 Open Access Article
Open Access ArticleDebondable phenoxy-based structural adhesives with β-amino amide containing reversible crosslinkers†
Francesca
Portone
a,
Loc Tan
Nguyen
 b,
Roberta
Pinalli
b,
Roberta
Pinalli
 a,
Alessandro
Pedrini
a,
Alessandro
Pedrini
 a,
Filip E.
Du Prez
a,
Filip E.
Du Prez
 *b and
Enrico
Dalcanale
*b and
Enrico
Dalcanale
 *a
*a
aDipartimento di Scienze Chimiche, della Vita e della Sostenibilità Ambientale, Università degli Studi di Parma, Parco Area delle Scienze 17/A, 43124, Parma, Italy. E-mail: enrico.dalcanale@unipr.it
bPolymer Chemistry Research Group, Centre of Macromolecular Chemistry (CMaC), Department of Organic and Macromolecular Chemistry, Faculty of Sciences, Ghent University, Krijgslaan 281 S4, 9000 Ghent, Belgium. E-mail: Filip.DuPrez@UGent.be
First published on 21st February 2025
Abstract
This study introduces dynamic phenoxy-based adhesives using β-aminoamide exchange chemistry, designed for durability, reprocessability and sustainability. Synthesized through a two-step process, the adhesive features linear poly-aminoamides and tailored amine formulations to optimize adhesion, flexibility, and the glass transition. The corresponding phenoxy-based adhesives demonstrated effective crosslinking, high thermal stability (Td5% ∼340–350 °C), and temperature-responsive viscoelastic properties. Notably, the materials with 5 mol% of TETA (E-BAAT5) exhibited ideal activation energy for stress relaxation, exceptional creep resistance, and retained up to 98% lap shear strength after recycling, with controlled debonding at elevated temperatures, making it ideal for high-performance, recyclable adhesive applications.
Introduction
The adhesive market is experiencing continuous growth, driven by the diverse and expanding range of applications for these materials.1 Among the various types of adhesives (e.g. hot-melt, pressure sensitive, contact adhesive), structural adhesives stand out for their ability to deliver high mechanical strength and long-term durability, making them indispensable in applications where the structural integrity of the bonded materials is of paramount importance.2 Structural adhesives for demanding engineering applications are typically defined by their capacity to achieve a lap shear strength exceeding the critical threshold of 7 MPa.3 Prominent examples of structural adhesives reported in literature include polyacrylates, polyurethanes, and especially epoxy thermosets, which are well-established for their robust performance in various conditions.4Despite their advantageous properties, traditional epoxy-based products face a lack of (re)processability, which leads to these materials being treated as permanent waste at the end of their useful life.5 This poses significant environmental challenges, as discarded epoxy-based products contribute to rapidly growing waste streams that are cumbersome to recycle or reuse.
Recently, however, promising advancements have been made in addressing this issue. For example, a reusable supramolecular crosslinked epoxy hot melt adhesive has been developed, achieving an adhesion strength of 10 MPa.6,7 This marks a significant step forward in creating materials that retain high performance while offering the possibility of reuse. Although early efforts in this field concentrated on supramolecular adhesives, due to their ability to form reversible bonds, covalent dynamic systems have emerged as a compelling alternative for applications requiring stronger, more durable connections yet being (re)processable.8,9
Over the past decade, several covalent dynamic chemistries have been investigated as potential solutions to enhance the reprocessability of adhesives without compromising their mechanical strength such as disulfide exchange,10,11 Diels–Alder chemistry,12,13 boronic ester methatesis,14 transesterification,15 vinylogous urethane,16 and β-amino ester chemistry.17
In 2023, Van Lijsebetten et al. reported an epoxy adhesive using amide–imide exchange chemistry.18 They prepared a new dynamic curing platform for epoxy resins using commercially available monomers to create polyglutaramide (PGA) and polysuccinamides (PSA) pre-polymers as dynamic hardeners. Rheology experiments demonstrated that heating triggered a shift in the dicarboxamide–imide equilibrium, allowing material flow at typical thermoplastic processing temperatures (230–280 °C). The modified epoxy resin improved bonding to metal substrates with a lap shear strength of up to 8 MPa and enabled controlled debonding and rebonding through reversible cohesion. This strategy offers a promising potential for industrial application, primarily due to the cost-effectiveness and scalability of the materials involved. Moreover, polyamides and amidoamines are well-established curing agents widely employed across various industries, including epoxy formulations for adhesives.19 These compounds significantly enhance both the strength and durability of the resulting adhesives, making them integral components in high-performance products.
Very recently, Du Prez and coworkers reported a related dissociative β-aminoamide dynamic exchange chemistry in which an amino group is positioned in proximity to an amide function.20 This type of exchange chemistry exhibits dynamic behavior at temperatures above 160 °C, resulting in reversible dissociation into acrylamides and amines (Fig. 1a). Furthermore, these materials exhibit creep resistance up to 100 °C, robust hydrolytic stability in acidic, basic, and neutral environments, combined with high thermal and dimensional stability.
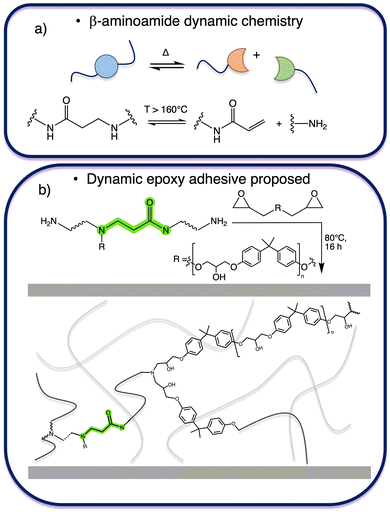 | ||
| Fig. 1 (a) Sketch of β-aminoamide moiety dissociation in the corresponding acrylamide and amine; (b) sketch of the proposed dynamic epoxy adhesive chemical structure and connectivity. | ||
To expand the range of dynamic chemistries available for the development of covalent adaptable networks (CANs) in structural adhesives, such β-aminoamide moieties have now been incorporated into a linear dynamic prepolymer as a curing agent for commercially available phenoxy-based resins (Fig. 1b). Specifically, the incorporation of a curing agent with dynamic covalent bonds in rigid epoxy curing systems offers an efficient method for producing dynamic epoxy-based materials while utilizing conventional reaction pathways and conditions traditionally used for crosslinked epoxy resins.21,22
In this work, we selected a commercially available phenoxy-based resin, Epikote™ 828LV, from Westlake Epoxy. This liquid epoxy resin was chosen due to its widespread application in adhesive formulations and its impressive mechanical performance. One of the key factors contributing to its superior properties is its low shrinkage during curing, as well as the presence of pendant hydroxyl groups that can interact with various substrates, thus enhancing the adhesive's bonding properties.
First, a scalable synthesis for covalent adaptable β-aminoamide crosslinkers, making use of a bifunctional amino-ester and various cost-effective bifunctional amine-containing building blocks, will be reported. Then, the impact of permanent crosslinking on the dynamic properties of the resulting epoxy networks will be investigated, including an analysis of dimensional stability through creep measurements. Additionally, the exceptional adhesive performance, alongside an evaluation of its heat-triggered debondability, will be described.
Results and discussion
Synthesis of the dynamic crosslinker
Following the preparation of β-aminoamides in a two-step approach,20 4,4′-trimethylene dipiperidine was in a first step reacted with methyl acrylate to obtain the corresponding β-aminoester 1 (BAE 1, see Fig. 2a) in a quantitative yield (Scheme S1†). The selection of such secondary amine was intentional to prevent a second Michael addition, which would have resulted in a branched crosslinker instead of a linear one. The formation of the Michael adduct was confirmed by 1H NMR (Fig. S1†). | ||
| Fig. 2 (a) Synthesis of the linear dynamic curing agent; (b) IR comparison of compound 1, BAA-T0, BAA-T5 and BAA-T10. | ||
In the second step, polycondensation of compound 1 at 120 °C for 24 h with a formulation of amines (priamine 1074, 2,2,4,4-trimethyl-1,6-hexanediamine (TMHD), triethylenetetramine (TETA), 2-(1-piperazinyl)ethylamine (PEA)) resulted in the viscous prepolymer BAA-T0, BAA-T5 and BAA-T10 (Fig. 2a). The BAA-T0 amine formulation consists only of priamine and PEA, designed to generate elastomeric materials. In addition, T5 and T10 make use of TMHD and 5 mol% and 10 mol% respectively of TETA to tune the Tg value. Equivalents and grams used for each curing agents are reported in Table S1.† Each amine was precisely selected to offer a good balance of adhesion, flexibility, and mechanical strength in the resulting materials. Specifically, TETA was chosen due to the presence of secondary amine groups in its structure, which reacts with the epoxy moiety, introducing permanent crosslinks. These crosslinks play a critical role in controlling the material properties, allowing the tuning of the glass transition temperature (Tg) values in the materials (vide infra). Consequently, different concentration of TETA was used in the preparation of the prepolymer. 2-(1-Piperazinyl)ethylamine (PEA), commonly employed as a reactive diluent in epoxy curing, was selected for two key factors: its ability to control the degree of polymerization by acting as a chain stopper, and the increased presence of secondary amine ends groups capable to react with the epoxy component.23 Priamine was selected to impart flexibility, thanks to its long aliphatic chains. Finally, TMHD was used by exploiting its low viscosity, which enables efficient mixing.
To produce a poly-aminoamides bearing two amine end groups, a molar ester-to-amine ratio (ester/NH2) of 0.83 was used. The slight excess of amine ensures that once the ester moieties of BAE 1 are consumed, no additional chain growth occurs, thus leading to an amine-terminated curing agent.
The complete consumption of the ester moieties was evaluated via FT-IR (Fig. 2b). The disappearance of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band of BAE 1 at 1731 cm−1 and the appearance of N–H and C
O stretching band of BAE 1 at 1731 cm−1 and the appearance of N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O amide stretching at 3297 cm−1 and 1636 cm−1, respectively, are clearly visible.
O amide stretching at 3297 cm−1 and 1636 cm−1, respectively, are clearly visible.
Preparation and characterization of the covalent adaptable network (CAN) polymers
The obtained pre-polymers were employed as curing agents in the reaction with the commercial liquid phenoxy component Epikote 828-LV (Fig. 3a), to synthesize E-BAAT0, E-BAAT5, and E-BAAT10 networks, respectively with no TETA, 5 mol% and 10 mol% of TETA. FT-IR was used to follow the crosslinking process (Fig. S2†), in which the disappearance of the stretching band of the epoxide groups (at 913 cm−1) was observed, thus confirming the successful network formation.The overall properties of the obtained materials have been summarized in Table 1. A gel fraction above 95% for all the networks further confirms the efficient crosslinking of the materials. Moreover, an increasing trend of Tg (Fig. S3†) values moving from E-BAAT0 (Tg = 14 °C) to E-BAAT10 (Tg = 45 °C) is observed. This observation is attributed to the presence of additional permanent crosslinking of E-BAAT5 and E-BAAT10, which affects both the backbone mobility and crosslink density. In addition, thermogravimetric analysis (TGA, Fig. S4a†) displayed a high degradation onset temperature (Td5%), ranging between 340 °C and 350 °C. Isothermal TGA experiments (Fig. S4b†) performed at 220 °C for 1 h in air revealed less than 2% of weight loss for all the networks (Table 1), resulting in negligible degradation of the materials at high temperatures.
| Swelling degreea (%) | Gel fractiona (%) |
T
g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (°C)
(°C) |
T
d5%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (°C)
(°C) |
m
iso 220 °C, 1 h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (%)
(%) |
|
|---|---|---|---|---|---|
| a Swelling degree and soluble fraction were obtained from solubility measurements in chloroform at room temperature, using five samples. b Determined from DSC analysis performed at a heating rate of 10 °C per minute. c TGA onset temperatures after 5% weight loss (Td5%) in air atmosphere. d Isothermal TGA for 1 h at 200 °C in air atmosphere. | |||||
| E-BAAT0 | 180 ± 5 | 95.7 ± 0.2 | 14 | 341 | 1.7 |
| E-BAAT5 | 156 ± 11 | 97.4 ± 0.3 | 35 | 345 | 0.9 |
| E-BAAT10 | 160 ± 7 | 97.7 ± 0.4 | 45 | 350 | 0.2 |
Subsequently, the processability of the networks was performed by cutting the materials into small pieces and compression molded at 200 °C under a pressure of 3 tonnes/m2 for 30 minutes (Fig. S5a†). However, pressing E-BAAT10 did not yield homogeneous samples, as illustrated in Fig. S5b.† This can be attributed to the high density of permanent crosslinks within the network, confirming an upper threshold for the number of permanent bonds24 and therefore is no longer consider in the following characterization discussion of the dynamic networks.
Viscoelastic properties of the synthetized polymer networks
The dynamic behavior of E-BAAT0 and E-BAAT5 was then examined by rheological frequency sweeps (Fig. S6†) and stress relaxation experiments (Fig. 3b and c). A noticeable decrease in the material's storage modulus (G′) with increasing temperature was observed in the frequency sweep experiment (Fig. S6a and b†). This observation provides additional evidence of the dissociative exchange25 in the dynamic amino-amide network via a reversible (retro) aza-Michael reaction (Fig. 1a). Stress relaxation measurements were performed in a temperature window ranging from 195 °C to 180 °C. The results, reported in Fig. 3b and c, show that all materials dissipate the stress via network rearrangements, showing their temperature-dependence viscoelastic properties. A high-temperature dependence of the dynamic behavior is observed, in which the applied stress could be relaxed completely at elevated temperatures (e.g. 180 °C), favoring (re)processability. As fitting stress relaxation curves with the single Maxwell model did not provide a good match, the stretched Maxwell exponential decay function26 was used to calculate the relaxation time (dotted line in Fig. 3b and c).Comparing the Arrhenius plots for E-BAAT0 and E-BAAT5, a large variation in activation energy, respectively 236 kJ mol−1 and 186 kJ mol−1 is observed. This aspect emphasizes that chain diffusion significantly affects the rate of network rearrangement. When diffusion is impeded, stress relaxation occurs more slowly than in a free system. The presence of permanent crosslinking contributes to this effect.
Unexpectedly, E-BAAT5 has the lowest activation energy despite the larger number of permanent crosslinks. This counterintuitive result can be rationalized assuming a proximity-like effect27 in the E-BAAT5 network. Indeed, the close spatial proximity of TETA's permanent cross-linking to the dissociated groups can facilitate a rapid bond exchange process. This proximity effect is responsible for lowering the activation energy of the bond exchange reaction, as the dissociated group can more readily encounter and reassociate with a nearby functional group.
Further characterizations were performed on the selected E-BAAT5 network. Its dimensional stability was assessed through a series of creep resistance experiments (Fig. S7a†) conducted across a temperature range from 60 °C to 140 °C. The material exhibited a remarkably low permanent deformation of approximately 0.2% after a recovery period of 3600 seconds in the absence of applied shear stress. Moreover, an examination of the creep compliance plot (Fig. S7b†) indicates that the power-law scaling exponent (m) reaches up to 0.11 (at 140 °C), implying that the material remains far from the characteristic steady-state regime (m ≈ 1). These results suggest that the observed residual strain is primarily attributed to the elastic responses of the material, rather than to the viscous behavior of the network. Consequently, it is not possible to further estimate creep rates under the given conditions.26
This minimal residual strain, observed consistently across all tested temperatures, indicates that E-BAAT5 possesses a high resistance to creep, even at elevated temperatures. Such stability suggests that E-BAAT5 could be a robust candidate for applications requiring long-term mechanical performance under thermal stress, as it demonstrates resilience against temperature-induced deformation, and a capacity to maintain structural integrity under conditions that might otherwise compromise other polymeric materials.
Additionally, tensile tests (Fig. S8†) showed ultimate stress different for E-BAAT0 and E-BAAT5, respectively 19 ± 3.6 MPa and 50 ± 7.5 MPa even though this latter is too brittle, as visible in Fig. S8b.†
Recyclability of the dynamic polymer networks
To assess the recyclability of this material, the performances on the recycled networks were evaluated by pressing E-BAAT5 at least three times (Fig. 4a), with no significant change observed in the FTIR spectra of the recycled sample compared to the initial one (Fig. 4b). However, stress relaxation measurements on the recycled samples (Fig. S9†) indicated deviations from the fitted curves, particularly in the initial phase of relaxation. Additionally, liberated acrylamide moieties during the dissociation process at elevated temperatures may undergo homopolymerization,20 potentially resulting in the observed increased activation energy (216 kJ mol−1). Despite these effects, the network retains its relaxation capability, confirming sufficient reprocessability of the material.Adhesive properties of the dynamic β-aminoamide networks
The adhesion properties of the networks were then determined using lap shear tests (Fig. 5a top). Experimentally, the formulations were coated using 0.1 g of the previously mixed formulations on the surface of two aluminum plates, that were overlapped in an area of 400 mm2 (16 mm × 24.8 mm) with a thickness of 0.2 mm according to ASTM D1002 procedure. The samples were then subsequently cured at 80 °C for 16 h, and post-cured at 100 °C for 1 h.When tensile force is applied to the sample, shear stress is distributed across the entire adhesive area, resulting in different types of failure (Fig. 5a bottom). The nature of the fracture is crucial to provide insights into the adhesive performance. In Fig. 5b, lap shear test results of E-BAAT0 and E-BAAT5 are reported compared to those of a static epoxy resin (ER) prepared using Ancamide™ and Epikote 828-LV with an NH/epoxy stoichiometric ratio. Combined adhesive and cohesive failures were observed for all specimens (Fig. S10†) with lap shear strengths of approximately 9.2 and 13.0 MPa for E-BAAT0 and E-BAAT5, respectively. Both values, obtained in the absence of any fillers, are remarkably higher compared to the ER reference material (≈7 MPa).
Based on the presence of reversible β-aminoamides in the formulations, these adhesives offer the potential for reconstruction (or rebonding) ability. Therefore, the broken specimens were subsequently bonded together by pressing the aluminum joints at 200 °C for 30 minutes under a pressure of 3 tonnes/m2 and cooled to 25 °C with a cooling rate of 5 °C min−1, resulting in rebonded specimens (Fig. S11†). The lap shear experiments were then repeated on the rebonded specimens (Fig. 5b). For the E-BAAT0 network, a partial recovery of the adhesive properties, reaching approximately 60% of the original lap shear strength, is observed. On the other hand, the E-BAAT5 adhesive exhibited an impressive recovery, almost fully restoring its initial lap shear strength (98%). This high retention of adhesive properties highlights the superior potential of E-BAAT5 for maintaining bonding performance after recycling.
Finally, to access debonding ability, the lap shear experiments were performed at 120 °C (Fig. 5c) to induce a complete debonding of the aluminum joints thanks to the presence of reversible β-aminoamide moieties. As a result, all specimens showed a drop in lap shear strength, which is almost zero for the dynamic epoxy adhesive while the reference material ER retained around 3 MPa of lap shear strength. These results show the heat-triggered bonding-debonding capabilities of the dynamic, structural epoxy adhesives, advantageously emphasizing their suitability in various applications.
Conclusions
Recent innovations in adhesive technology address the pressing need for reprocessable and environmentally sustainable materials, particularly for high-performance applications. This study successfully demonstrated the synthesis and comprehensive characterization of β-aminoamide-based dynamic curing agents and their application in the development of high-performance, reprocessable, and recyclable phenoxy-based networks. The results revealed that varying the curing agent composition, particularly the concentration of TETA, enabled fine-tuning of key material properties. All the networks exhibited a Tg between 14 to 45 °C.Among the synthesized networks, E-BAAT5 exhibited superior adhesive performance, resulting in a lap shear strength of 13 MPa and an impressive recovery of its adhesion properties (up to 98%) after the recycling of the broken specimens.
Furthermore, the dynamic networks demonstrated tunable heat-triggered debonding, with lap shear strengths dropping to near zero at elevated temperatures.
By combining excellent thermal stability, tunable mechanical properties, dynamic reprocessability, and robust adhesive capabilities, these materials pave the way for sustainable, cost effective and advanced applications in adhesives, coatings, and recyclable composite materials, meeting the growing demand for environmentally friendly polymer systems without compromising performance.
Author contributions
All authors have given approval to the final version of the manuscript. Francesca Portone: investigation, visualization, writing – original draft preparation. Loc Tan Nguyen: investigation, methodology. Roberta Pinalli: data curation, writing – review & editing. Alessandro Pedrini: methodology, data curation, writing – review & editing. Filip Du Prez: conceptualization, supervision, writing – review & editing. Enrico Dalcanale: resources, conceptualization, writing – review & editing, supervision.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Recovery and Resilience Plan (NRRP), Mission 04 Component 2 Investment 1.5 – NextGenerationEU, Call for tender no. 3277 dated 30/12/2021. Project title: Ecosystem for sustainable transition in Emilia-Romagna. Award Number: 0,001,052 dated 23/06/2022, within Spoke1 activities “Materials for sustainability and ecological transition” CUP D93C22000460001. E. D. and F. P. would like to thank the support of PNRR – M4C2 – I1.1 – PRIN 2022 REPRONET (2022TCJRCA) financed by NextGenerationEU. F. D. P. and L. T. N. would like to acknowledge the European Research Council (ERC) for support under the European Union's Horizon 2020 research and the innovation programme 101021081 (ERC-AdG-2020, CiMaC-project).This work benefited from the equipment and framework of the COMP-HUB Initiative, funded by the Departments of Excellence program of the Italian Ministry for Education, University and Research (MIUR, 2023–2027). Centro Interdipartimentale di Misure “G. Casnati” of the University of Parma is acknowledged for the use of NMR facilities.
References
- A. Marques, A. Mocanu, N. Tomić, S. Balos, E. Stammen, A. Lundevall, S. Abrahami, R. Günther, J. De Kok and S. Teixeira de Freitas, Materials, 2020, 13, 5590 CrossRef CAS PubMed.
- D. A. Dillard, in Advances in Structural Adhesive Bonding, Elsevier, Amsterdam, The Netherlands, 2010 Search PubMed.
- S. Ebnesajjad, Adhesives Technology Handbook, Elsevier, 2009, vol. 63 Search PubMed.
- S. Ebnesajjad, Handbook of Adhesives and Surface Preparation, Elsevier, 2011, vol. 3 Search PubMed.
- W. Post, A. Susa, R. Blaauw, K. Molenveld and R. J. I. Knoop, Polym. Rev., 2020, 60, 359 CrossRef CAS.
- P. Sun, Y. Li, B. Qin, J.-F. Xu and X. Zhang, ACS Mater. Lett., 2021, 3, 1003 CrossRef CAS.
- Z. Zhang, D. Lei, C. Zhang, Z. Wang, Y. Jin, W. Zhang, X. Liu and J. Sun, Adv. Mater., 2022, 35, 2208619 CrossRef PubMed.
- F. Van Lijsebetten, T. Debsharma, J. M. Winne and F. E. Du Prez, Angew. Chem., Int. Ed., 2022, 61, e202210405 CrossRef CAS PubMed.
- W. Zou, J. Dong, Y. Luo, Q. Zhao and T. Xie, Adv. Mater., 2017, 29, 1606100 CrossRef PubMed.
- H.-Y. Tsai, Y. Nakamura, T. Fujita and M. Naito, Mater. Adv., 2020, 1, 3182 RSC.
- L. Li, X. Chen and J. M. Torkelson, ACS Appl. Polym. Mater., 2020, 2, 4658 CrossRef CAS.
- W. Xiangjun, X. Li, Q. Lin, J. Xia and H. Xue, RSC Adv., 2021, 11, 32565 RSC.
- L. M. Sridhar, M. O. Oster, D. E. Herr, J. B. D. Gregg, J. A. Wilson and A. T. Slark, Green Chem., 2020, 22, 8669 RSC.
- Z. Zhao, P. Zhao, Y. Zhao, J. Zuo and C. Li, Adv. Funct. Mater., 2022, 32, 2201959 CrossRef CAS.
- S. Zhang, T. Liu, C. Hao, L. Wang, J. Han, H. Liu and J. Zhang, Green Chem., 2018, 20, 2995 RSC.
- G. Soavi, F. Portone, D. Battegazzore, C. Paravidino, R. Arrigo, A. Pedrini, R. Pinalli, A. Fina and E. Dalcanale, React. Funct. Polym., 2023, 191, 105681 CrossRef CAS.
- T. Maiheu, E. Laguzzi, A. T. Slark and F. E. Du Prez, ACS Appl. Mater. Interfaces, 2024, 16(46), 64050 CrossRef CAS PubMed.
- F. Van Lijsebetten, T. Maiheu, J. M. Winne and F. E. Du Prez, Adv. Mater., 2023, 35, 2300802 CrossRef CAS PubMed.
- W. R. Ashcroft, in Chemistry and Technology of Epoxy Resins, ed. B. Ellis, Springer, Berlin, Germany, 1993, vol. 37 Search PubMed.
- L. T. Nguyen, F. Portone and F. E. Du Prez, Polym. Chem., 2024, 15, 11 RSC.
- Y. Spiesschaert, M. Guerre, I. De Baere, W. Van Paepegem, J. M. Winne and F. E. Du Prez, Macromolecules, 2020, 53, 2485 CrossRef CAS.
- X.-L. Zhao, Y.-Y. Liu, Y. Weng, Y.-D. Li and J.-B. Zeng, ACS Sustainable Chem. Eng., 2020, 8, 15020 CrossRef CAS.
- K. Huang, J. Xia, X. Yang, M. Li and H. Ding, Polym. J., 2010, 42, 51 CrossRef CAS.
- L. Li, X. Chen, K. Jin and J. M. Torkelson, Macromolecules, 2018, 51(15), 5537 CrossRef CAS.
- P. J. Flory, Br. Polym. J., 1985, 17, 96 CrossRef CAS.
- R. G. Ricarte and S. Shanbhag, Polym. Chem., 2024, 15, 815 RSC.
- F. Van Lijsebetten, T. Debsharma, J. M. Winne and F. E. Du Prez, Angew. Chem., Int. Ed., 2022, 61, e202210405 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4lp00369a |
| This journal is © The Royal Society of Chemistry 2025 |



