, 118839 CrossRef.
- Q. Al-Yasiri and M. Szabo, Incorporation of Phase Change Materials into Building Envelope for Thermal Comfort and Energy Saving: A Comprehensive Analysis, J. Build. Eng., 2021, 36, 102122 CrossRef.
- Y. Su, W. Zhu, M. Tian, Y. Wang, X. Zhang and J. Li, Intelligent Bidirectional Thermal Regulation of Phase Change Material Incorporated in Thermal Protective Clothing, Appl. Therm. Eng., 2020, 174, 115340 CrossRef CAS.
- D. Cabaleiro, F. Agresti, L. Fedele, S. Barison, C. Hermida-Merino, S. Losada-Barreiro, S. Bobbo and M. M. Pineiro, Review on Phase Change Material Emulsions for Advanced Thermal Management: Design, Characterization and Thermal Performance, Renewable Sustainable Energy Rev., 2022, 159, 112238 CrossRef CAS.
- C. Xu, H. Zhang and G. Fang, Review on Thermal Conductivity Improvement of Phase Change Materials with Enhanced Additives for Thermal Energy Storage, J. Energy Storage, 2022, 51, 104568 Search PubMed.
- H. Ge, H. Li, S. Mei and J. Liu, Low Melting Point Liquid Metal as a New Class of Phase Change Material: An Emerging Frontier in Energy Area, Renewable Sustainable Energy Rev., 2013, 21, 331–346 CrossRef CAS.
- Y. Yao, W. Li and J. Liu, Liquid Metal Phase Change Materials for Thermal Management of Electronics, Adv. Phys.: X, 2024, 9, 2324910 Search PubMed.
- S.-Y. Tang, C. Tabor, K. Kalantar-Zadeh and M. D. Dickey, Gallium Liquid Metal: The Devil's Elixir, Annu. Rev. Mater. Res., 2021, 51, 381–408 Search PubMed.
- P. Fan, Z. Sun, Y. Wang, H. Chang, P. Zhang, S. Yao, C. Lu, W. Rao and J. Liu, Nano Liquid Metal for the Preparation of a Thermally Conductive and Electrically Insulating Material with High Stability, RSC Adv., 2018, 29, 16232–16242 RSC.
- M. H. Malakooti, N. Kazem, J. Yan, C. Pan, E. J. Markvicka, K. Matyjaszewski and C. Majidi, Liquid Metal Supercooling for Low-Temperature Thermoelectric Wearables, Adv. Func. Mater., 2019, 29, 1906098 CrossRef CAS.
- S. Kim, C. Song, S. Kim, S. Kang, H. G. Menge, Y. T. Park and J. Lee, Effects of a Liquid Metal Co-Filler on the Properties of Epoxy/Binary Filler Composites, J. Appl. Polym. Sci., 2024, 141, e55134 Search PubMed.
- S. Kim, S. Kang and J. Lee, High-Thermal-Conductivity and High-Fluidity Heat Transfer Emulsion with 89 wt% Suspended Liquid Metal Microdroplets, ACS Omega, 2023, 8, 17748–17757 CrossRef CAS PubMed.
- S. Y. Ryu, H. Lee, Y. Hong, H. Bandal, M. Goh, H. Kim and J. Lee, Vitrification of Liquid Metal-in-Oil Emulsions Using Nano-Mineral Oxides, Adv. Mater. Interfaces, 2023, 10, 2202527 CrossRef CAS.
- A. Uppal, M. Ralphs, W. Kong, M. Hart, K. Rykaczewski and R. Y. Wang, Pressure-Activated Thermal Transport via Oxide Shell Rupture in Liquid Metal Capsule Beds, ACS Appl. Mater. Interfaces, 2020, 12, 2625–2633 CrossRef CAS PubMed.
- A. Uppal, W. Kong, A. Rana, R. Y. Wang and K. Rykaczewski, Enhancing Thermal Transport in Silicone Composites via Bridging Liquid Metal Fillers with Reactive Metal Co-Fillers and Matrix Viscosity Tuning, ACS Appl. Mater. Interfaces, 2021, 13, 43348–43355 Search PubMed.
- S. Kim, J. Park, Y. H. Pi, J. S. Park, Y. S. Kim, K. Wu, G. Yu and J. Lee, Facile Low-Oxidation Emulsification of Liquid Metal Using Polyvinylpyrrolidone for Highly Viscoelastic Heat Conductive Pastes, ACS Appl. Eng. Mater. Interfaces, 2024, 7, 2705–2718 Search PubMed.
- J. Mingear, Z. Farrell, D. Hartl and C. Tabor, Gallium-Indium Nanoparticles as Phase Change Material Additives for Tunable Thermal Fluids, Nanoscale, 2021, 13, 730–738 Search PubMed.
- A. Yamaguchi, Y. Mashima and T. Iyoda, Reversible Size Control of Liquid-Metal Nanoparticles Under Ultrasonication, Angew. Chem., Int. Ed., 2015, 54, 12809–12813 Search PubMed.
- E. Lepercq-Bost, M.-L. Giorgi, A. Isambert and C. Arnaud, Use of the Capillary Number for the Prediction of Droplet Size in Membrane Emulsification, J. Membrane Sci., 2008, 314, 76–89 Search PubMed.
- Y. Liu, Q. Wang, S. Bi, W. Zhang, H. Zhou and X. Jiang, Water Processable Liquid Metal Nanoparticles by Single-Step Polymer Encapsulation, Nanoscale, 2020, 12, 13731–13741 RSC.
- D. Wu, D. Liu, X. Tian, C. Lei, X. Chen, S. Zhang, F. Chen, K. Wu and Q. Fu, A Universal Mechanochemistry Allows On Demand Synthesis of Stable and Processable Liquid Metal Composites, Small Methods, 2022, 6, 2200246 Search PubMed.
- S. Kang, W. Kim, C. Song, Y. Hong, S. Kim, M. Goh, S. K. Chung and J. Lee, Novel Latent Heat Storage Systems Based on Liquid Metal Matrices with Suspended Phase Change Material Microparticles, ACS Appl. Mater. Interfaces, 2023, 15, 36781–36791 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: Viscosities of glycerol solutions with varying PVP concentrations; Photographs of samples prepared under different conditions; Experimental confirmation of electrical insulation for PCMEs with various Ga contents; DSC heating and cooling curves of bulk Ga; Linear fits of the measured ΔHm and ΔHc values for PCMEs with various Ga contents. See DOI: https://doi.org/10.1039/d5ma00152h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |