Open Access Article
Open Access ArticleSolid-state aromatic nucleophilic fluorination: a rapid, practical, and environmentally friendly route to N-heteroaryl fluorides†
Koji
Kubota
 *ab,
Tetsu
Makino
a,
Keisuke
Kondo
a,
Tamae
Seo
a,
Mingoo
Jin
*ab,
Tetsu
Makino
a,
Keisuke
Kondo
a,
Tamae
Seo
a,
Mingoo
Jin
 b and
Hajime
Ito
b and
Hajime
Ito
 *ab
*ab
aDivision of Applied Chemistry, Graduate School of Engineering, Hokkaido University, Sapporo, Hokkaido 060-8628, Japan. E-mail: kbt@eng.hokudai.ac.jp; hajito@eng.hokudai.ac.jp
bInstitute for Chemical Reaction Design and Discovery (WPI-ICReDD), Hokkaido University, Sapporo, Hokkaido, Japan
First published on 4th January 2025
Abstract
A simple mechanochemical protocol for solid-state aromatic nucleophilic fluorination using potassium fluoride (KF) and quaternary ammonium salts was developed. This solid-state fluorination is fast and a variety of N-heteroaryl halides can be efficiently fluorinated within 1 h. Notably, highly polar and high-boiling solvents, which are often toxic and difficult to remove during purification, are not required for this protocol. Moreover, all the synthetic operations can be carried out under ambient conditions without complicated setups involving inert gases. The practical advantages of this mechanochemical protocol suggest potentially widespread applications for the preparation of valuable fluorine-containing molecules in a more efficient, cost-effective, and environmentally friendly manner than existing solution-based protocols.
Green foundation1. This work advances the field of green chemistry by introducing a solid-state mechanochemical protocol for aromatic nucleophilic fluorination that eliminates the need for toxic, high-boiling solvents, which are typically difficult to remove and environmentally harmful. The newly developed method operates under ambient conditions, without requiring complex setups or inert gases, making it more energy-efficient and reducing the overall environmental footprint compared to traditional solution-based fluorination processes.2. The specific achievement of this work is the development of a fast, solid-state nucleophilic aromatic fluorination method using a cost-effective combination of potassium fluoride and quaternary ammonium salts. This enables the efficient synthesis of a wide range of aromatic fluorides, which are crucial structural motifs in pharmaceuticals, agrochemicals, organic materials, and biological imaging agents. Notably, this approach eliminates the need for toxic, high-boiling solvents such as dimethylsulfoxide (DMSO), significantly reducing the environmental impact associated with their use and disposal. Using the E-factor evaluation, a metric for quantitatively assessing the environmental impact of chemical processes, it was found that this solid-state fluorination method is substantially more eco-friendly than conventional solution-based approaches. 3. A notable limitation of this method is the requirement for elevated temperatures to achieve efficient fluorination. However, we are confident that this study serves as an important proof of concept, demonstrating the feasibility of sustainable, solvent-free aromatic nucleophilic fluorination via mechanochemical methods. As a follow-up project, we aim to develop a room-temperature version of the aromatic nucleophilic fluorination under mechanochemical conditions, which represents the ultimate goal of our research. |
Introduction
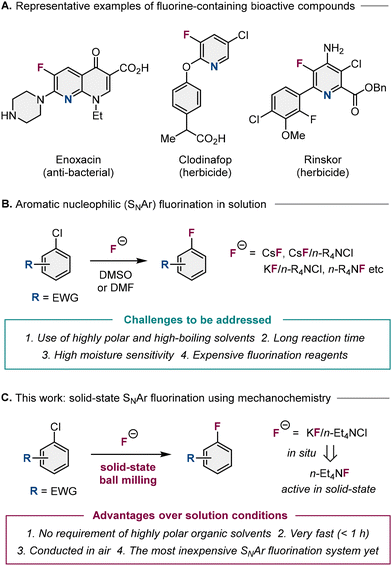
Aromatic fluorides are crucial structural motifs in pharmaceuticals, agrochemicals, organic materials, and biological imaging agents.1,2 An increasing number of fluorine-containing biologically active molecules have been developed for pharmaceutical and agrochemical applications (Fig. 1A),3 and to date, several useful procedures for the formation of Caryl–F bonds have been reported.1,2 One common approach for the formation of Caryl–F bonds are aromatic nucleophilic (SNAr) fluorination reactions, which use fluoride salts, i.e., reagents that are widely used in industrial-process chemistry (Fig. 1B).4–6 Traditionally, cesium fluoride (CsF) has been employed as a nucleophilic fluorination reagent at high temperatures.6 Since then, a combination of potassium fluoride (KF) and tetrabutylammonium chloride (n-Bu4NCl) has emerged as a more cost-effective alternative to CsF for SNAr fluorinations.6–9 More recently, Sanford has reported that the use of anhydrous tetramethylammonium fluoride (Me4NF) allows the reaction temperature of the SNAr fluorination to be lowered to room-temperature.10Despite this recent progress, SNAr fluorination chemistry still suffers from the following well-documented limitations (Fig. 1B):6 (1) a large amount of highly polar and high-boiling organic solvents, such as dimethyl sulfoxide (DMSO) or N,N-dimethyl formamide (DMF), which are difficult to remove during purification, are required. This makes the solution-based process wasteful and both time- and energy-consuming. In particular, DMF is a highly toxic solvent, and its use has been restricted in the European Union since 2023 on account of especially the hazards it poses to reproductive health;11 (2) the solution-based conditions require long reaction times (typically >24 h); (3) the solution-based reactions are highly moisture-sensitive, as water both attenuates the nucleophilicity of the fluoride and leads to hydrolysis byproducts; and (4) highly reactive but expensive fluorination reagents (e.g., CsF or Me4NF) are often required for the efficient formation of C–F bonds. Therefore, the exploration of reliable, efficient, low-cost, time-saving, and environmentally friendly methods for SNAr fluorination represents an important challenge in synthetic chemistry.
Recently, mechanochemical synthesis using ball milling has emerged as a more sustainable and efficient alternative to traditional solution-based approaches.12,13 This method allows organic reactions to be conducted using minimal amounts of solvent, and in most cases, all synthetic operations can be conducted under ambient conditions. Inspired by the attractive features of mechanochemistry, we envisioned that a mechanochemical protocol could allow the development of highly efficient solid-state SNAr fluorination reactions that overcome the aforementioned shortcomings associated with solution-based fluorination reactions (Fig. 1C). Elegant examples of mechanochemical fluorination have already been reported, but most of them are electrophilic fluorination protocols, and mechanochemical SNAr fluorination remained unexplored.14
Results and discussion
All mechanochemical reactions were conducted in a Retsch MM400 mill [stainless-steel milling jar (5 mL); 30 Hz; stainless-steel ball (diameter: 10 mm)] and were performed under ambient conditions. First, we investigated the SNAr fluorination of 2-fluoroquinoline derivative 1a with KF to give 2a (Table 1). In order to carry out the reaction at high temperature, we used a commercially available temperature-controllable heat gun, which was placed directly above the ball-milling jar (for details, see the ESI†).13g The reaction was conducted with the heat gun preset to 250 °C, and the internal temperature of the reaction mixture (130 °C) was determined by thermography immediately after opening the milling jar (for details, see the ESI†). The mechanochemical reaction did not yield the fluorination product (2a) (<1%; Table 1, entry 1) under the applied conditions. To accelerate the mechanochemical SNAr fluorination, we conducted the reaction in the presence of liquid additives (Table 1, entries 2–5).15 Although polar solvents such as DMSO and DMF, which are commonly used in solution-based conditions,6 were tested, 2a was not obtained (Table 1, entries 2–4). A reaction using toluene was also unsuccessful (Table 1, entry 5). Surprisingly, the addition of n-Bu4NCl, which is commonly used as a phase-transfer reagent under solution-based conditions,6–9 dramatically accelerated the solid-state SNAr fluorination to form 2a in 88% yield (Table 1, entry 6). The combination of n-Bu4NCl and DMSO was not as effective (72%; Table 1, entry 7). Next, other quaternary ammonium salts were examined (Table 1, entries 8–11). We found that the presence of an alkyl substituent on the ammonium salt affects the reactivity and the use of Et4NCl provided 2a quantitatively (Table 1, entry 9). Importantly, Et4NCl is much cheaper than the n-Bu4NCl reagent that is commonly employed in solution-based reactions.16 The reaction using n-Bu4NBr gave a poor result (7%; Table 1, entry 11), suggesting that the correct counter anion is also important to achieve high efficiency. The use of Ph4PCl did not promote the fluorination (6%; Table 1, entry 12). Next, we investigated the effect of the reaction temperature. Successively lowering the reaction temperature to 100 °C and 40 °C did not result in any reaction (Table 1, entries 13 and 14). Ultimately, we identified the optimized solid-state conditions as using an inexpensive KF/Et4NCl moisture-insensitive system without the requirement for any highly polar organic solvents (e.g., DMSO or DMF), thereby successfully addressing the aforementioned issues associated with typical solution-based protocols.| Entry | Additive | Activator | Temp. (°C) | Yield of 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
|---|---|---|---|---|
| a Conditions: 1a (0.5 mmol), KF (1.00 mmol), liquid additive (0.20 μL mg−1), activator (0.75 mmol) in a stainless-steel ball-milling jar (5 mL) with a stainless-steel ball (diameter: 10 mm). b Determined based on 19F NMR spectroscopy. Isolated yields are given in parenthesis. | ||||
| 1 | None | None | 130 | <1 |
| 2 | DMSO | None | 130 | <1 |
| 3 | DMF | None | 130 | <1 |
| 4 | DMA | None | 130 | <1 |
| 5 | Toluene | None | 130 | <1 |
| 6 | None | n-Bu4NCl | 130 | 88 |
| 7 | DMSO | n-Bu4NCl | 130 | 72 |
| 8 | None | Me4NCl | 130 | <1 |
| 9 | None | Et4NCl | 130 | >99 (85) |
| 10 | None | n-Pr4NCl | 130 | 89 |
| 11 | None | n-Bu4NBr | 130 | 7 |
| 12 | None | Ph4PCl | 130 | 6 |
| 13 | None | Et4NCl | 100 | <1 |
| 14 | None | Et4NCl | 40 | <1 |
We assumed that an anion exchange between KF and Et4NCl occurs to form the more reactive ion pair tetraethylammonium fluoride (Et4NF) under the applied solid-state conditions, thereby improving the reaction efficiency (Scheme 1A).6–9 To test this hypothesis, the reaction of 1a using pre-formed Et4NF was investigated (Scheme 1B). Because anhydrous Et4NF is not commercially available and difficult to prepare, commercial Et4NF hydrate was used. We found that the reaction proceeded to give 2a in 61% yield under our mechanochemical conditions. Although the yield was relatively low, which can probably be attributed to the presence of water,6 this result suggests that Et4NF is most likely the active fluorinating species in the present system. Under conventional solution-based conditions, quaternary ammonium salts act as phase-transfer reagents that improve the solubility of fluoride sources in organic solvents.9 Here, we found a different role for quaternary ammonium salts under mechanochemical conditions, i.e., they can tune and enhance the reactivity of fluoride anions in the solid-state reaction environment, thus enabling highly efficient SNAr fluorination reactions (Scheme 1A).
Next, we explored the substrate scope under the optimized conditions (Table 2). 2-Chloroquinoline derivatives (1a–1d) underwent the solid-state SNAr fluorination to give the corresponding products (2a–2d) in high yield (71–99%). The reaction of 4-chloroquinoline (1e) also proceeded efficiently to form 2e in 84% yield. This method also allows the synthesis of 1-fluoroisoquinoline (2f) in 89% yield. Next, we investigated the substrate scope of pyridine derivatives. We found that the reactions of 2-chloropyridines (1g–1i) with halogen groups selectively provided the 2-fluoropyridines (2g–2i) in good yield (66–83%). 2-Chloropyrazine (1k) also underwent the fluorination to give 2k in moderate yield (63%). For the reactions involving electron-deficient pyridines (1l–1o), adenine (1p), deazapurine (1q), and a pyrimidine (1r) derivative, the use of a smaller jar (1.5 mL) and ball (diameter: 7 mm) was crucial in order to achieve high yields of the corresponding products (2l–2r) (for details, see the ESI†). This mechanochemical approach is furthermore applicable to the electron-deficient benzene derivative 1s, which provided SNAr fluorination product 2s in 80% yield. Next, the robustness of the developed protocol was demonstrated via the efficient solvent-free SNAr fluorination of various bioactive molecules and their building blocks (1t–1o). The fluorination of 1t and 1u, synthetic intermediates for fluoroquinolone-based antimicrobial agents, proceeded smoothly to deliver the desired products (2t and 2u) in good yield (74% and 78%, respectively). The synthesis of a fluoro-analogue of Boscalid (2u), which is a carboxyamide-based fungicide, was accomplished in moderate yield (55%). Overall, the substrate scope of our newly developed reaction was found to be broad and comparable to established solution-based approaches.6–9
| a Unless otherwise noted, all mechanochemical reactions were conducted in a Retsch MM400 mill (stainless-steel milling jar (5 mL); 30 Hz; stainless-steel balls (diameter: 10 mm)). Conditions: 1 (0.5 mmol), KF (2.0 mmol), Et4NCl (0.75 mmol) in a stainless-steel ball-milling jar (5 mL) with a stainless-steel ball (diameter: 10 mm), heat gun set to 250 °C, ball milling (30 Hz) for 60 min. NMR yields were determined based on a 19F NMR analysis with an internal standard and are given in parentheses. b A stainless-steel ball-milling jar (1.5 mL) with a stainless-steel ball (diameter: 7 mm) was used. c Reaction time: 30 min. d Reaction time: 45 min. |
|---|

|
Subsequently, we investigated the solid-state reactions of substrates with different halide leaving groups (Table 3). We found that this method is not limited to arylchlorides, i.e., the reaction of arylbromides and aryliodides afforded the corresponding fluorination products (2b and 2f) in excellent yield (82–99%). The use of a nitro group as a leaving group in a simple cyanobenzene substrate facilitates the mechanochemical SNAr fluorination to give 2w in good yield (62%), while the corresponding chloride showed poor reactivity. This trend in reactivity is identical to the reported solution-based conditions.10
| a For details of the reaction conditions, see the ESI.† |
|---|

|
The utility of this protocol was demonstrated by conducting a scaled-up reaction (Scheme 2). The reaction of 1r on the 3.9 mmol scale was carried out in a 10 mL stainless-steel milling jar using a stainless-steel ball (diameter: 15 mm), which provided 2r in 76% isolated yield without any loss of yield compared to the small-scale reaction. This result emphasizes the practical utility of the protocol.
 | ||
| Scheme 2 Scaled-up reaction of 1r. For details of the reaction conditions, see the ESI.† | ||
Control experiments were performed using a test tube as a reaction vessel to confirm the effectiveness of the ball-milling process (Scheme 3). The SNAr fluorination of 1a was carried out under solvent-free neat conditions using a test tube with a stirring bar at 130 °C. We found that the test-tube reaction showed almost no conversion to 2a after 60 min (5% yield), while the mechanochemical reaction furnished 2a quantitatively after 60 min. Even after 24 h, the yield of the stirred test-tube reaction was still merely moderate (61%). This result clearly shows that strong mechanical agitation imparted by the ball-milling process is essential to achieve the remarkable efficiency of the solid-state SNAr fluorination.
 | ||
| Scheme 3 Reaction in a test tube with a magnetic stirring bar under solvent-free neat conditions. For details of the reaction conditions, see the ESI.† | ||
To quantify the environmental benefits of this solid-state mechanochemical approach, we compared the E-factor of the present solid-state conditions to those of previously reported representative solution-based conditions found in the literature (Table 4). The E-factor is an index for the quantitative evaluation of the environmental impact of a chemical process.17 For our solid-state SNAr fluorination, the E-factor is 2.6 (Table 4, entry 1), whereas the E-factors of representative solution-based methods reported by Bland9c and Sanford10a are 18.7 and 33.5, respectively (Table 4, entries 2 and 3). This difference is mainly due to the absence of bulk solvents under our solid-state conditions. According to these results, the present solid-state SNAr fluorination approach is substantially more eco-friendly than conventional solution-based approaches. However, it should also be noted here that the current workup/purification procedure is not optimal from a sustainability perspective. Even though this was not the focus of this study, it must be taken into account when developing industrial mechanochemical protocols.
| Entry | Conditions | E-factor |
|---|---|---|
| a For details of the E-factor calculations, see the ESI.† | ||
| 1 | KF, nBu4NCl, in DMSO (0.5 M), 130 °C, 24 h | 18.7 |
| 2 | Me4NF, in DMF (0.2 M), rt, 24 h | 33.5 |
| 3 | This work: KF, nEt4NCl, ball milling, 130 °C, 1 h | 2.6 |
Conclusions
In this study, we have developed the first solid-state protocol for SNAr fluorinations of N-heteroaryl halides using a combination of KF and Et4NCl. Remarkably, the reactions of a variety of substrates were completed within 1 h to give the desired fluorinated aromatic compounds in good to high yield. Our protocol is much quicker than previous solution-based methods (typically >24 h) and neither depends on significant quantities of highly polar solvents, which are often not easy to remove and can be highly toxic, nor complicated moisture-free reaction setups that use inert gases. We propose that the active fluorinating reagent under the solid-state conditions is in situ-formed Et4NF. To the best of our knowledge, this is the most inexpensive SNAr fluorination system reported to date (for details, see the ESI†). Given these benefits, our mechanochemical approach has the potential to inspire the development of industrially relevant, solvent-free fluorination technologies.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (JSPS) via KAKENHI grants 24H00453, 24H01050, 24H01832, 22H00318, and 22K18333; by the JST via CREST grant JPMJCR19R1; by FOREST grant JPMJFR201I; and by the Institute for Chemical Reaction Design and Discovery (ICReDD), which was established by the World Premier International Research Initiative (WPI), MEXT, Japan. We thank Mr Reon Hisazumi for his help in cross-checking experiments.References
- J. Wang, M. Sańchez-Rosello, J. L. Aceña, C. delPozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432 CrossRef CAS PubMed.
- (a) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC; (b) A. Haupt, Organic and inorganic fluorine chemistry, De Gruyter, Berlin, Boston, 2021 CrossRef; (c) E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly and N. A. Meanwell, J. Med. Chem., 2015, 58, 8315 CrossRef CAS PubMed.
- (a) T. Fujiwara and D. O'Hagan, J. Fluorine Chem., 2014, 167, 16 CrossRef CAS; (b) P. Jeschke, Pest Manage. Sci., 2010, 66, 10 CrossRef CAS PubMed; (c) M. Braun and J. Eicher, Prog. Fluorine Sci. Ser., 2017, 3, 7 Search PubMed.
- D. J. Adams and J. H. Clark, Chem. Soc. Rev., 1999, 28, 225 RSC.
- P. A. Champagne, J. Desroches, J. D. Hamel, M. Vandamme and J. F. Paquin, Chem. Rev., 2015, 115, 9073 CrossRef CAS PubMed.
- Y. Y. See, M. T. Morales-Colón, D. C. Bland and M. S. Sanford, Acc. Chem. Res., 2020, 53, 2372 CrossRef CAS PubMed.
- Y. Sasson, S. Negussie, M. Royz and N. Mushkin, Chem. Commun., 1996, 297 RSC.
- (a) H. R. Sun and S. G. DiMagno, J. Am. Chem. Soc., 2005, 127, 2050 CrossRef CAS PubMed; (b) H. Sun and S. G. DiMagno, Angew. Chem., Int. Ed., 2006, 45, 2720 CrossRef CAS PubMed.
- (a) L. J. Allen, J. M. Muhuhi, D. C. Bland, R. Merzel and M. S. Sanford, J. Org. Chem., 2014, 79, 5827 CrossRef CAS PubMed; (b) M. A. Cismesia, S. J. Ryan, D. C. Bland and M. S. Sanford, J. Org. Chem., 2017, 82, 5020 CrossRef CAS PubMed; (c) L. J. Allen, S. H. Lee, Y. Cheng, P. S. Hanley, J. M. Muhuhi, E. Kane, S. L. Powers, J. E. Anderson, B. M. Bell, G. A. Roth, M. S. Sanford and D. C. Bland, Org. Process Res. Dev., 2014, 18, 1045 CrossRef CAS; (d) C. M. Hong, A. M. Whittaker and D. M. Schultz, J. Org. Chem., 2021, 86, 3999 CrossRef CAS PubMed.
- (a) S. D. Schimler, S. J. Ryan, D. C. Bland, J. E. Anderson and M. S. Sanford, J. Org. Chem., 2015, 80, 12137 CrossRef CAS PubMed; (b) S. D. Schimler, M. A. Cismesia, P. S. Hanley, R. D. J. Froese, M. J. Jansma, D. C. Bland and M. S. Sanford, J. Am. Chem. Soc., 2017, 139, 1452 CrossRef CAS PubMed; (c) S. D. Schimler, R. D. J. Froese, D. C. Bland and M. S. Sanford, J. Org. Chem., 2018, 83, 11178 CrossRef CAS PubMed; (d) M. T. Morales-Colón, Y. Y. See, S. J. Lee, P. J. H. Scott, D. C. Bland and M. S. Sanford, Org. Lett., 2021, 23, 4493 CrossRef PubMed; (e) S. J. Lee, M. T. Morales-Colón, A. F. Brooks, K. J. Wright, P. J. H. Scott and M. S. Sanford, J. Org. Chem., 2021, 86, 14121 CrossRef CAS PubMed.
- (a) J. Sherwood, F. Albericio and B. G. de la Torre, ChemSusChem, 2024, 17, e202301639 CrossRef CAS PubMed; (b) A. Gescher, Chem. Res. Toxicol., 1993, 6, 245 Search PubMed.
- For selected reviews on the use of ball-milling for organic synthesis, see: (a) S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413 RSC; (b) G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7668 RSC; (c) J.-L. Do and T. Friščić, ACS Cent. Sci., 2017, 3, 13 CrossRef CAS PubMed; (d) J. G. Hernández and C. Bolm, J. Org. Chem., 2017, 82, 4007 CrossRef PubMed; (e) T.-X. Métro, J. Martinez and F. Lamaty, ACS Sustainable Chem. Eng., 2017, 5, 9599 CrossRef; (f) T. K. Achar, A. Bose and P. Mal, Beilstein J. Org. Chem., 2017, 13, 1907 CrossRef CAS PubMed; (g) O. Eguaogie, J. S. Vyle, P. F. Conlon, M. A. Gîlea and Y. Liang, Beilstein J. Org. Chem., 2018, 14, 955 CrossRef CAS PubMed; (h) J. L. Howard, Q. Cao and D. L. Browne, Chem. Sci., 2018, 9, 3080 RSC; (i) J. Andersen and J. Mack, Green Chem., 2018, 20, 1435 RSC; (j) C. Bolm and J. G. Hernández, Angew. Chem., Int. Ed., 2019, 58, 3285 CrossRef CAS PubMed; (k) T. Friščić, C. Mottillo and H. M. Titi, Angew. Chem., Int. Ed., 2020, 59, 1018 CrossRef PubMed; (l) K. Kubota and H. Ito, Trends Chem., 2020, 2, 1066 CrossRef CAS; (m) A. Porcheddu, E. Colacino, L. De Luca and F. Delogu, ACS Catal., 2020, 10, 8344 CrossRef CAS; (n) J. A. Leitch and D. L. Browne, Chem. – Eur. J., 2021, 27, 9721 CrossRef CAS PubMed; (o) K. J. Ardila-Fierro and J. G. Hernández, ChemSusChem, 2021, 14, 2145 CrossRef CAS PubMed; (p) V. Martinez, T. Stolar, B. Karadeniz, I. Brekalo and K. Užarević, Nat. Rev. Chem., 2023, 7, 51 CrossRef CAS PubMed; (q) K. Kubota, Bull. Chem. Soc. Jpn., 2023, 96, 913 CrossRef CAS; (r) N. Fantozzi, J.-N. Volle, A. Porcheddu, D. Virieux, F. García and E. Colacino, Chem. Soc. Rev., 2023, 52, 6680 RSC.
- For selected examples of organic transformations using ball milling from our group, see: (a) K. Kubota, T. Seo, K. Koide, Y. Hasegawa and H. Ito, Nat. Commun., 2019, 10, 111 CrossRef PubMed; (b) Y. Pang, T. Ishiyama, K. Kubota and H. Ito, Chem. – Eur. J., 2019, 25, 4654 CrossRef CAS PubMed; (c) K. Kubota, R. Takahashi and H. Ito, Chem. Sci., 2019, 10, 5837 RSC; (d) T. Seo, T. Ishiyama, K. Kubota and H. Ito, Chem. Sci., 2019, 10, 8202 RSC; (e) T. Kubota, Y. Pang, A. Miura and H. Ito, Science, 2019, 366, 1500 CrossRef PubMed; (f) T. Seo, K. Kubota and H. Ito, J. Am. Chem. Soc., 2020, 142, 9884 CrossRef CAS PubMed; (g) T. Seo, N. Toyoshima, K. Kubota and H. Ito, J. Am. Chem. Soc., 2021, 143, 6165 CrossRef CAS PubMed; (h) T. Seo, K. Kubota and H. Ito, Angew. Chem., Int. Ed., 2023, 62, e202311531 CrossRef CAS PubMed; (i) T. Seo, K. Kubota and H. Ito, J. Am. Chem. Soc., 2023, 145, 6823 CrossRef CAS PubMed.
- For selected examples of mechanochemical fluorination reactions, see: (a) Z. Zhao, S. Ikawa, S. Mori, Y. Sumii, H. Adachi, T. Kagawa and N. Shibata, ACS Sustainable Chem. Eng., 2024, 12, 3565 CrossRef CAS; (b) J. L. Howard, Y. Sagatov, L. Repusseau, C. Schotten and D. L. Browne, Green Chem., 2017, 19, 2798 RSC; (c) J. L. Howard, Y. Sagatov and D. L. Browne, Tetrahedron, 2018, 74, 3118–3123 CrossRef CAS; (d) J. G. Hernández, K. J. Ardila-Fierro, D. Barišić and H. Geneste, Beilstein J. Org. Chem., 2022, 18, 182 CrossRef PubMed; (e) S. Min, B. Park, J. Nedsaengtip and S. H. Hong, Adv. Synth. Catal., 2022, 364, 1975 CrossRef CAS; (f) D. Krištofíková, Ma. Mečiarová, E. Rakovský and R. Šebesta, ACS Sustainable Chem. Eng., 2020, 8, 14417 CrossRef; (g) X. Wang, X. Zhang, L. Xue, Q. Wang, F. You, L. Dai, J. Wu, S. Kramer and Z. Lian, Angew. Chem., Int. Ed., 2023, 62, e202307054 CrossRef CAS PubMed.
- P. Ying, J. Yu and W. Su, Adv. Synth. Catal., 2021, 363, 1246 CrossRef CAS.
- Retail price of Et4Cl in Feb 2024: ca. US $185 per mol (TCI Chemicals); retail price of n-Bu4Cl in Feb 2024: ca. US $643 per mol (TCI Chemicals).
- (a) R. A. Sheldon, Green Chem., 2007, 9, 1273 RSC; (b) V. K. Singh, A. Chamberlain-Clay, H. C. Ong, F. León, G. Hum, M. Y. Par, P. Daley-Dee and F. García, ACS Sustainable Chem. Eng., 2021, 9, 1152 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc06362g |
| This journal is © The Royal Society of Chemistry 2025 |