Asymmetric defective site-triggered triple synergistic modulation in nanoconfined aerogel for superior electrochemical conversion of low-concentration nitrate into ammonia †
Ke
Wang
,
Tong
Zhao
,
Shiyu
Zhang
,
Rupeng
Wang
,
Meng
Wang
,
Zixiang
He
and
Shih-Hsin
Ho
 *
*
State Key Laboratory of Urban Water Resource and Environment, School of Environment, Harbin Institute of Technology, Harbin, 150040, P. R. China. E-mail: stephen6949@hit.edu.cn
First published on 23rd May 2025
Abstract
Electrocatalytic conversion of low-concentration nitrate (NO3−) into ammonia (NH3) under ambient conditions is expected to provide an effective solution to the global nitrogen cycle imbalance. However, this process is hindered by slow reaction kinetics and the competing hydrogen evolution reaction (HER). Herein, we anchored oxygen vacancy-containing hollow Co3O4 nanoparticles on waste spirulina residue-derived reduced graphene oxide aerogel (Vo-HCo3O4@SRGA) for electrocatalytic low-concentration NO3− reduction. Finite element simulation demonstrates that the nanoconfined SRGA significantly increases the local concentration of NO3−, thereby accelerating the reaction kinetics. Moreover, the Vo is able to disrupt the local structural symmetry of Co–O–Co sites. The asymmetric active site (Vo) can simultaneously enhance NO3− adsorption, promote water dissociation, and inhibit hydrogen evolution. Thanks to the triple synergistic modulation of Vo and the nanoconfined effect of SRGA, Vo-HCo3O4@SRGA exhibits unprecedented activity (NH3–N yield rate: 1.53 mg h−1 cm−2; NH3–N Faraday efficiency: 96.5%) superior to most of the reported advanced electrocatalysts under low-concentration NO3− conditions. This work cleverly combines macroscopic modification with microscopic fine tuning of catalysts, which is expected to open up new opportunities in the direction of pollutant resourcing.
Green foundation1. This work provides an efficient solution for low-concentration nitrate reduction and ammonia synthesis under ambient conditions. By utilizing oxygen vacancy-rich hollow Co3O4 nanoparticles anchored on waste spirulina residue-derived graphene oxide aerogel (Vo-HCo3O4@SRGA), the combined effects of oxygen vacancies and confinement accelerate reaction kinetics, offering an energy-efficient solution for wastewater treatment and resource recovery. Importantly, the preparation process uses only deionized water instead of toxic organic solvents, making it more environmentally friendly.2. The catalyst achieves an NH3–N yield rate of 1.53 mg h−1 cm−2 and a Faraday efficiency of 96.5%, outperforming many previously reported electrocatalysts. 3. Future research will focus on replacing traditional power sources with solar panels, using renewable energy to create a zero-carbon system for sustainable nitrogen management. |
1. Introduction
Nitrate (NO3−) has been widely present in industrial wastewater and groundwater in recent years as a result of industrial emissions and agricultural fertilization, and its excessive emission and accumulation can disrupt ecological balance and the global nitrogen cycle.1–3 The electrocatalytic nitrate reduction reaction (eNO3−-RR) enables the conversion of aqueous NO3− pollutants into value-added ammonia (NH3) using renewable electricity.4–7 This approach represents a sustainable win–win strategy for environmental remediation and chemical production. The main research direction of the eNO3−-RR at present focuses on the exploration of the ideal conditions of high alkaline electrolyte and high NO3− concentration.8–11 Unfortunately, it is almost impossible to find ideal scenarios with both high alkalinity and high NO3− concentration in authentic polluted waters, greatly blocking the feasibility of eNO3−-RR technology in practical applications.12 Noteworthily, the majority of authentic contaminated water bodies around the globe are typically at low NO3− concentration levels (≤10 mM) with near-neutral pH values.13 From a practical perspective, directly reducing the low concentration of NO3− also helps to avoid high energy consumption that is typically associated with separating and concentrating NO3−. However, the slow reaction kinetics is caused by the decrease in the number of reactant molecules and the reduced utilization of active sites at low NO3− concentrations. Furthermore, the competing hydrogen evolution reaction (HER) severely limits efficient NO3− conversion. Consequently, the development of suitable catalysts to overcome the aforementioned bottlenecks and enhance the NH3 yield rate and selectivity is a critical issue that requires immediate attention.Inspired by the nanoconfined effect in natural biological systems (i.e., the cytoskeleton), a remarkable increase in reaction speed and specificity for efficient chemistry is expected to be achieved via constructing artificial microscopic nanoconfined spaces.14–16 Once reactants enter the nanoconfined space, reduced diffusion freedom significantly increases local concentration, thus accelerating the reaction kinetics.17–19 For example, the nanoconfined effect through graphene aerogel can effectively enrich the reducing intermediates and promote the reduction of Fe(III), resulting in a 208-fold enhancement in the phenol degradation rate constant compared with a system without nanoconfinement.20 Therefore, we envisage that the unavoidable slow kinetics at low NO3− concentrations can be expected to be solved by rationally designing an electrocatalytic system with nanoconfined effect. However, to the best of our knowledge, the application of the graphene aerogel-triggered nanoconfined effect in the eNO3−-RR has yet to be reported and validated.
In addition, the low catalytic activity due to the competitive HER cannot be ignored. An effective way to inhibit the HER is to regulate the adsorption and binding sites of the hydrogen proton (*H) on the surface of the catalysts. Usually, the asymmetric defect site (Vo) causes uneven spatial distribution of active sites, in turn leading to spatial mismatch between the adsorption and binding sites of *H. This geometric constraint further prevents the proximity and recombination of two *H intermediates into hydrogen.21 Moreover, this spatial mismatch likewise raises the reactive energy barrier for hydrogen generation, thereby effectively inhibiting the HER.22,23 In contrast, when *H is adsorbed on active sites with a symmetric structure, the symmetric structure is capable of effectively lowering the reactive energy barrier for the HER through a uniformly distributed electron cloud. Proverbially, the eNO3−-RR requires a large amount of *H to participate in the reaction, and these *H originate from the dissociation of water molecules. The asymmetry of Vo leads to a distortion of the geometrical configuration of the water molecule via altering the local electronic environment and creating an asymmetric electric field. This effect weakens the O–H bond energy and enhances H2O dissociation probability.24,25 This asymmetric electric field also exerts a force on NO3−, leading to polarity induction and thus enhancing the adsorption process. Here, we uncovered a principal fact that Vo can enhance NO3− adsorption, promote water dissociation, and inhibit the HER. Nevertheless, the current literature lacks sufficient information to support Vo-triggered triple synergistic modulation. Furthermore, it has also not been verified whether there is a synergistic co-operation between the nanoconfined effect and Vo.
Herein, we anchored Vo-HCo3O4 on the surface of the nanoconfined SRGA to address the kinetic limitations and competing HER issues for the electrocatalytic reduction of low concentrations of NO3− to NH3 under neutral conditions. Notably, the nanoconfined effect generated by the porous skeleton of SRGA significantly enhanced the local NO3− concentration, in turn effectively breaking through the slow bottleneck in the kinetic process. Then, the introduced Vo as an asymmetric active site induced a triple synergistic modulation: enhancement of NO3− adsorption, promotion of water dissociation and inhibition of the HER. Thanks to the synergistic co-operation of the above efficacies, the Vo-HCo3O4@SRGA exhibits remarkable catalytic activity for the conversion of low-concentration NO3− into NH3.
2. Experimental section
2.1. Chemicals
All chemical reagents are described in Text S1.†2.2. Catalyst preparation
2.3. Preparation of working electrodes of H-Co3O4
First, 21.5 mg of H-Co3O4 and 20 μL of 5 wt% Nafion solution were dispersed in a mixture of 655 μL of ethanol and 325 μL of deionized water via 60 min ultrasonication to prepare a homogeneous ink. Subsequently, 80 μL of the resulting ink was uniformly coated onto carbon paper (CP, 1 × 1 cm2) and air-dried at room temperature to complete the electrode fabrication.2.4. Characterization
More details on the characterization methods are provided in the ESI (Text S2).†2.5. Electrochemical measurements
The electrochemical measurements were conducted using a CHI 760e electrochemical workstation (Chenhua, Shanghai) in an H-type electrolytic cell. The as-prepared materials, Ag/AgCl electrode, and platinum foil were used as the working electrode, reference electrode, and counter electrode, respectively. The geometric area of the working electrode was set as 1 cm2. 0.1 M Na2SO4 solution (70 mL) was evenly distributed within the cathode and anode chambers, and NaNO3 was added into the cathode compartment containing 140 mg L−1 NO3−-N. All potentials were recorded against a reversible hydrogen electrode (RHE):| ERHE = EAg/AgCl + 0.197 V + 0.059 V × pH |
Prior to the NO3− electroreduction test, the electrolyte was purged with high-purity Ar for 30 min. The scan rates for linear sweep voltammetry (LSV) tests and Tafel slope tests were maintained at 10 mV s−1. Cyclic voltammetry (CV) curves acquired during electrochemical double-layer capacitance (Cdl) determinations were measured in a potential window nearly without faradaic processes at different scan rates of 20, 40, 60, 80, and 100 mV s−1. The plot of current density at a given potential against scan rate has a linear relationship, and its slope is Cdl. Electrochemical impedance spectra (EIS) were measured in a frequency domain ranging from 10 kHz to 0.1 Hz with a 5 mV perturbation. The chronoamperometry tests were conducted at a series of applied potentials.
2.6. Analytical methods
Detailed information on the determination of nitrogen species concentrations and the calculation of eNO3−-RR parameters is provided in Text S3 and Text S4,† respectively. Detailed steps of in situ Fourier transformed infrared (FT-IR) spectroscopy and online differential electrochemical mass spectrometry (DEMS) measurements are described in Text S5 and Text S6,† respectively. Detailed information on finite element method (FEM) simulations and density functional theory (DFT) calculations are presented in Text S7 and Text S8,† respectively.3. Results and discussion
3.1. Catalyst characterization
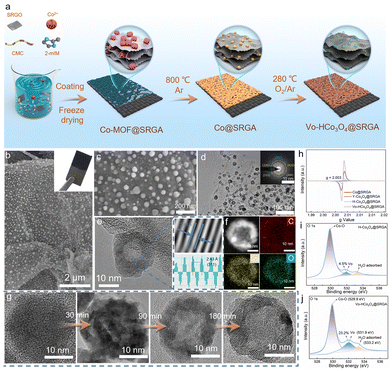
SRGO was prepared according to our previously reported work (Fig. S1†). The preparation procedure for in situ growth of Vo-HCo3O4@SRGA on carbon paper is shown in Fig. 1a. First, the Co-MOF@SRGO hydrogel was uniformly coated on the surface of carbon paper, and then freeze-dried to obtain Co-MOF@SRGA (Fig. S2†). Subsequently, the obtained Co-MOF@SRGA was subjected to argon pyrolysis (Co@SRGA) and slow oxidation in air to obtain Vo-HCo3O4@SRGA. In addition, we optimized the loading amounts of Vo-HCo3O4@SRGA on the carbon paper (Fig. S3 and S4†). Scanning electron microscopy (SEM) images clearly demonstrate that Vo-HCo3O4 nanoparticles present a highly dispersed distribution in the SRGA backbone without significant agglomeration (Fig. 1b and c and Fig. S5a†). In contrast, pure Vo-HCo3O4 undergoes significant sintering under the same conditions (Fig. S5b†), suggesting that the three-dimensional porous network of SRGA limits the migration of Vo-HCo3O4 particles, thus enhancing the structural stability of Vo-HCo3O4@SRGA. Transmission electron microscopy (TEM) images reveal that Vo-HCo3O4 is discretely embedded on the surface of SRGA in the form of hollow nanoparticles with a diameter of approximately 20 nm (Fig. 1d). High-resolution TEM (HRTEM) images show that Vo-HCo3O4 exhibited well-defined lattice diffraction fringes with a lattice spacing of 2.43 Å, consistent with the (311) plane of Co3O4 (Fig. 1e). Additionally, Vo-HCo3O4 contains uniformly distributed elements of Co and O, with the C element dispersed throughout the entire Vo-HCo3O4 structure (Fig. 1f). In order to reveal the evolution of the hollow structure of Vo-HCo3O4, Co@SRGA formed by pyrolysis under an argon atmosphere was oxidized in air for different times. As shown in Fig. 1g, Co@SRGA underwent an evolutionary process from solid to yolk–shell to a completely hollow structure. This interesting structural evolution process can be explained via the Kirkendall effect.26 Similarly, solid commercial Co nanoparticles were able to form hollow structures via the Kirkendall effect (Fig. S6†).X-ray diffraction (XRD) and Raman images further confirmed the formation of Co@SRGA consisting of a pure metallic Co phase, Y-Co3O4@SRGA consisting of a mixed phase of metallic Co and Co3O4, and Vo-HCo3O4@SRGA and H-Co3O4@SRGA consisting of a pure Co3O4 phase (Fig. S7†). In addition to the formation of hollow structures, the Kirkendall effect can produce structural disequilibria in metal oxides through the difference in diffusion rates between the metal and the oxygen, thus contributing to the generation of Vo.27 Electron paramagnetic resonance (EPR) techniques were applied to probe the presence of Vo in catalysts. Vo-HCo3O4@SRGA exhibited the highest EPR signal intensity, suggesting that the concentration of Vo could be effectively controlled by adjusting the oxidation times (Fig. 1h). The high-resolution X-ray photoelectron spectroscopy (XPS) of O 1s demonstrated that Vo-HCo3O4@SRGA (90 min) can reach the highest Vo content of 23.2%, which was higher than that for H-Co3O4@SRGA (180 min) of 4.5% and Y-Co3O4@SRGA (30 min) of 13.1% (Fig. 1i and j and Fig. S8a†). These results demonstrate that the controlled adjustment of oxidation time enables precise construction of different Vo concentrations in the catalysts. Meanwhile, according to the fine-scanned Co 2p XPS spectra, similarly high atomic ratios of Co2+/Co3+ can be observed in Y-Co3O4@SRGA (1.16) and Vo-HCo3O4@SRGA (1.34), which are ascribed to the incompletely oxidized conditions, being consistent with the large number of Vo (Fig. S8b–d†). In sharp contrast, the atomic ratio of Co2+/Co3+ in H-Co3O4@SRGA decreases to 1.05 because of the oxidation of Co2+ to Co3+, leading to the decrease of Vo. The above analyses are consistent with the EPR results, indicating that VoHCo3O4@SRGA indeed has the highest concentration of Vo. In addition, the Vo content of H-Co3O4 (5.1%) was not significantly different from that of H-Co3O4@SRGA, indicating that the introduction of SRGA did not affect the Vo content (Fig. S9†). The photoluminescence (PL) intensity of Vo-HCo3O4@SRGA is significantly lower than that of HCo3O4@SRGA. This suppression of radiative recombination is attributed to the presence of oxygen vacancies, which serve as electron-trapping centres and effectively inhibit the recombination of electron–hole pairs (Fig. S10†). As shown in Fig. S11,† the work function of Vo-HCo3O4@SRGA was significantly lower than that of H-Co3O4@SRGA, implying that the introduction of Vo can reduce the work function of the catalysts, thus enhancing the conductivity and reaction activity.28 The introduction of SRGA greatly extended the specific surface area of H-Co3O4@SRGA and Vo-HCo3O4@SRGA compared with H-Co3O4, providing theoretical support for the enrichment of NO3− through the nanoconfined effect (Fig. S12 and Table S2†). In addition, we measured the adsorption capacity of the three catalysts to elucidate the nanoconfined effect of the SRGA framework on NO3−. As shown in Fig. S13 and Table S3,† compared with the correlation coefficient values R2 of the two kinetic models, the pseudo-second-order kinetic model was closer to the experimental data than the pseudo-first-order kinetic model, suggesting that the adsorption process of NO3− by the three materials was consistent with the pseudo-second-order kinetic hypothesis. The adsorption capacity and kinetic constants of H-Co3O4@SRGA were 2.4 times and 1.9 times those of H-Co3O4, respectively. Therefore, SRGA showed an obvious nanoconfined effect on NO3−, increased the local concentration of NO3− on the catalyst surface, and promoted the reaction kinetics of the electric reduction of NO3− to NH3.
3.2. Electrocatalytic performance for the NO3−-RR
To evaluate the impact of Vo and the nanoconfined effect of SRGA on the eNO3−-RR performance, LSV tests were initially conducted in an H-type electrolytic cell. NO3−, NO2−, and the product NH3 were detected using an ultraviolet–visible (UV–vis) spectrophotometer (Fig. S14–S16†). The current density of Vo-HCo3O4@SRGA with the highest Vo concentration in the NO3− electrolyte was greater than that of H-Co3O4@SRGA, Co@SRGA, and Y-Co3O4@SRGA, indicating that Vo can effectively enhance the eNO3−-RR process (Fig. 2a). Additionally, the current density of H-Co3O4 was found to be significantly lower than that of H-Co3O4@SRGA, leading to the inference that SRGA with a nanoconfined effect can also contribute to the eNO3−-RR process. Notably, Vo-HCo3O4@SRGA also exhibited the smallest Tafel slope, indicating optimal reaction kinetics (Fig. S17a†). Subsequently, the NH3-N yield rate of the catalysts was examined at different operating voltages, with Vo-HCo3O4@SRGA demonstrating the highest NH3-N yield rate at all the corresponding voltages (Fig. 2b). The FE of Vo-HCo3O4@SRGA exhibited a volcano pattern with decreasing potential (Fig. 2c), indicating a certain starting potential is required for NO3− reduction to provide the driving force for the reaction. The subsequent FE decline at more negative potentials originates from the competing HER.29,30 The FE of Vo-HCo3O4@SRGA was the highest at −0.8 V vs. RHE (96.5%), corresponding to an NH3-N yield rate of 1.53 mg h−1 cm−2. CV curves at different scan rates were analysed in a non-FE potential window (Fig. S17b–f†). The corresponding Cdl values are shown in Fig. 2d, in which the Cdl value of Vo-HCo3O4@SRGA is larger than that of Y-Co3O4@SRGA, H-Co3O4@SRGA and Co@SRGA. Since the electrochemically active surface area is proportional to the value of Cdl, indicating that Vo increases the electrochemically active surface area, more active sites to facilitate the eNO3−-RR process are exposed. Furthermore, the Cdl value of H-Co3O4 was only 1.91 mF cm−2 in comparison with H-Co3O4@SRGA, indicating that the nanoconfined effect of SRGA also facilitates enhancing the amount of active sites. Vo-HCo3O4@SRGA exhibited the smallest Nyquist circle diameter, implying that Vo promotes the electron transfer rate in the eNO3−-RR process (Fig. S18†). Further comparison of H-Co3O4 and H-Co3O4@SRGA revealed that SRGA also accelerates the electron transfer rate to some extent. The product NH3-N was verified to be derived from NO3−-N by designing blank control experiments with bare carbon paper, an open circuit potential, and no NO3− addition. The negligible NH3-N yield rate under the conditions of the above blank control group experiments verified that the N in NH3 was derived from NO3−-N and not from other external contamination (Fig. S19†). The nitrogen changes and reaction processes were traced via1H-nuclear magnetic resonance (1H-NMR). The 1H-NMR peaks of 15NH4+ and 14NH4+ presented double and triple peaks when 15NO3− and 14NO3− were used as the N sources, further confirming that the NH3-N does indeed originate from NO3−-N rather than from external sources of pollution (Fig. 2e).31 Before advancing towards practical applications, the stability of the catalysts must be addressed. Fig. 2f illustrates the current versus time curves of Vo-HCo3O4@SRGA at different operating voltages, and the negligible current density decay indicates the appreciable stability of Vo-HCo3O4@SRGA. During 24 consecutive cycles, only the first three cycles revealed a reduced NH3-N yield rate and FE decay, before remaining largely stable (Fig. 2g). The crystal structure and microscopic morphology showed no obvious change before and after the reaction (Fig. S20†), further illustrating the stability of the structure of Vo-HCo3O4@SRGA during the eNO3−-RR process. To investigate the suitability of Vo-HCo3O4@SRGA for different water qualities, the effects of different inorganic anions, inorganic cations and HA on NH3-N yield rate and FE were also investigated (Fig. S21†). Only a high concentration of Mg2+ (10 mM) inhibited the NH3-N yield rate and FE, with no significant effect of other anions or even high concentrations of HA. In addition, Vo-HCo3O4@SRGA exhibited excellent activity in the pH range of 3–11 and NO3−-N of 50–500 mg L−1 (Fig. S22†). All these results indicated the potential applicability of Vo-HCo3O4@SRGA under real water conditions. Although the catalytic performance of Vo-HCo3O4@SRGA is greatly enhanced by defect and nanoconfined engineering, the difference in performance between Vo-HCo3O4@SRGA and other current state-of-the-art catalytic materials is unknown. Therefore, we summarized the performance of some advanced electrocatalytic systems reported in the literature at low concentrations of NO3− (Fig. 2h and Table S4†). Among these, Vo-HCo3O4@SRGA demonstrated the highest NH3-N yield rate and FE, highlighting its superior performance as an eNO3−-RR electrocatalyst.3.3. FEM and DFT investigation
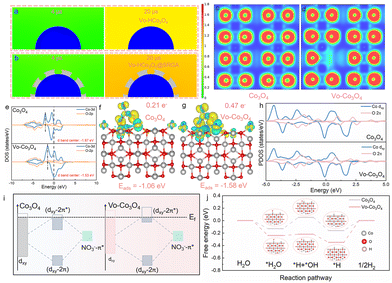
The nanoconfined effect of SRGA on NO3− was successfully verified by finite element method (FEM) simulations (Fig. 3a and b and Fig. S23†). Compared with pure Vo-HCo3O4, SRGA@Vo-HCo3O4 was able to significantly enhance the localized concentration of NO3− due to the nanoconfined effect, thus greatly increasing the accessibility of NO3−. This nanoconfined effect contributes to the enhancement of NO3− activity in the eNO3−-RR via enriching the localized concentration of NO3−, which further facilitates its conversion process to NH3. Electron localization function (ELF) calculations visualize that Vo formation induces a redistribution of the otherwise uniformly distributed electron density. This redistribution disrupts the original symmetry, generating a localized asymmetric electronic environment (Fig. 3c and d).32Moreover, the Vo induced inhomogeneity in the Co–O bond length, further reflecting the disruption of the symmetry of the geometry (Fig. S24†). The location of the d-band center is an important theoretical basis for understanding the interaction between the catalyst surface and the reactants.33,34 Vo raised the d-band center position of Co3O4 to −1.53 eV, leading to stronger electron exchange and NO3− adsorption (Fig. 3e). Further exploration is needed to elucidate the interaction between NO3− and the asymmetric defect sites. To investigate this, the adsorption configurations on the surfaces of Co3O4 and Vo-Co3O4 were constructed and analyzed (Fig. 3f and g). The adsorption energy (Eads) of NO3− on Vo-Co3O4 (−1.58 eV) was more negative than that of Co3O4 (−1.06 eV), and the charge transfer number between NO3− and Vo-Co3O4 (0.47 e−) was higher compared to Co3O4 (0.21 e−). These results demonstrated that Vo can strengthen NO3− adsorption and facilitate the electron transfer process.35 The dense energy band structure of Vo-Co3O4 implies a higher density of electronic states compared with Co3O4, contributing to a more efficient electron transfer with NO3− (Fig. S25†).36 Accordingly, the interaction between adsorbed NO3− and the metal-centered orbitals of Vo-Co3O4 was further explored. The dxy orbital of Co and the 2π orbitals of O in NO3− manifested a significant overlap in the partial density of states (PDOS) (Fig. 3h and Fig. S26†). This strong interaction triggers the splitting of the bonding and antibonding states. As the d-band center of Vo-Co3O4 was close to the Fermi energy level, the dxy–2π* energy levels of bonding and antibonding were subsequently elevated (* represents the antibonding energy level) (Fig. 3i). In this case, the electron occupation of the antibonding state in the NO3−/Vo-Co3O4 system decreased, enhancing the interaction between NO3− and Vo-Co3O4.37,38Fig. 3j and Fig. S27 and S28† show the Gibbs free energy change (ΔG) plots of Co3O4 and Vo-Co3O4 in the electrocatalytic HER and the optimized adsorption models of the corresponding intermediates. Vo drastically reduced the dissociation energy barrier of water molecules (0.22 eV to 0.05 eV), promoting easier dissociation of water molecules into H+ to provide the necessary intermediates for the reaction. In addition, the reaction energy barrier of *H → H2 for Vo-Co3O4 (0.35 eV) was significantly higher than that for Co3O4 (0.16 eV), indicating that Vo significantly inhibits the HER side reaction. In summary, the triple synergistic effect (enhanced NO3− adsorption, promoted H2O dissociation, and effectively inhibited HER) of the Vo effectively ensures the high activity and selectivity of Vo-Co3O4 in the eNO3−-RR process.
3.4. Reaction mechanisms and pathway analysis
Two main mechanisms are commonly thought to drive the electrochemical reduction of NO3− to NH3: direct electron transfer and bonding with H+, and indirect reduction facilitated by H*. The verification of whether H* plays a dominant role in the eNO3−-RR was done by adding various concentrations of tert-butanol (TBA) as a scavenger. Even with the addition of excess TBA, there was no significant change in NO3− removal, thus ruling out the H*-mediated pathway (Fig. 4a). Therefore, the main mechanism of Vo-HCo3O4@SRGA in the eNO3−-RR can be identified as direct electron transfer (Fig. 4b). The intermediate products adsorbed on the electrode surface were visualized using in situ FT-IR spectroscopy (Fig. 4c). There was no significant difference in the type of absorption peaks of H-Co3O4, H-Co3O4@SRGA, and Vo-HCo3O4@SRGA, suggesting that the presence of SRGA and Vo does not alter the reaction intermediate products (Fig. 4d–f). The broad bands observed at 1650 cm−1 and 3300 cm−1 are the O–H bending vibrations of water adsorption (δH–O–H) and cleavage (νH–O–H),39,40 while the bands located at around 1458 cm−1 and 1690 cm−1 belong to N–H and N–O stretching vibrations, confirming the formation of NH3 during the eNO3−-RR process.41,42 Compared with H-Co3O4@SRGA, Vo-HCo3O4@SRGA showed a more pronounced signal intensity change with H2O and related intermediates at more negative potentials. This result suggests that Vo can facilitate H2O dissociation and NH3 synthesis. Additionally, online DEMS further clarified that Vo-HCo3O4@SRGA generates intermediate products during the eNO3−-RR (Fig. 4g). The strongest m/z signal was 17, indicating that Vo-HCo3O4@SRGA is able to efficiently electrocatalyze the synthesis of NH3. Several less intense m/z signals, such as 14, 15, 16, and 30, which correspond to *N, *NH, *NH2, and *NO intermediates, confirm the occurrence of the *N hydrogenation pathway. Combined with the above detected intermediates and previous reports, the reaction pathways can be deduced and used for theoretical calculations (Fig. S29†). Fig. 4h shows the ΔG diagrams of Co3O4 and Vo-Co3O4 in the eNO3−-RR. For both Co3O4 and Vo-Co3O4, NH3 was synthesized by an 8-electron reduction of NO3−via the pathway of NO3− → *NO3 → *NO2 → *NO → *NOH → *N → *NH → *NH2 → *NH3 → NH3 (Fig. S30 and S31†).43,44 During the whole reaction process, there are two steps with positive ΔG values, which are the *NO hydrogenation (*NO → *NOH) and the desorption of *NH3 intermediates from the catalyst surface (*NH3 → NH3). Because of the high solubility of NH3 in aqueous solution and the low reaction energy barrier, NH3 can be readily desorbed from the surface of Co atoms, releasing additional reaction sites.45,46 Therefore, the rate-determining step (RDS) of the eNO3−-RR can be considered as *NO → *NOH. Obviously, Vo significantly reduced the reactive energy barrier of the RDS from 0.66 eV to 0.26 eV, improving the efficiency and selectivity of the eNO3−-RR.4. Conclusions
In summary, we successfully anchored Vo-HCo3O4 obtained via the Kirkendall effect to the surface of SRGA. The porous skeleton of SRGA can improve the accessibility of the active sites. It also significantly increases the local NO3− concentration due to a unique nanoconfined effect, thereby accelerating the reaction kinetics. In situ FT-IR spectroscopy and DFT calculations demonstrate that Vo induced a triple synergistic modulation: enhancement of NO3− adsorption, promotion of water dissociation, and inhibition of the HER. Furthermore, the introduction of Vo raised the bonding and antibonding energy levels of dxy–2π* in NO3−/Vo-Co3O4 and lowered the reaction energy barrier of RDS (*NO → *NOH), facilitating the adsorption and activation of NO3−. As a result, Vo-HCo3O4@SRGA exhibited a considerable NH3-N yield rate (1.53 mg h−1 cm−2), Faraday efficiency (96.5%), and selectivity (97.2%) in the conversion of low-concentration NO3− to NH3. This work highlights the design principles of advanced electrocatalysts for low-concentration NO3− conversion, providing solutions for resource recovery of NO3−-containing wastewater. We believe that this creative work has the opportunity to be extended to other electrocatalytic reactions in the aqueous phase, in addition to being generous in the eNO3−-RR.Author contributions
Ke Wang and Tong Zhao: data curation and writing – original draft. Shiyu Zhang: investigation. Rupeng Wang: formal analysis. Meng Wang: writing – review & editing. Zixiang He: supervision. Shih-Hsin Ho: funding acquisition.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 52070057).References
- D. R. Kanter, O. Chodos, O. Nordland, M. Rutigliano and W. Winiwarter, Nat. Sustainability, 2020, 3, 956–963 CrossRef.
- B. Zhang, Z. Dai, Y. Chen, M. Cheng, H. Zhang, P. Feng, B. Ke, Y. Zhang and G. Zhang, Nat. Commun., 2024, 15, 2816 CrossRef CAS PubMed.
- J. Sun, S. Garg, J. Xie, C. Zhang and T. D. Waite, Environ. Sci. Technol., 2022, 56, 17298–17309 CrossRef CAS PubMed.
- F.-Y. Chen, Z.-Y. Wu, S. Gupta, D. J. Rivera, S. V. Lambeets, S. Pecaut, J. Y. T. Kim, P. Zhu, Y. Z. Finfrock, D. M. Meira, G. King, G. Gao, W. Xu, D. A. Cullen, H. Zhou, Y. Han, D. E. Perea, C. L. Muhich and H. Wang, Nat. Nanotechnol., 2022, 17, 759–767 CrossRef CAS PubMed.
- S. Han, H. Li, T. Li, F. Chen, R. Yang, Y. Yu and B. Zhang, Nat. Catal., 2023, 6, 402–414 CrossRef CAS.
- X. Chen, Y. Cheng, B. Zhang, J. Zhou and S. He, Nat. Commun., 2024, 15, 6278 CrossRef CAS PubMed.
- B. Zhou, Y. Tong, Y. Yao, W. Zhang, G. Zhan, Q. Zheng, W. Hou, X.-K. Gu and L. Zhang, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2405236121 CrossRef PubMed.
- W. Liao, J. Wang, G. Ni, K. Liu, C. Liu, S. Chen, Q. Wang, Y. Chen, T. Luo, X. Wang, Y. Wang, W. Li, T.-S. Chan, C. Ma, H. Li, Y. Liang, W. Liu, J. Fu, B. Xi and M. Liu, Nat. Commun., 2024, 15, 1264 CrossRef CAS PubMed.
- J. Suh, H. Choi, Y. Kong and J. Oh, Adv. Sci., 2024, 11, 2407250 CrossRef CAS PubMed.
- J.-J. Zhang, Y.-Y. Lou, Z. Wu, X. J. Huang and S.-G. Sun, J. Am. Chem. Soc., 2024, 146, 24966–24977 CrossRef CAS PubMed.
- N. C. Kani, N. H. L. Nguyen, K. Markel, R. R. Bhawnani, B. Shindel, K. Sharma, S. Kim, V. P. Dravid, V. Berry, J. A. Gauthier and M. R. Singh, Adv. Energy Mater., 2023, 13, 2204236 CrossRef CAS.
- K.-H. Kim, H. Lee, X. Huang, J. H. Choi, C. Chen, J. K. Kang and D. O'Hare, Energy Environ. Sci., 2023, 16, 663–672 RSC.
- P. H. van Langevelde, I. Katsounaros and M. T. M. Koper, Joule, 2021, 5, 290–294 CrossRef.
- Y. Chen, M. Zuo, Y. Chen, P. Yu, X. Chen, X. Zhang, W. Yuan, Y. Wu, W. Zhu and Y. Zhao, Nat. Commun., 2023, 14, 5229 CrossRef CAS PubMed.
- Y. Huang, J. Ren and X. Qu, Chem. Rev., 2019, 119, 4357–4412 CrossRef CAS PubMed.
- J. Zhang, Z. Wang, X. Lin, X. Gao, Q. Wang, R. Huang, Y. Ruan, H. Xu, L. Tian, C. Ling, R. Shi, S. Xu, K. Chen and Y. Wu, Angew. Chem., Int. Ed., 2025, 64, e202416686 CrossRef CAS PubMed.
- W. Qu, M. Luo, Z. Tang, T. Zhong, H. Zhao, L. Hu, D. Xia, S. Tian, D. Shu and C. He, Environ. Sci. Technol., 2023, 57, 13205–13216 CrossRef CAS PubMed.
- S. Zhang, M. Sun, T. Hedtke, A. Deshmukh, X. Zhou, S. Weon, M. Elimelech and J.-H. Kim, Environ. Sci. Technol., 2020, 54, 10868–10875 CrossRef CAS PubMed.
- Q. V. Ly, L. Cui, M. B. Asif, W. Khan, L. D. Nghiem, Y. Hwang and Z. Zhang, Water Res., 2023, 230, 119577 CrossRef CAS PubMed.
- X. Zhang, J. Tang, L. Wang, C. Wang, L. Chen, X. Chen, J. Qian and B. Pan, Nat. Commun., 2024, 15, 917 CrossRef CAS PubMed.
- L. Wu, Z. Wang, J. Liu, C. Liu, X. Li, Y. Zhang, W. Wang, J. Ma and Z. Sun, Appl. Catal., B, 2024, 343, 123526 CrossRef CAS.
- Q. Cheng, M. Huang, L. Xiao, S. Mou, X. Zhao, Y. Xie, G. Jiang, X. Jiang and F. Dong, ACS Catal., 2023, 13, 4021–4029 CrossRef CAS.
- W. Zhong, Z. Gong, Z. He, N. Zhang, X. Kang, X. Mao and Y. Chen, J. Energy Chem., 2023, 78, 211–221 CrossRef CAS.
- X. Chen, J. Chen, H. Chen, Q. Zhang, J. Li, J. Cui, Y. Sun, D. Wang, J. Ye and L. Liu, Nat. Commun., 2023, 14, 751 CrossRef CAS PubMed.
- Z. Wei, M. Zhao, Z. Yang, X. Duan, G. Jiang, G. Li, F. Zhang and Z. Hao, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2217148120 CrossRef CAS PubMed.
- J. Zhu, W. Tu, H. Pan, H. Zhang, B. Liu, Y. Cheng, Z. Deng and H. Zhang, ACS Nano, 2020, 14, 5780–5787 CrossRef CAS PubMed.
- D. Ji, L. Fan, L. Tao, Y. Sun, M. Li, G. Yang, T. Q. Tran, S. Ramakrishna and S. Guo, Angew. Chem., Int. Ed., 2019, 58, 13840–13844 CrossRef CAS PubMed.
- Y. Zhang, Y. Lin, T. Duan and L. Song, Mater. Today, 2021, 48, 115–134 CrossRef CAS.
- K. Chu, W. Zong, G. Xue, H. Guo, J. Qin, H. Zhu, N. Zhang, Z. Tian, H. Dong, Y.-E. Miao, M. B. J. Roeffaers, J. Hofkens, F. Lai and T. Liu, J. Am. Chem. Soc., 2023, 145, 21387–21396 CrossRef CAS PubMed.
- Y. Xiong, Y. Wang, C. C. Tsang, J. Zhou, F. Hao, F. Liu, J. Wang, S. Xi, J. Zhao and Z. Fan, Environ. Sci. Technol., 2024, 58, 10863–10873 CrossRef CAS PubMed.
- Y. Wang, H. Li, W. Zhou, X. Zhang, B. Zhang and Y. Yu, Angew. Chem., Int. Ed., 2022, 61, e202202604 CrossRef CAS PubMed.
- W. Lyu, Y. Liu, J. Zhou, D. Chen, X. Zhao, R. Fang, F. Wang and Y. Li, Angew. Chem., Int. Ed., 2023, 62, e202310733 CrossRef CAS PubMed.
- S. Zhang, R. Wang, K. Wang, M. Wang, Z. He, H. Chen and S.-H. Ho, Environ. Sci. Technol., 2024, 58, 1921–1933 CrossRef CAS PubMed.
- L. Li, X. Zhang, M. Humayun, X. Xu, Z. Shang, Z. Li, M. F. Yuen, C. Hong, Z. Chen, J. Zeng, M. Bououdina, K. Temst, X. Wang and C. Wang, ACS Nano, 2024, 18, 1214–1225 CrossRef CAS PubMed.
- K. Wang, R. Mao, R. Liu, J. Zhang, H. Zhao, W. Ran and X. Zhao, Nat. Water, 2023, 1, 1068–1078 CrossRef CAS.
- H. Du, H. Guo, K. Wang, X. Du, B. A. Beshiwork, S. Sun, Y. Luo, Q. Liu, T. Li and X. Sun, Angew. Chem., 2023, 135, e202215782 CrossRef.
- S. Yang, X. Guo, X. Li, T. Wu, L. Zou, Z. He, Q. Xu, J. Zheng, L. Chen, Q. Wang and Z. J. Xu, Angew. Chem., Int. Ed., 2024, 63, e202317957 CrossRef CAS PubMed.
- G. Qian, W. Lyu, X. Zhao, J. Zhou, R. Fang, F. Wang and Y. Li, Angew. Chem., Int. Ed., 2022, 61, e202210576 CrossRef CAS PubMed.
- M. Xie, G. Zhu, H. Yang, B. Liu, M. Li, C. Qi, L. Wang, W. Jiang, P. Qiu and W. Luo, Adv. Energy Mater., 2024, 14, 2401717 CrossRef CAS.
- J. Li, H. Li, K. Fan, J. Y. Lee, W. Xie and M. Shao, Chem. Catal., 2023, 3, 100638 CrossRef CAS.
- Y. Huang, C. He, C. Cheng, S. Han, M. He, Y. Wang, N. Meng, B. Zhang, Q. Lu and Y. Yu, Nat. Commun., 2023, 14, 7368 CrossRef CAS PubMed.
- J. Ni, J. Yan, F. Li, H. Qi, Q. Xu, C. Su, L. Sun, H. Sun, J. Ding and B. Liu, Adv. Energy Mater., 2024, 14, 2400065 CrossRef CAS.
- A. Zhu, H. Liu, S. Bu, K. Liu, C. Luan, D. Lin, G. Gan, Y. Zhou, T. Zhang, K. Liu, G. Hong, H. Li and W. Zhang, ACS Nano, 2024, 18, 22344–22355 CrossRef CAS PubMed.
- S. Lu, G. Lin, H. Yan, Y. Li, T. Qi, Y. Li, S. Liang and L. Jiang, ACS Catal., 2024, 14, 14887–14894 CrossRef CAS.
- H. Y. Zhou, Y. B. Qu, J. C. Li, Z. L. Wang, C. C. Yang and Q. Jiang, Appl. Catal., B, 2022, 305, 121023 CrossRef CAS.
- Z. Meng, J.-X. Yao, C.-N. Sun, X. Kang, R. Gao, H.-R. Li, B. Bi, Y.-F. Zhu, J.-M. Yan and Q. Jiang, Adv. Energy Mater., 2022, 12, 2202105 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5gc00830a |
| This journal is © The Royal Society of Chemistry 2025 |