Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sustainable electro-organic synthesis of dicarboxylic acids from biogenic shellac
Edward P.
Rayner†
 a,
Tomas
Horsten†
a,
Tomas
Horsten†
 a and
Siegfried R.
Waldvogel
a and
Siegfried R.
Waldvogel
 *ab
*ab
aMax-Planck-Institute for Chemical Energy Conversion, Department of Electrosynthesis, Stiftstraße 34–36, Mülheim an der Ruhr 45470, Germany. E-mail: siegfried.waldvogel@cec.mpg.de
bKarlsruher Institut für Technologie (KIT), Institute of Biological and Chemical Systems – Functional Molecular Systems (IBCS-FMS), Kaiserstraße 12, Karlsruhe 76131, Germany
First published on 5th November 2025
Abstract
Shellac, a bioresin secreted by insects on trees is used as a coating in various applications. However, due to transesterification and polymerization, shellac and in particular its solutions have a limited shelf life. This work demonstrates the electrochemical degradation of this shellac waste stream to value-added dicarboxylic acids using activated nickel anodes. After optimisation of the shellac hydrolysis and electrolysis, we obtained up to 51% pimelic acid and 71% azelaic acid, with respect to the maximum theoretical amount. The reaction was successfully scaled to 12.5 g of shellac and isolated using distillation of the corresponding methyl esters. A green metrics comparison with the ozonolysis of oleic acid shows that our method is significantly safer.
Green foundation1. By recovering shellac waste-streams as a non-nutritional biomass, an electrochemical degradation toward dicarboxylic acids was established which are classically obtained from fossil resources or from nutritional biomass with environmentally harmful processes.2. Herein, a mild electrochemical oxidation was developed using a most simple, undivided setup and alkaline water as the solvent. Isolation of the corresponding esters, which can directly be used for the production of polymers, was done after a simple extraction and distillation. 3. Further research should focus on the use of efficient metal-free electrodes. Furthermore, alternative downstream processing could avoid the need of acidification, making the electrolyte mixture completely reusable. Lastly, an effective isolation of the oxidized sesquiterpene fraction could help finding an application for them. |
Introduction
Shellac, a natural resin secreted by the female Kerria lacca insects is a renewable resource primarily composed of aleuritic acid, jalaric acid, shellolic acid, and natural waxes.1 It is utilized as a food coating,2 in pharmaceuticals,3 as well as in cosmetics,4 and wood finisher.5 The structure of shellac is irregular and complex. However, its major building block is comprised of aleuritic acid 1 (9,10,16-trihydroxyhexadecanoic acid) with a reported weight content between 30–40%.6 Aleuritic acid links various sesquiterpene acids via inter- and intramolecular hydrogen and ester bonding (Fig. 1, hydrogen-bond donors depicted in green).7,8 Shellac comes in various warm colours, ranging from light blonde to dark brown due to the amount of lac dyes.9,10 Lighter or even colourless resin can be obtained by chemical bleaching with sodium hypochlorite11 or electrogenerated peroxodicarbonate,12 or by physical bleaching with activated charcoal.13 The properties of shellac can alter due to aging dependent on the conditions of storage. This occurs largely as a consequence of transesterification or esterification between free acids and alcohols.14 Furthermore, the activated double bond in sesquiterpene acids can cause polymerization, especially when chlorinated bleaching agents have been used.15 Therefore, shellac and in particular its solutions have a limited shelf lifetime and cannot be utilized for coating purposes after its expiration date. 1 can be isolated from shellac via alkaline hydrolysis for extended periods between 7 and 14 days followed by salting out the sodium aleurate.6 To obtain pure 1 at least one crystallisation is required after acidification with mineral acids. However, multiple crystallizations are necessary to obtain pure 1. It is worth noting that filtration and crystallization steps cause large losses of 1. Furthermore, around 80% of the original shellac weight ends up as a viscous chemical waste-stream that is clogging the drainage pipelines of industries and nearby areas.16 The market demand for pure 1 is limited to applications in perfume industry as a starting material for macrocyclic lactones.17 | ||
| Fig. 1 Degradation of shellac to dicarboxylic acids. Hydrogen bonding donors for inter- and intramolecular hydrogen bonding coloured in green. | ||
Dicarboxylic acids have a plethora of applications, e.g. the preparation of polyesters or polyamides.18 However, the industrial synthesis toward most dicarboxylic acids rely on petrochemical resources and environmentally harmful processes. Pimelic acid 2, a polymer building block and plasticizer, is industrially produced from the oxidative cleavage of petrochemical cycloheptane.19 Azelaic acid 3 has a broad range of applications in cosmetics, pharmaceuticals and industrial materials.20 Commonly, it is manufactured from oleic acid, a renewable and widely available feedstock. However, the currently required ozonolytic process is energy-intensive, sophisticated handling and uses toxic gasses.21 Furthermore, oleic acid production directly competes with nutrition purposes.
The direct use of green energy for large-scale electrosynthesis of platform molecules from renewable biomass appears as a promising approach to lower the environmental impact and petrochemical dependence of existing processes.22–26 This methodology can minimize waste generation by replacing oxidizers and reducing agents by electrical current served from reusable electrodes. Electrochemical processes instantaneously shut down when electrical current is interrupted making it an inherently safe process. Formation of hydrogen gas as the counter reaction of oxidations, delivers a valuable by-product in an environmentally benign way. Electrochemical oxidation of alcohols has relied on the use of nickel oxyhydroxide electrodes (Ni(O)OH).27–29 Already in the 1970s, Schäfer et al. showed the features of this heterogenous mediator for the oxidation of aliphatic alcohols.30 The required low current density is a limiting factor in the scaling-up. However, Waldvogel et al. have tackled this by the use of stacked electrodes setups and efficient flow-cell electrolysers.31,32 The utility of stable nickel oxyhydroxide electrodes has been proven on the electrochemical degradation of lignin33–36 and the oxidation of alkyl cyclohexanols towards adipic acid derivatives.29,31,32,37 The mechanism of the oxidation of alcohols on Ni(O)OH is thoroughly investigated.38,39 Furthermore, the mechanism of the C–C bond cleavage of a vicinal diol proceeds via oxidation of one alcohol to the ketone, which favours oxidation of the second alcohol to a dienone.27,40 Addition of hydroxide onto the carbonyls followed by another oxidation leads to the dicarboxylate.37 Anodic oxidation of 1 provides both 2 and 3via oxidative vicinal bond cleavage and oxidation of the primary alcohol. Schäfer et al. investigated the oxidation of both functional groups on nickel oxyhydroxide anodes, regenerated during electrolysis in alkaline media.41 However, Schäfer's findings illustrated that the required reaction conditions for different functional groups and substrates can change substantially due to different adsorbance rates and therefore can make the overall oxidation of 1 challenging.30 Herein, we describe the generation of 2 and 3 from the electro-oxidation of 1 in hydrolyzed shellac.
Results and discussion
Initial optimisation of electrolysis
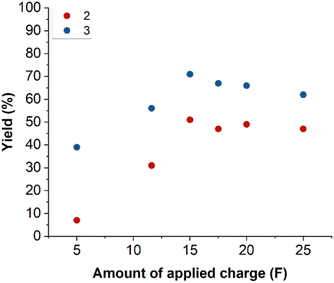
Commercially refined dewaxed bleached shellac was used for the optimisation. The content of 1 was estimated to be 35 wt% using periodic acid titration (see SI).42 The yield of 2 and 3 was calculated using GC-FID with dodecane as internal standard after esterification with trimethylsilyldiazomethane. Using the estimated aleuritic acid content, the concentration of 1 in a 7.5 wt% shellac solution is estimated to be 0.086 M. It was hypothesized that the hydrolysis parameters of shellac do not drastically influence the electrolysis outcome. Therefore, optimisation commenced with the electrolysis parameters, starting with a 7.5 wt% shellac solution which was hydrolyzed in KOH 1 M for 24 hours at 60 °C. The optimisation of the electrolysis parameters includes anode material, temperature, shellac concentration, solvent, supporting electrolyte and its concentration, amounts of applied charge and current density (Fig. 2). Anode material testing was carried out with an amount of applied charge of 11.6 F and an a geometrical current density of 5 mA cm−2 in KOH 1 M similar to a previous report from our group.32 Ni(O)OH activated anodes on various materials were tested including nickel plate, nickel foam, nickel mesh, graphite, graphite foil (SIGRAFLEX™), carbon felt, carbon paper (SIGRACELL™) as well as two non-activated anode materials graphite and nickel foam (Fig. 2a). The activated nickel foam anode material with a pore size of 0.4 mm (on RCM-Ni4753.03) exhibited the best results with a 36% and 47% yield for 2 and 3, respectively. The effect of applied charge was examined between the range of 5 to 25 F (Fig. 2b). An amount of applied charge of 15 F resulted in the best yields of 34% and 46% for 2 and 3, respectively. Increasing the geometric current density significantly lowered the yields, presumably due to the parasitic oxygen evolution reaction (Fig. 2c). Increasing the electrolysis temperature led to slightly decreased yields, although this effect is less significant (Fig. 2d). Next, a shellac concentration range of 5–10% was screened, with 5% resulting in slightly improved yields of 31% and 55% for 2 and 3, respectively (Fig. 2e). The choice of supporting electrolyte was also tested using solutions of 1 M NaOH, 1 M KOH and 1 M K2CO3 (Fig. 2f). As the supporting electrolyte plays the dual role as base during the hydrolysis, 1 M K2CO3 proved to be a poor choice. Much better results were obtained for both 1 M NaOH and 1 M KOH. However, 1 M KOH did exhibit the best yields for both 2 and 3 at 31% and 55%, respectively. Subsequently the effect of supporting electrolyte concentration was tested ranging from 0.5 M to 1.5 M with results showing that the initial concentration of 1 M KOH is optimal (Fig. 2g).Hydrolysis optimisation
The small variation in yields in the previous section led to the hypothesis that the shellac hydrolysis parameters may have a major impact in the electrolysis outcome. The previously optimized shellac concentration of 5 wt% in 1 M KOH was used moving forward. Hydrolysis temperature was tested between the ranges of 25 °C and 80 °C with the hydrolysis reaction times being examined over a 72-hour time span at 24-hour intervals. The results indicated that an increase in temperature led to an increase in hydrolysis efficiency until 60 °C was reached after which reduced yields were observed as in agreement with the previous hydrolysis experiments (Fig. 3a). It was observed that independent of the temperature, all hydrolysis experiments showed an increased yield of 2 and 3 during the first 48 hours, after which the yields started to decrease. The best results leading to the highest hydrolysis of shellac into free 1 was a 48-hour hydrolysis time at 60 °C leading to a yield of 51% for 2 and 71% for 3 as depicted in Fig. 3b after which diminishing yields were observed.Ensuing electrolysis optimisation
To ensure that the previously optimized electrolysis parameters are still optimal with the altered hydrolysis procedure, changes in optimal conditions (entry 1) have been explored (Table 1). Changing the shellac concentration to 2.5 wt% (entry 2) and 7.5 wt% (entry 3) both led to decreased yields which was more significant for 2 at higher concentrations. Increasing the geometrical current density (entry 4) caused a decreased yield for both 2 and 3, presumably due to the parasitic oxygen evolution reaction. Increasing the electrolysis temperature to 40 °C (entry 5) exhibited an approximate drop in yields of around 15% for 2 and 3. The impact of changing supporting electrolyte concentration was studied at 0.5 M (entry 6) and 1.5 M (entry 7). A significant decreased yield is observed at 0.5 M, due to incomplete hydrolysis of shellac, while the reason for the decreased yield with 1.5 M is less clear. Next, the amount of applied charge was decreased to 11.6 F (entry 8). A significant drop in yield of 20% and 15% for 2 and 3 was observed. Increasing the amount of applied charge to 20 F (entry 9) resulted in a significant drop of yield for 3 caused by oxidative decarboxylation reactions to suberic acid, while the yield of 2 is comparable to 15 F (Fig. 4). This result suggest that the electrolysis of the vicinal diol is occurring first, obtaining 3, followed by the oxidation of the primary alcohol to obtain 2.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| 1 | 2 | 3 | |
|---|---|---|---|
| 5 wt% hydrolyzed shellac | |||
| Entry | Deviation of optimal conditions | Yield 2 (%) | Yield 3 (%) |
| a Yields are determined via GC-FID after methylation with trimethylsilyldiazomethane. | |||
| 1 | None | 51 | 71 |
| 2 | 2.5 wt% shellac | 48 | 54 |
| 3 | 7.5 wt% shellac | 37 | 55 |
| 4 | 10 mA cm−2 | 30 | 46 |
| 5 | 40 °C | 32 | 50 |
| 6 | 0.5 M | 23 | 38 |
| 7 | 1.5 M | 33 | 55 |
| 8 | 11.6 F | 31 | 56 |
| 9 | 20 F | 49 | 66 |
Scale-up and isolation
A batch-type scale-up was performed in four-fold (cell B, Table 2) and ten-fold (cell C, Table 2). In the four-fold reaction, we observed that with the optimized amount of charge of 15 F, the yield was only 34% and 55% for 2 and 3, respectively. Increasing the amount of charge to 20 F resulted in a yield of 46% for 2 and 65% for 3. Further increase did let to more shorter chain dicarboxylic acids and did not increase the yields. Next, the reaction was performed on 12.5 g of shellac. Again, a slight decrease in yields was observed after 20 F (43% and 61% for 2 and 3, respectively). However, increasing the amount of charge more, did not lead to better results. This slight drop in yield is presumably due to the difference in ratio of the geographical anode area over the reaction volume leading to less efficient mass transfer. However, these batch-type cells have a limited anodic size, which hinders this upscaling. Nevertheless, we proved that the reaction is scalable and robust.| a Isolated yield of the corresponding methyl esters. |
|---|

|
Crystallization attempts to isolate the pure dicarboxylic acids was not successful and only small amounts of 3 could be isolated after acidification of the reaction mixture followed by a hot filtration of which 12% of 3 precipitated from the filtrate after cooling to room temperature. However, isolation of the corresponding methyl esters of 2 and 3 was possible after Fischer esterification of the crude mixture and subsequent fractional vacuum distillation gave 30% and 53% for 2 and 3, respectively.
Sustainability consideration
To quantify the advancements made by our novel developed protocol using shellac waste streams, we compared it with an oxidation of oleic acid using a mixture of ozone and oxygen. It must be noted that the production of pure oleic acid is using natural oils and is therefore directly competing with nutritional purposes. The atom economy of both, our approach and ozonolysis is excellent (Fig. 5). Furthermore, an economical factor was calculated as the ratio of costs for products and reagents employed. In both cases, this is very similar. However, no electricity costs are considered. Ozone generation is energy intensive and cooling of the reaction was required. Furthermore, the price of high-grade oleic acid is considerably more expensive. In the calculation, the price of ≥90% oleic acid was used, which will complicate the required purification. Therefore, it is expected that a deeper techno-economic analysis may be in our favour. When our method was performed using commercially available 1 (yield for 2 and 3 was 69% and 81%, respectively), the eco-factor was only 1.0. This clearly shows the benefit of electrolysing hydrolyzed shellac compared to the inefficient isolation of 1 which is using large amounts of sodium hydroxide and sulphuric acid followed by multiple crystallizations. The better yields obtained with ozonolysis is mainly due to the different reaction type and the use of complex reaction mixtures of shellac in our method. The most significant improvement of our procedure compared to ozonolysis is the safety. During ozonolysis, ozone and oxygen are mixed with acetone, a highly flammable solvent which can form explosive mixtures with strong oxidizers. The use of electricity is inherently safe as the current and consequently the reaction can be terminated instantly. Lastly, the EcoScale, a semi-quantitative tool to compare different processes, shows that our process is slightly better than ozonolysis.43 It must be noted that the lower yield of our process has a major influence on the EcoScale outcome while this is not a good comparison over different processes. However, the safety and mild reaction conditions make up for the yield penalty. | ||
| Fig. 5 Green metrics comparison of our method with ozonolysis of oleic acid. For details on the calculations, see the SI. | ||
Conclusions
A green and sustainable protocol at ambient conditions for the electro-organic synthesis of dicarboxylic acids from biogenic shellac via the oxidative cleavage of 1 to 2 and 3 was established. This electro-organic approach allows the valorisation of shellac waste streams to versatile C7 and C9 dicarboxylic acids. The optimized hydrolysis protocol requires a 5 wt% shellac solution in 1 M KOH treated over 48 h at 60 °C. The optimal conditions for the electrolysis of shellac were found to be a 5 wt% shellac solution using inexpensive stain-less steel cathode with nickel foam activated with Ni(O)OH as anode. The highest yields of 51% and 71% were observed for 2 and 3, respectively. This process serves both, taking advantage of shellac waste streams as well as a sustainable low-cost alternative for the production of dicarboxylic acids avoiding the use of conventional solvents and wasteful oxidants. Scale-up experiments were successful in demonstrating robustness and possible future industrial-scale application and the corresponding esters can be isolated via distillation. The feasibility and efficiency of this chemistry was demonstrated as a green alternative as well as economical beneficial.Author contributions
E. P. R. investigated the reaction and analysed the data. T. H. investigated the reaction, analysed the data and together with S. R. W. supervised the project. S. R. W. conceptualized the project. All authors wrote and revised the manuscript and agreed to this version of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5gc04392a.Acknowledgements
This work received funding by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy–EXC 2033-390677874–RESOLV and under project number 510228793 (CRC 1633. PCET, C05). The authors gratefully acknowledge technical support from the mechanical workshop at MPI CEC and MPG for covering Open Access fee. Furthermore, adobe stock is achnowledged for graphical material.References
- N. Thombare, S. Kumar, U. Kumari, P. Sakare, R. K. Yogi, N. Prasad and K. K. Sharma, Int. J. Biol. Macromol., 2022, 215, 203–223 CrossRef CAS PubMed
.
- Y. Yuan, N. He, Q. Xue, Q. Guo, L. Dong, M. H. Haruna, X. Zhang, B. Li and L. Li, Trends Food Sci. Technol., 2021, 109, 139–153 CrossRef CAS
.
- M. Rademaker, J. D. Kirby and I. R. White, Contact Dermatitis, 1986, 15, 307–308 CrossRef CAS PubMed
.
- Y. Yuan, N. He, L. Dong, Q. Guo, X. Zhang, B. Li and L. Li, ACS Nano, 2021, 15, 18794–18821 CrossRef CAS PubMed
.
- V. Landry, G. Boivin, D. Schorr, M. Mottoul, A. Mary, L. Abid, M. Carrère and B. Laratte, Curr. For. Rep., 2023, 9, 319–331 CrossRef
.
- M. Ali, D. K. Hazra, Y. B. Kumar and R. Karmakar, Sep. Sci. Technol., 2022, 57, 2916–2922 CrossRef CAS
.
- S. K. Sharma, S. K. Shukla and D. N. Vaid, Def. Sci. J., 2014, 33, 261–271 CrossRef
.
- D. Tamburini, J. Dyer and I. Bonaduce, Sci. Rep., 2017, 7, 14784 CrossRef PubMed
.
- R. Burwood, G. Read, K. Schofield and D. E. Wright, J. Chem. Soc. C, 1967, 842–851 RSC
.
- G. Shamim, K. S. Ranjan, M. D. Pandey and R. Ramani, Eur. J. Entomol., 2014, 111, 149–164 CrossRef CAS
.
- S. Saengsod, S. Limmatvapirat and M. Luangtana-anan, J. Food Process. Eng., 2019, 42, e13291 CrossRef
.
- T. Horsten and S. R. Waldvogel, RSC Sustainability, 2024, 2, 1963–1968 RSC
.
- Y. Farag and C. S. Leopold, Eur. J. Pharm. Sci., 2011, 42, 400–405 CrossRef CAS PubMed
.
- K. Li, H. Zheng, H. Zhang, W.-w. Zhang, K. Li and J. Xu, RSC Adv., 2016, 6, 55618–55625 RSC
.
- Y. Liao, J. Zhou and F. Huang, Trop. J. Pharm. Res., 2015, 14, 1953–1960 CrossRef CAS
.
- M. Ali, D. K. Hazra, G. Mondal, Y. B. Kumar, R. Karmakar and S. Saha, Chem. Pap., 2023, 77, 4995–5005 CrossRef CAS
.
- F. Elterlein, N. Bugdahn and P. Kraft, Chem. – Eur. J., 2024, 30, e202400006 CrossRef CAS PubMed
.
- F. Moeller and S. R. Waldvogel, Green Chem., 2025, 27, 2661–2665 RSC
.
-
B. Cornils and P. Lappe, in Ullmann's Encyclopedia of Industrial Chemistry, ed. B. Elvers, Wiley-VCH, Weinheim, 2000 Search PubMed
.
- S. King, J. Campbell, R. Rowe, M.-L. Daly, G. Moncrieff and C. Maybury, J. Cosmet. Dermatol., 2023, 22, 2650–2662 CrossRef PubMed
.
- R. S. Atapalkar, P. R. Athawale, D. Srinivasa Reddy and A. A. Kulkarni, Green Chem., 2021, 23, 2391–2396 RSC
.
- A. Wiebe, T. Gieshoff, S. Möhle, E. Rodrigo, M. Zirbes and S. R. Waldvogel, Angew. Chem., Int. Ed., 2018, 57, 5594–5619 CrossRef CAS PubMed
.
- D. Pollok and S. R. Waldvogel, Chem. Sci., 2020, 11, 12386–12400 RSC
.
- S. Arndt, D. Weis, K. Donsbach and S. R. Waldvogel, Angew. Chem., Int. Ed., 2020, 59, 8036–8041 CrossRef CAS PubMed
.
- S. R. Waldvogel and B. Janza, Angew. Chem., Int. Ed., 2014, 53, 7122–7123 CrossRef CAS PubMed
.
- M. Yan, Y. Kawamata and P. S. Baran, Chem. Rev., 2017, 117, 13230–13319 CrossRef CAS PubMed
.
- B. V. Lyalin and V. A. Petrosyan, Russ. J. Electrochem., 2010, 46, 1199–1214 CrossRef CAS
.
- M. Fleischmann, K. Korinek and D. Pletcher, J. Electroanal. Chem. Interf. Electrochem., 1971, 31, 39–49 CrossRef CAS
.
- B. V. Lyalin and V. A. Petrosyan, Russ. Chem. Bull., 2004, 53, 688–692 CrossRef CAS
.
- J. Kaulen and H.-J. Schäfer, Tetrahedron, 1982, 38, 3299–3308 CrossRef CAS
.
- R. J. R. Bednarz, A. S. Gold, J. Hammes, D. F. Rohrmann, S. Natalello, M. Mann, F. Weinelt, C. Brauer and S. R. Waldvogel, Org. Process Res. Dev., 2024, 28, 1529–1538 CrossRef CAS
.
- R. J.-R. Bednarz, P. Jiménez-Meneses, A. S. Gold, D. Monllor-Satoca, A. Stenglein, R. Gómez and S. R. Waldvogel, ChemCatChem, 2023, 15, e202300606 CrossRef CAS
.
- M. Breiner, M. Zirbes and S. R. Waldvogel, Green Chem., 2021, 23, 6449–6455 RSC
.
- M. Zirbes and S. R. Waldvogel, Curr. Opin. Green Sustainable Chem., 2018, 14, 19–25 CrossRef
.
- M. Zirbes, L. L. Quadri, M. Breiner, A. Stenglein, A. Bomm, W. Schade and S. R. Waldvogel, ACS Sustainable Chem. Eng., 2020, 8, 7300–7307 CrossRef CAS
.
- M. Zirbes, D. Schmitt, N. Beiser, D. Pitton, T. Hoffmann and S. R. Waldvogel, ChemElectroChem, 2019, 6, 155–161 CrossRef CAS
.
- A. L. Rauen, F. Weinelt and S. R. Waldvogel, Green Chem., 2020, 22, 5956–5960 RSC
.
- M. K. Goetz, M. T. Bender and K.-S. Choi, Nat. Commun., 2022, 13, 5848 CrossRef CAS PubMed
.
- P. C. M. Laan, F. J. de Zwart, E. M. Wilson, A. Troglia, O. C. M. Lugier, N. J. Geels, R. Bliem, J. N. H. Reek, B. de Bruin, G. Rothenberg and N. Yan, ACS Catal., 2023, 13, 8467–8476 CrossRef CAS PubMed
.
-
H.-J. Schäfer, in Electrochemistry I, ed. E. Steckhan, Springer, Berlin, 1987, vol. 142, pp. 101–129 Search PubMed
.
- H. Ruholl and H. J. Schäfer, Synthesis, 1988, 54–56 CrossRef CAS
.
- M. Mansur Rehman, Asian J. Chem., 2010, 11, 1155–1158 Search PubMed
.
- K. Van Aken, L. Strekowski and L. Patiny, Beilstein J. Org. Chem., 2006, 2, 3 Search PubMed
.
Footnote |
| † These authors have contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |