Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Small-molecule zwitterionic morpholinium sulfonates as non-cytotoxic materials exhibiting LCST thermo-responsive phase separation in water†
Yu-Hsin
Chung
a,
Jianbo
Jia
b,
Wen-Yi
Chen
a,
Pin-Hsuan
Chen
 a,
Bing
Yan
*b and
Yen-Ho
Chu
a,
Bing
Yan
*b and
Yen-Ho
Chu
 *a
*a
aDepartment of Chemistry and Biochemistry, National Chung Cheng University, Chiayi, Taiwan 62102, Republic of China. E-mail: cheyhc@ccu.edu.tw
bInstitute of Environmental Research at Greater Bay Area, Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education, Guangzhou University, Guangzhou 510006, China. E-mail: drbingyan@gzhu.edu.cn
First published on 28th December 2024
Abstract
In this work, for the first time, a fast and novel assembly of morpholinium-based zwitterionic materials (ZMs) is reported, with benzosultone 2 as the key component in the synthesis of zwitterionic smart materials. The developed materials exhibited LCST phase separation in water, and their structural engineering enabled the development of novel ZMs with attractive Tc values (ZM 2d, Tc = 15 °C) for proof-of-concept applications in biomolecules. Furthermore, to evaluate the potential health risks of the newly developed ZMs, experiments were conducted on both human normal gastric epithelial cells and human normal colon epithelial cells. Neither the thermo-responsive ZMs, such as ZM 2d, nor its untethered, non-thermo-responsive ionic salt IS 1, caused a decrease in cell viability in these two cell lines at concentrations as high as 2000 μM. To the best of our knowledge, this is the first report on morpholinium-based, small-molecule ZMs as non-toxic smart materials, with their temperature-triggered miscibility and immiscibility with water being fully reversible.
Introduction
Solvents are commonly used in academic laboratories and industrial processes. Many conventional molecular solvents are hazardous and have obvious shortcomings, including volatility, flammability, and inevitable toxicity. Due to these limitations, ionic liquids (ILs) have received significant attention as alternative substitutes for conventional solvents, offering potential for the development of sustainable processes in catalysis, synthesis, extraction, polymerization, and material dissolution and separation.1–4 While ILs have demonstrated their value and usefulness across a variety of research studies and applications, many still exhibit limitations, such as undesirable ion exchange during compound extraction and notable cytotoxicity.2,5,6 Recent advances in small-molecule zwitterionic liquids (ZILs) and zwitterionic materials (ZMs) have positioned these as promising candidate solvents and materials for chemical and biochemical processes.3,4,7–9Many ZMs, including ZILs and their polymers, are biocompatible.9 Compared to standard ILs and ionic salts, they exhibit distinct properties, such as very high polarity and dipole moments,10,11 strong hydration that leads to the formation of stable hydration shells,4 antifouling characteristics, no nonspecific protein adsorption on surfaces,9 inconsequential ion exchange, negligible individual ion partitioning, and minimal volatility. In addition to their strong affinity to water, these ZILs and ZMs readily associate with each other via interactions between their cationic and anionic groups. As a result, their intense intermolecular hydration and self-association abilities make them highly sensitive to external stimuli and responsive to temperature-induced phase changes in solvents.3,12 In this work, we report the combinatorial synthesis of a small-molecule library of zwitterionic morpholinium sulfonate materials (ZM 1a–f and ZM 2a–f), along with the screening of their ability to exhibit thermo-responsive phase separation in water, and the structural optimization of the candidate ZMs to ultimately achieve attractive smart materials (Fig. 1). Smart materials are compounds that possess one or more properties that are switchable or adaptable under the influence of external stimuli, such as stress, moisture, electric or magnetic fields, light, temperature, pH or chemicals. Characteristically, lower and upper critical solution temperature (LCST and UCST) systems are two typical phase-separation demeanours of thermo-responsive materials in solvents.1,12 Considering the recent advances and significant progress made in ZMs, we envisioned that small molecules such as ZMs could be developed with tuneable structures, particularly with the sulfonate motif being readily tailor-engineered. Consequently, these IL-based ZMs could serve as new candidate smart materials, potentially exhibiting LCST thermo-responsive properties (Fig. 1). These ZMs would be potentially valuable materials for studying biomolecules, among other applications.
 | ||
| Fig. 1 General structures of the small-molecule zwitterionic morpholinium sulfonate materials ZM 1a–f and ZM 2a–f. | ||
Experimental
Synthetic procedures
Detailed 1H and 13C NMR, as well as high-resolution mass spectrometry (HRMS), spectra of all 12 ZMs (ZM 1a–f, ZM 2a–f) and the ionic salt IS 1 are provided in the ESI.†Synthesis of 3H-benzo[c][1,2]oxathiole 1,1-dioxide (2)
Phosphorus oxychloride (1.0 equiv.) was added to sodium 2-formylbenzenesulfonate 1 (1.0 equiv.) in a reaction flask at ambient temperature. The mixture was heated up to 120 °C for 30 min before phosphorus oxychloride (1.0 equiv.) was added again and the solution was stirred for 1.5 h at the same temperature. Excessive POCl3 was removed under reduced pressure and the solid product was triturated with crushed ice. After stirring for 10 min in ice water, white crystals were formed, which were filtered off, washed with water, and then concentrated under reduced pressure to remove water to give 3-chloro-3H-benzo[c][1,2]oxathiole 1,1-dioxide, which was used in the next step without purification.
To a mixture containing the obtained solid 3-chloro-3H-benzo[c][1,2]oxathiole 1,1-dioxide (1.0 equiv.), zinc dust (2.5 equiv.) and THF (1 M), conc. aq. HCl was added dropwise at such a rate that the reaction solution slightly boiled. The mixture was stirred at room temperature for 5 h before filtering. The filtrate was concentrated under reduced pressure before it was triturated with cold water. The crystals formed were filtered, washed with water and n-hexane, and dried in air to afford the desired product 3H-benzo[c][1,2]oxathiole 1,1-dioxide (2): 70% yield, white solid; 1H NMR (400 MHz, CDCl3) δ 5.55 (s, ArCH2O, 2H), 7.45 (d, J = 8.0 Hz, aryl H, 1H), 7.63 (dd, J = 7.6, 7.6 Hz, aryl H, 1H), 7.73 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.86 (d, J = 8.0 Hz, aryl H, 1H); 13C NMR (100 MHz, CDCl3) δ 71.00, 121.92, 123.24, 130.01, 131.69, 133.68, 135.16; ESI-HRMS m/z [M + Na]+ calculated for C7H6O3NaS 199.9929, found 199.9932 ([M + Na]+).
Preparation of alkanolamines (3a, 3b)
To a mixture of morpholine (2.5 equiv.), potassium carbonate (1.2 equiv.), and potassium iodide (0.1 equiv.) in CH3CN (1 M) at 0 °C, chloroalkanol (1 equiv.) was slowly added. The mixture solution was stirred at the same temperature before it was heated to 90 °C for 12 h. After the alkylation reaction, the resulting solution was filtered, and concentrated under reduced pressure. The residue was purified by column chromatography (silica gel; CH2Cl2/MeOH) to afford the desired alkanolamines (3a, 3b).
4-Morpholinobutan-1-ol (3a): 73% yield, yellow liquid; 1H NMR (400 MHz, CDCl3) δ 1.66–1.68 (m, NCH2(CH2)2CH2OH, 4H), 2.39 (t, J = 5.4 Hz, NCH2(CH2)3OH, 2H), 2.51 (m, CH2CH2NCH2CH2, 4H), 3.57 (t, J = 4.8 Hz, N(CH2)3CH2OH, 2H), 3.72 (t, J = 4.8 Hz, CH2CH2OCH2CH2, 4H). 13C NMR (100 MHz, CDCl3) δ 24.59, 32.20, 53.35, 58.90, 62.50, 66.43; ESI-HRMS m/z [M + H]+ calculated for C8H18NO2 160.1332, found 160.1336 ([M + H]+).
6-Morpholinohexan-1-ol (3b): 81% yield, yellow liquid; 1H NMR (400 MHz, CDCl3) δ 1.30–1.42 (m, N(CH2)2(CH2)2(CH2)2OH, 4H), 1.47–1.60 (m, NCH2CH2(CH2)2CH2CH2OH, 4H), 2.29 (m, N(CH2)6OH, 12H), 2.33 (t, J = 7.8 Hz, NCH2(CH2)5OH, 2H), 2.43 (m, CH2CH2NCH2CH2, 4H), 3.62 (t, J = 6.6 Hz, N(CH2)5CH2OH, 2H), 3.72 (t, J = 4.6 Hz, CH2CH2OCH2CH2, 4H); 13C NMR (100 MHz, CDCl3) δ 25.57, 26.37, 27.16, 32.60, 53.74, 59.00, 62.69, 66.90; ESI-HRMS m/z [M + H]+ calculated for C10H22NO2 188.1645, found 188.1642 ([M + H]+).
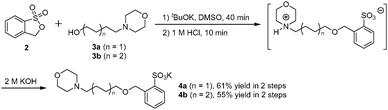
Synthesis of potassium morpholinobenzenesulfonates (4a, 4b)
To a solution of alkanolamine (3a, 3b; 1.2 equiv.) in DMSO (1.5 M), potassium tert-butoxide (1.1 equiv.) was added. The mixture was stirred for 10 min before 3H-benzo[c][1,2]oxathiole 1,1-dioxide (2) (1.0 equiv.) was added at 0 °C and then the ring-opening reaction was allowed to proceed at room temperature for 30 min. The resultant reaction mixture was quenched with 1 M HCl (1.1 equiv.) and stirred for 10 min before being concentrated under reduced pressure. The residue was purified by column chromatography (silica gel; CH2Cl2/MeOH) to give a white solid. Finally, the white solid intermediate isolated (1.0 equiv.) was added to aqueous KOH (2 M, 1.0 equiv.) solution and the resulting solution was stirred for 10 min. The solution was then concentrated under reduced pressure to afford the desired potassium morpholinobenzenesulfonates (4a, 4b).
Potassium 2-((4-morpholinobutoxy)methyl)benzenesulfonate (4a): 61% yield, white solid; 1H NMR (400 MHz, DMSO-d6) δ 1.49–1.63 (m, OCH2(CH2)2CH2N, 4H), 2.27–2.33 (m, N(CH2)3, 6H), 3.48 (t, J = 6.2 Hz, OCH2(CH2)5N, 2H), 3.56 (t, J = 4.6 Hz, CH2CH2OCH2CH2, 4H), 4.89 (s, OCH2Ar, 2H), 7.18 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.32 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.48 (d, J = 7.6 Hz, aryl H, 1H), 7.71 (d, J = 7.6 Hz, aryl H, 1H); 13C NMR (100 MHz, DMSO-d6) δ 13.43, 17.72, 19.10, 22.47, 25.92, 57.52, 59.56, 68.84, 125.77, 126.05, 126.27, 128.40, 136.36, 144.90; ESI-HRMS m/z [M + H]+ calculated for C15H23NO5SK 368.0928, found 368.0928 ([M + H]+).
Potassium 2-(((6-morpholinohexyl)oxy)methyl)benzenesulfonate (4b): 55% yield, white solid; 1H NMR (400 MHz, DMSO-d6) δ 1.30–1.48 (m, O(CH2)2(CH2)3CH2N, 6H), 1.55–1.62 (m, OCH2CH2(CH2)4N, 2H), 2.31–2.38 (m, N(CH2)3, 6H), 3.47 (t, J = 6.4 Hz, OCH2(CH2)5N, 2H), 3.57 (t, J = 4.2 Hz, CH2CH2OCH2CH2 4H), 4.89 (s, OCH2Ar, 2H), 7.19 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.32 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.48 (d, J = 7.6 Hz, aryl H, 1H), 7.71 (d, J = 7.6 Hz, aryl H, 1H); 13C NMR (100 MHz, DMSO-d6) δ 22.99, 25.22, 25.73, 28.91, 51.22, 56.08, 63.51, 68.66, 69.73, 125.67, 125.75, 126.23, 128.58, 136.70, 144.54; ESI-HRMS m/z [M + H]+ calculated for C17H27NO5SK 396.1241, found 396.1242 ([M + H]+).
Synthesis of zwitterionic morpholinium sulfonates (ZM 1a–f, ZM 2a–f)
To a solution of potassium morpholinobenzenesulfonate (4a, 4b; 1.0 equiv.) in CH3CN (1 M), the corresponding alkyl bromide (1.5 equiv. for butyl, pentyl, hexyl, heptyl, and octyl bromides, 3 equiv. for the volatile bromopropane) was added. The N-alkylation reaction was carried out at 100 °C for 24 h. The reaction mixture was directly purified by column chromatography (silica gel; EtOAc/MeOH) to finally afford the desired zwitterionic materials ZM 1a–f and ZM 2a–f.
2-((4-(4-Propylmorpholino-4-ium)butoxy)methyl)benzenesulfonate (ZM 1a): 54% yield, white solid, melting point: 220 °C; 1H NMR (400 MHz, DMSO-d6) δ 0.87 (t, J = 7.2 Hz, N(CH2)2CH3, 3H), 1.58–1.73 (m, NCH2CH2CH3 + OCH2(CH2)2CH2N, 6H), 3.42–3.47 (m, N(CH2)4, 8H), 3.55 (t, J = 5.8 Hz, OCH2(CH2)3N, 2H), 3.90 (m, CH2CH2OCH2CH2, 4H), 4.92 (s, OCH2Ar, 2H), 7.21 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.32 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.49 (d, J = 7.6 Hz, aryl H, 1H), 7.71 (d, J = 7.6 Hz, aryl H, 1H); 13C NMR (100 MHz, DMSO-d6) δ 10.42, 14.22, 17.70, 25.91, 57.31, 57.51, 58.96, 59.56, 68, 84, 68.85, 125.79, 126.14, 126.28, 128.45, 136.36, 144.92; ESI-HRMS m/z [M + H]+ calculated for C18H30NO5S 372.1839, found 372.1848 ([M + H]+), 394.1666 ([M + Na]+).
2-((4-(4-Butylmorpholino-4-ium)butoxy)methyl)benzenesulfonate (ZM 1b): 71% yield, white solid, melting point: 193 °C; 1H NMR (400 MHz, DMSO-d6) δ 0.90 (t, J = 7.2 Hz, N(CH2)3CH3, 3H), 1.28–1.33 (m, N(CH2)3CH2CH3, 2H), 1.58–1.75 (m, NCH2CH2CH2CH3 + OCH2(CH2)2CH2N, 6H), 3.49 (m, N(CH2)4, 8H), 3.56 (t, J = 5.4 Hz, OCH2(CH2)3N, 2H) 3.91 (m, CH2CH2OCH2CH2, 2H), 4.93 (s, OCH2Ar, 4H), 7.21 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.32 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.49 (d, J = 7.6 Hz, aryl H, 1H), 7.72 (d, J = 7.6 Hz, aryl H, 1H); 13C NMR (100 MHz, DMSO-d6) δ 13.42, 17.71, 19.09, 22.46, 25, 91, 57.15, 57.44, 57.51, 59.55, 68.82, 68.83, 125.76, 126.04, 126.28, 128.39, 136.35, 144.89; ESI-HRMS m/z [M + H]+ calculated for C19H32NO5S 386.1996, found 386.1986 ([M + H]+), 408.1808 ([M + Na]+).
2-((4-(4-Pentylmorpholino-4-ium)butoxy)methyl)benzenesulfonate (ZM 1c): 63% yield, white solid, melting point: 174 °C; 1H NMR (400 MHz, DMSO-d6) δ 0.85 (t, J = 7.2 Hz, N(CH2)4CH3, 3H), 1.24–1.31 (m, N(CH2)2(CH2)2CH3, 4H), 1.59–1.74 (m, NCH2CH2(CH2)CH3 + OCH2(CH2)2CH2N, 6H), 3.42–3.48 (m, N(CH2)4, 8H), 3.55 (t, J = 5.4 Hz, OCH2(CH2)3N, 2H) 3.90 (m, CH2CH2OCH2CH2, 4H), 4.92 (s, OCH2Ar, 2H), 7.20 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.30 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.49 (d, J = 7.6 Hz, aryl H, 1H), 7.72 (d, J = 7.6 Hz, aryl H, 1H); 13C NMR (100 MHz, DMSO-d6) δ 13.77, 17.75, 20.21, 21.61, 25.93, 27.86, 57.13, 57.52, 57.61, 59.57, 68.82, 68.86, 125.77, 126.05, 126.29, 128.40, 136.36, 144.90; ESI-HRMS m/z [M + H]+ calculated for C20H34NO5S 400.2152, found 400.2148 ([M + H]+), 422.1969 ([M + Na]+).
2-((4-(4-Hexylmorpholino-4-ium)butoxy)methyl)benzenesulfonate (ZM 1d): 61% yield, white solid, melting point: 174 °C; 1H NMR (400 MHz, DMSO-d6) δ 0.84 (t, J = 7.2 Hz, N(CH2)5CH3, 3H), 1.25 (m, N(CH2)2(CH2)3CH3, 6H), 1.58–1.73 (m, NCH2CH2(CH2)3CH3 + OCH2(CH2)2CH2N, 6H), 3.38–3.48 (m, N(CH2)4, 8H), 3.55 (t, J = 5.8 Hz, OCH2(CH2)3N, 2H) 3.90 (m, CH2CH2OCH2CH2, 4H), 4.92 (s, OCH2Ar, 2H), 7.20 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.30 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.48 (d, J = 7.6 Hz, aryl H, 1H), 7.71 (d, J = 7.6 Hz, aryl H, 1H); 13C NMR (100 MHz, DMSO-d6) δ 13.78, 17.72, 20.44, 21.91, 25.38, 25.91, 30.57, 57.11, 57.50, 57.64, 59.55, 68.80, 68.85, 125.74, 126, 00, 126.26, 128.36, 136.35, 144.89; ESI-HRMS m/z [M + H]+ calculated for C21H36NO5S 414.2309, found 414.2311 ([M + H]+), 436.2132 ([M + Na]+).
2-((4-(4-Heptylmorpholino-4-ium)butoxy)methyl)benzenesulfonate (ZM 1e): 67% yield, white solid, melting point: 160 °C; 1H NMR (400 MHz, DMSO-d6) δ 0.85 (t, J = 6.4 Hz, N(CH2)6CH3, 3H), 1.25 (m, N(CH2)2(CH2)4CH3, 8H), 1.59–1.73 (m, NCH2CH2(CH2)4CH3 + OCH2(CH2)2CH2N, 6H), 3.42–3.43 (m, N(CH2)4, 8H), 3.56 (t, J = 5.8 Hz, OCH2(CH2)3N, 2H) 3.90 (m, CH2CH2OCH2CH2, 4H), 4.92 (s, OCH2Ar, 2H), 7.20 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.30 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.48 (d, J = 7.6 Hz, aryl H, 1H), 7.71 (d, J = 7.6 Hz, aryl H, 1H); 13C NMR (100 MHz, DMSO-d6) δ 14.39, 18.21, 20.99, 22.43, 26.19, 26.41, 28.58, 31.55, 57.62, 57.98, 58.14, 60.04, 69.30, 69.33, 126.22, 126.50, 126.75, 128.85, 136.85, 145.39; ESI-HRMS m/z [M + H]+ calculated for C22H38NO5S 428.2465, found 428.2457 ([M + H]+), 450.2278 ([M + Na]+).
2-((4-(4-Octylmorpholino-4-ium)butoxy)methyl)benzenesulfonate (ZM 1f): 63% yield, white solid, melting point: 176 °C; 1H NMR (400 MHz, DMSO-d6) δ 0.85 (t, J = 6.8 Hz, N(CH2)7CH3, 3H), 1.25 (m, N(CH2)2(CH2)5CH3, 10H), 1.58–1.73 (m, NCH2CH2(CH2)5CH3 + OCH2(CH2)2CH2N, 6H), 3.36–3.50 (m, N(CH2)4, 8H), 3.55 (t, J = 5.8 Hz, OCH2(CH2)3N, 2H) 3.90 (m, CH2CH2OCH2CH2, 4H), 4.92 (s, OCH2Ar, 4H), 7.20 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.30 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.48 (d, J = 7.6 Hz, aryl H, 1H), 7.71 (d, J = 7.6 Hz, aryl H, 1H); 13C NMR (100 MHz, DMSO-d6) δ 13.93, 17.73, 20.50, 22.02, 25.74, 25.92, 28.39, 28.51, 31.12, 57.11, 57.49, 57.63, 59.55, 68.80, 68.85, 125.72, 126.01, 126.26, 128.35, 136.36, 144.92; ESI-HRMS m/z [M + H]+ calculated for C23H40NO5S 442.2622, found 442.2623 ([M + H]+), 464.2446 ([M + Na]+).
2-(((6-(4-Propylmorpholino-4-ium)hexyl)oxy)methyl)benzenesulfonate (ZM 2a): 66% yield, white solid, melting point: 204 °C; 1H NMR (400 MHz, DMSO-d6) δ 0.89 (t, J = 7.1 Hz, N(CH2)2CH3, 3H), 1.30–1.47 (m, O(CH2)2(CH2)2(CH2)2N, 4H), 1.58–1.62 (m, NCH2CH2CH3 + OCH2CH2(CH2)2CH2CH2N, 6H), 3.36–3.41 (m, N(CH2)4, 8H), 3.49 (t, J = 6.2 Hz, OCH2(CH2)5N, 2H), 3.90 (m, CH2CH2OCH2CH2, 4H), 4.90 (s, OCH2Ar, 2H), 7.19 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.31 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.48 (d, J = 7.6 Hz, aryl H, 1H), 7.70 (d, J = 7.6 Hz, aryl H, 1H); 13C NMR (100 MHz, DMSO-d6) δ 10.45, 14.27, 20.49, 25.26, 25.50, 29.00, 57.47, 57.54, 58.91, 59.55, 68.69, 69.68, 125.59, 125.62, 126.21, 128.40, 136.68, 144.75; ESI-HRMS m/z [M + H]+ calculated for C20H34NO5S 400.2152, found 400.2156 ([M + H]+), 422.1977 ([M + Na]+), 438.1714 ([M + K]+).
2-(((6-(4-Butylmorpholino-4-ium)hexyl)oxy)methyl)benzenesulfonate (ZM 2b): 57% yield, white solid, melting point: 179 °C; 1H NMR (400 MHz, DMSO-d6) δ 0.93 (t, J = 7.0 Hz, N(CH2)3CH3, 3H), 1.31–1.44 (m, O(CH2)2(CH2)2(CH2)2N + N(CH2)2CH2CH3, 6H), 1.60–1.61 (m, NCH2CH2CH2CH3 + OCH2CH2(CH2)2CH2CH2N, 6H), 3.41 (m, N(CH2)4, 8H), 3.49 (t, J = 5.8 Hz, OCH2(CH2)5N, 2H), 3.90 (m, CH2CH2OCH2CH2, 4H), 4.90 (s, OCH2Ar, 2H), 7.19 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.31 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.48 (d, J = 7.6 Hz, aryl H, 1H), 7.70 (d, J = 7.6 Hz, aryl H, 1H); 13C NMR (100 MHz, DMSO-d6) δ 13.49, 19.11, 20.50, 22.54, 25.26, 25.49, 29.01, 57.30, 57.42, 57.47, 59.56, 68.70, 69.69, 125.59, 125.62, 126.21, 128.41, 136.69, 144.74; ESI-HRMS m/z [M + H]+ calculated for C21H36NO5S 414.2309, found 414.2308 ([M + H]+), 436.2130 ([M + Na]+), 452.1875 ([M + K]+).
2-(((6-(4-Pentylmorpholino-4-ium)hexyl)oxy)methyl)benzenesulfonate (ZM 2c): 64% yield, white solid, melting point: 183 °C; 1H NMR (400 MHz, DMSO-d6) δ 0.89 (t, J = 7.0 Hz, N(CH2)4CH3, 3H), 1.24–1.47 (m, O(CH2)2(CH2)2(CH2)2N + N(CH2)2(CH2)2CH3, 8H), 1.60–1.63 (m, NCH2CH2(CH2)2CH3 + OCH2CH2(CH2)2CH2CH2N, 6H), 3.37–3.41 (m, N(CH2)4, 8H), 3.49 (t, J = 6.2 Hz, OCH2(CH2)5N, 2H), 3.90 (m, CH2CH2OCH2CH2, 4H), 4.90 (s, OCH2Ar, 2H), 7.19 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.31 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.48 (d, J = 7.6 Hz, aryl H, 1H), 7.70 (d, J = 7.6 Hz, aryl H, 1H); 13C NMR (100 MHz, DMSO-d6) δ 13.75, 20.23, 20.50, 21.63, 25.26, 25.49, 27.83, 29.01, 57.34, 57.45, 57.59, 59.56, 68.71, 69.69, 125.60, 125.60, 126.21, 128.41, 136.69, 144.73; ESI-HRMS m/z [M + H]+ calculated for C22H38NO5S 428.2465, found 428.2464 ([M + H]+), 450.2284 ([M + Na]+), 466.2022 ([M + K]+).
2-(((6-(4-Hexylmorpholino-4-ium)hexyl)oxy)methyl)benzenesulfonate (ZM 2d): 66% yield, white solid, melting point: 183 °C; 1H NMR (400 MHz, DMSO-d6) δ 0.87 (t, J = 6.4 Hz, N(CH2)5CH3, 3H), 1.29–1.47 (m, O(CH2)2(CH2)2(CH2)2N + N(CH2)2(CH2)3CH3, 10H), 1.60–1.63 (m, NCH2CH2(CH2)3CH3 + OCH2CH2(CH2)2CH2CH2N, 6H), 3.37–3.41 (m, N(CH2)4, 8H), 3.49 (t, J = 6.4 Hz, OCH2(CH2)5N, 2H), 3.89 (m, CH2CH2OCH2CH2, 4H), 4.90 (s, OCH2Ar, 2H), 7.19 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.31 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.48 (d, J = 7.6 Hz, aryl H, 1H), 7.70 (d, J = 7.6 Hz, aryl H, 1H); 13C NMR (100 MHz, DMSO-d6) δ 13.80, 20.47, 20.51, 21.89, 25.26, 25.35, 25.49, 29.01, 30.61, 57.37, 57.46, 57.61, 59.56, 68.71, 69.69, 125.59, 125.60, 126.21, 128.40, 136.69, 144.74; ESI-HRMS m/z [M + H]+ calculated for C23H40NO5S 442.2622, found 442.2628 ([M + H]+), 464.2448 ([M + Na]+), 480.2188 ([M + K]+).
2-(((6-(4-Heptylmorpholino-4-ium)hexyl)oxy)methyl)benzenesulfonate (ZM 2e): 67% yield, white solid, melting point: 183 °C; 1H NMR (400 MHz, DMSO-d6) δ 0.87 (t, J = 6.6 Hz, N(CH2)6CH3, 3H), 1.27–1.47 (m, O(CH2)2(CH2)2(CH2)2N + N(CH2)2(CH2)4CH3, 12H), 1.60–1.63 (m, NCH2CH2(CH2)4CH3 + OCH2CH2(CH2)2CH2CH2N, 6H), 3.41 (m, N(CH2)4, 8H), 3.49 (t, J = 6.2 Hz, OCH2(CH2)5N, 2H), 3.89 (m, CH2CH2OCH2CH2, 4H), 4.90 (s, OCH2Ar, 2H), 7.19 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.31 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.48 (d, J = 7.6 Hz, aryl H, 1H), 7.70 (d, J = 7.6 Hz, aryl H, 1H); 13C NMR (100 MHz, DMSO-d6) δ 13.92, 20.51, 20.51, 21.97, 25.27, 25.49, 25.65, 28.11, 29.01, 31.02, 57.38, 57.46, 57.58, 59.56, 68.71, 69.69, 125.60, 125.60, 126.22, 128.40, 136.69, 144.73; ESI-HRMS m/z [M + H]+ calculated for C24H42NO5S 456.2778, found 456.2785 ([M + H]+), 478.2605 ([M + Na]+), 494.2347 ([M + K]+).
2-(((6-(4-Octylmorpholino-4-ium)hexyl)oxy)methyl)benzenesulfonate (ZM 2f): 63% yield, white solid, melting point: 171 °C; 1H NMR (400 MHz, DMSO-d6) δ 0.86 (t, J = 6.8 Hz, N(CH2)7CH3, 3H), 1.26–1.47 (m, O(CH2)2(CH2)2(CH2)2N + N(CH2)2(CH2)5CH3, 14H), 1.60–1.63 (m, NCH2CH2(CH2)5CH3 + OCH2CH2(CH2)2CH2CH2N, 6H), 3.40–3.41 (m, N(CH2)4, 8H), 3.49 (t, J = 6.4 Hz, OCH2(CH2)5N, 2H), 3.89 (m, CH2CH2OCH2CH2, 4H), 4.90 (s, OCH2Ar, 2H), 7.19 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.31 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.48 (d, J = 7.6 Hz, aryl H, 1H), 7.70 (d, J = 7.6 Hz, aryl H, 1H); 13C NMR (100 MHz, DMSO-d6) δ 13.95, 20.51, 20.52, 22.05, 25.28, 25.50, 25.69, 28.40, 28.46, 29.02, 31.15, 57.38, 57.47, 57.60, 59.57, 68.72, 69.70, 125.60, 125.61, 126.23, 128.41, 136.70, 144.73; ESI-HRMS m/z [M + H]+ calculated for C25H44NO5S 470.2935, found 470.2927 ([M + H]+), 492.2746 ([M + Na]+), 508.2486 ([M + K]+).
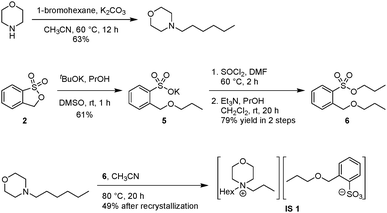
Synthesis of the non-zwitterionic ionic salt IS 1
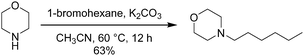
Preparation of N-hexylmorpholine
To a mixture of morpholine (1.1 equiv.) and potassium carbonate (1.1 equiv.) in CH3CN (1 M), 1-bromohexane (1.0 equiv.) was added. The N-alkylation reaction was carried out at 60 °C for 12 h. After the reaction, the mixture was filtered with a funnel, and concentrated under reduced pressure. The residue was diluted with CH2Cl2, then extracted with 10 wt% sodium bicarbonate (3×). The combined organic layers were dried over Na2SO4 and concentrated to afford N-hexylmorpholine as a yellow liquid: 63% yield, yellow liquid; 1H NMR (400 MHz, CDCl3) δ 0.90 (t, J = 6.7 Hz, N(CH2)5CH3, 3H), 1.29–1.36 (m, N(CH2)2(CH2)3CH3, 6H), 1.46–1.51 (m, NCH2CH2(CH2)3CH3, 2H), 2.3 (t, J = 7.8 Hz, NCH2(CH2)4CH3, 2H), 2.45 (m, CH2CH2NCH2CH2, 4H), 3.73 (t, J = 4.7 Hz, CH2CH2OCH2CH2, 4H).
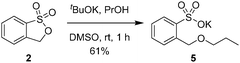
Preparation of potassium 2-(propoxymethyl)benzenesulfonate (5)
To a solution of propanol (1.2 equiv.) in DMSO (1 M). Potassium tert-butoxide (1.1 equiv.) was slowly added. The mixture was stirred for 30 min before 3H-benzo[c][1,2]oxathiole 1,1-dioxide (2) (1.0 equiv.) was added at 0 °C and then at room temperature for another 30 min. The residue was directly purified by column chromatography (silica gel; CH2Cl2/MeOH) to finally afford the desired potassium 2-(propoxymethyl)benzenesulfonate (5) as a white solid: 61% yield, white solid; 1H NMR (400 MHz, DMSO-d6) δ 0.92 (t, J = 7.4 Hz, O(CH2)2CH3, 3H), 1.55–1.64 (m, OCH2CH2CH3, 2H), 3.43 (t, J = 6.5 Hz, OCH2CH2CH3, 2H), 4.89 (s, OCH2Ar, 2H), 7.18 (dd, J = 7.4, 7.4 Hz, aryl H, 1H), 7.32 (dd, J = 7.2, 7.2 Hz, aryl H, 1H), 7.49 (d, J = 7.5 Hz, aryl H, 1H), 7.70 (d, J = 7.1 Hz, aryl H, 1H).
Preparation of propyl 2-(propoxymethyl)benzenesulfonate (6)
To a solution of thionyl chloride (2.5 equiv.) in DMF (0.1 equiv.), 5 (1.0 equiv.) was added to at room temperature. The mixture was heated to 60 °C for 2 h. Excess thionyl chloride was removed under reduced pressure to give the intermediate sulfonyl chloride.
To a solution of triethylamine (2.5 equiv.) in propanol (5 equiv.) at 0 °C, sulfonyl chloride (1 equiv.; 2 M in CH2Cl2) was added in a dropwise fashion. This nucleophilic acyl substitution reaction was then performed at room temperature for 20 h. Excess propanol and triethylamine were removed under reduced pressure. The residue was diluted with CH2Cl2, then washed with brine (3×). The combined organic layers were dried over Na2SO4 and concentrated to finally afford the desired propyl 2-(propoxymethyl)benzenesulfonate (6) as a yellow liquid: 79% yield, yellow liquid; 1H NMR (400 MHz, CDCl3) δ 0.92 (t, J = 7.4 Hz, SO3(CH2)2CH3, 3H), 0.98 (t, J = 7.4 Hz, CH2O(CH2)2CH3, 3H), 1.64–1.74 (m, SO3CH2CH2CH3 + CH2OCH2CH2CH3, 4H), 3.55 (t, J = 6.7 Hz, CH2OCH2CH2CH3, 2H), 3.98 (t, J = 6.6 Hz, SO3CH2CH2CH3, 2H), 4.91 (s, OCH2Ar, 2H), 7.42 (dd, J = 7.5, 7.5 Hz, aryl H, 1H), 7.65 (dd, J = 7.2, 7.2 Hz, aryl H, 1H), 7.84 (d, J = 7.8 Hz, aryl H, 1H), 7.97 (d, J = 7.9 Hz, aryl H, 1H).
Synthesis of IS 1
To a solution of propyl 2-(propoxymethyl)benzenesulfonate (6) (1.0 equiv.) in CH3CN (1 M), N-hexylmorpholine (1.3 equiv.) was added at room temperature. The quaternary ammonium forming reaction was carried out at 80 °C for 20 h, after the reaction, the reaction mixture was concentrated under reduced pressure to afford the desired product as a solid, which was further recrystallized using EA/hexane to finally obtain IS 1: 49% yield, white solid, melting point: 122 °C; 1H NMR (400 MHz, CDCl3) δ 0.87 (t, J = 6.6 Hz, N(CH2)5CH3, 3H), 0.94–0.98 (m, CH2O(CH2)2CH3 + N(CH2)2CH3, 6H), 1.26–1.29 (m, N(CH2)2(CH2)3CH3, 6H), 1.57–1.71 (m, NCH2CH2(CH2)3CH3 + NCH2CH2CH3 + SO3CH2CH2CH3, 6H), 3.33–3.39 (m, NCH2(CH2)4CH3 + NCH2CH2CH3, 4H), 3.51–3.55 (m, CH2CH2NCH2CH2 + CH2OCH2CH2CH3, 6H), 3.92–3.93 (m, CH2CH2OCH2CH2, 4H), 5.11 (s, OCH2Ar, 2H), 7.19 (dd, J = 7.5, 7.5 Hz, aryl H, 1H), 7.34 (dd, J = 7.5, 7.5 Hz, aryl H, 1H), 7.65 (d, J = 7.7 Hz, aryl H, 1H), 7.92 (d, J = 7.7 Hz, aryl H, 1H); 13C NMR (100 MHz, CDCl3) δ 10.80, 10.86, 13, 98, 15.23, 21.63, 22.52, 23.28, 26.04, 31.31, 58.18, 59.20, 60.59, 60.72, 69.46, 72.62, 125.95, 126.67, 126.94, 129.52, 137, 76, 143, 42; ESI-HRMS m/z [M + H]+ calculated for C13H28NO+ 214.2165, found 214.2168 ([M + H]+).
Cell culture and cytotoxicity measurements
Human normal gastric epithelial cells (GES-1) and human normal colon epithelial cells (FHC) were cultured at 37 °C in RPMI 1640 medium (Gibco), supplemented with 10% fetal bovine serum (ExCell Bio, Shanghai, China) and 1% penicillin–streptomycin (Sigma-Aldrich, St. Louis, USA). Cells at passages 5–7 post-thawing were selected for cytotoxicity assessment. After a 24 h culture period, the cells seeded in a 96-well plate were exposed to varying concentrations of ZIL or IL (0, 0.1, 0.5, 2.0, 7.8, 31.3, 125, 500, and 2000 μM) for 48 h. Cell viability was measured using the CellTiter-Lumi™ Plus Luminescent Cell Viability Assay Kit (Beyotime Biotechnology, Shanghai, China) as per the manufacturer's instructions.For the cell proliferation test, FHC cells seeded in a 24-well plate were exposed to 2000 μM IL for 48 h. The treated cells were washed with phosphate-buffered saline (PBS) and then stained with NucBlue™ Live ReadyProbes™ Reagent (Invitrogen™, Thermo Fisher Scientific Inc., Waltham, USA) for 15 min. Following removal of the staining solution, the cells were incubated with PBS and photos were taken of them using an Eclipse Ti2-U fluorescence microscope (Nikon Instruments Inc., Tokyo, Japan).
Results and discussion
Library synthesis of ZMs
Our structural design of the studied ZMs (ZM 1a–f and ZM 2a–f) is shown in Fig. 1. We selected morpholiniums as the cations in the ZMs, with an aim to ensure low toxicity and a practically harmless nature of the ZMs,13–16 to participate in interactions with very low-toxicity sulfonate anions.17,18 Accordingly, we synthesized a small-molecule ZM library to facilitate the discovery of potentially greener, temperature-switchable ionic liquid-based materials. Fig. 2 illustrates our concise synthesis of a library of 12 zwitterionic morpholinium sulfonate materials, namely ZM 1a–f and ZM 2a–f. Here, the critical element, benzosultone 2, was prepared from commercial, inexpensive sodium 2-formylbenzenesulfonate 1 following a modified literature procedure.19 Sultone scaffolds are well-known, valuable heterocycles for organic synthesis20 and material discovery.4 We commenced our library synthesis from 1, which underwent a sequence of four reactions: (1) 5-membered chlorosultone formation by POCl3 under heated conditions, followed by (2) zinc reduction, leading to the formation of 2, (3) a base-mediated O-alkylation reaction with N-morpholine-substituted alkanols (3a, 3b), which were straightforwardly prepared from commercial 4-chlorobutanol (for 3a) and 6-chlorohexanol (for 3b) with morpholine, and, finally, (4) zwitterion forming reactions with six alkyl (n-propyl to n-octyl) bromides under heated conditions to eventually afford the 12 desired products, ZM 1a–f and ZM 2a–f. In our experiments, the overall isolated yields for these 4-step syntheses of the zwitterions ZM 1a–f and ZM 2a–f were relatively moderate: 23–30% and 22–26%, respectively. The detailed 1H and 13C NMR, as well as high-resolution mass spectrometry (HRMS), spectra of all 12 ZILs are summarized in the ESI.†Small-molecule zwitterionic morpholinium sulfonates as LCST-type smart materials
Common salts exhibit good miscibility with water. However, ILs, ZILs, and other ionic materials with combinations of anions and cations can have characteristic miscibility or immiscibility with water. Recent advances3,12 have revealed that the water solubility of these materials can change dramatically upon temperature changes; that is, IL-based materials and solvents may be immiscible under certain molar or mass ratios but can be turned into homogeneous single-phase solutions by raising (i.e., UCST) or lowering (i.e., LCST) the temperature to reach their critical transition temperature (Tc).When they are thermo-responsive, a majority of binary mixtures exhibit UCST phase separation. The opposite LCST type (the material miscibility in water decreases upon temperature increases) is primarily observed and reported in polymer mixtures, and only a few examples of LCST phase behavior are known for small-molecule IL-based materials.3,12Fig. 3 shows our small library of 12 zwitterionic morpholinium benzenesulfonates (ZM 1a–f and ZM 2a–f) and their LCST phase behaviors toward temperature changes in water. To screen and successfully identify ZMs carrying temperature-switchable properties, each zwitterion product was mixed with water in a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
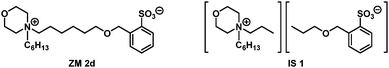
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (w/w), and then the mixture was placed in a water bath (4 °C) followed by gradual heating until it reached 90 °C. If present, the Tc for LCST transition was experimentally determined at a temperature point when the solution started turning misty during heating, as observed by the naked eye. To facilitate such Tc determination, Coomassie blue dye (0.006 wt% in water) was used to make the phase separation more visible. To our delight, in this library of 12 ZMs investigated (Fig. 3), three zwitterions, namely ZM 1e, ZM 1f, and ZM 2d (labeled in green), were found to exhibit the LCST property with Tc values of 45 °C, 23 °C, and 15 °C, respectively. In Fig. 3, the library screening results for the ZMs blotted in red and blue indicate the complete formation of a homogeneous single-phase solution and a heterogeneous two-phase mixture with water between 4 °C and 90 °C, respectively. It has been reported that the phase property of a temperature-sensitive material is a delicate balance between the hydrophobicity and hydrophilicity of the ionic salts investigated.3,12 Our results are consistent with such experimental observations, with ZM 1e, ZM 1f, and ZM 2d (green) found to reside on the rim between being hydrophilic (red) and hydrophobic (blue). In terms of the thermo-responsive ZM 1e and ZM 1f discovered, the structurally more hydrophobic ZM 1f (Tc = 23 °C) exhibited, as expected, a lower Tc value than that of ZM 1e (Tc = 45 °C) (Fig. 3). ZM 2d produced an attractive Tc (15 °C) and is considered to be a suitable candidate zwitterion for applications with biomolecules.
5 (w/w), and then the mixture was placed in a water bath (4 °C) followed by gradual heating until it reached 90 °C. If present, the Tc for LCST transition was experimentally determined at a temperature point when the solution started turning misty during heating, as observed by the naked eye. To facilitate such Tc determination, Coomassie blue dye (0.006 wt% in water) was used to make the phase separation more visible. To our delight, in this library of 12 ZMs investigated (Fig. 3), three zwitterions, namely ZM 1e, ZM 1f, and ZM 2d (labeled in green), were found to exhibit the LCST property with Tc values of 45 °C, 23 °C, and 15 °C, respectively. In Fig. 3, the library screening results for the ZMs blotted in red and blue indicate the complete formation of a homogeneous single-phase solution and a heterogeneous two-phase mixture with water between 4 °C and 90 °C, respectively. It has been reported that the phase property of a temperature-sensitive material is a delicate balance between the hydrophobicity and hydrophilicity of the ionic salts investigated.3,12 Our results are consistent with such experimental observations, with ZM 1e, ZM 1f, and ZM 2d (green) found to reside on the rim between being hydrophilic (red) and hydrophobic (blue). In terms of the thermo-responsive ZM 1e and ZM 1f discovered, the structurally more hydrophobic ZM 1f (Tc = 23 °C) exhibited, as expected, a lower Tc value than that of ZM 1e (Tc = 45 °C) (Fig. 3). ZM 2d produced an attractive Tc (15 °C) and is considered to be a suitable candidate zwitterion for applications with biomolecules.
Fig. 4A shows that, as a representative example, ZM 1f formed a single-phase homogeneous solution with water at a low temperature (4 °C). Upon heating above 23 °C, a two-phase system started to develop with this zwitterion in the bottom layer. A well-separated two-layer solution mixture was formed at a much higher temperature (45 °C). This temperature-triggered intermixing and separating with water were completely reversible, demonstrating that ZM 1f is a small-molecule zwitterion exhibiting LCST phase transition with water.
For any given LCST system in water, the Tc value is greatly influenced by the mass and mole fractions of the ZM studied. Fig. 4B shows the phase diagrams of a mixture of water with ZM 1f, showing a typical bowl-shaped curve with the lowest critical temperatures near its mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and mole ratio of 1
10 and mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 243 (ZM 1f/H2O). For the two other ZMs exhibiting thermo-responsive properties with water shown in Fig. 3 (i.e., ZM 1e and ZM 2d), their phase diagrams are given in the ESI† (see Fig. S1 and S2). We also experimentally investigated them to see if, upon phase separation in water, the temperature-sensitive ZMs (e.g., ZM 1e and ZM 2d) could be recovered effectively. To our delight, high recovery yields were obtained for both ZM 1e and ZM 2d after ZM layer separation, collection, and lyophilization: 95% and 98%, respectively. Furthermore, thermal stability is a prerequisite for temperature-sensitive ZMs application in water. Thermogravimetric analysis (TGA) was thus performed and the TGA results for the thermo-responsive ZM 1e, ZM 1f, and ZM 2d similarly showed they all experienced a 10% weight loss above 246 °C, which well exceeded water's boiling point (100 °C). They displayed major derivative thermogravimetry (DTG) peaks at 260 °C, 312 °C, and 332 °C, respectively (Fig. S1, ESI†). This successful development of small-molecule morpholinium arylsulfonates as LCST-type thermo-responsive ZMs highlights their structural tunability and engineering potential.
243 (ZM 1f/H2O). For the two other ZMs exhibiting thermo-responsive properties with water shown in Fig. 3 (i.e., ZM 1e and ZM 2d), their phase diagrams are given in the ESI† (see Fig. S1 and S2). We also experimentally investigated them to see if, upon phase separation in water, the temperature-sensitive ZMs (e.g., ZM 1e and ZM 2d) could be recovered effectively. To our delight, high recovery yields were obtained for both ZM 1e and ZM 2d after ZM layer separation, collection, and lyophilization: 95% and 98%, respectively. Furthermore, thermal stability is a prerequisite for temperature-sensitive ZMs application in water. Thermogravimetric analysis (TGA) was thus performed and the TGA results for the thermo-responsive ZM 1e, ZM 1f, and ZM 2d similarly showed they all experienced a 10% weight loss above 246 °C, which well exceeded water's boiling point (100 °C). They displayed major derivative thermogravimetry (DTG) peaks at 260 °C, 312 °C, and 332 °C, respectively (Fig. S1, ESI†). This successful development of small-molecule morpholinium arylsulfonates as LCST-type thermo-responsive ZMs highlights their structural tunability and engineering potential.
Non-cytotoxicity of zwitterionic morpholinium benzenesulfonate materials to human cells
Materials research focuses on the synthesis of new-fangled structures, property characterization, and targeted applications. Developing thermosensitive materials is aimed at benefiting from their property alteration (such as solvent-miscible transformation to solvent-immiscible) upon temperature changes in the environment. Many IL materials are known to be toxic, while ZILs are less toxic,2,5–8 and, accordingly, the zwitterionic structure is a key factor at developing low-toxicity, biocompatible smart materials.4,7–9 To produce more sustainable thermo-responsive ZMs, we selected ZM 2d as the representative material along with its homologous ionic salt IS 1, and investigated its cytotoxicity to human cells.Morpholinium benzenesulfonates are highly charged, organic ZMs that can sustain their overall electroneutrality (Fig. 1). These ZMs offer various potential advantages, including inconsequential ion exchange and non-fouling during bioprocessing.4,9 Both morpholinium cations and sulfonate anions in ZMs were selected because of their moderate to low cytotoxicity to mammalian cells, bacteria, and fungi, as reported in the literature.13–18 In addition, small-molecule ZMs featuring embedded morpholinyl substituent with temperature-switchable properties remain underexplored.
IL materials, such as ZMs, were developed in this work with negligible vapor pressure and total benignity to the atmosphere, which would lead to a decreased exposure risk by inhalation and therefore no pollution to the atmosphere. However, ILs can still be hazardous when released into the ecosystem by polluting soils and waters due to their increased accumulation, directly posing a threat and danger to human health.2 That is, their potential influence on human safety still needs to be studied and elucidated. Serving as the initial barrier against orally ingested IL-based materials, we decided to evaluate the cytotoxicity of ZMs in humans. We chose to test gastrointestinal tract-associated epithelial cell lines, GES-1 (human normal gastric epithelial cells) and FHC (human normal colon epithelial cells).8 The implications of this investigation are twofold. First, the “green impact” of these IL materials on environmental waters and soil can no longer be overlooked. Second, by investigating ZMs, biosafety optimization efforts will further enhance the broad applications and developments of the next generation of IL-based materials. The results in Fig. 5 demonstrate that neither ZM 2d nor its homolog IS 1 caused a decrease in the viability of these two cell lines at concentrations as high as 2000 μM. The median effective concentrations (EC50) of ZM 2d and IS 1 in these two cell lines were much higher than 2000 μM, suggesting their extremely low cytotoxicity. Interestingly, IS 1 administration at concentrations of 500 and 2000 μM increased the viability of FHC cells, but not GES-1 cells, by 16% and 44%, respectively (Fig. 5b).21 Cell viability was tested using the CellTiter method, which operates on the primary hypothesis that chemical treatment does not alter a cell's adenosine triphosphate (ATP) content.22 A qualitative cell proliferation test was performed to determine whether the increase in cell viability in FHC cells treated with high doses of IS 1 was due to an increased cellular ATP content or enhanced cell proliferation. The results, as shown in Fig. 5c, show that treatment with 2000 μM IS 1 significantly increased the density of FHC cells, suggesting a promotive effect on cell proliferation. The developed ZM 2d and IS 1 were thus found to be harmless and nontoxic to both GES-1 and FHC cells at concentrations as high as 2000 μM. Such low toxicity of the tested compounds was in accord with the low toxicity commonly attributed to ZILs.7,8 The detailed mechanism of the apparent promotion of human FHC cell proliferation by IS 1 is still under investigation and shall be disclosed in due course.
Preliminary and concept validation application
We designed and synthesized novel thermo-responsive zwitterionic materials to ensure that the ZMs developed would be compatible with biomolecule applications involving temperature-triggered phase separation. Protein separation and purification are typically carried out using time-consuming chromatographic procedures. Accordingly, developing a technologically straightforward and non-chromatographic method for concentrating very dilute aqueous solutions containing only small amounts of precious proteins is crucial.Since ZM 2d is non-cytotoxic to human cells, it is an ideal candidate material for analyzing biomolecules, such as proteins, can be used in studies to explore the potential of ZMs for direct application to enrich very dilute biomolecular solutions. ZM 2d (Tc = 15 °C) was selected because it exhibited an LCST phase transition well below body temperature (37 °C) in water. As for a proof-of-concept application, we selected the high molecular-weight Coomassie blue dye and the equine heart cytochrome c protein for their ease of naked-eye visualization to test if the concentration enrichment of a highly diluted solution could be realized by ZM 2d. As shown in Fig. 6, biomolecules were greatly enriched upon adding ZM 2d and mixing it with dilute dye and protein solutions. As a result, the Coomassie blue dye and cytochrome c protein were enriched substantially, 19- and 16-fold respectively, when a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 of ZM 2d/H2O was employed. According to the spectra shown in Fig. 6A, the upper aqueous phase (blue spectrum) was mostly free of the blue dye, and all the Coomassie dye was partitioned and concentrated in the ZM 2d-rich bottom layer. The ZM 2d also readily enriched cytochrome c protein (Fig. 6B). In the case of cytochrome c, its Soret band was preserved and no precipitation of the protein was observed during the entire temperature-triggered mixing and de-mixing process. On one hand, the protein examined in the enrichment experiments using ZM 2d could be recovered with excellent yield (98%).
10 of ZM 2d/H2O was employed. According to the spectra shown in Fig. 6A, the upper aqueous phase (blue spectrum) was mostly free of the blue dye, and all the Coomassie dye was partitioned and concentrated in the ZM 2d-rich bottom layer. The ZM 2d also readily enriched cytochrome c protein (Fig. 6B). In the case of cytochrome c, its Soret band was preserved and no precipitation of the protein was observed during the entire temperature-triggered mixing and de-mixing process. On one hand, the protein examined in the enrichment experiments using ZM 2d could be recovered with excellent yield (98%).
Conclusions
We reported in this work the concise 4-step synthesis of a small library of 12 zwitterionic morpholinium sulfonate materials (ZMs), and expeditiously discovered three new ZMs (ZM 1e, ZM 1f, and ZM 2d) that have good thermal stability and could exhibit desirable LCST phase separation in water. Using the discovered ZM 2d (Tc = 15 °C) as a candidate material, we evaluated the potential health risks from the new ZMs developed and experimentally found there was no decrease in viability of human normal GES-1 and FHC cells when exposed to ZM 2d. This is the first report on morpholinium-based, small-molecule ZMs as smart materials that are nontoxic, and where their miscibility and immiscibility with water triggered by temperature are entirely reversible. We concur that the combinatorial approach developed in this work is a highly convenient and effective technology platform to discover thermo-responsive materials promptly. It is still being developed, and more successful examples of ZM discovery may emerge in the future.Author contributions
Yen-Ho Chu, Bing Yan: conceived the idea, funding acquisition, designed experiments. Yu-Hsin Chung, Jianbo Jia, Wen-Yi Chen: performed experiments. Pin-Hsuan Chen: benzosultone 2 development. Yen-Ho Chu, Yu-Hsin Chung, Jianbo Jia: interpreted the data. Yen-Ho Chu: wrote the manuscript. All authors discussed and approved the paper.Data availability
The data supporting this article are included in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants from the Ministry of Science and Technology of Taiwan (NSTC 111-2113-M-194-010-MY2 to Y.-H.C.) and the introduced innovative R&D team project under the “The Pearl River Talent Recruitment Program” of Guangdong Province of China (2019ZT08L387 to B.Y.).Notes and references
- Y. Qiao, W. Ma, N. Theyssen, C. Chen and Z. Hou, Temperature-responsive ionic liquids: Fundamental behaviors and catalytic applications, Chem. Rev., 2017, 117, 6881–6928 CrossRef CAS PubMed.
- P. Wei, X. Pan, C.-Y. Chen, H.-Y. Li, X. Yan, C. Li, Y.-H. Chu and B. Yan, Emerging impacts of ionic liquids on eco-environmental safety and human health, Chem. Soc. Rev., 2021, 50, 13609–13627 RSC.
- H. Ohno, M. Yoshizawa-Fujita and Y. Kohno, Design and properties of functional zwitterions derived from ionic liquids, Phys. Chem. Chem. Phys., 2018, 20, 10978–10991 RSC.
- Q. Shao and S. Jiang, Molecular understanding and design of zwitterionic materials, Adv. Mater., 2015, 27, 15–26 CrossRef CAS PubMed.
- C.-W. Cho, T. P. T. Pham, Y. Zhao, S. Stolte and Y.-S. Yun, Review of the toxic effects of ionic liquids, Sci. Total Environ., 2021, 786, 147309 CrossRef CAS PubMed.
- N. Abramenko, L. Kustov, L. Metelytsia, V. Kovalishyn, I. Tetko and W. Peijnenburg, A review of recent advances towards the development of QSAR models for toxicity assessment of ionic liquids, J. Hazard. Mater., 2020, 384, 121429 CrossRef CAS PubMed.
- F. Jesus, H. Passos, A. M. Ferreira, K. Kuroda, J. L. Pereira, F. J. M. Goncalves, J. A. P. Coutinho and S. P. M. Ventura, Zwitterionic compounds are less ecotoxic than their analogous ionic liquids, Green Chem., 2021, 23, 3683–3692 RSC.
- H.-H. Huang, J. Jia, L. Ren, S. Wang, T. Yue, B. Yan and Y.-H. Chu, A zwitterionic solution for smart ionic liquids to evade cytotoxicity, J. Hazard. Mater., 2023, 453, 131430 CrossRef CAS PubMed.
- Q. Li, C. Wen, J. Yang, X. Zhou, Y. Zhu, J. Zheng, G. Cheng, J. Bai, T. Xu, J. Ji, S. Jiang, L. Zhang and P. Zhang, Zwitterionic biomaterials, Chem. Rev., 2022, 122, 17073–17154 CrossRef CAS PubMed.
- M. Galin, A. Chapoton and J.-C. Galin, Dielectric increments, intercharge distances and conformation of quaternary ammonioalkylsulfonates and alkoxydicyanoethenolates in aqueous and trifluoroethanol solutions, J. Chem. Soc., Perkin Trans. 2, 1993, 545–553 RSC.
- T. Wu, F. L. Beyer, R. H. Brown, R. B. Moore and T. E. Long, Influence of zwitterions on thermomechanical properties and morphology of acrylic copolymers: implications for electroactive applications, Macromolecules, 2011, 44, 8056–8063 CrossRef CAS.
- H.-Y. Li and Y.-H. Chu, Expeditious discovery of small-molecule thermoresponsive ionic liquid materials: a review, Molecules, 2023, 28, 6817 CrossRef CAS PubMed.
- K. S. Egorova and V. P. Ananikov, Toxicity of ionic liquids: eco(cyto)activity as complicated, but unavoidable parameter for task-specific optimization, ChemSusChem, 2014, 7, 336–360 CrossRef CAS PubMed.
- J. Pernak, N. Borucka, F. Walkiewicz, B. Markiewicz, P. Fochtman, S. Stolte, S. Steudtec and P. Stepnowski, Synthesis, toxicity, biodegradability and physicochemical properties of 4-benzyl-4-methylmorpholinium-based ionic liquids, Green Chem., 2011, 13, 2901–2910 RSC.
- C. Pretti, C. Chiappe, I. Baldetti, S. Brunini, G. Monni and L. Intorre, Acute toxicity of ionic liquids for three freshwater organisms: Pseudokirchneriella subcapitata, Daphnia megna and Danio rerio, Ecotoxicol. Environ. Saf., 2009, 72, 1170–1176 CrossRef CAS PubMed.
- M. H. Fatemi and P. Izadiyan, Cytotoxicity estimation of ionic liquids based on their effective structural features, Chemosphere, 2011, 84, 553–563 CrossRef CAS PubMed.
- A. Wibbertmann, I. Mangelsdorf, K. Gamon and R. Sedlak, Toxicological properties and risk assessment of the anionic surfactants category: alkyl sulfates, primary alkane sulfonates, and α-olefin sulfonates, Ecotoxicol. Environ. Saf., 2011, 74, 1089–1106 CrossRef CAS PubMed.
- L. Freeman, Toxicity thresholds of certain sodium sulfonates for daphnia magna straus, Sewage Ind. Wastes, 1953, 25, 1331–1335 CAS.
- O. Bakulina, D. Darin and M. Krasavin, Mixed carboxylic-sulfonic anhydride in reaction with imines: a straightforward route to water-soluble β-lactam vis a Staudinger-type reaction, Org. Biomol. Chem., 2018, 16, 3989–3998 RSC.
- S. Mondal, Recent developments in the synthesis and application of sultones, Chem. Rev., 2012, 112, 5339–5355 CrossRef CAS PubMed.
- S. Stolte, J. Arning, U. Bottin-Weber, M. Matzke, F. Stock, K. Thiele, M. Uerdingen, U. Welz-Biermann, B. Jastorffa and J. Ranke, Anion effects on the cytotoxicity of ionic liquids, Green Chem., 2006, 8, 621–629 RSC.
- S. Kamiloglu, G. Sari, T. Ozdal and E. Capanoglu, Guidelines for cell viability assays, Food Front., 2020, 1, 332–349 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1; detailed 1H and 13C NMR, as well as high-resolution mass spectrometry (HRMS), spectra of all 12 ZMs (ZM 1a–f, ZM 2a–f) and the ionic salt IS 1 a. See DOI: https://doi.org/10.1039/d4ma01029a |
| This journal is © The Royal Society of Chemistry 2025 |