Open Access Article
Open Access ArticleDopamine detection using leaf-shaped ZnO synthesized from zinc shells of recycled batteries
Md Yeasin
Pabel
a,
Md Humayun
Kabir
 *a,
Md. Sanwar
Hossain
a,
Fahima
Mojumder
a,
Sourav
Datta
a,
Muhammad Shahriar
Bashar
b and
Sabina
Yasmin
*a,
Md. Sanwar
Hossain
a,
Fahima
Mojumder
a,
Sourav
Datta
a,
Muhammad Shahriar
Bashar
b and
Sabina
Yasmin
 *a
*a
aInstitute of National Analytical Research and Service (INARS), Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhanmondi, Dhaka-1205, Bangladesh. E-mail: humayunkabir@bcsir.gov.bd; sabinayasmin@bcsir.gov.bd
bInstitute of Energy Research & Development (IERD), Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhanmondi, Dhaka-1205, Bangladesh
First published on 10th March 2025
Abstract
This study presents a cost-effective and sustainable method for synthesizing ZnO from electronic waste (e-waste) for the electrochemical detection of dopamine (DA) using a single metal oxide. The purity and various properties of the ZnO were confirmed using advanced techniques. The growth of ZnO clusters along the [0001] direction results in the formation of leaf-shaped ZnO with a direct band gap of 3.27 eV. Cyclic voltammetry, differential pulse voltammetry, and amperometric studies demonstrated successful DA detection at the ZnO–glassy carbon electrode in aqueous media, with an equal number of electron and proton pathways for DA oxidation. The sensor exhibited a linear response over a wide concentration range (0.01 to 100 μM), with a low limit of detection of 0.47 nM and a high sensitivity of 0.0389 A M−1. Additionally, the sensor showed high selectivity, repeatability and reproducibility with relative standard deviation of 4.80% in DA detection and proved effective in real sample analysis. These results suggest that the developed sensor holds great potential as a sensitive, practical, and cost-efficient tool for DA monitoring. Furthermore, the approach contributes to sustainable management of zinc–carbon battery waste by producing valuable ZnO.
1. Introduction
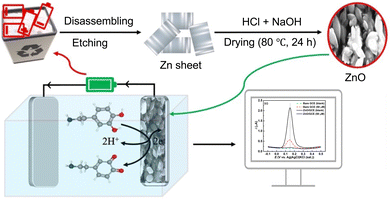
3,4-Dihydroxyphenethylamine, also known as dopamine (DA) and often referred to as the happiness molecule, functions as a neurotransmitter in the brain and nervous system, playing a vital role in regulating mood, motivation, reward, and motor control, playing a crucial role in various biological processes related to emotions and perception.1–3 Imbalances in DA levels in the blood serum are associated with diseases such as schizophrenia (excessive DA) and Parkinson's disease (deficient DA).4–6 While these diseases are incurable, early detection can facilitate management.5 Therefore, there is a pressing need for sensitive and selective detection of DA accurately for medical diagnosis. Among the various methods for DA detection, the electrochemical technique has gained popularity in sensing applications due to its numerous advantages, including ease of operation, real-time detection, and high sensitivity and selectivity. However, realizing these benefits relies on selecting suitable materials for modifying substrate electrodes.7–10 While many proposed sensors rely on composite materials for their synergistic properties,11–15 these often face challenges such as complex synthesis procedures and inconsistent material homogeneity, which can compromise sensor reliability and performance.16,17 In contrast, single metal oxides offer a simpler composition, ensuring greater reproducibility and uniformity in sensor performance—key factors for precise and consistent DA detection. Additionally, single metal oxides are typically more cost-effective and easier to fabricate, as they eliminate the need for intricate blending or multi-step preparation processes.18–20 These attributes make single metal oxides an attractive alternative for the development of efficient and reliable electrochemical sensors.In the digital era, electronic waste (e-waste) has become a critical environmental issue,21,22 with zinc–carbon batteries (ZCBs) being significant contributors due to their widespread use and inadequate disposal.23 However, their improper disposal exacerbates landfill congestion and environmental pollution, posing severe health and ecological risks.24,25 Addressing these challenges, sustainable solutions for ZCB recycling have gained attention, including extracting graphite, manganese oxides, and zinc for reuse.22,23,26,27 ZCB consists of a graphite positive electrode surrounded by manganese dioxide and carbon powder, encased within a zinc container acting as both packaging and a negative electrode.23,26 Graphite from spent ZCBs has been successfully repurposed for graphene-based materials,28–32 while zinc recovery efforts have focused on producing low-cost ZnO. Therefore, extracting zinc from waste ZCBs presents a sustainable and cost-effective alternative, addressing resource limitations while enhancing waste management and reducing environmental impact.
Recycling methods for e-waste primarily include pyrometallurgy, which uses high temperatures to extract metals but involves high energy consumption and environmental risks, and hydrometallurgy, which dissolves metals in chemical solutions but generates substantial waste requiring careful management.23,27 To address these limitations, simpler and more sustainable approaches for ZnO synthesis from e-waste-derived Zn are necessary. Here, we propose a straightforward precipitation method for synthesizing highly pure ZnO using zinc hulls recovered from discarded ZCBs. This approach offers a simple, cost-effective, and environmentally friendly solution for recycling e-waste while producing valuable ZnO.
In this study, we present a simple and efficient precipitation method to synthesize highly pure ZnO from zinc hulls recovered from discarded ZCBs for use as a sensitive and selective electrochemical sensor for DA. This approach minimizes energy consumption and environmental impact compared to conventional zinc extraction methods while enabling the development of single metal oxide-based electrochemical detectors for DA sensing. This method not only mitigates the environmental burden of e-waste but also transforms waste materials into valuable products such as ZnO, which hold economic potential for sensor applications and beyond.
2. Experimental
2.1. Reagents and chemicals
Analytical grade DA, uric acid, 4-nitrophenol, glucose, HCl, NaOH and other chemicals were purchased from Sigma-Aldrich (Merck), Germany. All chemicals were used as received, without further purification. Ultrapure deionized water (DI) (18 MΩ·cm) was used to prepare all solutions.2.2. Instruments
The morphology of the samples was analyzed using scanning electron microscopy (SEM) (EVO18, Carl Zeiss AG, Germany) at various magnifications with a 15 kV electron beam voltage. Elemental composition was determined by energy-dispersive X-ray spectroscopy (EDS) (TEAM EDS; EDAX, USA). The Fourier-transform infrared (FTIR) spectrum was acquired in transmittance mode using a PerkinElmer Frontier spectrophotometer (USA). X-ray diffraction (XRD) patterns were recorded with a Thermo Scientific ARL Equinox diffractometer (Cu Kα1, λ = 1.5406 Å, 40 kV, 30 mA). Ultraviolet-visible (UV-Vis) diffuse reflectance spectra were measured with a Shimadzu UV-1800 spectrophotometer (Japan) to determine the optical band gap (Eg). Photoluminescence (PL) spectra were obtained using a Hitachi F-7000 spectrophotometer with a 150 W xenon lamp at room temperature. Electrochemical analyses were performed using a CHI660 device (USA) with a glassy carbon (GC) working electrode (Φ = 3 mm), Ag/AgCl/KCl (sat.) reference electrode, and a spiral Pt wire counter electrode.2.3. Recovery of Zn–metal and synthesis of ZnO
To preserve the integrity of the Zn shell, the waste batteries were carefully dismantled. The Zn shell was then cleaned with deionized water to remove the attached mixture of MnO2, NH4Cl, carbon powder, and other substances. The Zn metal was polished using sandpaper and cleaned with Triton X-100 surfactant to remove impurities, resulting in a shiny surface. The Zn metal was dissolved in concentrated HCl, and 1 M NaOH was gradually added until Zn(OH)2 precipitated (Scheme 1). The product was separated via centrifugation, rinsed multiple times with DI water until the pH reached 7, and then washed with ethanol to remove any organic residue. Finally, the ZnO product was dried in a vacuum oven for 24 hours at 80 °C.2.4. Fabrication of the ZnO modified GC electrode
The GC electrode (CHI104, Φ = 3 mm) was meticulously polished using a polishing microcloth and an aqueous slurry of fine alumina particles. After polishing, the GC electrode was subjected to 5 minutes of ultrasonication in deionized water to remove any remaining alumina particles. The prepared ZnO was fully suspended in deionized water at a concentration of 1 mg mL−1 using sonication. A homogeneous suspension was then drop-cast onto the GC electrode, and the electrode was allowed to dry at room temperature, forming a uniform film of active components. This modified electrode was used for subsequent electrochemical measurements.3. Results and discussion
3.1. Characterization
 | ||
| Scheme 2 Reactions and mechanisms in ZnO formation. (A) Possible reactions involved in the synthesis of ZnO. (B) Proposed mechanism for the formation of ZnO leaf structures. | ||
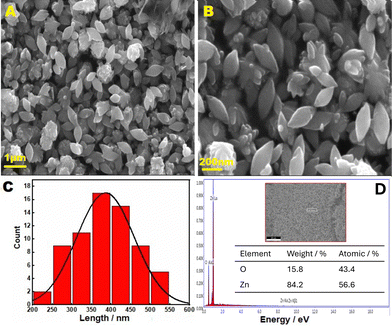
Then, dried ZnO particles were subjected to XRD analysis using Cu-Kα radiation (λ = 1.54060 Å), with relative intensity data collected over a 2θ range of 10–80° (Fig. 2B). The XRD pattern of ZnO exhibits sharp and intense peaks, indicative of the high purity and crystallinity of the synthesized ZnO (Fig. 2B). The diffraction bands at 2θ of 31.70°, 34.32°, 36.15°, 47.33°, 56.36°, 62.71°, 65.98°, 67.77°, 68.79°, 72.57°, and 76.85° correspond to the (100), (002), (101), (102), (110), (103), (200), (112), (201), (004), and (202) planes of the hexagonal wurtzite ZnO lattice.33–38 Importantly, no additional diffraction peaks were observed, indicating the purity of ZnO. The absence of characteristic peaks of Zn(OH)2, such as those at ∼19.0°, 35.6°, and 53.1°, further confirms the complete conversion of Zn(OH)2 to ZnO.39,40 Faisal et al. also reported that thermal treatment during the synthesis of ZnO facilitates the conversion of Zn(OH)2 to high-purity ZnO.36 Therefore, this evidence suggests the successful conversion of Zn(OH)2 to ZnO.
The optical band gap value of the produced ZnO was determined by calculating the absorption energy associated with the electron excitation from the valence band to the conduction band, as seen in Fig. 1D. The band gap energy was calculated using eqn (1) based on the Tauc plot (Fig. 1D).44
| (αhv)1/n = A(hv − Eg) | (1) |
The morphology of ZnO is influenced by a combination of kinetic factors, such as the growth rates of different crystal surfaces, and thermodynamic factors, which relate to the energetic stability of the overall structure.46–52 ZnO can form a wide range of morphologies, including nanorods, needles, leaf, flower, tubes, belts, wires, combs, etc., depending on the nature of the solvent, precursor materials, reaction temperature, reaction duration, and solution pH.36,46,50–52 In this study, Zn metal collected from a Zn–C battery was dissolved in concentrated HCl, and 1.0 M NaOH was gradually added until Zn(OH)2 precipitated. The precipitate was then dried in a vacuum oven for 24 hours at 80 °C (Scheme 1). The possible reactions involved in ZnO formation and a plausible mechanism for the formation of leaf-like ZnO are shown in Scheme 2. Generally, the excessive addition of NaOH to Zn2+ leads to the formation of zincate ions (Zn(OH)42−), which undergo dehydration upon heating to produce ZnO36 (Scheme 2A). As the solution is heated, the ions rearrange to form clusters and generate stable nuclei (Scheme 2B). The direction of growth of these nanostructures depends on the selective adsorption effect on certain facets. The presence of excess OH− generally promotes the growth of ZnO clusters along the [0001] direction, resulting in rod-shaped ZnO36 (Scheme 2B). The leaf-like structures are formed as multiple nanorods come together in an ordered array. This aggregation is driven by thermodynamic stability, where larger leaf-shaped structures are energetically more favorable than smaller nanorods.
3.2. Electrochemical dopamine sensing
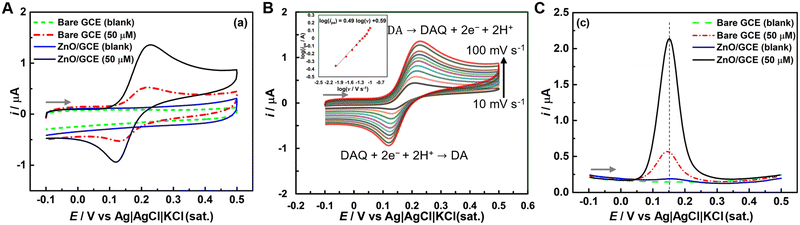
Fig. 3C shows the differential pulse voltammograms (DPVs) obtained at bare GC and ZnO/GC electrodes in the absence and presence of DA. Like CVs, no oxidation peak was detected in the absence of DA at either electrode (Fig. 3C). However, in the presence of DA, both electrodes displayed oxidation peaks corresponding to DA oxidation. The peak potential (Ep) at the ZnO/GC electrode was slightly shifted towards a more positive potential, and the peak current (ip) at the ZnO/GC electrode was ca. 4.5 times greater than that at the GC electrode. This significant increase in oxidation current at the ZnO/GC electrode compared to the GC electrode highlights again the superior electrocatalytic activity of the ZnO/GCE for DA sensing. The electrochemically active surface area (ECSA) of the ZnO/GC electrode was evaluated using the Randles–Sevcik equation in a standard ferri/ferrocyanide redox couple solution. The ECSA of the ZnO/GC electrode was determined to be ca. 2.1 times greater than the geometric surface area of the bare GC electrode (0.0707 cm2). Therefore, the substantial enhancement in the ip at the ZnO/GC electrode can be attributed to the increased ECSA of the modified electrode and the improved interaction between the ZnO and DA molecules, leading to greater DA accumulation on the electrode.
Fig. 4A presents DPVs for electrochemical DA sensing in PBS solution at various pHs using a ZnO/GC electrode. Shifts in peak potential and changes in peak current intensity for DA oxidation were observed with the pH medium (Fig. 4A and B). At lower pH, peak currents are observed at more positive potentials, whereas at higher pH, the peaks shift to more negative potentials (Fig. 4A and B). This indicates that the oxidation potential and rate of oxidation of DA at the ZnO/GC electrode are pH dependent. In acidic conditions (pH = 3 to 6), DA is relatively stable, primarily existing in its protonated form.53–57 As the pH increases to neutral and slightly basic levels (pH = 7 to 8), DA undergoes oxidation more easily, forming dopaminoquinone.53,54,57 In basic conditions (pH = 9 to 11), oxidation is further favored, leading to the formation of various oxidized products, such as quinones, and potentially polymerization into melanin-like substances.53,54,56 Consequently, DA is more susceptible to oxidation at higher pH levels, resulting in diverse chemical transformations. The Ep showed a linear relationship with the pH of the medium, following the equation Ep = 0.594–0.050 pH (Fig. 4B). For a Nernstian reaction involving an equal number of protons and electrons, the expected slope of Epversus pH is 59 mV pH−1. The observed slope in this study (50 mV pH−1), which is close to the theoretical value, suggests that an equal number of protons and electrons are involved in the oxidation of DA at the ZnO/GC electrode.
The highest peak current was observed at pH 6.9, indicating the optimal pH for DA oxidation at the ZnO/GC electrode. ZnO exhibits different surface dynamics in aqueous solutions across a broad pH range (3 to 11).33–35,37,38 ZnO maintains surface stability and exhibits a positive surface charge in acidic conditions (pH = 3 to 6), where DA exists in its protonated form. This results in a low oxidation rate (low peak current) due to coulombic repulsion between the like charges. At neutral to slightly basic pH (7 to 8), ZnO starts developing surface –OH groups,35,37,38 resulting in higher current due to possible H-bonding between these hydroxyl groups and DA. Under basic conditions (pH 9 to 11), zincate ions (Zn(OH)42−) are formed, which reduces the response to DA sensing.
A possible mechanism for the electrochemical oxidation of DA in PBS solution at the ZnO/GC electrode is illustrated in Fig. 4C. The cyclic voltammetric study indicated that the redox behavior of DA follows a quasi-reversible process, with electro-oxidation being more favorable than reduction (Fig. 3A and B), which aligns with findings in the literature.1,3,10,12 The oxidation process was diffusion-controlled (Fig. 3B) and involved an equal number of protons and electrons as discussed above. Typically, the electro-oxidation mechanism includes a two-step process where the two hydroxyl groups in DA are converted into dopaminequinone (Fig. 4C). This dopaminequinone is then transformed into 5,6-dihydroxyindole, which is further oxidized to 5,6-dihydroxy indolyl quinone.58 Repeated oxidation of DA ultimately leads to the formation of polydopamine, as shown in Fig. 4C.58
For the repeatability study, fifteen consecutive DPVs were recorded at a ZnO/GC electrode in 50 μM DA in 0.1 M PBS (inset of Fig. 5B). Fig. 5B shows minimal variation in peak current with increasing analysis count, with only ca. 13% of the peak current dropped after the 15th experiment. The reproducibility of the ZnO/GC electrode was assessed through electroanalysis of 50 μM DA at three distinct ZnO/GC electrodes. The relative standard deviation (RSD) of the current responses recorded at the modified electrodes was 4.80%. Therefore, the repeatability and reproducibility studies demonstrated that the ZnO/GC electrode produced a consistent DA oxidation peak current.
| Electrode | Method | Linear range (μM) | LOD (nM) | Ref. |
|---|---|---|---|---|
| CF: nanofiber; CN: carbon nanocomposites; DA-CS: dopamine-imprinted chitosan film; ErGO: electro-reduced graphene oxide; GMWCNTs: graphitized multi-walled carbon nanotubes; MC: mesoporous carbon; MWCNTs: multi-walled carbon nanotubes; NCAs: nanocone arrays; P-rGO: porous reduced graphene oxide; PC: porous carbon; SDLSV: second derivative linear sweep voltammetry; SPEEK: sulphonated polyether etherketone; SWV: square wave votammetry; 3D-KSC: three-dimensional kenaf stem-derived macroporous carbon. | ||||
| GO–ZnO/GCE | DPV | 1–70 | 330 | 9 |
| ZnO/CF | DPV | 6–20 | 402 | 14 |
| Au–ZnO NCAs/GF | DPV | 0–80 | 40 | 2 |
| SPEEK/ZnO | SWV | 5.4 × 10−7–1.9 × 10−3 | 0.89 | 71 |
| ZnO/ErGO/GCE | SDLSV | 0.01–80 | 3.6 | 10 |
| Ti3C2/GMWCNTs/ZnO/GCE | DPV | 0.01–30 | 3.2 | 11 |
| N-ZnO/N-rGO | DPV | 0.05–800 | 200 | 59 |
| Ag–Cu@ZnO | Amperometry | 0.1–10 | 210 | 60 |
| 3D-rGO–ZnO/GCE | DPV | 0.01–70 | 0.06 | 8 |
| ZnO–ZnFe2O4/Fe3O4/CN | DPV | 0.01–61.34 | 1.57 | 61 |
| ZnO@Au | DPV | 0.1–500 | 86 | 72 |
| ZnO/PC | DPV | 0.10–100.0 | 22 | 73 |
| β-Zn(OH)2/ZnO | DPV | 1.75–263 nM | 0.17 | 74 |
| In2O3ZnO@MC | Amperometry | 0.5–2056 | 24 | 75 |
| ZnO/GCE | DPV | 0.01–100 | 0.47 | This study |
Different concentrations of DA solution were prepared using DA hydrochloride injection in 0.1 M PBS to represent real sample analysis. Recovery experiments were performed by differential pulse voltammetry using a standard three electrode system. The background current was corrected to precisely determine the recovery (%) of DA, and three sequential experiments were performed. The recovery amounts for DA ranged from 92 to 107% (Table 2), demonstrating the practical reliability of the proposed sensor.
| Sample no. | Concentration of DA (μM) | Recovery (%) | |
|---|---|---|---|
| Added | Found | ||
| ±: Standard deviation (n = 3) | |||
| 1 | 1.0 | 1.03 ± 0.05 | 103 |
| 2 | 2.0 | 2.14 ± 0.02 | 107 |
| 3 | 5.0 | 4.60 ± 0.02 | 92 |
| 4 | 10.0 | 9.60 ± 0.03 | 96 |
| 5 | 15.0 | 14.70 ± 0.02 | 98 |
4. Conclusions
We demonstrate a low-cost method for synthesizing uniquely leaf-shaped ZnO using metallic Zn shells obtained from waste batteries via precipitation. The ZnO/GC electrode detects dopamine (DA) in the range of 0.01 to 100 μM, with a low limit of detection of 0.47 nM, which is below the physiological concentration. The developed sensor shows promising DA selectivity even in the presence of interfering species and exhibits good reproducibility. Moreover, the proposed ZnO/GC electrode can detect DA in real samples. The affordability of the precursors for synthesizing leaf-shaped ZnO offers an economical electrochemical sensor for detecting DA and other potential biomolecules, while also promoting environmentally sustainable e-waste management.Data availability
Data will be made available on request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the Institute of National Analytical Research and Service (INARS), Bangladesh Council of Scientific and Industrial Research (BCSIR) for financial support (R&D ref. no. 39.02.0000.011.14.169.2023/877, date 17.09. 2023) and facilities.References
- S. S. J. Aravind and S. Ramaprabhu, Dopamine Biosensor with Metal Oxide Nanoparticles Decorated Multi-Walled Carbon Nanotubes, Nanosci. Methods, 2012, 1(1), 102–114 CrossRef.
- H. Y. Yue, H. J. Zhang, S. Huang, X. X. Lu, X. Gao, S. S. Song, Z. Wang, W. Q. Wang and E. H. Guan, Highly Sensitive and Selective Dopamine Biosensor Using Au Nanoparticles-ZnO Nanocone Arrays/Graphene Foam Electrode, Mater. Sci. Eng., C, 2020, 108, 110490 CrossRef CAS PubMed.
- H. Y. Yue, H. J. Zhang, S. Huang, X. X. Lu, X. Gao, S. S. Song, Z. Wang, W. Q. Wang and E. H. Guan, Highly Sensitive and Selective Dopamine Biosensor Using Au Nanoparticles-ZnO Nanocone Arrays/Graphene Foam Electrode, Mater. Sci. Eng., C, 2020, 108, 110490 CrossRef CAS PubMed.
- U. Ungerstedt; M. Herrera-Marschitz and T. Zetterström, Dopamine Neurotransmission and Brain Function, in Progress in Brain Research, ed. Buijs, R. M., Pévet, P., Swaab, D. F., Chemical Transmission in the Brain: The Role of Amines, Amino Acids and Peptides, Elsevier, 1982, vol. 55, pp. 41–49 Search PubMed.
- J. Birtwistle and D. Baldwin, Role of Dopamine in Schizophrenia and Parkinson's Disease, Br. J. Nurs., 1998, 7(14), 832–841 CrossRef CAS PubMed.
- J. Segura-Aguilar, I. Paris, P. Muñoz, E. Ferrari, L. Zecca and F. A. Zucca, Protective and Toxic Roles of Dopamine in Parkinson's Disease, J. Neurochem., 2014, 129(6), 898–915 CrossRef CAS PubMed.
- Mounesh, K. V. Reddy and O. Nagaraja, Novel n-octadecylcarboxamide CoPc: amperometric detections for bioanalytes using modified GCE, Chem. Papers, 2021, 75, 2945–2956 CrossRef CAS.
- C. Nong, B. Yang, X. Li, S. Feng and H. Cui, Electrochemical Sensor Based on Three-Dimensional rGO/ZnO Composite for Dopamine Detection, Int. J. Electrochem. Sci., 2022, 17(3), 220331 CrossRef CAS.
- X. Zhang, Y.-C. Zhang and L.-X. Ma, One-Pot Facile Fabrication of Graphene-Zinc Oxide Composite and Its Enhanced Sensitivity for Simultaneous Electrochemical Detection of Ascorbic Acid, Dopamine and Uric Acid, Sens. Actuators, B, 2016, 227, 488–496 CrossRef CAS.
- F. Li, B. Ni, Y. Zheng, Y. Huang and G. Li, A Simple and Efficient Voltammetric Sensor for Dopamine Determination Based on ZnO Nanorods/Electro-Reduced Graphene Oxide Composite, Surf. Interfaces, 2021, 26, 101375 CrossRef CAS.
- M. Ni, J. Chen, C. Wang, Y. Wang, L. Huang, W. Xiong, P. Zhao, Y. Xie and J. Fei, A High-Sensitive Dopamine Electrochemical Sensor Based on Multilayer Ti3C2 MXene, Graphitized Multi-Walled Carbon Nanotubes and ZnO Nanospheres, Microchem. J., 2022, 178, 107410 CrossRef CAS.
- D. Balram, K.-Y. Lian and N. Sebastian, A Novel Electrochemical Sensor Based on Flower Shaped Zinc Oxide Nanoparticles for the Efficient Detection of Dopamine, Int. J. Electrochem. Sci., 2018, 13(2), 1542–1555 CrossRef CAS PubMed.
- K. V. Reddy, Novel garnished cobalt (II) phthalocyanine with MWCNTs on modified GCE: sensitive and reliable electrochemical investigation of paracetamol and dopamine, New J. Chem., 2020, 44(39), 16831–16844 RSC.
- C. Yang, C. Zhang, T. Huang, X. Dong and L. Hua, Ultra-Long ZnO/Carbon Nanofiber as Free-Standing Electrochemical Sensor for Dopamine in the Presence of Uric Acid, J. Mater. Sci., 2019, 54(24), 14897–14904 CrossRef CAS.
- B. S. Jilani, M. Pari, K. V. Reddy and K. S. Lokesh, Simultaneous and sensitive detection of ascorbic acid in presence of dopamine using MWCNTs-decorated cobalt (II) phthalocyanine modified GCE, Microchem. J., 2019, 147, 755–763 CrossRef.
- R. F. Gibson, A Review of Recent Research on Mechanics of Multifunctional Composite Materials and Structures, Compos. Struct., 2010, 92(12), 2793–2810 CrossRef.
- V. B. Mohan, K. Lau, D. Hui and D. Bhattacharyya, Graphene-Based Materials and Their Composites: A Review on Production, Applications and Product Limitations, Composites, Part B, 2018, 142, 200–220 CrossRef CAS.
- Z. S. Campbell, S. Baro, Y. Gao, F. Li and M. Abolhasani, Flow Synthesis of Single and Mixed Metal Oxides, Chem.:Methods, 2022, 2(8), e202200007 Search PubMed.
- T. P. Mabate, N. P. Maqunga, S. Ntshibongo, M. Maumela and N. Bingwa, Metal Oxides and Their Roles in Heterogeneous Catalysis: Special Emphasis on Synthesis Protocols, Intrinsic Properties, and Their Influence in Transfer Hydrogenation Reactions, SN Appl. Sci., 2023, 5(7), 196 CrossRef CAS.
- Y. Yoon, P. L. Truong, D. Lee and S. H. Ko, Metal-Oxide Nanomaterials Synthesis and Applications in Flexible and Wearable Sensors, ACS Nanosci. Au, 2022, 2(2), 64–92 CrossRef CAS PubMed.
- K. Zhang, J. L. Schnoor and E. Y. Zeng, E-Waste Recycling: Where Does It Go from Here?, Environ. Sci. Technol., 2012, 46(20), 10861–10867 CrossRef CAS PubMed.
- E. Sanchez Moran, D. Prodius, I. C. Nlebedim and M. Mba Wright, Rare-Earth Elements Recovery from Electronic Waste: Techno-Economic and Life Cycle Analysis, ACS Sustainable Chem. Eng., 2024, 12(38), 14164–14172 CrossRef CAS.
- M. H. Kabir, M. Y. Pabel, N. T. Bristy, M. A. Salam, M. S. Bashar and S. Yasmin, From e-waste to eco-sensors: synthesis of reduced graphene oxide/ZnO from discarded batteries for a rapid electrochemical bisphenol A sensor, RSC Adv., 2024, 14(48), 36073–36083 RSC.
- T. Zhang, B. Zhang, X. Bai, Y. Yao, L. Wang, Y. Shu, K. Kannan, X. Huang and H. Sun, Health Status of Elderly People Living Near E-Waste Recycling Sites: Association of E-Waste Dismantling Activities with Legacy Perfluoroalkyl Substances (PFASs), Environ. Sci. Technol. Lett., 2019, 6(3), 133–140 CrossRef CAS.
- M. Yan, Z. Cheng, Q. Zou, H. Zhao, L. Yang, H. Zhu, T. Zhang and H. Sun, Human Exposure Levels of Volatile Organic Compounds in E-Waste Recycling Area: Get Insight into Impacts of Manipulation Mode and Associations with Oxidative Stress Markers, Environ. Health, 2023, 1(6), 405–415 CAS.
- M. H. Kabir, M. J. Miah, A. K. Mohiuddin, M. S. Hossain, B. P. Upoma, M. A. A. Shaikh, M. Y. Pabel, F. Mojumder, R. Mahmud, N. I. Tanvir and S. Yasmin, Highly Effective Removal of Moxifloxacin from Aqueous Solutions Using Graphene Oxide Functionalized with Sodium Dodecyl Sulfate, ACS Sustainable Resour. Manage., 2025, 2(2), 256–266 CrossRef CAS.
- I. De Michelis, F. Ferella, E. Karakaya, F. Beolchini and F. Vegliò, Recovery of Zinc and Manganese from Alkaline and Zinc-Carbon Spent Batteries, J. Power Sources, 2007, 172(2), 975–983 CrossRef CAS.
- M. Y. Pabel, S. Yasmin, M. A. A. Shaikh and M. H. Kabir, Electronic Waste Derived Reduced Graphene Oxide Supported Silver Nanoparticles for the Electrochemical Sensing of Trace Level Arsenite in Aqueous Medium, Sens. Actuators, A, 2024, 366, 115028 CrossRef.
- F. Mojumder, S. Yasmin, M. A. A. Shaikh, P. Chowdhury and M. H. Kabir, Synthesis of Reusable Graphene Oxide Based Nickel-Iron Superparamagnetic Nanoadsorbent from Electronic Waste for the Removal of Doxycycline in Aqueous Media, J. Hazard. Mater. Adv., 2024, 14, 100429 Search PubMed.
- Md. S. Hossain, S. Yasmin and M. H. Kabir, Cost-Effective Synthesis of Magnetic Graphene Oxide Nanocomposite from Waste Battery for the Removal of Arsenic from Aqueous Solutions: Adsorption Mechanism with DFT Calculation, J. Saudi Chem. Soc., 2024, 28(3), 101873 Search PubMed.
- S. Yasmin, M. G. Azam, M. S. Hossain, U. S. Akhtar and M. H. Kabir, Efficient Removal of Ciprofloxacin from Aqueous Solution Using Zn–C Battery Derived Graphene Oxide Enhanced by Hydrogen Bonding, Electrostatic and π–π Interaction, Heliyon, 2024, 10(12), e33317 CrossRef CAS PubMed.
- M. H. Kabir, M. S. Hossain, M. M. Rahman, M. Ashrafuzzaman, M. Hasan, M. Y. Pabel, D. Islam, M. Shahriar Bashar, T. Faruque and S. Yasmin, Green Reduction of Waste-Battery-Derived Graphene Oxide by Jute Leaves and Its Application for the Removal of Tetracyclines from Aqueous Media, ACS Sustainable Resour. Manage., 2024, 1(8), 1812–1823 CrossRef CAS.
- M. Aliannezhadi, S. Z. Mirsanaee, M. Jamali and F. Shariatmadar Tehrani, The Physical Properties and Photocatalytic Activities of Green Synthesized ZnO Nanostructures Using Different Ginger Extract Concentrations, Sci. Rep., 2024, 14(1), 2035 CrossRef CAS PubMed.
- B. Naiel, M. Fawzy, M. W. A. Halmy and A. E. D. Mahmoud, Green Synthesis of Zinc Oxide Nanoparticles Using Sea Lavender (Limonium Pruinosum L. Chaz.) Extract: Characterization, Evaluation of Anti-Skin Cancer, Antimicrobial and Antioxidant Potentials, Sci. Rep., 2022, 12(1), 20370 CrossRef CAS PubMed.
- M. F. Ehsan, H. R. Barai, M. M. Islam, M. A. B. H. Susan, S. W. Joo and M. S. Miran, ZnO Nanocomposites Supported by Acid-Activated Kaolinite as Photocatalysts for the Enhanced Photodegradation of an Organic Dye, Mater. Today Commun., 2023, 36, 106563 CrossRef CAS.
- Md. A. Faisal, S. Ahmed and Md. A. B. H. Susan, Nanostructured ZnO with Tunable Morphology from Double-Salt Ionic Liquids as Soft Template, ACS Omega, 2024, 9(11), 12992–13005 CAS.
- M. Izzi, M. C. Sportelli, L. Torsi, R. A. Picca and N. Cioffi, Synthesis and Antimicrobial Applications of ZnO Nanostructures: A Review, ACS Appl. Nano Mater., 2023, 6(13), 10881–10902 CrossRef CAS.
- S. Maher, S. Nisar, S. M. Aslam, F. Saleem, F. Behlil, M. Imran, M. A. Assiri, A. Nouroz, N. Naheed, Z. A. Khan and P. Aslam, Synthesis and Characterization of ZnO Nanoparticles Derived from Biomass (Sisymbrium Irio) and Assessment of Potential Anticancer Activity, ACS Omega, 2023, 8(18), 15920–15931 CrossRef CAS PubMed.
- S. V. Nistor, D. Ghica, M. Stefan, I. Vlaicu, J. N. Barascu and C. Bartha, Magnetic Defects in Crystalline Zn(OH)2 and Nanocrystalline ZnO Resulting from Its Thermal Decomposition, J. Alloys Compd., 2013, 548, 222–227 CrossRef CAS.
- M. Ghaedi, H. Z. Khafri, A. Asfaram and A. Goudarzi, Response Surface Methodology Approach for Optimization of Adsorption of Janus Green B from Aqueous Solution onto ZnO/Zn(OH)2-NP-AC: Kinetic and Isotherm Study, Spectrochim. Acta, Part A, 2016, 152, 233–240 CrossRef CAS PubMed.
- V. Anand, A. Sakthivelu, K. D. A. Kumar, S. Valanarasu, V. Ganesh, M. Shkir, A. Kathalingam and S. AlFaify, Novel Rare Earth Gd and Al Co-Doped ZnO Thin Films Prepared by Nebulizer Spray Method for Optoelectronic Applications, Superlattices Microstruct., 2018, 123, 311–322 CrossRef CAS.
- L. Saikia, D. Bhuyan, M. Saikia, B. Malakar, D. K. Dutta and P. Sengupta, Photocatalytic Performance of ZnO Nanomaterials for Self Sensitized Degradation of Malachite Green Dye under Solar Light, Appl. Catal., A, 2015, 490, 42–49 CrossRef CAS.
- A. J. Santhosam, K. Ravichandran, M. Shkir and M. Sridharan, Effect of La Incorporation on the NH3 Sensing Behaviour of ZnO Thin Films Prepared Using Low-Cost Nebulizer Spray Technique, J. Mater. Sci. Mater. Electron., 2020, 31(16), 13240–13248 CrossRef CAS.
- J. Tauc, Optical Properties of Amorphous Semiconductors, in Amorphous and Liquid Semiconductors, ed. Tauc, J., Springer, US, Boston, MA, 1974, pp. 159–220 Search PubMed.
- D. Ponnusamy and S. Madanagurusamy, Nanostructured ZnO Films for Room Temperature Ammonia Sensing, J. Electron. Mater., 2014, 43(9), 3211–3216 CrossRef.
- H. F. Wilson, C. Tang and A. S. Barnard, Morphology of Zinc Oxide Nanoparticles and Nanowires: Role of Surface and Edge Energies, J. Phys. Chem. C, 2016, 120(17), 9498–9505 CrossRef CAS.
- A. Sulciute, K. Nishimura, E. Gilshtein, F. Cesano, G. Viscardi, A. G. Nasibulin, Y. Ohno and S. Rackauskas, ZnO Nanostructures Application in Electrochemistry: Influence of Morphology, J. Phys. Chem. C, 2021, 125(2), 1472–1482 CrossRef CAS.
- Q. C. Bui, G. Ardila, E. Sarigiannidou, H. Roussel, C. Jiménez, O. Chaix-Pluchery, Y. Guerfi, F. Bassani, F. Donatini, X. Mescot, B. Salem and V. Consonni, Morphology Transition of ZnO from Thin Film to Nanowires on Silicon and Its Correlated Enhanced Zinc Polarity Uniformity and Piezoelectric Responses, ACS Appl. Mater. Interfaces, 2020, 12(26), 29583–29593 CAS.
- L.-Y. Wang, B.-Y. Shi, C.-B. Yao, Z.-M. Wang, X. Wang, C.-H. Jiang, L.-F. Feng and Y.-L. Song, Size and Morphology Modulation in ZnO Nanostructures for Nonlinear Optical Applications: A Review, ACS Appl. Nano Mater., 2023, 6(12), 9975–10014 CrossRef CAS.
- A. Rezaei, E. Katoueizadeh and S. M. Zebarjad, Investigation of the Parameters Affecting the Morphology of Zinc Oxide (ZnO) Nanoparticles Synthesized by Precipitation Method, Mater. Today Chem., 2022, 26, 101239 CrossRef CAS.
- L. Xu, Y. Guo, Q. Liao, J. Zhang and D. Xu, Morphological Control of ZnO Nanostructures by Electrodeposition, J. Phys. Chem. B, 2005, 109(28), 13519–13522 CrossRef CAS PubMed.
- M. Søndergaard, E. D. Bøjesen, M. Christensen and B. B. Iversen, Size and Morphology Dependence of ZnO Nanoparticles Synthesized by a Fast Continuous Flow Hydrothermal Method, Cryst. Growth Des., 2011, 11(9), 4027–4033 CrossRef.
- V. Ball, D. D. Frari, V. Toniazzo and D. Ruch, Kinetics of Polydopamine Film Deposition as a Function of pH and Dopamine Concentration: Insights in the Polydopamine Deposition Mechanism, J. Colloid Interface Sci., 2012, 386(1), 366–372 CrossRef CAS PubMed.
- T.-P. Chen, T. Liu, T.-L. Su and J. Liang, Self-Polymerization of Dopamine in Acidic Environments without Oxygen, Langmuir, 2017, 33(23), 5863–5871 CrossRef CAS PubMed.
- X. Du, L. Li, F. Behboodi-Sadabad, A. Welle, J. Li, S. Heissler, H. Zhang, N. Plumeré and P. A. Levkin, Bio-Inspired Strategy for Controlled Dopamine Polymerization in Basic Solutions, Polym. Chem., 2017, 8(14), 2145–2151 RSC.
- C. Zhu, H. Xie, Y. Zhang, R. Zhang, S. Dai, X. Li, Y. Sun, Y. Zhang and M. Zhao, Exploring the Complex Impact of Proteins on Dopamine Polymerization: Mechanisms and Strategies for Modulation, J. Phys. Chem. B, 2024, 128(12), 2885–2896 CrossRef CAS PubMed.
- Y. Yang, P. Qi, Y. Ding, M. F. Maitz, Z. Yang, Q. Tu, K. Xiong, Y. Leng and N. Huang, A Biocompatible and Functional Adhesive Amine-Rich Coating Based on Dopamine Polymerization, J. Mater. Chem. B, 2015, 3(1), 72–81 RSC.
- N. Delmo, B. Mostafiz, A. E. Ross, J. Suni and E. Peltola, Developing an Electrochemical Sensor for the in Vivo Measurements of Dopamine, Sens. Diagn., 2023, 2(3), 559–581 RSC.
- S. Thareja and A. Kumar, In Situ Wet Synthesis of N-ZnO/N-rGO Nanohybrids as an Electrode Material for High-Performance Supercapacitors and Simultaneous Nonenzymatic Electrochemical Sensing of Ascorbic Acid, Dopamine, and Uric Acid at Their Interface, J. Phys. Chem. C, 2021, 125(45), 24837–24848 CrossRef CAS.
- S. Ponnada, D. B. Gorle, M. S. Kiai, S. Rajagopal, R. K. Sharma and A. Nowduri, A Facile, Cost-Effective, Rapid, Single-Step Synthesis of Ag–Cu Decorated ZnO Nanoflower-like Composites (NFLCs) for Electrochemical Sensing of Dopamine, Mater. Adv., 2021, 2(18), 5986–5996 RSC.
- R. Appiah-Ntiamoah, A. F. Baye and H. Kim, ZnO–ZnFe2O4/Fe3O4/Carbon Nanocomposites for Ultrasensitive and Selective Dopamine Detection, ACS Appl. Nano Mater., 2022, 5(4), 4754–4766 CrossRef CAS.
- H. Teymourian, A. Barfidokht and J. Wang, Electrochemical Glucose Sensors in Diabetes Management: An Updated Review (2010–2020), Chem. Soc. Rev., 2020, 49(21), 7671–7709 RSC.
- Z. Haghparas, Z. Kordrostami, M. Sorouri, M. Rajabzadeh and R. Khalifeh, Highly Sensitive Non-Enzymatic Electrochemical Glucose Sensor Based on Dumbbell-Shaped Double-Shelled Hollow Nanoporous CuO/ZnO Microstructures, Sci. Rep., 2021, 11(1), 344 CrossRef CAS PubMed.
- S. Yasmin, M. S. Ahmed, D. Park and S. Jeon, Nitrogen-Doped Graphene Supported Cobalt Oxide for Sensitive Determination of Dopamine in Presence of High Level Ascorbic Acid, J. Electrochem. Soc., 2016, 163(9), B491 CrossRef CAS.
- N. Roy, S. Yasmin and S. Jeon, Effective Electrochemical Detection of Dopamine with Highly Active Molybdenum Oxide Nanoparticles Decorated on 2, 6 Diaminopyridine/Reduced Graphene Oxide, Microchem. J., 2020, 153, 104501 CrossRef CAS.
- S. Yasmin, M. S. Ahmed and S. Jeon, Determination of Dopamine by Dual Doped Graphene-Fe2O3 in Presence of Ascorbic Acid, J. Electrochem. Soc., 2015, 162(14), B363 CrossRef CAS.
- Y. Tang, R. Huang, C. Liu, S. Yang, Z. Lu and S. Luo, Electrochemical Detection of 4-Nitrophenol Based on a Glassy Carbon Electrode Modified with a Reduced Graphene Oxide/Au Nanoparticle Composite, Anal. Methods, 2013, 5(20), 5508–5514 RSC.
- A. M. Visagamani, M. Harb, K. Kaviyarasu, A. Muthukrishnaraj, M. Ayyar, K. A. Alzahrani, R. H. Althomali and S. A. Althobaiti, Electrochemical Detection of 4-Nitrophenol Using a Novel SrTiO3/Ag/rGO Composite, ACS Omega, 2023, 8(45), 42479–42491 CrossRef PubMed.
- A. K. Mohiuddin, S. Yasmin and S. Jeon, CoxNi1-x Double Hydroxide Decorated Graphene NPs for Simultaneous Determination of Dopamine and Uric Acid, Sens. Actuators, A, 2023, 355, 114314 CrossRef CAS.
- S. Masrat, V. Nagal, M. Khan, A. Ahmad, M. B. Alshammari, S. Alam, U. T. Nakate, B. Lee, P. Mishra, K. S. Bhat and R. Ahmad, Electrochemical Sensing of Uric Acid with Zinc Oxide Nanorods Decorated with Copper Oxide Nanoseeds, ACS Appl. Nano Mater., 2023, 6(18), 16615–16624 CrossRef CAS.
- O. E. Fayemi, R. Baskar, A. S. Adekunle, E.-S. M. Sherif and E. E. Ebenso, SPEEK/ZnO Nanocomposite Modified Gold Electrode for Electrochemical Detection of Dopamine, Electroanalysis, 2020, 32(12), 2713–2722 CrossRef CAS.
- T. G. Beatto, W. E. Gomes, A. Etchegaray, R. Gupta and R. K. Mendes, Dopamine Levels Determined in Synthetic Urine Using an Electrochemical Tyrosinase Biosensor Based on ZnO@Au Core–Shell, RSC Adv., 2023, 13(47), 33424–33429 RSC.
- Y. Li, M. Xie, X. Kang, W. Hou and Y. Chen, Preparation of Rod-Zinc Oxide/Agaric Derived Porous Carbon Nanocomposites and Their Application in Electrochemical Sensing, New J. Chem., 2024, 48(37), 16289–16296 RSC.
- M. E. Guye, F. K. Egualle, R. Appiah-Ntiamoah, S. K. Kassahun and H. Kim, Synergetic Effect of Optimized β-Zn(OH)2/ZnO Heterostructure towards Electrochemical Dopamine Detection, J. Alloys Compd., 2024, 1002, 175184 CrossRef CAS.
- J. Ahmed, M. Faisal, J. S. Algethami, M. Alsaiari and F. A. Harraz, A Novel In2O3-Doped ZnO Decorated Mesoporous Carbon Nanocomposite as a Sensitive and Selective Dopamine Electrochemical Sensor, J. Mater. Res. Technol., 2024, 29, 540–549 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |