 Open Access Article
Open Access ArticleMn-substitution effects on the magnetic and zero-field ferromagnetic resonance properties of ε-Fe2O3 nanoparticles†
Jessica
MacDougall
 a,
Asuka
Namai
a,
Asuka
Namai
 *a,
Onno
Strolka‡
*a,
Onno
Strolka‡
 a and
Shin-ichi
Ohkoshi
a and
Shin-ichi
Ohkoshi
 *ab
*ab
aDepartment of Chemistry, School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. E-mail: Asuka@chem.s.u-tokyo.ac.jp; Ohkoshi@chem.s.u-tokyo.ac.jp
bCNRS International Research Laboratory DYNACOM, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan
First published on 18th December 2024
Abstract
Metal substitution is an important way to tune the magnetic properties of ferrites. In the present study, to investigate the effects of Mn substitution on the magnetic properties and millimeter wave absorption properties on ε-Fe2O3 for the first time, Mn-substituted epsilon iron oxides, ε-MnxFe2−xO3−x/2 (x = 0 (Mn0), 0.10 (Mn1), and 0.20 (Mn2)) were synthesized by sintering iron oxide hydroxide with manganese hydroxide in a silica matrix. Transmission electron microscopy shows particle sizes of 18.7 ± 5.8 nm (Mn0), 19.0 ± 6.2 nm (Mn1), and 19.8 ± 6.7 nm (Mn2). Energy dispersive X-ray spectroscopy confirms a uniform manganese distribution across all particles, while the powder X-ray diffraction patterns demonstrate that ε-MnxFe2−xO3−x/2 has an orthorhombic crystal structure with a space group of Pna21 (e.g., the lattice constants in Mn2 are a = 5.1031(4) Å, b = 8.7759(8) Å, and c = 9.4661(7) Å). As the Mn substitution ratio increases, the Curie temperature decreases from 487 K (Mn0) to 469 K (Mn2). As for the magnetic properties at 300 K, the coercive field increases from 17.2 kOe (Mn0) to 18.2 kOe (Mn2), while the saturation magnetisation decreases from 17.1 emu g−1 (Mn0) to 13.9 emu g−1 (Mn2), with increasing substitution ratio. Terahertz time-domain spectroscopy demonstrates that the samples exhibit electromagnetic wave absorption in the millimetre-wave region, due to zero-field ferromagnetic resonance. As the Mn substitution ratio increases, the resonance frequency increases from 174 GHz (Mn0) to 182 GHz (Mn1) and 187 GHz (Mn2). Due to the substitution of Fe3+ with Mn2+, the saturation magnetisation decreases and the coercive field and the resonance frequency increase.
Introduction
The demand for materials capable of absorbing electromagnetic waves has escalated due to recent advances in telecommunication technologies. As millimetre waves (30–300 GHz) become more common in applications such as high-speed wireless communications and radar systems, the development of efficient millimetre-wave absorbing materials is necessary to mitigate noise and interference.1–6 Electromagnetic wave absorbing materials are categorized by their absorbance mechanism, such as magnetic loss (e.g., spinel ferrites, barium ferrites) or dielectric loss. Several examples of absorbing materials in the millimetre-wave region have been reported.7–10 Amongst them, Epsilon iron(III) oxide (ε-Fe2O3) shows millimetre-wave absorption at a high frequency of 182 GHz due to its zero-field ferromagnetic resonance.11–17 Furthermore, ε-Fe2O3 exhibits a high coercivity, which is retained even with single-digit-diameters.18–20 Due to its unique functionalities, various other applications in addition to millimetre-wave absorption are anticipated to use ε-Fe2O3 such as magnetic recording,21–23 ferroelectric devices,24–28 magnetic hyperthermia,29,30 MRI contrast,31 Li-ion batteries,32 heavy metal ion detection,33 and photocatalytic applications.34 Moreover, ε-Fe2O3 was recently discovered on the surface of ancient glazed pottery, reportedly playing an important role as a pigment.35–42 Therefore, ε-Fe2O3 has been attracting attention, and various synthesis methods have been investigated.43–56Metal substitution can tailor the intrinsic properties of ε-Fe2O3. To date, ε-Fe2O3 has been substituted with gallium,14,57,58 aluminum,12,59–61 chromium,62,63 rhodium,13,64,65 ruthenium,66,67 indium,68,69 and scandium70 alongside co-substitution with titanium and cobalt.16,23,71 This approach can attune the coercive field and zero-field ferromagnetic resonance. So far, only rhodium and ruthenium substitution have been reported to increase magnetic anisotropy. In the present study, we investigated the effect of manganese substitution, which has not been explored previously. In this paper, we report the synthesis, crystal structure, magnetic properties, and millimetre-wave absorption properties of Mn-substituted ε-Fe2O3.
Results and discussion
Materials
Fig. 1 shows the synthetic scheme. Varying the feed ratio (=[Mn]/[Mn + Fe]) produced three samples: 0 (Mn0), 0.05 (Mn1), and 0.10 (Mn2). Iron oxy-hydroxide (β-FeO(OH)) nanoparticle dispersions and Mn(NO3)2 were added to 0.420 dm3 water. The molar amounts of Fe (nFe) in β-FeO(OH) and Mn (nMn) in Mn(NO3)2 were (nFe, nMn) = (10.0, 0 mmol) for Mn0, (9.52, 0.51 mmol) for Mn1, and (9.02, 1.01 mmol) for Mn2. While stirring the solution at 50 °C, aqueous ammonia (25%, 0.0192 dm3) was slowly added dropwise. Then the reaction mixture was stirred for an additional 30 minutes. Afterwards, tetraethyl orthosilicate (0.024 dm3) was added dropwise. Subsequent stirring at 50 °C for 20 hours yielded a colloidal solution. The addition of ammonium sulphate (∼10 g per 0.200 dm3) precipitated the product.The precipitated product was collected, washed by centrifugation, and dried at 60 °C. Next, the precipitate was ground into a fine powder and sintered in air for 4 hours at 1102 °C. The silica matrix was etched using a 5 mol dm−3 NaOH aqueous solution at 65 °C. Afterwards, the samples were collected by centrifugation, washed with water, and dried, providing a red-brown powder. Elemental analysis was performed with X-ray fluorescence spectroscopy (XRF). Table 1 shows that the observed [Mn]/[Mn + Fe] ratios were the consistent with the feed ratios (i.e., 0 for Mn0, 0.05 for Mn1, and 0.10 for Mn2).
| Sample | Feed [Mn]/[Mn + Fe] | Observed [Mn]/[Mn + Fe] |
|---|---|---|
| Mn0 | 0 | 0 |
| Mn1 | 0.05 | 0.05 |
| Mn2 | 0.10 | 0.10 |
Transmission electron microscopy (TEM) images were acquired using a JEM-1011 (JEOL). Fig. 2a shows the TEM images. The samples consisted of nanoparticles with sizes of 18.7 ± 5.8 nm (Mn0), 19.0 ± 6.2 nm (Mn1), and 19.8 ± 6.7 nm (Mn2). Scanning transmission electron microscopy with electron dispersive spectroscopy (STEM-EDS) images were taken with a Thermal ARM-200F. Fig. 2b shows the STEM-EDS images for Mn1. The distribution of Mn ions was consistent across all the particles, indicating that Mn was uniformly substituted into the iron oxide structure. Quantitative analysis of individual particles showed that the average [Mn]/[Mn + Fe] in the sample was 0.05 for Mn1. This value corresponds to the XRF results (Fig. S1, ESI†).
Crystal structure and composition analysis
Powder X-ray diffraction (PXRD) measurements were conducted using a Rigaku Ultima IV with Cu Kα = 1.5418 Å radiation, and Rietveld analyses were performed using Rigaku PDXL2 software. Fig. 3 shows the PXRD patterns of each sample. Mn0 consisted of ε-Fe2O3 (96%, orthorhombic, Pna21 space group) with lattice constants of a = 5.0907(4) Å, b = 8.7922(8) Å, c = 9.4764(5) Å, and V = 424.15(6) Å3 and a small impurity of α-Fe2O3 (4%, hexagonal, R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c space group). Mn1 was very similar to Mn0 as it was mostly ε-phase (94%) and α-phase (6%). However, there was a small amount of spinel-phase (1%, cubic, Fd
c space group). Mn1 was very similar to Mn0 as it was mostly ε-phase (94%) and α-phase (6%). However, there was a small amount of spinel-phase (1%, cubic, Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group). The major phase of Mn2 was also ε-phase (75%) along with smaller amounts of spinel-phase (14%), α-phase (4%), and β-phase (7%, cubic, Ia
m space group). The major phase of Mn2 was also ε-phase (75%) along with smaller amounts of spinel-phase (14%), α-phase (4%), and β-phase (7%, cubic, Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space group). The phase diagram is shown in Fig. S2 (ESI†).
space group). The phase diagram is shown in Fig. S2 (ESI†).
The lattice constants changed monotonically with manganese substitution (Fig. 4a and Table S1, ESI†). In the ε-phase, a increased from a = 5.0907(4) Å (Mn0) to 5.1031(4) Å (Mn2), whereas both b and c decreased from b = 8.7922(8) Å (Mn0) to 8.7759(8) Å (Mn2) and c = 9.4764(5) Å (Mn0) to 9.4661(7) Å (Mn2). Since the lattice volume was reduced (i.e., from V = 424.15(6) Å3 (Mn0) to 423.93(6) Å3 (Mn2)), the ε-phase showed an anisotropic contraction. X-ray photoelectron spectroscopy (XPS) measurements indicated that all samples had a Mn 2p peak at 641 eV. This peak was assigned to Mn2+.72 The change in the lattice constants of the unit cell can be considered as follows: replacing trivalent Fe3+ ions (ionic radius = 0.645 Å) with divalent Mn2+ (ionic radius = 0.830 Å) introduced oxygen vacancies into the structure, which anisotropically affected the lattice constants.73 There are several examples of hematite displaying such a volume contraction.74–76
The chemical compositions and corresponding oxygen vacancies for the Mn2+-substituted iron oxides were determined by charge balance considerations, assuming that each phase contained the same ratio of Mn2+ cations. The estimated compositions of each phase were ε-Fe2O3 (96%) and α-Fe2O3 (4%) for Mn0, ε-Mn0.10Fe1.90O2.95 (94%), α-Mn0.10Fe1.90O2.95 (6%), and spinel-Mn0.15Fe2.85O4 (1%) for Mn1, and ε-Mn0.20Fe1.80O2.90 (75%), α-Mn0.20Fe1.80O2.90 (4%), β-Mn0.20Fe1.80O2.90 (7%), and spinel-Mn0.30Fe2.70O4 (14%) for Mn2.
Fig. 4b shows the crystal structure of the ε-MnxFe2−xO3−x/2. The structure has four non-equivalent metal sites (i.e., two distorted octahedral sites (A and B sites)), one regular octahedral site (C site), and one tetrahedral site (D site). Rietveld analysis indicated that Mn doping selectively occurred at the distorted octahedral B site. Previous reports on metal-substituted ε-Fe2O3 indicated that large metal cations (In3+; 0.800 Å) substituted into the distorted octahedral A and B sites,68 while small metal cations (Al3+; 0.535 Å, Ga3+; 0.620 Å, Ti4+; 0.605 Å) substituted into the tetrahedral D sites12,14,22 and similar size cations (Rh3+; 0.665 Å, Ru3+; 0.68 Å) substituted into the regular octahedral C sites.13,64,66 In light of these reports, Mn2+ was considered to occupy the B site because it has a larger ionic radius than that of Fe3+.
Magnetic properties
Fig. 5 shows the field-cooled magnetisation (FCM) curves under an external field of 1 kOe. The Curie temperatures (TC) were 487 K for Mn0, 471 K for Mn1, and 469 K for Mn2. Mn1 and Mn2 had contributions from the spinel-phase (Fig. S3, ESI†). Fig. 6a left shows the magnetic hysteresis loops measured at 300 K. The ratio of Mn substitution affected the Hc value. The value increased slightly from 17.2 kOe for Mn0 to 17.3 kOe for Mn1. However, Mn2 exhibited a large distortion in the hysteresis loop, which had a negative effect on Hc (i.e., 0.35 kOe), due to the inclusion of the soft-magnetic spinel-phase.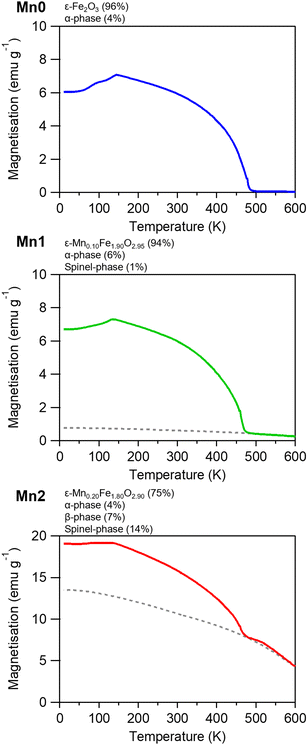 | ||
| Fig. 5 FCM curves in an external field of 1 kOe for Mn0, Mn1, and Mn2. Dotted line is to guide the eye for the contribution of the spinel-phase. | ||
To estimate the intrinsic Hc value of ε-MnxFe2−xO3−x/2, we applied a correction that considered the contributions of the spinel-phase and α-phase (Fig. 6a, right). The estimated Hc values for ε-MnxFe2−xO3−x/2 increased as the Mn ratio increased: 17.2 kOe for ε-Fe2O3, 18.0 kOe for ε-Mn0.10Fe1.90O2.95, and 18.2 kOe for ε-Mn0.20Fe1.80O2.90. By contrast, the saturation magnetisation (Ms) decreased: 17.1 emu g−1 for ε-Fe2O3, 15.6 emu g−1 for ε-Mn0.10Fe1.90O2.95, and 13.9 emu g−1 for ε-Mn0.20Fe1.80O2.90 (Fig. 6b and Table 2).
| Sample | T C (K) | H c (kOe) | M s (emu g−1) |
|---|---|---|---|
| Mn0 | 487 | 17.2 | 17.1 |
| Mn1 | 471 | 18.0 | 15.6 |
| Mn2 | 469 | 18.2 | 13.9 |
Millimetre wave absorption properties
An Advantest TAS7400 was used to perform terahertz time-domain spectroscopy (THz-TDS) measurements. A THz pulse was irradiated onto the sample and both the transmitted and reflected THz pulses were measured in the time domain. The spectra were obtained by a Fourier transformation. The measurement employed pellet samples (13 mmϕ). The pellets had a thickness (d) of approximately 1.11 mm and a volume filling ratio of 54 vol%. Fig. 7 shows the absorption spectra with the fringe patterns arising from multiple reflections removed.77 As the Mn substitution ratio increased, the absorption peak shifted to higher frequencies, the resonance frequency (fr) increased from 174 GHz (Mn0) to 182 GHz (Mn1) and 187 GHz (Mn2), and the full width at half maximum (FWHM) broadened from 9 GHz (Mn0) to 11 GHz (Mn1) and 13 GHz (Mn2). The observed increase in the fr value is apparently due to Mn-substitution since the fr value of ε-Fe2O3, which can be slightly affected by particle size and shape, is 182 GHz at most. A similar increase of fr in metal-substituted ε-Fe2O3 has only been reported with rhodium substitution. | ||
| Fig. 7 Millimetre-wave absorption spectra of Mn0, Mn1, and Mn2 normalized by the ε-phase fraction and filling ratio. Fringe patterns from multiple reflections are removed. | ||
Mechanism for the increased resonance frequency and coercive field by manganese substitution
In the present manganese substituted ε-Fe2O3, XPS measurements confirmed that Fe3+ was replaced by Mn2. O2− vacancies maintained the electrical neutrality. Therefore, the composition was ε-MnxFe2−xO3−x/2. The O2− vacancies reduced the number of superexchange interaction pathways, decreasing the TC value.78–80 In addition, B-site substitution of Mn2+ affected the magnetic structure of ε-Fe2O3. ε-Fe2O3 is a collinear ferrimagnet composed of positive sublattice magnetisations at B and C sites (MB and MC) and negative sublattice magnetisations at A and D sites (MA and MD).81–83 Since the superexchange interaction at tetrahedral D sites was smaller than those at the other octahedral sites (A–C sites), MD was smaller than the other sublattice magnetisation. Consequently, sublattice magnetisations did not compensate for each other and spontaneous magnetisation appeared in ε-Fe2O3. Mn2+ substitution at the B sites causes O2− vacancies around the substituted B sites, which reduced the number of superexchange interaction pathways and decreased MB. As a result, the total magnetisation decreased, which was experimentally observed by the 19% decrease in saturation magnetisation. On the other hand, the magnetic anisotropy Ha tended to be inversely proportional to magnetisation, while the coercive field and resonance frequency were proportional to Ha.84 Due to the decreased magnetisation, the coercive field and the resonance frequency increased by 7% and 8%, respectively.Conclusions
We prepared a series of Mn-substituted ε-Fe2O3, and ε-MnxFe2−xO3−x/2 (x = 0, 0.10, and 0.20). Replacing trivalent Fe3+ ions with divalent Mn2+ ions forms oxygen vacancies, causing anisotropic contraction of the unit cell. Mn-substitution increases not only the coercive field from 17.2 kOe (x = 0) to 18.2 kOe (x = 0.20) but also the zero-field ferromagnetic resonance frequency from 174 GHz (x = 0) to 187 GHz (x = 0.20). Although Rh-substituted ε-Fe2O3 shows a similar increase in the resonance frequency and coercive field, the mechanism differs from the present material. The enhancement in Rh-substituted ε-Fe2O3 is caused by the orbital angular momentum on Rh3+.13 From the viewpoint of sustainable development goals, manganese is the 12th most naturally abundant chemical element in the earth's crust (0.1 wt%). By contrast, rhodium is extremely rare (<0.001 wt%).85 Consequently, the present material has potential as an eco-friendly material in various applications such as millimetre-wave absorption, magnetic recordings, two-dimensional ferroelectric-ferroelectricity, and biomedical applications.Data availability
Crystallographic data for ε-MnxFe2−xO3−x/2, has been deposited at the Cambridge Crystallographic Data Centre under 2380300 – 2380302 and can be obtained from https://www.ccdc.cam.ac.uk/. The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research A from the Japan Society for the Promotion of Science (JSPS) (Grant Number 20H00369), a Grant-in-Aid for Scientific Research B from JSPS (Grant Numbers 23H01920 and 22H02046). We recognize the Cryogenic Research Center at The University of Tokyo, DOWA Technofund, the Tokyo Ohka Foundation for The Promotion of Science and Technology and the Center for Nano Lithography & Analysis at The University of Tokyo. We are grateful to Dr Marie Yoshikiyo at the University of Tokyo, and Dr Yasuto Miyamoto and Mr Kenji Sakane at DOWA Electronics Ltd., for their helpful discussions and for providing the TEM images.Notes and references
- Y. Yan, G. Xie, M. P. J. Lavery, H. Huang, N. Ahmed, C. Bao, Y. Ren, Y. Cao, L. Li, Z. Zhao, A. F. Molisch, M. Tur, M. J. Padgett and A. E. Willner, Nat. Commun., 2014, 5, 4876 CrossRef CAS.
- S. Rangan, T. Rappaport and E. Erkip, Proc. IEEE, 2024, 102, 366–385 Search PubMed.
- S. Koenig, D. Lopez-Diaz, J. Antes, F. Boes, R. Henneberger, A. Leuther, A. Tessmann, R. Schmogrow, D. Hillerkuss, R. Palmer, T. Zwick, C. Koos, W. Freude, O. Ambacher, J. Leuthold and I. Kallfass, Nat. Photonics, 2013, 7, 977–981 CrossRef CAS.
- A. Kumar, M. Gupta and R. Singh, Nat. Photon., 2021, 15, 715–716 CrossRef.
- S. Dang, O. Amin, B. Shihada and M. Alouini, Nat. Electron., 2020, 3, 20–29 CrossRef.
- J. Jornet, E. Knightley and D. Mittleman, Nat. Commun., 2023, 14, 841 CrossRef CAS PubMed.
- A. Goldman, Modern Ferrite Technology, Springer, New York, 2006 Search PubMed.
- S. Pawar, S. Biswas, G. Kar and S. Bose, Polymer, 2016, 84, 398–419 CrossRef CAS.
- A. Charles, A. Rider, S. Brown and C. Wang, Prog. Mater. Sci., 2021, 115, 10075 CrossRef.
- M. Qin, L. Zhang and H. Wu, Adv. Sci., 2022, 9, 2105553 CrossRef CAS.
- J. Tuček, R. Zbořil, A. Namai and S. Ohkoshi, Chem. Mater., 2010, 22, 6483–6505 CrossRef.
- A. Namai, S. Sakurai, M. Nakajima, T. Suemoto, K. Matsumoto, M. Goto, S. Sasaki and S. Ohkoshi, J. Am. Chem. Soc., 2009, 131, 1170–1173 CrossRef CAS PubMed.
- A. Namai, M. Yoshikiyo, K. Yamada, S. Sakurai, T. Goto, T. Yoshida, T. Miyazaki, M. Nakajima, T. Suemoto, H. Tokoro and S. Ohkoshi, Nat. Commun., 2012, 3, 1035 CrossRef.
- S. Ohkoshi, S. Kuroki, S. Sakurai, K. Matsumoto, K. Sato and S. Sasaki, Angew. Chem., Int. Ed., 2007, 46, 8392–8395 CrossRef CAS PubMed.
- G. Jo, M. Yun, Y. Hun Son, B. Park, J. Lee, Y. Kim, Y. Son and Y. Baek, Chem. Commun., 2022, 58, 11442–11445 RSC.
- R. Kinugawa, K. Imoto, Y. Futakawa, S. Shimizu, R. Fujiwara, M. Yoshikiyo, A. Namai and S. Ohkoshi, Adv. Eng. Mater., 2021, 23, 2001473 CrossRef CAS.
- E. Gorbachev, M. Soshnikov, M. Wu, L. Alyabyeva, D. Myakishev, E. Kozlyakova, V. Lebedev, E. Anokhin, B. Gorshunov, O. Brylev, P. Kazin and L. Trusov, J. Mater. Chem. C, 2021, 9, 6173–6179 RSC.
- J. Jin, S. Ohkoshi and K. Hashimoto, Adv. Mater., 2004, 16, 48–51 CrossRef CAS.
- S. Ohkoshi, A. Namai, K. Imoto, M. Yoshikiyo, W. Tarora, K. Nakagawa, M. Komine, Y. Miyamoto, T. Nasu, S. Oka and H. Tokoro, Sci. Rep., 2015, 5, 14414 CrossRef CAS PubMed.
- M. Gich, C. Frontera, A. Roig, E. Taboada, E. Molins, H. R. Rechenberg, J. D. Ardisson, W. A. A. Macedo, C. Ritter, V. Hardy, J. Sort, V. Skumryev and J. Nogués, Chem. Mater., 2006, 18, 3889–3897 CrossRef CAS.
- S. Ohkoshi, M. Yoshikiyo, K. Imoto, K. Nakagawa, A. Namai, H. Tokoro, Y. Yahagi, K. Takeuchi, F. Jia, S. Miyashita, M. Nakajima, H. Qiu, K. Kato, T. Yamaoka, M. Shirata, K. Naoi, K. Yagishita and H. Doshita, Adv. Mater., 2020, 32, 2004897 CrossRef CAS PubMed.
- S. Ohkoshi, A. Namai, M. Yoshikiyo, K. Imoto, K. Tamazaki, K. Matsuno, O. Inoue, T. Ide, K. Masada, M. Goto, T. Goto, T. Yoshida and T. Miyazaki, Angew. Chem., Int. Ed., 2016, 55, 11403 CrossRef CAS PubMed.
- T. Jussila, A. Philip, J. Lindén and M. Karppinen, Adv. Eng. Mater., 2023, 25, 2201262 CrossRef CAS.
- M. Gich, I. Fina, A. Morelli, F. Sánchez, M. Alexe, J. Gàzquez, J. Fontcuberta and A. Roig, Adv. Mater., 2014, 26, 4645–4652 CrossRef CAS PubMed.
- T. Wang, W. Xue, H. Yang, Y. Zhang, S. Cheng, Z. Fan, R.-W. Li, P. Zhou and X. Xu, Adv. Mater., 2024, 2311041 CrossRef CAS.
- Y. Hamasaki, S. Yasui, T. Katayama, T. Kiguchi, S. Sawai and M. Itoh, Appl. Phys. Lett., 2021, 119, 182904 CrossRef CAS.
- Y. Wang, P. Wang, H. Wang, B. Xu, H. Li, M. Cheng, W. Feng, R. Du, L. Song, X. Wen, X. Li, J. Yang, Y. Cai, J. He, Z. Wang and J. Shi, Adv. Mater., 2023, 35, 2209465 CrossRef CAS.
- V. Tiron, R. Jijie, I. Dumitru, N. Cimpoesu, I. Burducea, D. Iancu, A. Borhan, S. Gurlui and G. Bulai, Ceram. Int., 2023, 49, 20304–20314 CrossRef CAS.
- Y. Gu, M. Yoshikiyo, A. Namai, D. Bonvin, A. Martinez, R. Piñol, P. Téllez, N. J. O. Silva, F. Ahrentorp, C. Johansson, J. Marco-Brualla, R. Moreno-Loshuertos, P. Fernández-Silva, Y. Cui, S. Ohkoshi and A. Millán, RSC Adv., 2020, 10, 28786–28797 RSC.
- Y. Gu, N. J. O. Silva, M. Yoshikiyo, A. Namai, R. Piñol, G. Maurin-Pasturel, Y. Cui, S. Ohkoshi, A. Millán and A. Martínez, Chem. Commun., 2021, 57, 2285–2288 RSC.
- L. Kubíčková, P. Brázda, M. Veverka, O. Kaman, V. Herynek, M. Vosmanská, P. Dvořák, K. Bernášek and J. Kohout, J. Magn. Magn. Mater., 2019, 480, 154–163 CrossRef.
- D. Li, J. Liang, S. Song and L. Li, ACS Appl. Nano Mater., 2023, 6, 2356–2365 CrossRef CAS.
- D. Singh, S. Shaktawat, S. K. Yadav, R. Verma, K. R. B. Singh and J. Singh, Int. J. Biol. Macromol., 2024, 265, 130867 CrossRef CAS PubMed.
- G. Carraro, C. Maccato, A. Gasparotto, T. Montini, S. Turner, O. I. Lebedev, V. Gombac, G. Adami, G. Van Tendeloo, D. Barreca and P. Fornasiero, Adv. Funct. Mater., 2014, 24, 372–378 CrossRef CAS.
- S. Tao, Y. Zhu, S. Liu, J. Dong, Y. Yuan and Q. Li, Crystals, 2023, 13, 632 CrossRef CAS.
- G. Li, Z. Wang, J. Zhou, B. Kang, Y. Ding, M. Guan, X. Wei and Y. Lei, Herit. Sci., 2023, 11, 58 CrossRef CAS.
- C. Holé, M. Brunet, B. Joulié, Z. Ren, T. Wang, G. Wallez and P. Sciau, J. Appl. Crystallogr., 2024, 57, 431–439 CrossRef.
- M. Guan, Y. Guo, B. Kang, M. Wang, G. Li, Y. Zheng, Y. Ding, M. Wang, N. Wood, Y. Lei, X. Wei and D. Ma, J. Am. Ceram. Soc., 2024, 107, 522–533 CrossRef CAS.
- Z. Li, J. Liu, X. Jiang and J. Cui, J. Eur. Ceram. Soc., 2024, 44, 1856–1863 CrossRef CAS.
- T. Wang, S. Xia, F. Wang, Z. Ren, P. Sciau, C. Yang, J. Zhu, H. Luo, Q. Li and X. Fu, J. Eur. Ceram. Soc., 2024, 44, 3337–3343 CrossRef CAS.
- C. Holé, Z. Ren, F. Wang, J. Zhu, T. Wang and P. Sciau, Mater. Today Commun., 2022, 33, 104329 CrossRef.
- Y. Kusano, H. Nakata, Z. Peng, R. S. S. Maki, T. Ogawa and M. Fukuhara, ACS Appl. Mater. Interfaces, 2021, 13, 38491–38498 CrossRef CAS.
- H. Tokoro, W. Tarora, A. Namai, M. Yoshikiyo and S. Ohkoshi, Chem. Mater., 2018, 30, 2888–2894 CrossRef CAS.
- L. Altenschmidt, P. Beaunier, A. Bordage, E. Rivière, G. Fornasieri and A. Bleuzen, ChemNanoMat, 2023, 9, e202200469 CrossRef CAS.
- I. Khan, S. Morishita, R. Higashinaka, T. D. Matsuda, Y. Aoki, E. Kuzmann, Z. Homonnay, S. Katalin, L. Pavić and S. Kubuki, J. Magn. Magn. Mater., 2021, 538, 168264 CrossRef CAS.
- J. MacDougall, H. Tokoro, M. Yoshikiyo, A. Namai and S. Ohkoshi, Eur. J. Inorg. Chem., 2024, e202400148 CrossRef CAS.
- Y. Zhao and G. Wen, J. Magn. Magn. Mater., 2020, 512, 167039 CrossRef CAS.
- S. Sakurai, A. Namai, K. Hashimoto and S. Ohkoshi, J. Am. Chem. Soc., 2009, 131, 18299–18303 CrossRef CAS.
- M. Tadic, I. Milosevic, S. Kralj, D. Hanzel, T. Barudzija, L. Motte and D. Makovec, Acta Mater., 2020, 188, 16–22 CrossRef CAS.
- J. MacDougall, A. Namai, M. Yoshikiyo and S. Ohkoshi, Chem. Lett., 2023, 52, 229–232 CrossRef CAS.
- L. Corbellini, C. Lacroix, C. Harnagea, A. Korinek, G. A. Botton, D. Ménard and A. Pignolet, Sci. Rep., 2017, 7, 3712 CrossRef.
- S. Chen, Y. Jiang, T. Yao, A. Tao, X. Yan, F. Liu, C. Chen, X. Ma and H. Ye, Micron, 2022, 163, 103359 CrossRef CAS.
- S. Suturin, P. Dvortsova, L. Snigirev, V. Ukleev, T. Hanashima, M. Rosado and B. Ballesteros, Mater. Today Commun., 2022, 33, 104412 CrossRef CAS.
- J. Yuan, A. Balk, H. Guo, Q. Fang, S. Patel, X. Zhao, T. Terlier, D. Natelson, S. Crooker and J. Lou, Nano Lett., 2019, 19, 3777–3781 CrossRef CAS PubMed.
- T. Amrillah, L. T. Quynh, C. N. Van, T. H. Do, E. Arenholz, J.-Y. Juang and Y.-H. Chu, ACS Appl. Mater. Interfaces, 2021, 13, 17006–17012 CrossRef CAS.
- J. Cleron, A. A. Baker, T. Nakotte, A. Troksa and J. Han, J. Phys. Chem. C, 2022, 126, 7256–7263 CrossRef CAS.
- L. Kubíčková, O. Kaman, P. Veverka, V. Herynek, P. Brázda, K. Bernášek, M. Veverka and J. Kohout, J. Alloys Compd., 2021, 856, 158187 CrossRef.
- T. Katayama, S. Yasui, Y. Hamasaki and M. Itoh, Appl. Phys. Lett., 2017, 110, 212905 CrossRef.
- L. Kubíčková, O. Kaman, P. Veverka, V. Herynek, P. Brázda, M. Vosmanská, T. Kmječ, P. Dvořák, D. Kubániová and J. Kohout, Colloids Surf., A, 2020, 589, 124423 CrossRef.
- L. Corbellini, C. Lacroix, D. Ménard and A. Pignolet, Scr. Mater., 2017, 140, 63–66 CrossRef CAS.
- Q. Fu and G. Wen, J. Magn. Magn. Mater., 2023, 570, 170500 CrossRef CAS.
- Z. Ma, A. Romaguera, F. Fauth, J. Herrero-Martín, J. L. García-Muñoz and M. Gich, J. Magn. Magn. Mater., 2020, 506, 166764 CrossRef CAS.
- R. Nickel, C. Sun, D. Motta Meira, P. Shafer and J. van Lierop, Phys. Rev. Mater., 2024, 8, 024407 CrossRef CAS.
- S. Ohkoshi, K. Imoto, A. Namai, S. Anan, M. Yoshikiyo and H. Tokoro, J. Am. Chem. Soc., 2017, 139, 13268–13271 CrossRef CAS.
- S. Yasui, T. Katayama, T. Osakabe, Y. Hamasaki, T. Taniyama and M. Itoh, J. Ceram. Soc. Jpn., 2019, 127, 474–477 CrossRef CAS.
- A. Namai and S. Ohkoshi, Eur. J. Chem., 2018, 24, 11880–11884 CrossRef CAS.
- A. Tamm, A. Tarre, J. Kozlova, M. Rähn, T. Jõgiaas, T. Kahro, J. Link and R. Stern, RSC Adv., 2021, 11, 7521–7526 RSC.
- S. Sakurai, S. Kuroki, H. Tokoro, K. Hashimoto and S. Ohkoshi, Adv. Funct. Mater., 2007, 17, 2278–2282 CrossRef CAS.
- M. Yoshikiyo, A. Namai, M. Nakajima, K. Yamaguchi, T. Suemoto and S. Ohkoshi, J. Appl. Phys., 2014, 115, 172613 CrossRef.
- M. Polášková, O. Malina, J. Tuček and P. Jakubec, Nanoscale, 2022, 14, 5501–5513 RSC.
- S. Ohkoshi, K. Imoto, A. Namai, M. Yoshikiyo, S. Miyashita, H. Qiu, S. Kimoto, K. Kato and M. Nakajima, J. Am. Chem. Soc., 2019, 141, 1775–1780 CrossRef CAS.
- J. Moulder, W. Stickle, P. Sobol and K. Bomben, Handbook of X-ray Photoelectron Spectroscopy, PerkinElmer Corporation, Minnesota, 1992.
- R. Shannon, Acta Crystallogr., 1976, 32, 751 CrossRef.
- D. Varshney and A. Yogi, J. Mol. Struct., 2011, 995, 157–162 CrossRef CAS.
- P. Kumar, V. Sharma, J. P. Singh, A. Kumar, S. Chahal, K. Sachdev, K. H. Chae, A. Kumar, K. Asokan and D. Kanjilal, J. Magn. Magn. Mater., 2019, 489, 165398 CrossRef CAS.
- V. Sahoo, R. N. Bhowmik and S. Khan, Mater. Chem. Phys., 2023, 296, 127298 CrossRef CAS.
- A. Namai, Y. Oki, K. Imoto, H. Tokoro and S. Ohkoshi, J. Mater. Chem. C, 2022, 10, 10815–10822 RSC.
- Y. J. Song, G. B. Turpin, R. E. Bornfreund, H. Aoyama and P. E. Wigen, J. Magn. Magn. Mater., 1996, 154, 37–53 CrossRef CAS.
- S. Kumari, N. Mottaghi, C. Huang, R. Trappen, G. Bhandari, S. Yousefi, G. Cabrera, M. Seehra and M. Holcomb, Sci. Rep., 2020, 10, 3659 CrossRef CAS PubMed.
- A. Harbi, A. Azouaoui, S. Benmokhtar and M. Moutaabbid, J. Supercond. Novel Magn., 2022, 35, 1405–1412 CrossRef CAS.
- R. Nickel, J. Gibbs, J. Burgess, P. Shafer, D. M. Meira, C. Sun and J. Van Lierop, Nano Lett., 2023, 23, 7845–7851 CrossRef CAS.
- M. Yoshikiyo, K. Yamada, A. Namai and S. Ohkoshi, J. Phys. Chem. C, 2012, 116, 8688–8691 CrossRef CAS.
- S. Ohkoshi, A. Namai and S. Sakurai, J. Phys. Chem. C, 2009, 113, 11235–11238 CrossRef CAS.
- S. Chikazumi, Physics of Ferromagnetism, Oxford University Press, New York, 1997 Search PubMed.
- A. Yaroshevsky, Geochem. Int., 2006, 44, 48–55 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2380300–2380302. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ma00927d |
| ‡ Present address: Institute of Physical and Theoretical Chemistry, The University of Tübingen, Auf der Morgenstelle 18, D-72076 Tübingen, Germany. |
| This journal is © The Royal Society of Chemistry 2025 |





