 Open Access Article
Open Access ArticlePlasmonically optimized gold–gallia nanocomposites: a novel approach for high-temperature NO2 detection†
L.
Keerthana
a,
Mushtaq Ahmad
Dar
b,
R.
Sivasubramanian
 c and
Gnanaprakash
Dharmalingam
c and
Gnanaprakash
Dharmalingam
 *a
*a
aPlasmonic Nanomaterials Laboratory, PSG Institute of Advanced Studies, Coimbatore-641004, India. E-mail: dgp@psgias.ac.in
bCenter of Excellence for Research in Engineering Materials, Deanship of Scientific Research (DSR), King Saud University, Riyadh 11421, Saudi Arabia
cDepartment of Chemistry, Amrita School of Engineeering, Amrita Vishwa Vidyapeetham, Amaravati, Andhra Pradesh, India
First published on 21st April 2025
Abstract
Monitoring gases in harsh environments in real-time has become indispensable across various industries such as nuclear plants, turbines, and boiler plants. Materials capable of withstanding high temperatures are essential for sensing platforms, often operating at temperatures exceeding 300 °C. Localized surface plasmon resonance based optical gas sensing, although promising, has a glaring limitation when sensing analytes that themselves interact with light in wavelength regimes that overlap with the material's resonance, which is remedied in one manner in this report. In this study, we synthesize multiple gold–gallium oxide nanocomposites that were evaluated for their morphological and optical stabilities at high temperatures (800 °C), post which they were tested for NO2 detection at 800 °C, wherein temperature-dependent kinetic studies were conducted first to deconvolute the absorbance of NO2 itself at different temperatures. The findings suggest that gold–gallium oxide nanocomposites prepared using the described solution-based approach show promising applications in high-temperature and extreme environment gas sensing.
1. Introduction
Over the years, urbanization and industrialization have led to a rise in the prevalence of pollution from various sources, resulting in environmental degradation.1 High-temperature combustion sources produce pollutants like NO2, such as boiler plants (500–700 °C), gas turbines (>800 °C), steam turbines (400–600 °C), and glass-making industries (400–700 °C), in the concentration ranges 250–650 ppm, 2–20 ppm, 141–929 ppm, and 640 ppm, respectively.2 NO2 is one of the main contributors to the formation of tropospheric ozone and acid rain, affecting the quality of soil and water and human health, causing respiratory problems, nausea, dizziness, etc.3 Usually, real-time data pertaining to NO2 emissions from combustion sources are unavailable. To increase the accuracy of NO2 quantification from these sources, it is important to have sensors with materials that can operate reliably, even at elevated temperatures. Optical sensors have attributes such as non-contact detection, flexibility in monitoring multiple parameters (peak intensity, centroid, and peak position), high sensitivity,4 and being amenable to miniaturization through the use of narrow wavelength optics such as diodes and photodetectors, where for example generic solid-state semiconductors suffer from degradation of electrical contacts (when at temperatures >500 °C typically) though being cost-effective.5 Thus a suitable approach is proposed to employ plasmonic materials incorporated in metal oxides for high-temperature (800 °C) detection of NO2. Indeed, such an approach has already been reported by us and others. Among the plasmonic metals (Cu, Ag, Au, Pt, Pd, etc.), Au nanoparticles (AuNPs) are potential candidates owing to their high-temperature compositional stability and intricate tunability of the plasmon peak to achieve maximum sensitivity and being a good catalyst for multiple reactions,6–8 with sensitivity corresponding to the quantified changes in a material's observed property (the plasmon peak for plasmonic optical sensing applications, for example). Integrating AuNPs with metal oxides like TiO2, SiO2, ZrO2, etc. promotes thermal stability and creates oxygen vacancies for enhancing materials' sensitivity.9–15 In this work, Ga2O3 has been integrated as a matrix with AuNPs due to its high thermal stability (1100 °C), transparency in the visible region, and wide band gap (4.8 eV).16 Wide band gap materials allow for higher operating temperatures and better thermal conductivity since they have a relatively ordered crystal structure, which allows for more efficient phonon transport and hence a higher thermal conductivity.17–19 They also have higher phonon frequencies, which result in shorter mean free paths, carrying heat more effectively, thus making them resistant to higher temperatures.20 Thus these factors are crucial in high temperature sensing where precise control of material parameters is desired over long durations.21 Our group and others have fabricated such AuNP–metal oxide composites through physical vapor deposition, lithography, chemical vapor deposition etc., which make use of power intensive and expensive equipment.22–24 Here, we have explored a solution-based approach for synthesizing the same, wherein composites of Au and Ga2O3 (AGO) have been synthesized using two approaches viz., (i) AuNPs of different morphologies (nanospheres and nanorods) integrated with gallium oxide through a hydrothermal process and (ii) defined morphologies of Ga2O3 incorporated with Au nanospheres through a hydrothermal approach. The synthesized AGOs have been tested for their thermal stability and NO2 detection at 800 °C through a surface plasmon-based approach for the first time. Our group has already demonstrated that one of the AGOs is sensitive to concentration changes of CO at 800 °C and is morphologically and compositionally stable for at least 200 h of CO exposure at this temperature.25 Detecting NO2 through optical approaches has an inherent problem, in that NO2 itself absorbs in the visible range. Hence, a change in any optical metric (which is the scrutiny that is done to confirm a sensing response) cannot be only due to a change in the sample's optical properties and will be a convolution of the sample and the gas absorption changes. We present here a simple solution of measuring a baseline response with only NO2 wherein the degree of change in absorbance for wavelengths overlapping with the samples’ absorption is first quantified. After this, a similar measurement is done with the sample being exposed to NO2. By confirming the change in the degree of absorbance between the two, we confirm the presence of a distinct response of the sample to NO2. In addition to quantifying the sensing response, we have investigated the kinetics of the sensing mechanism substantiated by theory, experiments, and previous findings. We demonstrate thus that AGOs synthesized using simple solution-based approaches exhibit a multitude of hours of thermal stability, are responsive to NO2 at 800 °C, and can be utilized as potential candidates for real-time monitoring of such gases of concern in combustion environments.2. Materials and methods
2.1 Synthesis of AGO
The details of the synthesis of AuNPs, as well as defined morphologies of Ga2O3 such as hexagonal plates and nanorods, have been reported elsewhere.26,27 Briefly, AuNPs of various morphologies such as spheres, rods and flowers were synthesized using different reducing agents through irradiation with microwaves at a power of 425 W for 4 min. Similarly, Ga2O3 morphologies were synthesized using CTAB as a growth directing agent and microwave irradiation at a power of 425 W for 4 minutes.Two groups of AGOs have been investigated in this work. For the first group, AuNPs synthesized through a microwave method (resulting in spheres with average diameters of 5 nm and 25 nm and the samples are designated as 5S and 25S) and through chemical reduction (resulting in nanorods with average aspect ratios of 2R, 3R, and 4R) were incorporated into 10 mL of a 1 mM aqueous solution of gallium nitrate (GaNO3, Sigma Aldrich 99.9%) and placed in a hydrothermal reactor, where they were subsequently heated at 180 °C for 16 h. For the second group, defined gallium oxide morphologies (rods and hexagonal plates) as well as undefined Ga2O3 (prepared through a hydrothermal process) were synthesized using a combination of the microwave method and hydrothermal method, each of which was then added separately into separate 10 mL solutions of an aqueous solution containing 25S, transferred to a hydrothermal reactor, and subjected to heating at 180 °C for 16 h. Following this, the solutions obtained through both approaches were washed multiple times with water and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. Table 1 lists all the samples that have been investigated in this work and the nomenclatures that have been used for them. Group I comprises samples that involve different morphologies of Au NPs, which were combined with Ga2O3 through a hydrothermal approach, and group II comprises samples wherein defined as well as randomly formed morphologies of Ga2O3 were used to form composites with the same Au NPs’ morphology of spheres with an average diameter of 25 nm.
000 rpm. Table 1 lists all the samples that have been investigated in this work and the nomenclatures that have been used for them. Group I comprises samples that involve different morphologies of Au NPs, which were combined with Ga2O3 through a hydrothermal approach, and group II comprises samples wherein defined as well as randomly formed morphologies of Ga2O3 were used to form composites with the same Au NPs’ morphology of spheres with an average diameter of 25 nm.
| Group | Composite composition | Sample ID | Proportions of AuNPs/Ga2O3 taken to form the composites |
|---|---|---|---|
| Group-I | 5S + GaNO3 | 5SC | 10 mL of AuNPs + 10 mL of 1 mM GaNO3 |
| 2R + GaNO3 | 2RC | ||
| 3R + GaNO3 | 3RC | ||
| 4R + GaNO3 | 4RC | ||
| Group-II | 25S + GaNO3 | AIGO | |
| 25S + HGO | AHGO | 10 mL of 25S + 10 mg of Ga2O3 morphologies | |
| 25S + GOR | AGOR | ||
| 25S + GOH | AGOH | ||
| 25S + CGO | ACGO |
The AGOs were dispersed in isopropyl alcohol (IPA) and deposited onto quartz substrates at an optimized rotational speed of 1500 rpm to achieve maximum absorbance. Later, they were dried at 90 °C and finally subjected to annealing at 850 °C. A schematic representation of the synthesis of the two groups of plasmonic composites is shown in Fig. 1.
2.2 Gas sensing and data analysis
Sensing experiments involved monitoring the optical absorbances of both NO2 and the samples between the wavelength range of 350 nm and 1000 nm using a Flame-T-visible-NIR spectrometer from Ocean Optics. AGOs spun on quartz substrates were placed inside a horizontal split-type tubular furnace (Indfurr Superheat Furnaces, India), with provisions for flowing different concentrations of air as well as NO2 using Alicat Scientific mass flow controllers (MFCs). The percentage of NO2 present in the cylinder was 1% (air balance). Compressed air at appropriate flow rates was used as the background/carrier gas. The complete setup is shown in Fig. 2, wherein an absorbance measurement is made with air as the reference. The first tests were on the changes in absorbance detected by the spectrometer when only NO2 was flowed into the chamber at 800 °C. Multiple exposures were done at each concentration of 500 ppm, 1000 ppm, 2500 ppm, and 5000 ppm in order to get the average change in absorbance, presented in Section 3.5. The concentration of the gas (in ppm) was calculated based on the ratio between the gas flow (in SLPM) of the target gas (NO2) and that of the carrier gas (air). Flow vision software was used to program and control the gas flow. Post these trials and prior to the sensing tests, the composites were tested for their thermal stability (TS) i.e. their ability to withstand without degradation the temperature of 800 °C wherein they were heated to 800 °C at a ramp rate of 10 °C min−1 with continuous purging of air at a flow rate of 2 standard litres per minute (SLPM). Absorption spectra were collected every 5 s from room temperature to 800 °C and for 2 h (the typical duration of a TS study) at this temperature. The results of the TS studies have been reported elsewhere.28 Briefly, these studies revealed that AGOs 2RC and 3RC underwent a structural transformation (from rods to spheres, evident from the disappearance of the longitudinal plasmon band), whereas all other AGOs were found to be morphologically more stable at 800 °C for the tested duration of two hours. Even though 2RC and 3RC had undergone structural transformation, the resulting spherical particles were stable against further changes, as the plasmon bands were unvarying at 800 °C post this change. Hence, all the AGOs were tested to detect NO2.After the TS tests and ascertaining the suitability of these composites for high temperature sensing, they were exposed to NO2 at 800 °C. During the sensing experiments the sample was initially heated to 800 °C at a rate of 10 °C min−1, and this temperature was sustained for 1 h while a continuous airflow was maintained at a flow rate of 2 SLPM. Post this step NO2 was flowed into the setup with the aforementioned concentrations, wherein the NO2 stream coming from a separate mass flow controller was mixed with the already flowing stream of the carrier gas before entry into the sensing chamber. The initial purging with air allowed the sample to stabilize its response to O2 (present in the carrier air) at the temperature before NO2 was introduced, allowing confirmation to be made that any change to the absorbance spectra when the NO2/air mixture was flowed into the sensing chamber was due to reactions with NO2. Measurements of the changes to the absorbance intensities during these exposures were then performed using an in-house Python script and are discussed in Section 3.
2.3 Characterization techniques
UV-visible absorption spectra were obtained using a Flame-T-visible spectrometer (Ocean Optics). The crystalline structure of AGO was examined via X-ray diffraction (XRD) with an Empyrean device from Malvern Panalytical, utilising Cu-Kα radiation (λ = 1.54 Å). The diffractograms were recorded within a 2θ range of 20° and 90°. Morphological characteristics of the synthesized nanocomposites were analysed using transmission electron microscopy (TEM) images and energy dispersive X-ray spectra (EDS) with a JOEL JEM 2100 at an accelerating voltage of 200 kV. For TEM, colloidal suspensions were coated on copper grids and dried at room temperature. The surface morphology and elemental analyses were done using a ZEISS EVO 18 scanning electron microscope (SEM) operating at an accelerating voltage of 20 kV.3. Results and discussion
This section discusses the following topics, viz., results of optical absorbance investigations of the composites and TS tests (Section 3.1), the diffraction studies on the AGOs (Section 3.2), the morphological characterization (Section 3.3), and the scrutinization of the NO2 responses of the AGOs at 800 °C (Section 3.4).3.1 Optical absorbance investigations of the composites and TS studies
Optical studies have been carried out to analyze the absorbance of the synthesized AGOs and their corresponding thermal stability to confirm the presence of AuNPs in the composites. The synthesized AGOs exhibit their characteristic peaks in the region of 500–650 nm (in the case of spheres) due to the collective oscillation of conduction electrons in the AuNPs termed as localized surface plasmon resonance, and interband transitions are entrenched reasons for this absorption.29 These AGOs were tested for their thermal stability prior to gas sensing tests to evaluate their stability after exposure to 800 °C for 2 h. Upon integration of pristine Au nanostructures (AuNSs and AuNRs), with Ga2O3 using a hydrothermal process, AuNRs underwent a morphological transformation (rods to spheres) due to high-temperature exposure confirmed by the optical absorbance studies (monitored in situ) for all the composites, which showed a shift in the plasmon peak as well as the change of two distinct peaks into one. AuNRs tend to undergo thermal reshaping via surface diffusion as temperature increases, evolving towards a lower aspect ratio (i.e., a spherical shape). Kennedy et al. have studied this reshaping dynamics at elevated temperatures for example and reported that the AuNRs undergo shape transformations and that this behaviour was consistent across all the aspect ratios.30Unlike AuNRs, composites (2RC and 3RC) and AuNSs (5SC and group II AGOs) were found to be relatively more stable against changes to morphology, as post TS tests all AGOs exhibited the presence of the characteristic plasmon peak of AuNPs, albeit with varying degrees of shift in this peak towards longer wavelengths i.e., a redshift (to varying degrees between 2 nm and 10 nm) due to an increase in AuNP volume at high temperature.30 A representative absorbance graph as well as the TS graph of the best responding AGO (to NO2, i.e., AIGO, the reasons for which will be discussed in the subsequent sections) is presented in the ESI† as Fig. S1 and S2 respectively.
As shown in Fig. 3, following the TS tests, a 4 nm red shift has been noted, attributable to Ostwald ripening and/or aggregation characterized by the growth of larger particles at the expense of smaller ones and/or the clustering of particles, respectively, in addition to alterations in surface structure including changes in the type and concentration of roughness features/surface sites etc.31,32 These conclusions have been arrived at from the fact that a redshift in the plasmon wavelength post exposure to only temperature, without any changes in material compositions and the surrounding dielectric, is indicative of an increase in the average nanoparticle volume, and that the phenomena of ripening and/or agglomeration at high temperatures have been well-established. Although direct visualization of the samples would have confirmed these conclusions, limitations of the characterization techniques used (SEM and TEM) preclude imaging since the nanoparticles incorporated are below 50 nm (precluding SEM imaging) and are dispersed on a 1 mm thick quartz substrate (precluding TEM). Hence the confirmations regarding particle morphology changes have been arrived at from the optical absorbance changes, which have been valuable to judge the same, as confirmed through this report and others.
 | ||
| Fig. 3 Optical absorbance of the AIGO composite recorded before and after TS tests at room temperature. Reproduced with permission from ref. 30. | ||
3.2 Diffraction studies on the AGO composites
Diffraction studies have been performed to confirm the presence of Au and Ga2O3 before and after TS tests. The diffraction patterns exhibit the Bragg reflection planes (111), (110), (220), and (311) of Au for all the AGOs, whereas the reflection planes of Ga2O3 differ owing to formation of different phases such as β and γ. Among the polymorphs of Ga2O3, the β phase has high thermal and phase stability up to 1100 °C, which has been confirmed through thermal stability tests.33 All the reflection planes corresponding to the β phase have been observed except for the γ phase, which showed maximum lattice distortions. Since the lattice distortions are temperature-driven parameters wherein the atoms tend to shift from their equilibrium position by gaining kinetic energy and influence the stability and phase composition, they play a crucial role in tailoring the light–matter interactions and optical properties, which directly influence the sensing performance of the composites.34 Thus, to confirm the lattice distortions in the unit cell (after TS tests), Rietveld refinement (using Xpert HighScore software) has been performed for the AGOs comprising distinctive and discrete morphologies (AIGO, AGOH, AGOR, ACGO, and AHGO). From the analysis, it was confirmed that the AGOH exhibited maximum distortion compared to the pristine Ga2O3, whereas the AIGO had minimum distortion. Furthermore, statistical parameters derived from the reliable factors (R-value) confirm that AIGO had a lower goodness of fit (3.66) value compared to other AGOs due to minimum distortion in the crystal lattice. Since all the other AGOs (5SC, 2RC, 3RC, and 4RC) have been integrated through an in situ method similar to AIGO, these AGOs have been exempted from the analysis as they possess only the pure phase of β-Ga2O3. We observe a decrease in the crystallite sizes corresponding to the dominant (111) plane for all AGOs, which is envisaged as due to the high-temperature exposure (800 °C) tending to increase the crystallinity, which subsequently decreases the grain size.28,30 Also, no other peaks corresponding to crystalline/amorphous phases confirm the absence of contaminants in the samples. The diffraction patterns of the AGOs, Rietveld parameters, and the crystallite size corresponding to the (111) plane of Au are given in the ESI† (Fig. S3–S6). Diffraction patterns of AIGO before and after TS tests are presented in Fig. 4.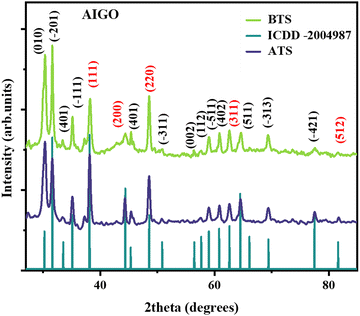 | ||
| Fig. 4 Diffraction patterns of AIGO before and after TS tests. Reproduced with permission from ref. 27. | ||
Based on the XRD analysis in Fig. 4, the peaks of Au have been retained. The % change in the crystallite size has been determined for the (111) plane of Au and is presented in the ESI† as Fig. S6.
3.3 Morphological characterization of the AGO composites
Morphologies of the AuNPs and Ga2O3 are important to achieve the tunable absorption desirable for gas sensing applications. The surface morphology of Ga2O3, as well as AuNP structures (spheres and rods), prepared through a microwave approach and their growth mechanisms have been studied by our group methodically.33 AGOs prepared using the hydrothermal approach have been subjected to morphological analysis to understand the dimensions and the interaction between the host and support matrix (Au–Ga2O3). SEM analyses were performed for AGOR, AGOH, and ACGO composites, and their elemental compositions were further confirmed using EDS. TEM analysis has been performed for other composites such as AIGO and ACGO, which confirmed the presence of discrete morphologies of Ga2O3 with the uniform dispersion of AuNPs. The SEM images and their corresponding EDS graphs, TEM images, and dimensions of all the composites obtained through SEM analysis using ImageJ software are given in Fig. S7 and S8 (ESI†), respectively. The TEM image and SAED pattern of the representative AIGO composite are presented in Fig. 5. | ||
| Fig. 5 (a) TEM images of the AIGO composite depicting the presence of Au and Ga2O3. (b) SAED diffraction pattern of AIGO with ring patterns and bright spots confirming the crystallinity of the composite. Reproduced with permission from ref. 28 and 30. | ||
3.4 NO2 sensing investigations on AGOs
Post a detailed morphological and structural analysis, we have investigated the response of the different sample architectures to NO2. Accordingly, this section has been divided into the determination of the changes in absorbance observed when only NO2 was present in the sensing chamber (i.e. without any sample), in Section 3.4.1, the changes in absorbance observed when the different samples were exposed to the same concentrations of NO2 (as in Section 3.4.1), as revealed in Section 3.4.2, the reaction mechanisms between the sample and NO2 as elaborated in 3.4.3, and finally examination of temperature driven kinetics as detailed in Section 3.4.4.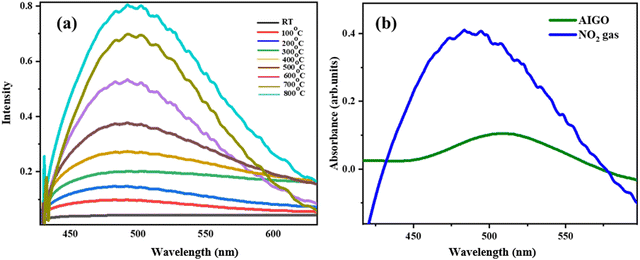 | ||
| Fig. 6 (a) Absorbance of NO2 gas as a function of temperature in the wavelength range of 450–600 nm and (b) superimposed optical absorbance of AIGO and NO2 gas. | ||
NO2 absorbance is extensively overlapped by that of its dimer N2O4 at any temperature and pressure. Experiments were carried out under conditions where N2O4 would not exist (since it absorbs below 400 nm and the acquisitions are not done at this wavelength), revealing that the absorbance of NO2 was at 420 nm.39 Another study reports that the absorbance of N2O4 alone was at 240 nm, wherein to minimize the absorbance of the dimer, measurements were performed at low pressures. A study analyzed the temperature dependent absorbance of NO2 and N2O4 in the temperature range of 213–298 K and between 310 and 570 nm using a diode array spectrometer wherein spectral changes were recorded with an increase in temperature in this region of interest. The changes in the absorbance spectral profile were observed with respect to temperature, whereas no such temperature effects were reported for N2O4.40 A tangible absorption cross-section of NO2 in the range of 300–500 nm, with that of N2O4 occurring below 400 nm, has also been reported.41
With this understanding of the contributions to the observed absorbance profile, the absorbance profile of N2O4 can be overlooked since the wavelength monitored is in the range of 400–1100 nm. It is to be noted that upon increasing the temperature, the equilibrium toward producing more NO2 gas molecules is likely,34 a result of which could be seen from the testing chamber changing to dark brown. Upon cooling, the equilibrium shifts backward towards N2O4 production. It is important to note that to date, there have been only two reports on the examination of absorbance of N2O4, at the wavelengths of 190 nm and 210 nm.34,41
From Fig. 6(b) it is clear that the optical absorbance of the gas molecules overlaps with the plasmonic band of the AIGO, which makes the deconvolution of the sensor response uncertain. In plasmonic sensing, apart from the commonly used parameter retrieving the response towards an analyte by monitoring the centroid/peak position, the intensity as a function of time/temperature/wavelength can also be monitored. The centroid/peak position monitoring has the benefit of offering a good signal-to-noise ratio (S/N) when used with techniques such as the polynomial fit function that works on the least squares principle.42 In our work, since the plasmon peak overlaps with the NO2 absorbance, it is quite inappropriate to monitor the centroid/peak position. It should be noted that the former approach has been carried out by our group in order to de-convolute the response of CO gas at 800 °C since the absorbance of CO (NIR region) does not overlap with the plasmon peak (visible region).25 Hence in the present work, intensity as a function of time has been monitored in the wavelength range of 400–700 nm as shown in Fig. 7 to examine for differences in the intensity changes when only NO2 was present within the monitoring cell.
In order to quantify the absorbance intensity changes with only NO2, two iterations have been performed at 800 °C for all gas concentrations (500 ppm, 1000 ppm, 2500 ppm, and 5000 ppm) as shown in Fig. 7(a). It has been observed that the absorbance intensity of NO2 has increased upon increasing the concentrations for both iterations. The change in intensity value has been plotted with standard deviations for the wavelength range of 400–700 nm as in Fig. 7(b). The wavelengths have been limited to 700 nm since the absorbance beyond this range was negligible (approaching baseline noise levels) for all concentrations and temperatures. If the response of the AGOs to NO2 at 800 °C goes beyond the values as in Fig. 4 we then can arrive at a conclusion that the composites have a distinct response to NO2.
 | ||
| Fig. 8 Sensing responses of AIGO towards NO2 at 800 °C at concentrations of (a) 500 ppm, (b) 1000 ppm, (c) 2500 ppm and (d) 5000 ppm as a function of wavelength. | ||
All AGOs were examined by this method to arrive at the optimal configuration, and the corresponding graphs are given in Fig. S9–S15 (ESI†).
After analysis of the sensing exposure data, calibration curves showing the changes in the intensity of all samples were determined and are shown in Fig. 9. The assessment has been carried out only in the plasmonic region from 500 to 650 nm due to the presence of the plasmonic peak of all AGOs in this region. Plausible mechanisms that occur upon gas exposure between the samples and NO2 are included in Section 3.4.3. It is noteworthy that across the plasmonic region, AIGO exhibits a maximum intensity change compared to other AGOs, although all the other composites were responsive to NO2 at 800 °C. A plausible explanation for the maximum response of AIGO may stem from the combination of a more uniform dispersion of AuNPs within the composite (as can be qualitatively confirmed from the TEM images of this sample), comparatively minimal alterations in the volume expansion of AuNPs post-high temperature exposure (as confirmed by the least changes to the plasmon peak shown in Fig. 3), a higher particle density on the substrate that has enhanced the sensitivity, and a higher consistency in crystallinity as observed in XRD data following high temperature exposure,28 apart from enhancements to sensitivity resulting possibly from a higher tri-phase boundary effect, a well-established governing authority in catalytic reactions.43–45
As noted before, the modifications to morphologies are difficult to directly visualize28,30 as the composites are coated onto quartz substrates measuring 10 × 10 × 1 mm, posing challenges during TEM sample preparation in terms of an arduous crimping process to reduce the substrate size to 3 × 3 mm (for imaging), often resulting in substrate breakage and sample loss.
 | (1) |
The reaction pathways for oxygen adsorption can be written as:
| O2(g) + Vo(s) + Ga(s)3+ → O2(ads) + Ga(s)4+ | (2) |
| O2(g) + Vo(s) + Au(s) → O2(ads) + Au(s)2+ | (3) |
| O2(ads) + VO(b) → O2(b) | (4) |
Upon NO2 exposure, the gas molecules extract chemisorbed oxygen ions from the vacancies, which ultimately decreases the free electron density and plasmon frequency and/or increases the polarizability of the matrix, and the wavelength shifts toward the red compared to the baseline spectrum. The reaction pathways upon NO2 exposure can be written as follows:50
| NO2 + 2O(s)− → NO2−(ads) + O2 + e− | (5) |
| NO2−(ads) + O2 + e− → NO + 1/2O2 + 2O− | (6) |
 | ||
| Fig. 11 Temperature-dependent sensing response of AIGO towards NO2 at 500 ppm at different temperatures: 400 °C, 600 °C and 800 °C. | ||
At higher temperatures, vacancy mediated reactions tend to increase due to higher ad/desorption reactions. Hence, the response to NO2 was envisaged to increase, but in contrast, the response linearly decreased, as revealed in Fig. 11. A similar trend has been reported by Rogers et al., wherein the authors have reported this change due to increased mass transport kinetics at high temperatures, i.e. when the gas molecules are heated, the volume of the gas increases according to the ideal gas equation PV = nRT.53,54 Hence the number of NO2 molecules/population decreases with the increase in temperature in the testing chamber, possibly causing reduced sensitivity (Fig. 12).
The response and recovery times have decreased as well with an increase in temperature. To explain these changes in time, it is necessary to take into consideration the temperature-dependent adsorption/desorption processes. Langmuir's theory predicts the adsorption equilibrium through the equation55
 | (7) |
4. Conclusions and outlook
To summarize, this report details a suitable plasmonic material incorporated metal oxide composite for high temperature detection of NO2, realized through an unsophisticated solution based approach for the first time. AGCs synthesized using a straightforward in situ hydrothermal approach can thus be used as potential candidates for real time monitoring of emissions of such gases in harsh environments, such as boiler plants, steam and gas turbines, glass making industries, food processing units, etc. The Au NPs presented in this study, when integrated with Ga2O3, exhibited hitherto unobserved morphological stability against temperature. Previous reports have employed the fabrication of such material combinations through physical vapor deposition, chemical vapor deposition, lithography, etc., involving expensive equipment and intensive power. We have realized the composites through relatively much simpler processes and proposed the hydrothermal approach for composite preparation as it allows repeatable results over size and morphology distributions, enabling the formation of uniform structures.A unique finding in this study is that the use of discrete wavelength-based investigations can significantly enable the use of plasmonic composites that have overlapping absorption with the analyte to be sensed. In particular, the confirmation of the sensitivity of the absorbance changes of the analyte to those when both the sample and analyte are present can be differentiated through this approach. Hitherto unusable Au and Ag based composites can hence be explored for the presence of a distinct response to relevant analytes. The necessity of careful analysis (to confirm a response magnitude above the average response with only the analyte) and repeated measurements of the sensing response (to ascertain the increased sensitivity of the sample compared to just the analyte) must be underscored, however. To the best of our knowledge, a plasmonically active composite has not been reported for NO2 sensing at a temperature of 800 °C to date. The approach of investigating individual wavelengths is the crucial aspect here, as contemplating changes in the SPR peak in its entirety will lead to errors in calculating the response due to the overlap in absorbance.
To suggest a few directions for furthering this work, integrating AuNPs of smaller particle sizes with Ga2O3 (for a higher rate of dissociation of the analyte due to a higher surface-to-volume ratio), encapsulating the plasmonic structures within the matrix in a core–shell architecture (with the matrix being the shell, possibly providing enhanced stability and protection for the NPs), optimizing the loading of Au NPs in the AGCs thereby enhancing, for example, the spillover effect leading to improved charge transfer, and fabricating sensor arrays made of different metal oxides that can have cross-sensitivity to multiple gases will all influence the choice of the analyte gas, the operating range, and the sensor performance metrics. For example, developing a sensing material with the plasmon band peaking beyond 600 nm can be a simpler option for sensing gases like NO2 that tend to absorb naturally in the visible region (the tendency for spheroidization at high temperatures notwithstanding) albeit presumably not for the temperatures reported here. Hence, fabrication of morphologies such as Au NRs, nanoflowers, and mixed polygons must be done keeping this in mind with steps such as functionalizing the NPs with ligands that can anchor the metal oxide for better morphological stability. These structures can still be realized when one chooses to work with simple solution-based material development.
Data availability
The authors confirm that all the data generated in this manuscript have been made available in the manuscript and/or the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors sincerely thank the Aeronautical Research & Development Board, Govt of India (sanction code: DGTM/TM/ARDB/GIA/18-19/0296, Project No: 2031895) for supporting this study.References
- S. Cheval, C. M. Adamescu, T. Georgiadis, M. Herrnegger, A. Piticar and D. R. Legates, Int. J. Environ. Res. Public Health, 2020, 17, 4140 CrossRef CAS PubMed.
- C. E. Baukal and P. B. Eleazer, Quantifying NOx for industrial combustion processes, J. Air Waste Manage. Assoc., 1998, 48, 52–58 CrossRef CAS PubMed.
- P. Saxena and S. Sonwani, Criteria Air Pollutants and their Impact on Environmental Health, Springer Nature, Springer Singapore, 2019, 1 Search PubMed.
- K. I. Jolic, C. R. Nagarajah and W. Thompson, Nom-contact, optically based measurement of surface roughness of ceramics, Meas. Sci. Technol., 1994, 5, 671 CrossRef.
- Y. K. Zhu, G. Y. Tian, R. S. Lu and H. Zhang, A review of optical NDT technologies, Sensors, 2011, 11, 7773–7798 CrossRef PubMed.
- S. B. Khan, M. T. S. Chani, K. S. Karimov, A. M. Asiri, M. Bashir and R. Tariq, Talanta, 2014, 120, 443–449 CrossRef CAS PubMed.
- M. P. Da Cunha, T. Moonlight, R. Lad, D. Frankel and G. Bernhardt, Proc. IEEE Sens., 2008, 205–208 Search PubMed.
- S. Sarma, S. Singh and A. Garg, Measurement, 2021, 172, 108876 CrossRef.
- L. Keerthana, M. Ahmad Dar and G. Dharmalingam, Chem. – Asian J., 2021, 16, 3558–3584 CrossRef CAS PubMed.
- H. Jin-Yong, H. Lei, H.-Y. Zhang, X.-X. Xue, X.-P. Wang, C.-H. Wang and Y. Zhang, Rare Met., 2024, 12(43), 6500–6515 Search PubMed.
- W. Ting-Ting, B.-S. Xing, C.-Y. Guo, J.-Y. Hao, Y. Wang, L.-H. Huo, X.-L. Cheng and Y.-M. Xu, Rare Metals, 2023, 12(42), 3897–3913 Search PubMed.
- Z. Yue, M.-Y. Wang, X.-G. San, Y.-B. Shen, G.-S. Wang, L. Zhang and D. Meng, Rare Met., 2024, 1(43), 267–279 Search PubMed.
- H. Jinyong, X. Liu, J. Zhang, X. Gu and Y. Zhang, Sens. Actuators, B, 2023, 382, 133505 CrossRef.
- H. Jinyong, X. Wang, H. Lei, M. Luo and Y. Zhang, Sens. Actuators, B, 2024, 407, 135422 CrossRef.
- W. Ding, D. Liu, J. Liu and J. Zhang, Chin. J. Chem., 2020, 38, 1832–1846 CrossRef CAS.
- L. Keerthana, M. Ahmad Dar and G. Dharmalingam, Chem. Asian J., 2021, 16, 3558–3584 CrossRef CAS PubMed.
- C. H. Liang, G. W. Meng, G. Z. Wang, Y. W. Wang, L. D. Zhang and S. Y. Zhang, Appl. Phys. Lett., 2001, 001(78), 3202–3204 CrossRef.
- Z. Liu, T. Yamazaki, Y. Shen, T. Kikuta, N. Nakatani and Y. Li, Sens. Actuators, B, 2008, 129, 666–670 CrossRef CAS.
- F. Safieddine, F. E. H. Hassan and M. Kazan, J. Solid State Chem., 2022, 312, 123272 CrossRef CAS.
- B. Zhao, X. Li, L. Yang, F. Wang, J. Li, W. Xia, W. Li, L. Zhou and C. Zhao, Photochem. Photobiol., 2015, 91, 42–47 CrossRef CAS PubMed.
- G. Dharmalingam and M. A. Carpenter, Sens. Actuators, B, 2017, 251, 1104–1111 CrossRef CAS.
- N. Karker, G. Dharmalingam and M. A. Carpenter, ACS Nano, 2014, 8, 10953–10962 CrossRef CAS PubMed.
- G. Dharmalingam, N. A. Joy, B. Grisafe and M. A. Carpenter, Beilstein J. Nanotechnol., 2012, 3(81), 712–721 CrossRef PubMed.
- L. Keerthana, A. R. Indhu and G. Dharmalingam, J. Mater. Res., 2023, 38, 497–506 CrossRef CAS.
- K. Narayanan and D. Gnanaprakash, J. Clust. Sci., 2022, 33, 227–240 CrossRef CAS.
- L. Keerthana, A. R. Indhu and G. Dharmalingam, App. Nano., 2022, 12, 2857–2871 CrossRef CAS.
- K. Narayanan and G. Dharmalingam, J. Phy. Chem. Sol., 2024, 185, 111800 CrossRef.
- J. R. Lakowicz, Plasmonics, 2006, 1, 5–33 CrossRef CAS PubMed.
- J. Kennedy, M. Soos, A. Vaccaro and M. Morbidelli, Chem. Eng. Process., 2006, 45(10), 936–943 CrossRef.
- L. Keerthana and G. Dharmalingam, Phys. Chem. Chem. Phys., 2024, 15018–15031 RSC.
- S. Shrestha, B. Wang and P. Dutta, Adv. Colloid Interface Sci., 2020, 279, 102162 CrossRef CAS PubMed.
- L. Fu, Y. Liu, P. Hu, K. Xiao, G. Yu and D. Zhu, Chem. Mater., 2003, 15, 4287–4291 CrossRef CAS.
- X. Wang, J. Wu, Y. Zhang, Y. Sun, K. Ma, Y. Xie, W. Zheng, Z. Tian, Z. Kang and Y. Zhang, Adv. Mater., 2023, 35, 2206576 CrossRef CAS PubMed.
- S. Park, M. Kim, Y. Lim, D. H. Oh, J. Ahn, C. Park, S. Woo, W. C. Jung, J. Kim and I. D. Kim, Adv. Mater., 2024, 2313731 CrossRef CAS PubMed.
- H. A. Bent, Dimers of Nitrogen Dioxide. II. Structure and Bonding, Inorg. Chem., 1963, 2, 747–752 CrossRef CAS.
- A. Amoruso, L. Crescentini, G. Fiocco and M. Volpe, J. Geophys. Res., 1993, 98, 816–857 CrossRef.
- J. A. Davidson, C. A. Cantrell, A. H. McDaniel, R. E. Shetter, S. Madronich and J. G. Calvert, J. Geophys. Res., 1988, 93, 7105–7112 CrossRef CAS.
- I. Zyrichidou, M. E. Koukouli, D. Balis, K. Markakis, A. Poupkou, E. Katragkou, I. Kioutsioukis, D. Melas, K. F. Boersma and M. van Roozendael, Atmos. Environ., 2015, 101, 82–93 CrossRef CAS.
- M. H. Harwood and R. L. Jones, J. Geophys. Res., 1994, 99, 22955–22964 CrossRef.
- M. F. Merienne, A. Jenouvrier and B. Coquart, J. Atmos. Chem., 1995, 20, 281–297 CrossRef CAS.
- A. B. Dahlin, J. O. Tegenfeldt and F. Hǒǒk, Improving the instrumental resolution of sensors based on localized surface plasmon resonance, Anal. Chem., 2006, 78, 4416–4423 CrossRef CAS PubMed.
- R. You, X. Hao, H. Yu, B. Wang, G. Lu, F. Liu and T. Cui, Sens. Actuators, B, 2018, 263, 445–451 CrossRef CAS.
- S. Lv, Y. Zhang, L. Jiang, L. Zhao, J. Wang, F. Liu, C. Wang, X. Yan, P. Sun, L. Wang and G. Lu, Sens. Actuators, B, 2022, 354, 131219 CrossRef CAS.
- X. Liang, S. Yang, J. Li, H. Zhang, Q. Diao, W. Zhao and G. Lu, Sens. Actuators, B, 2011, 158, 1–8 CrossRef CAS.
- M. Al-Hashem, S. Akbar and P. Morris, Sens. Actuators, B, 2019, 301, 126845 CrossRef CAS.
- P. K. Jain and M. A. El-Sayed, Chem. Phys. Lett., 2010, 487, 153–164 CrossRef CAS.
- C. David and F. J. García De Abajo, ACS Nano, 2014, 8, 9558–9566 CrossRef CAS PubMed.
- G. Dharmalingam, N. A. Joy, B. Grisafe and M. A. Carpenter, Beilstein J. Nanotechnol., 2012, 3, 712–721 CrossRef PubMed.
- G. Dharmalingam and M. A. Carpenter, Proceedings Volume Sensors for Extreme Harsh Environments II, SPIE, 2015, p. 949101 DOI:10.1117/12.2199541.
- I. Zyrichidou, M. E. Koukouli, D. Balis, K. Markakis, A. Poupkou, E. Katragkou, I. Kioutsioukis, D. Melas, K. F. Boersma and M. van Roozendael, Atmos. Environ., 2015, 101, 82–93 CrossRef CAS.
- R. J. van der A, H. J. Eskes, K. F. Boersma, T. P. C. van Noije, M. Van Roozendael, I. De Smedt, D. H. M. U. Peters and E. W. Meijer, J. Geo. Res. Atm, 2008, 113, D04302 Search PubMed.
- N. A. Joy, P. H. Rogers, M. I. Nandasiri, S. Thevuthasan and M. A. Carpenter, Anal. Chem., 2012, 84, 10437–10444 CrossRef CAS PubMed.
- N. A. Joy, M. I. Nandasiri, P. H. Rogers, W. Jiang, T. Varga, S. V. N. T. Kuchibhatla, S. Thevuthasan and M. A. Carpenter, Anal. Chem., 2012, 84, 5025–5034 CrossRef CAS PubMed.
- H. Swenson and N. P. Stadie, Langmuir, 2019, 35(16), 5409–5426 CrossRef CAS PubMed.
- Q. Chen, Y. Tian, P. Li, C. Yan, Y. Pang, L. Zheng, H. Deng, W. Zhou and X. Meng, J. Chem., 2017, 1, 1496463 Search PubMed.
- Patiha, E. Heraldy, Y. Hidayat and M. Firdaus, IOP Conf. Ser. Mater. Sci. Eng., 2016, 012067 CrossRef.
- D. A. H. Hanaor and C. C. Sorrell, J. Mater. Sci., 2011, 46, 855–874 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ma00151j |
| This journal is © The Royal Society of Chemistry 2025 |






