DOI:
10.1039/D4MA01122H
(Review Article)
Mater. Adv., 2025,
6, 3433-3454
Pharmaceutical pollution in the aquatic environment: advanced oxidation processes as efficient treatment approaches: a review
Received
11th November 2024
, Accepted 5th May 2025
First published on 20th May 2025
Abstract
Pharmaceutical residues in water are a major global concern, posing serious risks to aquatic ecosystems and human health. Pharmaceutical industry processes, such as washing and manufacturing, contribute notably to water pollution. Additional sources include drug manufacturing plants, landfill leachates, municipal wastewater, and medical and hospital wastes, introducing various pharmaceuticals into water bodies. Many of these substances are toxic, carcinogenic, and bioaccumulative, even at low concentrations, leading to chronic health issues. The removal of pharmaceutical residues from wastewater point sources is crucial for environmental remediation due to their toxic, non-biodegradable, and persistent properties. Recent research has focused on developing various strategies to address pharmaceutical contamination in wastewater. Advanced oxidation processes (AOPs) have proven to be promising and efficient methods for the degradation and mineralization of these pollutants. This review provides an overview of pharmaceutical pollution sources, water-related issues, their biological and chemical transformations, and associated human health impacts. It critically evaluates recent AOP methods for removing pharmaceutical residues from aquatic systems, including Fenton and Fenton-like processes, electrochemical oxidation, ozone-based AOPs, photocatalysis, non-thermal plasma, and hybrid combinations. The review also discusses green, low-cost activator catalysts used in various AOPs. It highlights the need for future research to enhance degradation and mineralization efficiency by developing cost-effective, efficient green activators and hybrid AOP systems. The review highlights the effectiveness of AOPs in pharmaceutical removal, addressing challenges, limitations, and future prospects, while suggesting the prioritization of environmentally friendly catalysts and large-scale AOP applications.
1. Introduction
Contamination of water sources with organic compounds, including pharmaceutical residues and pesticides, is recognized as a significant environmental issue. This challenge is intensified by rapid economic development and population growth, which contribute to worsening water pollution worldwide.1,2 The removal of toxic organic pollutants from wastewater has become a critical global challenge in environmental remediation. Contaminants of emerging concern include micropollutants, often found in trace concentrations in rivers, lakes, and even drinking water, that pose potential risks to human health and the environment. Among these are pharmaceutical residues, which have gained public attention due to their persistence and harmful effects.3 Pharmaceuticals are widely consumed and disposed of by industries, manufacturing plants, hospitals, farms, and institutions, making them a significant source of water pollution. Consequently, substantial amounts of pharmaceutical residues are released into the environment.4 Given that pharmaceutical use cannot be restricted due to population growth and industrial development, it is essential to minimize their environmental release to mitigate risks to human health, ecosystems, and climate. Studies have shown that these pharmaceutical compounds are highly recalcitrant and cannot be effectively removed by conventional wastewater treatment plants.5,6 Pharmaceutical residues in surface and groundwater have raised significant environmental concerns. The detection of pharmaceutical compounds at trace concentrations (ng to μg L−1) in the aquatic environment poses a major threat to the health of aquatic systems.7,8 Pharmaceutical sectors generate varied wastewater compositions due to the diverse range of medications produced using various manufacturing processes. The characteristics of pharmaceutical wastewater depend on the equipment, raw materials, and the specific production, compounding, and formulation processes employed.9,10 Alongside pharmaceutical residues, wastewater from pharmaceutical manufacturing frequently contains significant amounts of both biodegradable and non-biodegradable hazardous organic and inorganic chemicals.11
Pharmaceutical pollutants in wastewater are typically removed using physical, chemical, and biological methods. While physical methods are commonly applied to high-concentration organic wastewater, their effectiveness is often limited. Biological methods have been explored for pharmaceutical removal from wastewater, but studies suggest their effectiveness is limited. In contrast, advanced oxidation processes (AOPs) have gained significant attention due to their fast reaction rates and ease of control. AOPs utilize light, electricity, ozone, and other techniques to generate reactive oxygen species, effectively decomposing pharmaceutical pollutants. Various methods have been developed and implemented to eliminate pharmaceuticals from polluted water. These methods include biological degradation,11,12 membrane bioreactors,13 adsorption,14,15 nanofiltration,16 phytoremediation,17 deep eutectic solvents,18 biological treatment,19 AOPs,6,20 and the combination of AOPs with biological treatment.21 Adsorption techniques have been demonstrated to be an effective and cost-efficient approach for the removal of pollutants from wastewater.22 However, they require secondary treatment processes, as pharmaceuticals are transferred from the aqueous phase to the solid adsorbents, necessitating additional steps for the proper disposal or regeneration of the contaminated adsorbents. Biological treatment is an environmentally friendly option, but it demands substantial land area, has slower removal rates, and is often ineffective for pharmaceuticals that are non-biodegradable. AOPs offer a promising alternative, as they can degrade and mineralize pharmaceuticals into harmless byproducts, thereby eliminating the need for secondary treatment.23,24 Several review articles have addressed the application of AOPs for the removal of pharmaceuticals, including antibiotics25,26 and anti-inflammatory drugs.27 However, most of these reviews focus on a single AOP type or specific pharmaceutical pollutants. Therefore, a comprehensive review on recent advancements in the application of various AOPs and their combinations for removing diverse pharmaceutical pollutants is needed to address existing challenges, research gaps, and propose future research directions. A literature review on the topic of “Pharmaceutical & Removal” compared to “Pharmaceutical & Removal & Advanced Oxidation Processes” was conducted using the ScienceDirect database, covering publications from 2010 to 2025. The analysis shows a significant rise in research on AOPs for pharmaceutical removal over the past decade, contrasting with the slower growth in studies on pharmaceutical removal by other methods (Fig. 1). This trend underscores the increasing focus on and advancements in AOPs for addressing pharmaceutical contaminants. It highlights the relevance of this review in providing a structured overview of current knowledge, identifying research gaps, and suggesting future directions for pharmaceutical wastewater treatment. AOPs and their combinations present a promising approach for removing pharmaceuticals, which are persistent organic pollutants in water, as these methods can nearly mineralize pharmaceutical pollutants into harmless byproducts without secondary treatment or sludge formation.
 |
| | Fig. 1 Published articles from 2010 to 2025 on the topics “Pharmaceutical & Removal” and “Pharmaceutical & Removal & Advanced Oxidation Processes” Source: ScienceDirect (search conducted in November 2024). | |
Despite the utilization of various methods for treating pharmaceutical wastewater, each having its own merits and drawbacks, the most promising and effective approach involves combining AOPs with biological treatment. Among the array of treatment technologies designed for the efficient elimination of persistent emerging molecules, AOPs stand out due to their proven flexibility and high removal efficiency for pharmaceutical compounds.28,29 Notably, these processes have been identified as the most cost-effective techniques for treating water contaminated with pharmaceutical compounds that are poorly water-soluble and refractory.30 AOPs have become a viable solution for tackling industrial wastewater challenges. AOP shows potential in effectively eliminating difficult-to-break-down organic pollutants like dyes, pesticides, and pharmaceuticals, which may not be effectively treated using traditional wastewater methods. Common AOPs, such as photocatalysis, zone-based AOPs, Fenton, and Fenton-like processes, generate highly reactive and non-selective reactive oxygen species (ROS) through a catalytic activation reaction.31,32 Despite significant advancements in AOPs for pharmaceutical pollutant removal, a comprehensive review is needed to assess their large-scale applications, reactive species generation, catalyst development, and performance relationships among different AOPs. This review explores the role of AOPs in wastewater treatment, detailing reactive species mechanisms and their interactions with pharmaceuticals. It also critically examines current limitations, including energy consumption, scalability, operational costs, and challenges in achieving complete mineralization. This review outlines future research directions focused on sustainable AOP implementation, focusing on cost reduction and large-scale applicability. It emphasizes the need to improve pharmaceutical wastewater treatment by optimizing reaction conditions and enhancing catalyst performance. Furthermore, the review explores the use of hybrid AOP systems, which combine various oxidation techniques, to improve mineralization rates of recalcitrant pharmaceuticals. By integrating multiple AOP strategies, these systems enhance degradation efficiencies, effectively converting harmful pharmaceutical pollutants into environmentally benign byproducts, such as CO2, H2O, and mineral acids. This analysis provides insights into advancing sustainable pharmaceutical wastewater management using AOPs.
2. Sources of pharmaceutical pollution in aquatic environment
The term “pharmaceutical waste” typically refers to waste produced by pharmaceutical industries and hospitals. However, since the 1980s, pharmaceuticals have been acknowledged as chemical pollutants.33,34 Pharmaceutical pollutants pose an increasing threat to environmental safety, particularly within aquatic ecosystems. Pharmaceutical pollutants originate from human consumption, personal care products, improper medication disposal, pharmaceutical industries, hospital waste, and excretion via urine or feces from humans and animals. Their persistence in water systems is attributed to chemical stability and resistance to biodegradation.35,36 Pharmaceutical compounds include various classes, such as antibiotics, anti-inflammatory agents, blood-lipid regulators, and steroidal hormones. Their stability and biodegradability are influenced by their chemical nature, structure, and physicochemical properties. Previous studies have documented these characteristics for common pharmaceutical pollutants in aquatic environments.37–39 The persistent occurrence of pharmaceutical residues at low concentrations (ng L−1 to μg L−1) in wastewater, surface water, and even drinking water has become a significant global concern due to their documented environmental impacts.40 Advances in analytical technologies for the detection of pharmaceutical pollutants at trace levels (ng L−1) in aquatic environments have driven significant research in this area. Commonly detected pharmaceuticals in wastewater treatment plants include antibiotics such as sulfonamides (42.92 ng L−1), ciprofloxacin (390 ng L−1), and tetracycline (330 ng L−1) in China; amoxicillin (2027 ng L−1) in Italy; acetaminophen (36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 ng L−1) in Jordan; diclofenac (6300 ng L−1) in Germany; carbamazepine (2270 ng L−1) in Korea; and ibuprofen (2
700 ng L−1) in Jordan; diclofenac (6300 ng L−1) in Germany; carbamazepine (2270 ng L−1) in Korea; and ibuprofen (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 109
109![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 875 ng L−1) in India. These substances have been shown to have detrimental effects on public health and aquatic life.41 Pharmaceutical active compounds are increasingly concerning in environmental risk assessments due to their continuous release into water bodies, prompting the European Commission to classify them as emerging pollutants. Their presence disrupts ecosystems and contributes to antibiotic resistance, impacting aquatic organisms and ecosystem functions through bioconcentration from water and dietary exposure.42 Combined exposure, or bioaccumulation, arises from an imbalance between uptake and elimination. Measuring bioaccumulation is crucial for assessing the environmental risks of these compounds and their potential effects on human health.43 Pharmaceutical production facilities mainly utilize four industrial processes: fermentation, chemical synthesis, biological or natural extraction, and compounding or formulation.44 Active pharmaceutical products are manufactured through processes such as fermentation, chemical synthesis, and natural extraction. These products are then transformed into various usable forms such as tablets, syrups, ointments, and capsules. The manufacturing process involves crushing, combining, compressing, encapsulating, and packaging pharmaceutical ingredients using water, binders (such as starch or corn syrup), solvents (for tablet coatings), fillers (to dilute medicinal substances), preservatives, flavoring agents, antioxidants, and flavored syrups. The wastewater generated from these processes typically requires suitable treatment.45 The main sources of pharmaceutical pollution are manufacturing plants, hospitals, institutions, and landfill leachate, as shown in (Fig. 2). From production to disposal, pharmaceuticals including those from synthesis, excretion, and expired medications pose significant environmental and health risks. The high global demand for pharmaceuticals generates considerable waste.46 Pharmaceutical waste arises not only from the pharmaceutical industry but also from hospitals, patients, veterinary sectors, and agricultural areas in diverse forms. Inadequate disposal and improper management of pharmaceutical waste result in their release into the environment through soil contamination, and waster bodies.47
875 ng L−1) in India. These substances have been shown to have detrimental effects on public health and aquatic life.41 Pharmaceutical active compounds are increasingly concerning in environmental risk assessments due to their continuous release into water bodies, prompting the European Commission to classify them as emerging pollutants. Their presence disrupts ecosystems and contributes to antibiotic resistance, impacting aquatic organisms and ecosystem functions through bioconcentration from water and dietary exposure.42 Combined exposure, or bioaccumulation, arises from an imbalance between uptake and elimination. Measuring bioaccumulation is crucial for assessing the environmental risks of these compounds and their potential effects on human health.43 Pharmaceutical production facilities mainly utilize four industrial processes: fermentation, chemical synthesis, biological or natural extraction, and compounding or formulation.44 Active pharmaceutical products are manufactured through processes such as fermentation, chemical synthesis, and natural extraction. These products are then transformed into various usable forms such as tablets, syrups, ointments, and capsules. The manufacturing process involves crushing, combining, compressing, encapsulating, and packaging pharmaceutical ingredients using water, binders (such as starch or corn syrup), solvents (for tablet coatings), fillers (to dilute medicinal substances), preservatives, flavoring agents, antioxidants, and flavored syrups. The wastewater generated from these processes typically requires suitable treatment.45 The main sources of pharmaceutical pollution are manufacturing plants, hospitals, institutions, and landfill leachate, as shown in (Fig. 2). From production to disposal, pharmaceuticals including those from synthesis, excretion, and expired medications pose significant environmental and health risks. The high global demand for pharmaceuticals generates considerable waste.46 Pharmaceutical waste arises not only from the pharmaceutical industry but also from hospitals, patients, veterinary sectors, and agricultural areas in diverse forms. Inadequate disposal and improper management of pharmaceutical waste result in their release into the environment through soil contamination, and waster bodies.47
 |
| | Fig. 2 Sources and health risks of pharmaceutical residues in aquatic environment. | |
3. Pharmaceutical pollutants: impact on environment and human health
The widespread use of pharmaceuticals has raised concerns about their impact on both the environment and human health.48,49 As essential components of medical treatments, pharmaceuticals find their way into the environment through various pathways, including wastewater discharges from manufacturing plants, improper disposal, and excretion by individuals.50 This has led to the presence of pharmaceutical residues in water bodies, soil, and even drinking water. Pharmaceutical residues entering water bodies pose a threat to aquatic ecosystems. These compounds can affect aquatic organisms, potentially disrupting endocrine systems and leading to adverse effects on fish and other species.34,51 Disposing of pharmaceuticals, including unused medications, has the potential to contaminate the soil. This contamination can affect plant growth and soil microbial communities, potentially influencing ecosystems.52 The existence of antibiotics in the environment is linked to the emergence of antibiotic-resistant bacteria, presenting a substantial public health risk.53,54 Pharmaceuticals are specifically designed to yield desired therapeutic effects, and toxicity represents an undesired outcome associated with many pharmaceuticals. While pharmaceutical residues are present in trace concentrations in water bodies such as rivers, wells, ponds, and other water streams, continuous exposure to numerous pharmaceutical components, even in trace amounts, may lead to significant health hazards and collectively result in severe adverse reactions on health. Pharmaceuticals, including endocrine disruptors, may re-enter human life through various sources like drinking water, farm produce, livestock, and poultry products. This pharmaceutical waste component significantly affects internal biological processes by interfering with hormone-regulating activities related to reproduction, development, and growth. The alarming aspect is the disruption of sexual development in humans, posing a serious risk to human existence.34,55 Therefore, a crucial research area involves identifying and quantifying various pharmaceutical wastes that impact human health. Another major concern is the presence of modern chemotherapy agents or anticancer drugs, which exhibit cytotoxic activity. The detection of pharmaceutical residues in drinking water sources raises concerns about potential long-term health implications for humans. Prolonged exposure to low concentrations of pharmaceuticals may result in unexpected health effects.56,57 The environmental presence of antibiotics may contribute to the transmission of antibiotic resistance genes, compromising the efficacy of these drugs in treating infections. Additionally, certain pharmaceuticals display endocrine-disrupting properties, which may lead to hormonal imbalances and associated health issues in humans.58–60 Anti-inflammatory drugs, antibiotics, and painkillers are among the most widely used pharmaceuticals globally. Sewage treatment plants typically remove only 20–30% of diclofenac due to its low biodegradability and limited adsorption on activated sludge. Continuous release of these drugs into the environment results in chronic exposure for organisms, as they remain active and toxic even at low concentrations. Studies show that such exposure may lead to health issues like Alzheimer's, obesity, thyroid disorders, and cancer.61 Therefore, effective treatment of pharmaceuticals at their source is essential before discharge into aquatic environments. Addressing these challenges requires a multifaceted approach involving improved drug manufacturing practices, proper disposal methods, enhanced wastewater treatment, and increased awareness among healthcare professionals and the public. Ongoing research and regulatory measures aim to mitigate the environmental and human health impacts associated with pharmaceuticals. Fig. 2 illustrates the potential sources, pathways, and effects of pharmaceutical pollution on human health, environmental systems, and aquatic life.
4. Advanced oxidation processes (AOPs) for the removal of pharmaceuticals
Advanced oxidation processes (AOPs) were initially introduced for drinking water treatment in the 1980s, defined as methods that generate hydroxyl radicals (˙OH) in sufficient quantities to purify water.62 The concept of AOPs has broadened to include oxidative processes that use other reactive species, such as sulfate and superoxide radicals, along with singlet oxygen. These species act as powerful oxidants, efficiently breaking down pollutants in water and air.32,63 This approach differs from typical decontamination methods like adsorption, coagulation–flocculation, sedimentation, and membrane separation, which frequently transfer pollutants from water to another medium. Under optimal conditions, AOPs can decompose and mineralize a wide range of organic pollutants, such as pharmaceuticals, into CO2, H2O, and mineral salts. AOPs are designed to efficiently degrade and remove a wide range of organic and inorganic contaminants, including persistent and toxic emerging organic contaminants.24,64–66 A key advantage of AOPs is their capacity to degrade and mineralize persistent organic pollutants into harmless by-products via non-selective oxidation, regardless of the pollutants’ chemical structure or complexity. This makes AOPs especially effective for treating complex wastewater, such as pharmaceutical industrial effluents with diverse contaminant mixtures.67Table 1 summarizes several AOPs, detailing their strengths and limitations in the removal of pharmaceutical pollutants from aqueous systems.
Table 1 Various AOPs, along with their advantages and limitations, used for the removal of pharmaceuticals from aqueous solution
| Types of AOPs |
Operating conditions |
Advantages |
Limitations |
Ref. |
| Photocatalysis |
Photocatalyst type/dosage, light source/intensity, reaction time, initial concentration, pH and temperature |
Simple operation, eco-friendly, high efficiency, ease of use, and low cost |
Low mineralization efficiency, slow reaction rates, scalability, recovery of photocatalysts after treatment, reusability and stability |
68
|
| Fenton and Fenton-like |
Fenton or Fenton-like catalyst type, persulfate or H2O2 concentration, solution pH, reaction time, temperature, and initial concentration |
Wide pH range for heterogeneous persulfate and Fenton-like processes, high efficiency, low reaction costs, easy control, stable storage, and low selectivity |
High catalyst separation and reactivation costs, potential environmental impacts from metal leaching and sulfate, sludge production, and pH dependency in traditional Fenton |
69
|
| Ozone based AOPs |
O3 concentration, reaction time, pH, temperature, catalyst type, and the presence of additional oxidants |
Powerful oxidizing capabilities, fast reaction rates, and mild conditions |
Limited O3 solubility and mass transfer, high energy use and costs, pH dependency, and low mineralization |
26
|
| Non-thermal plasma |
Discharge type (DBD, corona, or gliding arc), voltage, frequency, gas composition, reactor design, initial concentration, and reaction time |
Generation of reactive oxygen species without chemical addition, achieving rapid removal efficiency |
Large-scale application, high cost, high energy consumption, and complex design |
70
|
| Electrochemical oxidation |
Electrode material, current density, voltage, electrolyte composition, pH, reaction time, and initial concentration |
Environmentally friendly, efficient, controllable, and broadly applicable, with no need for chemical reagents, precise control, and on-site reagent generation |
High application costs, toxic metal ions, challenges in enhancing pollutant mass transfer, electrode materials, and energy consumption |
71
|
| Sonolysis |
Ultrasound frequency and intensity, reaction time, medium composition, temperature, solution pH, and initial pollutant concentration |
Low operational costs, minimal sludge production, no chemical additives, environmentally friendly, precise control, and easily integrated with other AOPs |
Incomplete degradation and mineralization of recalcitrant and hydrophilic compounds, high energy consumption, and long processing times |
38
|
| Hybrid-AOPs |
AOP type combinations, oxidant and catalyst type/dosage, light source/intensity, reaction time |
High degradation efficiency, complete mineralization, and increased reactive species production |
Complex operational processes, high costs, and large-scale application |
72
|
AOPs are characterized by their ability to produce powerful oxidizing agents, such as hydroxyl and sulfate radicals, through various mechanisms like photocatalysis, Fenton and Fenton-like processes, sonochemistry, ozone-based AOPs, non-thermal plasma or their combinations. Fig. 3 illustrates the commonly applied AOPs for removing pharmaceutical pollutants through the generation of reactive oxygen species (ROS). These ROS include hydroxyl, sulfate, and superoxide radicals, which are highly reactive and effectively degrade contaminants. Additionally, singlet oxygen is generated as a non-radical oxidant, further enhancing the oxidative degradation of pharmaceuticals in water systems.
 |
| | Fig. 3 Commonly applied AOPs for the removal of pharmaceuticals from wastewater. | |
4.1. Photocatalysis
Photocatalysis is considered one of the most cost-effective and efficient methods for degrading and mineralizing pharmaceutical pollutants in wastewater. This innovative, renewable, and sustainable approach shows great potential for tackling the growing challenges of environmental remediation.73,74 In a photocatalytic system, the valence band (VB) and conduction band (CB) are responsible for producing ˙OH and superoxide anions, respectively (Fig. 4). These species are crucial for the degradation of pollutants.75 The process of photocatalysis is divided into two categories: homogeneous and heterogeneous. For oxidation processes, a variety of chalcogenides serve as dominant photocatalysts. These include oxides like TiO2, ZnO, ZrO2, and CeO2, as well as sulfides such as CdS and ZnS. Furthermore, non-metal compounds, particularly graphitic carbon nitride, are frequently employed as photocatalysts in solar energy applications.76 However, conventional metal oxide photocatalysts are limited by their large band gap energies, which restricts their ability to harness a significant portion of the solar spectrum, thereby extending the degradation process. To address this limitation, researchers have explored modifications such as doping with metals and non-metals or incorporating nanocomposites like carbon dots.77 These modifications reduce the band gap energies, allowing the photocatalysts to utilize a broader range of the solar spectrum. A primary challenge with photocatalysts that have smaller band gap energies is the rapid recombination of electron–hole pairs, which diminishes their efficiency.78 To address these challenges, modifications are essential to boost reactive oxygen species generation for effective photodegradation. Strategies like dye sensitization, doping, and heterojunction formation can enhance visible light photocatalytic activity and improve charge separation, which are crucial for pollutant degradation. Techniques such as metal and heteroatom doping and surface functionalization have been used to increase electron–hole pair generation and delay recombination, thereby improving overall degradation efficiency.79 Numerous studies have focused on various modification approaches to enhance the photocatalytic degradation of pharmaceutical pollutants, demonstrating the potential of these strategies in improving the performance of photocatalysts. Exploring the integration of photocatalysis with other advanced water treatment technologies is essential for maximizing their effectiveness. Tra and colleagues investigated the removal of the drug Diclofenac (DCF) from wastewater, utilizing advanced oxidation processes that focus on heterogeneous photocatalysis with H2O2 They found the most effective removal of DCF at a pH of 6.5 and a TiO2 concentration of 1 g L−1. This resulted in a 65.25% reduction in DCF concentration after 150 minutes. Additionally, the addition of H2O2 not only improved the process but also eliminated DCF, with an optimal initial concentration of 10 mg L−1 proving the most effective.80 Eskandari et al. synthesized and characterized L-arginine-TiO2/g-C3N4 nanoparticles (co-doped-TCN) to enhance photocatalysis by combining L-arginine with g-C3N4 and TiO2. This combination effectively lowered the bandgap energy (eV) and suppressed charge carrier recombination. Statistical analysis validated predictive models for the photodegradation of metronidazole (MNZ) and oxytetracycline (OTC). Using response surface methodology (RSM), the study showed that higher pollutant concentrations, lower catalyst amounts, and shorter irradiation times negatively affected the photocatalytic response. Key agents in degrading MNZ and OTC were superoxide radicals, hydroxyl radicals, singlet oxygen, and positive holes. Anions, especially NO3−, reduced degradation efficiency, while electron acceptors like H2O2, K2S2O8, and KBrO3 enhanced it. Humic acid (HA) adversely impacted photocatalytic efficiency. The co-doped-TCN-LED and co-doped-TCN/LED with electron acceptors emerged as promising for water and wastewater treatment, offering high reusability and efficiency. Complete degradation of MNZ and OTC under visible light was achieved, proving the catalyst's effectiveness and enhancing treated wastewater biodegradability.81 Oluwole et al. demonstrated that BWO/g-C3N4 heterojunction composites, prepared by wet impregnation of g-C3N4 with octahedron-shaped BWO, effectively photocatalytically degraded ibuprofen under visible light. The 7 wt% BWO/g-C3N4 composite achieved 94.8% degradation efficiency, outperforming pure BWO and g-C3N4. This improvement is attributed to a reduced bandgap and increased surface area of 46.15 m2 g−1, enhancing charge separation. Hydroxyl radicals and holes were crucial in the degradation process, underscoring photocatalysis's potential for treating pharmaceuticals.82Table 1 summarizes various studies on pharmaceutical degradation via photocatalysis. While photocatalysis shows great potential for degrading pharmaceuticals in water, its performance can be enhanced by combining it with methods like adsorption, membrane filtration, and other AOPs such as ozonation, photo-Fenton, and persulfate in hybrid approaches. Table 2 provides an overview of various photocatalysts applied under different reaction conditions for the removal of pharmaceutical pollutants from water.
 |
| | Fig. 4 Schematic illustration of photocatalytic degradation of pharmaceuticals in water. | |
Table 2 Photocatalysis for the degradation of pharmaceuticals in water
| Photocatalyst |
Reaction condition |
[Pharmaceuticals] |
% Removal |
Ref. |
| TiO2 |
UV irradiation (311 nm), pH (6.5), (150 min), catalyst dose (1 g L−1) |
Diclofenac, 50 mg L−1 |
65.25 |
80
|
| O-g-C3N4/ZnO/TiO2@halloysite nanotubes |
UVA light, pH (6.83), (50 min), catalyst dose (1 g L−1) |
Diclofenac, 10 mg L−1 |
100 |
83
|
| n-CeO2/p-CuS nanorods |
Halogen lamps (Philips, 54 W, 145 μW cm−2), catalyst dose (0.018 g), (25 min) |
Tetracycline |
98 |
84
|
| Ciprofloxacin |
93.1 |
| Capecitabine |
92.6 |
| 5.8 × 10−5 M |
| Co3O4/CdO/clinoptilolite |
Sunlight irradiation (250–270 KLux), (120 min), catalyst dose (2 g L−1), neutral pH |
Levofloxacin, 10 mg L−1 |
99.3 |
85
|
| Ti3C2 MXene/GQDs@ZnO |
Visible light (75 W), neutral pH, (180 min), Catalyst dose (50 mg) |
Amoxicillin, 10 mg L−1 |
99 |
86
|
| Zeolite/Fe3O4/CuS/CuWO4 nanocomposite |
Under direct sunlight, pH (6.8), (220 min), catalyst dose (2 g L−1), flow rate 5 mL min−1 |
Acetaminophen, 10 mg L−1 |
84.77 |
87
|
| Zn0.98Nd0.02O |
UV light (125 W), pH (5–13), (120 min), catalyst dose (0.5 g L−1) |
Ciprofloxacin, 10 mg L−1 |
97.5 |
88
|
| Ibuprofen, 20 mg L−1 |
74.1 |
| Peanut-like BiVO4 |
LED (blue and white) light (100–50/200 W), pH (9), (120 min), catalyst dose (100 mg L−1) |
Tetracycline, 50 mg L−1 |
96 |
89
|
| Fe2O3/g-C3N4 nanocomposite |
Visible light (150 W), neutral pH, (180 min), catalyst dose (1.6 g L−1) |
Metformin, 20 mg L−1 |
96 |
90
|
| Novel TiO2-orange peel-derived biochar |
Visible light (300 W Xe lamp), pH (4), (100 min), catalyst dose (0.8 g L−1) |
Acetaminophen, 20 mg L−1 |
94 |
91
|
4.2. Electrochemical oxidation
The electrochemical redox reaction is a highly effective advanced oxidation process for removing pharmaceutical contaminants from wastewater. This method uses an electrical current to initiate oxidation, breaking down contaminants into harmless products (Fig. 5). Electrochemical oxidation provides precise control and in situ reagent generation, ensuring an efficient and environmentally friendly approach for the degradation of various organic pollutants.92 It offers high removal efficiency, scalability, control, and reduced chemical use compared to conventional methods. However, its main limitation is the high cost associated with electrical energy consumption.93 Advanced electrochemical oxidation methods use electrical energy and redox reactions to break down and remove persistent organic contaminants through reactive species.94 Electrochemical treatment degrades pollutants through direct anodic oxidation and indirect oxidation via reactive species generation, along with cathodic reduction. Mediated indirect oxidation occurs through electro-generated oxidants from water discharge or electrolyte oxidation under high currents. Electrode porosity and surface-to-volume ratio significantly affect mass transfer limitations in the electrochemical removal of pharmaceutical pollutants.95 In some cases, electrochemical methods are combined with catalysts to activate the generated H2O2 in Fenton or Fenton-like processes, producing additional hydroxyl radicals and enhancing efficiency. These processes are promising due to their environmental friendliness, adaptability, simplicity, and cost-effectiveness. However, they also have drawbacks, including short electrode lifespan, low surface area-to-volume ratio, temperature increase during the process, low current density, and reduced efficiency. To address these limitations, three-dimensional electrochemical reactors have proven highly effective for removing emerging organic pollutants.61 These reactors build on the principles of two-dimensional systems, with significant improvements from integrating conductive particles between parallel electrodes, creating what is known as the third or particle electrode.
 |
| | Fig. 5 Electrochemical oxidation method for the removal of pharmaceuticals. | |
4.3. Fenton and Fenton-like oxidation
The combination of hydrogen peroxide and iron(II) is commonly known as “Fenton's reagent.” Fenton's reagent is recognized for its effectiveness in degrading various organic pollutants in water.96 In 1894, Fenton made the discovery of the oxidation of tartaric acid by hydrogen peroxide in the presence of iron(II). Subsequently, the combination of H2O2 and Fe has been recognized as the Fenton reagent.97 This reagent has proven successful in oxidizing a diverse range of organic compounds.6 However, low pH operation and the generation of sludge limit the practical application of the Fenton process. To overcome these drawbacks, researchers turned to Fenton-like processes which are modified versions of the Fenton process that use heterogeneous catalysts as an alternative to homogenous iron ions or different oxidants such as peroxymonosulfate (PMS), and peroxydisulfate (PDS) instead of H2O2.98 These can be combined with a source of energy (light, sound, heat) to generate ROS (Fig. 6). These reactions follow a similar mechanism to the traditional Fenton reaction but with varying redox potentials and kinetics. The Fenton-like systems have emerged as one of the effective strategies among AOPs, for the degradation of organic pollutants, particularly pharmaceuticals rapidly and completely, with minimal formation of harmful byproducts. Fenton-like systems offer a versatile and cost-effective alternative to traditional Fenton oxidation, featuring milder reaction conditions, wider applicability, lower costs, faster cycles, and easier catalyst recycling. As a result, they have become one of the most effective AOPs for rapidly and thoroughly degrading organic pollutants, particularly pharmaceuticals, with minimal harmful byproducts. These systems remove pharmaceuticals by generating ˙OH radicals, which act as strong oxidants that degrade pollutants through hydrogen atom abstraction, redox reactions, or electrophilic addition to π-systems. Recent research has shown promise in using heterogeneous Fenton and Fenton-like systems to remove pharmaceuticals from wastewater.99–101 Rahman and Hama Aziz utilized heterogeneous Fenton-like oxidation with a non-carbonized catalyst derived from scrap-printed circuit boards. Using 1 g L−1 of catalyst and 50 mg L−1 H2O2 at pH 4 for 30 minutes, 86% of Diclofenac and 66% of Ibuprofen were removed from an initial pharmaceutical concentration of 20 mg L−1.102 In recent research, Haroon et al. developed innovative nanocomposites by decorating Fe3O4 nanoparticles on graphitic carbon nitride nanosheets. These nanocomposites were applied in Fenton-like degradation of tetracycline, with the Fe3O4 nanoparticles decorated on metal-free graphitic carbon nitride/hydrogen peroxide system achieving an impressive 90% degradation efficiency within 60 minutes at neutral pH. This high efficiency was driven by the generation of reactive oxygen species through the Fe(II) and Fe(III) ions present in the nanocomposite.103 A novel MoS2@nZVI catalyst was synthesized by incorporating nano zero-valent iron with a multifunctional molybdenum disulfide film by Chundi Zhou et al. This material exhibited a complete 100% removal efficiency for sulfamethoxazole and achieved a rate constant (kobs of 0.4485 min−1) within just 10 minutes. The remarkable degradation performance is attributed to the MoS2 film, which not only facilitated Fe(II) regeneration but also assisted in proton accumulation and electron transfer, significantly enhancing the efficiency of sulfamethoxazole degradation across various pH levels.104Table 3 summarizes recent studies on the application of Fenton and Fenton-like AOPs for the removal of pharmaceutical contaminants from water.
 |
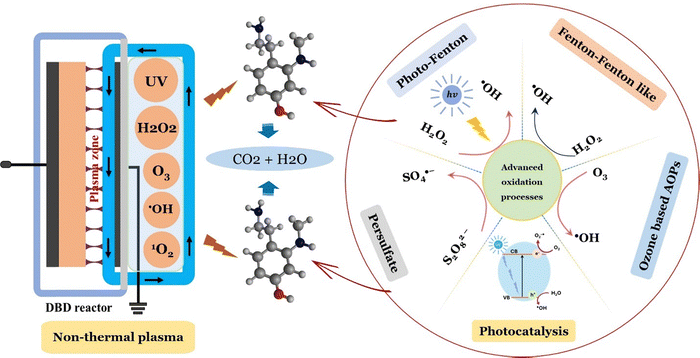
| | Fig. 6 Fenton, Fenton-like, ozone-based AOPs, and non-thermal plasma for the removal of pharmaceuticals. | |
Table 3 Fenton and Fenton-like processes for the removal of pharmaceuticals from water
| Methods |
Activation condition |
[Pharmaceuticals]o |
%Removal |
Ref. |
| Heterogenous Fenton-like oxidation |
[NC-PCB] = 1 g L−1, [H2O2] = 50 mg L−1, pH = 4, t = 30 min |
[DCF]o = 20 mg L−1 |
86 |
102
|
| [IBF]o = 20 mg L−1 |
66 |
| Heterogenous Fenton-like oxidation |
[FCN-20%] = 0.66 mg mL−1, [H2O2] = 2.45 mM, neutral pH, t = 60 min |
[TC]o = 10 mg L−1 |
90 |
103
|
| Persulfate based Fenton-like oxidation |
FeBS800 = 1.5 g L−1, [PDS] = 10 mM, neutral pH, t = 10 min |
[DCF]o = 20 mg L−1 |
88.3 |
105
|
| Persulfate based Fenton-like oxidation |
Non-carbonized catalyst = 0.75 g, [PDS] = 6 mM, neutral pH, t = 60 min |
[DCF]o = 20 mg L−1 |
76 |
8
|
| [IBF]o = 20 mg L−1 |
71 |
| Heterogenous Fenton-like catalyst |
Modified magnetic chitosan nanoparticles = 15 mg, [PS] = 20 mM, neutral pH = 3–8, t = 6 hours |
[TC]o = 50 mg L−1 |
94 |
106
|
| H2O2 based Fenton-like reaction |
Bimetallic Fe/Cu nanoparticles = 2.35 g L−1, [H2O2] = 10 g L−1, pH = 6, t = 60 min |
[Ceftriaxone]o = 60 μg L−1 |
99.5 |
107
|
| Heterogenous Fenton reaction |
Nano-Fe3O4 catalyst 1 g L−1, [H2O2] = 100 mM, neutral pH, t = 120 min |
[Carbamazepine]o = 15 mg L−1 |
99 |
108
|
| Fenton and Fenton-like reaction |
FeS2 catalyst = 2 g L−1, [H2O2] = 5 mM, pH (4), t = 180 min |
[Acetaminophen]o = 50 mg L−1 |
96.6 |
109
|
| Photo-Fenton-like reaction |
Fe3O4@MIL-100(Fe) = 0.33 g, [H2O2] = 5.27 g L−1, pH (6.68), light source = Xe (<420 nm), t = 180 min |
[Levofloxacin]o = 200 mg L−1 |
94.3 |
110
|
| Fenton oxidation |
Fe2+/H2O2 molar ratio of 0.125, pH of 3.5, t = 30 min |
Ciprofloxacin = 15 mg L−1 |
74.4 |
111
|
4.4. Non-thermal plasma
Plasma generation is typically classified into thermal and non-thermal types. Thermal plasma operates at higher temperatures and pressures, requires more power, and produces reactive species in thermodynamic equilibrium.112 Reactive species generate during plasma discharge through mechanisms like dissociation, ionization, excitation, neutralization, and photo-ionization.113 In contrast, non-thermal plasma (NTP), or cold plasma, is produced directly via electric discharge or passing gas such as He, Ar, N2, O2, air or a mixture of different gases through an electric field.114 NTP is a low-temperature plasma that generates reactive species with distinct properties. This process produces a variety of highly reactive species, including hydroxyl, sulfate, and superoxide radicals, along with H2O2, O3, UV light, and high-energy electrons without addition of chemicals.6,115 The exact species generated depend on the chosen carrier gas and applied high-voltage power. The concentration of H2O2 rises with increased discharge time and input energy until reaching a saturation point. Beyond this level, prolonged exposure to high energy results in a decrease in H2O2 concentration over time. This decline occurs due to H2O2 decomposition at higher concentrations through reactions with active species generated during the discharge process.116,117 Reactive species produced by cold plasma at atmospheric pressure, especially those formed through plasma–liquid interactions, offer diverse applications, such as the remediation of pharmaceutical contaminants in wastewater.118 A typical plasma discharge system includes a power source, electrodes, working gas, and a reactor design, all of which influence its performance (Fig. 6). The power supply and composition of the working gas are key factors affecting the energy yield of plasma-based devices.119 Common NTP methods for pollutant degradation involve dielectric barrier discharge (DBD) and corona discharge, generated either within the liquid or at the gas–liquid interface.120 In wastewater treatment, plasma discharge can ideally be organized into three types based on plasma distribution, reactor design, and operation: gas-phase discharge, liquid-phase discharge, and hybrid gas–liquid discharge.121 NTP, a part of AOPs, is generated through electrical discharges at liquid and gas–liquid interfaces. Plasma, a partially or fully ionized gas, consists of electrons, ions, free radicals, and neutral species. It is produced by discharges such as DBD, gliding arc, glow, spark, and corona discharges.122 The energetic electrons in plasma collide with molecules like N2, O2, and H2O, producing secondary electrons, photons, ions, radicals, and UV light. NTP is an effective and environmentally friendly AOP for removing pharmaceutical pollutants from wastewater.123 Studies show that NTP is a promising technique for water remediation, particularly in lab and pilot-scale applications. Plasma interacting with a thin water layer minimizes mass transfer limitations. DBDs with falling liquid films, in coaxial or planar configurations, have shown significant improvements in energy efficiency.66,124 This configuration can treat a broad range of water pollutants, including pharmaceuticals. Key factors affecting NTP performance include plasma type, reactor design, gas composition, and solution properties like pH and conductivity.125
The optimal parameter for evaluating NTP systems and comparing different reactor designs, particularly in terms of energy efficiency during pharmaceutical degradation, is the amount of selected pharmaceuticals degraded per unit of energy consumed. This can be calculated using eqn (1) for pharmaceutical pollutant degradation, with the energy yield preferably calculated at 50% degradation (G50). G50 is defined as grams of pharmaceuticals decomposed per kilowatt-hour and expressed in (g kW−1 h−1). This value is well established measure especially for non-thermal plasma experiments, to compare different plasma methods such as DBD, corona discharge, and electrohydraulic discharge in order to design the most effective experimental process. Comparing the degradation efficiency of pharmaceutical pollutants across studies on various NTP systems presents challenges, as energy yield estimates are influenced by multiple factors beyond reactor type. These include the degradation percentage achieved, presence of catalysts or additives, initial pollutant concentration, solution pH, gas atmosphere, reactor design, and the nature of pollutant by-products. G50 is chosen for two main reasons: at this point, the degradation of intermediate by-products during discharge is minimal, and it offers a standardized measure for assessing the efficiency of various NTP reactor designs.114
| |  | (1) |
where [
C]
0 is the initial concentration of pharmaceuticals (mg L
−1),
V is the volume of the treated solution in liters,
P is the average applied power (kW), and
K (min
−1) is the observed rate constant. The observed rate constant (k min
−1) for the degradation of the targeted pharmaceuticals is determined by plotting ln(
C0/
C) against time, based on the concentration profiles of the pharmaceutical degradation over time.
Table 4 illustrates the use of NTP alone, as well as in combination with other AOPs or catalysts, for the removal of pharmaceutical pollutants from water.
Table 2 presents recent NTP-based AOPs applied for the removal of various pharmaceuticals from water, along with their energy yields at
G50, which serve as an indicator of the performance for each discharge configuration.
Table 4 Non-thermal plasma to eliminate various pharmaceuticals from water
| Type of non-thermal plasma |
Reaction condition and reactor design |
[Pharmaceuticals]o |
Energy yields (g kW h−1) |
Ref. |
| DBD plasma |
pH 5.6, Ar/O2 (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) at a power of 200 W, DBD falling film reactor 20) at a power of 200 W, DBD falling film reactor |
[Diclofenac]o = 50 mg L−1 |
5.10 |
7
|
| [Ibuprofen]o = 50 mg L−1 |
0.75 |
| DBD plasma |
11 kV, 300 Hz, 15 W, DBD with falling liquid film inner tube; Ar/O2 (80/20) |
[Propranolol]o = 100 mg L−1 |
26.70 |
126
|
| DBD plasma |
DBD/UV system, high-voltage AC power supply with a frequency of 7 kHz and a voltage regulation range of 0–10 KV, in quartz glass tube |
[Enrofloxacin]o = 30 mg L−1 |
1.20 |
127
|
| Pulsed corona discharge/persulfate system |
PCD output power 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W with 0 to 880 pulses per second, solutions flow rate of 0.8 L h−1, pH = 3, drug/PDS molar ratios: 1/0.5 W with 0 to 880 pulses per second, solutions flow rate of 0.8 L h−1, pH = 3, drug/PDS molar ratios: 1/0.5 |
[Dexamethasone]o = 10 mg L−1 |
18.5 at G95 |
128
|
| Pulse DBD plasma/persulfate |
Frequency: 180 Hz, and input power of 85 W, liquid flow rate: 70 mL min−1, initial pH: 5.6 and drug/PS dose = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
[Sulfamethoxazole]o = 50 mg L−1 |
0.353 |
129
|
| DBD plasma/ZnO/g-C3N4/zeolite |
Coaxial WFFDBD plasma reactor, water flow rate 60–200 mL min−1, shock current of 0.8–1 A and 200 Hz |
[Tetracycline]o = 50 mg L−1 |
9.20 |
130
|
| DBD plasma |
Applied voltage of 8, 10, and 12 kV, water matrices containing chloride and carbonate ions |
[Carbamazepine]o = 25 mg L−1 |
0.19 |
131
|
| Submerged thermal plasma/LaMnO3 perovskite |
Ar/CO2 at 6.2 kW discharge power using a customized thermal plasma torch in water |
[Chloramphenicol]o = 20 mg L−1 |
0.144 |
132
|
| NTP-pulsed corona discharge |
Sequential flow plasma reactor, 51 W input power, and water flow rate 150-750 mL min−1 |
[Diclofenac]o = 10 mg L−1 |
0.256 |
133
|
| DBD plasma/persulfate |
Coaxial water falling film reactor design, water flow rate of 0.2 L min−1, input power 3.5–7.1 W at 24–28 kV and 0–300 Hz |
[Sulfamethoxazole]o = 0.08 mM |
1.44 |
134
|
4.5. Ozone based AOPs
Ozone, a highly toxic gas with a redox potential of 2.07 V, is a powerful oxidant commonly used in wastewater treatment for disinfection, bacteria sterilization, odor control, algae reduction, and organic pollutant degradation.135 However, the high cost of ozone production and its selective oxidation, which often results in only partial degradation, limit its widespread application. Ozonation occurs via two main pathways: direct oxidation in acidic conditions (pH ≤ 4) where it acts selectively with limited mineralization, and indirect oxidation in alkaline conditions (pH ≥ 9), where ˙OH radicals are generated to enhance pollutant breakdown.136,137 Under alkaline conditions or with catalysts and UV light, ozonation can act as AOPs, as ozone decomposes in the presence of OH− ions, or in combination with photocatalysis to produce highly reactive ˙OH radicals (Fig. 6). In ozone based AOPs, ozone (O3) reacts with organic molecules through electrophilic or radical chain reactions.135 Ozone rapidly decomposes, often triggered by ˙OH radicals, especially at wavelengths below 300 nm.124,138 The resulting oxygen atom is highly reactive, allowing it to oxidize various substrates, including pharmaceuticals. Direct ozonation in an acidic medium is not considered an AOP due to its selective degradation and low mineralization efficiency. However, when ozone interacts with water in the presence of a catalyst, UV light, or in an alkaline medium, hydroxyl radicals are produced, offering stronger oxidation capabilities for pharmaceutical degradation than ozone alone.137,139 Photocatalytic ozonation is a highly effective approach for breaking down toxic and persistent organic pollutants in water. Studies have shown that combining UV irradiation with ozonation is more efficient for removing certain organic pollutants than using either UV photolysis or ozonation alone.117 In water, ozone decomposes to produce hydroxyl radicals, and this process is significantly enhanced by UV light, making it particularly useful for eliminating pharmaceuticals in wastewater.140,141 Photocatalytic ozonation merges photocatalysis and ozonation, creating synergies that enhance pollutant removal. This approach, generating additional ROS in a single reactor with short residence times, improves removal compared to photolysis or ozonation alone.32 Photocatalytic ozonation effectively eliminate recalcitrant pollutants challenging for standard AOPs or direct ozonation. In the literature, combining ozonation and photocatalysis has demonstrated synergies ranging from 1.2 to 7.5 times.142 Catalytic ozonation has several advantages over direct ozonation, such as increased on-site production of hydroxyl radicals and the generation of biodegradable byproducts. However, it also presents challenges, including high energy consumption and limited ozone solubility in water. Additionally, catalytic ozonation may have limited effectiveness in fully mineralizing pharmaceutical compounds, emphasizing the need for further optimization and additional treatment methods.
4.6. Sonochemical process
Sonochemistry explores the chemical effects of ultrasound, which arise from acoustic cavitation. Sonolysis, a modern AOP technique, utilizes ultrasound to generate ˙OH radicals through water pyrolysis.143 Sonolysis presents advantages over conventional AOPs, such as the absence of chemical additives, low sludge generation, and broad applicability. It operates via acoustic cavitation, where ultrasound (US) induces bubble formation in aqueous solutions. These bubbles expand and collapse, releasing intense heat and pressure, generating reactive oxygen species that degrade pharmaceutical pollutants in water.144 Ultrasound irradiation of aqueous solutions induces acoustic cavitation, leading to the formation, growth, and violent implosion of high-energy cavitation bubbles, creating localized hot spots. In these regions of extreme temperature and pressure, ˙OH radicals are generated (reaction (a)). Additionally, ˙H react with O2 to form hydroperoxyl radicals (reaction (b)), ultimately producing H2O2 (eqn (c)). Pharmaceutical pollutants are degraded through three primary mechanisms: thermal decomposition within the cavitation bubbles, degradation at the bubble-liquid interface, and free radical-mediated reactions in the bulk solution (eqn (d)).145,146 The frequency of ultrasound waves plays a critical role, as the degradation rate of contaminants is influenced by factors such as ultrasound frequency, intensity, solution pH, and the physical and chemical properties of the pharmaceutical pollutants.147 The generated non-selective reactive species enhance the degradation of pharmaceutical pollutants, making sonolysis an environmentally friendly method for water remediation from contaminants without introducing additional chemicals.148 However, energy is necessary to sustain the acoustic cavitation of bubbles formed during ultrasound irradiation. The influence of sonication power on ultrasonic cavitation, sonochemistry, and paracetamol degradation was studied in single- and dual-frequency sonoreactors.149 Dual-frequency sonication exhibited a synergistic effect on degradation but did not significantly impact oxidant yield, regardless of horn power. The enhanced degradation rate is attributed to improved paracetamol transfer from the bulk to the cavitation bubbles, increasing its availability for reaction with ˙OH radicals. The degradation efficiency can be further improved by incorporating additives such as H2O2, persulfate, or heterogeneous solid catalysts like activated carbon, biochar, and semiconductor photocatalysts.150,151 While sonolysis offers advantages over conventional methods, it has drawbacks, including incomplete degradation of recalcitrant organic pollutants, high energy consumption, and long processing times. These challenges can be addressed by combining sonolysis with other oxidation processes like photocatalysis, Fenton, photo-Fenton, ozonolysis, and electrochemical methods. However, the use of sono-based AOPs in pilot and full-scale wastewater treatment is still in the early stages.| | | Pharmaceuticals + ˙OH → degradation on products → CO2 + H2O | (d) |
4.7. Hybrid AOPs
Conventional wastewater treatment methods are categorized into primary, secondary, and tertiary processes, encompassing sedimentation, flotation, activated sludge processes, membrane technologies, coagulation, and filtration. However, these traditional approaches face limitations in effectively removing intricate pollutants like micropollutants and emerging pharmaceutical contaminants.152 Therefore, there is a need for innovative treatment methods such as AOPs and their combinations to address these challenges. Integrating AOPs with other technologies can create synergistic effects that enhance efficiency, increase the range of pharmaceuticals degraded, and improve the sustainability of the treatment process.153 By combining AOPs with techniques such as membrane filtration, adsorption, biological treatments, or additional AOP methods, the treatment performance can surpass that of individual AOPs alone. This highlights the effectiveness of hybrid AOP systems as a promising approach for pharmaceutical wastewater treatment. Recent studies have increasingly focused on the development of hybrid AOPs, which combine chemical-based and energy-based systems, to explore their synergistic effects in the degradation of emerging contaminants. Combining two or more AOPs in a hybrid system offers the advantage of generating higher concentrations and diverse types of reactive species.32 This synergy addresses limitations of low mineralization efficiency in certain AOPs, especially for pharmaceuticals known for their recalcitrant nature against degradation and mineralization by single AOPs. For example, NTP can produce high concentrations of non-radical species such as H2O2 and O3. When suitable catalysts, like heterogeneous Fenton or ozone catalysts, or homogeneous Fenton and Fenton-like catalysts (e.g., Fe(II) or Cu(II)), are introduced, these non-radical species decompose into powerful, non-selective oxidative radicals, such as hydroxyl and superoxide radicals, which exhibit stronger oxidizing power than H2O2 or O3 alone. This principle also applies effectively to other hybrid systems, like combining persulfate with NTP, enhancing degradation efficiency and broadening treatment capabilities for various organic contaminants.154 A study155 investigated the removal of ibuprofen from wastewater using sonolysis and sono-Fenton oxidation. The combination of homogeneous Fenton oxidation with ultrasound irradiation demonstrated superior efficiency compared to the individual processes, particularly in terms of mineralization yield. The enhanced performance was due to the sono-regeneration of ferrous ions from ferric complexes, enabling continuous Fenton reactions. The process improved TOC removal from 25% to 50% within 3 hours using a low Fenton's reagent concentration, with iron levels below 10 mg L−1, highlighting sono-regeneration as the primary activation mechanism. The hybrid O3/UV/H2O2 system was optimized for carbamazepine removal (10 mg L−1), with O3 concentration at 0.91 mg L−1, H2O2 at 5.5 mg L−1, and UV intensity at 2.9 mW cm−2 for 5 minutes. Results showed 100% degradation of carbamazepine and 34.04% mineralization.156 The photocatalytic ozonation (O3/UVA/TiO2) system was studied for ciprofloxacin (10 mg L−1) removal under the conditions of ozone flow = 0.34 g h−1, TiO2 dosage = 1.0 g L−1, and an initial pH of 9.0 for 15 min. The results showed 98.5% degradation and 81.1% mineralization efficiency.157 Given their environmental friendliness and sustainability, hybrid AOPs utilizing solar irradiation, minimal chemical use, and reduced energy consumption present a promising solution for future large-scale industrial applications in removing pharmaceutical pollutants from wastewater. However, the choice of treatment process depends on pollutant characteristics, cost-effectiveness, and practical applicability.
4.8. Sustainable catalysts as an activator in AOPs
Recently, there has been a growing demand for eco-friendly solutions in cleaning up the aquatic environment.35 AOPs have emerged as effective methods for degradation and mineralization of pharmaceutical pollutants in water using a low-cost and green catalyst as an activator.158,159 A key element in AOP success is using catalysts to enhance oxidation reactions and improve pollutant removal efficiency. Sustainable catalysts have characteristic such as renewability, low energy usage, recyclability, non-toxicity, high catalytic activity, selectivity, compatibility with water, and cost-effectiveness.160 Green and sustainable catalysts could be prepared from a variety of materials, including agricultural, industrial, and animal wastes.161 Biochar-based materials, known for their large surface area and stability, have shown exceptional catalytic activity in AOPs.162 Similarly, metal–organic frameworks (MOFs) offer promise due to their adjustable structures and versatile catalytic properties for sustainable pollutant removal.163 Biochar and MOFs are considered promising, cost-effective, and environmentally friendly catalysts that serve as effective activators and adsorbents in AOPs such as photocatalysis, Fenton, persulfate, and ozone-based AOPs.164–167 Iron-impregnated biochar, derived from waste black seed pomace, was successfully modified and utilized to activate peroxydisulfate (PDS) for diclofenac degradation in water samples. This treatment achieved 88% diclofenac degradation within 10 minutes and a total organic carbon (TOC) removal rate of 90%, indicating effective mineralization, within 60 minutes.105 Biochar and MOFs can be sustainably synthesized from a wide range of waste materials, supporting the “waste-to-treat-waste” concept. This approach not only helps in waste management but also provides valuable adsorbents and catalysts for environmental applications. However, practical implementation in wastewater treatment introduces specific challenges. One major issue is the separation of fine powdered catalysts, such as biochar and MOFs, from the treated water, often requiring time-consuming filtration or centrifugation. To overcome this, incorporating magnetic materials (e.g., iron oxides) into biochar or MOFs can alter their surface properties, making separation with an external magnetic field feasible. This modification not only simplifies the recovery process but also enhances the catalytic activity of the materials in AOPs, such as Fenton and Fenton-like reactions, and in photocatalytic applications. The magnetic modification improves the overall efficiency and reusability of biochar and MOF catalysts, providing a more practical and scalable solution for pollutant degradation in wastewater treatment.
5. Mechanism of AOPs in generating reactive species for pharmaceutical degradation
AOPs are oxidation-based water treatment methods that use energy inputs (e.g., chemical agents like H2O2, persulfate, ozone, or catalysts; electrical power; ultrasound; or light) to generate highly reactive oxidizing species, primarily hydroxyl radicals (with an oxidizing potential of 2.80 V), sulfate radicals, superoxides, and singlet oxygen.168,169 These technologies effectively break down recalcitrant organic pollutants, such as pharmaceuticals, that are resistant to conventional wastewater treatment.20 AOPs commonly use oxidants like hydrogen peroxide, ozone, or persulfate, often combined with UV light, catalysts, or activators. They are effective in degrading complex, non-biodegradable contaminants into less harmful byproducts.67 The generation of reactive species varies depending on the AOP type, experimental conditions, and catalysts. In Fenton oxidation, hydroxyl radicals dominate, while persulfate-based AOPs generate sulfate and hydroxyl radicals, as well as singlet oxygen.63 Photocatalytic oxidation involves a photoactive catalyst, typically a semiconductor such as TiO2, which can be applied as a powder or coated onto a support like glass. When exposed to UV or visible light (depending on the photocatalyst's band-gap energy) in the presence of oxygen, the catalyst generates electron–hole pairs, initiating redox reactions with species adsorbed on its surface (Fig. 7). This process has proven effective in removing pharmaceuticals from water.170 The possible chemical reaction pathways in photocatalysis-based AOPs are illustrated in reactions (R1)–(R9).124,171| | | Photocatalyst + hv → Photocatalyst (e− + h+) | (R1) |
| | | ˙OH + Pharmaceuticals → H2O + CO2 | (R7) |
| | | O2˙− + Pharmaceuticals → H2O + CO2 | (R8) |
| | | ˙OOH + Pharmaceuticals →→→→ H2O + CO2 | (R9) |
 |
| | Fig. 7 Mechanism of ROS generation by various AOPs. | |
Ozonation can occur through two pathways: direct reaction in acidic solutions in the dark or indirect reaction via ozone decomposition, producing reactive species like OH radicals, which occurs in alkaline conditions or with a photocatalyst. In acidic conditions (pH ≤ 4) without light, direct ozonation is dominant. At pH levels between 4 and 9, both direct and indirect pathways are active, while at pH ≥ 9, the indirect route, or ozone-based AOPs, prevails (reactions (R10) and (R11)). In this pathway, generated radicals non-selectively degrade pharmaceuticals, achieving high TOC removal, an indicator of mineralization efficiency. The indirect pathway can be enhanced by UV light or by homogeneous and heterogeneous catalysts, either in darkness or in combination with light, as in photocatalytic ozonation. Photolytic ozonation has proven more effective in the degradation and mineralization of pharmaceuticals7 than UV photolysis or ozonation alone. The main chain reactions of ozone with photo-generated electrons on photocatalyst surfaces are shown in reactions (R12)–(R15).
| | | O3 + OH− → O2˙− + HO2˙ | (R10) |
| | | O3 + HO2˙ → 2O2 + ˙OH | (R11) |
| | | O3˙− + H2O → ˙OH + ˙OH + O2 | (R13) |
| | | ˙OH + Pharmaceuticals → H2O + CO2 | (R15) |
Non-thermal plasma (NTP)-based AOPs, generated by electrical discharge at the gas–liquid interface, in a gas atmosphere, or within a liquid, produce ultrasonic waves, UV radiation, and various reactive species under ambient conditions. These species include neutral molecules (H2, O2, O3, and H2O2), ions, free radicals (˙OH, ˙O, ˙H, and HO2˙), and singlet oxygen, which effectively degrade and mineralize pharmaceutical pollutants. The quantity and nature of these reactive species depend on factors such as discharge type, input power, gaseous atmosphere, water composition, pH, and conductivity. The mechanisms for the generation of these active species by discharge are outlined in reactions (R16)–(R28). Studies have shown that the gas atmosphere significantly influences the types of species generated. For instance, in an argon or helium atmosphere, H2O2 is predominant, whereas ozone formation is favored under oxygen and air atmospheres. In a nitrogen atmosphere, the solution becomes enriched with nitrates, leading to a decrease in the pH of the treated solution. The quantity of reactive species generated is directly proportional to the input power of the discharge. However, at very high input energy, reactive species may undergo scavenging, reducing their availability for pollutant degradation. Therefore, optimizing the input power and selecting an appropriate gas atmosphere are essential to maximize the efficiency of NTP for pharmaceutical pollutant removal.
| | | Energetic electron + H2O → ˙H + ˙OH + electron | (R16) |
| | | Energetic electron + 2H2O → H2O2 + H2 + electron | (R17) |
| | | H2O2 + ˙OH → HO2˙ + H2O | (R20) |
| | | HO2˙ + ˙OH → O2 + H2O | (R21) |
| | | Energetic electron + O2→ 2˙O + electron | (R23) |
| | | H2O + O3 + hv → O2 + H2O2 | (R25) |
| | | H2O2 + O3 → O2 + HO2˙ + ˙OH | (R26) |
| | | HO2˙ + O3 → O2 + O2˙ + ˙OH | (R27) |
The degradation of organic pollutants by hydrogen peroxide relies on ˙OH radical production via H2O2 decomposition, either through UV light or ferrous ions (the Fenton process) (Fig. 7). In the traditional Fenton process, H2O2 reacts with ferrous iron to produce hydroxyl radicals (˙OH), which are highly reactive oxidizers that break down a range of organic pollutants, including pharmaceuticals. The core mechanism starts with the generation of ˙OH from H2O2 by oxidizing Fe(II) to Fe(III) (reaction (29)). The ˙OH may then react with H2O2 to form HO2˙, a less potent oxidizer (reaction (30)). Fe(II) is regenerated by reacting with H2O2 (reaction (31)), enabling the cycle to produce additional ˙OH radicals. Under optimal conditions, this process can achieve complete mineralization of pharmaceuticals, converting them to harmless products like CO2 and H2O (reaction (32)).69
| | | Fe2+ + H2O2 →Fe3+ + ˙OH + OH− | (R29) |
| | | ˙OH + H2O2 →HO2˙ + H2O | (R30) |
| | | Fe3+ + H2O2 → Fe2+ + HO2˙ + H+ | (R31) |
| | | Organic compounds + ˙OH → degradation products → CO2 + H2O | (R32) |
Nonetheless, the process is highly pH-dependent (optimal at pH 2.8–3) and generates iron sludge, posing challenges for large-scale applications. To address these issues, researchers have developed Fenton-like processes, which are modified versions of the traditional Fenton process. These modifications include the use of heterogeneous catalysts as alternatives to homogeneous iron ions, integration with light (photo-Fenton), or employing electrochemistry to generate Fenton's reagent in situ (electro-Fenton).172,173 Fenton-like systems provide a more versatile and cost-effective alternative to traditional Fenton oxidation.
In hybrid AOPs, the mechanisms for generating reactive species vary in both the concentration and types of radical and non-radical oxidative species produced, depending on the specific combination of methods employed. Each method influences the generation pathways, leading to distinct profiles of reactive species that impact the overall oxidation efficiency and pollutant degradation pathways.
6. Factors affecting AOPs
The efficacy of AOPs is subject to numerous influencing factors, contingent upon the technique and environmental context. Key experimental parameters include the nature and characteristic of pharmaceuticals, solution pH, initial pollutant concentrations, water matrix composition, and ionic strength, among others.174 Notably, the duration of AOP application is important in determining its efficiency, as longer reaction times are positively associated with increased contaminant degradation. The pH of a solution is a crucial determinant, as certain processes function best under acidic or alkaline conditions. For instance, ozonation may not effectively eliminate certain organic pollutants due to its selective oxidant nature and low mineralization efficiency. However, when ozonation is conducted in an alkaline environment, it produces ˙OH radicals, which are non-selective and potent oxidants capable of decomposing most organic pollutants into water, CO2, and mineral acids, thus facilitating their complete mineralization.32,66 The initial concentration of pharmaceuticals influences the degradation efficiency in AOPs. Higher concentrations typically lead to lower degradation rates, as reactive species are consumed more rapidly, reducing their availability for further pollutant breakdown. In contrast, lower concentrations allow for more efficient use of reactive species, enhancing degradation efficiency. However, this trend does not apply to all AOPs, as it depends on reactor design, particularly in non-thermal plasma systems. For example, in a falling film reactor, reactive species are predominantly active at the water-gas interface, which can impact the overall degradation efficiency.24 The composition of the water matrix, including organic and inorganic constituents, impacts AOP efficiency. The impact of co-existing ions and catalyst dose in AOPs using catalysts as activators on degradation performance depends on the catalyst's surface electrochemistry and the ionic forms of pollutants and the water matrix. The influence of competing ions on degradation is determined by their type and concentration in the pharmaceutical solution. In photocatalytic AOPs, light source intensity and wavelength influence reaction rates. Understanding and optimizing these factors are crucial for the successful application of AOPs in water treatment and pollutant degradation (Fig. 8).
 |
| | Fig. 8 Factors influencing AOP performance in pharmaceutical removal from water. | |
7. AOPs: a promising solution with challenges and opportunities
The application of AOPs for removing pharmaceutical residues from water marks a significant progress in wastewater treatment technologies. AOPs offer key advantages, including high efficiency, no generation of secondary pollutants, the complete mineralization of pharmaceuticals into harmless byproducts like H2O, CO2, and short-chain acids.175,176 However, to fully realize the potential of AOPs, it is crucial to address challenges related to technological advancements, scaling for broader applications, commercialization, and closing existing research gaps. Despite technological advancements, significant challenges persist in scaling up AOPs to meet the demands of industrial pharmaceutical wastewater treatment. The complex and often costly operation of many AOPs, including high energy consumption and the instability of catalysts used in activation-based processes, hinder their large-scale application and commercialization. Additionally, the complex operations and high energy requirements of NTP and the stability and reusability issues of catalysts in processes such as Fenton, Fenton-like, ozonation, and photocatalysis remain critical barriers for AOPs in real-world applications.177 Sonolysis has limitations, such as incomplete degradation of persistent organic pollutants, high energy consumption, and prolonged processing times. These challenges can be overcome by integrating sonolysis with other oxidation processes. However, the application of ultrasound-based AOPs in pilot- and full-scale wastewater treatment remains in its early stages. Utilizing renewable energy sources, such as solar light, to power the electric discharge generation in non-thermal NTP systems has the potential to significantly reduce the operational costs of the treatments. Solar energy, being abundant and cost-efficient, can replace conventional energy inputs, making NTP-based AOPs more sustainable and economically viable for large-scale applications. Furthermore, the development of low-cost, sustainable catalysts such as biochar and MOFs offers another promising solution. These catalysts, which can be easily synthesized and modified from readily available agro-industrial wastes, not only reduce the material costs associated with AOPs but also enhance their environmental sustainability. The use of such catalysts can alleviate the limitations related to the large-scale implementation of AOPs for pharmaceutical wastewater remediation, thus contributing to the feasibility and affordability of these advanced treatment technologies in real-world applications. Pharmaceutical wastewater treatment commonly employs a single method for removing individual pharmaceuticals. However, cleaner production standards now call for tailored treatments that address the diverse chemical structures and properties of pharmaceuticals in various wastewater types, reflecting ongoing advancements in the field. Given that pharmaceutical compositions differ across manufacturing facilities, there is increasing interest in developing customized treatment processes for the distinct types of pharmaceutical wastewater generated. In practical applications, removing powdered catalysts from polluted solutions post-treatment, whether by filtration or centrifugation, can be challenging.178 Integrating magnetic materials into catalyst such as biochar and MOF, however, can enhance its surface properties, facilitating easier separation from aqueous solutions after wastewater treatment in AOPs, such as Fenton and Fenton-like oxidation, ozone-based AOPs, or when used as a photocatalyst. To enable large-scale, sustainable application of AOPs in pharmaceutical wastewater treatment, optimizing operational parameters is essential. Efficient techniques, such as machine learning and design of experiments (e.g., central composite design, Box-Behnken design, and Taguchi design), can enhance AOP efficiency for pharmaceutical removal in wastewater. Understanding the mechanisms of pharmaceutical degradation by AOPs is key to optimizing these processes through the development of more efficient and sustainable catalysts like biochar and MOFs. Although biochar and MOFs are increasingly used for pharmaceutical removal in AOPs, research gaps remain, requiring further study to enable the effective use of these sustainable catalysts in pharmaceutical wastewater treatment.
8. Conclusions and outlook
Pharmaceutical residues enter the environment from various sources, mainly from point sources like manufacturing plants, institutes, and hospitals, impacting the quality of aquatic ecosystems. These contaminants cause alterations and biological imbalances, posing significant risks to all living organisms. Research on monitoring pharmaceutical residues has shown a gradual increase in their concentrations in rivers, lakes, and even drinking water. AOPs have proven to be effective in eliminating pharmaceutical pollutants from the pollution point sources. The adoption of a waste-to-resource approach, using low-cost and sustainable catalysts such as biochar and MOFs as activators in various AOPs, including Fenton, persulfate, ozonation, and photocatalysis, greatly improves the degradation and mineralization of pharmaceuticals. Using these affordable, sustainable catalysts in AOPs improves the feasibility of these methods for commercial applications. These catalysts are easily prepared and modified, and they exhibit excellent stability and reusability under real wastewater conditions, making them a promising strategy for the efficient removal of pharmaceutical pollutants and suitable for large-scale water remediation applications. Additionally, the hybrid application of various AOPs shows potential for further increasing mineralization by boosting the production of ROS during the treatment processes. Future research should focus on the development of sustainable and efficient catalysts to act as activators in AOPs. The goal is to enhance mineralization efficiency, a critical factor for the successful treatment of wastewater using AOPs. Investigating the innovative combination of AOPs with other advanced water treatment technologies is crucial for optimizing their effectiveness in practical applications. Few studies have applied AOPs to treat actual pharmaceutical wastewater; most focus on removing pharmaceuticals from pure water. The complexity of contaminants in real wastewater and natural water matrices must be considered to assess pharmaceutical degradation selectivity in AOPs. Evaluating AOP techniques to effectively and simultaneously remove diverse pollutants from municipal, domestic, and industrial wastewater is crucial. Identifying reaction intermediates and byproducts is essential for effective pharmaceutical removal via AOPs. However, few studies report the toxicity of these degradation byproducts. Future research should focus on identifying reaction products and comparing their toxicity to the parent pharmaceuticals. TOC removal, indicating the degree of mineralization, is a crucial metric for assessing AOP effectiveness in wastewater treatment. Although AOPs have demonstrated significant potential for degrading pharmaceuticals, their performance can be improved by integrating them with additional water treatment methods, such as adsorption, membrane filtration, and other AOP processes in a hybrid approach.
Author contributions
K. H. H. A.: conceptualization, data curation, investigation, resources, software, validation, supervision, visualization, writing – original draft, writing – review & editing. F. S. M.: software, writing – review & editing. M. A. H.: writing – review & editing. S. H.: writing – review & editing.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgements
The authors are thankful to the University of Sulaimani for its assistance and collaboration throughout the review.
References
- M. Fida,
et al., Water contamination and human health risks in Pakistan: a review, Exposures Health, 2023, 15(3), 619–639 CrossRef CAS.
- X. Yu,
et al., Application of passive sampling device for exploring the occurrence, distribution, and risk of pharmaceuticals and pesticides in surface water, Sci. Total Environ., 2024, 908, 168393 CrossRef CAS PubMed.
- S. Khan,
et al., Emerging contaminants of high concern for the environment: Current trends and future research, Environ. Res., 2022, 207, 112609 CrossRef CAS PubMed.
- O. A. A. Falahi,
et al., Occurrence of pharmaceuticals and personal care products in domestic wastewater, available treatment technologies, and potential treatment using constructed wetland: a review, Process Saf. Environ. Prot., 2022, 168, 1067–1088 CrossRef.
- G. Mistry,
et al., New outlook on hazardous pollutants in the wastewater environment: Occurrence, risk assessment and elimination by electrodeionization technologies, Environ. Res., 2023, 219, 115112 CrossRef CAS PubMed.
- K. H. H. Aziz,
et al., Recent advances in water falling film reactor designs for the removal of organic pollutants by advanced oxidation processes: A review, Water Resour. Ind., 2023, 100227 CrossRef.
- K. H. H. Aziz,
et al., Degradation of pharmaceutical diclofenac and ibuprofen in aqueous solution, a direct comparison of ozonation, photocatalysis, and non-thermal plasma, Chem. Eng. J., 2017, 313, 1033–1041 CrossRef.
- K. H. H. Aziz, Heterogeneous catalytic activation of peroxydisulfate toward degradation of pharmaceuticals diclofenac and ibuprofen using scrap printed circuit board, RSC Adv., 2023, 13(1), 115–128 RSC.
- J. O. Eniola,
et al., A review on conventional and advanced hybrid technologies for pharmaceutical wastewater treatment, J. Cleaner Prod., 2022, 356, 131826 CrossRef CAS.
- X. Shi, K. Y. Leong and H. Y. Ng, Anaerobic treatment of pharmaceutical wastewater: a critical review, Bioresour. Technol., 2017, 245, 1238–1244 CrossRef CAS PubMed.
- A. Moghaddam,
et al., Biodegradation of pharmaceutical compounds in industrial wastewater using biological treatment: A comprehensive overview, Int. J. Environ. Sci. Technol., 2023, 20(5), 5659–5696 CrossRef.
- A. Khalidi-Idrissi,
et al., Recent advances in the biological treatment of wastewater rich in emerging pollutants produced by pharmaceutical industrial discharges, Int. J. Environ. Sci. Technol., 2023, 1–22 CAS.
- M. A. Gulamhussein, B. Saini and A. Dey, Removal of pharmaceutical contaminants through membrane bioreactor, Mater. Today: Proc., 2023, 77, 260–268 CAS.
- L. Sellaoui,
et al., Adsorption of pharmaceutical pollutants on activated carbon: Physicochemical assessment of the adsorption mechanism via advanced modelling, J. Mol. Liq., 2023, 389, 122929 CrossRef CAS.
- M. Zhai,
et al., Simultaneous removal of pharmaceuticals and heavy metals from aqueous phase via adsorptive strategy: A critical review, Water Res., 2023, 119924 CrossRef CAS PubMed.
- S. Kumar,
et al., Recent Advancement in Nanotechnology for the Treatment of Pharmaceutical Wastewater: Sources, Toxicity, and Remediation Technology, Curr. Pollut. Rep., 2023, 1–33 Search PubMed.
- S. Singh,
et al., Phytoremediation of pharmaceuticals and personal care products using the constructed wetland, Environ. Chem. Ecotoxicol., 2024, 6, 104–116 CrossRef CAS.
- S. Shafique,
et al., Deep eutectic solvents (DES): Structure, properties, and cutting-edge applications in green catalysis, J. Mol. Liq., 2024, 126769 Search PubMed.
- J. Park,
et al., Removal characteristics of pharmaceuticals and personal care products: Comparison between membrane bioreactor and various biological treatment processes, Chemosphere, 2017, 179, 347–358 CrossRef CAS PubMed.
- A. K. Thakur,
et al., Pharmaceutical waste-water treatment via advanced oxidation based integrated processes: An engineering and economic perspective, J. Water Process Eng., 2023, 54, 103977 CrossRef.
- R. Changotra, H. Rajput and A. Dhir, Treatment of real pharmaceutical wastewater using combined approach of Fenton applications and aerobic biological treatment, J. Photochem. Photobiol., A, 2019, 376, 175–184 CrossRef CAS.
- K. H. H. Aziz,
et al., Adsorptive removal of toxic heavy metals from aquatic environment by metal organic framework (MOF): A review, J. Water Process Eng., 2025, 70, 106867 CrossRef.
- V. Singh, S. Gupta and S. K. Samanta, Water resource rejuvenation via AOP based degradation of pharmaceuticals extensively used during COVID-19, J. Water Process Eng., 2024, 67, 106137 CrossRef.
- K. H. H. Aziz,
et al., Degradation of perfluorosurfactant in aqueous solution using non-thermal plasma generated by nano-second pulse corona discharge reactor, Arabian J. Chem., 2021, 14(10), 103366 CrossRef CAS.
- J. Xu,
et al., Progress of disinfection catalysts in advanced oxidation
processes, mechanisms and synergistic antibiotic degradation, Sci. Total Environ., 2024, 913, 169580 CrossRef CAS PubMed.
- Q. Zhang,
et al., Insight into antibiotic removal by advanced oxidation processes (AOPs): Performance, mechanism, degradation pathways, and ecotoxicity assessment, Chem. Eng. J., 2024, 157134 CrossRef CAS.
- T. L. da Silva,
et al., Overview of non-steroidal anti-inflammatory drugs degradation by advanced oxidation processes, J. Cleaner Prod., 2022, 346, 131226 CrossRef CAS.
- Y. D. Shahamat,
et al., Removal of azo red-60 dye by advanced oxidation process O3/UV from textile wastewaters using Box-Behnken design, Inorg. Chem. Commun., 2022, 143, 109785 CrossRef.
- A. E. Gahrouei,
et al., From wastewater to clean water: Recent advances on the removal of metronidazole, ciprofloxacin, and sulfamethoxazole antibiotics from water through adsorption and advanced oxidation processes (AOPs), Environ. Res., 2024, 119029 CrossRef CAS PubMed.
- X. A. Mutke,
et al., Efficiency of ozonation and sulfate radical-AOP for removal of pharmaceuticals, corrosion inhibitors, x-ray contrast media and perfluorinated compounds from reverse osmosis concentrates, Water Res., 2024, 255, 121346 CrossRef CAS PubMed.
- B. O. Ojo, O. A. Arotiba and N. Mabuba, A review on reactive oxygen species generation, anode materials and operating parameters in sonoelectrochemical oxidation for wastewater remediation, Chemosphere, 2024, 143218 CrossRef CAS PubMed.
- K. H. H. Aziz, Application of different advanced oxidation processes for the removal of chloroacetic acids using a planar falling film reactor, Chemosphere, 2019, 228, 377–383 CrossRef PubMed.
-
M. C. Néstor and C. Mariana, Impact of pharmaceutical waste on biodiversity, Ecopharmacovigilance: Multidisciplinary Approaches to Environmental Safety of Medicines, 2019, pp. 235–253 Search PubMed.
-
A. Dhawande, et al., Pharmaceutical waste: an emerging threat to the ecosystem, in 360-Degree Waste Management, Elsevier, 2023, vol. 2, pp. 3–37 Search PubMed.
- M. Hossein,
et al., Exploring eco-friendly approaches for mitigating pharmaceutical and personal care products in aquatic ecosystems: A sustainability assessment, Chemosphere, 2023, 316, 137715 CrossRef CAS PubMed.
- M. Mofijur,
et al., Advances in identifying and managing emerging contaminants in aquatic ecosystems: Analytical approaches, toxicity assessment, transformation pathways, environmental fate, and remediation strategies, Environ. Pollut., 2023, 122889 Search PubMed.
- H. Karimi-Maleh,
et al., Recent advances in using of chitosan-based adsorbents for removal of pharmaceutical contaminants: A review, J. Cleaner Prod., 2021, 291, 125880 CrossRef CAS.
- M. Pirsaheb, N. Moradi and H. Hossini, Sonochemical processes for antibiotics removal from water and wastewater: a systematic review, Chem. Eng. Res. Des., 2023, 189, 401–439 CrossRef CAS.
- A. Arumugam,
et al., Pharmaceuticals as Emerging Pollutants: Implications for Water Resource Management in Malaysia, Emerging Contam., 2025, 100470 CrossRef CAS.
- A. Puckowski,
et al., Bioaccumulation and analytics of pharmaceutical residues in the environment: A review, J. Pharm. Biomed. Anal., 2016, 127, 232–255 CrossRef CAS PubMed.
- A. Thakur,
et al., Pharmaceutical waste-water treatment via advanced oxidation based integrated processes: an engineering and economic perspective, J. Water Process Eng., 54, 103977, Cent. Eur. J. Chem., 2023, 10, 989–1027 Search PubMed.
- A. Singh, S. G. Pratap and A. Raj, Occurrence and dissemination of antibiotics and antibiotic resistance in aquatic environment and its ecological implications: a review, Environ. Sci. Pollut. Res., 2024, 31(35), 47505–47529 CrossRef CAS PubMed.
- M. del Carmen Gómez-Regalado,
et al., Bioaccumulation/bioconcentration of pharmaceutical active compounds in aquatic organisms: Assessment and factors database, Sci. Total Environ, 2023, 861, 160638 CrossRef PubMed.
-
C. O. Adetunji, et al., Integrated processes for production of pharmaceutical products from agro-wastes, Biomass, Biofuels, Biochemicals, Elsevier, 2022, pp. 439–461 Search PubMed.
- S. Muthusamy,
et al., Microbial pullulan for food, biomedicine, cosmetic, and water treatment: A review, Environ. Chem. Lett., 2022, 20(5), 3199–3234 CrossRef CAS.
- E. S. Okeke,
et al., Environmental and health impact of unrecovered API from pharmaceutical manufacturing wastes: A review of contemporary treatment, recycling and management strategies, Sustainable Chem. Pharm., 2022, 30, 100865 CrossRef CAS.
- M. Caban and P. Stepnowski, How to decrease pharmaceuticals in the environment? A review, Environ. Chem. Lett., 2021, 19, 3115–3138 CrossRef CAS.
- J. O. Osuoha, B. O. Anyanwu and C. Ejileugha, Pharmaceuticals and personal care products as emerging contaminants: Need for combined treatment strategy, J. Hazard. Mater. Adv., 2023, 9, 100206 CAS.
- A. Chakraborty,
et al., Pharmaceuticals and Personal Care Products as Emerging
Environmental Contaminants: Prevalence, Toxicity, and Remedial Approaches, ACS Chem. Health Saf., 2023, 30(6), 362–388 CrossRef CAS.
- M.-K. Nguyen,
et al., Occurrence, fate, and potential risk of pharmaceutical pollutants in agriculture: Challenges and environmentally friendly solutions, Sci. Total Environ., 2023, 899, 165323 CrossRef CAS PubMed.
- M. R. Letsoalo,
et al., Efficient detection and treatment of pharmaceutical contaminants to produce clean water for better health and environmental, J. Cleaner Prod., 2023, 387, 135798 CrossRef CAS.
- M. Paut Kusturica, A. Tomas and A. Sabo, Disposal of unused drugs: Knowledge and behavior among people around the world, Rev. Environ. Contam. Toxicol., 2017, 240, 71–104 CAS.
- S. M. Zainab,
et al., Antibiotics and antibiotic resistant genes (ARGs) in groundwater: A global review on dissemination, sources, interactions, environmental and human health risks, Water Res., 2020, 187, 116455 CrossRef CAS PubMed.
- Y. Ben,
et al., Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review, Environ. Res., 2019, 169, 483–493 CrossRef CAS PubMed.
- L. Wang, X. Ye and J. Liu, Effects of pharmaceutical and personal care products on pubertal development: Evidence from human and animal studies, Environ. Pollut., 2024, 123533 CrossRef CAS PubMed.
- J. O. Tijani, O. O. Fatoba and L. F. Petrik, A review of pharmaceuticals and endocrine-disrupting compounds: sources, effects, removal, and detections, Water, Air, Soil Pollut., 2013, 224, 1–29 CrossRef CAS.
- A. Khan,
et al., Impact, disease outbreak and the eco-hazards associated with pharmaceutical residues: a critical review, Int. J. Environ. Sci. Technol., 2021, 1–12 Search PubMed.
- E. R. Kabir, M. S. Rahman and I. Rahman, A review on endocrine disruptors and their possible impacts on human health, Environ. Toxicol. Pharmacol., 2015, 40(1), 241–258 CrossRef CAS PubMed.
- M. Puri, K. Gandhi and M. Suresh Kumar, A global overview of endocrine disrupting chemicals in the environment: Occurrence, effects, and treatment methods, Int. J. Environ. Sci. Technol., 2023, 20(11), 12875–12902 CrossRef.
- S. Hassan,
et al., Endocrine disruptors: Unravelling the link between chemical exposure and Women's reproductive health, Environ. Res., 2024, 241, 117385 CrossRef CAS PubMed.
- F. Rahimi,
et al., Advances in Three-Dimensional Electrochemical Degradation: A Comprehensive Review on Pharmaceutical Pollutants Removal from Aqueous Solution, Chemosphere, 2024, 142620 CrossRef CAS PubMed.
- Y. Deng and R. Zhao, Advanced Oxidation Processes (AOPs) in Wastewater Treatment, Curr. Pollut. Rep., 2015, 1(3), 167–176 CrossRef CAS.
- J. Wang and S. Wang, Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism, Chem. Eng. J., 2020, 401, 126158 CrossRef CAS.
- H. R. Ahmed,
et al., Utilizing sequence transformation of selective copper metal as an efficient heterogeneous Fenton-like catalyst for the degradation of aqueous methylene blue, React. Kinet., Mech. Catal., 2023, 1–18 Search PubMed.
- H. R. Ahmed,
et al., Iron-loaded carbon black prepared via chemical vapor deposition as an efficient peroxydisulfate activator for the removal of rhodamine B from water, RSC Adv., 2023, 13(37), 26252–26266 RSC.
- K. H. H. Aziz,
et al., Application of a planar falling film reactor for decomposition and mineralization of methylene blue in the aqueous media via ozonation, Fenton, photocatalysis and non-thermal plasma: A comparative study, Process Saf. Environ. Prot., 2018, 113, 319–329 CrossRef.
- P. Kumari and A. Kumar, Advanced oxidation process: A remediation technique for organic and non-biodegradable pollutant, Results Surf. Interfaces, 2023, 11, 100122 CrossRef.
- R. Ravi and A. K. Golder, Photocatalytic and biological degradation processes to mineralize pharmaceutically active compounds and catalyst recovery: a review, Coord. Chem. Rev., 2025, 523, 216267 CrossRef CAS.
- Y. Jiang,
et al., Recent progress in Fenton/Fenton-like reactions for the removal of antibiotics in aqueous environments, Ecotoxicol. Environ. Saf., 2022, 236, 113464 CrossRef CAS PubMed.
- M. Magureanu, N. B. Mandache and V. I. Parvulescu, Degradation of pharmaceutical compounds in water by non-thermal plasma treatment, Water Res., 2015, 81, 124–136 CrossRef CAS PubMed.
- S. W. da Silva,
et al., Advanced electrochemical oxidation processes in the treatment of pharmaceutical containing water and wastewater: A review, Curr. Pollut. Rep., 2021, 7, 146–159 CrossRef CAS.
-
S. Krishnan, et al., Removal of pharmaceutically active compounds from wastewater by hybrid advanced oxidation processes, Concept of Zero Liquid Discharge, Elsevier, 2023, pp. 187–223 Search PubMed.
- T. Wu,
et al., Construction of a novel S-scheme heterojunction piezoelectric photocatalyst V-BiOIO3/FTCN and immobilization with floatability for tetracycline degradation, J. Hazard. Mater., 2023, 443, 130251 CrossRef CAS PubMed.
- H. Yang, C.-G. Lee and J. Lee, Utilizing animal manure-derived biochar in catalytic advanced oxidation processes: A review, J. Water Process Eng., 2023, 56, 104545 CrossRef.
- M. Saeed,
et al., Synthesis of pn NiO-ZnO heterojunction for photodegradation of crystal violet dye, Alex. Eng. J, 2023, 65, 561–574 CrossRef.
- A. Kulišťáková, Removal of pharmaceutical micropollutants from real wastewater matrices by means of photochemical advanced oxidation processes–a review, J. Water Process Eng., 2023, 53, 103727 CrossRef.
- A. Mathew,
et al., Comprehensive understanding of biomedical usages of metal and non metal doped carbon dots, Mater. Today Commun., 2023, 37, 106991 CrossRef CAS.
- Y.-Y. Wang,
et al., Recent advances in non-metal doped titania for solar-driven photocatalytic/photoelectrochemical water-splitting, J. Energy Chem., 2022, 66, 529–559 CrossRef CAS.
- H. Khan and M. U. H. Shah, Modification strategies of TiO2 based photocatalysts for enhanced visible light activity and energy storage ability: A review, J. Environ. Chem. Eng., 2023, 111532 CrossRef CAS.
- T.-D. Tran,
et al., Enhance diclofenac removal in wastewater by photocatalyst process combination with hydrogen peroxide, Case Stud. Chem. Environ. Eng., 2023, 8, 100506 CrossRef.
- P. Eskandari,
et al., Photocatalytic degradation of metronidazole and oxytetracycline by novel l-Arginine (C, N codoped)-TiO2/g-C3N4: RSM optimization, photodegradation mechanism, biodegradability evaluation, Chemosphere, 2023, 337, 139282 CrossRef CAS PubMed.
- A. O. Oluwole and O. S. Olatunji, Synthesis and characterization of binary bismuth tungstate-graphitic carbon nitride (BWO/g-C3N4) heterojunction nanocomposites for efficient photodegradation of ibuprofen in aqueous media, J. Water Process Eng., 2023, 54, 104045 CrossRef.
- N. Aghababaei,
et al., Photocatalytic degradation of diclofenac using a novel double Z-scheme catalyst (Og-C3N4/ZnO/TiO2@ halloysite nanotubes): degradation mechanism, identification of by-products and environmental implementation, J. Water Process Eng., 2023, 53, 103702 CrossRef.
- V. Sumalatha,
et al., Hydrothermal fabrication of n-CeO2/p-CuS heterojunction nanocomposite for enhanced photodegradation of pharmaceutical drugs in wastewater under visible-light and fluorometric sensor for detection of uric acid, Inorg. Chem. Commun., 2023, 155, 110962 CrossRef CAS.
- M. Honarmand,
et al., Fabrication of Co3O4/CdO/clinoptilolite nanocomposite heterojunction with synergistically enhanced catalytic performance for photodegradation of pharmaceutical pollutants, Surf. Interfaces, 2024, 44, 103609 CrossRef CAS.
- D. Nugroho,
et al., Synergistic magnetic field effect to enhance photocatalytic effect for pharmaceutics degradation by Ti3C2 MXene/GQDs@ ZnO, J. Photochem. Photobiol., A, 2025, 462, 116224 CrossRef CAS.
- A. H. Ali and A. I. Alwared, Photocatalytic continuous degradation of pharmaceutical pollutants by zeolite/Fe3O4/CuS/CuWO4 nanocomposite under direct sunlight, Results Eng., 2024, 24, 103234 CrossRef CAS.
- A. Lins,
et al., Cashew gum-assisted synthesis of Zn0.98Nd0.02O photocatalyst: pH-dependent green approach and photocatalytic degradation of ciprofloxacin and ibuprofen pharmaceutical pollutants, Int. J. Biol. Macromol., 2025, 140720 CrossRef CAS PubMed.
- M. O. Qamar, K. Balu and Y.-H. Ahn, BiVO4-Driven photocatalytic degradation of pharmaceutical in Slurry Bubble Column Reactor: Influencing factors and toxicological profiling, Chem. Eng. J., 2024, 496, 153526 CrossRef.
- K. Kalantarian and S. Sheibani, Enhancement of photocatalytic efficiency of Fe2O3 through g-C3N4 nanosheet modification for degradation of organic and pharmaceutical pollutants and hydrogen production, Ceram. Int., 2024, 50(21), 42818–42834 CrossRef.
- M. S. Mohtaram,
et al., Photocatalytic degradation of acetaminophen using a novel TiO2-orange peel-derived biochar composite: Synthesize, characterization and optimization of key factors, J. Water Process Eng., 2024, 58, 104884 CrossRef.
- O. Ganzenko,
et al., Electrochemical advanced oxidation and biological processes for wastewater treatment: a review of the combined approaches, Environ. Sci. Pollut. Res., 2014, 21, 8493–8524 CrossRef CAS PubMed.
- C. Gong,
et al., Insights into degradation of pharmaceutical pollutant atenolol via electrochemical advanced oxidation processes with modified Ti4O7 electrode: Efficiency, stability and mechanism, Environ. Res., 2023, 228, 115920 CrossRef CAS PubMed.
- J. Qiao and Y. Xiong, Electrochemical oxidation technology: A review of its application in high-efficiency treatment of wastewater containing persistent organic pollutants, J. Water Process Eng., 2021, 44, 102308 CrossRef.
- V. Ganthavee and A. P. Trzcinski, Removal of pharmaceutically active compounds from wastewater using adsorption coupled with electrochemical oxidation technology: a critical
review, J. Ind. Eng. Chem., 2023, 126, 20–35 CrossRef CAS.
- A. Goi and M. Trapido, Hydrogen peroxide photolysis, Fenton reagent and photo-Fenton for the degradation of nitrophenols: a comparative study, Chemosphere, 2002, 46(6), 913–922 CrossRef CAS PubMed.
-
A. Santos Hernandez, Direct synthesis of H2O2 at ambient conditions and its applicability for water treatment with Fenton's catalysts, 2022, Cardiff University.
- H. H. P. Quang,
et al., Fe2+, Fe3+, Co2+ as highly efficient cocatalysts in the homogeneous electro-Fenton process for enhanced treatment of real pharmaceutical wastewater, J. Water Process Eng., 2022, 46, 102635 CrossRef.
- F. Rezaei and D. Vione, Effect of pH on zero valent iron performance in heterogeneous Fenton and Fenton-like processes: A review, Molecules, 2018, 23(12), 3127 CrossRef PubMed.
- X. Du,
et al., Derivatives of metal-organic frameworks for heterogeneous Fenton-like processes: From preparation to performance and mechanisms in wastewater purification–A mini review, Environ. Res., 2022, 206, 112414 CrossRef CAS PubMed.
- A. M. Díez,
et al., Nano-zero-valent particles synthesized with agroindustry wastes for pesticide degradation under real conditions, Process Saf. Environ. Prot., 2023, 176, 1089–1100 CrossRef.
- K. O. Rahman and K. H. H. Aziz, Utilizing scrap printed circuit boards to fabricate efficient Fenton-like catalysts for the removal of pharmaceutical diclofenac and ibuprofen from water, J. Environ. Chem. Eng., 2022, 10(6), 109015 CrossRef CAS.
- K. H. B. Haroon and S. K. Bhunia, Fenton-like catalysts based on graphitic carbon nitride nanosheets decorated with Fe3O4 nanoparticles for removal of colorless tetracycline, ACS Appl. Nano Mater., 2023, 7, 26356–26368 CrossRef.
- C. Zhou,
et al., Enhancing Fenton-like reaction through a multifunctional molybdenum disulfide film coating on nano zero valent iron surface (MoS2@ nZVI): Collaboration of radical and non-radical pathways, Sci. Total Environ., 2024, 920, 170818 CrossRef CAS PubMed.
- F. S. Mustafa and K. H. H. Aziz, Heterogeneous catalytic activation of persulfate for the removal of rhodamine B and diclofenac pollutants from water using iron-impregnated biochar derived from the waste of black seed pomace, Process Saf. Environ. Prot., 2023, 170, 436–448 CrossRef CAS.
- Y. Liu,
et al., Synergistic removal mechanism of tetracycline by ethylenediamine modified magnetic chitosan based Fenton-like catalyst, RSC Adv., 2024, 14(49), 36507–36516 RSC.
- M. Ayoub,
et al., Enhancing ceftriaxone degradation via an optimized Fenton-like reaction using ZVI/Cu nanoparticles, J. Water Process Eng., 2025, 69, 106860 CrossRef.
- S.-P. Sun,
et al., Enhanced heterogeneous and homogeneous Fenton-like degradation of carbamazepine by nano-Fe3O4/H2O2 with nitrilotriacetic acid, Chem. Eng. J., 2014, 244, 44–49 CrossRef CAS.
- S. Peng,
et al., Applicability study on the degradation of acetaminophen via an H2O2/PDS-based advanced oxidation process using pyrite, Chemosphere, 2018, 212, 438–446 CrossRef CAS PubMed.
- W. He,
et al., Facile synthesis of Fe3O4@ MIL-100 (Fe) towards enhancing photo-Fenton like degradation of levofloxacin via a synergistic effect between Fe3O4 and MIL-100 (Fe), Chem. Eng. J., 2021, 409, 128274 CrossRef CAS.
- A. S. Giri and A. K. Golder, Ciprofloxacin degradation from aqueous solution by Fenton oxidation: reaction kinetics and degradation mechanisms, RSC Adv., 2014, 4(13), 6738–6745 RSC.
- N. Belkessa,
et al., A review of Non-thermal plasma-catalysis: The mutual influence and sources of synergetic effect for boosting volatile organic compounds removal, Environ. Res., 2024, 119333 CrossRef CAS PubMed.
- R. C. Sanito, S.-J. You and Y.-F. Wang, Degradation of contaminants in plasma technology: An overview, J. Hazard. Mater., 2022, 424, 127390 CrossRef CAS PubMed.
- K. H. H. Aziz,
et al., Removal of dichloroacetic acid from aqueous solution using non-thermal plasma generated by dielectric barrier discharge and nano-pulse corona discharge, Sep. Purif. Technol., 2019, 216, 51–57 CrossRef.
- J. Sima,
et al., Complete degradation of polystyrene microplastics through non-thermal plasma-assisted catalytic oxidation, J. Hazard. Mater., 2024, 136313 CrossRef CAS PubMed.
- A. Ouzar,
et al., Enhanced removal of malachite green from wastewater using nonthermal plasma gliding arc discharge combined with ferrate oxidation, Desalin. Water Treat., 2024, 320, 100743 CrossRef CAS.
- K. H. H. Aziz,
et al., Comparative study on 2, 4-dichlorophenoxyacetic acid and 2, 4-dichlorophenol removal from aqueous solutions via ozonation, photocatalysis and non-thermal plasma using a planar falling film reactor, J. Hazard. Mater., 2018, 343, 107–115 CrossRef PubMed.
- K. Kyere-Yeboah, I. K. Bique and X.-C. Qiao, Advances of non-thermal plasma discharge technology in degrading recalcitrant wastewater pollutants, A comprehensive review, Chemosphere, 2023, 320, 138061 CrossRef CAS PubMed.
- L. Chandana,
et al., Non-thermal atmospheric pressure plasma jet for the bacterial inactivation in an aqueous medium, Sci. Total Environ., 2018, 640, 493–500 CrossRef PubMed.
- Y. He,
et al., Volatile organic compounds degradation by nonthermal plasma: a review, Environ. Sci. Pollut. Res., 2023, 30(12), 32123–32152 CrossRef CAS PubMed.
- B. Jiang,
et al., Review on electrical discharge plasma technology for wastewater remediation, Chem. Eng. J., 2014, 236, 348–368 CrossRef CAS.
- M. Ansari,
et al., A systematic review of non-thermal plasma (NTP) technologies for synthetic organic pollutants (SOPs) removal from water: Recent advances in energy yield aspects as their key limiting factor, J. Water Process Eng., 2023, 51, 103371 CrossRef.
- E. S. M. Mouele,
et al., A critical review on ozone and co-species, generation and reaction mechanisms in plasma induced by dielectric barrier discharge technologies for wastewater remediation, J. Environ. Chem. Eng., 2021, 9(5), 105758 CrossRef CAS.
-
K. H. H. Aziz, et al., Application of New Planar Falling Film Reactors for Degradation and Mineralization of Few Pharmaceuticals and Organic Pollutants in Aqueous Solutions by Means of Ozonation, Photocatalysis and Non-thermal plasma. Photocatalysi s and Non-thermal Plasma, Council of the College of Science at the University of Sulaimani in Partial, 2018.
- S. Kooshki,
et al., Selective reactive oxygen and nitrogen species production in plasma-activated water via dielectric barrier discharge reactor: An innovative method for tuning and its impact on dye degradation, J. Water Process Eng., 2024, 63, 105477 CrossRef.
- S. D. Savić,
et al., Complete degradation of propranolol by a water falling film non-thermal plasma reactor: The effects of input power and plasma gases on transformation pathway, Chem. Eng. J., 2024, 497, 154685 CrossRef.
- Y. Wei,
et al., Degradation of enrofloxacin in aqueous by DBD plasma and UV: degradation performance, mechanism and toxicity assessment, Chem. Eng. J., 2022, 431, 133360 CrossRef CAS.
- L. Onga,
et al., Degradation of anti-inflammatory drug dexamethasone by pulsed corona discharge: The effect of peroxycompounds addition, J. Environ. Chem. Eng., 2022, 10(3), 108042 CrossRef CAS.
- Y. Wang,
et al., Mechanism and process of sulfamethoxazole decomposition with persulfate activated by pulse dielectric barrier discharge plasma, Sep. Purif. Technol., 2022, 287, 120540 CrossRef CAS.
- L. Feyzi, N. Rahemi and S. Allahyari, Efficient degradation of tetracycline in aqueous solution using a coupled S-scheme ZnO/g-C3N4/zeolite P supported catalyst with water falling film plasma reactor, Process Saf. Environ. Prot., 2022, 161, 827–847 CrossRef CAS.
- M. P. Rayaroth,
et al., Degradation and transformation of carbamazepine in aqueous medium under non-thermal plasma oxidation process, Chemosphere, 2024, 352, 141449 CrossRef PubMed.
- N. Nandy,
et al., Antibiotic Chloramphenicol degradation using submerged thermal plasma synergized with LaMnO3 catalyst, Sep. Purif. Technol., 2024, 349, 127822 CrossRef CAS.
- N. Nippatlapalli, K. Ramakrishnan and L. Philip, Enhanced degradation of complex organic compounds in wastewater using different novel continuous flow non–thermal pulsed corona plasma discharge reactors, Environ. Res., 2022, 203, 111807 CrossRef CAS PubMed.
- K. Shang,
et al., Degradation of sulfamethoxazole (SMX) by water falling film DBD Plasma/Persulfate: Reactive species identification and their role in SMX degradation, Chem. Eng. J., 2022, 431, 133916 CrossRef CAS.
- C. V. Rekhate and J. Srivastava, Recent advances in ozone-based advanced oxidation processes for treatment of wastewater-A review, Chem. Eng. J. Adv., 2020, 3, 100031 CrossRef CAS.
- S. N. Malik,
et al., Hybrid ozonation process for industrial wastewater treatment: Principles and applications: A review, J. Water Process Eng., 2020, 35, 101193 CrossRef.
- P. P. Das,
et al., Advancements on ozonation process for wastewater treatment: A comprehensive review, Chem. Eng. Process., 2024, 109852 CrossRef CAS.
- Z. Ji,
et al., The degradation of polycyclic aromatic hydrocarbons (PAHs) by ozone-based advanced oxidation processes: A review, Ozone: Sci. Eng., 2024, 46(1), 26–42 CrossRef CAS.
-
K. H. H. Aziz, et al., Application of New Planar Falling Film Reactors for Degradation and Mineralization of Few Pharmaceuticals and Organic Pollutants in Aqueous Solutions by Means of Ozonation, Photocatalysis and Non-thermal plasma, Council of the College of Science at the University of Sulaimani in Partial, 2018.
-
P. P. Das, S. Dhara and M. K. Purkait, Ozone-based oxidation processes for the removal of pharmaceutical products from wastewater, Development in Wastewater Treatment Research and Processes, Elsevier, 2024, pp. 287–308 Search PubMed.
- F. A. Almomani,
et al., Removal of emerging pharmaceuticals from wastewater by ozone-based advanced oxidation processes, Environ. Prog. Sustainable Energy, 2016, 35(4), 982–995 CrossRef CAS.
- M. Mehrjouei, S. Müller and D. Möller, A review on photocatalytic ozonation used for the treatment of water and wastewater, Chem. Eng. J., 2015, 263, 209–219 CrossRef CAS.
- M. P. Rayaroth, U. K. Aravind and C. T. Aravindakumar, Degradation of pharmaceuticals by ultrasound-based advanced oxidation process, Environ. Chem. Lett., 2016, 14, 259–290 CrossRef CAS.
- R. F. Waris,
et al., Sonolysis-ozonation and peroxidation method for removal of atenolol and amoxicillin in wastewater, Desalin. Water Treat., 2024, 320, 100857 CrossRef CAS.
- M. H. Dehghani,
et al., Recent trends in the applications of sonochemical reactors as an advanced oxidation process for the remediation of microbial hazards associated with water and wastewater: A critical review, Ultrason. Sonochem., 2023, 94, 106302 CrossRef CAS PubMed.
- L. P. Vega, J. Soltan and G. A. Peñuela, Sonochemical degradation of triclosan in water in a multifrequency reactor, Environ. Sci. Pollut. Res., 2019, 26, 4450–4461 CrossRef CAS PubMed.
- T. Velempini, E. Prabakaran and K. Pillay, Recent developments in the use of metal oxides for photocatalytic degradation of pharmaceutical pollutants in water—a review, Mater. Today Chem., 2021, 19, 100380 CrossRef CAS.
- M. Sandaoui,
et al., The ultrasonic degradation of a pharmaceutical formulation including gentamicin sulfate and parabens: Optimization of operational parameters, antibacterial activity assessment, and analysis of resulting by-products, J. Water Process Eng., 2024, 58, 104875 CrossRef.
- M. Zare,
et al., Impact of sonication power on the degradation of paracetamol under single-and dual-frequency ultrasound, Ultrason. Sonochem., 2023, 99, 106564 CrossRef CAS PubMed.
- P. Qiu,
et al., A review on heterogeneous sonocatalyst for treatment of organic pollutants in aqueous phase based on catalytic mechanism, Ultrason. Sonochem., 2018, 45, 29–49 CrossRef CAS PubMed.
- N. S. Deshmukh and M. P. Deosarkar, A review on ultrasound and photocatalysis-based combined treatment processes for pesticide degradation, Mater. Today: Proc., 2022, 57, 1575–1584 CAS.
- A. Intisar,
et al., Adsorptive and photocatalytic degradation potential of porous polymeric materials for removal of pesticides, pharmaceuticals, and dyes-based emerging contaminants from water, Chemosphere, 2023, 336, 139203 CrossRef CAS PubMed.
- P. Koundle,
et al., Tetracycline degradation for wastewater treatment based on ozone nanobubbles advanced oxidation processes (AOPs)–Focus on nanobubbles formation, degradation kinetics, mechanism and effects of water composition, Chem. Eng. J., 2024, 156236 CrossRef CAS.
- H. Guo,
et al., Persulfate activated by non-thermal plasma for organic pollutants degradation: A review, Chem. Eng. J., 2023, 470, 144094 CrossRef CAS.
- S. Adityosulindro,
et al., Sonolysis and sono-Fenton oxidation for removal of ibuprofen in (waste) water, Ultrason. Sonochem., 2017, 39, 889–896 CrossRef CAS PubMed.
- J.-K. Im,
et al., Optimization of carbamazepine removal in O3/UV/H2O2 system using a response surface methodology with central composite design, Desalination, 2012, 285, 306–314 CrossRef CAS.
- E. Asgari,
et al., Degradation of ciprofloxacin by photocatalytic ozonation process under irradiation with UVA: comparative study, performance and mechanism, Process Saf. Environ. Prot., 2021, 147, 356–366 CrossRef CAS.
- N. N. Roslan,
et al., Recent Advances in Advanced Oxidation Processes for Degrading Pharmaceuticals in Wastewater—A Review, Catalysts, 2024, 14(3), 189 CrossRef CAS.
- M.-K. Nguyen,
et al., Occurrence and fate of pharmaceutical pollutants in wastewater: Insights on ecotoxicity, health risk, and state-of-the-art removal, Chemosphere, 2024, 141678 CrossRef CAS PubMed.
- P. Sudalaimuthu and R. Sathyamurthy, Forecast sustainable and renewable hydrogen production via circular bio-economy of agro waste, Int. J. Hydrogen Energy, 2024, 75, 179–199 CrossRef CAS.
- S. Kainth, P. Sharma and O. Pandey, Green sorbents from agricultural wastes: A review of sustainable adsorption materials, Appl. Surf. Sci. Adv., 2024, 19, 100562 CrossRef.
- K. H. H. Aziz and R. Kareem, Recent advances in water remediation from toxic heavy metals using biochar as a green and efficient adsorbent: a review, Case Stud. Chem. Environ. Eng., 2023, 100495 CrossRef.
- M. S. Khan,
et al., A Review of Metal-Organic Framework (MOF) Materials as an Effective Photocatalyst for Degradation of Organic Pollutants, Nanoscale Adv., 2023, 5, 6318–6348 RSC.
- C. Wang,
et al., Sulfate radicals-based advanced oxidation process (SR-AOPs): The future enabled by MOFs-based nanofibers, Chem. Eng. J., 2024, 154352 CrossRef CAS.
- P. Xia,
et al., MOF-derived single-atom catalysts: The next frontier in advanced oxidation for water treatment, Chem. Eng. J., 2023, 452, 139446 CrossRef CAS.
- K. H. Hama Aziz, N. M. Fatah and K. T. Muhammad, Advancements in application of modified biochar as a green and low-cost adsorbent for wastewater remediation from organic dyes, R. Soc. Open Sci., 2024, 11(5), 232033 CrossRef PubMed.
- Z. Kang,
et al., A review on application of biochar in the removal of pharmaceutical pollutants through adsorption and persulfate-based AOPs, Sustainability, 2022, 14(16), 10128 CrossRef CAS.
- E. Domingues,
et al., Sulfate radical based advanced oxidation processes for agro-industrial effluents treatment: A comparative review with Fenton's peroxidation, Sci. Total Environ., 2022, 832, 155029 CrossRef CAS PubMed.
- A. Giwa,
et al., Recent advances in advanced oxidation processes for removal of contaminants from water: A comprehensive review, Process Saf. Environ. Prot., 2021, 146, 220–256 CrossRef CAS.
- K. H. Hama Aziz,
et al., Application of photocatalytic falling film reactor to elucidate the degradation pathways of pharmaceutical diclofenac and ibuprofen in aqueous solutions, Coatings, 2019, 9(8), 465 CrossRef.
- S. Bagheri, A. TermehYousefi and T.-O. Do, Photocatalytic pathway toward degradation of environmental pharmaceutical pollutants: structure, kinetics and mechanism approach, Catal. Sci. Technol., 2017, 7(20), 4548–4569 RSC.
- Y. Yao,
et al., Bifunctional catalysts for heterogeneous electro-Fenton processes: a review, Environ. Chem. Lett., 2022, 20(6), 3837–3859 CrossRef CAS.
- A. Fdez-Sanromán,
et al., Heterogeneous electro-Fenton system using Fe-MOF as catalyst and electrocatalyst for degradation of pharmaceuticals, Chemosphere, 2023, 340, 139942 CrossRef PubMed.
- P. Sanga,
et al., A mechanistic view of removing pharmaceutical-induced pollutants by MXene and MXene-functionalized composites via adsorption and advanced oxidation process, J. Environ. Chem. Eng., 2023, 111685 Search PubMed.
- L. Liu,
et al., Treatment of industrial dye wastewater and pharmaceutical residue wastewater by advanced oxidation processes and its combination with nanocatalysts: A review, J. Water Process Eng., 2021, 42, 102122 CrossRef.
- Q. Gao,
et al., Advanced oxidation processes (AOPs)-based sludge pretreatment techniques for enhanced short-chain fatty acids production: A critical review, Chem. Eng. J., 2024, 151496 CrossRef CAS.
- A. G. Capodaglio, Critical perspective on advanced treatment processes for water and wastewater: AOPs, ARPs, and AORPs, Appl. Sci., 2020, 10(13), 4549 CrossRef CAS.
- K. H. H. Aziz, Removal of toxic heavy metals from aquatic systems using low-cost and sustainable biochar: A review, Desalin. Water Treat., 2024, 100757 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *ab,
Fryad S.
Mustafa
*ab,
Fryad S.
Mustafa
 a,
Mozart A. H.
Karim
a and
Sarkawt
Hama
a,
Mozart A. H.
Karim
a and
Sarkawt
Hama
 ac
ac
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 ng L−1) in Jordan; diclofenac (6300 ng L−1) in Germany; carbamazepine (2270 ng L−1) in Korea; and ibuprofen (2
700 ng L−1) in Jordan; diclofenac (6300 ng L−1) in Germany; carbamazepine (2270 ng L−1) in Korea; and ibuprofen (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 109
109![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 875 ng L−1) in India. These substances have been shown to have detrimental effects on public health and aquatic life.41 Pharmaceutical active compounds are increasingly concerning in environmental risk assessments due to their continuous release into water bodies, prompting the European Commission to classify them as emerging pollutants. Their presence disrupts ecosystems and contributes to antibiotic resistance, impacting aquatic organisms and ecosystem functions through bioconcentration from water and dietary exposure.42 Combined exposure, or bioaccumulation, arises from an imbalance between uptake and elimination. Measuring bioaccumulation is crucial for assessing the environmental risks of these compounds and their potential effects on human health.43 Pharmaceutical production facilities mainly utilize four industrial processes: fermentation, chemical synthesis, biological or natural extraction, and compounding or formulation.44 Active pharmaceutical products are manufactured through processes such as fermentation, chemical synthesis, and natural extraction. These products are then transformed into various usable forms such as tablets, syrups, ointments, and capsules. The manufacturing process involves crushing, combining, compressing, encapsulating, and packaging pharmaceutical ingredients using water, binders (such as starch or corn syrup), solvents (for tablet coatings), fillers (to dilute medicinal substances), preservatives, flavoring agents, antioxidants, and flavored syrups. The wastewater generated from these processes typically requires suitable treatment.45 The main sources of pharmaceutical pollution are manufacturing plants, hospitals, institutions, and landfill leachate, as shown in (Fig. 2). From production to disposal, pharmaceuticals including those from synthesis, excretion, and expired medications pose significant environmental and health risks. The high global demand for pharmaceuticals generates considerable waste.46 Pharmaceutical waste arises not only from the pharmaceutical industry but also from hospitals, patients, veterinary sectors, and agricultural areas in diverse forms. Inadequate disposal and improper management of pharmaceutical waste result in their release into the environment through soil contamination, and waster bodies.47
875 ng L−1) in India. These substances have been shown to have detrimental effects on public health and aquatic life.41 Pharmaceutical active compounds are increasingly concerning in environmental risk assessments due to their continuous release into water bodies, prompting the European Commission to classify them as emerging pollutants. Their presence disrupts ecosystems and contributes to antibiotic resistance, impacting aquatic organisms and ecosystem functions through bioconcentration from water and dietary exposure.42 Combined exposure, or bioaccumulation, arises from an imbalance between uptake and elimination. Measuring bioaccumulation is crucial for assessing the environmental risks of these compounds and their potential effects on human health.43 Pharmaceutical production facilities mainly utilize four industrial processes: fermentation, chemical synthesis, biological or natural extraction, and compounding or formulation.44 Active pharmaceutical products are manufactured through processes such as fermentation, chemical synthesis, and natural extraction. These products are then transformed into various usable forms such as tablets, syrups, ointments, and capsules. The manufacturing process involves crushing, combining, compressing, encapsulating, and packaging pharmaceutical ingredients using water, binders (such as starch or corn syrup), solvents (for tablet coatings), fillers (to dilute medicinal substances), preservatives, flavoring agents, antioxidants, and flavored syrups. The wastewater generated from these processes typically requires suitable treatment.45 The main sources of pharmaceutical pollution are manufacturing plants, hospitals, institutions, and landfill leachate, as shown in (Fig. 2). From production to disposal, pharmaceuticals including those from synthesis, excretion, and expired medications pose significant environmental and health risks. The high global demand for pharmaceuticals generates considerable waste.46 Pharmaceutical waste arises not only from the pharmaceutical industry but also from hospitals, patients, veterinary sectors, and agricultural areas in diverse forms. Inadequate disposal and improper management of pharmaceutical waste result in their release into the environment through soil contamination, and waster bodies.47


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) at a power of 200 W, DBD falling film reactor
20) at a power of 200 W, DBD falling film reactor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W with 0 to 880 pulses per second, solutions flow rate of 0.8 L h−1, pH = 3, drug/PDS molar ratios: 1/0.5
W with 0 to 880 pulses per second, solutions flow rate of 0.8 L h−1, pH = 3, drug/PDS molar ratios: 1/0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1






