Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
MoSe2-based room temperature gas sensor with a sub-parts-per-billion limit for ammonia and N,N-dimethylformamide†
Virendra Singh
Choudhary
a,
Ramandeep
Singh
a,
Ashok
Kumar
 a,
C. S.
Yadav
b,
Sandeep
Sharma
a,
C. S.
Yadav
b,
Sandeep
Sharma
 d,
Joel
Garcia
d,
Joel
Garcia
 *c and
Surender Kumar
Sharma
*c and
Surender Kumar
Sharma
 *a
*a
aDepartment of Physics, School of Basic Sciences, Central University of Punjab, Bathinda 151401, India. E-mail: surender.sharma@cup.edu.in
bDepartment of Physics, Indian Institute of Technology, Mandi, 175075, India
cDepartment of Chemistry, De La Salle University, Manila, Philippines. E-mail: joel.garcia@dlsu.edu.ph
dDepartment of Physics and Photonics Science, National Institute of Technology, Hamirpur, Himachal Pradesh 177005, India
First published on 7th March 2025
Abstract
A limit of detection of toxic gases at the level of ppb is critical for industrial safety. Here, we designed a room temperature MoSe2-based sensor for dual detection of ammonia (NH3) and N,N-dimethylformamide (DMF). The MoSe2/TiO2 composite exhibits a rapid and highly selective response to both NH3 and DMF compared to other industrial analytes. The MoSe2/TiO2 heterostructures exhibit a band gap of 0.31 eV, highlighting their electronic structure, adsorption energy, and fundamental gas sensing mechanism. NH3 and DMF demonstrated robust spontaneous adsorption on the below-MoSe2 surface, exhibiting the lowest adsorption energy (−0.12 eV) and (−0.09 eV) of NH3 and DMF, respectively. Bader charge analysis revealed charge transfer from the gas molecule to the heterostructure surface, enhancing its conductivity and gas detection sensitivity. The adsorption of NH3 on the MoSe2 site is exothermic whereas on the TiO2 side it is endothermic, indicating the potential of MoSe2/TiO2 composites for efficient room-temperature gas sensing. The sensor achieved an 85% higher response to NH3 and an 80% higher response to DMF, with density functional theory (DFT) simulations confirming a high negative adsorption energy. Detection limits were calculated at 4.91 ppb for NH3 and 7.82 ppb for DMF under 40% relative humidity, with robust sensitivity across varying humidity levels. Response times were reasonably stable, with NH3 detection at 150 s and recovery in 37–110 s, while DMF was detected in 150–160 s and recovered in 45–74 s. This study highlights the potential of the MoSe2/TiO2 composite in real-time, room-temperature monitoring of both NH3 and DMF, making it a valuable tool for industrial safety and environmental monitoring without the need for external recovery mechanisms.
1. Introduction
In recent years, gas sensors have become widely used in various fields, including industrial production,1 the automotive industry,2 medical applications,3 indoor air quality supervision,4 and environmental monitoring.5 These applications demand sensors that are sensitive and selective for specific gas analytes, as well as compact and easy to manufacture. Additionally, these sensors should operate at room temperature,6 be energy-efficient, and be cost-effective compared to existing technologies.7 The emission of volatile organic compounds (VOCs) and hazardous gases has contributed to air quality degradation and poses significant risks to human health due to increasing gas pollution.8 According to the World Health Organization, air pollution is a major contributor to illness and premature death, particularly in developing countries.9Ammonia (NH3) and N,N-dimethylformamide (DMF) are two common industrial chemicals that pose significant health risks.10 NH3 is a colorless gas with a pungent odor, produced by agricultural activities and industrial processes.11 It is used in various applications like refrigeration systems, water purification, and household cleaning products. However, exposure to NH3 can cause eye irritation, burns to the nose and throat, and, in severe cases, respiratory failure.12,13 Due to its high volatility, detecting low concentrations of NH3 is crucial for safety applications. DMF is widely used as a solvent in industries such as textiles and leather,14 but exposure to DMF vapors can cause hepatotoxicity and cancer, and poses particular risks to pregnant women.15 Thus, detecting DMF at low levels is equally important.
The development of faster, lower detection limit sensors has become a priority, with numerous methods being investigated,16 including chemiresistive sensors,17 which are favored for their simplicity, cost-effectiveness, and ease of integration.18 Metal oxide semiconductors (MOSs) are commonly used in chemiresistive sensors due to their stability and high sensitivity to gases.19 However, MOS-based sensors generally require high operating temperatures to achieve optimal gas responses,20 leading to high energy consumption and complicated sensor designs.21 Conducting polymers, which can operate at room temperature, have emerged as an alternative, but they suffer from stability issues and sensitivity degradation over time.22
Recent advancements in 2D materials, particularly transition metal dichalcogenides (TMDCs), such as MoS2, MoSe2, and WS2, have brought new possibilities to gas sensing technologies.23–25 TMDCs offer a large surface area, tunable electronic properties, and layer-dependent gas-sensing characteristics, making them ideal candidates for room temperature applications.26 MoSe2, in particular, has shown promise in detecting gases like NH3, H2S, and NO2. However, challenges remain in achieving high selectivity and stability under humid conditions, a crucial factor for real-world applications in industries and environmental monitoring.27,28
In this study, we address these challenges by utilizing MoSe2/TiO2 composites for the dual detection of NH3 and DMF at room temperature. By forming a heterostructure, we aim to enhance the sensitivity and selectivity of the sensor through their synergistic interaction. Unlike previous studies, which focused on high-temperature operation of TiO2-based sensors, our work demonstrates effective room temperature operation, low detection limits, and stability under varying humidity conditions. As highlighted in Table S1 (ESI†), most existing sensors show diminished performance in humid environments,29–33 whereas our sensor exhibits consistent performance, making it a viable candidate for real-world applications. This work presents a significant advancement in sub-parts-per-billion (ppb) gas detection for both NH3 and DMF, addressing a critical need for low-cost, energy-efficient sensors that operate effectively under ambient conditions.
2. Experimental section
2.1 Materials
Pure selenium powder (Se), sodium molybdate dihydrate (Na2MoO4·2H2O), hydrazine hydrate, titanium(IV) isopropoxide Ti[OCH(CH3)2]4, and sodium boro hydrate (NaBH4) were purchased from Sigma-Aldrich, India, and used without any further purification.2.2 Synthesis of the MoSe2/TiO2 nanocomposite
The MoSe2/TiO2 composite was synthesized by the hydrothermal method. The first step involved the synthesis of TiO2. Initially, 20 mL of titanium(IV) isopropoxide was added dropwise in DI-water and stirred continuously for 30 minutes to obtain a clear solution. The resultant solution was filtered with DI water and ethanol several times and dried at 65 °C. After collecting the white precipitates, the material was calcined at 500 °C for 2 hours in a tubular furnace and this way TiO2 powder was obtained.34 This procedure reliably produces ∼1 g of TiO2 powder per batch, and similar reproducibility was observed across multiple batches.In the second step, the MoSe2/TiO2 composite was synthesized using a hydrothermal method. First, 200 mg of TiO2 powder (obtained in the previous step) was bath-sonicated in 50 mL of distilled water to obtain a suspension of TiO2. To this suspension, after adding 0.4 gm of selenium powder and 0.6 gm of sodium molybdate dihydrate, the resulting mixture was bath sonicated for 10 minutes. 0.2 g of NaBH4 and 10 mL of hydrazine hydrate were added to the above mixture. The obtained mixture was bath-sonicated for an hour, and the resulting red-colored mixture was transferred into a 100 mL Teflon-lined stainless-steel autoclave. The autoclave was maintained at 200 °C in a furnace for 48 hours and then allowed to cool to room temperature naturally. After cooling, the black precipitates were collected by filtration using filter paper, followed by multiple washes with deionized water and ethanol. The final product was dried in a vacuum oven at 60 °C for 24 hours. This sample was designated as MT21 (∼1.2 g). Subsequently, using an identical procedure but changing the weight ratio, two more samples were synthesized namely MT11 and MT12. More details can be found in Table S2 of the ESI.† It is to be noted that the same name was assigned to two terminal devices that were obtained from the respective composites.
2.3 Characterization of sensing materials
The structural and morphological characteristics of the pure and composite materials were examined using field emission scanning electron microscopy (FESEM) with a Merlin Compact model, which is equipped with energy-dispersive X-ray spectroscopy (EDX). The crystallinity of the materials was confirmed through X-ray diffraction (XRD) using a PANalytical Empyrean system and Cu-Kα radiation (wavelength: 1.54 Å). Raman spectroscopy was conducted with a RIMS-U-DC spectrometer that utilizes a 532 nm laser source. Additionally, X-ray photoelectron spectroscopy (XPS) analysis was performed using an ESCALAB instrument coupled with Omicron nanotechnology to determine the elemental composition and oxidation states of the materials.2.4 Sensor fabrication and sensing measurements
To fabricate the sensing device, 10 mg of the dried MoSe2/TiO2 composite powder was combined with two to three drops of distilled water to create a uniform paste. This paste was then applied to an alumina substrate featuring gold electrodes spaced approximately 2 mm apart, using a paintbrush. Following the application of the sensing layer, the sensor was dried in a vacuum oven at 65 °C for 2 hours. A digital photograph of the two-terminal sensor device, measuring 1 cm × 1 cm, is provided in Fig. S1 (ESI†). Sensing measurements were conducted using a homemade setup, details of which have been previously described in other studies.33,35 The sensing measurements were performed at different natural relative humidity (RH) levels (40–80%), which were recorded using a digital hygrometer. Hence, the sensing measurements mimic the real-world situation. All VOC gases with variable concentration can be obtained by evaporation of pure ethanol, methanol, acetone, propanol, formaldehyde (40 wt%), N-methyl pyrrolidone (NMP), dimethylformamide (DMF) and ammonia (25 wt%) on a small hot plate placed at the corner of the test chamber. The known volume of the respective VOC was injected into the test chamber using a Hamilton microliter syringe. The required concentrations were calculated using eqn (1).33,35 | (1) |
In this equation, C represents the concentration of various gases in parts per million (ppm), ρ denotes the density of the concentrated liquid (g mL−1), ω indicates the purity of the liquid, T signifies the temperature (K), Vs refers to the volume of the evaporated liquid (μL), M is the molecular weight of the liquid (g mol−1), and V represents the volume of the test chamber (L).
2.5 Computational methodology
We investigated the gas sensing properties of MoSe2/TiO2 heterostructures, employing first principles calculations based on density functional theory (DFT). We utilized the Vienna Ab Initio Simulation Package (VASP) with the Perdew Burke–Ernzerhof (PBE) exchange–correlation functional and the generalized gradient approximation (GGA).36–39 A substantial vacuum distance of 20 Å in the z-direction prevented interaction between two periodic images. Monkhorst–Pack k points of 8 × 8 × 1 points were used to analyze the geometric optimization of the MoSe2/TiO2 heterostructure.40 We employed the conjugate gradient method during the geometrical relaxation, with a plane-wave basis set and cutoff energy of 450 eV. The criteria for energy convergence were set at 10−4 eV.3. Results and discussion
3.1 Structural characterizations
The XRD patterns of the MoSe2/TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), MoSe2/TiO2 (1
1), MoSe2/TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and MoSe2/TiO2 (2
2), and MoSe2/TiO2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
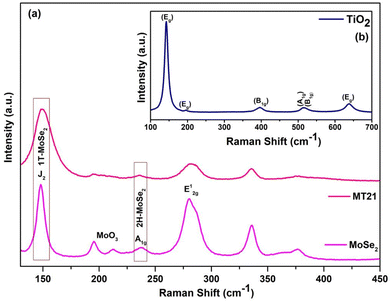
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composites were obtained in the range of 10°–80°. The XRD patterns of TiO2 calcined at 500 °C and MoSe2 are also given for comparison, as shown in Fig. S2 (ESI†). The diffraction peaks of TiO2 observed at 25.54°, 37.17°, 38.04°, 38.98°, 48.25°, 54.14°, 55.26°, 62.89°, 69.12°, 70.04°, and 75.30°, correspond to the (101), (103), (004), (112), (200), (105), (211), (204), (116), (220), and (215) crystal planes of the anatase phase of TiO2 (JCPDS 73-1764), respectively.41 Similarly, the diffraction peaks at 13.2°, 28.6°, 31.2°, 41.5°, 56.5°, and 65.3° correspond to the (002), (004), (100), (006), (008), and (108) planes, respectively, which are indexed to the hexagonal crystal structure of MoSe2 (JCPDS 29-0914).42,43 A few more peaks at 23.4°, 29.6°, 45.3°, 51.5°, and 61.6° corresponding to the (110), (130), (320), (410), and (160) planes confirm the presence of orthorhombic MoO3 (JCPDS 35-0609). In the MoSe2/TiO2 composites, the diffraction peaks corresponding to TiO2 and MoSe2 are present with a minor phase of MoO3, hence confirming the formation of a MoSe2/TiO2 composite with high purity. Fig. 1(a) shows the Raman spectrum of MoSe2 with five dominant peaks clearly observed at 149, 241, 280.8, 336, and 378 cm−1. Here, the peaks observed at 241 and 280.8 cm−1 correspond to the A1g and E12g modes of 2H-MoSe2 and the peak corresponding to 378 cm−1 predicts the interlayer modes of vibration of the Mo and Se atoms.44,45 Moreover, the additional resonance peaks in the as-prepared sample at 149 cm−1 have been identified as the J2 phonon modes of 1T-MoSe2. The peaks at 149 cm−1 and 196 cm−1 correspond to Se–Se bonding.42 The peaks at 336 and 212 cm−1 have been attributed to the O–Mo–O bending in MoO3.35 The Raman spectrum of TiO2 calcined at 500 °C is shown as an inset in Fig. 1(b). It matches with the anatase phase of TiO2, which has six Raman active modes: Eg(1) (144 cm−1), Eg(2) (197 cm−1), B1g(1) (399 cm−1), A1g/B1g(2) (overlapped at 519 cm−1), and Eg(3) (639 cm−1).46 The presence of the Eg(2) peak, which is usually very hard to detect, evidences the high degree of crystallinity of the samples. In the Raman spectrum of the MoSe2/TiO2 composite (MT21), the dominant peaks of MoSe2 are observed at 241, 282, 336, and 378 cm−1 and the peaks of TiO2 are observed at 146, 197, and 399 cm−1, respectively. Certain peaks of TiO2 have low intensity due to the larger content of MoSe2 phase. Moreover, all MoSe2 and TiO2 peaks are observed in the expected region.
1) composites were obtained in the range of 10°–80°. The XRD patterns of TiO2 calcined at 500 °C and MoSe2 are also given for comparison, as shown in Fig. S2 (ESI†). The diffraction peaks of TiO2 observed at 25.54°, 37.17°, 38.04°, 38.98°, 48.25°, 54.14°, 55.26°, 62.89°, 69.12°, 70.04°, and 75.30°, correspond to the (101), (103), (004), (112), (200), (105), (211), (204), (116), (220), and (215) crystal planes of the anatase phase of TiO2 (JCPDS 73-1764), respectively.41 Similarly, the diffraction peaks at 13.2°, 28.6°, 31.2°, 41.5°, 56.5°, and 65.3° correspond to the (002), (004), (100), (006), (008), and (108) planes, respectively, which are indexed to the hexagonal crystal structure of MoSe2 (JCPDS 29-0914).42,43 A few more peaks at 23.4°, 29.6°, 45.3°, 51.5°, and 61.6° corresponding to the (110), (130), (320), (410), and (160) planes confirm the presence of orthorhombic MoO3 (JCPDS 35-0609). In the MoSe2/TiO2 composites, the diffraction peaks corresponding to TiO2 and MoSe2 are present with a minor phase of MoO3, hence confirming the formation of a MoSe2/TiO2 composite with high purity. Fig. 1(a) shows the Raman spectrum of MoSe2 with five dominant peaks clearly observed at 149, 241, 280.8, 336, and 378 cm−1. Here, the peaks observed at 241 and 280.8 cm−1 correspond to the A1g and E12g modes of 2H-MoSe2 and the peak corresponding to 378 cm−1 predicts the interlayer modes of vibration of the Mo and Se atoms.44,45 Moreover, the additional resonance peaks in the as-prepared sample at 149 cm−1 have been identified as the J2 phonon modes of 1T-MoSe2. The peaks at 149 cm−1 and 196 cm−1 correspond to Se–Se bonding.42 The peaks at 336 and 212 cm−1 have been attributed to the O–Mo–O bending in MoO3.35 The Raman spectrum of TiO2 calcined at 500 °C is shown as an inset in Fig. 1(b). It matches with the anatase phase of TiO2, which has six Raman active modes: Eg(1) (144 cm−1), Eg(2) (197 cm−1), B1g(1) (399 cm−1), A1g/B1g(2) (overlapped at 519 cm−1), and Eg(3) (639 cm−1).46 The presence of the Eg(2) peak, which is usually very hard to detect, evidences the high degree of crystallinity of the samples. In the Raman spectrum of the MoSe2/TiO2 composite (MT21), the dominant peaks of MoSe2 are observed at 241, 282, 336, and 378 cm−1 and the peaks of TiO2 are observed at 146, 197, and 399 cm−1, respectively. Certain peaks of TiO2 have low intensity due to the larger content of MoSe2 phase. Moreover, all MoSe2 and TiO2 peaks are observed in the expected region.
X-ray photoelectron spectroscopy (XPS) was employed to investigate the chemical states and electronic structure of MoSe2, TiO2, and the MoSe2/TiO2 composite (Fig. 2 and Fig. S4, S5, ESI†). Fig. S4 (ESI†) features the complete survey spectra, confirming the presence of molybdenum (Mo), selenium (Se), titanium (Ti), and oxygen (O) in the MoSe2/TiO2 composite. The high-resolution spectra for Mo 3d, Se 3d, Ti 2p, and O 1s are illustrated in Fig. 2(a)–(d). The Mo signal shown in Fig. 2(a) can be fitted into three sets of doublet peaks. The lower binding energy doublet peaks (231.4 eV for Mo 3d3/2 and 228.3 eV for Mo 3d5/2) correspond to the 1T-MoSe2 phase.47–49 The doublet peaks at medium binding energies (232.1 eV for Mo 3d3/2 and 229 eV for Mo 3d5/2) are associated with the 2H-MoSe2 phase,47–49 while the peaks at higher energies (235.7 eV for Mo 3d3/2 and 232.6 eV for Mo 3d5/2) are attributed to Mo in the +6-oxidation state.50 This analysis indicates the presence of the 1T phase in the synthesized MoSe2 sample. The Se 3d spectrum, shown in Fig. 2(b), exhibits a significantly broadened peak profile, which can be fitted into two sets of doublet peaks. The lower energy doublet (54.7 eV for Se 3d3/2 and 53.7 eV for Se 3d5/2) corresponds to the 1T phase, while the higher energy doublet (55.3 eV for Se 3d3/2 and 54.3 eV for Se 3d5/2) represents the 2H phase.51Fig. 2(c) displays the Ti 2p spectra, where two peaks at 458.8 eV and 464.5 eV are assigned to Ti 2p3/2 and Ti 2p1/2, respectively, confirming the presence of Ti4+ and indicating the dominant valence state of +4 for titanium in TiO2. In the O 1s spectra shown in Fig. 2(d), peaks at 530.7 eV and 532 eV correspond to the oxygen-deficient structure of TiO2 and the O2− oxidation state in TiO2, respectively.51,52 Overall, the XPS analysis clearly indicates that the various samples contain traces of both 1T and 2H phases of MoSe2. The individual MoSe2 and TiO2 XPS data are provided in Fig. S5 (ESI†).
 | ||
Fig. 2 XPS spectra of MoSe2/TiO2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composites, including (a) Mo 3d spectrum, (b) Se 3d spectrum, (c) Ti 2p spectrum, and (d) O 1s spectrum. 1) composites, including (a) Mo 3d spectrum, (b) Se 3d spectrum, (c) Ti 2p spectrum, and (d) O 1s spectrum. | ||
3.2 Electrical and gas-sensing characteristics of the MoSe2/TiO2 composite
The electrical properties of the MoSe2/TiO2 composites and MoSe2 are studied using current–voltage (I–V) measurements at room temperature and shown in Fig. S6(a) (ESI†). Both devices exhibited linear curves in a fixed voltage range, suggesting their ohmic nature. This advocates that MoSe2 and the MoSe2/TiO2 composite exhibit semiconducting properties.13 Fig. S6(b) (ESI†) displays the I–V curves when a MoSe2 based two-terminal device was exposed to three different concentrations of DMF (3, 10, 20 ppm) at 30 °C. With increased DMF concentration, the current level increases, indicating a decrease in device resistance in the presence of DMF. Almost similar behavior with increased current levels was displayed by the MoSe2/TiO2-based devices, thus indicating that the latter has improved sensitivity towards DMF. Due to their sensitivity towards DMF, the devices were also tested with different levels of ammonia (see Fig. S6, ESI† panels (d) and (e)). The MoSe2-based devices have shown better sensitivity towards NH3 than DMF. In panel (f), a joint comparison between two different analytes is displayed for MoSe2/TiO2. As clear from the data, the device exhibited superior detection of NH3 than that of DMF at 30 °C. Therefore, it is clear that the devices exhibit ohmic behavior and selective behavior towards NH3 and DMF. More detailed sensing measurements were performed with different levels of NH3, as shown in Fig. 3. As we see, when a device (MoSe2/TiO2) is exposed to NH3, its resistance decreases. And then after removal of NH3 from the test chamber, the resistance again recovered to its initial value, which indicates that the MoSe2/TiO2 composite exhibits a n-type character. As evident from Fig. S6(f) (ESI†), in a separate I–V measurement at 30 °C, when a MoSe2/TiO2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) device was exposed to DMF and NH3, an increase in current level was observed at a fixed voltage, thereby indicating that the composites exhibit n-type conductivity.45 These changes in resistance in response to different analytes serve as a basis for their detection.
1) device was exposed to DMF and NH3, an increase in current level was observed at a fixed voltage, thereby indicating that the composites exhibit n-type conductivity.45 These changes in resistance in response to different analytes serve as a basis for their detection.
Prior to conducting detailed sensing experiments, a representative measurement was performed at 30 °C and 40% relative humidity (RH) with 1 ppm of NH3, as shown in Fig. 3 panel (a). The resistance of the device in air (Ra) decreased from 15.16 kΩ to 15.01 kΩ when exposed to NH3 (Rg). This change in resistance allows for the calculation of various sensor parameters. Specifically, the absolute change in resistance in the presence of gas molecules is defined as ΔR = Rg − Ra. The relative response, expressed as a percentage, is calculated using the formula [ΔR/Ra] × 100. The sensor response time (tresponse) is defined as the duration required for the sensor to reach 90% of its maximum response upon exposure to the target gas, while the recovery time (trecovery) is the time taken to return to a response value that is 10% above the maximum sensor response. A comparative analysis of the sensing performance between five different devices—MoSe2, TiO2, MoSe2/TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), MoSe2/TiO2 (1
1), MoSe2/TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and MoSe2/TiO2 (2
2), and MoSe2/TiO2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composites, is illustrated in Fig. 3(a) and (c) and Fig. S7 (ESI†). Fig. S7a (ESI†) shows that the MoSe2 sensor exhibits a 0.9% response at 20 ppm gas concentration under 40% humidity at room temperature. Fig. S7b (ESI†) represents the TiO2 sensor, where the lower intrinsic conductivity of TiO2 at room temperature might have contributed to the unstable response. However, the MoSe2/TiO2 (1
1) composites, is illustrated in Fig. 3(a) and (c) and Fig. S7 (ESI†). Fig. S7a (ESI†) shows that the MoSe2 sensor exhibits a 0.9% response at 20 ppm gas concentration under 40% humidity at room temperature. Fig. S7b (ESI†) represents the TiO2 sensor, where the lower intrinsic conductivity of TiO2 at room temperature might have contributed to the unstable response. However, the MoSe2/TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and MoSe2/TiO2 (1
1) and MoSe2/TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) composites exhibited unstable signals when exposed to NH3 gas, as shown in Fig. S7c and d (ESI†). This instability can be attributed to the improper balance between the components in the composite. The higher proportion of TiO2 in these ratios may have hindered the active sites required for NH3 adsorption and disrupted the charge transfer mechanism between MoSe2 and TiO2, leading to signal fluctuations. Additionally, the lower intrinsic conductivity of TiO2 at room temperature, particularly in higher TiO2 ratios, might have further contributed to the unstable response. In Fig. 3, these measurements were conducted for 1 ppm of NH3 at both room temperature and at 40% and 80% RH. The advantages of employing composite materials over pure MoSe2 or TiO2 are evident, attributed to factors such as increased specific surface area, the presence of defects, oxygen vacancies, and the synergistic effects that arise from composite formation.35,53,54Fig. 3(a) and (c) present the response transients at 40% and 80% RH, respectively. The baseline resistance of the sensor varies between the two RH conditions (40% and 80% RH) due to the presence of water molecules in the atmosphere. At higher humidity levels, more water molecules are adsorbed on the surface of the sensor, which can contribute to an increase in the baseline resistance due to the formation of a resistive water layer. This phenomenon is common in semiconducting metal oxides and TMDC-based sensors, where the adsorption of water molecules leads to changes in charge carrier density, impacting the sensor's conductivity. Panels Fig. 3(b) and (d) depict the relative response against NH3 concentration. The response showed a linear relationship from 1 to 100 ppm, with saturation observed at higher concentrations for both RH levels. The fitting curves for the sensor response versus NH3 concentration demonstrated strong linear correlations, with coefficients of determination (R2) of 0.95 and 0.99 for 40% and 80% RH, respectively. From the linear fitting, the theoretical limit of detection (LOD) can be calculated using the equation known as sensitivity, defined as three times the standard deviation (σ) of sensor noise divided by the slope (s) of the fitted curve, as expressed in eqn (2).
2) composites exhibited unstable signals when exposed to NH3 gas, as shown in Fig. S7c and d (ESI†). This instability can be attributed to the improper balance between the components in the composite. The higher proportion of TiO2 in these ratios may have hindered the active sites required for NH3 adsorption and disrupted the charge transfer mechanism between MoSe2 and TiO2, leading to signal fluctuations. Additionally, the lower intrinsic conductivity of TiO2 at room temperature, particularly in higher TiO2 ratios, might have further contributed to the unstable response. In Fig. 3, these measurements were conducted for 1 ppm of NH3 at both room temperature and at 40% and 80% RH. The advantages of employing composite materials over pure MoSe2 or TiO2 are evident, attributed to factors such as increased specific surface area, the presence of defects, oxygen vacancies, and the synergistic effects that arise from composite formation.35,53,54Fig. 3(a) and (c) present the response transients at 40% and 80% RH, respectively. The baseline resistance of the sensor varies between the two RH conditions (40% and 80% RH) due to the presence of water molecules in the atmosphere. At higher humidity levels, more water molecules are adsorbed on the surface of the sensor, which can contribute to an increase in the baseline resistance due to the formation of a resistive water layer. This phenomenon is common in semiconducting metal oxides and TMDC-based sensors, where the adsorption of water molecules leads to changes in charge carrier density, impacting the sensor's conductivity. Panels Fig. 3(b) and (d) depict the relative response against NH3 concentration. The response showed a linear relationship from 1 to 100 ppm, with saturation observed at higher concentrations for both RH levels. The fitting curves for the sensor response versus NH3 concentration demonstrated strong linear correlations, with coefficients of determination (R2) of 0.95 and 0.99 for 40% and 80% RH, respectively. From the linear fitting, the theoretical limit of detection (LOD) can be calculated using the equation known as sensitivity, defined as three times the standard deviation (σ) of sensor noise divided by the slope (s) of the fitted curve, as expressed in eqn (2).
 | (2) |
For ammonia (NH3), the limit of detection (LOD) was determined to be ∼4.91 ppb at 40% relative humidity (RH) and ∼10.02 ppb at 80% RH. The sensitivity of the sensor, represented by the slope (s), was calculated to be 0.049 per ppm at 40% RH and 0.02 per ppm at 80% RH. Notably, the response of the composite sensor exhibited minimal variation as humidity levels increased, highlighting the importance of response stability and consistency in practical sensor applications. Stability and repeatability, along with long-term durability, are critical parameters for assessing gas sensor performance. The sensor's response to 5, 10, and 40 ppm NH3 is illustrated in Fig. S8(c) (ESI†), where the device demonstrated consistent performance across multiple cycles with negligible changes in response transients. Recovery times varied, being shorter or longer than the response times at 40% and 80% RH.
To evaluate sensor reproducibility, we prepared two additional sensors using the same methodology. The response transient curves for these devices, which are MoSe2/TiO2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) sensors, are presented in Fig. S8(a) and (b) (ESI†). These figures represent the response to increasing NH3 concentrations from 1 ppm to 100 ppm and decreasing concentrations from 100 ppm to 1 ppm. Both sensors were tested under 40% and 80% humidity at room temperature, demonstrating consistent performance under varying humidity conditions. Over a continuous testing period of 10 weeks, the MoSe2/TiO2 (2
1) sensors, are presented in Fig. S8(a) and (b) (ESI†). These figures represent the response to increasing NH3 concentrations from 1 ppm to 100 ppm and decreasing concentrations from 100 ppm to 1 ppm. Both sensors were tested under 40% and 80% humidity at room temperature, demonstrating consistent performance under varying humidity conditions. Over a continuous testing period of 10 weeks, the MoSe2/TiO2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite sensor was exposed to 10 ppm NH3, as shown in Fig. S8(d) (ESI†). Remarkably, there was no significant decrease in response, with only slight variation, indicating excellent long-term durability and stability. As clearly seen, the sensing curves in Fig. S8d (ESI†) were constructed based on measurements taken at different time intervals-such as the 1st week, 2nd week, 3rd week, and so on. These measurements were then compiled into a single figure to provide a comprehensive overview of the sensor's performance over time. The variations observed in the curves can be attributed to slight fluctuations in humidity and temperature during each set of measurements conducted under real-world conditions. Additionally, the gas sensing experiments were carried out using a homemade gas sensing setup. In this setup, gas is injected using a syringe, which may introduce small variations in gas concentration due to manual handling. While we strive for precision, minor human error and variations in gas injection can occur, leading to slight differences in the sensing curves. Nevertheless, the robustness, accuracy, and reliability of the MoSe2/TiO2 (2
1) composite sensor was exposed to 10 ppm NH3, as shown in Fig. S8(d) (ESI†). Remarkably, there was no significant decrease in response, with only slight variation, indicating excellent long-term durability and stability. As clearly seen, the sensing curves in Fig. S8d (ESI†) were constructed based on measurements taken at different time intervals-such as the 1st week, 2nd week, 3rd week, and so on. These measurements were then compiled into a single figure to provide a comprehensive overview of the sensor's performance over time. The variations observed in the curves can be attributed to slight fluctuations in humidity and temperature during each set of measurements conducted under real-world conditions. Additionally, the gas sensing experiments were carried out using a homemade gas sensing setup. In this setup, gas is injected using a syringe, which may introduce small variations in gas concentration due to manual handling. While we strive for precision, minor human error and variations in gas injection can occur, leading to slight differences in the sensing curves. Nevertheless, the robustness, accuracy, and reliability of the MoSe2/TiO2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) sensor were further demonstrated by consistent responses across all tested conditions, reinforcing confidence in its ability to deliver accurate measurements in real-world applications. As depicted in Fig. 4 panels “a” and “b”, the response time remained consistent at 150 s across both humidity levels 40% and 80%, while recovery times were 37 s and 110 s, respectively. The response time under higher humidity (80% RH) is generally higher compared to lower humidity (40% RH). This is because the water molecules adsorbed on the surface of the sensor may act as a barrier, slowing down the adsorption of ammonia (NH3) molecules onto the active sites of the composite material. Water molecules can compete with ammonia for adsorption sites, leading to slower response times. Conversely, at lower humidity levels (40% RH), fewer water molecules are present, allowing NH3 molecules to interact more freely with the sensor's surface, thus reducing the response time. Selectivity and specificity are also vital for high-performance chemiresistive gas sensors. The MoSe2/TiO2 (2
1) sensor were further demonstrated by consistent responses across all tested conditions, reinforcing confidence in its ability to deliver accurate measurements in real-world applications. As depicted in Fig. 4 panels “a” and “b”, the response time remained consistent at 150 s across both humidity levels 40% and 80%, while recovery times were 37 s and 110 s, respectively. The response time under higher humidity (80% RH) is generally higher compared to lower humidity (40% RH). This is because the water molecules adsorbed on the surface of the sensor may act as a barrier, slowing down the adsorption of ammonia (NH3) molecules onto the active sites of the composite material. Water molecules can compete with ammonia for adsorption sites, leading to slower response times. Conversely, at lower humidity levels (40% RH), fewer water molecules are present, allowing NH3 molecules to interact more freely with the sensor's surface, thus reducing the response time. Selectivity and specificity are also vital for high-performance chemiresistive gas sensors. The MoSe2/TiO2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite sensor was tested at 30 °C and 40% RH against a variety of volatile organic compounds (VOCs), including NH3, N,N-dimethylformamide (DMF), acetone, ethanol, methanol, propanol, formaldehyde (FMD), and N-methyl-2-pyrrolidone (NMP), each at a concentration of 150 ppm, excluding NH3 and DMF. The sensor exhibited significantly higher responses to NH3 (≈2.6%) and DMF (≈2.4%) at 150 ppm compared to the other gases, underscoring its high selectivity towards NH3 and DMF, as shown in Fig. 4(c).
1) composite sensor was tested at 30 °C and 40% RH against a variety of volatile organic compounds (VOCs), including NH3, N,N-dimethylformamide (DMF), acetone, ethanol, methanol, propanol, formaldehyde (FMD), and N-methyl-2-pyrrolidone (NMP), each at a concentration of 150 ppm, excluding NH3 and DMF. The sensor exhibited significantly higher responses to NH3 (≈2.6%) and DMF (≈2.4%) at 150 ppm compared to the other gases, underscoring its high selectivity towards NH3 and DMF, as shown in Fig. 4(c).
Because the MoSe2/TiO2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite sensor is more sensitive to DMF, we evaluated its performance at low concentrations and compared it with other sensors, as shown in Fig. S9 (ESI†). One can see that the MoSe2 signal is visible, but TiO2 does not show any signal at room temperature. Additionally, no signal is observed for the MoSe2/TiO2 (1
1) composite sensor is more sensitive to DMF, we evaluated its performance at low concentrations and compared it with other sensors, as shown in Fig. S9 (ESI†). One can see that the MoSe2 signal is visible, but TiO2 does not show any signal at room temperature. Additionally, no signal is observed for the MoSe2/TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and MoSe2/TiO2 (1
1) and MoSe2/TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) composites with DMF. The reason that the TiO2, MoSe2/TiO2 (1
2) composites with DMF. The reason that the TiO2, MoSe2/TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and MoSe2/TiO2 (1
1), and MoSe2/TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) composites do not show any significant signal in DMF can be attributed to several factors. Firstly, TiO2 has low intrinsic conductivity at room temperature, which limits its ability to respond to DMF molecules, particularly without external stimuli such as elevated temperatures. In the case of MoSe2/TiO2 (1
2) composites do not show any significant signal in DMF can be attributed to several factors. Firstly, TiO2 has low intrinsic conductivity at room temperature, which limits its ability to respond to DMF molecules, particularly without external stimuli such as elevated temperatures. In the case of MoSe2/TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and (1
1) and (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) composites, the higher proportion of TiO2 in these composites may have reduced the number of active sites available for DMF adsorption on the MoSe2 surface. This imbalance in the composite structure likely disrupts the charge transfer process between MoSe2 and TiO2, making it less effective in detecting DMF. Furthermore, the presence of a higher amount of TiO2 in these ratios might hinder the overall sensor response by diluting the electroactive MoSe2 component, which is primarily responsible for detecting DMF molecules. As a result, no significant signal is observed for these composites at room temperature.
2) composites, the higher proportion of TiO2 in these composites may have reduced the number of active sites available for DMF adsorption on the MoSe2 surface. This imbalance in the composite structure likely disrupts the charge transfer process between MoSe2 and TiO2, making it less effective in detecting DMF. Furthermore, the presence of a higher amount of TiO2 in these ratios might hinder the overall sensor response by diluting the electroactive MoSe2 component, which is primarily responsible for detecting DMF molecules. As a result, no significant signal is observed for these composites at room temperature.
Interestingly, the relative response of the MoSe2/TiO2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite sensor with varying levels of DMF ranging is strikingly different (see Fig. 5 and Fig. S10, ESI†). The response exhibited a linear variation from 3 to 70 ppm and tended to saturate at higher concentrations at both RH (40% and 80%) levels. The fitting curves of the sensor response versus the DMF concentration (ppm) displayed a good linear correlation with R2 = 0.99, for different humidity levels. From linear fitting, one can calculate the theoretical LOD to be ∼7.82 and ∼7.71 ppb at RH 40% and RH 80%, respectively. To assess the reproducibility of the sensors, we fabricated two additional MoSe2/TiO2 (2
1) composite sensor with varying levels of DMF ranging is strikingly different (see Fig. 5 and Fig. S10, ESI†). The response exhibited a linear variation from 3 to 70 ppm and tended to saturate at higher concentrations at both RH (40% and 80%) levels. The fitting curves of the sensor response versus the DMF concentration (ppm) displayed a good linear correlation with R2 = 0.99, for different humidity levels. From linear fitting, one can calculate the theoretical LOD to be ∼7.82 and ∼7.71 ppb at RH 40% and RH 80%, respectively. To assess the reproducibility of the sensors, we fabricated two additional MoSe2/TiO2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) sensors using the same methodology. The response transient curves for these sensors are shown in Fig. S10(a) and (b) (ESI†). These figures illustrate the sensor responses to increasing DMF concentrations, ranging from 3 ppm to 70 ppm, and then decreasing concentrations from 70 ppm to 3 ppm. Both sensors were evaluated under 40% and 80% humidity at room temperature, demonstrating consistent and reliable performance across varying humidity levels. Fig. S10(c) (ESI†) presents repeatability cycles for the MoSe2/TiO2 (2
1) sensors using the same methodology. The response transient curves for these sensors are shown in Fig. S10(a) and (b) (ESI†). These figures illustrate the sensor responses to increasing DMF concentrations, ranging from 3 ppm to 70 ppm, and then decreasing concentrations from 70 ppm to 3 ppm. Both sensors were evaluated under 40% and 80% humidity at room temperature, demonstrating consistent and reliable performance across varying humidity levels. Fig. S10(c) (ESI†) presents repeatability cycles for the MoSe2/TiO2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite sensor response to 20 and 40 ppm DMF concentration. Over a continuous testing period of 10 weeks, the MoSe2/TiO2 (2
1) composite sensor response to 20 and 40 ppm DMF concentration. Over a continuous testing period of 10 weeks, the MoSe2/TiO2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite sensor was exposed to 10 ppm DMF, as shown in Fig. S10(d) (ESI†). Remarkably, there was no significant diminution in response, with only slight variation, indicating excellent long-term durability and stability. The robustness, accuracy, and reliability of the MoSe2/TiO2 (2
1) composite sensor was exposed to 10 ppm DMF, as shown in Fig. S10(d) (ESI†). Remarkably, there was no significant diminution in response, with only slight variation, indicating excellent long-term durability and stability. The robustness, accuracy, and reliability of the MoSe2/TiO2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) sensor were further demonstrated by consistent responses across all tested conditions, reinforcing confidence in its ability to deliver accurate measurements in real-world applications. Similar to NH3 sensing experiments, the measurements for DMF sensing were also taken at regular intervals such as the 1st week, 2nd week, 3rd week, and so on. These results were compiled into a single figure to provide a comprehensive view of the sensor's performance over time. The slight variations observed can be attributed to environmental factors such as fluctuations in humidity and temperature. As with the ammonia experiments, the gas was injected manually using a syringe in a homemade gas sensing setup, which may have introduced minor variations in gas concentration. Despite these small inconsistencies, the sensor's performance remained consistent, further demonstrating its reliability for real-world applications. Fig. 4(b) shows the DMF response time (160 s, and 150 s) and recovery time (74 s, 45 s) of different humidity conditions 40% and 80%. Fig. 4(d) shows the relative responses of four different composite sensors under varying humidity and analytes. We observed that the highest response occurs with NH3 when the room temperature is at 40% humidity, indicating that the sensor performs best under these conditions.
1) sensor were further demonstrated by consistent responses across all tested conditions, reinforcing confidence in its ability to deliver accurate measurements in real-world applications. Similar to NH3 sensing experiments, the measurements for DMF sensing were also taken at regular intervals such as the 1st week, 2nd week, 3rd week, and so on. These results were compiled into a single figure to provide a comprehensive view of the sensor's performance over time. The slight variations observed can be attributed to environmental factors such as fluctuations in humidity and temperature. As with the ammonia experiments, the gas was injected manually using a syringe in a homemade gas sensing setup, which may have introduced minor variations in gas concentration. Despite these small inconsistencies, the sensor's performance remained consistent, further demonstrating its reliability for real-world applications. Fig. 4(b) shows the DMF response time (160 s, and 150 s) and recovery time (74 s, 45 s) of different humidity conditions 40% and 80%. Fig. 4(d) shows the relative responses of four different composite sensors under varying humidity and analytes. We observed that the highest response occurs with NH3 when the room temperature is at 40% humidity, indicating that the sensor performs best under these conditions.
3.3 Density functional theory studies
We also studied the adsorption behavior of NH3 and DMF on these different surfaces of heterostructure and the value of relevant adsorption energy and optimum distance, as illustrated in Table 1.
| Gas species | Adsorption site | E ads (eV) | Optimum distance d (Å) |
|---|---|---|---|
| NH3 | Below-MoSe2 | −0.12 | 3.45 |
| top-TiO2 | 0.27 | 2.98 | |
| DMF | below-MoSe2 | −0.09 | 3.25 |
| top-TiO2 | −0.05 | 2.76 |
We determined the adsorption energy (Eads) using the below equation. The equation can be represented as:
| Eads = Ehetero+molecule − Ehetero − Emolecule |
We investigated the adsorption energy (Eads) of NH3 molecules and DMF at various adsorption sites on the MoSe2/TiO2 heterostructure. NH3 exhibits the lowest Eads value (−0.12 eV) and is most favourable at the below-MoSe2 surface, as compared to the top-TiO2 surface. Notably, the Eads value for DMF at both the below-MoSe2 and top-TiO2 sites is negative, indicating spontaneous and heat-releasing adsorption mechanisms. DMF exhibits lowest absorption energy (−0.09 eV) at the below-MoSe2 surface, illustrated in Table 1, highlighting its strong interaction with this substrate compared to the top-TiO2 surface.
To deeply examine the impact of the NH3 and DMF adsorption on the MoSe2/TiO2 heterostructures, we calculated the electronic band structure, which revealed that the heterostructure exhibits an indirect band gap of (0.31 eV), significantly increased by approximately (0.09 eV) after NH3 adsorption on the below-MoSe2 surface, as shown in Fig. 6(a) and (c). However, this change is attributed to the heterostructure's quantum confinement effects, surface dipoles, and charge redistribution. Additionally, we examined the projected density of states (PDOS) for each constituent atom. Notably, Mo and Ti atoms predominantly influence the electronic state near the Fermi level in the heterostructure, depicted in Fig. 6(b) and (d).
Furthermore, we examined the absorption behaviour of DMF on the below-MoSe2 surface, and the bandgap slightly increased (0.36 eV), as depicted in Fig. S13(a) (ESI†). Additionally, we computed the projected density of states (PDOS) as shown in Fig. S13(b) (ESI†), highlighting the intricate interaction between the constituent atom and adsorbate, emphasizing the importance of the heterostructure modulating the electronic behaviour of materials.
We analyzed the Bader charge of NH3 and DMF on the MoSe2/TiO2 heterostructure. A positive value of Bader charge (0.01e) is observed, confirming physisorption,55 highlighting the high sensitivity of the MoSe2 surface toward the NH3 molecule,53,56 as shown in Fig. 7(a) and (b). The small negative value of Bader charge (−0.09e) of DMF indicates a slight electron accumulation on the MoSe2 surface as depicted in Fig. 7(c) and (d), affecting the electronic properties of the heterostructure, crucial for gas sensing application.57
The charge transfer influences the surface charge distribution, impacting the material's overall reactivity and interactions with other adsorbates. The amount of charge transfer indicates the exchange of electrons within this system. The Bader charge analysis obtained the charge transfer (Q) outcomes, while a positive value of NH3 (electron are transfer from the monolayer to NH3 molecule) and a negative value of DMF (electron are transfer from DMF to the monolayer) suggests depletion and accumulation, respectively.57 The charge transfer was determined through Bader analysis, as shown in Table 2. Based on the adsorption energy and Bader charge analysis, we conclude that the below-MoSe2 surface of the heterostructure exhibits strong adsorption capability for NH3 and DMF.
| Gas species | Atom | Q transfer (e) | Bader charge (e) |
|---|---|---|---|
| NH3 | N | −1.25 | 0.01 |
| H | 0.43 | ||
| H | 0.43 | ||
| H | 0.40 | ||
| DMF | O | −0.85 | −0.09 |
| N | −0.59 | ||
| C | −0.32 | ||
| C | −0.34 | ||
| C | 0.73 | ||
| H | 0.18 | ||
| H | 0.20 | ||
| H | 0.19 | ||
| H | 0.18 | ||
| H | 0.19 | ||
| H | 0.22 | ||
| H | 0.15 |
3.4 Sensing mechanism and discussion
The experimental findings demonstrated that the MoSe2/TiO2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) hybrid material exhibited enhanced sensing properties, particularly as an NH3 gas sensor. The sensing mechanism for MoSe2/TiO2 (2
1) hybrid material exhibited enhanced sensing properties, particularly as an NH3 gas sensor. The sensing mechanism for MoSe2/TiO2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) based chemiresistive sensors relies on variations in electrical conductance caused by the adsorption and subsequent charge transfer between gas molecules and the material's surface. As discussed earlier, the MoSe2/TiO2 (2
1) based chemiresistive sensors relies on variations in electrical conductance caused by the adsorption and subsequent charge transfer between gas molecules and the material's surface. As discussed earlier, the MoSe2/TiO2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) sensor had the most pronounced response (negative relative response) to NH3. Additionally, density functional theory (DFT) simulations were employed to assess the adsorption energy (Eads) and the charge transfer (e) for reducing gases like NH3. The computed values for NH3 were Eads = −0.12 eV and e = 0.01e. The negative adsorption energy and significant charge transfer from the MoSe2 surface indicate a spontaneous, exothermic physisorption process. The operating principle of the MoSe2/TiO2 sensor is based on semiconductor behavior, where the surface resistance is regulated by the adsorption of gas molecules. When NH3 molecules adsorb and desorb from the MoSe2/TiO2 surface, it leads to a measurable change in resistance, enabling the detection of NH3.58 In semiconductors, O2 molecules may capture free electrons to form oxygen ions (such as O2−, O−, or O22−). This surface reaction can be described by the following equations:
1) sensor had the most pronounced response (negative relative response) to NH3. Additionally, density functional theory (DFT) simulations were employed to assess the adsorption energy (Eads) and the charge transfer (e) for reducing gases like NH3. The computed values for NH3 were Eads = −0.12 eV and e = 0.01e. The negative adsorption energy and significant charge transfer from the MoSe2 surface indicate a spontaneous, exothermic physisorption process. The operating principle of the MoSe2/TiO2 sensor is based on semiconductor behavior, where the surface resistance is regulated by the adsorption of gas molecules. When NH3 molecules adsorb and desorb from the MoSe2/TiO2 surface, it leads to a measurable change in resistance, enabling the detection of NH3.58 In semiconductors, O2 molecules may capture free electrons to form oxygen ions (such as O2−, O−, or O22−). This surface reaction can be described by the following equations:| O2(air) → O2(ads), | (3) |
| O2(ads) + e− → O2(ads)−, | (4) |
Based on the structural design of the sensing material and theoretical principles, when the MoSe2/TiO2 sensor is exposed to reducing gases like NH3, the surface-adsorbed oxygen species interact with NH3 molecules, leading to the production of N2, H2O, and the release of free electrons into the conduction band. These electrons recombine with holes, which lowers the Schottky barrier height and reduces the thickness of the electron depletion layer (EDL). As a result, the sensor experiences a decrease in resistance.
| 4NH3 + 3O2− → 2N2 + 6H2O + 3e− | (5) |
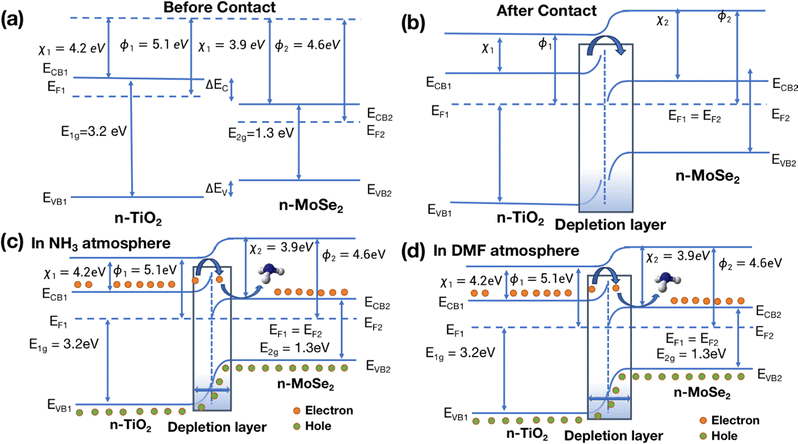
The MoSe2/TiO2 surface shows a distinct response to oxygen and ammonia gases. Both MoSe2 and TiO2 exhibit n-type semiconductor behavior, with TiO2 having a bandgap of 3.2 eV and a work function of 5.2 eV (anatase phase).59 In contrast, n-type MoSe2 has a bandgap of 1.3 eV and a work function of 4.6 eV.60 The formation of a composite leads to the creation of an n–n type heterostructure. As illustrated in Fig. 8(a), the lower work function of MoSe2 results in its Fermi level being higher than that of TiO2. When the two materials come into contact, electrons are transferred from MoSe2 (with a lower work function) to TiO2 (with a higher work function), resulting in the development of a depletion layer at the junction until the Fermi levels of both materials equalize as shown in Fig. 8(b). Electrons in the accumulation layer of TiO2 combine with oxygen from the environment to form O2− ions. When the MoSe2/TiO2 heterostructure is exposed to NH3, the gas interacts with the adsorbed oxygen species, releasing electrons into the MoSe2 conduction band. This leads to an increase in electron concentration in MoSe2 and a reduction in hole concentration in TiO2, thereby thinning the depletion layer and reducing the sensor's resistance, as shown in Fig. 8(c). A similar decrease in resistance (negative relative response) is observed when the sensor is exposed to DMF, showing a significant response, second only to NH3.
| 21O2− + 4(C3H7NO) → 12CO2 + 14H2O + 4NO2 + 21e− | (6) |
When the sensor is exposed to DMF vapors, the DMF molecules interact with the oxygen species adsorbed on the sensor's surface, leading to the release of electrons into the conduction band. This interaction increases the electron concentration and enhances the hole concentration in the n–n type junctions of the MoSe2/TiO2 composite. Consequently, as shown in Fig. 8(d), the depletion layer becomes thinner, resulting in a decrease in the sensor's resistance.
4. Conclusion
In this study, MoSe2/TiO2 composite-based sensors were successfully fabricated and tested for gas sensing at room temperature. Both experimental and theoretical analyses demonstrated selective and rapid responses to NH3 and DMF compared to other analytes. The sensor exhibited a significantly higher response, approximately 85% for NH3 and 80% for DMF, compared to other gases tested. Adsorption characteristics revealed a feasible exothermic physisorption process for both gases, supported by the negative adsorption energy values. DFT calculations further confirmed these results, with the computed bandgap of the MoSe2/TiO2 heterostructure slightly increasing from 0.31 eV to 0.36 eV after DMF adsorption, and to 0.40 eV after NH3 adsorption, indicating improved electronic properties for gas sensing applications. Bader charge analysis revealed a charge transfer of 0.01e from NH3 to the MoSe2/TiO2 heterostructure, enhancing its conductivity and sensitivity. The Bader charge value of −0.09e for DMF indicated weak physisorption, with minimal electron accumulation on the MoSe2 surface, affecting conductivity and overall reactivity. These results suggest that the MoSe2/TiO2 composite is well-suited for reversible gas sensing. The modulated band gap, alongside the negative adsorption energy results, underscores the potential of the MoSe2/TiO2 composite for NH3 and DMF detection. The sensor demonstrated impressive room-temperature performance, with calculated limits of detection for NH3 of approximately 4.91 ppb at 40% relative humidity and 10.02 ppb at 80% relative humidity, with sensitivity slopes of 0.049 and 0.02 per ppm, respectively. For DMF, the limits of detection were 7.82 ppb at 40% RH and 7.71 ppb at 80% RH. The sensor showed response times of 150 s and 160 s for DMF at 40% and 80% RH, respectively, with recovery times of 45 s and 74 s. For NH3, the response time was consistent at 150 s under both humidity conditions, while the recovery times were 37 s at 40% RH and 110 s at 80% RH.Moreover, the sensor exhibited stable and repeatable performance during long-term durability assessments. The ability to operate at room temperature without requiring additional recovery mechanisms confirms the practical usability of the device. These findings strongly support the potential of MoSe2/TiO2 heterostructures as highly efficient and selective sensors for NH3 and DMF detection in ambient conditions.
Author contributions
Virendra Singh Choudhary: conceptualization, methodology, investigation, formal analysis, writing – original draft, visualization. Ramandeep & Ashok Kumar: investigation, methodology, DFT calculations, data curation. C. S. Yadav: characterization, XPS data acquisition. Sandeep Sharma: manuscript editing. Joel Garcia: manuscript editing. Surender Kumar Sharma: supervision, manuscript editing, final review.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are very thankful to the Department of Physics, Central University of Punjab for providing these facilities and UGC-DAE Consortium for Scientific Research, India for providing financial support (CSR-IC-ISUM-54/CRS-337/2020-21/795).References
- S. Hong, M. Wu, Y. Hong, Y. Jeong, G. Jung, W. Shin, J. Park, D. Kim, D. Jang and J. H. Lee, FET-type gas sensors: A review, Sens. Actuators, B, 2021, 330, 129240 CAS.
- W. Li, Y. Zhang, X. Hao, Y. Zhang, X. Yang, X. Liang, F. Liu, X. Yan, S. Zhang and G. Lu, Nafion-based methanol gas sensor for fuel cell vehicles, Sens. Actuators, B, 2020, 311, 127905 CAS.
- L. Petani, L. Koker, J. Herrmann, V. Hagenmeyer, U. Gengenbach and C. Pylatiuk, Recent Developments in Ozone Sensor Technology for Medical Applications, Micromachines, 2020, 11, 624 Search PubMed.
- J. van den Broek, D. Klein Cerrejon, S. E. Pratsinis and A. T. Güntner, Selective formaldehyde detection at ppb in indoor air with a portable sensor, J. Hazard. Mater., 2020, 399, 123052 CAS.
- A. Bhardwaj, I. H. Kim, L. Mathur, J. Y. Park and S. J. Song, Ultrahigh-sensitive mixed-potential ammonia sensor using dual-functional NiWO4 electrocatalyst for exhaust environment monitoring, J. Hazard. Mater., 2021, 403, 123797 CAS.
- Y. Zhou, R. Zhang, X. She, J. Li, H. Zhao, Y. Wang, Y. Chen, L. Xie, C. Zou and X. Li, Alkalized Cellulose Nanofiber-Interweaved PEDOT:PSS Thin-Film Sensors via Layer-by-Layer Spraying Assembly for Ultrafast Molecular Ammonia Detection, ACS Appl. Mater. Interfaces, 2023, 15, 53802–53814 CrossRef CAS PubMed.
- M. Mayer and A. J. Baeumner, A Megatrend Challenging Analytical Chemistry: Biosensor and Chemosensor Concepts Ready for the Internet of Things, Chem. Rev., 2019, 119, 7996–8027 CrossRef CAS PubMed.
- C. Jia, S. Batterman and C. Godwin, VOCs in industrial, urban and suburban neighborhoods—Part 2: Factors affecting indoor and outdoor concentrations, Atmos. Environ., 2008, 42, 2101–2116 CrossRef CAS.
- J. J. Ye, S. S. Wang, Y. Fang, X. J. Zhang and C. Y. Hu, Ambient air pollution exposure and risk of chronic kidney disease: A systematic review of the literature and meta-analysis, Environ. Res., 2021, 195, 110867 CrossRef CAS PubMed.
- F. Y. Al-Aboosi, M. M. El-Halwagi, M. Moore and R. B. Nielsen, Renewable ammonia as an alternative fuel for the shipping industry, Curr. Opin. Chem. Eng., 2021, 31, 100670 CrossRef.
- K. Wetchakun, T. Samerjai, N. Tamaekong, C. Liewhiran, C. Siriwong, V. Kruefu, A. Wisitsoraat, A. Tuantranont and S. Phanichphant, Semiconducting metal oxides as sensors for environmentally hazardous gases, Sens. Actuators, B, 2011, 160, 580–591 CrossRef CAS.
- H. Tai, S. Wang, Z. Duan and Y. Jiang, Evolution of breath analysis based on humidity and gas sensors: Potential and challenges, Sens. Actuators, B, 2020, 318, 128104 CAS.
- S. Singh, J. Deb, U. Sarkar and S. Sharma, MoSe2 Crystalline Nanosheets for Room-Temperature Ammonia Sensing, ACS Appl. Nano Mater., 2020, 3, 9375–9384 CAS.
- Y. Li, S. Zhang and D. Song, A Luminescent Metal–Organic Framework as a Turn-On Sensor for DMF Vapor, Angew. Chem., Int. Ed., 2013, 52(2), 710–713, DOI:10.1002/anie.201207610.
- Q. Chen, J. Li, W. Fu, Y. Yang, W. Zhu and J. Zhang, Detection of N, N-dimethylformamide vapor down to ppb level using electrospunInYbO nanofibers field-effect transistor, Sens. Actuators, B, 2020, 323, 128676 CAS.
- J. Baranwal, B. Barse, G. Gatto, G. Broncova and A. Kumar, Electrochemical Sensors and Their Applications: A Review, Chemosensors, 2022, 10, 363 CAS.
- R. Zhang, Y. Wang, J. Li, H. Zhao, Y. Wang and Y. Zhou, Mesoporous cellulose nanofibers-interlaced PEDOT:PSS hybrids for chemiresistive ammonia detection, Microchim. Acta, 2022, 189, 308 CAS.
- R. Owyeung, M. Panzer and S. R. Sonkusale, Colorimetric gas sensing washable threads for smart textiles, Sci. Rep., 2019, 9(1), 5607 Search PubMed.
- Y. Zhou, Y. Wang, Y. Wang, H. Yu, R. Zhang, J. Li, Z. Zang and X. Li, MXene Ti3C2Tx-Derived Nitrogen-Functionalized Heterophase TiO2 Homojunctions for Room-Temperature Trace Ammonia Gas Sensing, ACS Appl. Mater. Interfaces, 2021, 13, 56485–56497 CAS.
- H. Zhao, J. Li, X. She, Y. Chen, M. Wang, Y. Wang, A. Du, C. Tang, C. Zou and Y. Zhou, Oxygen Vacancy-Rich Bimetallic Au@Pt Core–Shell Nanosphere-Functionalized Electrospun ZnFe2O4 Nanofibers for Chemiresistive Breath Acetone Detection, ACS Sens., 2024, 9, 2183–2193 CAS.
- C. Yu, J. Liu, H. Zhao, M. Wang, J. Li, X. She, Y. Chen, Y. Wang, B. Liu, C. Zou, Y. He and Y. Zhou, Sensitive Breath Acetone Detection Based on α-Fe2O3 Nanoparticles Modified WO3 Nanoplate Heterojunctions, IEEE Trans. Instrum. Meas., 2024, 73, 9513908 CAS.
- K. Wu, M. Debliquy and C. Zhang, Room temperature gas sensors based on Ce doped TiO2 nanocrystals for highly sensitive NH3 detection, Chem. Eng. J., 2022, 444, 136449 CAS.
- V. Schroeder, S. Savagatrup, M. He, S. Lin and T. M. Swager, Carbon nanotube chemical sensors, Chem. Rev., 2019, 119, 599–663 CrossRef CAS PubMed.
- X. Liu, T. Ma, N. Pinna and J. Zhang, Two-Dimensional Nanostructured Materials for Gas Sensing, Adv. Funct. Mater., 2017, 27, 1702168 CrossRef.
- H. Y. Li, S. N. Zhao, S. Q. Zang and J. Li, Functional metal–organic frameworks as effective sensors of gases and volatile compounds, Chem. Soc. Rev., 2020, 49, 6364–6401 RSC.
- Y. Zhou, G. Liu, X. Zhu and Y. Guo, Ultrasensitive NO2 gas sensing based on rGO/MoS2 nanocomposite film at low temperature, Sens. Actuators, B, 2017, 251, 280–290 CrossRef CAS.
- S. Singh, S. Sharma, R. C. Singh and S. Sharma, Hydrothermally synthesized MoS2-multi-walled carbon nanotube composite as a novel room-temperature ammonia sensing platform, Appl. Surf. Sci., 2020, 532, 147373 CAS.
- S. Singh, J. Deb, U. Sarkar and S. Sharma, MoS2/WO3 Nanosheets for Detection of Ammonia, ACS Appl. Nano Mater., 2021, 4, 2594–2605 CAS.
- S. Sharma, A. Kumar, N. Singh and D. Kaur, Excellent room temperature ammonia gas sensing properties of n-MoS2/p-CuO heterojunction nanoworms, Sens. Actuators, B, 2018, 275, 499–507 CrossRef CAS.
- D. Zhang, C. Jiang, P. Li and Y. Sun, Layer-by-Layer Self-assembly of Co3O4 Nanorod-Decorated MoS2 Nanosheet-Based Nanocomposite toward High-Performance Ammonia Detection, ACS Appl. Mater. Interfaces, 2017, 9, 6462–6471 CAS.
- Z. Pang, Z. Yang, Y. Chen, J. Zhang, Q. Wang, F. Huang and Q. Wei, A room temperature ammonia gas sensor based on cellulose/TiO2/PANI composite nanofibers, Colloids Surf., A, 2016, 494, 248–255 CAS.
- H. Tai, Z. Duan, Z. He, X. Li, J. Xu, B. Liu and Y. Jiang, Enhanced ammonia response of Ti3C2Tx nanosheets supported by TiO2 nanoparticles at room temperature, Sens. Actuators, B, 2019, 298, 126874 Search PubMed.
- N. Dogra, S. Gasso, A. Sharma, K. K. Sharma and S. Sharma, TiO2 decorated MXene nanosheets for high-performance ammonia gas sensing at room-temperature, Surf. Interfaces, 2024, 48, 104290 CAS.
- W. Buraso, V. Lachom, P. Siriya and P. Laokul, Synthesis of TiO2 nanoparticles via a simple precipitation method and photocatalytic performance, Mater. Res. Express, 2018, 5, 115003 Search PubMed.
- N. Dogra, S. S. Kushvaha and S. Sharma, Phase-Dependent Dual Discrimination of MoSe2/MoO3 Composites Toward N,N-Dimethylformamide and Triethylamine at Room Temperature, ACS Sens., 2023, 8, 3146–3157 CAS.
- G. Kresse and J. Hafner, Ab initio molecular dynamics for liquid metals, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CAS.
- D. Joubert, From ultrasoft pseudopotentials to the projector augmented-wave method, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 Search PubMed.
- G. Kresse and J. Furthmüller, Efficiency of ab initio total energy calculations for metals and semiconductors using a plane-wave basis set, Comput. Mater. Sci., 1996, 6, 15–50 Search PubMed.
- P. E. Blöchl, Projector augmented-wave method, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 Search PubMed.
- H. J. Monkhorst and J. D. Pack, Special points for Brillouin-zone integrations, Phys. Rev. B, 1976, 13, 5188–5192 Search PubMed.
- S. S. El-Deen, A. M. Hashem, A. E. A. Ghany, S. Indris, H. Ehrenberg, A. Mauger and C. M. Julien, Anatase TiO2 nanoparticles for lithium-ion batteries, Ionics, 2018, 24, 2925–2934 CAS.
- H. Tang, K. Dou, C. Kaun, Q. Kuang and S. Yang, MoSe2 nanosheets and their graphene hybrids: synthesis, characterization and hydrogen evolution reaction studies, J. Mater. Chem. A, 2014, 2, 360–364 Search PubMed.
- X. T. Tran, S. Poorahong and M. Siaj, One-pot hydrothermal synthesis and selective etching method of a porous MoSe 2 sand rose-like structure for electrocatalytic hydrogen evolution reaction, RSC Adv., 2017, 7, 52345–52351 RSC.
- Y. Lai, W. Chen, Z. Zhang, Y. Gan, X. Yang and J. Li, Two-dimensional graphene-like MoSe2 nanosheets anchored on hollow carbon nanofibers as a cathode catalyst for rechargeable Li–O2 batteries, RSC Adv., 2016, 6, 19843 RSC.
- S. Tongay, J. Zhou, C. Ataca, K. Lo, T. S. Matthews, J. Li, J. C. Grossman and J. Wu, Thermally driven crossover from indirect toward direct bandgap in 2D Semiconductors: MoSe2versus MoS2, Nano Lett., 2012, 12, 5576–5580 CrossRef CAS PubMed.
- U. Balachandran and N. G. Eror, Raman spectra of titanium dioxide, J. Solid State Chem., 1982, 42, 276–282 CrossRef CAS.
- G. Eda, H. Yamaguchi, D. Voiry, T. Fujita, M. Chen and M. Chhowalla, Photoluminescence from chemically exfoliated MoS2, Nano Lett., 2011, 11, 5111–5116 CrossRef CAS PubMed.
- X. Fan, P. Xu, D. Zhou, Y. Sun, Y. C. Li, M. A. T. Nguyen, M. Terrones and T. E. Mallouk, Fast and Efficient Preparation of Exfoliated 2H MoS2 Nanosheets by Sonication-Assisted Lithium Intercalation and Infrared Laser-Induced 1T to 2H Phase Reversion, Nano Lett., 2015, 15, 5956–5960 CrossRef CAS PubMed.
- C. Tan, W. Zhao, A. Chaturvedi, Z. Fei, Z. Zeng, J. Chen, Y. Huang, P. Ercius, Z. Luo, X. Qi, B. Chen, Z. Lai, B. Li, X. Zhang, J. Yang, Y. Zong, C. Jin, H. Zheng, C. Kloc and H. Zhang, Preparation of Single-Layer MoS2xSe2(1−x) and MoxW1−xS2 Nanosheets with High-Concentration Metallic 1T Phase, Small, 2016, 12, 1866–1874 CrossRef CAS PubMed.
- Z. T. Shi, W. Kang, J. Xu, Y. W. Sun, M. Jiang, T. W. Ng, H. T. Xue, D. Y. W. Yu, W. Zhang and C. S. Lee, Hierarchical nanotubes assembled from MoS2-carbon monolayer sandwiched superstructure nanosheets for high-performance sodium ion batteries, Nano Energy, 2016, 22, 27–37 CrossRef CAS.
- H. Wang, D. Kong, P. Johanes, J. J. Cha, G. Zheng, K. Yan, N. Liu and Y. Cui, MoSe2 and WSe2 nanofilms with vertically aligned molecular layers on curved and rough surfaces, Nano Lett., 2013, 13, 3426–3433 CrossRef CAS PubMed.
- L. Pan, S. Wang, J.-J. Zou, Z.-F. Huang, L. Wang and X. Zhang, Ti3+-defected and V-doped TiO2 quantum dots loaded on MCM-41, Chem. Commun., 2014, 50, 988 Search PubMed.
- S. Singh, J. Deb, U. Sarkar and S. Sharma, MoS2/MoO3 Nanocomposite for Selective NH3 Detection in a Humid Environment, ACS Sustain, Chem. Eng., 2021, 9, 7328–7340 Search PubMed.
- S. Singh, J. Deb, J. V. Singh, U. Sarkar and S. Sharma, Highly Selective Ethyl Mercaptan Sensing Using a MoSe2/SnO2 Composite at Room Temperature, ACS Appl. Mater. Interfaces, 2022, 14, 23916–23927 Search PubMed.
- J. Deb, B. Bhattacharya, D. Paul and U. Sarkar, Interaction of nitrogen molecule with pristine and doped graphyne nanotube, Phys. E, 2016, 84, 330 CrossRef CAS.
- J. Shen, Z. Yang, Y. Wang, L. Xu, R. Liu and X. Liu, The gas sensing performance of borophene/MoS2 heterostructure, Appl. Surf. Sci., 2020, 504, 144412 CrossRef CAS.
- J. A. Mehrez, S. Jiao, M. Zeng, N. Hu, T. Wang, J. Abdul-Aziz Mehrez, X. Chen, J. Yang, R. Liu, L. Xu, Y. Gonzá Lez-Alfaro and Z. Yang, MoTe2/InN van der Waals heterostructures for gas sensors: a DFT study, Phys. Chem. Chem. Phys., 2023, 25, 28677 Search PubMed.
- P. X. Zhao, Y. Tang, J. Mao, Y. X. Chen, H. Song, J. W. Wang, Y. Song, Y. Q. Liang and X. M. Zhang, One-Dimensional MoS2-Decorated TiO2 nanotube gas sensors for efficient alcohol sensing, J. Alloys Compd., 2016, 674, 252–258 CrossRef CAS.
- M. Setvin, J. Hulva, G. S. Parkinson, M. Schmid and U. Diebold, Electron transfer between anatase TiO2 and an O2 molecule directly observed by atomic force microscopy, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, E2556–E2562 CrossRef CAS PubMed.
- L. Zhou, Q. Mi, Y. Jin, T. Li and D. Zhang, Construction of MoO3/MoSe2 nanocomposite-based gas sensor for low detection limit trimethylamine sensing at room temperature, J. Mater. Sci.: Mater. Electron., 2021, 32, 17301–17310 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ma01169d |
| This journal is © The Royal Society of Chemistry 2025 |