Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
From Lab-on-a-Chip to Lab-on-a-Chip-in-the-Lab: a perspective of clinical laboratory medicine for the microtechnologist†
Kirby
Fibben
a,
Evelyn Kendall
Williams
b,
John D.
Roback
c,
Wilbur A.
Lam
 *ad and
David N.
Alter
*c
*ad and
David N.
Alter
*c
aThe Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology, Atlanta, Georgia, USA
bChildren's Healthcare of Atlanta Scottish Rite Hospital, Atlanta, Georgia, USA
cPathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, Georgia, USA. E-mail: DNALTER@emory.edu
dDepartment of Pediatrics, Emory University, Aflac Cancer Center and Blood Disorders Center at Children’s Healthcare of Atlanta, Atlanta, Georgia, USA
First published on 15th May 2025
Abstract
An overview of the evolving role of microfluidics within clinical laboratories and diagnostic settings. It explores how microfluidic technologies, initially envisioned to replace traditional lab practices, are instead integrating into established workflows. This integration is driven by advancements in miniaturization and automation, enhancing efficiency and expanding testing capabilities. Regulatory frameworks such as CLIA and FDA oversight shape the landscape for microfluidic adoption, emphasizing the need for rigorous validation and compliance. The total testing process (TTP) framework underscores the critical phases—pre-analytical, analytical, and post-analytical—where microfluidics must conform with to ensure accuracy and reliability in diagnostic outcomes. Automation emerges as pivotal by streamlining workflows and reducing errors, particularly in sample handling and result interpretation. Challenges persist including the complex categorization of tests and the push for tighter regulation of laboratory developed tests (LDTs). The challenges necessitate collaboration between researchers, clinicians, and regulatory bodies. This review highlights how automation and integration of microfluidic technologies in point-of-care settings are reshaping clinical diagnostics, offering rapid, personalized testing options while maintaining high standards of patient care. Despite advancements, mitigating diagnostic errors remains paramount, requiring continuous refinement of technologies and adherence to established clinical protocols. Ultimately, the successful integration of microfluidics into clinical laboratories hinges on balancing innovation with regulatory compliance, ensuring seamless usability and consistent diagnostic accuracy within existing healthcare infrastructures.
Introduction to the clinical modern laboratory
Since the advent of the field, a major goal of the lab-on-a-chip was to leverage microfabrication techniques to miniaturize clinical assays such that any diagnostic test can be performed at the point-of-care technologies (POCT) and in low resource settings, obviating the need for the complex clinical lab. While the last several decades have seen the successful implementation of various POCT outside of healthcare facilities, the clinical lab remains a stalwart part of clinical medicine. Moreover, few signs indicate that miniaturization will render the clinical lab obsolete. In fact, the opposite appears to be occurring – microtechnologies and microfluidic systems are integrating into assays of the clinical laboratory rather than replacing them. While POCT implies microfluidic testing; microfluidic testing shouldn't imply that the test is intended for the POC environment; hence, here we discuss implementation of microfluidic testing into a central/main laboratory, i.e. going from traditional macrofluidic testing to microfluidic incorporating concepts of the former into the latter.The hospital clinical laboratory presents significant opportunities for expansion into microfluidic testing (MFT), with sample size reduction as the common denominator driving its adoption. Smaller sample volumes translate to a reduced laboratory footprint, freedom from space constraints, lower costs due to decreased reagent use and waste production, and a less invasive, more patient-friendly collection process with reduced pain and infectious disease risk. The next major evolution in clinical laboratory medicine will be the shift from traditional “macrofluidic” testing—measuring in milliliters (mL) and microliters (μL)—to microfluidic methodologies operating at the nano-, pico-, and femtoliter scale. As diagnostic testing evolves to accommodate these smaller sample volumes, so too must clinical laboratory methodologies and instrumentation. The complete blood count (CBC) serves as a prime example, having progressively minimized required sample volumes while bringing clinical testing closer to the patient. This shift raises a critical question: how will current clinical laboratory strategies adapt to the microfluidic future? To navigate what lies ahead, we must first understand the lessons of the past.
The promise of Lab-on-a-Chip technology lies in its potential to revolutionize diagnostics by enabling rapid, precise, and decentralized testing. However, bringing a microfluidic Lab Chip device into a hospital setting presents a complex set of challenges that extend beyond technological innovation. Unlike research devices, clinical implementations must navigate stringent regulatory pathways, including compliance with CLIA and FDA guidelines, which dictate test classification, personnel requirements, and validation processes. Moreover, seamless integration into the existing clinical laboratory infrastructure demands consideration of pre- and post-analytical workflows—key elements of the total testing process (TTP) that ensure accuracy and reliability. Lessons learned from established automation in clinical laboratories, such as the evolution of complete blood counters, highlight the importance of usability, standardization, and error mitigation. As microfluidics advances, success will depend on aligning innovation with the structured realities of hospital laboratories, ensuring new technologies enhance rather than disrupt patient care.
Perspective 1: Miniaturization and multiplexing don't go well together
The holy grail of laboratory testing is, of course, a multi-plexed test “menu” performed on a drop of blood (or any other sample type) that will lead to a technological paradigm shift in laboratory medicine akin to the introduction of multichannel automated laboratory testing in the late 1960s. Unfortunately, as a field, we have not yet entered that era. For example, for a single test on a drop of blood, significant patient-to-patient variability exists on the final blood collection volume collected, which is dependent on the patient's distal perfusion, skin thickness (regardless of cause) and the technical skill of the person collecting the blood drop. Next, is the issue of precision, as miniaturized assays, by definition, involve small sample volumes and the smaller the volume of specimen, the lower the assay's precision as well as its ability to distinguish “signal from noise”. Finally, the field of laboratory medicine has not yet fully grasped how very small volume specimens may have different biophysical characteristics and how well they represent actual results.Indeed, one lesion learned from the Theranos™ debacle is that adding volume to single drop specimens for measurement using conventional assays leads to highly variable results. When taking these issues together and then multiplying them by the number of tests desired per droplet, the complexity of multiplex droplet testing is easily discernible.
Regulatory guidelines dominate the landscape of the clinical laboratory
Humans have been attempting to develop methods to diagnose and detect disease for centuries; however, the clinical lab did not become a mainstay of medical practice until the early 20th century.1,2 The move towards a centralized, clinical lab represented a shift away from more empirical forms of diagnosis and the desire for more quantitative, objective assessments of disease. As the clinical lab continued to grow with new discoveries and technological advances, a concomitant need for standardization, quality control and regulation also arose.Initially, pathologists and laboratory scientists themselves sought to provide their own oversight and set standards by forming institutions such as the College of American Pathologists (CAP) or the Joint Commission in the late 1940s and early 1950s. Soon after formation, these institutions established their own laboratory accreditation programs that are currently used today. In the US, federal oversight of clinical lab testing began with the Clinical Laboratory Improvement Amendment (CLIA) of 1967 and was vastly expanded into much of what the CLIA program now consists of in 1988.
Today, the CLIA program is managed by three agencies, including the Centers for Medicare & Medicaid Services (CMS), the Food and Drug Administration (FDA), and the Centers for Disease Control and Prevention (CDC). All laboratories that test specimens from the human body for any clinical purposes such as diagnosis, treatment and prevention of disease require a CLIA certificate. The type of CLIA certificate (waived, moderate or high) depends on the complexity of the performed tests. Labs that only perform waived tests must obtain a certificate of waiver, otherwise labs must obtain a certificate to perform moderate and/or high complexity testing. Labs performing moderate and/or high complexity testing must also obtain a certificate of accreditation from a CMS approved accreditation organization such as CAP or the Joint Commission, with follow up inspections every two years and enrollment in a proficiency testing program. Together, this ensures quality results at all phases of testing.
In the US, the FDA is responsible for designating the complexity of each test. In short, “waived” tests are designed to be operated by non-laboratory personnel near the person being tested (near patient testing = POCT) [Test Complexities under the Clinical Laboratory Improvement Amendments (CLIA)]. It is important to remember that not all POCT is waived; whereas, all waived tests are POCT [Test Complexities under the Clinical Laboratory Improvement Amendments (CLIA)]. Two classic examples of waived POC tests are urine pregnancy and fingerstick (capillary blood) glucose testing. Non-waived testing is divided up into three categories; provider performed microscopy (PPM), moderate complexity and high complexity testing. PPM is not within the scope of this review and will not be discussed. The FDA distinguishes between moderate and high complexity testing using a set of seven categorization criteria as well as the manufacturer's instructions for use [Test Complexities under the Clinical Laboratory Improvement Amendments (CLIA)]. These criteria consist of important features such as the degree of automation included in the test, the amount of training and experience required to perform the test, and the degree of knowledge required to successfully interpret its results. All current FDA cleared or approved tests are listed online with their associated CLIA categorizations.3–5
Any testing either modified from an existing FDA cleared or approved test, or developed for clinical use without FDA approval is considered a “laboratory developed test” (LDT). All LDTs are automatically categorized as high complexity tests. Any microfluidic tests translated to the clinical lab without FDA approval or clearance would all be classified as LDTs. There has been a constant and growing source of discussion and debate as the development and utilization of LDTs has flourished. Consequently, there has been a wide variation between test promises and test abilities, with some very close to each other and some far apart. In the last several years, tighter legislation equating LDT validation to medical device approval has been proposed ostensibly with the intent of closing that gap but with some unintended consequences. Unintended in that it would stifle laboratory ability to nimbly and efficiently develop tests in particularly for new diseases.6,7
As regulated by CLIA, the researcher must have a comprehensive understanding of the different laboratory activities and an understanding of each testing phase to determine what level of personnel is required for specific responsibilities [ECFR 2024]. CLIA standards range from highly specific requirements (see personnel requirements) to less specific (critical result requirements). The described process pertains specifically to the United States and may differ significantly in other jurisdictions, potentially warranting references to alternative policies. For the microfluidics researcher, understanding the intricacies of the CLIA standards can facilitate the transition of new microfluidic tests into the clinical space.8
However, integrating lab-on-a-chip devices into centralized laboratories could compromise their one of their advantages: being rapid, point-of-care diagnostics. While centralization offers benefits such as standardized quality control and streamlined regulatory compliance, it also introduces logistical bottlenecks that can delay real-time data provision. While not limited to, many microfluidic devices are designed for decentralized environments, enabling immediate diagnostic results at the site of care. Achieving the optimal balance between the centralized nature of the clinical lab and decentralization characteristics that microfluidics provide is essential to integrate microfluidics into clinical spaces outside the point-of-care.
Effective adoption of these technologies necessitates strong laboratory leadership and a strategic approach to implementation. Collaboration among technical experts, project managers, and regulatory professionals is critical in process development, standardization, and staff training. Ensuring laboratory personnel are adept at operating, troubleshooting, and maintaining microfluidic systems is vital to their successful deployment. Additionally, integration into high-throughput environments may require dedicated project management support to oversee logistics, workflow adaptation, and scalability while ensuring compliance with Clinical Laboratory Improvement Amendments (CLIA) and other regulatory frameworks (Fig. 1).
 | ||
| Fig. 1 Flow chart on the required steps to achieve CLIA approval for testing with samples from human bodies. Additionally, a table with CLIA complex descriptions and examples. | ||
Given the existing infrastructure and staff of the clinical lab, the regulatory burden to translate new moderate or high complexity tests, including new microfluidic assays, to the clinical lab is often lower as compared to new CLIA-waived tests including those for over-the-counter or at-home use. However, with new types of tests may come new personnel requirements and shifting responsibilities in the clinical lab.
The concept of total testing process determines all aspects of the clinical laboratory
Beyond introduction of new regulations, an important contribution towards improved standardization and quality of clinical lab testing was the concept of the “total testing process” (TTP), described by George D. Lundberg in a JAMA commentary in 1981. The TTP encompasses the entire testing process from conception of the test by the physician to the intervention based upon its results, or the “brain-to-brain loop”. It consists of nine distinct steps including “physician brain”, test ordering, patient identification, specimen collection, transportation, preparation, analysis, reporting, and action.9 These can be grouped into three major phases: pre-analytical, analytical, and post-analytical. Preanalytical refers to all processes that occur before testing (sample ordering, collection, and transport), the analytic phase refers to the assay itself, and post-analytic refers to reporting as well as end-user interpretation and utilization of the result. According to Lundberg, errors at any point during this process constituted a failure of the lab test and of the clinical laboratory. Indeed, errors to this day are most common in the pre-analytical and post-analytical phases of the TTP as opposed to the analytical phase, highlighting the need for focus on the complete testing process.For the microfluidics researcher, successfully translating a research test into one for clinical purposes requires consideration of the TTP, and the ability to easily integrate within existing pre-analytical and post-analytical workflows of the clinical lab. The key consideration is that the development of a high-quality microfluidic chip device alone is insufficient; robust pre- and post-processing of samples is equally critical. This functionality must either be integrated into the chip device's control equipment or necessitate the operation of the device within a centralized laboratory setting to ensure reliable and accurate results (Fig. 2).
The layout of clinical laboratory evolves alongside the assay workflow
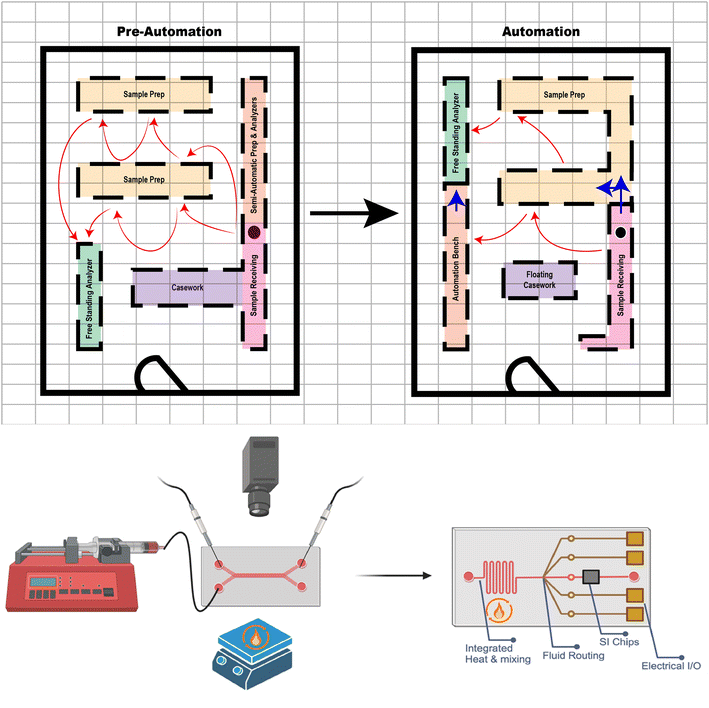
The physical space of the clinical lab has also become increasingly standardized, with distinct areas of the lab often designated for each part of the TTP. Currently, post-analytic processing is conducted virtually with results sent, post-verification, directly to the patient's electronic medical record. Prior to electronic medical record systems, labs required a more robust post-analytic section requiring space, personnel, and resources to manage hard copy results. Many laboratories still, however, maintain a call center for critical value notification and as a phone entry point.Pre-analytical sections are specimen entry points; referred to as “specimen receiving/processing” or similar. Here, specimens are received (accessed) into the lab and visually inspected for obvious pre-analytic errors such as correct ordering and proper labeling. Specimen manipulation such as centrifugation and aliquoting, as needed, also occurs here. Specimens are then transported to appropriate analytic sections for respective testing by either manually carrying the specimens or by using automated methods such as robot carriers or track systems. As such, the orientation of the preanalytical with respect to the analytic processes is currently a key laboratory design factor dependent on space availability.
This laboratory “geometry”, or the physical space that the clinical lab exists within, can be a crucial determinant of vendor and instrument(s) used. The geometry is often complicated by the presence of fixed obstacles such as load-bearing walls, electrical rooms, and pipe chases. Further integration of microfluidics into clinical lab diagnostics may help reduce the footprints of large instrument that microfluidics are replacing. The quantity of specimens, peripheral consumables, and the lab behind the chip might still require ample space. During the instrument request which is part of the proposal phase at the beginning of a new clinical laboratory space (either initially and/or over time), it is common practice to review the available laboratory space relative to an audit of desired processes and instrumentation to insure the best laboratory testing workflow within the available space. This can truly be a rate limiting step for implementation of any new testing.
This type of audit is necessary for all laboratory processes, whether they are macro- or micro-fluidic testing because, in high specimen volume situations, reduced specimen size and instruments don't necessarily imply that testing areas will have proportionately less space constraints. In many laboratories, it is discovered that delays are not as frequently the consequence of faults with the equipment as they are the result of workflow patterns that are not matched to the capabilities of the instrument.10 The development of microfluidic processes has the potential to reduce instrument backlogs, particularly in situations where testing in the clinical lab is still completed manually and this testing would benefit from automated phases (Fig. 3).
Error: The major barrier of entry for microfluidics into the clinical laboratory
According to the Institute of Medicine, diagnostic error is defined as “the failure to (a) establish an accurate and timely explanation of the patient's health problem(s) or (b) communicate that explanation to the patient”.11 Errors in clinical lab medicine are a major contributor to diagnostic error,12–14 and recent studies have demonstrated that 24–30% of laboratory errors have had an impact on patient care, while 3–12% of cases result in actual or potential patient harm.15Historically, much attention has been given to errors that occur during the analytical phase of testing. However, failures at any point during the clinical testing process, as defined by the TTP, can result in diagnostic error, and recent studies have indicated that the majority of these errors occur in the pre- and post-analytical phase of testing, where operator error is more likely to occur.13 In the pre-analytical phase, the most common errors include errors associated with specimen labeling, specimen collection, handling or processing, as well as ensuring the relevant test is ordered. In the post-analytical phases of testing, errors most commonly include incorrect interpretations of results, failures to inform patients of important aberrant results and/or following-up on these results, or additionally, failures to record the delivery of this information.13
New diagnostic assays entering the market must also contend with these errors. The use of microfluidics-based techniques may help to minimize these errors by further automating existing techniques and consolidating sample processing into fewer or single steps, but must fit within established clinical workflows. It is thus important for engineers to work closely with clinicians and laboratory scientists to ensure that their designs are clinically effective, precise, and dependable.16
Lab chip devices offer significant potential for minimizing errors by streamlining precision processes directly on-chip, thereby eliminating risks associated with sample transfer. This represents a significant leap forward in precision and reliability for analytical processes Additionally, microfluidics ability to multiplex and run multiple samples across many devices concurrently with small sample volumes ensures that results are not only consistent but also highly reliable. The evolution of lab chip technology promises to revolutionize various fields by streamlining workflows and improving data integrity, setting a new standard for laboratory efficiency and effectiveness. It is thus important for engineers to work closely with clinicians and laboratory scientists to ensure that their designs are clinically effective, precise, and dependable.
Perspective 3: Color-based, visual readouts are alluring but limited
Estimation of a laboratory test result by a visual color change is not an unreasonable request if the desired answer is purely qualitative: “DISEASE PRESENT” vs. “DISEASE ABSENT” but only if the color change is unequivocally detectable with the naked eye. Improvements can obviously be made by either providing a quantitative or semi-quantitative result. Either would require a spectrometer or reader of some sort to gauge the intensity of the color change. An alternative middle ground may be the development of a visual assay that is semi-quantitative leading to “DISEASE PRESENT” vs. ‘INDETERMINANT” vs. “DISEASE ABSENT” but that requires 3 distinct color-intensity regimes that are distinguishable with the naked eye (Fig. 4). | ||
| Fig. 4 A web map ‘case study’ on the CBC's quality indicators by testing phase. Highlights points of failure to account for in design. | ||
Automation has shaped the modern clinical laboratory
The future of clinical laboratory automation extends beyond the large-scale instruments that have dominated the field for decades. While automation has played a crucial role in improving efficiency, accuracy, and standardization, the next frontier lies in harnessing the potential of microfluidic devices to revolutionize diagnostic testing. Unlike traditional macroscale automation, which integrates pre- and post-analytical processes into large, centralized systems, microfluidic technology offers a paradigm shift by miniaturizing and decentralizing testing, paving the way for faster, more precise, and more accessible diagnostics.A classic example of laboratory automation's transformative impact is the evolution of the complete blood count (CBC). Before automation, CBCs were manually performed through blood smears, staining, and cell enumeration. The introduction of the Coulter counter in the mid-1900s automated this process, utilizing transient changes in electrical impedance to count cells as they passed through micro-scale apertures. This advancement revolutionized hematology by dramatically improving speed and accuracy. Today, many automated CBC analyzers are integrated into large-scale lab automation systems, streamlining the entire testing workflow.
However, while these large analyzers have enhanced clinical efficiency, they remain limited by their reliance on bulk instrumentation and centralized processing. Microfluidic technology presents a new opportunity to overcome these constraints. Unlike macroscale automation, microfluidic devices miniaturize entire workflows, allowing for sample collection, preparation, analysis, and detection to occur on a single chip. These devices use precise fluid manipulation techniques, enabling tests that traditionally required extensive sample handling to be performed rapidly with minimal reagents and sample volumes.
Sample collection and transport are critical considerations in laboratory automation. Traditional hospital networks rely on pneumatic tube systems and motorized sample tracks to transport and process specimens. While effective, these systems require significant infrastructure and still involve multiple handling steps. Microfluidic technology, however, streamlines these processes by integrating multiple test phases into a compact, self-contained system, reducing reliance on large-scale transport mechanisms. When combined with current automation capabilities, microfluidic devices offer a transformative approach to sample handling, minimizing errors and increasing efficiency.
Following the trajectory of large automated analyzers, microfluidic technology is poised to bring highly specialized tests such as cell adhesion assays and capillary electrophoresis directly into the clinical laboratory. By integrating automated sample handling, injection systems, and image processors into microfluidic platforms, these tests can transition from research settings to routine clinical use. Many microfluidic analyzers now incorporate pre-analytical, analytical, and post-analytical operations within a single instrument, much like traditional large-scale systems but with enhanced flexibility and efficiency.
For example, hemoglobin electrophoresis, a test traditionally conducted on bulk analyzers, can be fully automated on a microfluidic scale. In these systems, the sample is exposed to a hemolysis buffer, migrates across a microfluidic capillary, and generates hemoglobin curves that are all within a compact device. Such innovations demonstrate how microfluidics can refine and accelerate clinical workflows, offering a level of integration that surpasses traditional large-scale automation.
While established methodologies currently dominate the clinical landscape, microfluidic technology represents the future of laboratory automation. By transitioning from macroscale automation to microfluidic-based solutions, clinical laboratories can achieve greater efficiency, precision, and accessibility in diagnostic testing. As these technologies continue to evolve, they will play an increasingly central role in transforming clinical diagnostics beyond the limitations of conventional automation.
As with microfluidic testing, sample collection is a significant consideration when applying automation as with many cases, a macrofluidic specimen will still be needed. With the conventional laboratory, automation can reduce the number of phases requiring manual processing simplifying sample collection and introduction also. For example, microfluidics systems subject a sample to a series of tests as it moves down a track system after collection and processing integrating preanalytical, analytical, and post-analytical operations into a single instrument.
Beware that existing assays are evolving as well. Microfluidics represent a moving target that's opportunity more than a challenge that hasn't existed before.
Microfluidics and point-of-care have evolved alongside the clinical laboratory
Microfluidics and point-of-care technologies (POCT) provide significant potential for researchers and clinicians to develop devices that bridge the gap between what was historically done in a laboratory and what is now available at the patient's bedside. The field of microfluidics has emerged as an important contributor to point-of-care testing. POCT refers to near-patient testing at healthcare-patient clinical encounters such that a test result can be generated at the moment without involving the clinical laboratory. The growth of POCT has unknowingly preceded microfluidics as science e.g. test strips used in POCT glucose.With the advancements in microfluidics, many tests that formerly needed to be performed in a clinical laboratory may now be performed at the patient's bedside. As rapid diagnostic tests, like nucleic acid amplification tests (NAATs), continue to shrink in size, medical professionals are able to bring these tests straight to the patient's bedside in order to deliver a higher standard of care. Traditional research-based testing is currently being adapted for use in POCT settings including paper-based analytical devices. Other examples of research-based analysis include microfluidics or centrifugal microfluidics, specific sensor methodologies such as optical sensors and electrochemical transducers, and novel technologies geared toward use in settings with limited access to resources. Each phase of clinical testing may be optimized using microfluidics, and it is being done. At the POCT, microfluidics is transforming clinical tests at all phases of the TTP. For example, new microfluidics based techniques have enabled collection into micro-sample chambers (pre-analytical), bedside microfluidic blood testing (analytical), and app-based microfluidic reporting that is immediately available at the bedside (post-analytical).17
Perspective 3: Miniaturization may not be necessary in all cases
While the benefits of assay miniaturization are well known to the lab-on-chip community, for many use cases, especially for POC or home-based tests, there is a point of diminishing returns and smaller assays may not lead to increased value, especially when accounting for the potential drawbacks of miniaturization (see Perspective 1). Indeed, for the use case of POC and home testing, focus should be placed more on usability rather than shrinkage.During development, consideration of CLIA requirements, training needs, and ability for automation will ease translation of new POCT devices into waived spaces and eliminate some of the entry barriers. While personalized medicine catering to the clinician at the bedside is often at the forefront of a researcher's design philosophy, the small footprint and ease of use often account for seamless integration into a functional clinical laboratory instead. As the healthcare landscape changes, microfluidics is at the forefront of research-based analysis in point-of-care settings.
Development and integration of POCT into healthcare systems is broadening the conventional centralized model of clinical lab testing, increasing its scope and reach.
Recent microfluidic technologies that have integrated into the clinical lab
Microfluidics have become a pivotal technology in transforming diagnostic assays in clinical laboratories over the last 15 years. By enabling reduced reagent consumption, faster turnaround times, and multiplexed analyses in confined chip architectures, microfluidic platforms have permeated diverse domains such as immunoassays, molecular diagnostics, hematology, and microbiology. Notably, systems like Randox's Biochip Array (capable of ∼2940 tests per hour using only 7 μL of sample)14,18–20 and Gyros' centrifugal ELISA-on-disc21 have demonstrated that high-throughput clinical-grade multiplexing can be achieved in compact, integrated systems. ProteinSimple's Ella further exemplifies the transition from research to clinical deployment with its validated IL-6 immunoassay,22 and Seamaty's SMT780 illustrates how chip-based CLIA systems can scale down conventional workflows for mid-volume laboratories.23Nucleic acid diagnostics have particularly benefited from sample-to-answer microfluidic integration. Systems like the Cepheid GeneXpert use enclosed cartridges to automate sample lysis, nucleic acid extraction, nested PCR amplification, and real-time detection—an architecture that has made rapid TB, MRSA, and STI testing accessible in decentralized labs.24 FilmArray's pouch-based PCR platform extends this paradigm by integrating freeze-dried multiplex assays in microchannel-enabled cartridges, delivering 1-hour results across 20+ targets with moderate throughput (up to ∼175 samples per day)25 Rheonix Encompass MDx further pushes automation, combining multiple fluidic layers with micro-pumps and microarray detection in a single disposable unit.26 Meanwhile, droplet-based digital PCR (e.g., Bio-Rad QX200) has become a clinical mainstay for liquid biopsies and low-level viral detection, leveraging microdroplet partitioning for absolute quantification.27
In hematology, microfluidic advances are enabling miniaturized cell-based diagnostics traditionally performed by bulk cytometers. Lab-on-chip implementations of Coulter counters and 3D-focused flow cytometers now offer functional WBC differential counts using only microliters of whole blood, with optical detection on integrated photodiodes.28 In addition to cell counting, microfluidics is facilitating novel rheological assays: microchannel constriction platforms, such as those designed by Faustino et al., can differentiate red blood cell deformability and aggregation states with subcellular resolution and useful in sickle cell disease and inflammatory diagnostics.29 Chip-based sorters like On-chip Sort introduce air pressure-driven actuation to isolate fragile cells without shear stress,30 while FDA-cleared systems like Parsortix capture circulating tumor cells (CTCs) via size-based filtration for downstream oncological analysis,31 representing a milestone in liquid biopsy automation.
In clinical chemistry and microbiology, microfluidic platforms are accelerating analysis by confining enzymatic and culture reactions into miniaturized spaces. The Abaxis Piccolo Xpress exemplifies centrifugal microfluidics for panel-based chemistry, providing 14-analyte profiles in 12 minutes using self-calibrating reagent discs.32 Microfluidic AST platforms are perhaps the most transformative in microbiology: the Accelerate PhenoTest BC system, for example, performs ID and MIC determination within 7 hours using on-chip imaging of bacterial growth under drug exposure.33 Emerging AST systems like QuantaMatrix dRAST and Alifax Alfred 60AST further leverage microchannels and time-lapse microscopy to reduce diagnostic delays from days to hours.34 These systems demonstrate that microfluidics not only miniaturizes workflows but enables phenotypic assays to achieve clinical speed and automation, improving sepsis management and antibiotic stewardship.
Successful adoption of microfluidic systems in central labs depends on their integration with existing automation, throughput needs, and quality control protocols. Platforms vary from highly multiplexed batch processors like Randox Evidence to modular cartridge systems (e.g., FilmArray Torch, Rheonix Encompass) and fully automated disc systems like the Piccolo Xpress.32 Throughput can range from 4 samples per Rheonix run to >170 per day with Torch, to thousands of results per hour with multiplexed immunoassays. Most systems are LIS-compatible and offer onboard calibration (e.g., Abaxis iQC),32 minimizing the need for manual QC or staff retraining. While closed, proprietary cartridge systems may limit flexibility, their hands-off automation and contamination control offer strong benefits for high-complexity testing. Future directions will likely focus on enhancing openness, multiplexing, and integration. This brings microfluidics closer to becoming the core engine of next-generation clinical laboratories.
In the mid to late 1800s through the early years of the 20th century, clinical laboratory testing was limited to a handful of tests performed on urine specimens using, by even today's standards, sophisticated analyses; from the late 20s through mid-60s they resembled college chemistry labs with each bench dedicated to an analyte or two and investigated unknown – analyte being measured; this all changed in the late 1960s with introduction of the forerunner of today's modern autoanalyzer. The autoanalyzer and its successors allowed laboratories to move away from the single assay at a time mindset to multiple assays, in different combinations to be performed almost simultaneously. Data does not exist but it's safe to state that the volume of laboratory tested has expanded (and continues to expand) geometrically and now plays a pivotal role in patient care. Since then, the bells and whistles have significantly improved over time as demonstrated with total laboratory automation.
Automated multi-assay microfluidic clinical laboratory testing will be the next evolutionary step in patient diagnostic as the paradigm is re-invented in terms of a whole new class of testing that will surpass current simple enumerations of activity/concentration to analyses of the cellular and genetic levels; arguably these tests already exist clinically but microfluidics should allow them to expand to general clinical use. In addition, incorporation of microfluidics will significantly shrink the footprint of the conventional clinical laboratory and improve care by decreased specimen volume collection and its associated risks of anemia and infection (Fig. 5).
 | ||
| Fig. 5 Table of microfluidic technologies that have been integrated into the clinical lab with who, what, where, and how these assays are used in the clinical lab. | ||
Overall, the success of moving from Lab-on-a-Chip to Chip-in-the-Lab could be simplified to following existing rulesets, blueprints, and timelines. These factors generally fall under the understanding that the microfluidic tests should allow for translating it to be accessible within the space itself. It should be not only for the facility within the clinical laboratory but also for the geometry of the clinical laboratory itself. The tests need to have a high level of usability within the space by facilitating the use of the test. Without sacrificing their usability, narrowing in test consistency follows suit.
In conclusion, the journey from Lab-on-a-Chip to Chip-in-the-Lab has unfolded against the backdrop of the evolving landscape of clinical laboratories and diagnostic technologies. The original vision of obviating the need for traditional clinical labs in favor of microfluidic point-of-care testing has faced the reality that, rather than replacing clinical labs, microfluidic systems are integrating into the established workflows. As we venture into the microfluidic future, the shift from “macrofluidic” to “microfluidic” testing methods brings both opportunities and challenges.
The foundation of clinical laboratory testing, encapsulated in the total testing process (TTP), has shaped the contemporary approach to patient care. The TTP's three phases, pre-analytical, analytical, and post-analytical, provide a framework that is deeply entrenched in the clinical laboratory landscape. While the integration of microfluidic technologies holds promise, understanding and aligning with the TTP become paramount for the successful translation of research tests into clinically applicable solutions.
Regulatory frameworks, such as CLIA, guide the path for microfluidic researchers, necessitating a comprehensive understanding of laboratory activities and personnel requirements. The categorization of tests as waived or non-waived, and the distinction between FDA-cleared or approved tests and laboratory developed tests (LDTs), adds complexity to the regulatory landscape. The push for tighter legislation on LDTs highlights the challenges of aligning rapidly evolving technologies with regulatory oversight.
Perspective 4: Opportunities exist in developing “Lab-on-Chip-in-the-Lab” technologies
Existing conventional moderate to high complexity clinical laboratories may benefit significantly from new microtechnologies, enabling assays used in those environments to evolve from macrofluidic to microfluidic tests, which in turn, could lead to vast improvements in patient care, processes, and costs. Indeed, a significant need clinical laboratorians have that microtechnologists may not be aware of involves not new assays per se, but the need for miniaturized (yet robust) devices to transport small amounts of fluid from one component in a larger diagnostic system to another component. In addition, development of new microfluidic methods for simplified sample collection and sample preparation for downstream “macro” assays would also be valuable in a clinical laboratory. As such, lab-on-chip technologies need not attempt to obviate or replace the clinical lab but instead focus on improving it”.Automation emerges as a key player in the transformation of clinical laboratories, exemplified by the evolution of the complete blood counter (CBC). The quest for efficiency, standardized analysis, and reduced staffing requirements in the face of increasing test volumes is a driving force. However, automation introduces its own set of challenges, particularly in the pre- and post-analytical phases, emphasizing the need for continuous optimization and vigilance against potential errors.
Microfluidics and POCT have emerged as transformative forces, bridging the gap between traditional laboratory testing and on-the-spot diagnostics. The integration of microfluidic technologies into point-of-care settings, accompanied by the evolution of rapid diagnostic tests, presents new possibilities for personalized medicine and improved patient care.
Nevertheless, the specter of error looms large, demanding careful consideration and design adjustments to mitigate potential adverse impacts. Addressing error prevention not only within the laboratory but across the entire healthcare system becomes imperative. The role of diagnostic error, especially in the interpretation of test results, underscores the need for collaboration between microfluidic designers and healthcare professionals to ensure clinical effectiveness and patient safety.
In essence, the success of translating microfluidics in the clinical laboratory hinges on a nuanced understanding of the existing rules, blueprints, and timelines. The journey towards Chip-in-the-Lab requires a thoughtful balance between innovation and integration, usability and consistency, and adherence to established standards. While microfluidics continues to shape the future of diagnostic testing, the clinical laboratory, with its proven methodologies and infrastructure, remains an indispensable cornerstone of optimal patient care. As the healthcare landscape evolves, the microfluidics researcher's careful consideration of clinical laboratory components remains a crucial step in the successful integration of innovative testing solutions.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.Conflicts of interest
There are no conflicts to declare from any of the authors.References
- L. Landaverde, D. McIntyre, J. Robson, D. Fu, L. Ortiz and R. Chen, et al., Buildout and integration of an automated high-throughput CLIA laboratory for SARS-CoV-2 testing on a large urban campus, SLAS Technol., 2022, 27(5), 302–311 CrossRef PubMed.
- U.S. Food and Drug Administration, Laboratory Developed Tests. FDA, 2024, Jan 24 [cited 2024 Jan 31].
- U.S. Food and Drug Administration, CLIA Waived Tests. FDA, [cited 2024 Jan 31].
- Centers for Disease Control and Prevention. Test Complexities under the Clinical Laboratory Improvement Amendments (CLIA). CDC, 2018, Aug 6 [cited 2024 Jan 31].
- Centers for Medicare & Medicaid Services, Clinical Laboratory Improvement Amendments (CLIA) – How to Apply, https://Medicare.gov, 2024, Jan 23 [cited 2024 Jan 31] Search PubMed.
- D. J. Dietzen, Unleashing the Power of Laboratory Developed Tests: Closing Gaps in COVID Diagnosis and Beyond, J. Appl. Lab. Med., 2020, 5(5), 844–846 CrossRef PubMed.
- J. R. Genzen, Regulation of Laboratory-Developed Tests, Am. J. Clin. Pathol., 2019, 152(2), 122–131 CrossRef PubMed.
- U.S. Government, PART 493—LABORATORY REQUIREMENTS. e-CFR - Code of Federal Regulations, 2024, [cited 2024 Jan 31].
- G. D. Lundberg, Acting on significant laboratory results, JAMA, J. Am. Med. Assoc., 1981, 245(17), 1762–1763 CrossRef CAS PubMed.
- Advances in Patient Safety, Patient Safety and Quality: An Evidence-Based Handbook for Nurses, ed. R. G. Hughes, Agency for Healthcare Research and Quality, US, 2008, [cited 2024 Jan 31] Search PubMed.
- L. L. Leape, Errors in medicine, Clin. Chim. Acta, 2009, 404(1), 2–5 CrossRef CAS PubMed.
- P. Carraro and M. Plebani, Errors in a stat laboratory: types and frequencies 10 years later, Clin. Chem., 2007, 53(7), 1338–1342 CrossRef CAS PubMed.
- M. Plebani, Diagnostic Errors and Laboratory Medicine - Causes and Strategies, eJIFCC, 2015, 26(1), 7–14 Search PubMed.
- M. Plebani, M. Laposata and G. D. Lundberg, The brain-to-brain loop concept for laboratory testing 40 years after its introduction, Am. J. Clin. Pathol., 2011, 136(6), 829–833 CrossRef PubMed.
- M. Plebani, The detection and prevention of errors in laboratory medicine, Ann. Clin. Biochem., 2010, 47(Pt 2), 101–110 CrossRef PubMed.
- L. L. Leape, Striving for Perfection, Clin. Chem., 2002, 48(11), 1871–1872 CrossRef CAS.
- AACC, POCT How-To Guide for Non-Laboratorians. MyADLM, 2022, Sep [cited 2024 Jan 31].
- J. Y. Vis and A. Huisman, Verification and quality control of routine hematology analyzers, Int. J. Lab. Hematol., 2016, 38(Suppl 1), 100–109 CrossRef PubMed.
- S. Khan, R. A. Khan, S. Khan, S. Khan, S. Khan and S. Khan, et al., A comprehensive review on the role of artificial intelligence in healthcare, J. Innov. Knowl., 2023, 8(1), 100199 Search PubMed.
- Randox Laboratories, Lab Chip, 2022, 22, 254–262 Search PubMed.
- Gyros Protein, Lab Chip, 2021, 21, 987–995 RSC.
- Bio-Techne, ProteinSimple Ella IL-6 Assay Validation Report, 2023.
- Seamaty Diagnostics, SMT780 Technical Datasheet, 2023.
- Cepheid, Xpert MTB/RIF Assay Package Insert, 2021.
- BioFire Diagnostics, FilmArray System Overview. bioMérieux, 2022.
- Rheonix Inc, Encompass MDx Technical White Paper, 2021.
- Bio-Rad Laboratories, Droplet Digital PCR Applications Guide, 2022.
- K. Kim, et al. , Lab Chip, 2020, 20, 1122–1130 Search PubMed.
- R. Faustino, et al. , Lab Chip, 2019, 19, 1001–1012 Search PubMed.
- On-Chip Biotechnologies, On-chip Sort Product Manual, 2022.
- ANGLE PLC, Parsortix PC1 FDA Clearance Press Release, 2022.
- Abaxis/Zoetis, Piccolo Xpress Operator's Manual, 2020.
- Accelerate Diagnostics, PhenoTest BC FDA Summary, 2021.
- QuantaMatrix, dRAST Overview Brochure, 2023.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4lc00614c |
| This journal is © The Royal Society of Chemistry 2025 |