Open Access Article
Open Access ArticleFlexible polyurethane foam: materials, synthesis, recycling, and applications in energy harvesting – a review
Ahmed Abdelhamid
Maamoun
a,
Mustafa
Arafa
 b and
Amal M. K.
Esawi
b and
Amal M. K.
Esawi
 *b
*b
aDepartment of Engineering Physics and Mathematics, Chemistry Division, Faculty of Engineering, Ain Shams University, 1 EL-Sarayat Street – Abdo Basha Sq., Cairo, 11517, Egypt
bDepartment of Mechanical Engineering, The American University in Cairo, AUC Avenue, P.O. Box 74, New Cairo 11835, Egypt. E-mail: a_esawi@aucegypt.edu
First published on 3rd March 2025
Abstract
The increasing demand for sustainable and clean energy, driven by the finite supply of fossil fuels, has motivated researchers to explore alternative energy sources. Triboelectric nanogenerators (TENGs) are innovative devices that convert mechanical energy into electrical energy without the use of an external power source. The efficiency of TENG devices relies heavily on the materials employed. Polymeric materials with porous structures have proved particularly effective for TENG applications. Among these, polyurethanes (PUs) stand out as a versatile class of materials with significant potential across various applications, owing to their unique structure–property relationships. Flexible polyurethanes (FPUs) exhibit high elasticity, a three-dimensional pore network, and diverse densities that make them a promising material for energy harvesting applications. This review explores the materials, chemistry, recycling, and limitations of FPUs with a focus on their application in TENG devices. Furthermore, it compares the efficiency of FPUs in TENG devices with compact and other porous materials. The review concludes that FPU is a promising material for TENG devices across a wide range of applications, outperforming compact materials. This is mainly due to several advantages, such as high porosity, high elasticity, lightweight nature, versatility, durability, and cost-effectiveness. In addition, this review presents the future scope for the use of FPU in TENG applications.
1. Introduction
Polyurethane (PU) foams have gained recognition in a wide range of industries since their discovery by Otto Bayer and his co-workers in 1947.1 PU is a synthetic polymer formed through the reaction of polyol (R–(OH)n≥2) and isocyanate (R–(NCO)n≥2).2,3 These versatile materials find applications in coatings,4,5 adhesives,6,7 sealants,8,9 elastomers,10,11 construction,12 packaging,13,14 automobiles15 and energy harvesting.16 Nowadays, PUs have become ubiquitous in everyday products and are regarded as essential classes of polymers that enhance human well-being.17 According to statistics, it is estimated that by 2030, the global PU market will be worth approximately 112.45 billion USD.18The properties displayed by PUs are typically affected by the specific types of polyols and isocyanates used in their production.19 For instance, polyether polyols contribute flexibility, while polyester polyols enhance hardness and strength. Similarly, the choice of isocyanates, whether aliphatic or aromatic, influences the final properties of the PUs. This diversity in formulation allows for a wide range of applications, making the market for PU products virtually limitless.20,21 Flexible polyurethane (FPU) foam is characterized by its open cell morphology, high elasticity, light weight and versatility, and thus it can be employed in different applications such as bedding,22,23 automobiles,24–26 sound absorption,27–29 furniture,22 and energy harvesting.30 FPU is manufactured by introducing blowing agents, catalysts and stabilizers during the reaction between polyol and isocyanate, resulting in a cellular structure with a three-dimensional interconnected pore network.3Table 1 summarizes the role of each component in PU formulations. By adjusting the formulation and processing parameters, manufacturers customize the foam's physical and mechanical properties, such as density, and compression resistance to align to specific applications.
| Material | Role | Ref. |
|---|---|---|
| Polyol | Provides soft, flexible segments in PUs | 31 |
| Isocyanate | Enables the curing or hardening process of PUs | 32 |
| Surfactant | Stabilizes the foam's cellular structure | 33 |
| Catalysts | Accelerate the chemical reactions at lower temperatures, speeding up production | 34 |
| Blowing agent | Generates the foam architecture and controls the foam's level | 33 |
| Filler | Enhances mechanical properties like strength and durability, while reducing material costs | 35 |
| Flame retardancy | Reduces the flammability of the material, improving safety | 36 |
| Pigments | Add color to the material, enhancing its aesthetic appeal | 37 |
In recent years, many researchers have focused on utilizing FPU foams for energy harvesting applications to produce sustainable energy. Energy harvesting is a cutting-edge technology that captures energy from environmental sources, including those produced by natural or human activities, and transforms it into usable alternating current, thereby providing a sustainable power supply for portable systems.38 This approach effectively leverages various energy sources, including fluid,39 solar,40 wind,41 thermal,42–44 and mechanical energy.45 Among these, mechanical energy stands out due to its abundance and reliability, as it remains largely unaffected by fluctuations in weather conditions.46 Among the various forms of mechanical energy, triboelectric nanogenerators have garnered considerable interest from researchers due to their remarkable efficiency and adaptability. Using triboelectric nanogenerators (TENGs) is a novel approach that enables the conversion of mechanical energy, such as vibrations or movements, into electrical energy without relying on external power sources.47–49 The efficiency and performance of TENG devices are influenced by various factors, including the materials used, contact area, frequency, and applied force. The utilization of FPU foam as a triboelectric layer in TENGs represents a significant advancement in energy harvesting.16,48,50 FPU foam exhibits remarkable characteristics that offer compelling substitutes for conventional metals, which often face limitations such as susceptibility to corrosion and unsuitability for prolonged use in TENG applications.51 The foam's light weight, high elasticity, deformable nature and three dimensional pore structure enable more significant surface contact and friction between triboelectric layers, improving energy harvesting efficiency.52 These advantages make FPU foam suitable for applications such as wearable electronics, self-powered sensors, and portable devices, contributing to advancements in sustainable energy harvesting.
In this review, the application of FPU in TENGs is highlighted. Insights are provided into the materials and chemistry of FPU foams, aiming to deepen the understanding of the structure–property relationship. Furthermore, it covers recycling methods for polyurethane waste. This review also explores how FPU foams can effectively generate electricity through triboelectric effects. Additionally, it discusses the limitations and prospects of utilizing FPU in this application.
2. Materials
2.1. Polyols
Polyols are a versatile and innovative class of polymeric materials that have revolutionized the foam industry.31 They are widely used in the production of PU materials. They provide the fundamental framework for manufacturing high-quality, durable, and comfortable foam products that are integral to our daily lives.53 Typically, these compounds are derived from either crude oil or natural oil from renewable sources and contain two or more hydroxyl (–OH) groups, which provide the necessary reactive sites for forming PU foam.54 There are two primary categories of polyols: polyether and polyester polyols.55Fig. 1 illustrates the base-catalyzed ring opening polymerization reaction for ethylene oxide (EO) and propylene oxide (PO) that produces polyether polyol. On the other hand, polyester polyol is produced through the reaction of diacids and diols (as depicted in Fig. 2).56 There are numerous varieties of polyols, each with distinctive advantages and properties that make them suitable for various applications.The ability of polyols to impart flexibility and resilience to the final product is a major factor that makes them desirable to produce FPU foam. By carefully selecting the type and composition of polyols, manufacturers can tailor the foam's properties to satisfy specific needs,57 such as varying the densities, compression factors, and load-bearing abilities.
Polyester polyols' versatility extends to the PU elastomer industry as well. Polyester polyol-based elastomers exhibit remarkable mechanical properties, including high tensile strength and tear resistance. These elastomers are utilized in numerous industries, including automotive, footwear, and industrial manufacturing, where the combination of durability and flexibility is crucial.69 Therefore, the choice between polyether and polyester polyols for manufacturing PU foam depends on the specific needs of the application, such as the desired foam properties and environmental conditions. Table 2 provides a comparison between polyether and polyester polyols.
| Characteristic | Polyether polyols | Polyester polyols |
|---|---|---|
| Chemical structure | Linear or branched chain with ether (–O–) linkages | Linear chain with ester (–COO) linkages |
| Reactivity | Low viscosity, higher reactivity | Higher viscosity, slower reactivity |
| Hydrolytic stability | Excellent hydrolytic stability | Lower hydrolytic stability |
| Characteristics | Flexible | Stiffness |
| Markets | Furniture, sound absorption | Insulation, coating, adhesives |
Several processes, including transesterification,74 epoxidation,75 and ring-opening polymerization76 are required for the production of biobased polyols. These processes transform the biomass feedstock into polyol structures with desirable properties. Numerous advantages are associated with bio-based polyols, including reduced environmental impact, decreased reliance on fossil fuels, and the potential for enhanced biodegradability.77 In addition, their incorporation into PU formulations enhanced the mechanical properties, thermal stability, and overall performance of the material.
Numerous studies have explored the use of vegetable oil-based polyols, including castor oil,78,79 soybean oil,80,81 palm oil,82,83 rapeseed oil,84,85 tung oil,86,87 and sunflower oil88 in the production of PUs. Among them, castor and soybean oils are the most common feedstocks used in the PU industry to produce biobased polyols. Castor oil, extracted from the seeds of the castor plant, has become an essential raw material for biobased polyol synthesis. Castor oil, which is abundant in hydroxyl functional groups, is made suitable for direct use as a polyol precursor in the synthesis of PUs without the need for any thermochemical modification. Its distinctive chemical structure, which consists of a hydrophobic fatty acid chain and hydroxyl group functionality, imparts desirable properties to the resulting PU materials, such as increased crosslinking, enhanced mechanical performance, and superior water resistance. On the other hand, soybean oil derived from soybeans is a readily available and inexpensive feedstock that can be rapidly converted into polyols via epoxidation then ring opening.89
The epoxidation of double bonds, which are present in the backbone structure of soybean oil, is crucial for transforming an unsaturated oil into a polyol. The mechanism of soybean epoxidation is illustrated in Fig. 3a. Typically, soybean oil is epoxidized by reacting the double bonds of the oil with a peroxy acid, such as peroxyacetic acid (CH3CO3H). This acid is generated in situ by reacting hydrogen peroxide with acetic acid in the presence of a mineral acid catalyst.90 The formation of polyols from epoxides can occur via four distinct mechanisms: acid reaction, hydrolysis, alcoholysis, and hydrogenolysis, as shown in Fig. 3b. In the mechanism of the acidic reaction, the epoxide ring is cleaved, resulting in the formation of polyols with chlorohydrin, bromohydrin, or hydroxyalkyl ester structures. The epoxide undergoes this transformation by reacting with HCl, HBr, or various organic acids (R–COOH), respectively. On the other hand, the hydrolysis of epoxide rings with acidic catalysts such as sulfuric acid, p-toluene sulfonic acid, or phosphoric acid produces polyol with two vicinal hydroxyl groups. Additionally, the alcoholysis reaction utilizes excess alcohols and acidic catalysts to react with epoxidized rings, resulting in the formation of polyols. Finally, the hydrogenolysis of epoxidized oil to produce polyols entails exposing the compound to pressurized gaseous hydrogen (approximately 4.1–6.9 MPa) in the presence of a catalyst such as RANEY® Ni.91
2.2. Isocyanates
Isocyanates (–NCO), known for their versatility and high reactivity, play a central role in PU chemistry. Two of the most common diisocyanates used in the production of PU foam are toluene diisocyanate (TDI) and methylene diphenyl diisocyanate (MDI). TDI is a mixture of 2,4-TDI and 2,6-TDI, whereas MDI exists in various isomeric forms, including 2,2′-MDI, 2,4-MDI, and 4,4′-MDI. Isocyanates react with polyols in the presence of catalysts, surfactants, and a water-blowing agent to form a three-dimensional network structure with interconnected pores. This crosslinking reaction imparts FPU foam with its desired physical properties, such as elasticity, resilience, and durability.92The production of TDI typically involves three steps. Fig. 4 illustrates the protocol for preparing TDI. Initially, toluene is nitrated to produce a mixture of 2,4- and 2,6-nitrotoluene isomers. The nitration procedure involves reacting toluene with concentrated nitric acid (HNO3) and sulfuric acid (H2SO4), followed by separating and purifying the nitrotoluene isomers. The second step involves the catalytic hydrogenation of the nitrotoluene mixture, also known as the “reduction” process. Hydrogen gas and a metal catalyst, typically palladium, convert the nitrotoluene isomers into the corresponding toluene diamines. The toluene diamines are then reacted with phosgene (COCl2) to generate TDI, a mixture of 2,4- and 2,6-TDI isomers. In order to obtain the desired product, the resultant TDI mixture is purified to obtain specific isomer ratios.92,93
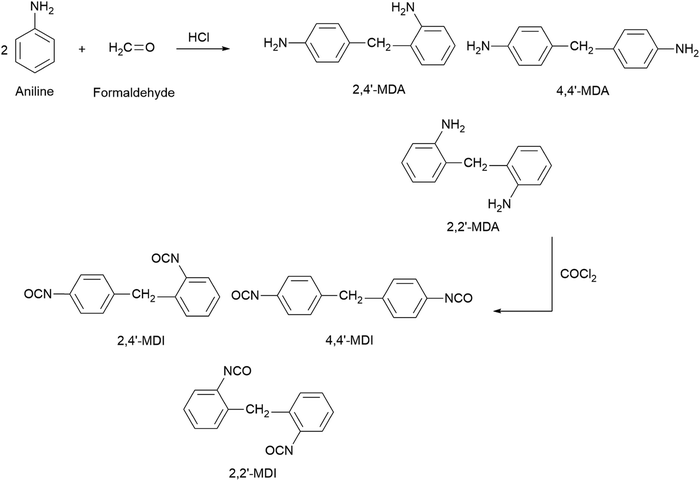
On the other hand, MDI is prepared through a two-step reaction of aniline and formaldehyde, followed by phosgenation, as demonstrated in Fig. 5. The first step is to condense aniline and formaldehyde to form methylene diphenyl diamines (MDAs). A suitable acid catalyst such as hydrochloric acid (HCl) is present during this stage under controlled temperature and pressure conditions. This reaction generates three principal isomers: 2,2′-MDA, 2,4′-MDA, and 4,4′-MDA. Depending on the concentration of the acid catalyst, the process temperature, and the aniline-to-formaldehyde ratio, various amounts of these products are produced. In the second phase of the process, the mixture of MDA isomers is phosgenated with COCl2, using chlorobenzene as a solvent, to form MDI. Phosgene and other chemical reactions are hazardous, so MDI synthesis is subject to strict safety and environmental regulations.94Table 3 compares the physical properties of TDI and MDI. MDI differs from TDI in terms of physical properties and isocyanate contents. Thus, it is essential to select the appropriate isocyanate based on the required degree of crosslinking, resilience, density, reactivity, and desired application.
| Characteristics | TDI | MDI |
|---|---|---|
| Apparent color | Colorless to pale yellow | Brown |
| M wt (g mol−1) | 174.20 | 250.25 |
| Structure | Mixture of 2,4- and 2,6-TDI | Mixture of 2,2′-, 2,4′- and 4,4′-MDI |
| Viscosity | Lower viscosity | Higher viscosity |
| Reactivity | More reactive | Less reactive |
| Toxicity | More toxic | Less toxic |
| Density | Moderate density | High density |
| Uses | Mainly to produce FPU foam | Mainly to produce semi-rigid and rigid PU foam |
2.3. Catalysts
The production of PU foam necessitates the use of catalysts to speed up the reaction between polyol and isocyanate, as well as to regulate the exothermic reaction and the polymerization process.95 The formulation of FPU foams involves two primary reactions: the gelling reaction and the blowing reaction. In the gelling (or polymerization) reaction, diisocyanates react with polyols to form urethane linkages. On the other hand, in the gas-producing (or blowing) reaction, the isocyanate reacts with water to form polyurea and carbon dioxide (CO2) gas. The kinetics of these reactions differ, dependent upon temperature, catalyst type, catalyst concentration, and various other factors. However, to produce high-quality foams, the rates of both reactions must be balanced and controlled.27,96–98Suppose the blowing reaction occurs faster than the gelling reaction. In that case, the gas produced by the reaction may expand before the polymer is strong enough to contain it, and internal splits and foam collapse can occur. In contrast, if the polymerization occurs faster than the gas-producing reaction, the foam cells remain closed, causing the foam to shrink as it cools.99 Uniform open cells will dominate the foam structure if these two reactions are balanced appropriately. Open cells offer little resistance to diffusion, and the cell pressures quickly equilibrate without significant foam shrinkage.99 Thus, there are two main types of catalysts typically used in the production of FPU foam to balance both reactions: metal-based catalysts and amine-based catalysts.
 | ||
| Fig. 6 Mechanisms of urethane formation catalyzed by tertiary amine: (a) Baker's mechanism, and (b) Farka's mechanism, adapted from ref. 97. | ||
2.4. Blowing agents
Water is essential as a chemical blowing agent in all FPU foam formulations. However, its application requires meticulous consideration due to potential safety implications arising from excessive heat generation and associated fire hazards.95 In the presence of isocyanate, water endures a chemical reaction that produces urea, biuret, and CO2 gas. This CO2 acts as a blowing agent, contributing to cell formation and expansion.31 On the other hand, physical blowing agents with lower boiling points are utilized to create foam due to their ability to generate gas bubbles and assist in the formation of cellular structures within the polymer matrix. However, traditional physical blowing agents such as hydrocarbons and halocarbons pose significant environmental risks. Due to their chemical composition and physical properties, these substances have the potential to exacerbate global climate change by contributing to the destruction of the ozone layer and the intensification of the effects of greenhouse gases.1012.5. Surfactant
A polydimethylsiloxane–polyether copolymer is widely employed as a surfactant in the manufacturing of FPU foams.102 This silicon-based surfactant plays a crucial role in the production of FPU foam due to its multifaceted functions. Initially, it reduces surface tension, which aids in creating a more uniform and stable foam structure. Additionally, it emulsifies the polyol–isocyanate interface, enhancing compatibility between the ingredients and improving overall foam quality. Furthermore, it stabilizes cell windows, ensuring that the foam structure maintains its integrity and does not collapse during curing. Lastly, it promotes the generation of bubbles during mixing, resulting in a more homogeneous and controlled distribution of air bubbles within the foam.1033. Basic chemistry of polyurethane
The urethane linkage (–NHCOO) is generated in PU chemistry by the reaction of an alcohol group (OH) and an isocyanate group (NCO). Understanding the isocyanate group is critical for understanding the entire process. Isocyanate molecules have the (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) group, and hydrogen atoms connected to atoms more electronegative than carbon are reactive to isocyanate. The isocyanate group's high reactivity with hydrogen-active compounds is explained by resonance configurations (Fig. 7a) in which the carbon atom has a low electron density, the oxygen atom has a high electron density, and the nitrogen atom has an intermediate negative charge. Isocyanates react with hydrogen-active substances through the carbon–nitrogen double bond (N
O) group, and hydrogen atoms connected to atoms more electronegative than carbon are reactive to isocyanate. The isocyanate group's high reactivity with hydrogen-active compounds is explained by resonance configurations (Fig. 7a) in which the carbon atom has a low electron density, the oxygen atom has a high electron density, and the nitrogen atom has an intermediate negative charge. Isocyanates react with hydrogen-active substances through the carbon–nitrogen double bond (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). The nucleophilic center of hydrogen-active compounds, such as the oxygen atom in hydroxyl groups or the nitrogen atoms in amines, attacks the electrophilic carbon atom. In contrast, hydrogen adds to the nitrogen atom in the –NCO groups. Additionally, electron-withdrawing groups increase –NCO group reactivity, whereas electron-donating groups decrease reactivity towards hydrogen-active substances. As a result, aromatic isocyanates (R = aryl) are more reactive than aliphatic isocyanates (R = alkyl). Furthermore, steric hindrance from adjacent groups can impair isocyanate reactivity.92,104
C). The nucleophilic center of hydrogen-active compounds, such as the oxygen atom in hydroxyl groups or the nitrogen atoms in amines, attacks the electrophilic carbon atom. In contrast, hydrogen adds to the nitrogen atom in the –NCO groups. Additionally, electron-withdrawing groups increase –NCO group reactivity, whereas electron-donating groups decrease reactivity towards hydrogen-active substances. As a result, aromatic isocyanates (R = aryl) are more reactive than aliphatic isocyanates (R = alkyl). Furthermore, steric hindrance from adjacent groups can impair isocyanate reactivity.92,104
 | ||
| Fig. 7 Chemistry of FPU foam: (a) resonance configurations of isocyanate, (b) gelling reaction, and (c) blowing reaction. | ||
Several important reactions contribute to FPU foam formation, including urethane formation, CO2 and urea generation, and biuret formation. Urethane formation, named gelling reaction, is a fundamental reaction in PU foam synthesis (Fig. 7b). It is an exothermic reaction that generates 24 kcal mol−1 of energy. It occurs when an NCO functional group reacts with a polyol's OH group. This reaction produces urethane linkages (–NHCOO–), contributing to the foam's polymer backbone.31
The second most crucial reaction is the isocyanate–water reaction, known as the blowing reaction (Fig. 7c). This reaction is highly exothermic, releasing 47 kcal mol−1 of energy. During this reaction, isocyanate groups react with water to generate carbamic acid, which rapidly decomposes into CO2 and an amine. The resulting amine then reacts with NCO to produce urea moieties. Additionally, in the presence of urea, isocyanate can form a biuret linkage. Meanwhile, the CO2 gas produced during this reaction is responsible for the nucleation of bubbles within the PU matrix. These bubbles subsequently expand and stabilize throughout the curing process, forming the cellular structure of the PU foam.105,106 The nucleation and growth of these bubbles determine the foam's final density, cell size, and mechanical properties.
4. Mechanism of cell formation and pore opening
Designing the pore structure of an FPU is crucial to meet the requirements of certain applications. The architecture of FPU foam consists of cavities (denoted by the orange circle) and pores, as shown in Fig. 8. The pores are categorized into three types: open, partially open, and closed pores. | ||
| Fig. 8 Schematic representation of the morphology of FPU foam, reproduced from ref. 107 with permission from Elsevier, copyright 2024. | ||
Each type has unique properties that influence the foam's overall behavior. The inflation of the cavities occurs due to the rapid generation of CO2 gases during the initial stages of the blowing process. These CO2 molecules continue to inflate the cavities until they encounter other existing ones. As a result of the thin cavity walls' inability to withstand pressure from both sides, pores are formed. At this stage, the characteristics of the pores are significantly influenced by both the thickness of the cavity wall and the drainage flow rate. Due to the low wall strength compared to the cavity pressures and the high drainage flow rate, narrow cavity walls are predominantly characterized by the presence of open pores. On the other hand, if the cavity walls become denser, they tend to solidify at a reduced drainage flow rate before forming fully open pores, leading to a predominance of partially open pores. Additionally, the pores remain closed if the gelling reaction is completed before the cavity walls break.94,107
5. Modification of PU foams
Modifying FPU foam is crucial to meet specific needs and enhance its overall performance. There are two common techniques for PU modification. The first strategy involves applying a surface coating, a versatile method to enhance the properties and performance of FPU foam. Surface coatings serve as a protective layer on PU substrates, thereby boosting thermal stability, resistance to chemical attack, and environmental degradation. Various coating techniques, such as dip coating,108–110 layer by layer self-assembly,111,112 hydrothermal113,114 or in situ polymerization115,116 can accomplish uniform and controlled deposition of the coating material. Moreover, the selection of coating material can be customized to meet specific requirements, enabling functionalities like flame retardancy, antimicrobial properties, and UV protection. By carefully choosing the coating materials and fine-tuning the coating parameters, the mechanical, thermal, and surface properties of PU can be substantially improved, thus broadening the potential applications of PU-based materials across multiple industries.77,117The second strategy focuses on adding fillers during FPU preparation. Fillers play a vital role in tailoring FPU properties by reinforcing the matrix and adding new functionalities.118 Strength, rigidity, and impact resistance can be enhanced by incorporating fillers into PU matrices. These fillers may be composed of either inorganic or organic substances, including nanoparticles and fibers. Inorganic fillers, such as montmorillonite,16 silica,119 alumina,120 and carbon black,121 are frequently used to improve mechanical properties, whereas organic fillers, such as cellulose122,123 and wood,107,124 offer benefits such as enhanced biodegradability and sustainability. It is important to optimize the filler type, particle size, and concentration to accomplish the desired cellular structure.125 In addition, functional additives, such as flame retardants,126,127 conductive materials,128 and antimicrobial agents,3,129,130 can be added to FPU matrices to impart specific properties for specialized applications. Overall, filler techniques provide an adaptable and effective method for customizing the properties of FPU-based materials to meet the requirements of various applications.
6. Mechanical performance of PU foams
The mechanical performance and durability of FPU foam are crucial considerations in various applications, especially in energy harvesting systems. The major drawback of PU foams is their relatively low mechanical strength, which restricts their usage.131 The mechanical properties of FPU, including compression strength, tensile strength, tearing, resilience rate and elongation at break, can be modified by incorporating fillers during foam synthesis. Several studies have been conducted using natural organic and inorganic fillers with specific percentages to enhance the foam's mechanical properties. Utilizing natural fillers in PU foams presents a dual advantage: it enhances mechanical performance and mitigates the environmental challenges linked to natural solid waste materials. Furthermore, natural organic fillers possess a significant number of hydroxyl groups on their surface, which can strengthen physical interactions and hydrogen bonding with the PU matrix, thereby improving the mechanical performance of PU foams. Moreover, the high surface area and aspect ratio of nanofillers enable them to form strong interfacial bonds with the FPU matrix, which in turn promotes effective stress transfer when subjected to mechanical loads. This reinforcement restricts the mobility of PU chains, resulting in increased stiffness and strength. Furthermore, the uniform distribution of nanofillers throughout the PU matrix enhances stress distribution, leading to an increase in the mechanical performance of the foam.132 However, the amount of filler must be carefully optimized, as an excessive quantity can lead to agglomeration, which compromises the flexibility and deformability of the FPU, adversely affecting the performance of the TENG during operation. Table 4 provides a summary of the impact of various natural fillers, including nanoparticles and fibers, on the mechanical properties of PU foams.| Filler | Optimal filler percentage (wt%) | Property enhanced | Enhancement (%) | Ref. |
|---|---|---|---|---|
| Palm oil fiber | 5 | Compressive strength | 14.28 | 133 |
| Nutmeg filler | 1 | Compressive strength | 19 | 134 |
| Flexural strength | 11 | |||
| Impact strength | 32 | |||
| Chitosan | 5 | Compressive strength | 305.64 | 3 |
| Tensile strength | 162.5 | |||
| Seashell | 25 | Compressive strength | 71.42 | 118 |
| Eggshell | 20 | Compressive strength | 13 | 57 |
| Chitin | 2.5 | Compressive strength | 4.17 | 135 |
| Tensile strength | 16.1 | |||
| Lignin | 5 | Tensile strength | 18.6 | 135 |
| Chitin-lignin | 10 | Compressive strength | 5.56 | 135 |
| Tensile strength | 14.4 | |||
| Rice plant waste | 5 | Tensile strength | 7.2 | 136 |
| Rice husk | 1 | Compressive strength | 77.9 | 137 |
| Silanized walnut shells | 1 | Compressive strength | 15 | 131 |
| Tensile strength | 9 | |||
| Impact strength | 6 | |||
| Artichoke stem waste fiber | 5 | Tensile strength | 102 | 138 |
| Sugar beet pulp | 0.5 | Compressive strength | 6 | 139 |
| Corncake waste | 2 | Tensile strength | 23.46 | 140 |
| Cellulose nanocrystals | 4 | Compressive strength | 116 | 141 |
| Na-MMT | 0.3 | Compressive strength | 27.75 | 16 |
| Sepiolite | 5 | Tensile strength | 69.4 | 142 |
| Organo modified MMT | 3 | Tensile strength | 31.94 | 143 |
| 5 | Tear properties | 284 | ||
| Organo modified bentonite | 3 | Tensile strength | 17.94 | 143 |
| 3 | Tear properties | 194 |
7. Recycling of PU waste
As environmental awareness grows, particularly in today's society which is focused on sustainable development, people are increasingly recognizing the strategic importance of resource conservation. Proper disposal and recycling of PU foam waste are essential for both environmental protection and cost reduction in production, as well as for improving material utilization. Due to its low density and high volume, PU foam waste is challenging to manage in landfills and incineration can produce hazardous gases. To address this issue, there are two primary methods for recycling PU foam waste (see Fig. 9): mechanical recycling and chemical recycling.1447.1. Mechanical recycling
Mechanical recycling is a simple and cost-effective approach for reusing PU waste without requiring chemical treatment. This process involves several methods, including pressing with a binder, pressing without a binder, and regrinding. In the pressing with a binder method, which is the most common method used in industry, PU scrap is shredded to suitable sizes and mixed with a binder, typically isocyanate (MDI), in the presence of steam. The mixture is then compressed to achieve the desired density. The pressing without binder method involves bonding PU waste in the presence of heat and pressure. PU materials have soft segments that exhibit a thermoplastic nature within a temperature range of 150–220 °C. When heated to this temperature and under pressure, these segments can form mutual bonds. Finally, the regrinding process involves grinding PU waste into a powder form and reusing it as a filler in PU foam formulations.1457.2. Chemical recycling
The urethane formation is a reversible reaction.31 Chemical recycling involves the depolymerization of urethane into monomers and low molecular weight oligomers in the presence of suitable reagents, heating, and catalysts. The polyol, isocyanate, and amines can be separated through a distillation process. The main techniques involved in the chemical recycling of PU waste, as shown in Fig. 9, are hydrolysis, acidolysis, aminolysis, and glycolysis.146When comparing the two primary methods of PU recycling, mechanical recycling stands out as a simpler, scalable, and cost-effective option that requires minimal energy compared to chemical recycling. The material obtained through mechanical recycling is characterized by a higher density and reduced flexibility and deformability when compared to FPU foam. This material is extensively employed in the fields of furniture, packaging, automotive manufacturing, sound attenuation, and impact resistance applications. However, mechanical recycling does not involve breaking down PU into its original chemical constituents. Instead, it repurposes shredded foam scraps to create new materials with varying densities, tailored to diverse industrial applications based on processing parameters. This technique is also environmentally sustainable, as it produces no detrimental emissions. In contrast, chemical recycling takes a fundamentally different approach, breaking down PU chains into their original chemical constituents, such as polyols, amines, and isocyanates. This method offers higher recycling efficiency by regenerating raw materials that can be reused to manufacture new PU products. However, chemical recycling is energy-intensive, requires precise control of reaction conditions, and relies on various chemicals, making it complex, costly, and less scalable. The quality and performance of FPU foam derived from recovered polyols are significantly influenced by the efficacy of the recovery process and the purity of the recovered polyols. Studies demonstrate that the characteristics of FPU foam made from recovered polyols are often comparable to,144 or even better than,151 those of virgin foam.
8. Application of FPU foam in energy harvesting
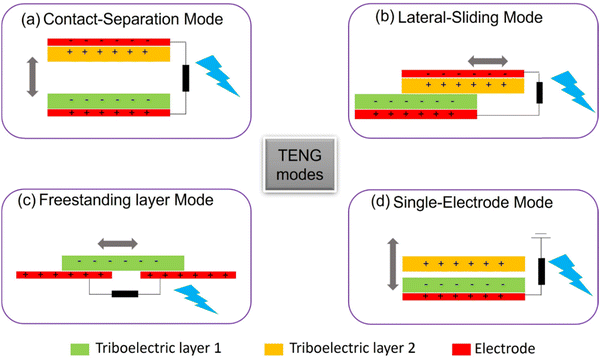
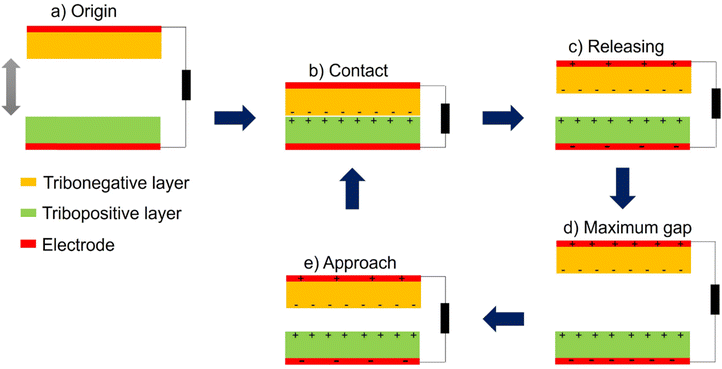
As the global economy continues to expand rapidly, coupled with the limited availability of fossil fuels and the escalating issue of environmental pollution, there is an ever-growing demand for energy sources that are efficient but also clean and sustainable. Nanogenerators (NGs) are an exciting new category of energy-harvesting devices that promise to harness energy from the surrounding environment, such as light, heat, or mechanical vibrations. Of these, the use of a triboelectric nanogenerator (TENG), developed by Wang in 2012,47 is a new approach in this field based on triboelectrification and electrostatic induction between two dissimilar materials. TENGs are distinguished by their high efficiency, significant output power, affordability, and simplicity of production. There are four operating modes of TENGs:154 vertical contact-separation mode (Fig. 10a), in-plane contact-sliding mode (Fig. 10b), freestanding triboelectric-layer mode (Fig. 10c), and single-electrode mode (Fig. 10d). Among them, vertical contact-separation is the most employed approach due to its ability to generate a high output voltage. The triboelectric effect is utilized to produce electrical energy by means of periodic vertical contact and separation between two distinct materials. Fig. 11 illustrates the working principle of a TENG employing a contact-separation mode. In the initial state, neither charge generation nor induction occurs, as demonstrated in Fig. 11a. When the surfaces of two distinct materials come into contact, triboelectric charges are produced, as shown in Fig. 11b. Subsequently, as the two contacted surfaces separate, a potential difference is established, leading to an instant flow of electrons from the bottom electrode to the top electrode, as depicted in Fig. 11c. Equilibrium is attained when the two surfaces are completely separated, as revealed in Fig. 11d. Upon pressing the two surfaces together once more, the electrostatic-induced charges will return through the external load to equalize the electric potential difference, as illustrated in Fig. 11e. The TENG can be operated repeatedly by periodically bringing the materials into contact and separating them, enabling continuous electrical energy generation.30,155–157 However, the output voltage of the TENG device is based on the applied force, contact frequency, contact area, and type of materials used.158,159The primary materials commonly used in TENG devices can be categorized into tribopositive and tribonegative materials. Tribopositive materials tend to donate electrons, while tribonegative materials tend to accept electrons.160 Metals like aluminum (Al), copper (Cu), and silver (Ag) are frequently employed as tribopositive materials, while polymeric-based materials such as polytetrafluoroethylene (PTFE), polyethyleneterephthalate (PET), polydimethylsiloxane (PDMS), polyimide (PI), and polyvinylidene fluoride (PVDF) are commonly used as tribonegative materials. However, metals are prone to oxidation and corrosion in harsh environments, which can adversely impact the performance and durability of TENG devices. To address this issue, various polymeric materials like polyurethanes (PUs),16 polypyrrole,161 cellulose,162 and polyamide163 have been utilized as tribopositive materials due to their adaptability, low cost, high ability to donate electrons and resistance to environmental degradation.164 For instance, Wang et al.164 successfully utilized polypyrrole as a tribopositive material paired with PTFE as the tribonegative material in a TENG device, achieving notable performance such as an output voltage of 52 V at 10 Hz, an output current density of 45 mA m−2 at 10 Hz, and a maximum power density of 5.8 W m−2 at a resistance of 400 MΩ. Although polymeric materials generally exhibit lower performance compared to metals in TENG devices, researchers have explored innovative approaches to enhance their efficiency. Zheng et al.165 demonstrated the use of porous materials, chitosan aerogel film and polyimide, in a TENG, resulting in an output voltage of 60.6 V, a current of 7.7 μA, and a power density of 2.33 W m−2. In another study, Wang et al.166 investigated the impact of porous PTFE on the electrical output of the TENG and compared it with the outputs obtained using solid PTFE (with no porosity) in the TENG. The results showed that the output voltage of the TENG utilizing porous PTFE was 1.8 times higher than that of the TENG using solid PTFE under identical oscillation conditions. Saadatnia et al.167 created a porous polyimide-based TENG, where the porosity of the polyimide varied from 0 to 50%. The results indicated that the output voltage, output current, and power were 40 V, 5 μA, and 47 μW, respectively, at 50% porosity. This was significantly higher than the solid polyimide film, with an enhancement of 8 times. These studies emphasized the greater efficiency of porous materials in TENG devices, as they facilitate charge generation not only on the surface as in dense materials but also within the inner pores, thereby enhancing electrostatic induction and overall device performance.
Different types of porous materials such as polystyrene foam,168 PDMS sponge,169 PVDF,170 PTFE166 and FPU foam171 have been investigated as triboelectric layers in TENG devices. Table 5 summarizes the output performance of TENG devices using different porous materials.
| Material | Output voltage (V) | Output current (μA) | Contact area (cm2) | Power density (mW cm−2) | Applied force/contact frequency (N/Hz) | Ref. |
|---|---|---|---|---|---|---|
| PDMS sponge | 181 | 2.26 | 3.7 × 3.7 | 0.089 | 6/1.6 | 169 |
| Polystyrene foam | 130 | 100 | 1 × 1 | — | 90/10 | 168 |
| Recycled polystyrene/ZnO | 8.2 | 10 | 2 × 1 | 0.0281 | 4/10 | 172 |
| Porous PVDF | 90 | 15 | 1 × 2 | 0.615 | 16/10 | 170 |
| Porous polyimide film | 40 | 5 | 2 × 2 | 0.01175 | —/5 | 167 |
| TPU nanofiber | 5.8 | 7.28 | 5 × 5 | 0.0009 | 5/8 | 173 |
| PANI NWs@FPU | 540 | 6 | 8 × 5 | 0.007 | —/6 | 171 |
| FPU/carbon nanocomposite | 130.6 | 15.6 | 2 × 2 | — | 20/6 | 48 |
| FPU/MMT nanocomposite | 164 | 11.5 | 9 × 5 | 0.0328 | 4.8/5 | 16 |
| FPU (density 33 kg m−3) | 273 | 10.2 | 8 × 5.5 | 0.025 | 4.8/5 | 174 |
| Rebonded FPU (density 60 kg m−3) | 117 | 28 | 7 × 5.5 | 0.085 | 4.7/5 | 175 |
In recent years, there has been a growing interest in incorporating FPU foam into TENG devices. FPU foam offers several advantages that make it well-suited for TENG applications including flexibility, lightweight, elasticity, deformability, versatility, tailored density, durability, and robustness.51,171,176,177 This adaptability enables the integration of TENG devices into wearable electronics, intelligent textiles, and other flexible devices, thereby expanding energy harvesting applications.48 Several studies have been conducted to improve the electrical properties of FPU foam. For instance, Weldemhret et al.48 demonstrated how carbon loading can influence the electrical performance of FPU foam when used in a TENG device. Their results showed that with a carbon loading of 40 wt%, FPU/carbon composites could be integrated into a shoe's insole, generating 36 V and 100 V when walking and running, respectively, at a contact force of 20 N, and a frequency of 6 Hz. Liu et al.171 developed a conductive elastic sponge (ES) coated with polyaniline nanowires (PANI NWs) to effectively harvest irregular and random mechanical energy using a TENG device. The study investigated the impact of deformation levels (2% to 60%) and PANI coating time (6 to 48 hours) on the ES-TENG's performance. The results showed that at a 60% deformation level, the ES-TENG achieved a maximum output of 540 V and 6 μA. As the PANI polymerization time increased, the output voltage, current, and charge density also increased, demonstrating the benefits of the porous sponge structure and PANI nanowires in enhancing triboelectric efficiency through a larger contact area. Furthermore, the conductive PANI coating allowed the ES-TENG to function as a self-powered ammonia (NH3) sensor, exhibiting high sensitivity and rapid response time. Weldemhret et al.30 studied the effect of a phosphorus-doped mesoporous carbon (PMC)-filled coating on FPU foam and its impact on the output performance of a TENG. The results demonstrated good energy harvesting performance, with an open-circuit voltage of 158 V and a short-circuit current density of 2.26 μA cm−2. Saadatnia et al.51 fabricated a high-performance TENG based on porous PU aerogel. The findings demonstrated significantly improved electrical output characteristics, 3.5 times higher compared to non-porous films, making it suitable for energy harvesting applications and as a biomechanical sensor for monitoring arm motion. The current authors modified FPU foam by adding varying amounts (0.1, 0.3, 0.5, and 0.7% by weight) of natural montmorillonite (Na-MMT) nanoclay. When the FPU/Na-MMT0.3 composite was subjected to a force of 4.8 N at a frequency of 5 Hz, it generated an output voltage of 164 V, representing a 62.37% increase compared to the unmodified FPU foam.16 In a separate study, the current authors explored how varying densities (17, 22, 26, and 33 kg m−3) and thicknesses (4, 6, 8, and 10 mm) of FPU foam affect the performance of a TENG device. The density of the FPU foam was adjusted by changing the isocyanate index (1.05, 1.10, 1.15, and 1.20) and water content. The findings revealed that the highest output voltage of 273 V was achieved with the highest density (33 kg m−3) and the lowest thickness (4 mm) at a contact frequency of 5 Hz and an applied force of 4.8 N. Under the same conditions, the maximum power density recorded was 0.025 mW cm−2 at a resistance of 20 MΩ. Additionally, the FPU-based TENG device successfully powered four white LEDs connected in series.174
The concept of waste-to-energy, as proposed by the US Environmental Protection Agency (USEPA) and the Energy Information Administration (EIA), promotes not only a cleaner environment but also clean and sustainable energy. In addition to virgin materials, numerous studies have highlighted the effectiveness of recycled waste materials as triboelectric layers, exhibiting high output performance in TENG applications.178–180 However, the chemical processing required for the fabrication of these materials often introduces additional complexity, increases costs, and extends the processing time. Therefore, the selection of waste materials and recycling technique employed are critical factors to consider prior to their utilization in TENG devices. The current authors used two distinct densities of rebonded FPU scraps, 60 and 70 kg m−3, as tribopositive sheets in a TENG device. The rebonded FPU was developed using mechanical compression with MDI as a binder. The results revealed that rebonded FPU with a density of 60 kg m−3 had a higher output voltage of 117 V than rebonded FPU with a density of 70 kg m−3. Furthermore, the power density was found to reach a maximum value of 0.085 mW cm−2 at a load resistance of 5 MΩ and frequency of 5 Hz contact frequency. The rebonded FPU-based TENG device charged a 10 μF capacitor to 16 V in 25 seconds, indicating fast charging.175 These findings showed an improvement over previously reported results using different waste materials.155,181,182 Furthermore, the technique of synthesizing rebonded FPU was simpler and less expensive than that of other waste materials discussed in the literature, which involve chemical treatments that complicate and increase the cost of production. Thus, the rebonded FPU demonstrated promising potential as a material for use in TENG devices, enabling efficient and sustainable energy harvesting for powering wearable and flexible devices.
9. Conclusions and future prospects
The versatility and adaptability of FPU foams have established them as a crucial material in diverse applications. A review of published studies on the utilization of FPU foam in energy harvesting highlights its significant potential in this field, primarily due to its unique characteristics, including its lightweight nature, deformability, adaptability, and elasticity, which collectively enhance its performance in energy harvesting applications. However, the review of the current literature revealed that the use of FPU foams in TENG devices is limited by several constraints that require future attention. The operational output of FPU based-TENG devices is susceptible to fluctuations due to environmental conditions such as temperature, humidity, and other external factors. Accordingly, it is essential to conduct research to assess the effect of environmental conditions on the FPU foam TENG performance as these factors are expected to directly influence the operational efficiency of TENGs. Additionally, the high volume-to-mass ratio of FPU foam results in larger device sizes, which can be a significant drawback and can limit its applications. This was highlighted by the current authors who reported that a thinner FPU material (4 mm) performed better in terms of generating higher output voltage from the TENG device compared to a thicker FPU material (10 mm). Therefore, it is crucial to carefully select and adjust the thickness of the FPU material to optimize the performance of the TENG device for specific application needs, ensuring maximum efficiency and effectiveness in energy harvesting. Moreover, the electrical outputs of the TENG device depend on the pore morphology of the FPU foam, making it crucial to control it during preparation. Therefore, the control of pore architecture during the synthesis process becomes a critical factor to consider when adjusting the formulations by balancing between gelling and blowing reactions. This requires expertise in the synthesis of FPU foam with well controlled density and pore structure. Additionally, the mechanical durability and longevity of the FPU foam can deteriorate over time due to bacterial attack, leading to mechanical loss over extended periods. Aerobic microorganisms can proliferate within FPU foams in environments characterized by high humidity and oxygen content, thereby compromising their elasticity and deformability. Given the increasing use of FPU foam, its applicability could be extended to situations where antibacterial properties are crucial for effective long-term durability. Therefore, researchers should consider this issue and thoroughly comprehend its influence.The use of various organic and inorganic fillers was reported to enhance the durability, thermal resistance, mechanical performance and electrical outputs of FPU foam. Fillers can also act as additional charges on the foam surface, increasing its electrical output. Despite the existing research incorporating various micron-sized and nanofillers, additional studies involving other nanofillers are desired.
Finally, it is important not to overlook that the production of PU foam relies heavily on petroleum feedstock, polyol, and isocyanate, necessitating a shift to renewable-based materials to protect the environment. Researchers are investigating alternative bio-based or recycled sources for polyol and diisocyanates to minimize the material's environmental impact. Furthermore, recycling of PU foams is crucial to mitigate the environmental consequences of PU waste disposal through landfill or incineration. The use of recycled FPU foam instead of virgin PU foam in energy harvesting applications should be encouraged as it offers additional energy savings linked to the substantial impact of virgin materials on the total embodied energy in PU foam.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the American University in Cairo under the internal grant agreement number SSE-MENG-A.E-FY22-RG-2022-Nov-10-19-38-38.References
- O. Bayer, Angew. Chem., 1947, 59, 257–272 CrossRef.
- A. Kemona and M. Piot, Polymers, 2020, 12, 1752 CrossRef CAS PubMed.
- A. Maamoun and A. Mahmoud, Cellulose, 2022, 29, 6323–6338 Search PubMed.
- S. Dutta and N. Karak, Prog. Org. Coat., 2005, 53, 147–152 CrossRef CAS.
- S. Thakur and N. Karak, Prog. Org. Coat., 2013, 76, 157–164 Search PubMed.
- S. D. Desai, J. V. Patel and V. K. Sinha, Int. J. Adhes. Adhes., 2003, 23, 393–399 Search PubMed.
- S. Sahoo, S. Mohanty and S. K. Nayak, J. Macromol. Sci., Part A: Pure Appl. Chem., 2018, 55, 36–48 Search PubMed.
- H. Ding, C. Xia, J. Wang, C. Wang and F. Chu, J. Mater. Sci., 2016, 51, 5008–5018 Search PubMed.
- D. Shen, S. Shi, T. Xu, X. Huang, G. Liao and J. Chen, Constr. Build. Mater., 2018, 174, 474–483 CrossRef CAS.
- V. Kanyanta and A. Ivankovic, J. Mech. Behav. Biomed. Mater., 2010, 3, 51–62 Search PubMed.
- Z. S. Petrović and J. Ferguson, Prog. Polym. Sci., 1991, 16, 695–836 CrossRef.
- H. Somarathna, S. Raman, D. Mohotti, A. Mutalib and K. Badri, Constr. Build. Mater., 2018, 190, 995–1014 CrossRef CAS.
- M. Zhao, L. Hu, L. Dai, Z. Wang, J. He, Z. Wang, J. Chen, D. Hrynsphan and S. Tatsiana, Bioresour. Technol., 2022, 345, 126427 CrossRef CAS PubMed.
- M. Indumathi and G. Rajarajeswari, Int. J. Biol. Macromol., 2019, 124, 163–174 Search PubMed.
- J. Moon, S. B. Kwak, J. Y. Lee and J. S. Oh, Elastomers Compos., 2017, 52, 249–256 CAS.
- A. A. Maamoun, D. M. Naeim, A. A. Mahmoud, A. M. Esawi and M. Arafa, Nano Energy, 2024, 124, 109426 CrossRef CAS.
- H. W. Engels, H. G. Pirkl, R. Albers, R. W. Albach, J. Krause, A. Hoffmann, H. Casselmann and J. Dormish, Angew. Chem., Int. Ed., 2013, 52, 9422–9441 CrossRef CAS PubMed.
- Precedence Research, Polyurethane Market Size and Companies, https://www.precedenceresearch.com/polyurethane-market, accessed 2nd April 2024.
- M. Charlon, B. Heinrich, Y. Matter, E. Couzigné, B. Donnio and L. Avérous, Eur. Polym. J., 2014, 61, 197–205 Search PubMed.
- M. Szycher, Szycher's handbook of polyurethanes, CRC Press, 1999 Search PubMed.
- A. Das and P. Mahanwar, Adv. Ind. Eng. Polym. Res., 2020, 3, 93–101 Search PubMed.
- A. B. Morgan, Fire Mater., 2021, 45, 68–80 CrossRef CAS.
- P. Scarfato, L. Di Maio and L. Incarnato, Composites, Part B, 2017, 109, 45–52 CrossRef CAS.
- R. Deng, P. Davies and A. Bajaj, J. Sound Vib., 2003, 262, 391–417 Search PubMed.
- S. D. Bote, A. Kiziltas, I. Scheper, D. Mielewski and R. Narayan, J. Appl. Polym. Sci., 2021, 138, 50690 CrossRef CAS.
- J. Moon, S. B. Kwak, J. Y. Lee, D. Kim, J. U. Ha and J. S. Oh, Waste Manage., 2019, 85, 557–562 Search PubMed.
- J. G. Gwon, S. K. Kim and J. H. Kim, Mater. Des., 2016, 89, 448–454 Search PubMed.
- G. Sung, S. K. Kim, J. W. Kim and J. H. Kim, Polym. Test., 2016, 53, 156–164 Search PubMed.
- R. Verdejo, R. Stämpfli, M. Alvarez-Lainez, S. Mourad, M. Rodriguez-Perez, P. Brühwiler and M. Shaffer, Compos. Sci. Technol., 2009, 69, 1564–1569 CrossRef CAS.
- T. G. Weldemhret, D.-W. Lee, M. Prabhakar, Y. T. Park and J. I. Song, ACS Appl. Nano Mater., 2022, 5, 12464–12476 CrossRef CAS.
- M. Ionescu, Chemistry and technology of polyols for polyurethanes, iSmithers Rapra Publishing, 2005 Search PubMed.
- C. Fang, X. Zhou, Q. Yu, S. Liu, D. Guo, R. Yu and J. Hu, Prog. Org. Coat., 2014, 77, 61–71 CrossRef CAS.
- Y. Savelyev, V. Veselov, L. Markovskaya, O. Savelyeva, E. Akhranovich, N. Galatenko, L. Robota and T. Travinskaya, Mater. Sci. Eng., C, 2014, 45, 127–135 Search PubMed.
- D. K. Chattopadhyay and K. Raju, Prog. Polym. Sci., 2007, 32, 352–418 CrossRef CAS.
- N. Taheri and S. Sayyahi, e-Polymers, 2016, 16, 65–73 CAS.
- R. Patel, M. Shah and H. Patel, Int. J. Polym. Anal. Charact., 2011, 16, 107–117 CrossRef CAS.
- D. Randall and S. Lee, The polyurethanes book, John Wiley & Sons, New York, NY, USA, 2002 Search PubMed.
- D. Choi, Y. Lee, Z.-H. Lin, S. Cho, M. Kim, C. K. Ao, S. Soh, C. Sohn, C. K. Jeong and J. Lee, ACS Nano, 2023, 17, 11087–11219 CrossRef CAS PubMed.
- M. Hamlehdar, A. Kasaeian and M. R. Safaei, Renewable Energy, 2019, 143, 1826–1838 CrossRef.
- X. Zhang, H. S. Chen and P. Yang, Nano Energy, 2024, 120, 109160 Search PubMed.
- J.-A. Choi, J. Jeong, M. Kang, H.-J. Ko, T. Kim, K. Park, J. Kim and S. Pyo, Nano Energy, 2024, 119, 109071 Search PubMed.
- Y. Cao, Y. Meng, Y. Jiang, S. Qian, D. Fan, X. Zhou, Y. Qian, S. Lin, T. Qian and Q. Pan, Chem. Eng. J., 2022, 433, 134549 CrossRef CAS.
- Y. Meng, Y. Liu, Z. Wan, Y. Huan, Q. Guo, D. Fan, X. Zhou, J. Liu, Y. Cao and X. Cao, Chem. Eng. J., 2023, 453, 139967 CrossRef CAS.
- H. Zhu, M. Gu, X. Dai, S. Feng, T. Yang, Y. Fan, J. Zhang, D. Fan, Y. Liu and Y. Lu, Chem. Eng. J., 2024, 494, 153235 CrossRef CAS.
- Z. Yu, Z. Zhu, Y. Zhang, X. Li, X. Liu, Y. Qin, Z. Zheng, L. Zhang and H. He, Carbohydr. Polym., 2024, 334, 122040 CrossRef CAS PubMed.
- X. Tang, X. Wang, R. Cattley, F. Gu and A. D. Ball, Sensors, 2018, 18, 4113 CrossRef PubMed.
- F.-R. Fan, Z.-Q. Tian and Z. L. Wang, Nano Energy, 2012, 1, 328–334 CrossRef CAS.
- T. G. Weldemhret, D.-W. Lee, Y. T. Park and J. I. Song, Chem. Eng. J., 2022, 450, 137982 CrossRef.
- Z. L. Wang, ACS Nano, 2013, 7, 9533–9557 CrossRef CAS PubMed.
- H. Zhang, Y. Lu, A. Ghaffarinejad and P. Basset, Nano Energy, 2018, 51, 10–18 CrossRef CAS.
- Z. Saadatnia, S. G. Mosanenzadeh, T. Li, E. Esmailzadeh and H. E. Naguib, Nano Energy, 2019, 65, 104019 CrossRef CAS.
- J. Zheng, X. Wei, Y. Li, W. Dong, X. Li, E. Shiju, Z. Wu and J. Wen, Nano Energy, 2021, 89, 106397 CrossRef CAS.
- C. Defonseka, Water-Blown Cellular Polymers: A Practical Guide, Walter de Gruyter GmbH & Co KG, 2019 Search PubMed.
- E. Sharmin and F. Zafar, Polyurethane, 2012, 3, 16 Search PubMed.
- T. Nakajima-Kambe, Y. Shigeno-Akutsu, N. Nomura, F. Onuma and T. Nakahara, Appl. Microbiol. Biotechnol., 1999, 51, 134–140 CrossRef CAS PubMed.
- J. O. Akindoyo, M. Beg, S. Ghazali, M. Islam, N. Jeyaratnam and A. Yuvaraj, RSC Adv., 2016, 6, 114453–114482 RSC.
- M. Zieleniewska, M. K. Leszczyński, L. Szczepkowski, A. Bryśkiewicz, M. Krzyżowska, K. Bień and J. Ryszkowska, Polym. Degrad. Stab., 2016, 132, 78–86 CrossRef CAS.
- T. Miyajima, K. Nishiyama, M. Satake and T. Tsuji, Polym. J., 2015, 47, 771–778 CrossRef CAS.
- J. Herzberger, K. Niederer, H. Pohlit, J. Seiwert, M. Worm, F. R. Wurm and H. Frey, Chem. Rev., 2016, 116, 2170–2243 CrossRef CAS PubMed.
- J. Blankenburg, E. Kersten, K. Maciol, M. Wagner, S. Zarbakhsh and H. Frey, Polym. Chem., 2019, 10, 2863–2871 RSC.
- M. Ates, S. Karadag, A. A. Eker and B. Eker, Polym. Int., 2022, 71, 1157–1163 CrossRef CAS.
- Z. Khan, F. Javed, Z. Shamair, A. Hafeez, T. Fazal, A. Aslam, W. B. Zimmerman and F. Rehman, J. Ind. Eng. Chem., 2021, 103, 80–101 CrossRef CAS.
- X. Zhang, D. A. MacDonald, M. F. Goosen and K. B. McAuley, J. Polym. Sci., Part A: Polym. Chem., 1994, 32, 2965–2970 CrossRef CAS.
- M. De Jong, R. Feijt, E. Zondervan, T. Nijhuis and A. De Haan, Appl. Catal., A, 2009, 365, 141–147 CrossRef CAS.
- C. Song, Y. Qi, T. Deng, X. Hou and Z. Qin, Renewable Energy, 2010, 35, 625–628 CrossRef CAS.
- F. E. Golling, R. Pires, A. Hecking, J. Weikard, F. Richter, K. Danielmeier and D. Dijkstra, Polym. Int., 2019, 68, 848–855 CrossRef CAS.
- S. Rabbani, E. Bakhshandeh, R. Jafari and G. Momen, Prog. Org. Coat., 2022, 165, 106715 CrossRef CAS.
- M. Malaki, Y. Hashemzadeh and M. Karevan, Prog. Org. Coat., 2016, 101, 477–485 CrossRef CAS.
- J. Zhang and C. P. Hu, Eur. Polym. J., 2008, 44, 3708–3714 CrossRef CAS.
- F. M. de Souza, P. K. Kahol and R. K. Gupta, Polyurethane Chemistry: Renewable Polyols and Isocyanates, ACS Publications, 2021, pp. 1–24 Search PubMed.
- Z. S. Petrović, Polym. Rev., 2008, 48, 109–155 CrossRef.
- Grand View Research, Bio-Based Polyurethane Industry Analysis, https://www.grandviewresearch.com/industry-analysis/bio-based-polyurethane-industry, accessed 20 May, 2024.
- M. A. Aristri, M. A. R. Lubis, S. M. Yadav, P. Antov, A. N. Papadopoulos, A. Pizzi, W. Fatriasari, M. Ismayati and A. H. Iswanto, Appl. Sci., 2021, 11, 4242 CrossRef CAS.
- A. Tenorio-Alfonso, M. C. Sánchez and J. M. Franco, J. Polym. Environ., 2020, 28, 749–774 CrossRef CAS.
- B. Freedman, E. Pryde and T. Mounts, J. Am. Oil Chem. Soc., 1984, 61, 1638–1643 CrossRef CAS.
- V. V. Goud, A. V. Patwardhan and N. C. Pradhan, Bioresour. Technol., 2006, 97, 1365–1371 CrossRef CAS PubMed.
- A. Noreen, K. M. Zia, M. Zuber, S. Tabasum and A. F. Zahoor, Prog. Org. Coat., 2016, 91, 25–32 CrossRef CAS.
- M. Spontón, N. Casis, P. Mazo, B. Raúd, A. Simonetta, L. Rios and D. Estenoz, Int. Biodeterior. Biodegrad., 2013, 85, 85–94 CrossRef.
- D. Gurgel, D. Bresolin, C. Sayer, L. Cardozo Filho and P. H. H. de Araújo, Ind. Crops Prod., 2021, 164, 113377 CrossRef CAS.
- J. John, M. Bhattacharya and R. B. Turner, J. Appl. Polym. Sci., 2002, 86, 3097–3107 CrossRef CAS.
- L. Zhang, H. K. Jeon, J. Malsam, R. Herrington and C. W. Macosko, Polymer, 2007, 48, 6656–6667 CrossRef CAS.
- A. Prociak, E. Malewska, M. Kurańska, S. Bąk and P. Budny, Ind. Crops Prod., 2018, 115, 69–77 CrossRef CAS.
- H. Pawlik and A. Prociak, J. Polym. Environ., 2012, 20, 438–445 CrossRef CAS.
- P. Rojek and A. Prociak, J. Appl. Polym. Sci., 2012, 125, 2936–2945 CrossRef CAS.
- M. Zieleniewska, M. K. Leszczyński, M. Kurańska, A. Prociak, L. Szczepkowski, M. Krzyżowska and J. Ryszkowska, Ind. Crops Prod., 2015, 74, 887–897 CrossRef CAS.
- V. R. Da Silva, M. A. Mosiewicki, M. I. Yoshida, M. C. Da Silva, P. M. Stefani and N. E. Marcovich, Polym. Test., 2013, 32, 438–445 CrossRef.
- W. Zhou, C. Bo, P. Jia, Y. Zhou and M. Zhang, Polymers, 2018, 11, 45 CrossRef PubMed.
- S. Dworakowska, A. Cornille, D. Bogdal, B. Boutevin and S. Caillol, Materials, 2022, 15, 628 CrossRef CAS PubMed.
- S. Miao, S. Zhang, Z. Su and P. Wang, J. Appl. Polym. Sci., 2013, 127, 1929–1936 CrossRef CAS.
- P. T. Wai, P. Jiang, Y. Shen, P. Zhang, Q. Gu and Y. Leng, RSC Adv., 2019, 9, 38119–38136 RSC.
- I. Singh, S. K. Samal, S. Mohanty and S. K. Nayak, Eur. J. Lipid Sci. Technol., 2020, 122, 1900225 CrossRef CAS.
- M. F. Sonnenschein, Polyurethanes: science, technology, markets, and trends, John Wiley & Sons, 2021 Search PubMed.
- R. H. Richter and R. D. Priester Jr, Kirk-Othmer Encyclopedia of Chemical Technology, 2000 Search PubMed.
- S.-T. Lee and N. S. Ramesh, Polymeric foams: mechanisms and materials, CRC Press, 2004 Search PubMed.
- C. Defonseka, Practical guide to flexible polyurethane foams, Smithers Rapra, 2013 Search PubMed.
- R. Van Maris, Y. Tamano, H. Yoshimura and K. M. Gay, J. Cell. Plast., 2005, 41, 305–322 CrossRef CAS.
- S. Dworakowska, D. Bogdał, F. Zaccheria and N. Ravasio, Catal. Today, 2014, 223, 148–156 CrossRef CAS.
- A. Strachota, B. Strachotová and M. Špírková, Mater. Manuf. Processes, 2008, 23, 566–570 CrossRef CAS.
- Y. Zhao, F. Zhong, A. Tekeei and G. J. Suppes, Appl. Catal., A, 2014, 469, 229–238 CrossRef CAS.
- A. Guo, I. Javni and Z. Petrovic, J. Appl. Polym. Sci., 2000, 77, 467–473 CrossRef CAS.
- T. Kattiyaboot and C. Thongpin, Energy Procedia, 2016, 89, 177–185 CrossRef CAS.
- B. Grüning and G. Koerner, Tenside, Surfactants, Deterg., 1989, 26, 312–317 CrossRef.
- X. Zhang, C. Macosko, H. Davis, A. Nikolov and D. Wasan, J. Colloid Interface Sci., 1999, 215, 270–279 CrossRef CAS PubMed.
- K. Ashida, Polyurethane and related foams: chemistry and technology, CRC Press, 2006 Search PubMed.
- N. Malwitz, S.-W. Wong, K. Frisch and P. Manis, J. Cell. Plast., 1987, 23, 461–502 CrossRef CAS.
- A. L. Silva and J. C. Bordado, Catal. Rev., 2004, 46, 31–51 CrossRef.
- H. Choe, G. Sung and J. H. Kim, Compos. Sci. Technol., 2018, 156, 19–27 CrossRef CAS.
- Q. Wu, J. Zhang, S. Wang, B. Chen, Y. Feng, Y. Pei, Y. Yan, L. Tang, H. Qiu and L. Wu, Front. Chem. Sci. Eng., 2021, 15, 969–983 CrossRef CAS.
- D. Fan, N. Li, M. Li, S. Wang, S. Li and T. Tang, Chem. Eng. J., 2022, 427, 131635 CrossRef CAS.
- T. G. Weldemhret, D. W. Lee, P. MN, A. Iqbal, C. M. Koo, Y. T. Park and J. I. Song, Adv. Mater. Interfaces, 2023, 10, 2300461 CrossRef CAS.
- Y. Pan, L. Liu, W. Cai, Y. Hu, S. Jiang and H. Zhao, Appl. Clay Sci., 2019, 168, 230–236 CrossRef CAS.
- J. Lu, C. Liao, L. Cheng, P. Jia, Z. Yin, L. Song, B. Wang and Y. Hu, J. Cleaner Prod., 2022, 333, 130172 CrossRef CAS.
- B.-X. Zhang, Z.-L. Hou, W. Yan, Q.-L. Zhao and K.-T. Zhan, Carbon, 2017, 125, 199–206 CrossRef CAS.
- Y. Shen, Z. Lin, J. Wei, Y. Xu, Y. Wan, T. Zhao, X. Zeng, Y. Hu and R. Sun, Carbon, 2022, 186, 9–18 CrossRef CAS.
- M. Beccatelli, M. Villani, F. Gentile, L. Bruno, D. Seletti, D. M. Nikolaidou, M. Culiolo, A. Zappettini and N. Coppede, ACS Appl. Polym. Mater., 2021, 3, 1563–1572 CrossRef CAS.
- Y. Ding, J. Yang, C. R. Tolle and Z. Zhu, ACS Appl. Mater. Interfaces, 2018, 10, 16077–16086 CrossRef CAS PubMed.
- J. Wicks, W. Zeno, F. N. Jones and S. Peppas, Drying Technol., 1993, 11, 1477 CrossRef.
- A. Maamoun, A. El-Wakil and T. M. El-Basheer, J. Cell. Plast., 2022, 58, 645–672 CrossRef CAS.
- J. W. Cho and S. H. Lee, Eur. Polym. J., 2004, 40, 1343–1348 CrossRef CAS.
- H. Etemadi, S. Afsharkia, S. Zinatloo-Ajabshir and E. Shokri, Polym. Eng. Sci., 2021, 61, 2364–2375 CrossRef CAS.
- D. Chen, J. Yang and G. Chen, Composites, Part A, 2010, 41, 1636–1638 CrossRef.
- T.-Y. Yu, Y.-K. Tseng, T.-H. Lin, T.-C. Wang, Y.-H. Tseng, Y.-H. Chang, M.-C. Wu and W.-F. Su, Carbohydr. Polym., 2022, 291, 119549 CrossRef CAS PubMed.
- A. Hadjadj, O. Jbara, A. Tara, M. Gilliot, F. Malek, E. M. Maafi and L. Tighzert, Compos. Struct., 2016, 135, 217–223 CrossRef.
- B. Krishnamurthi, S. Bharadwaj-Somaskandan, T. Sergeeva and F. Shutov, Cell. Polym., 2003, 22, 371–382 CrossRef CAS.
- I. Izarra, A. Borreguero, I. Garrido, J. Rodríguez and M. Carmona, Polym. Test., 2021, 100, 107268 CrossRef CAS.
- H. Liu, B. Zhang and J. Han, RSC Adv., 2017, 7, 35320–35329 RSC.
- F. Feng and L. Qian, Polym. Compos., 2014, 35, 301–309 CrossRef CAS.
- Y. J. Kim, H. J. Kang, C. T. Moerk, B.-T. Lee, J. S. Choi and J.-H. Yim, Sens. Actuators, B, 2021, 344, 130269 CrossRef CAS.
- C. Li, H. Ye, S. Ge, Y. Yao, B. Ashok, N. Hariram, H. Liu, H. Tian, Y. He and G. Guo, J. Mater. Res. Technol., 2022, 19, 3603–3615 CrossRef CAS.
- G. Kasi, S. Gnanasekar, K. Zhang, E. T. Kang and L. Q. Xu, J. Appl. Polym. Sci., 2022, 139, 52181 CrossRef CAS.
- S. Członka, A. Strąkowska and A. Kairytė, Polym. Test., 2020, 87, 106534 CrossRef.
- S. Kiddell, Y. Kazemi, J. Sorken and H. Naguib, Polym. Test., 2023, 119, 107919 CrossRef CAS.
- A. Alis, R. A. Majid, I. A. A. Nasir, N. S. Mustaffa and W. H. W. Hassan, AIP Conf. Proc., 2017, 1865, 040005 CrossRef.
- S. Członka, A. Strąkowska, A. Kairytė and A. Kremensas, Polym. Test., 2020, 86, 106479 CrossRef.
- P. Bartczak, M. Wysokowski, K. Szylińczuk, M. Odalanowska, T. Jesionowski and S. Borysiak, Ind. Crops Prod., 2023, 204, 117237 CrossRef CAS.
- H. Olcay and E. D. Kocak, Appl. Acoustics, 2021, 173, 107733 CrossRef.
- V. R. da Silva, M. A. Mosiewicki, M. I. Yoshida, M. C. da Silva, P. M. Stefani and N. E. Marcovich, Polym. Test., 2013, 32, 665–672 CrossRef.
- H. Olcay and E. D. Kocak, J. Ind. Text., 2020, 51, 8738S–8763S CrossRef.
- E. Akdogan and M. Erdem, J. Polym. Res., 2021, 28, 80 CrossRef CAS.
- J. Paciorek-Sadowska, M. Borowicz and M. Isbrandt, Polymers, 2023, 15, 3529 CrossRef CAS PubMed.
- X. Zhou, M. M. Sain and K. Oksman, Composites, Part A, 2016, 83, 56–62 CrossRef CAS.
- H. Chen, M. Zheng, H. Sun and Q. Jia, Mater. Sci. Eng., A, 2007, 445, 725–730 CrossRef.
- B. Adak, B. S. Butola and M. Joshi, Appl. Clay Sci., 2018, 161, 343–353 CrossRef CAS.
- T. Vanbergen, I. Verlent, J. De Geeter, B. Haelterman, L. Claes and D. De Vos, ChemSusChem, 2020, 13, 3835–3843 CrossRef CAS PubMed.
- D. Simón, A. Borreguero, A. De Lucas and J. Rodríguez, Waste Manage., 2018, 76, 147–171 CrossRef PubMed.
- K. M. Zia, H. N. Bhatti and I. A. Bhatti, React. Funct. Polym., 2007, 67, 675–692 CrossRef CAS.
- L. R. Mahoney, S. A. Weiner and F. C. Ferris, Environ. Sci. Technol., 1974, 8, 135–139 CrossRef CAS.
- N. Gama, B. Godinho, G. Marques, R. Silva, A. Barros-Timmons and A. Ferreira, Chem. Eng. J., 2020, 395, 125102 CrossRef CAS.
- N. V. Gama, A. Ferreira and A. Barros-Timmons, Materials, 2018, 11, 1841 CrossRef PubMed.
- M. Grdadolnik, A. Drincic, A. Oreski, O. C. Onder, P. Utrosa, D. Pahovnik and E. Zagar, ACS Sustainable Chem. Eng., 2022, 10, 1323–1332 CrossRef CAS PubMed.
- M. Grdadolnik, B. Zdovc, A. Drincic, O. C. Onder, P. Utrosa, S. G. Ramos, E. D. Ramos, D. Pahovnik and E. Zagar, ACS Sustainable Chem. Eng., 2023, 11, 10864–10873 CrossRef CAS PubMed.
- P. Zahedifar, L. Pazdur, C. M. Vande Velde and P. Billen, Sustainability, 2021, 13, 3583 CrossRef CAS.
- C. Molero, A. De Lucas, F. Romero and J. Rodríguez, J. Appl. Polym. Sci., 2008, 109, 617–626 CrossRef CAS.
- T. Cheng, Q. Gao and Z. L. Wang, Adv. Mater. Technol., 2019, 4, 1800588 CrossRef.
- G. M. Rani, C.-M. Wu, K. G. Motora and R. Umapathi, J. Cleaner Prod., 2022, 363, 132532 CrossRef.
- Y. Su, T. Yang, X. Zhao, Z. Cai, G. Chen, M. Yao, K. Chen, M. Bick, J. Wang and S. Li, Nano Energy, 2020, 74, 104941 CrossRef CAS.
- V. Singh and B. Singh, Polymer, 2023, 274, 125910 CrossRef CAS.
- Z. L. Wang, L. Lin, J. Chen, S. Niu, Y. Zi, Z. L. Wang, L. Lin, J. Chen, S. Niu and Y. Zi, Triboelectr, nanogener., 2016, 23–47 Search PubMed.
- R. K. Cheedarala and K. K. Ahn, Nano Energy, 2018, 44, 430–437 CrossRef CAS.
- P. Zhao, N. Soin, A. Kumar, L. Shi, S. Guan, C. Tsonos, Z. Yu, S. C. Ray, J. A. McLaughlin and Z. Zhu, Nano Energy, 2020, 67, 104291 CrossRef CAS.
- J. Wang, Z. Wen, Y. Zi, P. Zhou, J. Lin, H. Guo, Y. Xu and Z. L. Wang, Adv. Funct. Mater., 2016, 26, 1070–1076 CrossRef CAS.
- C. Yao, X. Yin, Y. Yu, Z. Cai and X. Wang, Adv. Funct. Mater., 2017, 27, 1700794 CrossRef.
- B. Cheng, C. Li, T. Ji, Y. Zhao, K. Gao, J. Hao and W. Xu, Nano Energy, 2024, 127, 109718 CrossRef CAS.
- J. Wang, Z. Wen, Y. Zi, L. Lin, C. Wu, H. Guo, Y. Xi, Y. Xu and Z. L. Wang, Adv. Funct. Mater., 2016, 26, 3542–3548 CrossRef CAS.
- Q. Zheng, L. Fang, H. Guo, K. Yang, Z. Cai, M. A. B. Meador and S. Gong, Adv. Funct. Mater., 2018, 28, 1706365 CrossRef.
- M. Wang, N. Zhang, Y. Tang, H. Zhang, C. Ning, L. Tian, W. Li, J. Zhang, Y. Mao and E. Liang, J. Mater. Chem. A, 2017, 5, 12252–12257 RSC.
- Z. Saadatnia, S. G. Mosanenzadeh, E. Esmailzadeh and H. E. Naguib, Sci. Rep., 2019, 9, 1370 CrossRef PubMed.
- K. Y. Lee, J. Chun, J.-H. Lee, K. N. Kim, N.-R. Kang, J.-Y. Kim, M. H. Kim, K.-S. Shin, M. K. Gupta and J. M. Baik, Adv. Mater., 2014, 26, 5037–5042 CrossRef CAS PubMed.
- Z. Peng, J. Song, Y. Gao, J. Liu, C. Lee, G. Chen, Z. Wang, J. Chen and M. K. Leung, Nano Energy, 2021, 85, 106021 CrossRef CAS.
- M. Wang, W. Liu, X. Shi, J. Pan, B. Zhou, J. Wang, T. Sun and Y. Tang, New J. Chem., 2021, 45, 1893–1898 RSC.
- Y. Liu, Y. Zheng, Z. Wu, L. Zhang, W. Sun, T. Li, D. Wang and F. Zhou, Nano Energy, 2021, 79, 105422 CrossRef CAS.
- D. Kamaruzaman, N. S. M. Mustakim, A. S. R. A. Subki, N. Parimon, M. K. Yaakob, M. F. Malek, N. Vasimalai, M. H. Abdullah, S. A. Bakar and M. K. Ahmad, Mater. Today Sustainability, 2024, 26, 100726 CrossRef.
- H. J. Oh, J. H. Bae, Y. K. Park, J. Song, D. K. Kim, W. Lee, M. Kim, K. J. Heo, Y. Kim and S. H. Kim, Polymers, 2020, 12, 1044 CrossRef CAS PubMed.
- A. A. Maamoun, A. A. Mahmoud, D. Naeim, M. Arafa and A. Esawi, Mater. Adv., 2024, 5, 6132–6144 RSC.
- A. A. Maamoun, A. M. Esawi, A. A. Mahmoud, D. M. Naeim and M. Arafa, Appl. Energy, 2025, 377, 124579 CrossRef CAS.
- Z. Wu, P. Wang, B. Zhang, H. Guo, C. Chen, Z. Lin, X. Cao and Z. L. Wang, Adv. Mater. Technol., 2021, 6, 2000737 CrossRef CAS.
- H. Li, T. K. Sinha, J. S. Oh and J. K. Kim, ACS Appl. Mater. Interfaces, 2018, 10, 14008–14016 CrossRef CAS PubMed.
- M. Sahu, S. Hajra, H.-G. Kim, H.-G. Rubahn, Y. K. Mishra and H. J. Kim, Nano Energy, 2021, 88, 106255 CrossRef CAS.
- M. Navaneeth, S. Potu, A. Babu, B. Lakshakoti, R. K. Rajaboina, U. Kumar K, H. Divi, P. Kodali and K. Balaji, ACS Sustainable Chem. Eng., 2023, 11, 12145–12154 CrossRef CAS.
- B. Dudem, R. I. G. Dharmasena, R. Riaz, V. Vivekananthan, K. Wijayantha, P. Lugli, L. Petti and S. R. P. Silva, ACS Appl. Mater. Interfaces, 2022, 14, 5328–5337 CrossRef CAS PubMed.
- N. R. Alluri, N. P. M. J. Raj, G. Khandelwal, V. Vivekananthan and S.-J. Kim, Nano Energy, 2020, 73, 104767 CrossRef CAS.
- G. Khandelwal, A. Chandrasekhar, N. R. Alluri, V. Vivekananthan, N. P. M. J. Raj and S.-J. Kim, Appl. Energy, 2018, 219, 338–349 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |