 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mechanochemically mediated electrosynthesis: unveiling a new pathway for redox reactions under mechanochemical conditions†
Mennatullah M.
Mokhtar
 *,
Tom
Heppler
and
James
Mack
*,
Tom
Heppler
and
James
Mack
 *
*
Department of Chemistry, University of Cincinnati, 301 Clifton Court, Cincinnati, Ohio 45221-0172, USA. E-mail: mackje@ucmail.uc.edu
First published on 15th March 2025
Abstract
Mechanochemistry has emerged as a powerful technique for conducting chemical transformations under minimal or solvent-free conditions, aligning with the global motivation for green protocols. Simultaneously, electrochemically-driven organic reactions have experienced a resurgence, leveraging electricity as a substitute for hazardous oxidants and reductants. While extensive research has focused on understanding both mechanochemistry and electrochemistry, less attention has been devoted to the use of an external power source in combination with mechanochemistry. Here, we explore the synergy between mechanochemistry and electrochemistry as a sustainable technique for organic transformations. We introduce a uniquely designed two-electrode mechano-electrochemical cell (MEC) connected to an external power source. Mechano-electrochemistry allows for precise control of applied potential during milling under minimal solvent conditions. Our investigation aims to unravel the impact of milling on electrochemically driven reactions and understand the parameters required to control electrochemically driven reactions under milling conditions. We present the design and optimization of the MEC, considering factors like electrode material, size, solvent volume, interelectrode gap, and milling motion. The developed MEC demonstrates its effectiveness in electrochemically reducing aromatic bromides and performing electrochemical oxidative coupling for sulfonamide synthesis with minimal solvent loading under milling conditions. This technique highlights the feasibility of performing mechanochemically mediated electrochemical reactions for substrates with low solubility, potentially leading to reduced solvent use, improved yields, and faster reaction times. The integration of electrochemistry and mechanochemistry paves the way for exploring mechanochemically mediated-electrochemistry as a powerful and sustainable tool for organic reactions, with implications for both academic research and industrial applications.
Green foundation1. By using less solvent and less chemical reagents our approach will advance the fields of green chemistry.2. We utilized green chemistry metrics to assess the mechano-electrochemical synthesis of sulfonamides, comparing our method to previously reported approaches. Our novel process achieves a comparable yield, maintains excellent atom economy, and significantly reduces process mass intensity (PMI)—by 51 g g−1 compared to the electrochemical batch reactor approach and 30 g g−1 relative to the microflow cell approach. 3. This study demonstrates a substantial improvement in mass efficiency for sulfonamide synthesis over previously reported methods. Notably, our work showcases the potential of conducting mechanochemically mediated electrochemical reactions with minimal solvent use. This eco-friendly and gentle synthetic methodology paves the way for a more sustainable future in organic synthesis. We envision that the innovative design and application of mechano-electrochemical cells will inspire further research, expanding the scope and applicability of this approach across diverse organic and inorganic transformations. |
Introduction
The field of synthetic organic chemistry has witnessed a significant paradigm shift with the emergence of green chemistry, which prioritizes the development of environmentally benign processes.1 A key focus in designing new synthetic methodologies is to mitigate the adverse impacts on human health and the environment. This growing emphasis on sustainable and eco-friendly techniques has given rise to mechanochemistry as a contemporary alternative to traditional solution-based chemistry, particularly for conducting synthetic transformations with minimal solvent usage. Mechanochemistry has garnered substantial interest across various chemistry domains, including polymer synthesis,2 cocrystal preparation,3 metal–organic frameworks,4,5 mechanoenzymatic synthesis,6,7 mechanoredox,8–12 and transition metal catalysis.13,14 Contrary to the perception that mechanochemistry primarily aims to enhance environmental friendliness by minimizing solvent usage, it is increasingly recognized as a valuable tool for research and exploration. Mechanochemical processes exhibit unique reaction selectivity and reactivity not observed in traditional methods, enabling the discovery of novel products and processes that may be unattainable through conventional solution-based approaches.15 Moreover, mechanochemistry facilitates reactions involving reactants with low solubility, a challenging task in solution-based setups, and allows for shorter reaction times and more convenient setups for synthetic transformations.16The expansion of research on synthetic mechanochemical reactions aligns with the resurgence of organic electrochemistry as another green and sustainable methodology for organic transformations. Organic electrochemistry offers advantages such as precise control over electrode potential, mild reaction compound synthesis.17–25 Unlike traditional methods requiring stoichiometric reagents, electrochemical processes operate with smaller quantities, reducing the overall environmental impact.26 However, organic electrosynthesis presents challenges, including the selection of suitable solvents and electrolytes,18,26 diffusion issues impacting reaction success,27 and potential problems like electrode fouling or degradation, reducing efficiency.28,29 Standardization of equipment and expertise in electrochemistry among the synthetic organic community is lacking, hindering widespread adoption.30 Several strategies have explored the convergence of mechanochemistry and electrochemistry, including electric discharge assisted mechanical milling (EDAMM) utilizing low-current discharge impulses for diverse synthetic pathways.9 Another method showcasing the mechanoredox approach involves the combination of dielectric barrier discharge plasma (DBDP) with milling, demonstrating its potential for applications in alloy synthesis and energy storage materials.10,31 conditions, reduced waste generation, improved safety, and access to unique reaction pathways for complex organic.
However, both approaches primarily focus on inorganic chemical transformations. An innovative technique involves the use of piezoelectric materials in mechanochemical reactions, leveraging their ability to induce polarization under mechanochemical conditions.8 Despite its success, certain limitations are associated with this approach. The homogeneous nature of this method diminishes the efficiency of the piezoelectric material under mechanical force, thereby affecting the overall efficiency of redox chemistry.32 Moreover, the widespread use of BaTiO3 as a piezoelectric material,33 which contains the toxic metal barium, raises environmental concerns. The generation of an AC signal from piezoelectric material also hampers its efficacy in chemical reactions, rendering it more suitable for irreversible reactions.32 Under the influence of mechanical forces, piezoelectric materials undergo division and random orientation, providing access to all modes of compression.32 This particular characteristic can present challenges for materials in which the developed potential may have different signs, complicating the fine-tuning of redox reactions. The precise control of mechanical force for activating the piezoelectric material and tuning the redox chemistry requires further investigation. The feasibility of establishing a galvanic system within mechanochemistry using metal vessels was explored.11 In this approach, a metal vial, accompanied by various metal powders or foils, was utilized along with ionized water to investigate the potential for electron transfer from the metal to organic substrates, resembling a galvanic cell. However, attempts to fine-tune metal combinations to reduce organic substrates inadvertently led to electron transfer from metal to water. Notably, water demonstrated a lower oxidation potential compared to the organic species, resulting in the generation of H2 gas and subsequent hydrogenation of organic compounds. These investigations have sparked further interest in delving deeper into the intersection of mechanochemistry and electrochemistry. Despite the challenges mentioned, the integration of electrochemistry and mechanochemistry shows great potential as an approach for conducting sustainable and environmentally friendly processes in organic chemistry. In this report, we delve into the synergy of mechanochemistry and electrochemistry using a uniquely designed two-electrode mechano-electrochemical cell (MEC) connected to an external power source (Fig. 1B). Our investigation aims to unravel the impact of milling on the performance of electrochemical reactions under conditions with limited solvent loading and understanding the parameters required to control the electrochemically driven reactions under mechanochemical conditions. This approach not only reduces overall reaction waste but also avoids the use of toxic redox agents, shortens reaction times, and ensures mild reaction conditions.‡
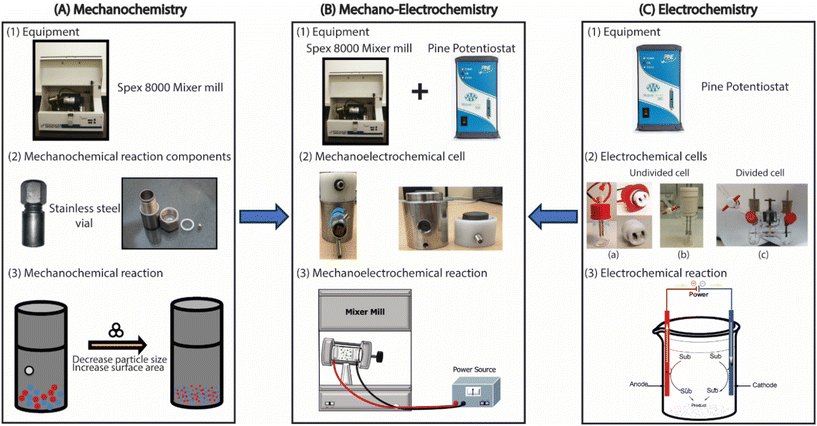 | ||
| Fig. 1 Illustration of mechanochemistry, electrochemistry and mechanochemically mediated electrochemistry: electrochemical cell (a) is reproduced from ref. 36 with permission from American Chemical Society, Electrochemical cell (b), and (c) are reproduced from ref. 37 with permission from John Wiley and Sons. | ||
Results and discussion
Mechano-electrochemical cell design
The conventional electrochemical setup involves a glass cell with electrodes immersed in an electrolyte solution (Fig. 1C). While effective for various electrochemical syntheses, this configuration is incompatible with the milling conditions required for mechanochemical reactions due to glass's inherent fragility. Similarly, the typical mechanochemical setup employs a metal vial (often stainless steel) with a ball bearing for reactant mixing (Fig. 1A). However, this metal vial is unsuitable for use in traditional electrochemical cells as a single electrode.Consequently, a modification and integration of these traditionally used setups in both mechanochemistry and electrochemistry were necessary to enable the execution of an electrochemical reaction under mechanochemical conditions. We developed a two-electrode mechano-electrochemical cell (MEC). This innovative design could be securely held within a Spex 8000 mixer mill and connected to an external power source, enabling a mechanochemically mediated-electrochemical reaction. The iterative process of designing and optimizing the MEC has led to the development of different MEC designs, which we thoroughly investigated for electrochemical reactions. These distinctive designs provided valuable insights into the essential parameters and factors required for MEC design (see ESI† for detailed information about the designs).
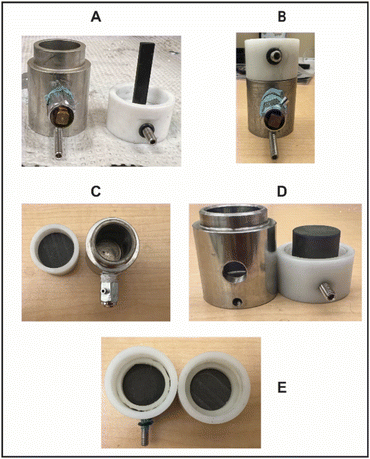
The current MEC's electrode system consists of a stainless-steel vial serving as the first electrode (Fig. 2A). This vial features a threaded hole in its body for attaching a vent to prevent pressure build up and leaks. Stainless steel was chosen for its cost-effectiveness and ease of machining. A blind threaded hole is featured at the bottom of the stainless-steel vial allows for the secure attachment of a crimp terminal, ensuring a robust connection during the milling process. This setup ensures a robust connection, preventing wire disconnection during the milling process. The second electrode is a graphite rod positioned in a slot within a Delrin cap, where the graphite rod is parallel to the wall of the stainless-steel vial (Fig. 2A and B). The Delrin cap's side integrates a threaded groove accommodating a set screw, this set screw is used to tighten the graphite rod securely in position within the Delrin cap. This design offers the parallel alignment, decreasing the interelectrode gap, in addition to the advantage of enclosing the graphite within the stainless-steel vial, minimizing porosity-related leakage concerns, and facilitating prolonged reaction durations. Based on the knowledge gained from these designs, we selected the benzophenone reduction to the diphenylmethanol as a model reaction to evaluate the functionality and viability of our design.
The selection of benzophenone 1 reduction as a model reaction was driven by an important consideration: when the potential of the working electrode is tuned to match benzophenone's reduction potential, electron transfer occurs, generating a characteristic deep blue colored benzophenone ketyl radical, subsequent hydration of this radical produces diphenylmethanol 2.34
This visually apparent color change serves as a reliable indicator of the reaction's initiation, effectively circumventing the need for a reference electrode. Which presented a challenge in precisely measuring the redox potential of the substrate in the current design. Furthermore, incorporating a reference electrode would introduce unwanted complexity to the mechano-electrochemical cell (MEC) design.
This strategic choice bypassed design limitations and established a platform for investigating the mechanochemical and the electrochemical aspects of the synthetic processes. Drawing from insights gained from previous design iterations detailed in the ESI,† we acknowledge the critical importance of various factors such as electrode material, size, solvent volume to electrode ratio, interelectrode gap, and electrode contact. Particularly when aiming to minimize solvent usage in the system and explore the influence of mechanochemistry on reducing solvent for electrochemically driven reactions, it becomes essential to synchronize solvent motion with the ball mill motion. This alignment ensures continuous contact among the electrodes, preventing splashing that could lead to a loss of electrode contact. In consideration of these factors in the current MEC design which has a two-electrode undivided cell system, the volume separating the two electrodes serves as the area for motion during the mechano-electrochemically driven reaction. Integrating a membrane to separate the counter and working electrodes presents design challenges. Using the MEC-1 design (Fig. 2A), we investigated the impact of milling frequency on the electrochemical reaction. We initially conducted the reaction in a solution-based system (η = 50 μL mg−1) by filling 90% of the stainless-steel vial's internal volume with solvent, totaling 8 mL. Without a milling ball, the milling motion alone was sufficient for mixing and mass transport. We conducted the reaction with varying the milling frequencies yielded intriguing results (Fig. 3). When we conducted the electrochemical reaction in a static system (0 Hz), it achieved a commendable 73% conversion, this result confirms efficient electrode contact.
Surprisingly, milling at 1 Hz produced an excellent 88% conversion, this suggests that low milling frequency facilitates the diffusion of electroactive species towards the electrode, and enhances the conversion rate. However, further increase in the milling frequency to 4 Hz and 13 Hz resulted in a substantial drop in conversion, reaching only 39% and 43% respectively. In contrast, elevating the frequency to 9 Hz, 17 Hz, 20 Hz, and 23 Hz exhibited a less pronounced decrease in conversion compared to 1 Hz. These findings indicate that while moderate milling can enhance mass transport and improve conversion; excessive agitation can disrupt the electrode–electrolyte interface and hinder the reaction. As the frequency increases, the electrode has limited time to maintain contact with the region featuring a small interelectrode gap. This results in a brief contact time, impacting the reaction rate. We aimed to investigate the feasibility of solvent-minimized and solvent-free electrochemical reactions under milling conditions, we conducted experiments with varying η (solvent-to-substrate ratio) which is used to assess the nature of the fluid used in the MEC-1 design. When the electrochemical reaction is conducted without adding any solvent (η = 0 μL mg−1), it did not result in the formation of any product and the observed current was negligible, (Table 1, entry 1), the milling of the neat reaction at 17 Hz did not result in the formation of any product, (Table 1, entry 2) this result confirms the electrochemical nature of the reaction. Increasing the solvent volume to assess the feasibility of mechanochemical-assisted electrochemical reactions under liquid assisted grinding (LAG) conditions did not yield any product, and the minimal current observed indicated high resistance due to insufficient conductive media or absence of electrode contact (Table 1, entries 3 and 4). A minimum solvent volume of 2 mL (η = 12.5 μL mg−1) was required for product formation and observable current, indicating a solution-based reaction. When the MEC-1 design was operated in a horizontal position at 0 Hz, a reduced product was observed with only 5% conversion (Table 1, entry 5) however when the milling frequency increased to 17 Hz with horizontal position with (η = 12.5 μL mg−1). (Table 1, entry 6), while milling can enhance mass transfer in some systems, our results indicate that higher milling frequencies in this specific case did not improve the diffusion of electroactive species towards the working electrode. Instead, the increased agitation led to intensified splashing, disrupting the electrode contact and resulting in a noticeable decrease in observed current and product formation. When the MEC-1 was shifted to a vertical orientation without milling, resembling the parallel plate alignment used in previous organic electrosynthesis studies,36 it yielded a moderately successful conversion of 49% (Table 1, entry 7). The decrease in conversion in the horizontal orientation with 2 mL of solvent volume can be attributed to the non-uniform interelectrode gap in the current design. The gap between the stainless-steel vial base and the graphite rod electrode bottom is smaller than the gap along the sidewalls, causing the solvent to spread more towards the wider gap areas. This reduces electrode contact and increases resistance, leading to a slower reaction rate compared to the vertical orientation. Furthermore, real-time current monitoring during milling reactions revealed significant current fluctuations at a low threshold, this emphasizes the importance of maintaining a minimum consistent electrode contact throughout the experiment. This is crucial when minimizing solvent usage and investigating the role of mechanochemistry in electrochemical reactions. A modification to the MEC-1 design was required to keep the solvent flow aligned with the motion of the ball mill. This co-alignment ensured the solvent continually maintains contact with the electrodes, preventing splashing that could disrupt electrode contact and potentially halt the reaction entirely.
To address the design limitations of the previous MEC-1, we replaced the graphite rod electrode with a flat graphite disc electrode (MEC-2, Fig. 2C). The disc is positioned within a Delrin cap, secured in place using a Delrin O-ring. The Delrin cap features a threaded groove for a set screw to securely fasten the graphite disc. The shift from a graphite rod to a graphite disc electrode offers several advantages. First, it allows for the exploration of solvent minimization without the concern of non-uniform interelectrode gap, which were problematic in the MEC-2 design. Second, the disc geometry and position facilitate consistent electrode contact during milling, enabling a more direct evaluation of the milling impact on the electrochemical reaction. The vial's milling motion ensures that the reaction mixture continuously aids in the diffusion of active species to the electrochemical sites on the graphite disc electrode.
To investigate the effect of solvent minimization using MEC-2, we varied the η value (solvent-to-substrate ratio) and monitored the conversion at 4 Hz. At a value of 40 μL mg−1 and 4 Hz, the solution-based electrochemical reaction achieved an 88% conversion (Table 2, entry 1). When the solvent volume was reduced to 1 mL (η = 20 μL mg−1), the conversion decreased to 77% (Table 2, entry 2), this result suggested that lowering the electrode surface exposure to the electroactive species affects the reaction rate. Notably, further reducing the solvent to 0.5 mL (η = 10 μL mg−1) resulted in a slurry-like reaction mixture and a lower conversion rate of 55%. (Table 2, entry 3) For comparison, running the same reaction at 0.5 mL without milling (0 Hz), even with applying a higher voltage, it produced a lower conversion (Table 2, entry 4). This suggests that milling aids in maintaining electrode contact and sustaining the reaction rate. However, when the solvent load was reduced to 0.25 mL (η = 5 μL mg−1), there was negligible current and no product formation (Table 2, entry 5). These results indicate that reducing the solvent volume limits the electrode's exposure to electroactive species, negatively affecting the necessary threshold for electrode contact, increasing resistance, and consequently slowing the reaction rate. Even when applying the maximum voltage available from the potentiostat to overcome system resistance, no reduced product was observed (Table 2, entry 6). This result confirms that increasing the applied voltage cannot compensate for the limitations imposed by insufficient solvent volume. The negligible current observed at low η values suggests difficulties in ion flow, which cannot be adequately detected or controlled due to the limited power capabilities of the system. Additionally, milling the reaction at 4 Hz under maximum voltage with a stainless-steel ball did not mitigate the loss of electrode connection at low solvent volumes or improve the diffusion of electroactive species to the electrode (Table 2, entry 7). A significant reproducibility challenge arose during our investigation with MEC-2, When we replaced the Delrin cap and graphite disc, and the Delrin O-ring with new components. Upon further inspection, we discovered that the original Delrin O-ring had specific gaps that exposed parts of the electrodes, allowing for a reduced interelectrode gap and facilitating electrochemical reactions by providing active areas for the electroactive species in the solvent (Fig. 2E). In contrast, the new O-ring lacked these gaps, effectively blocking the electroactive region and increasing the resistance of the electrochemical reaction (Fig. 2E). To address this challenge, we replaced the flat graphite electrode with a T-shaped graphite electrode for subsequent investigations (MEC-3, Fig. 2D). The T-shaped electrode has a larger surface area positioned parallel to the walls of the stainless-steel vial, maximizing the electrode–electrolyte interface and enhancing mass transport of electroactive species during milling. This larger surface area increases the probability of these species diffusing to the working electrode, potentially improving the reaction rate by reducing the interelectrode gap. Additionally, the T-shaped electrode design resolves the reproducibility issues and eliminates the O-ring's influence on the reaction.
Mechano-electroreduction of brominated aromatic compounds
To evaluate the feasibility of mechanochemically mediated electrochemistry for substrates with limited solubility, we screened several compounds to identify one that would precipitate from the solvent mixture. 4-Bromobenzophenone 3 was selected due to its precipitation behaviour when water was added to acetonitrile, providing an opportunity to investigate the effect of milling on its reduction (Scheme 1). With the MEC-3 design in place, we initially investigated the threshold for solvent minimization in the mechanochemically mediated electrochemical reduction of 3. Using an η value of 6.25 μL mg−1, which produced a slurry-like mixture, we observed the formation of the reduced product. However, further reduction of the solvent loading to neat conditions η value 0 μL mg−1 compromised the conversion, and no current was detected. Next, we explored the electroreduction of 3 at varying milling frequencies using a Spex 8000 mixer. A constant current of −100 mA was applied between a T-shaped graphite anode and a stainless-steel cathode in a total solvent volume of 1.5 mL. The highest conversion 49% to the desired product 1 was achieved at a milling frequency of 4 Hz. Interestingly, increasing the milling frequency to 9 Hz and 13 Hz resulted in lower conversions of 26% and 28%, respectively (Scheme 1). This suggests that milling intensity does not directly correlate with conversion efficiency. A small amount of product 1 4% was detected without milling at 0 Hz. (Table 3, entry 2) To elucidate the interplay between mechanical and electrical factors, a control experiment was conducted at 4 Hz without applying current (Table 3, entry 1). After 2 hours, no product formation was observed, confirming the electrochemical nature of the reaction. Upon applying current and milling at 4 Hz, the reaction resulted in 64% product conversion (Table 3, entry 3), demonstrating the significant enhancement achieved through milling. This result highlights how milling improves the efficiency of the electroreduction process, especially under solvent-minimized conditions. The crucial role of mechanochemistry in enhancing mixing and facilitating the transformation in slurry-based systems underscores the need for a combined mechanochemical and electrochemical approach. These findings confirm the critical importance of coupling mechanochemistry with electrochemistry to ensure successful reaction progression. The absence of either element significantly hinders product formation. To gain further insight into this system, we evaluated the impact of milling balls on mass transport and mixing under slurry conditions (Table 3, entry 4). The presence of two balls did not improve conversion. This suggest that our system does not require the ball to enhance mixing or diffusion of the electroactive species to the electrode. While milling balls are typically useful for enhancing mixing in solventless mechanochemical reactions via impacts and collisions, they are less critical in our electrochemical setup. In the current approach, the interelectrode gap distance and electrode surface area are the key factors driving the reaction. Without sufficient electrode contact, product formation would not occur. Although milling balls could aid when analyte species have difficulty reaching the electrode surface, this is not the case in our system. The analyte species in the slurry system are already in direct contact with the working electrode within the interelectrode gap space assisted by the milling process. Subsequently, we investigated the applicability of this mechano-electrochemical reduction to various bromo-substituted aryl substrates using the same setup and conditions (Scheme 2). Gratifyingly, electron-deficient aryl bromides underwent successful conversion to the desired products with high yields under the optimized conditions. Other aromatic substrates were also amenable to the reaction, albeit with somewhat lower conversion rates (Scheme 2). | ||
| Scheme 1 Screening the impact of milling on substrate with moderate solubility (0.9 mmol scale, 240 mg of 3, η = 6.25 μL mg−1, current applied = −100 mA). | ||
 | ||
| Scheme 2 Screening different brominated aromatic compounds for the mechano-electroreduction (0.9 mmol scale reaction, voltage applied = −4.8 V). | ||
The observed variation in conversion rates might be attributed to the substrates’ higher reduction potentials or potentially lower rates of electron transfer at the electrode surface due to their reduced solubility. Further research is warranted to fully elucidate the role of substrate solubility in these electrochemical reactions. Understanding the limitations of electron transfer to solid substrates is crucial. Even in scenarios where the substrate is surrounded by a conductive solution and subjected to mechanical force, intermixing the conductive solution with the insoluble organic compound might enhance its contact with the electrode material. This improved contact could consequently facilitate a more efficient electron transfer process. The underlying factor determining this phenomenon is yet to be conclusively identified. It is uncertain whether the constraint is rooted in the inherent physical characteristics of the substrates, necessitating the solubility of organic substrates in electrolyte solutions. Alternatively, it may be attributed to the elevated resistance encountered during electron transfer to solid substances, demanding higher voltages and detection capabilities beyond the norm provided by commercially available power sources or the limitation of the electrochemical window of available solvent to conduct electrochemical reactions at high voltages.
Mechano-electrosynthesis of sulfonamides
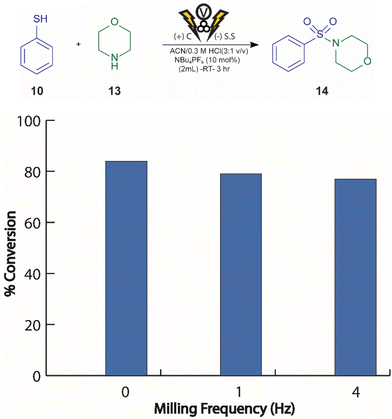
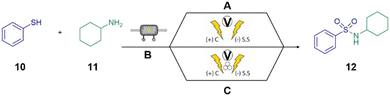
To investigate the potential of mechanochemically mediated electrochemistry for the synthesis of sulfonamides, we drew inspiration from recent advances in electrochemically driven thiol-amine couplings.35,36 Building on these findings, we explored the electrochemically driven oxidative coupling of thiols and amines under minimal solvent conditions, leveraging mechanochemical techniques to achieve faster reaction times. Sulfonamides hold a significant value in the pharmaceutical industry, motivating our focus on their synthesis. We focused our efforts on optimizing their synthesis. Cyclohexylamine 11, a commercially available primary amine, was selected for initial screening with thiophenol as the thiol substrate. Although the desired sulfonamide 12 was successfully formed, subsequent cleavage led to the formation of benzenesulfonamide as a byproduct (see ESI Table S5† for further details). To address this cleavage issue, we shifted to a secondary amine, morpholine 13, due to its commercial availability and stability. We evaluated the reaction between morpholine and thiophenol at varying milling frequencies (Fig. 4). The results demonstrated comparable yields of the desired sulfonamide 14 across all frequencies, suggesting that milling frequency had minimal influence under these specific reaction conditions.Prior to exploring substrate scope, we optimized reaction conditions by studying the influence of both acid type and concentration (Fig. 5). Notably, the absence of acid using deionized water as a solvent resulted in a moderate conversion of 47% to the desired product 14. Upon optimizing the hydrochloric acid (HCl) concentration, a significant improvement in yield was noted, with a peak conversion of 76% at 0.57 M HCl. However, further increase in the acid concentration 5.7 M led to a dramatic decrease in conversion 27%, this result is likely due to product hydrolysis. Further testing of different acids at the optimized HCl concentration (0.57 M) revealed that both toluene sulfonic acid and acetic acid provided lower conversions compared to HCl (Fig. 5).
 | ||
| Fig. 5 Optimizing the conditions for mechano-electrochemical oxidative coupling of thiols and amines (1 mmol scale reaction, 110 mg of 10, η = 8.3 μL mg−1, voltage applied = −3.4 V). | ||
Next, we examined the optimal amount of excess amine required. Incremental increases in amine equivalents yielded comparable results, with 1.7 equivalents achieving the highest conversion. This optimized approach significantly reduced reaction times compared to conventional electrochemically driven batch techniques reported in the literature.35,36 To demonstrate the approach's versatility, we applied it to a variety of aromatic thiols and amines (Scheme 3). Initially, piperidine was tested with various aromatic thiols under the optimized conditions. Thiophenols with electron-withdrawing substituents such as the chlorosubstituted thiophenol favoured sulfonamide 19 formation, while the trifluoromethyl-substituted thiophenol 20 exhibited a significantly lower conversion 20%. Interestingly, the methoxy substituted thiophenol 21 yielded comparable results to its methyl-substituted counterpart 22. Furthermore, the applicability of primary and secondary amines was investigated. Notably, Secondary amines such as morpholine and piperidine gave higher conversions, resulting in sulfonamides 14 and 15, respectively. In contrast, pyrrolidine and diethylamine led to moderate conversions, forming sulfonamides 16 and 17. While primary amines consistently showed lower conversions, with products like sulfonamides 12 and 18 predominantly undergoing cleavage of the alkyl group, producing benzenesulfonamide and other byproducts (see ESI, Table S5 for details†).
 | ||
| Scheme 3 Screening different thiols, and amines for the mechano-electrosynthesis of sulphonamide (1 mmol scale reaction, 1 mmol thiol, voltage applied = −3.4 V). | ||
A noteworthy observation emerged when evaluating the performance of the graphite electrode after multiple uses. The mechanochemically mediated electrochemical reaction of thiophenol and piperidine has encountered a dramatic drop in conversion from (82%) to (34%) after repeated reactions. This suggests a potential alteration in the graphite electrode surface, possibly due to repeated use in multiple reactions. This finding highlights the potential finite lifespan of the graphite electrode in the current approach. Future investigations should explore surface changes occurring in the graphite electrode during the milling process.
Green chemistry metrics
In pursuit of a more sustainable approach, we aimed to develop a greener synthesis of sulfonamides by replacing toxic reagents with electrons, minimizing solvent use, reducing reaction times, and improving overall efficiency. While reaction yield is a commonly used metric in comparing methodologies, it offers a limited perspective on the environmental impact, it solely focuses on the conversion of the limiting reactant without considering factors like solvent use and waste.To address this, several green chemistry metrics have been developed in recent years, providing a more comprehensive tool for evaluating the environmental impact and efficiency of chemical processes. We present comparative data highlighting the green credentials of our method, demonstrating its potential to advance green chemistry practices and achieve environmental improvement over previously reported approaches.35 We compared our method to previously reported approaches for sulfonamide synthesis.35 One earlier method (Table 4, route A) employed a batch reactor with electrochemical activation, using thiophenol and cyclohexylamine in the presence of tetramethylammonium tetrafluoroborate, acetonitrile, and 0.3 M HCl. Although this method achieved a 55% yield with a high atom economy (AE) of 100% and a reaction mass efficiency (RME) of 51%, it required a lengthy 24-hour reaction time and excessive solvent use, resulting in a high process mass intensity (PMI) of 68 g g−1 (excluding workup volumes). This highlights the method's inefficiency in terms of solvent consumption. Another reported method (Table 4, route B) used a microflow electrochemical cell, which achieved a higher yield of 80% and a shorter reaction time. However, it also suffered from excessive solvent usage, reflected in a PMI of 47 g g−1 and a reduced RME of 34%. However, achieved a higher reaction yield and a shorter reaction time. In contrast, our novel approach significantly reduces solvent consumption and achieves the desired transformation in a short timeframe (2 h). (Table 4, route C) Although the RME shows a slight decrease of 4.6% compared to the electrochemical batch reactor approach. This reduction in RME value is due to the slight decrease in the amount of product formed compared to the previous reported approach. The most significant advantage of our method lies in the dramatically reduced solvent consumption. The PMI (without workup) for our novel approach is a mere 17 g g−1, representing a remarkable enhancement in mass efficiency due to 75% solvent reduction over the batch reactor method and 64% reduction over the microflow cell approach (see ESI Table S6† for details on the green metrics calculations).
Our approach offers several key advantages over previously reported methods. First, it significantly enhances mass efficiency, as demonstrated by the substantially lower PMI. Second, the reaction time is dramatically reduced to just 2 hours. Third, unlike previous methods, our approach does not require complete solubility of the substrate in the reaction solvent, which expands its applicability to a wider range of substrates and potential compounds. These features collectively demonstrate the potential of our method to advance green chemistry practices while maintaining or improving on key performance metrics.
Conclusions
Mechanochemistry has emerged as a powerful technique for promoting chemical transformations under minimal or solvent-free conditions, aligning with global efforts to develop environmentally friendly chemical processes. In parallel, electrochemically driven organic reactions have gained renewed attention, utilizing electricity as a clean alternative to hazardous oxidants and reductants. While the individual potential of mechanochemistry and electrochemistry is well-documented, fewer studies have explored their convergence.In this work, we introduced an innovative mechanochemically assisted electrochemical approach, combining a mechanochemical setup with an external electrochemical power source. This novel design allows for the tuning of electrochemical potential under milling conditions, thereby expanding the scope of organic substrates that can be transformed. The optimization of the cell required careful consideration of various parameters, including electrode materials, solvent volume-to-electrode ratio, interelectrode gap, and the path of the milling motion. The developed cell demonstrated effective electrochemical reduction in slurry-based media, making it particularly advantageous for substrates with limited solubility. This approach highlights the potential for achieving higher reaction yields with minimal solvent usage, furthering the green chemistry agenda.
Our study underscores the critical role of mechanical action in facilitating product formation in slurry/paste-based electrochemical reactions. However, it also raises a fundamental question for the mechanochemical community: should a mechanochemical reaction be defined strictly as one driven solely by mechanical activation, or can reactions initiated by other energy sources—thermal, photochemical, or electrochemical—but facilitated by ball milling or mechanical mixing, still be considered mechanochemical? If not, how should such processes be classified, especially when they cannot be performed through conventional means? As highlighted by the article's reviewer, the distinction between mechanochemical activation and mechanical-assisted thermally, electrochemically, or photochemically driven transformations warrants further discussion.
To assess the sustainability of our approach, we employed green chemistry metrics to compare the mechano-electrochemical synthesis of sulfonamides to previously reported methods. Our novel process achieves comparable yields, excellent atom economy, and significantly reduces process mass intensity (PMI)—by 51 g g−1 compared to the electrochemical batch reactor approach and 30 g g−1 relative to the microflow cell approach. This notable reduction in PMI is primarily due to minimized solvent consumption, further underscoring the sustainability of our method.
This study demonstrates a substantial improvement in mass efficiency for sulfonamide synthesis over conventional electrochemical methods. Furthermore, it provides a framework for mechanochemically-assisted electrochemical reactions as a viable green synthetic strategy. We anticipate that the innovative design and application of mechano-electrochemical cells will drive further research, expanding their scope and refining the classification of mechanochemically assisted transformations in chemical synthesis.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
We gratefully acknowledge the generous support from the National Science Foundation (CHE-1900097). We extend our sincere appreciation to Matthew Spetz of the University of Cincinnati's 1819 Innovation Hub – Makerspace for his invaluable guidance and insights throughout this project. Special thanks are due to William Heineman for his insightful discussions, which greatly enriched our understanding. Additionally, we acknowledge Necati Kaval and Bob Voorhees for their assistance with various electronics-related inquiries crucial to this work.References
- O. V. Kharissova, B. I. Kharisov, C. M. O. González, Y. P. Méndez and I. López, R. Soc. Open Sci., 2019, 6(11), 191378 CrossRef PubMed.
- A. Krusenbaum, S. Grätz, G. T. Tigineh, L. Borchardt and J. G. Kim, Chem. Soc. Rev., 2022, 51(7), 2873–2905 RSC.
- D. Braga, L. Maini and F. Grepioni, Chem. Soc. Rev., 2013, 42(18), 7638–7648 RSC.
- F. Afshariazar and A. Morsali, J. Mater. Chem. A, 2022, 10(29), 15332–15369 RSC.
- G. Ayoub, B. Karadeniz, A. J. Howarth, O. K. Farha, I. Lilović, L. S. Germann, R. E. Dinnebier, K. Užarević and T. Friščić, Chem. Mater., 2019, 31(15), 5494–5501 CrossRef CAS.
- M. Pérez-Venegas, G. Reyes-Rangel, A. Neri, J. Escalante, E. Juaristi and J. Beilstein, Org. Chem., 2017, 13(1), 1728–1734 Search PubMed.
- U. Weißbach, S. Dabral, L. Konnert, C. Bolm and J. G. Hernández, Beilstein J. Org. Chem., 2017, 13(1), 1788–1795 CrossRef PubMed.
- K. Kubota, Y. Pang, A. Miura and H. Ito, Science, 2019, 366(6472), 1500–1504 CrossRef CAS PubMed.
- A. Calka and D. Wexler, Nature, 2002, 419(6903), 147–151 CrossRef CAS PubMed.
- M. Zhu, L. Y. Dai, N. S. Gu, B. Cao and L. Z. Ouyang, J. Alloys Compd., 2009, 478(1–2), 624–629 CrossRef CAS.
- M. M. Mokhtar, J. M. Andersen, E. A. Kister, J. X. Hopkins, T. Estier, F. Hamilton, H. Guan, J. Mack and R. A. Haley, Eur. J. Org. Chem, 2023, e202300149 CrossRef CAS.
- V. Martinez, T. Stolar, B. Karadeniz, I. Brekalo and K. Užarević, Nat. Rev. Chem., 2022, 7(1), 51–65 CrossRef PubMed.
- S. Hwang, S. Grätz and L. Borchardt, Chem. Commun., 2022, 58(11), 1661–1671 RSC.
- M. Wohlgemuth, S. Schmidt, M. Mayer, W. Pickhardt, S. Grätz and L. Borchardt, Chem. – Eur. J., 2023, 29, e202301714 CrossRef CAS PubMed.
- J. L. Do and T. Friščić, ACS Cent. Sci., 2017, 3(1), 13–19 CrossRef CAS PubMed.
- C. Wang, M. Hill, B. Theard and J. Mack, RSC Adv., 2019, 9(48), 27888–27891 RSC.
- C. Zhu, N. W. J. Ang, T. H. Meyer, Y. Qiu and L. Ackermann, ACS Cent. Sci., 2021, 7(3), 415–431 CrossRef CAS PubMed.
- Y. Yuan and A. Lei, Nat. Commun., 2020, 11(1), 802 CrossRef CAS PubMed.
- M. Yan, Y. Kawamata and P. S. Baran, Chem. Rev., 2017, 117(21), 13230–13319 CrossRef CAS PubMed.
- A. Wiebe, T. Gieshoff, S. Möhle, E. Rodrigo, M. Zirbes and S. R. Waldvogel, Angew. Chem., Int. Ed., 2018, 57(20), 5594–5619 CrossRef CAS.
- S. Cembellín and B. Batanero, Chem. Rec., 2021, 21(9), 2453–2471 CrossRef PubMed.
- C. Schotten, T. P. Nicholls, R. A. Bourne, N. Kapur, B. N. Nguyen and C. E. Willans, Green Chem., 2020, 3358–3375 RSC.
- S. D. Minteer and P. Baran, Acc. Chem. Res., 2020, 53(3), 545–546 CrossRef CAS PubMed.
- A. Shatskiy, H. Lundberg and M. D. Kärkäs, ChemElectroChem, 2019, 6(16), 4067–4092 CrossRef CAS.
- Y. H. Budnikova, E. L. Dolengovski, M. V. Tarasov and T. V. Gryaznova, J. Solid State Electrochem., 2023, 1, 1–18 Search PubMed.
- N. G. Connelly and W. E. Geiger, Chem. Rev., 1996, 96(2), 877–910 CrossRef CAS PubMed.
- R. E. Wilson and M. A. Youtz, Ind. Eng. Chem., 1923, 15(6), 603–606 CrossRef CAS.
- B. L. Hanssen, S. Siraj and D. K. Y. Wong, Rev. Anal. Chem., 2016, 35(1), 1–28 CAS.
- D. M. Heard and A. J. J. Lennox, Angew. Chem., Int. Ed., 2020, 59(43), 18866–18884 CrossRef CAS PubMed.
- E. J. Horn, B. R. Rosen and P. S. Baran, ACS Cent. Sci., 2016, 2(5), 302–308 CrossRef CAS PubMed.
- Z. Cao, L. Ouyang, Y. Wu, H. Wang, J. Liu, F. Fang, D. Sun, Q. Zhang and M. Zhu, J. Alloys Compd., 2015, 623, 354–358 CrossRef CAS.
- E. M. Marrero, C. J. Caprara, C. N. Gilbert, E. E. Blanco and R. G. Blair, Faraday Discuss., 2023, 241(0), 91–103 RSC.
- J. A. Leitch and D. L. Browne, Chem. – Eur. J., 2021, 27(38), 9721–9726 CrossRef CAS PubMed.
- R. Inoue, M. Yamaguchi, Y. Murakami, K. Okano and A. Mori, ACS Omega, 2018, 3(10), 12703–12706 CrossRef CAS PubMed.
- G. Laudadio, E. Barmpoutsis, C. Schotten, L. Struik, S. Govaerts, D. L. Browne and T. Noël, J. Am. Chem. Soc., 2019, 141(14), 5664–5668 CrossRef CAS PubMed.
- H. R. Stephen, C. Schotten, T. P. Nicholls, M. Woodward, R. A. Bourne, N. Kapur and C. E. Willans, Org. Process Res. Dev., 2020, 24(6), 1084–1089 CrossRef CAS.
- G. Hilt, ChemElectroChem, 2020, 7(2), 395–405 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc06293k |
| ‡ Although the combination of mechanical activation with other energetic sources—such as thermal, photochemical, and in this case, electrochemical—has been demonstrated in the literature, a question arose during the review process of this manuscript: is this process truly mechanochemical energetically, or is it simply mechanomixing? We appreciate this comment as it prompted us to consider whether we could distinguish between these two effects. While the majority of the energy in the system is derived from an electrical source, we believe, based on our results, that some mechanochemical energy is necessary to achieve the desired outcomes. This raises an important question: can a reaction be considered mechanochemical if the majority of the energy does not originate from mechanical impacts? We believe this is a topic for further exploration within the scientific community, and there is a need for better tools to determine the contribution of energy from each source. Therefore, we have chosen to describe the process as “mechanochemically assisted” to differentiate it from a purely mechanochemical reaction. |
| This journal is © The Royal Society of Chemistry 2025 |





![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [g g−1]
[g g−1]