 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Advances in nanoparticle synthesis assisted by microfluidics
Muhammad Mubashar
Saeed
 *abcd,
Eadaoin
Carthy
*abcd,
Eadaoin
Carthy
 bcd,
Nicholas
Dunne
bcefghijk and
David
Kinahan
bcd,
Nicholas
Dunne
bcefghijk and
David
Kinahan
 bcd
bcd
aML-Labs Centre for Research Training, Dublin City University, Ireland. E-mail: muhammad.saeed3@mail.dcu.ie
bSchool of Mechanical and Manufacturing Engineering, Dublin City University, Ireland
cBiodesign Europe, Dublin City University, Ireland
dRAPID Institute, Dublin City University, Ireland
eCentre for Medical Engineering Research, School of Mechanical and Manufacturing Engineering, Dublin City University, Dublin, Ireland
fAdvanced Manufacturing Research Centre (I-Form), School of Mechanical and Manufacturing Engineering, Dublin City University, Dublin, Ireland
gSFI Research Centre for Medical Devices (CÚRAM), University of Galway, Galway, Ireland
hAdvanced Materials and Bioengineering Research Centre (AMBER), Trinity College Dublin, Dublin, Ireland
iSchool of Pharmacy, McClay Research Centre, Medical Biology Centre, Queen's University Belfast, Belfast, UK
jDepartment of Mechanical and Manufacturing Engineering, School of Engineering, Trinity College Dublin, Dublin, Ireland
kTrinity Centre for Biomedical Engineering, Trinity Biomedical Sciences Institute, Trinity College Dublin, Dublin, Ireland
First published on 6th May 2025
Abstract
The synthesis of nanoparticles (NPs) has garnered significant interest due to their wide-ranging applications across various emerging industries, including pharmaceuticals, electronics, food engineering, agriculture, wastewater management, and medicine. Research efforts have focused on controlling key NP characteristics, such as size, polydispersity, zeta potential, drug release, and encapsulation efficiency, to meet the demands of these applications. In this review, we explore how microfluidics offers a superior alternative to conventional physical, chemical, and biological synthesis methods. We discuss various microfluidic NP manufacturing strategies, broadly classified into passive and active methods. Active methods utilise external energy sources, such as thermal, electrical, electromagnetic, and acoustic inputs. In contrast, passive methods do not use external energy sources and instead rely on techniques like hydrodynamic flow focusing, vortex generation, droplet generation, and chaotic advection. We also highlight the challenges associated with NP synthesis in microfluidic devices. Finally, we examine the potential of integrating microfluidics with machine learning algorithms to develop “intelligent microfluidics” for NP synthesis.
1. Introduction
The design and synthesis of nanoparticles (NPs) are active and innovative research areas, garnering significant attention from numerous academic groups.1–3 NPs have gained prominence due to their unique and tuneable properties,4 including thermal and electrical conductivity,5,6 melting point,7,8 and wettability,9 along with magnetic,10 catalytic,11 and optical properties,12 such as light absorption13 and scattering.14Traditionally, NPs are defined as materials ranging from 1 to 1000 nm in length in at least one dimension;15 however, the preferable diameter range is between 1 and 100 nm.16 The human body consists of nanostructures that play critical roles in body functionality. Therefore, artificially synthesised NPs that can interact with these nanostructures have great potential for applications in biotechnology and biomedical engineering. For example some carbon-based NPs are being used for antimicrobial activities.17 Lipid-based NPs are being employed for several drug delivery applications.18
Several research groups have reviewed different aspects of NP synthesis19–22 including reviews on microfluidics-based synthesis.18,23–27 Building on these reviews, this paper provides a comprehensive overview of NP synthesis using microfluidic devices utilizing different active and passive approaches. Section I introduces the fundamentals of NP technology, as well as focuses on their potential applications in medicines. Section II discusses critical factors in assessing NP performance in biomedical contexts. Section III examines the advantages and limitations of conventional NP synthesis and sorting methods, and section IV provides an in-depth discussion of microfluidic-based NP synthesis.
2. Nanoparticle technology
Nanoparticles can be applied to a diverse range of innovative applications, such as smart drug delivery systems, solar cells and environmental sensors. NPs have applications in biomedical sciences,28 as well as in mechanical,29 food,30 electronics,31 environmental,32,33 energy harvesting34 and aerospace industry.35 Different types of innovative applications of nanoparticles (NPs) can be seen in Fig. 1.Due to the excellent mechanical properties that NPs can offer, they have many applications in coating,36,37 lubricants,38 and adhesives.39 Zirconium carbide (ZrC) NPs have been employed in epoxy-based nanocomposite coatings, demonstrating enhanced mechanical strength and improved corrosion resistance for industrial applications.40 NPs have also proved useful in increasing the mechanical and thermal properties of fiber reinforced polymer composites.41 The performance of metals and polymer matrices also improved by embedding NPs in them.42 NP technology based on alumina, titania and carbon has laid the foundation for the development of desirable coatings.43–45
NPs have revolutionized the food industry in various ways.46 Silver (Ag), titanium dioxide (TiO2), and zinc oxide (ZnO) NPs have been employed in the food packaging industry.47,48 Nanofilms in the form of edible coatings also came up as an innovative strategy to extend the shelf life of perishable products and reduce food waste.49 NPs have also demonstrated potential applications in the field of electronics.50,51 Copper (Cu) and Ag NPs have been employed to make high-performance conductive inks for modern electronic chips52 and flexible printed electronic devices.53 ZnO NPs have been employed for enhancing electron transport and reducing trap density in P3HT-based hybrid thin-film transistors, leading to improved carrier mobility, threshold voltage, and potential use in low-cost non-volatile memory and sensing applications.54 Graphene-based ZnO NPs have been employed for developing thermal smart components with tunable thermal resistance, enabling efficient temperature regulation and energy conservation, showing strong potential for integration into thermal management systems in electronics, spacecraft, and power batteries.55 NPs also play a vital environmental role in a variety of ways.56 NPs have been employed for the mitigation of greenhouse gas emissions because of their ability to adsorb CO2 from industrial processes.57
NPs can also contribute to emerging technology areas such as energy harvesting.34 Zinc ferrite (ZnFe2O4) NPs have been employed in wearable nanogenerators as self-powered sensors.58 They can also be used to store energy at the nanoscale level.34 Silicon (Si) NPs have also been successfully utilized for improving the energy storage performance of lithium ion batteries.59
2.1. Nanoparticle technology in medicine
NPs have a wide range of applications in the biomedical and pharmaceutical industry, including diagnostics,60 magnetic resonance imaging (MRI),61 and drug62,63 and gene delivery.64,65 In Fig. 2, different modern applications of NPs are summarised. NPs enable diagnostics at a precise level, even at the single-cell and single-molecule levels. NPs have shown very successful results for diagnosis and treatment of different type of cancers including breast and pancreatic cancer.60,66,67 Diagnosis and treatment applications of NPs have been recently expanded to bone tumor68 and atherosclerosis treatment.69 Magnetic NPs have been used for enhancing MRI imaging as contrast agents due to their unique magnetic and physicochemical properties.70 Positively charged ZnFe2O NPs demonstrated superior MRI efficacy due to enhanced macrophage affinity, offering improved diagnosis of carotid atherosclerosis.71 Lipid-based NPs,72 peptide-based NPs,73 carbon nanotubes74 and inorganic NPs75 have brought the recent advancements in smart drug delivery systems. Using NP technology for drug delivery has a range of advantages, such as improved biocompatibility, stability, precise targeting and enhanced biocompatibility.76 In cancer treatment, NPs have demonstrated greater efficacy and reduced side effects compared to conventional chemotherapeutic medicines. They can deliver drugs with high precision within desired dose parameters and release profiles, as well as protect drugs from degradation, thereby enabling successful transport to the target.77,78 NPs have also shown potential for use in HIV therapy.79 The anti-viral drug Nevirapine, which is used for AIDS treatment, can be efficiently delivered using cellulose acetate butyrate (CAB) NPs.80 Additionally, dietary supplements can be made more effective through delivery via NPs.81,82 Therapeutics can be delivered using four methods: (1) dissolution, (2) encapsulation, (3) entrapment, or (4) attaching to a NP.83 Nanocapsules, which are reservoir-type devices, consist of a polymer surrounding a cavity where the drug can be contained In contrast, nanospheres, which are matrix-type nanodevices, have the drug uniformly dispersed throughout the structure.84,85 | ||
| Fig. 2 The expanding role of NPs in biomedical applications and industry that is revolutionising healthcare. | ||
While liposomes are commonly used as a drug delivery mechanism, they have drawbacks such as low drug encapsulation efficiency, poor stability in drug loading, and swift leakage of water-soluble components.86 NPs offer an alternative that can mitigate these issues, providing specific advantages in terms of drug loading stability and controlled release at the target site.87 Research on NP-based drug delivery systems focuses on targeted drug delivery while reducing toxicity. Fig. 3 illustrates the application of NPs in a smart drug delivery system. The major challenges in NP synthesis involve controlling particle size, surface characteristics, zeta potential, and the capacity for drug loading and release.88
 | ||
| Fig. 3 Schematic representation of an NP-driven drug delivery system for an advanced therapeutics application. | ||
3. Assessing the performance of nanoparticles in drug delivery systems and other biomedical applications
Various physical and chemical characteristics of NPs determine their suitability across diverse applications. In the biomedical field, particle size and surface properties significantly impact the effectiveness of NPs. For smart drug delivery systems utilising NPs, as shown in Fig. 3, factors such as drug loading and release capacity, stability within the body, biocompatibility, targeting ability, and therapeutic efficacy are essential. These factors are in addition to typical considerations like size, shape, and surface properties. This section highlights five critical factors that significantly influence NP performance: size, shape, surface properties, drug loading, and drug release.3.1. Particle size
The targeting ability and intercellular delivery of drugs in NP-based drug delivery systems depend highly on the NP size and the homogeneity of their size distribution.89 Particle size plays a pivotal role in determining the drug encapsulation and release efficiency,90 as well as their stability.91 Numerous studies have shown that smaller NPs are advantageous for drug release because they can target a wider range of cancer cells and exhibit a higher intercellular uptake compared to larger NPs.92 The smaller NP size also leads to faster drug release, while larger NPs provide a larger surface area for phagocytosis.93 However, larger NP can conjugate to a higher volume of drugs due to the larger surface area.Experimental verification using the Caco-2 cell line94 has shown that NPs with an average size of 100 nm can exhibit a two-three-fold improvement in drug uptake compared to particles that are 1 μm and a six-fold increase in drug uptake than particles of 10 μm size.95 The major risk associated with the small size of NPs is their tendency to aggregate during storage and transportation.96 NPs with a particle size ranging from 2 nm to 100 nm can affect cellular signalling processes. NPs with sizes ranging from 40 nm to 50 nm have been shown to have the greatest effect, especially inducing membrane receptors to down-regulate expression levels. Thus, these NPs can alter downstream signalling and cellular responses in breast cancer cells.97 The size of NPs can also induce phagocytosis, where NPs with a size greater than 200 nm are targeted by the body's lymphatic system and ingested, unlike relatively smaller size NPs.98
3.2. Nanoparticle morphology
NP morphology or shape also has a significant impact on their utilisation as a drug delivery agent. Different important factors, such as cellular uptake, circulation time and biodistribution, targeting efficiency and immune evasion, are directly connected with NP morphology.Investigation on the crucial role of NP morphology and surface chemistry in drug delivery effectiveness, focusing on ocular applications, has been performed. It has been found that the performance of biodegradable block copolymer NPs in cell uptake, drug release, and diffusion significantly depended on their shape and surface chemistry, with high aspect ratio NPs showing enhanced properties.99 In another study on understanding the impact of NP shape on cellular transport and drug release, using pair correlation microscopy, it was discovered that different polymeric-shaped NPs—namely, micelles, vesicles, rods, and worms with identical surface chemistries—navigated cellular barriers at varying rates, thus determining the drug release site. It was found that rods and worms, unlike micelles and vesicles, could passively diffuse into the nucleus. Enhancing nuclear access with a nuclear localisation signal increased the cytotoxicity of doxorubicin by enabling more drug release inside the nucleus, affirming the importance of the nuclear envelope as a critical barrier in drug delivery.100
3.3. Surface properties
Improving the surface properties of NPs can impact their performance and stability in vivo for various reasons. When administrated intravenously, NPs are recognised by the immune system, and phagocytes in circulation start clearing them through phagocytosis.101 To achieve effective targeted drug delivery, it is important to minimise the opsonisation and prolong the circulation of NPs in the bloodstream. Opsonisation refers to the attachment of opsonin protein on the surface of NPs,102 which helps the body's homeostasis system detect and clear NPs.103Fig. 4a illustrates the schematic depiction of the opsonisation-based immune response of the human body. Different techniques can be used to achieve this, including coating the NPs with a hydrophilic polymer or surfactant.104,105Fig. 4b defines how hydrophilic coating helps in the biocompatibility of the NPs. Another approach is to use some capping agent for sustained and targeted drug delivery. In this case, NPs are synthesised with biodegradable copolymers and hydrophilic components,106 such as polyethylene oxide,107 polyethylene glycol (PEG),108 polysorbate,109 and poloxamer.110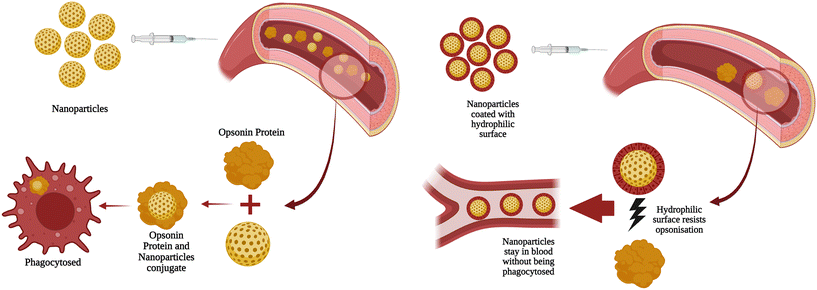 | ||
| Fig. 4 a Schematic depiction of the opsonization process that leads to phagocytosis, b: schematic illustration of the significant role of hydrophilic coating in enhancing biocompatibility. | ||
The zeta potential is the primary measurement associated with the surface charge properties of NPs.111 The surface charge can come from their core or ligand, and sometimes, the NP coating also plays an important role in defining their charge. Oppositely, charged particles are attracted to the surface of NPs, forming a thin layer known as the Stern layer. Since human body cell membranes are negatively charged, positively charged NPs tend to attach themselves to body cells.112 The penetration of NPs in the human body and their intracellular uptake depend on their surface charge. For this reason, the surface charge of NPs plays a crucial role in their applications in diagnostics and therapeutics. The electric potential at the surface boundary of the double layer ranges from +100 mV to −100 mV.113 NPs with a zeta potential exceeding +20 mV or falling below −20 mV are resistant to agglomeration and remain stable in solution due to electrostatic repulsion.114
3.4. Drug loading
The drug loading capacity is an important property in drug delivery systems using NPs. Inefficient drug loading of some nanomedicines poses a hurdle for their practical usage.115 If drug loading is poor, a greater concentration of NPs may be required, resulting in a highly viscous liquid that can cause issues for in vivo injections.116,117 Efficient drug loading reduces manufacturing costs.118 Drug loading capacity is the ratio of the mass of the drug to the total mass of NPs with the loaded drug. Encapsulation efficiency is the ratio of the weight of the drug in the NPs to the weight of the drug added initially during preparation.119 According to experimental data, drug loading and encapsulation efficiency are highly dependent on the polymer molecular weight,120,121 drug–polymer interaction, polymer composition,122 and ending functional groups of the polymer.123,124Three different strategies are employed for drug loading on NPs: (1) pre-loading, (2) co-loading, and (3) post-loading. Fig. 5 provides a visual representation of these three different strategies.
 | ||
| Fig. 5 Schematic illustration of different types of drug loading on the NPs: a) pre-loading, b) co-loading and c) post-loading. | ||
• Pre-loading: drug-loaded NPs are fabricated first, followed by the synthesis of a shell that protects the drug core.125 The drug loading capacity using this technique is proportional to the shell thickness.126
• Co-loading: the drug is loaded into the NPs during their synthesis.125 Covalent bonding plays a pivotal role along with hydrophobicity, π–π interactions, and electrostatic interactions.127
• Post-loading: nanocarriers such as carbon and silica are synthesised and mixed with the solution of the drug.125 The drug is then loaded through various mechanisms such as entrapment, hydrophobic forces, adsorption, and electrostatic interactions.128
3.5. Drug release
The drug release capacity is a crucial factor in the efficiency of NP-based drug delivery systems. Like poor drug loading, inefficient drug release presents a challenge for the practical implementation of various nanomedicines. The drug release efficiency of NPs is also influenced by the chosen drug loading strategy. For nanosphere-shaped NPs, drug release occurs through matrix erosion,129 while in the case of nanocapsules, diffusion of the drug through the polymeric layer controls the drug release.130 Other important factors that generally control the drug delivery are temperature, pH, solubility and desorption of the drug.131,132Auxiliary agents such as polyethylene oxide-propylene oxide (PEO-PPO) can be used to facilitate drug release by weakening the ionic interactions between the drug and NP.133 Recently, smart NP-based drug delivery systems have been developed. Aa an emerging area of research, these are capable of releasing drugs in response to particular physiological triggers.134
4. Nanoparticle synthesis
There is a wide range of different methods for NP synthesis, and the choice of method depends significantly on the desired properties of the NPs and their intended applications. Two main categories based on the starting point of the procedure are: (1) top-down methods and (2) bottom-up methods. In top-down methods, NPs are synthesised by the breaking down of their bulk constituents, whereas in bottom-up methods, atoms are clustered into NPs. Table 1 provides a comprehensive overview of the different NP synthesis methods with associated advantages and disadvantages.| Category | Advantages | Disadvantages | Synthesis method | Applications |
|---|---|---|---|---|
| Physical methods |
Free of solvent contamination
Uniform monodisperse NPs are synthesized No chemical purification is required Large scale production |
Abundant waste
Less economical Potential toxicity High energy consumption Difficult extraction procedure |
Laser ablation | Au, Mg, and Zn NPs synthesized using the pulsed laser ablation in liquid (PLAL) method for antibacterial and biocompatible functionalization of dental implant surfaces135 |
| TeO2 NPs synthesized using the top-ablation and bottom-ablation methods for ultrawide band gap (UWBG) applications136 | ||||
| Spray pyrolysis | NTO NPs for use as high-performance catalyst supports in polymer electrolyte fuel cells.137 | |||
| ZnO/Ag hollow NPs for enhanced antibacterial activity and visible light-assisted wound healing applications138 | ||||
| Magnetron sputtering | W NPs synthesized using magnetron sputtering combined with gas aggregation139 | |||
| ZrC NPs140 | ||||
| Ion implantation | MoC NPs for energy conversion applications.141 | |||
| Ag NPs142 | ||||
| Inert gas condensation | Zn NPs143 | |||
| Bimetallic FeCu for multifunctional magnetic-plasmonic applications in biosensing144 | ||||
| High-energy ball milling | Fe dopped ZnO NPs145 | |||
| Niobium-doped titanium dioxide NPs146 | ||||
| Chemical methods |
Controllable deposit parameters
Narrower size distribution Economical technique Production of NPs with thick coating is possible |
Usage of toxic and hazardous chemical reagents
Long synthesis time Limitation in increasing batch reactors Chemical purification is required Sometimes need to have a large amount of surfactants |
Precipitation methods | MnO NPs for antimicrobial applications against bacterial and fungal pathogens147 |
| CF NPs for enhanced biomedical performance148 | ||||
| Sol gel methods | Niobium-doped strontium titanate (SrTi1−xNbxO3, STNO) NPs for enhanced photocatalytic degradation of dyes and antibiotics under UV-A light irradiation149 | |||
| Electrochemical methods | Ag NPs for eco-friendly antibacterial applications in food, cosmetics, and medicine150 | |||
| Solvothermal synthesis | Ruthenium-doped CF NPs for enhanced nonlinear optical absorption in advanced optical limiting applications151 | |||
| Microemulsion methods | FeO NPs for advanced material applications.152 | |||
| Au NPs153 | ||||
| Biological methods |
Biocompatibility and environment-friendly
Non-toxic and safe reagents Greater stability of NPs Diverse nature of NPs |
Low yield
Long synthesis time Limited scalability |
Plant extract methods | FeO NPs154 |
| Ag NPs155 | ||||
| Microbial synthesis | ZnO NPs156 | |||
| Ag NPs157 | ||||
| Algal and fungal-mediated synthesis | Ag NPs158 | |||
| Au NPs159 | ||||
| ZnO NPs160 |
Based on the detailed working principles, these methods can also be categorised into: (1) physical, (2) chemical, and (3) biological methods. Physical methods of NP synthesis are typically top-down methods, while biological methods can be considered as bottom-up methods. Chemical methods can follow both bottom-up and top-down approaches. Each method has different advantages and limitations. Recently, researchers have focused on the green synthesis of NPs using non-toxic and environment-friendly reagents. Most biological and chemical synthesis methods are considered green methods, depending on their compatibility with green chemistry principles.161–163 In this section, we will briefly discuss these methods. Fig. 6 gives a comprehensive overview of different NP synthesis methods. After NP synthesis, different conventional techniques based on an external field (e.g., ultracentrifugation and gel electrophoresis) and sieving (e.g., chromatography and nanofiltration) techniques are employed for NP sorting with desired characteristics.
4.1. Physical methods
Physical methods employ different kinds of physical processes to apply high-energy radiation to different types of energy, such as thermal energy, electrical energy and mechanical pressure on the material. It results in condensation, abrasion, evaporation or melting of the substrate material, which leads towards the synthesis of NPs. These methods are categorised based on the physical process used and their impact on the substrate material. Laser ablation, spray pyrolysis, magnetron sputtering, ion implantation, inert gas condensation, and high-energy ball milling are very commonly used physical methods for NP synthesis.In the laser ablation method, a laser beam is focused on the target material, causing laser-induced vaporisation and condensation, which leads to the synthesis of NPs.164,165 Gold (Au), magnesium (Mg), and zinc (Zn) NPs have been synthesized using pulsed laser ablation in liquid (PLAL) for antibacterial and biocompatible functionalization of dental implant surfaces.135 Mercury iodide (HgI2) NPs have been synthesised with size 25–75 nm using laser ablation.166 Tellurium dioxide (TeO2) NPs have been synthesized using the top-ablation and bottom-ablation methods for ultrawide band gap (UWBG) applications.136 The spray pyrolysis method involves spraying a precursor material solution using a nano-porous atomiser on the hot substrate of the material.167 This method has been employed to generate iron–doped TiO2 NPs with an average size of 12 nm.168 Carbon-based NPs with an average size of 60 nm have also been prepared by spray pyrolysis.169 Niobium-doped tin oxide (NTO) NPs were synthesized using spray flame synthesis for use as high-performance catalyst supports in polymer electrolyte fuel cells.137 ZnO/Ag hollow NPs were synthesized using a one-step spray pyrolysis method for enhanced antibacterial activity and visible light-assisted wound healing applications.138 Magnetron sputtering involves subjecting a target material to high-energy ions, resulting in the ejection of atoms from the targeted material, and the deposition of these atoms leads to the synthesis of NPs.170 Tungsten NPs (W NPs) and Cu NPs with average sizes of 70 nm and 60 nm have also been synthesized using this method.171,172 Zirconium nitride (ZrN) NPs were synthesized using reactive direct current magnetron sputtering in an argon and nitrogen atmosphere for environmentally friendly, annealing-free production of plasmonic nanofluids with enhanced optoelectronic properties.140 The ion implantation method is based on principles of ion–solid interactions, where high-energy ions are bombarded on the substrate material for NP synthesis.173 Ag NPs and plasmonic-based NPs have been synthesized using this method.174,175 Molybdenum carbide (MoC) NPs were synthesized using ion implantation at room temperature for energy conversion applications.141 In the inert gas condensation method, evaporated materials are transported with inert gases, such as He, Ne, Ar, etc. Liquid nitrogen is used as a cooling medium for condensation.176 Highly size-controlled NPs can be synthesised using this method with particle sizes ranging from 1–5 nm.177 Bimetallic FeCu NPs were synthesized using the inert gas condensation technique for multifunctional magnetic–plasmonic applications in biosensing.144 NP fabrication using high-energy ball milling introduces kinetic energy from the moving balls to break the chemical bonds of the substrate material being milled.178 Otis and Chin fabricated ZnO NPs and iron sulphide-based NPs (e.g., FeS and FeS2 NPs) using high-energy ball milling.179,180
4.2. Chemical methods
Chemical methods for NP synthesis are based on the chemical reaction between different chemical reagents under various chemical and physical conditions that control the physical and chemical properties of NPs. Precipitation methods, sol–gel methods, electrochemical methods, solvothermal synthesis methods, and microemulsion methods are some of the most commonly used and practically feasible chemical methods for NP synthesis. Manganese oxide (MnO) NPs were synthesized using a simple and cost-effective co-precipitation method for antimicrobial applications against bacterial and fungal pathogens.147 Cobalt ferrite (CF) NPs were synthesized using this method with polyvinyl alcohol modulation for tunable morphology, magnetic behavior, and enhanced biomedical performance.148 Niobium-doped strontium titanate (SrTi1−xNbxO3, STNO) NPs were synthesized using a microwave-assisted sol–gel auto-combustion method for enhanced photocatalytic degradation of dyes and antibiotics under UV-A light irradiation.149Electrochemical methods use the electrochemical reaction for NP synthesis. An electric current is passed between the anode and cathode, which are immersed in the electrolyte, a solution of precursor material ions.181 ZnO NPs with a diameter range of 50–100 nm were synthesised using this method.182 Ag NPs were also synthesized using an electrochemical method with Camellia chrysantha flower extract as a green electrolyte, reducing agent, and stabilizer for eco-friendly antibacterial applications in food, cosmetics, and medicine.150 Solvothermal synthesis involves boiling the precursor material in a closed reaction system that creates a high-pressure environment, leading to NP synthesis.183 Iron-doped tin sulphide NPs (SnS NPs) with supercapacitive and magnetic properties have also been used.184 Ruthenium-doped cobalt ferrite NPs were synthesized using a modified solvothermal method for tunable magnetic behaviour and enhanced nonlinear optical absorption in advanced optical limiting applications.151 Microemulsion techniques use the concepts of interfacial chemistry, colloidal science, and thermodynamics for NP synthesis. This system consists of monodispersed spherical droplets of two immiscible liquids, depending on the surfactant.185 Size-controlled Ag NPs have been made by using water in an oil microemulsion.186 Iron oxide (FeO) NPs were synthesized using a microemulsion-hydrothermal method for controlled production of nanorods and nanospheres with tunable size, shape, and magnetic phase for advanced material applications.152
4.3. Biological methods
Biological methods for NP synthesis are the result of a synergistic interaction between nanotechnology and nanobiotechnology. These methods utilise extracts from living organisms—such as algae, fungi, plant extracts, bacteria, viruses, and yeast—or biomolecules like enzymes to synthesise NPs. Biological methods are considered to be green synthesis because they are non-toxic and environmentally friendly. Biological methods can be categorised into four sub-categories depending upon the biological system being used for NP synthesis: (1) plant extract methods, (2) enzyme-based synthesis, (3) microbial synthesis, and (4) algal and fungal mediated synthesis.Plant extract-based methods use plant biomass or plant extract as reducing, stabilizing, and capping agents, leading towards the synthesis of a wide array of inorganic NPs including Au, Ag, TiO2, ZnO, Fe, Cu, Se, and Pd.187 These reducing agents convert metal ions into metal NPs. Among the most promising green-synthesized nanoparticles are FeO NPs which have gained traction for their role in cancer diagnosis and treatment. Importantly, the utilization of waste plant materials makes this type of NP synthesis eco-friendly by addressing concerns related to resource efficiency and environmental impact.154
Biomolecule-based NP synthesis uses various biomolecules, such as nucleic acids, viruses, enzymes and membranes. Biomineralisation188 and biomimicry189 are the foundation phenomena for such synthesis techniques. Microbial-based synthesis employs different types of microorganisms, such as bacteria, yeast and actinomycetes, as bioreactors for NP formation. These microorganisms convert the metal ions to element metal through their cellular activities.190 Algal-mediated NP synthesis uses algae's ability to hyper-accumulate heavy metal ions and their extraordinary capability to transform these metal ions into more malleable forms.191 Fungal-mediated NP synthesis uses various biomolecules produced by fungi, such as enzymes, polysaccharides, and proteins, as reducing agents, stabilisers and templates for NP synthesis.192
4.4. Nanoparticle sorting techniques
While the synthesis is concerned with the fabrication of NPs, sorting is an essential subsequent step to ensure the homogeneity and consistency of NPs, especially for their practical applications. To create batches of NPs with uniform properties after their synthesis, different sorting techniques are applied, which separate NPs based on various physical properties, e.g., size, shape and surface charge. Several conventional methods have been employed for NP sorting that range from physical methods, e.g., centrifugation and electrophoresis, to sieving techniques, e.g., chromatography and nanofiltration. Table 2 provides a comparative overview of the different NP sorting techniques.| Technique | Working principle | Advantages | Disadvantages | NP sorted |
|---|---|---|---|---|
| Ultracentrifugation | Centrifugal force generated by rotation | Formation of concentration gradient | Sample loss | Analytical ultracentrifugation technique for polystyrene-ZIF-8 core–shell NPs193 |
| NPs are separated on the basis of size and shape. | Excellent results for ordering NPs as richer structures | Chances for aggregation are always high | Density-based separation of mRNA-loaded lipid NPs (LNPs)194 | |
| Stable and biocompatible NP synthesis | Expensive and unapplicable for commercial use due to the requirement for specialized equipment | |||
| NP morphology becomes tedious due to irreversible agglomeration or accumulation | ||||
| Gel electrophoresis | External electric field through a porous gel | Multiple runs in parallel | Batch-limited technique due to the requirement of multiple steps | Slab gel electrophoresis technique for TiO2 NPs195 |
| NP separation based on size, shape, charge and exact number of attached polymer chains | Long time required for sample separation | DNA-assisted electrophoresis technique for NPs196 | ||
| Sample retrieval is very complicated | ||||
| Chromatography | Sieving technique | High separation efficiency | Long time requirement | Bed chromatography technique for sorting of magnetic NPs197 |
| NPs are separated in a mobile phase through a stationary phase | Very few chances for NP agglomeration and accumulation | Multiple sample preparation | Size exclusion chromatography for metal-based NPs198 | |
| NPs are separated on the basis of size, shape and surface properties | No sample loss | Specialised beads, antibodies and buffers for separation | Optical chromatography technique using 3D-printed microfluidic devices for particle separation199 | |
| Less expensive technique | ||||
| Nanofiltration | Sieving technique based on membrane filtration | Fast, small amount of solvent required, and can be scaled up for commercialisation | Clogging of nanofiltration membrane | Graphene oxide nanofiltration membrane for Ag NPs200 |
| NPs are separated on the basis of size and shape | Physiochemical reactions between membrane and NPs | Supramolecular membrane for NP separation based on size201 | ||
| NP morphology becomes tedious |
In AUC-SV, complete sedimentation of the sample is achieved using a strong centrifugal field, while AUC-SE is performed in a comparatively low centrifugal field. Although both AUC-SV and AUC-SE are applicable to sort NPs into concentration gradients, AUC-SE has the advantage of inhibiting any transport as the concentration gradient stays constant in the equilibrium state.203–205 Sorting of Au nanorods has been performed by employing multiwavelength analytical ultracentrifugation.206 Optimal precursor concentrations identified by AUC have been used to get well-dispersed polystyren@ZIF-8 core–shell NPs.207 Recently, ultracentrifugation has also been successfully employed for density-based separation of mRNA-loaded lipid nanoparticles (LNPs), enabling resolution into subpopulations with distinct biophysical and functional properties, including differences in in vivo protein expression.194
Despite its widespread use for NP sorting, ultracentrifugation has several limitations that make it less efficient. Separation is based on a density gradient, and achieving a prerequisite density gradient is necessary to achieve higher-quality separation at the nanoscale. Additionally, sample loss is inevitable during the purification process, as large gravity force exposure to particles can result in aggregation. Furthermore, specialised equipment requirements make it impractical for large-scale industry use.208,209
Gel-EP allows parallel runs on the same gel, providing a significant advantage over other conventional techniques for NP synthesis, such as ultracentrifugation, chromatography, and nanofiltration. This advantage is particularly important for mechanism understanding and optimising conditions.215 Recently, Gel-EP has been used to purify photon-upconversion NPs for large-scale analogue and digital bioassays.216 However, this technique is batch-limited and requires multiple steps, making it time-consuming and challenging for sample retrieval.217
Chromatography. This technique achieves sample separation through a stationary phase suspended in a mobile solvent phase. The rate of separation is determined by the particle's partitioning speed through the stationary phase. There are several different types of chromatography for NP synthesis, such as ion-exchange chromatography, size-exclusion chromatography, high-performance liquid chromatography, and affinity chromatography.
A novel magnetic chromatography method has been employed for efficient and large-scale sorting of magnetic NPs.218 Pitkanen provided an extensive review of size-exclusion chromatographic separation and characterisation of noble metal-based NPs and quantum dots.219
Incorporating recent advances in lab on a chip technologies, 3D-printed microfluidic devices have been used to perform optical chromatography for the separation of micron-sized particles based on size and refractive index. These devices, integrated with single-mode optical fibers and designed with hydrodynamic focusing features, demonstrate efficient optofluidic trapping and sorting capabilities, pointing to a promising future for combining chromatography with additive microfluidic platforms.199 This technique can deliver high separation efficiency, but it is time-consuming and requires multiple preparation steps. Additionally, separation requires specialised beads and buffers.220
Nanofiltration. This sieving technique for NP synthesis uses a filtration membrane, and different membrane materials have been synthesised and used for nanofiltration. NPs that are smaller than a particular cut-off size of the nanofilter's pores are allowed to pass through. Yang examined the impact of silver NP size on the nanofiltration performance of graphene oxide/sAG composite membranes, finding the optimal NP size for water flux and dye rejection.221 A robust, reusable nanostructured supramolecular membrane has been developed by Krieg, which is capable of size-selective separation of metal and semiconductor-based NPs, utilising the strength of hydrophobic interactions and the reversible nature of noncovalent bonds for easy disassembly, cleaning, and reassembly.222
Recent advancements in membrane design further enhance this technique's performance. A study demonstrated the development of a polyethersulfone-based nanofiltration membrane functionalised with green-synthesised copper phosphide (CuxP) NPs, which achieved a 98.72% pesticide rejection rate and significantly improved water flux and antifouling properties, showcasing potential for agricultural waste water treatement.223 Additionally, incorporation of calcium carbonate (CaCO3) NPs into thin-film nanocomposite membranes has proven to overcome the traditional trade-off between permeability and selectivity. The resulting membranes demonstrated 1.7-fold higher permeability compared to conventional TFC membranes while maintaining high salt rejection.224
Nanofiltration is considered a fast technique for NP sorting and requires minimal solvent volume. It can be scaled for industrial applications to sort large samples. However, filtration membranes can become easily clogged, leading to particle aggregation and decreased sorting efficiency. Multiple steps are required to sort samples with different particle sizes.
Observing the data presented in Tables 1 and 2 which depicts different NP synthesis and sorting methods, and in light of the critical performance parameters of NPs for drug delivery systems outlined in section 2, it becomes clear that addressing the shortcomings of traditional NP synthesis and sorting techniques is of paramount importance. The concept of an optimal NP synthesis method would ideally allow for facile control of output properties such as size, shape, and surface characteristics while maintaining a minimal usage of reagents. Such a method would not only be economically efficient but also environmentally friendly. Microfluidics is a notable technique for making NPs with particular attributes. It offers a better way of creating and sorting NPs compared to other traditional techniques. The following section provides a comprehensive explanation of NP creation based on microfluidics.
5. Microfluidics
Microfluidics involves the design and fabrication of devices with various geometries of chambers and microchannels for precise control, processing, or manipulation of very small volumes of fluids (10−9 to 10−18 L).225,226 It can reduce and eliminate many of the limitations of conventional techniques, such as sample loss, long duration, and large volume requirement, and can offer better control on NP characteristics, e.g., size, morphology and surface properties, as well as better separation resolution.208 It falls into the chemical synthesis category as it mostly uses the nanoprecipitation principle that dissolved in water-miscible solvents, hydrophobic solutes precipitate when mixed with antisolvents.26,27,227,228Fig. 7 depicts a simple illustration of the microfluidic-based NP synthesis. Different reacting reagents are manipulated in microchannels that lead towards their mixing in a controlled way, which results in the synthesis of NPs. Different types of flow systems can be involved in the microfluidic chip for NP synthesis i.e. single phase flow system, gas liquid flow system, and liquid–liquid flow system.26Table 3 provides a brief overview of the benefits and drawbacks of NP synthesis using microfluidic-based technology.| Advantages | Limitations |
|---|---|
| Very highly reproductive synthesis technique229 | Sometimes fouling and channel clogging230 |
| Reagent consumption is lower than in other techniques | Diffusion of NPs in PDMS matrix231 |
| Easy to control experimental parameters e.g. flow | Not fully automated chip formation232 |
| Rate, pH, temperature, and concentration of reagents233,234 | |
| Less time consumption | |
| Easy to commercialise the production235 |
In a microfluidic system, different factors, such as fluidic resistivity, fluidic diffusivity, and viscosity, play important roles in performance rather than just dominant inertial force, as in the case of typical macrofluidic-based flow systems.236,237 Due to the relatively low velocity combined with the small-scale hydraulic diameter, a low Reynolds number (Re) causes intrinsic laminar flow within the microfluidic channels. Molecular diffusion and advection are two different phenomena responsible for the mass transfer. In molecular diffusion, molecules migrate across the fluidic interface, while in advection, mass is directly carried by fluid flow.238 Usually, molecular diffusion is the main mechanism for mixing, as laminar flows make mixing particularly difficult due to convective mass transfer in the flow direction.239 Mixing is promoted by using different methods, typically by manipulating the flow-through channel geometry or external disturbances.240 According to these methods, microfluidic-based technology is divided into two categories: (1) passive methods without external sources and (2) active methods requiring external sources. The mixing process is considered to be the most critical step for producing NPs with desired physical and chemical characteristics.241 Microfluidic systems have been increasingly investigated as methods to efficiently tune mixing. With exact control of parameters such as fluid concentration ratio, flow rates, and temperature, the ability to rapidly mix fluids in a homogeneous environment can be improved, resulting in improved product quality.242,243 Well-controlled tunability of the nucleation rate, homogeneity and physicochemical characteristics of NPs can be achieved by systematically screening the parameters used for micromixing in microfluidic devices.244,245
As this precise control of NP properties requires a continuous repetition of experiments to tune the flow parameters accordingly, which leads to the waste of time and resources, currently, microfluidic systems are being integrated with artificial intelligence.246 Deep learning and machine learning algorithms, artificial neural networks (ANN), and advanced graphics processing units (GPU) have led to very rapid structured data analysis to predict accurate complex outputs.247 Combining microfluidic chips with data-acquisition instrumentation, along with multimodal monitoring, can lead to advances in the field of online sensing of NP properties.246,248
5.1. Passive microfluidics
Passive microfluidic (PM) chips operate on the principle that embedded micromixers function without any external sources to aid the mixing process.249This type of chip is particularly suitable for fabricating NPs that are sensitive to external factors, such as thermal, acoustic, and electromagnetic conditions. Various geometries and techniques are employed to enhance the mixing of initial fluids in passive microfluidics, making it a cost-effective and efficient alternative to active microfluidic chips.
Four main methods are commonly used to enhance mixing in passive microfluidic systems: hydrodynamic flow focusing, vortex methods, chaotic advection, and droplet-based methods. Table 4 provides examples of NP synthesis using these different passive approaches.
| Passive microfluidic method | NP synthesised |
|---|---|
| Hydrodynamic flow focusing | PEG-PLGA NPs for controlled drug delivery synthesized using a sudden expansion microreactor250 |
| Liposomal NPs for cancer therapy251 | |
| Hybrid NPs for enhancing blood flow and heat transport in stenosed arteries252 | |
| Micro vortices | Salicylic acid-functionalized Fe3O4 core–shell NPs for applications in biomedicine and environmental remediation253 |
| ZnO NPs using spiral microfluidic reactor254 | |
| Chaotic advection | CuO NPs for enhanced antimicrobial and antioxidant activities255 |
| Cationic and stealth liposomes for high-throughput nanomedicine production synthesized using a 3D multihelical chaotic-advection microfluidic device256 | |
| Droplet-based methods | Calcium peroxide (CP)-based oxygen-releasing microgels for tissue engineering applications257 |
| Synthetic cells such as liposomes, polymersomes, and coacervate microdroplets for biomimetic and biomedical applications258 | |
| Cell-encapsulating microcarriers for tissue engineering and regenerative medicine259 |
 | ||
| Fig. 8 Schematic diagrams of a) single and b) double lamination-based HFF; c) schematic diagram of coaxial tube-based HFF; d) schematic diagram of 3D flow focusing, A: particles; B: sheath flow for vertical flow focusing; C and D: sheath flows for horizontal focusing adapted from261 Copyright (2009) Royal Society of Chemistry. | ||
5.1.1.1. Coaxial tube micromixers. Two concentric capillary tubes are used in this type of micromixer, which is connected at the central flow terminal. The inner capillary tube controls the central flow, while the outer tube facilitates the sheathed or side flow (Fig. 8c). The central flow size and pattern can be controlled by different parameters, such as tube geometry, fluid physical properties and FRR.260 As the FRR is directly proportional to the flow rate of the sheath flow, the flow in the central layer becomes narrower and narrower, leading to the formation of jetting flow. High-throughput coaxial tube micromixers have been used for siRNA-polyelectrolyte polyplex NP synthesis.262 Au and Ag NPs have also been synthesized by hydrodynamic flow focusing in coaxial tubes. The properties of these NPs can be tuned by controlling the mixing by varying the diameters of co-axial tubes.263
5.1.1.2. Chip-based hydrodynamic flow focusing. Chip-based flow synthesis is a cost-effective method due to its ease of mass manufacturability. Chip reactors are primarily fabricated using different techniques, such as soft-lithography and replica modelling. Integrating additional on-chip functions, including electrochemical and optical sensors, as well as microheaters, is also possible.264 2D HFF in microfluidic systems is achieved through a horizontally focused central flow.265 The most straightforward design involves a core flow sheathed by two side fluid streams.266 Using this geometry, mixing can be made highly efficient, with a mixing time of less than 50 μs. The sheath-flow channel can have different orientations and be tilted to a different inlet angle other than 90° to ensure stability in the flow profile. 3D HFF can further improve the size uniformity of the NPs by applying both horizontal and vertical focusing at the same time (Fig. 8d). Different techniques are used to make the 3D HFF system more economical by reducing the number of layers. This method is highly effective, fast, and economical as it does not require complex fabrication techniques other than soft lithography.267,268
Using 3D HFF, PLGA- PEG NPs with a size range of 50–150 nm have been successfully synthesised. HFF-based microfluidic systems are capable of providing a robust production rate for NP synthesis up to 288 mg h−1 using a single device, compared to conventional microfluidic devices without HFF.269 Doxorubicin (DOX/PCL) NPs with a uniform size range of 120–320 nm with high drug encapsulation efficiency between 48% and 87% have been achieved using an HFF microfluidic device. Additionally, the production process can result in highly stable encapsulation efficiency of the DOX drug, as the release profile of DOX/PCL NPs was sustained for 10 days at pH 7.4.270
 | ||
| Fig. 9 a) Schematic diagram of secondary flow in a microchannel; b) schematic cross-sectional view of a three-inlet microfluidic system used for the synthesis of lipid–polymer hybrid NPs with the help of microvortices adapted with permission from273 Copyright (2012) American Chemical Society. | ||
Another inertia-based mixing strategy is the introduction of secondary flow along the curved microchannels. A Dean vortex is generated due to the difference in inertia because of the parabolic velocity profile, which leads towards fast central flow and slow side flow. Dean vortices typically manifest as a pair of oppositely rotating vortices, which direct outwards at the centre of the microchannel while inwards near the bottom and top walls. Due to the intrinsic helical chirality in 3D vortices, self-assembly of molecules and hydrodynamic chiral effects are also exerted.274 The difference in flow velocities between the microchamber and the main channel creates laminar vortices (counterclockwise/clockwise) in the opposite microchamber.275 Microvortices can deliver productivity 1000 higher compared to simple diffusion-based mixing in the case of lipid–polymer NP synthesis. An efficient microfluidic platform for NP synthesis based on microvortices was proposed. It was suggested that the NP size can be controlled by regulating the mixing through microvortices (Fig. 9b).273 D microvortice-based microfluidic platforms have been employed for the synthesis of homogenous PLGA NPs with tunable properties.276
 | ||
| Fig. 10 Schematic diagram of different shaped microchannels for better mixing a) zig-zag b) square c) serpentine shaped microchannels. | ||
Modifications in the microchannel wall are a very well-established technique for chaotic advection creation when the flow is laminar with a low Re number. Incorporating slanted grooves and staggered herringbone grooves is commonly used for modifying a microchannel wall, which leads to transversal secondary flow. The stretching of fluidic elements occurs in a transverse direction due to the inclined orientation of the grooves about the channels. This increase in fluid interface enhances mixing through diffusion. Herringbone pattern-shaped microfluidic devices also provide a very controllable and reproducible NP synthesis platform. 3D printing of herringbone patterns is also proposed for lipid–polymer hybrid NP synthesis.285
 | ||
| Fig. 11 a) Co axial tubes, b) hydrodynamic flow focusing, and c) cross-flow based droplet generation in microfluidic devices for NP synthesis. | ||
1. Cross-flowing is based on a T-junction with two channels intersecting at a crossing angle of 90°. From the side of the microchannel, a discrete phase is introduced into an already running continuous phase. There can be different orientations for the introduction of side flow, leading to increased shear stress and accumulated pressure, converting the discrete phase into droplets. Research has been done on the dynamic behaviours of droplet formation in microfluidic cross-junction devices using a two-dimensional numerical model. It has been reported that the junction angle and viscosity ratio significantly influence the droplet size and frequency of generation, and these insights could be pivotal for NP synthesis.289
2. In co-flowing, the discrete and continuous phases are in a parallel manner and use either coaxial microchannel geometries or 3D configurations of tapered cylindrical capillaries that are inserted coaxially into rectangular or cylindrical capillaries. Investigation has been performed on the synthesis of Ag NPs in a coaxial flow reactor, finding that factors such as the flow rate of reagents and concentrations of trisodium citrate and silver nitrate played significant roles in reducing the NP size and dispersity, thereby affecting the resulting properties of the NPs.290
3. In a focusing structure, the discrete phase is compressed into a thin stream surrounded by the continuous phase at the inlet intersection. At the downstream, due to interfacial instability, it subsequently breaks up into droplets.291,292 Droplets have been used from flow-focusing structures for Au NP synthesis, and it found that NPs with smaller sizes can be synthesised compared to co-flowing and cross-flowing structures.293
5.2. Active microfluidics
External sources are involved in active microfluidic devices along with different channel geometries for NP synthesis. These external sources are mainly used to control the mixing of reagents that leads towards the generation of NPs with specific properties. In this section, six different types of active microfluidic devices have been discussed (Fig. 13). Table 5 summarises these six different active microfluidic approaches with some examples of synthesised NPs.| Active approach | Working principle | NP synthesised |
|---|---|---|
| Centrifugal microfluidics | Centrifugal force, along with other pseudo intrinsic forces e.g. Coriolis force and Euler force | Au NP-based colorimetric sensor for enantioselective detection of L-tryptophan294 |
| Cu hydride NPs for antitumor therapy295 | ||
| Pressure microfluidics | Pulsatile flow with the help of micropumps and microvalves | Finger-operated pumping based microfluidic system for PLGA NP and liposome synthesis296 |
| Thermal microfluidics | Thermal energy with the help of electrothermal effects, heat films and thermal bubbles | Microfluidic microreactor device with integrated heaters for temperature assisted synthesis of Au NPs297 |
| Ag NP-loaded alginate fibres for durable antibacterial application298 | ||
| Electromagnetic microfluidics | Electromagnetic field by employing a permanent magnet, integrated electrodes, electromagnets and magnetic stirrers | Magnetic carbon dot (M-CD) NPs for intelligent fluorescence/colorimetric detection of tetracycline299 |
| Chiral plasmonic Au NPs for rapid cancer exosome isolation and analysis300 | ||
| Acoustic microfluidics | Acoustic waves with the help of oscillatory microbubbles, sharp-edge structures, including oscillatory thin membranes, acoustic resonators, and acoustic metamaterials | Au NPs with the help of vibrating sharp-tip acoustic mixing301 |
| Liposome NPs for cancer drug delivery302 | ||
| Electrically-driven microfluidics | Electro-hydrodynamic (EHD), and electro-kinetic instability (EKI) with the methods e.g. electrophoresis, dielectrophoresis, and electroosmosis | Chitosan NPs for effective oral drug delivery and bone tissue regeneration303 |
| Ag NPs for scalable nanofabrication and advanced functional coatings304 |
Dielectrophoresis is the movement of neutral particles caused by an AC electric field. Bilateral motion of particles occurs due to the formation of a dipole moment between electrodes, resulting in stretching and folding of fluids and chaotic advection, thereby increasing the mixing index. Similar to electrophoresis-based micromixers, dielectrophoresis-based micromixers can employ conductive particles to enhance mixing.
Recent advancement in microfluidics for NP synthesis.
3D printed microfluidics. The convergence of 3D printing and microfluidic technologies has opened new frontiers in the controlled synthesis of NPs. These 3D printed microfluidic chips enabled the production of liposome MPs with tunable sizes and high stability by optimizing flow parameters such as the total flow rate and flow rate ratio.359 3D printed microreactors were also integrated with a UV-vis diagnostic system. It enabled droplet-based, high-throughput screening of Au nanoparticle synthesis. This system analysed over 1000 droplets in under an hour, demonstrating how 3D printed setups can rapidly optimize synthesis conditions through automation and multivariate analysis.360
Further advancing the field, hybrid systems combining 3D printing with micromilling techniques were established. It has enabled the design of impinging jet reactors (FIJRs) which offer superior mixing, temperature control, and scalability. These platforms have successfully produced colloidal Ag and FeO NPs with controlled sizes during continuous operation, highlighting their robustness and fouling resistance.361 Moreover, microfluidic chips fabricated for drug-loaded polymeric NP synthesis such as PLGA loaded with pentamidine demonstrated the potential of 3D printed systems to precisely tune formulation characteristics for biomedical applications. By adjusting flow rates and surfactant concentrations, researchers achieved monodisperse nanoparticles with therapeutic relevance and consistent performance.362 Altogether, these examples underscore the growing role of 3D printed microfluidic systems as versatile, scalable, and high-precision tools for nanoparticle synthesis in both industrial and biomedical domains (Table 6).
| Microfluidic technologies | NPs synthesised |
|---|---|
| 3D printed microfluidics | Ag NPs synthesized through high throughput continuous flow using a 3D helical microfluidic chip for enhanced flow-boiling heat transfer in electronic chip322 |
| Liposomal NPs synthesized for drug delivery applications using UV LCD 3D-printed microfluidic chips323 | |
| Modular microfluidics | Si NPs synthesized for photonic crystal applications324 |
| PLGA nanoparticles (33–180 nm) synthesized for drug and vaccine delivery using a low-cost, modular capillary-based microfluidic system325 | |
| Intelligent microfluidics | Coupling of machine learning with microfluidics for NP synthesis using RSM-differential evolution and RSM-NSGA-II enabled multi-objective optimization of micromixer design for enhanced mixing efficiency and energy reduction326 |
| Intelligent microfluidics for PLGA nanoparticle synthesis using TGAN-augmented datasets and predictive models such as decision tree, random forest, and deep neural networks enabled accurate NP size prediction and identification of key synthesis parameters327 | |
| Au NPs synthesized for multiplexed point-of-care detection of cysteamine using an AI-enhanced lab-on-a-disc (AI-LOAD) system328 |
Modular microfluidics. Modular microfluidic systems have emerged as a transformative platform for the precise and scalable synthesis of NPs. Their small reaction volumes and excellent control over fluid dynamics enable highly tunable reaction environments, particularly useful in the production of functional nanomaterials. Recent advancements have shown the strength of microfluidics in fabricating metal nano-electrocatalysts with tailored morphologies and electrochemical properties, offering better control over nucleation, growth, and reaction enhancement mechanisms.363 These systems allow for efficient parameter optimization, real-time control, and continuous operation key features for ensuring reproducibility and structural precision in nanoparticle production. The modularity of microfluidic setups further supports easy integration and reconfiguration of components, making them suitable for adapting to various synthesis protocols.
In drug delivery, modular microfluidics has proven equally impactful. Hydrodynamic focusing within continuous-flow microfluidic chips, such as PLA-based devices, enables the single-step synthesis of lipid nanoparticles under uniform conditions, resulting in consistent particle sizes (≤200 nm) ideal for biomedical applications.364 This streamlined approach not only enhances efficiency but also supports rapid parameter screening for formulation development. Additionally, microfluidic cartridge-based mixers (MCF) have enabled scalable and purification-free layer-by-layer (LbL) surface modification of NPs, essential for targeted and controlled drug delivery. These modular systems facilitate robust electrostatic assembly on diverse NP cores without the need for excess materials or laborious post-processing.365 Overall, modular microfluidic systems stand out as powerful, flexible, and scalable platforms for engineering next-generation nanoparticles across both therapeutic and catalytic domains (Table 6).
Intelligent microfluidics. Intelligent microfluidics has emerged as an advanced approach for the design and synthesis of NPs by leveraging artificial intelligence (AI), machine learning (ML), and computational modeling (Table 6). This integration of AI techniques into microfluidic platforms allows enhanced control over NP characteristics such as size, shape, surface charge, drug loading and release efficiency parameters critical for applications in drug delivery, biosensing, gene therapy, and diagnostics.
Recent advances include the application of data-driven surrogate modelling and multi-objective optimization to refine micromixer geometries, leading to more energy-efficient NP synthesis. These systems have demonstrated over 80% reduction in pressure drop and a 20% improvement in mixing efficiency at low Reynolds numbers, significantly improving the scalability and reproducibility of particle fabrication for hydrogel engineering and therapeutic delivery systems.326
Machine learning algorithms—such as decision trees, random forests, and deep neural networks—have been employed for predicting and tuning the properties of polymeric NPs. PLGA NPs have been modelled to achieve high prediction accuracy in particle size and zeta potential, with ensemble models like BAG-SVR and BAG-PR outperforming traditional regressors.327,366
The convergence of AI with microfluidics is further evident in the development of systems which integrates experimental microfluidic data with random forest models to predict nanomedicine attributes, including hydrodynamic diameter, polydispersity index, and encapsulation efficiency. This framework accelerates formulation development by enabling user-defined design of NP systems.367 Similarly, inverse modelling combined with Latin hypercube sampling and data assimilation has been successfully applied to the synthesis of Ag nanoparticles, facilitating precise control over size prediction and hydrodynamic conditions in microfluidic environments.367
The potential of intelligent microfluidics also extends into diagnostic platforms. The AI-enhanced lab-on-a-disc (AI-LOAD) system exemplifies this innovation, utilizing Au nanoparticles and ML-based pattern recognition to enable multiplexed, rapid colorimetric detection of cysteamine and structurally similar biothiols from minimal whole blood volumes.328 The integration of AI and additive manufacturing has enabled real-time tracking of droplets in droplet microfluidics by AI-assisted fluid simulations, and the fabrication of complex three-dimensional microfluidic architectures. This leads towards more accurate modeling of multiphase flows and designing more efficient microfluidic devices.368
Moreover, AI-assisted formulation strategies are advancing the design of smart, stimuli-responsive polymeric NPs capable of controlled and targeted drug release. These innovations, rooted in modern polymer chemistry and enabled by predictive modelling, offer new opportunities for personalized nanomedicine.369
6. Current challenges and future prospects
Although microfluidic platforms provide a superior alternative for NP synthesis, there are still a few challenges that need to be addressed to make microfluidic-based NP synthesis more efficient. These challenges range from the usage of the conventional world to chip connectors for connectivity of microfluidic devices with the reagents' reservoirs and tubing to the automation of the whole synthesis process based on machine learning-based predictive models. Further research in microfluidic systems aims to automate testing, reduce user intervention to minimise human error and increase NP replicability in production.Microfluidic platforms are primarily operated manually, but there is a growing trend towards coupling microfluidics with machine learning and optical probing. This integration holds the potential for intelligent microfluidic devices, revolutionising nanomaterials, drug discovery, and developmental biology. Integration of microfluidics with artificial intelligence can be a breakthrough in the field of NP synthesis with highly controlled desired properties. As evident from the literature, controllable synthesis of NPs is an area of great research interest, and this can enhance the application of NPs, especially in the field of smart drug delivery systems. To improve NP synthesis in microfluidics, there is a need to investigate further the impact of mixing parameters in microchannels on the properties of NPs. More control during the mixing can easily lead towards better control of NP properties. Machine learning has been coupled with microfluidic based synthesis of PLGA NPs to study the influence of different reaction parameters.370 PLGA NP production has also been optimized by combining design of experiment and machine learning.371 A tabular generative adversarial network has also been employed to predict NP size in microfluidics.327 These machine learning based predictive models for NP properties have laid the foundation for online sensing and automation of microfluidic based NP production.
The widespread adoption of microfluidic devices has been hindered by the high-cost and single-use nature of microfluidic connectors. Different researchers have introduced a variety of different types of microfluidic connectors to address these challenges.372,373 Universal and versatile magnetic microfluidic connectors have also been introduced.374 Along with magnetic connectors, fast plug and play mechanical connectors have also been developed for efficient connections.375 Other than connecting the microfluidic devices with the reservoir syringes, the overall handling and use of microfluidic devices with such small dimensions is also not an easy task. The micrometric scale size of microchannels makes it difficult to clean channels and often leads towards clogging. For instance, there are not enough suitable standards for microfluidic accessories, such as connectors, micropumps, and microvalves. This leads to issues such as misalignment, blockage, leakage, degradation, and dimensional errors. Furthermore, accurate methods for real-time flow rate fluctuation measurement, specifically for passive microfluidic devices where flow rate is critical, are lacking. This additional equipment, such as temperature sensors, ultrasound systems, magnetic fields and automated syringe pumps, adds an extra cost to the microfluidic-based NP synthesis system. Scalability of NP synthesis through microfluidics is also the main industrial challenge. These systems are typically characterised by very small reaction volumes and low flow rates, which results in very limited NP throughput. Complex fluid dynamics and control systems involved in microfluidic devices can be difficult to replicate and maintain while working with large volumes. Integrating microfluidic systems into existing pharmaceutical manufacturing processes and infrastructures can also be very challenging. Adapting and aligning this technology with current NP synthesis systems, quality control standards, and regulatory requirements may require significant modifications to the production setup and additional validation.376
Different strategies have been used to overcome this challenge for scaling up the NP synthesis in microfluidics. Scaled-up synthesis of hollow Au NPs using both batch and microfluidic approaches has been investigated. It has been found that the microfluidic approach offers a 10-fold increase in the throughput of hollow Au NPs with the same desirable features by increasing the diameter and length of the microchannels.377 A more appealing and practical approach, rather than increasing channel size, which can impair heat and mass transfer, is parallelisation, where multiple microfluidic reactors are operated simultaneously on a single microfluidic chip. A scalable parallelised microfluidic device featuring an array of 128 mixing channels was employed for lipid-based NPs. This innovative device incorporated an array of staggered herringbone micromixer channels and flow resistors to operate simultaneously. This approach resulted in a 100-fold throughput of lipid-based NPs compared to single microfluidic channels without compromising the desired properties of the lipid-based NPs.378 In recent years, there has been a significant increase in potential microfluidic devices, as reflected in the rising number of patent submissions to the US Food and Drug Administration. However, the lack of regulation and standardisation in microfluidic-based platform design has impeded the commercial success of these devices.
This gap has created a strong demand for universally accessible tools and software to evaluate microfluidic devices consistently. The microfluidic research community faces the challenge of establishing well-defined standards, which are essential for facilitating communication among various microfluidic professionals (i.e., academics, researchers, and industry) throughout different stages of product development. Addressing this challenge is crucial for mitigating a wide range of problems.
Overall, microfluidic technology revolutionises NP synthesis by overcoming the limitations of conventional methods—reducing sample loss, streamlining lengthy procedures, and eliminating the need for costly, multi-step equipment. By enabling precise control over NP characteristics, microfluidic platforms pave the way for highly efficient and targeted drug delivery systems, setting new standards in biomedical applications.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This publication has emanated from research conducted with the financial support of Taighde Éireann – Research Ireland under Grant number 18/CRT/6183. For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. Fig. 2 Created in BioRender. Saeed, M. (2025) https://BioRender.com/ob25b1x. Fig. 3 Created in BioRender. Saeed, M. (2025) https://BioRender.com/tscjlbw. Fig. 4 Created in BioRender. Saeed, M. (2025) https://BioRender.com/bejyyjn. Fig. 5 Created in BioRender. Saeed, M. (2025) https://BioRender.com/ajcyh5m. Fig. 6 Created in BioRender. Saeed, M. (2025) https://BioRender.com/kmfnokr. Fig. 8a–c Created in BioRender. Saeed, M. (2025) https://BioRender.com/3fez1ct. Fig. 11 Created in BioRender. Saeed, M. (2025) https://BioRender.com/p0ro0hg.References
- V. Mohanraj and Y. Chen, Nanoparticles-a review, Trop. J. Pharm. Res., 2006, 5(1), 561–573 Search PubMed.
- S. Vijayaram, et al., Applications of green synthesized metal nanoparticles—a review, Biol. Trace Elem. Res., 2024, 202(1), 360–386 CrossRef CAS.
- F. Eker, et al., A comprehensive review of nanoparticles: from classification to application and toxicity, Molecules, 2024, 29(15), 3482 CrossRef CAS.
- Q. Liu, J. Guan, R. Song, X. Zhang and S. Mao, Physicochemical properties of nanoparticles affecting their fate and the physiological function of pulmonary surfactants, Acta Biomater., 2022, 140, 76–87 CrossRef CAS.
- H. Ebadi-Dehaghani and M. Nazempour, Thermal Conductivity of Nanoparticles Filled Polymers, INTECH Open Access Publisher, 2012 Search PubMed.
- Y. Geng, et al., A comprehensive presentation on nanoparticles electrical conductivity of nanofluids: Statistical study concerned effects of temperature, nanoparticles type and solid volume concentration, Phys. A, 2020, 542, 123432 CrossRef CAS.
- Z. Qiao, H. Feng and J. Zhou, Molecular dynamics simulations on the melting of gold nanoparticles, Phase Transitions, 2014, 87(1), 59–70 CrossRef CAS.
- S. Alavi and D. L. Thompson, Molecular dynamics simulations of the melting of aluminum nanoparticles, J. Phys. Chem. A, 2006, 110(4), 1518–1523 CrossRef CAS.
- S. Kim, et al., Effects of nanoparticle deposition on surface wettability influencing boiling heat transfer in nanofluids, Appl. Phys. Lett., 2006, 89(15), 153107 CrossRef.
- L. Gloag, et al., Advances in the application of magnetic nanoparticles for sensing, Adv. Mater., 2019, 31(48), 1904385 CrossRef CAS.
- P. Suchomel, et al., Simple size-controlled synthesis of Au nanoparticles and their size-dependent catalytic activity, Sci. Rep., 2018, 8(1), 1–11 CAS.
- K. Naim, et al., Supramolecular confinement within chitosan nanocomposites enhances singlet oxygen generation, ChemPlusChem, 2018, 83(5), 418–422 CrossRef CAS PubMed.
- P. Yu, et al., Effects of plasmonic metal core-dielectric shell nanoparticles on the broadband light absorption enhancement in thin film solar cells, Sci. Rep., 2017, 7(1), 1–10 CrossRef CAS PubMed.
- P. M. Carvalho, et al., Application of light scattering techniques to nanoparticle characterization and development, Front. Chem., 2018, 6, 237 CrossRef.
- I. Khan, K. Saeed and I. Khan, Nanoparticles: properties, applications and toxicities, Arabian J. Chem., 2019, 12, 908–931 CrossRef CAS.
- J. Jeevanandam, et al., Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations, Beilstein J. Nanotechnol., 2018, 9(1), 1050–1074 CrossRef CAS.
- N. A. Lopes and A. Brandelli, Nanostructures for delivery of natural antimicrobials in food, Crit. Rev. Food Sci. Nutr., 2018, 58(13), 2202–2212 CrossRef CAS.
- S. Mehraji and D. L. DeVoe, Microfluidic synthesis of lipid-based nanoparticles for drug delivery: Recent advances and opportunities, Lab Chip, 2024, 24(5), 1154–1174 RSC.
- N. Rajput, Methods of preparation of nanoparticles-a review, Int. J. Adv. Eng. Technol., 2015, 7(6), 1806 Search PubMed.
- S. Hasan, A review on nanoparticles: their synthesis and types, Res. J. Recent Sci., 2015, 2277, 2502 Search PubMed.
- S. A. M. Ealia and M. P. Saravanakumar, A review on the classification, characterisation, synthesis of nanoparticles and their application, IOP Conf. Ser.: Mater. Sci. Eng., 2017, 263(3), 032019 CAS.
- W. H. De Jong and P. J. Borm, Drug delivery and nanoparticles: applications and hazards, Int. J. Nanomed., 2008, 3(2), 133–149 CrossRef.
- E. Chiesa, R. Dorati, S. Pisani, B. Conti, G. Bergamini, T. Modena and I. Genta, The microfluidic technique and the manufacturing of polysaccharide nanoparticles, Pharmaceutics, 2018, 10(4), 267 CrossRef CAS.
- R. Abedini-Nassab, M. Pouryosef Miandoab and M. Şaşmaz, Microfluidic synthesis, control, and sensing of magnetic nanoparticles: A review, Micromachines, 2021, 12(7), 768 CrossRef PubMed.
- S. Hettiarachchi, H. Cha, L. Ouyang, A. Mudugamuwa, H. An, G. Kijanka, N. Kashaninejad, N. T. Nguyen and J. Zhang, Recent microfluidic advances in submicron to nanoparticle manipulation and separation, Lab Chip, 2023, 23(5), 982–1010 RSC.
- A. Fabozzi, F. Della Sala, M. di Gennaro, M. Barretta, G. Longobardo, N. Solimando, M. Pagliuca and A. Borzacchiello, Design of functional nanoparticles by microfluidic platforms as advanced drug delivery systems for cancer therapy, Lab Chip, 2023, 23(5), 1389–1409 RSC.
- Y. Liu, G. Yang, Y. Hui, S. Ranaweera and C. X. Zhao, Microfluidic nanoparticles for drug delivery, Small, 2022, 18(36), 2106580 CrossRef CAS PubMed.
- A. Meher, A. Tandi, S. Moharana, S. Chakroborty, S. S. Mohapatra, A. Mondal, S. Dey and P. Chandra, Silver nanoparticle for biomedical applications: A review. Hybrid, Advances, 2024, 100184 Search PubMed.
- B. Mishra Sunil, K. S. S. Liyakat and K. K. S. Liyakat, Nanotechnology's Importance in Mechanical Engineering, Development, 2024, 4, 5 Search PubMed.
- S. S. Rout and K. C. Pradhan, A review on antimicrobial nano-based edible packaging: Sustainable applications and emerging trends in food industry, Food Control, 2024, 11047 Search PubMed.
- M. M. Ghobashy, S. A. Alkhursani, H. A. Alqahtani, T. K. El-damhougy and M. Madani, Gold nanoparticles in microelectronics advancements and biomedical applications, Mater. Sci. Eng., B, 2024, 301, 117191 CrossRef CAS.
- D. S. Chaudhari, R. P. Upadhyay, G. Y. Shinde, M. B. Gawande, J. Filip, R. S. Varma and R. Zboril, A review on sustainable iron oxide nanoparticles: synthesis and application in organic catalysis and environmental remediation, Green Chem., 2024, 2024(26), 7579–7655 RSC.
- S. Singh, H. Tiwari, A. Verma, P. Gupta, A. Chattopadhaya, A. Singh, S. Singh, B. Kumar, A. Mandal, R. Kumar and A. K. Yadav, Sustainable synthesis of novel green-based nanoparticles for therapeutic interventions and environmental remediation, ACS Synth. Biol., 2024, 13(7), 1994–2007 CrossRef CAS.
- W. Hussain, S. Algarni, G. Rasool, H. Shahzad, M. Abbas, T. Alqahtani and K. Irshad, Advances in nanoparticle-enhanced thermoelectric materials from synthesis to energy harvesting: a review, ACS Omega, 2024, 9(10), 11081–11109 CrossRef CAS PubMed.
- B. Monteiro and S. Simões, Recent Advances in Hybrid Nanocomposites for Aerospace Applications, Metals, 2024, 14(11), 1283 CrossRef CAS.
- P. Biehl, M. Von der Lühe, S. Dutz and F. H. Schacher, Synthesis, characterization, and applications of magnetic nanoparticles featuring polyzwitterionic coatings, Polymers, 2018, 10(1), 91 CrossRef PubMed.
- A. M. ElSawy, N. F. Attia, H. I. Mohamed, M. Mohsen and M. H. Talaat, Innovative coating based on graphene and their decorated nanoparticles for medical stent applications, Mater. Sci. Eng., C, 2019, 96, 708–715 CrossRef CAS PubMed.
- A. Singh, P. Chauhan and T. G. Mamatha, A review on tribological performance of lubricants with nanoparticles additives, Mater. Today: Proc., 2020, 25, 586–591 CAS.
- W. Yang, M. Rallini, M. Natali, J. Kenny, P. Ma, W. Dong, L. Torre and D. Puglia, Preparation and properties of adhesives based on phenolic resin containing lignin micro and nanoparticles: A comparative study, Mater. Des., 2019, 161, 55–63 CrossRef CAS.
- J. R. Xavier, Improvement in corrosion resistance and mechanical properties of epoxy coatings on steel with the addition of thiadiazole treated ZrC, J. Mater. Eng. Perform., 2023, 32(9), 3980–3994 CrossRef CAS.
- S. S. Selvan and I. R. Vedaraj, Effects of nanoparticles on the mechanical and thermal behavior of fiber reinforced polymer composites–A review, Mater. Today: Proc., 2023 DOI:10.1016/j.matpr.2023.07.008.
- N. H. Khdary, B. T. Almuarqab and G. El Enany, Nanoparticle-embedded polymers and their applications: a review, Membranes, 2023, 13(5), 537 CrossRef CAS PubMed.
- M. Kot, Ł. Major, J. M. Lackner, K. Chronowska-Przywara, M. Janusz and W. Rakowski, Mechanical and Tribological Properties of Carbon-Based Graded Coatings, J. Nanomater., 2016, 2016(1), 8306345 Search PubMed.
- S. Mallakpour and F. Sirous, Surface coating of α-Al2O3 nanoparticles with poly (vinyl alcohol) as biocompatible coupling agent for improving properties of bio-active poly (amide-imide) based nanocomposites having l-phenylalanine linkages, Prog. Org. Coat., 2015, 85, 138–145 CrossRef CAS.
- W. Shao, D. Nabb, N. Renevier, I. Sherrington, Y. Fu and J. Luo, Mechanical and anti-corrosion properties of TiO2 nanoparticle reinforced Ni coating by electrodeposition, J. Electrochem. Soc., 2012, 159(11), D671 CrossRef CAS.
- A. M. Brindhav, S. Sharma, S. Azizi and V. S. Rana, Unveiling the Cutting-edge Applications of Nanotechnology in the Food Industry-From Lab to Table-a comprehensive review, J. Agric. Food Res., 2025, 1018 Search PubMed.
- Q. Gao, Z. Feng, J. Wang, F. Zhao, C. Li and J. Ju, Application of nano-ZnO in the food preservation industry: antibacterial mechanisms, influencing factors, intelligent packaging, preservation film and safety, Crit. Rev. Food Sci. Nutr., 2024, 1–27 Search PubMed.
- H. G. El Gohary, I. A. Alhagri, T. F. Qahtan, A. N. Al-Hakimi, A. Saeed, F. Abolaban, E. M. Alshammari and G. M. Asnag, Reinforcement of structural, thermal and electrical properties and antibacterial activity of PVA/SA blend filled with hybrid nanoparticles (Ag and TiO2 NPs): Nanodielectric for energy storage and food packaging industries, Ceram. Int., 2023, 49(12), 20174–20184 CrossRef CAS.
- R. P. Mahato, P. Singh and S. Srivastava, Prospects and Challenges of Nanofilms-based Edible Food Coatings for Enhancement of Their Shelf Life, in The Nanotechnology Driven Agriculture, 2024, pp. 204–224 Search PubMed.
- N. Hossain, M. H. Mobarak, M. A. Mimona, M. A. Islam, A. Hossain, F. T. Zohura and M. A. Chowdhury, Advances and significances of nanoparticles in semiconductor applications–A review, Results Eng., 2023, 19, 101347 CrossRef CAS.
- S. Nazir, J. M. Zhang, M. Junaid, S. Saleem, A. Ali, A. Ullah and S. Khan, Metal-based nanoparticles: basics, types, fabrications and their electronic applications, Z. Phys. Chem., 2024, 238(6), 965–995 CrossRef CAS.
- A. Bobsin, T. C. Rodrigues, I. J. Fernandes, S. B. Ferreira, C. R. Peter, W. Hasenkamp and C. A. Moraes, Copper and silver microparticles for high-performance conductive inks in electronic chip shielding, Mater. Chem. Phys., 2024, 315, 129007 CrossRef CAS.
- L. Wang, F. Xia, W. Xu, G. Wang, S. Hong, F. Cheng, B. Wu and N. Zheng, Antioxidant High-Conductivity Copper Pastes Based on Core–Shell Copper Nanoparticles for Flexible Printed Electronics, Adv. Funct. Mater., 2023, 33(26), 2215127 CrossRef CAS.
- M. Ba, S. Mansouri, A. Jouili, Y. Yousfi, L. Chouiref, M. Jdir, M. Erouel, F. Yakuphanoglu and L. E. Mir, Controlling of hysteresis by varying ZnO-nanoparticles amount in P3HT: ZnO hybrid thin-film transistor: modeling, J. Electron. Mater., 2023, 52(2), 1203–1215 CrossRef CAS.
- Z. T. Zhang, Y. X. Dong and B. Y. Cao, Promoting temperature control and energy conservation by smart thermal management using nanoparticle suspensions with tunable thermal conductivity, Appl. Energy, 2024, 374, 124097 CrossRef CAS.
- D. Kirubakaran, J. B. A. Wahid, N. Karmegam, R. Jeevika, L. Sellapillai, M. Rajkumar and K. J. SenthilKumar, A comprehensive review on the green synthesis of nanoparticles: advancements in biomedical and environmental applications, Biomed. Mater. & Devices, 2025, 1–26 Search PubMed.
- Y. Sun, Y. Jiang, H. Wei, Z. Zhang, S. Irshad, X. Liu, Y. Xie, Y. Rui and P. Zhang, Nano-enabled strategies for greenhouse gases emission mitigation: a comprehensive review, Nano Today, 2024, 57, 102378 CrossRef CAS.
- N. K. Das, S. Veeralingam and S. Badhulika, Zinc ferrite nanoparticle-based wearable piezoelectric nanogenerators as self-powered sensors to monitor human motion, ACS Appl. Nano Mater., 2023, 6(14), 13431–13442 CrossRef.
- A. Fereydooni, C. Yue and Y. Chao, A Brief Overview of Silicon Nanoparticles as Anode Material: A Transition from Lithium-Ion to Sodium-Ion Batteries, Small, 2024, 20(17), 2307275 CrossRef CAS.
- N. Rashidi, M. Davidson, V. Apostolopoulos and K. Nurgali, Nanoparticles in cancer diagnosis and treatment: Progress, challenges, and opportunities, J. Drug Delivery Sci. Technol., 2024, 105599 CrossRef CAS.
- S. Wang, et al., Fe2+-Dominated Relaxometric Properties of Iron Oxide Nanoparticles as MRI Contrast Agents, J. Phys. Chem. Lett., 2024, 15(34), 8861–8866 CrossRef CAS.
- M. A. Beach, U. Nayanathara, Y. Gao, C. Zhang, Y. Xiong, Y. Wang and G. K. Such, Polymeric nanoparticles for drug delivery, Chem. Rev., 2024, 124(9), 5505–5616 CrossRef CAS.
- V. Rahimkhoei, et al., Advances in inorganic nanoparticles-based drug delivery in targeted breast cancer theranostics, Adv. Colloid Interface Sci., 2024, 103204 CrossRef CAS PubMed.
- S. M. Naghib, et al., Chitosan-based smart stimuli-responsive nanoparticles for gene delivery and gene therapy: Recent progresses on cancer therapy, Int. J. Biol. Macromol., 2024, 134542 CrossRef CAS PubMed.
- C. M. Jogdeo, et al., Beyond lipids: exploring advances in polymeric gene delivery in the lipid nanoparticles era, Adv. Mater., 2024, 36(31), 2404608 CrossRef CAS.
- X. Gu and T. Minko, Targeted nanoparticle-based diagnostic and treatment options for pancreatic cancer, Cancers, 2024, 16(8), 1589 CrossRef CAS PubMed.
- L. L. Panigrahi, et al., Nanoparticle-mediated diagnosis, treatment, and prevention of breast cancer, Nanoscale Adv., 2024, 6(15), 3699–3713 RSC.
- Y. Guan, et al., Nanoparticles and bone microenvironment: a comprehensive review for malignant bone tumor diagnosis and treatment, Mol. Cancer, 2024, 23(1), 246 CrossRef.
- T. Aili, et al., Recent advances of self-assembled nanoparticles in the diagnosis and treatment of atherosclerosis, Theranostics, 2024, 14(19), 7505 CrossRef CAS.
- Q. Zhang, et al., Renal clearable magnetic nanoparticles for magnetic resonance imaging and guided therapy, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2024, 16(1), e1929 CAS.
- L. Wen, et al., Tailoring zinc ferrite nanoparticle surface coating for macrophage-affinity magnetic resonance imaging of atherosclerosis, ACS Appl. Mater. Interfaces, 2024, 16(11), 13496–13508 CrossRef CAS.
- I. Waheed, et al., Lipid-based nanoparticles as drug delivery carriers for cancer therapy, Front. Oncol., 2024, 14, 1296091 CrossRef CAS.
- P. Kesharwani, et al., αvβ3 integrin targeting RGD peptide-based nanoparticles as an effective strategy for selective drug delivery to tumor microenvironment, J. Drug Delivery Sci. Technol., 2024, 105663 CrossRef CAS.
- L. Sonowal and S. Gautam, Advancements and challenges in carbon nanotube-based drug delivery systems, Nano-Struct. Nano-Objects, 2024, 38, 101117 CrossRef CAS.
- M. H. Karami, M. Abdouss and B. Maleki, The state of the art metal nanoparticles in drug delivery systems: A comprehensive review, Nanomed. J., 2024, 11(3), 222–249 CAS.
- Y. Dang and J. Guan, Nanoparticle-based drug delivery systems for cancer therapy, Smart Mater. Med., 2020, 1, 10–19 CrossRef PubMed.
- J. Dai, M. Ashrafizadeh, A. R. Aref, G. Sethi and Y. N. Ertas, Peptide-functionalized,-assembled and-loaded nanoparticles in cancer therapy, Drug Discovery Today, 2024, 103981 CrossRef CAS.
- X. Huang, T. He, X. Liang, Z. Xiang, C. Liu, S. Zhou, R. Luo, L. Bai, X. Kou, X. Li and R. Wu, Advances and applications of nanoparticles in cancer therapy, MedComm: Oncol., 2024, 3(1), e67 CAS.
- X. Wei, G. Zhang, D. Ran, N. Krishnan, R. H. Fang, W. Gao, S. A. Spector and L. Zhang, T-cell-mimicking nanoparticles can neutralize HIV infectivity, Adv. Mater., 2018, 30(45), 1802233 CrossRef PubMed.
- J. Varshosaz, S. Taymouri, E. Jafari, A. Jahanian-Najafabadi and A. Taheri, Formulation and characterization of cellulose acetate butyrate nanoparticles loaded with nevirapine for HIV treatment, J. Drug Delivery Sci. Technol., 2018, 48, 9–20 CrossRef CAS.
- R. F. Gonçalves, J. T. Martins, C. M. Duarte, A. A. Vicente and A. C. Pinheiro, Advances in nutraceutical delivery systems: From formulation design for bioavailability enhancement to efficacy and safety evaluation, Trends Food Sci. Technol., 2018, 78, 270–291 CrossRef.
- H. Rostamabadi, S. R. Falsafi and S. M. Jafari, Starch-based nanocarriers as cutting-edge natural cargos for nutraceutical delivery, Trends Food Sci. Technol., 2019, 88, 397–415 CrossRef CAS.
- A. P. Ramos, M. A. Cruz, C. B. Tovani and P. Ciancaglini, Biomedical applications of nanotechnology, Biophys. Rev., 2017, 9(2), 79–89 CrossRef CAS PubMed.
- Z. U. Rahman, N. Wei, Z. Li, W. Sun and D. Wang, Preparation of hollow mesoporous silica nanospheres: Controllable template synthesis and their application in drug delivery, New J. Chem., 2017, 41(23), 14122–14129 RSC.
- M. M. F. A. Baig, S. Khan, M. A. Naeem, G. J. Khan and M. T. Ansari, Vildagliptin loaded triangular DNA nanospheres coated with eudragit for oral delivery and better glycemic control in type 2 diabetes mellitus, Biomed. Pharmacother., 2018, 97, 1250–1258 CrossRef CAS PubMed.
- L. Sercombe, T. Veerati, F. Moheimani, S. Y. Wu, A. K. Sood and S. Hua, Advances and challenges of liposome assisted drug delivery, Front. Pharmacol., 2015, 6, 286 Search PubMed.
- M. Alavi and M. Hamidi, Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles, Drug Metab. Pers. Ther., 2019, 34(1), 20180032 Search PubMed.
- J. Safari and Z. Zarnegar, Advanced drug delivery systems: Nanotechnology of health design A review, J. Saudi Chem. Soc., 2014, 18(2), 85–99 CrossRef CAS.
- X. Y. Sun, Q. Z. Gan and J. M. Ouyang, Size-dependent cellular uptake mechanism and cytotoxicity toward calcium oxalate on Vero cells, Sci. Rep., 2017, 7(1), 41949 CrossRef CAS PubMed.
- Y. Hui, X. Yi, F. Hou, D. Wibowo, F. Zhang, D. Zhao, H. Gao and C. X. Zhao, Role of nanoparticle mechanical properties in cancer drug delivery, ACS Nano, 2019, 13(7), 7410–7424 CrossRef CAS PubMed.
- I. P. Mukha, A. M. Eremenko, N. P. Smirnova, A. I. Mikhienkova, G. I. Korchak, V. F. Gorchev and A. Y. Chunikhin, Antimicrobial activity of stable silver nanoparticles of a certain size, Appl. Biochem. Microbiol., 2013, 49, 199–206 CrossRef CAS.
- J. Panyam and V. Labhasetwar, Biodegradable nanoparticles for drug and gene delivery to cells and tissue, Adv. Drug Delivery Rev., 2003, 55(3), 329–347 CrossRef CAS PubMed.
- C. Buzea and I. Pacheco, Nanomaterial and nanoparticle: origin and activity, in Nanoscience and Plant–Soil Systems, 2017, pp. 71–112 Search PubMed.
- T. Lea, Caco-2 cell line, in The Impact of Food Bioactives on Health: In vitro and Ex vivo Models, 2015, pp. 103–111 Search PubMed.
- M. P. Desai, V. Labhasetwar, E. Walter, R. J. Levy and G. L. Amidon, The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent, Pharm. Res., 1997, 14, 1568–1573 CrossRef CAS PubMed.
- Y. Zare, Study of nanoparticles aggregation/agglomeration in polymer particulate nanocomposites by mechanical properties, Composites, Part A, 2016, 84, 158–164 CrossRef CAS.
- W. Jiang, B. Y. Kim, J. T. Rutka and W. C. Chan, Nanoparticle-mediated cellular response is size-dependent, Nat. Nanotechnol., 2008, 3(3), 145–150 CrossRef CAS.
- A. Prokop and J. M. Davidson, Nanovehicular intracellular delivery systems, J. Pharm. Sci., 2008, 97(9), 3518–3590 CrossRef CAS PubMed.
- R. Ridolfo, S. Tavakoli, V. Junnuthula, D. S. Williams, A. Urtti and J. C. van Hest, Exploring the impact of morphology on the properties of biodegradable nanoparticles and their diffusion in complex biological medium, Biomacromolecules, 2020, 22(1), 126–133 CrossRef PubMed.
- M. C. Arno, M. Inam, A. C. Weems, Z. Li, A. L. Binch, C. I. Platt, S. M. Richardson, J. A. Hoyland, A. P. Dove and R. K. O'Reilly, Exploiting the role of nanoparticle shape in enhancing hydrogel adhesive and mechanical properties, Nat. Commun., 2020, 11(1), 1420 CrossRef CAS PubMed.
- Y. Liu, J. Hardie, X. Zhang and V. M. Rotello, Effects of engineered nanoparticles on the innate immune system, Semin. Immunol., 2017, 34, 25–32 CrossRef CAS.
- M. H. Bakalar, et al., Size-dependent segregation controls macrophage phagocytosis of antibody- opsonized targets, Cell, 2018, 174(1), 131–142.e13 CrossRef CAS PubMed.
- D. A. Hume, K. M. Irvine and C. Pridans, The mononuclear phagocyte system: the relationship between monocytes and macrophages, Trends Immunol., 2019, 40(2), 98–112 CrossRef CAS PubMed.
- S. B. Somvanshi, et al., Hydrophobic to hydrophilic surface transformation of nano-scale zinc ferrite via oleic acid coating: magnetic hyperthermia study towards biomedical applications, Ceram. Int., 2020, 46(6), 7642–7653 CrossRef CAS.
- S. Y. Fam, et al., Stealth coating of nanoparticles in drug-delivery systems, Nanomaterials, 2020, 10(4), 787 CrossRef CAS.
- D. R. Perinelli, et al., PEGylated polylactide (PLA) and poly (lactic-co-glycolic acid)(PLGA) copolymers for the design of drug delivery systems, J. Pharm. Invest., 2019, 49(4), 443–458 CrossRef CAS.
- J. Hu, et al., Poly (ethylene oxide)-based composite polymer electrolytes embedding with ionic bond modified nanoparticles for all-solid-state lithium-ion battery, J. Membr. Sci., 2019, 575, 200–208 CrossRef CAS.
- N. Wilkosz, et al., Molecular insight into drug-loading capacity of PEG–PLGA nanoparticles for itraconazole, J. Phys. Chem. B, 2018, 122(28), 7080–7090 CrossRef CAS PubMed.
- S. Ray, et al., Polysorbate 80 coated crosslinked chitosan nanoparticles of ropinirole hydrochloride for brain targeting, J. Drug Delivery Sci. Technol., 2018, 48, 21–29 CrossRef CAS.
- G. L. Allotey-Babington, et al., Cancer chemotherapy: Effect of poloxamer modified nanoparticles on cellular function, J. Drug Delivery Sci. Technol., 2018, 47, 181–192 CrossRef CAS.
- J. D. Clogston and A. K. Patri, Zeta potential measurement, in Characterization of Nanoparticles Intended for Drug Delivery, Springer, 2011, pp. 63–70 Search PubMed.
- E. Fröhlich, The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles, Int. J. Nanomed., 2012, 5577–5591 CrossRef.
- R. Xu, Progress in nanoparticles characterization: Sizing and zeta potential measurement, Particuology, 2008, 6(2), 112–115 CrossRef CAS.
- M. C. Smith, et al., Zeta potential: a case study of cationic, anionic, and neutral liposomes, Anal. Bioanal. Chem., 2017, 409(24), 5779–5787 CrossRef CAS PubMed.
- G. Yang, et al., Bioinspired core–shell nanoparticles for hydrophobic drug delivery, Angew. Chem., 2019, 131(40), 14495–14502 CrossRef.
- C. Buzea, I. Pacheco and K. Robbie, Nanomaterials and nanoparticles: sources and toxicity, Biointerphases, 2007, 90(2), MR17–MR71, DOI:10.1116/1.28156.2007.90.
- Y. Liu, et al., Stable polymer nanoparticles with exceptionally high drug loading by sequential nanoprecipitation, Angew. Chem., 2020, 132(12), 4750–4758 CrossRef.
- S. Shen, et al., High drug-loading nanomedicines: progress, current status, and prospects, Int. J. Nanomed., 2017, 12, 4085 CrossRef CAS PubMed.
- Y. Liu, et al., Formulation of nanoparticles using mixing-induced nanoprecipitation for drug delivery, Ind. Eng. Chem. Res., 2019, 59(9), 4134–4149 CrossRef.
- V. Kumar and R. K. Prud'Homme, Thermodynamic limits on drug loading in nanoparticle cores, J. Pharm. Sci., 2008, 97(11), 4904–4914 CrossRef CAS PubMed.
- S. Sepassi, et al., Effect of polymer molecular weight on the production of drug nanoparticles, J. Pharm. Sci., 2007, 96(10), 2655–2666 CrossRef CAS PubMed.
- J. Wang and M. Windbergs, Influence of polymer composition and drug loading procedure on dual drug release from PLGA: PEG electrospun fibers, Eur. J. Pharm. Sci., 2018, 124, 71–79 CrossRef CAS PubMed.
- K. Kataoka, A. Harada and Y. Nagasaki, Block copolymer micelles for drug delivery: design, characterization and biological significance, Adv. Drug Delivery Rev., 2012, 64, 37–48 CrossRef.
- W.-H. Lee, C.-Y. Loo and R. Rohanizadeh, Functionalizing the surface of hydroxyapatite drug carrier with carboxylic acid groups to modulate the loading and release of curcumin nanoparticles, Mater. Sci. Eng., C, 2019, 99, 929–939 CrossRef CAS PubMed.
- Y. Liu, et al., Development of High-Drug-Loading Nanoparticles, ChemPlusChem, 2020, 85(9), 2143–2157 CrossRef CAS PubMed.
- G. Yang, et al., Development of core-shell nanoparticle drug delivery systems based on biomimetic mineralization, ChemBioChem, 2020, 21(20), 2871–2879 CrossRef CAS PubMed.
- L. Miao, et al., Nanoparticles with precise ratiometric co-loading and co-delivery of gemcitabine monophosphate and cisplatin for treatment of bladder cancer, Adv. Funct. Mater., 2014, 24(42), 6601–6611 CrossRef CAS PubMed.
- N. Wang, et al., Nanocarriers and their loading strategies, Adv. Healthcare Mater., 2019, 8(6), 1801002 CrossRef PubMed.
- J. H. Lee and Y. Yeo, Controlled drug release from pharmaceutical nanocarriers, Chem. Eng. Sci., 2015, 125, 75–84 CrossRef CAS PubMed.
- C. E. Mora-Huertas, H. Fessi and A. Elaissari, Polymer-based nanocapsules for drug delivery, Int. J. Pharm., 2010, 385(1–2), 113–142 CrossRef CAS PubMed.
- G.-H. Son, B.-J. Lee and C.-W. Cho, Mechanisms of drug release from advanced drug formulations such as polymeric-based drug-delivery systems and lipid nanoparticles, J. Pharm. Invest., 2017, 47(4), 287–296 CrossRef CAS.
- S. Mura, J. Nicolas and P. Couvreur, Stimuli-responsive nanocarriers for drug delivery, Nat. Mater., 2013, 12(11), 991–1003 CrossRef CAS PubMed.
- R. Singh and J. W. Lillard Jr, Nanoparticle-based targeted drug delivery, Exp. Mol. Pathol., 2009, 86(3), 215–223 CrossRef CAS PubMed.
- H. P. James, et al., Smart polymers for the controlled delivery of drugs–a concise overview, Acta Pharm. Sin. B, 2014, 4(2), 120–127 CrossRef PubMed.
- D. C. Costa, et al., Laser ablation in liquid-assisted synthesis of three types of nanoparticles for enhanced antibacterial applications, Int. J. Precis. Eng. Manuf. - Green Technol., 2025, 1–19 CAS.
- R. Subedi and G. Guisbiers, Synthesis of ultrawide band gap TeO2 nanoparticles by pulsed laser ablation in liquids: Top ablation versus bottom ablation, ACS Omega, 2024, 9(24), 25832–25840 CrossRef CAS PubMed.
- T. T. N. Ho, T. Hirano, R. Narui, S. Imaoka, A. Takano, S. Miyasaka, E. Tanabe, E. L. Septiani, K. L. A. Cao and T. Ogi, Flame Spray Pyrolysis Achieves Size-Tunable Niobium-doped Tin Oxide Nanoparticles for Improved Catalyst Performance in PEFCs, ACS Appl. Energy Mater., 2025, 8(7), 4640–4647 CrossRef CAS.
- H. Miao, et al., One-step spray pyrolysis synthesis of ZnO/Ag hollow spheres for enhanced visible-light-driven antibacterial applications and wound healing, Dalton Trans., 2025, 54(6), 2574–2583 RSC.
- T. Acsente, et al., Enhancement of W Nanoparticles Synthesis by Injecting H2 in a Magnetron Sputtering Gas Aggregation Cluster Source Operated in Ar, Plasma Chem. Plasma Process., 2024, 44(6), 2233–2246 CrossRef CAS.
- M. Protsak, et al., One-step synthesis of photoluminescent nanofluids by direct loading of reactively sputtered cubic ZrN nanoparticles into organic liquids, Nanoscale, 2024, 16(5), 2452–2465 RSC.
- H. Fiedler, et al., Room Temperature Ion Beam Synthesis of Ultra-Fine Molybdenum Carbide Nanoparticles: Toward a Scalable Fabrication Route for Earth-Abundant Electrodes, Small, 2024, 20(39), 2304118 CrossRef CAS PubMed.
- V. Paperny, et al., Enhancement of photoluminescence from rare-earth ions in fluoride crystals by ion-implanted silver nanoparticles, J. Lumin., 2025, 279, 121044 CrossRef CAS.
- A. N. Markov, et al., Synthesis of Zinc Nanoparticles by the Gas Condensation Method in a Non-Contact Crucible and Their Physical–Chemical Characterization, Nanomaterials, 2024, 14(2), 163 CrossRef CAS PubMed.
- K. Kollbek, et al., Inert gas condensation made bimetallic FeCu nanoparticles–plasmonic response and magnetic ordering, J. Mater. Chem. C, 2024, 12(7), 2593–2605 RSC.
- M. Zarei, et al., Fe-doped ZnO nanoparticles prepared by high energy ball milling with enhanced sonophotocatalytic performance, Adv. Powder Technol., 2024, 35(7), 104550 CrossRef CAS.
- G. T. Tractz, et al., Enhancing photovoltaic performance through high-energy ball milling of Nb-doped TiO2 nanoparticles, MRS Commun., 2024, 1–7 Search PubMed.
- S. Dhital, et al., Synthesis of manganese oxide nanoparticles using co-precipitation method and its antimicrobial activity, Int. J. New Chem., 2024, 11(3), 243–253 CAS.
- M. Piran, M. Kharaziha and S. Sheibani, PVA-Assisted Synthesis of Cobalt Ferrite Nanoparticles for Biomedical Applications, Ceram. Int., 2025, 02, 049 Search PubMed.
- P. Nunocha, et al., Efficient and novel synthesis of Nb-doped SrTiO3 nanoparticles via microwave-assisted sol-gel auto-combustion, Opt. Mater., 2025, 159, 116665 CrossRef CAS.
- H. Nguyen, et al., Electrochemical Synthesis of Silver Nanoparticles Using Camellia chrysantha Flower Extract: Characteristics and Antibacterial Activity, Colloid J., 2025, 1–10 Search PubMed.
- A. S. Joseph, et al., Solvothermal synthesis of ruthenium-doped cobalt ferrite nanoparticles–A feasible approach to modify the magnetic properties and nonlinear optical absorption, Mater. Sci. Semicond. Process., 2025, 188, 109246 CrossRef CAS.
- S. Ahmad, et al., Magnetic properties of different phases iron oxide nanoparticles prepared by micro emulsion-hydrothermal method, Sci. Rep., 2025, 15(1), 878 CrossRef CAS PubMed.
- A. Rajapantulu and R. Bandyopadhyaya, Role of Hydrazine and Size-Tuning Parameter in Gold Nanoparticle Synthesis by Water-in-Oil Microemulsion: Experiment and Simulation, Langmuir, 2025, 41(12), 8049–8059 CrossRef CAS PubMed.
- R. Soomro, et al., A novel plant-based approach for synthesis of iron oxide nanoparticles and cancer therapy, Discover Chemistry, 2025, 2(1), 25 CrossRef.
- R. Dhir, et al., Plant-mediated synthesis of silver nanoparticles: unlocking their pharmacological potential–a comprehensive review, Front. Bioeng. Biotechnol., 2024, 11, 1324805 CrossRef PubMed.
- M. Ebadi, et al., A bio-inspired strategy for the synthesis of zinc oxide nanoparticles (ZnO NPs) using the cell extract of cyanobacterium Nostoc sp. EA03: from biological function to toxicity evaluation, RSC Adv., 2019, 9(41), 23508–23525 RSC.
- N. Sani, B. Aminu and M. Mukhtar, Eco-friendly synthesis of silver nanoparticles using Lactobacillus delbrueckii subsp. bulgaricus isolated from kindrimo (locally fermented milk) in Kano State, Nigeria. Bayero, J. Pure Appl. Sci., 2017, 10(1), 481–488 Search PubMed.
- F. Ameen, Optimization of the synthesis of fungus-mediated bi-metallic Ag-Cu nanoparticles, Appl. Sci., 2022, 12(3), 1384 CrossRef CAS.
- K. Seku, et al., Fungal-mediated synthesis of gold nanoparticles and their biological applications, in Fungal Cell Factories for Sustainable Nanomaterials Productions and Agricultural Applications, Elsevier, 2023, pp. 23–58 Search PubMed.
- A. A. K. Elrefaey, et al., Algae-mediated biosynthesis of zinc oxide nanoparticles from Cystoseira crinite (Fucales; Sargassaceae) and it's antimicrobial and antioxidant activities, Egypt. J. Chem., 2022, 65(4), 231–240 Search PubMed.
- M. Afrouz, et al., Green synthesis of spermine coated iron nanoparticles and its effect on biochemical properties of Rosmarinus officinalis, Sci. Rep., 2023, 13(1), 775 CrossRef CAS.
- I. Hussain, et al., Green synthesis of nanoparticles and its potential application, Biotechnol. Lett., 2016, 38, 545–560 CrossRef CAS PubMed.
- A. Gour and N. K. Jain, Advances in green synthesis of nanoparticles, Artif. Cells, Nanomed., Biotechnol., 2019, 47(1), 844–851 CrossRef CAS PubMed.
- M. Kim, et al., Synthesis of nanoparticles by laser ablation: A review, Kona Powder Part. J., 2017, 34, 80–90 CrossRef CAS.
- N. Semaltianos, Nanoparticles by laser ablation, Crit. Rev. Solid State Mater. Sci., 2010, 35(2), 105–124 CrossRef CAS.
- A. S. Abd-Alrahman, R. A. Ismail and M. A. Mohammed, Synthesis of HgI 2 Nanoparticles and Nanorods by Laser Ablation in Liquid for Photodetector Applications: Effect of Laser Fluence, J. Inorg. Organomet. Polym. Mater., 2022, 1–12 Search PubMed.
- A. B. Workie, H. S. Ningsih and S.-J. Shih, An comprehensive review on the spray pyrolysis technique: historical context, operational factors, classifications, and product applications, J. Anal. Appl. Pyrolysis, 2023, 105915 CrossRef CAS.
- M. A. Ismail, et al., Synthesis and characterization of iron-doped TiO2 nanoparticles using ferrocene from flame spray pyrolysis, Catalysts, 2021, 11(4), 438 CrossRef CAS.
- W. Cheng, et al., Synthesis of ZnS/CoS/CoS2@ N-doped carbon nanoparticles derived from metal-organic frameworks via spray pyrolysis as anode for lithium-ion battery, J. Alloys Compd., 2020, 831, 154607 CrossRef CAS.
- F. Wang, et al., Magnetron sputtering enabled synthesis of nanostructured materials for electrochemical energy storage, J. Mater. Chem. A, 2020, 8(39), 20260–20285 RSC.
- T. Acsente, et al., Tungsten Nanoparticles Produced by Magnetron Sputtering Gas Aggregation: Process Characterization and Particle Properties, Prog. Fine Part. Plasmas, 2020, 117 Search PubMed.
- M. Verma, K. P. Singh and A. Kumar, Reactive magnetron sputtering based synthesis of WO3 nanoparticles and their use for the photocatalytic degradation of dyes, Solid State Sci., 2020, 99, 105847 CrossRef CAS.
- A. L. Stepanov, Synthesis of silver nanoparticles in dielectric matrix by ion implantation: A review, Rev. Adv. Mater. Sci., 2010, 26(1/2), 1–29 CAS.
- R. Li, et al., Plasmonic nanoparticles in dielectrics synthesized by ion beams: optical properties and photonic applications, Adv. Opt. Mater., 2020, 8(9), 1902087 CrossRef CAS.
- I. Lampé, et al., Investigation of silver nanoparticles on titanium surface created by ion implantation technology, Int. J. Nanomed., 2019, 4709–4721 CrossRef.
- K. Zheng and P. S. Branicio, Synthesis of metallic glass nanoparticles by inert gas condensation, Phys. Rev. Mater., 2020, 4(7), 076001 CrossRef CAS.
- E. Pérez-Tijerina, et al., Highly size-controlled synthesis of Au/Pd nanoparticles by inert-gas condensation, Faraday Discuss., 2008, 138, 353–362 RSC.
- S. S. Mughal and S. M. Hassan, Comparative study of AgO nanoparticles synthesize via biological, chemical and physical methods: a review, J. Mater. Synth. Process., 2022, 7(2), 15–28 Search PubMed.
- G. Otis, M. Ejgenberg and Y. Mastai, Solvent-free mechanochemical synthesis of ZnO nanoparticles by high-energy ball milling of ε-Zn (OH) 2 crystals, Nanomaterials, 2021, 11(1), 238 CrossRef CAS PubMed.
- P. Chin, et al., Synthesis of FeS2 and FeS nanoparticles by high-energy mechanical milling and mechanochemical processing, J. Alloys Compd., 2005, 390(1–2), 255–260 CrossRef CAS.
- D. Gao, et al., Electrochemical synthesis of catalytic materials for energy catalysis, Chin. J. Catal., 2022, 43(4), 1001–1016 CrossRef CAS.
- V. Anand and V. C. Srivastava, Zinc oxide nanoparticles synthesis by electrochemical method: Optimization of parameters for maximization of productivity and characterization, J. Alloys Compd., 2015, 636, 288–292 CrossRef CAS.
- Y. Huo, et al., Solvothermal synthesis and applications of micro/nano carbons: A review, Chem. Eng. J., 2022, 138572 Search PubMed.
- M. A. Dar, et al., Supercapacitor and magnetic properties of Fe doped SnS nanoparticles synthesized through solvothermal method, J. Energy Storage, 2022, 52, 105034 CrossRef.
- M. A. Malik, M. Y. Wani and M. A. Hashim, Microemulsion method: A novel route to synthesize organic and inorganic nanomaterials: 1st Nano Update, Arabian J. Chem., 2012, 5(4), 397–417 CrossRef CAS.
- J. N. Solanki and Z. V. P. Murthy, Controlled size silver nanoparticles synthesis with water-in-oil microemulsion method: a topical review, Ind. Eng. Chem. Res., 2011, 50(22), 12311–12323 CrossRef CAS.
- Z. Villagrán, et al., Plant-based extracts as reducing, capping, and stabilizing agents for the green synthesis of inorganic nanoparticles, Resources, 2024, 13(6), 70 CrossRef.
- W. Wang, et al., Biomineralization: an opportunity and challenge of nanoparticle drug delivery systems for cancer therapy, Adv. Healthcare Mater., 2020, 9(22), 2001117 CrossRef CAS PubMed.
- W. Yang, et al., Protein/peptide-templated biomimetic synthesis of inorganic nanoparticles for biomedical applications, J. Mater. Chem. B, 2017, 5(3), 401–417 RSC.
- X. Li, et al., Biosynthesis of nanoparticles by microorganisms and their applications, J. Nanomater., 2011, 2011, 1–16 Search PubMed.
- R. Chaudhary, et al., An overview of the algae-mediated biosynthesis of nanoparticles and their biomedical applications, Biomolecules, 2020, 10(11), 1498 CrossRef CAS PubMed.
- N. Feroze, et al., Fungal mediated synthesis of silver nanoparticles and evaluation of antibacterial activity, Microsc. Res. Tech., 2020, 83(1), 72–80 CrossRef CAS PubMed.
- M. Chen, S. Wang and B. Hu, Revealing the Formation of Well-Dispersed Polystyrene@ ZIF-8 Core–Shell Nanoparticles by Analytical Ultracentrifugation, Langmuir, 2020, 36(29), 8589–8596 CrossRef CAS PubMed.
- A. Vaidya, et al., Analytical characterization of heterogeneities in mRNA-lipid nanoparticles using sucrose density gradient ultracentrifugation, Anal. Chem., 2024, 96(14), 5570–5579 CrossRef CAS PubMed.
- S. H. Sajjadi, E. K. Goharshadi and H. Ahmadzadeh, Heat dissipation in slab gel electrophoresis: The effect of embedded TiO2 nanoparticles on the thermal profiles, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2019, 1118, 63–69 CrossRef PubMed.
- Y. Sun, et al., DNA-Assisted Separation of Nanoparticles, Small Methods, 2024, 2401389 CrossRef PubMed.
- L. Kuger, C. R. Arlt and M. Franzreb, Magnetic/flow controlled continuous size fractionation of magnetic nanoparticles using simulated moving bed chromatography, Talanta, 2022, 240, 123160 CrossRef CAS PubMed.
- L. Pitkänen and A. M. Striegel, Size-exclusion chromatography of metal nanoparticles and quantum dots, TrAC, Trends Anal. Chem., 2016, 80, 311–320 CrossRef PubMed.
- O. Milark, et al., Design and Fabrication of 3D-Printed Lab-On-A-Chip Devices for Fiber-Based Optical Chromatography and Sorting, Adv. Photonics Res., 2024, 5(10), 2400011 CrossRef CAS.
- K. Yang, L. J. Huang, Y. X. Wang, Y. C. Du, Z. J. Zhang, Y. Wang, M. J. Kipper, L. A. Belfiore and J. G. Tang, Graphene oxide nanofiltration membranes containing silver nanoparticles: Tuning separation efficiency via nanoparticle size, Nanomaterials, 2020, 10(3), 454 CrossRef CAS PubMed.
- E. Krieg, H. Weissman, E. Shirman, E. Shimoni and B. Rybtchinski, A recyclable supramolecular membrane for size-selective separation of nanoparticles, Nat. Nanotechnol., 2011, 6(3), 141–146 CrossRef CAS PubMed.
- X. Xu and H. Cölfen, Ultracentrifugation techniques for the ordering of nanoparticles, Nanomaterials, 2021, 11(2), 333 CrossRef PubMed.
- E. Karabudak, et al., Simultaneous identification of spectral properties and sizes of multiple particles in solution with subnanometer resolution, Angew. Chem., Int. Ed., 2016, 55(39), 11770–11774 CrossRef CAS PubMed.
- R. P. Carney, et al., Determination of nanoparticle size distribution together with density or molecular weight by 2D analytical ultracentrifugation, Nat. Commun., 2011, 2(1), 1–8 Search PubMed.
- H. Zhao, et al., Overview of current methods in sedimentation velocity and sedimentation equilibrium analytical ultracentrifugation, Curr. Protoc. Protein Sci., 2013, 71(1), 20.12.1–20.12.49 Search PubMed.
- S. E. Wawra, et al., Determination of the two-dimensional distributions of gold nanorods by multiwavelength analytical ultracentrifugation, Nat. Commun., 2018, 9(1), 4898 CrossRef PubMed.
- M. Chen, S. Wang and B. Hu, Revealing the Formation of Well-Dispersed Polystyrene@ ZIF-8 Core–Shell Nanoparticles by Analytical Ultracentrifugation, Langmuir, 2020, 36(29), 8589–8596 CrossRef CAS PubMed.
- T. Salafi, K. K. Zeming and Y. Zhang, Advancements in microfluidics for nanoparticle separation, Lab Chip, 2017, 17(1), 11–33 RSC.
- O. Akbulut, et al., Separation of nanoparticles in aqueous multiphase systems through centrifugation, Nano Lett., 2012, 12(8), 4060–4064 CrossRef CAS PubMed.
- S. A. Claridge, et al., Directed assembly of discrete gold nanoparticle groupings using branched DNA scaffolds, Chem. Mater., 2005, 17(7), 1628–1635 CrossRef CAS.
- X. Xu, et al., Size and shape separation of gold nanoparticles with preparative gel electrophoresis, J. Chromatogr. A, 2007, 1167(1), 35–41 CrossRef CAS PubMed.
- R. A. Sperling, et al., Electrophoretic separation of nanoparticles with a discrete number of functional groups, Adv. Funct. Mater., 2006, 16(7), 943–948 CrossRef CAS.
- X. Zhu and T. G. Mason, Separating nanoparticles by surface charge group using pH-controlled passivated gel electrophoresis, Soft Mater., 2016, 14(3), 204–209 CrossRef CAS.
- D. Montizaan, et al., Genome-wide forward genetic screening to identify receptors and proteins mediating nanoparticle uptake and intracellular processing, Nat. Nanotechnol., 2024, 19(7), 1022–1031 CrossRef CAS PubMed.
- M. Hanauer, et al., Separation of nanoparticles by gel electrophoresis according to size and shape, Nano Lett., 2007, 7(9), 2881–2885 CrossRef CAS PubMed.
- A. Hlavacek, et al., Large-scale purification of photon-upconversion nanoparticles by gel electrophoresis for analogue and digital bioassays, Anal. Chem., 2018, 91(2), 1241–1246 CrossRef PubMed.
- H. Zorbas, Bioanalytics: Methods on Molecular Biotechnology and Modern Biotechnology, Wiley, New York, 2010 Search PubMed.
- L. Kuger, C.-R. Arlt and M. Franzreb, Magnetic/flow controlled continuous size fractionation of magnetic nanoparticles using simulated moving bed chromatography, Talanta, 2022, 240, 123160 CrossRef CAS PubMed.
- L. Pitkänen and A. M. Striegel, Size-exclusion chromatography of metal nanoparticles and quantum dots, TrAC, Trends Anal. Chem., 2016, 80, 311–320 CrossRef PubMed.
- H. Block, B. Maertens, A. Spriestersbach, N. Brinker, J. Kubicek, R. Fabis, J. Labahn and F. Schäfer, Immobilized-metal affinity chromatography (IMAC): a review, Methods Enzymol., 2009, 463, 439–473 CAS.
- K. Yang, et al., Graphene oxide nanofiltration membranes containing silver nanoparticles: Tuning separation efficiency via nanoparticle size, Nanomaterials, 2020, 10(3), 454 CrossRef CAS PubMed.
- E. Krieg, et al., A recyclable supramolecular membrane for size-selective separation of nanoparticles, Nat. Nanotechnol., 2011, 6(3), 141–146 CrossRef CAS PubMed.
- M. Samari, R. Heydari and F. Gholami, Nanofiltration membrane functionalized with CuxP nanoparticles: a promising approach for enhanced pesticide removal and long-term stability, J. Cleaner Prod., 2024, 445, 141352 CrossRef CAS.
- X. Tang, et al., Theoretical and experimental study of high performance thin-film nanocomposite nanofiltration membranes by introducing calcium carbonate nanoparticles, J. Membr. Sci., 2025, 713, 123270 CrossRef CAS.
- G. M. Whitesides, The origins and the future of microfluidics, Nature, 2006, 442(7101), 368–373 CrossRef CAS PubMed.
- M. I. Hajam and M. M. Khan, Microfluidics: a concise review of the history, principles, design, applications, and future outlook. Biomaterials, Science, 2024, 12(2), 218–251 CAS.
- S. Gimondi, et al., Microfluidic mixing system for precise PLGA-PEG nanoparticles size control, Nanomed.: Nanotechnol., Biol. Med., 2022, 40, 102482 CrossRef CAS PubMed.
- T. Chen, Y. Peng, M. Qiu, C. Yi and Z. Xu, Recent Advances in Mixing-Induced Nanoprecipitation: From Creating Complex Nanostructures to Emerging Applications beyond Biomedicine, Nanoscale, 2023, 15(8), 3594–3609 RSC.
- J. Nette, P. D. Howes and A. J. deMello, Microfluidic Synthesis of Luminescent and Plasmonic Nanoparticles: Fast, Efficient, and Data-Rich, Adv. Mater. Technol., 2020, 5(7), 2000060 CrossRef CAS.
- N. Debnath, A. Kumar, T. Thundat and M. Sadrzadeh, Investigating fouling at the pore-scale using a microfluidic membrane mimic filtration system, Sci. Rep., 2019, 9(1), 10587 CrossRef PubMed.
- V. M. Gun'ko, E. M. Pakhlov, O. V. Goncharuk, L. S. Andriyko, Y. M. Nychiporuk, D. Y. Balakin, D. Sternik and A. Derylo-Marczewska, Nanosilica modified by polydimethylsiloxane depolymerized and chemically bound to nanoparticles or physically bound to unmodified or modified surfaces: Structure and interfacial phenomena, J. Colloid Interface Sci., 2018, 529, 273–282 CrossRef PubMed.
- X. Liu, et al., Microfluidics chips fabrication techniques comparison, Sci. Rep., 2024, 14(1), 28793 CrossRef CAS PubMed.
- B. Amoyav and O. Benny, Controlled and tunable polymer particles' production using a single microfluidic device, Appl. Nanosci., 2018, 8(4), 905–914 CrossRef CAS.
- A. A. Volk, R. W. Epps and M. Abolhasani, Accelerated development of colloidal nanomaterials enabled by modular microfluidic reactors: toward autonomous robotic experimentation, Adv. Mater., 2021, 33(4), 2004495 CrossRef CAS PubMed.
- B. K. Gale, A. R. Jafek, C. J. Lambert, B. L. Goenner, H. Moghimifam, U. C. Nze and S. K. Kamarapu, A review of current methods in microfluidic device fabrication and future commercialization prospects, Inventions, 2018, 3(3), 60 CrossRef.
- J. Ahn, et al., Microfluidics in nanoparticle drug delivery; From synthesis to pre-clinical screening, Adv. Drug Delivery Rev., 2018, 128, 29–53 CrossRef CAS PubMed.
- R. Riahi, et al., Microfluidics for advanced drug delivery systems, Curr. Opin. Chem. Eng., 2015, 7, 101–112 CrossRef PubMed.
- W. Raza, S.-B. Ma and K.-Y. Kim, Multi-objective optimizations of a serpentine micromixer with crossing channels at low and high Reynolds numbers, Micromachines, 2018, 9(3), 110 CrossRef PubMed.
- M. Rasouli, et al., Multi-criteria optimization of curved and baffle-embedded micromixers for bio-applications, Chem. Eng. Process., 2018, 132, 175–186 CrossRef CAS.
- N.-T. Nguyen, Micromixers: fundamentals, design and fabrication, William Andrew, 2011 Search PubMed.
- S. I. Hamdallah, et al., Microfluidics for pharmaceutical nanoparticle fabrication: The truth and the myth, Int. J. Pharm., 2020, 584, 119408 CrossRef CAS PubMed.
- J. D. Mello and A. D. Mello, FocusMicroscale reactors: nanoscale products, Lab Chip, 2004, 4(2), 11N–15N RSC.
- A. J. Demello, Control and detection of chemical reactions in microfluidic systems, Nature, 2006, 442(7101), 394–402 CrossRef CAS PubMed.
- Q. Feng, J. Sun and X. Jiang, Microfluidics-mediated assembly of functional nanoparticles for cancer-related pharmaceutical applications, Nanoscale, 2016, 8(25), 12430–12443 RSC.
- S. T. Sanjay, et al., Recent advances of controlled drug delivery using microfluidic platforms, Adv. Drug Delivery Rev., 2018, 128, 3–28 CrossRef CAS PubMed.
- G. Antonelli, et al., Integrating machine learning and biosensors in microfluidic devices: a review, Biosens. Bioelectron., 2024, 116632 CrossRef CAS PubMed.
- J. Riordon, et al., Deep learning with microfluidics for biotechnology, Trends Biotechnol., 2019, 37(3), 310–324 CrossRef CAS PubMed.
- X. Chen and H. Lv, Intelligent control of nanoparticle synthesis on microfluidic chips with machine learning, NPG Asia Mater., 2022, 14(1), 69 CrossRef.
- C.-Y. Lee, C. L. Chang, Y. N. Wang and L. M. Fu, Microfluidic mixing: a review, Int. J. Mol. Sci., 2011, 12(5), 3263–3287 CrossRef CAS PubMed.
- W. Yao, et al., Continuous synthesis of monodisperse polymer nanoparticles: New insights into the effects of experimental variables under controlled mixing conditions, Chem. Eng. Sci., 2024, 288, 119846 CrossRef CAS.
- S. M. Naghib and K. Mohammad-Jafari, Microfluidics-mediated Liposomal Nanoparticles for Cancer Therapy: Recent Developments on Advanced Devices and Technologies, Curr. Top. Med. Chem., 2024, 24(14), 1185–1211 CrossRef CAS PubMed.
- A. Hussain, et al., Investigating hybrid nanoparticles for drug delivery in multi-stenosed catheterized arteries under magnetic field effects, Sci. Rep., 2024, 14(1), 1170 CrossRef CAS PubMed.
- A.-G. Niculescu, et al., New 3D Vortex Microfluidic System Tested for Magnetic Core-Shell Fe3O4-SA Nanoparticle Synthesis, Nanomaterials, 2024, 14(11), 902 CrossRef CAS PubMed.
- M. Abaee, S. Sohrabi and M. K. Moraveji, CFD assisted engineering of spiral microfluidic reactors for room temperature synthesis of ZnO nanoparticles, Ceram. Int., 2024, 50(18), 32613–32623 CrossRef CAS.
- D. J. Magdalene and D. Muthuselvam, Microfluidics assisted green synthesis of copper oxide nanoparticles from the aqueous leaf extract of Ipomoea quamoclit L. with its antimicrobial and antioxidant activity, J. Herb. Med., 2024, 48, 100940 CrossRef.
- B. T. Ceccato, S. S. Vianna and L. G. de la Torre, Numerical and experimental investigation of chaotic advection and diffusion mixing effects in 3D multihelical microfluidics for liposome synthesis, Chem. Eng. Sci., 2024, 295, 120190 CrossRef CAS.
- D. Tomioka, et al., Fabrication of oxygen-releasing dextran microgels by droplet-based microfluidic method, RSC Adv., 2024, 14(36), 26544–26555 RSC.
- K. Ngocho, et al., Synthetic Cells from Droplet-Based Microfluidics for Biosensing and Biomedical Applications, Small, 2024, 20(33), 2400086 CrossRef CAS PubMed.
- H. Dortaj, et al., Droplet-based microfluidics: an efficient high-throughput portable system for cell encapsulation, J. Microencapsulation, 2024, 41(6), 479–501 CrossRef CAS PubMed.
- M. Lu, et al., Microfluidic hydrodynamic focusing for synthesis of nanomaterials, Nano Today, 2016, 11(6), 778–792 CrossRef CAS PubMed.
- X. Mao, S. C. S. Lin, C. Dong and T. J. Huang, Single-layer planar on-chip flow cytometer using microfluidic drifting based three-dimensional (3D) hydrodynamic focusing, Lab Chip, 2009, 9(11), 1583–1589 RSC.
- J.-M. Lim, et al., Ultra-high throughput synthesis of nanoparticles with homogeneous size distribution using a coaxial turbulent jet mixer, ACS Nano, 2014, 8(6), 6056–6065 CrossRef CAS PubMed.
- R. Baber, et al., An engineering approach to synthesis of gold and silver nanoparticles by controlling hydrodynamics and mixing based on a coaxial flow reactor, Nanoscale, 2017, 9(37), 14149–14161 RSC.
- M. A. Daniele, et al., 3D hydrodynamic focusing microfluidics for emerging sensing technologies, Biosens. Bioelectron., 2015, 67, 25–34 CrossRef CAS PubMed.
- G.-B. Lee, et al., The hydrodynamic focusing effect inside rectangular microchannels, J. Micromech. Microeng., 2006, 16(5), 1024 CrossRef.
- F. S. Majedi, et al., Microfluidic synthesis of chitosan-based nanoparticles for fuel cell applications, Chem. Commun., 2012, 48(62), 7744–7746 RSC.
- X. Mao, et al., Single-layer planar on-chip flow cytometer using microfluidic drifting based three-dimensional (3D) hydrodynamic focusing, Lab Chip, 2009, 9(11), 1583–1589 RSC.
- X. Mao, J. R. Waldeisen and T. J. Huang, Microfluidic drifting—implementing three-dimensional hydrodynamic focusing with a single-layer planar microfluidic device, Lab Chip, 2007, 7(10), 1260–1262 RSC.
- T. Baby, et al., Fundamental studies on throughput capacities of hydrodynamic flow-focusing microfluidics for producing monodisperse polymer nanoparticles, Chem. Eng. Sci., 2017, 169, 128–139 CrossRef CAS.
- F. Heshmatnezhad, et al., Production of doxorubicin-loaded PCL nanoparticles through a flow-focusing microfluidic device: encapsulation efficacy and drug release, Soft Matter, 2021, 17(47), 10675–10682 RSC.
- A. D. Stroock, et al., Chaotic mixer for microchannels, Science, 2002, 295(5555), 647–651 CrossRef CAS PubMed.
- H. Amini, W. Lee and D. Di Carlo, Inertial microfluidic physics, Lab Chip, 2014, 14(15), 2739–2761 RSC.
- Y. Kim, et al., Mass production and size control of lipid–polymer hybrid nanoparticles through controlled microvortices, Nano Lett., 2012, 12(7), 3587–3591 CrossRef CAS PubMed.
- H. Ahmed, S. Ramesan, L. Lee, A. R. Rezk and L. Y. Yeo, On-chip generation of vortical flows for microfluidic centrifugation, Small, 2020, 16(9), 1903605 CrossRef CAS PubMed.
- Y. Li, C. Liu, X. Bai, F. Tian, G. Hu and J. Sun, Enantiomorphic Microvortex-Enabled Supramolecular Sensing of Racemic Amino Acids by Using Achiral Building Blocks, Angew. Chem., Int. Ed., 2020, 59(9), 3486–3490 CrossRef CAS PubMed.
- D. Liu, et al., A versatile and robust microfluidic platform toward high throughput synthesis of homogeneous nanoparticles with tunable properties, Adv. Mater., 2015, 27(14), 2298–2304 CrossRef CAS PubMed.
- G. Karniadakis, A. Beskok and N. Aluru, Mixers and chaotic advection, Microflows and Nanoflows: Fundamentals and Simulation, 2005, pp. 343–363 Search PubMed.
- X. Zhang, et al., Continuous high-flux synthesis of gold nanoparticles with controllable sizes: a simple microfluidic system, Appl. Nanosci., 2020, 10, 661–669 CrossRef CAS.
- W. Fan, F. Zhao, M. Chen, J. Li and X. Guo, An efficient microreactor with continuous serially connected micromixers for the synthesis of superparamagnetic magnetite nanoparticles, Chin. J. Chem. Eng., 2023, 59, 85–91 CrossRef CAS.
- D. R. Kumar, B. Prasad and A. Kulkarni, Segmented flow synthesis of Ag nanoparticles in spiral microreactor: Role of continuous and dispersed phase, Chem. Eng. J., 2012, 192, 357–368 CrossRef.
- R. T. Tsai and C. Y. Wu, An efficient micromixer based on multidirectional vortices due to baffles and channel curvature, Biomicrofluidics, 2011, 5(1), 014103 CrossRef PubMed.
- C. K. Chung, T. R. Shih, C. K. Chang, C. W. Lai and B. H. Wu, Design and experiments of a short-mixing-length baffled microreactor and its application to microfluidic synthesis of nanoparticles, Chem. Eng. J., 2011, 168(2), 790–798 CrossRef CAS.
- J. Sun, Y. Xianyu, M. Li, W. Liu, L. Zhang, D. Liu, C. Liu, G. Hu and X. Jiang, A microfluidic origami chip for synthesis of functionalized polymeric nanoparticles, Nanoscale, 2013, 5(12), 5262–5265 RSC.
- B. Q. Ta, H. Le Thanh, T. Dong, T. N. Thoi and F. Karlsen, Geometric effects on mixing performance in a novel passive micromixer with trapezoidal-zigzag channels, J. Micromech. Microeng., 2015, 25(9), 094004 CrossRef.
- A. Bokare, A. Takami, J. H. Kim, A. Dong, A. Chen, R. Valerio, S. Gunn and F. Erogbogbo, Herringbone-patterned 3D-printed devices as alternatives to microfluidics for reproducible production of lipid polymer hybrid nanoparticles, ACS Omega, 2019, 4(3), 4650–4657 CrossRef CAS PubMed.
- W. W. Liu and Y. Zhu, Development and application of analytical detection techniques for droplet-based microfluidics-A review, Anal. Chim. Acta, 2020, 1113, 66–84 CrossRef CAS PubMed.
- Y. Zhang, H. F. Chan and K. W. Leong, Advanced materials and processing for drug delivery: the past and the future, Adv. Drug Delivery Rev., 2013, 65(1), 104–120 CrossRef CAS PubMed.
- I. Shestopalov, J. D. Tice and R. F. Ismagilov, Multi-step synthesis of nanoparticles performed on millisecond time scale in a microfluidic droplet-based system, Lab Chip, 2004, 4(4), 316–321 RSC.
- I.-L. Ngo, S. Woo Joo and C. Byon, Effects of junction angle and viscosity ratio on droplet formation in microfluidic cross-junction, J. Fluids Eng., 2016, 138(5), 051202 CrossRef.
- R. Baber, L. Mazzei, N. T. Thanh and A. Gavriilidis, Synthesis of silver nanoparticles in a microfluidic coaxial flow reactor, RSC Adv., 2015, 5(116), 95585–95591 RSC.
- S. Sohrabi and M. K. Moraveji, Droplet microfluidics: Fundamentals and its advanced applications, RSC Adv., 2020, 10(46), 27560–27574 RSC.
- Y. Ding, P. D. Howes and A. J. deMello, Recent advances in droplet microfluidics, Anal. Chem., 2019, 92(1), 132–149 CrossRef PubMed.
- M. V. Bandulasena, G. T. Vladisavljević and B. Benyahia, Droplet-based microfluidic method for robust preparation of gold nanoparticles in axisymmetric flow focusing device, Chem. Eng. Sci., 2019, 195, 657–664 CrossRef CAS.
- M. Karimian, K. Dashtian and R. Zare-Dorabei, Microfluidic chip and chiroptical gold nanoparticle-based colorimetric sensor for enantioselective detection of L-tryptophan, Talanta, 2024, 266, 125138 CrossRef CAS PubMed.
- G. He, et al., Microfluidic synthesis of CuH nanoparticles for antitumor therapy through hydrogen-enhanced apoptosis and cuproptosis, ACS Nano, 2024, 18(12), 9031–9042 CrossRef CAS PubMed.
- A. Azmeer, et al., Finger-operated pumping platform for microfluidic preparation of nanoparticles, Microfluid. Nanofluid., 2024, 28(7), 43 CrossRef CAS.
- T. N. Banavathi, M. K. Sivakumar, A. Balapure, S. Dudala, S. K. Dubey and S. Goel, Microfluidic Microreactor Device with Integrated Heaters for Temperature Assisted Synthesis of Gold Nanoparticles and Alkene, IEEE Open J. Nanotechnol., 2024,(5), 134–140 Search PubMed.
- H. Liu, X. Lan, Y. Yin, X. Wang, X. Chen, J. Zhou, K. Chen and X. Zhang, Green Synthesis and Durable Antibacterial AgNP-Loaded Alginate Fibers Enabled by Microfluidic Technology Coupled with Ultraviolet/Thermal Fields, ACS Appl. Polym. Mater., 2025, 7(8), 5259–5270 CrossRef CAS.
- L. Wang, et al., G-Quadruplex DNAzyme-Based Biocatalysis Combined with an Intelligent Electromagnetic-Actuated Microfluidic Chip for Tetracycline Detection, J. Agric. Food Chem., 2024, 73(2), 1598–1607 CrossRef PubMed.
- Y.-T. Kang, et al., Chiroptical detection and mutation analysis of cancer-associated extracellular vesicles using microfluidics with oriented chiral nanoparticles, Matter, 2024, 7(12), 4373–4389 CrossRef CAS.
- K. Curtin, T. Godary and P. Li, Synthesis of tunable gold nanostars via 3D-printed microfluidic device with vibrating sharp-tip acoustic mixing, Microfluid. Nanofluid., 2023, 27(11), 77 CrossRef CAS.
- A. P. Vardin, F. Aksoy and G. Yesiloz, A Novel Acoustic Modulation of Oscillating Thin Elastic Membrane for Enhanced Streaming in Microfluidics and Nanoscale Liposome Production, Small, 2024, 20(48), 2403463 CrossRef CAS PubMed.
- Z. Saifi, et al., Formulation approaches and pharmaceutical applications of chitosan and thiolated chitosan nanoparticles, J. Carbohydr. Chem., 2025, 1–30 CrossRef CAS.
- S. Karmakar, et al., Nanoparticle Deposition Techniques for Silica Nanoparticles: Synthesis, Electrophoretic Deposition, and Optimization-A review, arXiv, 2025, preprint, arXiv:2503.22593, DOI:10.48550/arXiv/2503.22593.
- O. Strohmeier, et al., Centrifugal microfluidic platforms: advanced unit operations and applications, Chem. Soc. Rev., 2015, 44(17), 6187–6229 RSC.
- H. Van Nguyen, et al., Serially diluting centrifugal microfluidics for high-throughput gold nanoparticle synthesis using an automated and portable workstation, Chem. Eng. J., 2023, 452, 139044 CrossRef.
- H. Van Nguyen, et al., Centrifugal microfluidic device for the high-throughput synthesis of Pd@ AuPt core–shell nanoparticles to evaluate the performance of hydrogen peroxide generation, Lab Chip, 2020, 20(18), 3293–3301 RSC.
- A. A. Deshmukh, D. Liepmann and A. P. Pisano, Continuous micromixer with pulsatile micropumps, in Technical Digest of the IEEE Solid State Sensor and Actuator Workshop, Hilton Head Island, SC, 2000 Search PubMed.
- J. W. Wu, H. M. Xia, Y. Y. Zhang, S. F. Zhao, P. Zhu and Z. P. Wang, An efficient micromixer combining oscillatory flow and divergent circular chambers, Microsyst. Technol., 2019, 25(7), 2741–2750 CrossRef.
- W.-B. Lee, et al., Biomedical microdevices synthesis of iron oxide nanoparticles using a microfluidic system, Biomed. Microdevices, 2009, 11, 161–171 CrossRef CAS PubMed.
- K. Sugano, et al., Mixing speed-controlled gold nanoparticle synthesis with pulsed mixing microfluidic system, Microfluid. Nanofluid., 2010, 9, 1165–1174 CrossRef CAS.
- S. M. Recktenwald, C. Wagner and T. John, Optimizing pressure-driven pulsatile flows in microfluidic devices, Lab Chip, 2021, 21(13), 2605–2613 RSC.
- Z. Li and S. J. Kim, Pulsatile micromixing using water-head-driven microfluidic oscillators, Chem. Eng. J., 2017, 313, 1364–1369 CrossRef CAS.
- G. Kunti, A. Bhattacharya and S. Chakraborty, Electrothermally actuated moving contact line dynamics over chemically patterned surfaces with resistive heaters, Phys. Fluids, 2018, 30(6), 2004 Search PubMed.
- J.-H. Tsai, L. J. S. Lin and A. A. Physical, Active microfluidic mixer and gas bubble filter driven by thermal bubble micropump, Sens. Actuators, A, 2002, 97, 665–671 CrossRef.
- H. Mao, T. Yang and P. S. Cremer, A microfluidic device with a linear temperature gradient for parallel and combinatorial measurements, J. Am. Chem. Soc., 2002, 124(16), 4432–4435 CrossRef CAS PubMed.
- H. Sun, et al., Three-fluid sequential micromixing-assisted nanoparticle synthesis utilizing alternating current electrothermal flow, Ind. Eng. Chem. Res., 2020, 59(27), 12514–12524 CrossRef CAS.
- H. Lv and X. Chen, New insights into the mechanism of fluid mixing in the micromixer based on alternating current electric heating with film heaters, Int. J. Heat Mass Transfer, 2021, 181, 121902 CrossRef.
- S. Srikanth, S. Dudala, U. S. Jayapiriya, J. M. Mohan, S. Raut, S. K. Dubey, I. Ishii, A. Javed and S. Goel, Droplet-based lab-on-chip platform integrated with laser ablated graphene heaters to synthesize gold nanoparticles for electrochemical sensing and fuel cell applications, Sci. Rep., 2021, 11(1), 9750 CrossRef CAS PubMed.
- H. Tan, Numerical study of a bubble driven micromixer based on thermal inkjet technology, Phys. Fluids, 2019, 31(6), 062006 CrossRef.
- J. Wu, W. Cao, W. Wen, D. C. Chang and P. Sheng, Polydimethylsiloxane microfluidic chip with integrated microheater and thermal sensor, Biomicrofluidics, 2009, 3(1), 012005 CrossRef PubMed.
- L. Huang, et al., Rational Design of 3D-Printed Microfluidic Chips for One-Step Production of Silver Nanofluids in Thermal Management of Power Electronic Chips, ACS Appl. Mater. Interfaces, 2025, 17(15), 23277–23285 CrossRef CAS PubMed.
- E. Weaver, et al., The manufacturing of 3D-printed microfluidic chips to analyse the effect upon particle size during the synthesis of lipid nanoparticles, J. Pharm. Pharmacol., 2023, 75(2), 245–252 CrossRef CAS PubMed.
- S. Dembski, et al., Establishing and testing a robot-based platform to enable the automated production of nanoparticles in a flexible and modular way, Sci. Rep., 2023, 13(1), 11440 CrossRef PubMed.
- M. A. Neustrup, et al., A versatile, low-cost modular microfluidic system to prepare poly (lactic-co-glycolic acid) nanoparticles with encapsulated protein, Pharm. Res., 2024, 41(12), 2347–2361 CrossRef CAS PubMed.
- A. Kouhkord, et al., Controllable microfluidic system through intelligent framework: Data-driven modeling, machine learning energy analysis, comparative multiobjective optimization, and experimental study, Ind. Eng. Chem. Res., 2024, 63(30), 13326–13344 CrossRef CAS.
- S. Mihandoost, et al., A Generative Adversarial Network Approach to Predict Nanoparticle Size in Microfluidics, ACS Biomater. Sci. Eng., 2024, 11(1), 268–279 CrossRef PubMed.
- S. Gao, et al., Artificial Intelligence Enhanced Microfluidic System for Multiplexed Point-of-care-testing of Biological Thiols, Talanta, 2025, 127619 CrossRef CAS PubMed.
- M. Ballard, et al., Orbiting magnetic microbeads enable rapid microfluidic mixing, Microfluid. Nanofluid., 2016, 20(6), 1–13 CrossRef CAS.
- A. N. Surendran and R. Zhou, Active High-Throughput Micromixer Using Injected Magnetic Mixture Underneath Microfluidic Channel, in International Conference on Nanochannels, Microchannels, and Minichannels, American Society of Mechanical Engineers, 2020, vol. 83693, p. V001T01A001 Search PubMed.
- J. Sun, Z. Shi, M. Li, J. Sha, M. Zhong, S. Chen, X. Liu and S. Jia, Numerical and experimental investigation of a magnetic micromixer under microwires and uniform magnetic field, J. Magn. Magn. Mater., 2022, 551, 169141 CrossRef CAS.
- D. Bahrami, A. A. Nadooshan and M. Bayareh, Effect of non-uniform magnetic field on mixing index of a sinusoidal micromixer, Korean J. Chem. Eng., 2022, 1–12 Search PubMed.
- A. Dehghan, A. Gholizadeh, M. Navidbakhsh, H. Sadeghi and E. Pishbin, Integrated microfluidic system for efficient DNA extraction using on-disk magnetic stirrer micromixer, Sens. Actuators, B, 2022, 351, 130919 CrossRef CAS.
- Y. A. Wu, B. Panigrahi, Y. H. Lu and C. Y. Chen, An integrated artificial cilia based microfluidic device for micropumping and micromixing applications, Micromachines, 2017, 8(9), 260 CrossRef PubMed.
- N. Banka, Y. L. Ng and S. Devasia, Individually controllable magnetic cilia: Mixing application, J. Med. Devices, 2017, 11(3), 031003 CrossRef.
- B. Panigrahi, V. Sahadevan and C.-Y. Chen, Shape-programmable artificial cilia for microfluidics, iScience, 2021, 24(12), 103367 CrossRef CAS PubMed.
- N. Veldurthi, P. Ghoderao, S. Sahare, V. Kumar, D. Bodas, A. Kulkarni and T. Bhave, Magnetically active micromixer assisted synthesis of drug nanocomplexes exhibiting strong bactericidal potential, Mater. Sci. Eng., C, 2016, 68, 455–464 CrossRef CAS PubMed.
- J. S. Bach and H. Bruus, Suppression of acoustic streaming in shape-optimized channels, Phys. Rev. Lett., 2020, 124(21), 214501 CrossRef CAS PubMed.
- P. Marmottant, J. P. Raven, H. J. G. E. Gardeniers, J. G. Bomer and S. Hilgenfeldt, Microfluidics with ultrasound-driven bubbles, J. Fluid Mech., 2006, 568, 109–118 CrossRef.
- A. Ozcelik, J. Rufo, F. Guo, Y. Gu, P. Li, J. Lata and T. J. Huang, Acoustic tweezers for the life sciences, Nat. Methods, 2018, 15(12), 1021–1028 CrossRef CAS PubMed.
- P. Li, Z. Mao, Z. Peng, L. Zhou, Y. Chen, P. H. Huang, C. I. Truica, J. J. Drabick, W. S. El-Deiry, M. Dao and S. Suresh, Acoustic separation of circulating tumor cells, Proc. Natl. Acad. Sci. U. S. A., 2015, 112(16), 4970–4975 CrossRef CAS PubMed.
- R. H. Liu, J. Yang, M. Z. Pindera, M. Athavale and P. Grodzinski, Bubble-induced acoustic micromixing, Lab Chip, 2002, 2(3), 151–157 RSC.
- A. Hashmi, G. Yu, M. Reilly-Collette, G. Heiman and J. Xu, Oscillating bubbles: a versatile tool for lab on a chip applications, Lab Chip, 2012, 12(21), 4216–4227 RSC.
- D. Ahmed, C. Y. Chan, S. C. S. Lin, H. S. Muddana, N. Nama, S. J. Benkovic and T. J. Huang, Tunable, pulsatile chemical gradient generation via acoustically driven oscillating bubbles, Lab Chip, 2013, 13(3), 328–331 RSC.
- P.-H. Huang, N. Nama, Z. Mao, P. Li, J. Rufo, Y. Chen, Y. Xie, C. H. Wei, L. Wang and T. J. Huang, A reliable and programmable acoustofluidic pump powered by oscillating sharp-edge structures, Lab Chip, 2014, 14(22), 4319–4323 RSC.
- M. R. Rasouli and M. Tabrizian, An ultra-rapid acoustic micromixer for synthesis of organic nanoparticles, Lab Chip, 2019, 19(19), 3316–3325 RSC.
- N. H. A. Le, et al., Ultrafast star-shaped acoustic micromixer for high throughput nanoparticle synthesis, Lab Chip, 2020, 20(3), 582–591 RSC.
- P. H. Huang, et al., Acoustofluidic synthesis of particulate nanomaterials, Adv. Sci., 2019, 6(19), 1900913 CrossRef CAS PubMed.
- H.-S. Kim, H.-O. Kim and Y.-J. Kim, Effect of electrode configurations on the performance of electro-hydrodynamic micromixer, in International Conference on Nanochannels, Microchannels, and Minichannels, American Society of Mechanical Engineers, 2018, vol. 51197, p. V001T02A005 Search PubMed.
- S. Rashidi, H. Bafekr, M. S. Valipour and J. A. Esfahani, A review on the application, simulation, and experiment of the electrokinetic mixers, Chem. Eng. Process., 2018, 126, 108–122 CrossRef CAS.
- X. Ou, P. Chen, X. Huang, S. Li and B. F. Liu, Microfluidic chip electrophoresis for biochemical analysis, J. Sep. Sci., 2020, 43(1), 258–270 CrossRef CAS PubMed.
- A. Alizadeh, W. L. Hsu, M. Wang and H. Daiguji, Electroosmotic flow: From microfluidics to nanofluidics, Electrophoresis, 2021, 42(7–8), 834–868 CrossRef CAS PubMed.
- H. Zhang, H. Chang and P. J. M. Neuzil, DEP-on-a-chip: Dielectrophoresis applied to microfluidic platforms, Micromachines, 2019, 10(6), 423 CrossRef PubMed.
- J. J. Huang, Y. J. Lo, C. M. Hsieh, U. Lei, C. I. Li and C. W. Huang, An electro-thermal micro mixer, in 2011 6th IEEE International Conference on Nano/Micro Engineered and Molecular Systems, IEEE, 2011 Search PubMed.
- T. Zhou, H. Wang, L. Shi, Z. Liu and S. W. Joo, An enhanced electroosmotic micromixer with an efficient asymmetric lateral structure, Micromachines, 2016, 7(12), 218 CrossRef PubMed.
- P. Modarres and M. Tabrizian, Nanoparticle Synthesis Using an Electrohydrodynamic Micromixer, in 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS), IEEE, 2020, pp. 1068–1070 Search PubMed.
- Y. Daghighi and D. Li, Numerical study of a novel induced-charge electrokinetic micro-mixer, Anal. Chim. Acta, 2013, 763, 28–37 CrossRef CAS PubMed.
- J. E. Ortiz-Castillo, M. Vazquez-Pinon, S. O. Martinez-Chapa and V. H. Perez-Gonzalez, A reactor-on-a-chip for cost-effective synthesis of gold nanoparticles, Mater. Today: Proc., 2020, 48, 10–15 Search PubMed.
- K. Rahali, A. G. Tabriz and D. Douroumis, The effect of 3D printed microfluidic array designs on the preparation of liposome nanoparticles, J. Drug Delivery Sci. Technol., 2024, 94, 105411 CrossRef CAS.
- N. V. Egil, et al., High-throughput screening of gold nanoparticle synthesis parameters in droplet microfluidics, Mendeleev Commun., 2024, 34(6), 783–785 CrossRef CAS.
- G. Gkogkos, et al., A compact 3D printed magnetically stirred tank reactor cascade coupled with a free impinging jet for continuous production of colloidal nanoparticles, Chem. Eng. Sci., 2024, 294, 120081 CrossRef CAS.
- I. Arduino, et al., The manufacturing and characterization of pentamidine-loaded poly (lactic-co-glycolic acid) nanoparticles produced by microfluidic method, J. Drug Delivery Sci. Technol., 2024, 100, 106063 CrossRef CAS.
- D. Zhang, et al., Microfluidic Technology: A New Strategy for Controllable Synthesis of Metal Nanomaterials, ChemElectroChem, 2025, e202400666 CrossRef CAS.
- T. P. Rosalba, et al., A modular flow process intensification towards lipid peptoids nano assembly formation, J. Flow Chem., 2024, 14(4), 677–689 CrossRef CAS.
- I. S. Pires, et al., High-Throughput Microfluidic-Mediated Assembly of Layer-By-Layer Nanoparticles, Adv. Funct. Mater., 2025, 2503965 CrossRef.
- B. Huwaimel and S. Alqarni, Design of Poly (lactic-co-glycolic acid) nanoparticles in drug delivery by artificial intelligence methods to find the conditions of nanoparticles synthesis, Chemom. Intell. Lab. Syst., 2025, 105335 CrossRef CAS.
- K. Nathanael, S. Cheng, N. M. Kovalchuk, R. Arcucci and M. J. Simmons, Optimisation of Microfluidic Synthesis of Silver Nanoparticles via Data-driven Inverse Modelling, Chem. Eng. Res. Des., 2025, 216, 523–530 CrossRef CAS.
- Y. Yuan, Y. Li, G. Li, L. Lei, X. Huang, M. Li and Y. Yao, Intelligent Design of Lipid Nanoparticles for Enhanced Gene Therapeutics, Mol. Pharmaceutics, 2025, 22(3), 1142–1159 CrossRef CAS PubMed.
- S. Orsini, M. Lauricella, A. Montessori, A. Tiribocchi, M. Durve, S. Succi, L. Persano, A. Camposeo and D. Pisignano, 3D printing and artificial intelligence tools for droplet microfluidics: Advances in the generation and analysis of emulsions, Appl. Phys. Rev., 2025, 12(1), 011306 CAS.
- S. Rezvantalab, S. Mihandoost and M. Rezaiee, Machine learning assisted exploration of the influential parameters on the PLGA nanoparticles, Sci. Rep., 2024, 14(1), 1114 CrossRef CAS PubMed.
- N. Seegobin, et al., Optimising the production of PLGA nanoparticles by combining design of experiment and machine learning, Int. J. Pharm., 2024, 667, 124905 CrossRef CAS PubMed.
- J. Atencia, et al., Magnetic connectors for microfluidic applications, Lab Chip, 2010, 10(2), 246–249 RSC.
- Q. Xu, J. C. C. Lo and S.-W. R. Lee, Directly printed hollow connectors for microfluidic interconnection with UV-assisted coaxial 3D printing, Appl. Sci., 2020, 10(10), 3384 CrossRef CAS.
- M. Alvarez-Amador, A. Salimov and E. Brouzes, Universal and Versatile Magnetic Connectors for Microfluidic Devices, Micromachines, 2024, 15(6), 803 CrossRef PubMed.
- Z. Shi, H. Wang and Y. Zhang, A fast and plug-and-play mechanical connector for microfluidic chips, J. Phys.: Conf. Ser., 2024, 2740, 012004 CrossRef CAS.
- X. Liu and H. Meng, Consideration for the scale-up manufacture of nanotherapeutics—A critical step for technology transfer, View, 2021, 2(5), 20200190 CrossRef CAS.
- L. Gomez, et al., Scaled-up production of plasmonic nanoparticles using microfluidics: from metal precursors to functionalized and sterilized nanoparticles, Lab Chip, 2014, 14(2), 325–332 RSC.
- S. J. Shepherd, et al., Scalable mRNA and siRNA lipid nanoparticle production using a parallelized microfluidic device, Nano Lett., 2021, 21(13), 5671–5680 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |





