Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fabrication and characterization of rare earth-free nanophosphor based devices for solid-state lighting applications†
M.
Rakshita
a,
Aachal A.
Sharma
a,
Payal P.
Pradhan
a,
K. A. K. Durga
Prasad
a,
M.
Srinivas
b and
D.
Haranath
 *a
*a
aDepartment of Physics, National Institute of Technology, Warangal 506 004, Telangana, India. E-mail: haranath@nitw.ac.in; Tel: +91 995 810 1115
bDepartment of Physics, Osmania University, Hyderabad-500007, Telangana, India
First published on 14th April 2025
Abstract
This study presents a novel, rare-earth-free Zn3V2O8 nanophosphor (ZnVO NP) with exceptional luminescent properties, making it ideal for phosphor-converted white light-emitting diodes (pc-WLEDs). When coupled with a 385 nm LED chip, ZnVO NP delivers white light with a correlated color temperature of approximately 4920 K, a high quantum yield of 74%, and an excellent color rendering index (CRI) of Ra = 91. Notably, the R9 value of 90.5 surpasses that of commercially available Y3Al5O12:Ce3+ (R9 = 14.3), highlighting superior red color rendering. The white light, excited at 385 nm, has CIE coordinates of (0.330, 0.301). Temperature-dependent photoluminescence spectra indicate high thermal stability, with emission peaking in the yellow region at CIE coordinates (0.43, 0.52) under 290 nm, 361 nm, and 385 nm excitation. This broadband yellow-emitting ZnVO NP offers a promising rare-earth-free alternative for pc-WLEDs, providing excellent color quality and stability under diverse operating conditions, demonstrating its practical potential in advanced lighting applications.
1. Introduction
Commercial solid-state lighting (SSL) has widely adapted white light-emitting diodes (WLEDs) due to their enhanced luminous efficiency (LE) and long lifespan. Traditionally, white light production in WLEDs involves coating a yellow-emitting phosphor (Y3Al5O12:Ce3+,YAG) onto a blue (InGaN) LED chip. However, differences in the aging properties of the blue (410–460 nm) LED chip and the yellow (570–590 nm) phosphor can lead to instability in white light production by the combination of these two complementary colors (yellow and blue).1–4 Furthermore, this type of white LED emits negligible red light, leading to a low color rendering index (CRI < 70) and a high correlated color temperature (CCT > 6000 K).5–7 To address these issues, a mixture of red, green, and blue (RGB) phosphors combined with a near-ultraviolet (NUV) LED chip (370–420 nm) can be employed to produce stable white light. In this setup, the visible components of white light are generated entirely by the phosphors, which exhibit minimal variation in color output under different forward-bias currents. NUV LED chips, particularly at 380 nm, are attractive due to their excellent energy conversion efficiency.8 All three phosphors (RGB) must efficiently absorb in the NUV region for optimal device performance. However, the commonly used rare-earth metal ion, Ce3+, is susceptible to oxidation, has limited availability, is costly, and is a non-renewable resource. Therefore, exploring rare-earth-free, yellow-emitting materials with excellent LE and stable thermal and chemical properties is essential.9 WLEDs that utilize a NUV LED chip, combined with UV LED and yellow-red emitting phosphors, are increasingly favored for their higher CRI, adjustable CCT, and ideal white CIE color coordinates. Consequently, there is a growing demand in the WLED industry for the design and development of tunable yellow-red emitting phosphors that can be efficiently activated by NUV light.In recent years, a wide range of inorganic materials, including tungstates, silicates, phosphates, borates, sulfides, molybdenites, and vanadates, have been explored for the development of high-performance luminescent materials.9–23 Among these, vanadates particularly those derived from alkali or alkaline earth materials have garnered significant attention due to their excellent chromatic properties and potential applications in electrochemistry, display technology, photocatalysis, triboelectric nanogenerators, and SSL devices.24–27 Vanadate-based, self-activated phosphors have emerged as promising candidates for WLED under near-ultraviolet (NUV) excitation.28,29 The photoluminescent quantum yield (PLQY) of vanadates is highly dependent on the spacing between V5+ luminescent centers.30,31 However, crystal defects such as oxygen vacancies inevitably occur during high-temperature oxide synthesis and play a crucial role in determining the optoelectrical properties of these materials.32,33 Studies have shown that oxygen vacancies influence the symmetry of octahedral structures, alter electron interactions, and contribute to structural disorder, resulting in changes to the optical, electrical, and magnetic properties of transition metal oxides (d-block elements).34 These vacancies act as donors, affecting the oxidation state of cations and creating local chemical pressure within the structure.
One significant advantage of self-activated vanadate phosphors over rare-earth-based phosphors is their cost-effectiveness, which helps conserve global rare-earth resources. These vanadate compounds have gained considerable attention due to their ability to self-activate and produce a broad emission spectrum in the visible region, primarily driven by the intense metal-to-oxygen charge transfer (CT) transition from V5+ to O2− within the VO4 tetrahedron. Their strong absorption bands in the NUV range make them highly suitable for SSL applications. By carefully selecting the luminescent center and composition, it is possible to tailor the luminescence properties of vanadates. The VO43− group, comprising four oxygen ions arranged in a tetrahedral configuration around a central vanadium ion, serves as the highly efficient light-emitting core.35–37
Nanophosphors (NPs) have gained significant attention due to their distinctive properties compared to bulk materials, including a large surface-to-volume ratio, drastically different physicochemical characteristics, and high porosity.38 Metal vanadates (MxVyOz, M = Co, Zn, Ni, Cu, Fe) represent a notable class of nanomaterials, attracting widespread research interest for their potential applications in WLEDs. The combination of the vanadate tetrahedral (Td) symmetry with various metal elements such as Ag, Cu, Fe, and Pb offers a wide array of optical properties, improved thermochemical stability, and an economical, simple synthesis process that eliminates the need for rare-earth elements. Oxygen vacancies in vanadium oxides contribute to a mixed valency state of V4+ and V5+, which adversely impacts PL.39 This phenomenon highlights the importance of understanding how oxygen vacancies in the [VO4] tetrahedron influence the self-activation of phosphors. Extensive research has been conducted on synthesizing metal vanadates through various methods, including wet chemical approaches, hydrothermal techniques, coprecipitation, sol–gel, and solid-state reactions.26,40,41 Among these, zinc vanadate nanomaterials have garnered significant interest due to zinc's environmental friendliness, abundance, low cost, and vanadium's multiple valence states, which enable the formation of complex architectures. Zinc vanadate (Zn3V2O8) has shown promise in several fields, including photocatalysis, lithium-ion batteries, supercapacitors, and luminescent materials for WLEDs, largely due to its unique crystal structure (porous framework) as shown in ESI,† Table S1.26,40
Nakajima et al. have reported the PL and color properties of M3V2O8 (M = Mg, Zn) and AVO3 (A = K, Rb, Cs), synthesized via the solid-state method (SSM).42 Similarly, Ni et al. described the hydrothermal synthesis of Zn3V2O8 microparticles using Zn3(OH)2V2O7·nH2O nanosheets as the starting material and investigated their optical properties.43 Hydrothermal synthesis of vanadium-based materials in the presence of long-chain alkylamines typically results in 1D and 2D structures such as rods, wires, fibers, sheets, or ribbons, but this process often requires extended reaction times (over 24 hours). For large-scale production, a simpler, cost-effective approach is needed.
In this study, Zn3V2O8 (ZnVO) NPs were synthesized using the sol–gel method (SGM), urea auto-combustion (UAC) method, coprecipitation method (CPM), and hydrothermal method (HTM). Among these, the CPM is one of the simplest and most economical techniques, where metal ions are transformed into the desired materials through a chemical reaction between metal nitrates and bases. This method offers advantages such as better homogeneity, a high surface-to-volume ratio, and higher purity. The broadband emission properties of Zn3(VO4)2 synthesized by CPM were extensively studied in terms of structural, morphological, elemental, and optical characteristics and compared with those produced using SGM, UAC, and HTM.
2. Experimental section
2.1 Sol–gel method (SGM)
ZnVO NP was synthesized using the SGM with NH4VO3, Zn(NO3)2·6H2O, and citric acid as starting materials. A solution with a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V molar ratio of 3
V molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was prepared by dissolving 3 mol% of Zn(NO3)2·6H2O and 2 mol% of NH4VO3 in 40 ml of deionized (DI) water. Citric acid, serving as the chelating agent, was added at a concentration of 0.05 mol%. The final solution was heated at 80 °C on a hot plate while being stirred continuously until all the water evaporated, forming a gel. This gel was then dried at 140 °C for 24 hours to yield Zn3V2O7(OH)2·2H2O powder. The resulting powder was ground and subsequently calcined in a preheated furnace at 600 °C for 30 minutes to remove any remaining organic and inorganic compounds, forming the ZnVO NP, which was then subjected to further characterization.
2 was prepared by dissolving 3 mol% of Zn(NO3)2·6H2O and 2 mol% of NH4VO3 in 40 ml of deionized (DI) water. Citric acid, serving as the chelating agent, was added at a concentration of 0.05 mol%. The final solution was heated at 80 °C on a hot plate while being stirred continuously until all the water evaporated, forming a gel. This gel was then dried at 140 °C for 24 hours to yield Zn3V2O7(OH)2·2H2O powder. The resulting powder was ground and subsequently calcined in a preheated furnace at 600 °C for 30 minutes to remove any remaining organic and inorganic compounds, forming the ZnVO NP, which was then subjected to further characterization.
2.2 Urea auto combustion method (UAC)
The UAC method involves rapidly heating a water-based solution containing stoichiometric proportions of ammonium nitrate, metal nitrates, and urea, which acts as a combustion fuel. ZnVO NP was synthesized using this method with Zn(NO3)2·6H2O, NH4VO3, and urea as starting precursors. These precursors were dissolved in DI water in their stoichiometric ratios and stirred at 80 °C until a clear, yellowish homogeneous mixture was obtained. The solution, placed in a quartz beaker, was then transferred to a preheated muffle furnace set at 600 °C for less than 30 minutes. During this process, the solution boiled, frothed, and ignited, resulting in the formation of the final product, which was subsequently ground and prepared for further characterization.2.3 Co-Precipitation method (CPM)
ZnVO NP was synthesized using the CPM. A zinc nitrate solution (solution 1) was prepared by dissolving 0.03 mol of Zn(NO3)2·6H2O in 30 ml of DI water. Simultaneously, an ammonium metavanadate solution (solution 2) with a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V molar ratio of 3
V molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was prepared by dissolving 0.02 mol of NH4VO3 in 20 ml of hot DI water (80 °C). The water facilitates hydrolysis, leading to the formation of metal hydroxides or intermediate compounds. After solution 2 cooled naturally to room temperature (RT), it was added dropwise to Solution 1 with continuous stirring. To adjust the pH to 10, 1 M NaOH was added gradually, and the mixture was stirred for 6 hours until a homogeneous yellow precipitate of Zn3V2O7(OH)2·2H2O formed. Upon the addition of the precipitating agent, a chemical reaction took place, resulting in the formation of insoluble nanoparticles. The precipitate was collected by centrifugation at 6000 rpm and thoroughly washed several times with DI water and ethanol to remove unreacted components. It was then dried at 80 °C for 24 hours. Finally, ZnVO NPs were obtained by sintering the dried powder (Zn3V2O7(OH)2·2H2O) in an air atmosphere at 600 °C for less than 30 minutes to remove hydroxide moieties and crystallization water.
2 was prepared by dissolving 0.02 mol of NH4VO3 in 20 ml of hot DI water (80 °C). The water facilitates hydrolysis, leading to the formation of metal hydroxides or intermediate compounds. After solution 2 cooled naturally to room temperature (RT), it was added dropwise to Solution 1 with continuous stirring. To adjust the pH to 10, 1 M NaOH was added gradually, and the mixture was stirred for 6 hours until a homogeneous yellow precipitate of Zn3V2O7(OH)2·2H2O formed. Upon the addition of the precipitating agent, a chemical reaction took place, resulting in the formation of insoluble nanoparticles. The precipitate was collected by centrifugation at 6000 rpm and thoroughly washed several times with DI water and ethanol to remove unreacted components. It was then dried at 80 °C for 24 hours. Finally, ZnVO NPs were obtained by sintering the dried powder (Zn3V2O7(OH)2·2H2O) in an air atmosphere at 600 °C for less than 30 minutes to remove hydroxide moieties and crystallization water.
2.4 Hydrothermal method (HTM)
ZnVO NP was synthesized using the HTM with Zn(NO3)2·6H2O and NH4VO3 as precursors. First, 0.03 mol of Zn(NO3)2·6H2O and 0.02 mol of NH4VO3 were dissolved in 100 ml of DI water and stirred continuously to form a homogeneous solution. To adjust the pH to approximately 10, 1 M NaOH was added dropwise while the mixture was stirred on a magnetic hot plate at 80 °C for 3 hours. The resulting solution was then transferred to a 250 ml Teflon-lined autoclave and maintained at 180 °C for 12 hours. After natural cooling to RT, the final product was collected by centrifugation at 6000 rpm for 30 minutes, followed by multiple washings with water and ethanol. The collected sample was then dried at 80 °C and annealed at 600 °C for 30 minutes in a muffle furnace. For comparison, a ZnVO sample was also prepared using the SSM. ZnO and V2O5 were thoroughly mixed in a mortar and pestle and annealed at 800 °C for 1 hour.It is understood that the synthesis method plays a crucial role in determining various properties of Zn3V2O8 phosphors by influencing factors such as crystal structure, particle size, and surface defects. Among the various synthesis approaches discussed above, the co-precipitation method (CPM) has a profound impact on the photoluminescence (PL) properties of self-activated Zn3V2O8, yielding the highest luminescence quantum yield (QY). This is primarily attributed to the controlled nucleation and rapid precipitation of Zn2+ and V5+ precursors, leading to ultrafine, homogeneous nanocrystals with minimal defects. The enhanced PL emission arises from the improved structural uniformity, reduced non-radiative recombination sites, and optimized charge transfer within the [VO4]3− luminescent centers. In contrast, the solid-state reaction method, despite ensuring excellent phase purity and crystallinity, often results in larger particle sizes and a lower surface area, leading to weaker luminescence due to limited exciton confinement and increased defect-induced quenching. The sol–gel method, while effective in producing fine particles with controlled stoichiometry, can introduce residual organic species or trapped solvent molecules, which act as quenching centers, reducing luminescence efficiency. Compared to hydrothermal and urea auto-combustion methods, which also produce nanostructured phosphors, co-precipitation provides superior control over particle growth and surface passivation, significantly enhancing quantum efficiency. The ability to tailor the defect density, crystallite size, and luminescent center distribution through this method makes it a promising route for optimizing the self-activated emission of Zn3V2O8 for high-performance phosphor-converted white light-emitting diodes.
2.5 Device fabrication of prototype LED
A WLED prototype was fabricated by combining ZnVO nanoparticles (NPs) with epoxy resin and a 385 nm LED chip. The resin and ZnVO NPs were mixed in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 ratio and applied as a 200 μm thick layer onto a thin glass slide. The mixture was dried at 70–90 °C for 24 hours. The devices were then encapsulated with ZnVO NPs and mounted directly on the headers. The electroluminescence (EL) of the packaged LED device, embedded with ZnVO NPs, was measured using an integrating sphere after final assembly.
7 ratio and applied as a 200 μm thick layer onto a thin glass slide. The mixture was dried at 70–90 °C for 24 hours. The devices were then encapsulated with ZnVO NPs and mounted directly on the headers. The electroluminescence (EL) of the packaged LED device, embedded with ZnVO NPs, was measured using an integrating sphere after final assembly.
2.6 Instrumentation tools
The powder X-ray diffraction (PXRD) patterns of the samples were acquired in the 2θ range from 10° to 80° using Ni-filtered Cu Kα (λ = 1.54 Å) as a source of X-ray at a scan rate of 0.01 s−1 with Rigaku. The Fourier transform infrared spectrum (FTIR) of the samples was measured from 4000 to 400 cm−1 range using PerkinElmer (100S FTIR spectrophotometer) in KBr pellets with 4 cm−1 resolutions for 20 scans. The morphology of the samples was carried out by scanning electron microscopy (SEM) using ZEISS. In the elemental study, the atomic and weight proportion of the component ions were measured by energy dispersive X-ray spectroscopy (EDAX) attached with SEM. The solid-state absorption spectroscopy was analyzed using UV/visible-diffuse reflectance spectroscopy (UV/vis-DRS) by Analytic Jena with a wavelength range of 200–1000 nm equipped with an integrating sphere using BaSO4 as standard. High-resolution X-ray photoelectron spectroscopy has been used to detect the ionic state of surface ions in hematite that uses a monochromatic Al Kα radiation source with an energy of ∼1500 eV in the Thermo Scientific K alpha system. The C 1s peak at ∼285 eV was used as a reference for sample charging correction of other elements. The photoluminescence excitation (PLE) and emission (PL) of the synthesized samples were recorded using HITACHI: fluorescence spectrophotometer F-4700. The Horiba Scientific Spectrophotometer: Fluorolog-3 is used to perform life decay and the TDPL of the samples. The EL of capped glass slides coated with ZnVO NPs was measured by using Spectra Wiz Integrated Sphere.3. Results and discussion
3.1 Structural, morphological, and optical characterization
 | (1) |
 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tan
tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, where ‘ε’ is the root mean square value of the micro-strain, d is the interplanar spacing, a, b, and c are the lattice constants and h, k, and l are the miller indices. As a result, the line broadening is a function of crystallite size and strain, as represented by the W–H equation using eqn (4),46,47 and the lattice parameters of the pure ZnVO were calculated using eqn (5),25 respectively as shown
θ, where ‘ε’ is the root mean square value of the micro-strain, d is the interplanar spacing, a, b, and c are the lattice constants and h, k, and l are the miller indices. As a result, the line broadening is a function of crystallite size and strain, as represented by the W–H equation using eqn (4),46,47 and the lattice parameters of the pure ZnVO were calculated using eqn (5),25 respectively as shown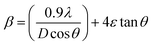 | (3) |
 | (4) |
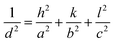 | (5) |
The microstrain can be determined by plotting the value of β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ as a function of 4
θ as a function of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ shown in Fig. 1(c), and the crystalline size from the intersection with the vertical axis. For SGM, UAC, CPM, and HTM synthesized samples, the strain of ZnVO NPs is 2.27 × 10−3, 1.56 × 10−3, 0.99 × 10−3, 1.3 × 10−3, and the crystalline size is 22.52 nm, 22.66 nm, 22. 02 nm, and 22.68 nm respectively. Table S2 (ESI†) shows that the estimated values are well-matched with the standard values without much variation. The micro-strain in the CPM synthesized sample is lower than in HTM, UAC, and SGM synthesized samples. The less strain in the CPM method synthesized samples shows the synthesized ZnVO sample has high crystallinity and better ionic bonding. To additional validate the purity of the phase and lattice characteristics of the synthesized material, the crystal structure of the ZnVO CPM NP was derived using a Rietveld refinement fullprof software.48Fig. 1(d) depicts both the observed and calculated XRD profiles, all peaks have been indexed and the refinement is satisfactory. The most straightforward discrepancy index, the weighted profile R-factor (Rwp), follows directly from the square root of the quantity minimized, scaled by the weighted intensities (eqn (6)):
θ shown in Fig. 1(c), and the crystalline size from the intersection with the vertical axis. For SGM, UAC, CPM, and HTM synthesized samples, the strain of ZnVO NPs is 2.27 × 10−3, 1.56 × 10−3, 0.99 × 10−3, 1.3 × 10−3, and the crystalline size is 22.52 nm, 22.66 nm, 22. 02 nm, and 22.68 nm respectively. Table S2 (ESI†) shows that the estimated values are well-matched with the standard values without much variation. The micro-strain in the CPM synthesized sample is lower than in HTM, UAC, and SGM synthesized samples. The less strain in the CPM method synthesized samples shows the synthesized ZnVO sample has high crystallinity and better ionic bonding. To additional validate the purity of the phase and lattice characteristics of the synthesized material, the crystal structure of the ZnVO CPM NP was derived using a Rietveld refinement fullprof software.48Fig. 1(d) depicts both the observed and calculated XRD profiles, all peaks have been indexed and the refinement is satisfactory. The most straightforward discrepancy index, the weighted profile R-factor (Rwp), follows directly from the square root of the quantity minimized, scaled by the weighted intensities (eqn (6)):
 | (6) |
This “best possible Rwp” quantity is a very useful concept and is called the expected R factor (Rexp) (eqn (7)). Using N as a label for the number of data points
 | (7) |
That χ2 (eqn (8)) can be determined from the weighted profile and expected R factors
 | (8) |
The single-crystal literature often uses the term goodness of fit (G) which is defined by “G2 = χ2”. The ultimate refinement, as observed, converges to Rwp = 2.05%, Rp = 5.24%, Rexp = 1.97%, and χ2 = 1.08%.
 | ||
| Fig. 2 FTIR spectra of ZnVO nanophosphors synthesized by different methods: (a) SGM, (b) UAC, (c) CPM, and (d) HTM. | ||
| S. No. | Bond | Wavelength (cm−1) | |||
|---|---|---|---|---|---|
| SGM | UAC | CPM | HTM | ||
| 1 | Zn–O | 413 | 417 | 420 | 426 |
| 2 | V–O–Zn | 549 | 449 | ||
| 3 | V–O–V | 620 | 620 | ||
| 4 | V–O–V | 661 | 667 | 658 | 662 |
| 5 | VO4 corner atoms | 791 | 788 | 793 | 789 |
| 6 | V–O–V | 843 | 850 | 843 | 848 |
| 7 | V–O–V | 900 | 917 | 901 | 907 |
| 8 | V–O–V | 966 | |||
| 9 | CH2 | 1116 | 1110 | ||
| 10 | CH2 | 1196 | 1189 | 1249 | |
| 11 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
1627 | 1626 | 1623 | 1623 |
| 12 | CH2 | 2842 | |||
| 13 | CH2 | 2937 | 2921 | ||
| 14 | H–O–H | 3458 | 3454 | 3430 | 3410 |
 | ||
| Fig. 3 SEM micrographs of ZnVO NPs synthesized by different methods: (a) SGM, (b) UAC, (c) CPM, (d) HTM, and (e) EDAX data with insets showing atomic and weight percentages of CPM ZnVO NP. | ||
| S. No. | Synthesis method | Particle size | Particle shape |
|---|---|---|---|
| 1 | SGM | 200–500 nm | Irregular |
| 2 | UAC | 100–600 nm | Irregular |
| 3 | CPM | 50–150 nm | Spherical |
| 4 | HTM | 50–100 nm thickness | Flacks |
| (F(R∞)hν)1/n = A(hν − Eg) | (9) |
 | (10) |
 | ||
| Fig. 5 UV/Vis-DRS spectra of ZnVO samples synthesized by different methods: (a) SGM, (b) UAC, (c) CPM, and (d) HTM. The inset shows the Kubelka–Munk function and Tauc plot for band gap estimation. | ||
3.2 Photoluminescence characterization
Fig. 6(a) and (b) present the PLE and PL spectra of ZnVO nanoparticles synthesized by various methods, measured at RT with an excitation wavelength of 361 nm. The spectra are consistent across all synthesis methods, indicating that similar excited centers are responsible for the broad PL emission observed. The larger FWHM of the emission spectra across different synthesis methods suggests that the (VO4)3− groups are not uniformly distributed within the crystal lattice, which implies the presence of defect centers. These defects lead to a redistribution of energy levels in the conduction and valence bands, affecting the band gap and intrinsic emission properties. The PLE spectrum of ZnVO nanoparticles results from the CT of an electron from the 2p orbital of oxygen to the unoccupied 3d orbital of vanadate ions in VO4 with Td symmetry.25,61–63 The PL spectra exhibit significant visible light emission spanning from 440 to 740 nm, with broadband emission peaks centered at 573 nm (SGM), 572 nm (UAC), 562 nm (CPM), 572 nm (HTM), and 575 nm (SSM). This broad emission is attributed to the self-trapped excitation radiative recombination process.The PLE spectrum of ZnVO NP is because of the CT of an electron from 2p orbital (O) to the unoccupied 3d orbital (vanadate) ions in VO4 with Td symmetry.25,61–63 The PL spectrum features two prominent peaks corresponding to emission from the higher energy 3T2 and 3T1 states to the ground 1A1 state of the V5+ ion. The intensity of the PL spectrum is notably higher, indicating efficient recombination of electrons and holes. Among the synthesized samples, those produced via the CPM method exhibit the highest PL intensity, followed by SSM, HTM, UAC, and SGM. Despite the consistent phase observed in all synthesis routes according to XRD (Fig. 1), the samples show significantly different morphologies. The PL emission in ZnVO nanoparticles is strongly influenced by particle size, oxygen vacancies, and the material's morphology. The observed PL band originates from defect states, as confirmed by FESEM and XPS analyses, which introduce intermediate energy levels within the band gap.
The photoluminescence (PL) characteristics of self-activated Zn3V2O8 originate from the intrinsic luminescent centers associated with the [VO4]3− tetrahedral units, which act as primary emission centers. Upon excitation, charge transfer occurs via the ligand-to-metal charge transfer (LMCT) mechanism, where electrons are promoted from the O 2p orbitals of the oxygen ligands to the empty V 3d orbitals of vanadium. This oxygen-to-vanadium charge transfer (O2− → V5+) results in a broad emission band, typically in the blue-green spectral region, attributed to the relaxation of excited electrons back to the ground state through radiative recombination. The presence of V4+ states due to surface defects, oxygen vacancies, or Zn2+ site substitution can introduce intermediate energy levels within the band gap, facilitating additional recombination pathways and modifying the emission profile. Moreover, the phonon-assisted relaxation process plays a critical role in spectral broadening, as energy dissipation through lattice vibrations can further modulate the emission characteristics. The intensity and position of the PL emission are strongly influenced by crystal field strength, structural distortions, and synthesis conditions, which dictate the extent of electronic delocalization and defect-related emission processes. The CPM-synthesized samples demonstrate the highest PL intensity, likely due to their spherical morphology and uniform particle size compared to those synthesized by SGM, UAC, HTM, and SSM. Accumulation of defects and additional oxygen vacancies lead to a higher number of inactive V4+ units, which contribute less to charge transfer and emission from V5+, thereby broadening the emission band and enhancing PL intensity. This occurs because the defects and oxygen vacancies alter the material's electronic structure, creating additional radiative recombination pathways that increase the PL intensity.
Although the charge transfer efficiency is reduced, the overall PL intensity is enhanced due to the increased number of radiative recombination pathways. As a result, the emission band broadens, and the PL intensity increases, indicating a complex interplay between defects, oxygen vacancies, and the material's optical properties. By tailoring the defect density, doping strategies, or nanostructuring, the luminescence efficiency of Zn3V2O8 can be fine-tuned for advanced solid-state lighting and optoelectronic applications.
The schematic model in Fig. 6(c) provides a clear representation of the energy diagram and transition states of ZnVO NPs during luminescence. It depicts the two primary relaxation mechanisms for an excited electron: nonradiative recombination and radiative processes. The excited electron, illustrated as a yellow ball, follows the paths indicated by the purple arrows. The green arc represents fluctuations in decay time due to the dynamic Jahn–Teller effect a time-dependent distortion of the VO4 tetrahedron. The blue curve illustrates the relative energy of the VO4 tetrahedra. The optical characteristics of phosphors are primarily determined by the luminescence center, but the coordination environment and local geometry also play significant roles. For the V5+ ion with Td symmetry, the molecular orbitals include one ground state (1A1) and four excited states 1T1, 1T2, 3T1, and 3T2.25,61–63
In the CPM-synthesized samples, the absorption band at 290 nm in the excitation spectrum corresponds to the 1A1 → 1T2 transition, while the excitation at 361 nm is attributed to the partially permitted 1A1 → 1T1 transition. The Gaussian deconvolution of the excitation spectra reveals two bands associated with these transitions. The ZnVO NPs synthesized via the CPM method exhibit a broadband excitation spectrum spanning 220 to 410 nm, with peaks at 290 nm (Ex1) and 361 nm (Ex2), and a smaller peak at approximately 385 nm (Fig. 6(d)). These absorption bands result from a partially permitted spin-forbidden transition in the Td symmetry of VO43−. The charge transfer (CT) of an electron from O2− to V5+ ions (from the 2p to the 3d orbital) in the VO43− group is confirmed to cause the broad emission of vanadate phosphors.
The emission spectrum of the ZnVO NPs, deconvoluted into two bands, corresponds to the energy transfers (ETs) from 3T1 and 3T2 → 1A1. These transitions are influenced by spin–orbital interactions in the defect structure (Fig. 6(e)). The FWHM values of 66 nm and 80 nm for the Em1 and Em2 bands indicate that the broadness of the PLE spectrum has increased along with its intensity. Additionally, the reduction in the crystallite size of the ZnVO NPs may contribute to the observed blue shift. The size, shape, and crystallinity of the phosphor particles significantly affect their luminous capabilities. We propose that the CT transitions from the 3T2 and 3T1 states to the 1A1 state play a crucial role in enhancing the peak intensity of the Em1 and Em2 bands.
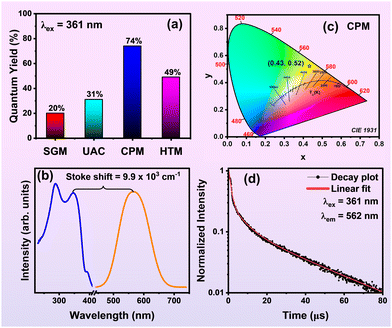
3.3 Quantum yield, colorimetric properties, and decay measurements
The absolute QY of ZnVO samples, measured under 361 nm excitation, was found to be 20%, 31%, 74%, and 49% for SGM, UAC, CPM, and HTM methods, respectively, as shown in Fig. 7(a). The higher QY of the CPM sample compared to SGM, UAC, HTM, and previously reported SSM19 is attributed to enhanced excitation diffusion, facilitated by the hybridization of the Zn 3d and O 2p orbitals in the lower energy band, and the Zn 4s and V 3d orbitals in the higher energy band.42 While commercial phosphors such as Y3Al5O12:Ce3+ (YAG:Ce3+) (70–90%),64 La3Si6N11:Ce3+ (85%),65 (Sr, Ba)2SiO4:Eu2+ (80%),66 Lu3Al5O12:Ce3+ (74%),67 BaMgAl10O17:Eu2+ (91%),68 exhibit QYs over 80%, the ZnVO CPM nanoparticles achieved a remarkable 74% efficiency under 361 nm excitation. This value is comparable to rare-earth-doped commercial phosphors and previously reported vanadate-based phosphors, as shown in ESI,† Table S3. When compared to commercial phosphors such as (Sr,Ca)AlSiN3:Eu3+ (2.6 × 103 cm−1)69 and (Ba,Sr)2SiO4:Eu2+ (3.2 × 103 cm−1),66 the Stokes shift (ΔS) of the ZnVO (CPM) NPs is significantly higher at 9.9 × 103 cm−1, as seen in Fig. 7(b). This higher ΔS value enables these phosphors to emit white light with improved CRI and reduced glare, making them ideal for indoor lighting applications. Therefore, ZnVO nanoparticles synthesized by the CPM method are well-suited for UV-based WLEDs with high CRI and LE, owing to their higher ΔS value and enhanced red emission. Fig. 7(c) displays the Commission Internationale de l'Éclairage (CIE) color coordinates of ZnVO CPM samples, under black light irradiation, at (0.43, 0.52). The emission color of phosphors synthesized using different methods varied slightly, as indicated by (x, y) values of (0.44, 0.51), (0.44, 0.51), and (0.42, 0.52) for the SGM, UAC, and HTM samples, respectively, as shown in ESI,† Fig. S1. In ZnVO nanoparticles, excitation with optimal Td symmetry is allowed through the 1A1 → 1T1 and 1A1 → 1T2 transitions. However, the intersystem crossing transitions (1T1, 1T2 → 3T1, 3T2) and the forbidden 3T1, 3T2 → 1A1 transition, due to the spin selection rule, also play a role in determining the observed luminescence.25,61–63The decay profile was analyzed to understand the mechanism behind the broad emission and its dependence on excitation wavelength, temperature, and emission. When the synthesized CPM nanoparticles were excited at UV wavelengths (290 nm and 361 nm), the emission intensity increased, reaching a maximum at 361 nm, and then decreased at higher excitation wavelengths (385 nm), as shown in ESI,† Fig. S2. The UV-vis spectrum confirms that the highest excitation maxima occur at 361 nm, which also corresponds to the second maximum emission intensity, attributed to bandgap energy absorption. At higher excitation wavelengths (385 nm), defect states contribute more significantly to the emissions, leading to a shift and an increase in the full width at half maximum (FWHM). The luminescence decay curves of ZnVO nanoparticles, observed with an excitation wavelength of 361 nm and an emission wavelength of 562 nm, are shown in Fig. 7(d). The extended lifetime in the microsecond range suggests that the emission originates from the excited triplet state. This indicates the involvement of a slow, radiative recombination process, likely influenced by the presence of defect states, contributing to the overall broad emission. The decay lifetime values obtained using eqn (11), individual lifetime, and contribution population are found to be 19%, 59%, and 22% for τ1, τ2, and τ370,71
 | (11) |
 | (12) |
The average lifetime of CPM-synthesized ZnVO nanoparticles is found to be 23.06 μs. This indicates that the lifetime decreases as the concentration of defect states increases, leading to more non-radiative relaxation pathways, transitioning from bulk to nanoscale, as confirmed by XPS analysis. Fig. 6(e) shows that an increase in oxygen vacancy density results in enhanced red emission while blue-green emission diminishes. Therefore, ZnVO nanoparticles, with their microsecond decay time, are highly suitable for WLED applications, comparable to the decay period of YAG:Ce3+ as reported by Guan et al.75 This suggests that in the ZnVO lattice, emission is primarily driven by the VO43− luminescent center. Moreover, ZnVO nanoparticles act as single-emitting-center phosphors, and materials with shorter decay times are ideal for use in WLEDs.
3.4 Thermal quenching properties and irradiation time PL
One of the critical factors affecting both the lifespan and performance of WLEDs, as well as the color stability of the emitted light, is the thermal stability of the phosphor.66,76 To ensure that the thermal quenching behavior of ZnVO NPs (CPM) aligns with the perating temperatures of WLEDs, we conducted temperature-dependent studies on the ZnVO NPs. In the liquid phase, molecules experience various motions—collisions, rotations, vibrations, and translations—which intensify when thermal energy is applied, leading to energy loss. However, in the solid state, where intermolecular and translational rotational motions are restricted, thermal quenching occurs less frequently. As the temperature increases, we observe a linear decrease in emission intensity from the ZnVO NPs in the solid state, from 25 °C to 275 °C, as shown in Fig. 8(a). The decline in PL intensity with increasing temperature is attributed to vibrational energy loss of excited electrons. A blue shift was noted with rising temperature, possibly due to the acceleration of non-radiative relaxation processes, defect-mediated emission, or predominated CT emission. The emission intensity of ZnVO CPM NPs retained 89% of its value at 150 °C and 78% at 275 °C, indicating excellent thermal stability. At RT, electrons are excited from the ground state 1A1 → 3T2 state before returning to 1A1. However, at higher temperatures, the emission decreases as electrons transition directly from 1A1 to the 1T2 state, bypassing the 3T1 state. As temperature increases, the excitation energy is transferred from the luminescent core to non-radiative thermal energy through lattice relaxation. This relaxation process results in a reduction of PL intensity due to non-radiative transitions, which manifest as a Stokes shift.77 Thermal quenching was observed at 180 °C for ZnVO NPs, slightly lower than the ZnVO bulk material, likely due to the larger Stokes shift. Despite a minor reduction in PL intensity, ZnVO NPs exhibit strong thermal stability and can be utilized at temperatures up to 210 °C, making them suitable for high-temperature applications in WLEDs. The Arrhenius equation was used to determine the activation energy (ΔE) for both phosphors to support the thermal quenching phenomenon as shown in eqn (13).25,73,74 | (13) |
Here, c is a constant, I0 and IT are the initial and various temperatures of PL intensity, and k is the Boltzmann constant in eV, respectively. Fig. 8(b) shows the relationship between ln[(I0/IT) − 1] and 1/(kT) for ZnVO NPs. The slope of the linear plot was used to calculate the activation energy, which was found to be approximately 0.158 eV for ZnVO NPs. The results indicate that as the Stokes shift increases, the ΔE decreases, leading to thermal quenching of ZnVO NPs at lower temperatures. Typically, a lower ΔE value increases the likelihood of nonradiative energy transfer. However, the ΔE value of ZnVO NPs synthesized via CPM (∼0.158 eV) is comparable to that of ZnVO synthesized by SSM (0.163 eV). UV-Vis and XPS analyses revealed a reduction in bandgap values and a rise in the valence band position, suggesting a shift in the band structure caused by increasing defect states. These changes reduce the distance between the luminescence center and the crossover point, resulting in a lower activation energy for quenching. The enhancement of the thermal quenching effect is attributed to the creation of trapping levels between the lowest excited level curve of VO43− (3T1) and the ground state curve of the VO43− group (1A1). This makes ZnVO NPs suitable for the production of WLEDs, where high thermal stability is essential for ensuring a long operating lifespan and higher LE. Additionally, the photostability of ZnVO NPs was evaluated over 4 hours of 361 nm illumination. As shown in Fig. 8(c) and (d), the normalized PL spectra remained unchanged in terms of both spectral position and intensity, even after extended irradiation at room temperature (RT) and elevated temperatures (150 °C). A comparison of the normalized PL emission intensity versus irradiation time (ESI,† Fig. S3a and b) at RT and 150 °C shows that after 4 hours of continuous exposure, the emission intensities remained at 99.46% at RT and 99.11% at 150 °C of their initial values. Thus, extended irradiation and elevated temperatures do not significantly affect the stability of ZnVO NPs, highlighting their robust photostability.
3.5 Application in WLEDs and colorimetric calculations
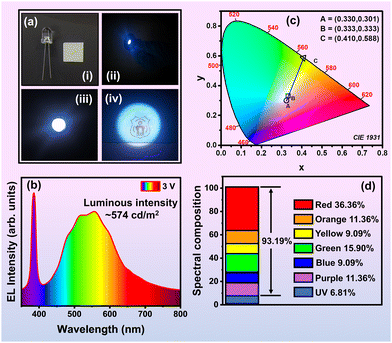
A WLED prototype was created by combining ZnVO CPM NPs with epoxy resin and a 385 nm, 0.5 WLED chip. Fig. 9(a(i)) illustrates the schematic of the ZnVO CPM NP LED package before applying current. Upon applying a current of ∼150 mA, the LED emitted a pleasant white light, as seen in Fig. 9(a(ii)). The LED produced a sharp peak at 385 nm, while the ZnVO CPM NPs generated a broad visible spectrum ranging from 400–800 nm, as shown in Fig. 9(b). The CIE color coordinates of the white emission from the ZnVO CPM NPs are (0.330, 0.301), close to ideal white light, with a CCT of ∼4920 K, outperforming YAG: Ce3+-based WLEDs, which typically have a CCT around 6500 K.78 The superior color quality of the ZnVO CPM NP and LED prototypes is highlighted in the CIE 1931 chromaticity diagram (Fig. 9(c)), where the CIE coordinates of the ZnVO CPM NP emission, excited at 385 nm, are plotted. The coordinates (0.330, 0.301) are close to the optimal white light equal energy point (0.333, 0.333), demonstrating excellent cool white light properties. It is noteworthy that the commercial YAG: Ce3+ phosphor, developed by Wolf Speed, Inc., exhibits a QY of 78% at 449 nm excitation. When compared to P-20 (LUMINOX), bulk YAG: Ce3+ (CREE), and bulk ZnVO, the ZnVO CPM NP shows similar or superior performance.79Table 4 outlines the relative efficiency of these bulk phosphors and ZnVO CPM NPs at various excitation wavelengths. The QY of ZnVO CPM NPs is comparable to these bulk phosphors and represents a significant improvement over the commonly used yellow-lighting P-20 phosphor. Further reduction of particle size into the quantum range could enhance the QY even more, as Bhargava et al.80 demonstrated for various NP systems. Fig. 9(d) presents the PL spectra for yellow-emitting P-20, bulk Zn3V2O8 phosphor, YAG: Ce3+, and ZnVO CPM NPs. The CIE color coordinates for the ideal white light, the 385 nm LED, ZnVO CPM NP, and the simulated white light (combining the 385 nm LED with ZnVO CPM NP) are listed in Table 4. These coordinates, derived using the equal-wavelength method,81 are crucial for determining the precise emission color of each sample, as visualized in the CIE chromaticity diagram.81| Company name | Product name | λ ex | QY (%) |
|---|---|---|---|
| CREE | YAG: Ce3+ | 449 | 78 |
| CREE | YAG: Ce3+ | 385 | 58 |
| LUMINOX | P-20 | 385 | 51 |
| Zn3V2O8-bulk | ZnVO | 385 | 68 |
| Zn3V2O8-NP | ZnVO-CPM | 385 | 76 |
| Sample | CIE (x, y) | ||
| IDEAL white | (0.333, 0.333) | ||
| ZnVO-CPM NP | (0.432, 0.522) | ||
| 385 nm LED + ZnVO-CPM NP (white light) | (0.330, 0.301) | ||
We are pleased to report that the WLED exhibited outstanding EL performance at a driving voltage of 3 V, producing cool white light with a CCT of 4920 K. Fig. 10(a) and the fabricated device shown in ESI† (Fig. S4) show images of the setup: (i) a 385 nm LED chip and a glass slide coated with ZnVO/epoxy resin used for fabrication, (ii) and (iii) the white light emission from the side and top views of the LED device, and (iv) the NIT Warangal (NITW) logo illuminated by the ZnVO and 385 nm LED at 3 V. The device's effective emission of polychromatic light upon excitation with 385 nm radiation confirms the ZnVO CPM NP's efficiency. The luminous value of the WLED device fabricated using our synthesized Zn3V2O8 phosphor is quite promising, with a luminous intensity of ∼574 cd m−2. This suggests that our material has good potential for application in solid-state lighting devices. Fig. 10(b) illustrates the spectral output from 360 nm to 800 nm for the WLED, combining a 385 nm LED with ZnVO CPM NP at 3 V. The resulting spectrum aligns closely with the “ideal white” region on the CIE chromaticity diagram, shown in Fig. 10(c). This suggests that ZnVO CPM NP is highly effective in converting 385 nm LED light into white light. The color temperature of a Planckian blackbody radiator with a similar white light color is used to define the CCT, highlighting the potential of ZnVO CPM NP for high-quality white light LED applications.82 The McCamy determined the value of CCT,83,84 using the following eqn (14):
| CCT = −499n3 + 3525n2 − 6823.3n + 5520.33 | (14) |
 | (15) |
4. Conclusions
Zn3V2O8 nanophosphors were successfully synthesized using the coprecipitation calcination method. These vanadate phosphors exhibited broad emission from 400 nm to over 750 nm due to charge transfer transitions in VO4 tetrahedra, with a yellow emission and CIE color coordinates of (0.43, 0.52). Among the synthesis methods, the coprecipitation method produced ZnVO NPs with the highest quantum yield, in the order of CPM > HTM > UAC > SGM, with corresponding values of 74%, 49%, 31%, and 20%. The ZnVO NPs displayed a broad yellow emission centered at 562 nm upon excitation at 361 nm, with FWHM values of 66 nm for Em1 and 80 nm for Em2. The thermal stability of the ZnVO NPs was demonstrated by their retention of 78% of room temperature emission at 275 °C and an activation energy (ΔE) of ∼0.158 eV. When integrated with a 385 nm LED chip, the ZnVO NPs achieved a cool CCT of ∼4920 K, a high CRI of 91%, and CIE coordinates of (0.330, 0.301). This work presents a cost-effective, rare earth-free, self-activated approach to enhancing vanadate phosphors for WLED applications. The WLEDs exhibited stability across various currents, operating times, and temperatures, maintaining their electroluminescence, CCT, CIE coordinates, and CRI values.Author contributions
M. Rakshita: data curing, methodology, writing – original draft. Aachal A. Sharma: data curation; investigation. Payal P. Pradhan: data curation; investigation. K. A. K. Durga Prasad: data curation; investigation. M. Srinivas: data curation, formal analysis. D. Haranath: conceptualization; funding acquisition; methodology; supervision; writing – review & editing.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors MR, AAS, MS and DH are grateful to the Council of Scientific & Industrial Research (CSIR) and Department of Science and Technology (DST), Government of India, for providing financial support under various projects viz CSIR – SRF #09/0922(11518)/2021-EMR-I, INSPIRE scheme #IF200233, #EEQ/2021/000329, and #CRG/2021/007142, respectively.Notes and references
- K. Jayanthi Rajan and S. V. Manorama, Formation of hierarchical macro porous YAlO:Ce multifunctional nanophosphors, J. Appl. Phys., 2016, 119, 114902, DOI:10.1063/1.4943418.
- R. Verstraete, H. F. Sijbom, J. J. Joos, K. Korthout, D. Poelman, C. Detavernier and P. F. Smet, Red Mn4+-Doped Fluoride Phosphors: Why Purity Matters, ACS Appl. Mater. Interfaces, 2018, 10, 18845–18856, DOI:10.1021/acsami.8b01269.
- P. Botella, F. Enrichi, A. Vomiero, J. E. Munõz-Santiuste, A. B. Garg, A. Arvind, F. J. Manjón, A. Segura and D. Errandonea, Investigation on the Luminescence Properties of InMO4 (M = V5+, Nb5+, Ta5+) Crystals Doped with Tb3+ or Yb3+ Rare Earth Ions, ACS Omega, 2020, 5, 2148–2158, DOI:10.1021/acsomega.9b02862.
- S. Elleuch, A. Lusson, S. Pillet, K. Boukheddaden and Y. Abid, White Light Emission from a Zero-Dimensional Lead Chloride Hybrid Material, ACS Photonics, 2020, 7, 1178–1187, DOI:10.1021/acsphotonics.9b01817.
- S. Hu, C. Lu, G. Zhou, X. Liu, X. Qin, G. Liu, S. Wang and Z. Xu, Transparent YAG:Ce ceramics for WLEDs with high CRI:Ce3+ concentration and sample thickness effects, Ceram. Int., 2016, 42, 6935–6941, DOI:10.1016/j.ceramint.2016.01.079.
- Y. Zhang, L. Li, X. Zhang and Q. Xi, Temperature effects on photoluminescence of YAG:Ce3+ phosphor and performance in white light-emitting diodes, J. Rare Earths, 2008, 26, 446–449, DOI:10.1016/S1002-0721(08)60115-5.
- Y. Wan, P. Dang, D. Liu, Q. Zhang, Y. Wei, H. Lian, G. Li and J. Lin, Highly Efficient Narrow-Band Green-Emitting Na3K5(Li3SiO4)8:Eu2+ Phosphor with Low Thermal Quenching, Chem. Mater., 2023, 5, 10702–10712, DOI:10.1021/acs.chemmater.3c02579.
- M. Cai, T. Lang, T. Han, D. Valiev, S. Fang, C. Guo, S. He, L. Peng, S. Cao, B. Liu, L. Du, Y. Zhong and E. Polisadova, Novel Cyan-Green-Emitting Bi3+-Doped BaScO2F, R+ (R = Na, K, Rb) Perovskite Used for Achieving Full-Visible-Spectrum LED Lighting, Inorg. Chem., 2021, 60, 15519–15528, DOI:10.1021/acs.inorgchem.1c02150.
- Y. Lin, G.-E. Wang, L. Li, C.-L. Hu, S. Lin and J.-G. Mao, Rare-Earth-Free Barium Borostannate with Deep-Blue Light Emission, Chem. Mater., 2021, 33, 1852–1859 CrossRef CAS.
- E. Pavitra, G. Seeta Rama Raju, L. Krishna Bharat, J. Y. Park, C. H. Kwak, J. W. Chung, Y. K. Han and Y. S. Huh, Evolution of highly efficient rare-earth free Cs(1−x)RbxVO3 phosphors as a single emitting component for NUV-based white LEDs, J. Mater. Chem. C, 2018, 6, 12746–12757, 10.1039/c8tc05110k.
- Y. Li, B. Yu, H. Wang and Y. Wang, Structural and optical characteristics of novel rare-earth-free red-emitting BaSn(PO4)2:Mn4+ phosphor, J. Mol. Struct., 2021, 1229, 129839, DOI:10.1016/j.molstruc.2020.129839.
- M. K. Jang, Y. S. Cho and Y. D. Huh, Preparation of red-emitting BaSiF6:Mn4+ phosphors for three-band white LEDs, Opt. Mater., 2020, 101, 109734, DOI:10.1016/j.optmat.2020.109734.
- R. Cao, X. Ceng, J. Huang, X. Xia, S. Guo and J. Fu, A double-perovskite Sr2ZnWO6:Mn4+ deep red phosphor: synthesis and luminescence properties, Ceram. Int., 2016, 42, 16817–16821, DOI:10.1016/j.ceramint.2016.07.173.
- A. A. G. Santiago, M. C. Oliveira, R. A. P. Ribeiro, R. L. Tranquilin, E. Longo, S. R. De Lázaro, F. V. Motta and M. R. D. Bomio, Atomistic Perspective on the Intrinsic White-Light Photoluminescence of Rare-Earth Free MgMoO4 Nanoparticles, Cryst. Growth Des., 2020, 20, 6592–6603, DOI:10.1021/acs.cgd.0c00757.
- A. A. Sharma, A. O. Chauhan, C. B. Palan and S. K. Omanwar, Synthesis and Photoluminescence study of Gd3+ doped YP3O9 phosphor prepared by Citric sol–gel method, IJRST, 2021, 8, 65–69 Search PubMed.
- R. Muniramaiah, G. Maharana, J. M. Fernandes, M. Manivel Raja, D. B. Padmanaban, P. Supraja, M. Rakshita, N. P. Reddy, M. Kovendhan, G. Laxminarayana, R. R. Kumar, D. Haranath and D. P. Joseph, Sputter-deposited highly flexible noble metal multi-layer electrode viable for energy and luminescent devices, Surf. Interfaces, 2023, 39, 102949, DOI:10.1016/j.surfin.2023.102949.
- R. Muniramaiah, J. M. Fernandes, M. M. Raja, D. B. Padmanaban, P. Supraja, M. Rakshita, N. P. Reddy, G. Maharana, M. Kovendhan, G. Veerappan, G. Laxminarayana, R. R. Kumar, D. Haranath and D. P. Joseph, Mechanically stable ultrathin flexible metallic Au/Pt/Au tri-layer as an alternative transparent conducting electrode for optoelectronic device applications, Vacuum, 2022, 206, 111487, DOI:10.1016/j.vacuum.2022.111487.
- K. Li, Z. Huang and D. Zhu, Novel Li2Ga0.5Sb0.5O3(Li4GaSbO6):Mn4+ phosphors with multisite occupancy for efficient FIR and luminescence lifetime dual-mode thermometers, Ceram. Int., 2025 DOI:10.1016/j.ceramint.2025.01.516.
- Z. Huang, K. Li, Z. Zhang, J. Liu and D. Zhu, Sr4GaNbO8:Mn4+: a novel perovskite-structured red-emitting phosphor for a luminescence lifetime thermometer with good relative sensitivity and repeatability, J. Mater. Chem. C, 2025, 13, 4564–4575, 10.1039/d4tc04916k.
- J. A. A. L. Jayarathna and K. R. Kaja, Energy-Harvesting Device Based on Lead-Free Perovskite, AI, Comput. Sci. Rob. Technol., 2024, 3, 1–9, DOI:10.5772/acrt.20240036.
- A. Panda, K. K. Das, K. R. Kaja, M. Belal and B. K. Panigrahi, Single electrode mode triboelectric nanogenerator for recognition of animal sounds, J. Met., Mater. Miner., 2024, 34, 2170, DOI:10.55713/JMMM.V34I4.2170.
- A. Panda, K. K. Das, K. R. Kaja, V. Gandi, S. Gourav Mohanty and B. K. Panigrahi, Low-cost high performance sustainable triboelectric nanogenerator based on laboratory waste, J. Met., Mater. Miner., 2025, 35, e2226, DOI:10.55713/jmmm.v35i1.e2226.
- K. R. Kaja, S. Hajra, S. Panda, M. A. Belal, P. Pakawanit, N. Vittayakorn, C. Bowen, H. Khanbareh and H. J. Kim, Triboelectrification Based on the Waste Waterproof Textiles for Multisource Energy Harvesting, Adv. Sustainable Syst., 2024, 2400678, DOI:10.1002/adsu.202400678.
- M. Rakshita, N. Madathil, A. A. Sharma, P. P. Pradhan, D. P. Kasireddi A. K., U. K. Khanapuram, R. K. Rajaboina and H. Divi, Phosphor-Based Triboelectric Nanogenerators for Mechanical Energy Harvesting and Self-Powered Systems, ACS Appl. Electron. Mater., 2024, 6, 1821–1828, DOI:10.1021/acsaelm.3c01728.
- M. Rakshita, A. A. Sharma, P. P. Pradhan, K. A. K. Durga Prasad, K. Jayanthi and D. Haranath, Highly efficient and self-activating Zn3V2O8 phosphor for the fabrication of cool-white light emitting devices, Ceram. Int., 2023, 49, 16775–16785, DOI:10.1016/j.ceramint.2023.02.038.
- S. Rajkumar, E. Elanthamilan and J. P. Merlin, Facile synthesis of Zn3V2O8 nanostructured material and its enhanced supercapacitive performance, J. Alloys Compd., 2021, 861, 157939, DOI:10.1016/j.jallcom.2020.157939.
- C. Liu, Z. Zhou and Y. Zhang, Synthesis and luminescence properties of rare-earth free narrow band green emitting Na2ZnSiO4:Mn2+ phosphor for white LEDs, J. Lumin., 2019, 213, 1–5, DOI:10.1016/j.jlumin.2019.04.060.
- L. K. Bharat, S. K. Jeon, K. G. Krishna and J. S. Yu, Rare-earth free self-luminescent Ca2KZn2(VO4)3 phosphors for intense white light-emitting diodes, Sci. Rep., 2017, 7, 1–9, DOI:10.1038/srep42348.
- K. Singh, P. Pradhan, S. Priya, S. Mund and S. Vaidyanathan, Recent progress in trivalent europium (Eu3+)-based inorganic phosphors for solid-state lighting: an overview, Dalton Trans., 2023, 52, 13027–13057, 10.1039/d3dt00303e.
- W. M. Yen, S. Shionoya and H. Yamamoto, Phosphor Handbook, 2006 Search PubMed.
- R. J. Xie, N. Hirosaki, T. Suehiro, F. F. Xu and M. Mitomo, A simple, efficient synthetic route to Sr2Si5N8:Eu2+-based red phosphors for white light-emitting diodes, Chem. Mater., 2006, 18, 5578–5583, DOI:10.1021/cm061010n.
- K. Ruthvik, A. Babu, P. Supraja, M. Navaneeth, V. Mahesh, K. Uday Kumar, R. Rakesh Kumar, B. Manmada Rao, D. Haranath and K. Prakash, High-performance triboelectric nanogenerator based on 2D graphitic carbon nitride for self-powered electronic devices, Mater. Lett., 2023, 350, 134947, DOI:10.1016/j.matlet.2023.134947.
- K. R. Kaja, S. Hajra, S. Panda, M. A. Belal, U. Pharino, H. Khanbareh, N. Vittayakorn, V. Vivekananthan, C. Bowen and H. J. Kim, Exploring liquid–solid interface based triboelectrification, structures, and applications, Nano Energy, 2024, 131, 110319, DOI:10.1016/J.NANOEN.2024.110319.
- A. A. Tedstone, D. J. Lewis and P. O’Brien, Synthesis, Properties, and Applications of Transition Metal-Doped Layered Transition Metal Dichalcogenides, Chem. Mater., 2016, 28, 1965–1974, DOI:10.1021/acs.chemmater.6b00430.
- P. Boutinaud, A. Barros and F. Kang, Luminescence depreciation in ScVO4:Bi3+ upon irradiation in the Bi3+-related absorption bands, J. Lumin., 2022, 248, 118941, DOI:10.1016/j.jlumin.2022.118941.
- V. Chauhan, P. Deshmukh, S. Satapathy and P. C. Pandey, Greenish-yellow emission from rare-earth free Li+ doped zinc vanadate phosphor, Results Phys., 2022, 39, 105689, DOI:10.1016/j.rinp.2022.105689.
- L. Wu, P. Dai and D. Wen, New Structural Design Strategy: Optical Center VO4-Activated Broadband Yellow Phosphate Phosphors for High-Color-Rendering WLEDs, ACS Sustainable Chem. Eng., 2022, 10, 3757–3765, DOI:10.1021/acssuschemeng.2c00515.
- N. Baig, I. Kammakakam, W. Falath and I. Kammakakam, Nanomaterials: a review of synthesis methods, properties, recent progress, and challenges, Mater. Adv., 2021, 2, 1821–1871, 10.1039/d0ma00807a.
- M. Nadolska, M. Szkoda, K. Trzciński, P. Niedziałkowski, J. Ryl, A. Mielewczyk-Gryń, K. Górnicka and M. Prześniak-Welenc, Insight into Potassium Vanadates as Visible-Light-Driven Photocatalysts: Synthesis of V(IV)-Rich Nano/Microstructures for the Photodegradation of Methylene Blue, Inorg. Chem., 2022, 61, 9433–9444, DOI:10.1021/acs.inorgchem.2c00136.
- P. Liu, J. Yi, R. Bao and D. Fang, A flower-like Zn3V2O8/Ag composite with enhanced visible light driven photocatalytic activity: the triple-functional roles of Ag nanoparticles, New J. Chem., 2019, 43, 7482, 10.1039/c9nj00211a.
- V. Chauhan, P. Dixit and P. C. Pandey, Enhancement in greenish-white photoluminescence of Zn3(VO4)2 phosphor by Bi3+ doping, Optik, 2021, 238, 166682, DOI:10.1016/j.ijleo.2021.166682.
- T. Nakajima, M. Isobe, T. Tsuchiya, Y. Ueda and T. Kumagai, A revisit of photoluminescence property for vanadate oxides AVO3 (A:K, Rb and Cs) and M3V2O8 (M: Mg and Zn), J. Lumin., 2009, 129, 1598–1601, DOI:10.1016/j.jlumin.2009.03.029.
- S. Ni, X. Wang, G. Zhou, F. Yang, J. Wang and D. He, Crystallized Zn3(VO4)2: synthesis, characterization and optical property, J. Alloys Compd., 2010, 491, 378–381, DOI:10.1016/j.jallcom.2009.10.188.
- F. Du, W. Zhuang, R. Liu, J. Zhong, Y. Liu, Y. Hu, W. Gao, X. Zhang, L. Chen and K. Lin, Site occupancy and photoluminescence tuning of La3Si6−xAlxN11−x/3:Ce3+ phosphors for high power white light-emitting diodes, CrystEngComm, 2017, 19, 2836–2843, 10.1039/c7ce00435d.
- M. Rakshita, A. Babu, K. Jayanthi, S. Bathula, K. Uday Kumar and D. Haranath, Studies on contact angle measurements in superoleophobic aluminum hydroxide nanoflakes, Mater. Lett., 2022, 315, 131938, DOI:10.1016/j.matlet.2022.131938.
- P. H. Kuo, N. A. Ley, M. L. Young and J. Du, Phase Evolution and Crystallization Mechanism of Glass Ceramic Solid-State Electrolyte from In Situ Synchrotron X-ray Diffraction, J. Phys. Chem. C, 2023, 127, 17051–17062, DOI:10.1021/acs.jpcc.3c02340.
- A. A. Sharma, M. Rakshita, P. P. Pradhan, K. A. K. D. Prasad, S. Mishra, K. Jayanthi and D. Haranath, Noninvasive treatment of psoriasis and skin rejuvenation using an akermanite-type narrowband emitting phosphor, Luminescence, 2023, 38, 1–10, DOI:10.1002/bio.4554.
- R. Devi Chandra, L. Veena and K. G. Gopchandran, Suppression of Visible Emission in Low-Temperature Synthesized Cobalt-Doped ZnO Nanoparticles and Their Photosensing Applications, Inorg. Chem., 2023, 62, 11360–11371, DOI:10.1021/acs.inorgchem.3c00846.
- P. Luo, W. Zhang, S. Wang, G. Liu, Y. Xiao, C. Zuo, W. Tang, X. Fu and S. Dong, Electroactivation-induced hydrated zinc vanadate as cathode for high-performance aqueous zinc-ion batteries, J. Alloys Compd., 2021, 884, 161147, DOI:10.1016/j.jallcom.2021.161147.
- D. A. Hoyos, A. Echavarría and C. Saldarriaga, Synthesis and structure of a porous zinc vanadate, Zn3(VO4)2·3H2O, J. Mater. Sci., 2001, 36, 5515–5518, DOI:10.1023/A:1012418706071.
- G. Maharana, R. Muniramaiah, J. Yuvashree, D. Mandal, S. Mondal, M. Kovendhan, J. M. Fernandes, G. Laxminarayana and D. P. Joseph, Tungsten and fluorine co-doping induced morphology change and textured growth of spray-pyrolyzed SnO2 thin films viable for photocatalytic application, Surf. Interfaces, 2023, 42, 103413, DOI:10.1016/j.surfin.2023.103413.
- A. A. Sharma, M. Rakshita, P. P. Pradhan, K. A. K. Durga Prasad, S. Mishra, K. Jayanthi and D. Haranath, Efficacy of photodynamic therapy using UVB radiation-emitting novel phosphor material for non-surgical treatment of psoriasis, J. Mater. Res., 2023, 38, 2812–2822, DOI:10.1557/s43578-023-01008-7.
- G. Chen, Y. Jin, L. Yuan, B. Wang, J. Huo, H. Suo, H. Wu, Y. Hu and F. Wang, Unlocking Cr3+–Cr3+ Coupling in Spinel: Ultrabroadband Near-Infrared Emission beyond 900 nm with High Efficiency and Thermal Stability, ACS Appl. Mater. Interfaces, 2024, 16, 30185–30195, DOI:10.1021/acsami.4c03419.
- S. Wu, L. Yuan, G. Chen, C. Peng and Y. Jin, All-inorganic Mn2+-doped metal halide perovskite crystals for the late-time detection of X-ray afterglow imaging, Nanoscale, 2023, 15, 13628–13634, 10.1039/d3nr02208k.
- R. Babu, I. López-Fernández, S. Prasanthkumar and L. Polavarapu, All-Inorganic Lead-Free Doped-Metal Halides for Bright Solid-State Emission from Primary Colors to White Light, ACS Appl. Mater. Interfaces, 2023, 15, 35206–35215, DOI:10.1021/acsami.3c06546.
- M. M. Sajid, N. A. Shad, S. B. Khan, Z. Zhang and N. Amin, Facile synthesis of Zinc vanadate Zn3(VO4)2 for highly efficient visible light assisted photocatalytic activity, J. Alloys Compd., 2019, 775, 281–289, DOI:10.1016/j.jallcom.2018.10.134.
- S. S. Kalanur, I. H. Yoo, I. S. Cho and H. Seo, Effect of oxygen vacancies on the band edge properties of WO3 producing enhanced photocurrents, Electrochim. Acta, 2019, 296, 517–527, DOI:10.1016/J.ELECTACTA.2018.11.061.
- A. Song, S. Liu, Q. Wang, D. Gao and J. Hu, Comprehensive evaluation of copper vanadate (α-CuV2O6) for use as a photoanode material for photoelectrochemical water splitting, J Environ. Chem. Eng., 2023, 11, 109892, DOI:10.1016/j.jece.2023.109892.
- J. K. Cooper, S. Gul, F. M. Toma, L. Chen, Y. S. Liu, J. Guo, J. W. Ager, J. Yano and I. D. Sharp, Indirect bandgap and optical properties of monoclinic bismuth vanadate, J. Phys. Chem. C, 2015, 119, 2969–2974, DOI:10.1021/jp512169w.
- M. V. Malashchonak, E. A. Streltsov, D. A. Kuliomin, A. I. Kulak and A. V. Mazanik, Monoclinic bismuth vanadate band gap determination by photoelectrochemical spectroscopy, Mater. Chem. Phys., 2017, 201, 189–193, DOI:10.1016/j.matchemphys.2017.08.053.
- S. Vijayakumar, S. H. Lee and K. S. Ryu, Synthesis of Zn3V2O8 nanoplatelets for lithium-ion battery and supercapacitor applications, RSC Adv., 2015, 5, 91822–91828, 10.1039/c5ra13904j.
- T. Jeyakumaran, P. Sriramachandran, R. Shunmugavel and S. Ramaswamy, Synthesis and characterization of Zn3V2O8 Yellow emitting nano phosphor by sol–gel method, AIP Conf. Proc., 2017, 1832, 10–13, DOI:10.1063/1.4980338.
- P. Botella, F. Enrichi, A. Vomiero, J. E. Munõz-Santiuste, A. B. Garg, A. Arvind, F. J. Manjón, A. Segura and D. Errandonea, Investigation on the Luminescence Properties of InMO4 (M = V5+, Nb5+, Ta5+) Crystals Doped with Tb3+ or Yb3+ Rare Earth Ions, ACS Omega, 2020, 5, 2148–2158, DOI:10.1021/acsomega.9b02862.
- W. Li, Y. Zhuang, P. Zheng, T. L. Zhou, J. Xu, J. Ueda, S. Tanabe, L. Wang and R. J. Xie, Tailoring Trap Depth and Emission Wavelength in Y3Al5−xGaxO12:Ce3+,V3+ Phosphor-in-Glass Films for Optical Information Storage, ACS Appl. Mater. Interfaces, 2018, 10, 27150–27159, DOI:10.1021/acsami.8b10713.
- D. Qu, D. Yang, Y. Sun, X. Wang and Z. Sun, White Emissive Carbon Dots Actuated by the H-/J-Aggregates and Förster Resonance Energy Transfer, J. Phys. Chem. Lett., 2019, 10, 3849–3857, DOI:10.1021/acs.jpclett.9b01575.
- K. A. Denault, J. Brgoch, M. W. Gaultois, A. Mikhailovsky, R. Petry, H. Winkler, S. P. Denbaars and R. Seshadri, Consequences of optimal bond valence on structural rigidity and improved luminescence properties in SrxBa2−xSiO4:Eu2+ orthosilicate phosphors, Chem. Mater., 2014, 26, 2275–2282, DOI:10.1021/cm500116u.
- A. Birkel, K. A. Denault, N. C. George, C. E. Doll, B. Héry, A. A. Mikhailovsky, C. S. Birkel, B. C. Hong and R. Seshadri, Rapid microwave preparation of highly efficient Ce3+-substituted garnet phosphors for solid state white lighting, Chem. Mater., 2012, 24, 1198–1204, DOI:10.1021/cm3000238.
- L. J. Yin, J. Dong, Y. Wang, B. Zhang, Z. Y. Zhou, X. Jian, M. Wu, X. Xu, J. R. Van Ommen and H. T. Hintzen, Enhanced Optical Performance of BaMgAl10O17:Eu2+ Phosphor by a Novel Method of Carbon Coating, J. Phys. Chem. C, 2016, 120, 2355–2361, DOI:10.1021/acs.jpcc.5b10215.
- H. S. Kim, K. Machida, M. Itoh and H. Hanzawa, Synthesis and Luminescence Properties of (Sr,Ca)AlSiN3:Eu2+ Phosphors under Atmospheric-Pressure, ECS J. Solid State Sci. Technol., 2014, 3, R234–R237, DOI:10.1149/2.0061412jss.
- K. A. K. D. Prasad, S. Puranjay, M. Rakshita, A. A. Sharma, P. P. Pradhan, K. U. Kumar, R. R. Kumar and D. Haranath, Simple and Cost-effective Synthesis of a Rare-earth Free Long Afterglow Phosphor for Dark Visual Markings, J. Fluoresc., 2024, 35, 867–875, DOI:10.1007/s10895-023-03566-9.
- N. Anitha, K. Jayanthi, M. Rakshita, A. A. Sharma, N. Jayarambabu, A. Akshaykranth, K. Babu, T. V. Rao, D. Dinakar and D. Haranath, Origin of the active luminescence from Sm3+-activated borate phosphors: a correlational study of trap states and decay kinetics, New J. Chem., 2022, 47, 1472–1478, 10.1039/d2nj04601f.
- P. Du, X. Wan, L. Luo, W. Li and L. Li, Thermally Stable Tb3+/Eu3+-Codoped K0.3Bi0.7F2.4 Nanoparticles with Multicolor Luminescence for White-Light-Emitting Diodes, ACS Appl. Nano Mater., 2021, 4, 7062–7071, DOI:10.1021/acsanm.1c01072.
- W. Liu, H. Wu, H. Dong, Y. Jin, L. Yuan and Y. Jin, Achieving broadband near-infrared emission with superior anti-thermal quenching by optimizing the excited-state population of Cr3+ in Gd3ScGa4O12 garnet phosphors, Mater. Horiz., 2024, 11, 6399–6407, 10.1039/d4mh01157k.
- D. Qian, Y. Jin, Z. Li, H. Wu and Y. Hu, Constructing Broadband Near-Infrared Garnet Emitters CaGd2Ga4SiO12:Cr3+ with Unity Quantum Efficiency and High Thermal Stability for Versatile Applications, Small, 2025, 21, e2411804, DOI:10.1002/smll.202411804.
- R. Guan, L. Cao, Y. You and Y. Cao, The Luminescence Properties and Energy Transfer from Ce3+ to Pr3+ for YAG:Ce3+,Pr3+ Phosphors, J. Nanomater., 2015, 1–8, DOI:10.1155/2015/549208.
- C. C. Lin, Y. T. Tsai, H. E. Johnston, M. H. Fang, F. Yu, W. Zhou, P. Whitfield, Y. Li, J. Wang, R. S. Liu and J. P. Attfield, Enhanced Photoluminescence Emission and Thermal Stability from Introduced Cation Disorder in Phosphors, J. Am. Chem. Soc., 2017, 139, 11766–11770, DOI:10.1021/jacs.7b04338.
- K. Saidi, I. Kachou, K. Soler-Carracedo, M. Dammak and I. R. Martín, Ba2YV3O11 Er3+/Yb3+ Nanostructures for Temperature Sensing in the Presence of Bismuth Ions, ACS Appl. Nano Mater., 2023, 6, 17681–17690, DOI:10.1021/acsanm.3c02911.
- Y. Zhuang, C. Li, C. Liu, Y. Fu, Q. Shi, Y. Liang and L. Xia, High-efficiency YAG:Ce3+ glass-ceramic phosphor by an organic-free screen-printing technique for high-power WLEDs, Opt. Mater., 2020, 107, 110118, DOI:10.1016/j.optmat.2020.110118.
- C. De Mello Donegá, S. J. L. Ribeiro, R. R. Gonçalves and G. Blasse, Luminescence and non-radiative processes in lanthanide squarate hydrates, J. Phys. Chem. Solids, 1996, 57, 1727–1734, DOI:10.1016/0022-3697(96)00032-7.
- R. N. Bhargava, D. Gallagher, X. Hong and A. Nurmikko, Optical properties of manganese-doped nanocrystals of ZnS, Phys. Rev. Lett., 1994, 72, 416–419, DOI:10.1103/PhysRevLett.72.416.
- D. Haranath, H. Chander, P. Sharma and S. Singh, Enhanced luminescence of Y3Al5O12:Ce3+ nanophosphor for white light-emitting diodes, Appl. Phys. Lett., 2006, 89, 1–4, DOI:10.1063/1.2367657.
- E. F. Schubert, Light Emitting Diodes and Solid-State Lighting, 2007, 12180, 1–82.
- J. Czajka and A. Szczeszak, Physicochemical Characterization of the Hybrid Molybdate-Tungstate Materials for Solid-State Lighting, J. Phys. Chem. C, 2023, 127, 17415–17424, DOI:10.1021/acs.jpcc.3c03482.
- P. P. Pradhan, R. Muddamalla, A. A. Sharma, D. P. Durga, U. K. Khanapuram, R. K. Rajaboina and H. Divi, Rare-Earth Ion-Activated Nanostructured Fluorescent Marker for Easy Naked Eye Detection and Swift Imaging of Latent Fingerprints, ACS Appl. Nano Mater., 2023, 6, 19767–19776, DOI:10.1021/acsanm.3c03578.
- S. Berman, M. Navvab, M. Martin, J. Sheedy and W. Tithof, Comment on “A Comparison of traditional and high colour temperature lighting on the near acuity of elementary school children” by S Berman, M Navvab, MJ Martin, J Sheedy, and W Tithof, Light: Res. Technol., 2006, 38, 41–52, DOI:10.1177/136578280603800114.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ma00117j |
| This journal is © The Royal Society of Chemistry 2025 |