Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Supramolecular assisted O-acylation of carbohydrates†
Soumyadip
Dey
 ,
Debabrata
Giri
,
Debabrata
Giri
 ,
Adrita
Nandy
,
Adrita
Nandy
 and
Abhijit
Sau
and
Abhijit
Sau
 *
*
Department of Chemistry, Indian Institute of Technology Hyderabad, Kandi, 502284, Sangareddy, Telangana, India. E-mail: asau@chy.iith.ac.in
First published on 9th April 2025
Abstract
The acylation of hydroxy groups serves as one of the most employed protecting group strategies in carbohydrate chemistry. Here, we present a base-free, supramolecular assisted approach for the O-acylation of carbohydrates under mild conditions, using 18-crown-6 in combination with a catalytic amount of potassium fluoride. This sustainable and useful method successfully converted various functional groups containing carbohydrate hydroxy groups into O-acyl, O-benzoyl, and O-propionyl derivatives with up to 99% yields.
Green foundation1. In this manuscript, we describe an efficient and green method via supramolecular assisted O-acylation of carbohydrate alcohols. This approach removes the use of hazardous and toxic pyridine and its derivatives, which are harmful to the ecosystem. Moreover, it is efficient and eco-friendly for acylation, including aliphatic and phenolic hydroxy groups under neat conditions, without using any complicated setups or inert gasses.2. This method requires very cheap and easily available chemicals: potassium fluoride (KF) and 18-crown-6. Our cost-effective acylation method achieves a very low E-factor (0.6) compared to other existing acylation techniques, which makes it more efficient and sustainable for synthesizing acylating substrates. 3. The long reaction time and the requirement of 40 °C for effective acylation are minor drawbacks of this synthetic method. However, we have confidence that this work provides a significant proof of concept, establishing supramolecular assisted acylation under mild conditions. |
Alcohols,1 amines,2 phenols,3 and thiols4 are often acylated for functional group transformations in organic synthesis.5–9 In carbohydrate chemistry, O-acetylation is one of the most commonly used techniques for protecting hydroxyl groups.10–12 Moreover, per-O-acetylated sugars are affordable and practically valuable intermediates for the synthesis of natural glycosides, glycoconjugates, and other oligosaccharides.13 Additionally, acylation is also useful in structural elucidations of various carbohydrate moieties containing natural products by converting them into their per-O-acetates.14 Apart from acetyl protection, benzoyl, pivaloyl, and chloroacetyl are also used in acyl-protecting groups during carbohydrate transformation. Generally, these common acyl protected sugars are synthesized with acetic anhydride or acetyl chloride, benzoyl chloride, pivaloyl chloride, and chloroacetyl chloride, respectively, in the presence of a base.15 Acetylation of sugar alcohols has been frequently accomplished with a significant excess of acetic anhydride in the presence of pyridine, acting as the base and solvent, despite its toxicity and disagreeable odour.16 In addition, different pyridine derivatives, like 4-(1-pyrrolidino)pyridine and 4-(N,N)-dimethylaminopyridine (DMAP), are largely used as co-catalysts to accelerate the reaction rate.17,18 Base-catalyzed acylation19 is often performed in the presence of imidazole or its derivatives.13,20,21 However, the preferred synthetic procedure for stereoselective synthesis of anomeric β-glycosyl acetates is Wolfrom's anhydrous sodium acetate method.22,23 In the literature, acid-catalyzed acylation has been reported using different Lewis acids such as In(OTf)3,24 InCl3,25 ZnCl2,26 FeCl3,27 Fe2(SO4)3,28 iodine,29,30 Cu(OTf)2,31 Sc(OTf)3,32 BiOCl–SOCl2,33 CoCl2,34 BiCl3,35 LiClO4,36etc. Perchloric acid,37 sulfuric acid,38 sulfamic acid,10 and HClO4–SiO239 have also been used in combination with acetic anhydride in various acetylations.40 In addition to these classical methods, various heterogeneous catalysts have been employed for acetylation, such as zeolites,41 montmorillonite K-10,14 and Nafion-H.42 Although different protocols, such as microwave irradiation conditions25,26,43,44 ionic liquids,45 and carbene-mediated46–49 selective acylation50 of saccharides, have also been investigated, these methods often face several challenges, including excessive use of acylating reagents, excessive environmental toxicity, moisture sensitivity, preparation of catalysts, incompatibility with acid-sensitive groups, high costs, handling the large amount of toxic pyridine and isomerization of unprotected reducing carbohydrate derivatives into their furanose and pyranose forms. To overcome these limitations, a quick, environmentally friendly, and clean reaction protocol that minimizes the need for traditional workup and purification processes is still desired for acetylation of carbohydrates. We sought a mild, efficient, non-toxic, green, and sustainable catalytic method for acylation. On the other hand, crown ethers have gained significant importance in the field of organic synthesis in recent years for numerous applications such as metal ion separations, phase transfer catalysis, metal–organic frameworks, and electron transfer reactions.51,52 Various types of crown ethers,53 such as 12-crown-4, 15-crown-5, 18-crown-6, etc., have been developed, and their own central hole size correlate with the diameters of different cations. The exceptional chemical property of excellent capacity to encapsulate metal ions from their salts with stability and high specificity makes crown ethers unique and significant in their applications.54 Furthermore, the strong interaction ability of crown ethers with metal cations has inspired the development of catalytic properties within the reaction mixture by activating the metal salts through enhancing their solubility.55 Several efforts have been made in the past for the acylation of carbohydrates, but still, there is a high demand for a less hazardous, cheaper, solvent-free new method for the synthesis of acyl-protected sugars that can avoid the extreme toxicity of pyridine. Keeping in mind the exciting properties of crown ethers, we envisioned exploring the catalytic ability of a metal salt–crown ether combined system for acylation in sugar molecules (Scheme 1).
In the preliminary screening, we started with four free hydroxyl groups containing the substrate 4-methoxyphenyl-β-D-glucopyranoside (1a) for the acylation reaction. During the first set of reactions, compound 1a was treated with 0.4 equiv. (0.1 equiv. per –OH) of potassium fluoride and 4.5 equiv. of acetic anhydride in acetonitrile, resulting in a trace amount of spot-on TLC at a temperature of 50 °C (Table 1, entry 1). After that, the use of a combination of 0.1 equiv. potassium fluoride and crown ether (0.1 equiv. per –OH) made a noticeable improvement in the formation of the desired product per-acetylated 4-methoxyphenyl-β-D-glucopyranoside (2a), yielding 43% under neat conditions after 12 hours (Table 1, entry 2). Next, increasing the loading of both potassium fluoride and crown ether to 0.8 equiv. (0.2 equiv. per –OH) at 25 °C under solvent free and DCM conditions after 12 hours resulted in good yields of our desired per-acylated product 2a, which were 68% and 71%, respectively (Table 1, entries 3 and 4). However, the yield was enhanced to 94% by increasing the temperature to 40 °C, while maintaining the same reagent and catalyst loading under neat conditions (Table 1, entry 5). Subsequently, we also explored the catalytic amount of other metal fluorides like sodium fluoride (NaF) and cesium fluoride (CsF), which resulted in 5% and 8% of desired compound 2a, respectively, at 40 °C (Table 1, entries 6 and 9). Moreover, reacting compound 1a with a combination of 0.8 equiv. of NaF and 15-crown-5 (0.2 equiv. per –OH) afforded up to 58% of per-acetylated 4-methoxyphenyl-β-D-glucopyranoside (2a) (Table 1, entries 7 and 8). In addition, we noticed that at 70 °C within 6 hours all starting materials were consumed and resulted in a similar isolated yield (Table 1, entry 10). After this extensive screening, we concluded that using catalytic amounts of potassium fluoride and 18-crown-6 (0.2 equiv. per –OH) with acetic anhydride (1.15 equiv. per –OH) at 40 °C under neat conditions is the best condition for the acylation reaction.
| Entry | Reagent (equiv. per –OH) | Temp (°C) | Solvent | Time (hours) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: substrate 1a (0.34 mmol) and acetic anhydride (1.61 mmol). b Isolated yield. | |||||
| 1 | KF (0.1) | 50 °C | MeCN | 12 h | Trace |
| 2 | KF (0.1), 18-C-6 (0.1) | 25 °C | Neat | 12 h | 43 |
| 3 | KF (0.2), 18-C-6 (0.2) | 25 °C | Neat | 12 h | 68 |
| 4 | KF (0.2), 18-C-6 (0.2) | 25 °C | DCM | 12 h | 71 |
| 5 | KF (0.2), 18-C-6 (0.2) | 40 °C | Neat | 12 h | 94 |
| 6 | NaF (0.2) | 50 °C | MeCN | 12 h | 5 |
| 7 | NaF (0.2), 15-C-5 (0.2) | 25 °C | Neat | 12 h | 43 |
| 8 | NaF (0.2), 15-C-5 (0.2) | 40 °C | Neat | 12 h | 58 |
| 9 | CsF (0.2) | 50 °C | MeCN | 6 h | 8 |
| 10 | KF (0.2), 18-C-6 (0.2) | 70 °C | Neat | 6 h | 92 |
Optimizing the standard reaction conditions, we further explored the substrate scope with various sugar molecules as well as by employing different acylating anhydrides (Scheme 2). Initially, we focused on the acylation reaction of methyl-α-D-galactopyranoside (1b), methyl-α-D-mannopyranoside (1c), and methyl-α-D-glucopyranoside (1d) with acetic anhydride under the optimized reaction conditions and obtained the corresponding peracetylated products (2b), (2c), and (2d) with excellent yields of 90%, 89%, and 87%, respectively. Next, acylation of 4-methoxyphenyl-β-D-galactopyranoside (1e) also resulted in an excellent yield of the desired peracetylated compound 2e (91%). After that, we aimed for acylation of different protected sugars such as methyl 3,4-O-isopropylidene-α-D-galactopyranoside (1f) and 3-hydroxy-l,2:5,6-di-O-isopropylidene-α-D-glucofuranoside (1g) with acetic anhydride and observed excellent yields of desired acetylated products 2f (98%) and 2g (99%), respectively. Similarly, methyl 4,6-O-benzylidene-α-D-galactopyranoside (1h) was successfully converted to the acylated product (2h) with 93% yield. These results suggest that acid-sensitive isopropylidene and benzylidene rings can tolerate the reaction conditions easily. Then, we obtained an excellent yield of the compound p-methoxyphenyl 2,3-di-O-benzyl-4,6-O-acetyl-β-D-glucopyranoside (2i, 95%) under the same reaction conditions. Besides, different thioglycosides, p-methoxyphenyl 1-thio-α-D-mannopyranoside (1j), p-methoxyphenyl 1-thio-β-D-xylopyranoside (1k) and p-methoxyphenyl 3-O-benzyl-1-thio-β-D-xylopyranoside (1l) were smoothly converted to the respective acylated products with good yields (2j, 86%; 2k, 88%; and 2l, 92%). Next, we attempted the acetylation of free sugar molecules such as galactose (1m) and arabinose (1n), and interestingly, observed impressive yields in both cases (83% and 86%, respectively). In addition, disaccharides sucralose (1o) and cellobiose (1p) were also smoothly converted to the per-acetylated form with good yields (2o, 85% and 2p, 82%) under the optimal reaction conditions. Furthermore, we also extended the acylation of the phenolic hydroxyl group of the essential natural product tocopherol (1q) and obtained an excellent yield (2q, 91%). Additionally, we expanded the substrate scope towards amino sugars such as glucosamine (1u), N-acetylglucosamine (1v) and galactosamine (1w) and obtained per-acetylated products 2r, 2r′ and 2s with excellent yields (93%, 97% and 98%, respectively, Scheme 2). To showcase the utility of this green approach, we further investigated acylation with different acylating reagents. Hence, compound 1f was reacted with 0.4 equiv. of benzoic anhydride in acetonitrile, resulting in the benzoylated product (2t) with a good yield (86%) under standard reaction conditions. Then, we executed a reaction of compound 1g with benzoic anhydride, which resulted in the corresponding benzoyl-protected product 2u with excellent yield (92%). In addition, disaccharide lactose and aliphatic alcohol l-menthol were easily converted to their corresponding benzoylated products 2v and 2w with good yields – 79% and 58%, respectively. We also observed good to excellent yields of 82% to 98% while extending the acylation scope with propionic anhydride and different sugar molecules, including disaccharide lactose (Scheme 2; 2x–2ad).
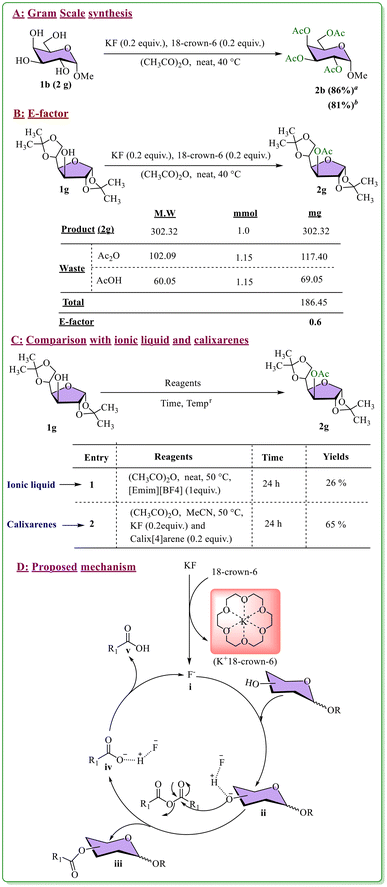
After exploring the substrate scope, we planned gram-scale synthesis of per-acylated carbohydrates under the optimized reaction conditions. For this purpose, compound 1b was easily converted to per-acetylated product 2b with a good yield (86% in a 2 g scale). Moreover, we recovered the crown ether from the reaction mixture following the reported work-up procedure54 (details in the ESI†) and utilized it in the further acylation reaction that provided a similar yield to the desired per-acylated product (81%). A commonly used indicator for the quantitative assessment of a synthetic reaction method's environmental impact is called the E-factor.56,57 The calculated E-factor of our supramolecular assisted acylation reaction was 0.6 (Scheme 3B). In addition, we checked the reaction in an ionic liquid ([Emim][BF4]) and calixarene (Calix[4]arene) and noticed 26% and 65% yields of compound 2g, respectively (Scheme 3C). The most probable mechanistic pathway is displayed in Scheme 3, where initially, crown ether encapsulates the potassium ion (K+) in the cavity, making free fluoride ions (F−) more nucleophilic in the reaction mixture. Then, F−, which abstracts the proton of sugar alcohols to form intermediate (ii), further reacts with the anhydride to yield the desired acylated product (iii). Next, R1COO− protonates in the medium to generate fluoride ions again to continue the catalytic cycle (Scheme 3D).
Conclusions
In conclusion, we have developed an efficient and practical method for acylation by using catalytic amounts of potassium fluoride (KF) and 18-crown-6 under solvent-free conditions, avoiding toxic pyridine and its derivatives. This supramolecular assisted approach was successfully applied to a wide range of carbohydrates and aliphatic alcohols, providing straightforward access to their acetyl, benzoyl, and propionyl derivatives. Importantly, functional groups commonly used for protecting and manipulating carbohydrates remained unaffected under the reaction conditions. Moreover, these mild reaction conditions are convenient, non-hazardous, and eco-friendly. We believe that this greener approach will be useful for the preparation of several common acylated carbohydrate building blocks in organic synthesis.Author contributions
SD: conceptualisation, methodology, validation, formal analysis, data curation and writing (original draft); DG: methodology, validation and formal analysis; AN: methodology, validation and formal analysis. AS: conceptualisation, funding acquisition, supervision, project administration, reviewing, and editing.Data availability
The data that support the findings of this study are available in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors appreciate the financial support from SERB and MoE-STARS, India (SRG/2023/000034 & MoE-STARS/STARS-2/2023-0126) and the Indian Institute of Technology Hyderabad (IITH). Instrumental support was provided by the IIT Hyderabad.References
- A. Orita, C. Tanahashi, A. Kakuda and J. Otera, Angew. Chem., Int. Ed., 2000, 39, 2877–2879 CrossRef CAS PubMed.
- F. Piazzolla and A. Temperini, Tetrahedron Lett., 2018, 59, 2615–2621 CrossRef CAS.
- T. G. Bonner and P. McNamara, J. Chem. Soc. B, 1968, 795–797 RSC.
- A. Temperini, D. Annesi, L. Testaferri and M. Tiecco, Tetrahedron Lett., 2010, 51, 5368–5371 CrossRef CAS.
- N. Anbu, N. Nagarjun, M. Jacob, J. M. V. K. Kalaiarasi and A. Dhakshinamoorthy, Chemistry, 2019, 1, 69–79 CrossRef.
- Z. Zhang, F. Cheng, X. Ma, K. Sun, X. Huang, J. An, M. Peng, X. Chen and B. Yu, Green Chem., 2024, 26, 7331–7336 RSC.
- Z. Zhang, Z. Zhao, M. Liu, H. Liu, Q. Li, J. Xiang, T. Wu and B. Han, Green Chem., 2022, 24, 9763–9771 RSC.
- Z. Zhong, H. Wu, X. Chen, Y. Luo, L. Yang, X. Feng and X. Liu, J. Am. Chem. Soc., 2024, 146, 20401–20413 CrossRef CAS PubMed.
- C. Li, J. Cheng, X. Wan, J. Li, W. Zu, Y. Xu, Y. Huang and H. Huo, J. Am. Chem. Soc., 2024, 146, 19909–19918 CrossRef CAS PubMed.
- A. Santra, G. Guchhait and A. K. Misra, Green Chem., 2011, 13, 1345–1351 RSC.
- S. Dey, S. Dwivedi, D. Giri and A. Sau, Org. Lett., 2024, 26, 10351–10355 CrossRef CAS PubMed.
- S. Dey, S. Dwivedi and A. Sau, Carbohydr. Res., 2025, 552, 109447 CrossRef CAS PubMed.
- P. Tiwari, R. Kumar, P. R. Maulik and A. K. Misra, Eur. J. Org. Chem., 2005, 2005, 4265–4270 CrossRef.
- P. M. Bhaskar and D. Loganathan, Tetrahedron Lett., 1998, 39, 2215–2218 CrossRef CAS.
- P. Tiwari and A. K. Misra, Carbohydr. Res., 2006, 341, 339–350 CrossRef CAS PubMed.
- B. Yu, J. Xie, S. Deng and Y. Hui, J. Am. Chem. Soc., 1999, 121, 12196–12197 CrossRef CAS.
- E. F. Scriven, Chem. Soc. Rev., 1983, 12, 129–161 RSC.
- G. Höfle, W. Steglich and H. Vorbrüggen, Angew. Chem., Int. Ed. Engl., 1978, 17, 569–583 CrossRef.
- R. Wang, Y. Chen, B. Fei, J. Hu, J. Chen, Y. Luo and Y. Xia, Org. Lett., 2023, 25, 2970–2974 CrossRef CAS PubMed.
- C. D. Hubbard and J. F. Kirsch, Biochemistry, 1972, 11, 2483–2493 CrossRef CAS PubMed.
- W. P. Jencks and J. Carriuolo, J. Biol. Chem., 1959, 234, 1272–1279 CrossRef CAS PubMed.
- J. Gelas, in Advances in Carbohydrate Chemistry and Biochemistry, ed. R. S. Tipson and D. Horton, Academic Press, 1981, vol. 39, pp. 71–156 Search PubMed.
- A. I. Vogel, A text-book of practical organic chemistry including qualitative organic analysis, Longmans, 1956, vol. 2, pp. 676–681 Search PubMed.
- N. P. Bizier, S. R. Atkins, L. C. Helland, S. F. Colvin, J. R. Twitchell and M. J. Cloninger, Carbohydr. Res., 2008, 343, 1814–1818 CrossRef CAS PubMed.
- S. K. Das, K. A. Reddy, V. L. N. R. Krovvidi and K. Mukkanti, Carbohydr. Res., 2005, 340, 1387–1392 CrossRef CAS PubMed.
- C. Limousin, J. Cléophax, A. Petit, A. Loupy and G. Lukacs, J. Carbohydr. Chem., 1997, 16, 327–342 CrossRef CAS.
- F. Dasgupta, P. P. Singh and H. C. Srivastava, Carbohydr. Res., 1980, 80, 346–349 CrossRef CAS.
- L. Shi, G. Zhang and F. Pan, Tetrahedron, 2008, 64, 2572–2575 CrossRef CAS.
- K. P. R. Kartha and R. A. Field, Tetrahedron, 1997, 53, 11753–11766 CrossRef CAS.
- S. Toorchi Roudsari and S. Sadjadi, ChemistrySelect, 2023, 8, e202204067 CrossRef CAS.
- C.-A. Tai, S. S. Kulkarni and S.-C. Hung, J. Org. Chem., 2003, 68, 8719–8722 CrossRef CAS PubMed.
- J.-C. Lee, C.-A. Tai and S.-C. Hung, Tetrahedron Lett., 2002, 43, 851–855 CrossRef CAS.
- R. Ghosh, A. Chakraborty and S. Maiti, Tetrahedron Lett., 2004, 45, 9631–9634 CrossRef CAS.
- S. Ahmad and J. Iqbal, J. Chem. Soc., Chem. Commun., 1987, 2, 114–115 RSC.
- J.-L. Montero, J.-Y. Winum, A. Leydet, M. Kamal, A. A. Pavia and J.-P. Roque, Carbohydr. Res., 1997, 297, 175–180 CrossRef CAS.
- K.-C. Lu, S.-Y. Hsieh, L. N. Patkar, C.-T. Chen and C.-C. Lin, Tetrahedron, 2004, 60, 8967–8973 CrossRef CAS.
- H. Binch, K. Stangier and J. Thiem, Carbohydr. Res., 1998, 306, 409–419 CrossRef CAS.
- B. Mukhopadhyay, Tetrahedron Lett., 2006, 47, 4337–4341 CrossRef CAS.
- A. K. Misra, P. Tiwari and S. K. Madhusudan, Carbohydr. Res., 2005, 340, 325–329 CrossRef CAS PubMed.
- J. Song and W.-H. Zheng, Org. Lett., 2022, 24, 2349–2353 CrossRef CAS PubMed.
- P. M. Bhaskar and M. Pallooru, Synlett, 1999, 129–131 CrossRef CAS.
- R. Kumareswaran, K. Pachamuthu and Y. D. Vankar, Synlett, 2000, 1652–1654 CAS.
- T. Hirose, B. G. Kopek, Z.-H. Wang, R. Yusa and B. W. Baldwin, Tetrahedron Lett., 2003, 44, 1831–1833 CrossRef CAS.
- M. H. S. Troftgruben, I. Oldenburg, A. M. Pama, B. Igawa, S. Deguara, M. Shields, N. K. Viswanathan, J. X. Silverio, C. Gleason, J. S.-M. Wang, J. S. Ford, H. Lazaro and S. Eagon, J. Org. Chem., 2024, 89, 17850–17852 CrossRef CAS PubMed.
- S. A. Forsyth, D. R. MacFarlane, R. J. Thomson and M. von Itzstein, Chem. Commun., 2002, 714–715 RSC.
- W.-X. Lv, H. Chen, X. Zhang, C. C. Ho, Y. Liu, S. Wu, H. Wang, Z. Jin and Y. R. Chi, Chem, 2022, 8, 1518–1534 CAS.
- Y.-G. Liu, Z. Zhong, Y. Tang, H. Wang, S. V. C. Vummaleti, X. Peng, P. Peng, X. Zhang and Y. R. Chi, Nat. Commun., 2025, 16, 54 CrossRef PubMed.
- G. Nie, T. Tu, T. Liao, D. Liu, W. Ye and S.-C. Ren, Green Chem., 2024, 26, 5397–5408 RSC.
- J. Jin, J. Guo, F. Xu, Y. Lv, G. Wang, J. Song, W.-X. Lv, T. Li and Y. R. Chi, Green Chem., 2024, 26, 5997–6004 RSC.
- G. Xiao, G. A. Cintron-Rosado, D. A. Glazier, B. Xi, C. Liu, P. Liu and W. Tang, J. Am. Chem. Soc., 2017, 139, 4346–4349 CrossRef CAS PubMed.
- C. J. Pedersen, Angew. Chem., Int. Ed. Engl., 1988, 27, 1021–1027 CrossRef.
- V. I. Lakshmanan and S. Vijayan, in Extraction 2018, ed. B. R. Davis, M. S. Moats, S. Wang, D. Gregurek, J. Kapusta, T. P. Battle, M. E. Schlesinger, G. R. Alvear Flores, E. Jak, G. Goodall, M. L. Free, E. Asselin, A. Chagnes, D. Dreisinger, M. Jeffrey, J. Lee, G. Miller, J. Petersen, V. S. T. Ciminelli, Q. Xu, R. Molnar, J. Adams, W. Liu, N. Verbaan, J. Goode, I. M. London, G. Azimi, A. Forstner, R. Kappes and T. Bhambhani, Springer International Publishing, Cham, 2018, pp. 1913–1930 Search PubMed.
- S. Blair, E. Kempen and J. Brodbelt, J. Am. Soc. Mass Spectrom., 1998, 9, 1049–1059 CrossRef CAS.
- Y. Song, H. Jing, B. Li and D. Bai, Chem. – Eur. J., 2011, 17, 8731–8738 CrossRef CAS PubMed.
- S. L. Silva, M. S. Valle and J. R. Pliego, J. Org. Chem., 2020, 85, 15457–15465 CrossRef CAS PubMed.
- R. A. Sheldon, Chem. Commun., 2008, 29, 3352–3365 RSC.
- R. A. Sheldon, Green Chem., 2016, 18, 3180–3183 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5gc00499c |
| This journal is © The Royal Society of Chemistry 2025 |