Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Optimizing ZIF-8 membrane growth on top of semiconductive Ga-doped ZnO sensitive layers†
Kevin
Dedecker
 *a,
Benjamin
Paret
b,
Lionel
Presmanes
*b,
Benjamin
Duployer
b,
Antoine
Barnabé
*a,
Benjamin
Paret
b,
Lionel
Presmanes
*b,
Benjamin
Duployer
b,
Antoine
Barnabé
 b,
Philippe
Menini
b,
Philippe
Menini
 c,
David
Farrusseng
c,
David
Farrusseng
 d,
Mikhael
Bechelany
d,
Mikhael
Bechelany
 ae,
Martin
Drobek
ae,
Martin
Drobek
 *a and
Anne
Julbe
*a and
Anne
Julbe
 a
a
aInstitut Européen des Membranes (IEM-UMR 5635) CNRS, ENSCM, Univ Montpellier, Place Eugène Bataillon, 34095 Montpellier Cedex 5, France. E-mail: kevin.dedecker@umontpellier.fr; martin.drobek@umontpellier.fr
bCentre Interuniversitaire de Recherche et d'Ingénierie des Matériaux (CIRIMAT-UMR 5085), Université de Toulouse, Université Toulouse 3 Paul Sabatier, Toulouse INP, CNRS, 118 Route de Narbonne, Cedex 9, 31062 Toulouse, France. E-mail: lionel.presmanes@univ-tlse3.fr
cLaboratoire d'Analyse et d'Architecture des Systèmes (LAAS-UPR 8001), Université de Toulouse, CNRS, UPS, 7 Avenue du Colonel Roche, Cedex 4, 31031 Toulouse, France
dInstitut de recherches sur la catalyse et l'environnement de Lyon (IRCELYON-UMR 5256), Université de Lyon, CNRS, 2 Avenue Albert Einstein, 69626 Villeurbanne Cedex, France
eFunctional Materials Group, Gulf University for Science and Technology, Mubarak Al-Abdullah, 32093, Kuwait
First published on 10th June 2025
Abstract
Functionalizing ZnO-based chemiresistive sensor surfaces with a ZIF-8 layer, which acts as a permselective membrane, is a well-established strategy to enhance sensor selectivity. This study examines the key factors influencing the conversion process, including the physicochemical properties of solvents or solvent mixtures and the thermal pretreatment, of Ga-doped ZnO (ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga). We have evidenced that the polarity, viscosity, and interfacial tension of the solvent significantly affect the dissolution of ZnO
Ga). We have evidenced that the polarity, viscosity, and interfacial tension of the solvent significantly affect the dissolution of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga and the crystallization of ZIF-8. Methanol–water mixtures were found to effectively control the conversion process, with a 3
Ga and the crystallization of ZIF-8. Methanol–water mixtures were found to effectively control the conversion process, with a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 MeOH/H2O ratio being optimal for producing continuous ZIF-8 membranes. Additionally, it has been demonstrated that annealing can greatly enhance the reactivity of the oxide. However, while it enhances the dissolution of ZnO
1 MeOH/H2O ratio being optimal for producing continuous ZIF-8 membranes. Additionally, it has been demonstrated that annealing can greatly enhance the reactivity of the oxide. However, while it enhances the dissolution of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga, excessively high temperatures can lead to over-dissolution, which hinders ZIF-8 formation. These insights are crucial for optimizing ZIF-8 layers on ZnO
Ga, excessively high temperatures can lead to over-dissolution, which hinders ZIF-8 formation. These insights are crucial for optimizing ZIF-8 layers on ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga, paving the way for the development of highly selective chemiresistive sensors.
Ga, paving the way for the development of highly selective chemiresistive sensors.
Introduction
Metal oxide (MOx) chemiresistive sensors,1,2 such as those based on SnO2, WO3, In2O3, and ZnO, are widely used for detecting gases and VOCs in industrial and domestic settings. They are valued for their low cost, portability, ease of use, real-time and very long-term monitoring capabilities. With sensitivity reaching levels below parts per billion, these devices often outperform traditional technologies like electrochemical sensors. Detection relies on redox reactions between MOx and target molecules, altering the electrical resistance.3 The performance of those sensors is mainly influenced by factors such as MOx layer thickness, grain boundaries, operating temperature, and doping levels.Zinc oxide (ZnO) is a key material for gas sensors due to its non-toxicity, low cost, and high electron mobility. As an n-type semiconductor with a wide bandgap (3.37 eV) and excellent carrier mobility,4–6 ZnO shows great potential for sensing applications. However, its high resistivity often limits the sensing performance.7 To overcome this limitation, doping with gallium (Ga) effectively reduces resistivity, enhances oxidation resistance, and improves thermal stability.8–10 Ga-doped ZnO (ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga) is therefore considered as one of the most promising materials for fabricating sensitive sensors,11 as demonstrated by Presmanes and coworkers in their sub-ppm NO2 sensing studies.12 Despite these advances, ZnO
Ga) is therefore considered as one of the most promising materials for fabricating sensitive sensors,11 as demonstrated by Presmanes and coworkers in their sub-ppm NO2 sensing studies.12 Despite these advances, ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga sensors, like their other MOx counterparts, continue to face challenges with selectivity, especially in the presence of interfering molecules. This necessitates further optimization for practical sensing applications.
Ga sensors, like their other MOx counterparts, continue to face challenges with selectivity, especially in the presence of interfering molecules. This necessitates further optimization for practical sensing applications.
To address the issue of limited selectivity in chemiresistive sensors, a promising solution involves applying a selective membrane as an overlayer on the MOx-based sensing material. This strategy has proven effective in various systems, including nanowire-based sensors like SnO2 coated with Al2O3 (SnO2@Al2O3)13 and ZnO combined with boron nitride and palladium (ZnO@boron nitride/Pd).14 These selective membranes not only enhance the selectivity of the sensors but also improve their stability and reliability in complex environments. It is worth noting that metal–organic frameworks (MOFs) have also been studied as membrane overlayers to enhance the selectivity of chemiresistive sensors15,16 forming interfaces conceptually similar to heterojunctions observed in certain metal-oxide clusters.17
MOFs are hybrid crystalline materials composed of metal nodes connected by coordinating ligands.18 Their versatility has generated significant interest in various fields, including adsorption, separation, catalysis, and sensing. This interest stems from the ability to fine-tune their properties by selecting specific metals,19–21 modifying ligand structures,22,23 and introducing functional groups24–26 to meet the demands of particular applications.27,28 ZIF-8 (zeolitic imidazolate framework),29 a type of MOF, has attracted considerable interest due to its exceptional properties. Constructed from zinc cations linked by 2-methylimidazolate ligands, ZIF-8 stands out for its thermal and chemical stability,30 ease of synthesis, and microporous structure. Its pore aperture of 3.4 Å and cavity size of 10 Å make it highly effective for molecular sieving and storage applications.31–33
To enhance the selectivity of chemiresistive sensors, ZIF-8 is often studied as a molecular sieving membrane that covers a sensitive ZnO layer. The ZnO layer serves a dual function: acting as a reservoir for Zn2+ ions and enabling in situ formation of the MOF. Various methods to convert ZnO into ZIF-8 have been reported, including mechanochemistry,34 sonochemistry,35 and solution-based chemistry,36 each offering unique advantages in efficiency and scalability. The direct conversion of ZnO layers deposited by atomic layer deposition (ALD),37 chemical vapor deposition (CVD)36 or sputtering37 onto various supports (e.g. alumina,38 silicon,39 PAN,..40) has been widely studied over the past decade. This research has also been extended to form advanced composites, such as 3D ZIF-8/CNT structures, with enhanced adsorption properties.41 This method is also applied to coat ZnO-based chemiresistive sensors with various morphologies,42 such as nanorods, nanofibers, tetrapods or supported thin films. Among various selective membrane approaches, ZIF-8 offers distinct advantages for improving gas-sensing applications thanks to its molecular sieving properties. Previous studies by our research group have demonstrated that ZIF-8 layers significantly enhance the selectivity of ZnO@ZIF-8 nanowire-based sensors.43 The presence of ZIF-8 (pore aperture of 3.4 Å), can effectively separate H2 from larger molecules like benzene and toluene, resulting in virtually zero gas sensing response to these larger molecules due to the size exclusion effect. Furthermore, Weber et al.44 demonstrated further improvement of selectivity for H2 over toluene, acetone, ethanol, and benzene using Pd-loaded ZIF-8 membranes on ZnO nanowire sensors showing high stability up to 300 °C during repetitive temperature cycling. Their results show that the addition of the ZIF-8 membrane layer achieved >1000% improvement in selectivity by completely eliminating sensor response to all interfering gases (toluene, acetone, ethanol, and benzene), while maintaining strong sensitivity to H2, effectively transforming a multi-gas responsive sensor into one exclusively selective for hydrogen.
Overall, the molecular sieving strategy significantly improves selectivity of metal oxide sensors compared to conventional approaches, such as catalyst loading in metal oxides, which show less efficient molecular discrimination.45,46 It also surpasses many other selective materials used directly as sensors like metal oxides or dense polymers, which often rely on other non-sieving mechanisms such as catalytic effects or differences in solution/diffusion rates.47
ZnO surfaces or films can be converted to ZIF-8 through two main methods: reaction with vaporized 2-methylimidazole48,49 or direct solution-based conversion.50 The solution-based approach is often preferred because it provides better control over ZIF-8 formation and allows ligand recycling (up to 50 times).51 It involves five stages: ZnO dissolution, Zn2+ ion release and diffusion, ZIF-8 nucleation, crystal growth, and Ostwald ripening. Imbalances in these steps can lead to defects or incomplete formation of the ZIF-8 layer. Factors such as temperature,52 ZnO deposition method,37 crystallinity, crystal orientation,53 solvent type,52 and reagent ratio54 critically influence the quality of ZIF-8 membranes.
While existing research offers valuable insights into optimizing the ZnO-to-ZIF-8 conversion process, several critical gaps remain. Specifically, the influence of solvent physicochemical properties - such as polarity, viscosity, and surface tension - has not been thoroughly investigated, leaving an incomplete understanding in this area. Additionally, studies on ZnO doping with other trivalent cations, such as aluminum have shown that solubility limits significantly affect material properties.55 These findings suggest that similar effects may occur in Ga-doped systems.
Furthermore, the importance of achieving controlled and partial consumption of the ZnO layer, which is essential for preserving its electrical properties and sensitivity during ZIF-8 membrane formation, has often been overlooked. In addition, the role of annealing treatments of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga, commonly applied to enhance sensor performance, has not been adequately studied as a factor impacting the conversion process. Lastly, although doping is widely recognized for significantly enhancing the sensitivity and functionality of sensors, most studies focus solely on pristine ZnO, providing limited information on the behavior of doped ZnO systems during the conversion to ZIF-8. Hence, addressing these gaps is crucial for advancing the development of high-performance, selective sensors.
Ga, commonly applied to enhance sensor performance, has not been adequately studied as a factor impacting the conversion process. Lastly, although doping is widely recognized for significantly enhancing the sensitivity and functionality of sensors, most studies focus solely on pristine ZnO, providing limited information on the behavior of doped ZnO systems during the conversion to ZIF-8. Hence, addressing these gaps is crucial for advancing the development of high-performance, selective sensors.
To tackle this issue, the present study aims to identify the critical factors affecting the controlled conversion of a high-performance ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga layer into a uniform ZIF-8 membrane while preserving the ZnO
Ga layer into a uniform ZIF-8 membrane while preserving the ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga layer to maintain its low electrical conductivity. Specifically, we have examined how solvent properties - such as polarity, surface tension, and viscosity-influence the ZnO-to-ZIF-8 conversion process. Additionally, we have explored the role of ZnO
Ga layer to maintain its low electrical conductivity. Specifically, we have examined how solvent properties - such as polarity, surface tension, and viscosity-influence the ZnO-to-ZIF-8 conversion process. Additionally, we have explored the role of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga annealing in determining the uniformity and of the resulting synthetized ZIF-8 membrane. This work thus provides valuable insights into optimizing the surface conversion of ZnO
Ga annealing in determining the uniformity and of the resulting synthetized ZIF-8 membrane. This work thus provides valuable insights into optimizing the surface conversion of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga to ZIF-8, which will be applied in our future works to improve the selectivity of ZnO
Ga to ZIF-8, which will be applied in our future works to improve the selectivity of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga-based gas sensing layers.
Ga-based gas sensing layers.
Experimental section
Materials and methods
All reagents were purchased from commercial suppliers and used as received, without any additional purification. The entire synthesis process, from ZnO deposition to ZIF-8 formation, is illustrated in ESI† (Fig. S23).Preparation of Si/SiO2 substrates
Standard 4-inch n-type silicon wafers (engineering wafers) were selected as substrates for the deposition and characterization of gallium-doped zinc oxide (ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga) thin films. Before deposition, the silicon wafers underwent a cleaning process involving immersion in buffered HF to remove native oxidation. They were then rinsed with deionized water and dried using a nitrogen gun to ensure a pristine, contaminant-free surface. To provide electrical insulation between the ZnO layer and the Si substrate, a 600 nm SiO2 layer was deposited using the plasma enhanced chemical vapor deposition (PECVD) technique, matching the configuration of future sensor on microhotplate.
Ga) thin films. Before deposition, the silicon wafers underwent a cleaning process involving immersion in buffered HF to remove native oxidation. They were then rinsed with deionized water and dried using a nitrogen gun to ensure a pristine, contaminant-free surface. To provide electrical insulation between the ZnO layer and the Si substrate, a 600 nm SiO2 layer was deposited using the plasma enhanced chemical vapor deposition (PECVD) technique, matching the configuration of future sensor on microhotplate.
Deposition of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) Ga thin films
Ga thin films
Gallium-doped zinc oxide (ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga) thin films were fabricated using physical vapor deposition (PVD) with an ALCATEL CIT SCM400 system operating in diode mode. The apparatus included a radio-frequency (RF) generator with a maximum power of 600 W for cathode polarization. The vacuum system, consisting of a primary pump and a secondary turbomolecular pump, achieved a base pressure of 10−5 Pa. The setup also featured a cooled target holder and two workstations equipped with a substrate cooling mechanism, ensuring precise control of the deposition parameters and film quality. Before deposition, the target underwent a 30-minute pre-sputtering process in an argon atmosphere containing 1% oxygen. This step was crucial for preventing target reduction during the subsequent deposition stages carried out in a pure argon environment. ZnO
Ga) thin films were fabricated using physical vapor deposition (PVD) with an ALCATEL CIT SCM400 system operating in diode mode. The apparatus included a radio-frequency (RF) generator with a maximum power of 600 W for cathode polarization. The vacuum system, consisting of a primary pump and a secondary turbomolecular pump, achieved a base pressure of 10−5 Pa. The setup also featured a cooled target holder and two workstations equipped with a substrate cooling mechanism, ensuring precise control of the deposition parameters and film quality. Before deposition, the target underwent a 30-minute pre-sputtering process in an argon atmosphere containing 1% oxygen. This step was crucial for preventing target reduction during the subsequent deposition stages carried out in a pure argon environment. ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga thin films were deposited under a working pressure of 1.8 × 10−2 mbar using RF power set to 30 W. The doping level, determined by CAMECA SXFiveFE electron probe microanalyser (EPMA) measurements, reached 4 at% of Ga, resulting in oxide films with the composition Zn0.96Ga0.04O. The film thickness, measured using a DEKTAK 3030 ST profilometer (VEECO), was around 50 nm with an uncertainty margin of ±10%. It should be noted that while Ga doping significantly enhances the electrical properties of ZnO for sensing applications, it does not notably affect the conversion process to ZIF-8. The small amount of Ga3+ cannot effectively coordinate with the imidazolate ligands to form stable MOF structures, thus having negligible impact on the dissolution-recrystallization mechanisms governing the conversion process.
Ga thin films were deposited under a working pressure of 1.8 × 10−2 mbar using RF power set to 30 W. The doping level, determined by CAMECA SXFiveFE electron probe microanalyser (EPMA) measurements, reached 4 at% of Ga, resulting in oxide films with the composition Zn0.96Ga0.04O. The film thickness, measured using a DEKTAK 3030 ST profilometer (VEECO), was around 50 nm with an uncertainty margin of ±10%. It should be noted that while Ga doping significantly enhances the electrical properties of ZnO for sensing applications, it does not notably affect the conversion process to ZIF-8. The small amount of Ga3+ cannot effectively coordinate with the imidazolate ligands to form stable MOF structures, thus having negligible impact on the dissolution-recrystallization mechanisms governing the conversion process.
Annealing of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) Ga films
Ga films
Annealed ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga thin films were prepared by subjecting the as-deposited films to a controlled thermal treatment to ensure their stability for subsequent processes and measurement conditions. The films were placed in a furnace and heated to 600 °C for 4 hours in an air atmosphere. After annealing, the films were gradually cooled to room temperature inside the furnace to prevent thermal shock and maintain their structural integrity.
Ga thin films were prepared by subjecting the as-deposited films to a controlled thermal treatment to ensure their stability for subsequent processes and measurement conditions. The films were placed in a furnace and heated to 600 °C for 4 hours in an air atmosphere. After annealing, the films were gradually cooled to room temperature inside the furnace to prevent thermal shock and maintain their structural integrity.
Conversion of ZnO-to-ZIF-8
The conversion of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga thin films into ZIF-8 was carried out using a (hydro)solvothermal treatment. The supported ZnO
Ga thin films into ZIF-8 was carried out using a (hydro)solvothermal treatment. The supported ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga films were placed in a Teflon-lined stainless steel autoclave with a 45 mL capacity. This autoclave was filled with a 1% w/v solution of 2-methylimidazole (99%, Sigma Aldrich) dissolved in selected pure solvents or solvent mixtures, including DMF (99.8%), ethanol (absolute), and methanol (>99.8%) from Sigma Aldrich. The autoclave was then sealed and heated in a conventional oven at either 100 °C or 200 °C for durations ranging from 1.5 up to 12 hours, depending on the experimental protocol. After the conversion, the films were washed three times with methanol to remove any unreacted reagents and then dried in an oven at 70 °C for 2 hours.
Ga films were placed in a Teflon-lined stainless steel autoclave with a 45 mL capacity. This autoclave was filled with a 1% w/v solution of 2-methylimidazole (99%, Sigma Aldrich) dissolved in selected pure solvents or solvent mixtures, including DMF (99.8%), ethanol (absolute), and methanol (>99.8%) from Sigma Aldrich. The autoclave was then sealed and heated in a conventional oven at either 100 °C or 200 °C for durations ranging from 1.5 up to 12 hours, depending on the experimental protocol. After the conversion, the films were washed three times with methanol to remove any unreacted reagents and then dried in an oven at 70 °C for 2 hours.
Characterization of supported films
The structure and purity of the prepared films were analyzed using various analytical techniques, including X-Ray Diffraction (XRD), Fourier-transform infrared (FTIR) spectroscopy, field emission scanning electron microscopy (FESEM), and energy-dispersive X-ray spectroscopy (EDS). XRD patterns were recorded using a Bruker D8 Advance diffractometer equipped with a LynxEye detector. Measurements were performed using CuKα radiation (λ (CuKα1) = 1.5405 Å). The diffractometer was configured in Bragg–Brentano geometry with an offset of 2° on θ, and data was collected with a step size of 0.02° in the 2θ range and a counting time of 1 second per step. Infrared spectra were obtained in the range of 600–2000 cm−1 with a resolution of 4 cm−1 using a Nicolet Nexus FT-IR in transmission mode.A Ga-Focused Ion Beam (FIB)-FESEM dual beam FEI Helios NanoLab600i apparatus, equipped with an Oxford Instruments Aztec Advanced EDS system featuring an X-Max 80 mm2 detector, was used for surface characterization and the preparation and analysis of film cross-sections. This system enabled high-resolution imaging and semi-quantitative chemical analysis of the thin films in both surface and cross-section views. To prevent damage to the samples during Ga-FIB milling, a platinum layer was deposited on the film surface. For surface imaging, a carbon layer was deposited to enhance conductivity and improve image clarity. Due to Ga milling, the analysis of Ga traces is not feasible on the cross-sections.
Results and discussion
Controlling the conversion of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga to ZIF-8 is essential for achieving a selective and sensitive membrane (permselectivity). This study investigates two main factors: the conversion rate and the quality of the resulting ZIF-8 membrane on the ZnO
Ga to ZIF-8 is essential for achieving a selective and sensitive membrane (permselectivity). This study investigates two main factors: the conversion rate and the quality of the resulting ZIF-8 membrane on the ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga layer surface. To identify optimal interfacial process conditions, encompassing ZnO
Ga layer surface. To identify optimal interfacial process conditions, encompassing ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga dissolution and ZIF-8 crystallization, a systematic analysis was performed on numerous samples prepared under different reaction conditions. The key parameters studied include: (i) solvent physicochemical properties, (ii) the MeOH/H2O volumetric ratio, and (iii) annealing of the ZnO
Ga dissolution and ZIF-8 crystallization, a systematic analysis was performed on numerous samples prepared under different reaction conditions. The key parameters studied include: (i) solvent physicochemical properties, (ii) the MeOH/H2O volumetric ratio, and (iii) annealing of the ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga layer. The findings are discussed in the following sections.
Ga layer. The findings are discussed in the following sections.
Effect of solvent physicochemical properties on ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) Ga conversion to ZIF-8
Ga conversion to ZIF-8
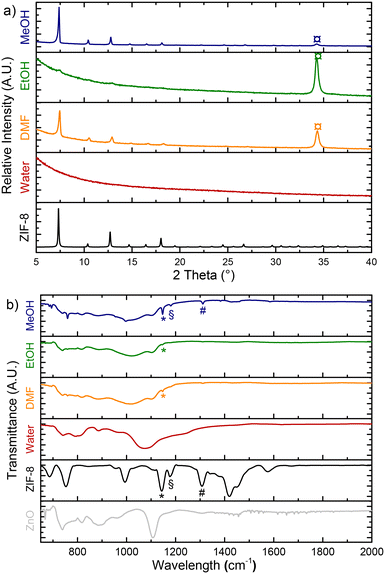
The choice of solvent significantly influences the formation of a continuous ZIF-8 membrane. A series of solvents with varying physicochemical characteristics (Table 1) were selected for ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga conversion under constant experimental conditions: ligand concentration (1 wt%), solvent volume (30 mL), reaction time (12 h), and temperature (100 °C). This enabled an exploration of the effects of key solvent parameters such as polarity, dynamic viscosity, and interfacial tension.
Ga conversion under constant experimental conditions: ligand concentration (1 wt%), solvent volume (30 mL), reaction time (12 h), and temperature (100 °C). This enabled an exploration of the effects of key solvent parameters such as polarity, dynamic viscosity, and interfacial tension.
| Solvent | Dielectric constant (F m−1) | Dipole moment (D) | Dynamic viscosity at 20 °C (mPa s) | Interfacial tension (mN m−1) |
|---|---|---|---|---|
| MeOH | 33.0 | 1.70 | 0.588 | 22.6 |
| EtOH | 25.3 | 1.69 | 1.095 | 22.3 |
| DMF | 36.7 | 3.82 | 0.92 | 36.4 |
| H2O | 78.4 | 1.86 | 0.354 | 72.6 |
Structural analysis
XRD patterns of the converted samples demonstrate the strong influence of solvent properties on the conversion rate and crystallinity of ZIF-8 (Fig. 1a). Notably, when water is used as the solvent, both ZnO and ZIF-8 peaks are absent, indicating complete removal of the ZnO layer under hydrothermal conditions, a finding confirmed by IR spectroscopy (Fig. 1b). Despite the high dielectric constant of water (78.4), which should theoretically favor the deprotonation of the 2-mim ligand and promote the formation of ZIF-8, it instead promotes the protonation of the ligand by autoprotolysis of water. This process inhibits ZIF crystallization by favoring the reaction of Zn2+ cations with OH− ions generated in solution, leading to the formation of zinc hydroxides. As a result, Zn2+ cations preferentially interact with water rather than with the ligand. Besides solvent effects, the passivation of grain boundaries is crucial in determining the final structure quality. This has been demonstrated in other systems where ZIF-8-based composites effectively passivate boundaries in perovskite photodetectors.56 For other solvents, ZnO undergoes partial conversion to ZIF-8, with different degrees of crystallinity. From the XRD patterns and IR spectra, the influence of the solvent on the conversion rate and ZIF-8 crystallinity, reflected by tighter and more intense XRD peaks, follows this order: EtOH < DMF < MeOH. This trend can be attributed to the differences in polarity, viscosity, and interfacial tension of the solvents.Application of ethanol leads to the lowest crystallinity among the solvents tested, mainly due to its relatively low polarity when compared to DMF. Indeed, polarity plays a critical role in dissolving ionic compounds and facilitating the nucleation process necessary for ZIF-8 formation. Due to its lower polarity, ethanol struggles to effectively deprotonate the 2-mim ligand, a crucial step in the generation of ZIF-8 nuclei. This limitation results in fewer nuclei forming, thereby reducing the overall crystallinity. Additionally, its higher viscosity hampers the diffusion of reactants, leading to their less frequent mutual collisions, thereby impeding crystal growth. The resulting ZIF-8 crystals are smaller in size and poorly defined. Moreover, the higher interfacial tension of ethanol compared to DMF imposes a significant surface barrier to crystallization, which further suppresses nucleation and limits crystal growth.
In contrast, DMF demonstrates better performance due to its higher polarity and lower viscosity. Its superior polarity enhances the deprotonation of the 2-mim ligand, promoting a higher level of nucleation. The lower viscosity of DMF allows for more rapid diffusion of reactants, increasing their frequency of collisions and thus accelerating crystal growth. However, despite these advantages, the interfacial tension and nucleation dynamics associated with DMF are less effective to achieve high crystallinity compared to methanol.
In fact, methanol appears as the most effective solvent for promoting high crystallinity in ZIF-8. The combination of its high polarity and low viscosity creates an optimal environment for efficient nucleation and robust crystal growth. Its high polarity ensures advanced deprotonation of the 2-mim ligand, resulting in the formation of abundant nuclei. Simultaneously, its low viscosity facilitates rapid diffusion of reactants, enabling frequent interactions that support sustained crystal growth. These synergistic properties of methanol produce larger, well-defined ZIF-8 crystals with the highest degree of crystallinity obtained among the solvents tested.
Morphological analysis
SEM micrographs (Fig. 2) offer valuable insights that complement the findings from XRD and IR spectroscopy, shedding light on how each solvent affects the conversion of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga layers on silicon wafers. These micrographs allow to clearly evidence the presence, shape and size of ZIF-8 crystals while also revealing surface defects, which can compromise the membrane efficiency.
Ga layers on silicon wafers. These micrographs allow to clearly evidence the presence, shape and size of ZIF-8 crystals while also revealing surface defects, which can compromise the membrane efficiency.
 | ||
Fig. 2 SEM observations (surfaces and cross-sections) of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga samples after reaction/conversion at 100 °C for 12 h in various solvents (water, DMF, ethanol or methanol). Ga samples after reaction/conversion at 100 °C for 12 h in various solvents (water, DMF, ethanol or methanol). | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga layer is entirely dissolved and removed from the silicon wafer during hydrothermal treatment (Fig. 2). This is in line with the absence of ZnO-related XRD peaks in Fig. 1a. The surface observed in Fig. 2 corresponds to the pristine silica coating (Fig. S1†). This is also confirmed by the EDX analysis (Fig. S2†). In contrast, ZIF-8 crystals are visibly formed when other solvents are used. The size of these crystals increases when using solvents in the following order: EtOH < DMF < MeOH, consistent with the sharper and narrower XRD peaks observed previously.
Ga layer is entirely dissolved and removed from the silicon wafer during hydrothermal treatment (Fig. 2). This is in line with the absence of ZnO-related XRD peaks in Fig. 1a. The surface observed in Fig. 2 corresponds to the pristine silica coating (Fig. S1†). This is also confirmed by the EDX analysis (Fig. S2†). In contrast, ZIF-8 crystals are visibly formed when other solvents are used. The size of these crystals increases when using solvents in the following order: EtOH < DMF < MeOH, consistent with the sharper and narrower XRD peaks observed previously.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga, and ii) superimposed bilayers of ZnO
Ga, and ii) superimposed bilayers of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga and ZIF-8.
Ga and ZIF-8.
For the ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga/ZIF-8 bilayer, the ZnO
Ga/ZIF-8 bilayer, the ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga layer thickness remains constant at approximately 55 nm, while the ZIF-8 layer grows to around 100 nm, indicating the consumption of about 36 nm of ZnO
Ga layer thickness remains constant at approximately 55 nm, while the ZIF-8 layer grows to around 100 nm, indicating the consumption of about 36 nm of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga during the transformation. The intercrystalline spaces, initially filled by the ZnO
Ga during the transformation. The intercrystalline spaces, initially filled by the ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga layer, appear to serve as reservoirs of metal ions, supplying Zn2+ for ZIF-8 formation in adjacent regions. This interpretation is supported by EDS spectra (Fig. S3 and S4†), which show that areas with intercrystalline defects have low zinc content compared to silicon and oxygen from the wafer. In contrast, neighboring regions exhibit higher concentrations of zinc, carbon, and nitrogen, consistent with contributions from the ZIF ligand.
Ga layer, appear to serve as reservoirs of metal ions, supplying Zn2+ for ZIF-8 formation in adjacent regions. This interpretation is supported by EDS spectra (Fig. S3 and S4†), which show that areas with intercrystalline defects have low zinc content compared to silicon and oxygen from the wafer. In contrast, neighboring regions exhibit higher concentrations of zinc, carbon, and nitrogen, consistent with contributions from the ZIF ligand.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga conversion results in a relatively compact surface composed of small ZIF-8 crystals. While the surface quality improves compared to that obtained with DMF, SEM images reveal the presence of pinhole-type defects measuring hundreds of nanometers (Fig. 2). These defects, distinct from the intercrystalline defects observed with DMF, suggest that the conversion mechanism varies depending on the solvent.
Ga conversion results in a relatively compact surface composed of small ZIF-8 crystals. While the surface quality improves compared to that obtained with DMF, SEM images reveal the presence of pinhole-type defects measuring hundreds of nanometers (Fig. 2). These defects, distinct from the intercrystalline defects observed with DMF, suggest that the conversion mechanism varies depending on the solvent.
Cross-sectional SEM micrographs show a well-defined ZnO/ZIF-8 stack (Fig. 2). The ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga layer thickness decreases to about 39 nm, while the ZIF-8 layer grows to approximately 48 nm, consistent with the partial consumption of ZnO
Ga layer thickness decreases to about 39 nm, while the ZIF-8 layer grows to approximately 48 nm, consistent with the partial consumption of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga during conversion. In some areas, only ZIF-8 is observed, as confirmed by EDS analysis (Fig. S5 and S6†), indicating that pinholes may result from the complete local conversion of ZnO
Ga during conversion. In some areas, only ZIF-8 is observed, as confirmed by EDS analysis (Fig. S5 and S6†), indicating that pinholes may result from the complete local conversion of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga into ZIF-8.
Ga into ZIF-8.
However, these observations raise additional questions. Theoretical calculations reported elsewhere57 predict that the ZIF-8 layer should be thicker than the original ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga layer after complete conversion (an expansion factor up to 17 was calculated for ZIF-8, corresponding to the ratio of metal ion densities in the crystalline structure of the oxide and the MOF). Yet, in the pinhole regions, the layer thickness remains around 50 nm, comparable to the original ZnO
Ga layer after complete conversion (an expansion factor up to 17 was calculated for ZIF-8, corresponding to the ratio of metal ion densities in the crystalline structure of the oxide and the MOF). Yet, in the pinhole regions, the layer thickness remains around 50 nm, comparable to the original ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga layer. This anomaly suggests that in these regions a complete conversion releases excess Zn2+ ions into the surrounding medium. These ions may accumulate at the interface, potentially driving additional ZIF-8 formation near the pinholes.
Ga layer. This anomaly suggests that in these regions a complete conversion releases excess Zn2+ ions into the surrounding medium. These ions may accumulate at the interface, potentially driving additional ZIF-8 formation near the pinholes.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga conversion, which diverges from conventional solution-state synthesis.
Ga conversion, which diverges from conventional solution-state synthesis.
The ZIF-8 crystals formed during methanol conversion display specific morphological features, notably their faceted geometry. SEM micrographs reveal that most crystals adopt a rhombic dodecahedral shape with 12 exposed {110} facets (Fig. 2). According to the well-established crystallography of ZIF-8,53,58 these facets correspond to {110} planes. This highly crystalline morphology is attributed to the slow growth rate of {110} facets,59 which, according to Wulff's rule, determines the final crystal structure. A small fraction of the crystals exhibits a truncated cubic shape, typically associated with slower growth of {100} facets relative to {110}. This shape is often induced by capping agents like surfactants, and its presence, even in small quantities, suggests that during the conversion process, the growth of {100} facets might be selectively decelerated. While direct visualization of these crystallographic planes would require advanced techniques such as HR-TEM, the observed external morphologies align with the crystallographic features of ZIF-8 reported in the literature.53,58,60
The formation of such large, faceted ZIF-8 crystals can be explained by the interfacial conversion mechanism. In the early stages, the release of Zn2+ ions from the ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga layer enriches the interfacial medium, temporarily creating a low ligand-to-metal molar ratio and a supersaturation regime. Similar conditions have been reported in the literature, where EDS spectroscopy showed elevated zinc concentrations on substrate surfaces outside ZIF-8 domains, peaking at 5.3% after three hours and declining gradually as Zn2+ is fully consumed after ten hours.61
Ga layer enriches the interfacial medium, temporarily creating a low ligand-to-metal molar ratio and a supersaturation regime. Similar conditions have been reported in the literature, where EDS spectroscopy showed elevated zinc concentrations on substrate surfaces outside ZIF-8 domains, peaking at 5.3% after three hours and declining gradually as Zn2+ is fully consumed after ten hours.61
This supersaturation regime enables two potential growth pathways. Both begin with the nucleation of crystalline primary particles (CPP), followed by growth through successive addition of either monomers (metal ions and ligands)62 or secondary building units (SBUs).63 These pathways contribute to the formation of the well-faceted crystals observed in SEM micrographs (Fig. 2). As the Zn2+ concentration diminishes over time, crystal intergrowth becomes more prominent, transitioning from monomer- or SBU-driven growth to processes like polynuclear growth, internal reorganization, and aggregative growth. These mechanisms further enhance the observed crystal morphology.64
The results indicate that among the solvents studied, methanol is the most effective for promoting ZIF-8 crystallization, while water primarily facilitates ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga dissolution. This highlights the dual importance of solvent selection in determining both the dissolution and crystallization dynamics of the conversion process. Other polar aprotic solvents with low viscosity, such as acetonitrile (dielectric constant: 37.5, dynamic viscosity: 0.369 mPa s, interfacial tension: 29.3 mN m−1) and acetone (dielectric constant: 20.7, dynamic viscosity: 0.306 mPa s, interfacial tension: 23.7 mN m−1), may exhibit intermediate behavior in this conversion process. The high polarity and very low viscosity of acetonitrile could potentially accelerate ZIF-8 nucleation while maintaining moderate dissolution rates, possibly resulting in smaller, and more numerous crystals with higher overall crystallinity compared to DMF. Conversely, acetone, with its lower polarity but similarly low viscosity, might favor controlled ZnO dissolution while promoting moderate crystal growth rates, potentially leading to a more uniform particle size distribution. Based on the trends observed in this study, these solvents could offer additional pathways for fine-tuning ZnO conversion kinetics. Future investigations should consider these solvents to expand the toolkit for ZIF-8 membrane engineering with tailored morphological properties.
Ga dissolution. This highlights the dual importance of solvent selection in determining both the dissolution and crystallization dynamics of the conversion process. Other polar aprotic solvents with low viscosity, such as acetonitrile (dielectric constant: 37.5, dynamic viscosity: 0.369 mPa s, interfacial tension: 29.3 mN m−1) and acetone (dielectric constant: 20.7, dynamic viscosity: 0.306 mPa s, interfacial tension: 23.7 mN m−1), may exhibit intermediate behavior in this conversion process. The high polarity and very low viscosity of acetonitrile could potentially accelerate ZIF-8 nucleation while maintaining moderate dissolution rates, possibly resulting in smaller, and more numerous crystals with higher overall crystallinity compared to DMF. Conversely, acetone, with its lower polarity but similarly low viscosity, might favor controlled ZnO dissolution while promoting moderate crystal growth rates, potentially leading to a more uniform particle size distribution. Based on the trends observed in this study, these solvents could offer additional pathways for fine-tuning ZnO conversion kinetics. Future investigations should consider these solvents to expand the toolkit for ZIF-8 membrane engineering with tailored morphological properties.
Effect of MeOH/H2O ratio on ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) Ga conversion to ZIF-8
Ga conversion to ZIF-8
The above studies using single solvents suggest that a solvent mixture might attractively combine their benefits while reducing inherent limitations. The solvent should be safe, eco-friendly, and ideally used under mild conditions with low temperatures and short processing times. Since the ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga layer sensitivity depends on its thickness, minimizing Zn2+ depletion is crucial. Optimizing reaction conditions should also eliminate intercrystalline defects and pinholes to maintain the ZnO
Ga layer sensitivity depends on its thickness, minimizing Zn2+ depletion is crucial. Optimizing reaction conditions should also eliminate intercrystalline defects and pinholes to maintain the ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga layer performance.
Ga layer performance.
As discussed previously, DMF exhibits notable potential in ZIF-8 synthesis but is unsuitable due to health and environmental risks. Ethanol forms high-quality layers but has limited impact on oxide dissolution and MOF crystallization process. Water effectively dissolves the ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga layer, providing Zn2+ ions for ZIF-8 formation, while methanol aids in crystallization, forming large, well-faceted ZIF-8 crystals. Both water and methanol influence ZnO
Ga layer, providing Zn2+ ions for ZIF-8 formation, while methanol aids in crystallization, forming large, well-faceted ZIF-8 crystals. Both water and methanol influence ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga dissolution and ZIF-8 formation, though neither component on its own can achieve the desired balance for optimal layer performance.
Ga dissolution and ZIF-8 formation, though neither component on its own can achieve the desired balance for optimal layer performance.
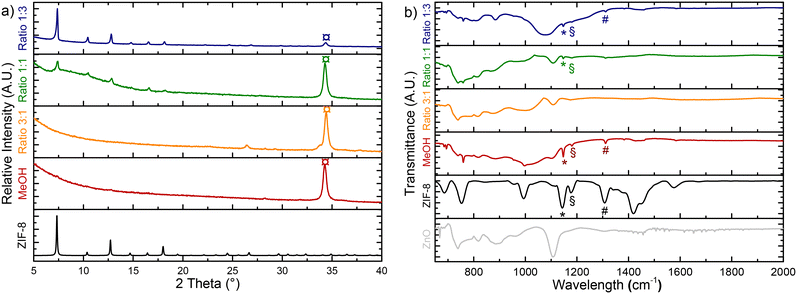
A water–methanol mixture was thus proposed to combine the strengths of both solvents while reducing their limitations. By adjusting methanol-to-water ratio, the process was optimized to enhance Zn2+ ion release or promote ZIF-8 crystallization. The conversion was optimized by maintaining a temperature of 100 °C, while reducing the reaction time from 12 hours to 1.5 hours. Three methanol-to-water ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 3
1, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were tested, along with pure methanol for comparison. The structural and morphological characteristics of the corresponding samples synthetized in water–methanol solvent mixtures are reported and discussed in the following sections.
1) were tested, along with pure methanol for comparison. The structural and morphological characteristics of the corresponding samples synthetized in water–methanol solvent mixtures are reported and discussed in the following sections.
Structural analysis
The XRD patterns of the converted samples highlight the high degree of tunability and control achievable in the process (Fig. 3a). When the solvent mixture contains a higher proportion of water (CH3OH/H2O ratio: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), the XRD peaks corresponding to ZIF-8 display higher intensity and sharpness than for the other samples. In addition, a significant reduction in the intensity of the ZnO
3), the XRD peaks corresponding to ZIF-8 display higher intensity and sharpness than for the other samples. In addition, a significant reduction in the intensity of the ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga XRD peaks indicates higher ZnO consumption and more advanced conversion.
Ga XRD peaks indicates higher ZnO consumption and more advanced conversion.
As the water content in the solvent mixture decreases in favor of methanol, the intensity of the ZIF-8 XRD peaks weakens, and the peaks become broader, while the relative intensity of the ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga peaks increases. Such result indicates a slower conversion rate and smaller ZIF-8 crystal sizes obtained in methanol solution with reduced water content. The infrared spectra (Fig. 3b) further confirm these observations, demonstrating that the MeOH/H2O solvent ratio can effectively regulate the conversion process. This allows for a precise control over the conversion rates, ranging from minimal to advanced, depending on the target reaction outcome.
Ga peaks increases. Such result indicates a slower conversion rate and smaller ZIF-8 crystal sizes obtained in methanol solution with reduced water content. The infrared spectra (Fig. 3b) further confirm these observations, demonstrating that the MeOH/H2O solvent ratio can effectively regulate the conversion process. This allows for a precise control over the conversion rates, ranging from minimal to advanced, depending on the target reaction outcome.
Morphological analysis
SEM micrographs (Fig. 4 and 5) confirm an enhanced quality of the formed ZIF-8 layers and reveal clear trends. Adjusting the solvent ratio enables a fine control over crucial properties such as homogeneity, crystal size, defect reduction, and conversion rate, thus demonstrating the flexibility and effectiveness of this approach in optimizing the reaction process. | ||
Fig. 4 SEM observations (surfaces) of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga samples after reaction/conversion at 100 °C for 1.5 h in CH3OH/H2O solvent mixtures with different ratios (1 Ga samples after reaction/conversion at 100 °C for 1.5 h in CH3OH/H2O solvent mixtures with different ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; 1 3; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3 1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
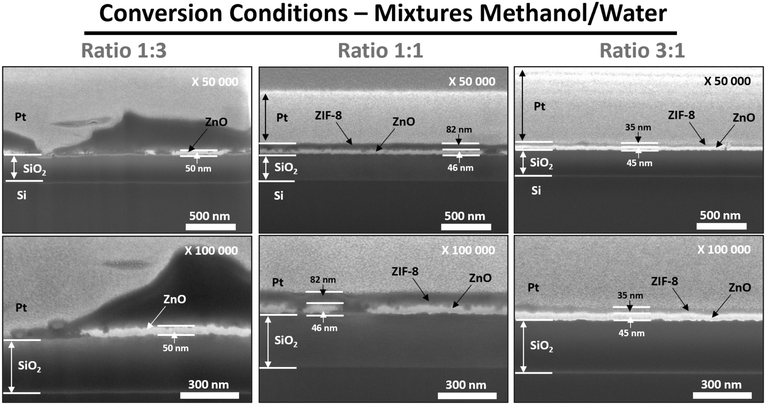 | ||
Fig. 5 SEM observations (cross-sections) of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga samples after reaction/conversion at 100 °C for 1.5 h in CH3OH/H2O solvent mixtures with different ratios (1 Ga samples after reaction/conversion at 100 °C for 1.5 h in CH3OH/H2O solvent mixtures with different ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; 1 3; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3 1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 3).
Using a water-enriched solvent mixture (MeOH/H2O ratio = 1
3).
Using a water-enriched solvent mixture (MeOH/H2O ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) offers valuable insights into the conversion process by significantly enhancing ZnO
3) offers valuable insights into the conversion process by significantly enhancing ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga dissolution. The higher water content facilitates ligand deprotonation due to its greater polarity, while its elevated surface tension reduces the likelihood of reactant collisions, delaying nucleation. Simultaneously, the lower viscosity improves diffusion, favoring crystal growth. These combined effects promote the formation of large ZIF-8 crystals, as evidenced by SEM micrographs and confirmed by EDS analysis (Fig. S10–S12†), which reveal micrometer-sized ZIF-8 crystals covering the surface of ZnO layers. However, such large crystals create intercrystalline defects at their boundaries (Fig. 4).
Ga dissolution. The higher water content facilitates ligand deprotonation due to its greater polarity, while its elevated surface tension reduces the likelihood of reactant collisions, delaying nucleation. Simultaneously, the lower viscosity improves diffusion, favoring crystal growth. These combined effects promote the formation of large ZIF-8 crystals, as evidenced by SEM micrographs and confirmed by EDS analysis (Fig. S10–S12†), which reveal micrometer-sized ZIF-8 crystals covering the surface of ZnO layers. However, such large crystals create intercrystalline defects at their boundaries (Fig. 4).
The ZIF-8 crystals feature an imperfect rhombic dodecahedral shape, coated with smaller spherical nanoparticles. This diminished crystal quality, compared to those formed in pure methanol, is attributed to faster dissolution kinetics and change in the physicochemical conditions at the crystal interface. Typically, a supersaturated environment with high Zn2+ concentration at the interface promotes the growth of well-defined, faceted crystals. However, in this scenario, the reduced viscosity allows Zn2+ ions to diffuse away from the interface more rapidly, disrupting the sustained high cation concentrations necessary for prolonged and precise crystal growth.
Two potential mechanisms could explain these observations:
1) Sequential growth and diffusion mechanism: water facilitates the rapid release of Zn2+ ions, creating a localized environment at the interface that favors the nucleation of crystalline primary particles (CPPs). These particles subsequently grow through the addition of monomers or secondary building units (SBUs). However, due to the low viscosity of water, Zn2+ ions quickly diffuse away from the interface, depleting the interfacial concentration. Such depletion prompts the formation of crystals via CPP nucleation in a metastable phase, followed by polynuclear growth, resulting in smaller particles with irregular crystal shapes.
2) Growth, erosion, and secondary nucleation mechanism: initially, the rapid release of Zn2+ ions supports the growth of large, well-defined rhombic dodecahedral crystals. However, the strong erosive properties of water accelerate Zn2+ depletion, partially dissolving the ZIF-8 crystals through hydrolysis of zinc-nitrogen bonds. This process releases Zn2+ ions near the larger crystals, triggering secondary nucleation and forming smaller spherical nanoparticles. This secondary nucleation compromises the morphological integrity of the larger crystals.
The latter hypothesis is supported by reports of ZIF-8 undergoing cycles of growth and dissolution under similar conditions.61 However, in the present study, these processes occur within 1.5 hours, hence significantly faster than the 10 hours reported elsewhere.61 Such accelerated kinetics is likely due to the high water content, which enhances the hydrolysis of zinc–ligand bonds and speeds up dissolution.
Cross-sectional micrographs (Fig. 5) reveal the advanced dissolution of the ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga layer caused by the high water content in the solvent mixture. Two distinct surface regions are evidenced: one where the ZnO
Ga layer caused by the high water content in the solvent mixture. Two distinct surface regions are evidenced: one where the ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga layer has been completely replaced by a ZIF-8 layer, and another where the ZnO
Ga layer has been completely replaced by a ZIF-8 layer, and another where the ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga layer remains intact at its original thickness. Additionally, the resulting ZIF-8 layer exhibits significant surface irregularities. These observations suggest that excessive ZnO
Ga layer remains intact at its original thickness. Additionally, the resulting ZIF-8 layer exhibits significant surface irregularities. These observations suggest that excessive ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga dissolution compromises sample quality, highlighting the need to reduce the water proportion in the solvent mixture.
Ga dissolution compromises sample quality, highlighting the need to reduce the water proportion in the solvent mixture.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1).
SEM micrographs (Fig. 4) and EDS analysis (Fig. S13 and S14) confirm that the conversion in the presence of reaction mixture with lower water quantity (MeOH/H2O ratio = 1
1).
SEM micrographs (Fig. 4) and EDS analysis (Fig. S13 and S14) confirm that the conversion in the presence of reaction mixture with lower water quantity (MeOH/H2O ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) leads to significantly better ZIF-8 layer quality. Indeed, the reduction in water content decreases ZIF-8 crystal size from micrometers to a few hundred nanometers. This effect arises from increased solvent viscosity, which slows crystal growth, and reduced interfacial tension facilitating the formation of a higher number of nuclei. Additionally, the solution enrichment in methanol affects the crystal morphology, yielding exclusively spherical particles instead of faceted crystals. Such observation suggests that slower ZnO
1) leads to significantly better ZIF-8 layer quality. Indeed, the reduction in water content decreases ZIF-8 crystal size from micrometers to a few hundred nanometers. This effect arises from increased solvent viscosity, which slows crystal growth, and reduced interfacial tension facilitating the formation of a higher number of nuclei. Additionally, the solution enrichment in methanol affects the crystal morphology, yielding exclusively spherical particles instead of faceted crystals. Such observation suggests that slower ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga dissolution lowers the Zn2+ concentration at the interface, promoting less defined morphologies. Crucially, the reduced water content minimizes intercrystalline defects, enhancing membrane quality.
Ga dissolution lowers the Zn2+ concentration at the interface, promoting less defined morphologies. Crucially, the reduced water content minimizes intercrystalline defects, enhancing membrane quality.
Cross-sectional SEM micrographs (Fig. 5) reveal uniform conversion across the sample surface. The remaining ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga layer retains a consistent thickness, indicating its controlled dissolution. However, isolated ZIF-8 regions are still observed on the surface, separated from the bilayer areas. These regions could affect system performance by increasing overall resistivity.
Ga layer retains a consistent thickness, indicating its controlled dissolution. However, isolated ZIF-8 regions are still observed on the surface, separated from the bilayer areas. These regions could affect system performance by increasing overall resistivity.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1).
The MeOH/H2O (3
1).
The MeOH/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture was used to study the conversion process under conditions intended to slow ZnO
1) mixture was used to study the conversion process under conditions intended to slow ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga dissolution while promoting ZIF-8 crystallization. SEM micrographs and EDS analysis (Fig. S15 and S16†) show that these conditions significantly improve surface homogeneity and compactness, aligning with trends observed for other solvent ratios (Fig. 4). The combination of increased viscosity and lower interfacial tension promotes the formation of smaller, more numerous crystals typically below 100 nm in size, hence significantly smaller than those formed in higher water content reaction mixtures. Additionally, reduced water content decreases surface defects resulting in enhanced ZIF-8 membrane quality.
Ga dissolution while promoting ZIF-8 crystallization. SEM micrographs and EDS analysis (Fig. S15 and S16†) show that these conditions significantly improve surface homogeneity and compactness, aligning with trends observed for other solvent ratios (Fig. 4). The combination of increased viscosity and lower interfacial tension promotes the formation of smaller, more numerous crystals typically below 100 nm in size, hence significantly smaller than those formed in higher water content reaction mixtures. Additionally, reduced water content decreases surface defects resulting in enhanced ZIF-8 membrane quality.
Cross-sectional SEM micrographs (Fig. 5) confirm these improvements. The ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga layer retains a constant thickness along the sample, similar to what was observed with the 1
Ga layer retains a constant thickness along the sample, similar to what was observed with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solvent mixture. The lower water content significantly minimizes ZnO
1 solvent mixture. The lower water content significantly minimizes ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga consumption during the conversion process. Importantly, the bilayer remains defect-free and continuous. Such optimized conditions allow for precise control of the ZnO
Ga consumption during the conversion process. Importantly, the bilayer remains defect-free and continuous. Such optimized conditions allow for precise control of the ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga dissolution rate, promoting effective ZIF-8 crystallization. This results in a high-quality bilayer system and a compact ZIF-8 membrane layer with only a slight reduction in ZnO
Ga dissolution rate, promoting effective ZIF-8 crystallization. This results in a high-quality bilayer system and a compact ZIF-8 membrane layer with only a slight reduction in ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga thickness.
Ga thickness.
Interestingly, while a high water content in solvent mixtures adversely affects membrane formation, the presence of a small amount of water is crucial for achieving optimal membrane quality. This observation is supported by SEM micrographs of samples converted in pure methanol under the same reaction conditions (Fig. S17 and S18†), which reveal suboptimal results in the absence of water.
Impact of the annealing treatment of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) Ga on its conversion to ZIF-8
Ga on its conversion to ZIF-8
Annealing temperature plays a crucial role in reaching the optimal sensitivity of metal oxides used in chemisensors, making thermal the treatment a common approach to enhance their sensing performance.12 To examine the impact of annealing temperature on ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga conversion, previously optimized conversion conditions were applied to ZnO
Ga conversion, previously optimized conversion conditions were applied to ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga films deposited on silicon wafers and annealed at 600 °C for 4 hours. Furthermore, the effect of more extreme conversion conditions was evaluated by increasing the conversion temperature from 100 °C to 200 °C.
Ga films deposited on silicon wafers and annealed at 600 °C for 4 hours. Furthermore, the effect of more extreme conversion conditions was evaluated by increasing the conversion temperature from 100 °C to 200 °C.
Structural analysis
The analysis of the recorded XRD patterns reveals significant differences compared to non-annealed ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga samples converted under similar conditions, highlighting the pronounced impact of the thermal treatment (Fig. 6a). The XRD patterns of the annealed sample converted at 100 °C for 1.5 h show peaks corresponding to the ZIF-8 structure, with higher intensity than those of the non-annealed sample, thus indicating better ZIF-8 formation on the surface. These peaks are broad, suggesting the presence of ZIF-8 nanodomains. However, the remaining ZnO
Ga samples converted under similar conditions, highlighting the pronounced impact of the thermal treatment (Fig. 6a). The XRD patterns of the annealed sample converted at 100 °C for 1.5 h show peaks corresponding to the ZIF-8 structure, with higher intensity than those of the non-annealed sample, thus indicating better ZIF-8 formation on the surface. These peaks are broad, suggesting the presence of ZIF-8 nanodomains. However, the remaining ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga is still significantly present. Interestingly, increasing the conversion temperature from 100 °C to 200 °C (for 1.5 h) results in no detectable XRD peaks corresponding to the ZIF-8 structure in the resulting sample. These findings are consistent with the recorded IR spectra (Fig. 6b).
Ga is still significantly present. Interestingly, increasing the conversion temperature from 100 °C to 200 °C (for 1.5 h) results in no detectable XRD peaks corresponding to the ZIF-8 structure in the resulting sample. These findings are consistent with the recorded IR spectra (Fig. 6b).
Morphological analysis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga reactivity induced by the annealing treatment. Studies have shown that ZnO annealed at 600 °C dissolves more quickly in water due to an increased number of oxygen vacancies on the surface and within the bulk of the metal oxide.65,66
Ga reactivity induced by the annealing treatment. Studies have shown that ZnO annealed at 600 °C dissolves more quickly in water due to an increased number of oxygen vacancies on the surface and within the bulk of the metal oxide.65,66
 | ||
Fig. 7 SEM observations (surfaces) of annealed ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga samples after reaction/conversion in a CH3OH/H2O mixture (3 Ga samples after reaction/conversion in a CH3OH/H2O mixture (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at different temperatures (100 °C and 200 °C) for 1.5 h. 1) at different temperatures (100 °C and 200 °C) for 1.5 h. | ||
When ZnO is annealed at 600 °C, several structural changes occur at the atomic level. The thermal energy causes oxygen atoms to migrate to the surface, where they can desorb as O2, creating oxygen vacancies within the metal oxide lattice.65 These vacancies generate localized regions of positive charge (defects) due to the missing O2− ions. During the subsequent conversion process, these defects serve as preferential dissolution sites, allowing water molecules to more readily weaken the Zn–O bonds and facilitate the release of Zn2+ ions into the solution. Additionally, the increased surface energy at defect sites promotes faster chemical reactions.
The higher concentration of Zn2+ ions at the interface then promotes the formation of larger ZIF-8 crystals. This finding is not surprising as for smaller ZIF-8 particles higher molar ratios of 2-methylimidazole to zinc ions is typically required. It should be mentioned that excessive 2-methylimidazole can coat ZIF-8 surfaces and slow the growth of crystal nuclei, but this effect is less significant in this case. The defects observed in the annealed sample are therefore caused by the accelerated ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga dissolution, which hinders the uniform crystallization of ZIF-8 at the interface.
Ga dissolution, which hinders the uniform crystallization of ZIF-8 at the interface.
Cross-sectional micrographs show that the regions with ZIF-8 islands consist of a bilayer of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga and ZIF-8, while the grain boundary defects reveal the absence of matter, exposing the silicon wafer below (Fig. 8). This indicates that the Zn2+ ions necessary for ZIF-8 crystallization are initially supplied by the ZnO
Ga and ZIF-8, while the grain boundary defects reveal the absence of matter, exposing the silicon wafer below (Fig. 8). This indicates that the Zn2+ ions necessary for ZIF-8 crystallization are initially supplied by the ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga surrounding the islands.
Ga surrounding the islands.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga dissolves more rapidly, and the increased conversion temperature accelerates the dissolution rate, thereby hindering the crystallization of ZIF-8 at the metal oxide interface.
Ga dissolves more rapidly, and the increased conversion temperature accelerates the dissolution rate, thereby hindering the crystallization of ZIF-8 at the metal oxide interface.
Cross-sectional micrographs provide further evidence for this hypothesis (Fig. 8). The images reveal a single layer of residual ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga surrounded by voids, exposing the silicon wafer to the environment. This confirms that under such reaction conditions, ZnO
Ga surrounded by voids, exposing the silicon wafer to the environment. This confirms that under such reaction conditions, ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga dissolution dominates over ZIF-8 crystallization, resulting in an incomplete conversion process.
Ga dissolution dominates over ZIF-8 crystallization, resulting in an incomplete conversion process.
These findings highlight the critical role of thermal treatment in influencing the dissolution kinetics of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga. They emphasize the importance of adjusting conversion conditions to slow down dissolution while enhancing ZIF-8 crystallization, ensuring a balanced dissolution/crystallization process and its effective control. The quality parameters of the ZIF-8 membranes optimized in this study should directly impact their molecular sieving performance in gas sensing applications, as demonstrated in our previous works.43,44 Furthermore, the literature extensively documents how ZIF-8 membrane characteristics influence sensing selectivity and response.16 The controlled membrane thickness (∼50–100 nm) achieved in our study represents an optimal balance between selectivity and response kinetics. Literature indicates that thicker ZIF-8 membranes (>200 nm) typically result in lower selectivity, preventing even H2 molecules from passing through.67 Conversely, an insufficient conversion rate may lead to cracks in the membrane or incomplete coverage, compromising selectivity.
Ga. They emphasize the importance of adjusting conversion conditions to slow down dissolution while enhancing ZIF-8 crystallization, ensuring a balanced dissolution/crystallization process and its effective control. The quality parameters of the ZIF-8 membranes optimized in this study should directly impact their molecular sieving performance in gas sensing applications, as demonstrated in our previous works.43,44 Furthermore, the literature extensively documents how ZIF-8 membrane characteristics influence sensing selectivity and response.16 The controlled membrane thickness (∼50–100 nm) achieved in our study represents an optimal balance between selectivity and response kinetics. Literature indicates that thicker ZIF-8 membranes (>200 nm) typically result in lower selectivity, preventing even H2 molecules from passing through.67 Conversely, an insufficient conversion rate may lead to cracks in the membrane or incomplete coverage, compromising selectivity.
The high crystallinity of the ZIF-8 layer optimized in this work, evidenced by sharp XRD peaks (Fig. 3a), ensures consistent 3.4 Å pore apertures across the membrane. This aperture size is ideal for selectively allowing H2 (kinetic diameter: 2.9 Å) to pass while blocking larger molecules such as acetone (4.6 Å), ethanol (4.5 Å), and benzene (5.9 Å).68 Finally, the proposed controlled conversion process preserves a significant portion of the underlying ZnO layer, which is critical for maintaining electrical conductivity for the sensing mechanism. The membrane quality parameters achieved in this study, particularly defect density, crystallinity, and thickness control, align well with those identified in the literature as determinants of superior molecular sieving performance.
This investigation thus demonstrates that solvent properties play a crucial role in ZIF-8 membrane engineering, while the characteristics of the metal oxide layer significantly influence the dissolution mechanism and, consequently, membrane quality. Our optimized synthesis conditions (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 MeOH/H2O ratio, 100 °C, 1.5 hours) provide precise control over ZnO dissolution and promote effective ZIF-8 crystallization. This results in a high-quality, uniform bilayer system with consistent thickness across the substrate and nanoscale crystals forming a compact layer, potentially suitable as a selective membrane barrier in chemiresistive sensor applications. Further examination of the metal oxide surface chemistry, which depends notably on deposition conditions and thermal treatment, is essential for a complete mechanistic understanding. These findings lay the groundwork for developing precise conversion protocols to engineer selective chemiresistive sensors, which will be further refined by additional studies of metal oxide chemistry. Our experimental approach has provided mechanistic insights through systematic investigation of solvent effects and thermal treatments. Recent advanced studies in the literature further support our mechanistic interpretations. Tao et al. investigated the interplay between organic linkers and ZnO substrates during ZIF-8 crystallization using in situ atomic force microscopy combined with ab initio molecular dynamics and density functional theory calculations. Their work revealed that organic ligands selectively bind to ZnO surface steps, controlling dissolution kinetics and thereby regulating MOF overgrowth.53 This selective binding mechanism aligns with our observations of how different solvents influence ZnO dissolution rates. Additionally, Sinnwell et al. utilized in situ X-ray diffraction to reveal the time-resolved formation of ZIF-8 and intermediary ZnO@ZIF-8 composites, demonstrating temperature-dependent mechanisms throughout the reaction process.69,70 These findings parallel our observations regarding the critical role of temperature in balancing dissolution and crystallization kinetics.
1 MeOH/H2O ratio, 100 °C, 1.5 hours) provide precise control over ZnO dissolution and promote effective ZIF-8 crystallization. This results in a high-quality, uniform bilayer system with consistent thickness across the substrate and nanoscale crystals forming a compact layer, potentially suitable as a selective membrane barrier in chemiresistive sensor applications. Further examination of the metal oxide surface chemistry, which depends notably on deposition conditions and thermal treatment, is essential for a complete mechanistic understanding. These findings lay the groundwork for developing precise conversion protocols to engineer selective chemiresistive sensors, which will be further refined by additional studies of metal oxide chemistry. Our experimental approach has provided mechanistic insights through systematic investigation of solvent effects and thermal treatments. Recent advanced studies in the literature further support our mechanistic interpretations. Tao et al. investigated the interplay between organic linkers and ZnO substrates during ZIF-8 crystallization using in situ atomic force microscopy combined with ab initio molecular dynamics and density functional theory calculations. Their work revealed that organic ligands selectively bind to ZnO surface steps, controlling dissolution kinetics and thereby regulating MOF overgrowth.53 This selective binding mechanism aligns with our observations of how different solvents influence ZnO dissolution rates. Additionally, Sinnwell et al. utilized in situ X-ray diffraction to reveal the time-resolved formation of ZIF-8 and intermediary ZnO@ZIF-8 composites, demonstrating temperature-dependent mechanisms throughout the reaction process.69,70 These findings parallel our observations regarding the critical role of temperature in balancing dissolution and crystallization kinetics.
While our study focuses on optimizing practical conversion conditions through systematic experimental investigation, these advanced mechanistic studies provide complementary theoretical foundations that support the proposed conversion mechanisms and the observed effects of solvent properties and ZnO thermal treatment on ZIF-8 growth.
Conclusions
This study systematically explores the liquid-phase conversion of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga to ZIF-8, examining the influence of the solvent physicochemical properties, solvent mixtures, reaction time and temperature including thermal treatment conditions of the metal oxide layers. It highlights key factors affecting the conversion process and provides valuable strategies for tuning chemiresistive sensor features, offering more versatility compared to vapor-phase conversions, which are limited by fewer variables.
Ga to ZIF-8, examining the influence of the solvent physicochemical properties, solvent mixtures, reaction time and temperature including thermal treatment conditions of the metal oxide layers. It highlights key factors affecting the conversion process and provides valuable strategies for tuning chemiresistive sensor features, offering more versatility compared to vapor-phase conversions, which are limited by fewer variables.
The results emphasize the importance of solvent physicochemical properties, such as polarity, viscosity, and interfacial tension, in controlling the ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga conversion rate and ZIF-8 crystallinity. Methanol, while effectively dissolving, excels in promoting ZIF-8 crystallization. Attractively, by utilizing methanol–water mixtures, the dissolution and crystallization processes can be precisely regulated and optimal balanced. For example, a 3
Ga conversion rate and ZIF-8 crystallinity. Methanol, while effectively dissolving, excels in promoting ZIF-8 crystallization. Attractively, by utilizing methanol–water mixtures, the dissolution and crystallization processes can be precisely regulated and optimal balanced. For example, a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CH3OH/H2O ratio minimizes defects formation and ensures a thin, uniform bilayer structure, making it ideal for the preparation of continuous ZIF-8 layers on ZnO
1 CH3OH/H2O ratio minimizes defects formation and ensures a thin, uniform bilayer structure, making it ideal for the preparation of continuous ZIF-8 layers on ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga thin films.
Ga thin films.
The effect of annealing on ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga conversion underscores the critical role of controlling dissolution kinetics. Annealing has been shown to enhance the reactivity of ZnO
Ga conversion underscores the critical role of controlling dissolution kinetics. Annealing has been shown to enhance the reactivity of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga by increasing oxygen vacancies, which accelerates dissolution, and influences MOF crystal growth. However, higher temperatures can lead to excessive dissolution, hindering ZIF-8 crystallization and compromising the structural integrity of the ZIF-8 layer.
Ga by increasing oxygen vacancies, which accelerates dissolution, and influences MOF crystal growth. However, higher temperatures can lead to excessive dissolution, hindering ZIF-8 crystallization and compromising the structural integrity of the ZIF-8 layer.
This study highlights the importance of optimizing solvent compositions, conversion parameters, and metal oxide thermal treatments to produce defect-free ZIF-8 membrane layers. In a broader context, these findings offer valuable insights for improving fabrication techniques in chemiresistive sensors, targeting superior sensitivity, selectivity, and environmental sustainability for practical applications. These insights are crucial for optimizing ZIF-8 selective films on metal oxide thin layers, paving the way for developing highly selective gas sensors based on sensitive semiconductors like ZnO. Our future research will focus on implementing these optimized ZIF-8 membranes in functional gas sensors based on ZnO semiconductor thin films and evaluating their selectivity enhancement in real-world conditions for specific target gases. Building on our previous work with ZnO@ZIF-8 systems, sensors fabricated using optimized conversion protocols are expected to demonstrate enhanced selectivity while maintaining high sensitivity.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Conceptualization, methodology and validation: KD, BP, AJ, LP. Investigation: KD, BP, LP and MD; data curation: KD, LP. Writing – original draft preparation: KD. Writing – review and editing: KD, BP, DF, PM, MB, MD, LP, AB, and AJ. Supervision: MD, LP and AJ. Project administration & funding acquisition: LP, AJ, DF, PM. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work received funding from the French ANR agency under grant agreements ANR-20-CE04-0012 and ANR-23-CE42-0029. It was supported by the LAAS-CNRS micro and nanotechnologies platform, a member of the Renatech French national network. The authors thank Claudie Josse and Sophie Gouy from the Centre de Microcaractérisation Raimond Castaing (UAR 3623) for their support with FIB and EPMA characterizations.Notes and references
- A. Kushwaha, R. Kumar and N. Goel, FlatChem, 2024, 43, 100584 CrossRef CAS.
- N. Ramgir, N. Datta, M. Kaur, S. Kailasaganapathi, A. K. Debnath, D. K. Aswal and S. K. Gupta, Colloids Surf., A, 2013, 439, 101–116 CrossRef CAS.
- P. Recum and T. Hirsch, Nanoscale Adv., 2024, 6, 11–31 RSC.
- S. K. Singh, D. Dutta, S. Das, A. Dhar and M. C. Paul, Mater. Sci. Semicond. Process., 2020, 107, 104819 CrossRef CAS.
- A. Dey, Mater. Sci. Eng., B, 2018, 229, 206–217 CrossRef CAS.
- M. A. Franco, P. P. Conti, R. S. Andre and D. S. Correa, Sens. Actuators Rep., 2022, 4, 100100 CrossRef.
- P. M. Bulemo, ACS Appl. Electron. Mater., 2023, 5, 2106–2114 CrossRef CAS.
- G. Paul and S. Sen, Mater. Lett., 2002, 57, 742–746 CrossRef CAS.
- R. Wang, A. W. Sleight and D. Cleary, Chem. Mater., 1996, 8, 433–439 CrossRef CAS.
- M. Lalanne, J. M. Soon, A. Barnabé, L. Presmanes, I. Pasquet and P. Tailhades, J. Mater. Res., 2010, 25, 2407–2414 CrossRef CAS.
- J. L. Noel, R. Udayabhaskar, B. Renganathan, S. Muthu Mariappan, D. Sastikumar and B. Karthikeyan, Spectrochim. Acta, Part A, 2014, 132, 634–638 CrossRef CAS PubMed.
- B. Paret, R. Monflier, P. Menini, T. Camps, Y. Thimont, A. Barnabé and L. Presmanes, Chemosensors, 2024, 13, 1 CrossRef.
- E. B. Kim, K. Y. Shin, W. Oum, S. Moon, A. Mirzaei, S. S. Kim and H. W. Kim, Sens. Actuators, B, 2025, 426, 137021 CrossRef CAS.
- M. Weber, J.-Y. Kim, J.-H. Lee, J.-H. Kim, I. Iatsunskyi, E. Coy, P. Miele, M. Bechelany and S. S. Kim, J. Mater. Chem. A, 2019, 7, 8107–8116 RSC.
- M. Lesego, D. T. Ndinteh, P. Ndungu and M. A. Mamo, Heliyon, 2023, 9, e22329 CrossRef CAS PubMed.
- T. Shi, S. Hussain, C. Ge, G. Liu, M. Wang and G. Qiao, Rev. Chem. Eng., 2023, 39, 911–939 CrossRef CAS.
- G. Zhang and Y. Wang, Polyoxometalates, 2023, 2, 9140020 CrossRef.
- W. Lu, Z. Wei, Z. Y. Gu, T. F. Liu, J. Park, J. Park, J. Tian, M. Zhang, Q. Zhang, T. Gentle, M. Bosch and H. C. Zhou, Chem. Soc. Rev., 2014, 43, 5561–5593 RSC.
- K. A. Forrest, T. Pham, S. K. Elsaidi, M. H. Mohamed, P. K. Thallapally, M. J. Zaworotko and B. Space, Cryst. Growth Des., 2019, 19, 3732–3743 CrossRef CAS.
- K. Dedecker, M. Drobek, V. Rouessac and A. Julbe, ACS Appl. Mater. Interfaces, 2023, 15, 6831–6838 CrossRef CAS PubMed.
- B. Van De Voorde, M. Hezinová, J. Lannoeye, A. Vandekerkhove, B. Marszalek, B. Gil, I. Beurroies, P. Nachtigall and D. De Vos, Phys. Chem. Chem. Phys., 2015, 17, 10759–10766 RSC.
- N. M. Padial, E. Quartapelle Procopio, C. Montoro, E. López, J. E. Oltra, V. Colombo, A. Maspero, N. Masciocchi, S. Galli, I. Senkovska, S. Kaskel, E. Barea and J. A. R. Navarro, Angew. Chem., Int. Ed., 2013, 52, 8290–8294 CrossRef CAS PubMed.
- K. Dedecker, M. Drobek and A. Julbe, J. Phys. Chem. B, 2023, 127, 11091–11099 CrossRef CAS PubMed.
- J. Pires, J. Fernandes, K. Dedecker, J. R. B. Gomes, G. Pérez-Sánchez, F. Nouar, C. Serre and M. L. Pinto, ACS Appl. Mater. Interfaces, 2019, 11, 27410–27421 CrossRef CAS PubMed.
- K. Dedecker, R. S. Pillai, F. Nouar, J. Pires, N. Steunou, E. Dumas, G. Maurin, C. Serre and M. L. Pinto, ACS Appl. Mater. Interfaces, 2018, 10, 13886–13894 CrossRef CAS PubMed.
- R. Banerjee, A. Phan, B. Wang, C. Knobler, H. Furukawa, M. O'Keeffe and O. M. Yaghi, Science, 2008, 319, 939–943 CrossRef CAS PubMed.
- K. Dedecker, E. Dumas, B. Lavédrine, N. Steunou and C. Serre, Metal-Organic Frameworks (MOFs) for Environmental Applications, Elsevier, 2019, pp. 141–178 Search PubMed.
- S. Dasgupta, S. Biswas, K. Dedecker, E. Dumas, N. Menguy, B. Berini, B. Lavedrine, C. Serre, C. Boissière and N. Steunou, ACS Appl. Mater. Interfaces, 2023, 15, 6069–6078 CrossRef CAS PubMed.
- A. Phan, C. J. Doonan, F. J. Uribe-Romo, C. B. Knobler, M. Okeeffe and O. M. Yaghi, Acc. Chem. Res., 2010, 43, 58–67 CrossRef CAS PubMed.
- K. S. Park, Z. Ni, A. P. Côté, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed.
- K. Dedecker, M. Drobek and A. Julbe, Molecules, 2024, 29, 5825 CrossRef CAS PubMed.
- K. Dedecker, M. Drobek and A. Julbe, RSC Appl. Interfaces, 2025, 2, 364–372 RSC.
- T.-M. T. Nguyen, J.-W. Chen, M.-T. Pham, H. M. Bui, C.-C. Hu, S.-J. You and Y.-F. Wang, Environ. Technol. Innovation, 2023, 31, 103169 CrossRef CAS.
- S. Tanaka, K. Kida, T. Nagaoka, T. Ota and Y. Miyake, Chem. Commun., 2013, 49, 7884–7886 RSC.
- L. Bazzi, I. Ayouch, H. Tachallait and S. EL Hankari, Results Eng., 2022, 13, 100378 CrossRef CAS.
- Y. Yue, Z.-A. Qiao, X. Li, A. J. Binder, E. Formo, Z. Pan, C. Tian, Z. Bi and S. Dai, Cryst. Growth Des., 2013, 13, 1002–1005 CrossRef CAS.
- K. Khaletskaya, S. Turner, M. Tu, S. Wannapaiboon, A. Schneemann, R. Meyer, A. Ludwig, G. Van Tendeloo and R. A. Fischer, Adv. Funct. Mater., 2014, 24, 4804–4811 CrossRef CAS.
- J. Yu, Y. Pan, C. Wang and Z. Lai, Chem. Eng. Sci., 2016, 141, 119–124 CrossRef CAS.
- A. J. Cruz, I. Stassen, M. Krishtab, K. Marcoen, T. Stassin, S. Rodríguez-Hermida, J. Teyssandier, S. Pletincx, R. Verbeke, V. Rubio-Giménez, S. Tatay, C. Martí-Gastaldo, J. Meersschaut, P. M. Vereecken, S. De Feyter, T. Hauffman and R. Ameloot, Chem. Mater., 2019, 31, 9462–9471 CrossRef CAS.
- M. Bechelany, M. Drobek, C. Vallicari, A. Abou Chaaya, A. Julbe and P. Miele, Nanoscale, 2015, 7, 5794–5802 RSC.
- I. Dönges, M. I. Büschges, C. Njel and J. J. Schneider, Dalton Trans., 2022, 51, 13725–13733 RSC.
- Z. L. Wang, Mater. Today, 2004, 7, 26–33 CrossRef CAS.
- M. Drobek, J. H. Kim, M. Bechelany, C. Vallicari, A. Julbe and S. S. Kim, ACS Appl. Mater. Interfaces, 2016, 8, 8323–8328 CrossRef CAS PubMed.
- M. Weber, J.-H. Kim, J.-H. Lee, J.-Y. Kim, I. Iatsunskyi, E. Coy, M. Drobek, A. Julbe, M. Bechelany and S. S. Kim, ACS Appl. Mater. Interfaces, 2018, 10, 34765–34773 CrossRef CAS PubMed.
- A. S. Mokrushin, I. A. Nagornov, T. L. Simonenko, N. P. Simonenko, P. Y. Gorobtsov, T. V. Khamova, G. P. Kopitsa, A. N. Evzrezov, E. P. Simonenko, V. G. Sevastyanov and N. T. Kuznetsov, Mater. Sci. Eng., B, 2021, 271, 115233 CrossRef CAS.
- J.-H. Kim, J.-H. Lee, Y. Park, J.-Y. Kim, A. Mirzaei, H. W. Kim and S. S. Kim, Sens. Actuators, B, 2019, 294, 78–88 CrossRef CAS.
- G. Eranna, B. C. Joshi, D. P. Runthala and R. P. Gupta, Crit. Rev. Solid State Mater. Sci., 2004, 29, 111–188 CrossRef CAS.
- I. Stassen, N. Campagnol, J. Fransaer, P. Vereecken, D. De Vos and R. Ameloot, CrystEngComm, 2013, 15, 9308 RSC.
- J.-B. Lin, R.-B. Lin, X.-N. Cheng, J.-P. Zhang and X.-M. Chen, Chem. Commun., 2011, 47, 9185 RSC.
- M. Drobek, M. Bechelany, C. Vallicari, A. Abou Chaaya, C. Charmette, C. Salvador-Levehang, P. Miele and A. Julbe, J. Membr. Sci., 2015, 475, 39–46 CrossRef CAS.
- Y. Li, C. Ma, P. Nian, H. Liu and X. Zhang, J. Membr. Sci., 2019, 581, 344–354 CrossRef CAS.
- Y. Wang, H. Zhang, X. Wang, C. Zou, B. Meng and X. Tan, Ind. Eng. Chem. Res., 2019, 58, 19511–19518 CrossRef CAS.
- J. Tao, M.-S. Lee, M. L. Sushko, J. J. De Yoreo, J. Liu, Z. Zhang, D. Banerjee, S. Akkineni, M. E. Bowden, P. K. Thallapally, Y. Shin and M. A. Sinnwell, Chem. Mater., 2020, 32, 6666–6675 CrossRef CAS.
- R. Freund, A. E. Lanza, S. Canossa, M. Gemmi, J. Goscianska, V. Cauda, M. Oschatz and S. Wuttke, Chem. Mater., 2023, 35, 1891–1900 CrossRef CAS.
- B. Mockenhaupt, J. K. Wied, S. Mangelsen, U. Schürmann, L. Kienle, J. Schmedt auf der Günne and M. Behrens, Dalton Trans., 2023, 52, 5321–5335 RSC.
- S. Duan, X. Xu, W. Chen, J. Zhi and F. Li, Polyoxometalates, 2022, 1, 9140003 CrossRef.
- K. Khaletskaya, S. Turner, M. Tu, S. Wannapaiboon, A. Schneemann, R. Meyer, A. Ludwig, G. Van Tendeloo and R. A. Fischer, Adv. Funct. Mater., 2014, 24, 4804–4811 CrossRef CAS.
- J. Cravillon, S. Münzer, S.-J. Lohmeier, A. Feldhoff, K. Huber and M. Wiebcke, Chem. Mater., 2009, 21, 1410–1412 CrossRef CAS.
- Y. Pan, D. Heryadi, F. Zhou, L. Zhao, G. Lestari, H. Su and Z. Lai, CrystEngComm, 2011, 13, 6937 RSC.
- K. Kida, M. Okita, K. Fujita, S. Tanaka and Y. Miyake, CrystEngComm, 2013, 15, 1794 RSC.
- H. Yoo and N. Shin, Appl. Surf. Sci., 2023, 625, 157211 CrossRef CAS.
- P. Y. Moh, P. Cubillas, M. W. Anderson and M. P. Attfield, J. Am. Chem. Soc., 2011, 133, 13304–13307 CrossRef CAS PubMed.
- J. Cravillon, C. A. Schröder, R. Nayuk, J. Gummel, K. Huber and M. Wiebcke, Angew. Chem., Int. Ed., 2011, 50, 8067–8071 CrossRef CAS PubMed.
- J. A. Allegretto, D. Onna, S. A. Bilmes, O. Azzaroni and M. Rafti, Chem. Mater., 2024, 36, 5814–5825 CrossRef CAS PubMed.
- H. He, J. Cao, X. Fei and N. Duan, Environ. Int., 2019, 130, 104930 CrossRef CAS PubMed.
- H. He, J. Cao, X. Fei and N. Duan, Sci. Total Environ., 2021, 787, 147545 CrossRef CAS PubMed.
- R. Lv, Q. Zhang, W. Wang, Y. Lin and S. Zhang, Sensors, 2021, 21, 4069 CrossRef CAS PubMed.
- T. Zhou, Y. Sang, X. Wang, C. Wu, D. Zeng and C. Xie, Sens. Actuators, B, 2018, 258, 1099–1106 CrossRef CAS.
- B. Jin, S. Wang, D. Boglaienko, Z. Zhang, Q. Zhao, X. Ma, X. Zhang and J. J. De Yoreo, J. Cryst. Growth, 2023, 603, 126989 CrossRef CAS.
- M. A. Sinnwell, Q. R. S. Miller, L. Liu, J. Tao, M. E. Bowden, L. Kovarik, D. Barpaga, Y. Han, R. Kishan Motkuri, M. L. Sushko, H. T. Schaef and P. K. Thallapally, ChemSusChem, 2020, 13, 2602–2612 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional material characterizations and computational details. See DOI: https://doi.org/10.1039/d5lf00054h |
| This journal is © The Royal Society of Chemistry 2025 |