Open Access Article
Open Access ArticleEfficient low-temperature depolymerization of polycarbonate catalyzed by lanthanum β-diketonate complexes†
Yuho
Kinbara
a,
Haruro
Ishitani
*b and
Shū
Kobayashi
 *ab
*ab
aDepartment of Chemistry, School of Science, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. E-mail: shu_kobayashi@chem.s.u-tokyo.ac.jp
bGreen & Sustainable Chemistry Social Cooperation Laboratory, Graduate School of Science, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. E-mail: hishitani@chem.s.u-tolup.ac.jp
First published on 25th March 2025
Abstract
The chemical recycling of polycarbonate is crucial for addressing plastic waste and promoting sustainability. Conventional methods often require high temperatures and harsh conditions, leading to energy consumption and potential degradation of the recovered monomers. In this study, we present a high-yielding depolymerization of BPA-based powdered polycarbonate, prepared through a pretreatment with toluene as the substrate using a series of lanthanum β-diketonate complexes as catalysts. Among the complexes investigated, lanthanum 3,4-dimethoxybenzoylacetonate exhibited the highest catalytic activity. This method enables depolymerization at significantly lower temperatures (e.g., 60 °C) compared to conventional approaches without using co-solvent. Under optimized conditions, we achieved a high depolymerization rate to give monomers in 96% and 93% yield within 8 h. This mild and high-yielding depolymerization process offers a promising approach to sustainable polycarbonate recycling and contributes to the development of a circular economy.
Green foundation1. We have developed a catalytic system that enables the depolymerization of polycarbonate with methanol as a sole solvent into dimethyl carbonate and bisphenol A at 60 °C, achieving over 93% monomer yield. This was achieved using an optimized ligand and a simple pre-treatment method involving only mixing with toluene at room temperature.2. We identified a highly active lanthanum benzoylacetonate complex and conducted a simultaneous analysis of both liquid and solid phases, providing new insights into the reaction mechanism of solidstate polymers. The slurry obtained can be separated by filtration into powdered PC and toluene, allowing the recovered toluene to be reused. 3. This study highlights the strong impact of initial polymer characteristics on depolymerization reactivity. A more detailed investigation into changes in molecular weight distribution during pretreatment is expected to contribute to more advanced plastic recycling technologies. |
Introduction
For thermoplastics like polycarbonate, chemical recycling presents a promising alternative to traditional mechanical recycling, enabling the recovery of monomers and the production of virgin-quality materials.1 However, despite this potential, the proportion of chemical recycling remains surprisingly low.2–4 This limited adoption can be attributed to several factors, including polymer degradation due to environmental exposure, the complexity of mixed plastic waste streams, and the economic challenges associated with sorting, depolymerization, and purification processes.5–8 To achieve sustainable chemical manufacturing and reduce reliance on fossil resources, overcoming the technical challenges associated with chemical recycling is of paramount importance. In particular, reducing the energy requirements of the depolymerization process is a crucial and urgent issue. We focused on polycarbonate (PC), an ester-based polymer relatively amenable to depolymerization reactions (Scheme 1). Polycarbonate is an engineering plastic widely used in applications such as low-refractive-index eyeglass lenses and protective goggles due to its high transparency and impact resistance.9 Various methods for PC depolymerization have been reported, including pyrolysis,10,11 hydrolysis,12–14 alcoholysis,15–20 and aminolysis.21–23 However, pyrolysis requires significant thermal energy and produces a mixture of low-molecular-weight compounds.10,11 While hydrolysis can yield bisphenol A (BPA) monomer with high efficiency, it necessitates reactors capable of withstanding high-pressure steam, which is a disadvantage. Additionally, the generation of carbon dioxide as a by-product is problematic.13,14Transesterification with alcohols, glycols, and amines enables depolymerization under relatively mild conditions. The nucleophile primarily influences this reaction. Among the various depolymerization methods available, this study focuses on methanolysis. Methanolysis simultaneously produces bisphenol A (BPA) and dimethyl carbonate (DMC). The use of DMC as an intermediate in polycarbonate synthesis is attracting attention because it eliminates the need for hazardous phosgene.24,25 This depolymerization process offers advantages beyond raw material recycling, including the high industrial value of DMC. DMC finds wide application in areas such as semiconductor manufacturing, coatings, adhesives, and as an electrolyte in lithium-ion batteries. This underscores the potential of this process to effectively convert waste plastics into high-value products.26–30
Numerous studies on the methanolysis of PC have been conducted over the past decade. Analysis of these studies reveals two main approaches: those using co-solvents and those using methanol as the sole solvent. Co-solvents can lower the depolymerization temperature to below 80 °C,15 while methanol-sole systems typically require temperatures of 100 °C16 or higher, often 120–140 °C.17–20 This trend suggests that PC solubility in the solvent significantly influences reactivity. When considering industrial-scale implementation of chemical recycling, the use of co-solvents should be minimized, and low-energy processes are preferable. From this perspective, achieving high-yielding depolymerization using methanol as the sole solvent, particularly below its boiling point, would be highly significant.
Moreover, to obtain high yields of both BPA and DMC, nearly neutral or mildly acidic conditions, typically achieved using Lewis acids, are required. While many metal Lewis acids are not well-suited for highly Lewis basic conditions, the high oxophilicity and rapid ligand-exchange capability of rare earth compounds might make this possible. Indeed, Hirano et al. have demonstrated the effectiveness of lanthanum acetylacetonate (La(acac)3, 1) in the methanolysis of PET and PC.16,31 Their research suggests the potential of rare earth Lewis acids, and optimizing catalyst structure is considered an effective approach to enable depolymerization at lower temperatures.
In this study, we carefully explored catalysts, especially the structure of ligands, for the methanolysis of PC. We focused particularly on the influence of rare earth 1,3-diketone and ketoester ligands on activity, identifying the most active complex from a total of 20 complexes.32,33 Furthermore, to deepen our understanding of PC methanolysis, we analyzed the molecular weight distribution in the initial stages of the reaction using GPC, investigating the macroscopic reaction process of polymer degradation. These findings provide interesting insights for catalyst and reaction design in the mild depolymerization of polycarbonate.
Results and discussion
As mentioned above, the depolymerization of PC at lower temperatures is highly dependent on the choice of solvent. Our strategy involves converting the polymer into its more reactive state through a pre-treatment process,34 enabling high-yielding depolymerization at lower temperatures and in a shorter reaction time. In this study, we incorporated a dispersion step in toluene as a pre-treatment method. While this pre-treatment step might appear inefficient, it should be noted that if the slurrying of PC in toluene proceeds almost quantitatively at room temperature, the heating required for depolymerization is expected to decrease. Furthermore, as will be discussed later, this method allows for the selective slurrying of PC from plastic products, making it advantageous from a separation and recycling perspective.When PC powder, obtained through toluene treatment, was treated in methanol without any catalyst at 60 °C, only trace amounts of the decomposition products BPA and DMC were detected (entry 1, Table 1). In contrast, the addition of 1 mol% of La(acac)3 (1) significantly accelerated the reaction, yielding BPA and DMC in 36% and 40% yields, respectively (entry 5). Although La(acac)3 is classified as a mild Lewis acid, its effectiveness in ester exchange reactions has been demonstrated not only by Hirano et al.16,28 but also by Neverov and Brown,35,36 making it noteworthy for this type of reaction.
| Entry | Catalyst | Temp. [°C] | Yieldb [%] | |
|---|---|---|---|---|
| BPA | DMC | |||
| a All reactions were performed using 1.02 g of powdered PC prepared through toluene pre-treatment (4 mmol, based on BPA-CO units) and 0.04 mmol of La complexes in 40 mL of methanol at 60 °C (for entries 1 to 7) or at 80 °C (for entries 8 to 10) for 2 h. b Determined by GC analysis. | ||||
| 1 | No | 60 | 3 | 3 |
| 2 | LaCl3 | 60 | 13 | 19 |
| 3 | La(OTf)3 | 60 | 26 | 28 |
| 4 | La(OAc)3 | 60 | 2 | 4 |
| 5 | La(acac)3 (1) | 60 | 36 | 40 |
| 6 | La(OiPr)3 | 60 | 9 | 8 |
| 7 | La(OH)3 | 60 | 3 | 3 |
| 8 | No | 80 | 20 | 20 |
| 9 | La(OTf)3 | 80 | 76 | 78 |
| 10 | La(acac)3 | 80 | 87 | 92 |
Remarkably, it outperformed LaCl3 and La(OTf)3, which were expected to exhibit higher Lewis acidity.28,37 Considering that La(OiPr)3 and La(OH)3, which are predicted to have basic characteristics, showed almost no activity, it is plausible that La-dicarbonyl-type complexes act in a concerted manner as Lewis acids, not only activating the carbonyl group in PC but also generating alkoxides in an alcoholic solvent, methanol. In fact, when La(acac)3 was used, BPA was obtained in an 87% yield at 80 °C (entry 10). This result represents one of the lowest temperature examples of PC depolymerization in methanol reported to date. Nevertheless, aiming to achieve even lower temperature conditions, we focused on optimizing the structure of 1,3-dicarbonyl-type ligands through systematic screening in this study.
To explore the effect of substituents on the 1,3-dicarbonyl scaffold, we prepared nine different diketones illustrated in Fig. 1, all based on the fundamental 1,3-diketone structure, and converted them into lanthanum complexes in basic methanol. Since each complex crystallized in methanol, we utilized the resulting solids as catalysts to investigate their activity in the depolymerization of PC at 60 °C in methanol (Table 2). This study primarily focused on examining the effects of substituent groups at the external carbon positions, using the acetylacetonate 2a complex, 1, as the reference. The results showed that the introduction of an iPr group slightly improved the yield, while bulkier substituents such as the tBu group significantly decreased the yield. This trend is likely due to steric hindrance caused by the bulky substituents near the carbonyl group, which serves as the coordination site for the La atom, making it more difficult to activate the carbonyl group of PC. In contrast, complexes with electron-withdrawing trifluoromethyl or heterocyclic groups (2d–g) did not exhibit a notable effect. However, an improvement in yield was observed with diketone 2h, leading to complex La-2h, which featured benzene rings at both sides of the diketone skeleton. Similarly, complex La-2i also showed positive results, although its performance was comparable to that of the isopropyl-substituted complex La-2b.
| Entry | Ligand | Catalyst | Yieldb [%] | |
|---|---|---|---|---|
| BPA | DMC | |||
| a All reactions were performed using 1.02 g of powdered PC prepared through toluene pre-treatment (4 mmol, based on BPA-CO units) and 0.04 mmol of La complexes in 40 mL of methanol at 60 °C for 2 h. b Determined by GC analysis. | ||||
| 1 | 2a | 1 | 36 | 40 |
| 2 | 2b | La-2b | 46 | 47 |
| 3 | 2c | La-2c | 11 | 11 |
| 4 | 2d | La-2d | 28 | 28 |
| 5 | 2e | La-2e | 13 | 12 |
| 6 | 2f | La-2f | 12 | 13 |
| 7 | 2g | La-2g | 26 | 26 |
| 8 | 2h | La-2h | 69 | 67 |
| 9 | 2i | La-2i | 45 | 45 |
Based on the above studies, it was determined that an ideal catalyst for the depolymerization of PC should have a La atom surrounded by a moderately spacious environment, coupled with electron-rich ligands. The above initial screening identified bis-aryl type ligands as the most effective. However, to prioritize ease of structural modification, we focused on benzoylacetone-type diketone ligands and examined the substituent effects on the aromatic ring of these ligands. Additionally, to evaluate the influence of electron-donating functional groups, we synthesized similar complexes using 1,3-ketoester ligands and assessed their catalytic activities (Fig. 2).
When using benzoylacetone (2i) as a standard, no clear trend was observed upon introducing either electron-withdrawing or electron-donating groups onto the aromatic ring. Notably, the use of ligand 3f resulted in the highest yield among complexes synthesized under identical temperature conditions, slightly surpassing the activity of La-2g. Comparing the benzoylacetoneate derivatives, ligands 4a and 4b exhibited higher yields than their corresponding 2a and 2i-ligated lanthanum. However, the La-4c complex, which was specifically designed to enhance performance, unfortunately resulted in a lower yield (Table 3).
| Entry | Ligand | Catalyst | Yieldb [%] | |
|---|---|---|---|---|
| BPA | DMC | |||
| a All reactions were performed using 1.02 g of powdered PC prepared through toluene pre-treatment (4 mmol, based on BPA-CO units) and 0.04 mmol of La complexes in 40 mL of methanol at 60 °C for 2 h. b Determined by GC analysis. c Yield at 8 h. d The reaction using untreated PC pellet. The methanolysis was conducted at 60 °C for 2 h. | ||||
| 1 | 2i | La-2i | 45 | 45 |
| 2 | 3a | La-3a | 34 | 35 |
| 3 | 3b | La-3b | 19 | 19 |
| 4 | 3c | La-3c | 23 | 24 |
| 5 | 3d | La-3d | 35 | 36 |
| 6 | 3e | La-3e | 37 | 38 |
| 7 | 3f | La-3f | 70(96c)[ndd] | 68 (93c)[ndd] |
| 8 | 3g | La-3g | 42 | 41 |
| 9 | 3h | La-3h | 51 | 48 |
| 10 | 4a | La-4a | 52 | 51 |
| 11 | 4b | La-4b | 61 | 61 |
| 12 | 4c | La-4c | 39 | 32 |
Additionally, the reaction profile of 2a in combination with 2h and 3f were monitored. The results indicated that using La-2h and La-3f, the reactions reached nearly quantitative yields of BPA within 8 h, whereas the yield was only 65% when using 1 under the same conditions (Fig. 3). These findings demonstrate that the synthesized complex exhibits superior catalytic activity.
 | ||
| Fig. 3 Comparison of reaction profile for the reactions using catalyst 1, La-2h, and La-3f. Symbols: white, 1; blue, La-2h; red, La-3f. | ||
To gain insight into the methanolysis process of PC, the reaction solution with catalyst La-2i was analyzed using Gel Permeation Chromatography (GPC) at various reaction times (Fig. 4). As the reaction progressed, we observed a gradual increase in peaks corresponding to degradation products with molecular weights ranging from approximately 200 to 300. In contrast, the polymer components with molecular weights between 1.0 × 104 and 1.0 × 105 showed a sharp decrease after the reaction started, indicating a rapid depolymerization of large polymer molecules. This change in average molecular weight suggested that depolymerization occurred not only at the terminals of polymer chains but also at random positions along the chain. Interestingly, a new peak with a molecular weight around 2500 began to appear at approximately 30 minutes from the beginning. Although the prepared PC powder remained largely insoluble in methanol, a low-polymerization PC prepared by us was found to be able to divide into methanol-insoluble (Component A, Fig. 5) and soluble components (Component B, Fig. 5). GPC analysis revealed that the components with molecular weights of 2500 or higher significantly decreased in solubility in methanol (see also Scheme S1†). The peak of this insoluble component is matched the newly appeared peak in the GPC profile. These observations suggest a mechanism in which the polymer undergoes random methanol attack along the chain, leading to the formation of low-molecular-weight oligomers with a molecular weight of around 2500 that are initially insoluble in methanol. These oligomers subsequently undergo further depolymerization into a homogeneous system, rapidly converting to monomers. In the same catalytic system, using PC pellets without toluene pre-treatment, methanolysis at 80 °C resulted in some degree of molecular weight reduction and monomerization (SI-6†). However, the rate of this process was extremely slow, and after 24 h, the majority of the polymer still maintained an average molecular weight of approximately 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. This observation underscores the significant impact of toluene pre-treatment on the outcomes of this study. These studies provide examples of observing the dynamic behaviour of polymer depolymerization using GPC. The findings offer valuable insights that could influence the design of future reactions and contribute significantly to the understanding of depolymerization and chemical recycling processes in another polymer.
000. This observation underscores the significant impact of toluene pre-treatment on the outcomes of this study. These studies provide examples of observing the dynamic behaviour of polymer depolymerization using GPC. The findings offer valuable insights that could influence the design of future reactions and contribute significantly to the understanding of depolymerization and chemical recycling processes in another polymer.
A key feature of this study is the successful low-temperature methanolysis achieved by leveraging the dispersibility of PC in toluene. This allows for initial powdering of the PC, which is then used as the starting material. While disposing of the toluene after each pre-treatment step would be inefficient, the recovered toluene can be reused in subsequent pre-treatment processes, addressing this concern. In our experiments, powdered PC was obtained after toluene evaporation. Notably, the PC after pre-treatment remains dispersed, not dissolved, in toluene, enabling separation via filtration. During filtration, impurities from the original PC and small amounts of soluble PC components will be present in the recovered toluene and may accumulate with repeated reuse (see ESI, SI-10†). Despite this, the energy requirements for toluene dispersion and recovery are estimated to be low. Furthermore, the addition of a pre-treatment step does not significantly hinder the chemical recycling of PC-based plastic products, as demonstrated with actual products.
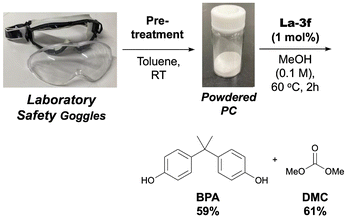
To illustrate this, we depolymerized the lens portion of polycarbonate safety goggles. After removing the band, the lens material was coarsely shredded. These fragments were soaked in toluene overnight, and following the procedures described earlier, a powder was obtained. IR spectroscopy analysis showed the powder's spectrum to be nearly identical to that of reagent-grade polycarbonate (SI-9†), indicating the lens material's equivalence to the reagent-grade PC. Subjecting this powder to methanolysis with catalyst La-3f for 2 h yielded 59% BPA and 61% DMC (Scheme 2), assuming complete PC conversion. This demonstrates equivalent reaction progress between recycled PC powder from products and reagent-grade PC powder. However, comparing our results with those of other studies using actual products is challenging due to difficulties in rigorously characterizing the diverse products involved.38 Our demonstration aims to highlight that the pre-treatment-induced reaction acceleration applies beyond virgin polycarbonate.
Conclusions
Using La complexes with 1,3-dicarbonyl-type ligands, we have demonstrated that the depolymerization of polycarbonate via transesterification in methanol proceeds under mild temperature conditions below the solvent boiling point. The structure of the ligand requires a moderate space for activation of the carbonyl moiety in the polymer yet possesses an electron-rich functional group. The results strongly suggest that the Lewis basicity of the formed La complex is also important for high catalytic activity. Optimized complexes achieved high conversion efficiencies of 69% in 4 h and 92% in 8 h, as determined by GC analysis, even at the low temperature of 60 °C. GPC analysis revealed a sharp drop in the molecular weight of polycarbonate immediately after the start of the reaction, indicating that cleavage of ester bonds occurred at random positions in the polymer chain. In addition, the appearance of a peak corresponding to a methanol-insoluble component with a molecular weight of about 2500 during the reaction progress indicates that the polymer-degradation reaction proceeds in a heterogeneous system. The reactivity of depolymerization in such a heterogeneous system is considered to be closely related to the molecular weight distribution. In future studies, we plan to further investigate the changes in molecular weight distribution during the pre-treatment process of polymers and their impact on reactivity, not limited to polycarbonate.Experimental
Preparation of PC powder
PC (20.1 g) in pellet form was added to toluene (2 L) and stirred at room temperature for more than 12 h. The obtained white suspension was filtered through a stainless steel mesh with an aperture of 500 μm. The solvent was then removed under reduced pressure to obtain a white solid. The white solid was ground in a mortar and dried under vacuum at 80 °C overnight to obtain a white powder (6.6 g). Particle size distribution measurement in methanol confirmed that the prepared PC powder possessed two peaks of 105 μm and 384 μm. This bimodal distribution likely arises from the aggregation of smaller primary particles rather than distinct size classes. Ultrasonic treatment induced a transition from larger aggregates to smaller particles. SEM images further suggest that the primary particles consist of even smaller sub-particles. Particle size distribution and SEM images are available in the ESI (SI-3 and SI-4,† respectively).Synthesis of La complexes28,29
1,3-Diketones and β-ketoesters were purchased or synthesized according to reported methods. In a typical experiment, a ligand (20 mmol, 10 equiv.) was placed in a 100 mL flask, and a solution of 0.5 M NaOMe in methanol (40 mL) was added with stirring until the ligand dissolved completely. L LaCl3 (2 mmol) dissolved in methanol (1 mL) was then added dropwise to the flask with stirring. After stirring for more than 6 h, the precipitated solid was collected by filtration and dried under vacuum at room temperature overnight.Methanolysis of PC powder
PC powder (101.7 mg, 0.4 mmol, based on the BPA-CO unit) and a La complex (4 μmol, 1 mol% relative to PC) were placed in a sealed tube reactor. Methanol (4 mL) was added to the reactor, and the atmosphere was replaced with argon. The reactor was then sealed tightly and stirred at 60 °C for 2 h. After this period, the reactor was allowed to cool to room temperature. Decane was added as an internal standard, and the mixture was diluted with methanol. After removing the reaction residue with a membrane filter, the methanol solution was analyzed by GC-FID.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge Mitsui Chemical Analysis & Consulting Service Inc. for helpful discussions and Particle Size Distribution measurements.References
- Plastics-The Fast Facts 2023, PlasticsEurope, 2023.
- C. Jehanno, J. W. Alty, M. Roosen, S. D. Meester, A. P. Dove, E. Y.-X. Chen, F. A. Leibfarth and H. Sardon, Nature, 2022, 603, 803 CAS.
- R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3, e1700782 CrossRef PubMed.
- R. A. Clark and M. P. Shaver, Chem. Rev., 2024, 124, 2617 CAS.
- K. N. Fotopoulou and H. K. Karapanagioti, Handb. Environ. Chem., 2017, 78, 71 Search PubMed.
- K. Kaiser, M. Schmid and M. Schlummer, Recycling, 2018, 3, 1 Search PubMed.
- J. P. Lange, ACS Sustainable Chem. Eng., 2021, 9, 15722 CAS.
- B. Ruj, V. Pandey, P. Jash and V. K. Srivastava, Int. J. Appl. Sci. Eng. Res., 2015, 4, 564 CAS.
- T. A. Ignatyev, W. Thielemans and B. V. Beke, ChemSusChem, 2014, 7, 1579 Search PubMed.
- E. V. Antonakou, K. G. Kalogiannis, S. D. Stefanidis, S. A. Karakoulia, K. S. Triantafyllidis, A. A. Lappas and D. S. Achilias, Polym. Degrad. Stab., 2014, 110, 482 CrossRef CAS.
- T. Yoshioka, K. Sugawara, T. Mizoguchi and A. Okuwaki, Chem. Lett., 2005, 34, 282 CrossRef CAS.
- G. Grause, N. Tuskada, W. Hall, T. Kameda, P. T. Williams and T. Yoshika, Polymer, 2010, 42, 438 CAS.
- E. Quaranta, Appl. Catal., B, 2017, 206, 233 CrossRef CAS.
- M. Taguchi, Y. Ishikawa, S. Kataoka, T. Naka and T. Funazukuri, Catal. Commun., 2016, 84, 93 CrossRef CAS.
- P. A. Krisbiantoro, M. Sato, T. M. Lin, Y. C. Chang, T. Y. Peng, Y. C. Wu, W. Liao, Y. Kamiya, R. Otomo and K. C.-W. Wu, Langmuir, 2024, 40, 5338 CrossRef CAS PubMed.
- K. Yamada, N. Komine and M. Hirano, ChemCatChem, 2024, 16, e202400870 CrossRef CAS.
- F. Liu, Z. Li, S. Yu, X. Cui and X. Ge, J. Hazard. Mater., 2010, 174, 872 CAS.
- Y. Zhao, X. Zhang, X. Song and F. Liu, Catal. Lett., 2017, 147, 2940 CrossRef CAS.
- F. Liu, J. Guo, P. Zhao, M. Jia, M. Liu and J. Gao, Polym. Degrad. Stab., 2019, 169, 108996 CrossRef.
- M. Liu, J. Guo, Y. Gu, J. Gao, F. Liu and S. Yu, ACS Sustainable Chem. Eng., 2018, 6, 13114 CrossRef CAS.
- F. Iannone, M. Casiello, A. Monopoli, P. Cotugno, M. C. Sportelli, R. A. Picca, N. Cioffi, M. M. Dell'Anna and A. Nacci, J. Mol. Catal. A: Chem., 2017, 426, 107 CrossRef CAS.
- M. Wang, G. Yuan and C. C. Han, Polymer, 2013, 54, 3612 CrossRef CAS.
- C.-H. Wu, L.-Y. Chen, R.-J. Jeng and S. A. Dai, ACS Sustainable Chem. Eng., 2018, 6, 8964 CAS.
- Y. Ono, Appl. Catal., A, 1997, 155, 133 CrossRef CAS.
- Y. Ono, Catal. Today, 1997, 35, 15 Search PubMed.
- M. P. Pacheco and C. L. Marchall, Energy Fuels, 1997, 11, 2 CAS.
- D. Delledonne, F. Rivetti and U. Romano, Appl. Catal., A, 2001, 221, 241 CAS.
- P. Tundo and M. Selva, Acc. Chem. Res., 2002, 35, 706 CAS.
- B. Schäffner, F. Schäffner, S. P. Verevkin and A. Börner, Chem. Rev., 2010, 110, 4554 Search PubMed.
- A. O. Esan, A. D. Adeyemi and S. Ganesan, J. Cleaner Prod., 2020, 257, 120561 CAS.
- R. Abe, N. Komine, K. Nomura and M. Hirano, Chem. Commun., 2022, 58, 8141 Search PubMed.
- T. Ahmed, A. Chakraborty, A. Paul and S. Baitalik, Dalton Trans., 2023, 52, 14027 CAS.
- P. C. Andrews, G. B. Deacon, R. Frank, B. H. Fraser, P. C. Junk, J. G. MacLellan, M. Massi, B. Moubaraki, K. S. Murray and M. Silberstein, Eur. J. Inorg. Chem., 2009, 2009, 744 Search PubMed.
- T. Kawase, H. Ishitani and S. Kobayashi, Chem. Lett., 2023, 52, 745 Search PubMed.
- A. A. Neverov and R. S. Brown, Can. J. Chem., 2000, 78, 1247 CAS.
- A. A. Neverov, P. J. Montoya-Pelaez and R. S. Brown, J. Am. Chem. Soc., 2001, 123, 210 CAS.
- N. Kobayashi, N. Komine, K. Nomura, H. Hirano and M. Hirano, Bull. Chem. Soc. Jpn., 2023, 96, 1324 CAS.
- L. D. Ellis, N. A. Rorrer, K. P. Sullivan, M. Otto, J. E. McGeehan, Y. Román-Leshkov, N. Wierckx and G. T. Beckam, Nat. Catal., 2021, 4, 539 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5gc00829h |
| This journal is © The Royal Society of Chemistry 2025 |