 Open Access Article
Open Access ArticleConnectivity and twist: engineering high-performance green phosphorescent OLEDs using unipolar symmetric bicarbazole regioisomers†
Kalidass
Kollimalaian
 a,
Jia-Fan
Wu‡
b,
Yu Hsuan
Lin‡
c,
Ya-Hsin
Cheng
b,
Premkumar
Gnanasekaran
c,
Sudhakar
Maddala
a,
Jia-Fan
Wu‡
b,
Yu Hsuan
Lin‡
c,
Ya-Hsin
Cheng
b,
Premkumar
Gnanasekaran
c,
Sudhakar
Maddala
 a,
Mandy M.
Lee
d,
Shih-Sheng
Sun
a,
Mandy M.
Lee
d,
Shih-Sheng
Sun
 d,
Chih-Hao
Chang
d,
Chih-Hao
Chang
 *b,
Yuan Jay
Chang
*b,
Yuan Jay
Chang
 *c and
Venkatakrishnan
Parthasarathy
*c and
Venkatakrishnan
Parthasarathy
 *a
*a
aDepartment of Chemistry, Indian Institute of Technology Madras, Chennai-600 036, Tamil Nadu, India. E-mail: pvenkat@zmail.iitm.ac.in
bDepartment of Electrical Engineering, Yuan Ze University, Chung-Li, 32003, Taiwan. E-mail: chc@saturn.yzu.edu.tw
cDepartment of Chemistry, Tunghai University, No. 1727, Sec. 4, Taiwan Boulevard, Xitun District, Taichung 40704, Taiwan. E-mail: jaychang@thu.edu.tw
dInstitute of Chemistry, Academia Sinica, Taipei 115201, Taiwan
First published on 3rd June 2025
Abstract
Molecular structural differences by position can be crucial in developing promising materials for device applications. We synthesized four regioisomeric symmetric bicarbazoles (BCzPh) with distinct dihedral angle twists using oxidative C–C coupling or transition-metal catalyzed Suzuki coupling methods. The structural differences in connectivity manifested in fine-tuning of the photophysical, thermal, and electrochemical properties of the materials, as well as the device performance. Particularly, understanding the balance between the resonance and conjugation effects seems crucial for manipulation of triplet energy levels. Our findings indicate that bicarbazoles with greater twist angles exhibit larger singlet–triplet/HOMO–LUMO energy gaps, and improved power and luminance efficiencies, benefiting phosphorescent organic light-emitting diode (PhOLED) devices. The external quantum efficiencies of PhOLEDs were over 23.4% and 23.9% for BCzPh-based devices B and D, with device C reaching a maximum brightness of 203![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 490 cd m−2, followed by device A at 96
490 cd m−2, followed by device A at 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 953 cd m−2. Notably, all BCzPh compounds served as excellent host materials, demonstrating stable, high-purity green-color emission in devices that turned on at voltages as low as 2.2 V.
953 cd m−2. Notably, all BCzPh compounds served as excellent host materials, demonstrating stable, high-purity green-color emission in devices that turned on at voltages as low as 2.2 V.
1. Introduction
Phosphorescent OLEDs (PhOLEDs) have garnered immense attention due to their potential to achieve nearly 100% internal quantum efficiency (IQE) by efficiently harnessing both singlet and triplet excitons.1,2 The development of high-triplet-energy organic host materials with suitable energy levels is crucial for ensuring optimal charge balance, exciton confinement, and efficient energy transfer to dopants.3,4 While various molecular hosts, including bipolar (donor–acceptor) and non-polar systems, have been extensively explored for their structure–property relationships,5–12 these studies have often lacked a systematic approach to molecular design and optimization.Positional isomerism has recently emerged as a powerful yet underutilized strategy for fine-tuning the electronic and optical properties of organic semiconductors, thereby enhancing optoelectronic device performance.5–19 Several studies with positional isomers have demonstrated that subtle variations in linkage positions can yield promising molecular candidates with high OLED efficiencies,11,13–17 tunable solid-state packing,20–22 tailored emission characteristics,12,20,23 ultralong organic phosphorescence,12,24–26 highly efficient perovskite solar cells,18,19,27etc. For instance, Poriel et al. demonstrated how variations in phenyl linkages (ortho, meta, and para) and steric congestion between spirobifluorene (SBF) units dictate the electrochemical and optical properties of regioisomeric SBF dimers, revealing distinct optical and electronic characteristics for each positional isomer.6,7,9,28,29 These studies underscore the profound impact of regioisomeric control on molecular properties, reinforcing the need for systematic investigations into positional effects across different molecular scaffolds for developing efficient devices.
Among various organic semiconductors, carbazole-based host materials have gained prominence due to their high triplet energy (ET > 2.95 eV), efficient hole transport, superior charge carrier mobility, and exceptional thermal, morphological, chemical, and photochemical stability.30–38 Their synthetic versatility, stable radical cations, and favourable electronic properties further enhance their potential in optoelectronic applications.3,4,39–45 Although extensive studies have explored carbazole functionalization at the 2,7- and 3,6-positions, as well as at the nitrogen atom, systematic studies correlating the structure and properties of bicarbazole (BCz) derivatives—comprising two carbazole units linked via C–C or C–N bonds—remain limited (Fig. 1).3,39,46–48 These bicarbazole architectures, depending on the connectivity of two carbazole units, provide a versatile platform for optimizing charge transport and triplet energy levels. Notably, 3,3′-bicarbazole (BCz, Fig. 1a) exhibits a small singlet–triplet energy gap (ΔEST = 0.46 eV) and a high triplet energy (ET = 2.8 eV),49 making it a promising candidate for PhOLED applications. While CBP (4,4′-bis(9-carbazolyl)-2,2′-biphenyl, ET = 2.6 eV; Fig. 1a) is widely employed as a green phosphor host,50–52 its relatively low ET can lead to reverse energy transfer from blue-emitting guests to hosts. In contrast, mCP (1,3-bis(9-carbazolyl)-benzene, ET ≈ 3.00 eV; Fig. 1a) offers a higher ET, making it more suitable for blue PhOLEDs.3,53–60 Diverse dipolar structural modifications of BCz, mCP and CBP through (i) substituents on nitrogen,61–63 (ii) reactive carbon center(s),62–64 and (iii) connectivity (symmetrical/unsymmetrical) differences and spacer linkages47,62,65–69 were explored, besides N–N bridged bicarbazoles reported recently (Fig. 1b).48,70,71 Despite these advances, regioisomeric effects in unipolar symmetric bicarbazoles remain largely unexplored, particularly in relation to charge transport and exciton management.
In this work, we systematically investigate four regioisomeric bicarbazole derivatives—1,1′-BCzPh, 2,2′-BCzPh, 3,3′-BCzPh, and 4,4′-BCzPh—where C–C bonds symmetrically link two carbazole units, leading to distinct molecular architectures (Chart 1). Although these isomers share the same molecular formula, variations in connectivity induce varying degrees of dihedral angles, π-conjugation, and resonance effects. Notably, 3,3′-BCzPh exhibits strong resonance due to para–para nitrogen connectivity, while 2,2′-BCzPh, with meta–meta nitrogen positioning, limits conjugation. The steric constraints in 1,1′-BCzPh and 4,4′-BCzPh result in greater twist angles, restricting extended conjugation and modifying their electronic properties. Moreover, 1,1′-BCzPh alone instils through-space conjugation between the N-phenyl and carbazole rings. These structural differences significantly influence electronic structure/properties, mesomeric effects, and triplet energy levels, making them ideal candidates for systematic study.72–75 We present a comprehensive structure–property analysis, integrating experimental and DFT studies to correlate molecular conformation with electronic, thermal, and photophysical properties. Furthermore, we evaluate their performance as host materials in green PhOLEDs, where notable differences emerge in luminescence and external quantum efficiencies (EQE). Remarkably, π-extended 3,3′-BCzPh exhibited the highest luminescence (203![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 490 cd m−2), while 2,2′-BCzPh and 4,4′-BCzPh achieved high EQEs of 23.4% and 23.9%, respectively—outperforming other isomers and conventional CBP-based hosts. These findings underscore the critical role of regioisomerism in tailoring material properties and advancing next-generation PhOLED design and applications.
490 cd m−2), while 2,2′-BCzPh and 4,4′-BCzPh achieved high EQEs of 23.4% and 23.9%, respectively—outperforming other isomers and conventional CBP-based hosts. These findings underscore the critical role of regioisomerism in tailoring material properties and advancing next-generation PhOLED design and applications.
2. Results and discussion
2.1. Synthesis and characterization
The positional isomers of bicarbazole (1,1′-BCzPh, 2,2′-BCzPh, 3,3′-BCzPh, and 4,4′-BCzPh, Chart 1) were synthesized from their respective carbazole derivatives (please refer to pages S4–S6, ESI†). In particular, 3,3′-BCzPh was synthesized from N-phenylcarbazole using our metal-free oxidative coupling reaction.74,75 The other regioisomers—1,1′-BCzPh, 2,2′-BCzPh, and 4,4′-BCzPh—were synthesized starting from the corresponding bromocarbazole derivatives via a transition metal-catalyzed Suzuki cross-coupling reaction.76 The synthesis of bicarbazoles typically involves the following steps: (i) N-arylation of 9H-carbazole using a copper catalyst,72,73,77 (ii) borylation of bromocarbazole with a boronate ester reagent in the presence of palladium or via lithium–halogen exchange,72,73 and (iii) Suzuki coupling between the appropriate bromocarbazole and carbazole boronate ester using a palladium catalyst. The synthesized symmetric bicarbazole isomers (BCz) were thoroughly characterized by spectroscopic (NMR and IR) and spectrometric (HRMS) analyses (Fig. S1–S3 and pages S3–S10, ESI†). Their structures were further confirmed by X-ray crystallography (Fig. S4–S6 and pages S11–S14, ESI†).2.2. Structural properties
The X-ray structure analysis of 1,1′-BCzPh (CCDC 2361782, triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ; Z = 2; Fig. 2a and Fig. S4, Table S1, ESI†) and 4,4′-BCzPh (CCDC 2361783, orthorhombic, Pbca; Z = 8; Fig. 2d and Fig. S6, Table S3, ESI†), revealed a non-planar, highly twisted geometry, in contrast to 2,2′-BCzPh (CCDC 2361784, monoclinic, P21/c; Z = 4; Fig. 2b and Fig. S5, Table S2, ESI†) and 3,3′-BCzPh (CCDC 1579239; Fig. 2c),78 which likely results from greater steric repulsion between the N-aryl and carbazole rings in 1,1′-BCzPh, and between the carbazole rings in 4,4′-BCzPh, compared to the steric hindrance caused by hydrogens in 2,2′-BCzPh and 3,3′-BCzPh. Consequently, the dihedral angles (θ) between the mean planes of the two carbazole rings were found to follow the order: 78.1° > 71.5° > 28.4° for 4,4′-BCzPh, 1,1′-BCzPh, and 2,2′-BCzPh, respectively (Fig. 2). This twist trend is comparable to analogous spirobiflorene (SBF) dimers.6,7,9,28,29 Besides, the new C–C bond length between the two carbazole rings in BCzPhs was measured to be ca. 1.486 Å, 1.490 Å, 1.496 Å for 1,1′-BCzPh, 2,2′-BCzPh, and 4,4′-BCzPh, respectively. Previous report74–78 has measured a torsion angle and C–C bond length of 0.77° and 1.491 Å, respectively, for 3,3′-BCzPh. It is worth noting that substituents can significantly modify these solid-state structural parameters.74,78 However, the C–C bond length measures suggest varying degrees of resonance in these systems, depending on their connectivity (steric inhibition of resonance), which aligns with the observed twist trend. Molecular packing analyses of the highly twisted 1,1′-BCzPh, 4,4′-BCzPh, and the relatively planar 2,2′-BCzPh reveal that their solid-state arrangement is primarily driven by weak C–H⋯π or/and π–π stacking interactions. Both 2,2′-BCzPh and 3,3′-BCzPh exhibited staggered arrangements of (bi)carbazoles, with the 2,2′-BCzPh stacking face-to-face in the same direction, while 3,3′-BCzPh adopted a slip-stacked arrangement in opposite directions.78 These highly planar structures and such packing configurations are advantageous for improving thermal stability and facilitating charge transport. In contrast, 1,1′-BCzPh and 4,4′-BCzPh lacked continuous carbazole–carbazole interactions, resulting in looser packing arrangements. Such twisted aryl systems are of great interest in inducing an amorphous character, which is crucial for generating morphologically stable, pin-hole-free thin films during OLED fabrication, enhancing efficient recombination and device stability.79,80 In contrast, more planar carbazole structures may function as efficient charge-transport systems.
; Z = 2; Fig. 2a and Fig. S4, Table S1, ESI†) and 4,4′-BCzPh (CCDC 2361783, orthorhombic, Pbca; Z = 8; Fig. 2d and Fig. S6, Table S3, ESI†), revealed a non-planar, highly twisted geometry, in contrast to 2,2′-BCzPh (CCDC 2361784, monoclinic, P21/c; Z = 4; Fig. 2b and Fig. S5, Table S2, ESI†) and 3,3′-BCzPh (CCDC 1579239; Fig. 2c),78 which likely results from greater steric repulsion between the N-aryl and carbazole rings in 1,1′-BCzPh, and between the carbazole rings in 4,4′-BCzPh, compared to the steric hindrance caused by hydrogens in 2,2′-BCzPh and 3,3′-BCzPh. Consequently, the dihedral angles (θ) between the mean planes of the two carbazole rings were found to follow the order: 78.1° > 71.5° > 28.4° for 4,4′-BCzPh, 1,1′-BCzPh, and 2,2′-BCzPh, respectively (Fig. 2). This twist trend is comparable to analogous spirobiflorene (SBF) dimers.6,7,9,28,29 Besides, the new C–C bond length between the two carbazole rings in BCzPhs was measured to be ca. 1.486 Å, 1.490 Å, 1.496 Å for 1,1′-BCzPh, 2,2′-BCzPh, and 4,4′-BCzPh, respectively. Previous report74–78 has measured a torsion angle and C–C bond length of 0.77° and 1.491 Å, respectively, for 3,3′-BCzPh. It is worth noting that substituents can significantly modify these solid-state structural parameters.74,78 However, the C–C bond length measures suggest varying degrees of resonance in these systems, depending on their connectivity (steric inhibition of resonance), which aligns with the observed twist trend. Molecular packing analyses of the highly twisted 1,1′-BCzPh, 4,4′-BCzPh, and the relatively planar 2,2′-BCzPh reveal that their solid-state arrangement is primarily driven by weak C–H⋯π or/and π–π stacking interactions. Both 2,2′-BCzPh and 3,3′-BCzPh exhibited staggered arrangements of (bi)carbazoles, with the 2,2′-BCzPh stacking face-to-face in the same direction, while 3,3′-BCzPh adopted a slip-stacked arrangement in opposite directions.78 These highly planar structures and such packing configurations are advantageous for improving thermal stability and facilitating charge transport. In contrast, 1,1′-BCzPh and 4,4′-BCzPh lacked continuous carbazole–carbazole interactions, resulting in looser packing arrangements. Such twisted aryl systems are of great interest in inducing an amorphous character, which is crucial for generating morphologically stable, pin-hole-free thin films during OLED fabrication, enhancing efficient recombination and device stability.79,80 In contrast, more planar carbazole structures may function as efficient charge-transport systems.
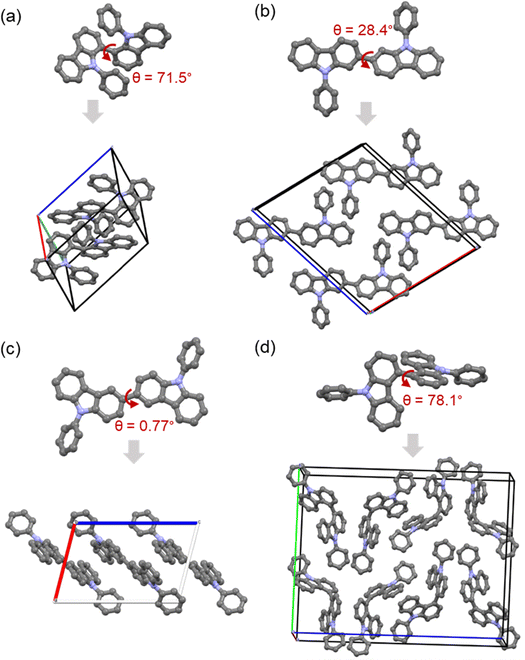 | ||
| Fig. 2 The X-ray molecular structure (top row) and unit cell packing diagram (bottom row) of (a) 1,1′-BCzPh, (b) 2,2′-BCzPh, (c) 3,3′-BCzPh,55 and (d) 4,4′-BCzPh. The dihedral angle (θ, °) between the carbazole planes is indicated and the hydrogen atoms are removed for clarity. | ||
2.3. Electrochemical properties
To understand the redox behavior, the cyclic voltammograms of the BCzs were recorded in dichloromethane solution (1.0 mM) using tetrabutylammonium hexafluorophosphate as the supporting electrolyte (Fig. 3 and Fig. S7, pages S15 and S16, ESI†). The BCzs display multiple (more than one electron) oxidation potentials, which are ascribed to the oxidation of two carbazole moieties connected distinctly. Their first oxidation potential increases in the order: 3,3′-BCzPh < 1,1′-BCzPh < 2,2′-BCzPh < 4,4′-BCzPh, indicating the influence of conjugation/mesomerism and variations in twist angles across these systems (Fig. 3 and Fig. S7, ESI†); mesomeric effects appear to dominate over π-conjugation in this context, highlighting the electron-rich nature of bicarbazole. The highest occupied molecular orbital (HOMO) energy levels (eV) of the BCzPhs were calculated from the onset oxidation potentials (EOX) derived from differential pulse voltammograms (DPV), using the relation EHOMO = EOX + 4.8 eV against Fc/Fc+. The lowest unoccupied molecular orbital (LUMO) energy values (eV) were obtained from the optical energy gap values (Eg, solution) and the corresponding HOMO energy values, using ELUMO = EHOMO + Eg (in eV). The oxidation and reduction onset values, along with the corresponding HOMO (−5.45 eV to 5.64 eV) and LUMO (−2.32 eV to −2.18 eV) energy values, are summarized in Table 1 and Table S4 (ESI†). Notably, 3,3′-BCzPh63,78 and 2,2′-BCzPh exhibit elevated HOMO energy levels compared to twisted 1,1′-BCzPh and 4,4′-BCzPh, likely due to extended π-conjugation and resonance in the former and steric inhibition of the same effects in the latter due to twisting. The HOMO energy level in 4,4′-BCzPh, closely resembling that of simple N-phenylcarbazole (−5.64 eV),78 suggests a lack of significant resonance and conjugation effects. This similarity implies that the extended electronic communication between the carbazole units is hindered, resulting in limited conjugation across the molecule. Overall, the HOMO energy values (−5.45 eV to −5.64 eV) observed for these bicarbazoles are comparable to that of CDBP80 and CBP,81 however lower than SBF dimers picturing nitrogen's role here.6,7,9,28,29 | ||
| Fig. 3 Cyclic voltammograms of BCzPhs (1.0 mM) recorded in dichloromethane using TBAPF6 (0.1 M) as the supporting electrolyte. | ||
| Molecule | Wavelengtha (nm) | φ f (%) | E g (eV) | E HOMO (eV) | E LUMO (eV) | T d/Tme (°C) | ||
|---|---|---|---|---|---|---|---|---|
| λ onset | λ abs | λ em | ||||||
| a Measured in dichloromethane solution at room temperature. b E g, the optical energy gap is estimated from the threshold of the UV-vis absorption spectra using the relationship, Eg = 1240/λonset. c HOMO and LUMO energy levels are calculated from the onset of the first oxidation potential and the optical energy gap from the absorption spectra. d Quantum yield was measured in dichloromethane solution at room temperature with the reference of 9,10-diphenylanthracene. e T d: decomposition temperature, Tm: melting point. | ||||||||
| 1,1′-BCzPh | 366 | 332, 347 | 363 | 60 | 3.39 | −5.56 | −2.22 | 281/— |
| 2,2′-BCzPh | 378 | 325, 349 | 390 | 76 | 3.28 | −5.60 | −2.32 | 329/234 |
| 3,3′-BCzPh 75 | 379 | 310, 361 | 406 | 21 | 3.27 | −5.45 | −2.18 | 366/205 |
| 4,4′-BCzPh | 373 | 330, 344 | 382 | 59 | 3.32 | −5.64 | −2.32 | 291/204 |
2.4. Thermal properties
To evaluate the functional utility of BCzPhs in PhOLED applications, their thermal stabilities were assessed through thermogravimetry (TGA) and differential scanning calorimetry (DSC) under a nitrogen gas atmosphere. The scans (Fig. 4 and Fig. S8, pages S16 and S17, ESI†) and data in Table 1 reveal that all BCzPhs exhibit high thermal stabilities, with decomposition temperatures (Td at 5% weight loss) exceeding 280 °C. Specifically, 2,2′-BCzPh has a Td of 329 °C, higher than 4,4′-BCzPh (291 °C) and 1,1′-BCzPh (281 °C). This is probably linked to the relatively planar molecular structure of 2,2′-BCzPh and 3,3′-BCzPh, which promotes closer packing in the solid state. Fortunately, the thermal decomposition temperature of these BCzPh compounds align with their twist angle: the smaller the twist angle, the higher the thermal decomposition, and vice versa. Notably, the thermal stability of BCzPhs is comparable to that of other bicarbazole-based derivatives (oCBP,82BCz1, BCz2, and BCz383) reported previously in the literature. The high thermal stability is attributed to the bicarbazole core, which increases the overall molecular size. Furthermore, the complete mass loss beyond Td, with no residual material, suggests that BCzPhs are promising candidates for fabricating devices under the vacuum-deposition method.The DSC scans for most BCzPhs (Fig. 4) reveal a sharp endothermic melting transition (Tm) in the first heating cycle, except for 1,1′-BCzPh, which showed no thermal transitions (up to 250 °C). Specifically, a melting transition was observed for 2,2′-BCzPh at 234 °C and 4,4′-BCzPh at 204 °C, alongside glass transitions (Tg) at ca. 175 and 160 °C, respectively (Fig. S8, ESI†). These Tg values are significantly higher than the simple unipolar bicarbazole (C–N or C–C based) systems known in the literature63,81–83 In comparison, previously reported 3,3′-BCzPh exhibits a Td of 366 °C and Tm of 205 °C, higher than the less-twisted counterpart 2,2′-BCzPh.63,75 The good thermal and amorphous properties of BCzPhs suggest they can offer enhanced morphological stability and uniform film-forming ability during vacuum deposition, making them suitable for device fabrication.
2.5. Optical properties in solution
The optical properties of isomeric 1,1′-BCzPh, 2,2′-BCzPh, 3,3′-BCzPh, and 4,4′-BCzPh were evaluated using UV-vis absorption and fluorescence spectroscopy in dilute dichloromethane solutions (ca. 10−5 M) at room temperature (Fig. 5 and Fig. S9, pages S17–S19, ESI†). The summarized results of all BCzPhs are gathered in Table 1 and Table S5 (ESI†). The BCzPhs revealed absorption bands tailing near the visible region up to ca. 380 nm, in contrast to simple carbazole (Cz).25,84 The red-shifted absorption in BCzPhs, when compared to simple Cz, is attributed to extended π-conjugation resulting from the establishment of a new C–C bond between the two carbazole rings. The absorption spectral profile for the twisted 1,1′-BCzPh and 4,4′-BCzPh is similar, exhibiting clear vibronic bands, likely due to sterically restricted carbazole rotations. However, 2,2′-BCzPh showed broader absorption bands with no vibronic features and a red-shift relative to 3,3′-BCzPh. The optical energy gap (Eg) for BCzPhs, estimated from the onset of the absorption spectrum, ranged from 3.27 to 3.39 eV, which is smaller than that of analogous SBF dimers, emphasizing the resonance contribution of nitrogen atoms.6,7,9,28,29 The highly twisted 1,1′-BCzPh exhibited the largest energy gap, followed by the relatively less twisted isomers (i.e., 4,4′-BCzPh > 2,2′-BCzPh > 3,3′-BCzPh). Supportively, this trend in Eg values is comparable to the theoretically derived ones; the electron density in the HOMO and LUMO was localized on the bicarbazole unit (Fig. S10 and Table S6, page S20, ESI†).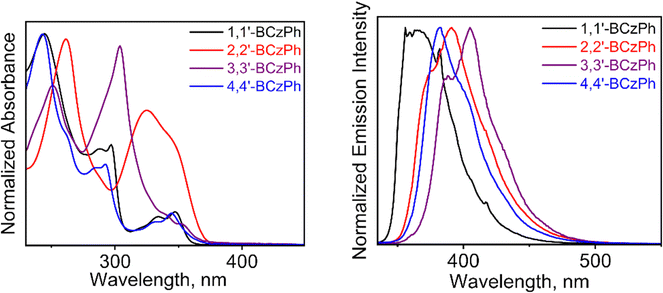 | ||
| Fig. 5 Normalized UV-vis absorption (left) and emission (right) spectra of isomeric BCzPhs in dichloromethane (ca. 10−5 M). | ||
The emission spectra of BCzPhs (recorded at λexc = 325 nm) presented characteristic vibronic patterns, typical of Cz, ranging from 350 to 450 nm.25,84 Both 3,3′-BCzPh and 2,2′-BCzPh exhibited a red-shifted emission maxima compared to their regioisomeric counterparts 1,1′-BCzPh and 4,4′-BCzPh, respectively (Fig. 5, Table 1 and Fig. S9, Table S5, ESI†). In other words, the more planar BCz structures displayed emission near the visible region, while the twisted structures emitted in the UV region. The fluorescence quantum yields (ϕf) measured in dichloromethane were in the range ca. 0.21–0.76 (Table 1 and Table S5, ESI†), with 2,2′-BCzPh exhibiting the highest value (0.76). The severe steric interactions between carbazole planes in the twisted BCz structures likely increase overall rigidity, promoting radiative energy loss and resulting in high ϕfs, as seen in 1,1′-BCzPh and 4,4′-BCzPh (ϕf, ca. 0.60). In sharp contrast, the relatively less-twisted 3,3′-BCzPh and 2,2′-BCzPh likely permit free-rotation along the bridging C–C bonds, facilitating non-radiative decay and reducing their PLQYs. Interestingly, 2,2′-BCzPh exhibits a ca. 3-fold increase in PLQY compared to 3,3′-BCzPh, though the origin of this difference remains unclear. However, it may be discerned from the fact that the increment in PLQY (up to 62%) of 3,3′-BCzPh when mixed with FIrpic dopant emitter, suggesting a possible non-radiative decay pathway in 3,3′-BCzPh.63 The optical properties of BCzPhs are consistent with those reported for BCz analogs.53,81
2.6. Photophysical properties in thin films
To replicate device-like conditions, the fluorescence and phosphorescence emission spectra were also examined in the thin-film state (ca. 60 nm) and in 77 K glassy matrices, respectively. The complete spectrum and associated data are presented in Fig. 6 and Table 2, respectively. When comparing the fluorescence spectrum of all synthesized compounds, it becomes clear that the 1,1′-BCzPh film displays apparent vibronic features and a broader spectral profile, resembling the solution phase behavior. The full-width at half-maximum (FWHM) values for 1,1′-BCzPh, 2,2′-BCzPh, 3,3′-BCzPh, and 4,4′-BCzPh were estimated as 73, 53, 48, and 42 nm, respectively. The positioning of the two carbazole entities in BCzPhs significantly impacts their spectral profiles in both solution and film phases. The increased molecular symmetry and restricted molecular motion in 1,1′-BCzPh probably contribute to clear vibronic features, reflecting enhanced rigidity.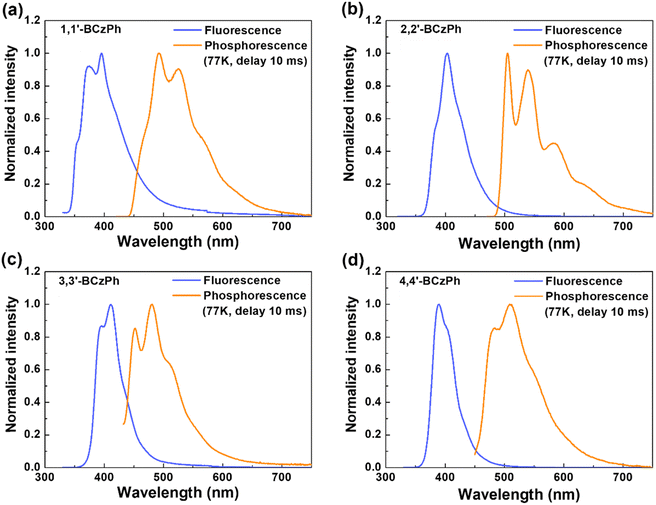 | ||
| Fig. 6 Fluorescence (blue line, thin-film) and phosphorescence (orange line, in 2-MeTHF at 77 K) spectra of (a) 1,1′-BCzPh; (b) 2,2′-BCzPh; (c) 3,3′-BCzPh; and (d) 4,4′-BCzPh. | ||
| Molecule | Fluorescenceaλpeak (nm) | Phosphorescencebλpeak (nm) | S1c (eV) | T1d (eV) |
|---|---|---|---|---|
| a Fluorescence spectrum measured in neat films. b Phosphorescence spectrum measured in 2-MeTHF at 77 K. c Estimated from the fluorescence onset. d Estimated from the phosphorescence onset. | ||||
| 1,1′-BCzPh | 353 (sh), 374, 395, 422 (sh) | 493, 525, 564 (sh) | 3.46 | 2.78 |
| 2,2′-BCzPh | 382 (sh), 403, 426 (sh) | 505, 540, 583, 635 (sh) | 3.27 | 2.54 |
| 3,3′-BCzPh | 395, 411, 433 (sh) | 451, 481, 510 (sh) | 3.36 | 2.8763 |
| 4,4′-BCzPh | 390, 403 (sh) | 583, 509, 548 (sh) | 3.33 | 2.73 |
The singlet energies (S1) of the thin film samples did not fully match the calculated values derived from solution, which might result from the molecular packing effects and film morphology. Similarly, the triplet energies (T1), determined from the onset of the phosphorescence spectrum, were found to be 2.78, 2.54, 2.87,63 and 2.73 eV for 1,1′-BCzPh, 2,2′-BCzPh, 3,3′-BCzPh, and 4,4′-BCzPh, respectively. Although the more twisted BCzPh isomers (1,1′-BCzPh and 4,4′-BCzPh) generally exhibited higher triplet and singlet energy levels, 3,3′-BCzPh surprisingly exhibited the highest triplet energy in the series. This suggests that factors beyond molecular twisting, such as enhanced resonance effects, significantly contribute to the elevation of excited-state energy levels. Resonance and conjugation effects were both found to effectively elevate the singlet and triplet energy levels in 3,3′-BCzPh, likely due to improved π-orbital overlap and charge delocalization. On the other hand, 2,2′-BCzPh, which primarily benefits from conjugation but lacks substantial resonance stabilization, showed the lowest energy levels among the four isomers. The T1 energy values of these isomers are comparable to unipolar bicarbazoles like mCP,63CBP,81 or oCDBP,82 although lower than 3-(9-carbazolyl)carbazoles83 and SBF isomers.6,7,9,28,29 Because efficient energy transfer in green phosphorescent OLEDs requires host materials with triplet energy levels typically above 2.5 eV, these results indicate that triplet energy levels can be effectively fine-tuned by engineering the twist angles via C–C coupling (in contrast to C–N coupling), which significantly impacts the molecular conformation and π–π interactions. Therefore, the BCz-based compounds investigated here demonstrate strong potential as host materials for green phosphorescent emitters.
2.7. Film morphology and carrier transport properties
We conducted the atomic force microscopy (AFM) measurements of the thin film samples fabricated on the glass substrate to assess whether the twisted aryl system could form amorphous, morphologically stable, and pinhole-free films for OLED applications. The results indicated that all four samples using synthesized BCzPh-based compounds could exhibit smooth morphologies, as shown in Fig. S11 (ESI†). The average roughness values, ranging from 1 to 2 nm, suggest that these materials have the potential to serve as ideal host materials.The charge mobility of host materials significantly impacts electroluminescence (EL) performance in OLEDs and warrants careful consideration during device design. To evaluate the hole transport capabilities of the synthesized compounds, we fabricated single-carrier devices and conducted space-charge-limited current (SCLC) measurements.85 The architecture of the hole-only devices (HOD) was set to ITO (120 nm)/MoO3 (5 nm)/TAPC (10 nm)/BCzPh (150 nm)/MoO3 (10 nm)/Al (120 nm). Here, MoO3 functions as the hole injection and electron blocking layer, while TAPC served as the hole transport layer. The electron-only devices (EOD) adopted the following architecture: ITO (120 nm)/CN-T2T (10 nm)/BCzPh (150 nm)/CN-T2T (10 nm)/CN-T2T:Li2CO3 10 wt% (5 nm)/Al (120 nm), where CN-T2T and CN-T2T:Li2CO3 acted as the hole blocking layer and the electron transport/injection layer, respectively.
Fig. 7(a) displays the J–V curves for the hole-only devices incorporating the synthesized BCzPh-based compounds. Given the comparable HOMO/LUMO energy levels of the target compounds, the outcome of the current density primarily reflects their charge transport capabilities. For the hole-only devices, the current density follows the trend 3,3′-BCzPh > 2,2′-BCzPh > 1,1′-BCzPh > 4,4′-BCzPh. In sharp contrast, the electron-only devices presented ultra-low current density values, indicating that these compounds could primarily transport holes. Hole mobilities were calculated based on the SCLC theory, presuming the evaporated organic solid films possess an inherently disordered morphology. The corresponding formula for carrier mobility is provided in the ESI† (page S28). A comparison of field-dependent hole mobility among the BCzPh-based compounds is illustrated in Fig. 7(b). The trend in hole mobility values at a field of 0.25 MV cm−1 mirrored the current density trend, with values of (μ(1,1′-BCzPh) = 1.6 × 10−4 cm2 V−1 s−1, μ(2,2′-BCzPh) = 4.6 × 10−4 cm2 V−1 s−1, μ(3,3′-BCzPh) = 8.5 × 10−3 cm2 V−1 s−1, and μ(4,4′-BCzPh) = 1.9 × 10−5 cm2 V−1 s−1). The hole mobility values of the first three compounds are comparable to those of commonly used hole transport materials,86,87 enabling efficient hole injection from the HTL to the emitting layer (EML) without significant deceleration. The connectivity and twist angle variation in the BCzPh series significantly alter the electronic communication, as revealed by the correlatable trends in current density/hole-mobility. These results, in alignment with the X-ray packing arrangement, suggest that well-designed devices, combined with this new set of BCz-based synthetic compounds, can achieve satisfactory electroluminescent (EL) performance.
 | ||
| Fig. 7 (a) Current density–voltage (J–V) curves of the hole-only devices and (b) comparative field dependence mobility of BCzPh-based compounds. | ||
To further understand the carrier transport capabilities of the EML, we fabricated HOD and EOD using the synthesized compounds blended with B3PyMPM in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, as shown in Fig. 8. The hole transport properties of the blended samples closely mirrored those of the pure synthesized compounds (cf.Fig. 7 and 8(a), (b)). The current density in the blended hole-only devices followed the trend 3,3′-BCzPh > 2,2′-BCzPh > 1,1′-BCzPh > 4,4′-BCzPh. This trend was also reflected in hole mobility values at an electric field of 0.25 MV cm−1, with values of (μ(1,1′-BCzPh) = 2.3 × 10−6 cm2 V−1 s−1, μ(2,2′-BCzPh) = 5.4 × 10−6 cm2 V−1 s−1, μ(3,3′-BCzPh) = 2.2 × 10−4 cm2 V−1 s−1, and μ(4,4′-BCzPh) = 1.7 × 10−6 cm2 V−1 s−1). Interestingly, the hole mobility values for the blended hole-only devices were lower than those of pure hole-only devices, suggesting that the addition of B3PyMPM influences the morphology of the EML, thereby reducing hole transport capabilities. In sharp contrast, the current density of the blended electron-only devices was significantly higher than that of the pure electron-only devices (Fig. 8(c) and (d)), demonstrating the enhanced electron transport ability achieved by incorporating the electron transport material into the EML. The current density in the blended electron-only devices followed the trend 2,2′-BCzPh > 4,4′-BCzPh > 1,1′-BCzPh > 3,3′-BCzPh, which was consistent with the electron mobility values at a field of 0.25 MV cm−1: (μ(1,1′-BCzPh) = 2.7 × 10−6 cm2 V−1 s−1, μ(2,2′-BCzPh) = 1.8 × 10−5 cm2 V−1 s−1, μ(3,3′-BCzPh) = 2.0 × 10−9 cm2 V−1 s−1, and μ(4,4′-BCzPh) = 1.2 × 10−5 cm2 V−1 s−1). Notably, the reported electron mobility of B3PyMPM is ca. 1.5 × 10−5 cm2 V−1 s−1, which aligns closely with the electron mobility of the blended devices, except for the 4,4′-BCzPh case. Clearly, the electron transport capability of the blended sample was significantly mitigated by 4,4′-BCzPh. Overall, these results indicate that the blended samples function as bipolar host systems, enabling easier-to-achieve carrier balance in the devices, improving their overall performance.
1 ratio, as shown in Fig. 8. The hole transport properties of the blended samples closely mirrored those of the pure synthesized compounds (cf.Fig. 7 and 8(a), (b)). The current density in the blended hole-only devices followed the trend 3,3′-BCzPh > 2,2′-BCzPh > 1,1′-BCzPh > 4,4′-BCzPh. This trend was also reflected in hole mobility values at an electric field of 0.25 MV cm−1, with values of (μ(1,1′-BCzPh) = 2.3 × 10−6 cm2 V−1 s−1, μ(2,2′-BCzPh) = 5.4 × 10−6 cm2 V−1 s−1, μ(3,3′-BCzPh) = 2.2 × 10−4 cm2 V−1 s−1, and μ(4,4′-BCzPh) = 1.7 × 10−6 cm2 V−1 s−1). Interestingly, the hole mobility values for the blended hole-only devices were lower than those of pure hole-only devices, suggesting that the addition of B3PyMPM influences the morphology of the EML, thereby reducing hole transport capabilities. In sharp contrast, the current density of the blended electron-only devices was significantly higher than that of the pure electron-only devices (Fig. 8(c) and (d)), demonstrating the enhanced electron transport ability achieved by incorporating the electron transport material into the EML. The current density in the blended electron-only devices followed the trend 2,2′-BCzPh > 4,4′-BCzPh > 1,1′-BCzPh > 3,3′-BCzPh, which was consistent with the electron mobility values at a field of 0.25 MV cm−1: (μ(1,1′-BCzPh) = 2.7 × 10−6 cm2 V−1 s−1, μ(2,2′-BCzPh) = 1.8 × 10−5 cm2 V−1 s−1, μ(3,3′-BCzPh) = 2.0 × 10−9 cm2 V−1 s−1, and μ(4,4′-BCzPh) = 1.2 × 10−5 cm2 V−1 s−1). Notably, the reported electron mobility of B3PyMPM is ca. 1.5 × 10−5 cm2 V−1 s−1, which aligns closely with the electron mobility of the blended devices, except for the 4,4′-BCzPh case. Clearly, the electron transport capability of the blended sample was significantly mitigated by 4,4′-BCzPh. Overall, these results indicate that the blended samples function as bipolar host systems, enabling easier-to-achieve carrier balance in the devices, improving their overall performance.
2.8. OLED device performance
To evaluate the performance of bicarbazole-based host materials in the EL devices, we incorporated the highly efficient green-emitting Ir(ppy)3 (ET = 2.4 eV) as the emitter. The selected BCzPh compounds possess only hole transport capabilities. Therefore, we designed an EML using a co-host system that blends two materials with complementary carrier transport properties. For the electron transport part of the EML, we chose B3PyMPM, a material with a wide triplet energy band gap and suitable electron transport capability. We constructed the EML of the devices by including the synthesized compounds and B3PyMPM in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (wt/wt) blending ratio. Furthermore, the HOMO energy levels of the synthesized BCzPh compounds align closely with those of 1,1-bis[(di-4-tolylamino)phenyl]cyclohexane (TAPC), thus obviating the need for a conventional step-wise HTL design in green-emitting devices.88 The energy barrier at the interface between HTL and EML is minimal, facilitating hole injection into the EML. On the electron transport side, to address the energy barrier between ETL and EML, B3PyMPM was selected for the ETL, positioned between the EML and the lithium fluoride (LiF)-electron injection layer (EIL). This straightforward configuration enables effective exciton confinement and efficient carrier transport within the device. Furthermore, considering the slightly different carrier transport capabilities and energy gaps of the target compounds, we carefully adjusted the device architectures and the thicknesses of each layer to optimize EL efficiency. Consequently, the green-emitting OLEDs were fabricated using the following architecture: indium tin oxide (ITO) (120 nm)/TAPC (30 nm)/host doped with 8 wt% Ir(ppy)3 (25 nm)/B3PyMPM (50 nm)/LiF (1.5 nm)/Al (150 nm), with ITO and aluminum, functioning as the anode and cathode, respectively. The host materials in the EML were assigned as follows: 1,1′-BCzPh
1 (wt/wt) blending ratio. Furthermore, the HOMO energy levels of the synthesized BCzPh compounds align closely with those of 1,1-bis[(di-4-tolylamino)phenyl]cyclohexane (TAPC), thus obviating the need for a conventional step-wise HTL design in green-emitting devices.88 The energy barrier at the interface between HTL and EML is minimal, facilitating hole injection into the EML. On the electron transport side, to address the energy barrier between ETL and EML, B3PyMPM was selected for the ETL, positioned between the EML and the lithium fluoride (LiF)-electron injection layer (EIL). This straightforward configuration enables effective exciton confinement and efficient carrier transport within the device. Furthermore, considering the slightly different carrier transport capabilities and energy gaps of the target compounds, we carefully adjusted the device architectures and the thicknesses of each layer to optimize EL efficiency. Consequently, the green-emitting OLEDs were fabricated using the following architecture: indium tin oxide (ITO) (120 nm)/TAPC (30 nm)/host doped with 8 wt% Ir(ppy)3 (25 nm)/B3PyMPM (50 nm)/LiF (1.5 nm)/Al (150 nm), with ITO and aluminum, functioning as the anode and cathode, respectively. The host materials in the EML were assigned as follows: 1,1′-BCzPh![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B3PyMPM (1
B3PyMPM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for Device A, 2,2′-BCzPh
1) for Device A, 2,2′-BCzPh![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B3PyMPM (1
B3PyMPM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for Device B, 3,3′-BCzPh
1) for Device B, 3,3′-BCzPh![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B3PyMPM (1
B3PyMPM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for Device C, and 4,4′-BCzPh
1) for Device C, and 4,4′-BCzPh![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B3PyMPM (1
B3PyMPM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for Device D. Fig. 9 provides the materials used, schematic architectures, and energy level diagrams of the fabricated green-emitting OLEDs.
1) for Device D. Fig. 9 provides the materials used, schematic architectures, and energy level diagrams of the fabricated green-emitting OLEDs.
Fig. 10 presents the EL characteristics of the devices, and Table 3 provides the corresponding numerical data. As shown in Fig. 10(a), the EL spectra of all devices, recorded at a luminance of 1000 cd m−2, exhibit pure Ir(ppy)3 emission. No emissions from the host or the carrier transport materials were detected, implying efficient energy transfer from the co-host to the guest in each green-emitting device, as well as effective exciton confinement within the EML.89 Moreover, variations in optical path differences among the examined devices arose from the diverse positions of exciton formation regions within the EML, leading to minor discrepancies in the spectral profiles that occur in the longer wavelength regions.90Fig. 10(b) presents the current density–voltage (J–V) curves for the devices. The current density of devices follows the trend A > D > C > B. For example, at an operating voltage of 6 V, the respective current density values for devices A, B, C, and D are 73.0, 1.5, 12.8, and 23.4 mA cm−2, respectively. This trend is inconsistent from the behavior observed in the blended carrier-only devices discussed earlier. Device A, employing a 1,1′-BCzPh:B3PyMPM EML, demonstrated the most balanced carrier transport mobility, favoring a balanced charge transport condition and minimizing the number of trapped carriers within the EML. However, the current densities of the other devices did not directly align with the carrier mobility results, likely due to the influence of additional emissive dopants within the EML. The luminance–voltage (L–V) characteristics of the devices are depicted in Fig. 10(c). All tested devices exhibited a low turn-on voltage of approximately 2.2–2.3 V. A similar turn-on voltage may be attributed to the combined effects of current density and efficiency across the devices. Notably, device C, featuring 3,3′-BCzPh, achieved an impressive maximum luminance exceeding 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2, highlighting its potential for diverse practical applications.
000 cd m−2, highlighting its potential for diverse practical applications.
| Device | A | B | C | D | |
|---|---|---|---|---|---|
BCzPh![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B3PyMPM (1 B3PyMPM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1,1′-BCzPh | 2,2′-BCzPh | 3,3′-BCzPh | 4,4′-BCzPh | |
| a Maximum efficiency. b Recorded at 102 cd m−2. c Turn-on voltage measurement at 1 cd m−2. d Recorded at 103 cd m−2. | |||||
| External quantum efficiency (%) | 20.1 | 23.4 | 20.1 | 23.9 | |
| 19.9 | 22.9 | 20.0 | 22.7 | ||
| Luminance efficiency (cd A−1) | 70.5 | 81.0 | 69.6 | 83.1 | |
| 69.8 | 79.4 | 69.1 | 79.0 | ||
| Power efficiency (lm W−1) | 91.9 | 108.6 | 82.1 | 108.8 | |
| 81.9 | 66.5 | 74.1 | 87.6 | ||
| V on (V) | 2.22 | 2.22 | 2.23 | 2.25 | |
| λ max (nm) | 516.5 | 519.0 | 520.0 | 513.0 | |
| Max. luminance (cd m−2) [V] | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 953 [9.4] 953 [9.4] |
92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 593 [11.8] 593 [11.8] |
203![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 494 [10.6] 494 [10.6] |
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 158 [10.4] 158 [10.4] |
|
| CIE 1931 coordinates (x,y) | (0.34, 0.60) | (0.36, 0.60) | (0.35, 0.60) | (0.34, 0.61) | |
| (0.34, 0.60) | (0.35, 0.60) | (0.35, 0.60) | (0.33, 0.61) | ||
Fig. 10(d)–(f) respectively show the external quantum efficiency, luminance efficiency, and power efficiency versus luminance for the tested devices (A–D). The corresponding maximum efficiencies of devices A, B, C, and D reached 20.1% (70.5 cd A−1 and 91.9 lm W−1), 23.4% (81.0 cd A−1 and 108.6 lm W−1), 20.1% (69.6 cd A−1 and 82.1 lm W−1), and 23.9% (83.1 cd A−1 and 108.8 lm W−1). Essentially, devices using host materials based on BCzPh demonstrated a maximum efficiency surpassing 20% (Table 3), suggesting the ease of achieving carrier balance despite differing carrier transport capabilities. Devices B and D, in particular, exhibited superior efficiency, indicating that both 2,2′-BCzPh and 4,4′-BCzPh with B3PyMPM effectively create superior carrier balance conditions in the EML. At a higher practical luminance of 100 cd m−2, the efficiency of device B remained at 22.9% (79.4 cd A−1 and 66.5 lm W−1), while device D sustained an efficiency of 22.7% (79.0 cd A−1 and 87.6 lm W−1). Thus, the EQE values of devices B and D showed efficiency drops of 2.1% and 5.0% from their respective peak values compared to those recorded at 100 cd m−2. This indicates that the mitigated efficiency roll-off can be attributed to the extended exciton formation zone and stable carrier balance in these co-host systems, which minimize triplet–triplet annihilation (TTA).91 In general, incorporating 2,2′-BCzPh and 4,4′-BCzPh (devices B and D, respectively) with B3PyMPM as the co-host EML yielded strong EL performance, underscoring the effectiveness of our molecular structure and device designs for achieving efficient green PhOLEDs. It is important to note that 2,2′-BCzPh, 1,1′-BCzPh, and 4,4′-BCzPh have not been investigated as hosts in green PhOLEDs, in contrast to the well-known 3,3′-BCzPh. The performance observed here for regioisomeric BCzPh-based unipolar host materials are exemplary when compared to those already reported (see Table S10 for comparison, page S31, ESI†). For instance, the hosts 3,3′-BCzPh and mCP with dopant emitter FIrpic produced blue-light emission with maximum external efficiencies of 16.4 and 13.8%, respectively.63 Pure-hydrocarbon hosts 1,1′-(SBF)2 and 3,3′-(SBF)2 with FIrpic emitter yielded blue emitting PhOLED with external quantum efficiencies 20.1% and 11.4%, respectively.9,92,93 Bipolar host material CBP with Ir(ppy)3 and BCzs with Ir(ppy)2(acac), resulted in green emission with external quantum efficiencies 17.10%81 and 14.6–16.8%,70 respectively. Correlating the twist angle with electronic, carrier transport and device performance characteristics (Fig. S12, ESI†) clearly reveals that the extended π-conjugation in 3,3′-BCzPh contributes to improved hole mobility and outstanding luminance. Meanwhile, connectivity- or steric-inhibited resonance in 2,2′-BCzPh and 4,4′-BCzPh yield excellent luminance, power, and external quantum efficiencies. If one looks at higher brightness levels of 5000 cd m−2, the efficiency roll-off behavior showed a difference. The efficiency roll-off values evaluated from the peak to the luminance of 5000 cd m−2 are as follows: 29.9% for device A, 28.1% for device B, 7.5% for device C, and 36.9% for device D. The significantly reduced efficiency roll-off for device C (with 3,3′-BCzPh) illustrates the advantage of balanced carrier transport, relaxing exciton quenching. The notably higher maximum luminance in device C further demonstrates superior carrier transport capabilities of 3,3′-BCzPh. This design insight is crucial for developing successful next-generation molecular materials.
3. Conclusions
In conclusion, we successfully synthesized regioisomeric bicarbazoles (BCzPhs) with varying degrees of twist using oxidative/transition-metal catalyzed Suzuki coupling methods. The connectivity differences between the isomers imparted significant structural modifications that can be correlated to variations in photophysical, thermal, and electrochemical properties, as well as in device performance data. Resonance and conjugation effects operate in BCzs mutually contribute to altering the triplet energy levels. Notably, increasing the twist angle between the carbazole planes resulted in larger singlet–triplet/HOMO–LUMO energy gaps, leading to improved power and luminance efficiencies. Despite these differences, external quantum efficiencies were comparable across devices (A and C, and B and D), with devices B and D with BCzPh having meta–meta nitrogens realizing outstanding external quantum efficiencies up to 23.9%. Device C exhibited exceptional maximum brightness, followed closely by device A, both with BCzPh having para–para nitrogens. These results suggest that connectivity differences play a pivotal role in determining device performance. All BCzPh compounds demonstrated excellent performance as host materials, delivering stable, high-purity green emission in devices that turned on at voltages as low as 2.2–2.3 V.4. Experimental section
All detailed experimental procedures for the synthesis, OLED device fabrication and characterization of this work are in the ESI.†Data availability
The data (detailed synthetic procedure and characterization, structural characterization details, theoretical calculations, spectroscopic, electrochemical and thermal, and device fabrication and performance details) supporting this article is available in ESI.† Crystallographic data for 1,1′-BCzPh (CCDC no. 2361782), 2,2′-BCzPh (CCDC no. 2361784), and 4,4′-BCzPh (CCDC no. 2361783) can be obtained from ESI.†Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
PVK gratefully acknowledges IIT Madras for the generous funding support through IoE-EC-ACESC (SP22231245CPMOEXEGYHOC), Ministry of Education, Government of India. KK and SM wishes to thank IIT Madras for a senior research fellowship (SRF). We thank Department of Chemistry, Indian Institute of Technology Madras for the infrastructural facility. This work was financially supported by the National Science and Technology Council of Taiwan (NSTC 111-2221-E-155-012-MY2, NSTC 112-2923-E-155-002-MY4, NSTC 112-2113-M-029-004, NSTC 113-2113-M-029-001, and NSTC 113-2113-M-001-005). Institute of Chemistry, Academia Sinica, Yuan Ze University and Tunghai University are gratefully acknowledged.References
- C. Adachi, M. A. Baldo, M. E. Thompson and S. R. Forrest, J. Appl. Phys., 2001, 90, 5048–5051 CrossRef CAS.
- Q. Wang, J. Ding, D. Ma, Y. Cheng, L. Wang, X. Jing and F. Wang, Adv. Funct. Mater., 2009, 19, 84–95 Search PubMed.
- L. Xiao, Z. Chen, B. Qu, J. Luo, S. Kong, Q. Gong and J. Kido, Adv. Mater., 2011, 23, 926–952 CrossRef CAS PubMed.
- D. Blazevicius and S. Grigalevicius, Nanomaterials, 2024, 14, 356 CrossRef CAS PubMed.
- G. Fan and D. Yan, Sci. Rep., 2014, 4, 1–8 Search PubMed.
- C. Poriel and J. Rault-Berthelot, Acc. Chem. Res., 2018, 51, 1818–1830 CrossRef CAS PubMed.
- C. Poriel, L. Sicard and J. Rault-Berthelot, Chem. Commun., 2019, 55, 14238–14254 RSC.
- P. Ledwon, Org. Electron., 2019, 75, 105422 CrossRef CAS.
- C. Poriel, C. Quinton, F. Lucas, J. Rault-Berthelot, Z.-Q. Jiang and O. Jeannin, Adv. Funct. Mater., 2021, 31, 2104980 CrossRef CAS.
- Y. Xiong, J. Gong, J. Liu, D. Wang, H. Wu, Z. Zhao, M. Fang, Z. Li, D. Wang and B. Z. Tang, J. Mater. Chem. C, 2022, 10, 10009–10016 RSC.
- G. Yuan, Y. Z. Yang, D. H. Wang, F. M. Xie, Q. Zhang, Y. Q. Li, Y. Y. Hu, J. X. Tang and X. Zhao, Dyes Pigm., 2024, 224, 111981 CrossRef CAS.
- Z. Wu, Z. Xu, D. Wang, G. Deng, S. Lin, Z. Zhao, D. Wang, Y. Xiong and B. Z. Tang, Adv. Funct. Mater., 2025, 35, 2415285 CrossRef CAS.
- D. Thakur, M. R. Nagar, A. Tomar, D. K. Dubey, S. Kumar, S. S. Swayamprabha, S. Banik, J. H. Jou and S. Ghosh, ACS Appl. Electron. Mater., 2021, 3, 2317–2332 CrossRef CAS.
- C. Brouillac, J. Rault-Berthelot, C. Quinton, C. Poriel, F.-C. Kong and Z.-Q. Jiang, Adv. Mater. Technol., 2023, 8, 2300763 CrossRef CAS.
- R. Wang, Y. Zhu, D. Hu, J. Hu, S. Wei Chen, J. Lin, L. Xing, Y. Huo and S. Ji, Chem. Eng. J., 2025, 506, 160269 CrossRef CAS.
- M. Chen, Y. Chen, T. Zhang, H. Zhang, Z. Xiao, Z. Su and Y. Wu, RSC Adv., 2024, 14, 32221–32228 RSC.
- Y. Liu, J. Yang, Z. Mao, Y. Wang, J. Zhao, S.-J. Su and Z. Chi, Chem. Sci., 2023, 14, 1551–1556 RSC.
- A. A. Sutanto, V. Joseph, C. Igci, O. A. Syzgantseva, M. A. Syzgantseva, V. Jankauskas, K. Rakstys, V. I. E. Queloz, P.-Y. Huang, J.-S. Ni, S. Kinge, A. M. Asiri, M.-C. Chen and M. K. Nazeeruddin, Chem. Mater., 2021, 33, 3286–3296 CrossRef CAS.
- X. Zhang, T. Xu, Z. Tian, X. He, S. Zhang, L. Ai, W. Zhang, S. Liu and W. Song, Chem. Commun., 2023, 59, 5874–5877 RSC.
- J. W. Chung, Y. You, H. S. Huh, B.-K. An, S.-J. Yoon, S. H. Kim, S. W. Lee and S. Y. Park, J. Am. Chem. Soc., 2009, 131(23), 8163–8172 CrossRef CAS PubMed.
- G. Fan and D. Yan, Sci. Rep., 2014, 4, 4933 CrossRef CAS PubMed.
- A. V. Monika, M. K. Tiwari, B. Show and S. Saha, ACS Omega, 2020, 5, 448 CrossRef CAS PubMed.
- H. Yi, X. Qin, L. Zhai, H. Duan, H. Chen, Y. Zuo, X. Lian, K. Tian, J. Zhang, Z. Liu and P. Xu, Precis. Chem., 2023, 1, 548–554 CrossRef CAS.
- Y. Xiong, Z. Zhao, W. Zhao, H. Ma, Q. Peng, Z. He, X. Zhang, Y. Chen, X. He, J. W. Y. Lam and B. Z. Tang, Angew. Chem., Int. Ed., 2018, 57, 7997–8001 CrossRef CAS PubMed.
- C. Chen, Z. Chi, K. C. Chong, A. S. Batsanov, Z. Yang, Z. Mao, Z. Yang and B. Liu, Nat. Mater., 2021, 20, 175–180 CrossRef CAS PubMed.
- K. Zheng, X. Yang, F. Ni, Z. Chen, C. Zhong and C. Yang, Chem. Eng. J., 2021, 408, 12730 CrossRef.
- R. Wang, C. Wu, J. Qi, W. Shen, F. Wu, M. Li, R. He, X. Liu, R. Wang, C. Wu, J. Qi, W. Shen, M. Li, R. He, X. Liu and F. Wu, Adv. Funct. Mater., 2023, 33, 2213843 CrossRef CAS.
- L. Sicard, C. Quinton, J.-D. Peltier, D. Tondelier, B. Geffroy, U. Biapo, R. Métivier, O. Jeannin, J. Rault-Berthelot and C. Poriel, Chem. – Eur. J., 2017, 23, 7719–7727 CrossRef CAS PubMed.
- C. Poriel and J. Rault-Berthelot, Chem. Soc. Rev., 2023, 52, 6754–6805 RSC.
- N. Blouin and M. Leclerc, Acc. Chem. Res., 2008, 41, 1110–1119 CrossRef CAS PubMed.
- Z. Xu, D. Wu, C. Fang and Y. Li, Des. Monomers Polym., 2023, 26, 90–105 CrossRef CAS PubMed.
- N. Chopra, J. Lee, Y. Zheng, S.-H. Eom, J. Xue and F. So, ACS Appl. Mater. Interfaces, 2009, 1, 1169–1172 CrossRef CAS PubMed.
- S. O. Jeon, K. S. Yook, C. W. Joo and J. Y. Lee, Adv. Mater., 2010, 22, 1872–1876 CrossRef CAS PubMed.
- S. J. Lee, J. S. Park, K.-J. Yoon, Y.-I. Kim, S.-H. Jin, S. K. Kang, Y.-S. Gal, S. Kang, J. Y. Lee, J.-W. Kang, S.-H. Lee, H.-D. Park and J.-J. Kim, Adv. Funct. Mater., 2008, 18, 3922–3930 CrossRef CAS.
- Y. J. Cho, K. S. Yook and J. Y. Lee, Adv. Mater., 2014, 26, 4050–4055 CrossRef CAS PubMed.
- M. H. Tsai, H. W. Lin, H. C. Su, T. H. Ke, C. C. Wu, F. C. Fang, Y. L. Liao, K. T. Wong and C. I. Wu, Adv. Mater., 2006, 18, 1216–1220 CrossRef CAS.
- R. J. Holmes, S. R. Forrest, Y. J. Tung, R. C. Kwong, J. J. Brown, S. Garon and M. E. Thompson, Appl. Phys. Lett., 2003, 82, 2422–2424 CrossRef CAS.
- K. L. Woon, Z. A. Hasan, B. K. Ong, A. Ariffin, R. Griniene, S. Grigalevicius and S.-A. Chen, RSC Adv., 2015, 5, 59960–59969 RSC.
- K. S. Yook and J. Y. Lee, Adv. Mater., 2012, 24, 3169–3190 CrossRef CAS PubMed.
- N. Thejo Kalyani and S. J. Dhoble, Renewable Sustainable Energy Rev., 2015, 44, 319–347 CrossRef.
- Y. Wang, J. H. Yun, L. Wang and J. Y. Lee, Adv. Funct. Mater., 2021, 31, 2008332 CrossRef CAS.
- S. Oner and M. R. Bryce, Mater. Chem. Front., 2023, 7, 4304–4338 RSC.
- S. Kasemthaveechok, L. Abella, M. Jean, M. Cordier, N. Van-thuyne, T. Guizouarn, O. Cador, J. Autschbach, J. Crassous and L. Favereau, J. Am. Chem. Soc., 2022, 144, 7253–7263 CrossRef CAS PubMed.
- G. Krucaite and S. Grigalevicius, Synth. Met., 2019, 247, 90–108 CrossRef CAS.
- K. Brunner, A. van Dijken, H. Börner, J. J. A. M. Bastiaansen, N. M. M. Kiggen and B. M. W. Langeveld, J. Am. Chem. Soc., 2004, 126, 6035–6042 CrossRef CAS PubMed.
- D. Li, J. Li, D. Liu, W. Li, C.-L. Ko, W.-Y. Hung and C. Duan, ACS Appl. Mater. Interfaces, 2021, 13, 13459–13469 CrossRef CAS PubMed.
- S.-i Kato, H. Noguchi, A. Kobayashi, T. Yoshihara, S. Tobita and Y. Nakamura, J. Org. Chem., 2012, 77, 9120–9133 CrossRef CAS PubMed.
- C. Jiang, X. Huang, B. Sun, Y. Li, M. Gao, L. Ye, H. Ade, S. R. Forrest and J. Fan, Org. Electron., 2020, 84, 105784 CrossRef CAS.
- S.-O. Jeon, K. S. Yook, C. W. Joo, J. Y. Lee, K.-Y. Ko, J.-Y. Park and Y. G. Baek, Appl. Phys. Lett., 2008, 93, 063306 CrossRef.
- M. A. Baldo, S. Lamansky, P. E. Burrows, M. E. Thompson and S. R. Forrest, Appl. Phys. Lett., 1999, 75, 4–6 CrossRef CAS.
- M. A. Baldo and S. R. Forrest, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 62, 10958–10966 CrossRef CAS.
- C. Adachi, R. C. Kwong, P. Djurovich, V. Adamovich, M. A. Baldo, M. E. Thompson and S. R. Forrest, Appl. Phys. Lett., 2001, 79, 2082–2084 CrossRef CAS.
- H. Mu, Y. Jiang and H. Xie, Org. Electron., 2019, 66, 195–205 CrossRef CAS.
- Y. H. Jeong, C. W. Joo, H. Jeong, J. Lee and Y.-H. Kim, J. Inf. Disp., 2022, 23, 273–279 CrossRef CAS.
- D. Sun, X. Zhou, J. Liu, X. Sun, H. Li, Z. Ren, D. Ma, M. R. Bryce and S. Yan, ACS Appl. Mater. Interfaces, 2015, 7, 27989–27998 CrossRef CAS PubMed.
- W. Jiang, L. Duan, J. Qiao, D. Zhang, G. Dong, L. Wang and Y. Qiu, J. Mater. Chem., 2010, 20, 6131–6137 RSC.
- J. S. Swensen, E. Polikarpov, A. Von Ruden, L. Wang, L. S. Sapochak and A. B. Padmaperuma, Adv. Funct. Mater., 2011, 21, 3250–3258 CrossRef CAS.
- T. Tsuboi, H. Murayama, S.-J. Yeh, M.-F. Wu and C.-T. Chen, Opt. Mater., 2008, 31, 366–371 CrossRef CAS.
- F.-M. Hsu, C.-H. Chien, P.-I. Shih and C.-F. Shu, Chem. Mater., 2009, 21, 1017–1022 CrossRef CAS.
- C.-H. Chang, Z.-J. Wu, C.-H. Chiu, Y.-H. Liang, Y.-S. Tsai, J.-L. Liao, Y. Chi, H.-Y. Hsieh, T.-Y. Kuo, G.-H. Lee, H.-A. Pan, P.-T. Chou, J.-S. Lin and M.-R. Tseng, ACS Appl. Mater. Interfaces, 2013, 5, 7341–7351 CrossRef CAS PubMed.
- O. Bezvikonnyi, G. Grybauskaite-Kaminskiene, D. Volyniuk, J. Simokaitiene, Y. Danyliv, R. Durgaryan, A. Bucinskas, E. Jatautienė, I. Hladka, J. Scholz, H. Starykov and J. V. Grazulevicius, Mater. Sci. Eng., B, 2020, 261, 114662 CrossRef CAS.
- M. Kim and J. Y. Lee, ACS Appl. Mater. Interfaces, 2014, 6, 14874–14880 CrossRef CAS PubMed.
- H. Sasabe, N. Toyota, H. Nakanishi, T. Ishizaka, Y.-J. Pu and J. Kido, Adv. Mater., 2012, 24, 3212–3217 CrossRef CAS PubMed.
- L.-S. Cui, Y. Liu, X.-D. Yuan, Q. Li, Z.-Q. Jiang and L.-S. Liao, J. Mater. Chem. C, 2013, 1, 8177–8185 RSC.
- M. Kim and J. Y. Lee, Org. Electron., 2013, 14, 67–73 CrossRef CAS.
- S. M. Kim, S. Y. Byeon, S. H. Hwang and J. Y. Lee, Chem. Commun., 2015, 51, 10672–10675 RSC.
- J. Hwang, J. Yoon, C. Y. Kim, S. Choi, H. Kang, J. Y. Kim, D.-W. Yoon, C. W. Han, S. Park, M. J. Cho and D. H. Choi, Dyes Pigm., 2020, 179, 108403 CrossRef CAS.
- H.-G. Jang, B. S. Kim, J. Y. Lee and S.-H. Hwang, Dalton Trans., 2014, 43, 7712–7715 RSC.
- Y.-J. Na, W. Song, J. Y. Lee and S.-H. Hwang, Dalton Trans., 2015, 44, 8360–8363 RSC.
- X.-Y. Liu, Y.-L. Zhang, X. Fei, L.-S. Liao and J. Fan, Chem. – Eur. J., 2019, 25, 4501–4508 CrossRef CAS PubMed.
- D. Zhou, B. Zhang, Z. Yu, Q. Liao and H. Fu, New J. Chem., 2020, 44, 10472–10478 RSC.
- S. Kang, H. Jung, H. Lee, S. Lee, M. Jung, J. Lee, Y. C. Kim and J. Park, Dyes Pigm., 2018, 156, 369–378 CrossRef CAS.
- P. Pandit, T. Nakamura and S. Higashibayashi, Chem. Lett., 2015, 44, 1336–1338 CrossRef CAS.
- S. Mallick, S. Maddala, K. Kollimalayan and P. Venkatakrishnan, J. Org. Chem., 2019, 84, 73–93 CrossRef CAS PubMed.
- S. Maddala, C.-L. Chung, S.-Y. Wang, K. Kollimalayan, H.-L. Hsu, P. Venkatakrishnan, C.-P. Chen and Y. J. Chang, Chem. Mater., 2020, 32, 127–138 CrossRef CAS.
- N. Miyaura, K. Yamada and A. Suzuki, Tetrahedron Lett., 1979, 20, 3437–3440 CrossRef.
- C.-L. Ho, L.-C. Chi, W.-Y. Hung, W.-J. Chen, Y.-C. Lin, H. Wu, E. Mondal, G.-J. Zhou, K.-T. Wong and W.-Y. Wong, J. Mater. Chem., 2012, 22, 215–224 RSC.
- J. Yuan, L. Jin, R. Chen, X. Tang, X. Xie, Y. Tang and W. Huang, New J. Chem., 2018, 42, 14704–14708 RSC.
- W. Li, J. Li, D. Liu, F. Wang and S. Zhang, J. Mater. Chem. C, 2015, 3, 12529–12538 RSC.
- Z. He, C. Wang, J. Zhao, X. Du, H. Yang, P. Zhong, C. Zheng, H. Lin, S. Tao and X. Zhang, J. Mater. Chem. C, 2019, 7, 11806–11812 RSC.
- T. Zhang, Y. Liang, J. Cheng and J. Li, J. Mater. Chem. C, 2013, 1, 757–764 RSC.
- J. He, H. Liu, Y. Dai, X. Ou, J. Wang, S. Tao, X. Zhang, P. Wang and D. Ma, J. Phys. Chem. C, 2009, 113, 6761 CrossRef CAS.
- M.-H. Tsai, Y.-H. Hong, C.-H. Chang, H.-C. Su, C.-C. Wu, A. Matoliukstyte, J. Simokaitiene, S. Grigalevicius, J. V. Grazulevicius and C.-P. Hsu, Adv. Mater., 2007, 19, 862 CrossRef CAS.
- C. Chen, K. C. Chong, Y. Pan, G. Qi, S. Xu and B. Liu, ACS Mater. Lett., 2021, 3, 1081–1087 CrossRef CAS.
- Y. H. Lin, W.-H. Lin, Y.-S. Huang, C.-H. Wu, P. Gnanasekaran, Y.-M. Chang, S.-W. Teng, C.-W. Lu, C.-H. Chang and Y. J. Chang, J. Mater. Chem. C, 2023, 11, 3101–3111 RSC.
- S. K. So, S. C. Tse and K. L. Tong, J. Disp. Technol., 2007, 3, 225–232 CAS.
- S. Grigalevicius, D. Tavgeniene, G. Krucaite, R. Griniene, W.-C. Li, D. Luo and C.-H. Chang, Dyes Pigm., 2018, 152, 100–104 CrossRef CAS.
- Y.-J. Lien, T.-C. Lin, C.-C. Yang, Y.-C. Chiang, C.-H. Chang, S.-H. Liu, Y.-T. Chen, G.-H. Lee, P.-T. Chou, C.-W. Lu and Y. Chi, ACS Appl. Mater. Interfaces, 2017, 9, 27090–27101 CrossRef CAS PubMed.
- J.-M. Wang, T.-C. Lee, C.-C. Chung, W.-Y. Chen, S.-Y. Wu, Y.-D. Lin, Y. J. Chang, C.-W. Lu and C.-H. Chang, Chem. Eng. J., 2023, 472, 145023 CrossRef CAS.
- C.-W. Lu, C.-C. Tsai, Y.-S. Huang, H.-Y. Chih, W.-C. Li, T.-Y. Chiu and C.-H. Chang, Dyes Pigm., 2019, 163, 145–152 CrossRef CAS.
- G. Ganesan, P. Balasubramaniam, J. Lade, K.-H. Wang, K.-W. Chen, A. C. Chaskar and C.-H. Chang, ACS Appl. Opt. Mater., 2023, 1, 1546–1558 CrossRef CAS.
- L.-S. Cui, Y.-M. Xie, Y.-K. Wang, C. Zhong, Y.-L. Deng, X.-Y. Liu, Z.-Q. Jiang and L.-S. Liao, Adv. Mater., 2015, 27, 4213–4217 CrossRef CAS PubMed.
- L. J. Sicard, H.-C. Li, Q. Wang, X.-Y. Liu, O. Jeannin, J. Rault-Berthelot, L.-S. Liao, Z.-Q. Jiang and C. Poriel, Angew. Chem., Int. Ed., 2019, 58, 3848–3853 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2361782–2361784. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ma01003e |
| ‡ Equal contribution first author. |
| This journal is © The Royal Society of Chemistry 2025 |






