Open Access Article
Open Access ArticleEfficient stacking of iso-butene in sulfonate functional metal–organic frameworks for efficient iso-butene/iso-butane separation†
Zhensong
Qiu‡
a,
Jiyu
Cui‡
a,
Dengzhuo
Zhou
a,
Zhenglu
Yang
ab,
Xiaofei
Lu
b,
Xian
Suo
b,
Anyun
Zhang
 a,
Xili
Cui
a,
Xili
Cui
 ab,
Lifeng
Yang
*ac and
Huabin
Xing
ab,
Lifeng
Yang
*ac and
Huabin
Xing
 *ab
*ab
aEngineering Research Center of Functional Materials Intelligent Manufacturing of Zhejiang Province, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou, Zhejiang 310058, PR China. E-mail: lifeng_yang@zju.edu.cn; xinghb@zju.edu.cn
bZJU-Hangzhou Global Scientific and Technological Innovation Center, Hangzhou 311215, China
cState Key Laboratory of Silicon Materials, School of Materials Science and Engineering, Zhejiang University, Hangzhou 310027, China
First published on 2nd July 2025
Abstract
Separation of iso-butene and iso-butane is vital to producing high purity iso-butene feedstock, but is challenging because of their close molecular size and properties. Adsorptive separation using porous materials like metal organic frameworks (MOFs) is emerging as a potential energy-efficient alternative. But it's hindered by the lack of porous materials that exhibit satisfactory iso-butene/iso-butane separation performance. In this study, a novel sulfonate functionalized material, ZU-603, is reported to achieve the benchmark separation performance of iso-butene/iso-butane via exploiting the geometric difference of the carbon backbone between the planar iso-butene and tetrahedral iso-butane. Single-crystal analysis of ZU-603 loaded with iso-butene and simulation studies reveal that the sulfonate sites bound the iso-butene via Sδ−⋯Hδ+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C interactions, meanwhile iso-butene molecules are efficiently stacked via π–π interactions within the confined space, realizing higher stacking efficiency of iso-butene than iso-butane. ZU-603 shows an exceptionally high iso-butene adsorption uptake of 2.30 mmol g−1 (298 K, 1 bar) and a record high iso-butene/iso-butane uptake ratio of 2.77 at 1 bar, outperforming previously reported benchmarking materials (1.2). Fixed-bed breakthrough experiments confirm the impressive iso-butene/iso-butane dynamic separation ability of ZU-603. The work provides a potential shape-recognition strategy in designing functional materials for the efficient separation of hydrocarbons with similar physicochemical properties.
C interactions, meanwhile iso-butene molecules are efficiently stacked via π–π interactions within the confined space, realizing higher stacking efficiency of iso-butene than iso-butane. ZU-603 shows an exceptionally high iso-butene adsorption uptake of 2.30 mmol g−1 (298 K, 1 bar) and a record high iso-butene/iso-butane uptake ratio of 2.77 at 1 bar, outperforming previously reported benchmarking materials (1.2). Fixed-bed breakthrough experiments confirm the impressive iso-butene/iso-butane dynamic separation ability of ZU-603. The work provides a potential shape-recognition strategy in designing functional materials for the efficient separation of hydrocarbons with similar physicochemical properties.
Keywords: Adsorptive separation; Hydrocarbon; Metal-organic frameworks; Iso-butene/iso-butane; Purification.
1 Introduction
Iso-butene (iso-C4H8) is an important feedstock in the petroleum industry, which could be utilized in producing butyl rubber, methyl methacrylate (MMA) and other high-performance materials.1–4 One of the primary industrial sources of iso-C4H8 is the catalytic dehydrogenation of iso-butane (iso-C4H10), the product of which typically consists of approximately equal mole fractions of iso-C4H8 and iso-C4H10 isomers due to the limited conversion rate.5–7 To obtain high purity iso-C4H8 from the mixture, an industrial practice is to react the mixture with methanol to convert iso-C4H8 into methyl tert-butyl ether (MTBE).8–10 Then MTBE is separated from iso-C4H10 and cracked back into iso-C4H8 and methanol.11 This complicated purification process may be simplified by the more convenient technology of adsorption separation.12–14 The potential application of adsorption separation has been widely investigated in separating chemicals with similar physiochemical properties.15–21 The key to adsorption separation is the design and application of high-performance porous materials. In recent years, metal organic frameworks (MOFs) with their extremely variable pore structures and highly tunable nature have shown promising application potential in the separation of hydrocarbon gas mixtures with very close physiochemical properties,22–26 including ethane/ethylene,27–31 ethylene/acetylene,32–37 propane/propylene,38–44 C6 isomers,45–48etc. HKUST-1 shows preferential adsorption of iso-C4H8 over iso-C4H10 in the very low-pressure range of 0.5 kPa. However, it demonstrates negligible selectivity at higher pressures, limiting its separation performance.49 Other than that, little attention has been paid to investigating the adsorption separation of iso-C4H8 and iso-C4H10 using MOFs.The properties of iso-C4H8 and iso-C4H10 are very similar, where the most significant difference lies in the presence of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond in the iso-C4H8 molecule. As a result, the 4 carbon atoms of iso-C4H8 are in the same plane while the 4 carbon atoms of the iso-C4H10 molecule form a triangular pyramid. If this shape difference in the carbon backbone of the 2 isomers could be utilized to discriminate one another, the adsorption separation of iso-C4H8 and iso-C4H10 could be achieved with high selectivity. Shape selective materials have been developed in the literature for the separation of butadiene,50 xylene isomers,51,52etc. A feasible strategy is to construct pore cages with limited space and suitable shape to accommodate the desired molecule. The single pore cage of the material could simultaneously accommodate multiple target molecules with suitable molecular shape when the molecules are densely packed. But for the undesired molecules with inappropriate molecular shape, a single pore cage could hardly accommodate multiple molecules, realizing the preferential adsorption of the target molecule.
C double bond in the iso-C4H8 molecule. As a result, the 4 carbon atoms of iso-C4H8 are in the same plane while the 4 carbon atoms of the iso-C4H10 molecule form a triangular pyramid. If this shape difference in the carbon backbone of the 2 isomers could be utilized to discriminate one another, the adsorption separation of iso-C4H8 and iso-C4H10 could be achieved with high selectivity. Shape selective materials have been developed in the literature for the separation of butadiene,50 xylene isomers,51,52etc. A feasible strategy is to construct pore cages with limited space and suitable shape to accommodate the desired molecule. The single pore cage of the material could simultaneously accommodate multiple target molecules with suitable molecular shape when the molecules are densely packed. But for the undesired molecules with inappropriate molecular shape, a single pore cage could hardly accommodate multiple molecules, realizing the preferential adsorption of the target molecule.
In this work, a shape-recognizing sulfonate functional material, ZU-603, was found to be capable of separating the iso-C4H8 and iso-C4H10 mixture. Pure component adsorption isotherms show that ZU-603 exhibits higher adsorption affinity toward iso-C4H8 over iso-C4H10 with an iso-C4H8/iso-C4H10 uptake ratio of 2.77, superior to previously reported HKUST-1 (1.2).49 Fixed bed breakthrough experiments further prove the separation performance of ZU-603. The structures of single crystals loaded with iso-C4H8 molecules were solved to unveil the adsorption behavior. Coupled with the DFT-D simulation, the separation mechanism was revealed.
2 Results and discussion
Mild conditions of ambient temperature and pressure are utilized for the synthesis of ZU-603, with methanol and water as solvents. The mild synthesis conditions enable ZU-603 to be further scaled-up. The Cu metal node is coordinated with the 4,4′-dipyridyl disulfide ligand to form the pore windows of ZU-603, with an opening of approximately 5.2 Å × 5.2 Å (Fig. 1), close to the molecular dimensions of iso-C4H8 and iso-C4H10 (Fig. S1†). The sulfonate anion, 1,2-ethanedisulfonate, serves as the connecting unit of the pore windows, which further forms the pore cage (as illustrated by the orange sphere in Fig. 1) of ZU-603. It's revealed that the pore cages are connected to form a 2D layered structure (Fig. S2†). And then multiple 2D layered structures are stacked together to form the 3D structure of ZU-603, which endows it with the sql topology (Fig. S3†). The powder X-ray diffraction patterns of the synthesized samples match well with the simulated ones, confirming the high purity of the synthesized materials (Fig. 2f). Besides, the permanent porosity of ZU-603 is investigated via N2 adsorption experiments at 77 K (Fig. S4†), which displays a Brunauer–Emmett–Teller (BET) surface area of 214 m2 g−1 (Fig. S5†). Thermogravimetric analysis shows that ZU-603 is stable up to 240 °C (Fig. S6†). The SEM image of ZU-603 shows a flake-like morphology with a narrow size distribution in the range of 0.9–1.2 μm (Fig. S7†).To explore the adsorptive properties of ZU-603 for iso-C4H8 and iso-C4H10, pure component adsorption–desorption isotherms are collected at 298 K and 313 K (Fig. 2a and b and S8†). It's displayed that ZU-603 shows higher adsorption affinity toward iso-C4H8 over iso-C4H10 at both temperatures. At 298 K, ZU-603 exhibits a steep iso-C4H8 uptake at the pressure as low as 0.003 bar. The iso-C4H8 uptake is 2.30 mmol g−1 at 1 bar. For iso-C4H10, the uptake at 1 bar is 0.83 mmol g−1. The iso-C4H8/iso-C4H10 uptake at 1 bar is 2.77, higher than that of HKUST-1 (1.2).49 Ideal adsorbed solution theory was applied to calculate the adsorption selectivity (IAST selectivity). At infinity dilution, the IAST selectivity is 39 at 298 K and 14 at 313 K (Fig. 2c and S9 and S10†). It's noted that ZU-603 not only possesses a very high iso-C4H8 uptake of 2.10 mmol g−1 at 0.5 bar, but also shows the highest iso-C4H8/iso-C4H10 uptake ratio of 3.33 at 0.5 bar compared with other reported materials (Fig. 2d): SD-65 (1.29),53 Mg-gallate (1.27),54 ZU-609 (1.20),55 and HKUST-1 (1.23).49 The time dependent adsorption profiles of both isomers were further measured to investigate the diffusion rate of iso-C4H8 and iso-C4H10 within the pores of ZU-603 (Fig. 2e). The diffusion time constants of iso-C4H8 and iso-C4H10 were calculated using the micropore diffusion model, which are 7.42 × 10−4 s−1 and 6.43 × 10−4 s−1 for iso-C4H8 and iso-C4H10, respectively (Fig. S11 and S12†). The result indicates that both isomers show a close diffusion rate within the pores of ZU-603.
Grand Canonical Monte Carlo (GCMC) simulation was used to elucidate the preferential adsorption of iso-C4H8 over iso-C4H10 at a fixed pressure of 1 atm. Snapshots at different steps are taken to understand the adsorption process (Fig. S13 and S14†). The two images compare the adsorption behaviour of iso-C4H8 and iso-C4H10 in ZU-603 at 1 bar. Iso-C4H8 shows significantly higher uptake, with dense and progressive filling of the framework cavities from panel (1) to (10), while iso-C4H10 adsorption remains sparse even at saturation. This contrast highlights ZU-603's selective adsorption driven by stronger interactions with the unsaturated iso-C4H8 likely due to π-complexation or better shape compatibility. In addition, the low energy snapshot of iso-C4H8 also shows a higher packing density than that of iso-C4H10 (Fig. S15†). The average loading of iso-C4H8 is 0.77 molecules per unit cell and iso-C4H10 is 0.21 molecules per unit cell, yielding an uptake ratio of 3.66 at 1 bar, which is very similar to the experimental result of 2.77. To further unveil the higher adsorption uptake of iso-C4H8 over iso-C4H10, the structures of the single crystals loaded with iso-C4H8 were successfully obtained. It's found that 2 iso-C4H8 molecules could be simultaneously adsorbed within the same pore cage of ZU-603 (Fig. 3a). This allows the maximized occupation of the pore space. Further investigation into the packing diagram of the 2 iso-C4H8 molecules adsorbed reveals the reason behind such a phenomenon. Due to the double bond of iso-C4H8 molecules, the 4 carbon atoms of iso-C4H8 are distributed in the same plane, which could be utilized to pack two iso-C4H8 molecules in a space efficient way. In detail, the 2 iso-C4H8 molecules are stacked in a confined pore cage of ZU-603 in a way that the aforementioned planes within each iso-C4H8 run parallel to each other, with a distance of 3.4 Å between the two planes (Fig. 3b). This facilitates the space-efficient packing of iso-C4H8 in the limited space of the pore cages within ZU-603, which could not be achieved by iso-C4H10. The O atoms of the sulfonate anions are the primary binding site of iso-C4H8, with the C–H⋯O hydrogen bond in the range of 1.7–2.9 Å (Fig. 3c). DFT-D calculation was applied to calculate the specific binding sites of iso-C4H10 within ZU-603. The result show that like iso-C4H8, O atoms of the sulfonate anions are also the main binding sites of iso-C4H10, where the C–H⋯O hydrogen bond is in the range of 2.6–2.9 Å (Fig. 3d).
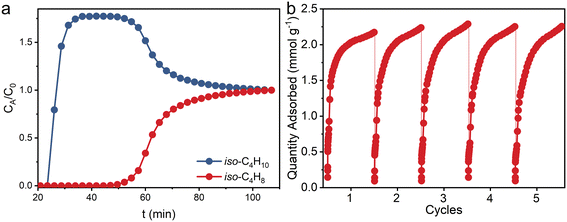
A fixed bed breakthrough experiment with a gas mixture of iso-C4H8/iso-C4H10 (50/50, v/v) was carried out to investigate the dynamic separation performance of ZU-603. The results show that ZU-603 could selectively adsorb iso-C4H8 in the binary mixture with a breakthrough sequence of iso-C4H8 < iso-C4H10. After the introduction of the gas mixture, both isomers were adsorbed within the column packed with ZU-603 until 23.5 min, where iso-C4H10 first elutes. Iso-C4H8 did not breakthrough until 50 min, with an effective dynamic adsorption capacity of 1.06 mmol g−1. The results show a good dynamic separation performance of ZU-603 for the binary mixture (Fig. 4a). Cycling adsorption experiments of pure component iso-C4H8 show no obvious gas uptake loss after 5 cycles, exhibiting the good cycling capacity of ZU-603 (Fig. 4b).
3 Conclusions
In summary, a novel sulfonate functionalized ultramicroporous material, ZU-603, was designed and it exhibits an excellent iso-C4H8/iso-C4H10 separation performance because of its selective shape-recognizing ability toward iso-C4H8 molecules over iso-C4H10. Through exploiting the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond and the planar configuration of iso-C4H8, ZU-603 with suitable pore size and distributed sulfonate functional sites induces the effective packing of 2 iso-C4H8 molecules within the limited volume of 1 pore cage of the material via Sδ−⋯Hδ+
C double bond and the planar configuration of iso-C4H8, ZU-603 with suitable pore size and distributed sulfonate functional sites induces the effective packing of 2 iso-C4H8 molecules within the limited volume of 1 pore cage of the material via Sδ−⋯Hδ+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C interactions and π–π guest–guest interactions. This work not only provides an important case of iso-C4H8/iso-C4H10 porous materials with both high capacity and selectivity, but also demonstrates the shape-recognizing strategy in the separation of C4 hydrocarbons, which may also be applied in designing high-performance materials for other challenging separations.
C interactions and π–π guest–guest interactions. This work not only provides an important case of iso-C4H8/iso-C4H10 porous materials with both high capacity and selectivity, but also demonstrates the shape-recognizing strategy in the separation of C4 hydrocarbons, which may also be applied in designing high-performance materials for other challenging separations.
4 Experimental section
Materials
All chemicals were used as received without further purification. 4,4′-Dipyridyl disulfide (>97%) was purchased from TCI Chemicals, and 1,2-ethanedisulfonate disodium salt (98%) was purchased from Energy Chemical. Cu(NO3)2·3H2O (AR) and methanol (AR) were purchased from Sinopharm Chemical Reagent Co., Ltd.Synthesis of ZU-603 (powder)
4,4′-Dipyridyl disulfide (1 mmol, 0.220 g) is dissolved in 20 mL anhydrous methanol, yielding the ligand solution. Cu(NO3)2·3H2O (0.5 mmol, 0.121 g) and 1,2-ethanedisulfonate disodium salt (0.5 mmol, 0.117 g) are dissolved in 3 mL deionized water, after which 10 mL anhydrous methanol is added to the salt solution. The salt solution is then slowly added to the ligand solution under constant stirring. The composite is then stirred for 24 h at 25 °C. The purple product is obtained by filtration and purified by washing with 250 mL anhydrous methanol. The product is then soaked in anhydrous methanol for 3 days.Synthesis of ZU-603 (single crystal)
Cu(NO3)2·3H2O (0.02 g) and 1,2-ethanedisulfonate disodium salt (0.02 g) are dissolved in 20 mL deionized water. 4,4′-Dipyridyl disulfide (0.02 g) is dissolved in 20 mL methanol. Then the methanol solution is carefully layered onto the water solution at ambient temperature. The single crystals of ZU-603 are obtained after two weeks.Single crystal X-ray crystallography
The crystal structures of ZU-603 and iso-C4H8 loaded ZU-603 are determined by single crystal X-ray diffraction experiments. The X-ray diffraction experiments are conducted using a Bruker D8 Venture diffractometer equipped with a PHOTONII/CMOS detector (GaKα, λ = 1.34139 Å). Data collection is performed using APEX3, and the dataset of each sample is integrated and reduced using SaintPlus 6.01. The space group of the material is determined using XPREP in APEX3. Structure solutions and refinements are carried out with SHELXS-201 and SHELXL-2018 with APEX3 for the samples described above. The CIF file of ZU-603 has been deposited at CCDC (2453159).Thermogravimetric analysis (TGA)
The thermal gravimetric analysis is performed on a TGA Q500 V20.13 Build 39. Experiments are carried out using a platinum pan under nitrogen atmosphere which is conducted by a flow rate of 60 mL min−1 nitrogen gas. The data are collected in the temperature range of 55 °C to 900 °C with a ramp of 10 °C min−1.Scanning electron microscopy (SEM)
The morphology of ZU-603 was characterized using a Hitachi SU-8010 scanning electron microscope.N2 adsorption measurements
N2 adsorption and desorption isotherms on activated materials are measured on a Micromeritics ASAP 2460 surface area analyzer at 77 K.C4 gas equilibrium adsorption measurements
Iso-C4H8 and iso-C4H10 isotherms are collected on a Micromeritics ASAP 2050 surface area analyzer at 298 K.Kinetic adsorption measurement
The time-dependent adsorption profiles of different C4 isomers on ZU-603 are measured using a BEL-SORP-max II at 0.6 bar and 298 K or 313 K. For the experiments at the specified pressure, a fixed amount of target gas is introduced into the sample chamber, and then the equipment monitors the pressure in the chamber until it becomes stable.Breakthrough experiments
The fixed-bed breakthrough experiments and cycling tests are carried out on dynamic gas breakthrough equipment. All experiments are conducted using stainless-steel columns with an inner diameter of 4.6 mm and a length of 10 cm. The ZU-603 sample packed in the column is 0.8409 g. The packing density is calculated to be 0.506 g cm−3, which remained the same after constant N2 purging at 20 mL min−1 for 24 hours. The gas mixture consists of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio iso-C4H8, and iso-C4H10. The column packed with porous materials are regenerated by purging dry N2 with a flow rate of 20 mL min−1 at 100 °C overnight.
1 mole ratio iso-C4H8, and iso-C4H10. The column packed with porous materials are regenerated by purging dry N2 with a flow rate of 20 mL min−1 at 100 °C overnight.
Grand Canonical Monte Carlo (GCMC) simulation
The gradual packing of iso-C4H8 and iso-C4H10 was determined through GCMC simulations in the sorption module. The framework of ZU-603 was first optimized by DFT-D calculations, and considered to be rigid during the simulation. The charges for atoms of ZU-603 were derived from Qeq method and Qeq_charged 1.1 parameters. The simulations adopted the task of fixed pressure at 100 kPa, the Metropolis method in the sorption module, the universal force field (UFF) for ZU-603, and the configurational bias method in the sorption module. The interaction energy between the adsorbed molecules and the framework was computed through the Coulomb and Lennard-Jones 6–12 (LJ) potentials. The cutoff radius was chosen as 18.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Å for LJ potential and the long range electrostatic interactions were handled using the Ewald summation method. The loading steps and the equilibration steps were 1 × 107; the production steps were 1 × 107.
Å for LJ potential and the long range electrostatic interactions were handled using the Ewald summation method. The loading steps and the equilibration steps were 1 × 107; the production steps were 1 × 107.
Dispersion-corrected density functional theory (DFT-D) calculations
The Quantum-Espresso package is applied for dispersion-corrected density-functional theory (DFT-D) calculations. The van der Waals interactions are accounted for by the addition of semi-empirical dispersive forces to conventional DFT-D. Vanderbilt-type ultrasoft pseudopotentials and generalized gradient approximation (GGA) with Perdew–Burk–Ernzerhof (PBE) exchange corrections are utilized. A cutoff energy of 544 eV and a 3 × 3 × 3 k-point mesh (generated using the Monkhorst–Pack scheme) are enough for the total energy to converge within 0.01 meV per atom. The structure of ZU-603 is first optimized and the results are a good match for the experimentally determined crystal structure of the material. Iso-C4H10 molecules are then introduced to various locations of the pore channels, followed by a full structural relaxation.Calculation of ideal adsorbed solution theory (IAST) selectivity
The software IAST++ is used for the calculation of IAST selectivity. Using the Dual-Site Langmuir–Freundlich (DSLF) model, the adsorption isotherms of iso-C4H8 and iso-C4H10 on ZU-603 at 298 K are fitted. The fitted model parameters are input into the software to calculate the IAST selectivity at 298 K for an iso-C4H8/iso-C4H10 mixture with a 0.5/0.5 composition ratio. The employed model equation is shown in eqn (1): | (1) |
The equation for IAST selectivity is given by eqn (2):
 | (2) |
Calculation of diffusion time constants
The time dependent adsorption profiles of iso-C4H8 and iso-C4H10 on ZU-603 are fitted using the micropore diffusion model,56,57 from which the diffusion time constant Dc/rc2 of the gas was obtained. The specific equation is as follows: | (3) |
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
H. X. and L. Y. initiated and supervised the research. Z. Q., J. C., and D. Z. performed material synthesis and data collection. Z. Y. performed DFT-D calculation. Z. Q., J. C., L. Y., X. L., X. S., and X. C. engaged in data analysis. A. Z., X. S., X. C., and X. L., engaged in discussion and draft editing. Z. Q., J. C., L. Y. and H. X. engaged in manuscript preparation and revision.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. U24A20531, 22438011), the National Center for International Research on Intelligent Nano-Materials and Detection Technology in Environmental Protection (No. SDGH2401), and the Natural Science Foundation of Zhejiang Province (No. LD24B010001).References
- P. Yao, H. Wu, N. Ning, L. Zhang, H. Tian, Y. Wu, G. Hu, T. W. Chan and M. Tian, Properties and unique morphological evolution of dynamically vulcanized bromo-isobutylene-isoprene rubber/polypropylene thermoplastic elastomer, RSC Adv., 2016, 6, 11151–11160 RSC.
- K. Gong, H. Tian, H. Liu, X. Liu, G.-H. Hu, B. Yu, N. Ning, M. Tian and L. Zhang, Grafting of isobutylene–isoprene rubber with glycidyl methacrylate and its reactive compatibilization effect on isobutylene–isoprene rubber/polyamides 12 blends, Ind. Eng. Chem. Res., 2021, 60, 16258–16266 CrossRef CAS.
- M. J. D. Mahboub, J.-L. Dubois, F. Cavani, M. Rostamizadeh and G. S. Patience, Catalysis for the synthesis of methacrylic acid and methyl methacrylate, Chem. Soc. Rev., 2018, 47, 7703–7738 RSC.
- W. E. Luttrell, Isobutylene, J. Chem. Health Saf., 2013, 20, 35–37 Search PubMed.
- U. Rodemerck, E. V. Kondratenko, T. Otroshchenko and D. Linke, Unexpectedly high activity of bare alumina for non-oxidative isobutane dehydrogenation, Chem. Commun., 2016, 52, 12222–12225 RSC.
- G. Wang, C. Li and H. Shan, Highly efficient metal sulfide catalysts for selective dehydrogenation of isobutane to isobutene, ACS Catal., 2014, 4, 1139–1143 CrossRef CAS.
- E. Cheng, L. McCullough, H. Noh, O. Farha, J. Hupp and J. Notestein, Isobutane dehydrogenation over bulk and supported molybdenum sulfide catalysts, Ind. Eng. Chem. Res., 2019, 59, 1113–1122 CrossRef.
- F. Collignon, R. Loenders, J. Martens, P. Jacobs and G. Poncelet, Liquid phase synthesis of MTBE from methanol and isobutene over acid zeolites and amberlyst-15, J. Catal., 1999, 182, 302–312 CrossRef CAS.
- P. Chu and G. H. Kuhl, Preparation of methyl tert-butyl ether (MTBE) over zeolite catalysts, Ind. Eng. Chem. Res., 1987, 26, 365–369 CrossRef CAS.
- I. Stepchuk, M. Pérez-Fortes and A. Ramírez, Assessing impacts of deploying bio-based isobutene for MTBE production in an existing petrochemical cluster, J. Cleaner Prod., 2025, 145114 CrossRef CAS.
- F. Lombardi and A. Compagnone, MTBE catalytic cracking for the production of high purity isobutene, Master's thesis, Politecnico di milano, 2019 Search PubMed.
- X. Peng, W. Wang, R. Xue and Z. Shen, Adsorption separation of CH4/CO2 on mesocarbon microbeads: Experiment and modeling, AIChE J., 2006, 52, 994–1003 CrossRef CAS.
- J.-X. Li, Y.-J. Zhao, Y.-F. Yu, Q.-Q. Huang, S.-C. Qi, X.-Q. Liu and L.-B. Sun, The selective adsorption of anionic dyes over 3D printing composite sorbents of aerogel and porous carbon, Sep. Purif. Technol., 2025, 360, 130945 CrossRef CAS.
- H. Zhang, K. Zhao, W. Guo, K. Liang, J. Li, X. Li, Q. Deng, X. Xu, H. Chao, H. Xi and C. Duan, Room-temperature rapid synthesis of hierarchically porous ZIF-93 for effective adsorption of volatile organic compounds, Ind. Chem. Mater., 2025, 3, 109–121 RSC.
- Z. R. Herm, E. D. Bloch and J. R. Long, Hydrocarbon separations in metal–organic frameworks, Chem. Mater., 2014, 26, 323–338 CrossRef CAS.
- J.-W. Cao, T. Zhang, Y.-Q. Liu, Y. Wang, F.-P. Pan, J. Chen and K.-J. Chen, Precise C2H2 adsorption affinity modulation by nitrogen functionalization in isostructural coordination networks, Small, 2025, 21, 2501924 CrossRef CAS PubMed.
- R. Yang, Y. Wang, J.-W. Cao, Z.-M. Ye, T. Pham, K. A. Forrest, R. Krishna, H. Chen, L. Li, B.-K. Ling, T. Zhang, T. Gao, X. Jiang, X.-O. Xu, Q.-H. Ye and K.-J. Chen, Hydrogen bond unlocking-driven pore structure control for shifting multi-component gas separation function, Nat. Commun., 2024, 15, 804 CrossRef CAS PubMed.
- Y. Jiang, T. Yang, X.-Q. Liu, P. Cui and L.-B. Sun, A metal–organic cage with light-switchable motifs for controllable CO2 adsorption, J. Mater. Chem. A, 2024, 12, 892–898 RSC.
- Z. Xu, S. Chen, B. Yang, H. Zhou, L. Wang, B. E. Keshta, W. Zhu and Y. Zhang, Breakthrough developments in Metal-organic frameworks (MOFs) for efficient C4 hydrocarbon separation, Chem. Eng. J., 2025, 508, 160992 CrossRef CAS.
- S. Luo, T. Han, C. Wang, Y. Sun, H. Zhang, R. Guo and S. Zhang, Hierarchically microporous membranes for highly energy-efficient gas separations, Ind. Chem. Mater., 2023, 1, 376–387 RSC.
- H. Yang, N. U. Afsar, Q. Chen, X. Ge, X. Li, L. Ge and T. Xu, Poly(alkyl-biphenyl pyridinium) anion exchange membranes with a hydrophobic side chain for mono−/divalent anion separation, Ind. Chem. Mater., 2023, 1, 129–139 RSC.
- L. Yang, P. Zhang, J. Cui, X. Cui and H. Xing, The chemistry of metal–organic frameworks for multicomponent gas separation, Angew. Chem., 2024, 136, e202414503 CrossRef.
- L. Yang, S. Qian, X. Wang, X. Cui, B. Chen and H. Xing, Energy-efficient separation alternatives: Metal–organic frameworks and membranes for hydrocarbon separation, Chem. Soc. Rev., 2020, 49, 5359–5406 RSC.
- J.-R. Li, R. J. Kuppler and H.-C. Zhou, Selective gas adsorption and separation in metal–organic frameworks, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- J.-J. Li, S.-Y. Liu, G. Liu, Y.-G. Liu, G.-Z. Wu, H.-D. Li, R. Krishna, X.-Q. Liu and L.-B. Sun, A robust perylene diimide-based zirconium metal–organic framework for preferential adsorption of ethane over ethylene, Sep. Purif. Technol., 2023, 320, 124109 CrossRef CAS.
- S. Du, X. Wang, J. Huang, K. Kent, B. Huang, I. Karam, Z. Li and J. Xiao, Ultramicroporous carbons featuring sub-Ångstrom tunable apertures for the selective separation of light hydrocarbon, AIChE J., 2021, 67, e17285 CrossRef CAS.
- L. Li, R.-B. Lin, R. Krishna, H. Li, S. Xiang, H. Wu, J. Li, W. Zhou and B. Chen, Ethane/ethylene separation in a metal-organic framework with iron-peroxo sites, Science, 2018, 362, 443–446 CrossRef CAS PubMed.
- Y. Ma, C. Yu, L. Yang, R. You, Y. Bo, Q. Gong, H. Xing and X. Cui, Boosting kinetic separation of ethylene and ethane on microporous materials via crystal size control, Chin. J. Chem. Eng., 2024, 65, 85–91 CrossRef CAS.
- H. Yang, Y. Wang, R. Krishna, X. Jia, Y. Wang, A. N. Hong, C. Dang, H. E. Castillo, X. Bu and P. Feng, Pore-space-partition-enabled exceptional ethane uptake and ethane-selective ethane–ethylene separation, J. Am. Chem. Soc., 2020, 142, 2222–2227 CrossRef CAS PubMed.
- S.-M. Wang, H.-R. Liu, S.-T. Zheng, H.-L. Lan, Q.-Y. Yang and Y.-Z. Zheng, Control of pore structure by the solvent effect for efficient ethane/ethylene separation, Sep. Purif. Technol., 2023, 304, 122378 CrossRef CAS.
- Y. Peng, H. Xiong, P. Zhang, Z. Zhao, X. Liu, S. Tang, Y. Liu, Z. Zhu, W. Zhou, Z. Deng, J. Liu, Y. Zhong, Z. Wu, J. Chen, Z. Zhou, S. Chen, S. Deng and J. Wang, Interaction-selective molecular sieving adsorbent for direct separation of ethylene from senary C2-C4 olefin/paraffin mixture, Nat. Commun., 2024, 15, 625 CrossRef CAS PubMed.
- X. Cui, K. Chen, H. Xing, Q. Yang, R. Krishna, Z. Bao, H. Wu, W. Zhou, X. Dong and Y. Han, Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene, Science, 2016, 353, 141–144 CrossRef CAS PubMed.
- Y.-Y. Xiong, H. Chen, C.-X. Chen, B. Lan, T. Pham, K. A. Forrest, Z.-W. Wei, M. Pan and C.-Y. Su, Enhancing acetylene/ethylene separation through cation exchange in an anion-pillared hybrid ultramicroporous MOF, Ind. Eng. Chem. Res., 2024, 63, 13826–13833 CrossRef CAS.
- Q.-L. Qian, X.-W. Gu, J. Pei, H.-M. Wen, H. Wu, W. Zhou, B. Li and G. Qian, A novel anion-pillared metal–organic framework for highly efficient separation of acetylene from ethylene and carbon dioxide, J. Mater. Chem. A, 2021, 9, 9248–9255 RSC.
- P. Wang, S. Shang, H. Xiong, X. Liu, J. Liu, H. Shuai, L. Wang, Z. Zhu, Z. Zhao, Y. Peng, J. Chen, S. Chen, Z. Zhou and J. Wang, Engineering the pore size of interpenetrated metal–organic frameworks for molecular sieving separation of C2H2/C2H4, J. Mater. Chem. A, 2025, 13, 1915–1922 RSC.
- Y. Jiang, L. Wang, G. Xing, C. Liu, G. Xiong, D. Sun, J. Hu, W. Zhu, Z. Gu, B. Chen, T. Ben and Y. Zhang, Optimizing charge separated synergistic binding sites in self-Healing crystalline porous organic salts for benchmark trace alkyne/alkene separation, Angew. Chem., Int. Ed., 2025, 64, e202507442 CrossRef CAS PubMed.
- Y. Zhang, W. Sun, B. Luan, J. Li, D. Luo, Y. Jiang, L. Wang and B. Chen, Topological design of unprecedented metal-organic frameworks featuring multiple anion functionalities and hierarchical porosity for benchmark acetylene separation, Angew. Chem., 2023, 62, e202309925 CrossRef CAS PubMed.
- J. Cui, Z. Zhang, L. Yang, J. Hu, A. Jin, Z. Yang, Y. Zhao, B. Meng, Y. Zhou and J. Wang, A molecular sieve with ultrafast adsorption kinetics for propylene separation, Science, 2024, 383, 179–183 CrossRef CAS PubMed.
- A. Cadiau, K. Adil, P. Bhatt, Y. Belmabkhout and M. Eddaoudi, A metal-organic framework–based splitter for separating propylene from propane, Science, 2016, 353, 137–140 CrossRef CAS PubMed.
- R. A. Klein, L. W. Bingel, A. Halder, M. Carter, B. A. Trump, E. D. Bloch, W. Zhou, K. S. Walton, C. M. Brown and C. M. McGuirk, Adaptive pore opening to form tailored adsorption sites in a cooperatively flexible framework enables record inverse propane/propylene separation, J. Am. Chem. Soc., 2023, 145, 21955–21965 CrossRef CAS PubMed.
- Y. Zhong, Z.-M. Ye, F. Sha, H. Xie, X. Wang, C. Zhang, D. Lv, Y. Chen, Z. Li and O. K. Farha, Reduced-symmetry ligand constructed Y-Based metal–organic framework for Inverse propane/propylene separation, ACS Mater. Lett., 2024, 6, 5348–5353 CrossRef CAS.
- Z. Deng, L. Yang, H. Xiong, J. Liu, X. Liu, Z. Zhou, S. Chen, Y. Wang, H. Wang, J. Chen, S. Deng, B. Chen and J. Wang, Green and scalable preparation of an isomeric CALF-20 adsorbent with tailored pore size for molecular sieving of propylene from propane, Small Methods, 2025, 9, 2400838 CrossRef CAS PubMed.
- S. Du, J. Huang, M. R. Ryder, L. L. Daemen, C. Yang, H. Zhang, P. Yin, Y. Lai, J. Xiao, S. Dai and B. Chen, Probing sub-5 Ångstrom micropores in carbon for precise light olefin/paraffin separation, Nat. Commun., 2023, 14, 1197 CrossRef CAS PubMed.
- S. Du, B. Huang, L. Zhu, Y. Wu, J. Huang, Z. Li, H. Yu and J. Xiao, Optimizing gas transport for C3H6/C3H8 size sieving separation via fine pore engineering of carbon microspheres, Carbon, 2023, 215, 118451 CrossRef CAS.
- L. Li, Z. Yang, Q. Wang, L. Yang, X. Suo, X. Cui and H. Xing, Efficient separation of dibranched hexane from its linear and monobranched isomers via the synergistic molecular sieving and pore shape-matching strategy, Small, 2025, 21, 2412724 CrossRef CAS PubMed.
- Q. Wang, L. Yang, T. Ke, J. Hu, X. Suo, X. Cui and H. Xing, Selective sorting of hexane isomers by anion-functionalized metal-organic frameworks with optimal energy regulation, Nat. Commun., 2024, 15, 2620 CrossRef CAS PubMed.
- E. Velasco, S. Xian, H. Wang, S. J. Teat, D. H. Olson, K. Tan, S. Ullah, T. M. Osborn Popp, A. D. Bernstein and K. A. Oyekan, Flexible Zn-MOF with rare underlying scu topology for effective separation of C6 alkane isomers, ACS Appl. Mater. Interfaces, 2021, 13, 51997–52005 CrossRef CAS PubMed.
- B. Lal, K. B. Idrees, H. Xie, C. S. Smoljan, S. Shafaie, T. Islamoglu and O. K. Farha, Pore aperture control toward size-exclusion-based hydrocarbon separations, Angew. Chem., Int. Ed., 2023, 135, e202219053 Search PubMed.
- M. Hartmann, S. Kunz, D. Himsl, O. Tangermann, S. Ernst and A. Wagener, Adsorptive separation of isobutene and isobutane on Cu3 (BTC) 2, Langmuir, 2008, 24, 8634–8642 CrossRef CAS PubMed.
- P.-Q. Liao, N.-Y. Huang, W.-X. Zhang, J.-P. Zhang and X.-M. Chen, Controlling guest conformation for efficient purification of butadiene, Science, 2017, 356, 1193–1196 CrossRef CAS PubMed.
- Q. Wang, Y. Li, Z. Qiu, D. Zhou, L. Yang, X. Suo, X. Cui and H. Xing, Highly efficient separation of intermediate-size m-xylene from xylenes via a length-matched metal–organic framework with optimal oxygen sites distribution, Angew. Chem., Int. Ed., 2024, 136, e202408817 CrossRef.
- Y. Li, J. Cui, Q. Wang, L. Yang, L. Chen, H. Xing and X. Cui, Responsive shape recognition of styrene over ethylbenzene with excellent selectivity and capacity in a hybrid porous material, Chem. Eng. J., 2023, 453, 139756 CrossRef CAS.
- K. Kishida, Y. Okumura, Y. Watanabe, M. Mukoyoshi, S. Bracco, A. Comotti, P. Sozzani, S. Horike and S. Kitagawa, Recognition of 1, 3-butadiene by a porous coordination polymer, Angew. Chem., Int. Ed., 2016, 55, 13784–13788 CrossRef CAS PubMed.
- J. Chen, J. Wang, L. Guo, L. Li, Q. Yang, Z. Zhang, Y. Yang, Z. Bao and Q. Ren, Adsorptive Separation of Geometric Isomers of 2-Butene on Gallate-Based Metal–Organic Frameworks, ACS Appl. Mater. Interfaces, 2020, 12, 9609–9616 CrossRef CAS PubMed.
- J. Cui, Z. Qiu, Z. Yang, A. Jin, X. Cui, L. Yang and H. Xing, One-step butadiene purification in a sulfonate-functionalized metal–organic framework through synergistic separation mechanism, Angew. Chem., Int. Ed., 2024, 63, e202403345 CrossRef CAS PubMed.
- N. S. Wilkins, J. A. Sawada and A. Rajendran, Diffusion of CH4 and N2 in Barium-exchanged reduced pore zorite (Ba-RPZ) and zeolite 4A, Ind. Eng. Chem. Res., 2021, 60, 10777–10790 CrossRef CAS.
- Z. Bao, S. Alnemrat, L. Yu, I. Vasiliev, Q. Ren, X. Lu and S. Deng, Adsorption of ethane, ethylene, propane, and propylene on a magnesium-based metal–organic framework, Langmuir, 2011, 27, 13554–13562 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional figures, crystal information. See DOI: https://doi.org/10.1039/d5im00077g |
| ‡ These authors contributed equally. |
| This journal is © Institute of Process Engineering of CAS 2025 |