Open Access Article
Open Access ArticleHigh-capacity iodine adsorption from diverse media using iron-based metal–organic copolymer networks synthesized via a microwave-assisted Buchwald–Hartwig cross-coupling reaction†
Suchetha
Shetty
 ab,
Noorullah
Baig
ab,
Noorullah
Baig
 ab,
Badvel
Pallavi
ab,
Rupa
Bargakshatriya
c,
Sumit Kumar
Pramanik
ab,
Badvel
Pallavi
ab,
Rupa
Bargakshatriya
c,
Sumit Kumar
Pramanik
 c and
Bassam
Alameddine
c and
Bassam
Alameddine
 *ab
*ab
aDepartment of Mathematics and Natural Sciences, Gulf University for Science and Technology, Mubarak Al-Abdullah, Hawally 32093, Kuwait. E-mail: alameddine.b@gust.edu.kw; Tel: +965 2530 7111
bFunctional Materials Group, Gulf University for Science and Technology, Mubarak Al-Abdullah, Hawally 32093, Kuwait
cCSIR-Central Salt and Marine Chemicals Research Institute, Gijubhai Badheka Marg, Bhavnagar, Gujarat 364002, India
First published on 10th April 2025
Abstract
Three-dimensional iron(II) clathrochelate-bridged secondary arylamine copolymers (TAC1–3) were synthesized via a facile microwave-assisted Buchwald–Hartwig cross-coupling reaction, utilizing a custom-designed diamine clathrochelate precursor along with a range of tribrominated aryl surrogates. The target copolymers exhibited impressive iodine vapor adsorption capabilities, with TAC3 achieving an outstanding uptake of 1500 wt% (15 g g−1). Kinetic analysis identified a predominant pseudo-2nd-order adsorption model, whilst recyclability tests confirmed the materials’ durability, sustaining high efficiency albeit over multiple adsorption–desorption cycles. The TAC1–3 copolymers demonstrated notable iodine uptake from cyclohexane solutions with TAC3 achieving a maximum capacity of 1.11 g g−1 at an initial iodine concentration of 1000 mg L−1. Moreover, TAC1–3 demonstrated exceptional performance in aqueous systems, achieving adsorption capacities up to 5.95 g g−1 in I2 solutions and 5.34 g g−1 in I3− (KI/I2) solutions.
Introduction
Radioactive nuclides present an intricate challenge as they offer the advantage of producing clean nuclear energy with greenhouse-gas-free emissions, on the one hand, but they generate hazardous waste that poses substantial health and environmental risks if left untreated and released into the atmosphere, on the other hand.1,2 Among others, iodine nuclides are ominous byproducts resulting from plutonium-239 and uranium-235 fission in nuclear reactors; notably, iodine-129 is particularly dangerous due to its toxicity and extremely long half-life of ∼16 million years, in addition to its capacity to easily pass through a wide variety of geological environments.3,4 Meanwhile, despite the much shorter half-life of iodine-131 estimated to be ∼8 days, it poses an imminent threat to human health by disrupting metabolic processes through its severe radiation.5 Therefore, managing nuclear waste is deemed crucial, especially when analyzing past nuclear accidents like Chernobyl in 1986 and Fukushima in 2011, where large amounts of radioactive iodine, including iodine-129 and iodine-131, were released into the atmosphere as gaseous iodine (I2) or into water bodies as I2 and I3−.6 These incidents highlight the urgent need for advanced materials that can efficiently and rapidly capture and remediate iodine from various sources, namely, aqueous effluents.Several materials have been identified as potent iodine adsorbents, including zeolites,7 ceramics,8 aerogels,9,10 metal–organic frameworks (MOFs),11–13 covalent organic frameworks (COFs),14,15 activated carbon (AC),16 and porous organic polymers.17,18 Nonporous materials have also demonstrated significant capture abilities.19,20 Among these, porous organic polymers have been of particular interest due to their prominent adsorption capacity, high physicochemical stability, lower synthesis costs, and robust linkages. In addition, organic polymers offer an on-demand structural flexibility depending on specific needs.21 It is noteworthy that there are several factors which depict the iodine capture by porous materials, such as their surface area and extended conjugation, and more importantly, the presence of binding sites and/or heteroatoms in the polymer's structure.22–25 Nevertheless, despite the high performance that these materials display in capturing gaseous iodine (I2), most of them exhibit limited iodine uptake in aqueous environments, typically less than 1.0 g g−1, namely because of their interference with water.26–30 Only a select few materials have demonstrated a high iodine uptake capacity from water.31–35
Iron(II) clathrochelate-based materials are exploited to a great extent across various applications owing to their structural modularity, exceptional chemical and physical stability, straightforward synthesis and ease of isolation, as well as their potential for post-functionalization.36–40 Various Fe(II) clathrochelate derivatives were synthesized and tested as adsorbents for different gases and dyes revealing astounding uptake capacities.41–46 Recently, our research group has developed a versatile synthetic methodology to make polyimide materials bearing Fe(II) clathrochelate as intercalators, portraying an impressive iodine vapor uptake of 680 wt%.47 In this study, we report the synthesis of a series of three-dimensional polyarylamine copolymers incorporating iron(II) clathrochelate building blocks through a conventional Buchwald–Hartwig cross-coupling reaction. Iodine uptake tests reveal the exceptional adsorption properties of these 3D copolymers reaching 15 g g−1 in addition to the effective removal of iodine from aqueous and cyclohexane solutions revealing prominent capacities of 5.95 g g−1 and 1.11 g g−1, respectively.
Experimental section
Synthesis
Synthesis of TAC2
TAC2 was synthesized using procedure A with tris(4-bromophenyl)amine (0.2 g, 0.41 mmol, 1 equivalent), DAC (0.43 g, 0.62 mmol, 1.5 equivalents), Pd2(dba)3 (0.03 g, 0.033 mmol, 8 mol%), XPhos (0.03 g, 0.066 mmol, 16 mol%), and sodium tert-butoxide (0.119 g, 1.24 mmol, 3 equivalents) dissolved in 8.3 mL of degassed DMF. The reaction yielded a brown solid (356 mg, 93%). 13C-NMR (solid state, 125.8 MHz): δ 153.33, 148.41, 136.51, 129.12, 124.88, 117.07, 25.45 and 20.17. FTIR (KBr pellet, cm−1): 3333 (N–H stretching), 2935 (aliphatic C–H stretching), 1584 (N–O stretching), 1490 (aliphatic C–H bending), 770 and 810 (aromatic C–H bending).Synthesis of TAC3
TAC3 was synthesized using procedure A with 1,3,5-tris(4-bromophenyl)benzene (0.2 g, 0.37 mmol, 1 equivalent), DAC (0.38 g, 0.55 mmol, 1.5 equivalents), Pd2(dba)3 (0.027 g, 0.029 mmol, 8 mol%), XPhos (0.027 g, 0.058 mmol, 16 mol%), and sodium tert-butoxide (0.106 g, 1.1 mmol, 3 equivalents) dissolved in 7.4 mL of degassed DMF. This reaction produced a brown solid (343 mg, 95%). 13C-NMR (solid state, 125.8 MHz): δ 155.21, 150.14, 136.55, 128.21, 122.33, 117.09, 112.71, 21.09 and 14.88. FTIR (KBr pellet, cm−1): 3352 (N–H stretching), 2919 (aliphatic C–H stretching), 1591 (N–O stretching), 1488 (aliphatic C–H bending), 747 and 811 (aromatic C–H bending).Iodine adsorption studies
The iodine vapor adsorption of TAC1–3 was analyzed using gravimetric techniques with 10 mg of each sample being used as an adsorbent by placing it in small weighing vials, which were then deposited in a larger vial to avoid any direct surface contamination during testing. The entire setup was tightly sealed in a container having iodine pellets at its bottom and the temperature was maintained at 353 K to promote adsorption. At various time intervals, the vials with the adsorbents were weighed and the percentage of I2 uptake was calculated using the following formula: | (1) |
Kinetics and adsorption equilibrium of iodine
The kinetics of iodine adsorption were quantitatively analyzed using the pseudo-1st-order, pseudo-2nd-order, and intra-particle diffusion models as represented by eqn (2)–(4), respectively:ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t | (2) |
 | (3) |
| qt = kpt0.5 + C | (4) |
Iodine uptake capability from cyclohexane
A 20 mL cyclohexane solution containing 100 mg L−1 of iodine was introduced to a 10 mg sample of TAC1–3, placed in a tightly closed glass vial, and kept in the dark for 24 hours. Subsequently, 2 mL of the supernatant was extracted and transferred into a quartz cuvette to measure its absorbance and determine the iodine concentration. This procedure was repeated for each polymer using cyclohexane solutions with iodine concentrations of 300 mg L−1, 500 mg L−1 and 1000 mg L−1. The iodine uptake by the TAC1–3 samples was calculated using the following formula: | (5) |
In this formula, V represents the volume of the iodine-cyclohexane solution (L), m is the mass of the TAC1–3 sample (g), and C0 is the initial iodine concentration in the cyclohexane solution (mg L−1). The iodine concentration in cyclohexane after 24 hours of adsorption is denoted as Ct.5
The following formula is used to calculate the efficiency of iodine removal by the TAC1–3 samples:
 | (6) |
Iodine uptake capacity from water
TAC1–3 samples were immersed in an aqueous iodine solution, either containing I2 (3 mg of adsorbent in 100 mL of 1 mM solution) or I3− (5 mg of adsorbent in a solution of 300 mg KI and 150 mg I2 in 100 mL of water). The solution was analyzed using UV-Vis spectroscopy, and the iodine absorbed by the TAC1–3 samples was calculated using eqn (5), where V represents the volume of the aqueous iodine solution (L), m is the mass of the TAC1–3 sample (g), and C0 (g L−1) and Ct (g L−1) are the iodine concentrations in the solution before and after adsorption, respectively.Results and discussion
It is worthwhile to mention that while our current work employed the previously reported diamino iron(II) clathrochelate building block, DAC,47 the latter was utilized as a surrogate for the preparation of clathrochelate-based imide copolymers (ACP1–3) through a cyclocondensation reaction under microwave heating, as illustrated in Scheme 1. Copolymers ACP1–3 have demonstrated remarkable iodine vapor uptake of 6.8 g g−1 and exhibited an uptake of up to 0.65 g g−1 in cyclohexane solution. These exceptional iodine adsorption capacities prompted us to design new copolymers bearing DAC with the prospect of enhancing iodine adsorption through the incorporating of secondary amino groups into the polymer backbone. It is noteworthy that in previous work, we synthesized linear secondary arylamine copolymers containing iron(II) clathrochelate units CLP1–338 before we had modified them into tertiary arylamine derivatives PCLP1–3 using a Buchwald–Hartwig cross-coupling reaction. Therefore, this motivated us to develop three-dimensional secondary arylamine copolymers as potential adsorbents of iodine. | ||
| Scheme 1 Synthesis of copolymers TAC1–3 and comparison with the previous work ACP1–3. Reaction conditions: (i) Pd2(dba)3, Xphos, NaOtBu, DMF, MW, 180 °C, 2 h; (ii) imidazole, DMF, MW, 180 °C, 2 h. | ||
In the current work secondary arylamine copolymers containing clathrochelate units, TAC1–3, were synthesized via a Buchwald–Hartwig amination cross-coupling reaction, which was performed in DMF at 180 °C for 2 hours under microwave heating, utilizing the diamine iron(II) clathrochelate synthon (DAC) in combination with different tribrominated aryl derivatives, including 1,3,5-tribromobenzene, tris(4-bromophenyl)amine, and 1,3,5-tris(4-bromophenyl)benzene, as shown in Scheme 1. The resulting copolymers were obtained in excellent yields and were purified using simple filtration followed by extensive washing of the solid with demineralized water, acetone, methanol (MeOH), dichloromethane, and naphtha (petroleum ether). The copolymers TAC1–3 were found to be insoluble in various organic solvents, such as dimethylformamide (DMF), DCM, chloroform, THF, and methanol. Consequently, the formation and purity of the target copolymers were confirmed by carrying out a careful structural analysis using various solid-state techniques, such as 13C-NMR spectroscopy, Fourier-transform infrared spectroscopy (FTIR), and X-ray photoelectron spectroscopy (XPS) (see Fig. 1–3 and Fig. S1–S7 in the ESI†).
Fig. 1 represents the 13C-NMR spectrum (solid-state) of the target copolymer TAC1, clearly displaying all the expected peaks. The peaks between 152.11 ppm to 116.43 ppm correspond to the aromatic (sp2) carbons while the signals at 22.67 ppm and 16.93 ppm (Fig. 1, peaks a and b) are fingerprints of aliphatic sp3 carbon atoms of the cyclohexyl groups present in the clathrochelate unit. Similarly, the solid-state 13C-NMR spectra of TAC2 and TAC3 display similar distinct chemical shifts, as can be noticed in Fig. S1 and S2 of the ESI.†
As illustrated in Fig. 2, the relative FT-IR absorption spectra of the two precursor materials TBB and DAC compared to the target copolymer TAC3 exhibit all the expected differences between the peaks of the starting materials and target compound, thus confirming the successful synthesis of the latter. The N–H stretching vibrations observed in DAC at 3412 cm−1 are shifted to 3352 cm−1 along with a noticeable decrease in the peak intensity in TAC3, which confirms the formation of the secondary amine copolymers.51 On the other hand, the fingerprint C–Br stretching vibrations observed in TBB at 695 cm−1 disappeared in the TAC3 spectrum, signifying the total consumption of all the reactants, and confirming the successful formation of the copolymers.52 Additionally, TAC3 reveals other distinctive aromatic C–H vibrations at 3032 cm−1 (stretching), and at 811 cm−1 and 747 cm−1 (vibrations).53 Furthermore, the characteristic frequencies of the aliphatic C–H groups are observed at 2919 cm−1 (str) and 1488 cm−1 (ben).54 Likewise, the absorption band at 1591 cm−1 is attributed to the stretching frequency of the N–O groups,55 supporting further evidence of the effective formation of TAC3. It is also worth mentioning that both TAC1 and TAC2 display their distinctive FT-IR peaks, proving their efficacious formation as well (Fig. S3 and S4 in the ESI†).
Survey-scan X-ray photoelectron spectroscopy (XPS) data of TAC1–3 corroborate the existence of the desired elements. The binding energies for C 1s, O 1s, and N 1s were determined to range between 284.4–284.7 eV, 531.9–532.2 eV, and 399.3–399.4 eV, correspondingly. Meanwhile, the B 1s and Fe 2p binding energies were observed in the range of 190.6–190.7 eV and 709.1–722.1 eV, respectively56 (Fig. 3 and Fig. S6, S7 in the ESI†).
BET surface area studies
The microporous nature of TAC1–3 was examined at low relative pressure using N2 adsorption tests conducted at 77 K (Fig. S8 in the ESI†). The surface areas and pore volumes of the target copolymers were determined from the Brunauer–Emmett–Teller (BET) theory based on the obtained isotherms. TAC1–3 demonstrated BET surface areas of up to 69 m2 g−1 and pore volumes ranging from 0.096 cm3 g−1 to 0.6 cm3 g−1 (Table S1 in the ESI†). The limited BET surface areas of TAC1–3 can be attributed to kinetic restrictions at the cryogenic temperature of 77 K, which impede molecular nitrogen diffusion into the micropores.47,57Iodine capture
Experimental gravimetric tests were carried out to investigate the iodine vapor adsorption characteristics of TAC1–3. These studies involved exposing a 10 mg sample of a particular copolymer to excess I2(s) in a sealed container at 353 K under atmospheric pressure. The amount of adsorbed iodine was monitored at different intervals until saturation was reached (Fig. 4). The results disclosed remarkable iodine uptakes, namely for TAC3, which achieved an adsorption capacity of 1500 wt% (15 g g−1) after 5 days of exposure (Table 1 and Fig. 4). This exceptionally high iodine vapor absorption underscores TAC3 as a prominent adsorbent, especially considering its comparatively easy and adaptable production in comparison to other materials documented in the literature (Table S2, ESI†).23,57–66 Both TAC1 and TAC2 displayed very good iodine capture capacities of 860 wt% and 490 wt%, respectively. The extraordinary iodine adsorption values observed in TAC1–3, ranging from 490 to 1500 wt%, are attributed to the complex structural properties of the copolymers featuring numerous adsorption sites,31,47,67,68 which allows for an efficient iodine capture capacity that is not only influenced by the copolymers’ specific surface areas but also the existence of binding sites. Moreover, the presence of terphenyl groups, which are known to enhance the aromatic π interactions with iodine,69 in the TAC3 copolymer's skeleton boosts its iodine capacity when compared to TAC1 and TAC2.As it can be perceived from the comparative thermograms for the iodine-loaded samples, designated as TAC1–3@I2, and those for the pure copolymers, there is a clear decline in the 10% weight loss temperatures in the range of 125–134 °C for the iodine-bearing copolymers from the initial 288–335 °C temperature values recorded for the TAC1–3 (Fig. S9 and S10 in the ESI†).70,71 Furthermore, FTIR spectroscopy was utilized to analyze the characteristics of TAC1–3@I2, which were found to exhibit conspicuous variations in their vibrational bands when compared to their pure counterparts TAC1–3. As shown in Fig. 5, the FTIR spectrum of TAC3@I2 displays significant shifts attributed to iodine adsorption, particularly in the aliphatic C–H stretching (∼14 cm−1) and bending bands (∼5 cm−1), as well as the aromatic C–H stretching (∼68 cm−1) and bending bands (∼31 cm−1). Additionally, notable shifts are observed in the stretching frequencies of the functional groups such as N–H (∼50 cm−1) and N–O (∼6 cm−1) in the iodine adsorbed copolymer TAC3@I2. These changes strongly indicate interactions between the hitherto mentioned copolymer and iodine molecules, especially involving the aromatic rings, amine groups, and N–O units. These aforementioned binding interaction sites which contain electron lone pairs can effectively interact with iodine to form polyiodide anions, thus resulting in the observed shifts in the corresponding groups68,72 (Fig. S14 in the ESI†). Furthermore, the electrostatic potential of the amino groups decreases the conjugation with the aromatic rings, which enhances their interaction with iodine molecules, consequently, further improving their I2 absorption capacity.73 It is noteworthy that the observed FTIR peak shifts confirm the presence of weak interactions between the copolymers and I2, indicating a physisorption behavior which occurs at the surface of TAC1–3.74
To gain more insight into the adsorption of iodine species by TAC1–3, XPS analysis was performed after iodine adsorption and the spectra were compared to those prior to adsorption (Fig. 6 and Fig. S11, S12 in the ESI†). The XPS survey scan spectrum of TAC3 (Fig. 6) lacks the characteristic binding energy peaks corresponding to iodine's 3d orbital, whereas that of I2@TAC3 discloses the effective adsorption of iodine moieties, as evidenced by the existence of iodine 3d orbital signals in the range of 619–634 eV.75,76 Two pronounced peaks, ascribed to the I3d,3/2 and I3d,5/2 energy levels, were also observed at 632.58 eV and 621.02 eV, respectively. Additionally, the appearance of two more peaks alongside the I3d,3/2 and I3d,5/2 peaks represents the manifestation of polyiodide species (I− and I3−) along with molecular iodine (I2).77 Furthermore, the slight shifts in the binding energies of all the copolymers’ constituting elements after iodine adsorption suggest that the adsorption affinity of the latter is prompted not only by the copolymers’ surface areas but also by the presence of active binding sites.31
 | ||
| Fig. 6 XPS analysis spectra of TAC3 prior and post iodine capture (a) and high resolution (HR)-XPS spectra of I3d of TAC3@I2 (b). | ||
Experimental tests were conducted to investigate the mechanism of iodine adsorption by TAC1–3 through kinetic studies using pseudo-1st-order, pseudo-2nd-order, and intra-particle diffusion models, as outlined in eqn (2)–(4). Fig. 7 presents the calculated equilibrium uptake capacity (qe,cal) for the pseudo-1st-order model, obtained by plotting ln(qe − qt) against time (t). Conversely, the qe,cal values for the pseudo-2nd-order model were determined by plotting t/qtvs. time (t) for TAC3. Table 2 summarizes the iodine adsorption data for TAC3 based on both models, clearly showing a higher correlation coefficient (R2) of 0.9784 for the pseudo-2nd-order model, compared to 0.9595 for the pseudo-1st-order model. The correlation coefficient obtained from the intra-particle diffusion model for TAC3 (R2 = 0.9595) appears to be lower than that of the pseudo-2nd-order kinetic model (Fig. S15 in the ESI†). This clearly indicates a better fit of the experimental data with the pseudo-2nd-order model, especially when the experimental iodine adsorption capacity at equilibrium (qe,exp) of 15 g g−1 is benchmarked against the calculated value (qe,cal) of 14.3 g g−1 from the pseudo-2nd-order model. These results strongly suggest that iodine adsorption by TAC3 follows a pseudo-2nd-order kinetic model. Likewise, the other copolymers, TAC1 and TAC2, were also found to conform to the pseudo-2nd-order kinetic model (Table 2 and Fig. S13–S15 in the ESI†). This finding proposes an adsorption behavior that is strongly influenced by the prerequisite for the adsorbate and adsorbent-free sites to interact in order for the uptake to take place, thus leading to a relatively longer time for the process, which affects the iodine sorption overall rate.78
| Polymer | q e,exp (g g−1) | Pseudo 1st order model | Pseudo 2nd order model | ||||
|---|---|---|---|---|---|---|---|
| q e,cal (g g−1) | k 1 (h−1) | R 2 | q e,cal (g g−1) | k 2 (h−1) | R 2 | ||
| TAC1@I2 | 8.6 | 5.4 | −5.7 × 10−4 | 9.49 × 10−1 | 8.3 | 1.4 × 10−4 | 9.93 × 10−1 |
| TAC2@I2 | 4.9 | 3.1 | −9.9 × 10−3 | 9.69 × 10−1 | 5.0 | 2.0 × 10−4 | 9.93 × 10−1 |
| TAC3@I2 | 15 | 11.8 | −2.0 × 10−4 | 9.59 × 10−1 | 14.3 | 9.8 × 10−6 | 9.78 × 10−1 |
Desorption trials were made by exposing the iodine-loaded copolymers TAC1–3@I2 to heat (120 °C), as shown in Fig. 4 and outlined in Table 1. This method lead to the release of ∼98% of the captured iodine in the very first 6 hours followed by achieving quantitative desorption after 24 hours of continuous heating. Further investigation into iodine desorption was conducted by immersing the TAC3@I2 copolymer in ethanol (EtOH), a well-known solvent of I2, which released the latter from TAC3@I2 as proven from the UV-Vis spectra recorded for ethanolic solution at various time periods (Fig. 8).79 Most of the adsorbed iodine was released in the ethanol medium during the first 40 minutes of immersion followed by a slower release rate attaining equilibrium after 1 hour. These results emphasize the ease with which TAC3 can be regenerated, either by heating or immersion in ethanol, hence underscoring its potential usage as a renewable material.
Investigation of TAC3 regeneration was examined by heating the copolymer TAC3@I2 to 120 °C for one day to completely release the captured iodine and isolate the regenerated copolymer TAC3(R), which was subsequently exposed to iodine vapors before measuring its uptake capacity employing the previously described gravimetric method. This capture-release cyclic procedure was repeated using the same copolymer sample for six consecutive times showing only a minor drop of 3.8%, which has proven the sturdy iodine capacity of TAC3 (Fig. 9).
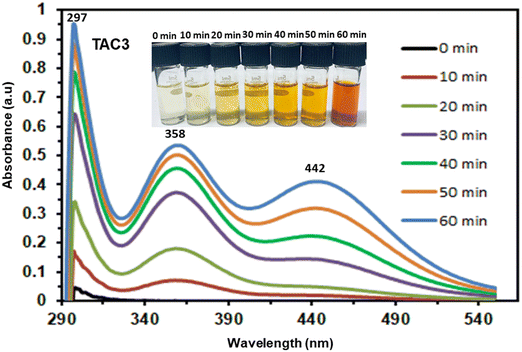
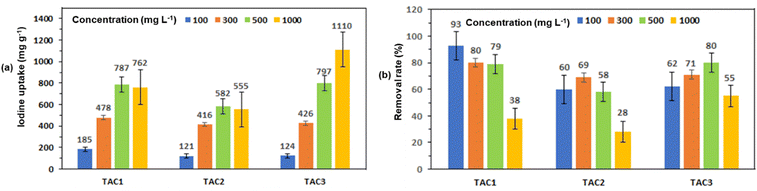
The iodine adsorption efficiency of copolymers TAC1–3 in cyclohexane mixtures was studied at various dilutions (100 mg L−1, 300 mg L−1, 500 mg L−1 and 1000 mg L−1) during 24 hours (Fig. 10, 11, and Fig. S16 in the ESI†). The mother liquors’ absorbance was monitored with a UV spectrophotometer, to determine the amount of iodine absorbed by TAC1–3 against a standardized absorption spectrum, as shown in Fig. 10(a)–(d). Fig. 11(a) depicts the quantity of iodine adsorbed for TAC1, TAC2, and TAC3 from 100 mg L−1 iodine in cyclohexane preliminary concentration, revealing uptake capacities of 185 mg g−1, 121 mg g−1, and 124 mg g−1, respectively. When testing the copolymers’ iodine adsorption capacity from 300 mg L−1 of I2 in cyclohexane, the adsorbed amounts were found to be 478 mg g−1, 416 mg g−1, and 426 mg g−1 for TAC1, TAC2, and TAC3, respectively. Similarly, when using an initial concentration of 500 mg L−1, the adsorption amounts were 787 mg g−1, 582 mg g−1, and 797 mg g−1 for TAC1, TAC2, and TAC3, respectively. Furthermore, when using a solution with an initial concentration of 1000 mg L−1, the adsorption amounts were found to be 762 mg g−1, 555 mg g−1, and 1110 mg g−1 for TAC1, TAC2, and TAC3, respectively. Fig. 11(b) illustrates the iodine elimination efficacy of TAC samples in the different iodine in cyclohexane solutions (i.e., 100 mg L−1, 300 mg L−1,500 mg L−1 and 1000 mg L−1), where TAC1 exhibited removal efficiencies of 93%, 80%,79% and 38% at different dilutions, whereas TAC2 portrayed efficiencies of 60%, 69%, 58% and 28%. Likewise, TAC3 displayed exclusion efficiencies of 62%, 71%, 80% and 55% for iodine in cyclohexane solutions whose concentrations ranged from 100 mg L−1 to 1000 mg L−1, as shown in Table 3. The differences in iodine adsorption and removal rates among TAC1, TAC2, and TAC3 can be attributed to their structural properties, the distribution of active sites, and the initial iodine concentration. TAC3 appears to be the most efficient polymer for iodine adsorption from cyclohexane solutions, especially at higher concentrations, due to its superior structural characteristics and higher number of active sites. However, beyond 1000 mg L−1 concentrations, the removal efficiency of TAC3 drops significantly, indicating saturation of the active sites.22,80TAC3 showed the maximum iodine uptake capacity of 1110 mg g−1 at a concentration of 1000 mg L−1 (Fig. 11(a)), which highlights the copolymer's exceptional ability to remove iodine from cyclohexane solutions, surpassing other adsorptive materials reported in the literature (Table S3, ESI†).15,81–85
 | ||
| Fig. 11 The amount of iodine adsorbed (a) and removed (b) by TAC1–3 from cyclohexane solutions with various iodine concentrations. | ||
| Polymer | Initial concentration (mg L−1) | Adsorption capacity (mg g−1) | Removal efficiency (%) |
|---|---|---|---|
| TAC1 | 100 | 185 | 93 |
| 300 | 478 | 80 | |
| 500 | 787 | 79 | |
| 1000 | 762 | 38 | |
| TAC2 | 100 | 121 | 60 |
| 300 | 416 | 69 | |
| 500 | 582 | 58 | |
| 1000 | 555 | 28 | |
| TAC3 | 100 | 124 | 62 |
| 300 | 426 | 71 | |
| 500 | 797 | 80 | |
| 1000 | 1110 | 55 |
The remarkable iodine adsorption performance of TAC1–3 from the vapor phase and cyclohexane prompted testing their ability to capture I2 from aqueous media. Therefore, the copolymers were added to 1 mM solutions of iodine in water and their uptake capacities were measured by UV/Vis spectroscopic analysis (Fig. 12a–c), which revealed efficient iodine adsorption within the first 30 minutes. It is worthwhile to note that the aqueous solution color changed from dark yellow to colorless (inset of Fig. 12d). The iodine uptake reached equilibrium within 24 hours and the maximum I2 uptake capacities recorded for TAC1, TAC2, and TAC3 were found to be 4.85 g g−1, 4.58 g g−1, and 5.95 g g−1, respectively (Table 4 and Fig. 12). This exceptional iodine (I2) adsorption from aqueous solution for TAC3 is very promising, especially when benchmarked against other adsorbents reported in the literature (Table S4, ESI†).29,68,86–88
| Entry | Polymer | I2 adsorption after 24 h (g g−1) |
|---|---|---|
| 1 | TAC1 | 4.85 |
| 2 | TAC2 | 4.58 |
| 3 | TAC3 | 5.95 |
In addition to neutral I2, both iodide (I−) and triiodide (I3−) are two significant anionic iodine species. In aqueous media, I2 tends to form triiodide (I3−) species by the dynamic equilibrium I2 + I− ⇄ I3−, especially when I− is present. Therefore, the adsorption of triiodide is crucial for effective iodine remediation. To evaluate the effectiveness of the copolymers TAC1–3 in adsorbing I3− (also referred to as I2/I−), adsorption experiments were conducted by immersing the copolymers in a KI3 solution, which was prepared by dissolving 300 mg KI and 150 mg I2 in 100 mL of water, for 48 hours and the iodine removal was monitored by UV-Vis spectroscopy (Fig. 13a–c). Interestingly, the iodine uptake capacities were found to be 4.89 g g−1 for TAC1, 3.90 g g−1 for TAC2, and 5.34 g g−1 for TAC3 (Table 5 and Fig. 13d). These results demonstrate the exceptional ability of copolymers TAC1–3 to adsorb I3− from aqueous media, surpassing the performance of other materials reported in the literature (Table S5, ESI†).89–94
| Entry | Copolymer | I3− adsorption after 24 h (g g−1) |
|---|---|---|
| 1 | TAC1 | 4.89 |
| 2 | TAC2 | 3.90 |
| 3 | TAC3 | 5.34 |
Conclusion
In summary, we have successfully synthesized a novel class of 3D metal–organic copolymers, TAC1–3, featuring Fe(II) clathrochelate units with various secondary arylamine linkages, demonstrating strong potential for sustainable applications. Iodine gas adsorption findings revealed an impressive sorption capacity of 1500 wt% and conformed to a pseudo-2nd-order kinetic model. Additionally, these copolymers demonstrated significant iodine uptake in cyclohexane, achieving an extreme adsorption capacity of 1.11 g g−1 at an initial iodine concentration of 1000 mg L−1. Regeneration tests confirmed the efficient recyclability of TAC3, retaining high performance across six successive adsorption–desorption cycles. In aqueous media, the copolymers also showed promising iodine adsorption, with capacities reaching 5.95 g g−1 in I2 solutions and 5.34 g g−1 in I3− (KI/I2) solutions. These conjugated copolymers offer multiple benefits, including ease of synthesis, outstanding chemical and thermal stability, and substantial potential for environmental remediation, as evidenced by their high iodine adsorption capacities.Data availability
On behalf of my co-authors, I hereby attest that the datasets generated and analyzed during the current study are available from the corresponding author upon request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The project was partially supported by the Kuwait Foundation for the Advancement of Sciences (KFAS) under project code PN18-14SC-03.References
- N. N. Ponomarev-Stepnoi, V. V. Kuznetsov, A. Y. Gagarinskii, E. J. Moniz, R. Gottemoeller and D. Poneman, The Future of Nuclear Power: Energy, Ecology, and Safety, At. Energy, 2002, 93(5), 855–865, DOI:10.1023/A:1022400419098.
- U. Davies, T. Kunchev, P. Cosgrove, N. Read, M. Kowalski and E. Shwageraus, Nuclear energy: Between global electricity demand, worldwide decarbonisation imperativeness, and planetary environmental implications, J. Environ. Manage., 2019, 249, 109208, DOI:10.1016/j.jenvman.2019.06.109.
- A. Saiz-Lopez, J. M. C. Plane, A. R. Baker, L. J. Carpenter, R. von Glasow, J. C. Gómez Martín, G. McFiggans and R. W. Saunders, Atmospheric Chemistry of Iodine, Chem. Rev., 2012, 112(3), 1773–1804, DOI:10.1021/cr200029u.
- F. C. Küpper, M. C. Feiters, B. Olofsson, T. Kaiho, S. Yanagida, M. B. Zimmermann, L. J. Carpenter, G. W. Luther Iii, Z. Lu and M. Jonsson, et al., Commemorating Two Centuries of Iodine Research: An Interdisciplinary Overview of Current Research, Angew. Chem., Int. Ed., 2011, 50(49), 11598–11620, DOI:10.1002/anie.201100028.
- L. Thurakkal, S. R. Cheekatla and M. Porel, Superfast Capture of Iodine from Air, Water, and Organic Solvent by Potential Dithiocarbamate-Based Organic Polymer, Int. J. Mol. Sci., 2023, 24(2), 1466, DOI:10.3390/ijms24021466.
- M. Little, G. Kendall, A. Bouville and S. Simon, Measurement of Fukushima-related radioactive contamination in aquatic species, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 3720–3721, DOI:10.1073/pnas.1602648113.
- J. Zhou, T. Lan, T. Li, Q. Chen, P. Bai, F. Liu, Z. Yuan, W. Zheng, X. Luo and W. Yan, et al., Highly efficient capture of iodine in spent fuel reprocessing off-gas by novelly porous copper-doped silica zeolites, Sep. Purif. Technol., 2022, 290, 120895, DOI:10.1016/j.seppur.2022.120895.
- H. Xiao, H. Zhou, S. Feng, D. B. Gore, Z. Zhong and W. Xing, In situ growth of two-dimensional ZIF-L nanoflakes on ceramic membrane for efficient removal of iodine, J. Membr. Sci., 2021, 619, 118782, DOI:10.1016/j.memsci.2020.118782.
- R. Gao, Y. Lu, S. Xiao and J. Li, Facile Fabrication of Nanofibrillated Chitin/Ag2O Heterostructured Aerogels with High Iodine Capture Efficiency, Sci. Rep., 2017, 7(1), 4303, DOI:10.1038/s41598-017-04436-8.
- A. P. Katsoulidis, J. He and M. G. Kanatzidis, Functional Monolithic Polymeric Organic Framework Aerogel as Reducing and Hosting Media for Ag nanoparticles and Application in Capturing of Iodine Vapors, Chem. Mater., 2012, 24(10), 1937–1943, DOI:10.1021/cm300696g.
- X. Zhang, J. Maddock, T. M. Nenoff, M. A. Denecke, S. Yang and M. Schröder, Adsorption of iodine in metal–organic framework materials, Chem. Soc. Rev., 2022, 51(8), 3243–3262, 10.1039/D0CS01192D.
- D. F. Sava, M. A. Rodriguez, K. W. Chapman, P. J. Chupas, J. A. Greathouse, P. S. Crozier and T. M. Nenoff, Capture of volatile iodine, a gaseous fission product, by zeolitic imidazolate framework-8, J. Am. Chem. Soc., 2011, 133(32), 12398–12401, DOI:10.1021/ja204757x.
- Z. Yin, Q.-X. Wang and M.-H. Zeng, Iodine Release and Recovery, Influence of Polyiodide Anions on Electrical Conductivity and Nonlinear Optical Activity in an Interdigitated and Interpenetrated Bipillared-Bilayer Metal–Organic Framework, J. Am. Chem. Soc., 2012, 134(10), 4857–4863, DOI:10.1021/ja211381e.
- P. Wang, Q. Xu, Z. Li, W. Jiang, Q. Jiang and D. Jiang, Exceptional Iodine Capture in 2D Covalent Organic Frameworks, Adv. Mater., 2018, 30(29), 1801991, DOI:10.1002/adma.201801991.
- S. Huang, J. Y. Choi, Q. Xu, Y. Jin, J. Park and W. Zhang, Carbazolylene-Ethynylene Macrocycle based Conductive Covalent Organic Frameworks, Angew. Chem., Int. Ed., 2023, 62(22), e202303538, DOI:10.1002/anie.202303538.
- J. Zhou, S. Hao, L. Gao and Y. Zhang, Study on Adsorption Performance of Coal Based Activated Carbon to Radioactive Iodine and Stable Iodine, Ann. Nucl. Energy, 2014, 72, 237–241, DOI:10.1016/j.anucene.2014.05.028.
- K. Su, W. Wang, B. Li and D. Yuan, Azo-Bridged Calix[4]resorcinarene-Based Porous Organic Frameworks with Highly Efficient Enrichment of Volatile Iodine, ACS Sustainable Chem. Eng., 2018, 6(12), 17402–17409, DOI:10.1021/acssuschemeng.8b05203.
- J. F. Kurisingal, H. Yun and C. S. Hong, Porous Organic Materials for Iodine Adsorption, J. Hazard. Mater., 2023, 458, 131835, DOI:10.1016/j.jhazmat.2023.131835.
- K. Jie, Y. Zhou, E. Li, Z. Li, R. Zhao and F. Huang, Reversible Iodine Capture by Nonporous Pillar[6]arene Crystals, J. Am. Chem. Soc., 2017, 139(43), 15320–15323, DOI:10.1021/jacs.7b09850.
- D. Luo, Y. He, J. Tian, J. L. Sessler and X. Chi, Reversible Iodine Capture by Nonporous Adaptive Crystals of a Bipyridine Cage, J. Am. Chem. Soc., 2022, 144(1), 113–117, DOI:10.1021/jacs.1c11731.
- S. Xiong, X. Tang, C. Pan, L. Li, J. Tang and G. Yu, Carbazole-Bearing Porous Organic Polymers with a Mulberry-Like Morphology for Efficient Iodine Capture, ACS Appl. Mater. Interfaces, 2019, 11(30), 27335–27342, DOI:10.1021/acsami.9b07679.
- X. Pan, C. Ding, Z. Zhang, H. Ke and G. Cheng, Functional Porous Organic Polymer with High S and N for Reversible Iodine Capture, Microporous Mesoporous Mater., 2020, 300, 110161, DOI:10.1016/j.micromeso.2020.110161.
- Y. Wu, Y. Guo, R. Su, X. Ma, Q. Wu, Z. Zeng, L. Li, X. Yao and S. Wang, Hierarchical Porous Carbon with an Ultrahigh Surface Area for High-efficient Iodine Capture: Insights into adsorption mechanisms through experiments, simulations and modeling, Sep. Purif. Technol., 2022, 303, 122237, DOI:10.1016/j.seppur.2022.122237.
- S. Pourebrahimi, M. Pirooz, A. De Visscher and G. H. Peslherbe, Highly Efficient and Reversible Iodine Capture Utilizing Amorphous Conjugated Covalent Triazine-based Porous Polymers: Experimental and computational studies, J. Environ. Chem. Eng., 2022, 10(3), 107805, DOI:10.1016/j.jece.2022.107805.
- L. Zhang, Y.-T. Luo, J.-Q. Fan, S.-J. Xiao, Q.-Q. Zheng, X.-L. Liu, Q.-G. Tan, C. Sun, Q. Shi and R.-P. Liang, et al., Efficient Capture of Iodine in Steam and Water Media by Hydrogen Bond-driven Charge Transfer Complexes, J. Hazard. Mater., 2024, 465, 133488, DOI:10.1016/j.jhazmat.2024.133488.
- J. Huve, A. Ryzhikov, H. Nouali, V. Lalia, G. Augé and T. J. Daou, Porous Sorbents for the Capture of Radioactive Iodine Compounds: a review, RSC Adv., 2018, 8(51), 29248–29273, 10.1039/C8RA04775H.
- T. J. Robshaw, J. Turner, S. Kearney, B. Walkley, C. A. Sharrad and M. D. Ogden, Capture of Aqueous Radioiodine Species by Metallated Adsorbents from Wastestreams of the Nuclear Power Industry: a review, SN Appl. Sci., 2021, 3(11), 843, DOI:10.1007/s42452-021-04818-8.
- Y.-N. Yu, Z. Yin, L.-H. Cao and Y.-M. Ma, Organic Porous Solid as Promising Iodine Capture Materials, J. Inclusion Phenom. Macrocyclic Chem., 2022, 102, 1–33, DOI:10.1007/s10847-022-01128-3.
- X. Li, Z. Jia, J. Zhang, Y. Zou, B. Jiang, Y. Zhang, K. Shu, N. Liu, Y. Li and L. Ma, Moderate and Universal Synthesis of Undoped Covalent Organic Framework Aerogels for Enhanced Iodine Uptake, Chem. Mater., 2022, 34(24), 11062–11071, DOI:10.1021/acs.chemmater.2c03117.
- X. Yang, C. Li, M. Giorgi, D. Siri, X. Bugaut, B. Chatelet, D. Gigmes, M. Yemloul, V. Hornebecq and A. Kermagoret, et al., Energy-Efficient Iodine Uptake by a Molecular Host Guest Crystal, Angew. Chem., Int. Ed., 2022, 61(49), e202214039, DOI:10.1002/anie.202214039.
- W. Zhou, A. Li, M. Zhou, Y. Xu, Y. Zhang and Q. He, Nonporous Amorphous Superadsorbents for Highly Effective and Selective Adsorption of Iodine in Water, Nat. Commun., 2023, 14(1), 5388, DOI:10.1038/s41467-023-41056-5.
- Y. Lin, X. Jiang, S. T. Kim, S. B. Alahakoon, X. Hou, Z. Zhang, C. M. Thompson, R. A. Smaldone and C. Ke, An Elastic Hydrogen-Bonded Cross-Linked Organic Framework for Effective Iodine Capture in Water, J. Am. Chem. Soc., 2017, 139(21), 7172–7175, DOI:10.1021/jacs.7b03204.
- A. Gogia, P. Das and S. Mandal, Tunable Strategies Involving Flexibility and Angularity of Dual Linkers for a 3D Metal-Organic Framework Capable of Multimedia Iodine Capture, ACS Appl. Mater. Interfaces, 2020, 12(41), 46107–46118, DOI:10.1021/acsami.0c13094.
- A. Sen, S. Sharma, S. Dutta, M. M. Shirolkar, G. K. Dam, S. Let and S. K. Ghosh, Functionalized Ionic Porous Organic Polymers Exhibiting High Iodine Uptake from Both the Vapor and Aqueous Medium, ACS Appl. Mater. Interfaces, 2021, 13(29), 34188–34196, DOI:10.1021/acsami.1c07178.
- D. An, L. Li, Z. Zhang, A. M. Asiri, K. A. Alamry and X. Zhang, Amino-bridged Covalent Organic Polycalix[4]arenes for Ultra Efficient Adsorption of Iodine in Water, Mater. Chem. Phys., 2020, 239, 122328, DOI:10.1016/j.matchemphys.2019.122328.
- Z. Chen, K. B. Idrees, S. Shetty, H. Xie, M. C. Wasson, W. Gong, X. Zhang, B. Alameddine and O. K. Farha, Regulation of Catenation in Metal–Organic Frameworks with Tunable Clathrochelate-Based Building Blocks, Cryst. Growth Des., 2021, 21(12), 6665–6670, DOI:10.1021/acs.cgd.1c01151.
- B. Alameddine, S. Shetty, R. S. Anju, F. Al-Sagheer and S. Al-Mousawi, Highly Soluble Metal-organic Polymers Based on Iron(II) clathrochelates and their Gelation Induced by Sonication, Eur. Polym. J., 2017, 95, 566–574, DOI:10.1016/j.eurpolymj.2017.08.049.
- S. Shetty, N. Baig, S. Al-Mousawi, F. Al-Sagheer and B. Alameddine, Synthesis of Secondary Arylamine Copolymers with Iron(II) Clathrochelate Units and their Functionalization into Tertiary Polyarylamines via Buchwald–Hartwig Cross-coupling reaction, Polymer, 2019, 178, 121606, DOI:10.1016/j.polymer.2019.121606.
- S. M. Jansze and K. Severin, Clathrochelate Metalloligands in Supramolecular Chemistry and Materials Science, Acc. Chem. Res., 2018, 51(9), 2139–2147, DOI:10.1021/acs.accounts.8b00306.
- O. A. Varzatskii, I. N. Denisenko, S. V. Volkov, A. V. Dolganov, A. V. Vologzhanina, Y. N. Bubnov and Y. Z. Voloshin, First Example of Perfluoroalkylation of a Quasi-aromatic Encapsulating ligand: 2,5-Dithiahexane-assisted Reaction of the Iron(II) diiodoclathrochelate with Trifluoromethylcopper(I), Inorg. Chem. Commun., 2013, 33, 147–150, DOI:10.1016/j.inoche.2013.04.024.
- B. Alameddine, S. Shetty, N. Baig, S. Al-Mousawi and F. Al-Sagheer, Synthesis and Characterization of Metalorganic Polymers of Intrinsic Microporosity Based on Iron(II) Clathrochelate, Polymer, 2017, 122, 200–207, DOI:10.1016/j.polymer.2017.06.048.
- S. Shetty, K. B. Idrees, H. Xie, B. Alameddine and O. K. Farha, Synthesis of Zirconium-based Metal–organic Frameworks with Iron(II) Clathrochelate Ligands, CrystEngComm, 2023, 25(10), 1550–1555, 10.1039/D2CE01686A.
- S. Shetty, N. Baig, A. Hassan, S. Al-Mousawi, N. Das and B. Alameddine, Fluorinated Iron(II) Clathrochelate Units in Metalorganic Based Copolymers: Improved Porosity, Iodine Uptake, and Dye Adsorption Properties, RSC Adv., 2021, 11(25), 14986–14995, 10.1039/D1RA02357H.
- S. Shetty, N. Baig, S. Al-Mousawi and B. Alameddine, Removal of Anionic and Cationic Dyes Using Porous Copolymer Networks Made from a Sonogashira Cross-coupling Reaction of Diethynyl Iron(II) clathrochelate with Various Arylamines, J. Appl. Polym. Sci., 2022, 139(43), e52966, DOI:10.1002/app.52966.
- W. Gong, Y. Xie, T. D. Pham, S. Shetty, F. A. Son, K. B. Idrees, Z. Chen, H. Xie, Y. Liu and R. Q. Snurr, et al., Creating Optimal Pockets in a Clathrochelate-Based Metal–Organic Framework for Gas Adsorption and Separation: Experimental and Computational Studies, J. Am. Chem. Soc., 2022, 144(8), 3737–3745, DOI:10.1021/jacs.2c00011.
- N. Baig, S. Shetty, R. Bargakshatriya, S. K. Pramanik and B. Alameddine, Efficient Removal of Carcinogenic Azo Dyes from Water Using Iron(II) Clathrochelate Derived Metalorganic Copolymers Made from a Copper-Catalyzed [4+2] Cyclobenzannulation Reaction, Polymers, 2023, 15(13), 2948, DOI:10.3390/polym15132948.
- S. Shetty, N. Baig, M. Bechelany and B. Alameddine, Construction of Polyimide Structures Containing Iron(II) Clathrochelate Intercalators: Promising Materials for CO2 Gas Uptake and Salient Adsorbents of Iodine from Gaseous and Liquid Phases, J. Mater. Chem. A, 2024, 12(23), 14059–14071, 10.1039/D4TA02194K.
- C. Zhao, L. Ge, M. Zuo, L. Mai, S. Chen, X. Li, Q. Li, Y. Wang and C. Xu, Study on the mechanical strength and iodine adsorption behavior of coal-based activated carbon based on orthogonal experiments, Energy, 2023, 282, 128450, DOI:10.1016/j.energy.2023.128450.
- J. Cheng, B. Gao, H. Tang, Z. Sun, L. Xu, L. Wang and D. Cao, Hexnut[12]arene and its derivatives: Synthesis, host-guest properties, and application as nonporous adaptive crystals, Sci. China:Chem., 2022, 65(3), 539–545, DOI:10.1007/s11426-021-1186-2.
- A. Mohan, M. Al-Sayah, A. Ahmed and O. El-Kadri, Triazine-based porous organic polymers for reversible capture of iodine and utilization in antibacterial application, Sci. Rep., 2022, 12, 2638, DOI:10.1038/s41598-022-06671-0.
- Y. Matsumoto and K. Honma, NH stretching vibrations of pyrrole clusters studied by infrared cavity ringdown spectroscopy, J. Chem. Phys., 2007, 127, 184310, DOI:10.1063/1.2790894.
- S. Shetty, N. Baig, D. Sengupta, O. K. Farha and B. Alameddine, Tröger's Base-Enriched Conjugated Cyclopentannulated Copolymers: Prominent Adsorbents of CO2, H2, and Iodine, ACS Appl. Mater. Interfaces, 2024, 16(6), 8130–8139, DOI:10.1021/acsami.3c18055.
- M. Tommasini, A. Lucotti, M. Alfè, A. Ciajolo and G. Zerbi, Fingerprints of polycyclic aromatic hydrocarbons (PAHs) in infrared absorption spectroscopy, Spectrochim. Acta, Part A, 2016, 152, 134–148, DOI:10.1016/j.saa.2015.07.070.
- M. Slaný, L. Jankovič and J. Madejová, Near-IR study of the impact of alkyl-ammonium and -phosphonium cations on the hydration of montmorillonite, J. Mol. Struct., 2022, 1256, 132568, DOI:10.1016/j.molstruc.2022.132568.
- L. Rintoul, A. S. Micallef and S. E. Bottle, The vibrational group frequency of the N–O stretching band of nitroxide stable free radicals, Spectrochim. Acta, Part A, 2008, 70(4), 713–717, DOI:10.1016/j.saa.2007.08.017.
- N. Baig, S. Shetty, S. S. Habib, A. A. Husain, S. Al-Mousawi and B. Alameddine, Synthesis of Iron(II) Clathrochelate-Based Poly(vinylene sulfide) with Tetraphenylbenzene Bridging Units and Their Selective Oxidation into Their Corresponding Poly(vinylene sulfone) Copolymers: Promising Materials for Iodine Capture, Polymers, 2022, 14(18), 3727, DOI:10.3390/polym14183727.
- O. Yildirim, A. Tsaturyan, A. Damin, S. Nejrotti, V. Crocellà, A. Gallo, M. R. Chierotti, M. Bonomo and C. Barolo, Quinoid-Thiophene-Based Covalent Organic Polymers for High Iodine Uptake: When Rational Chemical Design Counterbalances the Low Surface Area and Pore Volume, ACS Appl. Mater. Interfaces, 2023, 15(12), 15819–15831, DOI:10.1021/acsami.2c20853.
- S. S. AlNeyadi, M. T. Alhassani, A. S. Aleissaee, Sultan J., A. H. Khalaf, A. A. Alteneij and Y. Y. Alyaarbi, Synthesizing covalent organic frameworks for unprecedented iodine capture performance, Heliyon, 2024, 10(4), e25921, DOI:10.1016/j.heliyon.2024.e25921.
- Y. Xie, T. Pan, Q. Lei, C. Chen, X. Dong, Y. Youyou, J. Shen, Y. Cai, C. Zhou and I. Pinnau, et al., Ionic Functionalization of Multivariate Covalent Organic Frameworks to Achieve Exceptionally High Iodine Capture Capacity, Angew. Chem., 2021, 133(41), 22606–22614, DOI:10.1002/ange.202108522.
- J. Wang, T. Wu, X. Wang, J. Chen, M. Fan, Z. Shi, J. Liu, L. Xu and Y. Zang, Construction of hydroxyl-functionalized hyper-crosslinked networks from polyimide for highly efficient iodine adsorption, iScience, 2024, 27(2), 108993, DOI:10.1016/j.isci.2024.108993.
- C. Li, Q. Yan, H. Xu, S. Luo, H. Hu, S. Wang, X. Su, S. Xiao and Y. Gao, Highly Efficient Capture of Volatile Iodine by Conjugated Microporous Polymers Constructed Using Planar 3- and 4-Connected Organic Monomers, Molecules, 2024, 29(10), 2242, DOI:10.3390/molecules29102242.
- F. Begar, M. Erdogmus, Y. Gecalp, U. C. Canakci and O. Buyukcakir, Synthesis of Triazole-Linked Porous Cage Polymers: Modulating Cage Size for Tailored Iodine Adsorption, ACS Appl. Polym. Mater., 2024, 6(9), 5358–5365, DOI:10.1021/acsapm.4c00560.
- S. Luo, Q. Yan, S. Wang, H. Hu, S. Xiao, X. Su, H. Xu and Y. Gao, Conjugated Microporous Polymers Based on Octet and Tetratopic Linkers for Efficient Iodine Capture, ACS Appl. Mater. Interfaces, 2023, 15(39), 46408–46416, DOI:10.1021/acsami.3c10786.
- C.-H. Zhang, B.-X. Zhou, X. Lin, J.-X. Wu, L.-H. Wu, S. Cai, J. Fan, W.-G. Zhang, Y. Yan and S.-R. Zheng, High-Capacity Iodine Adsorption and Nonporous to Porous Structural Transformation in an Originally Nonporous Coordination Polymer, Inorg. Chem. Front., 2024, 11(3), 769–778, 10.1039/D3QI01881D.
- H. Zhu, Y. Qin, Y. Guo, Z. Shen, M. Imran, M. Asim Mushtaq, Z. Zhang, C. Ni, Y. Chen and Y. Ding, et al., Covalent organic polymers for efficient removal of iodine from gas- and liquid-phase environments, Chem. Eng. J., 2024, 484, 149739, DOI:10.1016/j.cej.2024.149739.
- B. Liu, C. Mao, Z. Zhou, Q. Wang, X. Zhou, Z. Liao, R. Deng, D. Liu, J. Beiyuan and D. Lv, et al., Two Facile Aniline-Based Hypercrosslinked Polymer Adsorbents for Highly Efficient Iodine Capture and Removal, Int. J. Mol. Sci., 2023, 24(1), 370, DOI:10.3390/ijms24010370.
- Y. H. Abdelmoaty, T. D. Tessema, F. A. Choudhury, O. M. El-Kadri and H. M. El-Kaderi, Nitrogen-Rich Porous Polymers for Carbon Dioxide and Iodine Sequestration for Environmental Remediation, ACS Appl. Mater. Interfaces, 2018, 10(18), 16049–16058, DOI:10.1021/acsami.8b03772.
- N. Arora, T. Debnath, M. C. Senarathna, R. M. Johnson, I. G. Roske, G. A. Cisneros and R. A. Smaldone, Rapid, high-capacity adsorption of iodine from aqueous environments with amide functionalized covalent organic frameworks, Chem. Sci., 2024, 15(10), 3571–3577, 10.1039/D3SC06004G.
- A. Mohan, M. H. Al-Sayah, A. Ahmed and O. M. El-Kadri, Triazine-based porous organic polymers for reversible capture of iodine and utilization in antibacterial application, Sci. Rep., 2022, 12(1), 2638, DOI:10.1038/s41598-022-06671-0.
- Z. Yan, Y. Yuan, Y. Tian, D. Zhang and G. Zhu, Highly Efficient Enrichment of Volatile Iodine by Charged Porous Aromatic Frameworks with Three Sorption Sites, Angew. Chem., Int. Ed., 2015, 54(43), 12733–12737, DOI:10.1002/anie.201503362.
- M. Ansari, A. Alam, R. Bera, A. Hassan, S. Goswami and N. Das, Synthesis, characterization and adsorption studies of a novel triptycene based hydroxyl azo- nanoporous polymer for environmental remediation, J. Environ. Chem. Eng., 2020, 8(2), 103558, DOI:10.1016/j.jece.2019.103558.
- B. Liu, C. Mao, Z. Zhou, Q. Wang, X. Zhou, Z. Liao, R. Deng, D. Liu, J. Beiyuan and D. Lv, et al., Two Facile Aniline-Based Hypercrosslinked Polymer Adsorbents for Highly Efficient Iodine Capture and Removal, Int. J. Mol. Sci., 2022, 24(1), 370, DOI:10.3390/ijms24010370.
- T. Geng, M. Liu, C. Zhang, C. Hu and H. Xu, Synthesis of secondary amine-based fluorescent porous organic polymers via Friedel–Crafts polymerization reaction for adsorbing and fluorescent sensing iodine, J. Appl. Polym. Sci., 2020, 137(41), 49255, DOI:10.1002/app.49255.
- S. Pourebrahimi and M. Pirooz, Functionalized covalent triazine frameworks as promising platforms for environmental remediation: A review, Cleaner Chem. Eng., 2022, 2, 100012, DOI:10.1016/j.clce.2022.100012.
- C. Feng, G. Xu, W. Xie, S. Zhang, C. Yao and Y. Xu, Polytriazine porous networks for effective iodine capture, Polym. Chem., 2020, 11(16), 2786–2790, 10.1039/C9PY01948K.
- H. Zuo, W. Lyu, W. Zhang, Y. Li and Y. Liao, High-Yield Synthesis of Pyridyl Conjugated Microporous Polymer Networks with Large Surface Areas: From Molecular Iodine Capture to Metal-Free Heterogeneous Catalysis, Macromol. Rapid Commun., 2020, 41(22), 2000489, DOI:10.1002/marc.202000489.
- A. Hassan, A. Alam, S. Chandra, O. Prince and N. Das, Triptycene-based and imine linked porous uniform microspheres for efficient and reversible scavenging of iodine from various media: a systematic study, Environ. Sci.:Adv., 2022, 1(3), 320–330, 10.1039/D2VA00024E.
- T. Wang, M. Jiang, X. Yu, N. Niu and L. Chen, Application of lignin adsorbent in wastewater Treatment: A review, Sep. Purif. Technol., 2022, 302, 122116, DOI:10.1016/j.seppur.2022.122116.
- Z.-b Liu, J. Tian, W. Zang, W.-Y. Zhou, F. Song, C.-P. Zhang, J.-Y. Zheng and H. Xu, Flexible alteration of optical nonlinearities of iodine charge-transfer complexes in solutions, Opt. Lett., 2004, 29, 1099–1101, DOI:10.1364/OL.29.001099.
- Y. Ji, J. Huang, C. Liu, C. Ni, H. Yan, Y. David and Y. Qin, Introgression of Lone Electron Pairs into π-Conjugated Structures Resulting in Giant Electron-Rich Clusters with High Trapping Capacity for Iodine Molecules, ACS Appl. Polym. Mater., 2024, 6(13), 7478–7487, DOI:10.1021/acsapm.4c00816.
- H. Fu, Y. Tang, Q. Yuan, J. Chang, F. Liao, J. Zhang, H. Gao, Y. Yang and Y. Liao, Understanding iodine adsorption sites on monolithic N/O co-doped carbon fibers with scaffolding structure, Fuel, 2024, 371, 132035, DOI:10.1016/j.fuel.2024.132035.
- S. Bera, K. Garg and S. K. Samanta, Amide-Linked cis,cis-1,3,5-Cyclohexanetricarbonyl-Based Porous Organic Polymers for the Uptake of Iodine in Solution and Vapor Phase, ACS Appl. Nano Mater., 2024, 7(2), 1797–1803, DOI:10.1021/acsanm.3c04973.
- L.-L. Li, M. Huang, T. Chen, X.-F. Xu, Z. Zhuo, W. Wang and Y.-G. Huang, A Porous π-Stacked Self-Assembly of Cup-Shaped Palladium Complex for Iodine Capture, Molecules, 2023, 28(7), 2881, DOI:10.3390/molecules28072881.
- F. Khosravi Esmaeiltarkhani, M. Dinari and N. Mokhtari, Nitrogen-rich porous organic polymer as a promising adsorbent for iodine capture from organic solvents, New J. Chem., 2024, 48(5), 1943–1951, 10.1039/D3NJ04674E.
- Q. Tao, X. Zhang, L. Jing, L. Sun and P. Dang, Construction of Ketoenamine-Based Covalent Organic Frameworks with Electron-Rich Sites for Efficient and Rapid Removal of Iodine from Solution, Molecules, 2023, 28(24), 8151, DOI:10.3390/molecules28248151.
- L. Xie, Z. Zheng, Q. Lin, H. Zhou, X. Ji, J. L. Sessler and H. Wang, Calix[4]pyrrole-based Crosslinked Polymer Networks for Highly Effective Iodine Adsorption from Water, Angew. Chem., Int. Ed., 2022, 61(1), e202113724, DOI:10.1002/anie.202113724.
- E. Yazdankish, M. Foroughi and M. H. A. Azqhandi, Capture of I131 from medical-based wastewater using the highly effective and recyclable adsorbent of g-C3N4 assembled with Mg-Co-Al-layered double hydroxide, J. Hazard. Mater., 2020, 389, 122151, DOI:10.1016/j.jhazmat.2020.122151.
- Y. Yin, Y. Yang, G. Liu, H. Chen, D. Gong, Y. Ying, J. Fan, S. Liu, Z. Li and C. Wang, et al., Ultrafast solid-phase synthesis of 2D pyrene-alkadiyne frameworks towards efficient capture of radioactive iodine, Chem. Eng. J., 2022, 441, 135996, DOI:10.1016/j.cej.2022.135996.
- M. Avais and S. Chattopadhyay, Porous polyaminoamides via an exotemplate synthesis approach for ultrahigh multimedia iodine adsorption, J. Mater. Chem. A, 2022, 10(37), 20090–20100, 10.1039/D2TA02708A.
- C. X. Yu, X. J. Li, J. S. Zong, D. J. You, A. P. Liang, Y. L. Zhou, X. Q. Li and L. L. Liu, Fabrication of Protonated Two-Dimensional Metal-Organic Framework Nanosheets for Highly Efficient Iodine Capture from Water, Inorg. Chem., 2022, 61(35), 13883–13892, DOI:10.1021/acs.inorgchem.2c01886.
- D. Chen, T. Ma, X. Zhao, X. Jing, R. Zhao and G. Zhu, Multi-Functionalization Integration into the Electrospun Nanofibers Exhibiting Effective Iodine Capture from Water, ACS Appl. Mater. Interfaces, 2022, 14(41), 47126–47135, DOI:10.1021/acsami.2c14724.
- X.-H. Xu, Y.-X. Li, L. Zhou, N. Liu and Z.-Q. Wu, Precise fabrication of porous polymer frameworks using rigid polyisocyanides as building blocks: from structural regulation to efficient iodine capture, Chem. Sci., 2022, 13(4), 1111–1118, 10.1039/D1SC05361B.
- P. Samanta, S. Dutta, S. Let, A. Sen, M. M. Shirolkar and S. K. Ghosh, Hydroxy-Functionalized Hypercrosslinked Polymers (HCPs) as Dual Phase Radioactive Iodine Scavengers: Synergy of Porosity and Functionality, ChemPlusChem, 2022, 87(11), e202200212, DOI:10.1002/cplu.202200212.
- M. Das, S. Sarkar, Y. Patra, A. Manna, S. Mukherjee and S. Das, Soft Self-Templating Approach-Derived Covalent Triazine Framework with Bimodal Nanoporosity for Efficient Radioactive Iodine Capture for Safe Nuclear Energy, ACS Appl. Nano Mater., 2022, 5(7), 8783–8793, DOI:10.1021/acsanm.2c00564.
Footnote |
| † Electronic supplementary information (ESI) available: Analysis of all compounds, and spectral data of NMR, FTIR, XPS, N2 adsorption, TGA, kinetic studies, proposed I2 adsorption mechanism and comparison of I2 capture with different adsorbent materials. See DOI: https://doi.org/10.1039/d5ma00061k |
| This journal is © The Royal Society of Chemistry 2025 |