 Open Access Article
Open Access ArticleAdvanced dual-mode Er3+/Yb3+ phosphors for high-precision optical thermometry across broad temperature ranges†
Zein El
Abidine Aly Taleb
 a,
Ikhlas
Kachou
a,
Kamel
Saidi
a,
Ikhlas
Kachou
a,
Kamel
Saidi
 ab,
Mohamed
Dammak
ab,
Mohamed
Dammak
 *a,
Irene
Mediavilla
c and
Juan
Jiménez
c
*a,
Irene
Mediavilla
c and
Juan
Jiménez
c
aLaboratoire de Physique Appliquée, Faculté des Sciences de Sfax, Département de Physique, Université de Sfax, BP 1171 Sfax, Tunisia. E-mail: madidammak@yahoo.fr; mohamed.dammak@fss.usf.tn
bDepartment of Physics, Sfax Preparatory Engineering Institute, University of Sfax, 1172-3000 Sfax, Tunisia
cGdS Optronlab, Department of Condensed Matter Physics, University of Valladolid, LUCIA Building, Paseo de Belén 19, Valladolid, 47011, Spain
First published on 20th March 2025
Abstract
Dual-mode light-emitting phosphors play a vital role in advanced technologies and functions as they constitute optical thermometers for a wide range of temperature environments. This study presents a novel Er3+/Yb3+ co-doped NaCaY(MoO4)3 (NCYM) phosphor synthesized via the sol–gel method for precise optical thermometry across a broad temperature range (300–510 K). The research includes an in-depth analysis of the crystal structure, morphology, optical properties, and decay kinetics. The luminescence mechanism and energy transfer processes were elucidated, with NCYM:Er3+/Yb3+ phosphors efficiently activated under 980 nm and 325 nm laser excitation. These excitations produced 2H11/2/4S3/2 → 4I15/2 transitions via up-conversion (UC) and down-conversion (DC) mechanisms, respectively. A dual-mode optical thermometry system was developed, combining DC and UC approaches for simultaneous evaluation. At 300 K, the maximum relative sensitivities (Sr-max) were 1.2% K−1 (DC) and 1.045% K−1 (UC), while at 510 K, the maximum absolute sensitivities (Sa-max) reached 15.17 × 10−3 K (DC) and 12.15 × 10−3 K (UC). The system demonstrated exceptional temperature resolution, with uncertainties (δT) below 0.313 K, covering the full range of 300 to 510 K. This work positions NCYM:Er3+/Yb3+ phosphors as highly promising materials for precise optical temperature sensing in a variety of advanced applications.
1. Introduction
Temperature is a necessary parameter that affects many aspects of daily life, including industry, aerospace, bioengineering, infrared detectors, scientific research activities, and other areas.1–3 Initially, traditional contact-based temperature measurement methods were employed, relying on the expansion and contraction of liquids (typically mercury) in response to temperature changes.4,5 However, these techniques have limitations, especially in challenging environments such as fast-moving objects, high-temperature reactors, and underground mines.6–8 Consequently, traditional methods are becoming increasingly inadequate for the needs of evolving technologies, driving a transition toward non-contact temperature measurement techniques. These innovative approaches allow remote temperature monitoring, in contrast to the limitations of direct-contact thermometers.Luminescent materials capable of both down-conversion (DC) and up-conversion (UC) processes have garnered significant interest due to their wide-range of applications. In DC processes, high-energy photons are absorbed and re-emitted as lower-energy photons, whereas UC processes involve the absorption of multiple low-energy photons to emit a higher-energy photon. Rare-earth (RE) luminescent materials, particularly Er3+/Yb3+ co-doped hosts, are frequently explored for their ability to facilitate these processes through efficient energy transfer between Yb3+ and Er3+ ions.
In recent studies, we have systematically investigated the potential of Yb/Ln3+ ion couples across various applications, with a special focus on their utility in optical thermometry. Notably, our first series of articles9–12 explored the broad applicability and versatility of these couples in diverse material systems. Building on this foundation, the second ensemble of our work specifically delved into the exceptional performance and unique advantages of the Er/Yb pair in advanced optical sensing and high-precision thermometry applications.13–21 Together, these investigations underscore the critical role of Yb/Ln3+ dopant combinations in advancing functional materials for next-generation technologies.
The photoluminescence behavior of RE elements is primarily determined by intra-4f electronic transitions. Er3+ ions are especially desirable for UC and DC luminescence due to their multiple metastable energy states and wide emission spectra, including visible emissions at 550 nm and 670 nm, as well as near-infrared (NIR) emissions at 800 nm. Nonetheless, the inherently low absorption cross-section of Er3+ ions poses a challenge, limiting the efficiency of direct photon absorption in both UC and DC processes.
Yb3+ ions are commonly selected as sensitizers for UC processes due to their high absorption cross-section at 980 nm. This choice enhances the efficiency of detecting the green emission from Er3+ ions when excited at this wavelength. The energy states of Er3+, particularly 2H11/2 and 4S3/2, are separated by approximately 700 to 800 cm−1, facilitating efficient non-radiative transitions and contributing to their distinct luminescence properties,22,23 leading to a Boltzmann-type distribution of these states. This makes the 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transitions ideal for luminescent ratiometric thermal detection.
Optical temperature parameters are determined using Boltzmann's distribution law and the fluorescence intensity ratio (LIR) technique, applied to two thermally coupled energy levels (TCL) with an energy gap of less than 2000 cm−1.24–26
The choice of a host material is also important, as it influences the emission color through the varying lattice environments. The UC process is strongly affected by the host lattice properties and its interaction with doped rare-earth ions.27–29 Molybdate compounds have garnered interest as fluorescent media due to their broad and intense absorption bands, high emission intensity, and excellent dispersibility of RE ions.30,31 Recently, NCYM has gained attention as a host material for RE-ion-doped phosphors, offering good physical and chemical stability, low cutoff phonon energy, and high solubility for RE ions, making it ideal for solid-state applications.
We selected NCYM as the host material in our study due to its potential for enhanced detection performance, tunable optical properties, and the opportunity to explore a novel material system. The incorporation of supplementary elements, specifically sodium (Na), calcium (Ca), and yttrium (Y), introduces additional energy levels and modifies the host lattice's properties. This enables finer control over luminescence behavior and improves detection capabilities. When compared to existing systems (e.g., SrTiO3:Er3+/Yb3+,32 SrTiO3:Er3+,33 CaTiO3:Er3+/Yb3+,34 CaTiO3:Er3+,35 Ca3Y2Ge3O12:Er3+/Yb3+,36 Sr2YTaO6:Er3+, Yb3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 and SrWO4:Er3+/Yb3+
37 and SrWO4:Er3+/Yb3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38), the Er3+/Yb3+-co-doped NCYM material system emerges as a promising candidate for precise optical temperature sensing applications.
38), the Er3+/Yb3+-co-doped NCYM material system emerges as a promising candidate for precise optical temperature sensing applications.
In this study, NCYM:Er3+/Yb3+ phosphors were excited using both 980 nm and 325 nm lasers. The green emission from Er3+ ions, corresponding to the 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transitions, was observed for both excitation wavelengths. These emissions resulted from up-conversion and down-conversion processes, respectively. The thermometric properties of the NCYM:Er3+/Yb3+ phosphors were systematically evaluated under both excitation conditions, with key temperature sensor parameters, such as relative sensitivity and temperature resolution, being compared. This study emphasizes the development of advanced luminescent thermometers with outstanding relative sensitivity, leveraging the unique properties of Er3+/Yb3+ co-doped NCYM materials.
2. Experimental section
2.1 Materials
The starting materials used for the synthesis of NCYM:0.02Er3+/0.2Yb3+ phosphors included sodium nitrate (NaNO3, 99.0%), calcium nitrate tetrahydrate (Ca(NO3)2·4H2O, 99.0%), yttrium(III) nitrate hexahydrate (Y(NO3)3·6H2O, 99.9%), erbium nitrate pentahydrate (Er(NO3)3·5H2O, 99.9%), ytterbium nitrate pentahydrate (Yb(NO3)3·5H2O, 99.9%), ammonium molybdate tetrahydrate ((NH4)6Mo7O24·4H2O, 99.9%), and citric acid (C6H8O7, 99.0%). All chemical reagents were obtained from Sigma-Aldrich and used as received, without further purification.2.2 Sample synthesis
The sol–gel method was employed to synthesize Er3+/Yb3+ co-doped NaCaY(MoO4)3 (NCYM). Initially, stoichiometric amounts of sodium nitrate, calcium nitrate tetrahydrate, yttrium nitrate hexahydrate, erbium nitrate pentahydrate, ytterbium nitrate pentahydrate, and ammonium molybdate tetrahydrate were dissolved in 200 mL of deionized water. To facilitate complexation, 5 mol of citric acid was added as a chelating agent, maintaining a citric acid to metal ion ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The resulting mixture was stirred for one hour at room temperature, producing a solution that changed color from transparent to blue as it was heated. As the reaction progressed, a blue wet gel began to form. The stopper was then removed, allowing the liquid to evaporate. The gel was dried in an oven at 423 K for 12 hours, resulting in a stable, porous xerogel. This xerogel was subsequently annealed at 673 K for 3 hours, yielding black particles. Finally, the sample was sintered in air at 973 K for 3 hours, resulting in the formation of pure-phase crystalline particles. This technique was utilized to make NCYM structures doped with the following doping ion concentrations: 20%Yb3+/2%Er3+. To prevent UC luminescence quenching, we utilized certain quantities of dopant ions based on our experience and general understanding. Indeed, for UC systems, 20% sensitizer (Yb3+) and low concentrations of emitters (around 2%) are commonly employed, since they are appropriate conditions that typically result in strong UC for strong emission.17,18,39,40
2. The resulting mixture was stirred for one hour at room temperature, producing a solution that changed color from transparent to blue as it was heated. As the reaction progressed, a blue wet gel began to form. The stopper was then removed, allowing the liquid to evaporate. The gel was dried in an oven at 423 K for 12 hours, resulting in a stable, porous xerogel. This xerogel was subsequently annealed at 673 K for 3 hours, yielding black particles. Finally, the sample was sintered in air at 973 K for 3 hours, resulting in the formation of pure-phase crystalline particles. This technique was utilized to make NCYM structures doped with the following doping ion concentrations: 20%Yb3+/2%Er3+. To prevent UC luminescence quenching, we utilized certain quantities of dopant ions based on our experience and general understanding. Indeed, for UC systems, 20% sensitizer (Yb3+) and low concentrations of emitters (around 2%) are commonly employed, since they are appropriate conditions that typically result in strong UC for strong emission.17,18,39,40
3. Characterization techniques
X-ray powder diffraction (PXRD) patterns were obtained at room temperature using a Bruker D8-Advance X-ray powder diffractometer, with monochromatic CuKa1 copper radiation (1.5406 Å) in the range of 5–70 degrees 2θ. The morphology of the samples was examined using a Zeiss Supra55VP FEG-SEM field emission scanning electron microscope and a Bruker XFlash 5030 detector. The photoluminescence (PL) spectra were recorded using a He–Cd laser (325 nm) with a Labram UV-HR 800 Raman spectrometer (Horiba-Jobin Yvon) equipped with a low-dispersion 150 g mm−1 grating. Emission spectra were also obtained under excitation with a diode laser (980 nm) at a constant pump power of 30 mW. These measurements were carried out using a monochromator (Horiba Jobin Yvon, iHR320) equipped with an 1800 g mm−1 grating blazed at 500 nm and a photomultiplier tube (Hamamatsu, R928) to detect luminescence in the green–red spectral window. The chemicals and equipment used for this study were sourced through the facilities of the University of Valladolid, Spain, and the University of Sfax, Tunisia.4. Result and discussion
4.1 X-ray diffraction analysis
X-ray diffraction (XRD) patterns were used to study the crystal structure and phase purity of the samples. Fig. 1 shows XRD patterns of NCYM:Er3+/Yb3+ nanocrystals in the 5–70 2θ° range. All diffraction peaks in the XRD curves are easily indexed to a pure tetragonal phase with a scheelite structure. Its powder X-ray diffraction (PXRD) pattern matches the standard Joint Committee on Powder Diffraction Standards (JCPDS 25-0828). There are no impurity diffraction peaks observed, which suggests that Er3+/Yb3+ ions were successfully incorporated at the Y3+ sites and the obtained sample is composed of the pure molybdate structure. The Rietveld refinement was used to check the phase purity (Fig. S1, ESI†) and determine the unit cell parameters, which are listed in Table S1 (ESI†), confirming the phase purity of the synthesized material. X-ray powder diffraction (XRPD) was employed for structural analysis.Considering the similar ionic radii of Y3+ (1.019 Å, C.N. = 8), Er3+ (1.004 Å, C.N. = 8) and Yb3+ (0.985 Å, C.N. = 8),41,42 Er3+ and Yb3+ ions are most likely to replace Y3+ ions and enter the Er3+ and Yb3+ positions. As known, for a new solid-state solution, the radius percentage difference (Dr) between the potential substituted ions and dopants should be less than 30% by using eqn (1):43
 | (1) |
The morphology of the phosphors was also investigated by SEM, as shown in Fig. 2(a)–(d). It can be seen clearly from the images that the particles are well formed, with a homogeneous morphology and a size of about 1–2 μm. In general, the tightly packed particles contribute to a reduction in light scattering, which ultimately results in a high luminous efficiency. During the calcination process, the crystallites are of nanoscale size and therefore they have high surface energy. This causes the particles to agglomerate into larger structures and, finally, bulk materials.9,44,45
4.2 Photoluminescence properties
The plots of [F(R∞)hν]2versus photon energy (hν) for NCYM:Er3+/Yb3+ are shown in Fig. 3(b). The direct optical bandgap values (Eg) can be determined using the Kubelka–Munk (K–M) function and Tauc's relationship:45,46
 | (2) |
| [F(R∞)hν] = B(hν − Eg)n | (3) |
Fig. S2 (ESI†) shows the photoluminescence excitation (PLE) spectrum of NSGM:Er3+/Yb3+ microcrystals, measured by monitoring the emission at 550 nm. This spectrum reveals a broad excitation band along with several narrow peaks. The broad band, ranging from 250 to 348 nm with a maximum centered around 300 nm, is attributed to the charge transfer band (CTB). The narrow peaks observed at approximately 365, 378, 406, 451, and 488 nm correspond to the 4f–4f transitions of Er3+ ions, from the ground state 4I15/2 to the excited states 4G9/2, 4G11/2, 2H9/2, 4F5/2, and 4F7/2, respectively. This novel phosphor can be excited well by using a UV source (250–348 nm).
 | ||
| Fig. 4 (a) and (b) Emission spectra of NCYM:Er3+/Yb3+ under 325 nm and 980 nm excitation, respectively. (c) and (d) CIE chromaticity coordinates of the phosphors. | ||
The color coordinates of the phosphors were calculated using the CIE chromaticity diagram,49,50 which is shown in Fig. 4(c) and (d). In addition, under both 325 nm and 980 nm laser excitations, the final color is green, with CIE chromaticity coordinates: (x = 0.2339, y = 0.7414) and (x = 0.2736, y = 0.6947) for DC and UC, respectively. These coordinates are calculated based on the corresponding DC and UC emission spectra measured under ambient conditions (Fig. 3a and b). The CCT of the phosphor material is equal to the temperature of an ideal body radiator that emits light of the same chromaticity as the phosphor does. The McCamy formula is followed to determine the CCT of the light emitted from the synthesized material:51
| CCT = −449n3 + 3525n2 − 6823n + 5520.33 | (4) |
To investigate the UC mechanisms involved, a pump power-dependent UC analysis was performed on phosphorus NCYM:Er3+/Yb3+, as shown in Fig. 5. The UC emission intensity is related to the excitation power according to the following equation:52,53
| I ∝ Pn | (5) |
To gain a better understanding of the mechanism involved in light emission via UC emission modes, a study of the lifetime was carried out. The decay time was estimated by fitting the observed emission decay profiles with the simple exponential function mentioned below:54,55
| I(t) = A + I0e(−t/τ) | (6) |
Based on the photoluminescence properties observed in the visible and red regions, and the analysis of the UC emission process, a proposed energy level diagram for NCYM co-doped with Er3+/Yb3+ was built up, as shown in Fig. 6. Therein the emission mechanisms for both UC and DC processes are described in detail.
Under 325 nm excitation (DC), the molybdate ion in its ground state 1A1 can be excited to the 1T2 state, and then relaxes non-radiatively to the 1T1 state. The excited energy level 1T1 of the molybdate is nearly aligned with the excitable 4G11/2 state of Er3+, leading to a non-radiative resonance energy transfer from the molybdate to the Er3+ ions. The excited 4G11/2 state of Er3+ undergoes non-radiative relaxation to the emitting 2H11/2, 4S3/2, and 4F9/2 states of Er3+, resulting in green and red emissions at 533, 553, and 657 nm, respectively, thus explaining the DC mechanism.56–59
The UC mechanisms of the green and red emissions of Er3+ ions in the co-doped Er3+/Yb3+ NCYM follow the absorption of two photons. This result is similar to that of Er3+/Yb3+ doping in various other media, such as NaYF4 glass ceramic,60 NaYF4,61 Y2O3,62 SnO2,63 TiO2,64 and so on. Green light is emitted from the excited states 2H11/2 and 4S3/2. First, the electrons transit from the 4I15/2 ground state to the 4I11/2 state via ground state absorption (GSA), followed by excited state absorption (ESA1) to the 4F7/2 state. Then, by multi-phonon relaxation, they reach the 2H11/2 and 4S3/2 states, and emit green light by decaying to the 4I15/2 ground state. Simultaneously, the red emission is linked to the population of the 4F9/2 state. NCYM co-doped Er3+/Yb3+ and Yb3+ ions are excited from the ground state to the 2F5/2 level under excitation with 980 nm by GSA (2F7/2 → 2F5/2). As Yb3+ ions have a much larger absorption cross section than Er3+ ions in the 980 nm spectral range, the ET process becomes dominant over GSA for excitation of the Er3+ 4I11/2 level. A second Yb3+ ion transfers its energy to the 4I11/2 level to populate the 4F7/2 Er3+ level, which then de-excites to the 2H11/2, 4S3/2, 4F9/2 and 4I9/2 levels.65,66 At the end of these UC processes, green and red emissions are obtained by up-conversion. Yb3+ acts as a sensitizer and enriches the population of all emitting levels by transferring their absorbed energy to the activating ion.
4.3 Temperature sensing properties of NCYM:Er3+/Yb3+ (DC)
To evaluate the potential application of the Er3+/Yb3+ co-doped NCYM phosphor in optical thermometry, the temperature-dependent luminescence behavior of the sample was studied in detail under 325 nm excitation. Fig. 7 illustrates the temperature-dependent emission spectra in the range of 450–850 nm for the NCYM:Er3+/Yb3+ phosphor excited at 325 nm. The obtained spectra show two peaks attributed to the Er3+ ion transitions, located in the green visible region: 533 nm (2H11/2 → 4I15/2) and 553 nm (4S3/2 → 4I15/2), along with a relatively weaker red band centered at 657 nm, attributed to the 4F9/2 → 4I15/2 transition of Er3+ ions. Compared to the 4S3/2 → 4I15/2 transition, the emission intensity of the 2H11/2 → 4I15/2 transition exhibits a monotonic increase with no apparent shift in the band position as the temperature rises. This indicates that the 2H11/2 level can be effectively populated from the 4S3/2 level through a thermal excitation process.67 Furthermore, the increase in temperature is also beneficial in reducing structural defects and surface ligands. Therefore, the thermally coupled levels (TCLs) method is assumed to be applicable for optical thermometry.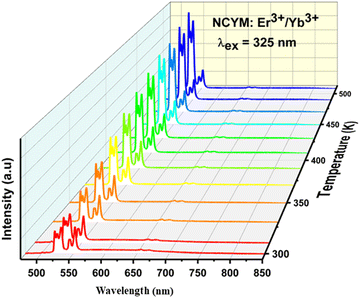 | ||
| Fig. 7 DC emission spectra of the Er3+/Yb3+ co-doped NCYM sample measured under 325 nm laser irradiation in the 300 to 510 K temperature range. | ||
Among the various techniques used to study temperature-sensing behavior, the luminescence intensity ratio (LIR) technique is widely recognized. Several studies have investigated the temperature sensing behavior of different rare-earth doped materials using the LIR technique. As the temperature increases, the peak positions of the emission bands remain unchanged; however, the intensities of the bands change, resulting in variations in the luminescence intensity ratio (LIR) between the 533 nm and 553 nm bands. The LIR varies according to the following relationship:68,69
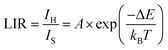 | (7) |
 | (8) |
 | (9) |
 | ||
| Fig. 8 (a) and (b) Temperature dependence of the LIR1 value between 2H11/2 and 4S3/2 TCL of Er3+ ions, and (b) the calculated Sr and Sa by LIR1 from TCL based on down-conversion. | ||
4.4 Temperature sensing properties of NCYM:Er3+/Yb3+ (UC)
The optical temperature sensing capability was also investigated in this case by measuring the temperature-dependent UC emission spectra of NCYM:Er3+/Yb3+ in the 450–850 nm range. Fig. 9 shows the UC emission spectra of the Er3+/Yb3+ co-doped NCYM material at different temperatures. Luminescence emission intensity is highly dependent on temperature. | ||
| Fig. 9 UC emission spectra of the Er3+/Yb3+-co-doped NCYM sample measured under 980 nm laser irradiation in the temperature range from 300 to 510 K. | ||
To minimize the potential effects of temperature increase during high power density excitations, appropriate measures must be implemented. It is essential to maintain relatively low power levels in LIR thermometry to mitigate self-heating effects. Consequently, the spectra were acquired at different powers of the excitation laser, selecting the maximum power at which there are no spectral changes, thus the measurements were carried out with a pump power of 30 mW, corresponding to a power density of 0.2 W cm−2.
It was observed that most of the emissions from the sample, except for the emissions at 533 nm, decreased with increasing temperature. As the temperature rises, the lattice vibrations are enhanced and accelerate the rate of non-radiative relaxation between closely spaced energy levels. This process leads to thermal equilibrium between the energy levels, suggesting that the 2H11/2 and 4S3/2 states are thermally coupled. The relative population of the two thermally coupled electronic states can be mathematically described using the Boltzmann-distribution law, as defined previously.
The calculated LIR values, as shown in Fig. 10(a), are in good agreement with the Boltzmann-distribution. The LIR was calculated using eqn (7). LIR values for the thermally coupled levels 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 (LIR2 = 533/553 nm) were determined and presented in Fig. 10(a). The LIR data fit very well, with a high degree of correlation (R2 = 0.996).
 | ||
| Fig. 10 (a) and (b) Temperature dependence of the LIR2 value between 2H11/2 and 4S3/2 TCL of Er3+ ions, and (b) the calculated Sr and Sa by LIR2 from TCL based on up-conversion. | ||
The performance of the thermometer was further assessed using both the absolute and relative thermal sensitivity, as previously defined (eqn (8) and (9)). Fig. 10(b) displays the temperature-dependent variations in the values of Sa and Sr over the range from 300 to 510 K for LIR2. The observed data reveal contrasting trends for the absolute and relative sensitivities as a function of temperature. The study indicated that the highest absolute sensitivities occurred at different temperatures, with a maximum value of 12.15 × 10−3 K−1 observed at room temperature for LIR2. In contrast, the relative sensitivity (Sr) decreases with increasing temperature, reaching its maximum value of 1.045% K−1 at 300 K (Fig. 10(b)).
Finally, aiming to provide the reader with an understanding of the current state of the art, a summary of various luminescent thermometers based on up/down conversion is presented in Table 1. It can be concluded that, in comparison with the detection sensitivity of other phosphors based on TCLs (2H11/2/4S3/2), our phosphor demonstrates superior performance using both methods.
| Samples | Temperature range (K) | S r-max (% K−1) | Excitation (nm) | Ref. |
|---|---|---|---|---|
| Ca2MgWO6:Yb3+/Er3+ | 300–600 | 0.92 (at 303 K) | 980 | 74 |
| La2O3:Er3+/Yb3+ | 303–600 | 0.91 (at 303 K) | 980 | 75 |
| BiVO4:Er3+/Yb3+ | 283–483 | 1.39 (at 283 K) | 980 | 76 |
| KYb2F7:Er3+ | 300–480 | 0.45 (at 300 K) | 980 | 77 |
| Sr2YbF7:Er3+ | 300–500 | 0.62 (at 300 K) | 980 | 78 |
| SrMoO4:Er3+/Yb3+ | 300–543 | 1.18 (at 300 K) | 980 | 79 |
| LaGdO3:Er3+/Yb3+ | 283–393 | 1.27 (at 283 K) | 980 | 80 |
| NaBiF4:Er3+/Yb3+ | 248–498 | 0.40 (at 498 K) | 980 | 81 |
| β-NaYF4:Er3+/Yb3+ | 303–678 | 0.30 (at 580 K) | 980 | 82 |
| YOF:Er3+/Yb3+ | 260–490 | 0.60 (at 490 K) | 980 | 83 |
| LaGdO3:Er3+ | 298–873 | 0.43 (at 300 K) | 488 | 84 |
| La2MoO6:Er3+ | 303–463 | 0.97 (at 480 K) | 379 | 85 |
| NCYM:Er 3+ /Yb 3+ | 300–510 | 1.20 (at 300 K) | 325 | This Work |
| NCYM:Er 3+ /Yb 3+ | 300–510 | 1.04 (at 300 K) | 980 | This Work |
Temperature resolution, also known as uncertainty (δT), is another key parameter that determines the smallest temperature change that can be detected by an optical thermometer. It also describes the possible error in temperature readings. The temperature uncertainty (δT) using luminescent thermometry can be calculated using the equation as follows:23,59,86
 | (10) |
 | ||
| Fig. 11 (a)–(d) Temperature resolution values, δT, corresponding to (a) LIR1 (DC) and (b) LIR2 (UC) for NCYM:Er3+/Yb3+. | ||
Overall, our results suggest that NCYM materials, which utilize both up-conversion and down-conversion processes, exhibit high thermal sensitivity and low measurement uncertainty, making them highly promising for optical thermometry applications using the LIR approach.
5. Conclusions
NCYM phosphors co-doped with Er3+/Yb3+, emitting in dual modes, were successfully synthesized using the sol–gel method. X-ray diffraction (XRD) and Rietveld refinement confirm that all prepared phosphors exhibit a pure phase with a tetragonal scheelite structure (space group I41/a). The phosphors show intense green and red light emissions due to the electronic transitions of Er3+ ions, including 2H11/2 → 4I15/2, 4S3/2 → 4I15/2, 4F9/2 → 4I15/2, and 4I9/2 → 4I15/2 under both near UV (325 nm) and NIR (980 nm) excitation. The chromaticity coordinates are in the green region, with CIE coordinates (x = 0.2339; y = 0.7414) for down-conversion (DC) and (x = 0.2736; y = 0.6947) for up-conversion (UC). The power-dependent UC emission analysis reveals that two photons are involved in the UC emission process. The luminescence intensity ratio (LIR) of the green bands corresponding to the 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transitions were studied as a function of temperature to assess their potential for temperature sensing. Maximum sensitivity values were found to be 1.2% K−1 and 1.045% K−1 at 300 K for DC and UC, respectively. The luminescence thermometry results demonstrated excellent temperature accuracy, with a calculated temperature uncertainty (δT) of less than 0.313 K. These findings underscore the remarkable potential of NCYM:Yb3+/Er3+ phosphors as optical temperature sensors, offering high sensitivity and a broad temperature detection range in both DC and UC modes.Data availability
All data underlying the results are available as part of the article and no additional source data are required.Conflicts of interest
There are no conflicts to declare.References
- Y. Chen, et al., Temperature-dependent luminescence of Bi3+, Eu3+ co-activated La2MgGeO6 phosphor for dual-mode optical thermometry, J. Lumin., 2022, 249, 118995 CrossRef CAS.
- M. Pokhrel, et al., Up- and Down-Convertible LaF3:Yb,Er Nanocrystals with a Broad Emission Window from 350 nm to 2.8 μm: Implications for Lighting Applications, ACS Appl. Nano Mater., 2021, 4, 13562–13572 CrossRef CAS.
- P. Singh, et al., Lanthanide doped ultrafine hybrid nanostructures: multicolour luminescence, upconversion based energy transfer and luminescent solar collector applications, Nanoscale, 2017, 9, 696–705 RSC.
- Y. Zhou, et al., Abnormal thermally enhanced upconversion luminescence of lanthanide-doped phosphors: proposed mechanisms and potential applications, J. Mater. Chem. C, 2021, 9, 2220–2230 RSC.
- F. Jahanbazi and Y. Mao, Recent advances on metal oxide-based luminescence thermometry, J. Mater. Chem. C, 2021, 9, 16410–16439 RSC.
- L. Huang, et al., Near-Infrared Persistent Luminescence in a Cr3+-Doped Perovskite for Low-Irradiance Imaging, Chem. Mater., 2020, 32, 5579–5588 CrossRef CAS.
- Z. Tang, G. Zhang and Y. Wang, Design and Development of a Bluish-Green Luminescent Material (K2HfSi3O9:Eu2+) with Robust Thermal Stability for White Light-Emitting Diodes, ACS Photonics, 2018, 5, 3801–3813 CrossRef CAS.
- Q. Tang, et al., Luminous tuning in Eu3+/Mn4+ co-doped double perovskite structure by designing the site-occupancy strategy for solid-state lighting and optical temperature sensing, Mater. Res. Bull., 2022, 149, 111704 CrossRef CAS.
- N. B. Amar, K. Saidi, C. Hernández-Álvarez, M. Dammak and I. R. Martin, Ultra-high-sensitive temperature sensing based on emission Pr3+ and Yb3+ codoped Y2Mo3O12 nanostructures, Mater. Adv., 2024, 6, 827–838 RSC.
- M. Fhoula, et al., Unlocking the luminescent potential of Pr3+/Yb3+ Co-doped Y2Mo4O15 for advanced thermometry applications, J. Alloys Compd., 2024, 979, 173537 CrossRef CAS.
- I. Kachou, K. Saidi, C. Hernández-Álvarez, M. Dammak and I. R. Martín, Enhancing thermometric precision: modulating the temperature of maximum sensitivity via erbium dopant addition in Ba2GdV3O11:Tm3+/Yb3+ nano phosphors, Mater. Adv., 2024, 5, 8280–8293 RSC.
- K. Saidi, M. Dammak, K. Soler-Carracedo and I. R. Martín, A novel optical thermometry strategy based on emission of Tm3+/Yb3+ codoped Na3GdV2O8 phosphors, Dalton Trans., 2022, 51, 5108–5117 RSC.
- F. Ayachi, K. Saidi, M. Dammak, I. Mediavilla and J. Jiménez, Unlocking advanced thermometric capabilities: BiVO4:Er3+/Yb3+ nanophosphors with dual-mode up-conversion and down-shifting features, RSC Adv., 2025, 15, 655–664 RSC.
- M. Fhoula, M. Khitouni and M. Dammak, Comparative optical thermometry analysis using Na2SrP2O7:Er3+/Yb3+ phosphors: evaluation of LIR TCL and LIR NTCL methods for high-resolution temperature sensing, RSC Adv., 2024, 14, 39373–39380 RSC.
- I. Kachou, et al., Advanced temperature sensing with Er3+/Yb3+ co-doped Ba2GdV3O11 phosphors through upconversion luminescence, Dalton Trans., 2024, 53, 2357–2372 RSC.
- K. Saidi, C. Hernández-Álvarez, M. Runowski, M. Dammak and I. Rafael Martín Benenzuela, Temperature and Pressure Sensing Using an Optical Platform Based on Upconversion Luminescence in NaSrY(MoO4)3 Codoped with Er3+ and Yb3+ Nanophosphors, ACS Appl. Nano Mater., 2023, 6, 19431–19442 CrossRef CAS.
- K. Saidi, I. Kachou, K. Soler-Carracedo, M. Dammak and I. R. Martín, Ba2YV3O11 Er3+/Yb3+ Nanostructures for Temperature Sensing in the Presence of Bismuth Ions, ACS Appl. Nano Mater., 2023, 6, 17681–17690 CrossRef CAS.
- K. Saidi, C. Hernández-Álvarez, M. Runowski, M. Dammak and I. R. Martín, Ultralow pressure sensing and luminescence thermometry based on the emissions of Er3+/Yb3+ codoped Y2Mo4O15 phosphors, Dalton Trans., 2023, 52, 14904–14916 RSC.
- N. M. Bhiri, et al., Excitation power density dependence of a primary luminescent thermometer based on Er3+,Yb3+:GdVO4 microcrystals operating in the visible, J. Alloys Compd., 2022, 921, 166020 CrossRef CAS.
- N. M. Bhiri, et al., Stoichiometric dependence and laser heating effect on the luminescence thermometric performance of Er3+, Yb3+:YuGdwVO4 microparticles in the non-saturation regime, Mater. Res. Bull., 2022, 151, 111801 CrossRef CAS.
- F. Ayachi, K. Saidi, W. Chaabani and M. Dammak, Synthesis and luminescence properties of Er3+ doped and Er3+–Yb3+ codoped phosphovanadate YP0.5V0.5O4 phosphors, J. Lumin., 2021, 240, 118451 CrossRef CAS.
- K. N. Kumar, G. Kang, J. Lim and J. Choi, Biocompatible Yb3+/Er3+ Co-activated La2(WO4)3 Upconversion Nanophosphors for Optical Thermometry, Biofluorescent, and Anticancer Agents, Inorg. Chem., 2022, 61, 3851–3865 CrossRef CAS PubMed.
- F. Ayachi, et al., Dual-mode luminescence of Er3+/Yb3+ codoped LnP0.5V0.5O4 (Ln = Y, Gd, La) for highly sensitive optical nanothermometry, Mater. Today Chem., 2023, 27, 101352 CrossRef CAS.
- K. Pavani, et al., Highly efficient upconversion of Er3+ in Yb3+ codoped non-cytotoxic strontium lanthanum aluminate phosphor for low temperature sensors, Sci. Rep., 2017, 7, 17646 CrossRef CAS.
- C. Hernández-Álvarez, et al., Multifunctional optical sensing platform of temperature, pressure (vacuum) and laser power density: NaYF4:Gd3+,Yb3+,Er3+ nanomaterial as luminescent thermometer, manometer and power meter, J. Mater. Chem. C, 2023, 11, 10221–10229 RSC.
- S. Tomar, et al., Dual-Mode Light Emission and Dynamic Studies of Er3+/Yb3+-Doped NaLa(MoO4)2 Phosphor for Optical Thermometry Operating from Cryogenic to above Room Temperatures, ACS Appl. Opt. Mater., 2024, 2, 1965–1984 CrossRef CAS.
- S. K. Singh, K. Kumar and S. B. Rai, Diode laser pumped Gd2O3:Er3+/Yb3+ phosphor as optical nano-heater, Appl. Phys. B: Lasers Opt., 2010, 100, 443–446 CrossRef CAS.
- A. K. Singh, et al., Light-into-heat conversion in La2O3:Er3+–Yb3+ phosphor: an incandescent emission, Opt. Lett., 2012, 37, 776–778 CrossRef CAS PubMed.
- S. K. Singh, K. Kumar and S. B. Rai, Multifunctional Er3+–Yb3+ codoped Gd2O3 nanocrystalline phosphor synthesized through optimized combustion route, Appl. Phys. B: Lasers Opt., 2009, 94, 165–173 CrossRef CAS.
- X. He, J. Zhou, N. Lian, J. Sun and M. Guan, Sm3+-activated gadolinium molybdate: an intense red-emitting phosphor for solid-state lighting based on InGaN LEDs, J. Lumin., 2010, 130, 743–747 CrossRef CAS.
- X. Zhao, et al., Novel Eu3+-doped red-emitting phosphor Gd2Mo3O9 for white-light-emitting-diodes (WLEDs) application, J. Alloys Compd., 2007, 433, 352–355 CrossRef CAS.
- I. Kumar, et al., Simultaneous realization of FIR-based multimode optical thermometry and photonic molecular logic gates in Er3+ and Yb3+ co-doped SrTiO3 phosphor, Phys. Scr., 2023, 98, 105532 CrossRef CAS.
- I. Kumar, et al., Charge compensation-driven downconverted luminescence enhancement in Er3+-doped SrTiO3 phosphors by co-doping with alkali ions (M+ = Li, Na, K) for solid-state lighting applications, Indian J. Phys., 2024, 1–10, DOI:10.1007/s12648-024-03432-9.
- I. Kumar, et al., NIR Light-triggered Green Emitting Perovskite-based Phosphor for Optical Thermometry and Future Molecular Logic Gate Applications, J. Fluoresc., 2024, 1–13, DOI:10.1007/s10895-024-04044-6.
- I. Kumar, A. Kumar, S. Kumar, V. Sangwan and A. K. Gathania, Green Emission in Thermally Stable Er3+ Doped Lead-Free Perovskite Phosphor for Solid-State Lighting and Optical Thermometry Applications, IEEE Photonics J., 2024, 16, 1–7 Search PubMed.
- X. Chen, et al., A multi-mode optical thermometer based on the up-conversion Ca3Y2Ge3O12:Er3+,Yb3+ phosphor, J. Lumin., 2023, 261, 119907 CrossRef CAS.
- Y. Bu, et al., A dual-mode self-referenced optical thermometry with high sensitivity based on Er3+–Yb3+ co-doped Sr2YTaO6 thermochromic phosphor, J. Lumin., 2022, 248, 118923 CrossRef CAS.
- A. Pandey, V. K. Rai, V. Kumar, V. Kumar and H. C. Swart, Upconversion based temperature sensing ability of Er3+–Yb3+ codoped SrWO4: an optical heating phosphor, Sens. Actuators, B, 2015, 209, 352–358 CrossRef CAS.
- M. Runowski, P. Woźny, S. Lis, V. Lavín and I. R. Martín, Optical Vacuum Sensor Based on Lanthanide Upconversion—Luminescence Thermometry as a Tool for Ultralow Pressure Sensing, Adv. Mater. Technol., 2020, 5, 1901091 CrossRef CAS.
- F. Paz-Buclatin, et al., GdVO4:Er3+/Yb3+ nanocrystalline powder as fluorescence temperature sensor. Application to monitor the temperature of an electrical component, Sens. Actuators, A, 2019, 299, 111628 CrossRef CAS.
- J. Liu, et al., Synthesis and luminescence properties of CaMoO4:Er3+/Yb3+ nanoparticles, J. Mater. Sci.: Mater. Electron., 2015, 26, 3380–3383 CrossRef CAS.
- A. Kumar and J. Manam, Optical thermometry using up and down conversion photoluminescence mechanism in Y2Zr2O7:Er3+ phosphors with excellent sensing sensitivity, J. Alloys Compd., 2020, 829, 154610 CrossRef CAS.
- M. Wei, et al., Albumin assisted sol-gel synthesized SrSnO3:Pr3+ red persistent phosphors for temperature sensing, J. Lumin., 2021, 239, 118328 CrossRef CAS.
- X. Yang, et al., Optical Temperature Sensing Behavior of High-Efficiency Upconversion: Er3+ –Yb3+ Co-Doped NaY(MoO4)2 Phosphor, J. Am. Ceram. Soc., 2015, 98, 2595–2600 CrossRef CAS.
- Z. E. A. A. Taleb, K. Saidi and M. Dammak, High-precision optical thermometry using Pr3+-doped NaCaY(MoO4)3 luminophores: a multi-spectral and chromaticity-based approach to non-contact temperature sensing, RSC Adv., 2025, 15, 5327–5337 RSC.
- M. Enneffati, M. Rasheed, B. Louati, K. Guidara and R. Barillé, Morphology, UV-visible and ellipsometric studies of sodium lithium orthovanadate, Opt. Quantum Electron., 2019, 51, 299 CrossRef.
- Y. Jiang, et al., A three-mode self-referenced optical thermometry based on up-conversion luminescence of Ca2MgWO6:Er3+,Yb3+ phosphors, Chem. Eng. J., 2021, 413, 127470 CrossRef CAS.
- S. Tabanli and G. Eryurek, Optical investigation of Er3+ and Er3+/Yb3+ doped zinc-tellurite glass for solid-state lighting and optical thermometry, Sens. Actuators, A, 2019, 285, 448–455 CrossRef CAS.
- S. Agrawal and V. Dubey, Down conversion luminescence behavior of Er and Yb doped Y2O3 phosphor, J. Radiat. Res. Appl. Sci., 2014, 7, 601–606 Search PubMed.
- K. Saidi, et al., Multifunctional Optical Sensing with Lanthanide-Doped Upconverting Nanomaterials: Improving Detection Performance of Temperature and Pressure in the Visible and NIR Ranges, ACS Appl. Mater. Interfaces, 2024, 16, 19137–19149 CrossRef CAS.
- I. Kachou, K. Saidi, R. Salhi and M. Dammak, Synthesis and optical spectroscopy of Na3Y(VO4)2:Eu3+ phosphors for thermometry and display applications, RSC Adv., 2022, 12, 7529–7539 RSC.
- S. Su, et al., KYb2F7:Er3+ based nanothermometers: controlled synthesis, enhanced red emission, and improved sensitivities via crystal-site engineering, J. Mater. Chem. C, 2023, 11, 2375–2388 RSC.
- V. Kesarwani and V. K. Rai, Fluorescence intensity ratio technique and its reliability, Methods Appl. Fluoresc., 2022, 10, 034006 CrossRef CAS PubMed.
- M. M. Upadhyay, N. K. Mishra and K. Kumar, Upconversion luminescence based temperature sensing properties and anti-counterfeiting applications of GdNbO4:Tm3+/Yb3+ phosphor, Spectrochim. Acta, Part A, 2024, 304, 123333 CrossRef CAS PubMed.
- Z. E. A. A. Taleb, K. Saidi, M. Dammak, D. Przybylska and T. Grzyb, Ultrasensitive optical thermometry using Tb3+ doped NaSrGd(MoO4)3 based on single band ratiometric luminescence, Dalton Trans., 2023, 52, 4954–4963 RSC.
- K. Bouras, et al., Photon management properties of Yb-doped SnO2 nanoparticles synthesized by the sol–gel technique, Phys. Chem. Chem. Phys., 2019, 21, 21407–21417 RSC.
- H. Cui, et al., Extremely intense green up-conversion luminescent and ultra-high temperature sensitivity in Er3+/Yb3+ co-doped BiTa7O19 phosphors, J. Lumin., 2022, 241, 118484 CrossRef CAS.
- Y. Chen, et al., Up-conversion luminescence and temperature sensing properties based on Y2GeO5:Er3+,Yb3+ phosphors for three-mode optical thermometry, J. Lumin., 2022, 250, 119121 CrossRef CAS.
- Z. E. A. A. Taleb, K. Saidi and M. Dammak, The dual-model up/down-conversion green luminescence of NaSrGd(MoO4)3:Er3+ and its application for temperature sensing, RSC Adv., 2024, 14, 8366–8377 RSC.
- X. Li, L. Yang, Y. Zhu, J. Zhong and D. Chen, Upconversion of transparent glass ceramics containing β-NaYF4:Yb3+,Er3+ nanocrystals for optical thermometry, RSC Adv., 2019, 9, 7948–7954 RSC.
- J. Zhao, et al., Upconversion luminescence with tunable lifetime in NaYF4:Yb,Er nanocrystals: role of nanocrystal size, Nanoscale, 2013, 5, 944–952 RSC.
- C. D. Mayrinck, et al., Downconversion and upconversion observed from Er3+/Yb3+/Eu3+ tri-doped-Y2O3 for application in energy conversion, J. Alloys Compd., 2020, 816, 152591 CrossRef CAS.
- C. T. M. Dung, L. Van Hieu, L. Q. Vinh and T. T. T. Van, Remarkable enhancement of Er3+ emission at 1.54 μm in Er/Yb co-doped SiO2–SnO2 glass-ceramics, J. Alloys Compd., 2018, 757, 489–495 CrossRef.
- R. Salhi and J.-L. Deschanvres, Efficient green and red up-conversion emissions in Er/Yb co-doped TiO2 nanopowders prepared by hydrothermal-assisted sol–gel process, J. Lumin., 2016, 176, 250–259 CrossRef CAS.
- S. Tabanli and G. Eryurek, Excitation power and Er3+ concentration effect on the color quality parameters in Y2O3:Er3+/Yb3+/Tm3+ nanophosphors, J. Nanophotonics, 2018, 12, 026008 Search PubMed.
- J. Yang, et al., Controllable Red, Green, Blue (RGB) and Bright White Upconversion Luminescence of Lu2O3:Yb3+/Er3+/Tm3+ Nanocrystals through Single Laser Excitation at 980 nm, Chem. – Eur. J., 2009, 15, 4649–4655 CrossRef CAS PubMed.
- W. Xu, et al., Optical temperature sensing in Er3+–Yb3+ codoped CaWO4 and the laser induced heating effect on the luminescence intensity saturation, J. Alloys Compd., 2017, 726, 547–555 CrossRef CAS.
- Z. E. A. A. Taleb, K. Saidi and M. Dammak, Dual-mode optical ratiometric thermometry using Pr3+-doped NaSrGd(MoO4)3 phosphors with tunable sensitivity, Dalton Trans., 2023, 52, 18069–18081 RSC.
- V. Kesarwani and V. K. Rai, Non-contact optical thermometry via non-thermally coupled levels in upconverting glass, J. Appl. Phys., 2022, 132, 113102 CrossRef CAS.
- Z. Chen, et al., Mn4+-Activated Double-Perovskite-Type Sr2LuNbO6 Multifunctional Phosphor for Optical Probing and Lighting, ACS Appl. Mater. Interfaces, 2023, 15, 28193–28203 CrossRef CAS.
- Y. Ding, B. So, J. Cao, F. Langenhorst and L. Wondraczek, Light Delivery, Acoustic Read-Out, and Optical Thermometry Using Ultrasound-Induced Mechanoluminescence and the Near-Infrared Persistent Luminescence of CaZnOS:Nd3+, Adv. Opt. Mater., 2023, 11, 2300331 CrossRef CAS.
- P. Du, L. Luo and J. S. Yu, Energy Back Transfer Induced Color Controllable Upconversion Emissions in La2MoO6:Er3+/Yb3+ Nanocrystals for Versatile Applications, Part. Part. Syst. Charact., 2018, 35, 1700416 CrossRef.
- J. Drabik, R. Kowalski and L. Marciniak, Enhancement of the sensitivity of single band ratiometric luminescent nanothermometers based on Tb3+ ions through activation of the cross relaxation process|, Sci. Rep., 2020, 10, 11190 CrossRef CAS.
- Y. Jiang, et al., A three-mode self-referenced optical thermometry based on up-conversion luminescence of Ca2MgWO6:Er3+,Yb3+ phosphors, Chem. Eng. J., 2021, 413, 127470 CrossRef CAS.
- R. Dey and V. K. Rai, Yb3+ sensitized Er3+ doped La2O3 phosphor in temperature sensors and display devices, Dalton Trans., 2014, 43, 111–118 RSC.
- J. Sun, et al., Temperature self-monitoring photothermal nano-particles of Er3+/Yb3+ Co-doped zircon-tetragonal BiVO4, Ceram. Int., 2021, 47, 409–415 CrossRef CAS.
- J. Cao, et al., Optical thermometry based on up-conversion luminescence behavior of Er3+-doped KYb2F7 nano-crystals in bulk glass ceramics, J. Alloys Compd., 2017, 693, 326–331 CrossRef CAS.
- X. Li, J. Cao, Y. Wei, Z. Yang and H. Guo, Optical Thermometry Based on Up-Conversion Luminescence Behavior of Er3+-Doped Transparent Sr2YbF7 Glass-Ceramics, J. Am. Ceram. Soc., 2015, 98, 3824–3830 CrossRef CAS.
- X. Liu, et al., Comparison study on the different strategies designed for ratiometric luminescence thermometry in Er3+/Yb3+:SrMoO4 phosphor, Sens. Actuators, A, 2020, 315, 112287 CrossRef CAS.
- A. Siaï, P. Haro-González, K. Horchani Naifer and M. Férid, Optical temperature sensing of Er3+/Yb3+ doped LaGdO3 based on fluorescence intensity ratio and lifetime thermometry, Opt. Mater., 2018, 76, 34–41 CrossRef.
- O. A. Savchuk, J. J. Carvajal, C. Cascales, M. Aguiló and F. Díaz, Benefits of Silica Core–Shell Structures on the Temperature Sensing Properties of Er,Yb:GdVO4 Up-Conversion Nanoparticles, ACS Appl. Mater. Interfaces, 2016, 8, 7266–7273 CrossRef CAS PubMed.
- D. Chen, et al., Bulk glass ceramics containing Yb3+/Er3+:β-NaGdF4 nanocrystals: Phase-separation-controlled crystallization, optical spectroscopy and upconverted temperature sensing behavior, J. Alloys Compd., 2015, 638, 21–28 CrossRef CAS.
- H. Suo, et al., Sensitivity Modulation of Upconverting Thermometry through Engineering Phonon Energy of a Matrix, ACS Appl. Mater. Interfaces, 2016, 8, 30312–30319 CrossRef CAS PubMed.
- V. Gutiérrez-Cano, F. Rodríguez, J. A. González and R. Valiente, Upconversion and Optical Nanothermometry in LaGdO3:Er3+ Nanocrystals in the RT to 900 K Range, J. Phys. Chem. C, 2019, 123, 29818–29828 CrossRef.
- P. Du and J. S. Yu, Near-ultraviolet light induced visible emissions in Er3+-activated La2MoO6 nanoparticles for solid-state lighting and non-contact thermometry, Chem. Eng. J., 2017, 327, 109–119 CrossRef CAS.
- J. Stefanska, K. Maciejewska and L. Marciniak, Blue-emitting single band ratiometric luminescent thermometry based on LaF3:Pr3+, New J. Chem., 2021, 45, 11898–11904 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ma00108k |
| This journal is © The Royal Society of Chemistry 2025 |





