Open Access Article
Open Access ArticleDFO-modified polydopamine sulfonated PEEK enhances osseointegration through macrophage immunomodulation and osteogenic differentiation of BMSCs†
Shengjie
Wang‡
a,
Wei
Liu‡
a,
Chao
Yang
a,
Xianlong
Zhang
*a and
Chunming
Lyu
 *bc
*bc
aDepartment of Orthopaedic Surgery, Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, 600 Yishan Road, Shanghai 200233, China. E-mail: dr_zhangxianlong@sjtu.edu.cn; Fax: +86 021-24058101; Tel: +86 021-24058101
bExperiment Center for Science and Technology, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. E-mail: chunming83g@126.com; Tel: +86 021-51323044
cQinghai Province Key Laboratory of Tibetan Medicine Pharmacology and Safety Evaluation, Northwest Institute of Plateau Biology, Chinese Academy of Sciences, Xining 810008, China
First published on 29th March 2025
Abstract
This study aimed to develop a novel artificial joint prosthesis material with osteogenic properties. Deferoxamine mesylate (DFO) was immobilized on the porous surface of sulfonated polyetheretherketone (SPEEK) using polydopamine (PDA), resulting in a novel material designated as DFO-PDA@SPEEK (DFO-PS). DFO-PS induced macrophage M2 phenotype polarization, reduced inflammatory factor expression, promoted osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs), and enhanced implant osseointegration and osteogenic capacity. In vitro evaluation demonstrated that DFO-PS significantly modulated immune and inflammatory responses, promoted angiogenesis, and enhanced osteogenic differentiation. In the rat model with femoral bone defects, in comparison to the control group, the DFO-PS group exhibited a 1.22-fold increase in trabecular thickness and a 1.51-fold enhancement in maximum pull-out force. This work demonstrates that DFO-PS is a promising material for constructing multifunctional implants with biomineralization and immunomodulation properties for bone joint replacement.
1. Introduction
Joint replacement surgery is the most crucial intervention for end-stage bone and joint disorders, including osteoarthritis, deformities, and femoral head necrosis.1,2 However, approximately 18% of patients experience aseptic loosening postoperatively, imposing substantial economic burdens on both society and patients, highlighting the urgent need for developing next-generation prosthetic materials that enhance osseointegration between implants and surrounding bone tissue.3 Polyetheretherketone (PEEK), an aromatic polymer compound containing chain segments in its molecular backbone, has emerged as one of the most promising polymeric implant materials in orthopedics due to its excellent biocompatibility, the absence of imaging artifacts, its bone-like elastic modulus, and its superior wear resistance.4,5 Nevertheless, the long-term in vivo application of PEEK implants is limited by its poor osseointegration and weak anti-infection capabilities due to surface bioinertness.6,7 Polydopamine (PDA) exhibits multiple advantageous properties, including hydrophilicity, appropriate roughness, biocompatibility, antibacterial activity, cell adhesion, and osteogenic potential. These characteristics make it suitable for modifying orthopedic implant surfaces, promoting bone reconstruction through its strong adhesive properties and dopamine (DA) release.8 The high adhesive properties of PDA enable it to serve as an intermediate layer facilitating “dual modification” with other functional bone reconstruction materials, such as growth factors, nanoparticles, peptides, and hydrogels.9 During bone remodeling, PDA degradation leads to the release of DA into the surrounding microenvironment, playing a crucial role in regulating DA receptors on osteoblasts and osteoclasts.10Deferoxamine mesylate (DFO), a hexadentate molecule that binds to plasma iron in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio and forms aluminoxanes with plasma aluminum, is a chelating agent used to treat iron and aluminum toxicity and is eliminated through biliary or renal excretion.11 It was reported that DFO reduces the release of local inflammatory factors (TNF-α, IL-1β, and IL-6) and fibrosis following radiation or surgery while increasing local vascular endothelial growth factor (VEGF) expression.12 Momeni et al.13 documented the clinical application of DFO in a young male patient undergoing maxillary distraction osteogenesis, observing greater new bone area and density in the DFO treatment group. Lintel et al.14 found that DFO promotes local collagen deposition and microvessel growth, improving radiation therapy-induced wound healing in mice. It was also reported that DFO, in conjunction with three-dimensional scaffolds, enhances human BMSC osteogenic gene expression and vascularization at fracture sites.15
1 molar ratio and forms aluminoxanes with plasma aluminum, is a chelating agent used to treat iron and aluminum toxicity and is eliminated through biliary or renal excretion.11 It was reported that DFO reduces the release of local inflammatory factors (TNF-α, IL-1β, and IL-6) and fibrosis following radiation or surgery while increasing local vascular endothelial growth factor (VEGF) expression.12 Momeni et al.13 documented the clinical application of DFO in a young male patient undergoing maxillary distraction osteogenesis, observing greater new bone area and density in the DFO treatment group. Lintel et al.14 found that DFO promotes local collagen deposition and microvessel growth, improving radiation therapy-induced wound healing in mice. It was also reported that DFO, in conjunction with three-dimensional scaffolds, enhances human BMSC osteogenic gene expression and vascularization at fracture sites.15
Based on these previous research studies, a scientific hypothesis is proposed in this study: DFO loaded PDA-SPEEK may achieve dual advantages. First, PDA's adhesive properties serving as an intermediate layer could facilitate the attachment of functional bone reconstruction materials (such as DFO), thereby enhancing the osseointegration of SPEEK implants. Second, exogenously released DFO could promote macrophage M2 polarization, protecting bone formation from inflammatory inhibition in the implant microenvironment while enhancing osteogenic differentiation of BMSCs. Consequently, functional bone reconstruction materials may achieve better contact and fusion with bone tissue following artificial joint implantation, improving the stability and durability of prosthetic-bone connections.
2. Materials and methods
The animal experiments were conducted at the Shanghai Laboratory Animal Center, Chinese Academy of Sciences, under the approval of the Animal Care and Experimentation Committee (approval number: 20211201039).2.1. Preparation, characterization, and biocompatibility of DFO-PS
2.2. Effects of DFO-PS on the M2 polarization of the macrophage
In vitro and in vivo experiments were conducted to evaluate the effects of DFO-PS on macrophage M2 polarization and its osteogenic mechanisms.For in vitro studies, five groups of materials (10 mm × 10 mm × 1 mm discs) were placed in 24-well plates (n = 3 per group). RAW264.7 cells (5 × 105 cells per well) were seeded and cultured in DMEM supplemented with 100 U mL−1 penicillin, 100 U mL−1 streptomycin, and 10% FBS at 37 °C with 5% CO2. Culture medium was replaced after 24 h of cell attachment. After 3 days of culture (80% confluence), the secretion of IL-4, IL-10, TNF-α, and IL-6 was measured using ELISA. The mRNA expression levels of polarization markers (CD86 and CD163) and osteogenic factors (BMP-2, VEGF) were analyzed by RT-PCR. Immunofluorescence staining was performed to assess iNOS and CD206 (Mannose receptor) protein expression.
For in vivo studies, air pouches were created by subcutaneous injection of 5 mL of sterile air into BALB/c mice dorsa. After 3 days, sterilized material discs from the five groups were implanted into the air pouches under sterile conditions, respectively. Animals were maintained under SPF conditions with free access to water and food. After 1 week, the implant sites were irrigated with 1 mL of PBS, and the lavage fluid was collected for IL-4, IL-10, TNF-α, and IL-6 analysis by ELISA. Surrounding tissues were harvested for RT-PCR analysis of CD86, CD163, BMP-2, and VEGF expression, and immunofluorescence examination of iNOS and CD206.
2.3. DFO-PS enhanced rBMSC osteogenic differentiation in vitro
Two sets of in vitro experiments were conducted: direct osteogenic differentiation and macrophage polarization-induced osteogenic differentiation.Material discs (10 mm × 10 mm × 1 mm) from five groups (Con, PS, 1DFO-PS, 5DFO-PS, and 25DFO-PS) were placed in a 24-well plate (n = 3 per group). RAW264.7 cells or rBMSCs (5 × 105 cells per well) were cultured in DMEM supplemented with 100 U mL−1 penicillin, 100 U mL−1 streptomycin, and 10% FBS at 37 °C with 5% CO2. Conditioned medium (CM) was prepared by mixing the RAW264.7 culture supernatant with complete DMEM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
For the transwell migration assay, rBMSCs (1 × 105 cells per sample) were seeded in the upper chamber of transwells. Two experimental setups were designed to evaluate both direct and indirect effects on rBMSC migration. Direct effects: material discs from different experimental groups were placed in the lower chamber containing DMEM to assess their direct influence on rBMSC migration. Indirect macrophage-mediated effects on cell migration: conditioned medium collected from RAW264.7 cells previously cultured with different material groups was added to the lower chamber, without the presence of material discs, to evaluate the macrophage-mediated effects on rBMSC migration. After 24 hours of incubation, migrated cells on the bottom surface of the transwell membrane were quantified using optical microscopy.
For osteogenic differentiation studies, rBMSCs were cultured with either DMEM or CM for 14 days. Osteogenic gene expression (ALP, OCN, COL-1, BMP-2, OPN, RUNX2, BSP, and OC) was analyzed by RT–PCR. ALP staining and Alizarin Red S (ARS) staining were performed to assess mineralization capacity and calcium deposition, respectively.
2.4. DFO-PS enhanced rBMSC osteogenic differentiation in vivo
2.5. Real-time quantitative PCR
Total RNA was isolated from dorsal skin tissues, RAW264.7 cells, or rBMSCs using Trizol (12183555, Invitrogen, California, USA) and reverse transcribed using reverse transcriptase (A48571, Thermo Fisher Scientific Inc., Waltham, USA). RT-PCR was performed in 96-well plates using the SYBR Premix Ex Taq kit (RR001B, TaKaRa, Dalian, China) with 20 μL reaction volume on an ABI 750 system (Applied Biosystems, Carlsbad, USA). PCR conditions were 5 min initial denaturation at 94 °C, followed by 36 cycles at 94 °C for 30 s, annealing for 40 s, and extension at 72 °C for 40 s. The reaction was completed with a final extension at 72 °C for 5 min. Data were normalized to GAPDH and analyzed using the 2−ΔΔCT method (n = 3). All primer sequences were obtained from Sangon (Shanghai Shengong Biological Engineering Co., LTD Shanghai, China) (Table 1).| Target gene | Oligonucleotide sequence |
|---|---|
| Mice CD89 | 5′-TGGGCGCAGAGAAACTTGAT-3′ (forward) |
| 5′-AAGCCCGTGTCCTTGATCTG-3′ (reverse) | |
| Mice CD163 | 5′-GTGGTCAACTCCGCTTGGTA-3′ (forward) |
| 5′-CTTGGGGCACCATCTGTGAT-3′ (reverse) | |
| Mice BMP-2 | 5′-AACGAGAAAAGCGTCAAGCC-3′ (forward) |
| 5′-AGGTGCCACGATCCAGTCAT-3′ (reverse) | |
| Mice VEGF | 5′-GCAAGAGAAGACACGGTGGT-3′ (forward) |
| 5′-CAGGAGGTGGGGTAAGGAG-3′ (reverse) | |
| Rats BMP-2 | 5′-AGTAGTTTCCAGCACCGAATTA-3′ (forward) |
| 5′-CACTAACCTGGTGTCCAATAGT-3′ (reverse) | |
| Rats OPN | 5′-CTTGAGCATTCCAAAGAGAGC-3′ (forward) |
| 5′-CTTGTGGCTGTGAAACTTGTG-3′ (reverse) | |
| Rats RUNX-2 | 5′-GCTGTTGTGATGCGTATTCCC-3′ (forward) |
| 5′-TTGAACCTGGCCACTTGGTT-3′ (reverse) | |
| Rats BSP | 5′-ACAACACTGCGTATGAAACCTATGAC-3′ (forward) |
| 5′-AGTAATAATCCTGACCCTCGTAGCC-3′ (reverse) | |
| Rats OC | 5′-AGGGATGAAGCGTTTCTTAGGTTTTG-3′ (forward) |
| 5′-AGGATGCTGTGGTTGGTGACTG-3′ (reverse) | |
| Rats GAPDH | 5′-AGTGCCAGCCTCGTCTCATG-3′ (forward) |
| 5′-GATGGTGATGGGTTTCCCGT-3′ (reverse) |
2.6. Statistical analysis
Graphpad Prism 9 (Graphpad Software, La Jolla, California) was used to perform the statistical analysis. The data were presented as the mean ± standard deviation (mean ± SD). Unpaired Student's t test was used to determine significances between two groups, while multiple groups were compared by one-way ANOVA. Statistical significance was defined as p < 0.05 for all analyses.3. Results
3.1. Material characterization of DFO-PS
With increasing DFO concentration, the drug accumulation on the porous surface became denser, leading to faster release rates and higher maximum release concentrations of DFO-PS (Fig. 1B). Due to the poor solubility of DFO, the liquid contact angles of DFO-PS gradually increased with higher DFO concentrations (Fig. 1C). Fig. 1D shows the scanning electron microscopy images of DFO-PS. Through surface modification methods, DFO was successfully loaded onto the surface and within the pores of SPEEK. In summary, DFO was effectively loaded onto both the surface and internal pores of SPEEK, providing a good foundation for the osteogenic modification of SPEEK.3.2. Biocompatibility analysis of DFO-PS in RAW264.7 macrophages and rBMSCs and in rats
To evaluate the potential cytotoxicity of DFO-PS, RAW264.7 macrophages and rBMSCs were selected as experimental models. LDH release and cell viability assays revealed that DFO concentrations exceeding 64 mg mL−1 resulted in elevated LDH release and decreased cell numbers (Fig. 2B). Based on these findings, DFO concentrations of 1, 5, and 25 mg mL−1 were selected to modify PS materials, yielding 1DFO-PS, 5DFO-PS, and 25DFO-PS, respectively.As illustrated in Fig. 2A, both RAW264.7 and rBMSCs exhibited favorable adhesion characteristics on Con (SPEEK), PS (PDA-SPEEK), and 25DFO-PS (25DFO-PDA-SPEEK) surfaces, with extensive pseudopod formation. Additionally, immunofluorescence analysis revealed well-developed and uniformly distributed cytoskeletal structures (Fig. 2C). While high DFO concentrations could lead to cell injury and death, the maximum DFO-PS concentration was established at 25 mg mL−1 to ensure biocompatibility.
In vivo biocompatibility was assessed using a murine model. A subcutaneous implantation of 25 mg mL−1 DFO-PS showed no significant reduction in body weight over a 4-week observation period (Fig. 2D). Histological examination of major organs using H&E staining at days 0, 7, and 28 revealed no pathological abnormalities across all experimental groups (Fig. 2E). At 4 weeks post-implantation, peripheral blood analysis was conducted to evaluate hematological parameters, including RDW, lymphocytes, WBC, HGB, granulocytes, HCT, PLT, RBC, MPV, MCV, monocytes, and MCH (Fig. 2F). Furthermore, serum biochemical markers including TBIL, AST, CREA, UA, CK, ALP, BUN, ALT, and LDH were monitored (Fig. 2G) and no statistically significant differences were observed among groups.
These comprehensive analyses demonstrate that, while high concentrations of DFO-PS exhibited cytotoxicity toward RAW264.7 and rBMSCs, the optimized concentration of 25 mg mL−1 DFO-PS showed no significant toxicity in both cellular and animal models. Statistical analysis was performed using one-way ANOVA followed by Tukey's post hoc test (p < 0.05 considered statistically significant).
3.3. Modulation of DFO-PS on macrophage polarization in vitro
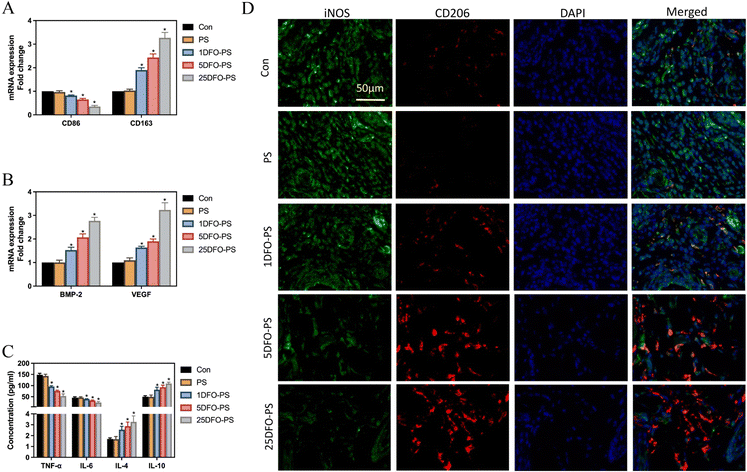
In vitro studies demonstrated that after 3-day co-culture with RAW264.7 macrophages, DFO-PS significantly enhanced the secretion of anti-inflammatory cytokines, IL-4 and IL-10 (Fig. 3A), while simultaneously suppressing the production of pro-inflammatory cytokines, TNF-α and IL-6 (Fig. 3B). The 25DFO-PS group exhibited the most pronounced anti-inflammatory effects. Additionally, DFO-PS significantly upregulated the expression of osteoinductive factor BMP-2 and tissue repair factor VEGF in macrophages (Fig. 3C).Further investigations revealed that DFO-PS remarkably induced phenotypic alterations in macrophages. Specifically, DFO-PS treatment resulted in decreased expression of the M1 polarization marker CD86 while increasing the expression of the M2 polarization marker CD163. This phenomenon demonstrated a concentration-dependent manner, where higher DFO loading corresponded to lower CD86 expression and higher CD163 expression levels (Fig. 3D). Immunofluorescence analysis further confirmed that DFO-PS suppressed the expression of the M1 polarization marker iNOS (Fig. 3E) while enhancing the expression of the M2 polarization marker CD206 (Fig. 3F).
These findings demonstrate that DFO-PS effectively promotes M1-to-M2 macrophage polarization in vitro, resulting in decreased pro-inflammatory cytokine secretion, enhanced anti-inflammatory cytokine production, and elevated expression of osteoinductive and tissue repair factors.
3.4. Modulation of DFO-PS on macrophage polarization in vivo
In vivo studies conducted on peri-implant tissue at 2 weeks post-implantation demonstrated that DFO-PS modulated macrophage polarization in a dose-dependent manner. RT-PCR analysis revealed suppressed expression of M1 marker CD86 mRNA concurrent with enhanced expression of M2 marker CD163 mRNA, exhibiting a clear dose-dependent relationship (Fig. 4A). Analysis of peri-implant tissue demonstrated upregulated expression of osteoinductive factor BMP-2 and tissue repair factor VEGF mRNA levels in DFO-PS treated groups (Fig. 4B).ELISA analysis of peri-implant tissue revealed that DFO-PS significantly attenuated the secretion of pro-inflammatory cytokines TNF-α and IL-6 while enhancing the production of anti-inflammatory cytokines IL-4 and IL-10 (Fig. 4C). The 25DFO-PS group exhibited the most pronounced effects among all treatment groups. Immunofluorescence analysis demonstrated markedly decreased expression of M1 marker iNOS accompanied by significantly enhanced expression of M2 marker CD206. These alterations showed a positive correlation with DFO concentration, where higher DFO loading resulted in more pronounced phenotypic changes (Fig. 4D).
These findings collectively demonstrate that subcutaneous implantation of DFO-PS effectively promotes M1-to-M2 macrophage polarization in vivo, characterized by reduced pro-inflammatory cytokine secretion, enhanced anti-inflammatory cytokine production, and elevated expression of osteoinductive and tissue repair factors.
3.5. Direct and indirect macrophage-mediated effects of DFO-PS on rBMSC osteogenic differentiation in vitro
The in vitro studies demonstrated both direct and macrophage-mediated effects of DFO-PS on rBMSC osteogenic differentiation. Direct co-culture with DFO-PS significantly enhanced rBMSC migration capacity (Fig. 5A) and upregulated the expression of osteogenic-related genes, including ALP, OCN, COL-1, BMP-2, OPN, RUNX2, BSP, and OC (Fig. 5B). The osteogenic potential demonstrated a positive correlation with DFO loading concentration, as evidenced by enhanced ALP activity and increased calcium deposition through ARS staining at day 14 (Fig. 5C).The indirect effects mediated through macrophage-conditioned medium revealed that RAW264.7 cells treated with DFO-PS secreted factors that significantly enhanced rBMSC chemotaxis (Fig. 5D) and promoted the expression of osteogenic markers (ALP, OCN, COL-1, BMP-2, OPN, RUNX2, BSP, and OC) (Fig. 5E). Furthermore, rBMSCs cultured in macrophage-conditioned medium exhibited enhanced ALP activity and increased matrix mineralization, as demonstrated by intensified ALP and ARS staining at day 14 (Fig. 5F).
These findings demonstrate that DFO-PS promotes osteogenic differentiation of rBMSCs through both direct effects, potentially mediated by surface-enriched DFO, and indirect macrophage-mediated effects via modulation of macrophage secretory profiles.
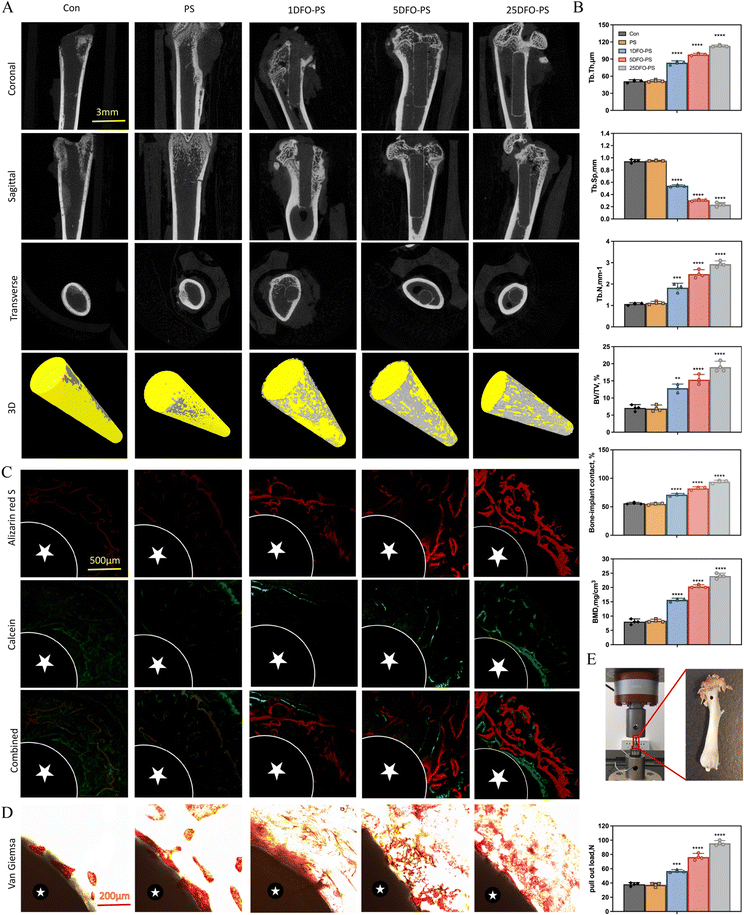
3.6. Osteogenic effects of DFO-PS after 8-week femoral implantation
The osseointegration efficacy of DFO-PS was evaluated in a rat femoral defect model over an 8-week period. Micro-CT analysis revealed enhanced peri-implant bone formation in DFO-PS treated groups (Fig. 6A). Quantitative assessment demonstrated increased BMD, BIC, BV/TV, Tb.N, and Tb.Th values, accompanied by decreased Tb.Sp, indicating enhanced bone growth and remodeling in DFO-PS treated specimens (Fig. 6B).Sequential fluorescence labeling demonstrated a concentration-dependent increase in new bone formation, with the 25DFO-PS group exhibiting the most substantial osteogenic response (Fig. 6C). Van Gieson staining revealed enhanced collagen fiber formation in the peri-implant region (Fig. 6D), indicating improved extracellular matrix organization and bone formation.
Biomechanical testing demonstrated a significant dose-dependent increase in maximum pull-out force with increasing DFO concentration (Fig. 6E), indicating enhanced implant stability and osseointegration. These findings collectively demonstrate that DFO-PS effectively enhances new bone formation in vivo, while simultaneously improving implant stability through enhanced bone-implant integration.
4. Discussion
PEEK has emerged as an outstanding biomaterial for orthopedic applications, offering several crucial advantages. Its mechanical properties closely match human cortical bone, which helps reduce stress shielding, while providing excellent wear resistance and long-term stability. PEEK's clinical benefits include radiolucent properties allowing artifact-free medical imaging, outstanding chemical stability, and proven biocompatibility.18 Additionally, PEEK's ability to be modified through sulfonation (SPEEK) and its suitability for surface functionalization make it ideal for our application where both mechanical performance and surface modification capability are essential.19 The incorporation of PDA into our material design strategy plays a crucial role in modulating macrophage responses. PDA's unique surface chemistry, characterized by catechol and quinone groups, enables specific interactions with cell membrane proteins and extracellular matrix components.20 Studies have demonstrated that PDA-modified surfaces exhibit excellent biocompatibility and can regulate immune cell response.21 PDA surface modification can effectively reduce the immune response to implanted biomaterials. Specifically, PDA coating decreased macrophage adhesion and activation, leading to reduced inflammatory cell infiltration and fibrous capsule formation around the implants. These findings suggest that PDA surface modification can serve as a simple yet effective strategy to modulate immune responses and improve the biological performance of implanted materials.22 In this study, DFO-PS was synthesized by combining PDA with SPEEK and loading DFO, utilizing the adhesive properties of the intermediate layer to facilitate functional bone remodeling material formation while employing exogenous stimulation of PDA and DFO to modulate immune responses and promote osseointegration. The findings demonstrate that DFO-PS enhances new bone formation and prosthetic integration through multiple pathways and mechanisms. These results contribute significantly to improving artificial joint adaptability, biocompatibility, and surgical success rates, potentially prolonging post-operative joint functionality, thereby providing crucial guidance and innovation for artificial joint technology development and patient rehabilitation.This study demonstrated that DFO-PS exhibited excellent properties and biocompatibility. Loading DFO onto the porous surface or within SPEEK effectively enhanced the surface bioactivity and drug release characteristics of DFO-PS. Superior biocompatibility is fundamental for developing next-generation artificial joint prosthesis materials. DFO-PS prepared at concentrations ≤25 mg mL−1 exhibited favorable cellular biocompatibility with both RAW264.7 and rBMSCs. Furthermore, weight monitoring, major organ H&E staining, and peripheral blood cell and biochemical parameter analyses confirmed the excellent in vivo biocompatibility of DFO-PS.
Investigation of the synergistic mechanisms between DFO-PS and rBMSCs revealed both direct and indirect osteogenic effects. DFO-PS directly stimulated peri-implant bone growth in rat femur by upregulating target genes involved in rBMSC osteogenic differentiation, including ALP, OCN, COL-1, BMP-2, OPN, RUNX2, BSP, and OC. These genes encode matrix proteins and transcription factors crucial for osteoblast proliferation, differentiation, and matrix formation. Dey et al.23 demonstrated that Symphytum officinale promotes BMSC osteogenic differentiation genes RUNX2, OPN, OC, and ALP, suggesting its potential use in orthopedic implant surface coatings. Ding et al.24 found that ginsenoside compound K induces rBMSC osteogenic differentiation genes and increases new bone BMD, BV/TV, and callus volume. Akshaya et al.25 identified valproic acid as a promising surface modification coating for artificial joint prostheses, showing good affinity with mouse BMSCs and promoting osteogenic differentiation. In this study, it was found that DFO-PS could promote osteogenesis through indirect mechanisms by interacting with RAW264.7 macrophages, releasing osteoinductive factor BMP-2 and tissue repair factor VEGF, which promoted peri-implant bone formation by inducing adjacent mesenchymal stem cell differentiation.
DFO-PS demonstrated significant modulatory effects on macrophage polarization by suppressing M1 markers (CD86 and iNOS) while enhancing M2 markers (CD163 and CD206), thereby facilitating the M1-to-M2 polarization of RAW264.7 macrophages. M1 macrophages are primarily involved in inflammatory responses and early tissue repair, whereas M2 macrophages exhibit anti-inflammatory and tissue-regenerative properties. The M2 macrophage-induced microenvironment has been shown to promote BMSC osteogenic differentiation.26–28 Recent studies have demonstrated various approaches to enhance osteogenesis through macrophage modulation. Wang et al.29 identified adrenomedullin 2 as an effective joint prosthesis coating that induces M2 polarization and subsequent BMSC osteogenic differentiation, showing favourable bone healing properties in type 1 diabetic rat tibial fracture models. Hamlet et al.30 demonstrated that titanium alloy surface modification significantly upregulated CD163 expression, promoting M2 polarization and enhancing TGF-BMP signaling pathway-related gene expression. β-Tricalcium phosphate (β-TCP), as reported by Zheng,31 promoted M2 polarization through CD206 upregulation, subsequently enhancing the expression of osteogenic genes including ALP, OPN, OC, Runx2, COL-1, and ATF in BMSCs. Additionally, Romero-Lopez et al.32 found that IL-4-supplemented photocrosslinked methacrylated gelatin 3D hydrogel scaffolds increased CD206, CCL17, and CCL18 expression, creating an osteogenic microenvironment through M2 polarization.
DFO-PS enhanced new bone formation and osseointegration by promoting calcium deposition and collagen fiber formation, thereby strengthening the bone–implant interface. This effect was achieved through reduced pro-inflammatory cytokine secretion (TNF-α and IL-6) and increased anti-inflammatory factors (IL-4 and IL-10), while simultaneously stimulating osteoinductive factor BMP-2 and tissue repair factor VEGF production. Geng et al.33 demonstrated strontium ranelate's ability to suppress TNF-α and IL-1β secretion while promoting RunX2, OCN, and OPG expression. Kamboj et al.34 developed a 400-μm pore size scaffold using silicon powder and wollastonite, which enhanced IL-8 and TGF-β expression, creating an osteoimmune environment conducive to BMSC differentiation. Croes et al.35 identified TLR-2 activators derived from inactivated S. aureus as potential joint interface coatings, demonstrating reduced inflammatory infiltration and enhanced bone formation.
While our current study demonstrates encouraging functional outcomes, including enhanced M2 macrophage polarization, improved angiogenesis, and superior osseointegration by DFO-PS implantation, further mechanistic investigations would strengthen our findings. Building on other researchers’ groundbreaking works,36 our future work will focus on (1) exploring detailed molecular mechanisms, particularly the HIF-1α pathway and its downstream effects; (2) investigating the cross-talk between immune cells and BMSCs in response to DFO-PS; (3) incorporating advanced characterization techniques for surface–biological interactions; (4) examining the temporal relationship between immunomodulation and enhanced bone formation.
In summary, DFO-PS promotes bone regeneration and prosthetic integration through multiple mechanisms as shown in Fig. 7: (1) enhancing osteogenic gene expression (ALP, OCN, COL-1, BMP-2, OPN, RUNX2, BSP, and OC), (2) modulating macrophage polarization from M1 to M2 phenotype, and (3) creating a pro-regenerative microenvironment through cytokine modulation. These findings suggest DFO-PS as a promising component for next-generation joint prostheses with significant clinical translation potential.
5. Conclusions
This study successfully developed DFO-PS, a novel multifunctional material, for artificial joint prostheses, by immobilizing DFO on SPEEK through PDA-mediated modification. DFO-PS demonstrated enhanced osseointegration through multiple mechanisms, including upregulation of osteogenic genes, M1-to-M2 macrophage polarization, and optimization of the local microenvironment. In vivo evaluation revealed a 1.22-fold increase in trabecular thickness and a 1.51-fold enhancement in maximum pull-out force compared to controls. These findings establish DFO-PS as a promising candidate for next-generation artificial joint prostheses with integrated biomineralization and immunomodulation properties, offering significant potential for clinical translation.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
All authors declare that they have no competing financial interests or personal relationships that could appear to influence the work reported in this paper.Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (grant no. 82102539 and 82272513) and the Shanghai Sailing Program (grant no. 20YF1436100 and 21YF1433800).References
- U. G. Longo, R. Papalia, G. Salvatore, S. M. Tecce, A. Jedrzejczak, M. Marcozzi, I. Piergentili and V. Denaro, BMC Surg., 2022, 22, 355 CrossRef PubMed.
- D. N. Bracey, K. Barry, H. S. Khanuja and V. Hegde, J. Am. Acad. Orthop. Surg., 2022, 30, 443–447 Search PubMed.
- H. M. Kremers, J. L. Howard, Y. Loechler, C. D. Schleck, W. S. Harmsen, D. J. Berry, M. E. Cabanela, A. D. Hanssen, M. W. Pagnano, R. T. Trousdale and D. G. Lewallen, J. Bone Jt. Surg., Am. Vol., 2012, 94, e82 CrossRef PubMed.
- P. Soares Machado, A. C. Cadore Rodrigues, E. T. Chaves, A. H. Susin, L. F. Valandro, G. K. R. Pereira and M. P. Rippe, J. Adhes. Dent., 2022, 24, 233–245 Search PubMed.
- B. Pidhatika, V. T. Widyaya, P. C. Nalam, Y. A. Swasono and R. Ardhani, Polymers, 2022, 14 Search PubMed.
- M. Paglia, M. Beretta, V. Quinzi and S. Colombo, Eur. J. Paediatr. Dent., 2022, 23, 137–139 Search PubMed.
- N. Muthiah, Y. U. Yolcu, N. Alan, N. Agarwal, D. K. Hamilton and A. Ozpinar, Eur. Spine J., 2022, 31, 2547–2556 Search PubMed.
- M. L. Alfieri, T. Weil, D. Y. W. Ng and V. Ball, Adv. Colloid Interface Sci., 2022, 305, 102689 CrossRef PubMed.
- S. El Yakhlifi and V. Ball, Colloids Surf., B, 2020, 186, 110719 CrossRef CAS PubMed.
- E. Schwendich, L. Salinas Tejedor, G. Schmitz, M. Rickert, J. Steinmeyer, S. Rehart, S. Tsiami, J. Braun, X. Baraliakos, J. Reinders, E. Neumann, U. Muller-Ladner and S. Capellino, Cells, 2022, 11, 1609 CrossRef CAS PubMed.
- J. Velasquez and A. A. Wray, StatPearls, StatPearls Publishing, Copyright © 2023, Treasure Island (FL), 2023.
- R. Tevlin, M. T. Longaker and D. C. Wan, Adv. Wound Care, 2022, 11, 548–559 CrossRef PubMed.
- A. Momeni, S. Rapp, A. Donneys, S. R. Buchman and D. C. Wan, J. Craniofac. Surg., 2016, 27, 880–882 CrossRef PubMed.
- H. Lintel, D. B. Abbas, C. V. Lavin, M. Griffin, J. L. Guo, N. Guardino, A. Churukian, G. C. Gurtner, A. Momeni, M. T. Longaker and D. C. Wan, J. Transl. Med., 2022, 20, 274 CrossRef CAS PubMed.
- M. Pfeiffenberger, A. Damerau, I. Ponomarev, C. H. Bucher, Y. L. Chen, D. Barnewitz, C. Thone-Reineke, P. Hoff, F. Buttgereit, T. Gaber and A. Lang, J. Bone Miner. Res., 2021, 36, 1189–1201 CrossRef CAS.
- W. Liu, J. H. Li, M. Q. Cheng, Q. J. Wang, K. W. K. Yeung, P. K. Chu and X. L. Zhang, Adv. Sci., 2018, 5, 1800749 CrossRef.
- S. Sang, C. Yang, H. Chai, X. Yuan, W. Liu and X. Zhang, Chem. Eng. J., 2021, 420, 130059 CrossRef.
- P. Sikder, Acta Biomater., 2025, 191, 29–52 CrossRef PubMed.
- M. Kauke-Navarro, L. Knoedler, S. Knoedler, C. Deniz and A. F. Safi, Front. Surg., 2024, 11, 1351749 CrossRef PubMed.
- Y. L. Liu, K. L. Ai and L. H. Lu, Chem. Rev., 2014, 114, 5057–5115 CrossRef PubMed.
- N. Wang, Y. Yang, X. Y. Wang, X. H. Tian, W. X. Qin, X. J. Wang, J. S. Liang, H. J. Zhang and X. F. Leng, ACS Biomater. Sci. Eng., 2019, 5, 2330–2342 CrossRef PubMed.
- S. K. Hong, K. Y. Kim, H. J. Wook, S. Y. Park, K. D. Lee, D. Y. Lee and H. S. Lee, Nanomedicine, 2011, 6, 793–801 CrossRef CAS.
- D. Dey, P. Jingar, S. Agrawal, V. Shrivastava, A. Bhattacharya, J. Manhas, B. Garg, M. T. Ansari, A. R. Mridha, V. Sreenivas, A. Khurana and S. Sen, J. Ethnopharmacol., 2020, 248, 112329 CrossRef CAS.
- L. Ding, S. Gu, B. Zhou, M. Wang, Y. Zhang, S. Wu, H. Zou, G. Zhao, Z. Gao and L. Xu, Front. Pharmacol., 2022, 13, 855393 CrossRef CAS PubMed.
- N. Akshaya, P. Prasith, B. Abinaya, B. Ashwin, S. V. Chandran and N. Selvamurugan, Curr. Mol. Pharmacol., 2021, 14, 27–35 CrossRef CAS.
- E. Oba, N. Y. Aung, R. Ohe, M. Sadahiro and M. Yamakawa, Am. J. Transl. Res., 2020, 12, 1728–1740 CAS.
- D. Naot, B. Pool, A. Chhana, R. Gao, J. T. Munro, J. Cornish and N. Dalbeth, Arthritis Res. Ther., 2022, 24, 212 CrossRef CAS PubMed.
- J. Munoz, N. S. Akhavan, A. P. Mullins and B. H. Arjmandi, Nutrients, 2020, 12, 2999 Search PubMed.
- F. Wang, L. Kong, W. Wang, L. Shi, M. Wang, Y. Chai, J. Xu and Q. Kang, Stem Cell Res. Ther., 2021, 12, 288 CrossRef CAS PubMed.
- S. M. Hamlet, R. S. B. Lee, H. J. Moon, M. A. Alfarsi and S. Ivanovski, Clin. Oral. Implants Res., 2019, 30, 1085–1096 CrossRef.
- M. Zheng, M. Weng, X. Zhang, R. Li, Q. Tong and Z. Chen, Biomed. Mater., 2021, 16, 025005 CrossRef CAS.
- M. Romero-Lopez, Z. Li, C. Rhee, M. Maruyama, J. Pajarinen, B. O'Donnell, T. H. Lin, C. W. Lo, J. Hanlon, R. Dubowitz, Z. Yao, B. A. Bunnell, H. Lin, R. S. Tuan and S. B. Goodman, Tissue Eng., Part A, 2020, 26, 1099–1111 CrossRef CAS.
- T. Geng, X. Chen, M. Zheng, H. Yu, S. Zhang, S. Sun, H. Guo and Q. Jin, Mol. Med. Rep., 2018, 18, 1849–1857 CAS.
- N. Kamboj, J. Kazantseva, R. Rahmani, M. A. Rodriguez and I. Hussainova, Mater. Sci. Eng., C, 2020, 116, 111223 CrossRef CAS PubMed.
- M. Croes, M. C. Kruyt, W. Boot, B. Pouran, M. V. Braham, S. A. Pakpahan, H. Weinans, H. C. Vogely, A. C. Fluit, W. J. Dhert, J. Alblas and F. C. Oner, Eur. Cells Mater., 2019, 37, 402–419 CrossRef CAS.
- X. Shi, Z. W. Liu, X. Y. Ren, W. X. Wang, H. Zhang, Y. G. Wang, M. Liu, Q. Yao and W. G. Wu, Int. J. Biol. Macromol., 2025, 284, 137968 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ma01179a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |