Open Access Article
Open Access ArticleGreen synthesis of building blocks, drug candidates and fine chemicals by barochemistry: application of high pressure in organic synthesis
Guoshu
Xie
a,
Valerie
Wright
a,
Alexander
Lazarev
b,
Gary
Smejkal
b,
Vera
Gross
b and
Béla
Török
 *a
*a
aDepartment of Chemistry, University of Massachusetts Boston, 100 Morrissey Blvd, Boston, MA, USA. E-mail: Bela.Torok@umb.edu
bPressure BioSciences Inc., 480 Neponset St, Unit 10B, Canton, MA 02021, USA
First published on 20th May 2025
Abstract
While there are many areas of green chemistry that affect contemporary synthesis, the development of non-traditional activation methods, such as microwaves, ultrasound, mechanochemistry or high hydrostatic pressure (HHP) is considered as one the most important contributors to the development of green synthetic processes. Among these methods HHP, which, by analogy with the other methods, e.g. sonochemistry or mechanochemistry, can be referred to as barochemistry, is well-suited for industrial production; the large scale instrumentation is broadly available, at this time focusing on food processing applications. HHP instruments are safe and easy to handle, robust, and are a good fit for batch and (stopped)-flow operations. The same instruments could be used for large scale chemical synthesis as well, however, the high pressure synthesis of organic compounds, including Active Pharmaceutical Ingredients (APIs), is still in its infancy with extensive developments expected in the near future. HHP applies mechanical compression force to initiate transformations, such as the inactivation of pathogens and enzymes, or activation of chemical reactions. The pressure range of these reactions (2–20 kbar) significantly exceeds that of the typical chemistry using pressurized gases (0.01–0.1 kbar), such as hydrogenations. This tutorial review provides a succinct introduction to the theory and use of barochemistry, with particular emphasis on its current applications and great potential in green synthesis.
Green foundation1. We discuss the use of high hydrostatic pressure (HHP) that applies mechanical compression force to activate chemical processes is an emerging field in chemistry. We describe the fundamentals of high pressure activation, give an overview of available equipment and survey representative green organic synthesis applications.2. Non-traditional activation methods, such as microwaves, ultrasounds, mechanochemistry or high hydrostatic pressure (HHP) is considered as one the most important contributors to the development of green synthetic processes. High pressure activation utilizes high hydrostatic pressure (HHP) to apply mechanical compression force to activate chemical reactions, organic and inorganic alike. The physical closeness of the reacting partners can create favorable orientations for the active centers to react, resulting in improved yields and selectivity, often combined with shorter reaction times, and easier workup as compared to reactions activated by traditional convective heating. 3. The green benefits of high pressure activation in organic syntheses suggests that broader applications of the HHP or barochemistry would bring extensive benefits to the green synthesis of pharmaceuticals, building blocks, and fine chemicals at the laboratory and likely at the industrial scale. |
1. Introduction
The industrial production of chemicals and pharmaceuticals has led to great achievements in fulfilling important societal needs. The 20th and 21st centuries can be called the golden age of chemical synthesis. However, these unprecedented developments can have a dark side such as environmental toxicity of the product itself, e.g. DDT, PFAS and other “forever chemicals”, or the generation of highly toxic waste during manufacturing.1 By the end of the 20th century a growing sentiment developed that the traditional methods of chemical synthesis were not sustainable, effectively initiating the green chemistry movement.2 This movement resulted in unparalleled advances in this field developing effective green tools with the aim of reforming the effective but outdated protocols in the chemical and pharmaceutical industries to provide fine chemicals and pharmaceuticals safely at low cost with no, or minimal negative effects on the environment.3 Today the environmentally benign synthesis of active pharmaceutical ingredients (APIs) and fine chemicals4 is one of the most important pillars of Green Chemistry.5,6 There are several major contributors to these developments, such as catalysis,7 solvents, and the so-called nontraditional activation methods that offer new perspectives for the chemical industry and new advances in the activation of chemical reactions.8 Often these nontraditional activation methods are utilized together with other green tools.91.1. Green synthesis by non-traditional activation methods
An overwhelming majority of chemical reactions require some sort of activation to proceed. Although the use of catalytic approaches brings down the activation energy barrier, the use of non-traditional activation methods offers further improvements compared to conventional convective heating in activating chemical processes. The term non-traditional activation methods, includes protocols that use energy forms other than simple heat. The major areas are microwave, ultrasound, photo-, electro-, mechanochemistry and high pressure.8 These methods possess a wide variety of energy forms and transmission to a reaction system. However, despite the different modes of energy transfer, the common attribute of these methods is that they activate certain chemical reactions more specifically and more efficiently than convective heating.High pressure activation, or barochemistry, the topic of this work, uses mechanical compression force to initiate reactions. Definition: The term barochemistry refers to the use of externally applied hydrostatic pressure to initiate or accelerate chemical transformations. The name is analogous to other non-traditional activation methods such as photochemistry, sonochemistry or mechanochemistry.
The major advantages and green benefits of barochemistry are multifaceted and will be illustrated in the work: (i) catalyst-free and solvent-free conditions eliminate catalyst and solvent handling, and can be used for a wide range of reactions from Diels–Alder reaction or cycloadditions, to multistep cyclizations, (ii) such conditions help achieve higher yields and selectivities, (iii) high atom economy and low waste generation (depending on the reactions), (iv) and guarantee energy efficiency (once pressurized, a system can hold pressure for an extended period of time without continued energy input, and many reactions occur at room temperature), (v) the tunable features of the pressure instruments (pressure, temperature, static pressure or cycling) allow broad applicability, (vi) water is used as a pressure transmitting fluid, (non-flammable, non-toxic, inexpensive, widely available, and has low compressibility), (vii) and finally, barochemistry offers scalability; unlike with many non-traditional activation methods, the large scale instruments are already available should the need for scale up arise. Definitions: (i) The term static pressure refers to a system being pressurized and kept at that constant desired pressure for a period of time, after which the system gets decompressed. (ii) The term cycling or pressure cycling refers to system being pressurized and kept at that desired pressure for a period of time, decompressed, kept decompressed for a period of time, and these action repeated for a number of desired times as illustrated in Fig. 1.
The pressure cycling (Fig. 1b) is a practical approach that often produces higher yields. While the exact nature of the cycling phenomenon is not fully understood, it is hypothesized that the pressure cycling protocol causes periodic change in the volume of the reaction vessel that could lead to some mass transfer and molecular re-alignments during compression and decompression steps that are beneficial for reaction kinetics.
The significance of organic synthesis in the preparation of pharmaceuticals, fine chemicals, and other consumer goods is indisputable. It is also vital that these synthetic operations are carried out in accordance with the green chemistry principles, to ensure that they have zero or negligible negative impact on the environment. In this tutorial, we will outline and discuss the beneficial features of high hydrostatic pressure for the synthesis of valuable products, including fine chemicals and APIs.
2. The theory and practice of high pressure reactions
High pressure activation utilizes high hydrostatic pressure (HHP) to apply mechanical compression force to activate chemical reactions, organic and inorganic alike. The physical proximity of the reacting partners can create favorable orientations for the active centers to react, resulting in improved yields and selectivity, often combined with shorter reaction times, and easier workup as compared to reactions activated by traditional convective heating. The application of HHP has more than a century of history. Röntgen's early work in 1892 appears to be the first use of high pressure.10 Further applications followed in the early 1900s in food chemistry or general food science, and biology, including the preservation of milk,11 or the denaturation of egg white albumin.12,13 With the development of reliable and safe high pressure equipment such applications have become a mainstream tool in the food industry.14,15 Despite the early developments, the first HHP-activated synthetic procedure was only reported in the 1970s.16 Since then, HHP-assisted synthesis has become more common, and has been the subject of regular reviews.17–21A survey of the literature reveals that the number of publications in the high pressure area steadily increased since the 1970s. Fig. 2 illustrates the distribution of pressure-related publications based on gradually narrowing down the topic.
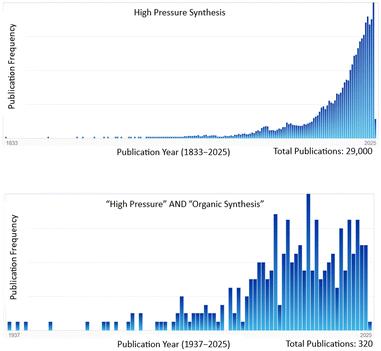 | ||
| Fig. 2 Illustrations of the publication frequency over the years and total number of publications for generic search terms by SciFindern searches as of February 2025. | ||
As shown, the search term “high pressure synthesis” finds 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 papers published, while an intersection of “high pressure” and “organic synthesis” keywords yields only 320 publications over the course of nearly a century. Fig. 2 also indicates that the publication frequency in both graphs significantly increased in the past few decades, perhaps suggesting that the commercial availability of reliable instrumentation accelerates new discoveries. Although these literature searches do not account for the exact numbers due to the occasional obscure mention of high pressure in synthesis (e.g. in medicinal chemistry synthesis of anti-hypertensive drugs), Fig. 2 still provides a comparison within different areas. Moreover, the vast majority of relevant publications to date are related to the application of high pressure in solid state reaction systems, both homogeneous and heterogeneous, for the preparation of inorganic compounds and materials, rather than applications in organic synthesis. The examples of typical inorganic/material science applications include gallium nitride semiconductors, perovskite solar cells, and low-temperature superconductors, just to name a few.22 In contrast, high pressure organic synthesis often involves the use of HHP, i.e. utilizing liquids as pressure transmitting media. Compared to solid state applications, HHP-initiated organic synthesis, despite continuous developments,17,18 is still in its comparative infancy.
000 papers published, while an intersection of “high pressure” and “organic synthesis” keywords yields only 320 publications over the course of nearly a century. Fig. 2 also indicates that the publication frequency in both graphs significantly increased in the past few decades, perhaps suggesting that the commercial availability of reliable instrumentation accelerates new discoveries. Although these literature searches do not account for the exact numbers due to the occasional obscure mention of high pressure in synthesis (e.g. in medicinal chemistry synthesis of anti-hypertensive drugs), Fig. 2 still provides a comparison within different areas. Moreover, the vast majority of relevant publications to date are related to the application of high pressure in solid state reaction systems, both homogeneous and heterogeneous, for the preparation of inorganic compounds and materials, rather than applications in organic synthesis. The examples of typical inorganic/material science applications include gallium nitride semiconductors, perovskite solar cells, and low-temperature superconductors, just to name a few.22 In contrast, high pressure organic synthesis often involves the use of HHP, i.e. utilizing liquids as pressure transmitting media. Compared to solid state applications, HHP-initiated organic synthesis, despite continuous developments,17,18 is still in its comparative infancy.
As one of the important thermodynamic parameters, pressure contributes to controlling the equilibrium and rate of chemical reactions.23 Despite being a potentially useful tool, high pressure chemistry has been somewhat overlooked by organic chemists. Typical organic syntheses are carried out at or near atmospheric pressure (1 bar) or using pressurized gases (up to about 150 bar at the most), while under HHP conditions, reactions experience much higher pressures.24 The typical pressure range of HHP reactions (2–20 kbar) is significantly higher than what is used in general organic synthesis, e.g. hydrogenations (0.01–0.1 kbar). Fig. 3 illustrates a logarithmic scale of the pressures encountered on Earth and elsewhere in the Universe, highlighting the relevant pressure range for organic synthesis (2–20 kbar). Pressure levels beyond this region (ultra-high pressure up to 7000 kbar) deal with materials in solid state and, while being routinely used in geology, material science, and engineering, and typically require Diamond Anvil Cells (DAC) instrumentation and specialized analytical approaches, are not applicable in organic chemistry and are not being covered in this work.
 | ||
| Fig. 3 A logarithmic scale of selected pressures in our Solar System (blue) as well as the pressure range used in organic synthesis (green).25 | ||
2.1. Understanding pressure effects: reaction volume ΔV and activation volume ΔV‡
Although the beneficial effects of high pressure have been reported in several publications, the underlying phenomena of its effects on chemical reactions are still not fully understood.The three major contributors that affect the rate of chemical reaction under high pressure conditions are (i) the increase in concentration of the reacting molecules in the medium; (ii) the change in the rate of molecular diffusion; and (iii) the compression of the reactants at the molecular level which can alter the shape of the “electron clouds” and thus change the rate of effective collisions, phase transitions and specific rearrangements of hydrogen bonding networks in solvents as well.
There is broad agreement that changes in the activation volume (ΔV‡) and reaction volume (ΔV) are the main driving forces of the reactions under pressure. Definitions: (i) Activation volume – The term refers to the volume change between the total volume of the reactant molecules and the volume of the transition state (ΔV‡ = Vtransition state − Vstarting materials). (ii) Reaction volume – The term refers to the volume change between the total volume of the reactants and the total volume of the products (ΔV = Vproducts − Vstarting materials). The volume of starting materials and products include every participant (e.g. even minor byproducts) in the reaction scheme.
Negative values indicate that pressure is going to improve the reaction. In contrast, positive values point in the other direction, i.e., slowing down the given reaction.26 When the values are around zero, pressure is expected to have little effect on reaction rate. The activation volume (ΔV‡) and reaction volume (ΔV) data can be predictive of reaction behaviour under pressurized conditions. Several theoretical methods are available to calculate these values, among these the XP-PCM (extreme pressure polarizable continuum model) method was specifically developed for high pressure conditions.27
2.2. Adiabatic compression heating
Adiabatic compression heating is a well-characterized phenomenon, which has a significant effect on the temperature of materials in gas phase, as the extent of heating is proportional to compressibility. In this tutorial, however, we are going to review compression of reagents in their liquid phase. Compressibility of most liquids is generally small, resulting in relatively insignificant adiabatic heat generation. Water, in particular, exhibits very low compressibility relative to most organic solvents (the bulk modulus of water at room temperature is about 22 kbar28). As a result, temperature rise (expressed in °C) due to compression heating in most aqueous systems typically does not exceed single digits if the starting temperature is near or at room temperature and the pressure is increased up to 7 kbar. Upon decompression the process is reversed and under ideal adiabatic (thermally insulated) conditions the system returns to the starting temperature. In real-world systems, however, compression heat tends to rapidly dissipate into the massive pressure vessel hardware surrounding the reaction mixture, thus reaching thermal equilibrium closer to the starting temperature than anticipated for the true adiabatic conditions.29 It is also important to note that compression heat is being produced simultaneously within the entire volume of the pressurized liquid independent of the volume; therefore, it is possible to predict heat generation in any system, independent of its scale. However, large vessels tend to trap some heat in the middle, while heat dissipation occurs rapidly near the vessel walls, therefore, special engineering solutions may be necessary to ensure uniform temperature conditions in large scale equipment, if precise temperature control is important for the process at hand.303. Contemporary synthetic applications in barochemistry
3.1. High pressure technology in practice
HHP uses water as a pressure transfer medium that makes it possible to reach high pressures safely. Water is not very compressible, and the danger of pressure release in case of a sudden hardware failure is much lower compared to the gas-phase systems. Pressure cookers achieve significantly less pressure yet are far more dangerous as they rely on steam for pressure, which is highly compressible and could expand with explosive force. Water is about 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times less compressible than air or steam, so it can be compressed to much greater levels of pressure without a risk of explosive decompression.31 Accordingly, the high pressure technology is widely used, even at the industrial level, in food processing,14,15 and in material science.22
000 times less compressible than air or steam, so it can be compressed to much greater levels of pressure without a risk of explosive decompression.31 Accordingly, the high pressure technology is widely used, even at the industrial level, in food processing,14,15 and in material science.22
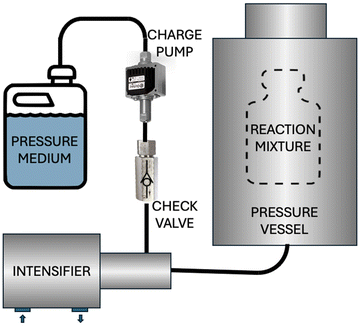
A schematic description of a typical high pressure device is shown in Fig. 4. A relatively modest pressure is provided by a pneumatic or hydraulic source, which is amplified by an intensifier that, via a pressure medium, will generate the desired pressure for the pressure vessel, where the reaction occurs.
3.2. High pressure equipment
One of the most crucial parts of high pressure synthesis is the equipment itself. The first such equipment was reported in 1899 by Hite11 to preserve milk in the dairy industry. The food industry is still the major user of large-scale high-pressure equipment. Similar reactors could be dedicated to barochemical synthesis as well, translating the laboratory protocols to large scale. There are several companies producing safe and reliable high-pressure equipment today.High pressure in liquid phase is generated by high pressure intensifiers (Fig. 5). Low-to-moderate pressure levels are being applied to a larger piston, which, in turn, is pushing a smaller diameter piston to compress the pressure media. The cross-sectional ratio between the two pistons is equal to the pressure amplification factor. Intensifiers can be driven pneumatically or hydraulically. Small pneumatic intensifiers are less expensive; however, they rely on air compressors, which become increasingly expensive and difficult to maintain as their scale grows. Hydraulic intensifiers, on the other hand, typically require lower cross-sectional ratios and tend to scale much easier.
 | ||
| Fig. 5 High pressure intensifier amplifies pneumatic or hydraulic input pressure P1 resulting in output pressure P2 being greater by a factor determined as a ratio of piston areas (ϕ12/ϕ22). | ||
A specific model, a Barocycler 23020EXT (Pressure BioSciences) shown in Fig. 6, is a bench-top instrument which is a space-saving alternative to larger models. The stand-alone instrument is shown in Fig. 6(A), connected to an air compressor underneath (Fig. 6(B)). For small scale reactions, individually sealed fluoropolymer reaction vials (fluorinated ethylene propylene (FEP) 150 μl MicroTubes, Fig. 6(C)) are used, that are placed into metal sample cassettes which are then immersed in the pressure vessel. Up to 16 reactions (Fig. 6(D)) can be carried out under identical conditions, enabling the screening of a large set of reactions in a timely manner.
Continuous-flow systems have also been reported, which frequently consist of a modified HPLC-style pump, a flow-through reactor, e.g., an aging tube or a capillary, and a downstream flow restrictor device capable of sustained restriction to the flow to maintain desired pressure level in the system.51 All such systems are presently research-scale.52
A recently introduced manufacturing-scale “In-Bulk” HHP equipment by Hyperbaric SA53 is a flow-through system that offers a semi-discontinuous treatment of fruit/vegetable juices and other pumpable liquids at a throughput of 4000 L per hour and pressure up to 7 kbar. The system is pressurized in a batch mode but filled and emptied in a flow-through manner, thus eliminating a need of manual handling of bags or bottles of packaged product.
The sample cuvette can be placed into the high pressure chamber and monitored using the AvaSpec Spectrophotometer attached via fiber optic cables. The spectrophotometer is coupled to a Barocycler HUB440, a compact, programmable high-pressure generator that can reach pressure of 4 kbar.56
The HiPOS system offers an ability to monitor the sample using optical spectroscopic techniques under HHP in real time. The system also has the benefit of being able to control the sample temperature. The HHP approach is safe and green, while the UV-Vis or fluorescence detection is sensitive, widely used, and well understood.
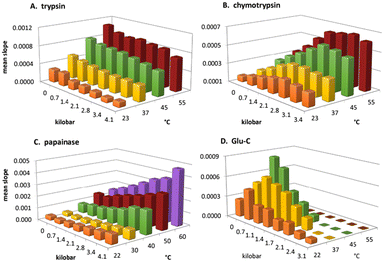
3.2.6.1. Mechanistic investigations of enzyme reactions by the HiPOS system. As enzyme-catalyzed reactions are strong contributors to green synthesis, thus mechanistic investigations by HiPOS could help the design of enzymatic synthetic processes. As Fig. 8 indicates, some enzymes exhibit dramatic increase of their activity, while others show significant inhibition as a function of pressure. Real-time kinetics of several proteolytic enzymes was studied in a HiPOS system as a function of temperature and pressure following the spectral change due to release of a chromophore the suitable chromogenic substrates. Trypsin showed consistent performance over a wide range of pressure and a marked increase in proteolytic activity with temperature. Both chymotrypsin and papainase (traditionally known as papain) showed marked activation with pressure and temperature, while endopeptidase Glu-C was rapidly inactivated with elevated pressure.54
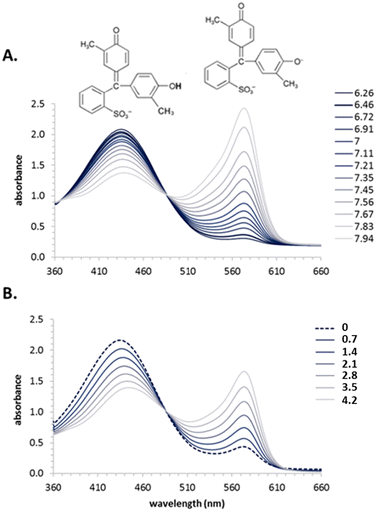
Further, the HiPOS system has demonstrated the barosensitivity of several Good's buffers where high pressure induces a shift in pH. Such changes can be measured spectrophotometrically in real time using pH indicator dyes which undergo color transition (Fig. 9 and 10, original data, experimental details available in figure legends). Definition: Barosensitivity refers to the sensitivity of a system (such as a molecule, buffer, protein, or reaction) to changes in hydrostatic pressure, typically manifested as structural, functional, or physicochemical changes under elevated pressure.
3.3. Synthetic applications
Synthetic applications of high pressure were employed for several common types of reactions in organic synthesis. The following sections focus on some of the reaction types. In most cases a typical HHP instrument, as described in 3.1 and 3.2, was used, sometimes commercially available instruments, in some others, custom-made machines that were overwhelmingly used in following reactions. In a few examples an innovative use of water freezing was applied to circumvent the need for special apparatus, that could generate up to 2 kbar pressure, which is specifically noted at these examples.3.3.2.1. Diels–Alder reactions. Cyclization reactions generally benefit from high pressure conditions due to their activation volume ΔV‡ and reaction volume ΔV values, that are sufficiently negative to ensure a beneficial contribution of pressure.
The Diels–Alder cycloadditions61 are likely the most well-known cyclizations having high significance as a synthetic source of large variety of invaluable chemicals. A high-pressure protocol described the Diels–Alder reaction of thiophene62 that was generally considered more as a heteroaromatic compound than a diene prohibiting its use in these reactions even with strong dienophiles such as maleic anhydride.63 Kotsuki's group disclosed the use of high pressure as an activation method for this reaction, significantly improving the product yield. The authors carried out the reaction at 8 kbar under solvent-free conditions and obtained the products as a mixture of endo and exo isomers in good to excellent yields (81–99%) as shown in Scheme 2. Further investigations were also carried out to determine the dependence of the yield on the level of pressure (Scheme 2). At 100 °C, the yields considerably increased with pressure, suggesting high pressure improved the kinetics of the reaction.
 | ||
| Scheme 2 High pressure-initiated Diels–Alder reaction of thiophene with maleimide dienophiles and the dependence of the yield on the applied pressure. | ||
The Diels–Alder reaction of cis-1,2-dihydrocatechols (X = Me and Cl) with a variety of activated alkenes as dienophiles is another successful example of HHP-initiated cyclization (Scheme 3). The reaction carried out at 19 kbar,64 as described by Stewart et al., provided two different bicyclo[2.2.2]octene products via syn-addition pathway and anti-selective addition pathway at the same pressure with good to excellent yields for the Me analog. Conversely, the Cl analog performed poorly in the reaction.
 | ||
| Scheme 3 High-pressure-promoted selective Diels–Alder reactions of cis-1,2-dihydrocatechols with electron-deficient dienophiles. | ||
High pressure–assisted Diels–Alder reactions have shown promise in the synthesis of complex molecules such as antibiotics by the Rutjes group. Due to the quick adaptation of bacteria to resist antibiotics, these drugs are constantly being designed or modified to ensure their efficacy. An HHP-initiated Diels–Alder cyclization of sterically hindered substrates served as a key step for the synthesis of a new antibiotic, platencin (Scheme 4). Traditional methods of high heat or acid catalysis can only overcome steric hindrance when a compatible reagent is used. The Danishefsky's diene is not one of those reagents. Nevertheless, the high-pressure protocol produced good isolated yield of 81%.65 It is hypothesized, that pressure caused molecular re-alignments during compression, that overcame the steric hindrance represented by the cyclic dienophile.
 | ||
| Scheme 4 High-pressure-promoted selective Diels–Alder reaction for the synthesis of a key intermediate of platencin. | ||
The same research group, Rutjes and co-workers, applied another Diels–Alder cycloaddition for the synthesis of an intermediate for the preparation of a privileged steroid scaffold (Scheme 5).66
 | ||
| Scheme 5 High-pressure-promoted selective Diels–Alder reaction for the synthesis of a privileged steroid scaffold. | ||
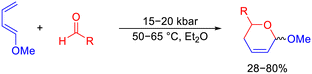
In addition, hetero-Diels–Alder reactions were also investigated by Chmielewski and Jurczak under high pressure conditions. In a series of reactions to generate unsaturated δ-lactones, high pressure was required for the Diels–Alder cyclization between 1-methoxybuta-1,3-diene with aldehydes that do not normally perform well at ambient pressure. High pressure allowed a new series of δ-lactone rings to be produced under mild reaction conditions with yield of up to 80% with some aldehydes (Scheme 6).67
3.3.2.2. [2 + 2] Cycloadditions. [2 + 2] Cycloadditions provide excellent opportunities for the synthesis of strained products containing 4-membered rings, such as cyclobutanes. High pressure has been applied in derivatizing steroid structures to unlock new functionality. To obtain the end product, the carbonyl group at C-17 of dehydroepiandrosterone (DHEA) must undergo a Knoevenagel condensation, which product was subjected to a high pressure-initiated [2 + 2] cycloaddition (Scheme 7). DHEA had reduced reactivity to both reaction types due to steric hindrance. To overcome the obstacle, 15 kbar of pressure was applied to the reaction and quantitative yields were obtained by the Rutjes group.66
 | ||
| Scheme 7 High-pressure-promoted [2 + 2] cycloaddition for the synthesis of a privileged steroid scaffold. | ||
3.3.2.3. [3 + 2] Cycloadditions. Similarly to the above cyclizations [3 + 2] cycloadditions are another branch of concerted reactions. The [3 + 2]-cycloaddition of thiocarbonyl ylides with alkenes and alkynes is another application of high-pressure chemistry for the synthesis of complex molecules, such as tetracyclic meroterpenoids.68 The reaction was described by Magauer's group69 in a comprehensive study (Scheme 8). In the control experiments under thermal conditions the reaction yielded only minute amounts of the cycloadduct.70 In contrast, high pressure appeared to be effective in initiating this pericyclic reaction, likely due to the negative activation and reaction volumes. The authors compared the reaction under thermal and high-pressure conditions. Thermal conditions resulted in up to 40% yield, while high pressure was much more effective giving up to 86% yield. When testing the effect of pressure, a significant improvement (16%–85%) in yield was observed with the increase in pressure (1 bar–5 kbar), while in higher pressure range (up to 14 kbar), only negligible increase in yield (1%) was found.
 | ||
| Scheme 10 Michael addition of activated acyclic donors with disubstituted enone acceptors under high pressure. | ||
In a similar organocatalytic addition of nitromethane to α,β-unsaturated substrates high hydrostatic pressure was used to improve the outcome of the reaction by Kwiatkowski et al. HHP appeared to add significant benefits to this otherwise difficult process that is mainly due to the sterically congested enones.74 The high pressure protocol ensured the preparation of γ-nitroketones in good yields (73–90%), and excellent enantioselectivity (96–99% ee). The authors selected the addition of nitromethane to 3-methylcyclohexenone (Scheme 11(a)) as a test reaction to determine the pressure dependence of the reaction rate as well as the enantiomeric excess. Scheme 11(b) demonstrates that up to 8 kbar, increasing pressure significantly improved the yield of the product. Above 8 kbar, however, pressure increase only provided limited enhancement, the yield essentially plateauing at 9 kbar. Interestingly, the ee appeared to be the highest (99% ee) at lower pressures (3–6 kbar) and showed some decline, although minimal, at higher pressures (97.5–98% ee). A similar Michael addition of α-substituted cyclic ketones with acrylates was also carried out under high pressure by Kotsuki's group using a chiral 1-methylbenzyl amine organocatalysis providing good yields and enantioselection, although in quite long reaction times (48 h).75
High pressure has been instrumental to improve aza-Michael additions. The addition of amines to α-dialkoxymethyl α,β-unsaturated ester was successfully carried out by Rulev, Kotsuki, and Maddaluno to produce the expected aza-Michael adducts. The reaction conditions were mild using 15 kbar pressure at ambient temperature, yielding 66% product.76 While it is only a moderate yield, it must be noted, that the same reaction did not produce any product under atmospheric pressure despite the 24 h long reflux (Scheme 12). It appears that the high pressure activation is an effective method to carry out the conjugate addition of nitrogen nucleophiles to alkenes.
 | ||
| Scheme 12 Comparison of the aza-Michael addition of amines onto α-dialkoxymethyl α,β-unsaturated ester under high pressure (15 kbar) and ambient pressure (1 bar). | ||
A similar, direct aza-Michael addition of anilines to activated alkenes was carried out by Fedotova et al. with the aid of hexafluoroisopropanol (HFIP),77 which was applied as a catalyst and a solvent as well. The effects of HFIP and HHP were found to be synergistic in activating the Michael acceptor. This dual enhancement ensured the efficient 1,4-addition of anilines of poor nucleophilicity onto Michael acceptors for the first time. The protocol resulted in up to 100% yield for the corresponding products (Scheme 13).
The synthesis of β-lactams often starts with a β-aminoester intermediate. β-Lactams are a common structure in antibiotics. Diversity of them and, therefore, their intermediates are important in creating new antibiotics. Previously, the creation of β-aminoesters was limited by steric hindrance; only the simplest un-substituted reagents were shown to react. Scheme 14 reports a high pressure application by Jenner that shows not only how a new lanthanide triflate catalyst can encourage the reaction, but also, when combined with high pressure, such reaction yields a whole new array of compounds at excellent yields using mild reaction conditions. While neither catalyst alone, nor pressure alone resulted in any extraordinary outcomes, when both were applied together, the yields were up to 100%, and created products that under other circumstances would not be attained through this scheme.78
 | ||
| Scheme 17 High-pressure accelerated asymmetric organocatalytic Friedel–Crafts alkylation of indoles with enones (a) and enone-carboxylic acid esters (b). | ||
Harrington and Kerr found high pressure conditions to be effective at overcoming steric hindrance in additional similar applications as well. Scheme 18 is another example of coming across the obstacle of steric hindrance despite having an effective catalyst in this conjugate addition of indole to olefins. As complexity of reagents rose, a decrease in yield was observed. 13 kbar of pressure was applied to increase yields to quantitative amounts that couldn't be observed at ambient pressures.85
 | ||
| Scheme 19 HHP-assisted catalyst- and solvent-free Paal–Knorr reactions for the green synthesis of pyrrole derivatives. | ||
The calculation of the ΔV values by the Joback fragmentation method88 for the reaction steps revealed reasonably negative values for the cyclization providing a theoretical explanation of the obtained results.
High pressure was used in basic trans-esterification reactions by the Sałański group to show its efficacy in producing increasing yields of well-known reactions. Alkyl acetates and benzoates were reacted at 1.1 kbar for 2 h with methanol using triethyl amine catalysis to afford the trans-esterification products seen in Scheme 21 at high yields.90
 | ||
| Scheme 22 HHP-assisted synthesis of 5,10,15,20-tetrakis[4-(substituted amino)-2,3,5,6-tetrafluorophenyl]porphyrins. | ||
Three factors have been noted for their influence on conformational changes and dynamics of polymers: temperature, solvent, and high hydrostatic pressure. Martinho et al. reported the effect of high pressure on polystyrene chains with pyrene derivative end labels by following the rate of excimer formation (Scheme 23). In experiments employing several solvents and temperature levels to study pressure effects in a multitude of conditions, higher pressure was found to reduce the excimer formation. High pressure was found to increase solvent viscosity, leading to a decreased likelihood of cyclization.92
Hydrazones are valuable compounds in biomedical applications, for example, as multifunctional anti-Alzheimer disease agents,93 or antioxidants applied in mitochondrial antioxidant therapy in preeclampsia.94 Thus, their synthesis, and especially the green alternatives of synthetic protocols, attracted significant attention. A new, catalyst- and solvent-free synthesis was described by Costa et al. via the condensation of benzaldehydes and phenylhydrazones, which was initiated by HHP, providing moderate to excellent yields (Scheme 24).95 The authors also carried out control experiments at ambient pressure, and it was found that the pressurized reactions usually provided 10–30% increase in product yields. During the syntheses the so-called pressure cycling was used, when compression–decompression cycles were applied in a predetermined sequence (Fig. 1b). Under the current conditions cycling improved the yields by about 20% compared to the static system.
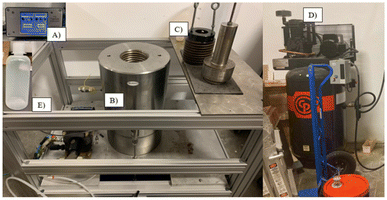 | ||
| Fig. 11 Pressure BioSciences Inc. pilot scale high pressure reactor indicating (A) pressure indicator, (B) 5 L chamber, (C) screw cap, (D) air compressor, (E) 1000 mL LDPE reaction bottle. | ||
The scale-up reactions yielded excellent results, as shown in Scheme 25. Based on the data obtained in the pilot experiment, it appears that the reaction scale can be readily increased. The successful completion of reactions with up to 2100-fold increase in the mass of the starting materials demonstrated that the application of the reaction parameters from the mg scale reactions to the several hundred-gram level did not negatively affect the outcome of the reactions. Reaction times and yields remained essentially the same providing quantitative yields without any by-product formation, thus purification was not necessary, further improving the green advantages of the methodology.
4. Potential disadvantages and limitations of barochemistry
Similarly to any methodology, barochemistry also has its disadvantages and limitations. These aspects originate from multiple issues such as the instruments and technical aspects, and the reactions themselves.4.1. Instrument and other technical issues
(i) The first such issue is the generation of the pressure. Pneumatic intensifiers are less expensive; they require an air compressor or a gas tank as pressure input. However, they are difficult to scale up due to the limits of power that can be delivered by highly compressible gas, as well as due to the safety concerns arising from large compressible volume of gas being used.(ii) The pressure vessels of the smaller instruments are commonly made from a single metal block. A disadvantage of the single block pressure vessels is an inability to predict when the material fatigue will cause a failure. Most of the stress occurs initially on the inner surface of the pressure vessel, initiating hairline cracks that will eventually propagate outward. Therefore a high quality surface finish on the inner surface is critical to eliminate any possible stress concentrators. Periodic inspection of the vessel ID surface by a dye penetrant technique42 can reveal small hairline cracks that would eventually propagate to the outside of the vessel.
(iii) Although the scale up of barochemical syntheses at static pressure appears to be relatively straightforward,96 it must be mentioned that scaling up is significantly more difficult when doing pressure cycles rather than static pressure. It is much easier to hold pressure at the large scale for a given time, rather than constantly pressurize and decompress with relatively short holding times.
(iv) The reaction vessel, that holds the reaction mixture and is immersed into the pressure vessel, could also represent a challenge. It must be chemically inert, and flexible. Although the compressibility of water, used as a pressure transmitting fluid, is low, no liquid is completely noncompressible. Thus, a rigid reaction vessel can easily break under high pressure condition, losing the reaction mixture and contaminating the pressure vessel. This limitation excludes all crystalline polymeric reaction vessels. In addition, some reaction vessels might not be compatible with the solvent use. Some solvents swell, and sometimes even dissolve certain polymers.
4.2. Potential issues derived from the reactions
(i) Since the beneficial effects of high pressure organic synthesis (different from the diamond-anvil-cells used in solid phase materials applications) are mainly derived from the low compressibility of liquids, the reaction mixture must be in the liquid state. That certainly results in some limitations. For example, if solvent-free reactions are attempted, at least one of the components must be liquid, and it should dissolve the other components. If all starting materials are solids, then either a solvent must be used, leading to another limitation, or one can shift to solid state synthesis using diamon anvil cells.(ii) The solvent compatibility is important considering the flexible nature of the reaction vessels, that are made of one form of plastic. The solvent must not react with or swell the reaction vessel, otherwise leaks could occur compromising the outcome of the reaction. One must consult with the literature to make sure that an appropriate solvent is selected. Commonly, Teflon vessels are resistant to solvents, but other common materials such as polyethylene might present challenges when the solvent selection is in question.
(iii) As we pointed out above, the effect of pressure on a process is dependent upon the ΔV‡ and ΔV values of the reaction. When both are negative, the reaction benefits from pressure, when both are positive, then pressure works against the reaction. Any other combination is an individual case. When the sum of the changes amount to zero, then the reaction will occur similarly to the non-pressurized reaction. In short, not all reactions improve under pressure, and one should calculate the potential volume changes to predict the direction of the pressure effect or consult the literature for similar examples.
5. Relevant green chemistry metrics97
(i) Mass-related descriptors are either dependent upon the reaction itself, or the conditions applied. The atom economy (AE) as a theoretical descriptor is independent from the conditions and only related to the reaction itself. More practical mass-related metrics, such as the E-factor, environmental quotient (EQ), mass efficiency (ME), reaction mass efficiency (RME), and process mass intensity (PMI), however, are significantly dependent on the reaction conditions. These metrics are strongly dependent on practical factors such as chemical yield, selectivity (chemo-, regio, or stereo), since these descriptors determine the amount of waste, the use of solvent and auxiliary materials, the potentially required purification method and the likes. When a barochemical synthesis occurs with excellent yields and selectivity under potentially catalyst- and solvent-free conditions then the process will produce highly desirable mass-related metrics. However, every reaction is unique, and the benefits of high pressure must be evaluated on a case-by-case basis.(ii) Solvents also play an important role in any process, especially in barochemistry, where the reaction mixture must be in the liquid phase. The compatibility of the solvent with the reaction vessel is an obvious issue, as was discussed above. Another parameter is the compressibility of the solvent. When it is high, the use of pressure is less than ideal due to large volume changes or higher adiabatic thermal effect. The third issue is the solvent intensity, i.e. how much solvent a process requires. Not considering purification (that is independent of the use of pressure during the reaction) the solvent intensity of the actual reaction will be dependent on the solubility of the reactants in each solvent. Once a process/reaction vessel is selected the applicable solvents must be evaluated based on these parameters, and their overall greenness.
(iii) Energy efficiency. The amount of energy used in a reaction is also crucial in determining the overall greenness of a process. Different forms of energy (convective heating vs. non-traditional activation methods) will result in different efficiency in each system. However, just like in the case of different other descriptors, there is no ultimate best. The polarity, viscosity, chemical nature, boiling point, compressibility etc. all contribute to the energy efficiency of a reaction. In addition, various reactions respond quite differently to the type of activation. In a recent study the effect of different activation methods was investigated on the catalyst- and solvent-free Paal–Knorr reaction.98 It was observed, that the barochemical synthesis was the most energy efficient, due to the extremely short reaction times. Even conventional heating appeared to be more energy efficient than microwave irradiation. However, the opposite was observed, namely microwave activation was superior, when the reaction was catalyzed by a solid acid, K-10 montmorillonite.99 However, in this case the solid acid acted as a strong microwave absorber providing internal heating for the reaction, while in the catalyst-free approach, the starting materials appeared to be poor microwave absorbers, i.e. most of the energy input was wasted. This example shows, how complex the appropriate selection of the activation method could be, to ensure the most energy efficient conditions.
6. Conclusions and outlook
In this tutorial review the fundamental characteristics of high hydrostatic pressure-induced organic synthesis have been described. After a brief introduction about history and theory, the application side has been highlighted, from equipment to applicable reactions. We believe that the broader applications of HHP or barochemistry would bring extensive benefits to the green synthesis of APIs, building blocks, and fine chemicals at the laboratory and likely at the industrial scale. Based on the major findings, the key benefits of barochemistry can be summarized in the following main points: (i) many reactions (cyclizations in particular) occur in a catalyst- and solvent-free environment, eliminating catalyst separation/recycling and reagent disposal steps; (ii) the reactions produce higher yields and often improved selectivities; in the ideal cases quantitative yields and exclusive selectivity were obtained, thus no product purification is required; (iii) the majority of reactions occur at room temperature or moderate temperatures, which is convenient, safe and energy efficient (iv) the commercially available HHP instruments allow safe protocols with tuneable characteristics, such as pressure, temperature, reaction time, or pressure cycles, if needed, (v) the instruments use water as a pressure transmitting fluid, that is non-flammable, non-toxic, inexpensive, widely available, and also has the lowest compressibility of most liquids, in short, ideal from both engineering and green chemistry point of views.Despite the above summarized advantages, efforts were also made to address the drawbacks and limitations of barochemistry. There are still multiple gaps in our understanding of the effect of HHP on chemical reactions. Although the mechanistic understanding of pericyclic reactions appears more advanced than that of some other types, one must not forget, that those reaction proceed in one step. In contrast, many catalytic reactions, including multicomponent reactions proceed in many steps, thus the pressure effect should be investigated on each of those elementary steps to explain the final experimental outcome. However, as more advances are made in high pressure synthesis, more data will be available to corroborate experimental findings with the theoretical fundamentals. This progress will help overcome the existing gaps and will undoubtedly help apply HHP to a wide range of reactions.
The high-pressure methodology is a promising activation strategy that is applicable to many reaction types. Based on the examples listed in this work, it might enable other reactions as well, such as multicomponent cyclizations that produce important heterocycles, Pd-catalyzed cross-coupling reactions, or alkene metathesis among other possibilities.
Data availability
The data supporting this article have been included in the References.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R15GM157702 (BT). Support of our work by the University of Massachusetts Boston is also gratefully acknowledged.References
- Green Chemistry: An Inclusive Approach, ed. B. Török and T. Dransfield, Elsevier, Oxford-Cambridge, MA, 2018 Search PubMed.
- P. Anastas and J. Warner, Green Chemistry: Theory and Practice, Oxford University Press, Oxford, UK, 1998 Search PubMed.
- Contemporary Chemical Approaches for Green and Sustainable Drugs, ed. M. Török, Elsevier, Amsterdam, Oxford, Cambridge, MA, 2022 Search PubMed.
- (a) S. Datta, A. Sood and M. Török, Curr. Org. Synth., 2011, 8, 262–280 CrossRef CAS; (b) M. Mishra and B. Török, Sustainable large-scale synthesis of fine chemicals, active pharmaceutical ingredients and biologically active compounds by non-traditional activation methods and biocatalysis, in Mechanochemistry and Emerging Technologies for Sustainable Chemical Manufacturing, ed. E. Colacino, D. Crawford and F. Garcia, CRC Press/Taylor & Frances, Boca Raton, FL, 2023 Search PubMed.
- Handbook of Green Chemistry – Green Solvents, ed. W. Leitner, P. G. Jessop, C. J. Li, P. Wasserscheid and A. Stark, Wiley-VCH, Weinhheim, 2010 Search PubMed.
- Encyclopedia of Sustainable Science and Technology, ed. R. A. Meyers, Springer-Nature, 2018. DOI:10.1007/978-1-4939-2493-6_1008-1.
- B. Török, C. Schäfer and A. Kokel, Heterogeneous Catalysis in Sustainable Synthesis, Elsevier, Cambridge, Oxford, 2021 Search PubMed.
- Non-traditional Activation Methods in Green and Sustainable Applications: Microwaves, Ultrasounds, Photo, Electro and Mechanochemistry and High Hydrostatic Pressure, ed. B. Török and C. Schäfer, Elsevier, Cambridge, Oxford, 2021 Search PubMed.
- R. B. Vlocskó, G. Xie and B. Török, Molecules, 2023, 28, 4153 CrossRef PubMed.
- W. C. Röntgen, Annu. Rev. Phys. Chem., 1892, 281, 98–107 CrossRef.
- B. H. Hite, Bull. - W. Va. Univ., Agric. Exp. Stn., 1899, 58, 15–35 Search PubMed.
- P. W. Bridgman, J. Biol. Chem., 1914, 19, 511–512 CrossRef CAS.
- R. J. Hemley, High Pressure Res., 2010, 30, 581–619 CrossRef CAS.
- T. Grauwet, I. Van der Plancken, L. Vervoort, M. Hendrickx and A. Van Loey, High-pressure processing uniformity, in High Pressure Processing of Foods, ed. V. M. Balasubramaniam, G. V. Barbosa-Cánovas and H. L. M. Lelieved, Springer, New York, 2016, pp. 253–268 Search PubMed.
- K. Yamamoto, High hydrostatic pressure in food industry applications, in Non-traditional Activation Methods in Green and Sustainable Applications: Microwaves, Ultrasounds, Photo, Electro and Mechanochemistry and High Hydrostatic Pressure, ed. B. Török and C. Schäfer, Elsevier, Cambridge, Oxford, 2021, pp. 559–574 Search PubMed.
- W. G. Dauben and H. O. Krabbenhoft, J. Am. Chem. Soc., 1976, 98, 1992–1993 CrossRef CAS.
- A. Y. Rulev and F. I. Zubkov, Org. Biomol. Chem., 2022, 20, 2320–2355 RSC.
- D. Margetic, High Pressure Organic Synthesis, De Gruyter, Berlin, Boston, 2019. DOI:10.1515/9783110556841.
- C. L. Hugelshofer and T. Magauer, Synthesis, 2014, 1279–1296 Search PubMed.
- W. Holzapfel and N. Isaacs, High Pressure Techniques in Chemistry and Physics. A Practical Approach, Oxford University Press, 1997, p. 400 Search PubMed.
- (a) T. Asano and W. J. Le Noble, Chem. Rev., 1978, 78, 407–489 CrossRef CAS; (b) R. Van Eldik, T. Asano and W. J. Le Noble, Chem. Rev., 1989, 89, 549–688 CrossRef CAS; (c) A. Drljaca, C. D. Hubbard, R. Van Eldik, T. Asano, M. V. Basilevsky and W. J. Le Noble, Chem. Rev., 1998, 98, 2167–2290 CrossRef CAS PubMed.
- M. Miao, Y. Sun, E. Zurek and H. Lin, Nat. Rev. Chem., 2020, 4, 508–527 CrossRef CAS.
- V. Schettino and R. Bini, Chem. Soc. Rev., 2007, 36, 869–880 RSC.
- P. F. McMillan, Chem. Soc. Rev., 2006, 35, 855 RSC.
- G. Xie, A. Lazarev and B. Török, Organic Synthesis With High Hydrostatic Pressure, in Encyclopedia of Green Chemistry, Elsevier, 2024. DOI:10.1016/B978-0-443-15742-4.00113-7.
- I. Chataigner and J. Maddaluno, High-Pressure Synthesis: An Eco-friendly Chemistry, in Activation Methods: Sonochemistry and High Pressure, ed. J.-P. Goddard, M. Malacria and C. Ollivier, ISTE Ltd and John Wiley & Sons, Inc., 1st edn, 2019, pp. 95–149 Search PubMed.
- B. Chen, R. Hoffmann and R. Cammi, Angew. Chem., Int. Ed., 2017, 56, 11126–11142 CrossRef CAS PubMed.
- The Engineering ToolBox https://www.engineeringtoolbox.com/water-thermal-properties-d_162.html (accessed: 02/25/2025).
- K. Knoerzer, R. Buckow and C. Versteeg, J. Food Eng., 2010, 98, 110–119 CrossRef and the references cited therein.
- T. Grauwet, C. Rauh, I. Van der Plancken, L. Vervoort, M. Hendrickx, A. Delgado and A. Van Loey, Trends Food Sci. Technol., 2012, 23, 97–110 CrossRef CAS.
- Objectives_template. https://archive.nptel.ac.in/content/storage2/courses/112104118/lecture-2/2-4-compressibility.htm (accessed 02/25/2025).
- D. F. Farkas, A Short History and Development Efforts leading to eth Commercialization of High-Pressure Processing of Food, in High Pressure Processing of Food, Technology and Applications, ed. V. M. Balasubramaniam, G. V. Barbosa-Canovas and H. L. M. Lelleveld, Springer, 2016, pp. 19–36 Search PubMed.
- https://www.hiperbaric.com/en/ (accessed 02/25/2025).
- https://www.jbtc.com/foodtech/products-and-solutions/brands/avure-technologies/ (accessed 02/25/2025).
- https://www.thyssenkrupp-uhde.com/ (accessed: 02/25/2025).
- https://www.kobelco.co.jp/english/ (accessed: 02/25/2025).
- https://btkf-hpp.com/ (accessed: 02/25/2025).
- https://www.pressurebiosciences.com/ (accessed: 02/25/2025).
- https://www.highpressure.com/ (accessed: 02/25/2025).
- https://www.parker.com/us/en/home.html (accessed: 02/25/2025).
- https://highpressuretech.com/ (accessed: 02/25/2025).
- ASME Boiler and Pressure Vessel Code, Section V, Art. 24 Standard Test Method for Liquid Penetrant Examination SE-165.
- T. Johannisson and K. Zander, Wire Wound High Pressure Vessels for Industrial Applications, in High-Pressure Science and Technology, ed. K. D. and Timmerhaus, M. S. Barber, Springer, Boston, MA, 1979 Search PubMed.
- H. Herberhold, S. Marchal, R. Lange, C. H. Scheyhing, R. F. Vogel and R. Winter, J. Mol. Biol., 2003, 330, 1153–1164 CrossRef CAS PubMed.
- A. C. Bourges, A. Lazarev, N. Declerck, K. L. Rogers and C. A. Royer, Biophys. J., 2020, 118, 2670–2679 CrossRef CAS PubMed.
- J. Koehler, M. B. Erlach, E. Crusca Jr., W. Kremer, C. E. Munte and H. R. Kalbitzer, Materials, 2012, 5, 1774–1786 CrossRef CAS.
- M. T. Lerch, Z. Yang, C. Altenbach and W. L. Hubbell, Methods Enzymol., 2015, 564, 29–57 CAS.
- D. R. Davydov, Z. Yang, N. Davydova, J. R. Halpert and W. L. Hubbell, Biophys. J., 2016, 110, 1485–1498 CrossRef CAS PubMed.
- Y. O. Kamatari, R. Kitahara, H. Yamada, S. Yokoyama and K. Akasaka, Methods, 2004, 34, 133–143 CrossRef CAS PubMed.
- D. K. Rai, R. E. Gillilan, Q. Huang, R. Miller, E. Ting, A. Lazarev, M. W. Tate and S. M. Gruner, J. Appl. Crystallogr., 2021, 54, 111–122 CrossRef CAS PubMed.
- E. Y. Ting, A. Lazarev and J. Ma, US Pat., 11156295B2, 2021 Search PubMed.
- B. J. Deschner, D. E. Doronkin, T. L. Sheppard, G. Rabsch, J. D. Grunwaldt and R. Dittmeyer, Rev. Sci. Instrum., 2021, 92, 124101 CrossRef CAS PubMed.
- https://www.hiperbaric.com/en/hpp-technology/equipment/hpp-in-bulk/ (accessed 02/22/2025).
- G. Smejkal, E. Ting, V. Gross, N. Cutri and A. Lazarev, High, Pressure Optical Spectroscopy (HiPOS) and Real-Time Analysis of Enzyme Kinetics at Elevated Hydrostatic Pressure, South Easton, 2021 Search PubMed.
- H. Vass, S. L. Black, E. M. Herzig, F. B. Ward, P. S. Clegg and R. J. Allen, A Multipurpose Modular System for High-Resolution Microscopy at High Hydrostatic Pressure, American Institute of Pysics, 2010, p. 81 Search PubMed.
- HUB High Pressure Generators and Accessories – Pressure BioSciences, Inc. https://products.pressurebiosciences.com/collections/hub-high-pressure-generators-and-accessories (accessed 02/25/2025).
- B. Török, C. Schäfer and A. Kokel, Hydrogenation, in Heterogeneous Catalysis in Sustainable Synthesis, Elsevier, Cambridge, Oxford, 2021, ch. 3.1 Search PubMed.
- A. Kulkarni and B. Török, Heterogeneous Catalytic Hydrogenation as an Environmentally Benign Tool for Organic Synthesis, Curr. Org. Synth., 2011, 8, 187–207 CrossRef CAS.
- A. Tomin, A. Lazarev, M. P. Bere, H. Redjeb and B. Török, Selective Reduction of Ketones Using Water as a Hydrogen Source under High Hydrostatic Pressure, Org. Biomol. Chem., 2012, 10, 7321–7326 RSC.
- H. Cho, C. Schäfer and B. Török, Hydrogenations and Deuterium Labeling with Aluminum-based Metal Alloys under Aqueous Conditions, Curr. Org. Synth., 2016, 13, 255–277 CrossRef CAS.
- O. Diels, Ber. Dtsch. Chem. Ges. A, B, 1936, 69, 11 Search PubMed.
- (a) K. Kumamoto, I. Fukada and H. Kotsuki, Angew. Chem., Int. Ed., 2004, 43, 2015–2017 CrossRef CAS PubMed; (b) D. Loco, R. Spezia, F. Cartier, I. Chataigner and J.-P. Piquemal, Chem. Commun., 2020, 56, 6632–6635 RSC.
- Thiophene and Its Derivatives, Part 1, ed. S. Gronowitz, Wiley, New York, 1985, pp. 697–705 Search PubMed.
- S. G. Stewart, J. H. Gwion, K. J. McRae, Y. Teng, L.-J. Yu, B. Chen, R. Cammi, M. L. Coote, M. G. Banwell and A. C. Willis, J. Org. Chem., 2020, 85, 13080–13095 CrossRef CAS PubMed.
- D. C. J. Waalboer, M. C. Schaapman, F. L. Van Delft and F. P. J. T. Rutjes, Angew. Chem., Int. Ed., 2008, 47(35), 6576–6578 CrossRef CAS PubMed.
- D. Blanco-Ania, R. W. M. Aben, L. W. A. Van Berkom, H. W. Scheeren and F. P. J. T. Rutjes, Eur. J. Org. Chem., 2014, 1438–1444 CrossRef CAS.
- M. Chmielewski and J. Jurczak, J. Org. Chem., 1981, 46, 2230–2233 CrossRef CAS.
- R. Wildermuth, K. Speck, F.-L. Haut, P. Mayer, B. Karge, M. Brönstrup and T. Magauer, Nat. Commun., 2017, 8, 2083 Search PubMed.
- F.-L. Haut, C. Habiger, K. Speck, K. Wurst, P. Mayer, J. N. Korber, T. Müller and T. Magauer, J. Am. Chem. Soc., 2019, 141, 13352–13357 CrossRef CAS PubMed.
- K. Speck and T. Magauer, Chem. – Eur. J., 2017, 23, 1157–1165 CrossRef CAS PubMed.
- K. Matsumoto, Angew. Chem., Int. Ed. Engl., 1984, 23, 617–618 CrossRef.
- Y. Misumi and K. Matsumoto, Angew. Chem., Int. Ed., 2002, 41, 1031–1033 CrossRef CAS PubMed.
- W. G. Dauben and J. M. Gerdes, Tetrahedron Lett., 1983, 24, 3841–3844 CrossRef CAS.
- P. Kwiatkowski, K. Dudzinski and D. Lyzwa, Org. Lett., 2011, 13, 3624–3627 CrossRef CAS PubMed.
- R. Horinouchi, K. Kamei, R. Watanabe, N. Hieda, N. Tatsumi, K. Nakano, Y. Ichikawa and H. Kotsuki, Eur. J. Org. Chem., 2015, 4457–4463 CrossRef CAS.
- A. Y. Rulev, H. Kotsuki and J. Maddaluno, Green Chem., 2012, 14, 503–508 RSC.
- A. Fedotova, B. Crousse, I. Chataigner, J. Maddaluno, A. Y. Rulev and J. Legros, J. Org. Chem., 2015, 80, 10375–10379 CrossRef CAS PubMed.
- G. Jenner, Tetrahedron Lett., 1995, 36, 233–236 CrossRef CAS.
- R. A. Bunce, M. F. Schlecht, W. G. Dauben and C. H. Heathcock, Tetrahedron Lett., 1983, 24, 4943–4946 CrossRef CAS.
- (a) Y. Hayashi, W. Tsuboi, M. Shoji and N. Suzuki, J. Am. Chem. Soc., 2003, 125, 11208–11209 CrossRef CAS PubMed; (b) Y. Hayashi, J. Synth. Org. Chem., 2014, 72, 1228–1238 CrossRef CAS.
- (a) Y. Hayashi and K. Nishimura, Chem. Lett., 2002, 296–297 CrossRef CAS; (b) Y. Hayashi, K. Okado, I. Ashimine and M. Shoji, Tetrahedron Lett., 2002, 43, 8683–8686 CrossRef CAS.
- D. Lewis, Synform, 2018, 4, A49–A52 Search PubMed.
- B. Török, C. Schäfer and A. Kokel, Friedel-Crafts and related reactions catalyzed by solid acids, in Heterogeneous Catalysis in Sustainable Synthesis, Elsevier, Cambridge, Oxford, 2021, ch. 3.5 Search PubMed.
- D. Łyżwa, K. Dudzinski and P. Kwiatkowski, Org. Lett., 2012, 14, 1540–1543 CrossRef PubMed.
- P. Harrington and M. A. Kerr, Can. J. Chem., 1998, 76, 1256–1265 CrossRef CAS.
- H. Cho, R. Madden, B. Nisanci and B. Török, Green Chem., 2015, 17, 1088–1099 RSC.
- G. Xie, A. Lazarev and B. Török, Green Chem., 2023, 25, 1582–1587 RSC.
- K. G. Joback and R. C. Reid, Chem. Eng. Commun., 1987, 57, 233–243 CrossRef CAS.
- M. J. Eisenmenger and J. I. Reyes-De-Corcuera, J. Mol. Catal. B: Enzym., 2010, 67, 36–40 CrossRef CAS.
- J. Jurczak, D. T. Gryko, P. Lipkowski and P. Salanski, Rev. High Pressure Sci. Technol., 1998, 7, 1236–1240 CrossRef CAS.
- A. T. P. C. Gomes, P. C. Freire, C. R. M. Domingos, M. G. P. M. S. Neves, J. A. S. Cavaleiro, F. A. Almeida Paz, J. A. Saraiva and A. C. Tomé, J. Porphyrins Phthalocyanines, 2016, 20, 1377–1389 CrossRef CAS.
- J. M. G. Martinho, E. M. S. Castanheira, A. T. Reis e Sousa, S. Saghbini, J. C. André and M. A. Winnik, Macromolecules, 1995, 28, 1167–1171 CrossRef CAS.
- B. Török, A. Sood, S. Bag, R. Tulsan, S. Ghosh, D. Borkin, A. R. Kennedy, M. Melanson, R. Madden, W. Zhou, H. LeVine III and M. Török, Biochemistry, 2013, 52, 1137–1148 CrossRef PubMed.
- M. Mastyugin, R. B. Vlocskó, Z. K. Zsengellér, B. Török and M. Török, J. Med. Chem., 2025 DOI:10.1021/acs.jmedchem.5c00010.
- M. Costa, F. Adhamidhi, M. Mastyugin, A. R. Fusco, A. Lazarev, Z. K. Zsengeller, M. Török and B. Török, Molecules, 2024, 29, 5287 CrossRef CAS PubMed.
- V. Wright, G. Xie, M. Costa, A. Lazarev and B. Török, High Hydrostatic Pressure-assisted reactions at a large scale: Scale-up of HHP-initiated solvent-free and catalyst-free Paal-Knorr reactions from milligram to kilogram scale. ACS Spring 2024 National Meeting - Many Flavors of Chemistry, Division of Organic Chemistry, New Orleans, LA, March 17–21, 2024, Paper ID: 3981077.
- D. Daggett, Y. Shi and B. Török, Characterizing the Environmentally Benign Nature of Chemical Processes: Green Chemistry Metrics, in Contemporary Chemical Approaches for Green and Sustainable Drugs, ed. M. Török, Elsevier, Amsterdam, Oxford, Cambridge, MA, 2022 Search PubMed.
- M. Costa, S. Baoengan, A. Lazarev, B. Török and M. Török, Energy Efficiency of Paal-Knorr Reactions using Traditional and Non-traditional Activation Methods: Ultrasonication, Microwave-Assisted Heating, and High Hydrostatic Pressure, ACS Spring 2025 National Meeting, Division of Organic Chemistry, San Diego, CA, March 22–27, 2025, Paper ID: 4192162.
- H. Cho, F. Török and B. Török, Green Chem., 2014, 16, 3623–3634 RSC.
| This journal is © The Royal Society of Chemistry 2025 |