Open Access Article
Open Access ArticleInfluence of hemicellulose and lignin on the effect of drying of cellulose and the subsequent enzymatic hydrolysis†
Tian-Jie
Ao
abe,
Jie
Wu
 *bc,
Richard
Chandra
*bc,
Richard
Chandra
 *bd,
Huai-Yu
Zhang
*bd,
Huai-Yu
Zhang
 c,
Yu-Feng
Yuan
b,
Yi-Ping
Luo
c,
Yu-Feng
Yuan
b,
Yi-Ping
Luo
 a,
Dong
Li
a,
Dong
Li
 a,
Chen-Guang
Liu
a,
Chen-Guang
Liu
 e,
Scott
Renneckar
e,
Scott
Renneckar
 c and
Jack
Saddler
c and
Jack
Saddler
 b
b
aAgricultural Microbial Agents Key Laboratory of Sichuan Province, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610213, PR China. E-mail: adam.wu@ubc.ca; richard.chandra@twu.ca
bForest Product Biotechnology/Bioenergy Group, Department of Wood Science, Faculty of Forestry, University of British Columbia, 2424 Main Mall, Vancouver, BC V6T 1Z4, Canada
cAdvanced Renewable Materials Lab, Department of Wood Science, University of British Columbia, 2424 Main Mall, Vancouver, BC V6T 1Z4, Canada
dDepartment of Biology, Trinity Western University, 22500 University Dr, Langley, BC V2Y 1Y1, Canada
eState Key Laboratory of Microbial Metabolism, Joint International Research Laboratory of Metabolic & Developmental Sciences, School of Life Sciences and Biotechnology, Shanghai Jiao Tong University, Shanghai 200240, China
First published on 27th June 2025
Abstract
Transporting water contained in lignocellulosic biomass is both costly and impractical. Thus, the inevitable increase in the utilization of biomass derived products such as hygroscopic nanocellulose and dissolving pulp cellulose prior to downstream chemical/enzymatic processing will necessitate a greater understanding of the potential drying induced impacts on the reactivity/accessibility of cellulose. To assess the effects of hemicellulose and lignin on the drying behavior and enzymatic hydrolysis of cellulose, corn stover was subjected to steam pretreatment, bleaching, and LiBr·3H2O treatment to produce model substrates rich in holocellulose, cellulose-lignin, pure cellulose and the original composition. The model substrates were freeze-dried, air-dried, and oven-dried, and were subjected to Simons’ staining (both wet and dried samples) and N2 adsorption analysis (dried samples) to assess cellulose accessibility and surface area. Drying-induced hornification reduced cellulose accessibility, with freeze-drying preserving the structure more effectively than oven or air drying. The presence of hemicellulose and lignin influenced drying-induced hornification by significantly increasing cellulose accessibility. Hemicellulose removal was as effective as lignin removal in enhancing enzymatic hydrolysis at low enzyme loading, but its presence played a key role in mitigating drying effects. Additionally, cellulose properties, such as the degree of polymerization, affected drying responses, as seen in the reduction of hydrolysis yield in endoglucanase-treated dissolving pulp.
Green foundation1. This study enhances understanding of how lignin, hemicellulose, and cellulose affect biomass drying and enzymatic hydrolysis, offering insights for low-chemical, energy-efficient biofuel production. It supports the development of biorefinery processes that maximize resource utilization, minimize waste, and reduce reliance on harsh chemical pretreatments, which are core principles of green chemistry.2. We found that natural components like hemicellulose and lignin can mitigate the negative effects of drying on cellulose accessibility. These findings pave the way for greener, milder pretreatment methods that preserve native structures of biomass and reduce energy consumption. 3. Future work could focus on biological or enzymatic approaches for selective component modification. Combining structural insights with techno-economic and life cycle analyses will help develop scalable, sustainable biorefinery processes. |
1 Introduction
Cellulose accessibility remains a key parameter affecting the ultimate utility of lignocellulosic biomass for producing renewable bioproducts. Areas such as the pulp and paper industry, cellulose utilization in textiles, the development of cellulose nanoproducts and the hydrolysis of cellulose for the production of sugars all depend on the accessibility of cellulose to meet product requirements and specifications.1–4 The accessibility of the cellulose macromolecule itself is controlled by several factors including its own properties such as crystallinity, degree of polymerization and surface area.5 When cellulose is embedded with lignin and hemicellulose as part of the lignocellulosic matrix that comprises biomass structure, it can be argued that the study of the complex factors affecting the cellulose accessibility has evolved into a research field all on its own, focusing on areas such as assessing measurement techniques, enzyme/physiochemical treatment facilitated cellulose accessibility enhancement.6–10The accessibility of cellulose in biomass, substrates pretreated for enzymatic hydrolysis, pulps and nano-products is also heavily influenced by the amount of drying undergone by the cellulosic material. Due to the abundance of hydroxyl groups that decorate its molecular backbone, cellulose is typically associated with water in plant/woody tissues in vivo and after processing at varying levels of moisture.11 However, due to the costs and impracticality associated with shipping water contained in wet pulp slurries, in many cases, pulps are dewatered or flash dried.12 For the same reasons, drying methods for nanofibrillated and nanocrystalline cellulose is an area currently under significant investigation.13–15 Biomass is typically dried and densified to a density of 650–700 kg m−3 in the case of pellets used for combustion.16 However, the methods utilized for drying and densifying pellets cannot yet be utilized for cellulosic products such as pulps and nanocellulose due to the irreversible drying induced changes undergone by cellulose during the drying process referred to as “hornification”. Hornification is defined as the stiffening of the polymer structure that takes place in lignocellulosic materials upon drying or water removal.17 Hornification involves intramolecular and intermolecular hydrogen bonding between cellulose molecules. A recent study analyzing hornification of pulps varying in cellulose, hemicellulose and lignin content illustrated 4 stages of hornification as moisture is removed from the lignocellulosic matrix, including an initial crystallization of cellulose, co-crystallization with hemicellulose, a period of hornification controlled by hemicellulose and a second crystallization period.12 It was shown that lignin and hemicellulose inhibited what the authors referred to as cellulose crystallization.12 Even in the wet-state cellulose is susceptible to loss of accessibility.18 Dissolving pulps are a highly purified cellulose (>95% pure) utilized for dissolution/derivatization that can be utilized for studying the changes in cellulose during drying and through the removal of lignin and hemicellulose.19 Cellulose microfibril aggregation has been discussed as a form of hornification where cellulose collapses upon itself even in the wet-state thereby reducing its accessibility as lignin and particularly hemicellulose are being removed during pulp processing.20,21 This aggregation of cellulose microfibrils is highly resistant to mechanical treatment and it reduces the access of cellulose to the extent that it impedes the access to water molecules. It has been shown that once the lignin content decreases below 1%, reducing the hemicellulose content below 5% results in significant cellulose microfibril aggregation.22,23
Similar to the hornification related issues discussed above, it would be expected that drying substrates produced via pretreatment processes prior to enzymatic hydrolysis by cellulases would significantly impede cellulose hydrolysis presumably via drying induced reductions in cellulose accessibility. It is understandable that any compromise in cellulose accessibility would influence the ease of hydrolysis of cellulose by cellulases as it has been estimated that the rate limiting pore size for cellulose hydrolysis is 51 nm.24 Based on the relative size of cellulase enzyme components, it can be hypothesized that the accessibility requirements for cellulases would be higher than those required for a molecule of water required for cellulose swelling and/or chemical processes such as cellulose dissolution thus drying would play a significant role in reducing substate accessibility to cellulases.25 However, in the case of pelletized steam pretreated softwood biomass it has been shown that drying and densification had little to no effect when the pellets were subsequently rehydrated and subjected to enzymatic hydrolysis.26,27 It should be noted that the authors in the aforementioned studies attributed the inhibition of hornifcation and resistance to enzymatic hydrolysis after drying and resuspending the pelletized substrates to the presence of soluble hemicelluloses since the steam pretreated substrates did not undergo subsequent washing.26 In contrast, when utilizing washed substrates rich in hemicellulose and lignin, previous work has shown that drying, and in particular the mode of drying (freeze-drying, air-drying, oven-drying) played a significant role influencing enzymatic accessibility to cellulose.12,28–30
Typically, substrates pretreated for cellulose hydrolysis contain significant amounts of hemicellulose and lignin that have been associated with limiting cellulose accessibility and thus inhibiting enzymatic hydrolysis. Based on the studies referred to above, the presence of hemicellulose and lignin can also play a positive role in mitigating the negative effects of substrate drying on cellulose accessibility in cellulose rich pulps. However, the influence of hemicellulose and lignin on inhibiting the drying induced reduction in cellulose accessibility of lignocellulosic substrates pretreated for subsequent enzymatic hydrolysis remains unclear. The relative role and magnitude of lignin and hemicellulose in potentially reducing cellulose accessibility in never dried substrates also remains unclear, likely due to the challenges associated with isolating the effects of hemicellulose and lignin within the heterogeneous lignocellulosic substrate. Therefore, the current work aims to utilize a set of model substrates to assess and deconvolute the influence of hemicellulose and lignin on: (1) reducing the accessibility of cellulose in never dried substrates; and (2) mitigating drying-induced reduction in cellulose accessibility. It is clear from the data that a compromise between hemicellulose/lignin removal and retention must be considered if it is necessary to dry pretreated lignocellulosic substrates.
2 Materials and methods
2.1 Materials and chemicals
Corn stover was generously provided by Novozymes (Davis, California) and used without prior washing. Dissolving pulp was provided by Fortress Global Enterprises Inc. as pulp sheets, soaked overnight in water at 2% solid, and subsequently disintegrated using a pulp disintegrator for 15 min. The disintegrated pulp was then filtered through P8 filter paper using a Buchner funnel, and the wet solid fraction was stored at 4 °C before analysis. The cellulosic enzyme cTec 3, with a protein content of 266 mg mL−1 and the endoglucanase NS510330, with a protein content of 1 mg mL−1, were all provided by Novonesis (Denmark). Chemicals, including oxalic acid, sodium acetate, acetic acid, sodium chlorite, magnesium chloride, lithium bromide and cupriethylenediamine, were sourced from Fisher Scientific (Canada). Direct Orange 15 dye was purchased from Pylam Products (USA).2.2 Preparation of various model substrates
The overall procedure for preparing various model substrates rich in cellulose, along with different levels of hemicellulose and lignin is presented in Fig. 1. To obtain cellulose- and lignin-rich pulp (CL-rich), oxalic acid-assisted steam pretreatment of corn stover was performed using a 2 L Stake Tech II steam gun (SunOpta formerly Stake Technologies, Norval, ON, Canada) to primarily remove hemicellulose in corn stover, following Chandra, et al.31 Briefly, 200 g of dry corn stover was mixed with 200 g of an oxalic acid/MgCl solution (equimolar to 6% H2SO4, containing 5.5 g oxalic acid and 5.8 g MgCl), soaked overnight at room temperature. The impregnated corn stover was then pretreated in steam gun at 140 °C for 20 min. All substrates were mechanically refined by Vitamix E310 Explorian Blender at 1 wt% consistency for 15 min to improve homogeneity. The resulting slurry was vacuum-filtered through a P8 filter paper with the filtrate recycled twice over the filter cake, and the solid fraction was washed extensively with 10 L of deionized water to remove impurities and adjust the pH to neutral. The washed solid was stored at 4 °C until further use.To obtain cellulose- and hemicellulose-rich pulp (CH-rich), corn stover were delignified with sodium chlorite at room temperature according to the Pulp and Paper Technical Association of Canada (PAPTAC) Useful Method G10.U.32 In brief, 30 g of corn stover, 24 g of sodium chlorite, and 3 mL of acetic acid were combined in water at a 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 liquor to corn stover ratio. The mixture was stirred every 30 min and allowed to react for 3 h at room temperature in a fume hood. The slurry was filtered and washed extensively with 10 L of deionized water, then bleached two more times. The final wet solid fraction was stored at 4 °C before analysis.
1 liquor to corn stover ratio. The mixture was stirred every 30 min and allowed to react for 3 h at room temperature in a fume hood. The slurry was filtered and washed extensively with 10 L of deionized water, then bleached two more times. The final wet solid fraction was stored at 4 °C before analysis.
To isolate cellulose-rich pulp (C-rich), the steam explosion pretreated corn stover was treated with lithium bromide trihydrate (LBTH) to further remove hemicellulose, following Gong, et al.33 Briefly, LBTH solvent was prepared by mixing LiBr and deionized water in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio. Then, 5 g of the pretreated sample was added to 100 g of this solvent in a glass vial (200 mL capacity) and stirred at 450 rpm in an 80 °C water bath for 5 h. The mixture was filtered, washed, and then subjected to three rounds of bleaching (as described above). The final washed and filtered solid fraction was stored at 4 °C until analysis.
3 molar ratio. Then, 5 g of the pretreated sample was added to 100 g of this solvent in a glass vial (200 mL capacity) and stirred at 450 rpm in an 80 °C water bath for 5 h. The mixture was filtered, washed, and then subjected to three rounds of bleaching (as described above). The final washed and filtered solid fraction was stored at 4 °C until analysis.
To isolate lignin from steam explosion-pretreated corn stover, the pretreated corn stover underwent enzymatic hydrolysis using cTec 3 at a solid loading rate of 2%. Protein loading was 100 mg g−1 cellulase in a 0.05 M sodium citrate buffer solution (pH = 4.8). The hydrolysis was performed in a shaker at 50 °C and 200 rpm for 72 h. Following hydrolysis, the solid residue was extensively washed with 10 L of deionized water and subsequently air dried. The dried residue was then mixed with a solution of dioxane and 0.05 M H2SO4 (pH = 2) in a 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (v/v) ratio at a solid-to-liquid ratio of 1
4 (v/v) ratio at a solid-to-liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. Lignin extraction was carried out at 87 °C under a continuous N2 flow reflux for 3 h using a single-neck round-bottom flask in a fume hood. After extraction, the mixture was centrifuged to obtain a supernatant containing lignin and dioxane, which was neutralized with NaHCO3. The neutralized supernatant was gradually added dropwise into 0.05 M H2SO4 (pH = 2) to precipitate lignin. The precipitated lignin was separated, washed extensively with acidic and distilled water, and then freeze-dried. The final lignin product was stored at 4 °C for further use. For control experiments, the raw corn stover was milled using a rotary ball mill (Retsch PM200) at 600 rpm for 6 h, with 10 min milling intervals and 5 min breaks. The milled corn stover then underwent the same enzymatic hydrolysis and lignin extraction process as described above.
20. Lignin extraction was carried out at 87 °C under a continuous N2 flow reflux for 3 h using a single-neck round-bottom flask in a fume hood. After extraction, the mixture was centrifuged to obtain a supernatant containing lignin and dioxane, which was neutralized with NaHCO3. The neutralized supernatant was gradually added dropwise into 0.05 M H2SO4 (pH = 2) to precipitate lignin. The precipitated lignin was separated, washed extensively with acidic and distilled water, and then freeze-dried. The final lignin product was stored at 4 °C for further use. For control experiments, the raw corn stover was milled using a rotary ball mill (Retsch PM200) at 600 rpm for 6 h, with 10 min milling intervals and 5 min breaks. The milled corn stover then underwent the same enzymatic hydrolysis and lignin extraction process as described above.
2.3 Lignocellulosic materials drying
The prepared never-dried model substrates, including CL-rich, CH-rich, C-rich, and the control (corn stover, CHL-rich), were subjected to three drying methods: air-drying, oven-drying, and freeze-drying. For air-drying, the substrates were placed in a fume hood at room temperature for 5 days. For oven-drying, they were kept in an oven at 105 °C overnight. For freeze-drying, the substrates underwent lyophilization using a vacuum freeze-dryer for 3 days, followed by sublimation. In each case, the drying process continued until the substrates reached a constant weight. The dried substrates were stored at 4 °C until further use.2.4 Enzymatic hydrolysis
The never dried and dried materials were enzymatically hydrolyzed using cTec 3 at protein loadings of 2, 5, and 10 mg g−1 cellulose with a 2% solid loading. A 2% solids loading was selected to avoid the influence of mixing limitations and changes in substrate viscosity. The enzyme activity of this cocktail using the filter paper assay (FPA), which was 148 FPU mL−1. The hydrolysis was performed in 2 mL centrifuge tubes containing sodium acetate buffer (50 mM, pH = 4.8), the samples, and cTec 3 (triplicate) with a working volume of 1 mL. The tubes were incubated in a rotating incubator at 50 °C for 72 h. Released sugars were analyzed using high performance liquid chromatography (HPLC) equipped with a Biorad Aminex column (HPX-87H, 300 mm × 7.8 mm, Hercules, CA) with a refractive detector (Waters 410). The mobile phase consisted of 0.04 M at a flow rate of 0.6 mL min−1. The RID and column temperatures were maintained at 55 °C and 65 °C, respectively.2.5 Endoglucanase treatment of dissolving pulp
Dissolving pulp with a 2% of solid loading was hydrolyzed using endoglucanase at a protein loading of 10 mg g−1 endoglucanase in 0.05 M sodium citrate buffer solution (pH = 4.8). The hydrolysis was carried out in a 250 mL flask with a working volume of 100 mL and incubated in a rotary shaker at 50 °C with 200 rpm for 24 h. After hydrolysis, the enzymatic solution was centrifuged, and the solid fraction was stored at 4 °C for further use.2.6 Analysis methods
The main components in dried materials were analyzed using two-step acid hydrolysis following the methods provided by the National Renewable Energy Laboratory (NREL).34 The surface morphology of the dried materials was observed by scanning electron microscopy (SEM) using a Helios Nanolab 650 FIB-SEM (Field Electron and Ion Company, United States). To determine the accessibility of cellulose, direct orange (Pontamine Fast Orange 6RN, lot no. 814071) dye was obtained from Pylam Products Co. Inc. (Garden Cicy, NY). The specific methods for Direct Orange dye was provided following the modified method by Chandra and Saddler.35 During this measurement, the substrates were soaked in buffer overnight to allow full swelling prior to the addition of DO dye. To determine the pore volume, pore surface area, and pore size distribution of the dried materials, the nitrogen (N2, 77 K) absorption–desorption and carbon dioxide (CO2, 273 K) adsorption isotherms were conducted using Micromertics 3Flex physisorption analyzer. Dried materials were degassed under vacuum at 60 °C overnight before gas sorption experiments. The specific surface area was determined using the Brunauer–Emmett–Teller (BET) method and the total pore volume was collected from the nitrogen adsorption at p/p0 ∼ 0.99.36 The viscosity of dissolving pulp was determined using the procedures described in TAPPI T230 om-08.To confirm the effect of steam explosion pretreatment on the structure of lignin in corn stover, the molecular weight of the extracted lignin samples was analyzed by Gel permeation chromatography (GPC) using an Agilent 1100 equipped with an optilab T-rEX differential refractive index detector (dRID, Wyatt Tech. CA, United States) and poly (styrenesulfonate) as standard and DMSO/LiBr (0.5% w/v) as eluent at a flow rate of 0.5 ml min−1.37 The functional groups located on the extracted lignin from corn stover were analyzed by 31P nuclear magnetic resonance (31P NMR) using a Bruker Avance 300 MHz spectrometer, according to the method described by Song, et al.38
3 Results and discussion
3.1 Characterization of various model substrates rich in cellulose
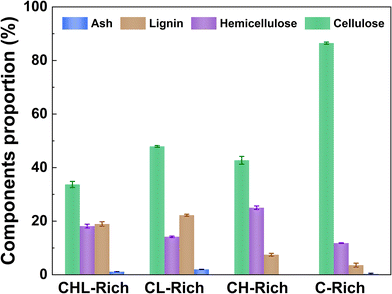
The objective of this study was to utilize model substrates to assess the relative effects of hemicellulose and lignin on influencing cellulose accessibility in never dried substrates and in substrates subjected to various modes of drying. In order to deconvolute and assess the influence of hemicellulose and lignin, various model substrates rich in cellulose, with different levels of hemicellulose and lignin, were prepared (Fig. 1). Corn stover was selected as the feedstock for these studies since it contained virtually the same amount of lignin and hemicellulose (18% hemicellulose and 19% lignin) (Fig. 2), while also being more amenable to hemicellulose removal and size reduction via the mechanical refining treatment.39,40In order to maximize the retention of hemicellulose and lignin in the corn stover biomass, the corn stover was subjected to mechanical size reduction (CHL-rich substrate). To obtain cellulose-rich (C-rich), cellulose-hemicellulose-rich (CH-rich), and cellulose-lignin-rich (CL-rich) substrates, corn stover underwent different chemical treatments, with subsequent mechanical refining to enhance homogeneity. Typically, either acidic or alkaline pH can be used to remove hemicellulose from biomass. Unlike alkali which also solubilizes lignin, acidic treatment can selectively hydrolyze and remove hemicellulose.41 However, the removal of lignin under acidic conditions is frequently accompanied by an acid induced fragmentation of lignin through acidolysis with the likelihood of lignin condensation increasing as the severity of the pretreatment is raised.42 Another reason that corn stover was chosen was because its lignin component has been shown to be less prone to lignin condensation reactions.43 To limit lignin modification/condensation, a mild steam explosion pretreatment with oxalic acid was employed, as previous work showed that oxalic acid was effective in selectively removing hemicellulose at lower steam pretreatment temperatures (140 °C).31 The oxalic acid catalyzed steam pretreatment solubilized hemicellulose to produce a cellulose (48%) and lignin (22%) rich substrate (CL-rich) (Fig. 2). To assess structural changes undergone by the lignin during the mild steam pretreatment, the residual lignin in the CL-rich substrate was isolated using the enzymatic mild acidolysis lignin (EMAL) technique with subsequent analysis (Table S1†) using 31P nuclear magnetic resonance (NMR) and gel permeation chromatography (GPC). The analyses of the EMAL samples from the untreated and steam pretreated lignin indicated lignin depolymerization with limited condensation during oxalic acid-catalyzed steam pretreatment. There was a reduction in aliphatic OH groups, a slight increase in condensed 5–5 phenolics with an overall decrease in molecular weight (GPC) when comparing the EMAL isolated from steam-pretreated corn stover to the untreated corn stover (Table S1†). This lignin is less condensed than technical lignins, such as softwood kraft lignin (Table S1†). To produce a substrate rich in cellulose and hemicellulose, lignin was selectively removed from corn stover using sodium chlorite treatment at room temperature. The sodium chlorite treatment resulted in a substrate that contained cellulose (43%) and hemicellulose (25%) which was designated the CH-rich substrate. To obtain a cellulose rich substrate, the residual hemicellulose in the CL-rich substrate was further removed using a sequential treatment with lithium bromide (LiBr),33 achieving 17% hemicellulose removal, followed by sodium chlorite bleaching, resulting in a cellulose (87%) rich substrate (C-rich).
Each of the corn stover substrates (C-rich, CH-rich, CL-rich, and CHL-rich) were subsequently mechanically refined and then subjected to oven-drying, air-drying, and freeze-drying to assess the influence of hemicellulose and lignin on the drying induced alteration of enzymatic hydrolysis of each substrate.
3.2 Morphology of various model substrates rich in cellulose
Scanning electron microscopy (SEM) was used to examine freeze-dried model substrates and assess morphological changes resulting from different pretreatment methods. Freeze-drying was selected as the drying approach as it is known to preserve the structure of substrates. The SEM analysis served as an initial investigation into how the removal of hemicellulose and lignin affects cellulose accessibility to cellulase. Biomass morphology plays a significant role in cellulose accessibility, as it determines the available surface area for enzymatic action.1,44 In raw biomass, cellulose is intricately cross-linked with lignin and hemicellulose, contributing to its recalcitrance. Various pretreatment methods can modify this structure, potentially enhancing cellulose accessibility and enzymatic hydrolysis efficiency. Cellulose accessibility is a key factor in effective enzymatic hydrolysis of cellulose, as hydrolysis efficiency depends on the intimate contact between cellulose and cellulase enzymes.45,46 By altering biomass morphology, pretreatment can increase the exposed surface area of cellulose, facilitating better enzyme–substrate interaction and improving hydrolysis rates.5In this study, raw corn stover (CHL-rich) exhibited a smooth and intact surface structure, where cellulose accessibility was likely hindered by the presence of lignin and hemicellulose (Fig. 3a). Mild steam explosion pretreatment with oxalic acid in CL-rich substrates led to some degree of lignin relocation, as evidenced by the formation of lignin droplets (Fig. 3b). This occurred because the pretreatment temperature (140 °C) exceeded the glass transition temperature of lignin, allowing it to migrate from the secondary cell wall to the biomass surface in an aqueous environment, where it reorganizes into globules upon cooling. As a result, cellulose fibers become more exposed, leading to a moderate increase in cellulose accessibility and enzymatic hydrolysis efficiency.47 Delignified CH-rich substrates exhibited a greater presence of exposed cellulose microfibrils derived from the secondary cell wall (Fig. 3c), indicating an increased cellulose surface area compared to the CHL-rich and likely the CL-rich substrates. The C-rich substrate, obtained by further removing hemicellulose from the CH-rich substrate, displayed the most prominent cellulose microfibrils, significantly enhancing cellulase-cellulose interactions (Fig. 3d). However, cellulose accessibility is influenced more by internal surface area, which is associated with porosity, than by external surface area.45
 | ||
| Fig. 3 Morphology of freeze-dried (a) CHL-rich, (b) CL-rich, (c) CH-rich and (d) C-rich substrates determined by SEM microscopy. | ||
3.3 Assessing substrate accessibility
The reduction in cellulose accessibility that results from the drying of lignocellulosic substrates and the consequential influence on enzymatic hydrolysis has been assessed by several methods that typically aim to measure the accessible surface area of cellulose. Simons’ stain uses high-molecular weight fraction of direct orange (DO) dye as the probe (in mg g−1) to assess the accessibility of cellulose to enzymes.48 This method has been shown successful in estimating cellulose hydrolysis yield of biomass substrates before and after drying as the DO dye has high affinity for cellulose over hemicellulose and lignin, and size similar to cellulase enzymes.35,49 It was quite apparent that the partial removal of either hemicellulose or lignin from the never dried corn stover increased the maximum dye absorption of DO dye, confirming enhanced cellulose accessibility upon selective removal of these components (Fig. 4). However, when compared to the C-rich substrate, where both hemicellulose and lignin were removed, the presence of either hemicellulose or lignin had a similarly adverse effect on accessibility. Notably, the negative impacts of hemicellulose and lignin on cellulose accessibility were comparable, suggesting that, at least in the case of corn stover, hemicellulose and lignin play equally critical roles in the biomass recalcitrance that limits enzymatic hydrolysis. Recent work comparing the steam pretreatment of softwood to corn stover, has shown that, due to structural differences in the biomass and lignin, corn stover lignin is more prone to relocation during steam pretreatment. It was hypothesized that the increase in lignin relocation was at least partially responsible for the reduction in the negative influence of lignin on the enzymatic hydrolysis of steam pretreated corn stover.47 | ||
| Fig. 4 Maximum DO dye adsorption of CHL-rich, CL-rich, CH-rich, and C-rich substrates at wet and various dried stages, determined using the Simons’ staining technique. | ||
It was anticipated that drying induced hornification would result in an irreversible reduction of cellulose accessibility to enzymes.15,50 In this study, the Direct Orange component of the Simons’ stain indicated a reduction in cellulose accessibility after the substrates were subjected to various modes of drying. However, it was quite apparent that the general trend was that the drying of the cellulose-rich substrate underwent the more significant reductions in cellulose accessibility indicating that the retention of either hemicellulose, lignin or both components helps to mitigate drying-induced reductions in cellulose accessibility (Fig. 4). When assessing different drying methods, freeze drying was the most effective in maintaining cellulose accessibility suggesting that freeze-drying retained a greater amount of the inherent fibre morphology of the pretreated corn stover biomass. The reduction in cellulose accessibility upon drying, especially oven and air drying was consistent with the previous findings using steam pretreated softwood and pure cellulose as the substrates.48,51
The surface area in dried model substrates was further assessed using BET N2 absorption analysis. Unlike Simons staining that utilizes the Direct Orange dye which is large (>100 kDa) and has a high substantivity for cellulose, using the small N2 probe provides the ability to estimate the total surface area of a biomass sample.52 In almost all cases, freeze drying resulted in highest surface area and pore volume of pretreated biomass, consistent with the results from Simons’ staining (Fig. 5). However, interestingly air drying and oven drying did not negatively impact the surface area of the hemicellulose-rich substrate, unlike the other cases, suggesting that hemicellulose plays a key role in mitigating hornification of corn stover fibers.15 When comparing pretreatment methods, it was apparent that hemicellulose removal notably increased the total surface area and pore volume of the biomass, achieving levels similar to those observed in the cellulose-rich substrates.
 | ||
| Fig. 5 BET surface area (a) and total pore volume (b) of CHL-rich, CL-rich, CH-rich and C-rich substrates after various drying methods, determined by nitrogen adsorption analysis. | ||
Interestingly, while the removal of lignin caused minimal changes in the total surface area and pore volumes as measured by BET for dried substrates, Simons’ staining showed improvements in cellulose accessibility. This discrepancy can be attributed to the molecular size and specificity differences between the N2 molecules are significantly smaller than cellulase enzymes and are not specific for cellulose, thus, leading to overestimations of surface area and pore volumes in BET measurements.53 The adsorption of the Direct Orange dye that comprises the Simons’ stain would indicate the larger areas of accessible cellulose in the substrate with higher specificity but would likely be unable to estimate smaller pores in the substrate that would be preserved upon drying a hemicellulose-rich substrates. Previous work has demonstrated that the retention of hemicellulose can aid in preserving cellulose accessibility by reducing cellulose microfibril coalescence during drying processes. Unlike N2, it is likely that the Direct Orange dye was not sufficiently sensitive to detect prevention of coalescence at the level of cellulose microfibrils. In contrast, despite the negligible differences between the measured pore volumes and surface areas of the CH-rich and the raw corn stover using N2, lignin removal in the case of the CH-rich substrate likely increased accessibility to large pores accessible to cellulases that were not differentiated by the N2 adsorption, but were effectively estimated by the Direct Orange dye of the Simons’ stain. In addition, Direct Orange dye is known for its high affinity for cellulose compared to hemicellulose and lignin, whereas N2 used in BET analysis is a non-specific probe, which may further contribute to the discrepancy. Therefore, it was anticipated that the results of the surface area/cellulose accessibility estimated by the Direct Orange dye would better reflect the accessibility of the substrates to cellulase enzymes during subsequent enzymatic hydrolysis.
3.4 Enzymatic hydrolysis yield of various model substrates rich in cellulose
The enzymatic hydrolysis of the never dried and dried substrates was performed at a range of enzyme loadings from 2–10 mg g−1 of cellulose. At the lower enzyme loadings of 2 and 5 mg g−1 cellulose, the impact of mild steam pretreatment (CL-rich substrate) on enhancing hydrolysis was similar to that of bleaching (CH-rich substrate) (Fig. 6), which reflected the results observed during the Simons’ staining measurement (Fig. 4). The results suggested that, in the case of corn stover, hemicellulose removal is just as important as lignin removal for enhancing cellulose accessibility at the lower enzyme loading (2 and 5 mg g−1 cellulose). However, when the enzyme loading was increased to 10 mg protein per g cellulose, the enzymatic hydrolysis yield of the never dried CH-rich substrates was significantly higher than that of the never dried CL-rich substrates. Despite their similar cellulose accessibility, the higher lignin content in the CL-rich substrate likely caused unproductive lignin–cellulose binding, thereby reducing enzymatic hydrolysis yield.45 It should also be noted that as the enzyme loading was raised, the amount of hemicellulases that were presumably present in the CTec 3 may have increased to a threshold level that facilitated the synergistic hydrolysis of both of the cellulose and hemicellulose contained in the CH-rich substrate but the hemicellulases could not enhance the hydrolysis of the lignin rich CL-rich substrate.It was apparent that all types of drying resulted in a reduction in enzymatic hydrolysis yields with freeze drying helped to reduce the negative effects of drying (Fig. 6). However, it was quite evident that raising the enzyme loading from 2 mg to 10 mg reduced the negative impact of drying on cellulose hydrolysis of the C-rich substrate, while the drying still compromised the hydrolysis of the CH- and CL- rich substrates at the 10 mg g−1 cellulose enzyme loading (Fig. 6). The results indicate that the drying in the presence of hemicellulose and lignin could not preserve the required amount of cellulose accessibility to facilitate enzymatic hydrolysis to the same yield as the never dried substrate (Fig. 6) which is in contrast to the results of the Direct Orange dye adsorption results that indicated hemicellulose and lignin helped to preserve cellulose accessibility when the CL-rich and CH-rich substrates were subjected to drying. These results indicate the limitations of both of the Direct Orange dye and N2 adsorption and other techniques for estimating accessible surface area to cellulases during subsequent enzymatic hydrolysis. Methods that estimate cellulose surface area do not account for the dynamic nature of enzymatic hydrolysis that involves the action of accessory enzymes such as hemicellulases and lytic polysaccharide monooxygenase (LPMO). These accessory enzymes may have acted to modify and/or alter the accessibility of cellulose during the enzymatic hydrolysis reaction of the C-rich substrate that helped to overcome the negative effects of drying. However, the enzyme action appeared to be unable to overcome the effects of drying in the CH-rich and CL-rich substrates likely due to the lower overall cellulose accessibility of these substrates which is in alignment the Direct Orange Dye adsorption measurements (Fig. 4).
3.5 Effect of cellulose DP on drying and enzymatic hydrolysis
While hemicellulose and lignin can influence the drying behavior of cellulose-rich substrates, we hypothesize that the intrinsic properties of the cellulose molecule itself can also play a crucial role in influencing the effects of hornification upon drying. For example, a recent study suggested that cellulose in dissolving pulp with shorter chain lengths is more susceptible to tighter packing during drying.54 To further investigate the effects of the degree of polymerization of cellulose on drying induced hornification, a commercial dissolving pulp was selected as a model “pure cellulose”, due to its high cellulose content and high accessibility to enzymes resulted from reduced DP during the production process. The pulp was treated with endoglucanase, a mono-component enzyme known to cleave the β-1,4-glycosidic linkages of cellulose.55 A reduction in DP was confirmed by a decrease in pulp viscosity (dropping from 5.5 to 4.8 mPa S) when dissolved in Cupriethylenediamine (CED) (Fig. 7), a parameter widely used in the pulp and paper industry to indicate cellulose DP.56 Subsequent enzymatic hydrolysis of the never-dried dissolving pulp control and endoglucanase treated substrate indicated that the endoglucanase treatment enhanced the enzymatic hydrolysis yields of the dissolving pulp which was likely due to the increased numbers of cellulose chain ends available for the reaction with cellobiohydrolases (exoglucanases) contained in the cellulase cocktail. In the previous sections, oven drying was shown to have the most severe impact on cellulose accessibility (Fig. 4 and 5), thus, the samples were subjected to oven drying with subsequent enzymatic hydrolysis. It was evident that compared to the drying of the control sample, the endoglucanase-treated dissolving pulp exhibited a more pronounced decrease in the cellulose hydrolysis yield, particularly at a low enzyme loading of 5 mg cellulase per g cellulose (dropping from 27% to 15% in the endoglucanase-treated pulp, compared to a decline from 8.2% to 7.9% in the untreated dissolving pulp). The results indicate that, in addition to pretreatment induced changes to the nature and content of hemicellulose and lignin, the ability of pretreatments such as acidic steam/organosolv to modify the molecular properties of cellulose such as the degree of polymerization, may also play a role in influencing the drying induced changes to pretreated substrates that compromise enzymatic hydrolysis yields.57,584 Conclusions
This study aimed to assess the effects of the presence of hemicellulose and lignin on the changes undergone by cellulose during drying that reduce accessibility to cellulases during subsequent enzymatic hydrolysis. In all cases, drying-induced hornification reduced cellulose accessibility, with freeze-drying preserving cellulose structure better than oven- or air- drying. The removal of hemicellulose and lignin significantly increased cellulose accessibility and influenced its response to drying-induced hornification. Hemicellulose removal was as effective as lignin removal in enhancing cellulose hydrolysis at low enzyme loading. However, the presence of hemicellulose in biomass appeared to be more important in mitigating the negative effects of drying on the cellulose pore structure that may not have been accessible to cellulases but presumably would be important for accessibility to chemicals and for cellulose dissolution. In addition to hemicellulose and lignin, intrinsic cellulose properties, such as the degree of polymerization that are frequently altered during pretreatment can influence the drying induced changes to cellulose accessibility. This work highlights the need for optimized lignocellulose processing strategies that balance the compromise between the benefits of hemicellulose and lignin retention on the drying of cellulose against the drawbacks of retaining hemicellulose and lignin on cellulose accessibility. Our findings suggest that partial retention of hemicellulose and lignin helps to prevent pore collapse and preserves substrate structure during drying, which is important for downstream processing. In industrial biorefineries, this could guide pretreatment strategies to avoid excessive removal of these components, improving drying efficiency and reducing energy use. However, retained hemicellulose and lignin can hinder enzymatic hydrolysis, while their complete removal may cause substrate collapse and loss of porosity. Therefore, it is essential to find an optimal balance by adjusting pretreatment severity or applying sequential processes such as partial delignification followed by controlled drying to maximize both structural integrity and digestibility.Author contributions
TJA: investigation, data curation, methodology, formal analysis, writing – original draft. JW: conceptualization, investigation, methodology, supervision, validation, writing – original draft, writing – review and editing. RC: conceptualization, methodology, supervision, validation, writing – original draft, writing – review and editing. HYZ, YFY, YPL, DL: methodology. CGL, SR: methodology, resources. JS: conceptualization, resources, supervision.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
The data supporting this article have been included as part of the ESI.†Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (32301538). Work by R Chandra on this project was supported by the Supporting Structures grant from the John Templeton Foundation and the MJ Murdock Charitable Trust. The authors express their gratitude to Novozymes (now Novonesis) for providing the enzymes used in this study.References
- Y. Yu, J. Wu, X. Ren, A. Lau, H. Rezaei, M. Takada, X. Bi and S. Sokhansanj, Steam explosion of lignocellulosic biomass for multiple advanced bioenergy processes: a review, Renewable Sustainable Energy Rev., 2022, 154, 111871, DOI:10.1016/j.rser.2021.111871.
- G. Banvillet, C. Grange, D. Curtil, J.-L. Putaux, G. Depres, N. Belgacem and J. Bras, Cellulose nanofibril production by the combined use of four mechanical fibrillation processes with different destructuration effects, Cellulose, 2023, 30, 2123–2146, DOI:10.1007/s10570-022-05016-4.
- T. Li, C. Chen, A. H. Brozena, J. Y. Zhu, L. Xu, C. Driemeier, J. Dai, O. J. Rojas, A. Isogai, L. Wagberg and L. Hu, Developing fibrillated cellulose as a sustainable technological material, Nature, 2021, 590, 47–56, DOI:10.1038/s41586-020-03167-7.
- S. Acharya, S. Liyanage, P. Parajuli, S. S. Rumi, J. L. Shamshina and N. Abidi, Utilization of cellulose to its full potential: A review on cellulose dissolution, regeneration, and applications, Polymers, 2021, 13, 4344, DOI:10.3390/polym13244344.
- R. P. Chandra, R. Bura, W. E. Mabee, A. Berlin, X. Pan and J. N. Saddler, in Substrate pretreatment: The key to effective enzymatic hydrolysis of lignocellulosics?, ed. L. Olsson, Biofuels, Springer Berlin Heidelberg, Berlin, Heidelberg, 2007, pp. 67–93 Search PubMed.
- T. Jeoh, J. D. Nill, W. Zhao, S. R. Narayanasamy, L. Chen and H.-Y. N. Holman, Spatiotemporal dynamics of cellulose during enzymatic hydrolysis studied by infrared spectromicroscopy, Green Chem., 2024, 26, 396–411, 10.1039/d3gc03279e.
- Z. K. Haviland, D. Nong, N. Zexer, M. Tien, C. T. Anderson and W. O. Hancock, Lignin impairs Cel7A degradation of in vitro lignified cellulose by impeding enzyme movement and not by acting as a sink, Biotechnol. Biofuels Bioprod., 2024, 17, 7, DOI:10.1186/s13068-023-02456-3.
- A. A. Vaidya, K. D. Murton, D. A. Smith and G. Dedual, A review on organosolv pretreatment of softwood with a focus on enzymatic hydrolysis of cellulose, Biomass Convers. Biorefin., 2022, 12, 5427–5442, DOI:10.1007/s13399-022-02373-9.
- M. Zeng and X. Pan, Insights into solid acid catalysts for efficient cellulose hydrolysis to glucose: progress, challenges, and future opportunities, Catal. Rev., 2022, 64, 445–490, DOI:10.1080/01614940.2020.1819936.
- S. Henríquez-Gallegos, G. Albornoz-Palma, A. Andrade, D. Filgueira, A. Méndez-Miranda, R. Teixeira Mendonça and M. Pereira, Effect of enzyme lignin oxidation by laccase on the enzymatic-mechanical production process of lignocellulose nanofibrils from mechanical pulp, Cellulose, 2024, 31, 3545–3560, DOI:10.1007/s10570-024-05784-1.
- N. Sweygers, D. E. C. Depuydt, S. Eyley, W. Thielemans, Y. Mosleh, J. Ivens, R. Dewil, L. Appels and A. W. Van Vuure, Prediction of the equilibrium moisture content based on the chemical composition and crystallinity of natural fibres, Ind. Crops Prod., 2022, 186, 115187, DOI:10.1016/j.indcrop.2022.115187.
- W. Mo, K. Chen, X. Yang, F. Kong, J. Liu and B. Li, Elucidating the hornification mechanism of cellulosic fibers during the process of thermal drying, Carbohydr. Polym., 2022, 289, 119434, DOI:10.1016/j.carbpol.2022.119434.
- S. Sinquefield, P. N. Ciesielski, K. Li, D. J. Gardner and S. Ozcan, Nanocellulose dewatering and drying: Current state and future perspectives, ACS Sustainable Chem. Eng., 2020, 8, 9601–9615, DOI:10.1021/acssuschemeng.0c01797.
- Y. Xu, Y. Xu, H. Chen, M. Gao, X. Yue and Y. Ni, Redispersion of dried plant nanocellulose: A review, Carbohydr. Polym., 2022, 294, 119830, DOI:10.1016/j.carbpol.2022.119830.
- F. A. Sellman, T. Benselfelt, P. T. Larsson and L. Wagberg, Hornification of cellulose-rich materials - A kinetically trapped state, Carbohydr. Polym., 2023, 318, 121132, DOI:10.1016/j.carbpol.2023.121132.
- T. R. Sarker, S. Nanda, V. Meda and A. K. Dalai, Densification of waste biomass for manufacturing solid biofuel pellets: A review, Environ. Chem. Lett., 2022, 21, 231–264, DOI:10.1007/s10311-022-01510-0.
- G. V. Laivins, A. M. Scallan and C. F. Baker, The Mechanism of Hornification of Wood Pulps, in Trans. of the Xth Fund. Res. Symp, Oxford, 1993, vol. 1993, pp. 1235–1260 Search PubMed.
- S. Grönqvist, T. K. Hakala, T. Kamppuri, M. Vehviläinen, T. Hänninen, T. Liitiä, T. Maloney and A. Suurnäkki, Fibre porosity development of dissolving pulp during mechanical and enzymatic processing, Cellulose, 2014, 21, 3667–3676, DOI:10.1007/s10570-014-0352-x.
- C. Duan, Y. Long, J. Li, X. Ma and Y. Ni, Changes of cellulose accessibility to cellulase due to fiber hornification and its impact on enzymatic viscosity control of dissolving pulp, Cellulose, 2015, 22, 2729–2736, DOI:10.1007/s10570-015-0636-9.
- A.-C. Engström, M. Ek and G. Henriksson, Improved accessibility and reactivity of dissolving pulp for the viscose process: pretreatment with monocomponent endoglucanase, Biomacromolecules, 2006, 7, 2027–2031, DOI:10.1021/bm0509725.
- H. Sixta, M. Iakovlev, L. Testova, A. Roselli, M. Hummel, M. Borrega, A. van Heiningen, C. Froschauer and H. Schottenberger, Novel concepts of dissolving pulp production, Cellulose, 2013, 20, 1547–1561, DOI:10.1007/s10570-013-9943-1.
- J. Wan, Y. Wang and Q. Xiao, Effects of hemicellulose removal on cellulose fiber structure and recycling characteristics of eucalyptus pulp, Bioresour. Technol., 2010, 101, 4577–4583, DOI:10.1016/j.biortech.2010.01.026.
- A. Kumagai and T. Endo, Effects of hemicellulose composition and content on the interaction between cellulose nanofibers, Cellulose, 2020, 28, 259–271, DOI:10.1007/s10570-020-03530-x.
- H. E. Grethlein, The effect of pore size distribution on the rate of enzymatic hydrolysis of cellulosic substrates, Bio/Technology, 1985, 3, 155–160 CrossRef CAS.
- A. Vera-Loor, E. Walger, N. Marlin and G. Mortha, Evaluation of the dissolving ability of cellulosic pulps: investigation of a novel method using light scattering follow-up during classical cellulose carbanilation, Holzforschung, 2023, 77, 139–148, DOI:10.1515/hf-2022-0154.
- L. Kumar, Z. Tooyserkani, S. Sokhansanj and J. N. Saddler, Does densification influence the steam pretreatment and enzymatic hydrolysis of softwoods to sugars?, Bioresour. Technol., 2012, 121, 190–198, DOI:10.1016/j.biortech.2012.06.049.
- J. Wu, M. Ebadian, K. H. Kim, C. S. Kim and J. Saddler, The use of steam pretreatment to enhance pellet durability and the enzyme-mediated hydrolysis of pellets to fermentable sugars, Bioresour. Technol., 2022, 347, 126731, DOI:10.1016/j.biortech.2022.126731.
- X. Luo and J. Y. Zhu, Effects of drying-induced fiber hornification on enzymatic saccharification of lignocelluloses, Enzyme Microb. Technol., 2011, 48, 92–99, DOI:10.1016/j.enzmictec.2010.09.014.
- R. Chandra, S. Ewanick, C. Hsieh and J. N. Saddler, The characterization of pretreated lignocellulosic substrates prior to enzymatic hydrolysis, part 1: a modified Simons’ staining technique, Biotechnol. Prog., 2008, 24, 1178–1185, DOI:10.1002/btpr.33.
- B. Koo, J. Jo and S.-M. Cho, Drying effect on enzymatic hydrolysis of cellulose associated with porosity and crystallinity, Appl. Sci., 2020, 10, 5545, DOI:10.3390/app10165545.
- R. P. Chandra, K. Gourlay, C.-S. Kim and J. N. Saddler, Enhancing hemicellulose recovery and the enzymatic hydrolysis of cellulose by adding lignosulfonates during the two-stage steam pretreatment of poplar, ACS Sustainable Chem. Eng., 2015, 3, 986–991, DOI:10.1021/acssuschemeng.5b00124.
- L. Kumar, V. Arantes, R. Chandra and J. Saddler, The lignin present in steam pretreated softwood binds enzymes and limits cellulose accessibility, Bioresour. Technol., 2012, 103, 201–208, DOI:10.1016/j.biortech.2011.09.091.
- R. Gong, C. Liu, M. Wu, R. Tian, G. Yu, X. Luo, B. Li, F. Peng and Y. Tang, Efficient fractionation of pure hemicellulose with high DP from bleached hardwood pulp using LiBr·3H2O and co-production of dissolving pulp, Green Chem., 2024, 26, 4622–4632, 10.1039/d4gc00335g.
- A. Sluiter, B. Hames, R. O. Ruiz, C. Scarlata, J. Sluiter and D. Templeton, Determination of structural carbohydrates and lignin in biomass, in Biomass Analysis Technology Team Laboratory Analytical Procedure, 2004, vol. 2011, pp. 1–14 Search PubMed.
- R. P. Chandra and J. N. Saddler, Use of the simons’ staining technique to assess cellulose accessibility in pretreated substrates, Ind. Biotechnol., 2012, 8, 230–237, DOI:10.1089/ind.2012.0016.
- M. A. Karaaslan, L.-T. Lin, F. Ko and S. Renneckar, Carbon aerogels from softwood kraft lignin for high performance supercapacitor electrodes, Front. Mater., 2022, 9, 894061, DOI:10.3389/fmats.2022.894061.
- J. Wu, R. P. Chandra, M. Takada, L. Y. Liu, S. Renneckar, K. H. Kim, C. S. Kim and J. N. Saddler, Enhancing enzyme-mediated cellulose hydrolysis by incorporating acid groups onto the lignin during biomass pretreatment, Front. Bioeng. Biotechnol., 2020, 8, 608835, DOI:10.3389/fbioe.2020.608835.
- Y. Song, R. P. Chandra, X. Zhang, T. Tan and J. N. Saddler, Comparing a deep eutectic solvent (DES) to a hydrotrope for their ability to enhance the fractionation and enzymatic hydrolysis of willow and corn stover, Sustainable Energy Fuels, 2019, 3, 1329–1337, 10.1039/c8se00617b.
- S. M. Kim, B. S. Dien and V. Singh, Promise of combined hydrothermal/chemical and mechanical refining for pretreatment of woody and herbaceous biomass, Biotechnol. Biofuels, 2016, 9, 97, DOI:10.1186/s13068-016-0505-2.
- C. E. Wyman, B. E. Dale, V. Balan, R. T. Elander, M. T. Holtzapple, R. S. Ramirez, M. R. Ladisch, N. S. Mosier, Y. Y. Lee, R. Gupta, S. R. Thomas, B. R. Hames, R. Warner and R. Kumar, Comparative performance of leading pretreatment technologies for biological conversion of corn stover, poplar wood, and switchgrass to sugars, in Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals, 2013, pp. 239–259 Search PubMed.
- K. Ohgren, R. Bura, J. Saddler and G. Zacchi, Effect of hemicellulose and lignin removal on enzymatic hydrolysis of steam pretreated corn stover, Bioresour. Technol., 2007, 98, 2503–2510, DOI:10.1016/j.biortech.2006.09.003.
- J. Li, G. Henriksson and G. Gellerstedt, Lignin depolymerization/repolymerization and its critical role for delignification of aspen wood by steam explosion, Bioresour. Technol., 2007, 98, 3061–3068, DOI:10.1016/j.biortech.2006.10.018.
- T. Mouthier, M. M. Appeldoorn, H. Pel, H. A. Schols, H. Gruppen and M. A. Kabel, Corn stover lignin is modified differently by acetic acid compared to sulfuric acid, Ind. Crops Prod., 2018, 121, 160–168, DOI:10.1016/j.indcrop.2018.05.008.
- V. Novy, K. Aissa, F. Nielsen, S. K. Straus, P. Ciesielski, C. G. Hunt and J. Saddler, Quantifying cellulose accessibility during enzyme-mediated deconstruction using 2 fluorescence-tagged carbohydrate-binding modules, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 22545–22551, DOI:10.1073/pnas.1912354116.
- X. Meng and A. J. Ragauskas, Recent advances in understanding the role of cellulose accessibility in enzymatic hydrolysis of lignocellulosic substrates, Curr. Opin. Biotechnol., 2014, 27, 150–158, DOI:10.1016/j.copbio.2014.01.014.
- J. Wu, Y. Dong, H. Zhang, J. Liu, S. Renneckar and J. Saddler, Reduced cellulose accessibility slows down enzyme-mediated hydrolysis of cellulose, Bioresour. Technol., 2023, 371, 128647, DOI:10.1016/j.biortech.2023.128647.
- M. Takada, R. P. Chandra and J. N. Saddler, The influence of lignin migration and relocation during steam pretreatment on the enzymatic hydrolysis of softwood and corn stover biomass substrates, Biotechnol. Bioeng., 2019, 116, 2864–2873, DOI:10.1002/bit.27137.
- R. Chandra, S. Ewanick, C. Hsieh and J. N. Saddler, The characterization of pretreated lignocellulosic substrates prior to enzymatic hydrolysis, part 1: A modified Simons’ staining technique, Biotechnol. Prog., 2008, 24, 1178–1185, DOI:10.1002/btpr.33.
- X. Yu, J. L. Minor and R. H. Atalla, Mechanism of action of Simons’ stain, Tappi J., 1995, 78, 175–180 CAS.
- A. R. Esteghlalian, M. Bilodeau, S. D. Mansfield and J. N. Saddler, Do enzymatic hydrolyzability and Simons’ stain reflect the changes in the accessibility of lignocellulosic substrates to cellulase enzymes?, Biotechnol. Prog., 2001, 17, 1049–1054, DOI:10.1021/bp0101177.
- D. Mboowa, V. Khatri and J. N. Saddler, The use of fluorescent protein-tagged carbohydrate-binding modules to evaluate the influence of drying on cellulose accessibility and enzymatic hydrolysis, RSC Adv., 2020, 10, 27152–27160, 10.1039/d0ra05333c.
- Y. Yuan, J. Wu, F. Wei, Z. Wan, Y. Dong, Y. Lu, P. Yang, Y. Jin and J. Saddler, Elucidating the synergistic action between sulfonated lignin and lytic polysaccharide monooxygenases (LPMOs) in enhancing cellulose hydrolysis, Int. J. Biol. Macromol., 2025, 296, 139674, DOI:10.1016/j.ijbiomac.2025.139674.
- Q. Q. Wang, Z. He, Z. Zhu, Y. H. P. Zhang, Y. Ni, X. L. Luo and J. Y. Zhu, Evaluations of cellulose accessibilities of lignocelluloses by solute exclusion and protein adsorption techniques, Biotechnol. Bioeng., 2011, 109, 381–389, DOI:10.1002/bit.23330.
- A. Koistinen, J. Phiri, K. K. Kesari, T. Vuorinen and T. Maloney, Effect of pulp prehydrolysis conditions on dissolution and regenerated cellulose pore structure, Cellulose, 2023, 30, 2827–2840, DOI:10.1007/s10570-023-05050-w.
- J. Wu, Y. Yuan, Q. Hua, T. Zou, Z. Wan, G. F. Bautista, O. Rojas, S. Renneckar and J. Saddler, Comparing enzymatic post-treatments by endoglucanase (EG) and lytic polysaccharide monooxygenase (LPMO) on microfibrillated cellulose (MFC) to enhance cellulose film fabrication, Carbohydr. Polym., 2025, 349, 123037, DOI:10.1016/j.carbpol.2024.123037.
- J. Wu, Y. Dong, X. Sun, P. Wang, J. Zhu, Y. Zhu, F. Jiang and J. Saddler, Production of lignin containing cellulose nanofibrils (CNF) after enzymatic treatment of curl-induced, unbleached kraft pulps, Green Chem., 2024, 26, 5477–5484, 10.1039/D4GC00834K.
- B. B. Hallac and A. J. Ragauskas, Analyzing cellulose degree of polymerization and its relevancy to cellulosic ethanol, Biofuels, Bioprod. Biorefin., 2011, 5, 215–225, DOI:10.1002/bbb.269.
- L. F. Del Rio, R. P. Chandra and J. N. Saddler, Fibre size does not appear to influence the ease of enzymatic hydrolysis of organosolv-pretreated softwoods, Bioresour. Technol., 2012, 107, 235–242, DOI:10.1016/j.biortech.2011.12.057.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5gc02029h |
| This journal is © The Royal Society of Chemistry 2025 |