Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solid-supported polymer–lipid hybrid membrane for bioelectrochemistry of a membrane redox enzyme†
Rosa
Catania
 ab,
George R.
Heath
ab,
George R.
Heath
 bc,
Michael
Rappolt
bc,
Michael
Rappolt
 d,
Stephen P.
Muench
d,
Stephen P.
Muench
 be,
Paul A.
Beales
be,
Paul A.
Beales
 *ab and
Lars J. C.
Jeuken
*ab and
Lars J. C.
Jeuken
 *f
*f
aSchool of Chemistry, University of Leeds, Leeds LS2 9JT, UK. E-mail: P.A.Beales@leeds.ac.uk
bAstbury Centre for Structural Molecular Biology, University of Leeds, Leeds LS2 9JT, UK
cSchool of Physics and Astronomy, University of Leeds, Leeds LS2 9JT, UK
dSchool of Food Science and Nutrition, University of Leeds, Leeds LS2 9JT, UK
eSchool of Biomedical Sciences, Faculty of Biological Sciences, University of Leeds, Leeds LS2 9JT, UK
fLeiden Institute of Chemistry, Leiden University, PO Box 9502, 2300 RA, Leiden, The Netherlands. E-mail: L.J.C.Jeuken@lic.leidenuniv.nl
First published on 11th February 2025
Abstract
Hybrid membranes, consisting of phospholipids and amphiphilic block polymers, offer enhanced stability compared to liposomes and greater biocompatibility than polymersomes. These qualities make them a versatile platform for a wide range of applications across various fields. In this study, we have investigated the ability of solid-supported polymer–lipid hybrid membranes (SSHM) to act as a platform for bioelectrochemistry of membrane proteins. The redox enzyme, cytochrome bo3 (cyt bo3), a terminal oxidase in Escherichia coli, was reconstituted into hybrid vesicles (HVs), which were subsequently tested for their ability to form SSHMs on different self-assembled monolayers (SAMs) on gold electrodes. SSHM formation was monitored with electrochemical impedance spectroscopy (EIS), quartz crystal microbalance with dissipation (QCM-D), and atomic force microscopy (AFM). SSHMs were successfully formed on gold electrodes with mixed SAMs of 6-mercapto-1-hexanol and 1-hexanethiol at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The activity of cyt bo3 was confirmed using cyclic voltammetry (CV), with electron transfer to cyt bo3 mediated by a lipophilic substrate-analogue decylubiquinone (DQ). SSHMs formed with HVs-cyt bo3 samples, stored for more than one year before use, remain bioelectrocatalytically active, confirming our previously established longevity and stability of HV systems.
1 ratio. The activity of cyt bo3 was confirmed using cyclic voltammetry (CV), with electron transfer to cyt bo3 mediated by a lipophilic substrate-analogue decylubiquinone (DQ). SSHMs formed with HVs-cyt bo3 samples, stored for more than one year before use, remain bioelectrocatalytically active, confirming our previously established longevity and stability of HV systems.
Introduction
Hybrid polymer–lipid membranes consist of a blend of phospholipids and amphiphilic block copolymers. The resulting mixture exhibits synergistic properties that surpass those of the individual components.1 When in the form of hybrid vesicles (HVs), they combine the advantages of pure lipids (liposomes) and pure polymer (polymersomes) systems, offering biocompatibility to a diverse range of biomolecules along with enhanced stability and tuneable membrane properties.2 These combined properties make HVs highly versatile for applications such as drug delivery,3 biosensors4 and artificial cell development.5 HVs have also emerged as a promising platform for reconstituting membrane proteins.2,6–8Solid-supported hybrid membranes (SSHMs) extend the concept of HVs to solid-supported systems, combining lipid bilayer properties with polymeric support.9 In bioelectrochemistry, solid-supported lipid membranes (SSLMs) have been widely used to study membrane redox enzymes,10 as they provide a functional lipid environment for electrochemical characterisation.11 However, their low mechanical stability limits their long-term applicability. In contrast, solid-supported polymer membranes (SSPLMs) offer greater membrane stability and can be chemically functionalised, enabling tailored surface properties for specific applications.12
So far, only a few studies have explored the properties of the hybrid membranes on solid supports and their combinations with membrane proteins. Indeed, their potential in fundamental studies of hybrid membranes and membrane proteins or applications in bioelectrocatalysis remains largely underexplored.
In 2014, Gettel et al. demonstrated that hybrid vesicles composed of POPC and PBd22–PEO14 can form homogeneous SSHM on solid surfaces through fusion, producing different structures depending on the surface energy: bilayers on hydrophilic surfaces, monolayers on hydrophobic surfaces, and mixed morphologies on amphiphilic surfaces.13 Further investigation in 2018 by Paxton et al. explored SSHMs formed using HVs composed of DOPC and PBd37–PEO22 on both planar and non-planar silica surfaces.14 They found that the hybrid bilayers maintained a continuous and homogeneous structure at lower polymer content. However, when the polymer content exceeded 50 mol%, the films became increasingly heterogeneous, showing a tendency toward vesicle adsorption rather than fusion. In 2020, Mumtaz Virk et al. similarly observed that HVs composed of POPC and PBd22-b-PEO23, with polymer content up to 50% w/w, formed smooth, defect-free planar surfaces on silica supports. However, similar weight fractions of DPPC lipids resulted in hybrid bilayers with nanoscale defects.15 Balestri et al. also confirmed micrometre-scale phase separation in SSHM composed of PBd46-b-PEO30 (from 10% to 65%) and DPPC onto silica substrates via spontaneous rupture and fusion of HVs.16 In 2021, Bello et al. investigated the behaviour of low molecular weight PBd12-b-PEO9 polymers mixed with POPC in supported hybrid bilayers.17 They found that stable bilayer formation and “lipid-like” properties were achieved with up to 50 mol% polymers, consistent with findings using higher molecular weight polymers. Vesicles with 50 mol% polymer content were more prone to deformation but effectively stabilized and homogenized the POPC distribution. Higher polymer concentrations resulted in phase separation. In 2024, Cardellini et al. found that gold nanoparticles (AuNP) preferentially clustered in the polymer-rich regions of the heterogeneous SSHM made of PBd46-b-PEO34 and DPPC.18 Recently, Schafer et al. demonstrated that mixing PBd22–PEO14 with POPC to form a SSHM enhances bilayer resilience, while maintaining biocompatibility, as evidenced by the successful incorporation of α-hemolysin.19 The Meier group studied SSHM monolayers and bilayers made from lipids (DPPC, DPPE, DOPC, POPE) and PDMS-b-PMOXA copolymers with varying PDMS lengths (16, 37, and 65 units).20–22 They found that phase separation and domain formation were most distinct with longer PDMS chains and saturated lipids, with the lipid headgroups affecting domain size and shape.22 By adjusting the polymer–lipid composition, they could control the insertion and distribution of the membrane protein MloK1. In these mixtures, membrane proteins preferentially inserted into the more fluid phase. Specifically, in mixtures with saturated lipids, the proteins localised in the polymer-rich phase, whereas with unsaturated lipids, they favoured the lipid-rich phase.
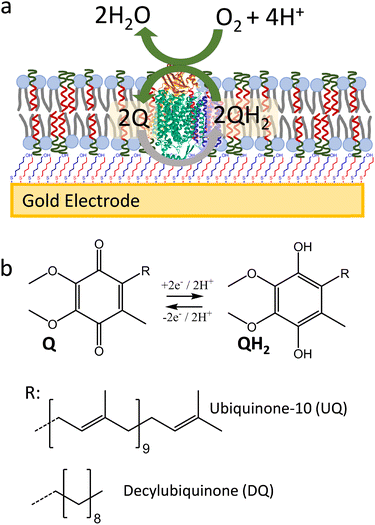
Here, we show that SSHMs can be used as a platform to study the bioelectrocatalytic activity from a membrane redox enzyme, cytochrome bo3 (cyt bo3) from Escherichia coli (E. coli) (Fig. 1). Cyt bo3 is a four-subunit terminal oxidase complex (∼143 kDa) that belongs to the haem-copper oxidase enzyme family. It oxidises ubiquinol molecules while reducing molecular oxygen to water.23 The SSHM consisted of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of poly(butadiene-b-ethylene oxide) (PBd22-b-PEO14; MW 1.8 kDa) amphiphilic block copolymer and E. coli polar lipid extracts. The SSHMs were formed onto a gold electrode coated with self-assembled monolayer (SAM) made of an optimised ratio of 6-mercapto-1-hexanol (MH) to hydrophobic 1-hexanethiol (HT) mixture (1
1 molar ratio of poly(butadiene-b-ethylene oxide) (PBd22-b-PEO14; MW 1.8 kDa) amphiphilic block copolymer and E. coli polar lipid extracts. The SSHMs were formed onto a gold electrode coated with self-assembled monolayer (SAM) made of an optimised ratio of 6-mercapto-1-hexanol (MH) to hydrophobic 1-hexanethiol (HT) mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio). These hybrid bilayers were shown to efficiently cover the SAM-coated gold electrode, while allowing electrochemical oxidation and reduction of lipophilic quinones in the membranes. Cyt bo3 could be incorporated into the SSHM in an active form, as confirmed by its quinone
1 molar ratio). These hybrid bilayers were shown to efficiently cover the SAM-coated gold electrode, while allowing electrochemical oxidation and reduction of lipophilic quinones in the membranes. Cyt bo3 could be incorporated into the SSHM in an active form, as confirmed by its quinone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxygen oxidoreductase activity.
oxygen oxidoreductase activity.
Experimental
Materials
E. coli polar lipids were obtained from Avanti Polar Lipids (AL, U.S.A.). Block-copolymer poly(butadiene-b-ethylene oxide) (PBd22-b-PEO14, P9089–BdEO) was purchased from Polymer Source (Canada). If not otherwise stated, all the other chemicals were purchased from Merck. The compound mercapto-(ethylene-oxy)3-carbamate cholesterol (EO3C) was synthesized as previously described.24Cytochrome bo3 extraction and purification
Membrane protein cytochrome bo3 (cyt bo3) was expressed in E. coli GO105/pJRhisA as previously described.25 A freshly plated colony of E. coli GO105/pJRhisA was inoculated in LB containing 100 μg mL−1 carbenicillin and cultured at 37 °C at an agitation rate of 200 rpm for ∼16 h. This starter culture was then inoculated in LB medium (2% v/v) also supplemented with 100 μg mL−1 carbenicillin and 0.1 mM CuSO4. E. coli was grown to mid-logarithmic phase at 37 °C with shaking at 200 rpm for ∼6 h, until the optical density at 600 nm (OD600nm) reached 1.5. The cells were harvested by centrifugation at 7000g for 20 min at 4 °C and resuspended in W1 buffer (20 mM MOPS, 5 mM Mg2SO4, 30 mM Na2SO4) at a concentration of 0.25 g of wet cells per mL. E. coli cells suspension was passed twice through a cell disrupter (Constant Systems) at 30 kPsi. Cell debris was removed by centrifugation at 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500g for 10 min at 4 °C. Cell membranes in the supernatant were then pelleted by ultracentrifugation at 200
500g for 10 min at 4 °C. Cell membranes in the supernatant were then pelleted by ultracentrifugation at 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 90 min at 4 °C. To purify cyt bo3 in SMALPs, the membrane pellet was resuspended in 50 mM Tris–HCl (pH 8), 500 mM NaCl and 10% glycerol at a ‘wet weight’ concentration of 40 mg mL−1 (protein content ∼4 mg mL−1 as determine using a bicinchoninic acid assay (BCA) assay). Styrene maleic acid (SMA) copolymer (Cray Valley, SMA 2000 – MW 7.5 kDa) was added at a concentration of 2% w/v.26 The suspension was incubated for 2 h on a rotary shaker at RT and then centrifuged at 100
000g for 90 min at 4 °C. To purify cyt bo3 in SMALPs, the membrane pellet was resuspended in 50 mM Tris–HCl (pH 8), 500 mM NaCl and 10% glycerol at a ‘wet weight’ concentration of 40 mg mL−1 (protein content ∼4 mg mL−1 as determine using a bicinchoninic acid assay (BCA) assay). Styrene maleic acid (SMA) copolymer (Cray Valley, SMA 2000 – MW 7.5 kDa) was added at a concentration of 2% w/v.26 The suspension was incubated for 2 h on a rotary shaker at RT and then centrifuged at 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 45 min at 4 °C to remove any non-solubilised proteins. SMA-solubilized proteins were incubated with pre-equilibrated Ni2+–NTA resin (Neo Biotech) for ∼16 h on a rotary shaker at 4 °C. The resin suspensions were loaded onto a gravity column, and the SMALPcyt bo3 was eluted with 200 mM imidazole, 50 mM Tris–HCl (pH 8), 500 mM NaCl and 10% (vol/vol) glycerol. The imidazole was immediately removed after elution by performing 3 cycles of dilution in imidazole-free storage buffer (50 mM Tris and 150 mM NaCl, pH 8.0), and concentration using 100 kDa MW cut-off concentrator (VivaSpin). The purified SMALPcyt bo3 were snap frozen in liquid nitrogen and stored at −20 °C until use. Protein concentration of purified cyt bo3 was determined via Soret Band at 409 nm (Nanodrop DeNovix DS-11) using extinction coefficient value ε408 nm = 188 mM−1 cm−1.27
000g for 45 min at 4 °C to remove any non-solubilised proteins. SMA-solubilized proteins were incubated with pre-equilibrated Ni2+–NTA resin (Neo Biotech) for ∼16 h on a rotary shaker at 4 °C. The resin suspensions were loaded onto a gravity column, and the SMALPcyt bo3 was eluted with 200 mM imidazole, 50 mM Tris–HCl (pH 8), 500 mM NaCl and 10% (vol/vol) glycerol. The imidazole was immediately removed after elution by performing 3 cycles of dilution in imidazole-free storage buffer (50 mM Tris and 150 mM NaCl, pH 8.0), and concentration using 100 kDa MW cut-off concentrator (VivaSpin). The purified SMALPcyt bo3 were snap frozen in liquid nitrogen and stored at −20 °C until use. Protein concentration of purified cyt bo3 was determined via Soret Band at 409 nm (Nanodrop DeNovix DS-11) using extinction coefficient value ε408 nm = 188 mM−1 cm−1.27
Vesicles preparation
HVs were prepared using an adaptation of our previously described method.28E. coli polar lipid extracts (5 mg, 6.6 μmol) and PBd22-b-PEO14 (11.84 mg, 6.6 μmol) were each solubilised in chloroform, in 200 and 500 μL respectively, and mixed together in a glass vial. The organic solution was supplemented with decylubiquinone (DQ) (62.83 μg, 0.19 μmol, added as an aliquot taken from a stock solution 1 mg mL−1 in chloroform) or ubiquinol (UQ, 168.62 μg, 0.19 μmol, from a stock solution 1 mg mL−1 in chloroform). The mixture was then dried in a vacuumed desiccator for at least 2 h to form a thin lipid–copolymer. The lipid–copolymer films were resuspended in 1 mL of 20 mM 3-(N-morpholino)propanesulfonic acid (MOPS), 30 mM Na2SO4 (pH 7.4) to achieve a final concentration of 16.84 mg mL−1 (total lipid and copolymer mass). The suspension was incubated at 50 °C for 5 min and vortex for 1 min followed by five freeze–thaw–vortex cycles. The HVs were subsequently extruded 11 times through a 100 nm pore size polycarbonate membrane filter using an Avanti Mini-Extruder to form vesicles. Liposomes, made of E. coli lipids polar extract (Avanti Polar Lipids, AL, U.S.A.) were prepared by extrusion using similar methods at a final lipid concentration of 5 mg mL−1.Reconstitution of cyt bo3 into HVs
Incorporation of cyt bo3 into HVs was performed according to our previously published detergent-free reconstitution method.29 SMALPcyt bo3 and HVs were incubated on ice for 30 min at a protein to lipid content ratio (w/w) of ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. MgCl2 was then added to a concentration of 10 mM and incubated with gentle shaking overnight at 4 °C. Mg2+ promotes SMA precipitation from solution forming a non-soluble complex that can be removed by centrifugation. The samples were subsequently centrifuged at 17
100. MgCl2 was then added to a concentration of 10 mM and incubated with gentle shaking overnight at 4 °C. Mg2+ promotes SMA precipitation from solution forming a non-soluble complex that can be removed by centrifugation. The samples were subsequently centrifuged at 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 15 min to pellet SMA and non-reconstituted SMALPcyt bo3. The supernatant, containing HVs with reconstituted cyt bo3, was collected and stored at 4 °C until use.
000g for 15 min to pellet SMA and non-reconstituted SMALPcyt bo3. The supernatant, containing HVs with reconstituted cyt bo3, was collected and stored at 4 °C until use.
Electrochemical measurements
Template stripped gold (TSG) and SAM surfaces were prepared as previously described.30 SAMs of mixed MH/EO3C (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60) were prepared by incubation of TSG surface in 0.88 mM MH and 0.12 mM EO3C in propanol for 16 h. Mixed SAMs of MH/HT were formed by incubation of TSG surfaces with different molar ratios of 6-mercapto-1-hexanol (MH) and 1-hexanethiol (HT) to give a total of 1 mM thiol compounds in isopropanol (∼16 h). The SAM-coated TSG were rinsed with isopropanol and dried under nitrogen before being incorporated into a home-built electrochemical cell.31 Voltammetry and electrochemical impedance spectroscopy were carried out using an Autolab (Eco-chemie) electrochemical analyser equipped with a PGSTAT30 potentiostat, SCANGEN module and an FRA2 frequency analyser. All electrochemical measurements were performed in 2 mL 20 mM MOPS, 30 mM Na2SO4 buffer, pH 7.4. Electrochemical measurements were carried out in a three-electrode configuration electrochemical cell using mercury–mercury sulfate reference electrode and a Pt wire as counter electrode, as described previously.31 The electrochemical cell was housed in a Faraday cage and purged with nitrogen to remove oxygen. All reported potentials are quoted versus SHE (ESHE = EHg2SO4 + 651 mV at 25 °C).
60) were prepared by incubation of TSG surface in 0.88 mM MH and 0.12 mM EO3C in propanol for 16 h. Mixed SAMs of MH/HT were formed by incubation of TSG surfaces with different molar ratios of 6-mercapto-1-hexanol (MH) and 1-hexanethiol (HT) to give a total of 1 mM thiol compounds in isopropanol (∼16 h). The SAM-coated TSG were rinsed with isopropanol and dried under nitrogen before being incorporated into a home-built electrochemical cell.31 Voltammetry and electrochemical impedance spectroscopy were carried out using an Autolab (Eco-chemie) electrochemical analyser equipped with a PGSTAT30 potentiostat, SCANGEN module and an FRA2 frequency analyser. All electrochemical measurements were performed in 2 mL 20 mM MOPS, 30 mM Na2SO4 buffer, pH 7.4. Electrochemical measurements were carried out in a three-electrode configuration electrochemical cell using mercury–mercury sulfate reference electrode and a Pt wire as counter electrode, as described previously.31 The electrochemical cell was housed in a Faraday cage and purged with nitrogen to remove oxygen. All reported potentials are quoted versus SHE (ESHE = EHg2SO4 + 651 mV at 25 °C).
Solid-supported polymer–lipid hybrid membranes were formed adding liposomes or HVs into the electrochemical cell to final concentration of 0.5 mg mL−1 in 2 mL 20 mM MOPS, 30 mM Na2SO4 buffer supplemented with 10 mM CaCl2 at room temperature for 1 h. The HVs or liposome suspension was removed by rinsing the electrochemical cell three times with deionised water, three times with 1 mM ethylenediaminetetraacetic acid (EDTA) followed by 5 washes with 20 mM MOPS, 30 mM Na2SO4 buffer at pH 7.0.
Quartz crystal microbalance with dissipation monitoring
QCM-D measurements were conducted on a Q-Sense E4 multifrequency QCM-D Instrument. Au crystals were cleaned by ultrasonication in 2% sodium dodecyl sulfate (SDS) detergent for 15 min, followed by rinsing and further ultrasonication in Milli-Q water for 15 min. The crystals were then dried under nitrogen and incubated in a UV-ozone (ultraviolet ozone cleaning system, low-pressure quartz-mercury vapor lamp emitting 254 and 185 nm UV, UVOCS) for 30 min. After UV-ozone treatment, the crystals were incubated in isopropanol for 30 min to reduce gold oxide after which the crystals then were transferred into the thiol solutions for 16 h followed by rinsing with isopropanol, as described under Electrochemical measurements. Prior to the measurements, the QCM-D chambers were filled with buffer to find the resonant frequencies of each overtone used. Experiments were performed at 22 °C at flow rate of 40 μL min−1 and a HV or liposome concentration of 0.5 mg mL−1 in 20 mM MOPS, 30 mM Na2SO4 pH 7.4. All data shown represent recordings at the seventh overtone.Atomic force microscopy sample preparation and imaging
MH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HT (50
HT (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) SAM coated TSG electrodes, as described under electrochemical measurements, were glued to metal stubs using a layer of epoxy glue. AFM imaging was performed in liquid in PeakForce tapping mode using a Bruker Dimension FastScan Bio with Bruker ScanAsyst Fluid+ probes. Imaging was performed at room temperature in 20 mM MOPS, 30 mM Na2SO4 pH 7.4 buffer. HVs were incubated on the TSG electrodes at a concentration of 0.4 mg mL−1 in 10 mM CaCl2, 20 mM MOPS, 30 mM Na2SO4 pH 7.4 for 15 min. Excess vesicles were rinsed via fluid exchange using 5 exchanges of milli Q water followed by 5 exchanges of buffer whilst maintaining the membranes in a liquid environment.
50) SAM coated TSG electrodes, as described under electrochemical measurements, were glued to metal stubs using a layer of epoxy glue. AFM imaging was performed in liquid in PeakForce tapping mode using a Bruker Dimension FastScan Bio with Bruker ScanAsyst Fluid+ probes. Imaging was performed at room temperature in 20 mM MOPS, 30 mM Na2SO4 pH 7.4 buffer. HVs were incubated on the TSG electrodes at a concentration of 0.4 mg mL−1 in 10 mM CaCl2, 20 mM MOPS, 30 mM Na2SO4 pH 7.4 for 15 min. Excess vesicles were rinsed via fluid exchange using 5 exchanges of milli Q water followed by 5 exchanges of buffer whilst maintaining the membranes in a liquid environment.
Results and discussion
Solid-supported hybrid membranes (SSHMs)
Different methods have been developed to ‘support’ or couple lipid membranes to electrode surfaces for the study of redox-active membrane proteins.32–35 One common method is to ‘tether’ the membrane to the surface using lipid analogues that are chemically bonded to the electrode via a short linker, usually a poly–ethylene-glycol group. A second common approach is to ‘suspend’ the membrane above the electrode using non-covalent interactions. While various self-assembled monolayers (SAMs) on gold electrodes have been investigated for their ability to form such solid-supported lipid membranes (SSLMs), there is limited knowledge about which SAM is most suitable to form a solid-supported hybrid membrane (SSHM). Here, we tested both the tethered and suspended approach.To test the tethered approach, a self-assembled monolayer (SAM) was prepared of a mixture of hydrophilic 6-mercapto-1-hexanol (MH) and the lipid ‘tether’, mercapto-(ethylene-oxy)3-carbamate cholesterol (EO3C) in a ∼40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
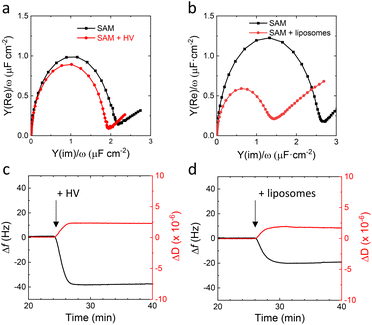
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 ratio in the surface.30 We previously showed that EO3C-based SAMs are suitable to form well-defined tethered SSLMs, which can be used to electrochemically study cytochrome bo3 in E. coli lipid extracts.25,31,36,37 Well-formed tethered SSLMs on EO3C/MH surfaces are characterised by double layer capacitance of around 1 μF cm−2 by electrochemical impedance spectroscopy (EIS) and a frequency change of around −20 Hz with quartz crystal microbalance with dissipation (QCM-D) (Fig. 2b and d). Fig. 2b presents the EIS data in Cole–Cole plots, also known as normalised admittance plots, in which the diameter of the semicircle directly signifies the double layer capacitance, Cdl. The −20 Hz frequency shift (Δf) observed by QCM-D in Fig. 2d is slightly less than the ∼25 Hz typically observed for SSLM formation on SiO2 surfaces.38 This is because the EO3C ‘tether’ occupies part of the lower lipid leaflet in the tethered SSLM system.
60 ratio in the surface.30 We previously showed that EO3C-based SAMs are suitable to form well-defined tethered SSLMs, which can be used to electrochemically study cytochrome bo3 in E. coli lipid extracts.25,31,36,37 Well-formed tethered SSLMs on EO3C/MH surfaces are characterised by double layer capacitance of around 1 μF cm−2 by electrochemical impedance spectroscopy (EIS) and a frequency change of around −20 Hz with quartz crystal microbalance with dissipation (QCM-D) (Fig. 2b and d). Fig. 2b presents the EIS data in Cole–Cole plots, also known as normalised admittance plots, in which the diameter of the semicircle directly signifies the double layer capacitance, Cdl. The −20 Hz frequency shift (Δf) observed by QCM-D in Fig. 2d is slightly less than the ∼25 Hz typically observed for SSLM formation on SiO2 surfaces.38 This is because the EO3C ‘tether’ occupies part of the lower lipid leaflet in the tethered SSLM system.
In contrast to the tethered lipid systems, EIS and QCM-D indicate that HVs do not form impermeable planar SSHMs on EO3C-based SAMs (Fig. 2). After addition of HVs, no change in Cdl is observed with EIS and a higher Δf (∼−40 Hz) is recorded by QCM-D (Fig. 2a and c). SSHMs are reported to have higher Δf and ΔD values than those observed in SSLMs because membranes of HVs prepared with PBd22-b-PEO14 are thicker than lipid membranes and have different viscoelastic properties.15 The increase in viscoelasticity has been attributed to the extended poly(ethylene oxide) (PEO) chains interacting with the surrounding water and the more disordered hydrophobic core caused by the PBd chains.39 The QCM-D is thus not inconsistent with SSHM formation (see also below), but the EIS data indicate that the HVs are unable to form an impermeable SSHM on the surface. We hypothesize this is due to the length of the hydrophilic PEO chain of PBd22-b-PEO14, which is mismatched in size with the relatively short linker of the EO3C tether.
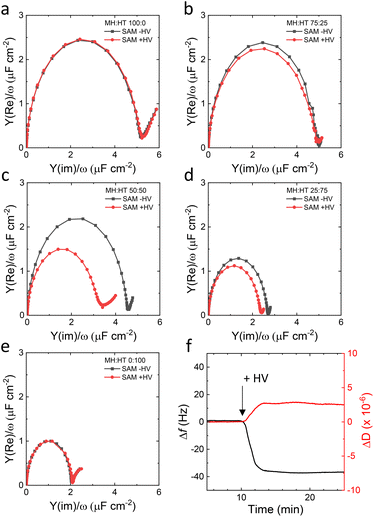
As the tethered systems could not form impermeable SSHMs, a non-tethered approach was considered. We chose to investigate the formation of SSHM on mixed SAMs of hydrophilic 6-mercapto-1-hexanol (MH) and hydrophobic 1-hexanethiol (HT). Fig. 3a–e (black traces) show EIS results of SAMs prepared from different stoichiometric ratios of MH and HT in solution (MH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HT 100
HT 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 75
0, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 50
25, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 25
50, 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75, 0
75, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100). It is known that Cdl differs greatly between hydrophobic and hydrophilic surfaces40 and the Cdl values obtained here for pure MH and HT SAM (5 μF cm−2 and 2 μF cm−2, respectively) are in line with previous values reported by us and others.30,41 Notably, Cdl decreases with an increase in the fraction of the hydrophobic HT in the SAM, indicating that mixed monolayers are formed (Fig. 3a–e, black traces). Elucidating why Cdl decreases non-linearly with the MH
100). It is known that Cdl differs greatly between hydrophobic and hydrophilic surfaces40 and the Cdl values obtained here for pure MH and HT SAM (5 μF cm−2 and 2 μF cm−2, respectively) are in line with previous values reported by us and others.30,41 Notably, Cdl decreases with an increase in the fraction of the hydrophobic HT in the SAM, indicating that mixed monolayers are formed (Fig. 3a–e, black traces). Elucidating why Cdl decreases non-linearly with the MH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HT ratio is beyond the remit of this study. Fig. 3a–e also include the EIS results of MH
HT ratio is beyond the remit of this study. Fig. 3a–e also include the EIS results of MH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HT surfaces of different ratios after incubation with HVs (red symbols and lines). For all SAMs except for the 50
HT surfaces of different ratios after incubation with HVs (red symbols and lines). For all SAMs except for the 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio, following deposition of the hybrid membrane, no or marginal reductions in Cdl are observed. This implies that an incomplete or no SSHM forms on SAM compositions of MH
50 ratio, following deposition of the hybrid membrane, no or marginal reductions in Cdl are observed. This implies that an incomplete or no SSHM forms on SAM compositions of MH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HT with 100
HT with 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 75
0, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 27
25, 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 and 0
75 and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 ratio (see also Fig. S1†). In contrast, for the 50
100 ratio (see also Fig. S1†). In contrast, for the 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio, a significant reduction in Cdl is observed (Fig. 3c), which is indicative of SSHM formation. Cdl of the 50
50 ratio, a significant reduction in Cdl is observed (Fig. 3c), which is indicative of SSHM formation. Cdl of the 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 MH
50 MH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HT SAM, as determined from the diameter of the semi-circle in the Cole–Cole plot, is measured to be 4.17 ± 0.13 μF cm−2, decreasing to 2.80 ± 0.08 μF cm−2 after incubation with HVs. Interestingly, the same 50
HT SAM, as determined from the diameter of the semi-circle in the Cole–Cole plot, is measured to be 4.17 ± 0.13 μF cm−2, decreasing to 2.80 ± 0.08 μF cm−2 after incubation with HVs. Interestingly, the same 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 MH
50 MH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TH SAM interface does not support the formation of impermeable planar SSLM by liposomes (Fig. S2†). Upon liposome addition, EIS shows no noticeable change in Cdl (Fig. S2a†), and QCM-D instead detects a substantial frequency shift (∼−175 Hz), indicating vesicle adsorption rather than fusion into a continuous bilayer (Fig. S2b†).
TH SAM interface does not support the formation of impermeable planar SSLM by liposomes (Fig. S2†). Upon liposome addition, EIS shows no noticeable change in Cdl (Fig. S2a†), and QCM-D instead detects a substantial frequency shift (∼−175 Hz), indicating vesicle adsorption rather than fusion into a continuous bilayer (Fig. S2b†).
To assess the role of polymer content, we attempted to form SSHMs using HVs with a PBd22-b-PEO14 polymer molar ratio of 25% on both EO3C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MH and MH
MH and MH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HT SAMs. In both cases, no significant reduction in Cdl was observed, indicating that this composition does not effectively support SSHM formation (Fig. S3†). Higher polymer content (≥75%) was not investigated, as our previous work has shown that cyt bo3 exhibits little activity in such compositions.28
HT SAMs. In both cases, no significant reduction in Cdl was observed, indicating that this composition does not effectively support SSHM formation (Fig. S3†). Higher polymer content (≥75%) was not investigated, as our previous work has shown that cyt bo3 exhibits little activity in such compositions.28
Confirmation of SSHM formation on 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 MH
50 MH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HT electrodes was sought by QCM-D and atomic force microscopy (AFM). In Fig. 3f, the addition of HV (50% PBd22-b-PEO14) to the MH
HT electrodes was sought by QCM-D and atomic force microscopy (AFM). In Fig. 3f, the addition of HV (50% PBd22-b-PEO14) to the MH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HT (50
HT (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) SAM coated QCM-D sensors is consistent with SSHM formation: a sharp decrease is followed by an almost constant frequency with Δf = −37 Hz. Similarly, in the dissipation profiles, a sharp increase in dissipation, followed by an almost constant value, is observed, with ΔD of 2.5 × 10−6. These profiles and changes in Δf and ΔD agree with previous similar reports on SSHM formation.18,42
50) SAM coated QCM-D sensors is consistent with SSHM formation: a sharp decrease is followed by an almost constant frequency with Δf = −37 Hz. Similarly, in the dissipation profiles, a sharp increase in dissipation, followed by an almost constant value, is observed, with ΔD of 2.5 × 10−6. These profiles and changes in Δf and ΔD agree with previous similar reports on SSHM formation.18,42
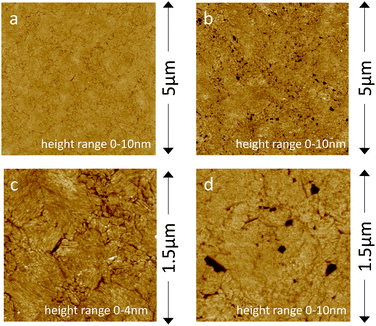
Fig. 4a and c show the planar and homogenous surface morphology of the MH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HT (50
HT (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) SAM coated electrodes as studied with AFM. AFM confirms that upon addition of HVs, a planar SSHM is formed (Fig. 4b and d). The SSHM displays a planar and uniform surface structure with some defects. No visible signs of phase separation were observed, unlike hybrid membranes based on different copolymers.20,21 We have previously demonstrated that HVs made of PBd22-b-PEO14 exhibit homogeneous membrane structures without evidence of phase-separated domains.43,44
50) SAM coated electrodes as studied with AFM. AFM confirms that upon addition of HVs, a planar SSHM is formed (Fig. 4b and d). The SSHM displays a planar and uniform surface structure with some defects. No visible signs of phase separation were observed, unlike hybrid membranes based on different copolymers.20,21 We have previously demonstrated that HVs made of PBd22-b-PEO14 exhibit homogeneous membrane structures without evidence of phase-separated domains.43,44
Electrochemical study of cytochrome bo3 in SSHM
The activity of membrane proteins in SSHM was assessed using the model enzyme, cytochrome bo3 (cyt bo3), which is an E. coli ubiquinol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxygen oxidoreductase. We have previously shown that the activity of cyt bo3 can be studied by the electrochemical reduction of the lipophilic ubiquinone-10 substrate in the SSLM.25,31,36,37 As the electrochemically formed ubiquinol-10 is catalytically re-oxidised to ubiquinone-10 by cyt bo3, cyclic voltammetry shows a characteristic catalytic wave, confirming cyt bo3 activity.
oxygen oxidoreductase. We have previously shown that the activity of cyt bo3 can be studied by the electrochemical reduction of the lipophilic ubiquinone-10 substrate in the SSLM.25,31,36,37 As the electrochemically formed ubiquinol-10 is catalytically re-oxidised to ubiquinone-10 by cyt bo3, cyclic voltammetry shows a characteristic catalytic wave, confirming cyt bo3 activity.
We investigated the electrochemical behaviour of SSHM (on SAMs of MH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HT, 50
HT, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) enriched with ubiquinone-10 (UQ) and decylubiquinone (DQ) using cyclic voltammetry (CV) (Fig. 5a). Comparison of peak area of the redox peaks of UQ and DQ shows much higher electroactive coverage of DQ compared to UQ, even though their concentrations in the SSHM are approximately the same (Fig. 5a). We hypothesize that the PEG ‘layer’ in the SSHM, which is formed by PBd22-b-PEO14 at the interface between SAM and the planer membrane, might increase the distance between the quinone headgroup and the electrode surface. It is possible that DQ, which is less hydrophobic than UQ (Fig. 1b), more readily diffuses into the PEG layer and therefore shows improved electrochemical behaviour. As DQ displayed better electrochemical performance compared to UQ, we used this quinone to study the activity of cyt bo3.
50) enriched with ubiquinone-10 (UQ) and decylubiquinone (DQ) using cyclic voltammetry (CV) (Fig. 5a). Comparison of peak area of the redox peaks of UQ and DQ shows much higher electroactive coverage of DQ compared to UQ, even though their concentrations in the SSHM are approximately the same (Fig. 5a). We hypothesize that the PEG ‘layer’ in the SSHM, which is formed by PBd22-b-PEO14 at the interface between SAM and the planer membrane, might increase the distance between the quinone headgroup and the electrode surface. It is possible that DQ, which is less hydrophobic than UQ (Fig. 1b), more readily diffuses into the PEG layer and therefore shows improved electrochemical behaviour. As DQ displayed better electrochemical performance compared to UQ, we used this quinone to study the activity of cyt bo3.
Cyt bo3 was purified from E. coli in styrene maleic acid lipid particles (SMALPs) and reconstituted in HVs using our recently developed detergent-free reconstitution method.29 The cyt bo3-containing HVs were subsequently used to form the SSHM on MH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HT SAMs. Comparison of EIS of SSHM from HV with and without cyt bo3 (Fig. 5b) shows that the inclusion of cyt bo3 has almost no effect on the Cdl of the formed SSHM, suggesting that cyt bo3 does not majorly affect the permeability or structure of the SSHM. Cyclic voltammetry (CV) of the cyt bo3-containing SSHM shows the expected catalytic wave, with catalytic currents in the same order of magnitude as those previously obtained on the ‘tethered’ SSLM with similar amounts of cyt bo3.37 Inhibition with HQNO confirms the activity is due to cyt bo3 (Fig. 5c). We estimated the surface coverage of cyt bo3 in SSHMs using two independent approaches. First, we calculated the enzyme density based on the surface area and mass composition of the membrane. The membranes were prepared with a 1
HT SAMs. Comparison of EIS of SSHM from HV with and without cyt bo3 (Fig. 5b) shows that the inclusion of cyt bo3 has almost no effect on the Cdl of the formed SSHM, suggesting that cyt bo3 does not majorly affect the permeability or structure of the SSHM. Cyclic voltammetry (CV) of the cyt bo3-containing SSHM shows the expected catalytic wave, with catalytic currents in the same order of magnitude as those previously obtained on the ‘tethered’ SSLM with similar amounts of cyt bo3.37 Inhibition with HQNO confirms the activity is due to cyt bo3 (Fig. 5c). We estimated the surface coverage of cyt bo3 in SSHMs using two independent approaches. First, we calculated the enzyme density based on the surface area and mass composition of the membrane. The membranes were prepared with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of E. coli lipids and PBd22–PEO14, with molecular areas of 0.61 nm2 for POPE, as E. coli lipids are ∼80% POPE,45 and 0.63 nm2 for PBd–PEO.39 Given that cyt bo3 accounts for 0.74 mass percent of the total membrane mass,29 this analysis yields an estimated coverage of 18 fmol cm−2 (ranging from 14–21 fmol cm−2).
1 molar ratio of E. coli lipids and PBd22–PEO14, with molecular areas of 0.61 nm2 for POPE, as E. coli lipids are ∼80% POPE,45 and 0.63 nm2 for PBd–PEO.39 Given that cyt bo3 accounts for 0.74 mass percent of the total membrane mass,29 this analysis yields an estimated coverage of 18 fmol cm−2 (ranging from 14–21 fmol cm−2).
Second, we estimated the enzyme density from the catalytic activity on the electrode (∼450 nA cm−2, Fig. 5c) and the known maximum activity of cyt bo3 in solution (540 e− per s).46 This calculation gives an enzyme coverage of 8.6 fmol cm−2.
Both estimates are in good agreement with our previous findings for cyt bo3 (ref. 37 and 47) in SSLMs and provide confidence that ∼50% of the incorporated cyt bo3 remains electrochemically active in SSHMs.
Our SSHM approach overcomes the limitations observed in fully polymer-based solid supported membrane for membrane protein. Previous studies on PBd–PEO solid-supported membranes reported a fivefold lower reconstitution efficiency for αHL.48 This highlights the advantage of hybrid lipid–polymer systems in balancing membrane stability with protein functionality, overcoming the reduced incorporation typically seen in polymer-only platforms.
We have previously shown that cyt bo3 displays a greatly increased durability at 4 °C, when reconstituted in HV compared to reconstitution in liposomes.28,49 Here, we confirmed that cyt bo3 in HV stored at 4 °C for over one year can still form SSHM with a similar EIS profile to a freshly prepared sample, and retains over 50% of its original activity (Fig. 5d and S4†). We note, however, that when monitoring the activity of cyt bo3 on the surface within SSHM, all activity is lost after storing the SSHM for >16 h at 4 °C, although the Cdl remains relatively unchanged (Fig. S5a†). Notably, the quinone peak, which is critical for the catalytic activity of cyt bo3, is diminished after overnight storage as SSHM (Fig. S5b†). This reduction may indicate a loss of redox mediators from the SSHM, contributing significantly to the observed loss of activity. Indeed, we observed that quinone depletion occurs in SSHMs even without cyt bo3 (Fig. S5d†), while Cdl remains nearly unchanged (Fig. S5c†), indicating that the membrane remains stable. Attempts to replenish quinones within the membrane by adding additional quinones to the bulk media above did not recover the lost function. As some quinone molecules remain while cyt bo3 activity is entirely lost, quinone depletion alone does not fully explain the loss of enzymatic function. We therefore hypothesize that, although the HV stabilizes cyt bo3, it is unable to compensate for the destabilizing effect that a gold/SAM interface might impose on proteins. Hence, there is significant scope for the future engineering of the hard-soft, electrode–membrane interface of SSHMs that can retain the longevity of membrane enzyme activity, which the hybrid membrane environment is capable of providing.
Conclusions
We have demonstrated that hybrid vesicles prepared from PBd22-b-PEO14 and E. coli lipids extract can form planar solid-supported hybrid membranes (SSHM) on mixed self-assembled monolayers from 6-mercapto-1-hexanol and 1-hexanethiol (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio). Membrane proteins in SSHM remain active as shown by the redox activity of the membrane enzyme cyt bo3, which was detected electrochemically using lipophilic quinone substrates as redox mediator.
50 ratio). Membrane proteins in SSHM remain active as shown by the redox activity of the membrane enzyme cyt bo3, which was detected electrochemically using lipophilic quinone substrates as redox mediator.
Importantly, our findings highlight the non-interchangeability of SAMs between lipid and polymer–lipid hybrid membranes. While the MH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HT SAM at a 50
HT SAM at a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio was highly effective for forming homogenous and impermeable SSHMs with HVs, it did not support the formation of solid-supported lipid membranes (SSLM) from liposomes. In contrast, SAMs optimized for liposomes, such as those containing mercapto-(ethylene-oxy)3-carbamate cholesterol (EO3C), failed to form impermeable SSHMs with HVs. This shows the need to tailor surface chemistries specifically for each membrane system. Building on this, further investigations into SAMs formed from thiols with varying chain lengths or headgroup functionalities could provide additional insights into expanding SSHM applicability across different membrane architectures.
50 ratio was highly effective for forming homogenous and impermeable SSHMs with HVs, it did not support the formation of solid-supported lipid membranes (SSLM) from liposomes. In contrast, SAMs optimized for liposomes, such as those containing mercapto-(ethylene-oxy)3-carbamate cholesterol (EO3C), failed to form impermeable SSHMs with HVs. This shows the need to tailor surface chemistries specifically for each membrane system. Building on this, further investigations into SAMs formed from thiols with varying chain lengths or headgroup functionalities could provide additional insights into expanding SSHM applicability across different membrane architectures.
Our study also provides new insights into the stability and longevity of cyt bo3 reconstituted in HVs. We found that HV with cyt bo3 maintain their capability to form SSHMs with active enzyme even after one year of storage at 4 °C. However, despite this enhanced stability, a complete loss of activity was observed when SSHMs containing cyt bo3 were stored for more than 16 hours at 4 °C. Looking forward, there is significant potential to improve SSHM designs to better preserve membrane enzyme activity. The potential tunability of the physical and chemical properties of SSHMs opens new avenues to study complex cellular processes and broaden the applications of hybrid membranes in biosensing and bioelectrocatalysis.
Data availability
The data files supporting figures in the manuscript and the ESI† are available in the Research Data Leeds repository at https://doi.org/10.5518/1592.Author contributions
R. C., designed and performed all experiments and analysed the resulting data. G. R. H. performed the AFM experiments and analysed the resulting data. M. R., S. P. M., P. A. B. and L. J. C. J. conceived the project. P. A. B. and L. J. C. J. directed the work. R. C., P. A. B. and L. J. C. J. wrote the initial manuscript. All authors have contributed and given approval to the final version of the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was funded by the Biotechnology and Biological Sciences Research Council (BBSRC), grant number BB/T000546/1. We gratefully acknowledge Dr. Benjamin Johnson for the assistance with the gold-coating of the silicon wafers.References
- Y. K. Go and C. Leal, Chem. Rev., 2021, 121, 13996–14030 CrossRef CAS PubMed.
- P. A. Beales, S. Khan, S. P. Muench and L. J. Jeuken, Biochem. Soc. Trans., 2017, 45, 15–26 Search PubMed.
- E. Reimhult and M. M. Virk, J. Biomed. Res., 2021, 35, 301–309 CrossRef CAS PubMed.
- L. Wang, X. Zeng, W. Shen, S. Tang and H. K. Lee, TrAC, Trends Anal. Chem., 2023, 168, 117343 CrossRef CAS.
- Y. Lu, G. Allegri and J. Huskens, Mater. Horiz., 2022, 9, 892–907 RSC.
- E. Brodszkij and B. Städler, Chem. Sci., 2024, 15, 10724–10744 RSC.
- C. G. Palivan, R. Goers, A. Najer, X. Zhang, A. Car and W. Meier, Chem. Soc. Rev., 2016, 45, 377–411 RSC.
- J. Shin, B. D. Cole, T. Shan and Y. Jang, Biomacromolecules, 2022, 23, 1505–1518 CrossRef CAS PubMed.
- O. M. Eggenberger, P. Jaśko, S. Tarvirdipour, C.-A. Schoenenberger and C. G. Palivan, Helv. Chim. Acta, 2023, 106, e202200164 CrossRef CAS.
- A. Küchler, M. Yoshimoto, S. Luginbühl, F. Mavelli and P. Walde, Nat. Nanotechnol., 2016, 11, 409–420 CrossRef PubMed.
- E. T. Castellana and P. S. Cremer, Surf. Sci. Rep., 2006, 61, 429–444 CrossRef CAS PubMed.
- S. Belegrinou, S. Menon, D. Dobrunz and W. Meier, Soft Matter, 2011, 7, 2202–2210 RSC.
- D. L. Gettel, J. Sanborn, M. A. Patel, H.-P. de Hoog, B. Liedberg, M. Nallani and A. N. Parikh, J. Am. Chem. Soc., 2014, 136, 10186–10189 CrossRef CAS PubMed.
- W. F. Paxton, P. T. McAninch, S. H. R. Shin and M. T. Brumbach, Soft Matter, 2018, 14, 8112–8118 RSC.
- M. Mumtaz Virk, B. Hofmann and E. Reimhult, Langmuir, 2019, 35, 739–749 CrossRef CAS PubMed.
- A. Balestri, L. Chiappisi, C. Montis, S. Micciulla, B. Lonetti and D. Berti, Langmuir, 2020, 36, 10941–10951 CrossRef CAS PubMed.
- G. Bello, F. Cavallini, L. A. Dailey and E.-K. Ehmoser, Biochim. Biophys. Acta, Biomembr., 2021, 1863, 183472 CrossRef CAS PubMed.
- J. Cardellini, A. Balestri, L. Comparini, B. Lonetti, M. Brucale, F. Valle, D. Berti and C. Montis, J. Colloid Interface Sci., 2024, 654, 848–858 CrossRef CAS PubMed.
- E. A. Schafer, E. Davis, Z. Manzer, S. Daniel and J. Rivnay, ACS Appl. Mater. Interfaces, 2023, 15, 24638–24647 CrossRef CAS PubMed.
- S. Di Leone, S. Y. Avsar, A. Belluati, R. Wehr, C. G. Palivan and W. Meier, J. Phys. Chem. B, 2020, 124, 4454–4465 CrossRef CAS PubMed.
- S. Di Leone, M. Kyropoulou, J. Köchlin, R. Wehr, W. P. Meier and C. G. Palivan, Langmuir, 2022, 38, 6561–6570 CrossRef CAS PubMed.
- J. Kowal, D. Wu, V. Mikhalevich, C. G. Palivan and W. Meier, Langmuir, 2015, 31, 4868–4877 CrossRef CAS PubMed.
- J. A. García-Horsman, B. Barquera, J. Rumbley, J. Ma and R. B. Gennis, J. Bacteriol., 1994, 176, 5587–5600 CrossRef PubMed.
- N. Boden, R. J. Bushby, Q. Liu, S. D. Evans, A. T. A. Jenkins, P. F. Knowles and R. E. Miles, Tetrahedron, 1998, 54, 11537–11548 CrossRef CAS.
- S. A. Weiss, R. J. Bushby, S. D. Evans and L. J. C. Jeuken, Biochim. Biophys. Acta, Bioenerg., 2010, 1797, 1917–1923 CrossRef CAS PubMed.
- S. C. Lee, T. J. Knowles, V. L. G. Postis, M. Jamshad, R. A. Parslow, Y.-P. Lin, A. Goldman, P. Sridhar, M. Overduin, S. P. Muench and T. R. Dafforn, Nat. Protoc., 2016, 11, 1149–1162 CrossRef CAS PubMed.
- J. P. Osborne, N. J. Cosper, C. M. V. Stälhandske, R. A. Scott, J. O. Alben and R. B. Gennis, Biochemistry, 1999, 38, 4526–4532 CrossRef CAS PubMed.
- S. Khan, M. Li, S. P. Muench, L. J. Jeuken and P. A. Beales, Chem. Commun., 2016, 52, 11020–11023 RSC.
- R. Catania, J. Machin, M. Rappolt, S. P. Muench, P. A. Beales and L. J. C. Jeuken, Macromolecules, 2022, 55, 3415–3422 CrossRef CAS PubMed.
- L. J. C. Jeuken, N. N. Daskalakis, X. Han, K. Sheikh, A. Erbe, R. J. Bushby and S. D. Evans, Sens. Actuators, B, 2007, 124, 501–509 CrossRef CAS.
- S. A. Weiss, R. J. Bushby, S. D. Evans, P. J. F. Henderson and L. J. C. Jeuken, Biochem. J., 2009, 417, 555–560 CrossRef CAS PubMed.
- J. Alvarez-Malmagro, G. García-Molina and A. López De Lacey, Sensors, 2020, 20, 3393 CrossRef CAS PubMed.
- L. J. C. Jeuken, Nat. Prod. Rep., 2009, 26, 1234–1240 RSC.
- Z. Su, J. J. Leitch and J. Lipkowski, Curr. Opin. Electrochem., 2018, 12, 60–72 CrossRef CAS.
- H. Zhang, R. Catania and L. J. C. Jeuken, Catalysts, 2020, 10, 1427 CrossRef CAS.
- G. R. Heath, M. Li, H. Rong, V. Radu, S. Frielingsdorf, O. Lenz, J. N. Butt and L. J. C. Jeuken, Adv. Funct. Mater., 2017, 27, 1606265 CrossRef.
- L. J. C. Jeuken, S. D. Connell, P. J. F. Henderson, R. B. Gennis, S. D. Evans and R. J. Bushby, J. Am. Chem. Soc., 2006, 128, 1711–1716 CrossRef CAS PubMed.
- R. P. Richter, R. Berat and A. R. Brisson, Langmuir, 2006, 22, 3497–3505 CrossRef CAS PubMed.
- W. A. Müller, P. A. Beales, A. R. Muniz and L. J. C. Jeuken, Biomacromolecules, 2023, 24, 4156–4169 CrossRef PubMed.
- U. K. Sur and V. Lakshminarayanan, J. Colloid Interface Sci., 2002, 254, 410–413 CrossRef CAS PubMed.
- A. K. Gaigalas and G. Niaura, J. Colloid Interface Sci., 1997, 193, 60–70 CrossRef CAS PubMed.
- K. L. Willes, J. R. Genchev and W. F. Paxton, Polymer, 2020, 12, 745 CAS.
- R. Seneviratne, R. Catania, M. Rappolt, L. J. C. Jeuken and P. A. Beales, Soft Matter, 2022, 18, 1294–1301 RSC.
- R. Seneviratne, G. Coates, Z. Xu, C. E. Cornell, R. F. Thompson, A. Sadeghpour, D. P. Maskell, L. J. C. Jeuken, M. Rappolt and P. A. Beales, Small, 2023, 19, 2206267 CrossRef CAS PubMed.
- K. Murzyn, T. Róg and M. Pasenkiewicz-Gierula, Biophys. J., 2005, 88, 1091–1103 CrossRef CAS PubMed.
- J. N. Rumbley, E. F. Nickels and R. B. Gennis, Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol., 1997, 1340, 131–142 CrossRef CAS PubMed.
- L. J. C. Jeuken, S. A. Weiss, P. J. F. Henderson, S. D. Evans and R. J. Bushby, Anal. Chem., 2008, 80, 9084–9090 CrossRef CAS PubMed.
- X. Zhang, W. Fu, C. G. Palivan and W. Meier, Sci. Rep., 2013, 3, 2196 CrossRef PubMed.
- R. Seneviratne, S. Khan, E. Moscrop, M. Rappolt, S. P. Muench, L. J. C. Jeuken and P. A. Beales, Methods, 2018, 147, 142–149 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4lf00362d |
| This journal is © The Royal Society of Chemistry 2025 |