 Open Access Article
Open Access ArticleZinc-ion batteries: pioneering the future of sustainable energy storage through advanced materials and mechanisms
Zixuan
Chen†
a,
Liang
Zhang†
c,
Tianyu
Yu
f,
Huancheng
Yang
a,
Yao
Lu
 *b,
Xiaodan
Wang
*d,
Rui
Li
*e,
Zonglun
Ye
g,
Yue
Wang
d,
Pengwei
Li
d,
Bowen
Zheng
hj,
Yukun
Sun
i,
Depeng
Wang
i,
Guoqiang
Xu
i and
Wenchao
Gao
*b
*b,
Xiaodan
Wang
*d,
Rui
Li
*e,
Zonglun
Ye
g,
Yue
Wang
d,
Pengwei
Li
d,
Bowen
Zheng
hj,
Yukun
Sun
i,
Depeng
Wang
i,
Guoqiang
Xu
i and
Wenchao
Gao
*b
aSchool of Mechanical Engineering, University of Shanghai for Science and Technology, Shanghai 200093, P. R. China
bCAS Center for Excellence in Nanoscience, Beijing Key Laboratory of Micro-Nano Energy and Sensor, Beijing Institute of Nanoenergy and Nanosystems, Chinese Academy of Sciences, Beijing 101400, P. R. China. E-mail: Luyao@binn.cas.cn
cCollege of Physics and Mathematics and Beijing Key Laboratory for Magneto-Photoelectrical Composite and Interface Science, University of Science and Technology Beijing, Beijing 100083, P. R. China
dChina Astronaut Research and Training Center, Beijing 100094, P. R. China
eBeijing Key Laboratory of Environmental, Science and Engineering School of Materials Science & Engineering Beijing Institute of Technology, Beijing 100081, P. R. China
fShanghai Suochen Information Technology Co., Ltd., Shanghai 200030, P. R. China
gCapital University of Economics and Business, Beijing 100070, P. R. China
hOrdos New Energy Research Institute, Ordos 017000, Inner Mongolia, P. R. China
iSichuan New Energy Vehicle Innovation Center, Co., Ltd., Yibin 644000, Sichuan, P. R. China
jState Key Laboratory of Intelligent Green Vehicle and Mobility, School of Vehicle and Mobility, Tsinghua University, Beijing 100084, P. R. China
First published on 3rd July 2025
Abstract
The growing global demand for sustainable energy storage has positioned zinc-ion batteries (ZIBs) as a promising alternative to lithium-ion batteries (LIBs), offering inherent advantages in safety, cost, and environmental compatibility. Despite challenges like dendrite formation and cathode dissolution, recent advancements in electrode materials and electrolytes show significant progress. Anode innovations focus on surface modification and structural engineering to mitigate dendrites, while cathode development explores manganese/vanadium oxides, Prussian blue analogs, and emerging materials like Chevrel phases and MXenes. Electrolyte optimization, including aqueous, non-aqueous, and hybrid systems, has improved ion transport and interfacial stability. Mechanistic studies reveal complex redox processes involving cations, anions, and functional groups, guiding material design. ZIBs demonstrate potential for grid storage, flexible electronics, and electric vehicles, though challenges in energy density and cycle life remain. Addressing these through advanced characterization, computational modeling, and scalable fabrication could accelerate ZIB commercialization, establishing them as key players in sustainable energy storage and supporting global decarbonization efforts. Future research should focus on interdisciplinary approaches to overcome existing limitations and unlock their full potential. This review consolidates current knowledge while outlining pathways for ZIB development toward practical implementation.
1. Introduction
In the quest for carbon neutrality, energy storage technologies are increasingly recognized as essential to building a sustainable energy ecosystem. The transition from fossil fuels to renewable sources such as wind and solar requires efficient and scalable storage solutions to handle their inherent intermittency.LIBs, currently the backbone of the energy storage market, have facilitated the proliferation of electric vehicles, the construction of smart grids, and the evolution of portable electronic devices with their high energy density, long cycle life, and wide range of applications. Regardless of the notable benefits offered by LIBs, such as their impressive capacity, durability, and efficiency, their associated shortcomings including safety issues, environmental concerns, and shortage of raw materials are unneglectable problems. In tandem with the aggressive surge in energy density over the past decade, incidents stemming from LIBs have assumed greater significance. These accidents result from inadvertent chemical energy release, potentially leading to substantial property loss and, in some cases, even posing threats to human life.1–3 Furthermore, the disposal of LIBs can give rise to significant environmental issues, including the destruction of ecosystems and the highly polluting processes involved in metal extraction.4 The unregulated extraction of critical components like lithium, cobalt, and nickel, which are integral to LIBs, exacerbates the scarcity of these naturally limited metals. This, in turn, is likely to result in foreseeable spikes in raw material prices and intensify competition for these resources. As society's pursuit of cleaner energy and a lower-carbon lifestyle intensifies, the high carbon emissions associated with the production, usage, and recycling of LIBs have gradually come into focus, prompting the search for more environmentally friendly and efficient energy storage solutions. Thus, although LIBs currently dominate the market, their limitations including safety issues, limited resources, and high costs have driven the exploration of alternative technologies.
In view of this dilemma, rechargeable aqueous zinc-ion batteries (ZIBs) have gained attention for their enhanced safety, sustainability, and cost-effectiveness. Specifically, zinc is a kind of abundant element in the Earth's crust, and its extraction and processing would be both economically viable and environmentally favorable, with notably lower carbon emissions compared to the extraction and refinement of lithium. This intrinsic advantage results in a lower carbon footprint for ZIBs from the outset. Moreover, the manufacturing process of ZIBs is simpler and less energy-intensive compared to the complex production chain of LIBs, thereby further reducing energy consumption and associated emissions. This streamlined production not only enhances the environmental friendliness of ZIBs but also supports a significant reduction in carbon emissions across the entire industrial supply chain. Besides, ZIBs demonstrate superior stability and safety during cycling, which helps to minimize carbon emissions associated with battery failures or premature retirements. These attributes collectively position ZIBs as a promising low-carbon alternative in the field of energy storage, supporting the transition to more sustainable energy systems.5–8
However, ZIBs have encountered obstacles that hinder their practical application such as dendrite growth, limited voltage window and possible corrosion reaction.8–11 To overcome these issues, recent research has focused on electrode materials regulation and electrolyte optimization. With regard to the anode side, initiatives are concentrated on growth control, structural design, and additives selection, aiming for alleviating dendrite formation and enhancing plating/stripping coulombic efficiency. Concerning the cathode aspect, efforts focus on regulation of electronic structure, optimization of crystal structure, defect engineering, and control of microstructure. Meanwhile, development of electrolytes has led to products with excellent Zn2+ conductivity and highly reversible Zn stripping/plating behavior.12,13 Organic,14 ionic liquids,15 aqueous,16 and solid-state17 electrolytes have made significant strides in recent years, these advancements are crucial for enhancing the safety, energy density, and overall performance of ZIBs.18
In addition to the development of components for ZIBs, research on the actual operating mechanisms would be crucial as well.19 It aids in more effectively investigating the failure mechanism during the service life, enabling the design of enhanced electrochemical performance. Recent advances in characterization techniques have offered unprecedented opportunities to investigate the electrochemical reaction of ZIBs. In particular, in situ characterization techniques can provide real-time observations of the structural and electronic changes during the charge/discharge process.20–26
Despite the significant advancements, several challenges remain in the journey towards the commercialization of ZIBs. For instance, the practical energy density of ZIBs is still lower than that of LIBs, which is mainly due to the sluggish kinetics of Zn2+ insertion/extraction.27–30 Additionally, while ZIBs have been proposed for various applications including grid-scale energy storage, portable electronics, flexible or wearable devices, and electric vehicles, but their widespread adoption necessitates further improvements in cycle life, energy density, and power density.31 Furthermore, to realize the full potential of ZIBs, more effort should be devoted to fundamental research,30,32 for instance, unraveling the intrinsic process of Zn ions storage in various host materials holds significance, because the understanding of the reaction mechanism at the atomic or molecular level is the key to guide the rational design of better materials.33 In addition, the accelerated discovery and development of novel materials via high-throughput computational methods and machine learning could be game-changers in the field.34,35 Although ZIBs are attracting increasing attention, it is imperative to thoroughly understand the advantages and limitations of the technology. With the continuous development of materials, electrolytes, and cell designs, coupled with a deep understanding of the operating mechanisms, ZIBs could indeed provide a viable and competitive alternative to current battery technologies in the foreseeable future.
At present, aqueous ZIBs still face enormous challenges such as low energy density and short cycle life. There is an urgent need to continue to strengthen basic research and technology development in this field. Focusing on the basic scientific issues existing in aqueous ZIBs, this paper systematically summarizes the latest progress of anode materials, cathode materials, electrochemical mechanism and electrolyte design for aqueous ZIBs, as well as their respective core issues and research strategies, as shown in Fig. 1. Additionally, we provide an evaluation of potential application avenues and projected market growth for ZIBs with a particular emphasis on their prospective commercialization and deployment in stationary energy resources. Through presenting this comprehensive review, our aim is to offer an up-to-date perspective on the current status and future directions of ZIB technology.
2. Anode electrode of ZIBs
The design and optimization of anode materials are pivotal in advancing the performance metrics of ZIBs. An ideal anode material must exhibit a combination of high electrical conductivity, fast zinc-ion diffusion kinetics, and a structurally stable framework capable of mitigating issues related to volume expansion and morphological degradation during repeated charge–discharge cycles. According to the electrochemical behavior of Zn2+ in the charge and discharge processes, the anode materials can be roughly classified into two categories: (1) materials that can demonstrate Zn2+ stripping/plating behavior, such as metal Zn anode, which would be widely utilized and (2) materials with Zn2+ intercalation behavior.362.1. Metal Zn anode
Metallic zinc is a common choice for ZIB anode due to the high theoretical capacity and low redox potential.37 However, the actual application process is fraught with numerous issues such as Zn dendrites,38 corrosion,39,40 and passivation.41In aqueous ZIBs, zinc dendrite growth is a complex process. During charging, zinc ions migrate and deposit on the electrode surface under the influence of an electric field. Due to surface irregularities, zinc ions preferentially deposit in certain areas, forming initial zinc nuclei. As deposition continues, zinc atoms accumulate and diffuse, leading to the formation of dendritic structures. The growth of these dendrites is influenced by factors such as electric field distribution, ion concentration gradients, current density, and electrolyte composition. The uneven growth of dendrites may cause short circuits, reduce coulombic efficiency, and pose safety risks. Thus, inhibiting dendrite growth is crucial for enhancing the performance and safety of ZIBs.42
As for corrosion of Zn metal, it mainly occurs during the electrochemical reaction. When Zn sheds electrons, it transforms into Zn2+, eventually dissolving in the electrolyte upon the discharge process. The hydrogen evolution reactions would elevate the concentration of OH− locally and lead to the formation of insoluble compounds (Zn oxides and hydroxides in neutral or slightly alkaline solutions and zincate ions in high pH (pH > 9) solutions) throughout the charging process. Moreover, as dendrites grow, the surface area of the Zn anode grows. This growth is further intensified by the corrosion reaction, leading to an accelerated depletion of the Zn anode and declining battery efficiency.36,43,44
Besides, the issue of passivation on the surface demands attention and cannot be overlooked. The byproducts resulting from the detrimental interaction of Zn2+ and OH− would induce passivation on the Zn surface, influencing dissolution of Zn anode and the movement of carriers. The process of passivation creates a protective layer on the electrode's surface, which inhibits the release of discharge products and/or OH−, preventing further discharge. As the discharge products attains its maximum solubility during the discharge, ZnO would form as precipitate on the electrode's surface. Pore dimensions in porous Zn electrodes diminish due to the precipitation of ZnO prior to passivation. As soon as the newly released discharge products significantly surpasses the solubility threshold, it promptly precipitates, which can fully obstruct the residual pore space, leading to passivation.45–47
In response to these issues, numerous scientists have conducted thorough investigations into preparing advanced Zn anodes, which can be summarized as three main design strategies as following:
 | ||
| Fig. 2 (a) Schematic illustration of the surface evolution of Zn. Reproduced with permission from ref. 48. Copyright 2020, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (b) The schematic illustrations of Zn deposition on CC and CNT electrodes. Reproduced with permission from ref. 49. Copyright 2019, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (c) Schematic illustration of fabrication procedure and mechanism simulations of Ni–Zn alloy. Reproduced with permission from ref. 50. Copyright 2023, Springer. (d) Mo6S8 for anode in ZIBs. Reproduced with permission from ref. 51. Copyright 2016, American Chemical Society. (e) Mo2.5+yVO9+z host framework. Reproduced with permission from ref. 52. Copyright 2016, Royal Society of Chemistry. | ||
Lately, extensive research has been conducted on creating viable methods for structuring three-dimensional Zn anodes, which is capable of enlarging the Zn anode's surface area and diminishing the overpotential in Zn deposition, thereby decreasing the chances of dendrite development and passivation.60,61 Parker et al.62 developed a 3D porous sponge Zn electrode, which enhances Zn utilization and charging capacity, and this design enlarges the active area and diminishes the local current density, thereby mitigating Zn dendrite formation. Besides, Zn can be also capable of being applied as an active material to deposited on different base materials with a certain spatial configuration. Zeng et al.49 achieved dendrites-free Zn anodes by incorporating a 3D structure of carbon nanotubes (CNTs) into the surface of a carbon cloth substrate (CC) to serve as a scaffold for Zn plating and stripping (Fig. 2b). In contrast to the pristine deposited Zn electrode, the as-fabricated anode affords lower overpotential for Zn nucleation and more homogeneously distributed electric field, making it more favorable for highly reversible Zn plating/stripping with satisfactory coulombic efficiency rather than the formation of Zn dendrites or other byproducts. Besides, metal-based materials,63 MOF materials,64 and 2D MXene65 can be good choices for Zn depositing as well.
2.2. Zn ions intercalated anode
Just as its name implies, Zn ions intercalated anode would be a kind of anode based on the insertion/extraction mechanism. Drawing inspiration from the electrochemical behavior of Zn2+ intercalation in diverse cathode materials, employing an anode material with an appropriate tunnel structure or larger interlayer spacing is a logical and promising strategy.36,68,69 At present, Zn2+-intercalation anodes primarily consist of sulfides (Fig. 2d)51 and molybdenum–vanadium oxides (Fig. 2e).52 The advancement of these anodes offers valuable insights and strategies for the exploration of novel anode materials in aqueous ZIBs.The study of zinc anode materials has consistently faced challenges, including the proliferation of zinc dendrites, limited reversibility suffered, and a variety of adverse reactions. A range of tactics have been formulated to tackle these difficulties. This encompasses the structural architecture and interface safeguarding of zinc anodes, both primarily targeting the stabilization of the zinc stripping/plating layer. Eliminating in situ dendrites, along with corrosion and passivation, are also efficient methods for extending battery duration. Additionally, scientists have developed appropriate low-voltage Zn2+-intercalated anodes as substitutes for the existing metal zinc anode. Intercalated anodes demonstrate substantial storage for Zn2+, offering superior rate efficiency and cycle steadiness in ZIBs. Even with considerable advancements in high-performance zinc anode research, existing optimization methods fall short in addressing issues like dendrite development and various side reactions. Ongoing goal for research is to achieve extended lifespans, greater capacity, and enhanced coulombic efficiency in the zinc anode.
2.3. Summary of this chapter
The anode design in zinc-ion batteries (ZIBs) is crucial for performance, with materials classified into (1) Zn stripping/plating anodes (e.g., metallic Zn) and (2) Zn2+-intercalation anodes. Metallic Zn offers high capacity but suffers from dendrite growth, corrosion, and passivation, which degrade efficiency and safety. To address these issues, three key strategies have been developed: (1) surface modification (e.g., MOFs, carbon coatings) to homogenize Zn deposition and suppress side reactions; (2) structural design (e.g., 3D porous scaffolds) to reduce local current density and dendrite formation; and (3) Zn alloying (e.g., Zn–Al, Ni–Zn) to enhance cycling stability. Alternatively, Zn2+-intercalation anodes (e.g., sulfides, Mo–V oxides) provide dendrite-free operation with improved rate capability. Despite progress, challenges remain in achieving long-term stability and high capacity, driving ongoing research into advanced materials and interfacial engineering for next-generation ZIBs.3. Cathode materials of ZIBs
Selecting an appropriate cathode material is vital as it significantly influences the operating voltage and capacity of ZIBs. An ideal cathode material should fulfill these criteria: Selecting an appropriate cathode material is crucial, as it significantly affects the operating voltage and capacity of ZIBs. An ideal cathode material should meet several key criteria to ensure optimal performance, including high electrochemical stability, high electrode potential, favorable energy density, abundant Zn2+ storage sites, and compatibility with the aqueous electrolyte system commonly used in ZIBs.8,70,71At present, the choices of cathode materials for ZIBs primarily concentrates on manganese/vanadium-based oxides, Prussian blue analogues, spinel-structured oxides, organic materials, Chevrel phase compounds, layered sulfides, polyanionic compounds, and a few others. In this section, the selection of cathode materials according to the type would be elaborated.
3.1. Manganese-based oxides
The fascination with manganese-based oxides in the realm of electrochemical domain stems from their abundant natural presence, affordability, ecological suitability, and notably abundant redox chemical properties (Fig. 3a).72 Owing to the unique electronic structure of manganese, manganese oxides with several valence states exhibit diverse crystal formations. Taking MnO2 for a case study, it possesses an abundant crystalline formation, including α-, β-, γ-, δ-, λ-, and R-types (Fig. 3b).73 | ||
| Fig. 3 (a) Characterizations of MnO2/polypyrrole electrode for ZIBs, including TEM (inset), HRTEM, Raman, and XPS. Reproduced with permission from ref. 72. Copyright 2020. Elsevier. (b) Different MnO2 polymorphs. Reproduced with permission from ref. 73. Copyright 2018, American Chemical Society. | ||
The orange and blue octahedra surround spin up and down Mn atoms, respectively. The red atoms represent oxygen atom.73 In essence, basic coordination polyhedron is an octahedral unit composed of six oxygen atoms and one Mn atom. Various types of polymorphs can be established by connecting MnO6 octahedral units through edges and/or corners, which can be further divided into three categories:
All the aforementioned MnO2 polymorphs, including tunnel-type, layered-type, and spinel-type, can serve as cathode materials for ZIBs. MnO2 in different polymorphism adhere to distinct electrochemical reaction processes across diverse electrochemical systems,74 which would be encapsulated in Table 1.
| Materials | Reaction path | Ref. | ||
|---|---|---|---|---|
| α-MnO2 | 1 | Anode | Zn ↔ Zn2+ + 2e− | 75 |
| Cathode | Zn2 + 2e− + 2MnO2 ↔ ZnMn2O4 | |||
| 2 | Anode | Zn ↔ Zn2+ + 2e− | 76 | |
| Cathode | MnO2 + H+ + e− ↔ MnOOH | |||
| MnOOH + Zn2+ + e− → ZnMn2O4 | ||||
| 3 | Anode | Zn ↔ Zn2+ + 2e− | 77 | |
| Cathode | MnO2 + xZn2+ + 2xe− → ZnxMnO2 | |||
| MnO2 + H+ + e− → MnOOH | ||||
| 4Zn2+ + 6OH− + SO42− + 5H2O → ZnSO4·3Zn(OH)2·5H2O | ||||
| 2MnO2 + 2H+ + 2e− → Mn2O3 + H2O | ||||
| 3(ZnSO4·3Zn(OH)2·5H2O) + 3Mn2+ + 8e− → ZnMn3O7·3H2O + 8Zn2+ + 18OH− + 3ZnSO4 + 12H2O | ||||
| ZnxMnO2 + (0.5 − x)Zn2+ + (1 − 2x)e− → 0.5ZnMn2O4 | ||||
| Zn2Mn3O8 + Mn2+ → 2ZnMn2O4 | ||||
| β-MnO2 | 1 | Anode | Zn ↔ Zn2+ + 2e− | 78 |
| Cathode | MnO2 + H+ + e− ↔ MnOOH | |||
| MnOOH + 3H+ + e− ↔ Mn2+ + H2O | ||||
| 4Zn2+ + SO42− + 8H2O ↔ Zn4SO4(OH)6·5H2O + 6H+ | ||||
| γ-MnO2 | 1 | Anode | Zn ↔ Zn2+ + 2e− | 79 |
| Cathode | 2γ-MnO2 + Zn2+ + 2e− ↔ spinel ZnMn2O4 | |||
| Spinel ZnMn2O4 + (2x − 1) Zn2+ + (2x − 1)e− ↔ 2γ-ZnxMnO2 tunnel-type | ||||
| γ-ZnxMnO2 tunnel-type ↔ L-ZnxMnO2 (layered-type) | ||||
| δ-MnO2 | 1 | Anode | Zn ↔ Zn2+ + 2e− | 80 |
| Cathode | Zn + 2MnO2 ↔ ZnMn2O4 | |||
| Zn + MnO2 ↔ ZnMnO2 | ||||
| 2 | Anode | (1/2 + x)Zn ↔ (1/2 + x)Zn2+ + (1 + 2x)e− | 81 | |
| Cathode | MnO2 + xZn2+ + 2xe− ↔ ZnxMnO2 (non-diffusion controlled) | |||
| H2O ↔ H+ + OH− | ||||
| MnO2 + H+ + e− ↔ MnOOH (diffusion controlled) | ||||
| 1/2Zn2+ + OH− + 1/6Zn(TFSI)2 + x/6H2O ↔ 1/6Zn(TFSI)2[Zn(OH)2]3·xH2O | ||||
Each polymorph offers distinct advantages, such as enhanced ion diffusion, structural stability, or higher capacity retention, depending on the nature of the MnO2 structure. However, despite their potential, there remain critical challenges that need to be addressed. Issues such as limited cycle stability, dissolution of Mn in the electrolyte, and relatively sluggish ion diffusion in certain structures must be overcome to optimize their performance in practical ZIB applications. Further research is required to improve the durability, efficiency, and scalability of these MnO2-based cathode materials for the next generation of sustainable energy storage solutions.
3.2. Vanadium-based compounds
The diverse valence states of vanadium, ranging from +2 to +5, have facilitated the exploration of a wide range of chemical compositions and crystal structures. Various vanadium-based compounds, including vanadium oxides,90 vanadium phosphates,91 vanadates,92 vanadium sulfides,93 and vanadium nitrides,94 have been investigated as potential cathode materials for ZIBs. In this review, we provide a comprehensive overview of recent advancements in vanadium-based electrode materials for aqueous ZIBs, highlighting their structural characteristics, electrochemical performance, and the challenges that remain for their practical application. | ||
| Fig. 4 Various vanadium coordination polyhedrons (red O atoms, green V atoms, and pink V–O bonds). Reproduced with permission from ref. 97. Copyright 2023. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
 | ||
| Fig. 5 (a) Schematic illustration of the Zn2+ (de)intercalation mechanism in the bilayer VOPO4 nanosheets. Reproduced with permission from ref. 104. Copyright 2021. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (b) VOPO4·2H2O for ZIBs. Reproduced with permission from ref. 102. Copyright 2018, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (c) KVOPO4 electrodes for ZIBs. Reproduced with permission from ref. 105. Copyright 2021, Elsevier Ltd. | ||
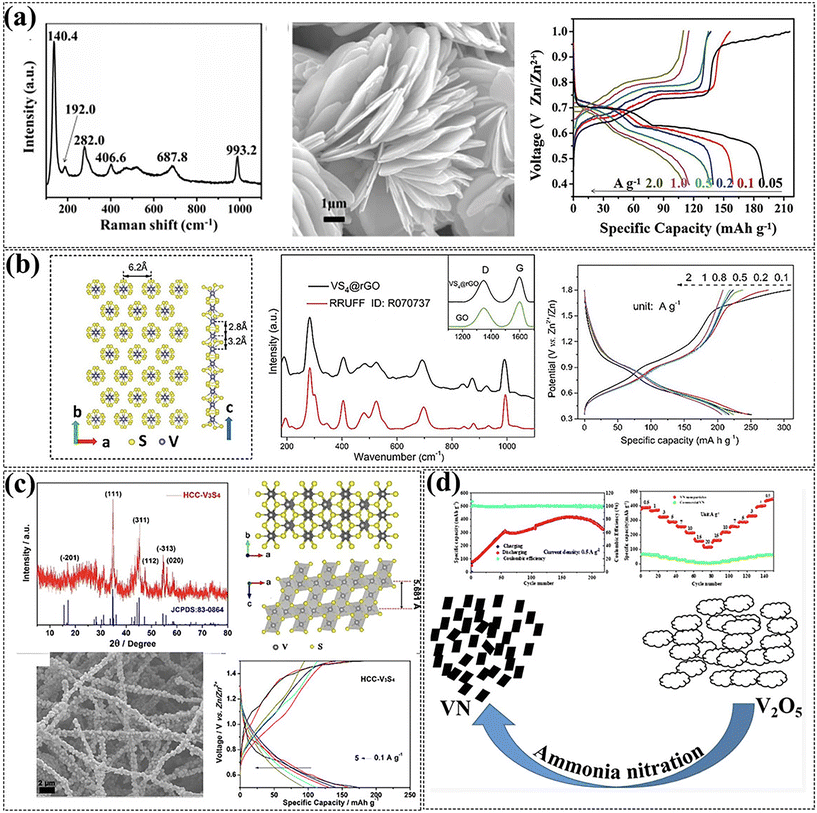 | ||
| Fig. 6 (a) VS2 cathodes for ZIBs. Reproduced with permission from ref. 113. Copyright 2017, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (b) VS4 cathodes for ZIBs. Reproduced with permission from ref. 114. Copyright 2018, Royal Society of Chemistry. (c) V3S4 cathodes for ZIBs. Reproduced with permission from ref. 115. Copyright 2019, American Chemical Society. (d) VN cathodes for ZIBs. Reproduced with permission from ref. 116. Copyright 2021, American Chemical Society. | ||
3.3. Prussian blue analogues
Extensive research has been conducted on Prussian blue analogues (PBAs) as hosts for metal-ion batteries, characterized by their adoption of three-dimensional open-structure configurations, numerous redox-active sites, and significant structural steadiness.117,118 The ideal PBA is indicated by the equation A2MAMB(CN)6 (Fig. 7a), where A denotes as Li+, Na+, K+, zeolite water, and MA symbolizes Fe, Ni, Mn, V, Mo, Cu, Co, Zn, and MB signifies Fe, Co, Cr, Ru. Owing to its robustness in aqueous solutions, ferricyanide stands as the main raw material for synthesis, resulting in a majority of MB sites within this structure being filled by Fe atmos. PBA represents a kind of metallic compound with a body-centered cubic configuration, where nitrogen and carbon atoms from C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N ligand link with MB and MA atoms to create open ion channels and expansive interstitial gaps.119 Zhang et al.120 demonstrated the feasibility for zinc hexacyanoferrate (Zn3[Fe(CN)6]2, ZnHCF) as cathode for aqueous ZIBs, showing high operating voltage (Fig. 7b). In PBAs, the MB site exemplifies the redox active site in this configuration. That is to say, replacing a distinct transition metal MA affects the reaction potential and the ability of PBAs. Different or the same metal atoms inhabit the MA and MB locations, leading to unique combination and reaction potentials.121,122 The elements Fe, Mn, Co, and V exhibit electrochemical activity within the stable range of the organic electrolyte and aqueous system in PBAs. A double-electron redox reaction may take place when these metal ions fill the MA site.
N ligand link with MB and MA atoms to create open ion channels and expansive interstitial gaps.119 Zhang et al.120 demonstrated the feasibility for zinc hexacyanoferrate (Zn3[Fe(CN)6]2, ZnHCF) as cathode for aqueous ZIBs, showing high operating voltage (Fig. 7b). In PBAs, the MB site exemplifies the redox active site in this configuration. That is to say, replacing a distinct transition metal MA affects the reaction potential and the ability of PBAs. Different or the same metal atoms inhabit the MA and MB locations, leading to unique combination and reaction potentials.121,122 The elements Fe, Mn, Co, and V exhibit electrochemical activity within the stable range of the organic electrolyte and aqueous system in PBAs. A double-electron redox reaction may take place when these metal ions fill the MA site.
 | ||
| Fig. 7 (a) Schematic representation of the crystal structure of the class of materials belonging to the PBAs. Reproduced with permission from ref. 117. Copyright 2020, Elsevier Ltd. (b) Prussian blue ideal framework and its coordination form. Reproduced with permission from ref. 119. Copyright 2024, Royal Society of Chemistry. | ||
Based on the redox sites involved in the reaction, PBA-based cathode materials can be divided into two categories: a single redox pair and double redox pair. As for a single redox pair PBA-based cathode, it shows a pair of anodic/cathodic react ion peaks, while two pairs presented for the cathode of double redox pair. Advances in in situ characterization techniques have helped researchers better understand the presence of double REDOX pairs in electrochemical mechanisms. Yang et al.123 presents innovative insights into the electrochemical mechanisms of vanadium hexacyanoferrate (VOHCF) as a cathode material. As the charge impelling, the strength of the V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O between 864–921 cm−1 steadily reduces, suggesting a gradual process of ion insertion. When discharged, the intensity for V
O between 864–921 cm−1 steadily reduces, suggesting a gradual process of ion insertion. When discharged, the intensity for V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak gradually intensifies, indicating the possibility of reversible ion intercalation and de-intercalation into VOHCF. Besides, the [Fe(CN)6]4− group gradually shifts to higher wavenumbers, followed by a reverse migration that corresponds to Fe2+ to Fe3+, thereby demonstrating the pivotal role of [Fe(CN)6]4− as an additional REDOX active site.
O peak gradually intensifies, indicating the possibility of reversible ion intercalation and de-intercalation into VOHCF. Besides, the [Fe(CN)6]4− group gradually shifts to higher wavenumbers, followed by a reverse migration that corresponds to Fe2+ to Fe3+, thereby demonstrating the pivotal role of [Fe(CN)6]4− as an additional REDOX active site.
PBAs are emerging as highly promising cathode for ZIBs due to their unique combination of high theoretical capacity, excellent cycling stability, and rapid ion diffusion kinetics enabled by their open-framework structures.124 These materials offer significant advantages, including cost-effectiveness, environmental benignity, and compatibility with aqueous electrolytes, which collectively support their potential for ideal cathode. However, PBAs also face challenges such as relatively low electrical conductivity, which can impede rate performance, and potential structural degradation in highly acidic or alkaline electrolytes over prolonged cycling. Despite these limitations, ongoing research efforts aimed at enhancing conductivity through composite formulations and optimizing structural stability are paving the way for PBAs to overcome these hurdles, thereby positioning them as a viable and sustainable solution for next-generation ZIBs.
3.4. Organic materials
The appeal of organic compounds, including small molecules and polymers, as electrode materials for aqueous ZIBs lies in their eco-friendly nature, structural versatility, cost-effectiveness, and tunable electrochemical properties.125,126 Unlike conventional inorganic cathodes, organic materials are inherently sustainable, composed of abundant light elements such as carbon (C), hydrogen (H), oxygen (O), nitrogen (N), and sulfur (S), eliminating reliance on scarce or toxic heavy metals (e.g., Co, Ni). These materials can be synthesized through scalable artificial routes or derived from renewable biomass resources, aligning with green chemistry principles. Critically, their molecular and polymeric frameworks allow for precise customization of physical properties (e.g., solubility, porosity, and electronic conductivity) and electrochemical performance (e.g., redox potentials, ion-binding affinity) via functional group engineering or conjugation extension.127 Furthermore, the redox mechanisms in organic electrodes differ fundamentally from inorganic counterparts: instead of Zn2+ intercalation/deintercalation, which induces structural strain and phase transitions, organic materials undergo reversible bond reorganization (e.g., enolization, proton-coupled electron transfer) during charge/discharge, enabling smoother ion accommodation and reduced mechanical degradation.126Organic electrode materials are classified into three categories based on their redox-active functional groups and charge-compensation mechanisms during cycling (Fig. 8):
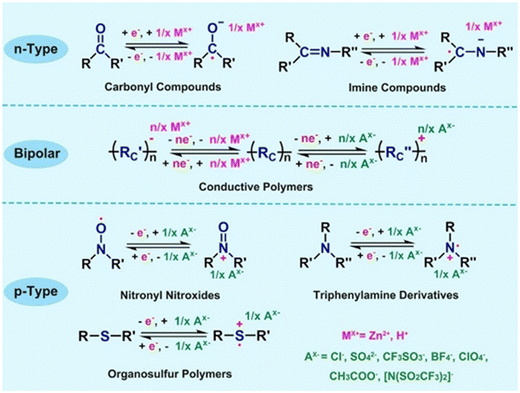 | ||
| Fig. 8 The energy storage mechanisms of different types of organic electrode materials in aqueous ZIBs. Reproduced with permission from ref. 126. Copyright 2017, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) undergo reduction, generating negatively charged enolate or radical anions. These anions stabilize through coordination with cations (e.g., Zn2+, H+) from the electrolyte, enabling reversible Zn2+ storage. For example, in a quinone-based cathode, the reduction of C
O) undergo reduction, generating negatively charged enolate or radical anions. These anions stabilize through coordination with cations (e.g., Zn2+, H+) from the electrolyte, enabling reversible Zn2+ storage. For example, in a quinone-based cathode, the reduction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O to C–O− during discharge attracts Zn2+ ions, forming a Zn–organic complex. Upon charging, the process reverses, releasing Zn2+ back into the electrolyte. A key advantage of n-type materials is their high theoretical capacity (often >300 mA h g−1) due to multi-electron redox reactions. However, challenges include solubility in aqueous electrolytes (leading to capacity fade) and sluggish kinetics caused by strong electrostatic interactions between Zn2+ and the organic matrix. Strategies to mitigate these issues involve polymerization, cross-linking, or hybridization with conductive carbon networks to enhance stability and charge transfer.
O to C–O− during discharge attracts Zn2+ ions, forming a Zn–organic complex. Upon charging, the process reverses, releasing Zn2+ back into the electrolyte. A key advantage of n-type materials is their high theoretical capacity (often >300 mA h g−1) due to multi-electron redox reactions. However, challenges include solubility in aqueous electrolytes (leading to capacity fade) and sluggish kinetics caused by strong electrostatic interactions between Zn2+ and the organic matrix. Strategies to mitigate these issues involve polymerization, cross-linking, or hybridization with conductive carbon networks to enhance stability and charge transfer.
While organic electrodes offer unique advantages for ZIBs, practical deployment requires addressing intrinsic limitations. Their low electronic conductivity often necessitates composite architectures with conductive additives (e.g., carbon nanotubes, graphene). Solubility in aqueous electrolytes remains a persistent issue, particularly for small-molecule organics, driving research into covalent immobilization strategies or gel-based electrolytes. Furthermore, the redox mechanisms of many organic materials involve H+ co-insertion, which complicates Zn2+ storage quantification and may accelerate hydrogen evolution at the anode. Future work should prioritize in operando characterization (e.g., X-ray diffraction, Raman spectroscopy) to decouple Zn2+/H+ contributions and machine learning-guided molecular design to optimize ion selectivity. By synergizing molecular engineering with electrolyte innovation, organic materials could emerge as high-performance, sustainable cathodes for next-generation ZIBs.
3.5. Chevrel phase compounds
Chevrel-phase materials (with the chemical formula Mo6T8, where T = S, Se, or their composites) have attracted increasing attention since their discovery in the 1970s due to their unique rigid open-framework structure and excellent ion-insertion capability, making them a research hotspot for multivalent-ion batteries (such as Mg2+ and Zn2+).130 In terms of crystal structure, Chevrel phase Mo6T8 with a three-dimensional array of Mo6T8 units in which each Mo6T8 unit is composed of the octahedral cluster of molybdenum atoms inside the anion cube to form tri-directional channels for metal cations.131,132 As can be seen in Fig. 9a, there are three different cation locations for Mo6T8 (cavities 1, 2 and 3). Cavity 1 is the site far away from Mo atom and share corner with the Mo6T8 cube. Cavity 2 and 3 share the edges and faces with Mo6T8, respectively. Only the cavities 1 and 2 can be filled by metal cation when cavity 3 is blocked by a strong repulsion of the Mo atoms. Metal cations in cavities 1 or 2 are primarily positioned based on size of M, with M cations of greater atomic size typically situated at the cavity's center. Taking Mg2+ as a typical example (Fig. 9b), it insertion into Mo6T8 (i.e., T = S) firstly occupies the inner six sites in cavity 1 with an inner ring and the cation occupies in cavity 2 with another six sites with an outer ring. The potential energy of the cation site in cavity 1 is less compared to that in insertion cavity 2. Research has been conducted on various monovalent (like Li, Na) and multi-valent cation (such as Zn, Mg, Al) intercalation-type batteries, grounded in the Chevrel phase's crystal structure. | ||
| Fig. 9 (a) Different schematic presentation of graphic structure of Mo6T8 and Mo6S8 and typical discharge–charge profiles of Mo6Se8 and Mo6S8 electrode. Reproduced with permission from ref. 130. Copyright 2017, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (b) ZnxMo6S8 cathode for ZIBs. Reproduced with permission from ref. 136. Copyright 2016, American Chemical Society. | ||
In recent years, with the rise of aqueous ZIBs, Chevrel-phase materials have been considered as a highly promising cathode material system for ZIBs due to their efficient and reversible storage characteristics for Zn2+. More importantly, the working voltage range of Chevrel-phase materials (0.4–1.2 V vs. Zn2+/Zn) highly match the stability window of aqueous electrolytes, avoiding hydrogen-evolution and oxygen-evolution side reactions. Moreover, their intrinsic oxidation-resistance (Mo–S bond energy > 300 kJ mol−1) ensures chemical stability at high temperatures, meeting the stringent safety requirements of ZIBs.51,133–135 Hong's research team136 achieved the reversible insertion/extraction of Zn2+ in Mo6S8 through electrochemical methods, demonstrating the feasibility of Chevrel-phase materials in aqueous ZIBs.
The Chevrel phase garners more interest as a potential electrode for ZIBs due to its distinctive open crystal formation and robust electrochemical behavior.130 Yet, currently, the synthesis conditions for Chevrel phase remain somewhat stringent relative to other cathode materials, and the challenge of optimizing the preparation conditions deserves a consideration in the future, the employment of synthesis methods such as Joule heating or microwave-assisted strategy may be the remedies.135
3.6. MXenes
Two-dimensional transition metal carbides, nitrides and carbonitrides, also termed as MXenes, are recognized for their adaptability owing to their high electrical conductivity and rich surface chemistry.137,138 Like many two-dimensional materials, MXene follows a typical intercalation mechanism when applied directly to the cathode for the ZIBs, offering inherent benefits as a cathode material for ZIBs, not only can it be used directly as an active material to host Zn2+, but also as a stable conductive network to be combined with other materials.137,139,140 Huang et al.141 delved into the intricate characteristics of different MXene materials containing different halogen groups in the molt salt, it is found that the electrochemical properties of MXene cathodes for ZIBs can be optimized effectively by adjusting the types of terminal groups. Besides, owing to the superior conductivity and hydrophilicity, it's feasible to composite MXene with active material possessing greater theoretical capacity. Using transition metal oxides as a case study, while their conductivity is typically low, but electrochemical performance can be enhanced after composited with MXene. Yan et al.142 prepared an innovative 3D MXene–MnO2 composite cathode using a gas-phase spray drying technique, encapsulating MnO2 nanoparticles within the wrinkled MXene nanosheets, resulting in a sturdy, conductive structure resembling a 3D micro-flower, advantageous for quick ion/electron movement and enhanced structural stability. When used as cathode for ZIBs, the Ti3C2Tx@MnO2 micro-flowers delivers a high reversible capacity, which can be superior to the pure MnO2 electrode.Despite the promising properties of MXenes, several challenges need to be addressed for their practical application in ZIBs. These include ensuring structural stability during long-term cycling, improving ion diffusion kinetics, and addressing the potential toxicity of certain transition metals. Future research should focus on developing advanced synthesis methods to enhance the stability and performance of MXenes, as well as exploring new MXene-based composites to further improve their electrochemical properties. Additionally, the oxidation of MXenes under reactive conditions remains a critical challenge.143,144 Furthermore, MXene's present production technique primarily involves hydrofluoric acid, where the acid's corrosive nature poses extra safety hazards. Future studies should focus on developing safer and more environmentally friendly methods, which can be the foundation to advance the MXene-based cathode materials for ZIBs.140,145,146
In recent years, significant progress has been made in the development of cathode materials for ZIBs. Researchers have explored a wide range of materials, from traditional inorganic compounds to emerging organic and composite materials, each offering unique advantages in terms of voltage, capacity, and cycling stability (Table 3). While some materials have demonstrated high operating voltages and excellent rate capabilities, others have shown remarkable discharge capacities and long-term cyclability. These advancements highlight the potential for ZIBs to address key challenges in energy storage applications.
| Cathode materials | Electrolyte component | Testing voltage (V) | Cycling performance (retention/cycles/rates) | Ref. |
|---|---|---|---|---|
| α-MnO2 | 2 M ZnSO4 + 0.1 M MnSO4 | 1.0–1.8 | 92%/5000/1540 mA g−1 | 83 |
| β-MnO2 | 3 M Zn(CF3SO3)2 + 0.1 M Mn(CF3SO3)2 | 0.8–1.9 | 94%/2000/2000 mA g−1 | 147 |
| V2O5 | 3 M ZnSO4 | 0.4–1.4 | 75%/400/100 mA g−1 | 148 |
| VO2(B)/RGO | 3 M Zn(CH3F3SO3) | 0.2–1.4 | 90%/1000/5 A g−1 | 149 |
| CuHCF | 1 M ZnSO4 | 0.2–1.2 | 77%/20/20 mA g−1 | 150 |
| ZnHCF | 1 M ZnSO4 | 0.8–1.9 | 81%/100/300 mA g−1 | 120 |
| Quinone(C4Q) | 3 M Zn(CF3SO3)2 | 0.2–1.8 | 87%/1000/500 mA g−1 | 151 |
| p-Chloranil | 1 M Zn(CF3SO3)2 | 0.8–1.4 | 70%/200/0.2 C | 152 |
| VS2 | 1 M ZnSO4 | 0.4–1.4 | 98%/200/50 mA g−1 | 113 |
| VS2-RGO | 3 M Zn(CF3SO3)2 | 0.2–1.8 | 93%/1000/5 A g−1 | 153 |
| MO6S8 | 1 M ZnSO4 | 0.25–1.0 | 95%/150/180 mA g−1 | 51 |
| V2CTx | 21 M LiTFSI/1 M Zn(CF3SO3)2 | 0.1–2.0 | ∼100%/2000/10 A g−1 | 154 |
| VO2/Ti3C2 | 2 M Zn(CF3SO3)2 | 0.2–1.4 | 76%/5000/6 A g−1 | 155 |
However, despite these achievements, several challenges remain. Many cathode materials still suffer from issues such as complex preparation process, harsh storage conditions and so on. Additionally, the complex interplay between material properties and electrolyte interactions limits the practical application of these materials to some extent. Going forward, the architecture of cathode materials for ZIBs must take into account its compatibility with the electrolyte stability window and the theoretical capacity for anode. Moreover, the development of new reaction mechanisms and the exploration of sustainable, cost-effective cathode materials are crucial for the long-term success of ZIBs.
3.7. Summary of this chapter
Cathode materials play a pivotal role in determining the performance of zinc-ion batteries (ZIBs), with ideal candidates requiring high electrochemical stability, favorable energy density, and efficient Zn2+ storage. Major cathode categories include (1) manganese-based oxides (α-, β-, γ-, δ-, λ-MnO2), which offer tunable structures but face challenges like Mn dissolution and sluggish kinetics; (2) vanadium-based compounds (V2O5, VOPO4, NASICON-type phosphates), leveraging multiple redox states for high capacity yet hindered by structural instability; (3) Prussian blue analogues (PBAs), prized for their open frameworks and dual redox sites but limited by low conductivity; (4) organic materials (n-type, p-type, bipolar), which are sustainable and tunable but suffer from solubility and poor conductivity; (5) Chevrel-phase compounds (Mo6S8), enabling reversible Zn2+ intercalation with high thermal stability but complex synthesis; and (6) MXenes, valued for conductivity and compositing potential but challenged by oxidation and toxicity. While advancements in structural engineering (e.g., 3D architectures, surface coatings) and electrolyte optimization (e.g., MnSO4 additives) have improved performance, issues like capacity fade, side reactions, and scalability persist. Future research must focus on mechanistic insights, sustainable designs, and compatibility with aqueous electrolytes to realize practical ZIBs.4. Electrolyte research
The scarcity of resources required for LIBs, flammable and toxic organic electrolytes that are harmful to the environment and safety, and the fact that they cannot be easily recycled limit the further development of LIBs. In contrast, ZIBs are rich in resources, are less prone to thermal runaway at high temperatures or short circuits, and are highly secure, while having a low environmental impact and resources that can be efficiently recovered and recycled. Liquid electrolytes and solid electrolytes are used in different types of ZIBs depending on the application requirements and design decisions of the ZIBs.4.1. Liquid electrolytes
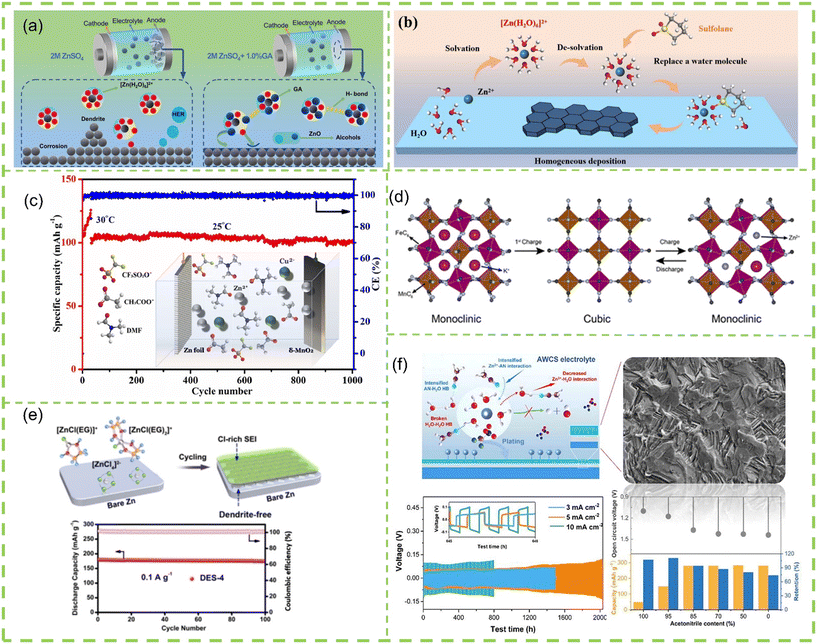 | ||
| Fig. 10 Liquid electrolytes: (a) a natural multifunctional additive gum arabic into the ZnSO4-based electrolyte. Reproduced with permission from ref. 156. Copyright 2023, Elsevier Ltd. (b) Schematic illustration for Zn deposition cycled in sulfolane/ZnSO4 electrolyte. Reproduced with permission from ref. 157. Copyright 2023, Elsevier Ltd. (c) DMF based nonaqueous electrolyte containing Cu2+ as an additive. Reproduced with permission from ref. 158. Copyright, Elsevier Ltd. (d) Schematic diagram of the redox reaction in the Zn(TFA)2-AN-TEP nonaqueous electrolyte. Reproduced with permission from ref. 159. Copyright 2022, American Chemical Society. (e) A new eutectic electrolyte consisting of EG and ZnCl2 for dendrite-free and long-lifespan ZIBs. Reproduced with permission from ref. 160. Copyright 2022, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (f) Acetonitrile is introduced into Zn(OTf)2 electrolyte as co-solvent reproduced with permission from ref. 161. Copyright 2022, Elsevier Ltd. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles (Fig. 10e). Similarly, Meng et al.161 introduced an electrolyte that combines AN and water as co-solvents, demonstrating its applicability across various battery systems. The resultant electrolyte interface, formed by AN, effectively controlled zinc deposition, prevented hydrogen formation, and notably enhanced the cycling stability and rate performance, Zn//Zn symmetric cell with hybrid electrolyte has an ultra-long cycle of 2100 hours at a current density of 5 mA cm−2 (Fig. 10f). In a recent breakthrough, Mei et al.166 unveiled an organic-aqueous hybrid electrolyte incorporating Zn(OTf)2-TEP/H2O. This formulation showcased the remarkable capability to enable the anode to exhibit outstanding zinc storage capacity, consequently elevating the stability of ZIBs to new heights. The capacity at a current density of 0.5 A g−1 was 117.8 mA h g−1, and the capacity retention after 4000 cycles reached 78.6%. The advent of these hybrid electrolytes signifies a pivotal advancement in ZIBs technology, offering tailored solutions to overcome existing challenges and elevating the prospects for safer, more stable, and higher-performing energy storage systems.
000 cycles (Fig. 10e). Similarly, Meng et al.161 introduced an electrolyte that combines AN and water as co-solvents, demonstrating its applicability across various battery systems. The resultant electrolyte interface, formed by AN, effectively controlled zinc deposition, prevented hydrogen formation, and notably enhanced the cycling stability and rate performance, Zn//Zn symmetric cell with hybrid electrolyte has an ultra-long cycle of 2100 hours at a current density of 5 mA cm−2 (Fig. 10f). In a recent breakthrough, Mei et al.166 unveiled an organic-aqueous hybrid electrolyte incorporating Zn(OTf)2-TEP/H2O. This formulation showcased the remarkable capability to enable the anode to exhibit outstanding zinc storage capacity, consequently elevating the stability of ZIBs to new heights. The capacity at a current density of 0.5 A g−1 was 117.8 mA h g−1, and the capacity retention after 4000 cycles reached 78.6%. The advent of these hybrid electrolytes signifies a pivotal advancement in ZIBs technology, offering tailored solutions to overcome existing challenges and elevating the prospects for safer, more stable, and higher-performing energy storage systems.
4.2. Solid electrolyte
The surge in demand for flexible ZIBs in the context of flexible electronic devices has fueled extensive research into the development of appropriate solid electrolytes.167–169 In contrast to liquid electrolytes, solid electrolytes offer multiple advantages. They not only eliminate the issue of electrolyte leakage but also function as a diaphragm to avoid cathode and anode contact while transporting zinc ions. Additionally, solid electrolytes possess distinct mechanical properties that enable them to withstand various mechanical operations such as stretching, bending, and twisting. In terms of physical state, solid electrolytes are typically categorized into two main types: all-solid-state electrolytes and quasi-solid-state electrolytes applied to solid-state and quasi-solid-state batteries.17,170–172 | ||
| Fig. 11 Solid electrolyte: (a) fabricate flexible all-solid-state ZIBs through stencil printing combined with UV-assisted curing technology. Reproduced with permission from ref. 173. Copyright 2023, Elsevier Ltd. (b) A solid polymer electrolyte based on poly (methyl acrylate) grafted MXenes. Reproduced with permission from ref. 171. Copyright 2022, Elsevier Ltd. (c) DMSO was combined with H2O to prepare a gel electrolyte (PAM-DMSO/H2O). Reproduced with permission from ref. 174. Copyright 2023, Elsevier Ltd. (d) A lean water hydrogel electrolyte. Reproduced with permission from ref. 175. Copyright 2023, Elsevier Ltd. | ||
4.3. Summary of this chapter
Electrolytes play a critical role in zinc-ion batteries (ZIBs), influencing ion transport, stability, and interfacial compatibility. Aqueous electrolytes (e.g., ZnSO4, Zn(CF3SO3)2) are cost-effective and safe but suffer from water-induced side reactions like hydrogen evolution and dendrite growth, which additives (e.g., gum arabic, sulfolane) can mitigate. Non-aqueous electrolytes (e.g., AN, DMF-based systems) offer wider voltage windows and high-temperature stability but face challenges in ionic conductivity and interfacial compatibility. Hybrid electrolytes (aqueous/non-aqueous blends, e.g., EG-ZnCl2, AN-H2O) combine the advantages of both, enhancing cycling stability and suppressing parasitic reactions. Solid-state electrolytes (all-solid and quasi-solid) eliminate leakage and dendrite issues while enabling flexible ZIBs. All-solid-state electrolytes (e.g., polymer-MXene composites) improve safety but require enhanced conductivity, whereas quasi-solid gels (e.g., PAM-DMSO/H2O hydrogels) balance ionic transport and mechanical flexibility. Future research should focus on optimizing electrolyte formulations (additives, hybrid systems) and interfacial engineering to achieve high-performance, durable, and scalable ZIBs. In addition, we summarize the key parameters of the cathode, anode, and electrolyte in Table 4 to achieve more effective matching for higher performance of zinc-ion batteries.| Type | Material | Specific capacity | Cycle life (retention) | Notable features |
|---|---|---|---|---|
| Anode | Zn metal | ∼820 mAh g−1 (theoretical); practical ∼300 mAh g−1 | 100–300 cycles (rapid degradation if unprotected) | Dendrite formation; requires additives/coatings for stability |
| Anode | Zn–Al alloy18 | ∼320 mAh g−1 (at 0.5 mA cm−2) | 1000+ hours in symmetric cell (>80% capacity retention) | Lamellar alloy structure suppresses dendrites and prolongs Zn plating life |
| Anode | Zn@carbon foam15 | ∼300 mAh g−1 (at 2 mAh cm−2) | 3000 h stable Zn plating (no shorting) | 3D porous carbon host ensures uniform Zn deposition |
| Cathode | α-MnO2 (nanowire)27 | 280 mAh g−1 (0.3 A g−1) | 94% capacity after 2000 cycles | MnSO4 additive used to suppress Mn dissolution |
| Cathode | V2O5 (hydrated)30 | 350 mAh g−1 (0.1 A g−1) | 91% after 4000 cycles (5 A g−1) | Pre-inserted H2O in structure enables fast Zn2+ diffusion and stability |
| Cathode | ZnHCF (Prussian blue analog)42 | ∼65 mAh g−1 (0.1 A g−1) | 80% after 5000 cycles (5 C rate) | Open framework with minimal strain; excellent high-rate capability |
| Cathode | PANI (polymer)45 | ∼110 mAh g−1 (0.5 A g−1) | 85% after 1000 cycles | Conductive polymer, stores charge via protonation/deprotonation; requires carbon composite for conductivity |
| Cathode | Mo6S8 (Chevrel)51 | ∼100 mAh g−1 (0.2 A g−1) | 90% after 400 cycles | 3D cluster framework allows rapid Zn2+ intercalation with low strain |
| Electrolyte | 2 M ZnSO4 (aqueous) | — | — | Baseline electrolyte; pH ∼4–5; voltage window ∼1.8 V; prone to Zn dendrites and HER at Zn anode |
| Electrolyte | “Water-in-salt” (e.g. 30 m ZnCl2)16 | — | — | Super-concentrated electrolyte extends stability window to ∼2.3 V; suppresses water activity and dendrites; higher viscosity/cost |
| Electrolyte | Zn(OTf)2-Acetonitrile160 | — | — | Non-aqueous/aqueous hybrid electrolyte; widens voltage window, eliminates water-induced side reactions; flammable organic content |
| Electrolyte | PVA–ZnSO4 gel (quasi-solid)4 | — | 95% after 500 cycles (bendable cell) | Gel polymer electrolyte with ionic conductivity ∼10−3 S cm−1; improves safety and enables flexible ZIB designs |
5. Working mechanism for ZIBs
Research on the working mechanism of ZIBs is of paramount importance, which not only helps in optimizing their electrochemical performance, such as energy density and cycling stability, but also provides a foundation for developing safer and more sustainable energy storage solutions. Understanding how zinc ions interact with electrode materials during charge and discharge can lead to the design of novel electrode materials with enhanced conductivity and capacity.19 Additionally, it aids in identifying degradation mechanisms and developing strategies to mitigate them, thereby extending the lifespan of the batteries.33 As for most anode side in ZIBs, the electrochemical process entails a straightforward reversible redox reaction of Zn (Zn0 − 2e− ⇌ Zn2+).19 However, the electrochemical reaction for the cathode would be much more complex. According to the redox chemistry, the working mechanisms can be categorized as cationic redox, anionic redox, and redox of active functional groups.5.1. Cationic redox
Cationic redox refers to the reduction/oxidation of transition metals, such as Mn4+ ⇌ Mn3+ ⇌ Mn2+, Fe3+ ⇌ Fe2+ and so on. Given that Zn2+ (0.74 Å) have a similar ionic radius to that of Li+ (0.76 Å), which can be favorable for the insertion and extraction of carriers within the host material. Due to the existence of solvation effect, Zn2+ typically undergoes hydration with six water molecules to form hydrated cations with a radius of 4.3 Å, which means large interlayer distances and/or tunnel sizes should be considered for cathode material design. Remarkably, the surrounded H2O can be capable of effectively shielding the electrostatic interactions of the intercalated Zn2+, facilitating the insertion and extraction.In the mildly acidic electrolytes, the directional movement of zinc ions would be often accompanied with the movement of protons, which may or may not be a simultaneous process. This process largely depends on the choice of material system. Chen et al. demonstrated that H+ and Zn2+ could be simultaneously inserted and extracted into/from the NaV3O8·1.5H2O cathode, which renders excellent electrochemical performance.112 For MnO2 cathodes, the reversible insertion and extraction of H+ and Zn2+ have been shown to be a two-step process. Wang and colleagues76 proposed this stepwise mechanism based on an electrodeposited ε-MnO2. From the galvanostatic intermittent titration technique (GITT) curve, the rapid ionic diffusion with minimal voltage fluctuation indicated the intercalation of H+. As the depth of discharge continues, the significant overpotential corresponded to the Zn2+ intercalation due to its slower diffusion rate. It is worth noting that the insertion of H+ increases the concentration of OH− near the cathode, leading to the precipitation of ZnSO4[Zn(OH)2]3·xH2O flakes on the cathode surface. The extraction of H+ after recharging dissolves the ZnSO4[Zn(OH)2]3·xH2O products.
5.2. Anionic redox
The redox process of anions, such as oxygen, sulfur, and nitrogen, has been observed as an accompanying mechanism in ZIBs. Chen and his team101 discovered a dual-redox process involving both cationic V and anionic oxygen using a VOPO4 cathode. The oxidation of oxygen occurred at the end of the first charge, indicating the oxidation of lattice oxygen from O2− to O1−. In reverse, O1− was reduced back to O2− during the subsequent discharge from 2.1 V to 1.7 V. The reduced oxygen intensity in the X-ray absorption spectra (SXAS) at the fully discharged state was attributed to the reduction of V. Conversely, the stronger signals at the fully charged state compared to the initial state indicated the oxidation of oxygen. It is important to note that the oxygen redox chemistry not only contributes additional specific capacity but also increases the average working voltage of Zn/VOPO4 batteries. It is important to note that the oxygen redox chemistry not only contributes additional specific capacity but also increases the average working voltage of Zn/VOPO4 batteries. A similar dual-ion mechanism was also observed in a surface-oxidized vanadium nitride (VNxOy) cathode, which involved a simultaneous reversible reaction of cationic redox (V3+ ↔ V2+) during Zn2+/H+ de-/intercalation and pseudo-capacitance-like surface anionic redox (N3+ ↔ N2+) during OH− release/uptake.183 Notably, the lower electronegativity of nitrogen compared to oxygen can weaken the ionic bonds between Zn2+ and oxygen, thereby enabling rapid and reversible diffusion of Zn2+. As a result, the VNxOy cathode exhibited outstanding rate performance of 200 mA h g−1 at 30 A g−1 and superior 2000-cycling durability.5.3. Redox of active functional groups
In recent years, researchers have explored a variety of organic compounds with abundant functional groups as alternative cathodes for ZIBs, such as PANI,184 quinone,185 calix[4]quinone (C4Q),151 sulfur heterocyclic quinone dibenzo[b,i]thianthrene-5,7,12,14-tetraone186 and pyrene-4,5,9,10-tetraone (PTO).187 The reaction mechanism of these organic compounds typically involves the redox activity of functional groups within their chain structure, such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– and carbonyl groups (C
N– and carbonyl groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).188 For example, in PANI, the C
O).188 For example, in PANI, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N−– and protonated C
N−– and protonated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH+– groups are recognized as the electrochemically active sites for Zn2+ storage.189 During the initial discharge, the protonated
NH+– groups are recognized as the electrochemically active sites for Zn2+ storage.189 During the initial discharge, the protonated ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH+ groups are reduced to –NH–. At the same time, the C
NH+ groups are reduced to –NH–. At the same time, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– in the polymer backbone is reduced to electronegative –N– species, which can attract Zn2+. After partial charging, both the –NH– and –N– groups return to their original
N– in the polymer backbone is reduced to electronegative –N– species, which can attract Zn2+. After partial charging, both the –NH– and –N– groups return to their original ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH+– and C
NH+– and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– states, respectively. It is worth noting that the surplus –NH– groups in PANI can be further oxidized to
N– states, respectively. It is worth noting that the surplus –NH– groups in PANI can be further oxidized to ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH+– when fully charged. In addition to nitrogen-containing species, the electronegative oxygen in C
NH+– when fully charged. In addition to nitrogen-containing species, the electronegative oxygen in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups can also serve as active centers to coordinate with Zn ions. Thanks to the abundance of C
O groups can also serve as active centers to coordinate with Zn ions. Thanks to the abundance of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O active centers on the surface, each C4Q molecule can coordinate with three Zn2+.151 Although still in the early stages, these innovative preliminary studies may pave a new way for the development of novel organic compounds with rich functional groups that can actively participate in redox reactions with Zn2+.
O active centers on the surface, each C4Q molecule can coordinate with three Zn2+.151 Although still in the early stages, these innovative preliminary studies may pave a new way for the development of novel organic compounds with rich functional groups that can actively participate in redox reactions with Zn2+.
5.4. Summary of this chapter
The working mechanisms of zinc-ion batteries (ZIBs) primarily involve three redox pathways: cationic redox, anionic redox, and redox of active functional groups. The anode typically undergoes a simple Zn2+ plating/stripping process (Zn0 ↔ Zn2+), while cathode reactions are more complex. Cationic redox involves transition metal valence changes (e.g., Mn4+/Mn3+, V5+/V4+), often coupled with H+/Zn2+ co-intercalation, influenced by solvation effects and host material structure. Anionic redox (e.g., O2−/O−, N3−/N2−) contributes additional capacity and higher voltages, as seen in VOPO4 and VNxOy cathodes. Organic cathodes leverage redox-active functional groups (e.g., C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) for Zn2+ coordination, enabling high capacity but facing challenges in stability. Understanding these mechanisms is crucial for optimizing electrode design, enhancing ion transport, and mitigating degradation, paving the way for high-performance, sustainable ZIBs.
N) for Zn2+ coordination, enabling high capacity but facing challenges in stability. Understanding these mechanisms is crucial for optimizing electrode design, enhancing ion transport, and mitigating degradation, paving the way for high-performance, sustainable ZIBs.
6. Applications of zinc-ion batteries
Leveraging their inherent advantages including exceptional safety profiles, abundant zinc reserves, cost-efficiency, and remarkable energy density, ZIBs have emerged as a frontrunner in next-generation energy storage technologies with unparalleled research and development potential. This electrochemical energy storage system demonstrates transformative capabilities across diverse application fields, with continuous technological breakthroughs driving their rapid deployment in innovative configurations that yield impressive operational outcomes.11As global demand surges for sustainable energy solutions, ZIBs distinguish themselves through their unique compatibility with energy harvesting technologies – a critical advantage for ensuring reliable power supply integration. Their superior energy capture capabilities span multiple domains including photoelectric conversion, mechanical energy harvesting, and thermal energy utilization, with experimental systems already achieving groundbreaking performance. Kaur et al.190 revolutionized solar integration by developing a dual-functional ZIB system incorporating natural beetroot dye and chlorine-doped quantum dots, achieving exceptional 1 V charging under solar exposure with 240 mA h g−1 discharge capacity. Concurrently, Wang et al.191 demonstrated the mechanical energy superiority of ZIBs through a textile-integrated triboelectric nanogenerator system that converts human motion into stored energy, boosting battery voltage by 37% while delivering 10.9 μAh discharge capacity-a testament to their mechanical–electrical conversion efficiency (Fig. 12).
 | ||
| Fig. 12 Applications in energy harvesting and energy utilization: (a) a unique rapid-charging photo-battery of TiO2/BT/Cl-GQDs/MoO3-NRs/Zn2+/Zn-AC to harvest light energy. Reproduced with permission from ref. 190. Copyright 2023, Elsevier Ltd. (b) Integration and the charging of the flexible ZIB by the triboelectric nano-generator fabric. Reproduced with permission from ref. 191. Copyright 2018, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (c) Flexible planar ZIBs for a self-powered wearable sensing system. Reproduced with permission from ref. 192. Copyright 2023, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (d) Printed ZIBs for a self-powered smart watch. Reproduced with permission from ref. 193. Copyright 2023, Royal Society of Chemistry. | ||
The operational versatility of ZIBs shines particularly in flexible energy solutions, where their mechanical resilience and high energy retention enable transformative wearable applications. Cai et al.192 engineered a self-powered sensing system combining printed flexible ZIBs with solar cells, overcoming intermittent renewable supply limitations while enabling continuous environmental monitoring. Yan et al.193 further extended these advantages through 3D-printed micro-batteries integrated into smart wearables, achieving full-day physiological monitoring capabilities. Beyond portable electronics, ZIBs demonstrate exceptional scalability for industrial applications including seawater electrolysis, leveraging their superior corrosion resistance and catalytic efficiency in hydrogen production.
The strategic advantages of ZIBs become particularly pronounced in electric vehicle (EV) and grid-scale applications, where they outpace conventional LIBs through multiple performance parameters. Their aqueous electrolyte systems eliminate combustion risks while providing enhanced thermal stability – critical safety advantages for collision-prone EV applications. Coupled with rapid charging capabilities and theoretical energy densities exceeding 400 W h kg−1, ZIBs present a formidable alternative to LIBs in transportation electrification. For grid-scale deployment, ZIBs offer unmatched economic and operational benefits: Their low-cost zinc-based chemistry enables massive energy arbitrage capabilities, effectively balancing supply–demand fluctuations while providing emergency backup resilience. These grid systems capitalize on ZIBs' unique ability to store surplus renewable energy during off-peak periods for high-value discharge during demand peaks, significantly improving grid reliability and operational economics.
The commercial trajectory of ZIBs reflects their compelling value proposition, with global markets projected to expand from $0.2 billion to $28.1 billion by 2030. Their commercialization strengths stem from three core advantages: (1) abundant zinc reserves ensuring material security and price stability; (2) simplified recycling processes enabling >95% metal recovery rates; (3) inherent non-flammability addressing critical safety concerns in energy storage. Current production optimizations focus on enhancing their natural advantages through advanced electrode architectures and electrolyte formulations, targeting 50% cost reductions versus LIBs while maintaining competitive energy densities.
While technical challenges in zinc dendrite suppression and electrolyte optimization remain, ZIBs' fundamental advantages position them as a cornerstone technology for sustainable energy transitions. Their development directly supports global decarbonization goals through reduced resource intensity (60% lower than LIBs in lifecycle assessments) and enhanced renewable integration capabilities. As research breakthroughs continue to amplify their innate strengths, ZIBs are poised to redefine energy storage paradigms across consumer electronics, transportation, and grid infrastructure sectors-establishing zinc as the keystone material for next-generation electrochemical storage solutions.
7. Bright prospects of ZIBs
Zinc-ion batteries offer a combination of high safety, low cost, environmental friendliness, excellent electrochemical performance, and broad applicability, making them highly promising for future energy storage applications. With ongoing technological advancements, ZIBs are poised to replace traditional battery technologies in various fields, providing significant support for global energy transitions and sustainable development. The specific advantages would be demonstrated as following:(1) High safety
ZIBs are considered as the ideal energy-supply equipment for wearable electronics. In the standard configuration, they utilize non-noble materials such as zinc foil, carbon-based materials, and inorganic salts, combined with aqueous electrolytes, which are inherently less reactive and more stable than the organic electrolytes used in LIBs. This significantly reduces the risk of thermal runaway, combustion, or explosion, which are common safety concerns in lithium-based systems.170(2) Cost-effectiveness
ZIBs offer significant cost advantages over LIBs in general. Firstly, the abundance of zinc in the Earth's crust is approximately four times that of lithium, making zinc a more readily available and cheaper raw material. The price of zinc (0.5–1.5$ per lb) is much lower than that of lithium (8–11$ per lb).194 Additionally, ZIBs can be manufactured in open-air environments, whereas LIBs often require inert atmospheres for production, which increases the manufacturing cost. Furthermore, the electrolytes used in ZIBs are typically aqueous and less expensive compared to the organic electrolytes used in LIBs. These combined factors make ZIBs a more cost-effective energy storage solution, especially for cost-sensitive devices.(3) Compatibility
The manufacturing processes for ZIBs are similar to those for LIBs. First of all, both ZIBs and LIBs utilize electrodes that can be fabricated using common methods such as slurry coating, drying, and calendaring. The active materials for ZIBs (e.g., zinc metal for the anode and various transition metal oxides or Prussian blue analogs for the cathode) can be processed into slurries and coated onto current collectors in a manner similar to that used for LIB electrodes. And ZIBs typically use aqueous electrolytes, the principles of electrolyte formulation and handling are analogous to those in LIBs. The aqueous electrolytes used in ZIBs can be integrated into existing battery assembly lines with minimal modifications, as they are compatible with the same types of separators and packaging materials used in LIBs. At the same time, the assembly of ZIBs follows similar steps to those of LIBs, including stacking or winding of electrodes, electrolyte filling, and cell sealing. The flexible configurations of ZIBs, such as planar or cable structures, can be adapted to existing manufacturing equipment used for LIBs, making it feasible to switch between the two battery types with minimal investment in new machinery.195–198 Moreover, ZIBs offer the intrinsic potential for scalability and industrial compatibility. The manufacturing processes for ZIBs can be scaled up using the same principles as those for LIBs. Techniques like roll-to-roll processing, which are commonly used for LIB production, can also be applied to ZIBs, ensuring that large-scale production is feasible and cost-effective.Overall, the combination of safety, cost-effectiveness, and compatibility with the existing industrial foundation makes ZIBs a highly attractive option for next-generation energy storage solutions.
8. Main challenges and possible solutions
ZIBs have garnered significant attention as a promising alternative for sustainable energy storage, thanks to their numerous advantages. However, despite these promising attributes, ZIBs face several challenges that need to be addressed for their practical application. As for anode, one significant concern is the formation of zinc dendrites during repeated charge–discharge cycles, which can lead to short circuits and reduced battery lifespan. This problem would be exacerbated by the inhomogeneous deposition of zinc ions, causing uneven growth on the anode surface. Additionally, the aqueous electrolytes used in ZIBs can suffer from a narrow electrochemical stability window, leading to hydrogen evolution reactions and corrosion of the zinc anode. These side reactions not only decrease the coulombic efficiency but also result in the formation of by-products that disturb the uniform deposition of zinc. Another challenge lies in the cathode materials, which often experience dissolution of active materials and structural degradation during cycling, leading to poor rate capability and cycling stability. For instance, transition metal-based compounds like manganese and vanadium oxides, as well as Prussian blue analogs, are prone to dissolution of metal ions, further compromising their performance. Moreover, our understanding of the electrochemical mechanisms of ZIBs would be still incomplete and inaccurate, and the reasons for this would be varied. Firstly, the aqueous electrolytes commonly used in ZIBs may trigger various side reactions, which not only brings difficulties to the phase analysis of electrochemical processes, but also impedes the detection of target products. Besides, the solvation and de-solvation processes of zinc ions are intricate, which would be difficult to perceive due to the restricted characterization techniques.Herein, we propose some perspectives in view of the issues for ZIBs as shown in Fig. 13:
(1) Design and optimization of anode materials
As discussed above, surface modification with protective layers, such as carbon-based materials, polymers, or metal oxides, can effectively inhibit zinc dendrite growth and corrosion, thereby improving the anode's stability and cycle life. And the significance of structural design would be taken into account as well, including the establishment of three-dimensional porous frameworks and conductive substrates, to promote uniform zinc deposition and reduce local current density. While these standard modification techniques merit the scrutiny of scholars, the influence of different components in ZIBs, particularly the electrolyte, on the anode should not be overlooked. Improving the compatibility of electrolyte and anode material is also an important direction worth studying in the future.(2) Advanced electrolyte design
The development of aqueous electrolyte and solid electrolyte is never a contradictory relationship, and it is the most important to choose the appropriate electrolyte system for the actual service environment of the battery. As for aqueous electrolyte, future research should focus on expanding the electrochemical stability window and minimizing adverse reactions as much as possible. The expertise can be gained from the design strategy of LIBs, such as establishing water-in-salt one. When facing flexible wearable devices or implantable devices, solid phase electrolytes are bound to become the best choice for energy sources due to their excellent safety performance. In this moment, establishing appropriate safety standards and researching on failure mechanisms in real service environments should be the top priority.(3) Design and optimization of cathode materials
The choice of cathode materials is obviously more than that of anode materials, offering researchers diverse choices but clearly escalating the workload. Future studies will focus not only on improving current materials but also on searching and screen more advanced ones via high-throughput computing method or artificial intelligence technology. It is worth mentioning that the optimization of the cathode material still needs to consider compatibility with other components, such as the electrochemical platform of the cathode material within the electrochemical window of the electrolyte and compatibility with the anode material capacity output.(4) In-depth understanding of the electrochemical mechanism
By delving into their fundamental electrochemical processes, researchers can further optimize performance, enhance safety, and extend cycle life, ultimately making ZIBs more competitive in the market, which would be crucial for developing cost-effective and sustainable energy storage solutions that can support the growing demand for renewable energy, reduce dependence on fossil fuels, and contribute to global energy security. However, the exploration of electrochemical mechanisms is often inseparable from advanced characterization techniques and in situ characterization methods, which is an interdisciplinary matter that often involves the development of devices.9. Conclusion
ZIBs are promising as sustainable alternatives in the realm of energy storage. The urgent global need for decarbonization, emphasized in the introduction, underscores the significance of advanced Electrical Energy Storage (EES) systems. Throughout the paper, we meticulously investigate the various components of ZIBs, including anode materials such as metallic zinc, carbon-based structures, and transition metal oxides/sulfides/phosphides. Simultaneously, our analysis delved into cathode materials, ranging from transition metal oxides to organic cathode materials and emerging cathode nanomaterials, emphasizing recent advancements and challenges in their applications.The pivotal role of electrolyte materials in determining battery performance was dissected, considering both liquid and solid electrolytes. Understanding the nuances of these components is vital, as highlighted in our detailed examination. Through this exploration, it becomes evident that ZIBs offer distinct advantages, notably their safety, high energy density, and eco-friendly attributes, owing to the abundance and low cost of zinc.
This paper serves as a significant contribution to ongoing discourse on energy storage technologies. By elucidating the current state of ZIB technology and its potential future trajectories, we provide researchers, policymakers, and industry stakeholders with valuable insights. The discussion on fundamental operating mechanisms, coupled with innovative approaches in electrode and electrolyte materials, illuminates a path toward overcoming challenges such as low cycle life and energy efficiency. Additionally, our focus on potential applications and market growth, especially in stationary energy resources, outlines a tangible future for ZIBs in the energy landscape.
As we venture into an era where sustainable energy solutions are imperative, the knowledge encapsulated in this review propels the development of ZIBs, offering a viable and competitive avenue for the future of energy storage. With continuous advancements, collaborative efforts, and a keen focus on addressing existing limitations, ZIBs stand poised to revolutionize the energy storage sector, steering us toward a more sustainable and environmentally conscious future.
Data availability
This review article does not generate new data. All data and information discussed are derived from previously published studies, which are appropriately cited in the manuscript.Author contributions
Z. Chen, L. Zhang and T. Yu wrote the paper. H. Yang, Zonglun Ye, Y. Wang, B. Zheng, Y. Sun, D. Wang, G. Xu and R. Li sorted out the relevant literature. Y. Wang contributed contents to the Conclusion. W. Gao, Y. Lu, and X. Wang reviewed and revised the paper. W. Gao, R. Li, Y. Lu and X. Wang proposed the concept and the structure of this review.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This review is supported by the China Postdoctoral Science Foundation (No. 2021M691729), the National Natural Science Foundation of China (No. 52177217), and the Beijing Natural Science Foundation (No. 3212031).References
- W. Huang, X. Feng, X. Han, W. Zhang and F. Jiang, Cell Rep. Phys. Sci., 2021, 2, 100285 CrossRef CAS.
- Z. Zhu, T. Jiang, M. Ali, Y. Meng, Y. Jin, Y. Cui and W. Chen, Chem. Rev., 2022, 122, 16610–16751 CrossRef CAS.
- X. B. Cheng, R. Zhang, C. Z. Zhao and Q. Zhang, Chem. Rev., 2017, 117, 10403–10473 CrossRef CAS PubMed.
- M. Zhou, B. Li, J. Li and Z. Xu, ACS ES&T Eng., 2021, 1, 1369–1382 Search PubMed.
- P. Hu, T. Zhu, X. Wang, X. Wei, M. Yan, J. Li, W. Luo, W. Yang, W. Zhang, L. Zhou, Z. Zhou and L. Mai, Nano Lett., 2018, 18, 1758–1763 CrossRef CAS PubMed.
- P. Canepa, G. Sai Gautam, D. C. Hannah, R. Malik, M. Liu, K. G. Gallagher, K. A. Persson and G. Ceder, Chem. Rev., 2017, 117, 4287–4341 CrossRef CAS PubMed.
- K. E. K. Sun, T. K. A. Hoang, T. N. L. Doan, Y. Yu, X. Zhu, Y. Tian and P. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 9681–9687 CrossRef CAS.
- N. Zhang, X. Chen, M. Yu, Z. Niu, F. Cheng and J. Chen, Chem. Soc. Rev., 2020, 49, 4203–4219 RSC.
- B. Tang, L. Shan, S. Liang and J. Zhou, Energy Environ. Sci., 2019, 12, 3288–3304 RSC.
- Q. Ni, B. Kim, C. Wu and K. Kang, Adv. Mater., 2022, 34, 1–30 Search PubMed.
- J. Ming, J. Guo, C. Xia, W. Wang and H. N. Alshareef, Mater. Sci. Eng., R, 2019, 135, 58–84 CrossRef.
- R. Puttaswamy, H. Lee, H. W. Bae, D. Y. Kim and D. Kim, Small, 2024, 20, 1–11 CrossRef.
- R. He, F. Yu, K. Wu, H. X. Liu, Z. Li, H. K. Liu, S. X. Dou and C. Wu, Nano Lett., 2023, 23, 6050–6058 CrossRef CAS PubMed.
- Y. Lv, Y. Xiao, L. Ma, C. Zhi and S. Chen, Adv. Mater., 2022, 34, 1–31 Search PubMed.
- C. Su, X. Gao, K. Liu, A. He, H. He, J. Zhu, Y. Liu, Z. Chen, Y. Zhao, W. Zong, Y. Dai, J. Lin and H. Dong, Green Energy Intell. Transp., 2023, 2, 100126 CrossRef.
- S. Huang, J. Zhu, J. Tian and Z. Niu, Chem. – Eur. J., 2019, 25, 14480–14494 CrossRef CAS PubMed.
- K. Wu, J. Huang, J. Yi, X. Liu, Y. Liu, Y. Wang, J. Zhang and Y. Xia, Adv. Energy Mater., 2020, 10, 1–32 CAS.
- G. Yaman Uzunoglu and R. Yuksel, Small, 2025, 21, 1–9 CrossRef.
- H. Liu, J. G. Wang, Z. You, C. Wei, F. Kang and B. Wei, Mater. Today, 2021, 42, 73–98 CrossRef CAS.
- F. Shao, Y. Huang, X. Wang, Z. Li, X. Huang, W. Huang, L. Dong, F. Kang, W. Liu and C. Xu, Chem. Eng. J., 2022, 448, 137688 CrossRef CAS.
- Y. Zhu, H. Li, Y. Rao, S. Guo and H. Zhou, Adv. Energy Mater., 2024, 14, 1–27 Search PubMed.
- J. Cao, D. Zhang, Y. Yue, X. Wang, T. Pakornchote, T. Bovornratanaraks, X. Zhang, Z. S. Wu and J. Qin, Nano Energy, 2021, 84, 105876 CrossRef CAS.
- Y. Y. Liu, G. Q. Yuan, X. Y. Wang, J. P. Liu, Q. Y. Zeng, X. T. Guo, H. Wang, C. Sen Liu and H. Pang, Chem. Eng. J., 2022, 428, 132538 CrossRef CAS.
- F. Wu, Y. Chen, Y. Chen, R. Yin, Y. Feng, D. Zheng, X. Xu, W. Shi, W. Liu and X. Cao, Small, 2022, 18, 1–9 Search PubMed.
- X. Xu, Y. Chen, W. Li, R. Yin, D. Zheng, X. Niu, X. Dai, W. Shi, W. Liu, F. Wu, M. Wu, S. Lu and X. Cao, Small, 2023, 19, 1–8 Search PubMed.
- R. Li, L. Li, R. Jia, K. Jiang, G. Shen and D. Chen, Small Methods, 2020, 4, 1–9 Search PubMed.
- X. Li, J. Qu, J. Xu, S. Zhang, X. Wang, X. Wang and S. Dai, J. Electroanal. Chem., 2021, 895, 115529 CrossRef CAS.
- S. Liu, H. Zhu, B. Zhang, G. Li, H. Zhu, Y. Ren, H. Geng, Y. Yang, Q. Liu and C. C. Li, Adv. Mater., 2020, 32, 1–10 Search PubMed.
- D. Zhang, J. Cao, Y. Yue, T. Pakornchote, T. Bovornratanaraks, J. Han, X. Zhang, J. Qin and Y. Huang, ACS Appl. Mater. Interfaces, 2021, 13, 38416–38424 CrossRef CAS.
- L. E. Blanc, D. Kundu and L. F. Nazar, Joule, 2020, 4, 771–799 CrossRef CAS.
- P. Yu, Y. Zeng, H. Zhang, M. Yu, Y. Tong and X. Lu, Small, 2019, 15, 1–27 Search PubMed.
- S. W. D. Gourley, R. Brown, B. D. Adams and D. Higgins, Joule, 2023, 7, 1415–1436 CrossRef CAS.
- D. Chen, M. Lu, D. Cai, H. Yang and W. Han, J. Energy Chem., 2021, 54, 712–726 CrossRef CAS.
- H. Luo, Q. Gou, Y. Zheng, K. Wang, R. Yuan, S. Zhang, W. Fang, Z. Luogu, Y. Hu, H. Mei, B. Song, K. Sun, J. Wang and M. Li, ACS Nano, 2025, 19, 2427–2443 CrossRef CAS PubMed.
- G. Xu, Y. Zhang, M. Jiang, J. Li, H. Sun, J. Li, T. Lu, C. Wang, G. Yang and L. Pan, Chem. Eng. J., 2023, 476, 146676 CrossRef CAS.
- T. Wang, C. Li, X. Xie, B. Lu, Z. He, S. Liang and J. Zhou, ACS Nano, 2020, 14, 16321–16347 CrossRef CAS PubMed.
- A. Yu, W. Zhang, N. Joshi and Y. Yang, Energy Stor. Mater., 2024, 64, 103075 Search PubMed.
- V. C. Ho, H. Lim, M. J. Kim and J. Mun, Chem. – Asian J., 2022, 17, e202200289 CrossRef CAS PubMed.
- Y. Liu, H. He, A. Gao, J. Ling, F. Yi, J. Hao, Q. Li and D. Shu, Chem. Eng. J., 2022, 446, 137021 CrossRef CAS.
- A. Bayaguud, Y. Fu and C. Zhu, J. Energy Chem., 2022, 64, 246–262 CrossRef CAS.
- J. Yang, R. Zhao, Z. Hu, Y. Wang, K. Zhang, Y. Wang, X. Han, A. Zhang, C. Wu and Y. Bai, Energy Stor. Mater., 2024, 70, 103449 Search PubMed.
- Y. Zuo, K. Wang, P. Pei, M. Wei, X. Liu, Y. Xiao and P. Zhang, Mater. Today Energy, 2021, 20, 100692 CrossRef CAS.
- W. Xiong, D. Yang, T. K. A. Hoang, M. Ahmed, J. Zhi, X. Qiu and P. Chen, Energy Stor. Mater., 2018, 15, 131–138 Search PubMed.
- H. Kim, G. Jeong, Y.-U. Kim, J.-H. Kim, C.-M. Park and H.-J. Sohn, Chem. Soc. Rev., 2013, 42, 9011 RSC.
- N. Diomidis and J.-P. Celis, J. Electrochem. Soc., 2007, 154, C711 CrossRef CAS.
- W. G. Sunu and D. N. Bennion, J. Electrochem. Soc., 1980, 127, 2017–2025 CrossRef CAS.
- C. Y. Jung, T. H. Kim, W. J. Kim and S. C. Yi, Energy, 2016, 102, 694–704 CrossRef CAS.
- H. Yang, Z. Chang, Y. Qiao, H. Deng, X. Mu, P. He and H. Zhou, Angew. Chem., 2020, 132, 9463–9467 CrossRef.
- Y. Zeng, X. Zhang, R. Qin, X. Liu, P. Fang, D. Zheng, Y. Tong and X. Lu, Adv. Mater., 2019, 31, 1–8 Search PubMed.
- Q. Zhang, Y. Dai, K. Zhao, C. Zhang, R. Lu, J. Li, S. Jin, L. Zhang, Q. An and L. Mai, Nano Res., 2023, 16, 11604–11611 CrossRef CAS.
- Y. Cheng, L. Luo, L. Zhong, J. Chen, B. Li, W. Wang, S. X. Mao, C. Wang, V. L. Sprenkle, G. Li and J. Liu, ACS Appl. Mater. Interfaces, 2016, 8, 13673–13677 CrossRef CAS PubMed.
- W. Kaveevivitchai and A. Manthiram, J. Mater. Chem. A, 2016, 4, 18737–18741 RSC.
- M. Zhou, S. Guo, G. Fang, H. Sun, X. Cao, J. Zhou, A. Pan and S. Liang, J. Energy Chem., 2021, 55, 549–556 CrossRef CAS.
- W. Li, K. Wang, M. Zhou, H. Zhan, S. Cheng and K. Jiang, ACS Appl. Mater. Interfaces, 2018, 10, 22059–22066 CrossRef CAS PubMed.
- Y. Da Cho and G. T. K. Fey, J. Power Sources, 2008, 184, 610–616 CrossRef.
- Z. Zhao, J. Zhao, Z. Hu, J. Li, J. Li, Y. Zhang, C. Wang and G. Cui, Energy Environ. Sci., 2019, 12, 1938–1949 RSC.
- M. Cui, Y. Xiao, L. Kang, W. Du, Y. Gao, X. Sun, Y. Zhou, X. Li, H. Li, F. Jiang and C. Zhi, ACS Appl. Energy Mater., 2019, 2, 6490–6496 CrossRef CAS.
- M. Liu, L. Yang, H. Liu, A. Amine, Q. Zhao, Y. Song, J. Yang, K. Wang and F. Pan, ACS Appl. Mater. Interfaces, 2019, 11, 32046–32051 CrossRef CAS PubMed.
- H. Li, L. Ma, C. Han, Z. Wang, Z. Liu, Z. Tang and C. Zhi, Nano Energy, 2019, 62, 550–587 CrossRef CAS.
- Y. Liu, X. Zhou, R. Liu, X. Li, Y. Bai, H. Xiao, Y. Wang and G. Yuan, ACS Appl. Mater. Interfaces, 2019, 11, 19191–19199 CrossRef CAS.
- Y. Cheng, H. Zhang, Q. Lai, X. Li, D. Shi and L. Zhang, J. Power Sources, 2013, 241, 196–202 CrossRef CAS.
- J. F. Parker, C. N. Chervin, E. S. Nelson, D. R. Rolison and J. W. Long, Energy Environ. Sci., 2014, 7, 1117–1124 RSC.
- X. Shi, G. Xu, S. Liang, C. Li, S. Guo, X. Xie, X. Ma and J. Zhou, ACS Sustainable Chem. Eng., 2019, 7, 17737–17746 CrossRef CAS.
- R. Yuksel, O. Buyukcakir, W. K. Seong and R. S. Ruoff, Adv. Energy Mater., 2020, 10, 1–8 Search PubMed.
- Y. Tian, Y. An, C. Wei, B. Xi, S. Xiong, J. Feng and Y. Qian, ACS Nano, 2019, 13, 11676–11685 CrossRef CAS.
- S.-B. Wang, Q. Ran, R.-Q. Yao, H. Shi, Z. Wen, M. Zhao, X.-Y. Lang and Q. Jiang, Nat. Commun., 2020, 11, 1634 CrossRef CAS.
- L. Chang, J. Li, Q. Sun, X. Liu, X. Lu and H. Cheng, Small, 2024, 20, 202408124 Search PubMed.
- H. Zhang, X. Liu, B. Qin and S. Passerini, J. Power Sources, 2020, 449, 227594 CrossRef CAS.
- W. Liu, L. Dong, B. Jiang, Y. Huang, X. Wang, C. Xu, Z. Kang, J. Mou and F. Kang, Electrochim. Acta, 2019, 320, 134565 CrossRef CAS.
- G. Li, L. Sun, S. Zhang, C. Zhang, H. Jin, K. Davey, G. Liang, S. Liu, J. Mao and Z. Guo, Adv. Funct. Mater., 2024, 34 DOI:10.1002/adfm.202301291.
- J. Hao, L. Yuan, B. Johannessen, Y. Zhu, Y. Jiao, C. Ye, F. Xie and S. Qiao, Angew. Chem., 2021, 133, 25318–25325 CrossRef.
- J. Huang, X. Tang, K. Liu, G. Fang, Z. He and Z. Li, Mater. Today Energy, 2020, 17, 1–9 Search PubMed.
- T. R. Juran, J. Young and M. Smeu, J. Phys. Chem. C, 2018, 122, 8788–8795 CrossRef CAS.
- X. Liu, J. Yi, K. Wu, Y. Jiang, Y. Liu, B. Zhao, W. Li and J. Zhang, Nanotechnology, 2020, 31, 122001 CrossRef CAS PubMed.
- C. Xu, B. Li, H. Du and F. Kang, Angew. Chem., Int. Ed., 2012, 51, 933–935 CrossRef CAS PubMed.
- W. Sun, F. Wang, S. Hou, C. Yang, X. Fan, Z. Ma, T. Gao, F. Han, R. Hu, M. Zhu and C. Wang, J. Am. Chem. Soc., 2017, 139, 9775–9778 CrossRef CAS PubMed.
- Y. Huang, J. Mou, W. Liu, X. Wang, L. Dong, F. Kang and C. Xu, Nano-Micro Lett., 2019, 11, 49–62 CrossRef CAS PubMed.
- W. Liu, X. Zhang, Y. Huang, B. Jiang, Z. Chang, C. Xu and F. Kang, J. Energy Chem., 2021, 56, 365–373 CrossRef CAS.
- M. H. Alfaruqi, V. Mathew, J. Gim, S. Kim, J. Song, J. P. Baboo, S. H. Choi and J. Kim, Chem. Mater., 2015, 27, 3609–3620 CrossRef CAS.
- M. H. Alfaruqi, J. Gim, S. Kim, J. Song, D. T. Pham, J. Jo, Z. Xiu, V. Mathew and J. Kim, Electrochem. Commun., 2015, 60, 121–125 CrossRef CAS.
- Y. Jin, L. Zou, L. Liu, M. H. Engelhard, R. L. Patel, Z. Nie, K. S. Han, Y. Shao, C. Wang, J. Zhu, H. Pan and J. Liu, Adv. Mater., 2019, 31, 1–8 Search PubMed.
- Z. Azmi, K. C. Senapati, A. K. Goswami and S. R. Mohapatra, J. Power Sources, 2024, 613, 234816 CrossRef CAS.
- H. Pan, Y. Shao, P. Yan, Y. Cheng, K. S. Han, Z. Nie, C. Wang, J. Yang, X. Li, P. Bhattacharya, K. T. Mueller and J. Liu, Nat. Energy, 2016, 1, 1–7 Search PubMed.
- J. Lu, C. Zhan, T. Wu, J. Wen, Y. Lei, A. J. Kropf, H. Wu, D. J. Miller, J. W. Elam, Y. K. Sun, X. Qiu and K. Amine, Nat. Commun., 2014, 5, 1–8 Search PubMed.
- S. Guo, Q. Li, P. Liu, M. Chen and H. Zhou, Nat. Commun., 2017, 8, 1–9 CrossRef.
- X. Wang, Z. Zhang, B. Xi, W. Chen, Y. Jia, J. Feng and S. Xiong, ACS Nano, 2021, 15, 9244–9272 CrossRef CAS PubMed.
- B. Yong, D. Ma, Y. Wang, H. Mi, C. He and P. Zhang, Adv. Energy Mater., 2020, 10, 1–38 Search PubMed.
- B. Jiang, C. Xu, C. Wu, L. Dong, J. Li and F. Kang, Electrochim. Acta, 2017, 229, 422–428 CrossRef CAS.
- J. Hao, J. Mou, J. Zhang, L. Dong, W. Liu, C. Xu and F. Kang, Electrochim. Acta, 2018, 259, 170–178 CrossRef CAS.
- C. Liu, R. Li, W. Liu, G. Shen and D. Chen, ACS Appl. Mater. Interfaces, 2021, 13, 37194–37200 CrossRef CAS PubMed.
- Y. H. Liu, W. H. Li, H. Y. Lü, X. X. Luo, Z. X. Huang, Z. Y. Gu, X. X. Zhao and X. L. Wu, ACS Appl. Mater. Interfaces, 2022, 14, 45494–45502 CrossRef CAS PubMed.
- T. Zhou, L. Xie, Q. Han, X. Qiu, Y. Xiao, X. Yang, X. Liu, S. Yang, L. Zhu and X. Cao, Coord. Chem. Rev., 2024, 498, 215461 CrossRef CAS.
- Y. Y. Liu, L. Xu, X. T. Guo, T. T. Lv and H. Pang, J. Mater. Chem. A, 2020, 8, 20781–20802 RSC.
- Y. Zhang, S. Jiang, Y. Li, X. Ren, P. Zhang, L. Sun and H. Y. Yang, Adv. Energy Mater., 2023, 13, 1–10 Search PubMed.
- G. Li, L. Sun, S. Zhang, C. Zhang, H. Jin, K. Davey, G. Liang, S. Liu, J. Mao and Z. Guo, Adv. Funct. Mater., 2024, 34, 2301291 CrossRef CAS.
- N. Zhang, X. Chen, M. Yu, Z. Niu, F. Cheng and J. Chen, Chem. Soc. Rev., 2020, 49, 4203–4219 RSC.
- T. Lv, Y. Peng, G. Zhang, S. Jiang, Z. Yang, S. Yang and H. Pang, Adv. Sci., 2023, 10, 1–49 Search PubMed.
- X. Li, Z. Chen, Y. Yang, S. Liang, B. Lu and J. Zhou, Inorg. Chem. Front., 2022, 9, 3986–3998 RSC.
- C. Li, W. Wu, H. Y. Shi, Z. Qin, D. Yang, X. Yang, Y. Song, D. Guo, X. X. Liu and X. Sun, Chem. Commun., 2021, 57, 6253–6256 RSC.
- A. K. Padhi, K. S. Nanjundaswamy and J. B. Goodenough, J. Electrochem. Soc., 1997, 144, 1188–1194 CrossRef CAS.
- F. Wan, Y. Zhang, L. Zhang, D. Liu, C. Wang, L. Song, Z. Niu and J. Chen, Angew. Chem., Int. Ed., 2019, 58, 7062–7067 CrossRef CAS PubMed.
- F. Wang, W. Sun, Z. Shadike, E. Hu, X. Ji, T. Gao, X. Yang, K. Xu and C. Wang, Angew. Chem., 2018, 130, 12154–12157 CrossRef.
- K. Zhu, Z. Sun, P. Liu, H. Li, Y. Wang, K. Cao and L. Jiao, J. Energy Chem., 2021, 63, 239–245 CrossRef CAS.
- Z. Wu, C. Lu, F. Ye, L. Zhang, L. Jiang, Q. Liu, H. Dong, Z. Sun and L. Hu, Adv. Funct. Mater., 2021, 31, 1–12 Search PubMed.
- K. Zhu, Z. Sun, P. Liu, H. Li, Y. Wang, K. Cao and L. Jiao, J. Energy Chem., 2021, 63, 239–245 CrossRef CAS.
- Z. Jian, W. Han, X. Lu, H. Yang, Y. S. Hu, J. Zhou, Z. Zhou, J. Li, W. Chen, D. Chen and L. Chen, Adv. Energy Mater., 2013, 3, 156–160 CrossRef CAS.
- L. Wang and J. Zheng, Mater. Today Adv., 2020, 7, 100078 CrossRef.
- Y. Marcus, Chem. Rev., 1988, 88, 1475–1498 CrossRef CAS.
- G. Li, Z. Yang, Y. Jiang, C. Jin, W. Huang, X. Ding and Y. Huang, Nano Energy, 2016, 25, 211–217 CrossRef CAS.
- S. Pavithra, K. Pramoda and R. S. Keri, Chem. Eng. J., 2024, 495, 153423 CrossRef CAS.
- X. Guo, G. Fang, W. Zhang, J. Zhou, L. Shan, L. Wang, C. Wang, T. Lin, Y. Tang and S. Liang, Adv. Energy Mater., 2018, 8, 1–7 Search PubMed.
- F. Wan, L. Zhang, X. Dai, X. Wang, Z. Niu and J. Chen, Nat. Commun., 2018, 9, 1–11 CrossRef CAS PubMed.
- P. He, M. Yan, G. Zhang, R. Sun, L. Chen, Q. An and L. Mai, Adv. Energy Mater., 2017, 7, 1–5 Search PubMed.
- H. Qin, Z. Yang, L. Chen, X. Chen and L. Wang, J. Mater. Chem. A, 2018, 6, 23757–23765 RSC.
- S. Liu, X. Chen, Q. Zhang, J. Zhou, Z. Cai and A. Pan, ACS Appl. Mater. Interfaces, 2019, 11, 36676–36684 CrossRef CAS PubMed.
- Y. Rong, H. Chen, J. Wu, Z. Yang, L. Deng and Z. Fu, Ind. Eng. Chem. Res., 2021, 60, 8649–8658 CrossRef CAS.
- G. Zampardi and F. La Mantia, Curr. Opin. Electrochem., 2020, 21, 84–92 CrossRef CAS.
- M. B. Zakaria and T. Chikyow, Coord. Chem. Rev., 2017, 352, 328–345 CrossRef CAS.
- J. Liu, Z. Shen and C. Z. Lu, J. Mater. Chem. A, 2024, 12, 2647–2672 RSC.
- L. Zhang, L. Chen, X. Zhou and Z. Liu, Adv. Energy Mater., 2015, 5, 1–5 Search PubMed.
- F. Scholz and A. Dostal, Angew. Chem., Int. Ed. Engl., 1996, 34, 2685–2687 CrossRef.
- K. Hurlbutt, S. Wheeler, I. Capone and M. Pasta, Joule, 2018, 2, 1950–1960 CrossRef CAS.
- J. Yang, W. Hou, L. Ye, G. Hou, C. Yan and Y. Zhang, Small, 2024, 20, 1–9 Search PubMed.
- X. Y. Fu, L. L. Zhang, C. C. Wang, H. Bin Sun and X. L. Yang, Rare Met., 2024, 44, 34–59 CrossRef.
- H. Cui, L. Ma, Z. Huang, Z. Chen and C. Zhi, SmartMat, 2022, 3, 565–581 CrossRef CAS.
- Z. Tie and Z. Niu, Angew. Chem., Int. Ed., 2020, 59, 21293–21303 CrossRef CAS PubMed.
- Y. Liang, Z. Tao and J. Chen, Adv. Energy Mater., 2012, 2, 742–769 CrossRef CAS.
- Z. Tie and Z. Niu, Angew. Chem., Int. Ed., 2020, 59, 21293–21303 CrossRef CAS PubMed.
- X. Deng, J. K. Sarpong, G. Zhang, J. Hao, X. Zhao, L. Li, H. Li, C. Han and B. Li, InfoMat, 2023, 5, 1–21 CrossRef.
- L. Mei, J. Xu, Z. Wei, H. Liu, Y. Li, J. Ma and S. Dou, Small, 2017, 13, 1–11 Search PubMed.
- M. M. Huie, D. C. Bock, E. S. Takeuchi, A. C. Marschilok and K. J. Takeuchi, Coord. Chem. Rev., 2015, 287, 15–27 CrossRef CAS.
- E. Levi, E. Lancry, A. Mitelman, D. Aurbach, G. Ceder, D. Morgan and O. Isnard, Chem. Mater., 2006, 18, 5492–5503 CrossRef CAS.
- A. Elgendy, A. A. Papaderakis, A. Ejigu, K. Helmbrecht, B. F. Spencer, A. Groß, A. S. Walton, D. J. Lewis and R. A. W. Dryfe, Nanoscale, 2024, 16, 13597–13612 RSC.
- X. Jia, C. Liu, Z. G. Neale, J. Yang and G. Cao, Chem. Rev., 2020, 120, 7795–7866 CrossRef CAS.
- M. Liu, G. Lv, T. Liu, H. Liu, L. Kong, L. Tian, W. Rao, Y. Li, L. Liao and J. Guo, Prog. Nat. Sci.:Mater. Int., 2023, 33, 8–15 CrossRef CAS.
- M. S. Chae, J. W. Heo, S. C. Lim and S. T. Hong, Inorg. Chem., 2016, 55, 3294–3301 CrossRef CAS.
- Y. Wei, P. Zhang, R. A. Soomro, Q. Zhu and B. Xu, Adv. Mater., 2021, 33, 1–30 Search PubMed.
- P. Ma, D. Fang, Y. Liu, Y. Shang, Y. Shi and H. Y. Yang, Adv. Sci., 2021, 8, 1–25 Search PubMed.
- M. Shekhirev, C. E. Shuck, A. Sarycheva and Y. Gogotsi, Prog. Mater. Sci., 2021, 120, 100757 CrossRef CAS.
- H. Liu, Z. Xin, B. Cao, B. Zhang, H. J. Fan and S. Guo, Adv. Sci., 2024, 11, 1–18 Search PubMed.
- M. Li, X. Li, G. Qin, K. Luo, J. Lu, Y. Li, G. Liang, Z. Huang, J. Zhou, L. Hultman, P. Eklund, P. O. Å. Persson, S. Du, Z. Chai, C. Zhi and Q. Huang, ACS Nano, 2021, 15, 1077–1085 CrossRef CAS PubMed.
- M. Shi, B. Wang, C. Chen, J. Lang, C. Yan and X. Yan, J. Mater. Chem. A, 2020, 8, 24635–24644 RSC.
- M. S. Javed, A. Mateen, S. Ali, X. Zhang, I. Hussain, M. Imran, S. S. A. Shah and W. Han, Small, 2022, 18, 1–39 CrossRef PubMed.
- R. Zhao, C. Liu, Y. Zhu, G. Zou, H. Hou and X. Ji, Adv. Funct. Mater., 2024, 34, 1–28 Search PubMed.
- Q. Li, Y. Zhao, F. Mo, D. Wang, Q. Yang, Z. Huang, G. Liang, A. Chen and C. Zhi, EcoMat, 2020, 2, 1–14 CAS.
- X. Li, X. Ma, Y. Hou, Z. Zhang, Y. Lu, Z. Huang, G. Liang, M. Li, Q. Yang, J. Ma, N. Li, B. Dong, Q. Huang, F. Chen, J. Fan and C. Zhi, Joule, 2021, 5, 2993–3005 CrossRef CAS.
- N. Zhang, F. Cheng, J. Liu, L. Wang, X. Long, X. Liu, F. Li and J. Chen, Nat. Commun., 2017, 8, 1–9 CrossRef.
- J. Zhou, L. Shan, Z. Wu, X. Guo, G. Fang and S. Liang, Chem. Commun., 2018, 54, 4457–4460 RSC.
- F. Cui, J. Zhao, D. Zhang, Y. Fang, F. Hu and K. Zhu, Chem. Eng. J., 2020, 390, 124118 CrossRef CAS.
- Z. Jia, B. Wang and Y. Wang, Mater. Chem. Phys., 2015, 149–150, 601–606 CrossRef CAS.
- Q. Zhao, W. Huang, Z. Luo, L. Liu, Y. Lu, Y. Li, L. Li, J. Hu, H. Ma and J. Chen, Sci. Adv., 2018, 4, eaao1761 CrossRef PubMed.
- D. Kundu, P. Oberholzer, C. Glaros, A. Bouzid, E. Tervoort, A. Pasquarello and M. Niederberger, Chem. Mater., 2018, 30, 3874–3881 CrossRef CAS.
- T. Chen, X. Zhu, X. Chen, Q. Zhang, Y. Li, W. Peng, F. Zhang and X. Fan, J. Power Sources, 2020, 477, 228652 CrossRef CAS.
- X. Li, M. Li, Q. Yang, H. Li, H. Xu, Z. Chai, K. Chen, Z. Liu, Z. Tang, L. Ma, Z. Huang, B. Dong, X. Yin, Q. Huang and C. Zhi, ACS Nano, 2020, 14, 541–551 CrossRef CAS.
- Z. Xu, X. Li, Y. Jin, Q. Dong, J. Ye, X. Zhang and Y. Qian, Nanoscale, 2022, 14, 11655–11663 RSC.
- H. Zheng, Y. Huang, J. Xiao, W. Zeng, X. Li, X. Li, M. Wang and Y. Lin, Chem. Eng. J., 2023, 468, 143834 CrossRef CAS.
- H. Cao, X. Huang, Y. Li, Y. Liu, Q. Zheng, Y. Huo, R. Zhao, J. Zhao and D. Lin, Chem. Eng. J., 2023, 455, 140538 CrossRef CAS.
- B. Raza, A. Naveed, J. Chen, H. Lu, T. Rasheed, J. Yang, Y. NuLi and J. Wang, Energy Stor. Mater., 2022, 46, 523–534 Search PubMed.
- G. Ni, G. Zou, M. Sun, F. Xu, Z. Pan, F. Cao and C. Zhou, ACS Appl. Energy Mater., 2022, 5, 12437–12447 CrossRef CAS.
- L. Geng, J. Meng, X. Wang, C. Han, K. Han, Z. Xiao, M. Huang, P. Xu, L. Zhang, L. Zhou and L. Mai, Angew. Chem., Int. Ed., 2022, 61, 1–9 Search PubMed.
- C. Meng, W. He, Z. Kong, Z. Liang, H. Zhao, Y. Lei, Y. Wu and X. Hao, Chem. Eng. J., 2022, 450, 138265 CrossRef CAS.
- T. Zhang, Y. Tang, S. Guo, X. Cao, A. Pan, G. Fang, J. Zhou and S. Liang, Energy Environ. Sci., 2020, 13, 4625–4665 RSC.
- Z. Chen, F. Mo, T. Wang, Q. Yang, Z. Huang, D. Wang, G. Liang, A. Chen, Q. Li, Y. Guo, X. Li, J. Fan and C. Zhi, Energy Environ. Sci., 2021, 14, 2441–2450 RSC.
- W. Kao-ian, M. T. Nguyen, T. Yonezawa, R. Pornprasertsuk, J. Qin, S. Siwamogsatham and S. Kheawhom, Mater. Today Energy, 2021, 21, 100738 CrossRef CAS.
- A. Naveed, H. Yang, Y. Shao, J. Yang, N. Yanna, J. Liu, S. Shi, L. Zhang, A. Ye, B. He and J. Wang, Adv. Mater., 2019, 31, 1–9 CrossRef PubMed.
- Y. Mei, Y. Liu, W. Xu, M. Zhang, Y. Dong and J. Qiu, Chem. Eng. J., 2023, 452, 139574 CrossRef CAS.
- W. Bin Tu, S. Liang, L. N. Song, X. X. Wang, G. J. Ji and J. J. Xu, Adv. Funct. Mater., 2024, 34, 1–11 Search PubMed.
- S. Liu, W. Liu, D. Ba, Y. Zhao, Y. Ye, Y. Li and J. Liu, Adv. Mater., 2023, 35, 1–23 Search PubMed.
- X. Wang, X. Li, H. Fan and L. Ma, Nano-Micro Lett., 2022, 14, 1–24 CrossRef PubMed.
- S. Lei, Z. Liu, C. Liu, J. Li, B. Lu, S. Liang and J. Zhou, Energy Environ. Sci., 2022, 15, 4911–4927 RSC.
- Z. Chen, X. Li, D. Wang, Q. Yang, L. Ma, Z. Huang, G. Liang, A. Chen, Y. Guo, B. Dong, X. Huang, C. Yang and C. Zhi, Energy Environ. Sci., 2021, 14, 3492–3501 RSC.
- L. Ma, S. Chen, X. Li, A. Chen, B. Dong and C. Zhi, Angew. Chem., Int. Ed., 2020, 59, 23836–23844 CrossRef CAS PubMed.
- F. Bu, C. Li, Q. Wang and X. Liu, Chem. Eng. J., 2022, 449, 137710 CrossRef CAS.
- P. D. Kimilita, M. Hayashi, H. M. Nkomba, H. Fukunishi, N. Lobo, T. Mizuno, L. E. Eale and E. K. Mwilambwe, Electrochim. Acta, 2023, 462, 142702 CrossRef CAS.
- Y. Wang, Q. Li, H. Hong, S. Yang, R. Zhang, X. Wang, X. Jin, B. Xiong, S. Bai and C. Zhi, Nat. Commun., 2023, 14, 1–10 Search PubMed.
- W. Qiu, Y. Tian, Z. Lin, S. Lin, Z. Geng, K. Huang, A. Lei, F. Huang, H. Feng, F. Ding, Y. Li and X. Lu, J. Energy Chem., 2022, 70, 283–291 CrossRef CAS.
- L. Ma, S. Chen, N. Li, Z. Liu, Z. Tang, J. A. Zapien, S. Chen, J. Fan and C. Zhi, Adv. Mater., 2020, 32, 1–10 Search PubMed.
- Z. Zhao, J. Wang, Z. Lv, Q. Wang, Y. Zhang, G. Lu, J. Zhao and G. Cui, Chem. Eng. J., 2021, 417, 128096 CrossRef CAS.
- H. Dong, J. Li, S. Zhao, F. Zhao, S. Xiong, D. J. L. Brett, G. He and I. P. Parkin, J. Mater. Chem. A, 2020, 8, 22637–22644 RSC.
- N. Sun, H. Sun, D. Tan, Q. Guo, Z. Zhang, Z. Tao, C. Fang, J. Bu, J. Huang and C. Jiang, Chem. Eng. J., 2023, 469, 143997 CrossRef CAS.
- B. Sun, Y. Zong, K. Bao, M. Wang, P. Wang, H. Xu and Y. Jin, ACS Appl. Mater. Interfaces, 2023, 15, 37916–37924 CrossRef CAS PubMed.
- R. Puttaswamy, Z. Tian, H. Lee, D. Y. Kim, A. Le Mong and D. Kim, J. Mater. Chem. A, 2023, 11, 14075–14085 RSC.
- G. Fang, S. Liang, Z. Chen, P. Cui, X. Zheng, A. Pan, B. Lu, X. Lu and J. Zhou, Adv. Funct. Mater., 2019, 29, 1–9 Search PubMed.
- W. Du, J. Xiao, H. Geng, Y. Yang, Y. Zhang, E. H. Ang, M. Ye and C. C. Li, J. Power Sources, 2020, 450, 227716 CrossRef CAS.
- Y. Liang, Y. Jing, S. Gheytani, K. Y. Lee, P. Liu, A. Facchetti and Y. Yao, Nat. Mater., 2017, 16, 841–848 CrossRef CAS PubMed.
- Y. Wang, C. Wang, Z. Ni, Y. Gu, B. Wang, Z. Guo, Z. Wang, D. Bin, J. Ma and Y. Wang, Adv. Mater., 2020, 32, 1–8 Search PubMed.
- Z. Guo, Y. Ma, X. Dong, J. Huang, Y. Wang and Y. Xia, Angew. Chem., 2018, 130, 11911–11915 CrossRef.
- M. Zhang, R. Liang, T. Or, Y. P. Deng, A. Yu and Z. Chen, Small Struct., 2021, 2, 1–21 Search PubMed.
- F. Wan, L. Zhang, X. Wang, S. Bi, Z. Niu and J. Chen, Adv. Funct. Mater., 2018, 28, 1–8 Search PubMed.
- B. Kaur, D. Maity, P. Y. Naidu and M. Deepa, Chem. Eng. J., 2023, 468, 143835 CrossRef CAS.
- Z. Wang, Z. Ruan, W. S. Ng, H. Li, Z. Tang, Z. Liu, Y. Wang, H. Hu and C. Zhi, Small Methods, 2018, 2, 1–8 CAS.
- X. Cai, Y. Liu, J. Zha, F. Tan, B. Zhang, W. Yan, J. Zhao, B. Lu, J. Zhou and C. Tan, Adv. Funct. Mater., 2023, 33, 1–11 Search PubMed.
- W. Yan, X. Cai, F. Tan, J. Liang, J. Zhao and C. Tan, Chem. Commun., 2023, 59, 1661–1664 RSC.
- J. Yan, E. H. Ang, Y. Yang, Y. Zhang, M. Ye, W. Du and C. C. Li, Adv. Funct. Mater., 2021, 31, 1–30 Search PubMed.
- Y. A. Kumar, S. Vignesh, T. Ramachandran, A. M. Fouda, H. H. Hegazy, M. Moniruzzaman and T. H. Oh, J. Ind. Eng. Chem., 2025, 145, 191–215 CrossRef.
- T. Ramachandran, R. K. Raji, S. Palanisamy, N. Renuka and K. Karuppasamy, J. Ind. Eng. Chem., 2025, 145, 144–168 CrossRef CAS.
- T. Ramachandran, N. Roy, H. H. Hegazy, I. S. Yahia, Y. A. Kumar, M. Moniruzzaman and S. W. Joo, J. Alloys Compd., 2025, 1010, 112478 CrossRef.
- T. Ramachandran, H. Butt, L. X. Zheng and M. Rezeq, J. Energy Storage, 2024, 99, 113425 CrossRef.
Footnote |
| † Z. C. and L. Z. contributed equally to this paper. |
| This journal is © The Royal Society of Chemistry 2025 |




