Open Access Article
Open Access ArticleAutonomous wearable sensing enabled by capillary microfluidics: a review
Kiran
Kuruvinashetti
ab,
Amin
Komeili
*ab and
Amir
Sanati Nezhad
 *ab
*ab
aDepartment of Biomedical Engineering, University of Calgary, Calgary, Alberta T2N 1N4, Canada. E-mail: amin.komeili@ucalgary.ca; amir.sanatinezhad@ucalgary.ca
bDepartment of Mechanical and Manufacturing Engineering, University of Calgary, Calgary, Alberta T2N 1N4, Canada
First published on 10th July 2025
Abstract
Capillary microfluidic wearables have emerged as a versatile class of autonomous biosensing platforms for continuous, non-invasive monitoring of biofluids such as sweat, saliva, tears, and interstitial fluid. This review critically examines recent advances in skin-conformal device architectures that enable passive, power-free fluid sampling and integration with biochemical sensing modalities. Systems are classified by fluid handling strategy chrono-sampling versus continuous flow and sensing mode on-body versus off-body analysis. Key design principles including the use of burst valves, evaporative reservoirs, multilayer channel networks, and hydrogel-assisted interfaces are discussed in the context of minimizing evaporation, backflow, and biofouling. Advances in electrochemical and optical biosensing for real-time quantification of physiologically relevant analytes such as cortisol, glucose, lactate, pH, and electrolytes are evaluated alongside emerging trends in multiplexing and closed-loop therapeutic integration. Finally, the review highlights translational challenges in clinical validation, biocompatibility, and manufacturing scalability, outlining a roadmap for future development of lab-on-skin diagnostics and personalized health monitoring.
1. Introduction
Wearable sensors have transformed personal health monitoring by enabling continuous, real-time, and non-invasive tracking of physiological and biochemical markers.1–7 These platforms address limitations of centralized diagnostic systems by offering decentralized, user-centric solutions for health assessment at home or the point of care.1 Advances in material science, wireless electronics, and rapid prototyping have supported the development of lightweight, skin-interfaced devices with integrated sensing, fluidics, and data transmission capabilities.8–11First-generation wearable devices were primarily designed to monitor physical signals such as body motion, heart rate, respiration rate, blood pressure, temperature, sweat rate, and conductivity1,11,12 (Fig. 1a). As technology advanced, second-generation wearables emerged with multifunctional capabilities for biochemical sensing, enabling analysis of electrolytes, glucose, metabolites, hormones, pH, and other molecular markers present in sweat, saliva, interstitial fluid (ISF), and tears.1–3 This evolution has brought new diagnostic potential but also introduced challenges in power-free sample handling, miniaturized integration, and consistent bio signal extraction (Fig. 1b).
Capillary microfluidics addresses many of these limitations by enabling autonomous, self-powered transport of biofluids through engineered wettability and channel design.13–18 Typically composed of layered hydrophilic or hydrophobic materials, these systems guide fluid through defined paths without external pumps, often using skin-contacting inlets or wicking textiles9,11,19 (Fig. 1c). Collected biofluids are routed to sensing reservoirs sequentially, ensuring signal fidelity and preventing backflow or cross-contamination.14,20 While flow saturation can impair prolonged operation, optimized geometries and evaporative disposal strategies mitigate these effects and support continuous sensing.15
Wearable biosensors primarily rely on optical or electrochemical transduction mechanisms.6,11,21,22 Optical systems often integrate colorimetric or fluorescence reagents into microfluidic channels, while electrochemical systems incorporate working electrodes between the skin and fluidic reservoirs. Electrochemical detection operates via direct redox activity or indirect signal amplification using redox mediators.23,24 For long-term monitoring, reversible binding elements such as aptamers25 or electrode regeneration protocols26 are essential. Clinical examples such as the Eversense E3 glucose monitor demonstrate successful multi-month deployment.27 Molecularly imprinted polymers (MIPs) have become attractive transduction elements due to their specificity, chemical stability, and facile fabrication.28–30 These synthetic receptors mimic natural binding sites using template molecules, offering sensitive and selective detection across a range of analytes while requiring minimal power and offering excellent shelf-life.31–33
Despite significant advances in wearable biosensing, many recent reviews have focused narrowly on sensor materials, microneedle integration, AI-driven data analytics, or adaptive bioelectronics, with limited emphasis on the fluidic subsystems essential for power-free operation, reliability, and device longevity.2,34–36 These works offer valuable insights into emerging sensing modalities and system-level designs, but they often address capillary microfluidics only peripherally—if at all.37–39
In contrast, this review provides a systematic and cross-cutting assessment of capillary microfluidics as the core fluid-handling strategy for wearable biosensors. We uniquely focus on autonomous, power-free sample acquisition and routing, highlighting design distinctions between chrono-sampling versus continuous flow and on-body versus off-body sensing—dimensions often overlooked in prior literature. Importantly, we cover all major non-invasive biofluids (sweat, saliva, tears, ISF), and evaluate their distinct properties in the context of fluid handling and sensor integration. This review also integrates recent advances in electrochemical, optical, and biomolecular biosensing—including aptamers, immunosensors, and MIPs—to demonstrate how capillary systems support robust, multimodal sensing. By bridging sensing and fluidic domains within a unified capillary microfluidic framework, we address a critical gap left by earlier reviews and advance the design of next-generation wearable diagnostics that are truly autonomous, miniaturized, and clinically relevant. Fig. 1d summarizes the architectural integration of capillary elements with sensing and data modules, underscoring their importance in ensuring robust fluid delivery and sensor functionality across diverse health applications.
2. Biofluid composition, advantages, and limitations
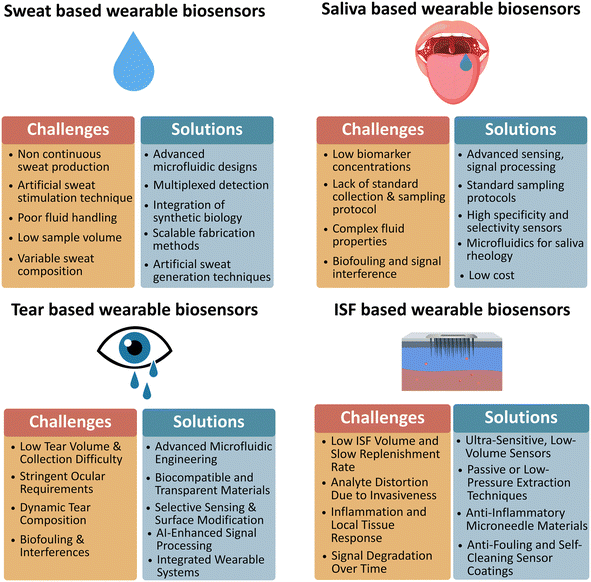
While blood is a gold standard for clinical diagnostics, its invasive collection, need for trained personnel, and risk of clotting limit its use in wearable applications.40,41 In contrast, alternative biofluids such as sweat, saliva, tears, and ISF are more suitable for continuous and minimally invasive monitoring.2,422.1 Sweat as a bodily fluid
Sweat, secreted by eccrine glands, comprises 99% water and 1% solutes, including electrolytes (sodium Na+, chloride Cl−, and potassium K+), metabolites (lactate, glucose, urea), proteins (cytokines, peptides), and trace elements (zinc, copper, iron).43–45 Hormones like cortisol and disease biomarkers such as elevated chloride (cystic fibrosis) are also detectable.7,46 Its diagnostic versatility has led to widespread integration of sweat into wearable platforms for analytes such as cortisol, chloride, and lactate.19 However, individual variability, surface contamination, low biomarker concentrations, and limited correlation to blood levels present analytical challenges.3,9 The emerging field of “iSudorology” emphasizes sweat's potential for real-time digital diagnostics.472.2 Saliva as a bodily fluid
Saliva, composed of 99% water, contains a diverse biomolecular profile, including electrolytes (Na+, K+, Cl−), enzymes (amylase, lysozyme), immunoglobulins, mucins, hormones (cortisol, melatonin), metabolites (glucose, urea), nucleic acids, and microbial content.48–50 Its continuous secretion, non-invasive collection, and good correlation with serum levels make it promising for wearable monitoring.51 Saliva-based sensing has shown utility in monitoring oral, systemic, and neurological diseases, stress, and hormonal changes.50,52 Nevertheless, intra- and inter-individual variability, along with sensor stability in the oral environment, remain barriers to deployment.532.3 Tears as a bodily fluid
Tears are a continuously secreted, minimally invasive biofluid with growing potential in wearable sensing. Many tear biomarkers correlate well with blood concentrations, making them suitable for real-time health monitoring.43 Smart contact lenses, for example, have been developed for glucose monitoring in diabetes.54,55 Proteomic and inflammatory markers in tears have also been utilized for diagnosing dry eye disease,5 while neurodegenerative biomarkers for Alzheimer's and Parkinson's have shown promise for early detection.56 Tear analysis supports intraocular pressure monitoring and systemic disease detection, including cancer and multiple sclerosis.57 However, low analyte concentrations demand highly sensitive detection platforms,56 and long-term sensor stability within the ocular environment remains a key challenge57 Establishing standardized tear biomarker reference ranges is also essential for clinical utility.552.4 ISF as a bodily fluid
ISF, occupying ∼45% of human skin volume, serves as an intermediary between blood and tissues and is an abundant source of biomarkers.58,59 Its composition includes metabolites, proteins, nucleic acids, exosomes, drugs, and cytokines.60–62 While many ISF biomarkers mirror those in plasma, diffusion barriers and electrostatic exclusion mechanisms influence analyte concentrations based on size and charge.58 ISF contains over 92% of RNA species found in blood and is especially enriched in exosomes, making it a rich matrix for molecular diagnostics.58 ISF offers several advantages for continuous biosensing: it is non-clotting, reflects local tissue states, and enables real-time monitoring using minimally invasive tools like microneedles.59,61,62 However, dynamic dilution effects, cellular uptake, and biological variability can complicate analyte interpretation.58,62 Affinity-based sensors, though promising, require improvements in longevity and fouling resistance for extended use.62 ISF, like sweat and saliva, reinforces the importance of advanced microfluidic integration. Achieving real-time, autonomous biosensing depends on engineering capillary microfluidic systems that allow continuous, pump-free fluid handling. These platforms will be central to the next generation of personalized, real-time healthcare diagnostics.A comparative analysis of metabolites, hormones, and electrolytes across sweat, saliva, ISF, and blood reveals distinct concentration profiles shaped by molecular weight, membrane permeability, and transport mechanisms. Among these fluids, metabolite concentrations are consistently highest in blood and ISF, reflecting their direct roles in systemic homeostasis. In contrast, sweat and saliva typically present more diluted analyte levels due to glandular filtration and extracellular exchange processes. Table 1 summarizes representative biomarker concentrations across these major biofluids, while Table 2 provides physiological and pathological relevance for each analyte. These values are critical for the rational design of wearable biosensors, where high selectivity and sensitivity are essential for accurate detection in low-concentration matrices.
| Biomolecule | Sweat (μM) | Saliva (μM) | ISF (μM) | Blood (μM) | Ref. |
|---|---|---|---|---|---|
| Cortisol | 22.08–386.37 | 0.331–82.79 | 0.276–27.6 | 11.04–1104 | 63–67 |
| Glucose | 10–1000 | 30–80 | 3900–6900 | 3900–6900 | 68–70 |
| Lactate | 3700–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0–3500 | 500–2000 | 1600–12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
71–74 |
| Sodium | 10–90 × 103 | 2–21 × 103 | 135–145 × 103 | 135–145 × 103 | 75–79 |
| Potassium | 4–16 × 103 | 1–36 × 103 | 3.5–5 × 103 | 3.5–5 × 103 | 80–83 |
| Chloride | 10–70 × 103 | 10–50 × 103 | 98–107 × 103 | 98–107 × 103 | 84–88 |
| Uric acid | 2–10 × 103 | 0.1–0.3 × 103 | 0.2–0.4 × 103 | 0.2–0.4 × 103 | 89–92 |
| Immunoglobulin A | 0.0000625–0.00625 | 0.0167–4.0 | 0.0000667–0.00667 | 0.0107–0.6667 | 93–96 |
| α-Amylase | 714 × 10−5–714 × 10−3 | 143 × 10−3–0.0286 | 714 × 10−5–714 × 10−3 | 2.86 × 10−6–1.0 × 10−5 | 97–100 |
| Chromogranin A | 0.00204–0.2044 | 0.00204–0.2044 | 0.00204–0.2044 | 0.511–2.862 | 101–105 |
| Brain-derived neurotrophic factor | 0.000370–0.0370 | 0.00248–0.1015 | 0.000370–0.0370 | 0.00222–0.5926 | 106–109 |
| Albumin | 1.50–75.19 | 1.50–7.52 | 300.75–451.13 | 526.32–751.88 | 110–114 |
| Creatinine | 50–200 | 4–30 | 60–100 | 60–100 | 60, 115–117 |
| Zinc | 0.4–1.2 | 1–10 | 10–18 | 10–18 | 118–120 |
| Copper | 0.3–1 | 0.1–1.5 | 11–22 | 11–22 | 119, 121–125 |
| Iron | 0.1–1 | 1–10 | 10–30 | 10–30 | 126–128 |
| Bicarbonate | 1–40 × 103 | 5–40 × 103 | 22–29 × 103 | 22–29 × 103 | 43, 44, 129, 130 |
| Phosphate | 0.1–1 × 103 | 2–10 × 103 | 0.8–1.4 × 103 | 0.8–1.4 × 103 | 131–133 |
| Amino acids (total) | 1–10 × 103 | 1–5 × 103 | 2–5 × 103 | 2–5 × 103 | 134–137 |
| Urea | 2–10 × 103 | 2–10 × 103 | 2.5–7.1 × 103 | 2.5–7.1 × 103 | 138–140 |
| Cholesterol | 0.01–0.1 × 103 | 0.01–0.1 × 103 | 3.9–5.2 × 103 | 3.9–5.2 × 103 | 141–143 |
| Triglycerides | 0.01–0.1 × 103 | 0.01–0.1 × 103 | 0.4–1.8 × 103 | 0.4–1.8 × 103 | 144–146 |
| Testosterone | 0.0347–3.47 | 0.0347–0.6935 | 10.4–34.68 | 10.4–34.68 | 147–150 |
| Estradiol | 3.67 × 10−6–3.67 × 10−4 | 1.84 × 10−6–1.84 × 10−5 | 3.67 × 10−5–1.84 × 10−4 | 3.67 × 10−5–1.84 × 10−4 | 151–154 |
| Progesterone | 0.318–3.18 | 0.159–1.59 | 0.636–63.6 | 0.636–63.6 | 155–158 |
| C-reactive protein | 0.00087–0.0870 | 0.00043–0.0435 | 0.00087–0.0870 | 0.00087–0.0870 | 159–161 |
| Interleukin-6 | 4.76 × 10−9–4.76 × 10−7 | 4.76 × 10−9–2.38 × 10−7 | 0–4.76 × 10−7 | 0–4.76 × 10−7 | 162–165 |
| Tumor necrosis factor-α | 5.88 × 10−9–5.88 × 10−7 | 5.88 × 10−9–2.94 × 10−7 | 0–1.18 × 10−6 | 0–1.18 × 10−6 | 166–169 |
| Leptin | 0.00625–0.625 | 0.00625–0.3125 | 0.0625–6.25 | 0.0625–6.25 | 170–173 |
| Adiponectin | 0.00333–0.333 | 0.00333–0.1667 | 0.0667–1.0 | 0.0667–1.0 | 171, 174–176 |
| Ghrelin | 3.03 × 10−6–3.03 × 10−5 | 3.03 × 10−6–1.52 × 10−5 | 3.03 × 10−5–3.03 × 10−4 | 3.03 × 10−5–3.03 × 10−4 | 171, 177–179 |
| Melatonin | 4.30 × 10−6–4.30 × 10−5 | 4.30 × 10−6–2.15 × 10−5 | 4.30 × 10−5–2.15 × 10−4 | 4.30 × 10−5–2.15 × 10−4 | 155, 180–182 |
| Serotonin | 0.568–5.68 | 0.568–2.84 | 283.7–1134.9 | 283.7–1134.9 | 183–186 |
| Dopamine | 0.653–6.53 | 0.653–3.27 | 6.53 × 10−5–6.53 × 10−4 | 6.53 × 10−5–6.53 × 10−4 | 187–191 |
| Epinephrine | 0.0546–0.546 | 0.0546–0.273 | 5.46 × 10−5–5.46 × 10−4 | 5.46 × 10−5–5.46 × 10−4 | 192–197 |
| Vitamin C | 5.68–56.78 | 5.68–28.39 | 22.71–113.54 | 22.71–113.54 | 198–203 |
| Folate | 2.27–22.66 | 2.27–11.33 | 4.53–45.33 | 4.53–45.33 | 204–206 |
| Thyroid-stimulating hormone | 3.57 × 10−4–3.57 × 10−3 | 3.57 × 10−4–1.79 × 10−3 | 0.0143–0.143 | 0.0143–0.143 | 207–209 |
| Biomolecule | Physiological conditions | Pathological conditions |
|---|---|---|
| Cortisol | Stress response, immune modulation, maintaining homeostasis. Regulation of blood pressure, metabolism, and inflammatory responses | Cushing's syndrome, characterized by excessive cortisol levels; Addison's disease, caused by insufficient cortisol production. Mental health disorders like depression and anxiety |
| Glucose | Glycolysis, glycogen storage, and glucose homeostasis, regulated by hormones like insulin and glucagon. Essential for ATP production, oxidative stress management, and neurotransmitter synthesis210 | Diabetes mellitus (type 1 and type 2), hyperglycemia, hypoglycemia, diabetic ketoacidosis211 |
| Lactate | An energy source for the myocardium, regulates smooth vascular muscle cells (VSMCs), promotes angiogenesis, and modulates immune cell functions212 | Lactic acidosis, cardiovascular diseases like heart failure and pulmonary hypertension, cancer progression, and inflammatory conditions. It is also associated with metabolic disorders and mitochondrial dysfunction213 |
| Sodium | Maintaining fluid balance, nerve conduction, muscle contraction, and blood pressure regulation. It is essential for the functioning of the nervous system and muscle activity, as well as for maintaining cellular osmotic pressure and blood volume | Hyponatremia (low sodium levels), hypernatremia (high sodium levels), heart failure, kidney diseases, and hypertension214 |
| Potassium | Maintaining normal levels of fluid inside cells, muscle contraction, nerve function, and blood pressure regulation | Hypokalemia (low potassium levels) and hyperkalemia (high potassium levels) are associated with muscle weakness, cardiac arrhythmias, and other health issues215 |
| Chloride | Maintaining ionic homeostasis, osmotic pressure, acid–base balance, and electrical excitability. It is involved in cell volume regulation, transepithelial transport, and the regulation of membrane potential in various tissues216 | Cystic fibrosis, myotonia congenita, Bartter's syndrome, hyperchloremia, and hypochloremia216 |
| Uric acid | A major antioxidant in human plasma, scavenging reactive oxygen species and protecting against oxidative stress. It also plays a role in immune responses and tissue healing217 | Elevated uric acid levels are associated with gout, kidney stones, cardiovascular diseases, and metabolic syndrome. Low levels are linked to neurological disorders such as multiple sclerosis and Parkinson's disease217 |
| Immunoglobulin A (IgA) | (IgA) is the most abundant antibody at mucosal surfaces, playing a key role in host–pathogen defense, immune exclusion, and maintaining homeostasis. It interacts with the intestinal microbiota to enhance diversity and provides protection against pathogens by immune exclusion. IgA also contributes to mucosal immunity by neutralizing toxins and viruses, blocking colonization and penetration of pathogenic bacteria, and promoting antigen sampling218 | IgA nephropathy, IgA vasculitis, celiac disease, inflammatory bowel disease, dermatitis herpetiformis, and autoimmune diseases like rheumatoid arthritis and Sjögren's syndrome. Aberrant IgA immune complexes can lead to excessive immune cell activation, causing tissue damage in autoimmune diseases218 |
| α-Amylase | α-Amylase plays a critical role in carbohydrate digestion by breaking down starch into simpler sugars. It is involved in glucose metabolism and has been studied as a stress biomarker due to its activity in response to adrenergic system activation | Acute and chronic pancreatitis, diabetes, Alzheimer's disease, and stress-related conditions. Elevated levels are often diagnostic markers for pancreatic disorders, while low levels are linked to metabolic syndromes and other chronic conditions |
| Chromogranin A | Chromogranin A (CgA) plays a role in endocrine, cardiovascular, metabolic, and immune system regulation. It is involved in the formation of dense core secretory vesicles in neuroendocrine cells and regulates calcium homeostasis, glucose metabolism, and vascular physiology. CgA-derived peptides such as vasostatin, catestatin, and pancreastatin contribute to these physiological roles219 | Neuroendocrine tumors, cardiovascular diseases, inflammatory diseases (e.g., rheumatoid arthritis, systemic inflammatory response syndrome), and neuropsychiatric conditions. It is also linked to conditions like chronic atrophic gastritis, renal insufficiency, and prostate cancer220 |
| Brain-derived neurotrophic factor (BDNF) | BDNF plays a critical role in neuronal survival, differentiation, synaptic plasticity, and memory formation. It is involved in the growth and maintenance of neurons, synaptic transmission, and neurogenesis, particularly in the hippocampus and cortex221 | Several neurological and psychiatric disorders, including Alzheimer's disease, Parkinson's disease, depression, Huntington's disease, and multiple sclerosis. Abnormal BDNF signaling is associated with neurodegeneration, cognitive decline, and mood disorders222 |
| Albumin | Maintaining oncotic pressure, preventing fluid leakage into extravascular spaces. It acts as a carrier for various endogenous and exogenous compounds, including fatty acids, hormones, and drugs223 | Hypoalbuminemia, which can result from liver diseases (e.g., cirrhosis), kidney diseases (e.g., nephrotic syndrome), malnutrition, burns, sepsis, and critical illnesses. Hypoalbuminemia is linked to increased morbidity and mortality, as it affects vascular homeostasis and immune responses223 |
| Creatinine | It is a byproduct of muscle metabolism, specifically from the breakdown of creatine phosphate, which is involved in energy production in muscles. It is excreted by the kidneys and serves as a marker for kidney function | Elevated levels are associated with kidney dysfunction, including chronic kidney disease, acute kidney injury, and conditions like diabetes, hypertension, and glomerulonephritis. Low creatinine levels may indicate reduced muscle mass or liver disease |
| Calcium | Calcium plays a critical role in bone structure, muscle contraction, nerve function, enzymatic processes, and blood coagulation. It is also essential for cell signaling and maintaining the potential difference across excitable cell membranes | Pathological conditions associated with calcium include hypocalcemia, hypercalcemia, osteoporosis, kidney stones, and cardiovascular issues related to abnormal calcium levels224 |
| Magnesium | Magnesium is essential for over 300 biochemical reactions in the body, including maintaining normal nerve and muscle function, supporting a healthy immune system, keeping the heartbeat steady, and aiding in bone health and energy production225 | Magnesium deficiency is associated with cardiovascular diseases, type 2 diabetes, osteoporosis, migraines, depression, and neurological disorders. It can also lead to hypocalcemia, hypokalemia, and cardiac arrhythmias226 |
| Zinc | Zinc is essential for enzymatic reactions, immune function, cell division, wound healing, and growth. It plays a role in the synthesis of DNA, RNA, and proteins, and is critical for the function of over 300 enzymes. Zinc also supports the senses of taste and smell, and is involved in hormonal regulation, including insulin and thyroid hormones227 | Zinc deficiency is associated with conditions such as acrodermatitis enteropathica, immune dysfunction, delayed wound healing, growth retardation, and increased susceptibility to infections. Chronic diseases like diabetes, neurodegenerative disorders, and certain cancers are also linked to zinc imbalance228 |
| Copper | Copper plays a critical role in oxidative metabolism, antioxidant defense, neurotransmitter synthesis, immune system functioning, angiogenesis, and regulation of gene expression. It is essential for the production of red blood cells, maintenance of nerve cells, and energy production229 | Copper dysregulation is associated with Menkes disease, Wilson's disease, Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, cardiovascular diseases, and certain cancers. It can lead to oxidative stress, neurodegeneration, and other systemic dysfunctions229 |
| Iron | Iron plays a critical role in oxygen transport (as hemoglobin), muscle oxygenation (as myoglobin), DNA synthesis, cellular respiration (as cytochromes), immune function, and myelin sheath formation | Pathological conditions associated with iron include hemochromatosis (iron overload), anemia (iron deficiency), organ damage due to iron deposits in the liver, heart, and pancreas, and conditions like diabetes and cirrhosis |
| Bicarbonate | Bicarbonate plays a vital role in the physiological pH buffering system, maintaining acid–base homeostasis. It is involved in the regulation of pH in the stomach and small intestine, neutralizing acidic chyme, and is essential for metabolic functions such as carbon dioxide transport and excretion | Pathological conditions associated with bicarbonate include metabolic acidosis, systemic acidosis, kidney stones, hypertension, and brain dysfunction. It is also implicated in cancer progression and chronic kidney disease230 |
| Phosphate | Phosphate is essential for bone and teeth formation, energy metabolism, cellular signaling, and pH buffering. It is also involved in the synthesis of DNA and RNA, and the regulation of protein phosphorylation231 | Hypophosphatemia can lead to osteomalacia, rickets, and muscle weakness, while hyperphosphatemia is associated with chronic kidney disease, cardiovascular complications, and vascular calcification232 |
| Amino acids (total) | Amino acids serve as building blocks of proteins, regulators of metabolic pathways, and precursors for hormones, neurotransmitters, and other biomolecules. They are essential for growth, repair, immune function, and maintaining homeostasis | Disorders in amino acid metabolism are associated with metabolic diseases, cardiovascular diseases, immune dysfunctions, and cancer. Specific conditions include phenylketonuria, maple syrup urine disease, and homocystinuria233 |
| Urea | Urea plays a key role in osmoregulation, nitrogen excretion, and urine concentration. It is involved in the urea cycle, which detoxifies ammonia into urea for excretion. Urea transporters facilitate its movement across membranes, aiding in water balance and nutrient recycling | Pathological conditions associated with urea include chronic kidney disease (CKD), uremia, and cardiovascular diseases. High urea levels can lead to oxidative stress, endothelial dysfunction, and protein carbamylation, contributing to these diseases234 |
| Cholesterol | Cholesterol is essential for cell membrane structure and fluidity, serves as a precursor for steroid hormones (e.g., cortisol, aldosterone, testosterone, estrogens), vitamin D, and bile acids, and facilitates the absorption of fat-soluble vitamins (A, D, E, K)235 | High levels of LDL cholesterol are associated with atherosclerosis, coronary artery disease, stroke, and peripheral arterial disease. Other conditions include familial hypercholesterolemia and hypothyroidism235 |
| Triglycerides | Triglycerides serve as the body's primary energy storage molecules, stored in adipose tissue and released during periods of energy demand. They are also involved in the transport of dietary fats via lipoproteins like VLDL and chylomicrons | High triglyceride levels (hypertriglyceridemia) are associated with cardiovascular diseases, pancreatitis, metabolic syndrome, and atherosclerosis. Extremely high levels can lead to acute pancreatitis |
| Testosterone | Testosterone plays a key role in male reproductive development, including the formation of male reproductive tissues such as the testicles and prostate. It promotes secondary sexual characteristics like increased muscle and bone mass, body hair growth, and deepening of the voice. It also regulates libido, sperm production, and erythropoiesis (red blood cell production) | Pathological conditions associated with testosterone include hypogonadism, characterized by low testosterone levels leading to symptoms like reduced libido, erectile dysfunction, and decreased muscle mass. High testosterone levels can be linked to prostate cancer, cardiovascular risks, and metabolic syndrome. Other conditions include polycystic ovary syndrome (PCOS) in women and androgenic alopecia |
| Estradiol | Estradiol plays a critical role in the reproductive system, including the development and maintenance of female secondary sexual characteristics and the menstrual cycle. It also contributes to cardiovascular health by improving lipid profiles and promoting vasodilation, supports bone density by inhibiting bone resorption, and has neuroprotective effects, including anti-inflammatory actions and stimulation of neurogenesis236 | Estradiol is implicated in pathological conditions such as breast cancer, cardiovascular diseases (e.g., stroke, deep vein thrombosis), and neurodegenerative disorders like Alzheimer's disease. It is also associated with conditions like endometriosis, uterine fibroids, and estrogen-induced cholestasis237 |
| Progesterone | Progesterone plays a critical role in the menstrual cycle, pregnancy, and lactation. It prepares the endometrium for implantation, maintains pregnancy by suppressing uterine contractions, and supports mammary gland development. Additionally, it has neuroprotective effects, modulates immune responses, and regulates bone mass | Progesterone is implicated in pathological conditions such as endometriosis, breast cancer, endometrial hyperplasia, and progesterone resistance. It is also associated with neurodegenerative diseases and certain gynecological disorders238 |
| C-reactive protein | C-reactive protein (CRP) is an acute-phase protein synthesized by the liver in response to inflammatory cytokines. It plays a role in the innate immune system by binding to damaged tissue, nuclear antigens, and certain pathogens. CRP activates the complement system, enhances phagocytosis, and promotes the clearance of cellular debris and pathogens. It also regulates platelet adhesion and chemotaxis during inflammation239 | CRP is associated with various pathological conditions, including cardiovascular diseases (e.g., atherosclerosis, myocardial infarction), rheumatoid arthritis, systemic lupus erythematosus, type 2 diabetes, Alzheimer's disease, and Parkinson's disease. Elevated CRP levels are indicative of acute and chronic inflammation, and it is used as a biomarker for these conditions240 |
| Interleukin-6 | Interleukin-6 (IL-6) plays a role in immune responses, hematopoiesis, metabolism, and acts as both a pro-inflammatory cytokine and an anti-inflammatory myokine. It is involved in acute-phase responses, B and T lymphocyte activation, and metabolic regulation during exercise | IL-6 is implicated in autoimmune diseases such as rheumatoid arthritis, Castleman disease, and juvenile idiopathic arthritis. It is also associated with chronic inflammation, cytokine release syndrome, and conditions like Alzheimer's disease and multiple sclerosis241 |
| Tumor necrosis factor-α | Tumor necrosis factor-α (TNF-α) plays roles in immunomodulation, fever, inflammatory response, inhibition of tumor formation, and inhibition of viral replication. It regulates survival, proliferation, and apoptosis of embryonic stem cells and progenitor cells, and is involved in neurogenesis, myelination, and synaptic plasticity in the central nervous system242 | TNF-α is associated with autoimmune diseases such as rheumatoid arthritis, inflammatory bowel disease, and psoriasis. It is also linked to chronic inflammation, neuroinflammation, and diseases like multiple sclerosis, asthma, and chronic obstructive pulmonary disease243 |
| Leptin | Leptin regulates energy homeostasis, neuroendocrine function, and metabolism. It signals the hypothalamus to suppress appetite, increase energy expenditure, and regulate body weight. It also plays roles in thermogenesis, glucose homeostasis, and reproductive function | Leptin is implicated in obesity, leptin resistance, hypothalamic amenorrhea, metabolic syndrome, and cardiovascular diseases. It is also associated with immune dysfunctions and certain infectious diseases due to its role in cytokine production244 |
| Melatonin | Melatonin regulates sleep cycles, circadian rhythms, and acts as an antioxidant. It modulates immune function, reduces oxidative stress, and influences mitochondrial dynamics, including biogenesis, fission, fusion, and mitophagy245 | Melatonin is associated with sleep disorders (e.g., insomnia, circadian rhythm sleep disorders), neurodegenerative diseases (e.g., Alzheimer's, Parkinson's), metabolic disorders (e.g., type 2 diabetes), and certain cancers245 |
| Serotonin | Serotonin plays a role in mood regulation, gastrointestinal motility, cardiovascular function, sleep, appetite, and blood clotting. It acts as a neurotransmitter in the central nervous system and as a hormone in peripheral systems | Conditions associated with serotonin include serotonin syndrome, depression, anxiety disorders, schizophrenia, and gastrointestinal disorders like irritable bowel syndrome |
| Dopamine | Dopamine plays a role in motor control, reward and pleasure systems, mood regulation, attention, learning, memory, sleep, kidney function, and cardiovascular regulation | Dopamine is associated with Parkinson's disease, schizophrenia, ADHD, depression, addiction, and bipolar disorder246 |
| Epinephrine | Epinephrine plays a key role in the fight-or-flight response, increasing cardiac output, raising glucose levels in the blood, and enhancing alertness. It also causes bronchodilation and vasodilation in skeletal muscles and the liver | Pathological conditions associated with epinephrine include pheochromocytoma, hypoglycemia, myocardial infarction, and cardiac arrhythmias |
In support of these insights, a wireless smart contact lens was recently developed for continuous, high-resolution monitoring of basal tear glucose.55 The device showed a strong correlation between tear and blood glucose levels in both human and animal studies, particularly when subject-specific lag times—typically 5 to 15 min—were appropriately calibrated. Despite significantly lower glucose concentrations in tears (approximately 0.1–0.6 mM) compared to blood (0.5–3 mM), consistent glycemic patterns emerged once the effects of diffusion delays and reflex tearing were addressed. Real-time electrochemical sensing with sub-minute resolution confirmed that tear glucose levels reliably mirrored blood glucose dynamics, underscoring the feasibility of tear-based biosensing as a non-invasive and practical approach for glycemic monitoring.
3. Capillary microfluidics: material selection and fabrication strategies
Capillary microfluidics enable self-driven fluid manipulation via surface tension and wettability, making them ideally suited for wearable biosensors where compact, power-free operation is critical.13,16,17 The selection of materials and fabrication methods must account for the target biofluid, device geometry, and intended use (disposable versus long-term monitoring), as these parameters influence extraction efficiency, flow dynamics, and signal reliability. For example, capillary channel dimensions must match the active sensing area to ensure uniform biofluid distribution and minimal sample loss. Material stability under mechanical stress and environmental exposure is also essential, particularly for skin-interfacing devices.Materials with tailored hydrophilic/hydrophobic properties, electrostatic behavior, and biocompatibility are key to optimizing capillary action and ensuring consistent fluid transport.13,14 Paper, polymer films, hydrogels, and hybrid composites have been used extensively, each offering specific advantages in wettability, flexibility, and integration with sensors. Advances in laser cutting and 3D printing have democratized the fabrication of capillary networks, supporting low-cost and rapid prototyping without cleanroom infrastructure.247 Laser-cut films enable precise channel patterning, while 3D printing facilitates complex multilayered designs with embedded capillary elements. These techniques streamline development of capillary microfluidic components tailored for diverse wearable applications. The section below details material types and fabrication methods, comparing their functional benefits and limitations for wearable implementation.
3.1 Material selection
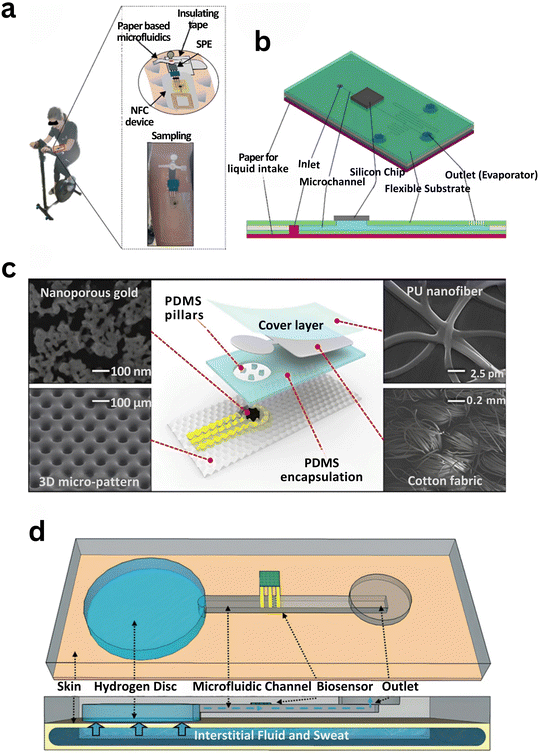 | ||
| Fig. 2 Representative materials used in wearable capillary microfluidic systems: (a) paper-based microfluidic patch with a screen-printed electrode for sweat cortisol sensing.247 (b) Silicon-based microfluidic device for electrochemical sweat pH detection, integrating a paper inlet and laser-fabricated microchannels.276 (c) Polymer-based stretchable patch for glucose monitoring using a nanoporous gold electrode and passive microfluidics.261 (d) Hydrogel-disc-driven osmotic microfluidic system enabling fluid transport for biosensing through solute-induced flow.266 | ||
3.2 Key design principles for capillary microfluidics in wearable and point-of-care applications
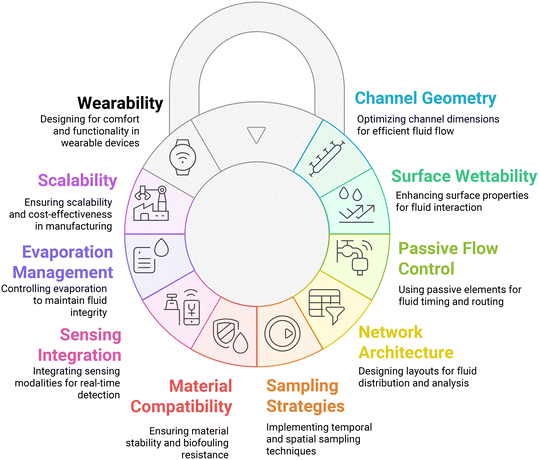
A critical aspect of capillary microfluidic design is selecting materials suited to the target biofluid and application. Materials such as paper and fabric enable passive fluid movement by capillary action without requiring external pumps.15,20 Their inherent porosity and hydrophilicity facilitate efficient wicking of small fluid volumes, making them well-suited for wearable sampling of sweat, saliva, tears, and interstitial fluid.277 Fabrics, in particular, offer capillary channels formed by fiber gaps that allow liquid wicking along threads, which is advantageous for on-body applications due to their comfort and flexibility.278,279 Biocompatible materials like PDMS, acrylics, and hydrogels are also widely used in wearable systems.13 These materials are soft, stretchable, and conformable to skin, which helps maintain device integrity during movement. Material selection must be guided by both the physicochemical properties of the interface—such as wettability, swelling behavior, and fouling resistance—and the geometric features of the fluidic network, such as channel width, depth, and orientation.13,16 Fluid transport performance is also strongly influenced by the layout of the microfluidic system. Design considerations include the shape and size of the channels, the surface properties that govern fluid spreading (wettability),280 and the ability to sustain passive flow without external power.13 Key components such as inlets, reservoirs, burst valves, and retention zones must be optimized to enable regulated fluid distribution and efficient use of small sample volumes. Minimizing the need for external actuators not only enhances portability but also improves usability in decentralized or low-resource settings.13,16 In addition, effective designs must reduce evaporation losses and resist clogging or contamination from biofouling.281 Sensors should be seamlessly integrated for real-time biomarker monitoring, and their performance should remain stable across varying hydration states and environmental exposures.282 For manufacturing and deployment, systems must be cost-effective and robust. In wearable applications, the device should also be soft, lightweight, breathable, and safe for prolonged skin contact.262Fig. 3 summarizes these key design principles, outlining how materials, fluidic geometry, sensor integration, and human factors together determine the functionality and translational success of wearable capillary microfluidic platforms.4. Fabrication of wearable capillary microfluidic devices
The fabrication of capillary microfluidic devices plays a central role in defining their precision, scalability, and compatibility with wearable systems. Key fabrication strategies include rapid prototyping methods such as laser cutting and 3D printing, as well as cleanroom-based microfabrication approaches like photolithography, soft lithography, and deep reactive ion etching.5,13,17,32,44–46 Laser cutting and 3D printing offer fast, cost-effective solutions for prototyping and scale-up, as shown in Fig. 4a, where laser-cut channels and 3D-printed molds demonstrate their utility in creating fluid conduits with varying volumes.11,19,199,283 These methods support quick iterations but may be limited by resolution and material choices.17,251,254 In contrast, photolithography and deep etching provide high fidelity and fine features but require expensive cleanroom infrastructure and are less suited for flexible or wearable formats.13 Soft lithography presents a compromise between precision and accessibility, supporting fabrication of flexible PDMS-based microfluidic systems (Fig. 4b), though it can still be time-intensive and not ideal for high-throughput production. The integration of capillary elements including flow resistors, stop valves, trigger valves, and burst valves enables passive and self-regulated flow, eliminating the need for external pumps.13 Notably, affordable stereolithography (SLA)-based 3D printing now enables the high-resolution, low-cost fabrication of microneedles, with sensor manufacturing costs reported as low as €0.10 per patch. This cost-effective approach facilitates scalable production of microneedle arrays for both biofluid extraction and continuous biochemical monitoring, significantly improving the accessibility and translational potential of MN-based sensors for wearable and point-of-care diagnostics.284,285 Together, these fabrication strategies enable the realization of autonomous microfluidic circuits tailored for wearable sensing applications.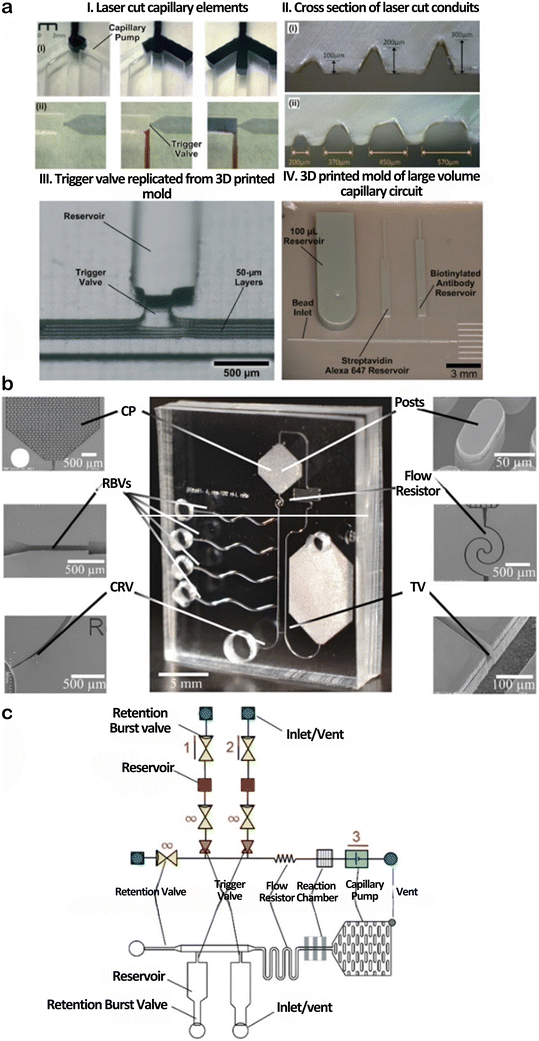 | ||
| Fig. 4 Fabrication approaches wearable capillary microfluidic devices. (a) Prototyping using carbon dioxide (CO2) laser cutting and 3D printing: (I) laser-cut capillary components,283 (II) cross-sectional view of laser-cut conduits,283 (III) trigger valve formed using a 3D-printed mold,286 and (IV) mold for a large-volume capillary circuit.287 (b) Optical image of a PDMS-based microfluidic circuit with side reservoirs, flow reversal, and venting via a PDMS cover.14 (c) Schematic of integrated capillary elements (e.g., valves and resistors) for self-regulated fluid flow; see ref. 13 for detailed descriptions. | ||
Capillary microfluidics leverages physical forces such as surface tension, viscosity, and pressure to drive fluid flow through passive means.13,16,274,275 This flow behavior is modulated by integrated capillary elements including retention burst valves, retention valves, trigger valves, capillary pumps, and vents which control timing, directionality, and flow rates (Fig. 4c). While numerous other components exist, these elements represent core building blocks in self-powered systems. For a more detailed treatment of capillary elements and their mechanisms, refer to comprehensive sources in ref. 13 and 14.
5. Capillary microfluidic-based wearable sensors and biosensors
Capillary microfluidic-enabled wearables can be systematically categorized by their sample acquisition strategy chrono-sampling or continuous sampling and by the location of analysis on-body or off-body. This framework aids in mapping specific biosensing formats to corresponding cases in health monitoring and diagnostics.5.1 Chrono sampling and off-body analysis (CHOFF)
Chrono-sampling systems enable periodic fluid collection for downstream laboratory testing. They are particularly useful for applications that do not require continuous monitoring and benefit from simpler, disposable, and user-friendly platforms. By limiting contact time and minimizing on-device processing, CHOFF systems reduce contamination risk and are compatible with sweat, saliva, ISF, and tears. | ||
| Fig. 5 Representative designs of capillary microfluidic sweat patches developed for chrono-sampling and off-body analysis. (a) Schematic of the ten-layer MicroSweat patch designed for passive sweat collection, directional flow, and storage in fiber layers. (b) (i–vi) Images of the patch showing front, back, isometric, twisted, and bent configurations, demonstrating flexibility (scale bar: 10 mm).19 (c) Integration of a capillary microfluidic patch on skin with layered encapsulation, sensors, and colorimetric chambers for offline biomarker analysis. (d) Layout of sequential sweat chambers with integrated capillary pumps for temporal glucose, Na+, and pH sensing.288 (e) Exploded and assembled views of a hydrophilic film-based microfluidic patch with funnel inlet, air vents, and channel architecture. (f) On-body validation during exercise with biometric monitoring setup.289 | ||
Capillary microfluidics have also been adapted with Tesla valves to promote unidirectional flow, reduce backflow, and spatially separate sweat samples. Filter paper-based chambers functionalized with colorimetric reagents enable glucose and pH detection, with PDMS microchannels and polyvinylpyrrolidone (PVP)-treated surfaces improving wettability.292 Sweat patches for environmental exposure monitoring such as bisphenol A (BPA) have also been explored.293 However, challenges like low sweat volume under typical conditions, variable patch placement, and matrix effects limit their sensitivity. Still, these studies highlight sweat's value as a non-invasive matrix for temporal exposure tracking. Programmable sweat capture has also been demonstrated using electrowetting valves. In one example, silver electrodes were inkjet-printed on hydrophilic polyethylene terephthalate (PET), with one surface rendered hydrophobic via a perfluorodecanethiol (PFDT) monolayer.294 The valve held fluid for up to 9 h before actuated release, allowing selective time-point sampling for biomarker analysis. Finally, a low-cost wearable patch with integrated Ag/AgCl electrodes enabled both continuous conductivity sensing and chronological sweat collection in passive capillary reservoirs. Fabricated with PET and adhesive layers, this system captured sweat into 5–10 chambers (∼50 μL each) and enabled ion chromatography-based Na+/Cl− analysis.295 However, challenges like low secretion rates and contamination from skin residues remain and highlight the need for improved interface design.
 | ||
| Fig. 6 Capillary microfluidics enable minimally invasive chrono-sampling of tears and ISF using diverse techniques integrated with wearable platforms. (a) Schematic of tear fluid absorption via Schirmer strip, a passive method for chrono-sampling.312 (b) Stepwise Schirmer strip application and removal: (i and ii) placement at lower eyelid, (iii and iv) capillary absorption, (v and vi) retrieval for downstream analysis. (c) (i–iii) Tear sampling with a capillary tube positioned at the canthus for passive fluid uptake.297 (d) Microneedle patch penetrating skin layers to access ISF.305 (e) Sonophoresis approach using low-frequency ultrasound to create transient microchannels for ISF extraction. (f) Reverse iontophoresis mechanism enabling migration of charged ISF constituents across the skin via mild electrical current. (g) Standard microneedle-based ISF sampling through skin-generated microchannels. (h) Hydrogel-based microneedle patch: (top) insertion and swelling, (middle) biomarker diffusion, (bottom) patch removal for analysis.306 (i) Continuous ISF flow enabled by porous microneedles coupled with a glucose biosensor and open capillary pump for real-time analysis.308 | ||
| S. no. | Analyte | Target biomarker | Sample volume | Concentration range | Sensing method | Number of participants | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Sweat | Glucose | 70 μL | 0 to 610 μM | Colorimetric analysis | 1 | 292 |
| 2 | Sweat | pH | 70 μL | 5 | Colorimetric analysis | 1 | 292 |
| 3 | Sweat | Cocaine-d5, benzoylecgonine-d5, ecgonine methyl ester-d5 | — | 2 ng per patch | Gas chromatographic-mass spectrometric (GC-MS) assay | 12 | 290 |
| 4 | Sweat | Cortisol | 120 μL | 25 to 125 ng ml−1 | ELISA | 11 | 19 |
| 100–1000 ng ml−1 (armpit) | |||||||
| 5 | Sweat | Na+ | 5.9 μL | 20–200 mm | Electrochemical | 6 | 288 |
| 6 | Sweat | Cl− | 5.9 μL | 20–200 mm | Colorimetric analysis | 6 | 288 |
| 7 | Sweat | Glucose | 5.9 μL | 50–200 μM | Potentiostatic | 6 | 288 |
| 8 | Sweat | pH | 5.9 μL | Colorimetric analysis | 6 | 288 | |
| 9 | Sweat | Na | >100 μL | — | Ion chromatography | 6 | 289 |
| 10 | Sweat | Cl | >100 μL | — | Ion chromatography | 6 | 289 |
| 11 | Sweat | K | >100 μL | — | Ion chromatography | 6 | 289 |
| 12 | Sweat | Bisphenol-A (BPA) | — | 195 ng ml−1 | Liquid chromatography-tandem mass spectrometry | 50 | 293 |
| 13 | Sweat | Na+ | 14 μL | 15–60 mM | Electrical impedance (ion chromatography) | 5 | 313 |
| 14 | Artificial sweat | — | 50 μL | — | Electrowetting | In vitro | 294 |
| 15 | Sweat | Na+, Cl− | 70 μL | 10–150 mM | Ion chromatography | 6 | 295 |
| 16 | Tear film | Proteins, nucleic acids, cytokines | 5–10 μL | — | BCA assay – protein | 10 | 312 |
| Proteomics – mass spectroscopy | |||||||
| Western blot – protein | |||||||
| IL-17 A – MSD immunoassay | |||||||
| RT-qPCR-gene expression | |||||||
| 17 | Tears | Protein (surfactant protein) | — | — | Bradford assay | 9 | 297 |
| Western blot – detection of surfactant protein | |||||||
| 18 | Tears | K+ | — | 0.010–0.050 M | Ionophores and chromoionophores | 1 | 299 |
| 19 | Tears | Na+ | — | 0.120–0.160 M | Ionophores and chromoionophores | 1 | 299 |
| 20 | Tears | pH | — | 6.8–7.5 | Bromophenol blue-doped sol–gel film | 1 | 299 |
| 21 | Tears | Glucose | — | — | Amperometry | 1 | 298 |
| 22 | Mice tears | Arginine, histamine, alanine, taurine, glutamate, and aspartate | 70 ± 25 nL | — | Capillary electrophoresis with light-emitting diode-induced fluorescence detection (CE-LEDIF) | 1 | 301 |
| 23 | Tears | Glucose | 6 μL | 0.1–2 mM | Colorimetric | — | 302 |
| 24 | Tears | Total protein, immunoglobulin G (IgG) | 10 μL | — | Total protein – Bradford assay | 15 | 303 |
| ELISA – IgG | |||||||
| 25 | ISF | Glucose | 80–120 mg dL−1 | Commercial blood glucose test | 1 | 304 | |
| 26 | ISF | Glucose | 16.22 μL | 0–16 mM | Colorimetric | 1 | 305 |
| 27 | ISF | Lactate | 16.22 μL | 0–3.2 mM | Colorimetric | 1 | 305 |
| 28 | ISF | Cholesterol | 16.22 μL | 0–12 mM | Colorimetric | 1 | 305 |
| 29 | ISF | pH | 16.22 μL | 5–8 | Colorimetric | 1 | 305 |
| 30 | ISF | 6-Monoacetylmorphine | 1 μL | — | Liquid chromatography-mass spectrometry | 3 | 307 |
| 31 | ISF | Glucose | 50–400 mg dL−1 | Colorimetric | Rat models, rat skin | 314 | |
| 32 | ISF | miRNA-21, miRNA-141, miRNA-155 (biomarkers for psoriasis) | 21.34 μL in 30 min | — | Fluorescence | Mouse | 315 |
| 33 | ISF | Cytokines (IL-6) | — | — | Ultrasensitive fluorescent immunoassay | Mice | 301 |
| 34 | ISF | Matricellular protein periostin | — | — | Plasmonic fluor-enhanced microneedles | Mice | 301 |
5.2 Chrono sampling and on-body analysis (CHON)
Chrono sampling with on-body analysis integrates time-resolved biospecimen collection with real-time sensing, enabling dynamic health monitoring without requiring sample removal. These platforms utilize capillary microfluidics to autonomously route biofluid to integrated sensors for in situ biochemical detection. | ||
| Fig. 7 Wearable microfluidic platforms developed for chrono-sampling and on-body analysis of sweat biomarkers. (a) Schematic of a multilayered sweat patch comprising graphics, capping, microfluidic, and skin-adhesive layers. (b) (i) Microchannels for simultaneous sweat rate and chloride sensing; (ii) on-body application with smartphone readout; (iii) sensing regions and patch boundaries.317 (c) (i) Layout for ion-selective sweat detection (Ca2+, K+, Na+); (ii) assembled patch; (iii) on-skin deployment with labeled cross-section.318 (d) Exploded view showing nutrient sensing, colorimetric, blocking, adhesive, and channel layers. (e) (i) Embedded micro-reservoir design; (ii and iii) fingertip and pill-size comparison; (iv) annotated sensing locations.321 (f) 3D paper-based evaporator with transverse/vertical fluidics, central collector, and electrode layer. (g) Operational diagram: sweat uptake, guided evaporation, and electrode-based sensing.21 (h) Schematic of electrochemical glucose sensor using Nafion, chitosan, and GOx for enzymatic detection.21 (i) (i) Exploded patch view with sweat transport, colorimetric assay (ammonia/alcohol), superabsorbent polymer retention, and adhesive layers; (ii) assay layout with reference zones; (iii) assembled patch image.327 (j) Mechanism of reference channel operation: SAP activation, capillary flow of standard solution, and calibration reaction sequence. | ||
Biodegradable microfluidics employing porous paper and capillary routing have emerged for eco-friendly hydration monitoring.322 Waterproof epidermal patches now enable real-time biomarker detection even during aquatic activity. Spectroscopy-enabled 3D-printed devices with microcuvettes offer optical precision, while ketone-sensing patches expand metabolic tracking via colorimetric sensing.323,324 To address sweat accumulation beneath sensors, a 3D paper-based electrochemical device (3D-PMED) was developed with layered microfluidics for vertical and lateral sweat flow, continuous refresh, and real-time glucose detection (Fig. 7f–h).21 Iontophoresis-assisted systems stimulate sweat secretion for consistent sampling,325 while soft microfluidic devices store sweat chronologically for retrospective analysis.7 Further innovations include potentiometric ion sensors for electrolyte monitoring,326 skin-mounted enzymatic sensors for alcohol and ammonia detection,327 and multilayer patches for colorimetric analysis with embedded standard references (Fig. 7i and j). Printable microfluidics now support simultaneous cortisol and glucose detection,328 and tattoo-based electrochemical biosensors enable discreet alcohol monitoring in real-time.329
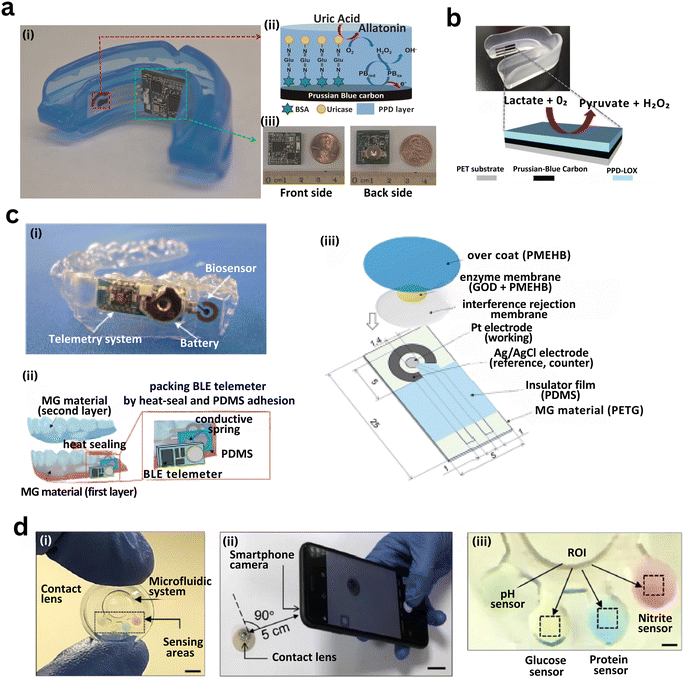 | ||
| Fig. 8 Capillary-integrated biosensing strategies have been implemented for real-time, on-body salivary and tear fluid analysis using wearable platforms such as mouthguards and smart contact lenses. (a) Wearable mouthguard biosensor for uric acid detection: (i) photograph of the mouthguard integrating a miniaturized electronic module for signal acquisition and wireless data transmission. (ii) Enzymatic detection mechanism: uricase catalyzes uric acid to allantoin, producing H2O2 detected via a Prussian blue-modified carbon electrode. (iii) Front and rear views of the fabricated mouthguard with coin scale for size comparison.331 (b) Schematic of lactate detection using a mouthguard biosensor: lactate oxidase (LOX) converts lactate to pyruvate and H2O2, which is quantified electrochemically via a Prussian blue-modified electrode.330 (c) Fully integrated wireless glucose biosensor system: (i) photograph of the assembled device including glucose sensor, BLE telemetry, and battery. (ii) Assembly steps showing PDMS encapsulation and conductive spring contacts. (iii) Exploded view of multilayer biosensor components including enzyme membrane, interference rejection layer, electrode system, insulating film, and MG substrate.333 (d) Smart contact lens with integrated microfluidic biosensors for multiplexed tear analysis: (i) lens with structured capillary microchannels and embedded biosensing zones. (ii) Illustration of smartphone-based optical readout positioned at 90° and 5 cm from the eye. (iii) Annotated sensing zones for detection of pH, glucose, protein, and nitrite biomarkers within tear fluid.334 | ||
 | ||
| Fig. 9 Wearable microneedle-based and iontophoretic platforms for chrono-sampling and on-body analysis of ISF biomarkers. (a) Microneedle biosensor: (i) schematic of Au-coated polyamide microneedles integrated with a 3D-printed holder; (ii) skin insertion showing electrode positioning; (iii) photo of fabricated biosensor.341 (b) Dual-mode EBV cfDNA patch: (i) Au@CNT microneedles on Ecoflex; (ii) point of care testing mechanism using [Ru(phen)2dppz]2+; (iii) I–V curves with/without target; (iv) EBV cfDNA separation schematic; (v) patch on forearm.342 (c) Tattoo-based dual-fluid biosensor: (i) screen-printed electrodes; (ii) iontophoretic extraction mechanism; (iii) alcohol and glucose sensing via enzymatic pathways.343 (d) 3D-printed hydrogel microneedle array: (i) fabrication process; (ii) glucose/pH sensing in skin; (iii) device photo; (iv) microneedle microscopy.344 (e) CRISPR microneedle patch: (i) graphene biointerfaces for cfDNA/dRNP capture; (ii) patch on mouse; (iii) current–time plots indicating target detection.345 (f) Integrated BP/metabolite patch: (i) device layout with agarose electrodes; (ii) on-body application; (iii) metabolite sensing via redox current.346 (g) Wireless microneedle sensor: (i) device front view; (ii) smartphone interface; (iii) microneedle array detail. (h) Exploded view of wearable system with labeled components.347 (i) Electrode configuration and device dimensions.347 | ||
| Analyte | Target biomarker | Sample volume | Concentration range | Sensing methods | On-body location | Number of participants | Ref. |
|---|---|---|---|---|---|---|---|
| Sweat | Cortisol | N/A | 0.1–100 ng mL−1 | Electrochemical | Portable | N/A | 348 |
| Sweat | Ethyl glucuronide (EtG) | N/A | 0.003–0.3 ng mL−1 | Electrochemical | Wrist | 5 | 349 |
| Sweat | Na+ | N/A | 10–200 mM | Potentiometric | Forearm | 12 | 350 |
| Sweat | Na+ | 20 μL | 10–100 mM | Potentiometric | Forearm | 10 | 319 |
| Sweat | K+ | 20 μL | N/A | Potentiometric | Forearm | 10 | 319 |
| Sweat | Glucose | 5 μL | 0–1 mM | Colorimetric | Arm | 5 | 351 |
| Sweat | Lactate | 1.6 μL | 1–20 mM | Electrochemical | Forearm | 7 | 325 |
| Sweat | Glucose | N/A | 0–1 mM | Amperometric | Arm | 10 | 352 |
| Sweat | Na+ | N/A | 10–100 mM | Colorimetric | “Glove fingers” | 3 | 353 |
| Sweat | K+ | N/A | N/A | Colorimetric | “Glove fingers” | 3 | 353 |
| Sweat | pH | N/A | N/A | Colorimetric | “Glove fingers” | 3 | 353 |
| Sweat | Lactate | N/A | 0–25 mM | Electrochemical | “Textile patch” | 6 | 354 |
| Sweat | Cl− | 30 μL | 10–100 mM | Colorimetric | Forearm | 15 | 355 |
| Sweat | Cortisol | 1.5 μL | 1–200 ng mL−1 | Colorimetric | Forearm | 10 | 328 |
| Sweat | Glucose | 1.5 μL | N/A | Colorimetric | Forearm | 10 | 328 |
| Sweat | Ketones | N/A | 0–10 mM | Colorimetric | Forearm | 5 | 324 |
| Sweat | Cl− | 25 μL | 10–100 mM | Colorimetric | “Forearm, back” | 12 | 317 |
| Sweat | Sweat rate | 1.0 μL | N/A | Colorimetric | Forearm | 8 | 322 |
| Sweat | Ammonia | N/A | 0.05–1 mM | “Enzymatic assays” | Forearm | 5 | 327 |
| Sweat | Ethanol | N/A | 0.05–1 mM | “Enzymatic assays” | Forearm | 5 | 327 |
| Sweat | Na+ | N/A | 10–100 mM | Potentiometric | Arm | 5 | 356 |
| Sweat | K+ | N/A | N/A | Potentiometric | Arm | 5 | 356 |
| Sweat | pH | N/A | N/A | Potentiometric | Arm | 5 | 356 |
| Sweat | Glucose | 0.5 μL | 0–2 mM | Electrochemical | Arm | 6 | 21 |
| Sweat | Lactate | 1.2 μL | 1–20 mM | Microfluidic | Various | 9 | 357 |
| Sweat | Cortisol | N/A | “1 pg mL−1–1 μg mL−1” | “Aptamer-FET” | Forearm | 10 | 358 |
| Sweat | Na+ | N/A | 1–100 mM | Potentiometric | Forearm | 8 | 359 |
| Sweat | K+ | N/A | N/A | Potentiometric | Forearm | 8 | 359 |
| Sweat | Glucose | 15 μL | 0–0.5 mM | Electrochemical | Arm | 12 | 360 |
| Sweat | pH | 15 μL | N/A | Electrochemical | Arm | 12 | 360 |
| Sweat | Cortisol | 5 μL | 0.01–1000 ng mL−1 | Molecularly imprinted | Arm | 7 | 320 |
| Sweat | pH | N/A | 4–8 pH units | Piezoelectric | Arm | 5 | 361 |
| Sweat | Na+ | N/A | 10–100 mM | Potentiometric | Arm | 10 | 326 |
| Sweat | Glucose | 20 μL | 0–1 mM | Amperometric | Forearm | 8 | 362 |
| Sweat | Glucose | 20 μL | 0–1 mM | Amperometric | Forearm | 8 | 362 |
| ISF | Glucose | — | 0–20 mM | Colorimetric – enzymatic | Porcine skin ex vivo (mimicking human skin) | Pig skin | 344 |
| ISF | pH | — | 5–8 | Bromothymol blue dye – colorimetric | Porcine skin ex vivo (mimicking human skin) | Pig skin | 344 |
| ISF | Lactate | 100 μL (for in vitro) | 1–5 mM | Amperometry – enzymatic biosensor | Forearm | 4 | 339 |
| ISF | Apomorphine | 100 mL (in vitro) | 5–60 μM | Chronoamperometry | Mimicked on phantom gel model | — | 340 |
| ISF | pH | Ex vivo | 4–8.6 | Potentiometry | Mouse skin | — | 341 |
| ISF | Na+ | In vivo | 10–160 mM | Gate-field effect transistor | Arm or forearm | — | 363 |
| Tear | pH | In situ | 6.5–7.6 | Colorimetric | Eye | — | 334 |
| Tear | Glucose | In situ | 0–5 mmol L−1 | Colorimetric | Eye | — | 334 |
| Tear | Protein | In situ | 3–7 μg μL−1 | Colorimetric | Eye | — | 334 |
| Tear | Nitrite ion | In situ | 120 μmol L−1 | Colorimetric | Eye | — | 334 |
| Tear | Cortisol | In situ | 1 to 40 ng ml−1 | Graphene – FET | Eye | Human and animal (rabbit) | 338 |
5.3 Continuous sampling and off-body analysis (COFF)
COFF leverages the continuous fluid acquisition capabilities of wearable systems while relying on off-body instrumentation for high-accuracy biomarker analysis. This hybrid model enables long-duration monitoring and facilitates personalized health tracking in both clinical and at-home contexts. Paper-based microfluidic systems are particularly advantageous for COFF applications due to their inherent ability to wick, store, and evaporate biofluids without pumps. Integrated with osmotic extraction, these systems can harvest and dispose of sweat over extended periods (≥10 days), using linear paper strips for capillary transport and high-surface-area pads for evaporation. Sampling is passive and sustained, and collected analytes such as sweat lactate can be quantified offline via laboratory analysis.15,295 To reduce labor and contamination risks associated with traditional absorbent patches, a simplified sweat collection system was introduced featuring an onboard conductivity sensor and multiple reservoirs with fill-level indicators. The system enabled both continuous conductivity tracking and off-body Na+ and Cl− quantification via ion chromatography.295 Despite advancements, conventional biosensors often require large sweat volumes due to limited sensitivity of bio-recognition components such as antibodies,364 aptamers,365,366 enzymes,367,368 or molecularly imprinted polymers.28 Reducing assay volumes not only accelerates response times by mitigating sweat evaporation delays but also facilitates rapid re-priming for subsequent tests. Hydrogel-paper hybrid patches have addressed this issue by coupling osmotic extraction through skin-integrated hydrogel membranes with paper-based capillary microchannels. The sweat extraction rate is tunable via osmolyte concentration and evaporation pad size, enabling controlled and sustained biomarker delivery to test zones. On-skin validation revealed successful lactate sampling at rest (11 mM in 2 h) and during exercise (20 mM in 1 h), demonstrating efficacy without inducing perspiration.369To support dynamic profiling of biomarker fluctuations, a compact wearable integrating continuous fluid sampling with in situ chemical assays was developed for simultaneous glucose and lactate tracking.370 The platform combines peristaltic nanoliter droplet generation, optical detection, and microfluidic flow control within a single patch. A screen-driven micropump circulates reagents and biofluid to an optical flow cell, enabling continuous, colorimetric readout. Clinical testing in five volunteers during an oral glucose tolerance test demonstrated strong correlation between dermal interstitial glucose and blood glucose levels, validating the system's utility for longitudinal, non-invasive diagnostics.
5.4 Continuous sampling and on-body analysis (CONN)
Continuous sampling combined with on-body analysis enables real-time monitoring of sweat biomarkers, eliminating reliance on bulky external systems and providing timely feedback for dynamic physiological and pathological conditions. This approach requires two key components: efficient and sustained sample acquisition and robust sensor integration for uninterrupted biomarker detection.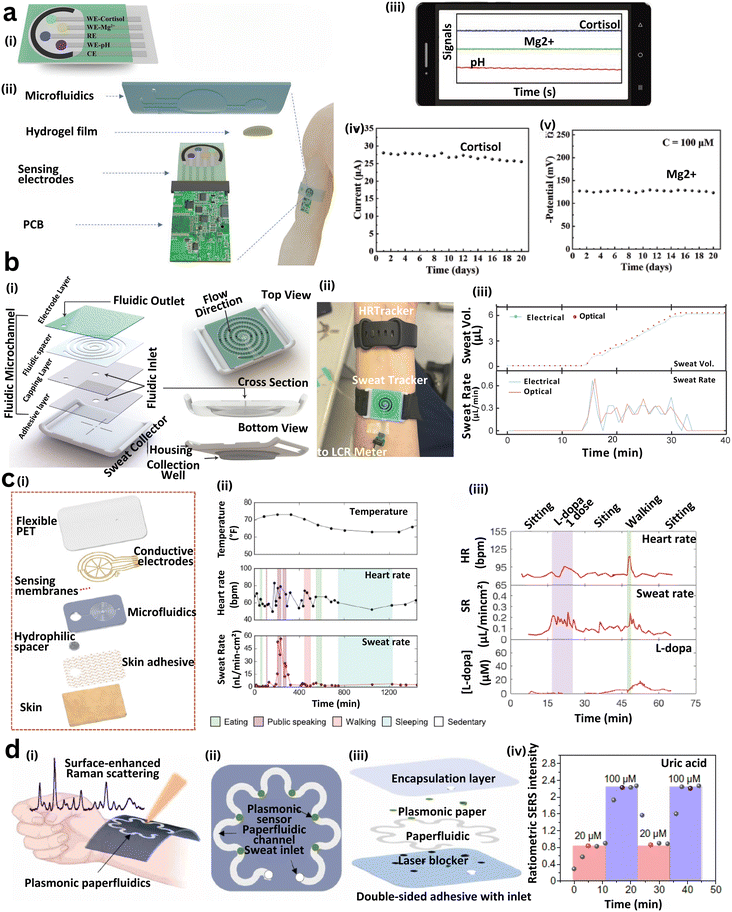 | ||
| Fig. 10 Capillary microfluidic systems integrated into wearable platforms enable continuous on-body sweat sampling and real-time multiplexed biochemical analysis. (a) Wearable microfluidic patch for simultaneous detection of cortisol, Mg2+, and pH. (i) Schematic of the skin-mounted patch showing sweat routing to sensing zones. (ii) Exploded view of layered construction, including microfluidic, sensing, and electronic components. (iii) Real-time mobile interface for monitoring biomarkers. (iv) Long-term performance of the cortisol sensor. (v) Mg2+ sensor stability during prolonged operation.371 (b) Spiral microfluidic collector system. (i) Structural views of a 3D-printed concave sweat collector guiding fluid through a spiral path. (ii) Device embedded in a digital band for sweat and heart rate tracking. (iii) Electrical and optical data showing time-resolved sweat rate and volume.375 (c) Flexible epidermal patch for physiological monitoring. (i) Multilayer assembly with PET, electrodes, hydrogel, and adhesive. (ii) Tracking of temperature, HR, and sweat rate during exertion. (iii) Comparison of HR, sweat rate, and L-dopa concentration.376 (d) Paper-based plasmonic SERS patch. (i) Schematic showing gold nanorod-enabled SERS detection of uric acid. (ii) Channel structure with sweat inlet and sensor region. (iii) Cross-section of patch architecture with PDMS encapsulation and laser protection layers. (iv) Quantified SERS signal response over time and analyte concentration.252 | ||
Understanding thermoregulatory sweat behavior is critical for evaluating hydration status and metabolic function. A PDMS-based microfluidic patch incorporating a patterned SU-8 filler and a hydrogel uptake layer was developed for continuous sweat analysis in resting conditions.376 The device integrates electrochemical sensors for pH, chloride, and levodopa, and uses an interdigitated wheel-shaped electrode array to monitor sweat flow dynamics. It enables 24-hour on-body monitoring across various body locations, capturing physiological changes in sweat composition. However, its reusability and multiplexing capacity for additional biomarkers require further investigation. To improve fluid control and storage, a three-layered microfluidic system using interconnected reservoirs and capillary burst valves was introduced.325 The patch features electrochemical sensors targeting glucose, creatinine, lactate, chloride, and pH, and is designed for continuous monitoring under exercise-induced sweating. The system maintains strong skin adhesion and leak-proof performance, with reservoir-stored samples remaining stable for up to 125 hours, facilitating delayed or ex situ biochemical analysis. Advancing multimodal sensing, a wearable patch was developed that couples biochemical and electrophysiological monitoring with microfluidic sweat handling.377 The system integrates a reduced graphene oxide-based glucose biosensor with MXene–polyvinylidene fluoride (PVDF)-based carbon nanofiber dry electrodes for electrocardiogram (ECG) recording. Wireless communication enables real-time data transmission to a smartphone app, allowing simultaneous tracking of glucose, ECG, pH, and temperature. On-body exercise trials confirmed the sensor's ability to dynamically adjust readings based on sweat composition and skin temperature, improving measurement accuracy and contextual insight.
 | ||
| Fig. 11 Overview of capillary microfluidic and microneedle-based systems for continuous glucose monitoring via ISF and their integration into wearable formats. (a) (i) Schematic representation of skin cross-section showing microneedle insertion into the dermis for direct ISF access. (ii) Microneedle-based sensor with integrated working, reference, and counter electrodes. (iii) Photograph of the assembled glucose-sensing patch. (iv) In vivo application of the patch on mouse skin demonstrating real-time monitoring.382 (b) Wearable smartwatch system incorporating a glucose sensor patch with built-in LED display, printed circuit board (PCB), and battery within the strap for standalone operation.384 (c) Electrode design on flexible polyimide substrate displaying glucose extraction, sensing, and reference components. (d) Structural composition of the sensor including glucose oxidase (GOx) membrane, Ag/AgCl reference layer, and Prussian blue mediator. (e) Enzymatic glucose sensing mechanism: glucose is catalyzed to gluconic acid and H2O2 by GOx, and the H2O2 is electrochemically reduced at the PB electrode, generating a quantifiable signal.384 | ||
Despite these advances, current ISF biosensors predominantly rely on passive capillary forces, which may limit performance under variable skin conditions. Incorporating active microfluidic strategies such as electroosmotic or vacuum-assisted extraction could enhance fluid control. Furthermore, maintaining long-term sensor calibration and stability remains a critical barrier for clinical deployment. Future directions should include integrating flexible electronics, machine learning-based analytics, and closed-loop drug delivery to enable autonomous, real-time health monitoring in wearable formats. For instance, in one study, a closed-loop system was developed by integrating a microneedle-based electrochemical sensor for real-time methotrexate (MTX) monitoring with an iontophoretic microneedle patch for on-demand transdermal drug delivery, enabling continuous feedback-based therapeutic regulation. This approach was shown to ensure precise, minimally invasive drug dosing within the therapeutic range, significantly advancing personalized treatment for conditions such as cancer and rheumatoid arthritis.385 In another example, a wearable closed-loop microneedle system was developed, in which interstitial glucose levels were continuously monitored and, upon exceeding the normal range, an ultrasonic pump was automatically activated to deliver insulin, enabling effective glycemic control (Table 5).386
| Analyte | Target biomarker | Sample volume | Concentration range | Detection methods | On-body location | Duration | Number of participants | Ref. |
|---|---|---|---|---|---|---|---|---|
| Sweat | Lactate, glucose, Cl−, pH | — | Lactate: 0–20 mM; Cl−: 10–100 mM | Colorimetric assays with smartphone image analysis | Skin (cycling) | Exercise duration | — | 7 |
| Sweat | pH, Cl−, levodopa | <5 μL | pH: 4–8; Cl−: 10–200 mM; levodopa: 1–50 μM | Electrochemical (pH, Cl−), enzymatic (levodopa) | Shoulder, chest, wrist | Up to 24 h | — | 376 |
| Sweat | Glucose | — | 0–25 mM | Electrochemical (enzyme-based) | Not specified | Not specified | — | 68 |
| Sweat | Electrolytes, metabolites | <10 μL | Na+: 10–100 mM; K+: 1–20 mM | Electrochemical (ion-selective electrodes) | Forearm | 2 h | — | 387 |
| Sweat | Cortisol, IL-6 | ∼20 μL | Cortisol: 1–100 ng mL−1; IL-6: 0.1–100 pg mL−1 | Electrochemical immunosensors | Wrist | 8 h | 15 | 371 |
| Sweat | Glucose | — | 2–20 mM | Reverse iontophoresis + electrochemical | Wrist | Continuous | 12 | 384 |
| Sweat | Lactate, EEG signals | — | Lactate: 0–25 mM | Electrochemical (lactate), electrophysiological (EEG) | In-ear | 6 h | 20 | 388 |
| Sweat | Glucose, lactate, Na+, K+, pH | 1–3 μL | Glucose: 0–0.5 mM; lactate: 0–25 mM | Electrochemical (enzyme-based), impedance (Na+/K+), potentiometric (pH) | Arm | 6 h | 10 | 377 |
| Sweat | Skin hydration, sweat loss | — | Hydration: 0–100 a.u.; sweat loss: 0–500 mL | Optical reflectance/fluorescence | Wrist | 8 h | 25 | 389 |
| Sweat | Ascorbate (vitamin C) | — | 10–500 μM | Colorimetric (natural oxidase-mimicking copper-organic frameworks) | Not specified | Not specified | — | 390 |
| Sweat | Glucose | 0.5–2 μL | 0.01–1 mM | Non-enzymatic electrochemical (Pt/MXene) | Forearm | 2 h | 8 | 373 |
| Sweat | Sweat rate, Na+, K+ | Not specified | Na+: 10–100 mM; K+: 1–20 mM | Microfluidic impedance sensing | Back | 1 h | 10 | 374 |
| Sweat | Na+, K+, pH, glucose | ∼5 μL | Na+: 10–100 mM; glucose: 0–1 mM | Plasmonic colorimetry (paper-based microfluidics) | Forearm | 3 h | 12 | 252 |
| ISF | Glucose | ∼500 nL | 2–20 mM | Electrochemical (graphene microneedles with thermos-responsive drug release) | Skin (microneedle array) | 72 h | 5 | 381 |
| Tear | Intraocular pressure (IOP) | NA | 10–50 mmHg | Capacitive sensing (wireless LC resonance circuit) | Contact lens | 24 h | 12 | 391 |
| ISF | Glucose, lactate | 1–2 μL | Glucose: 2–20 mM; lactate: 0.5–25 mM | Electrochemical (hollow microneedles with microfluidic chip) | Forearm (wearable patch) | 12 h | 8 | 347 |
| ISF | Anti-SARS-CoV-2 IgM/IgG | — | — | Paper-based lateral flow immunoassay (porous microneedles) | Skin (patch) | Single use | 25 | 392 |
| Tear | Corneal temperature | — | 30–40 °C | Thermochromic liquid crystals (colorimetric analysis) | Contact lens | 4 h | 10 | 393 |
| ISF | Glucose | 0.5–1 μL | 3–20 mM | Electrochemical (3D printed microneedle with GOx enzyme) | Arm | 48 h | 7 | 382 |
| Tear | Glucose | — | 0–2 mM | Fluorescence resonance energy transfer (FRET-based hydrogel lens) | Contact lens | 6 h | — | 378 |
| Tear | Glucose | — | 0.1–0.6 mM | Colorimetric (glucose oxidase/tetramethylbenzidine) | Contact lens | — | 20 | 54 |
| Tear | Glucose | — | 0.05–1 mM | Reflectance spectroscopy (gold nanoparticle-embedded lens) | Contact lens | 8 h | 12 | 394 |
| Tear | Glucose | — | 0–2 mM | Fluorescent nanosensors (smartphone camera detection) | Contact lens | Continuous | — | 395 |
| Saliva | Uric acid | 20–100 μL | 50–1000 μM | Amperometric (screen-printed electrode with wireless BLE) | Mouthguard | Real-time | 10 | 330 |
| Tear | Glucose | — | 0.1–1 mM | Electrochemical (wireless potentiostat with drug delivery) | Contact lens | Continuous | 6 | 337 |
6. Clinical applications consideration and clinical evaluation studies
6.1 Clinical studies
Wearable biosensing devices are transforming healthcare by enabling continuous, non-invasive monitoring of physiological and biochemical parameters. To translate their promise into clinical reality, rigorous clinical trials are essential—not only to validate device accuracy and safety, but also to understand their impact on real-world health outcomes and support regulatory approvals approval.396,397 Historically, initial evaluations of integrated wearable systems have relied on feasibility studies with fewer than 10 participants,398 which serve as proof-of-concept demonstrations. However, broader clinical acceptance requires large-scale, statistically powered studies that meet regulatory standards and ethical frameworks.199,398–400 These include validated performance metrics, safety and risk assessments, transparent informed consent processes, unbiased cohort selection, and robust data security protocols. Crucially, developers must consider whether the wearable is intended for medical-grade use or consumer wellness, as this distinction impacts the design, regulatory pathway, and required clinical evidence.To gain approval for clinical use, wearable sensors must demonstrate analytical and clinical performance comparable to existing diagnostic methods. For example, glucose sensors require detection limits of 0.06–0.11 mM for non-diabetic patients and 0.01–1 mM for diabetic patients.22 Such benchmarks necessitate both technical optimization and validation in diverse populations. A key component of clinical validation is establishing strong correlations between sweat or saliva biomarkers and reference fluids like blood or plasma, often requiring large study cohorts.358 Minimally invasive designs improve user adherence. Innovations such as skin-like flexible electronics and microneedles under 200 μm reduce discomfort while enabling biofluid access.34,401 These features are vital as biosensors are increasingly deployed on sensitive skin areas and used for extended durations.
Recent clinical studies underscore growing confidence in wearable biosensing. Baker et al.317 demonstrated large-scale clinical deployment of a microfluidic colorimetric sweat patch in over 300 athletes, monitoring sweat chloride and rate. Choi et al.402 evaluated sweat chloride dynamics in 50 individuals during physical activity, while Ray et al.403 assessed a sweat-based diagnostic sticker for cystic fibrosis across 51 participants aged 2 months to 51 years. However, broader trials involving infants and children are still needed. Clinical trials often involve moderate physical exertion, with devices placed on sweat-prone regions such as the forearm or lower back. Environmental factors—including body temperature, evaporation rates under the patch, and user movement—must be accounted for during design and interpretation.7
Beyond biomarker monitoring, wearable systems are gaining traction in neurological and chronic disease management. A smartwatch-based system for Parkinson's disease symptom tracking was evaluated in 343 participants, with a six-month longitudinal trial on 225 patients.404 Such large cohorts provide critical evidence for digital therapeutics and behavioral phenotyping. From a regulatory standpoint, wearable sensors may be classified as in vitro diagnostic devices (IVDs), with classification determined by invasiveness and intended use. Clinical validation, scalability, reproducibility, and patient benefit must be demonstrated for regulatory clearance.396 Several platforms, such as the Apple Watch and VitalPatch, have received FDA clearance for monitoring vital signs using embedded ECG sensors.405 Interestingly, industries such as fitness, cosmetics, and sports have embraced wearable biosensors more rapidly, offering a streamlined pathway to mass production and broader public acceptance. Manufacturing costs for some devices have dropped to as low as $0.50 per chip, enabling scalable production exceeding 10 million units. As more wearable platforms achieve regulatory clearance, the foundation is being laid for comprehensive clinical trials, long-term real-world evidence generation, and mainstream clinical adoption.
6.2 Regulatory requirements for wearable biosensors
To achieve clinical use, wearable biosensors must navigate structured regulatory pathways to ensure safety, performance, and reliability. In the United States, the Food and Drug Administration (FDA) classifies these devices under the Federal Food, Drug, and Cosmetic Act (FD&C Act), with most diagnostic wearables falling into Class II and requiring a 510(k) premarket notification that demonstrates substantial equivalence to an existing legally marketed device.Several devices have successfully obtained FDA clearance through this pathway. Dexcom's G6 and G7 continuous glucose monitors (CGMs) were cleared under 510(k) submissions (K182041 and K193371), as was Abbott's FreeStyle Libre 2 flash glucose monitor.406 VitalConnect's VitalPatch, which tracks ECG, heart rate, respiratory rate, and other metrics, was also cleared under 510(k) (K152139), reflecting regulatory acceptance of multi-analyte wearable biosensors.407
In contrast, novel sensing platforms often face greater regulatory hurdles. Biolinq's intradermal microneedle-based glucose sensor, which represents a departure from conventional designs, has not yet received FDA clearance and remains in clinical trials. Without a clear predicate device, it is likely to require de novo classification or even premarket approval (PMA)—both more rigorous routes requiring extensive safety and efficacy data.408
Devices incorporating high-risk features (e.g., implantable biosensors) fall under class III and must go through the PMA process. This includes clinical trials, manufacturing audits, and FDA inspections, as seen with implantable cardiac pacemakers.409
Beyond classification, all medical biosensors must comply with FDA's Quality System Regulation (21 CFR Part 820), which sets standards for design controls, risk management, and manufacturing quality.410 Biosensors containing software, wireless communication, or AI algorithms must also follow Software as a Medical Device (SaMD) guidelines. These include validation, cybersecurity safeguards (e.g., threat modeling, encryption), and compliance with HIPAA for data protection.410,411
In Europe, wearable biosensors must meet the Medical Device Regulation (MDR) and obtain CE marking for distribution in the European Economic Area. Most diagnostic devices are classified as class IIa or IIb, requiring technical documentation, risk assessments, and clinical evaluation reports (CERs).396 These submissions are assessed by Notified Bodies, adding time and complexity to approval.
Globally, ISO standards play a central role in regulatory compliance. ISO 13485:2016 specifies quality management systems for medical devices,412 while the ISO 10993 series outlines biocompatibility requirements for materials in contact with the body—covering cytotoxicity, irritation, and systemic toxicity. Devices with embedded software must follow ISO/IEC 62304, and electrical and usability standards are addressed by IEC 60601-1 and IEC 62366, respectively.
One of the primary challenges in wearable biosensor approval is demonstrating analytical and clinical validity. Devices must detect analytes accurately at physiological levels, particularly in alternative biofluids like sweat or tears where concentrations are lower and more variable than in blood. Rigorous validation is necessary to ensure consistent performance across populations and environmental conditions. Clinical trials are essential to support FDA or CE submissions. These must adhere to Good Clinical Practice (GCP), receive IRB approval, and—for significant-risk devices—comply with FDA Investigational Device Exemption (IDE) regulations (21 CFR Part 812).412 Trials should include both clinical and at-home scenarios and address safety, usability (as guided by IEC 62366), statistical rigor, and adverse event reporting.
Overall, several wearable biosensors—such as Dexcom's CGMs, FreeStyle Libre, and VitalPatch—have successfully passed through established regulatory pathways.406,407 However, next-generation platforms with novel sensing modalities face additional barriers, including lack of predicates, technical complexity, and demands for robust validation. Overcoming these challenges requires coordinated efforts across regulatory strategy, quality systems, cybersecurity, biocompatibility, and clinical design to bring safe, reliable biosensors to market.
6.3 AI and machine learning for wearable applications
Recent developments in AI and machine learning (ML) have significantly enhanced the utility of wearable biosensors, particularly in processing data from sweat, saliva, ISF, and other non-invasive fluids. AI algorithms such as support vector machines (SVM), artificial neural networks (ANN), and convolutional neural networks (CNN) have been employed to extract meaningful patterns, remove noise, and compensate for fluid variability and environmental interferences, thereby improving prediction accuracy and reliability in real-time biosensing.413–416 For instance, Moon et al.417 integrated ML into a meta-plasmonic DNA biosensor, achieving a 13-fold sensitivity enhancement through neural network-guided design. Similarly, CNNs trained on simulated data improved ultrasonic biosensor output by reducing noise 15 dB and achieving high signal-to-noise ratios in real-time protein detection.418 Other fluidics-focused platforms have incorporated LS-SVM to resolve fluorescence signal overlap in dual-analyte sweat biosensors,418 and ANN models to enhance multiplex detection of tyrosine and uric acid in sweat and saliva using molybdenum polysulfide electrodes.419Capillary microfluidic systems have also benefited from ML-assisted design optimization. Using supervised models trained on large datasets mapping channel geometry to delivery profiles, researchers achieved sub-percent fluidic prediction errors—accelerating design cycles and enhancing biosensor functionality. In colorimetric biosensing, CNNs trained on over 4600 images of microarray outputs enabled precise analysis of glucose, lactate, and pH in sweat with >91% accuracy.420 Smartphone-integrated ML platforms like “DeepLactate” further expanded accessibility, achieving >99% lactate classification accuracy in sweat using image-based deep learning.421
These examples underscore the transformative potential of ML in wearable diagnostics—especially in overcoming the unique challenges of fluid-derived biosensing such as low analyte levels, matrix complexity, and motion artifacts. Integration of ML with flexible substrates, neuromorphic edge processors, and multimodal sensing architectures is driving the next generation of autonomous, real-time, and personalized health monitoring systems.
7. Outlook and future directions
Wearable sensors and technologies hold immense potential to revolutionize health monitoring in both clinical trials and outpatient care by enabling continuous, non-invasive or minimally invasive tracking of physiological and pathological parameters. Despite advances in detecting a wide range of analytes including glucose, lactate, uric acid, tyrosine, cortisol, ethanol, pH, and electrolytes like potassium, chloride, and sodium further studies are needed to characterize their detection capabilities across both normal and disease states. While much of the existing research focuses on in vitro or early-stage validation, bridging the gap between wearable data and standard blood chemistry is essential for clinical translation.7.1 Sweat
Sweat-based biosensors are particularly attractive due to their non-invasive nature and rich biomarker content, offering a comprehensive window into an individual's health. However, several technical barriers must be overcome before these devices can be widely adopted. Current microfluidic platforms utilizing capillary circuits face challenges such as sweat contamination, poor fluid handling under variable evaporation rates, and limitations in sample transport. A key issue is the reliable, high-sensitivity detection of multiple analytes over time, particularly in low-volume and fluctuating sweat flows. A fundamental bottleneck in sweat biosensing is the need for continuous sweat production. Most current studies depend on exercise-induced sweating for 30–40 minutes, which limits usability outside of athletic contexts.19,313 For commercialization, sweat generation must be achievable at rest and they must be able to generate enough to perform sensing several times. Strategies such as localized skin heating using Peltier elements422 or iontophoresis with pilocarpine-containing hydrogels,423 have been explored, but these methods may alter analyte concentrations due to artificial stimulation. Further investigation is required to validate these techniques for clinical use. Most existing wearable systems provide single-point or short-duration measurements. To be clinically impactful, wearable devices must deliver continuous, non-invasive analyte sensing over extended periods, enabling longitudinal monitoring and integration into personalized medicine frameworks. This shift requires significant improvements in microfluidic design. Current systems based on absorbent pads and cellulose fibers are limited in their ability to channel microliter sweat volumes into sensing zones with the precision needed for multiplexed real-time analysis. Next-generation wearable platforms should incorporate capillary microfluidic circuits optimized for passive flow control, burst valve regulation, and multi-analyte integration. Further, leveraging synthetic biology and molecular engineering could expand the biosensing repertoire to include nucleic acids, bacteria, and viral targets,12 unlocking new applications in infectious disease surveillance and pandemic response. Scalability is another critical factor. Advancements in rapid prototyping such as laser cutting and roll-to-roll manufacturing combined with the use of low-cost, modular materials will be key to achieving the reproducibility and throughput needed for mass production. Fig. 12a highlights the significant challenges and solutions that must be addressed to realize the sweat based wearable biosensors as next generation health monitoring devices. By addressing these technical, biological, and manufacturing challenges, wearable biosensors can evolve from proof-of-concept tools to clinically robust systems capable of reshaping diagnostics, chronic disease management, and precision healthcare.7.2 Saliva
One of the primary challenges in developing saliva-based wearable devices lies in the significantly lower concentration of biomarkers compared to blood. Several analytes are present in saliva at levels up to 1000-fold lower than in blood, necessitating highly sensitive detection methods to achieve clinically meaningful results.424 This vast concentration disparity poses a substantial obstacle for accurate quantification, especially within the constrained form factors of wearable platforms. While modern analytical technologies can now detect biomolecules at picogram-per-milliliter concentrations, translating this sensitivity into compact, energy-efficient, and cost-effective wearable devices remains technically demanding.425 Another key barrier is the lack of standardized saliva collection protocols, which compromises measurement reproducibility and inter-study comparability. Saliva sampling is influenced by numerous factors, including circadian rhythm, oral hygiene, hydration status, recent food intake, and the method used to stimulate glandular secretion.424 Even with commercial collection devices and predefined protocols, inconsistencies persist. Standard practices often involve mouth rinsing, absorbent pad placement, time-controlled collection, membrane filtration, and tube-based transfer.426 Each step introduces variability and contamination risks, which can distort biomarker levels and affect downstream analysis. Consequently, there is a pressing need for unified, clinically validated collection procedures to support reliable salivary biomarker sensing in wearable applications.Microfluidic integration further complicates saliva-based wearables due to the unique rheological characteristics of saliva. Its variable viscosity and heterogeneous molecular composition often result in microchannel clogging, erratic flow, and uneven delivery to detection zones.427 Moreover, salivary mucins and proteins readily adsorb onto microchannel surfaces, altering fluid dynamics and compromising sensor performance.425 These effects demand microfluidic designs that are resilient to biofouling, ensure steady flow, and deliver precise sample volumes to sensor interfaces.
Electrochemical biosensors, a common modality in wearables, face additional complications due to the presence of numerous electroactive species in saliva. These endogenous molecules can interfere with detection signals, necessitating the use of selective membranes or advanced filtering strategies.428 However, even conventional filtration techniques such as using 0.22 μm or 0.45 μm filters have been shown to disrupt sample composition, particularly by removing extracellular vesicles that carry critical diagnostic information.429 Transitioning from laboratory-scale prototypes to commercially viable saliva-based wearables are further hindered by operational complexity, cost, and regulatory challenges. Most current systems require supervised operation, involve multi-step protocols, and demand user training.428 The absence of inter-laboratory standardization adds to the difficulty of clinical validation and regulatory approval.424 Additionally, the high manufacturing costs of sensitive biosensors and microfluidic systems can make commercial pricing untenable. These barriers collectively explain why, despite significant scientific progress, only a limited number of saliva-based wearable platforms have reached the market. Fig. 12b highlights the significant challenges and solutions that must be addressed for saliva-based wearables. Overcoming these limitations will require innovations in ultra-sensitive detection, standardized sample handling, biofouling-resistant microfluidics, and scalable manufacturing processes.
7.3 Tears
Current tear collection strategies include flexible epidermal microfluidic patches placed beneath the eye and contact lens-based platforms that interface directly with the ocular surface.430 While these approaches provide promising routes for tear-based biosensing, both face significant challenges in consistently collecting sufficient tear volumes without compromising ocular health or user comfort particularly in the context of continuous, long-term monitoring. Microfluidic channels for such applications must be precisely engineered at microscale dimensions to accommodate the inherently limited tear volume, minimize evaporation, and ensure accurate sample delivery to sensing regions.431 The ocular environment imposes strict requirements for biocompatibility, mechanical compliance, and transparency. Contact lens-based sensors must simultaneously preserve essential characteristics such as oxygen permeability and optical clarity while incorporating functional microfluidic and sensing components.430 These concurrent demands constrain material choices and complicate device design. Even slight deviations from baseline comfort can stimulate reflex tearing, diluting tear analytes and introducing variability that affects diagnostic reliability. Thus, sustained biocompatibility, particularly for devices intended for prolonged wear, remains a central concern.Tear composition is inherently dynamic, subject to fluctuations driven by environmental exposure, emotional responses, circadian rhythms, and other physiological variables. These factors introduce significant variability in biomarker levels, necessitating advanced signal processing methods to differentiate between pathophysiological signals and normal physiological noise.431 Additionally, tear fluid contains proteins, lipids, and electrolytes that can interfere with sensor readouts, requiring selective sensing strategies and surface modifications to improve detection fidelity. To address these complexities, emerging tear biosensing systems are integrating machine learning and AI algorithms—such as deep neural networks—to correct for pH shifts, ambient light conditions, and colorimetric response variability.431 Despite their considerable potential, tear-based wearable biosensors face significant commercialization hurdles, including regulatory, manufacturing, and end-user challenges. Ocular devices are subject to stringent oversight due to their proximity to sensitive tissues, lengthening development cycles and increasing compliance costs. On the manufacturing front, producing microscales, transparent, and biocompatible devices with integrated sensing and communication capabilities poses non-trivial fabrication challenges. Moreover, user adoption is heavily dependent on achieving contact lens-level comfort while delivering reliable, actionable health insights. Fig. 12c highlights the significant challenges and corresponding solutions that need to be addressed, which help explain the limited commercial availability of tear-based wearables, despite increasing research interest and technological progress in this field.
7.4 ISF
ISF offers a promising biofluid for continuous health monitoring; however, it presents intrinsic volume limitations that severely constrain its usability in wearable diagnostics. In even the most ISF-rich regions of the dermis, only about 120 μL of ISF is present per cm2 of skin, and most current extraction methods can yield only 1–10 μL at a time.432 Compounding the issue, ISF replenishment occurs at extremely low rates typically 5 to 50 nL min−1 cm−2 making continuous or high-frequency sampling particularly challenging.41 These low volumes necessitate the development of ultra-sensitive detection technologies and impose strict limitations on sensor design, microfluidic handling, and device longevity. Extraction methods for ISF face a difficult trade-off between invasiveness and sample fidelity. Traditional approaches such as suction blisters, reverse iontophoresis, sonophoresis, micro dialysis, and thermal ablation often cause significant tissue disruption or inflammatory responses that can in turn alter the biomarker landscape of ISF itself.306 Although microneedles are widely regarded as a minimally invasive alternative, they still penetrate the skin and may induce localized inflammation, which can distort analyte concentrations for hours post-insertion complicating longitudinal monitoring and undermining data reliability. Microfluidic integration with ISF extraction technologies introduces a distinct set of engineering and physiological challenges. Channels must be optimized to accommodate nanoliter-scale flow rates while preventing analyte degradation from adsorption or evaporation, and avoiding blockage caused by biofouling or particulate matter.41,432 Furthermore, fluidic interfaces between extraction sites and microchannels must minimize dead volume and transfer delays, while maintaining mechanical flexibility to withstand skin deformation and movement without compromising performance. One critical issue often overlooked is analyte distortion due to filtration effects during forced ISF extraction. When pressure-based techniques such as vacuum or iontophoresis are used, small molecules like water and electrolytes pass through tissue barriers more readily than larger biomarkers. This results in sample compositions that may no longer reflect true ISF physiology.41,432 For example, vacuum methods can extract fluid at rates up to 5 μL min−1 cm−2, but the resulting samples exhibit significant deviations from serum composition, particularly for proteins and larger macromolecules. Such filtration artifacts necessitate complex calibration strategies or correction algorithms to restore measurement accuracy. Sensor integration in ISF platforms poses further hurdles. Devices must be capable of operating reliably with minimal sample volumes, all while compensating for variability in extraction efficiency and analyte distortion.In long-term applications, biofouling and foreign body responses often degrade signal quality and sensor accuracy. Although surface coatings and anti-fouling strategies have shown short-term efficacy, ensuring stable performance over days or weeks remains an unresolved challenge in most ISF-based wearable systems.41,432 From a commercialization standpoint, ISF-based wearables face high barriers. These include the manufacturing complexity of miniaturized extraction and sensing components, as well as stringent regulatory hurdles tied to their minimally invasive nature. Compared to non-invasive sweat or saliva-based systems, ISF wearables typically require more rigorous clinical validation and longer approval timelines. Fig. 12 outlines the key challenges and proposed solutions for ISF-based wearable devices. In addition to technical hurdles, issues related to user comfort, skin puncture, and the risk of irritation or visible marks significantly influence consumer acceptance. Together, these factors help explain the limited commercial adoption of ISF-based monitors, despite their strong potential in targeted applications such as continuous glucose monitoring.41
7.5 Commercial landscape and translation outlook: wearable biosensors
From needle-free glucose sensors to sweat-based hydration patches, a new generation of wearable biosensing technologies is reshaping personalized healthcare. This section highlights key innovators and commercially promising platforms across different biofluids. Biolinq, a San Diego-based startup, is advancing a next-generation intradermal biosensor platform for continuous metabolic monitoring, with a focus on non-invasive, needle-free glucose sensing.433 Its skin-conformal patch contains microscale electrochemical sensors that continuously measure glucose beneath the skin, while also tracking metrics such as activity and sleep. The device uses a user-friendly, color-coded interface that eliminates the need for external readers. Currently undergoing pivotal clinical trials in the U.S., Biolinq is preparing for FDA submission and commercial release. VitalConnect, based in San Jose, offers the VitalPatch RTM®, a single-lead, wearable biosensor patch that continuously monitors up to 11 vital signs, including ECG, heart rate, respiratory rate, skin temperature, posture, activity, and fall detection.405 FDA 510(k)-cleared and deployed in both inpatient and outpatient settings, VitalPatch has demonstrated strong clinical utility and market adoption.Profusa has developed a novel class of tissue-integrating biosensors composed of flexible hydrogel fibers implanted beneath the skin. These sensors monitor key analytes like oxygen and, in the future, aim to track glucose, lactate, and ions through a wearable optical reader linked to a smartphone.434 Profusa's Lumee Oxygen Platform™ is already CE-marked in Europe and under FDA investigation in the U.S., with its minimally invasive, tissue-compatible design holding promise for long-term biochemical monitoring. Allez Health contributes to the CGM ecosystem with a next-generation wearable sensor designed for 15-day use.435 Using a minimally invasive electrochemical filament, Allez's CGM system offers real-time glucose monitoring with medical-grade accuracy and smartphone connectivity. The system is currently under FDA investigation and backed by funding to support clinical trials and manufacturing scale-up, with a focus on affordability and access. Dexcom, a market leader in CGM, offers a robust suite of FDA-cleared systems including the G6, G7, and the recently launched Stelo—an over-the-counter CGM with a 15-day wear period.436 Dexcom sensors use a subcutaneous electrochemical filament to measure interstitial glucose every minute, transmitting data wirelessly to mobile devices or receivers. With wide clinical adoption, Dexcom sets the benchmark for CGM performance and integration.
Epicore Biosystems is leading innovations in sweat-based biosensing, with several platforms tailored to athletic, industrial, and clinical use cases.437 The Gx Sweat Patch, developed in partnership with Gatorade, leverages soft microfluidics to monitor fluid and electrolyte loss in athletes, offering real-time feedback via a connected app. The Discovery Patch is FDA-registered for remote sweat collection in clinical trials, while the Connected Hydration system targets industrial workers, tracking sweat rate, electrolyte loss, and skin temperature to mitigate heat stress. With over $32m in funding and industry collaborations (e.g., DuPont, Chevron), Epicore is a recognized leader in sweat biosensing. Nutromics offers a minimally invasive platform called the Lab on a Patch®, which uses DNA aptamer-functionalized microneedles to continuously monitor biomarkers in interstitial fluid.438 The coin-sized wearable has demonstrated real-time vancomycin tracking in clinical trials. Based on electrochemical aptamer sensing, the platform enables multiplex monitoring of drug levels, organ function, and inflammatory markers. Although regulatory approval is pending, Nutromics' approach holds significant promise for precision medicine applications such as therapeutic drug monitoring and sepsis detection.
These companies illustrate the diversity and momentum of the wearable biosensor ecosystem. Their platforms span multiple biofluids, sensing modalities, and clinical use cases—from metabolic tracking and hydration monitoring to drug dosing and remote patient care. Looking forward, the convergence of advanced microfluidics, stretchable electronics, and AI-driven analytics is expected to propel the next generation of smart biosensors. Future systems will likely integrate multimodal sensing, closed-loop feedback, and secure wireless transmission, enabling continuous, autonomous, and predictive health monitoring across both clinical and consumer domains.
Conclusion
The integration of wearable biosensing technologies with microfluidic platforms is rapidly advancing the capabilities of non-invasive health monitoring. By enabling continuous, real-time analysis of biomarkers in sweat, saliva, tears, and ISF, these systems present a compelling alternative to conventional diagnostic modalities. Each biofluid offers specific advantages—such as the non-invasiveness of sweat and saliva or the close correlation of ISF with systemic biomarkers—yet poses distinct challenges in terms of sampling volume, analyte stability, and device integration. Recent innovations in soft materials, microfabrication techniques, capillary and active microfluidics, and biochemical sensing have collectively improved the feasibility of chrono-sampling and multiplexed analysis in wearable formats. Nevertheless, widespread clinical translation is impeded by several critical limitations, including insufficient fluid volumes (particularly in ISF and tears), variability in biofluid composition, limited long-term sensor stability, and lack of standardized sampling protocols. Furthermore, achieving continuous sampling at rest, addressing signal drift over time, and ensuring compatibility with dynamic skin movements remain non-trivial. To advance the field, future efforts should prioritize the integration of adaptive microfluidic interfaces, robust anti-fouling sensor chemistries, and machine learning-enabled data processing to enhance sensitivity, specificity, and interpretability. Rigorous clinical validation through well-designed cohort studies and alignment with regulatory standards will also be essential to facilitate real-world implementation. Overall, wearable microfluidic biosensors represent a transformative class of analytical platforms that may redefine point-of-care diagnostics, personalized medicine, and digital health. Continued interdisciplinary collaboration will be key to overcoming current limitations and unlocking their full diagnostic and therapeutic potential.Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Mitacs Canada and Canada Research Chairs for funding this work and the University of Calgary for their support.References
- H. C. Ates, P. Q. Nguyen, L. Gonzalez-Macia, E. Morales-Narváez, F. Güder, J. J. Collins and C. Dincer, Nat. Rev. Mater., 2022, 7, 887–907 CrossRef PubMed.
- J. Kim, A. S. Campbell, B. E.-F. De Ávila and J. Wang, Nat. Biotechnol., 2019, 37, 389–406 CrossRef CAS PubMed.
- S. M. Mugo, W. Lu, M. Wood and S. Lemieux, Electrochem. Sci. Adv., 2022, 2, e2100039 CrossRef CAS.
- M. Smuck, C. A. Odonkor, J. K. Wilt, N. Schmidt and M. A. Swiernik, npj Digit. Med., 2021, 4, 45 CrossRef PubMed.
- J. Choi, D. Kang, S. Han, S. B. Kim and J. A. Rogers, Adv. Healthcare Mater., 2017, 6, 1601355 CrossRef PubMed.
- R. Ghaffari, J. Choi, M. S. Raj, S. Chen, S. P. Lee, J. T. Reeder, A. J. Aranyosi, A. Leech, W. Li, S. Schon, J. B. Model and J. A. Rogers, Adv. Funct. Mater., 2020, 30, 1907269 CrossRef CAS.
- A. Koh, D. Kang, Y. Xue, S. Lee, R. M. Pielak, J. Kim, T. Hwang, S. Min, A. Banks, P. Bastien, M. C. Manco, L. Wang, K. R. Ammann, K.-I. Jang, P. Won, S. Han, R. Ghaffari, U. Paik, M. J. Slepian, G. Balooch, Y. Huang and J. A. Rogers, Sci. Transl. Med., 2016, 8(366), 366ra165 Search PubMed.
- L. Tai, W. Gao, M. Chao, M. Bariya, Q. P. Ngo, Z. Shahpar, H. Y. Y. Nyein, H. Park, J. Sun, Y. Jung, E. Wu, H. M. Fahad, D. Lien, H. Ota, G. Cho and A. Javey, Adv. Mater., 2018, 30, 1707442 CrossRef PubMed.
- M. Wang, Y. Yang, J. Min, Y. Song, J. Tu, D. Mukasa, C. Ye, C. Xu, N. Heflin, J. S. McCune, T. K. Hsiai, Z. Li and W. Gao, Nat. Biomed. Eng., 2022, 6, 1225–1235 CrossRef CAS PubMed.
- S. Imani, A. J. Bandodkar, A. M. V. Mohan, R. Kumar, S. Yu, J. Wang and P. P. Mercier, Nat. Commun., 2016, 7, 11650 CrossRef CAS PubMed.
- Y. Yang, Y. Song, X. Bo, J. Min, O. S. Pak, L. Zhu, M. Wang, J. Tu, A. Kogan, H. Zhang, T. K. Hsiai, Z. Li and W. Gao, Nat. Biotechnol., 2020, 38, 217–224 CrossRef CAS PubMed.
- P. Q. Nguyen, L. R. Soenksen, N. M. Donghia, N. M. Angenent-Mari, H. De Puig, A. Huang, R. Lee, S. Slomovic, T. Galbersanini, G. Lansberry, H. M. Sallum, E. M. Zhao, J. B. Niemi and J. J. Collins, Nat. Biotechnol., 2021, 39, 1366–1374 CrossRef CAS PubMed.
- A. Olanrewaju, M. Beaugrand, M. Yafia and D. Juncker, Lab Chip, 2018, 18, 2323–2347 RSC.
- R. Safavieh and D. Juncker, Lab Chip, 2013, 13, 4180 RSC.
- T. Shay, T. Saha, M. D. Dickey and O. D. Velev, Biomicrofluidics, 2020, 14, 034112 CrossRef CAS PubMed.
- J. Guerrero, Y. Chang, A. A. Fragkopoulos and A. Fernandez-Nieves, Small, 2020, 16, 1904344 CrossRef CAS PubMed.
- J. Songok, M. Tuominen, H. Teisala, J. Haapanen, J. Mäkelä, J. Kuusipalo and M. Toivakka, ACS Appl. Mater. Interfaces, 2014, 6, 20060–20066 CrossRef CAS PubMed.
- S. Hassan, A. Tariq, Z. Noreen, A. Donia, S. Z. J. Zaidi, H. Bokhari and X. Zhang, Diagnostics, 2020, 10, 509 CrossRef CAS PubMed.
- S. Shajari, R. Salahandish, A. Zare, M. Hassani, S. Moossavi, E. Munro, R. Rashid, D. Rosenegger, J. S. Bains and A. Sanati Nezhad, Adv. Sci., 2023, 10, 2204171 CrossRef CAS PubMed.
- T. Abbasiasl, F. Mirlou, E. Istif, H. Ceylan Koydemir and L. Beker, Sens. Diagn., 2022, 1, 775–786 RSC.
- Q. Cao, B. Liang, T. Tu, J. Wei, L. Fang and X. Ye, RSC Adv., 2019, 9, 5674–5681 RSC.
- L. Zheng, Y. Liu and C. Zhang, Sens. Actuators, B, 2021, 343, 130131 CrossRef CAS.
- E. VanArsdale, J. Pitzer, G. F. Payne and W. E. Bentley, iScience, 2020, 23, 101545 CrossRef CAS PubMed.
- K. Manibalan, P. Arul, H.-J. Wu, S.-T. Huang and V. Mani, ACS Meas. Sci. Au, 2024, 4, 163–183 CrossRef CAS PubMed.
- G. Wu, E. T. Zhang, Y. Qiang, C. Esmonde, X. Chen, Z. Wei, Y. Song, X. Zhang, M. J. Schneider, H. Li, H. Sun, Z. Weng, S. Santaniello, J. He, R. Y. Lai, Y. Li, M. R. Bruchas and Y. Zhang, bioRxiv, 2023, preprint, DOI:10.1101/2023.10.18.562080.
- J. Kim, R. Kumar, A. J. Bandodkar and J. Wang, Adv. Electron. Mater., 2017, 3, 1600260 CrossRef.
- FDA extends use of implantable CGM sensor to 6 months, https://www.healio.com/news/endocrinology/20220211/fda-extends-use-of-implantable-cgm-sensor-to-6-months, (accessed March 25, 2025).
- Q. Zhang, D. Jiang, C. Xu, Y. Ge, X. Liu, Q. Wei, L. Huang, X. Ren, C. Wang and Y. Wang, Sens. Actuators, B, 2020, 320, 128325 CrossRef CAS.
- K. Spychalska, D. Zając, S. Baluta, K. Halicka and J. Cabaj, Polymers, 2020, 12, 1154 CrossRef CAS PubMed.
- B. Babamiri, M. Farrokhnia, M. Mohammadi and A. Sanati Nezhad, Sci. Rep., 2025, 15, 8859 CrossRef CAS PubMed.
- F. Lopes, J. G. Pacheco, P. Rebelo and C. Delerue-Matos, Sens. Actuators, B, 2017, 243, 745–752 CrossRef CAS.
- O. S. Ahmad, T. S. Bedwell, C. Esen, A. Garcia-Cruz and S. A. Piletsky, Trends Biotechnol., 2019, 37, 294–309 CrossRef CAS PubMed.
- Y. Li, L. Zhang, Y. Dang, Z. Chen, R. Zhang, Y. Li and B.-C. Ye, Biosens. Bioelectron., 2019, 127, 207–214 CrossRef CAS PubMed.
- H. Park, W. Park and C. H. Lee, NPG Asia Mater., 2021, 13, 23 CrossRef CAS.
- M. Padash, C. Enz and S. Carrara, Sensors, 2020, 20, 4236 CrossRef CAS PubMed.
- N. Brasier, J. Wang, W. Gao, J. R. Sempionatto, C. Dincer, H. C. Ates, F. Güder, S. Olenik, I. Schauwecker, D. Schaffarczyk, E. Vayena, N. Ritz, M. Weisser, S. Mtenga, R. Ghaffari, J. A. Rogers and J. Goldhahn, Nature, 2024, 636, 57–68 CrossRef CAS PubMed.
- A. Childs, B. Mayol, J. A. Lasalde-Ramírez, Y. Song, J. R. Sempionatto and W. Gao, ACS Nano, 2024, 18, 24605–24616 CrossRef CAS PubMed.
- N. Davis, J. Heikenfeld, C. Milla and A. Javey, Nat. Biotechnol., 2024, 42, 860–871 CrossRef CAS PubMed.
- R. F. R. Ursem, A. Steijlen, M. Parrilla, J. Bastemeijer, A. Bossche and K. De Wael, Lab Chip, 2025, 25, 1296–1315 RSC.
- A. A. Smith, R. Li and Z. T. H. Tse, Sci. Rep., 2023, 13, 4998 CrossRef CAS PubMed.
- J. Heikenfeld, A. Jajack, J. Rogers, P. Gutruf, L. Tian, T. Pan, R. Li, M. Khine, J. Kim, J. Wang and J. Kim, Lab Chip, 2018, 18, 217–248 RSC.
- J. Min, J. Tu, C. Xu, H. Lukas, S. Shin, Y. Yang, S. A. Solomon, D. Mukasa and W. Gao, Chem. Rev., 2023, 123, 5049–5138 CrossRef CAS PubMed.
- F. Gao, C. Liu, L. Zhang, T. Liu, Z. Wang, Z. Song, H. Cai, Z. Fang, J. Chen, J. Wang, M. Han, J. Wang, K. Lin, R. Wang, M. Li, Q. Mei, X. Ma, S. Liang, G. Gou and N. Xue, Microsyst. Nanoeng., 2023, 9, 1 CrossRef PubMed.
- B. Lindsay, D. M. Engel and A. S. Wolfe, Gatorade Sports Science Institute, 2022.
- B. A. Katchman, M. Zhu, J. Blain Christen and K. S. Anderson, Proteomics: Clin. Appl., 2018, 12(6), 1800010 Search PubMed.
- L. Lyzwinski, M. Elgendi, A. V. Shokurov, T. J. Cuthbert, C. Ahmadizadeh and C. Menon, Commun. Eng., 2023, 2, 48 CrossRef.
- N. Brasier and J. Eckstein, Digit. Biomark, 2019, 3, 155–165 CrossRef PubMed.
- P. Pandit, B. Crewther, C. Cook, C. Punyadeera and A. K. Pandey, Mater. Adv., 2024, 5, 5339–5350 RSC.
- M. Song, H. Bai, P. Zhang, X. Zhou and B. Ying, Int. J. Oral Sci., 2023, 15, 2 CrossRef CAS PubMed.
- A. K. Ghosh, A. Nath, E. Elangovan, A. Banerjee, K. Ramalingam and S. Sethuraman, Cureus, 2024, 16, e65725 Search PubMed.
- S. Williamson, C. Munro, R. Pickler, M. J. Grap and R. K. Elswick, Nurs. Res. Pract., 2012, 2012, 246178 Search PubMed.
- M. Elgendi, L. Lyzwinski, E. Kübler, A. V. Shokurov, N. Howard and C. Menon, npj Biosensing, 2024, 1, 1–10 CrossRef.
- L. Anchidin-Norocel, W. K. Savage, A. Nemţoi and C. Cobuz, Chemosensors, 2024, 12(12), 269 CrossRef CAS.
- F. J. Ascaso and V. Huerva, Optom. Vis. Sci., 2016, 93, 426–434 CrossRef PubMed.
- W. Park, H. Seo, J. Kim, Y.-M. Hong, H. Song, B. J. Joo, S. Kim, E. Kim, C.-G. Yae, J. Kim, J. Jin, J. Kim, Y. Lee, J. Kim, H. K. Kim and J.-U. Park, Nat. Commun., 2024, 15, 2828 CrossRef CAS PubMed.
- A. Rajan, J. Vishnu and B. Shankar, Biosensors, 2024, 14, 483 CrossRef CAS PubMed.
- N. L. Kazanskiy, S. N. Khonina and M. A. Butt, Biosensors, 2023, 13, 933 CrossRef PubMed.
- H. Haslene-Hox, E. Oveland, K. C. Berg, O. Kolmannskog, K. Woie, H. B. Salvesen, O. Tenstad and H. Wiig, PLoS One, 2011, 6, e19217 CrossRef CAS PubMed.
- P. P. Samant, M. M. Niedzwiecki, N. Raviele, V. Tran, J. Mena-Lapaix, D. I. Walker, E. I. Felner, D. P. Jones, G. W. Miller and M. R. Prausnitz, Sci. Transl. Med., 2020, 12, eaaw0285 CrossRef CAS PubMed.
- A. Oharazawa, G. Maimaituxun, K. Watanabe, T. Nishiyasu and N. Fujii, J. Dermatol. Sci., 2024, 114, 141–147 CrossRef CAS PubMed.
- Y. Kim and M. R. Prausnitz, Nat. Biomed. Eng., 2021, 5, 3–5 CrossRef CAS PubMed.
- Z. Wu, Z. Qiao, S. Chen, S. Fan, Y. Liu, J. Qi and C. T. Lim, Commun. Mater., 2024, 5, 1–15 CrossRef CAS.
- P. A. Kusov, Y. V. Kotelevtsev and V. P. Drachev, Molecules, 2023, 28, 2353 CrossRef CAS PubMed.
- C.-E. Karachaliou, G. Koukouvinos, D. Goustouridis, I. Raptis, S. Kakabakos, P. Petrou and E. Livaniou, Biosensors, 2023, 13, 285 CrossRef CAS PubMed.
- M. Jia, W. M. Chew, Y. Feinstein, P. Skeath and E. M. Sternberg, Analyst, 2016, 141, 2053–2060 RSC.
- V. Vignesh, B. Castro-Dominguez, T. D. James, J. M. Gamble-Turner, S. Lightman and N. M. Reis, ACS Sens., 2024, 9, 1666–1681 CrossRef CAS PubMed.
- M. Imamovic, N. Bäcklund, S. Lundstedt, G. Brattsand, E. Aardal, T. Olsson and P. Dahlqvist, Endocr. Connect., 2022, 12(1), e220324 Search PubMed.
- L. Johnston, G. Wang, K. Hu, C. Qian and G. Liu, Front. Bioeng. Biotechnol., 2021, 9, 733810 CrossRef PubMed.
- J. Giaretta, R. Zulli, T. Prabhakar, R. J. Rath, S. Naficy, S. Spilimbergo, P. S. Weiss, S. Farajikhah and F. Dehghani, Adv. Sens. Res., 2024, 3, 2400065 CrossRef CAS.
- T. I. L. Chan, Y. W. Y. Yip, T. T. C. Man, C. P. Pang and M. E. Brelén, Transl. Vis. Sci. Technol., 2022, 11, 3 CrossRef PubMed.
- S. Dobashi, D. Funabashi, K. Sameshima, N. Tsuruoka and T. Matsui, bioRxiv, 2023, preprint, DOI:10.1101/2023.10.23.563585.
- T.-T. Luo, Z.-H. Sun, C.-X. Li, J.-L. Feng, Z.-X. Xiao and W.-D. Li, J. Physiol. Sci., 2021, 71, 26 CrossRef CAS PubMed.
- X. Xuan, C. Pérez-Ràfols, C. Chen, M. Cuartero and G. A. Crespo, ACS Sens., 2021, 6, 2763–2771 CrossRef CAS PubMed.
- A.-M. Spehar-Délèze, S. Anastasova and P. Vadgama, Chemosensors, 2021, 9, 195 CrossRef.
- L. B. Baker, Sports Med., 2017, 47, 111–128 CrossRef PubMed.
- L. B. Baker, P. J. D. De Chavez, R. P. Nuccio, S. D. Brown, M. A. King, B. C. Sopeña and K. A. Barnes, J. Appl. Physiol., 2022, 133, 1250–1259 CrossRef CAS PubMed.
- P. Pampani, S. Shenoy, R. V. Anegundi, M. K. Shekar, K. Dharani and L. Fathima, J. Indian Soc. Periodontol., 2024, 28, 569–574 CrossRef PubMed.
- I. M. Thowsen, T. V. Karlsen, E. Nikpey, H. Haslene-Hox, T. Skogstrand, G. J. Randolph, B. H. Zinselmeyer, O. Tenstad and H. Wiig, J. Physiol., 2022, 600, 2293–2309 CrossRef CAS PubMed.
- N. I. Dmitrieva, D. Liu, C. O. Wu and M. Boehm, Eur. Heart J., 2022, 43, 3335–3348 CrossRef CAS PubMed.
- D. Vairo, L. Bruzzese, M. Marlinge, L. Fuster, N. Adjriou, N. Kipson, P. Brunet, J. Cautela, Y. Jammes, G. Mottola, S. Burtey, J. Ruf, R. Guieu and E. Fenouillet, Sci. Rep., 2017, 7, 11801 CrossRef PubMed.
- B. Kallapur, K. Ramalingam, Bastian, A. Mujib, A. Sarkar and S. Sethuraman, J. Nat. Sci., Biol. Med., 2013, 4, 341–345 CrossRef PubMed.
- Body Fluids and Fluid Compartments | Anatomy and Physiology II, https://courses.lumenlearning.com/suny-ap2/chapter/body-fluids-and-fluid-compartments-no-content/, (accessed June 30, 2025).
- Office of Dietary Supplements - Potassium, https://ods.od.nih.gov/factsheets/Potassium-HealthProfessional/, (accessed June 30, 2025).
- A. G. Faria, F. A. L. Marson, C. C. S. Gomez, M. de F. Servidoni, A. F. Ribeiro and J. D. Ribeiro, Front. Pediatr., 2017, 5, 222 CrossRef PubMed.
- A. C. Gonçalves, F. A. de L. Marson, R. M. de H. Mendonça, J. D. Ribeiro, A. F. Ribeiro, I. A. Paschoal and C. E. Levy, Diagn. Pathol., 2013, 8, 46 CrossRef PubMed.
- A. C. Gonçalves, F. A. L. Marson, R. M. H. Mendonça, C. S. Bertuzzo, I. A. Paschoal, J. D. Ribeiro, A. F. Ribeiro and C. E. Levy, J. Pediatr., 2019, 95, 443–450 CrossRef PubMed.
- S. K. Raut, K. Singh, S. Sanghvi, V. Loyo-Celis, L. Varghese, E. R. Singh, S. Gururaja Rao and H. Singh, Biosci. Rep., 2024, 44, BSR20240029 CrossRef CAS PubMed.
- N. Sagar and S. Lohiya, Cureus, 2024, 16, e55625 Search PubMed.
- C.-T. Huang, M.-L. Chen, L.-L. Huang and I.-F. Mao, Chin. J. Physiol., 2002, 45, 109–115 CAS.
- A. Jaiswal, S. Madaan, N. Acharya, S. Kumar, D. Talwar and D. Dewani, Cureus, 2021, 13, e19649 Search PubMed.
- L. Du, Y. Zong, H. Li, Q. Wang, L. Xie, B. Yang, Y. Pang, C. Zhang, Z. Zhong and J. Gao, Signal Transduction Targeted Ther., 2024, 9, 212 CrossRef CAS PubMed.
- J. Maiuolo, F. Oppedisano, S. Gratteri, C. Muscoli and V. Mollace, Int. J. Cardiol., 2016, 213, 8–14 CrossRef PubMed.
- C. O. Page and J. S. Remington, J. Lab. Clin. Med., 1967, 69, 634–650 CAS.
- T. Okada, H. Konishi, M. Ito, H. Nagura and J. Asai, J. Invest. Dermatol., 1988, 90, 648–651 CrossRef CAS PubMed.
- K. Matsuzaki, N. Sugimoto, R. Islam, M. E. Hossain, E. Sumiyoshi, M. Katakura and O. Shido, Int. J. Mol. Sci., 2020, 21, 815 CrossRef CAS PubMed.
- P. Patel, Z. Jamal and K. Ramphul, in StatPearls, StatPearls Publishing, Treasure Island (FL), 2025 Search PubMed.
- W. P. Nikolaejek and H. M. Emrich, Eur. J. Pediatr., 1976, 122, 289–291 CrossRef CAS PubMed.
- A. L. Mandel, C. Peyrot des Gachons, K. L. Plank, S. Alarcon and P. A. S. Breslin, PLoS One, 2010, 5, e13352 CrossRef PubMed.
- K. Pierzynowska, P. Wychowański, K. Zaworski, J. Woliński, J. Donaldson, D. Szkopek, K. Roszkowicz-Ostrowska, A. Kondej and S. G. Pierzynowski, World J. Exp. Med., 2024, 14, 92589 CrossRef PubMed.
- Amylase - blood Information | Mount Sinai - New York, https://www.mountsinai.org/health-library/tests/amylase-blood, (accessed June 30, 2025).
- Chromogranin A Test, https://www.quironsalud.com/en/diagnostic-tests/chromogranin-test, (accessed June 30, 2025).
- E. C. Wyatt, E. L. Wyatt and R. L. Graham, JAAD Case Rep., 2025, 60, 41–43 CrossRef PubMed.
- Y. Kanamaru, A. Kikukawa and K. Shimamura, Stress, 2006, 9, 127–131 CrossRef CAS PubMed.
- M. Tammayan, N. Jantaratnotai and P. Pachimsawat, PLoS One, 2021, 16, e0256172 CrossRef CAS PubMed.
- A. Corti, Cell. Mol. Neurobiol., 2010, 30, 1163–1170 CrossRef CAS PubMed.
- A. J. Steckl and P. Ray, ACS Sens., 2018, 3, 2025–2044 CrossRef CAS PubMed.
- F. Akutsu, S. Sugino, M. Watanabe, Y.-A. Barde and M. Kojima, F1000Research, 2025, 14, 161 Search PubMed.
- Y. K. Yoo, J. Lee, J. Kim, G. Kim, S. Kim, J. Kim, H. Chun, J. H. Lee, C. J. Lee and K. S. Hwang, Sci. Rep., 2016, 6, 33694 CrossRef CAS PubMed.
- K. Wójtowicz, K. Czarzasta, L. Przepiorka, S. Kujawski, A. Cudnoch-Jedrzejewska, A. Marchel and P. Kunert, Cureus, 2023, 15, e48237 Search PubMed.
- É. Csősz, G. Emri, G. Kalló, G. Tsaprailis and J. Tőzsér, J. Eur. Acad. Dermatol. Venereol., 2015, 29, 2024–2031 CrossRef PubMed.
- M. K. Mahmood, H. A. Kurda, B. H. Qadir, H. Tassery, R. Lan, D. Tardivo and M. A. Abdulghafor, Saudi Dent. J., 2024, 36, 698–707 CrossRef PubMed.
- M. Ellmerer, L. Schaupp, G. A. Brunner, G. Sendlhofer, A. Wutte, P. Wach and T. R. Pieber, Am. J. Physiol., 2000, 278, E352–E356 CrossRef CAS PubMed.
- H. L. Poulsen, Scand. J. Clin. Lab. Invest., 1974, 34(2), 119–122 CrossRef CAS PubMed.
- Albumin Blood Test, https://medlineplus.gov/lab-tests/albumin-blood-test/, (accessed June 30, 2025).
- S. Adelaars, C. S. M. Lapré, P. Raaijmakers, C. J. A. M. Konings, M. Mischi, R. A. Bouwman and D. van de Kerkhof, J. Chromatogr., B, 2025, 1252, 124444 CrossRef CAS PubMed.
- Y. Zhang, H. Guo, S. B. Kim, Y. Wu, D. Ostojich, S. H. Park, X. Wang, Z. Weng, R. Li, A. J. Bandodkar, Y. Sekine, J. Choi, S. Xu, S. Quaggin, R. Ghaffari and J. A. Rogers, Lab Chip, 2019, 19, 1545–1555 RSC.
- Comparing creatinine levels in blood and interstitial fluid, https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/comparing-creatinine-levels-in-blood-and-interstitial-fluid/, (accessed June 30, 2025).
- A. Córdova and F. J. Navas, Ann. Nutr. Metab., 1998, 42, 274–282 CrossRef PubMed.
- J. L. Stauber and T. M. Florence, Sci. Total Environ., 1988, 74, 235–247 CrossRef CAS PubMed.
- A. Mathur, K. Wallenius and M. Abdulla, Scand. J. Clin. Lab. Invest., 1977, 37, 469–472 CrossRef CAS PubMed.
- J. L. Stauber and T. M. Florence, Sci. Total Environ., 1987, 60, 263–271 CrossRef CAS PubMed.
- M. Schaefer, M. Schellenberg, U. Merle, K. H. Weiss and W. Stremmel, BMC Gastroenterol., 2008, 8, 29 CrossRef PubMed.
- V. R. Reddy, S. Devakar, N. Chowdhary, S. M. Chaitan, R. Peddi and P. S. Kumar, Int. J. Clin. Pediatr. Dent., 2021, 14, 235–237 CrossRef PubMed.
- E. W. Rice and N. P. Goldstein, Metabolism, 1966, 15, 1050–1053 CrossRef CAS PubMed.
- C. Muñoz-Bravo, E. Soler-Iborte, M. Lozano-Lorca, M. Kouiti, C. González-Palacios Torres, R. Barrios-Rodríguez and J. J. Jiménez-Moleón, Front. Cardiovasc. Med., 2023, 10 DOI:10.3389/fcvm.2023.1217748.
- C. A. Coltman and N. J. Rowe, Am. J. Clin. Nutr., 1966, 18, 270–274 CrossRef CAS PubMed.
- A. M. Gawaly, A. A. Y. El-Naby and G. M. Alghazaly, Egypt. J. Haematol., 2020, 45, 156 CrossRef.
- Anemia - Iron-Deficiency Anemia | NHLBI, NIH, https://www.nhlbi.nih.gov/health/anemia/iron-deficiency-anemia, (accessed June 30, 2025).
- A. Bardow, J. Madsen and B. Nauntofte, Clin. Oral Investig., 2000, 4, 245–253 CrossRef CAS PubMed.
- Serum Bicarbonate - Range, levels, testing | National Kidney Foundation, https://www.kidney.org/kidney-failure-risk-factor-serum-bicarbonate, (accessed June 30, 2025).
- C. A. Prompt, P. M. Quinton and C. R. Kleeman, Nephron, 1978, 20, 4–9 CrossRef CAS PubMed.
- M. S. Razzaque, FASEB BioAdv., 2022, 4, 102–108 CrossRef CAS PubMed.
- What Is Hypophosphatemia?, https://my.clevelandclinic.org/health/diseases/24040-hypophosphatemia, (accessed June 30, 2025).
- S. Itoh and T. Nakayama, Jpn. J. Physiol., 1951, 2, 248–253 CrossRef PubMed.
- Y. Nakamura, H. Kodama, T. Satoh, K. Adachi, S. Watanabe, Y. Yokote and H. Sakagami, In Vivo, 2010, 24, 837–842 CAS.
- Gutierrez, Anderstam and Alvestrand, Eur. J. Clin. Invest., 1999, 29, 947–952 CrossRef CAS PubMed.
- J. A. Schmidt, S. Rinaldi, A. Scalbert, P. Ferrari, D. Achaintre, M. J. Gunter, P. N. Appleby, T. J. Key and R. C. Travis, Eur. J. Clin. Nutr., 2016, 70, 306–312 CrossRef CAS PubMed.
- R. W. Keller, J. L. Bailey, Y. Wang, J. D. Klein and J. M. Sands, Physiol. Rep., 2016, 4, e12825 CrossRef PubMed.
- A. G. Kovalčíková, K. Pavlov, R. Lipták, M. Hladová, E. Renczés, P. Boor, Ľ. Podracká, K. Šebeková, J. Hodosy, Ľ. Tóthová and P. Celec, Sci. Rep., 2020, 10, 21260 CrossRef PubMed.
- T. J. Lasisi, Y. R. Raji and B. L. Salako, BMC Nephrol., 2016, 17, 10 CrossRef PubMed.
- T. Takemura, P. W. Wertz and K. Sato, Br. J. Dermatol., 1989, 120, 43–47 CrossRef CAS PubMed.
- S. Karjalainen, L. Sewón, E. Söderling, B. Larsson, I. Johansson, O. Simell, H. Lapinleimu and R. Seppänen, J. Dent. Res., 1997, 76, 1637–1643 CrossRef CAS PubMed.
- What Should My Cholesterol Levels Be?, https://my.clevelandclinic.org/health/articles/11920-cholesterol-numbers-what-do-they-mean, (accessed June 30, 2025).
- K. Agrawal, R. K. Sivamani and J. W. Newman, Sking Res. Technol., 2019, 25, 3–11 CrossRef PubMed.
- V. Singh, R. Patil, S. Singh, A. Tripathi, V. Khanna and W. Ali, Natl. J. Maxillofac. Surg., 2021, 12, 188–192 CrossRef PubMed.
- B. Larsson, G. Olivecrona and T. Ericson, Arch. Oral Biol., 1996, 41, 105–110 CrossRef CAS PubMed.
- O. Zagoory-Sharon, A. Levine and R. Feldman, Psychoneuroendocrinology, 2023, 158, 106407 CrossRef CAS PubMed.
- B. Keevil, P. MacDonald, W. Macdowall, D. Lee and F. Wu, Ann. Clin. Biochem., 2014, 51, 368–378 CrossRef CAS PubMed.
- I.-S. Huang, L.-H. Li, W.-J. Chen, E. Y.-H. Huang, C.-C. Juan and W. J. Huang, Eur. Urol. Open Sci., 2023, 54, 88–96 CrossRef PubMed.
- M. Diver, Front. Horm. Res., 2009, 37, 21–31 CAS.
- C. Ye, M. Wang, J. Min, R. Y. Tay, H. Lukas, J. R. Sempionatto, J. Li, C. Xu and W. Gao, Nat. Nanotechnol., 2024, 330–337 CrossRef CAS PubMed.
- B. K. Gandara, L. Leresche and L. Mancl, Ann. N. Y. Acad. Sci., 2007, 1098, 446–450 CrossRef CAS PubMed.
- H. T. Depypere, S. Bolca, M. Bracke, J. Delanghe, F. Comhaire and Ph. Blondeel, Maturitas, 2015, 81, 42–45 CrossRef CAS PubMed.
- S. Glynne, D. Reisel, A. Kamal, A. Neville, L. McColl, R. Lewis and L. Newson, Menopause, 2025, 32, 103 CrossRef PubMed.
- J. Brunmair, M. Gotsmy, L. Niederstaetter, B. Neuditschko, A. Bileck, A. Slany, M. L. Feuerstein, C. Langbauer, L. Janker, J. Zanghellini, S. M. Meier-Menches and C. Gerner, Nat. Commun., 2021, 12, 5993 CrossRef CAS PubMed.
- C. C. Muir, K. Treasurywala, S. McAllister, J. Sutherland, L. Dukas, R. G. Berger, A. Khan and D. deCatanzaro, Horm. Metab. Res., 2008, 40, 819–826 CrossRef CAS PubMed.
- E. Ferrer, G. Rodas, G. Casals, A. Trilla, L. Balagué-Dobon, J. R. González, K. Ridley, R. White and R. J. Burden, Front. Sports Act. Living, 2024, 6 DOI:10.3389/fspor.2024.1430158.
- Y. Lu, G. R. Bentley, P. H. Gann, K. R. Hodges and R. T. Chatterton, Fertil. Steril., 1999, 71, 863–868 CrossRef CAS PubMed.
- J. Tu, J. Min, Y. Song, C. Xu, J. Li, J. Moore, J. Hanson, E. Hu, T. Parimon, T.-Y. Wang, E. Davoodi, T.-F. Chou, P. Chen, J. J. Hsu, H. B. Rossiter and W. Gao, Nat. Biomed. Eng., 2023, 7, 1293–1306 CrossRef CAS PubMed.
- J. B. Pay and A. M. Shaw, Clin. Biochem., 2019, 68, 1–8 CrossRef CAS PubMed.
- A. Grammoustianou, A. Saeidi, J. Longo, F. Risch and A. M. Ionescu, arXiv, 2024, preprint, arXiv:2407.16734, DOI:10.48550/arXiv.2407.16734.
- L. E. McCrae, W.-T. Ting and M. M. R. Howlader, Biosens. Bioelectron.: X, 2023, 13, 100288 CAS.
- M. Hladek, S. L. Szanton, Y.-E. Cho, C. Lai, C. Sacko, L. Roberts and J. Gill, J. Immunol. Methods, 2018, 454, 1–5 CrossRef CAS PubMed.
- M. M. Grisius, D. K. Bermudez and P. C. Fox, J. Rheumatol., 1997, 24, 1089–1091 CAS.
- E. A. Said, I. Al-Reesi, N. Al-Shizawi, S. Jaju, M. S. Al-Balushi, C. Y. Koh, A. A. Al-Jabri and L. Jeyaseelan, J. Med. Virol., 2021, 93, 3915–3924 CrossRef CAS PubMed.
- R. P. Hirten, K.-C. Lin, J. Whang, S. Shahub, D. Helmus, S. Muthukumar, B. E. Sands and S. Prasad, Sci. Rep., 2024, 14, 2833 CrossRef CAS PubMed.
- H. R. Mozaffari, M. Ramezani, M. Mahmoudiahmadabadi, N. Omidpanah and M. Sadeghi, Oral Surg. Oral Med. Oral Pathol., 2017, 124, e183–e189 CrossRef PubMed.
- Y. Nakai, S. Hamagaki, R. Takagi, A. Taniguchi and F. Kurimoto, J. Clin. Endocrinol. Metab., 1999, 84, 1226–1228 CAS.
- B. Zinman, A. J. Hanley, S. B. Harris, J. Kwan and I. G. Fantus, J. Clin. Endocrinol. Metab., 1999, 84, 272–278 CAS.
- C. Alexander, C. J. Cochran, L. Gallicchio, S. R. Miller, J. A. Flaws and H. Zacur, Fertil. Steril., 2010, 94, 1037–1043 CrossRef CAS PubMed.
- T. L. Laursen, R. B. Zak, R. J. Shute, M. W. S. Heesch, N. E. Dinan, M. P. Bubak, D. T. La Salle and D. R. Slivka, Temp. Multidiscip. Biomed. J., 2017, 4, 166–175 Search PubMed.
- A. Khorsand, M. Bayani, S. Yaghobee, S. Torabi, M. J. Kharrazifard and F. Mohammadnejhad, J. Dent., 2016, 13, 1–9 Search PubMed.
- G. Sendlhofer, G. Brunner, L. Schaupp, A. Wutte, M. Ellmerer and T. R. Pieber, Eur. J. Clin. Invest., 2015, 45, 445–451 CrossRef CAS PubMed.
- M. Toda, R. Tsukinoki and K. Morimoto, Acta Diabetol., 2007, 44, 20–22 CrossRef CAS PubMed.
- F.-Y. Lin, H. C. Wu, K. C. Cheng, C. L. Tung, C. P. Chang and Y. H. Feng, Int. J. Hematol., 2015, 102, 312–317 CrossRef CAS PubMed.
- K. Hotta, T. Funahashi, Y. Arita, M. Takahashi, M. Matsuda, Y. Okamoto, H. Iwahashi, H. Kuriyama, N. Ouchi, K. Maeda, M. Nishida, S. Kihara, N. Sakai, T. Nakajima, K. Hasegawa, M. Muraguchi, Y. Ohmoto, T. Nakamura, S. Yamashita, T. Hanafusa and Y. Matsuzawa, Arterioscler., Thromb., Vasc. Biol., 2000, 20, 1595–1599 CrossRef CAS PubMed.
- R. Dall, J. Kanaley, T. K. Hansen, N. Møller, J. S. Christiansen, H. Hosoda, K. Kangawa and J. O. L. Jørgensen, Eur. J. Endocrinol., 2002, 147, 65–70 CAS.
- C. Stylianou, A. Galli-Tsinopoulou, G. Koliakos, M. Fotoulaki and S. Nousia-Arvanitakis, J. Cystic Fibrosis, 2007, 6, 293–296 CrossRef CAS PubMed.
- T. Shiiya, M. Nakazato, M. Mizuta, Y. Date, M. S. Mondal, M. Tanaka, S.-I. Nozoe, H. Hosoda, K. Kangawa and S. Matsukura, J. Clin. Endocrinol. Metab., 2002, 87, 240–244 CrossRef CAS PubMed.
- I. Salarić, I. Karmelić, J. Lovrić, K. Baždarić, M. Rožman, I. Čvrljević, I. Zajc, D. Brajdić and D. Macan, Sci. Rep., 2021, 11, 13201 CrossRef PubMed.
- D. J. Kennaway, J. Pineal Res., 2019, 67, e12572 CrossRef PubMed.
- A. W. Hsing, T. E. Meyer, S. Niwa, S. M. Quraishi and L. W. Chu, Cancer Epidemiol., Biomarkers Prev., 2010, 19, 932–937 CrossRef CAS PubMed.
- M. J. Nunes, J. J. G. Moura, J. P. Noronha, L. C. Branco, A. Samhan-Arias, J. P. Sousa, C. Rouco and C. M. Cordas, Analytica, 2022, 3, 178–194 CrossRef CAS.
- M. S. Karbownik and S. D. Hicks, Front. Psychiatry, 2022, 13, 788153 CrossRef PubMed.
- N. M. Kushnir-Sukhov, E. Brittain, L. Scott and D. D. Metcalfe, Eur. J. Clin. Invest., 2008, 38, 953–958 CrossRef CAS PubMed.
- Serotonin | University Hospitals, https://www.uhhospitals.org/health-information/health-and-wellness-library/article/lab-tests-v1/serotonin, (accessed June 30, 2025).
- G. R. Van Loon, Fed. Proc., 1983, 42, 3012–3018 CAS.
- X. Zeng, Y. Zhang, X. Li, C. Wang, X. Xia, C. Jin, D. Huo and C. Hou, Anal. Chem., 2025, 97, 12090–12099 CrossRef PubMed.
- D. Tomassoni, E. Traini, M. Mancini, V. Bramanti, S. S. Mahdi and F. Amenta, Am. J. Physiol., 2015, 309, R585–R593 CAS.
- N. Blohm, Theses Diss. Proj, Smith College, 2017 Search PubMed.
- M. R. Keerthanaa, L. R. Panicker, R. Narayan and Y. G. Kotagiri, RSC Adv., 2024, 14, 7131–7141 RSC.
- Y. Ding, K. Tan, S. Zhang, S. Wang, X. Zhang and P. Hu, Chem. Eng. J., 2023, 477, 146844 CrossRef CAS.
- H. J. Hurley and J. A. Witkowski, J. Appl. Physiol., 1961, 16, 652–654 CrossRef CAS PubMed.
- K. O. Schwab, G. Heubel and H. Bartels, Eur. J. Clin. Chem. Clin. Biochem., 1992, 30, 541–544 CAS.
- B. Kennedy, E. Dillon, P. J. Mills and M. G. Ziegler, Life Sci., 2001, 69, 87–99 CrossRef CAS PubMed.
- O. Rachid, M. M. Rawas-Qalaji, F. E. R. Simons and K. J. Simons, J. Allergy Clin. Immunol., 2013, 131, 236–238 CrossRef CAS PubMed.
- Catecholamine blood test Information | Mount Sinai - New York, https://www.mountsinai.org/health-library/tests/catecholamine-blood-test, (accessed June 30, 2025).
- L. Wang, L. Pan, X. Han, M. N. Ha, K. Li, H. Yu, Q. Zhang, Y. Li, C. Hou and H. Wang, J. Colloid Interface Sci., 2022, 616, 326–337 CrossRef CAS PubMed.
- J. Zhao, H. Y. Y. Nyein, L. Hou, Y. Lin, M. Bariya, C. H. Ahn, W. Ji, Z. Fan and A. Javey, Adv. Mater., 2021, 33, 2006444 CrossRef CAS PubMed.
- E. Mäkilä and P. Kirveskari, Arch. Oral Biol., 1969, 14, 1285–1292 CrossRef PubMed.
- L. W. Evans and S. T. Omaye, Antioxidants, 2017, 6, 5 CrossRef PubMed.
- A. F. Hagel, H. Albrecht, W. Dauth, W. Hagel, F. Vitali, I. Ganzleben, H. W. Schultis, P. C. Konturek, J. Stein, M. F. Neurath and M. Raithel, J. Int. Med. Res., 2018, 46, 168–174 CrossRef CAS PubMed.
- Q. Chen, M. G. Espey, A. Y. Sun, J.-H. Lee, M. C. Krishna, E. Shacter, P. L. Choyke, C. Pooput, K. L. Kirk, G. R. Buettner and M. Levine, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 8749–8754 CrossRef CAS PubMed.
- Folic acid - test Information | Mount Sinai - New York, https://www.mountsinai.org/health-library/tests/folic-acid-test, (accessed June 30, 2025).
- E. Mäkilä, Arch. Oral Biol., 1966, 11, 839–844 CrossRef PubMed.
- B. C. Johnson, T. S. Hamilton and H. H. Mitchell, J. Biol. Chem., 1945, 159, 425–429 CrossRef CAS.
- TSH test, https://medlineplus.gov/ency/article/003684.htm, (accessed June 30, 2025).
- N. Brasier, C. Niederberger and G. A. Salvatore, Soft Sci., 2024, 4, 6 CAS.
- N. Sawicka-Gutaj, P. Glinicki, K. Nijakowski, B. Bromińska, M. Ostrowska, A. Szatko, Z. Sobol, K. Kowalski, P. Wilk, W. Zgliczyński and M. Ruchala, in Endocrine Abstracts, Bioscientifica, 2023, p. 90 Search PubMed.
- F. M. Matschinsky and D. F. Wilson, Front. Physiol., 2019, 10 DOI:10.3389/fphys.2019.00148.
- M. N. Nakrani, R. H. Wineland and F. Anjum, in StatPearls, StatPearls Publishing, Treasure Island (FL), 2025 Search PubMed.
- P. Wu, T. Zhu, Y. Huang, Z. Fang and F. Luo, Front. Endocrinol., 2023, 14, 1205442 CrossRef PubMed.
- X. Li, Y. Yang, B. Zhang, X. Lin, X. Fu, Y. An, Y. Zou, J. Wang, Z. Wang and T. Yu, Signal Transduction Targeted Ther., 2022, 7, 305 CrossRef CAS PubMed.
- P. Strazzullo and C. Leclercq, Adv. Nutr., 2014, 5, 188–190 CrossRef PubMed.
- A. J. Viera and N. Wouk, Am. Fam. Physician, 2015, 92, 487–495 Search PubMed.
- T. J. Jentsch, V. Stein, F. Weinreich and A. A. Zdebik, Physiol. Rev., 2002, 82, 503–568 CrossRef CAS PubMed.
- R. El Ridi and H. Tallima, J. Adv. Res., 2017, 8, 487–493 CrossRef CAS PubMed.
- A. Breedveld and M. van Egmond, Front. Immunol., 2019, 10, 553 CrossRef CAS PubMed.
- B. Tota, T. Angelone and M. C. Cerra, Front. Chem., 2014, 14(2), 64 Search PubMed.
- M. A. D'amico, B. Ghinassi, P. Izzicupo, L. Manzoli and A. Di Baldassarre, Endocr. Connect., 2014, 3, R45–R54 Search PubMed.
- L. Colucci-D'Amato, L. Speranza and F. Volpicelli, Int. J. Mol. Sci., 2020, 21, 7777 CrossRef PubMed.
- S.-H. Dou, Y. Cui, S.-M. Huang and B. Zhang, Front. Hum. Neurosci., 2022, 16, 1–11 Search PubMed.
- V. Gounden, R. Vashisht and I. Jialal, in StatPearls, StatPearls Publishing, Treasure Island (FL), 2025 Search PubMed.
- Calcium disorders | Medical Council of Canada, https://mcc.ca/objectives/medical-expert/calcium-disorders/, (accessed February 24, 2025).
- Magnesium in diet, https://medlineplus.gov/ency/article/002423.htm, (accessed February 24, 2025).
- R. Swaminathan, Clin. Biochem. Rev., 2003, 24, 47–66 CAS.
- A. Hussain, W. Jiang, X. Wang, S. Shahid, N. Saba, M. Ahmad, A. Dar, S. U. Masood, M. Imran and A. Mustafa, Front. Nutr., 2022, 9, 717064 CrossRef PubMed.
- M. Maywald and L. Rink, Biomolecules, 2022, 12, 1748 CrossRef CAS PubMed.
- J. Gale and E. Aizenman, Eur. J. Neurosci., 2024, 60, 3505–3543 CrossRef CAS PubMed.
- K. Alka and J. R. Casey, IUBMB Life, 2014, 66, 596–615 CrossRef CAS PubMed.
- C. A. Wagner, Nephrol., Dial., Transplant., 2024, 39, 190–201 CrossRef CAS PubMed.
- I. Portales-Castillo, T. Rieg, S. B. Khalid, S. U. Nigwekar and J. A. Neyra, Adv. Kidney Dis. Health, 2023, 30, 177–188 CrossRef PubMed.
- Z.-N. Ling, Y.-F. Jiang, J.-N. Ru, J.-H. Lu, B. Ding and J. Wu, Signal Transduction Targeted Ther., 2023, 8, 1–32 CrossRef PubMed.
- O. I. Adeyomoye, C. O. Akintayo, K. P. Omotuyi and A. N. Adewumi, Indian J. Nephrol., 2022, 32, 539–545 CrossRef PubMed.
- T. Huff, B. Boyd and I. Jialal, in StatPearls, StatPearls Publishing, Treasure Island (FL), 2025 Search PubMed.
- B. J. Delgado and W. Lopez-Ojeda, in StatPearls, StatPearls Publishing, Treasure Island (FL), 2025 Search PubMed.
- P. M. Wise, S. Suzuki and C. M. Brown, Dialogues Clin. Neurosci., 2009, 11, 297–303 CrossRef PubMed.
- J. A. MacLean and K. Hayashi, Cells, 2022, 11, 647 CrossRef CAS PubMed.
- T. W. Du Clos, Ann. Med., 2000, 32, 274–278 CrossRef CAS PubMed.
- Y. Luan and Y. Yao, Front. Immunol., 2018, 7(9), 1302 CrossRef PubMed.
- E. Grebenciucova and S. VanHaerents, Front. Immunol., 2023, 4, 1255533 CrossRef PubMed.
- K. You, H. Gu, Z. Yuan and X. Xu, Front. Cell Dev. Biol., 2021, 9, 8–13 Search PubMed.
- D. Jang, A.-H. Lee, H.-Y. Shin, H.-R. Song, J.-H. Park, T.-B. Kang, S.-R. Lee and S.-H. Yang, Int. J. Mol. Sci., 2021, 22, 2719 CrossRef CAS PubMed.
- R. Maurya, P. Bhattacharya, R. Dey and H. L. Nakhasi, Front. Immunol., 2018, 9, 2741 CrossRef PubMed.
- R. Hardeland, Sci. World J., 2012, 2012, 640389 Search PubMed.
- A. Mishra, S. Singh and S. Shukla, J. Exp. Neurosci., 2018, 12, 1179069518779829 CrossRef PubMed.
- L. Fiore, V. Mazzaracchio, A. Serani, G. Fabiani, L. Fabiani, G. Volpe, D. Moscone, G. M. Bianco, C. Occhiuzzi, G. Marrocco and F. Arduini, Sens. Actuators, B, 2023, 379, 133258 CrossRef CAS.
- A. K. Yetisen, M. S. Akram and C. R. Lowe, Lab Chip, 2013, 13, 2210 RSC.
- J. Liu, X. Kong, H. Wang, Y. Zhang and Y. Fan, Microfluid. Nanofluid., 2020, 24, 6 CrossRef.
- S. Nishat, A. T. Jafry, A. W. Martinez and F. R. Awan, Sens. Actuators, B, 2021, 336, 129681 CrossRef CAS.
- A.-G. Niculescu, C. Chircov, A. C. Bîrcă and A. M. Grumezescu, Int. J. Mol. Sci., 2021, 22, 2011 CrossRef CAS PubMed.
- U. Mogera, H. Guo, M. Namkoong, M. S. Rahman, T. Nguyen and L. Tian, Sci. Adv., 2022, 8, eabn1736 CrossRef CAS PubMed.
- Microfluidics and Bio-MEMS: devices and applications, ed. T. S. Santra, Jenny Stanford Publishing, Singapore, 2021 Search PubMed.
- A. N. Nordin and A. Abd Manaf, in Microfluidic Biosensors, Elsevier, 2023, pp. 41–85 Search PubMed.
- L. DeFrancesco and I. Jarchum, Nat. Biotechnol., 2019, 37, 329–329 CrossRef CAS PubMed.
- J. C. Yeo, K. Kenry and C. T. Lim, Lab Chip, 2016, 16, 4082–4090 RSC.
- G. Chen, J. Zheng, L. Liu and L. Xu, Small Methods, 2019, 3, 1900688 CrossRef CAS.
- L. Meng, I. Jeerapan and W. C. Mak, in Microfluidic Biosensors, Elsevier, 2023, pp. 107–157 Search PubMed.
- C. B. Goy, R. E. Chaile and R. E. Madrid, React. Funct. Polym., 2019, 145, 104314 CrossRef CAS.
- L. Gervais and E. Delamarche, Lab Chip, 2009, 9, 3330 RSC.
- C. W. Bae, P. T. Toi, B. Y. Kim, W. I. Lee, H. B. Lee, A. Hanif, E. H. Lee and N.-E. Lee, ACS Appl. Mater. Interfaces, 2019, 11, 14567–14575 CrossRef CAS PubMed.
- S. Jo, D. Sung, S. Kim and J. Koo, Biomed. Eng. Lett., 2021, 11, 117–129 CrossRef PubMed.
- M. Sekar, R. Sriramprabha, P. K. Sekhar, S. Bhansali, N. Ponpandian, M. Pandiaraj and C. Viswanathan, J. Electrochem. Soc., 2020, 167, 067508 CrossRef CAS.
- J. G. Turner, L. R. White, P. Estrela and H. S. Leese, Macromol. Biosci., 2021, 21, 2000307 CrossRef CAS PubMed.
- X. Zhang, L. Li and C. Luo, Lab Chip, 2016, 16, 1757–1776 RSC.
- T. Shay, M. D. Dickey and O. D. Velev, Lab Chip, 2017, 17, 710–716 RSC.
- Y. K. Jung, J. Kim and R. A. Mathies, Biosens. Bioelectron., 2016, 79, 371–378 CrossRef CAS PubMed.
- T. Biswal, S. K. BadJena and D. Pradhan, Mater. Today: Proc., 2020, 30, 305–315 CAS.
- K. Markandan and C. Q. Lai, Composites, Part B, 2023, 256, 110661 CrossRef CAS.
- Y. Fu, J. Dai, Y. Ge, Y. Zhang, H. Ke and W. Zhang, Molecules, 2018, 23, 2552 CrossRef PubMed.
- S. Peng, Y. Yu, S. Wu and C.-H. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 43831–43854 CrossRef CAS PubMed.
- Md. A. Ali, S. Srivastava, P. R. Solanki, V. Reddy, V. V. Agrawal, C. Kim, R. John and B. D. Malhotra, Sci. Rep., 2013, 3, 2661 CrossRef PubMed.
- H. Tavakoli, S. Mohammadi, X. Li, G. Fu and X. Li, TrAC, Trends Anal. Chem., 2022, 157, 116806 CrossRef CAS PubMed.
- S. Wang, X. Zhang, C. Ma, S. Yan, D. Inglis and S. Feng, Biosensors, 2021, 11, 405 CrossRef CAS PubMed.
- Y. Yu, L. Shang, J. Guo, J. Wang and Y. Zhao, Nat. Protoc., 2018, 13, 2557–2579 CrossRef CAS PubMed.
- C. Nie, A. Frijns, M. Zevenbergen and J. D. Toonder, Sens. Actuators, B, 2016, 227, 427–437 CrossRef CAS.
- M. Rovira, C. Fernández-Sánchez and C. Jiménez-Jorquera, Biosensors, 2021, 11, 303 CrossRef PubMed.
- M. Naeimirad, R. Abuzade, V. Babaahmadi and F. Dabirian, Mater. Des. Process. Commun., 2019, 1, e78 Search PubMed.
- C. Zhang, Y. Su, Y. Liang and W. Lai, Biosens. Bioelectron., 2020, 168, 112391 CrossRef CAS PubMed.
- M. S. Maria, P. E. Rakesh, T. S. Chandra and A. K. Sen, Sci. Rep., 2017, 7, 43457 CrossRef PubMed.
- Z. He, X. Yang, N. Wang, L. Mu, J. Pan, X. Lan, H. Li and F. Deng, Front. Bioeng. Biotechnol., 2021, 9, 807357 CrossRef PubMed.
- C. Chen, P. Duru, P. Joseph, S. Geoffroy and M. Prat, Sci. Rep., 2017, 7, 15110 CrossRef PubMed.
- M. I. Mohammed, E. Abraham and M. P. Y. Desmulliez, J. Micromech. Microeng., 2013, 23, 035034 CrossRef CAS.
- A. Sena-Torralba, M. Parrilla, A. Hernanz-Grimalt, A. Steijlen, E. Ortiz-Zapater, C. Cabaleiro-Otero, N. López-Riquelme, S. Cerveró-Ferragut, Á. Maquieira, K. De Wael and S. Morais, Anal. Chem., 2024, 96, 20684–20692 CrossRef CAS PubMed.
- M. Parrilla, A. Steijlen, R. Kerremans, J. Jacobs, L. den Haan, J. De Vreese, Y. Van Noten Géron, P. Clerx, R. Watts and K. De Wael, Chem. Eng. J., 2024, 500, 157254 CrossRef CAS.
- A. O. Olanrewaju, A. Robillard, M. Dagher and D. Juncker, Lab Chip, 2016, 16, 3804–3814 RSC.
- A. O. Olanrewaju, A. Ng, P. DeCorwin-Martin, A. Robillard and D. Juncker, Anal. Chem., 2017, 89, 6846–6853 CrossRef CAS PubMed.
- H. Zhang, Y. Qiu, S. Yu, C. Ding, J. Hu, H. Qi, Y. Tian, Z. Zhang, A. Liu and H. Wu, Biomicrofluidics, 2022, 16, 044104 CrossRef CAS PubMed.
- A. S. M. Steijlen, J. Bastemeijer, P. Groen, K. M. B. Jansen, P. J. French and A. Bossche, Anal. Methods, 2020, 12, 5885–5892 RSC.
- N. Uemura, R. P. Nath, M. R. Harkey, G. L. Henderson, J. Mendelson and R. T. Jones, J. Anal. Toxicol., 2004, 28, 253–259 CrossRef CAS PubMed.
- I. PharmChem, The Comprehensive Guide to Sweat Patches, https://www.pharmchek.com/resources/blog/the-comprehensive-guide-to-sweat-patches-how-the-patch-works-and-how-to-use-it, (accessed March 25, 2025).
- H. Shi, Y. Cao, Y. Zeng, Y. Zhou, W. Wen, C. Zhang, Y. Zhao and Z. Chen, Talanta, 2022, 240, 123208 CrossRef CAS PubMed.
- C. A. Porucznik, K. J. Cox, D. G. Wilkins, D. J. Anderson, N. M. Bailey, K. M. Szczotka and J. B. Stanford, J. Anal. Toxicol., 2015, 39, 562–566 CrossRef CAS PubMed.
- A. R. Naik, B. Warren, A. Burns, R. Lenigk, J. Morse, A. Alizadeh and J. J. Watkins, Microfluid. Nanofluid., 2021, 25, 2 CrossRef CAS.
- A. S. M. Steijlen, K. M. B. Jansen, J. Bastemeijer, P. J. French and A. Bossche, Anal. Chem., 2022, 94, 6893–6901 CrossRef CAS PubMed.
- J. Pieczyński, U. Szulc, J. Harazna, A. Szulc and J. Kiewisz, Eur. J. Ophthalmol., 2021, 31, 2245–2251 CrossRef PubMed.
- A. Posa, L. Bräuer, M. Schicht, F. Garreis, S. Beileke and F. Paulsen, Ann. Anat., 2013, 195, 137–142 CrossRef CAS PubMed.
- A. K. Yetisen, B. Soylemezoglu, J. Dong, Y. Montelongo, H. Butt, M. Jakobi and A. W. Koch, RSC Adv., 2019, 9, 11186–11193 RSC.
- E. Lindner, D. Bordelon, M. D. Kim, S. A. Dergunov, E. Pinkhassik and E. Chaum, Electroanalysis, 2012, 24, 42–52 CrossRef CAS.
- E. Ponzini, C. Santambrogio, A. De Palma, P. Mauri, S. Tavazzi and R. Grandori, Mass Spectrom. Rev., 2022, 41, 842–860 CrossRef CAS PubMed.
- A. Barmada and S. A. Shippy, Anal. Bioanal. Chem., 2019, 411, 329–338 CrossRef CAS PubMed.
- B.-H. Kang, M. Park and K.-H. Jeong, BioChip J., 2017, 11, 294–299 CrossRef CAS.
- F. Bachhuber, A. Huss, M. Senel and H. Tumani, Sci. Rep., 2021, 11, 10064 CrossRef CAS PubMed.
- E. V. Mukerjee, S. D. Collins, R. R. Isseroff and R. L. Smith, Sens. Actuators, A, 2004, 114, 267–275 CrossRef CAS.
- D. D. Zhu, L. W. Zheng, P. K. Duong, R. H. Cheah, X. Y. Liu, J. R. Wong, W. J. Wang, S. T. Tien Guan, X. T. Zheng and P. Chen, Biosens. Bioelectron., 2022, 212, 114412 CrossRef CAS PubMed.
- K. M. Saifullah and Z. Faraji Rad, Adv. Mater. Interfaces, 2023, 10, 2201763 CrossRef.
- D. J. O'Brien, D. Mills, J. Farina and M. Paranjape, IEEE Trans. Biomed. Eng., 2023, 70, 2573–2580 Search PubMed.
- K. Takeuchi, N. Takama, K. Sharma, O. Paul, P. Ruther, T. Suga and B. Kim, Drug Delivery Transl. Res., 2022, 12, 435–443 CrossRef CAS PubMed.
- X. Jiang and P. B. Lillehoj, Microsyst. Nanoeng., 2020, 6, 96 CrossRef CAS PubMed.
- T. A. Hakala, A. García Pérez, M. Wardale, I. A. Ruuth, R. T. Vänskä, T. A. Nurminen, E. Kemp, Z. A. Boeva, J.-M. Alakoskela, K. Pettersson-Fernholm, E. Hæggström and J. Bobacka, Sci. Rep., 2021, 11, 7609 CrossRef CAS PubMed.
- R. Raman, E. B. Rousseau, M. Wade, A. Tong, M. J. Cotler, J. Kuang, A. A. Lugo, E. Zhang, A. M. Graybiel, F. M. White, R. Langer and M. J. Cima, Sci. Adv., 2020, 6, eabb0657 CrossRef CAS PubMed.
- M. Akkurt Arslan, G. Rabut, S. Chardonnet, C. Pionneau, A. Kobal, M. Gratas Pelletier, N. Harfouche, A. Réaux La Goazigo, C. Baudouin, F. Brignole-Baudouin and K. Kessal, Exp. Eye Res., 2023, 237, 109679 CrossRef CAS PubMed.
- H. Y. Y. Nyein, L.-C. Tai, Q. P. Ngo, M. Chao, G. B. Zhang, W. Gao, M. Bariya, J. Bullock, H. Kim, H. M. Fahad and A. Javey, ACS Sens., 2018, 3, 944–952 CrossRef CAS PubMed.
- Q. Zeng, M. Xu, W. Hu, W. Cao, Y. Zhan, Y. Zhang, Q. Wang and T. Ma, Biosensors, 2023, 13, 537 CrossRef CAS PubMed.
- Y. Qiao, J. Du, R. Ge, H. Lu, C. Wu, J. Li, S. Yang, S. Zada, H. Dong and X. Zhang, Anal. Chem., 2022, 94, 5538–5545 CrossRef CAS PubMed.
- A. He, X. Wang, L. Zhang, H. Zhang, X. Xu, C. Yu, Y. Ma, W. Wei and P. Niu, Nanotechnol. Precis. Eng., 2024, 8, 013006 CrossRef.
- L. B. Baker, J. B. Model, K. A. Barnes, M. L. Anderson, S. P. Lee, K. A. Lee, S. D. Brown, A. J. Reimel, T. J. Roberts, R. P. Nuccio, J. L. Bonsignore, C. T. Ungaro, J. M. Carter, W. Li, M. S. Seib, J. T. Reeder, A. J. Aranyosi, J. A. Rogers and R. Ghaffari, Sci. Adv., 2020, 6, eabe3929 CrossRef CAS PubMed.
- T. Kim, Q. Yi, E. Hoang and R. Esfandyarpour, Adv. Mater. Technol., 2021, 6, 2001021 CrossRef CAS.
- P. Pirovano, M. Dorrian, A. Shinde, A. Donohoe, A. J. Brady, N. M. Moyna, G. Wallace, D. Diamond and M. McCaul, Talanta, 2020, 219, 121145 CrossRef CAS PubMed.
- X. Mei, J. Yang, X. Yu, Z. Peng, G. Zhang and Y. Li, Sens. Actuators, B, 2023, 381, 133451 CrossRef CAS.
- J. Kim, Y. Wu, H. Luan, D. S. Yang, D. Cho, S. S. Kwak, S. Liu, H. Ryu, R. Ghaffari and J. A. Rogers, Adv. Sci., 2022, 9, 2103331 CrossRef CAS PubMed.
- S. Liu, D. S. Yang, S. Wang, H. Luan, Y. Sekine, J. B. Model, A. J. Aranyosi, R. Ghaffari and J. A. Rogers, EcoMat, 2023, 5, e12270 CrossRef CAS.
- D. S. Yang, Y. Wu, E. E. Kanatzidis, R. Avila, M. Zhou, Y. Bai, S. Chen, Y. Sekine, J. Kim, Y. Deng, H. Guo, Y. Zhang, R. Ghaffari, Y. Huang and J. A. Rogers, Mater. Horiz., 2023, 10, 4992–5003 RSC.
- Y. Wu, X. Li, K. E. Madsen, H. Zhang, S. Cho, R. Song, R. F. Nuxoll, Y. Xiong, J. Liu, J. Feng, T. Yang, K. Zhang, A. J. Aranyosi, D. E. Wright, R. Ghaffari, Y. Huang, R. G. Nuzzo and J. A. Rogers, Lab Chip, 2024, 24, 4288–4295 RSC.
- A. Martín, J. Kim, J. F. Kurniawan, J. R. Sempionatto, J. R. Moreto, G. Tang, A. S. Campbell, A. Shin, M. Y. Lee, X. Liu and J. Wang, ACS Sens., 2017, 2, 1860–1868 CrossRef PubMed.
- M. Parrilla, I. Ortiz-Gómez, R. Cánovas, A. Salinas-Castillo, M. Cuartero and G. A. Crespo, Anal. Chem., 2019, 91, 8644–8651 CrossRef CAS PubMed.
- S. B. Kim, J. Koo, J. Yoon, A. Hourlier-Fargette, B. Lee, S. Chen, S. Jo, J. Choi, Y. S. Oh, G. Lee, S. M. Won, A. J. Aranyosi, S. P. Lee, J. B. Model, P. V. Braun, R. Ghaffari, C. Park and J. A. Rogers, Lab Chip, 2020, 20, 84–92 RSC.
- A. R. Naik, Y. Zhou, A. A. Dey, D. L. G. Arellano, U. Okoroanyanwu, E. B. Secor, M. C. Hersam, J. Morse, J. P. Rothstein, K. R. Carter and J. J. Watkins, Lab Chip, 2022, 22, 156–169 RSC.
- J. Kim, I. Jeerapan, S. Imani, T. N. Cho, A. Bandodkar, S. Cinti, P. P. Mercier and J. Wang, ACS Sens., 2016, 1, 1011–1019 CrossRef CAS.
- J. Kim, G. Valdés-Ramírez, A. J. Bandodkar, W. Jia, A. G. Martinez, J. Ramírez, P. Mercier and J. Wang, Analyst, 2014, 139, 1632–1636 RSC.
- J. Kim, S. Imani, W. R. De Araujo, J. Warchall, G. Valdés-Ramírez, T. R. L. C. Paixão, P. P. Mercier and J. Wang, Biosens. Bioelectron., 2015, 74, 1061–1068 CrossRef CAS PubMed.
- T. Arakawa, Y. Kuroki, H. Nitta, P. Chouhan, K. Toma, S. Sawada, S. Takeuchi, T. Sekita, K. Akiyoshi, S. Minakuchi and K. Mitsubayashi, Biosens. Bioelectron., 2016, 84, 106–111 CrossRef CAS PubMed.
- T. Arakawa, K. Tomoto, H. Nitta, K. Toma, S. Takeuchi, T. Sekita, S. Minakuchi and K. Mitsubayashi, Anal. Chem., 2020, 92, 12201–12207 CrossRef CAS PubMed.
- R. Moreddu, J. S. Wolffsohn, D. Vigolo and A. K. Yetisen, Sens. Actuators, B, 2020, 317, 128183 CrossRef CAS.
- Z. Li, J. Yun, X. Li, M. Kim, J. Li, D. Lee, A. Wu and S. W. Lee, Adv. Funct. Mater., 2023, 33, 2304647 CrossRef CAS.
- J. Park, J. Kim, S.-Y. Kim, W. H. Cheong, J. Jang, Y.-G. Park, K. Na, Y.-T. Kim, J. H. Heo, C. Y. Lee, J. H. Lee, F. Bien and J.-U. Park, Sci. Adv., 2018, 4, eaap9841 CrossRef PubMed.
- D. Hee, J. Won, B. Ho, K. Jae, S. Hyun and S. Kwang, Sci. Adv., 2020, 6(17), eaba3252 CrossRef PubMed.
- M. Ku, J. Kim, J.-E. Won, W. Kang, Y.-G. Park, J. Park, J.-H. Lee, J. Cheon, H. H. Lee and J.-U. Park, Sci. Adv., 2020, 6, eabb2891 CrossRef CAS PubMed.
- E. De La Paz, T. Saha, R. Del Caño, S. Seker, N. Kshirsagar and J. Wang, Talanta, 2023, 254, 124122 CrossRef CAS PubMed.
- K. Y. Goud, K. Mahato, H. Teymourian, K. Longardner, I. Litvan and J. Wang, Sens. Actuators, B, 2022, 354, 131234 CrossRef CAS.
- M. Dervisevic, E. Dervisevic, L. Esser, C. D. Easton, V. J. Cadarso and N. H. Voelcker, Biosens. Bioelectron., 2023, 222, 114955 CrossRef CAS PubMed.
- B. Yang, X. Fang and J. Kong, Adv. Funct. Mater., 2020, 30, 2000591 CrossRef CAS.
- J. Kim, J. R. Sempionatto, S. Imani, M. C. Hartel, A. Barfidokht, G. Tang, A. S. Campbell, P. P. Mercier and J. Wang, Adv. Sci., 2018, 5, 1800880 CrossRef PubMed.
- M. Razzaghi, A. Seyfoori, E. Pagan, E. Askari, A. Hassani Najafabadi and M. Akbari, Polymers, 2023, 15, 1389 CrossRef CAS PubMed.
- B. Yang, J. Kong and X. Fang, Nat. Commun., 2022, 13, 3999 CrossRef CAS PubMed.
- J. R. Sempionatto, M. Lin, L. Yin, E. De La Paz, K. Pei, T. Sonsa-ard, A. N. De Loyola Silva, A. A. Khorshed, F. Zhang, N. Tostado, S. Xu and J. Wang, Nat. Biomed. Eng., 2021, 5, 737–748 CrossRef CAS PubMed.
- F. Tehrani, H. Teymourian, B. Wuerstle, J. Kavner, R. Patel, A. Furmidge, R. Aghavali, H. Hosseini-Toudeshki, C. Brown, F. Zhang, K. Mahato, Z. Li, A. Barfidokht, L. Yin, P. Warren, N. Huang, Z. Patel, P. P. Mercier and J. Wang, Nat. Biomed. Eng., 2022, 6, 1214–1224 CrossRef CAS PubMed.
- X. Weng, Z. Fu, C. Zhang, W. Jiang and H. Jiang, Anal. Chem., 2022, 94, 3526–3534 CrossRef CAS PubMed.
- A. Panneer Selvam, S. Muthukumar, V. Kamakoti and S. Prasad, Sci. Rep., 2016, 6, 23111 CrossRef CAS PubMed.
- A. Alizadeh, A. Burns, R. Lenigk, R. Gettings, J. Ashe, A. Porter, M. McCaul, R. Barrett, D. Diamond, P. White, P. Skeath and M. Tomczak, Lab Chip, 2018, 18, 2632–2641 RSC.
- Y. Cheng, S. Feng, Q. Ning, T. Li, H. Xu, Q. Sun, D. Cui and K. Wang, Microsyst. Nanoeng., 2023, 9, 36 CrossRef CAS PubMed.
- A. Wiorek, M. Parrilla, M. Cuartero and G. A. Crespo, Anal. Chem., 2020, 92, 10153–10161 CrossRef CAS PubMed.
- M. Bariya, L. Li, R. Ghattamaneni, C. H. Ahn, H. Y. Y. Nyein, L.-C. Tai and A. Javey, Sci. Adv., 2020, 6, eabb8308 CrossRef CAS PubMed.
- W. He, C. Wang, H. Wang, M. Jian, W. Lu, X. Liang, X. Zhang, F. Yang and Y. Zhang, Sci. Adv., 2019, 5, eaax0649 CrossRef CAS PubMed.
- D.-H. Choi, G. B. Kitchen, M. T. Jennings, G. R. Cutting and P. C. Searson, npj Digit. Med., 2020, 3, 49 CrossRef PubMed.
- T. Terse-Thakoor, M. Punjiya, Z. Matharu, B. Lyu, M. Ahmad, G. E. Giles, R. Owyeung, F. Alaimo, M. Shojaei Baghini, T. T. Brunyé and S. Sonkusale, npj Flexible Electron., 2020, 4, 18 CrossRef.
- J. T. Reeder, J. Choi, Y. Xue, P. Gutruf, J. Hanson, M. Liu, T. Ray, A. J. Bandodkar, R. Avila, W. Xia, S. Krishnan, S. Xu, K. Barnes, M. Pahnke, R. Ghaffari, Y. Huang and J. A. Rogers, Sci. Adv., 2019, 5, eaau6356 CrossRef PubMed.
- B. Wang, C. Zhao, Z. Wang, K.-A. Yang, X. Cheng, W. Liu, W. Yu, S. Lin, Y. Zhao, K. M. Cheung, H. Lin, H. Hojaiji, P. S. Weiss, M. N. Stojanović, A. J. Tomiyama, A. M. Andrews and S. Emaminejad, Sci. Adv., 2022, 8, eabk0967 CrossRef CAS PubMed.
- I. Shitanda, N. Muramatsu, R. Kimura, N. Takahashi, K. Watanabe, H. Matsui, N. Loew, M. Motosuke, T. Mukaimoto, M. Kobayashi, T. Mitsuhara, Y. Sugita, K. Matsuo, S. Yanagita, T. Suzuki, H. Watanabe and M. Itagaki, ACS Sens., 2023, 8, 2889–2895 CrossRef CAS PubMed.
- D. Liu, Z. Liu, S. Feng, Z. Gao, R. Chen, G. Cai and S. Bian, Biosensors, 2023, 13, 157 CrossRef CAS PubMed.
- E. Scarpa, V. M. Mastronardi, F. Guido, L. Algieri, A. Qualtieri, R. Fiammengo, F. Rizzi and M. De Vittorio, Sci. Rep., 2020, 10, 10854 CrossRef CAS PubMed.
- G. Bolat, E. De La Paz, N. F. Azeredo, M. Kartolo, J. Kim, A. N. De Loyola, E. Silva, R. Rueda, C. Brown, L. Angnes, J. Wang and J. R. Sempionatto, Anal. Bioanal. Chem., 2022, 414, 5411–5421 CrossRef CAS PubMed.
- Y. Zheng, R. Omar, R. Zhang, N. Tang, M. Khatib, Q. Xu, Y. Milyutin, W. Saliba, Y. Y. Broza, W. Wu, M. Yuan and H. Haick, Adv. Mater., 2022, 34, 2108607 CrossRef CAS PubMed.
- S. Sharma, H. Byrne and R. J. O'Kennedy, Essays Biochem., 2016, 60, 9–18 CrossRef PubMed.
- Y. Wu, F. Tehrani, H. Teymourian, J. Mack, A. Shaver, M. Reynoso, J. Kavner, N. Huang, A. Furmidge, A. Duvvuri, Y. Nie, L. M. Laffel, F. J. Doyle, M.-E. Patti, E. Dassau, J. Wang and N. Arroyo-Currás, Anal. Chem., 2022, 94, 8335–8345 CrossRef CAS PubMed.
- N. K. Singh, S. Chung, A.-Y. Chang, J. Wang and D. A. Hall, Biosens. Bioelectron., 2023, 227, 115097 CrossRef CAS PubMed.
- S. Mross, S. Pierrat, T. Zimmermann and M. Kraft, Biosens. Bioelectron., 2015, 70, 376–391 CrossRef CAS PubMed.
- K. Sadani, P. Nag, X. Y. Thian and S. Mukherji, Biosens. Bioelectron.: X, 2022, 12, 100278 CAS.
- T. Saha, J. Fang, S. Mukherjee, M. D. Dickey and O. D. Velev, ACS Appl. Mater. Interfaces, 2021, 13, 8071–8081 CrossRef CAS PubMed.
- A. M. Nightingale, C. L. Leong, R. A. Burnish, S. Hassan, Y. Zhang, G. F. Clough, M. G. Boutelle, D. Voegeli and X. Niu, Nat. Commun., 2019, 10, 2741 CrossRef PubMed.
- H. Zhao, X. Zhang, Y. Qin, Y. Xia, X. Xu, X. Sun, D. Yu, S. M. Mugo, D. Wang and Q. Zhang, Adv. Funct. Mater., 2023, 33, 2212083 CrossRef CAS.
- M. Dautta, L. Fernando Ayala-Cardona, N. Davis, A. Aggarwal, J. Park, S. Wang, L. Gillan, E. Jansson, M. Hietala, H. Ko, J. Hiltunen and A. Javey, Adv. Mater. Technol., 2023, 8(6), 2201187 CrossRef CAS.
- Q.-F. Li, X. Chen, H. Wang, M. Liu and H.-L. Peng, ACS Appl. Mater. Interfaces, 2023, 15, 13290–13298 CrossRef CAS PubMed.
- Y. Hashimoto, T. Ishihara, K. Kuwabara, T. Amano and H. Togo, Micromachines, 2022, 13, 575 CrossRef PubMed.
- M. Dautta, L. F. Ayala-Cardona, N. Davis, A. Aggarwal, J. Park, S. Wang, L. Gillan, E. Jansson, M. Hietala, H. Ko, J. Hiltunen and A. Javey, Adv. Mater. Technol., 2023, 8, 2201187 CrossRef CAS.
- H. Y. Y. Nyein, M. Bariya, B. Tran, C. H. Ahn, B. J. Brown, W. Ji, N. Davis and A. Javey, Nat. Commun., 2021, 12, 1823 CrossRef CAS PubMed.
- M. A. Zahed, D. K. Kim, S. H. Jeong, M. Selim Reza, M. Sharifuzzaman, G. B. Pradhan, H. Song, M. Asaduzzaman and J. Y. Park, ACS Sens., 2023, 8, 2960–2974 CrossRef CAS PubMed.
- R. Badugu, E. A. Reece and J. R. Lakowicz, J. Biomed. Opt., 2018, 23, 1 Search PubMed.
- A. Barmada and S. A. Shippy, Eye, 2020, 34, 1731–1733 CrossRef PubMed.
- J. Zhang, K. Kim, H. J. Kim, D. Meyer, W. Park, S. A. Lee, Y. Dai, B. Kim, H. Moon, J. V. Shah, K. E. Harris, B. Collar, K. Liu, P. Irazoqui, H. Lee, S. A. Park, P. S. Kollbaum, B. W. Boudouris and C. H. Lee, Nat. Commun., 2022, 13, 5518 CrossRef CAS PubMed.
- H. Lee, T. K. Choi, Y. B. Lee, H. R. Cho, R. Ghaffari, L. Wang, H. J. Choi, T. D. Chung, N. Lu, T. Hyeon, S. H. Choi and D.-H. Kim, Nat. Nanotechnol., 2016, 11, 566–572 CrossRef CAS PubMed.
- Y. Liu, Q. Yu, X. Luo, L. Yang and Y. Cui, Microsyst. Nanoeng., 2021, 7, 75 CrossRef PubMed.
- L. Bao, J. Park, B. Qin and B. Kim, Sci. Rep., 2022, 12, 10693 CrossRef CAS PubMed.
- T. Chang, H. Li, N. Zhang, X. Jiang, X. Yu, Q. Yang, Z. Jin, H. Meng and L. Chang, Microsyst. Nanoeng., 2022, 8, 25 CrossRef CAS PubMed.
- M. Parrilla, U. Detamornrat, J. Domínguez-Robles, S. Tunca, R. F. Donnelly and K. De Wael, ACS Sens., 2023, 8, 4161–4170 CrossRef CAS PubMed.
- X. Luo, Q. Yu, L. Yang and Y. Cui, ACS Sens., 2023, 8, 1710–1722 CrossRef CAS PubMed.
- S. Kim, S. Park, J. Choi, W. Hwang, S. Kim, I.-S. Choi, H. Yi and R. Kwak, Nat. Commun., 2022, 13, 6705 CrossRef CAS PubMed.
- Y. Xu, E. De La Paz, A. Paul, K. Mahato, J. R. Sempionatto, N. Tostado, M. Lee, G. Hota, M. Lin, A. Uppal, W. Chen, S. Dua, L. Yin, B. L. Wuerstle, S. Deiss, P. Mercier, S. Xu, J. Wang and G. Cauwenberghs, Nat. Biomed. Eng., 2023, 7, 1307–1320 CrossRef PubMed.
- E. Volkova, A. Perchik, K. Pavlov, E. Nikolaev, A. Ayuev, J. Park, N. Chang, W. Lee, J. Y. Kim, A. Doronin and M. Vilenskii, Sci. Rep., 2023, 13, 13371 CrossRef CAS PubMed.
- Z. Wang, Y. Huang, K. Xu, Y. Zhong, C. He, L. Jiang, J. Sun, Z. Rao, J. Zhu, J. Huang, F. Xiao, H. Liu and B. Y. Xia, Nat. Commun., 2023, 14, 69 CrossRef CAS PubMed.
- J. Kim, J. Park, Y.-G. Park, E. Cha, M. Ku, H. S. An, K.-P. Lee, M.-I. Huh, J. Kim, T.-S. Kim, D. W. Kim, H. K. Kim and J.-U. Park, Nat. Biomed. Eng., 2021, 5, 772–782 CrossRef PubMed.
- L. Bao, J. Park, B. Qin and B. Kim, Sci. Rep., 2022, 12, 10693 CrossRef CAS PubMed.
- R. Moreddu, M. Elsherif, H. Butt, D. Vigolo and A. K. Yetisen, RSC Adv., 2019, 9, 11433–11442 RSC.
- S. Kim, H.-J. Jeon, S. Park, D. Y. Lee and E. Chung, Sci. Rep., 2020, 10, 8254 CrossRef CAS PubMed.
- M. Elsherif, M. U. Hassan, A. K. Yetisen and H. Butt, ACS Nano, 2018, 12, 5452–5462 CrossRef CAS PubMed.
- A. Ravizza, C. De Maria, L. Di Pietro, F. Sternini, A. L. Audenino and C. Bignardi, Front. Bioeng. Biotechnol., 2019, 7, 313 CrossRef PubMed.
- J. B. Brönneke, J. Müller, K. Mouratis, J. Hagen and A. D. Stern, Sensors, 2021, 21, 4937 CrossRef PubMed.
- W. Gao, S. Emaminejad, H. Y. Y. Nyein, S. Challa, K. Chen, A. Peck, H. M. Fahad, H. Ota, H. Shiraki, D. Kiriya, D.-H. Lien, G. A. Brooks, R. W. Davis and A. Javey, Nature, 2016, 529, 509–514 CrossRef CAS PubMed.
- J. Zhao, Y. Lin, J. Wu, H. Y. Y. Nyein, M. Bariya, L.-C. Tai, M. Chao, W. Ji, G. Zhang, Z. Fan and A. Javey, ACS Sens., 2019, 4, 1925–1933 CrossRef CAS PubMed.
- J. Tu and W. Gao, Adv. Healthcare Mater., 2021, 10, 2100127 CrossRef CAS PubMed.
- Y. Chen, S. Lu, S. Zhang, Y. Li, Z. Qu, Y. Chen, B. Lu, X. Wang and X. Feng, Sci. Adv., 2017, 3, e1701629 CrossRef PubMed.
- D.-H. Choi, G. Kitchen, J. S. Kim, Y. Li, K. Kim, I. C. Jeong, J. Nguyen, K. J. Stewart, S. L. Zeger and P. C. Searson, Sci. Rep., 2019, 9, 17877 CrossRef PubMed.
- T. R. Ray, M. Ivanovic, P. M. Curtis, D. Franklin, K. Guventurk, W. J. Jeang, J. Chafetz, H. Gaertner, G. Young, S. Rebollo, J. B. Model, S. P. Lee, J. Ciraldo, J. T. Reeder, A. Hourlier-Fargette, A. J. Bandodkar, J. Choi, A. J. Aranyosi, R. Ghaffari, S. A. McColley, S. Haymond and J. A. Rogers, Sci. Transl. Med., 2021, 13, eabd8109 CrossRef CAS PubMed.
- F. Lipsmeier, K. I. Taylor, T. Kilchenmann, D. Wolf, A. Scotland, J. Schjodt-Eriksen, W.-Y. Cheng, I. Fernandez-Garcia, J. Siebourg-Polster, L. Jin, J. Soto, L. Verselis, F. Boess, M. Koller, M. Grundman, A. U. Monsch, R. B. Postuma, A. Ghosh, T. Kremer, C. Czech, C. Gossens and M. Lindemann, Mov. Disord., 2018, 33(8), 1287–1297 CrossRef PubMed.
- Home, https://vitalconnect.com/, (accessed March 25, 2025), 406 510(k) Premarket Notification.
- https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=k182041, (accessed June 29, 2025).
- CDRH Medical Device Databases, https://www.fda.gov/medical-devices/device-advice-comprehensive-regulatory-assistance/medical-device-databases, (accessed June 29, 2025).
- U.S. Food and Drug Administration (FDA), ( 2020), Classify Your Medical Device, Retrieved from https://www.fda.gov/medical-devices/overview-device-regulation/classify-your-medical-device.
- O. of the Commissioner, FDA.
- C. for D. and R. Health, Quality System (QS) Regulation/Medical Device Current Good Manufacturing Practices (CGMP), https://www.fda.gov/medical-devices/postmarket-requirements-devices/quality-system-qs-regulationmedical-device-current-good-manufacturing-practices-cgmp, (accessed June 29, 2025).
- C. for D. and R. Health, Cybersecurity in Medical Devices, https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cybersecurity-medical-devices-quality-system-considerations-and-content-premarket-submissions, (accessed June 29, 2025).
- ISO 13485, https://www.iso.org/standard/59752.html, (accessed June 29, 2025).
- S. S. Arya, S. B. Dias, H. F. Jelinek, L. J. Hadjileontiadis and A.-M. Pappa, Biosens. Bioelectron., 2023, 235, 115387 CrossRef CAS PubMed.
- S. Hamedi, H. D. Jahromi and A. Lotfiani, Eng. Appl. Artif. Intell., 2023, 118, 105646 CrossRef.
- S. Shajari, K. Kuruvinashetti, A. Komeili and U. Sundararaj, Sensors, 2023, 23, 9498 CrossRef CAS PubMed.
- S. Kadian, P. Kumari, S. Shukla and R. Narayan, Talanta Open, 2023, 8, 100267 CrossRef.
- G. Moon, J. Choi, C. Lee, Y. Oh, K. H. Kim and D. Kim, Biosens. Bioelectron., 2020, 164, 112335 CrossRef CAS PubMed.
- D. Pelenis, D. Barauskas, G. Vanagas, M. Dzikaras and D. Viržonis, Ultrasonics, 2019, 99, 105956 CrossRef CAS PubMed.
- V. Kammarchedu, D. Butler and A. Ebrahimi, Anal. Chim. Acta, 2022, 1232, 340447 CrossRef CAS PubMed.
- S. Wang, C. Lafaye, M. Saubade, C. Besson, J. M. Margarit-Taulé, V. Gremeaux and S.-C. Liu, IEEE J. Biomed. Health Inform., 2022, 26, 4725–4732 Search PubMed.
- E. Yüzer, V. Doğan, V. Kılıç and M. Şen, Sens. Actuators, B, 2022, 371, 132489 CrossRef.
- S. Hong, Y. Gu, J. K. Seo, J. Wang, P. Liu, Y. S. Meng, S. Xu and R. Chen, Sci. Adv., 2019, 5, eaaw0536 CrossRef CAS PubMed.
- S. Li, K. Hart, N. Norton, C. A. Ryan, L. Guglani and M. R. Prausnitz, Bioeng. Transl. Med., 2021, 6, e10222 CrossRef CAS PubMed.
- Y. Li, Y. Ou, K. Fan and G. Liu, Theranostics, 2024, 14, 6969–6990 CrossRef CAS PubMed.
- T. W. Pittman, D. B. Decsi, C. Punyadeera and C. S. Henry, Theranostics, 2023, 13, 1091–1108 CrossRef CAS PubMed.
- N. Farsaeivahid, C. Grenier and M. L. Wang, Diagnostics, 2024, 14, 1088 CrossRef CAS PubMed.
- S. Apoorva, N.-T. Nguyen and K. R. Sreejith, Lab Chip, 2024, 24, 1833–1866 RSC.
- R. S. P. Malon, S. Sadir, M. Balakrishnan and E. P. Córcoles, BioMed Res. Int., 2014, 2014, 962903 Search PubMed.
- J. Boulestreau, L. Molina, A. Ouedraogo, L. Laramy, I. Grich, T. N. N. Van, F. Molina and M. Kahli, Sci. Rep., 2024, 14, 31233 CrossRef CAS PubMed.
- A. Rajan, J. Vishnu and B. Shankar, Biosensors, 2024, 14, 483 CrossRef CAS PubMed.
- Z. Wang, Y. Dong, X. Sui, X. Shao, K. Li, H. Zhang, Z. Xu and D. Zhang, npj Flexible Electron., 2024, 8, 1–11 CrossRef.
- M. Friedel, I. A. P. Thompson, G. Kasting, R. Polsky, D. Cunningham, H. T. Soh and J. Heikenfeld, Nat. Biomed. Eng., 2023, 7, 1541–1555 CrossRef PubMed.
- Biolinq | Sensing your path to better health, https://www.biolinq.com/, (accessed June 29, 2025).
- Profusa, Inc. | Join The Conversation With Your Body, https://profusa.com/, (accessed June 29, 2025).
- Allez Health, https://www.allezhealth.com/, (accessed June 29, 2025).
- Dexcom Continuous Glucose Monitoring - The most accurate CGM system1, https://www.dexcom.com, (accessed June 29, 2025).
- Epicore Biosystems | Wearable Hydration & Heat Stress Monitor, https://www.epicorebiosystems.com/, (accessed June 29, 2025).
- Nutromics – Solving some of the biggest healthcare challenges, https://www.nutromics.com/, (accessed June 29, 2025).
| This journal is © The Royal Society of Chemistry 2025 |