Open Access Article
Open Access ArticleImproved CO2 capture performance of CeO2-doped CaO-based pellets: effects of particle size and steam treatment†
Yong
Li
a,
Wuhao
Sun
a,
Xilei
Liu
a,
Jian
Chen
 *a,
Hedan
Tang
a,
Youshi
Li
a,
Mingdi
Li
*a,
Hedan
Tang
a,
Youshi
Li
a,
Mingdi
Li
 a and
Lunbo
Duan
a and
Lunbo
Duan
 *b
*b
aSchool of Automotive Engineering, Changshu Institute of Technology, Changshu 215500, China. E-mail: chenjian@cslg.edu.cn
bKey Laboratory of Energy Thermal Conversion and Control, Ministry of Education, School of Energy and Environment, Southeast University, Nanjing 210096, China. E-mail: duanlunbo@seu.edu.cn
First published on 17th April 2025
Abstract
Rapid deactivation of CaO-based sorbents remains a major challenge for the calcium looping (CaL) process. Recently, CeO2, known for its high Tammann temperature and abundant oxygen vacancies, has been extensively investigated for CaO-based sorbents to mitigate performance degradation. In this study, CeO2-doped CaO-based pellets, prepared in a more industrially relevant form for the first time, were investigated. The incorporation of CeO2 alleviated the negative impact of pelletization and particle size on performance. CeO2-doped CaO-based pellets with different particle sizes (106–180, 180–250, 250–355, and 355–500 μm) exhibited nearly identical CO2 capture performance, closely matching the reactivity of powdery CeO2-doped CaO-based sorbents. Furthermore, the effects of two steam-based strategies—steam hydration and steam injection—on the reactivity of the CeO2-doped pellets were explored. Hydration after the sixth calcination significantly enhanced the reactivity of CeO2-doped CaO-based pellets. Hydration at 650 °C resulted in a conversion of 86.0% at the sixth cycle, surpassing the non-hydrated pellets by 55.4%. In contrast, steam hydration had minimal impact on the performance of undoped CaO-based pellets, indicating that CeO2 greatly enhanced the improvement from steam hydration. The effect of steam injection was more complex and highly dependent on the steam concentration. Only a moderate steam concentration (10–15%) enhanced the reactivity, leading to higher carbonation conversions. With 10% steam during carbonation, the initial conversion surged to 93.8%, representing a 22.0% improvement over the counterpart without steam.
Keywords: CO2 capture; Calcium looping (CaL); Stabilizer; Oxygen vacancy; Particle size; Steam.
1 Introduction
The calcium looping (CaL) process, which relies on the reversible carbonation/calcination reaction between CO2 and CaO-based sorbents, stands out as a highly effective method for reducing anthropogenic CO2 emissions.1–3 As depicted in Fig. 1, the carbonation reaction takes place in a carbonator, where CO2 from flue gas (e.g., emitted by coal-fired power plants) reacts with CaO to form CaCO3 (reaction 1). The produced CaCO3 is then moved to a calciner, where it is heated to high temperatures, regenerating CaO and releasing CO2 (reaction 2). Oxyfuel combustion is often employed in calciners, which helps produce a concentrated CO2 stream at the outlet.4,5 CaO-based sorbents are an attractive option for CO2 capture due to their abundant availability, high theoretical CO2 uptake (∼786 gCO2 kgCaO−1), and cost-effectiveness. As a result, substantial research has been focused on advancing the CaL process to make it more commercially viable.6,7 However, a significant challenge remains: the rapid performance decay of CaO-based sorbents.8 Consequently, enhancing the reactivity and stability of sorbents has become a central research focus.| CaO + CO2 → CaCO3 ΔH = −178 kJ mol−1 | (Reaction 1) |
| CaCO3 + CaO → CO2 ΔH = +178 kJ mol−1 | (Reaction 2) |
An alternative approach to improve the performance of sorbents is the doping of additives.9,10 Different inert stabilizers (e.g., Al2O3,11 ZrO2,12 MgO,13 Y2O3,14 and SiO215) and active promoters (e.g., Na2CO3,16 KCl,17 and K2CO318) have been investigated as additives for CaO-based sorbents. Recent studies show that the carbonation process itself involves three subreactions: CO2 adsorption, surface reaction with O2− to CO32−, and CaCO3 production (reactions 3–5). The diffusion of CO2 and mobility of O2− are critical factors influencing the rate and efficiency of the carbonation reaction.19 A density functional theory study confirmed that oxygen vacancies facilitated the diffusion of CO2.19 Yi et al. added oxygen vacancy-containing additives (CexZr1−xO2 and LaAlyMg1−yO3) into CaO-based sorbents, and found that CO2 capture stability was directly related to the presence of oxygen vacancies in the additives.20 These results underscored the potential for enhancing the reactivity of CaO-based sorbents by introducing oxygen vacancies.19,21
CeO2 has emerged as an excellent candidate for doping into CaO-based sorbents. It has a high Tammann temperature of 1064 °C22 and abundant oxygen vacancies from the coexistence of Ce3+ and Ce4+.23 The oxygen vacancies are essential for promoting the carbonation reaction, potentially resulting in improved CO2 capture ability.21 Research demonstrated that adding CeO2 to CaO-based sorbents boosted the number of oxygen vacancies, promoting CO2 sorption.24,25 Yanase et al. observed a decrease in the activation energy of the carbonation reaction from 107 to 41 kJ mol−1 when the CeO2/CaO mass ratio increased from 0 to 2, confirming the promotion effect of CeO2.26 Similarly, Wang et al. claimed that adding CeO2 accelerated the carbonation reaction rate and increased the CO2 uptake capacity within a short reaction duration.22 Furthermore, several researchers, including Liu et al.,24 Jiang et al.,25 Yan et al.,27 and Guo et al.,28 prepared CeO2-doped CaO-based sorbents using various synthesis approaches. These studies all confirmed the beneficial role of CeO2 in enhancing sorbent reactivity, primarily attributed to the presence of oxygen vacancies, as verified by X-ray photoelectron spectroscopy (XPS) analysis. However, it is important to note that these studies mainly focused on the preparation and evaluation of powdery sorbents.
| CO2(g) ↔ CO2(ads) | (Reaction 3) |
| CO2(ads) + O2− → CO2−3 | (Reaction 4) |
| CO2−3 + CaO → CaCO3 + O2− | (Reaction 5) |
In addition, using steam is an effective method for enhancing the reactivity of CaO-based sorbents.29 Steam can be introduced into the system through two primary strategies: (i) adjusting the steam concentration in the carbonator and (ii) introducing an external reactor between the calciner and carbonator, where steam hydration takes place (reaction 6). The first approach takes advantage of the steam naturally present in flue gas, which typically contains steam concentrations ranging from 5% to 20%.30 For instance, Manovic et al. found that introducing 15% steam during carbonation significantly increased the CO2 uptake capacity, achieving 0.33 gCO2 gsorbent−1 after ten cycles.31 This was 120% higher than the capacity achieved in the counterpart without steam. The second approach involves the use of an external reactor to hydrate the spent sorbents, thus recovering their performance. Yu et al. reported that after hydration, the capacity of spent pellets boosted from 0.11 to 0.42 gCO2 gsorbent−1.32 This improvement is attributed to the higher molar volume of Ca(OH)2 (∼33.7 cm3 mol−1) compared to CaO (∼16.9 cm3 mol−1).33 The formation of Ca(OH)2 leads to particle expansion and surface cracking, ultimately increasing the porosity.34
| CaO + H2O → Ca(OH)2 ΔH = −109 kJ mol−1 | (Reaction 6) |
Fluidized bed reactors are often recommended for industrial applications of the CaL process, necessitating the use of CaO-based sorbents in pellet form.35,36 However, most recent studies have primarily focused on developing powdery CeO2-doped CaO-based sorbents, followed by evaluations of their cyclic performance.22,24,25,37 Nevertheless, using pelletized sorbents in the CaL process is crucial for practical applications. Therefore, in this study, CeO2-doped CaO-based pellets with varying particle sizes were prepared for the first time, aiming to investigate their performance in a more industrially relevant form. Additionally, the impacts of two steam-based strategies—steam hydration and steam injection—on the performance of these pellets were explored. These methods were tested to assess their potential for enhancing the cyclic reactivity of pelletized sorbents in the context of the CaL process.
2 Results and discussion
2.1 Effect of CeO2 content on the reactivity of powdery CeO2-doped CaO-based sorbents
A series of CaO-based sorbents doped with varying CeO2 contents were prepared using the Pechini technique. The crystalline phase compositions of the resulting sorbents were characterized by XRD, and the results are shown in Fig. 2a. For the undoped CaO-based sorbent (i.e., CaO-PO), the XRD pattern revealed the presence of only the CaO crystalline phase, indicating the successful synthesis of pure calcium oxide. Upon doping with CeO2, the XRD patterns of the CeO2-doped CaO-based sorbents showed two distinct crystalline phases: CaO and CeO2. The intensity of the CeO2 peaks increased as the CeO2 content increased. Additionally, ICP-OES measurements were conducted to quantify the exact CeO2 content incorporated into the sorbents. The ICP-OES analysis revealed that the actual mass ratio of CeO2 to CaO matched the theoretical values, validating the precision of the doping process.Fig. 2b shows the performance of the undoped sorbent and those doped with different CeO2 contents over ten testing cycles. The undoped CaO-based sorbent, CaO-PO, demonstrated an initial conversion of 57.5%, dropping to 35.2% in the tenth cycle and corresponding to a reactivity loss of 38.8%. In contrast, the addition of 5 wt% CeO2 resulted in a slightly lower initial carbonation conversion for 5Ce-CaO-PO (∼53.4%) compared to CaO-PO (∼57.5%). Nevertheless, 5Ce-CaO-PO retained a conversion of 44.2% after ten cycles, maintaining 82.7% of the original performance and exceeding CaO-PO by 30%. Increasing the CeO2 content to 10 wt% yielded 10Ce-CaO-PO with initial and final carbonation conversions of 57.1% and 40.4%, respectively. When the CeO2 content reached 15 wt%, 15Ce-CaO-PO achieved an initial carbonation conversion of 75.9%, 30% higher than that of CaO-PO. It retained a conversion of 54.2% after ten cycles, outperforming CaO-PO by 50%. At 20 wt% CeO2 doping, 20Ce-CaO-PO exhibited the highest initial carbonation conversion of 82.3%, which decreased to 60.1% after ten cycles, preserving 73% of its original reactivity. Given the high cost of the CeO2 precursor, the optimal CeO2 content was found to be 20 wt%, which provided high CO2 uptake capacity and excellent cyclic stability. In addition, the CO2 capture experiments conducted in the vertical fixed bed reactor revealed that CeO2 doping significantly enhanced the CO2 sorption rate during carbonation (Fig. S1†).
SEM and TEM images of fresh CaO-PO and 20Ce-CaO-PO revealed that CeO2 doping effectively reduced the grain size of the sorbents, as shown in Fig. 2c and e and 3a and b. Incorporating CeO2 into the CaO-based sorbents resulted in a more refined microstructure, contributing to improved structural stability. A homogeneous distribution of Ca, Ce, and O elements was also observed in the fresh 20Ce-CaO-PO, confirming the uniform dispersion of CeO2 throughout the material. This homogeneous elemental distribution was crucial as it enabled CeO2 to function as a structural framework. After ten cycles, the spent 20Ce-CaO-PO exhibited less sintering than the spent CaO-PO, as demonstrated in Fig. 2d and f. This observation suggested that CeO2 doping played a significant role in mitigating sintering during cyclic operations.
In addition, XPS characterization was performed to further investigate the surface properties of fresh 20Ce-CaO-PO. As shown in Fig. 4a, six distinct peaks appeared in the Ce 3d spectra, which corresponded to both Ce3+ and Ce4+. According to the charge balance principle, the formation of oxygen vacancies was typically accompanied by the reduction of Ce4+ to Ce3+.38 Therefore, the presence of Ce3+ in the spectrum provided direct evidence for the formation of oxygen vacancies within the sorbent. Moreover, two distinct peaks were observed in the O 1s spectra (Fig. 4b). The peak at 531.2 eV was associated with surface-adsorbed oxygen species (i.e., Oα), while that present at 528.5 eV was related to lattice oxygen species (i.e., Oβ). Oxygen vacancies were closely associated with the Oα species, as these vacancies increased the concentration of Oα species on the surface.38,39 The Oα/(Oα + Oβ) ratio, which was found to be 94%, indicated a high concentration of oxygen vacancies.
2.2 Effect of particle size on the reactivity of CeO2-doped CaO-based pellets
The most effective powdery sorbent, i.e., 20Ce-CaO-PO, was used to prepare pellets with varying particle sizes, including 106–180, 180–250, 250–355, and 355–500 μm. These pellets were then subjected to cyclic experiments to assess their reactivity over multiple cycles. As presented in Fig. 5, after the pelletization process, a slight reduction in reactivity was observed for all CeO2-doped CaO-based pellets compared to the powdery form. Specifically, 20Ce-CaO-PE-1, which had a particle size range of 106–180 μm, exhibited an initial carbonation conversion of 79.3%, which was close to that of 20Ce-CaO-PO (∼82.3%). By the tenth cycle, the reactivity of 20Ce-CaO-PE-1 decreased to 51.0%, retaining 64.4% of its initial reactivity. This represented a decrease of 15.1% in comparison to the reactivity of 20Ce-CaO-PO.Interestingly, particle size had minimal impact on the performance of CeO2-doped CaO-based pellets. For instance, 20Ce-CaO-PE-4, with a larger particle size range of 355–500 μm, showed initial and final carbonation conversions of 76.9% and 50.6%, respectively. These values were nearly identical to those of 20Ce-CaO-PE-1, suggesting that the particle size did not significantly influence the carbonation performance. This finding aligned with the results from previous studies.40–44 The negligible impact of particle size on carbonation performance can be attributed to the slight reduction in the specific surface area and pore volume as the particle size increased, as detailed in Table S1.† Moreover, when comparing Fig. 6, it was evident that all CeO2-doped CaO-based pellets maintained higher porosity than their undoped counterparts after repeated cycles. This finding is especially important for industrial applications of CeO2-doped pellets in fluidized bed systems, where particle size effects on reactivity are common concerns.40
As previously mentioned, oxyfuel combustion is often recommended to produce a concentrated CO2 stream at the calciner outlet. However, the occurrence of the calcination reaction in a CO2-rich atmosphere increases the calcination temperature and accelerates material sintering. In recent years, O2/H2O combustion has been performed in calciners to reduce the calcination temperature by lowering CO2 concentration. Therefore, the reactivity of 20Ce-CaO-PE-4 was further assessed under these industrially relevant calcination conditions. As shown in Fig. 7, when calcination was performed at 920 °C in CO2, the reactivity of 20Ce-CaO-PE-4 dropped significantly. It had a final conversion of 25.8% by the tenth cycle, about 50% of the N2 counterpart. In contrast, when calcination was performed at 850 °C in steam, 20Ce-CaO-PE-4 demonstrated a moderate performance decline. It had an initial carbonation conversion of 75.1%, which dropped to 29.9% by the 20th cycle, retaining 40% of the original performance. These results suggested that the O2/H2O combustion method offered significant advantages in reducing the calcination temperature while maintaining relatively stable performance. Table S2† summarizes the carbonation performance of the CaO-based pellets prepared in this study and those reported previously. As shown in Table S2,† 20Ce-CaO-PE-4 exhibited competitive carbonation performance, making it a promising candidate for industrial applications.
 | ||
| Fig. 7 Reactivity of 20Ce-CaO-PE-4 under different calcination conditions in the dual-packed bed reactor. Reaction conditions: calcination: 10 min; carbonation: 15% CO2, 85% N2, 650 °C, 15 min. | ||
2.3 Effect of steam hydration on the reactivity of CeO2-doped CaO-based pellets
To enhance the CO2 capture performance of CeO2-doped CaO-based pellets, a steam hydration strategy was employed following the sixth calcination. The pellets were subjected to steam treatment in an external hydrator to investigate how hydration affected their reactivity. The reactivity of these hydrated pellets at different temperatures was then evaluated, with results shown in Fig. 8. Since the decomposition of Ca(OH)2 starts at approximately 420 °C,34 the hydration process was primarily conducted at 120–400 °C. Hydration at 120 °C significantly improved the carbonation conversion of 20Ce-CaO-PE-4, with the conversion increasing to 79.6% in the sixth cycle, representing a 45% improvement over the fifth cycle. At 200 and 400 °C, carbonation conversion similarly improved in the sixth cycle, with both temperatures showing higher conversions than in the fifth cycle. However, as the hydration temperature increased from 120 °C to 400 °C, the effect of steam hydration weakened, leading to smaller improvements in carbonation conversion. When the hydration temperature was further increased to 650 °C, the conversion in the sixth cycle increased significantly, surpassing the improvement observed at 120 °C. Despite the initial increase in capacity, the enhanced performance of 20Ce-CaO-PE-4 declined significantly after the sixth cycle, with notable reactivity reduction over the subsequent four cycles. By the tenth cycle, the carbonation conversion approached that of the non-hydrated counterpart, indicating that the positive impact of steam hydration was temporary for these CeO2-doped CaO-based pellets. Similar findings were also reported by Yu et al.,32 Rong et al.,45,46 and Manovic et al.44 In contrast, steam hydration had little effect on the reactivity of CaO-PE-4, as indicated by the overlapping performance curves across the cycle. This was likely due to the low porosity of CaO-PE-4 after five cycles, with the improvement in porosity from steam hydration in the sixth cycle insufficient to enhance reactivity effectively. This suggested that CeO2 in the CaO matrix significantly amplified the improvement from steam hydration, highlighting the role of CeO2 in enhancing its reactivity.XRD characterization was performed on 20Ce-CaO-PE-4 hydrated at different temperatures (120, 200, 400, and 650 °C) to investigate the structural changes induced by steam hydration. As shown in Fig. S2,† the crystalline phases of 20Ce-CaO-PE-4 after hydration at 120, 200, and 400 °C were identified as CeO2 and Ca(OH)2, confirming that hydration resulted in the formation of Ca(OH)2. However, hydration at 650 °C resulted in crystalline phases of CeO2 and CaO, as the hydration reaction (reaction 6) was unfavorable at temperatures above 500 °C.47,48 SEM analysis (Fig. 9) provided further insight into the structural changes of the hydrated pellets. Significant improvements in pellet porosity were observed after steam hydration, especially at 650 °C, contributing to the increased CO2 uptake capacity in the sixth cycle. However, increasing the hydration temperature from 120 to 400 °C accelerated the sintering of Ca(OH)2, reducing porosity and decreasing the CO2 uptake capacity in the sixth cycle. A similar finding was reported by Rong et al., who attributed the reduced effectiveness of steam hydration at 200–500 °C to the sintering effect of Ca(OH)2.45 While steam hydration resulted in an initial improvement in the porosity of the pellets, sintering continued to occur when the hydrated pellets were subjected to subsequent carbonation/calcination cycles. As a result, the porosity decreased again during cycling, ultimately reaching levels similar to those of the counterparts without hydration (see Fig. 9). Consequently, it seemed that the performance improvement by steam hydration was temporary.
 | ||
| Fig. 9 SEM images of 20Ce-CaO-PE-4 at different hydration temperatures with a hydration duration of 60 min in the dual-packed bed reactor. | ||
2.4 Effect of steam injection on the reactivity of CeO2-doped CaO-based pellets
A steam injection strategy was implemented to improve the CO2 capture performance of CeO2-doped CaO-based pellets. The pellets were carbonated in 15% CO2 with varying steam concentrations (i.e., 5%, 10%, 15%, 20%, balanced with N2) in each carbonation reaction, aiming to study the effect of steam injection on their reactivity. As shown in Fig. 10a, introducing 5% steam into the carbonation process did not significantly improve pellet performance compared to the counterpart without steam. The performance curves for pellets with 5% steam and those without steam overlapped almost completely across all cycles. This suggested that at this low steam concentration, steam did not notably alter the reactivity of the pellets. In contrast, increasing the steam concentration to 10% resulted in a noticeable enhancement of carbonation performance. The initial carbonation conversion in the first cycle surged to 93.8%, representing a 22.0% improvement over the counterpart without steam. This was due to the catalytic effect of H2O by forming Ca(OH)2 during the chemical reaction controlled stage, and diffusion enhancement in the CaCO3 product layer mainly during the diffusion controlled stage.49,50 However, despite the improved initial performance, the reactivity of the pellets declined significantly in the subsequent cycles. By the tenth cycle, the carbonation conversion dropped to 53.4%, slightly higher than the 50.6% conversion observed for pellets without steam, indicating that the effect of steam was not sustained over multiple cycles. This was because the presence of steam during carbonation also promoted the sintering of the sorbents.51 Further increasing the steam concentration to 15% resulted in a reduction in the carbonation performance compared to the 10% steam condition. The initial carbonation conversion decreased to 77.1%, which, although higher than the counterpart without steam, was lower than that achieved with 10% steam. This implied that while 15% steam offered some performance improvement, it was less effective than 10% steam at sustaining high reactivity across multiple cycles. Finally, introducing 20% steam caused a significant deterioration in carbonation performance. Both initial and final carbonation conversions were much lower than those observed for the counterparts without steam. This indicated that a higher steam concentration had a detrimental effect on the reactivity of the pellets. This was mainly due to that the high steam concentration promoted sintering of the pellets. This result was consistent with previous studies.51,52To further assess the impact of steam injection, a cyclic experiment with 10% steam was conducted on CaO-PE-4. As shown in Fig. 10b, the introduction of 10% steam significantly improved the initial CO2 capture performance of CaO-PE-4. In the first cycle, the carbonation conversion reached 29.1%, which was 1.34 times higher than that of the counterpart without steam. However, despite this initial boost, a sharp decline in reactivity was observed in subsequent cycles. By the tenth cycle, the carbonation conversion dropped to 12.2%, only slightly higher than the conversion of the counterpart without steam (∼12.1%). These results implied that steam injection had a greater effect during the initial cycles for both CeO2-doped and undoped CaO-based pellets, with the reactivity declining as the cycles progressed. Overall, CeO2-doped CaO-based pellets consistently outperformed their undoped counterparts, regardless of steam injection. This implied that the presence of CeO2 in the pellets enhanced their ability to capture CO2, regardless of the steam concentration. To investigate further, SEM analysis was conducted on spent 20Ce-CaO-PE-4 and spent CaO-PE-4 after ten cycles with steam injection. As shown in Fig. 11, the porosity of spent 20Ce-CaO-PE-4 was significantly higher than that of spent CaO-PE-4. Additionally, both spent 20Ce-CaO-PE-4 and spent CaO-PE-4 exhibited a more porous surface structure compared to their counterparts without steam injection (see Fig. 6f, 9, and 11). This increased porosity was likely responsible for the higher activity observed in the initial cycles, as a more porous surface allowed greater access to CO2 during carbonation.
3 Conclusions
In this study, powdery CeO2-doped CaO-based sorbents with varying CeO2 contents were prepared using the Pechini method. As the CeO2 content increased from 5 wt% to 20 wt%, the CO2 capture performance of CeO2-doped CaO-based sorbents improved significantly. At 20 wt% CeO2 doping, 20Ce-CaO-PO exhibited the highest initial carbonation conversion of 82.3%, which decreased to 60.1% after ten cycles, preserving 73% of its original reactivity. SEM and TEM analysis confirmed that CeO2 doping effectively reduced the grain size and mitigated the sintering during cycling. The presence of oxygen vacancies in the CeO2-doped CaO-based sorbents was confirmed by XPS characterization.20Ce-CaO-PO was then used to prepare pellets in an industrially relevant form for the first time, with varying particle sizes, including 106–180, 180–250, 250–355, and 355–500 μm. The incorporation of CeO2 alleviated the negative impact of pelletization and particle size on performance. CeO2-doped CaO-based pellets with different particle sizes exhibited nearly identical CO2 capture performance, closely matching the reactivity of powdery CeO2-doped CaO-based sorbents. The negligible impact of particle size on carbonation performance can be attributed to the slight reduction in the specific surface area and pore volume as the particle size increased. This finding is especially important for industrial applications of CeO2-doped pellets in fluidized bed systems, where particle size effects on reactivity are a common concern. The reactivity of 20Ce-CaO-PE-4 was further assessed under the industrially relevant calcination conditions. When calcination was performed at 850 °C in steam, 20Ce-CaO-PE-4 had an initial carbonation conversion of 75.1%, which dropped to 29.9% by the 20th cycle, retaining 40% of the original performance.
Furthermore, the effects of two steam-based strategies—steam hydration and steam injection—on the reactivity of the CeO2-doped pellets were explored. Following the sixth calcination, steam hydration was conducted and significantly improved the reactivity of CeO2-doped CaO-based pellets. Hydration at 650 °C resulted in a carbonation conversion of 86.0% in the sixth cycle, surpassing the non-hydrated pellets by 55.4%. However, the performance improvement by steam hydration was temporary. Although steam hydration resulted in an initial improvement in the porosity of the pellets, sintering continued to occur when the hydrated pellets were subjected to subsequent carbonation/calcination cycles. As a result, the porosity decreased again during cycling, ultimately reaching levels similar to those of the counterparts without hydration. The effect of steam injection was more complex and highly dependent on the steam concentration. Only a moderate steam concentration (10–15%) enhanced the reactivity, leading to higher carbonation conversions. With 10% steam during carbonation, the initial conversion in the first cycle surged to 93.8%, representing a 22.0% improvement over the counterpart without steam. However, steam injection had a greater effect during the initial cycles, with reactivity declining as the cycles progressed.
4 Experimental
4.1 Synthetic procedures
Powdery CaO-based sorbents were first made using the Pechini method. Specific amounts of Ca(NO3)2·4H2O, Ce(NO3)3·6H2O, citric acid, and polyethylene glycol 6000 were dissolved in deionized water. The mixed solution was placed in an electrically heated oil bath at 85 °C and maintained for more than 3 h to obtain a viscous gel. The gel was then transferred to an oven for further drying at 120 °C overnight. The dried sample was carefully ground and calcined in a muffle furnace at 850 °C for 2 h. After calcination, the powdery sample was collected and pelletized into small discs using a manual hydraulic press (DF-4, Gangdong). The pellets were then crushed and sieved to obtain CaO-based pellets with various particle sizes: 106–180 μm, 180–250 μm, 250–355 μm, and 355–500 μm.For simplicity, xCe-CaO-PO is used to denote powdery CeO2-doped CaO-based sorbents, where x represents the mass fraction of CeO2. 20Ce-CaO-PE-Y denotes CeO2-doped CaO-based pellets with a 20 wt% CeO2 mass fraction, where Y means the particle size range. Four particle size ranges were prepared: 106–180, 180–250, 250–355, and 355–500 μm, corresponding to Y values of 1, 2, 3, and 4, respectively. Additionally, CaO-PO and CaO-PE-4 denote undoped powdery CaO-based sorbents and undoped CaO-based pellets sieved to 355–500 μm, respectively.
4.2 Characterization
Elemental composition was determined using inductively coupled plasma optical emission spectroscopy (ICP-OES, Avio 200). The crystalline phases were analyzed using an X-ray powder diffractometer (XRD, SmartLab). The microstructure and elemental distribution were evaluated using scanning electron microscopy (SEM, Regulus 8100) and transmission electron microscopy (TEM) coupled with energy-dispersive X-ray spectroscopy (EDX). Surface properties were analyzed using an X-ray photoelectron spectrometer (XPS, Nexsa). The specific surface area and pore volume were determined using N2 physisorption analysis (TriStar II 3020).4.3 Reactivity measurement
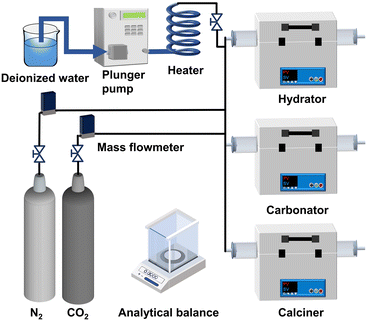
The reactivity of sorbents was assessed in a dual-packed bed reactor. As shown in Fig. 12, the dual-packed bed reactor primarily consisted of a hydrator, carbonator, calciner, plunger pump (EPP010S, Elite), heater, gas cylinders (N2 and CO2, respectively), and mass flow meters (CS200A, Sevenstar). The plunger pump regulated the flow of deionized water, which was then directed to a heater and vaporized into steam. The sample (∼0.15 g) was loaded into a porcelain boat and placed in the calciner. Calcination was carried out at 850 °C for 10 min in N2 or steam or at 920 °C for 10 min in CO2. The carbonation reaction was performed at 650 °C for 15 min in 15% CO2, mixed with varying amounts of steam (balanced with N2). Steam hydration was conducted at temperatures of 120, 200, 400, and 650 °C for durations ranging from 30 to 240 min in pure steam. To simulate multiple carbonation/calcination cycles, the samples were alternately subjected to the calcination and carbonation reactions. After each reaction, the sample was removed and cooled for 10 min in a dryer. The cooled sample's mass was then measured precisely using an analytical balance (ME104) with a precision of 0.0001 g.In addition, the CO2 sorption rate during the carbonation reaction was also investigated in a vertical fixed bed reactor. Details of the setup are described in our previous study.25 Typically, the carbonation was performed at 650 °C for 15 min in 15% CO2 (balanced with N2), while the calcination was conducted at 850 °C for 10 min in N2. A total gas flow rate of 400 mL min−1 was used in each stage. The CO2 concentration in the off-gas was measured using a gas analyzer.
The carbonation conversion (eqn (1) and (2)) was used to evaluate the performance of sorbents, as defined by the following equations:
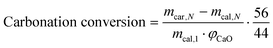 | (1) |
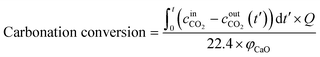 | (2) |
 is the inlet CO2 concentration;
is the inlet CO2 concentration;  (t′) is the outlet CO2 concentration at time t′; and Q denotes the total gas flow rate.
(t′) is the outlet CO2 concentration at time t′; and Q denotes the total gas flow rate.
Data availability
The data supporting this article are available within the article and its ESI.† If necessary, the raw data are available upon request to the corresponding author (Dr. Jian Chen and Prof. Lunbo Duan).Author contributions
Yong Li: methodology, investigation, data curation, and writing – original draft. Wuhao Sun: methodology and investigation. Xilei Liu: methodology and investigation. Hedan Tang: methodology and investigation. Jian Chen: conceptualization, funding acquisition, supervision, and writing – review & editing. Youshi Li: methodology and investigation. Mingdi Li: methodology and investigation. Lunbo Duan: conceptualization, supervision, and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support from the National Natural Science Foundation of China (No. 52206230) and the Natural Science Research of Jiangsu Higher Education Institutions of China (22KJB480001).References
- M. T. Dunstan, F. Donat, A. H. Bork, C. P. Grey and C. R. Müller, CO2 capture at medium to high temperature using solid oxide-based sorbents: Fundamental aspects, mechanistic insights, and recent advances, Chem. Rev., 2021, 121, 12681–12745 CrossRef CAS PubMed.
- J. Chen, L. Duan, Y. Ma, Y. Jiang, A. Huang, H. Zhu, H. Jiao, M. Li, Y. Hu, H. Zhou, Y. Xu, F. Donat, M. Awais Naeem and O. Kröcher, Recent progress in calcium looping integrated with chemical looping combustion (CaL-CLC) using bifunctional CaO/CuO composites for CO2 capture: A state-of-the-art review, Fuel, 2023, 334, 126630 CrossRef CAS.
- I. Martínez, G. Grasa, J. Parkkinen, T. Tynjälä, T. Hyppänen, R. Murillo and M. C. Romano, Review and research needs of Ca-Looping systems modelling for post-combustion CO2 capture applications, Int. J. Greenhouse Gas Control, 2016, 50, 271–304 CrossRef.
- B. Arias, M. E. Diego, A. Méndez, M. Alonso and J. C. Abanades, Calcium looping performance under extreme oxy-fuel combustion conditions in the calciner, Fuel, 2018, 222, 711–717 CrossRef CAS.
- M. Hornberger, J. Moreno, M. Schmid and G. Scheffknecht, Experimental investigation of the calcination reactor in a tail-end calcium looping configuration for CO2 capture from cement plants, Fuel, 2021, 284, 118927 CrossRef CAS.
- H. Sun, C. Wu, B. Shen, X. Zhang, Y. Zhang and J. Huang, Progress in the development and application of CaO-based adsorbents for CO2 capture—A review, Mater. Today Sustain., 2018, 1–2, 1–27 CrossRef.
- J. Chen, L. Duan and Z. Sun, Review on the development of sorbents for calcium looping, Energy Fuels, 2020, 34, 7806–7836 Search PubMed.
- S. M. Hashemi, M. H. Sedghkerdar and N. Mahinpey, Calcium looping carbon capture: Progress and prospects, Can. J. Chem. Eng., 2022, 100, 2140–2171 CrossRef CAS.
- J. Chen, A. Huang, C. Huang, W. Xia, H. Tang, C. Liu, Y. Xu, Y. Li, Z. Wang and B. Qian, Stabilizer-coated combined Ca/Cu pellets with controllable particle sizes for the Ca/Cu chemical loop, Sep. Purif. Technol., 2024, 338, 126535 CrossRef CAS.
- Y. Hu, H. Lu, W. Liu, Y. Yang and H. Li, Incorporation of CaO into inert supports for enhanced CO2 capture: A review, Chem. Eng. J., 2020, 396, 125253 CrossRef CAS.
- P. Teixeira, C. Bacariza, I. Mohamed and C. I. C. Pinheiro, Improved performance of modified CaO-Al2O3 based pellets for CO2 capture under realistic Ca-looping conditions, J. CO2 Util., 2022, 61, 102007 CrossRef CAS.
- H. J. Yoon and K. B. Lee, Introduction of chemically bonded zirconium oxide in CaO-based high-temperature CO2 sorbents for enhanced cyclic sorption, Chem. Eng. J., 2019, 355, 850–857 CrossRef CAS.
- M. A. Naeem, A. Armutlulu, Q. Imtiaz, F. Donat, R. Schaublin, A. Kierzkowska and C. R. Muller, Optimization of the structural characteristics of CaO and its effective stabilization yield high-capacity CO2 sorbents, Nat. Commun., 2018, 9, 2408 CrossRef PubMed.
- D. He, C. Qin, Z. Zhang, S. Pi, J. Ran and G. Pu, Investigation of Y2O3/MxOy-incorporated Ca-based sorbents for efficient and stable CO2 capture at high temperature, Ind. Eng. Chem. Res., 2018, 57, 11625–11635 CrossRef CAS.
- M. Imani, M. Tahmasebpoor, P. Enrique Sánchez-Jiménez, J. Manuel Valverde and V. Moreno, A novel, green, cost-effective and fluidizable SiO2-decorated calcium-based adsorbent recovered from eggshell waste for the CO2 capture process, Sep. Purif. Technol., 2023, 305, 122523 CrossRef CAS.
- M. Krödel, L. Abduly, M. Nadjafi, A. Kierzkowska, A. Yakimov, A. H. Bork, F. Donat, C. Copéret, P. M. Abdala and C. R. Müller, Structure of Na species in promoted CaO-based sorbents and their effect on the rate and extent of the CO2 uptake, Adv. Funct. Mater., 2023, 33, 2302916 CrossRef.
- D. Choi, A.-H. Alissa Park and Y. Park, Effects of eutectic alkali chloride salts on the carbonation reaction of CaO-based composites for potential application to a thermochemical energy storage system, Chem. Eng. J., 2022, 437, 135481 CrossRef CAS.
- Y. Xu, F. Donat, C. Luo, J. Chen, A. Kierzkowska, M. Awais Naeem, L. Zhang and C. R. Müller, Investigation of K2CO3-modified CaO sorbents for CO2 capture using in-situ X-ray diffraction, Chem. Eng. J., 2023, 453, 139913 CrossRef CAS.
- X. Yan, C. Duan, S. Yu, B. Dai, C. Sun and H. Chu, Revealing the mechanism of oxygen vacancy defect for CO2 adsorption and diffusion on CaO: DFT and experimental study, J. CO2 Util., 2024, 79, 102648 CrossRef CAS.
- K. B. Yi, C. H. Ko, J.-H. Park and J.-N. Kim, Improvement of the cyclic stability of high temperature CO2 absorbent by the addition of oxygen vacancy possessing material, Catal. Today, 2009, 146, 241–247 CrossRef CAS.
- Z. Yuyuan, G. Zhiwei, S. Haocheng, W. Liang, Z. Shuang, L. Xipeng, C. Qicheng and C. Haisheng, The role of oxygen vacancy in CaO-Ca12Al14O33 materials derived from hydrocalumite for enhanced CO2 capture cyclic performance, Chem. Eng. J., 2024, 481, 147955 CrossRef.
- S. Wang, S. Fan, L. Fan, Y. Zhao and X. Ma, Effect of cerium oxide doping on the performance of CaO-based sorbents during calcium looping cycles, Environ. Sci. Technol., 2015, 49, 5021–5027 CrossRef CAS PubMed.
- H. Guo, X. Kou, Y. Zhao, S. Wang and X. Ma, Role of microstructure, electron transfer, and coordination state in the CO2 capture of calcium-based sorbent by doping (Zr-Mn), Chem. Eng. J., 2018, 336, 376–385 CrossRef CAS.
- X. Liu, J. Chen, Y. Ma, C. Liu, A. Huang, J. He, M. Wang, H. Tang, W. Zuo and Y. Li, Synergistic effects of CeO2 and Al2O3 on reactivity of CaO-based sorbents for CO2 capture, Sep. Purif. Technol., 2024, 347, 127660 CrossRef CAS.
- Y. Jiang, J. Chen, F. Chen, M. Wang, Y. Li, M. Li, B. Qian, Z. Wang, S. Zhang and H. Zhou, Synergistic enhancement of Ca-based materials via CeO2 and Al2O3 co-doping for enhanced CO2 capture and thermochemical energy storage in calcium looping technology, Sep. Purif. Technol., 2025, 358, 130264 CrossRef CAS.
- I. Yanase, T. Maeda and H. Kobayashi, The effect of addition of a large amount of CeO2 on the CO2 adsorption properties of CaO powder, Chem. Eng. J., 2017, 327, 548–554 CrossRef CAS.
- X. Yan, Y. Li, X. Ma, Z. Bian, J. Zhao and Z. Wang, CeO2-modified CaO/Ca12Al14O33 bi-functional material for CO2 capture and H2 production in sorption-enhanced steam gasification of biomass, Energy, 2020, 192, 116664 CrossRef CAS.
- H. Guo, J. Feng, Y. Zhao, S. Wang and X. Ma, Effect of micro-structure and oxygen vacancy on the stability of (Zr-Ce)-additive CaO-based sorbent in CO2 adsorption, J. CO2 Util., 2017, 19, 165–176 Search PubMed.
- J. Dong, Y. Tang, A. Nzihou and E. Weiss-Hortala, Effect of steam addition during carbonation, calcination or hydration on re-activation of CaO sorbent for CO2 capture, J. CO2 Util., 2020, 39, 101167 CrossRef CAS.
- L. Chen and N. Qian, The effects of water vapor and coal ash on the carbonation behavior of CaO-sorbent supported by γ-Al2O3 for CO2 capture, Fuel Process. Technol., 2018, 177, 200–209 CrossRef CAS.
- V. Manovic, P. S. Fennell, M. J. Al-Jeboori and E. J. Anthony, Steam-enhanced calcium looping cycles with calcium aluminate pellets doped with bromides, Ind. Eng. Chem. Res., 2013, 52, 7677–7683 CrossRef CAS.
- Z. Yu, L. Duan, C. Su, Y. Li and E. J. Anthony, Effect of steam hydration on reactivity and strength of cement-supported calcium sorbents for CO2 capture, Greenhouse Gases:Sci. Technol., 2017, 7, 915–926 CrossRef CAS.
- S. Zhang, L. Zhang and F. Duan, Sulfation, pore, and fractal properties of hydrated spent calcium magnesium acetate from calcium-based looping, Greenhouse Gases:Sci. Technol., 2019, 9, 539–552 CrossRef CAS.
- L. Zhang, B. Zhang, Z. Yang and M. Guo, The role of water on the performance of calcium oxide-based sorbents for carbon dioxide capture: A review, Energy Technol., 2014, 3, 10–19 CrossRef.
- Y. Long, J. Sun, C. Mo, X. She, P. Zeng, H. Xia, J. Zhang, Z. Zhou, X. Nie and C. Zhao, One-step fabricated Zr-supported, CaO-based pellets via graphite-moulding method for regenerable CO2 capture, Sci. Total Environ., 2022, 851, 158357 CrossRef CAS PubMed.
- J. Chen, Y. Jiang, X. Liu, W. Xia, A. Huang, J. Zong, Z. Wang, B. Qian and F. Donat, One-step synthesis of CaO/CuO composite pellets for enhanced CO2 capture performance in a combined Ca/Cu looping process via a facile gel-casting technique, Sep. Purif. Technol., 2024, 328, 125057 CrossRef CAS.
- J. Phanthasri, T. Saelee, N. Sosa, S. Youngjan, N. Samart, M. Rittiruam, P. Khajondetchairit, S. Chomchin, T. Chankhanittha, K. Prasitnok, S. Kiatphuengporn, M. Chareonpanich, N. Chanlek, S. Nijpanich, P. Kidkhunthod, P. Praserthdam, S. Praserthdam and P. Khemthong, Integrated experimental and theoretical studies for unravelling CO2 capture of dual function CeO2-CaO bio-based sorbents, J. Environ. Chem. Eng., 2024, 12, 112412 CrossRef CAS.
- J. Lin, W. Kong, C. Qiu, X. Zhong, Y. Li, M. Fu and Z. Li, New insight into catalytic performance and reaction mechanism for butyl acetate over CeO2 catalysts: Oxygen vacancy and surface reactive oxygen species, J. Environ. Chem. Eng., 2024, 12, 114046 CrossRef CAS.
- S. Wu, H. Liu, Z. Huang, H. Xu and W. Shen, Mn1ZrxOy mixed oxides with abundant oxygen vacancies for propane catalytic oxidation: Insights into the contribution of Zr doping, Chem. Eng. J., 2023, 452, 139341 CrossRef CAS.
- J. Sun, W. Liu, Y. Hu, J. Wu, M. Li, X. Yang, W. Wang and M. Xu, Enhanced performance of extruded-spheronized carbide slag pellets for high temperature CO2 capture, Chem. Eng. J., 2016, 285, 293–303 CrossRef CAS.
- C. Qin, H. Du, L. Liu, J. Yin and B. Feng, CO2 capture performance and attrition property of CaO-based pellets manufactured from organometallic calcium precursors by extrusion, Energy Fuels, 2013, 28, 329–339 CrossRef.
- F. N. Ridha, V. Manovic, A. Macchi and E. J. Anthony, High-temperature CO2 capture cycles for CaO-based pellets with kaolin-based binders, Int. J. Greenhouse Gas Control, 2012, 6, 164–170 CrossRef CAS.
- Y. Wu, V. Manovic, I. He and E. J. Anthony, Modified lime-based pellet sorbents for high-temperature CO2 capture: Reactivity and attrition behavior, Fuel, 2012, 96, 454–461 CrossRef CAS.
- V. Manovic and E. J. Anthony, Reactivation and remaking of calcium aluminate pellets for CO2 capture, Fuel, 2011, 90, 233–239 CrossRef CAS.
- N. Rong, Q. Wang, M. Fang, L. Cheng, Z. Luo and K. Cen, Steam hydration reactivation of CaO-based sorbent in cyclic carbonation/calcination for CO2 capture, Energy Fuels, 2013, 27, 5332–5340 CrossRef CAS.
- N. Rong, Y. Wu, J. Wang, L. Han and G. Wang, Steam reactivation of biotemplated CaO-based pellets for cyclic CO2 capture, Energy Fuels, 2021, 35, 6056–6067 CrossRef CAS.
- K. Wang, T. Yan, R. K. Li and W. G. Pan, A review for Ca(OH)2/CaO thermochemical energy storage systems, J. Energy Storage, 2022, 50, 104612 CrossRef.
- S. Funayama, H. Takasu, S. T. Kim and Y. Kato, Thermochemical storage performance of a packed bed of calcium hydroxide composite with a silicon-based ceramic honeycomb support, Energy, 2020, 201, 117673 CrossRef CAS.
- S. Yang and Y. Xiao, Steam catalysis in CaO carbonation under low steam partial pressure, Ind. Eng. Chem. Res., 2008, 47, 4043–4048 CrossRef CAS.
- V. Manovic and E. J. Anthony, Carbonation of CaO-based sorbents enhanced by steam addition, Ind. Eng. Chem. Res., 2010, 49, 9105–9110 CrossRef CAS.
- C. Li, C. Zhang and X. Guo, Sintering mechanism of CaO during carbonation reaction in the presence of water vapor, Proc. Combust. Inst., 2023, 39, 4467–4476 CrossRef.
- Z. He, Y. Li, X. Ma, W. Zhang, C. Chi and Z. Wang, Influence of steam in carbonation stage on CO2 capture by Ca-based industrial waste during calcium looping cycles, Int. J. Hydrogen Energy, 2016, 41, 4296–4304 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5im00017c |
| This journal is © Institute of Process Engineering of CAS 2025 |