Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Waste-based value-added feedstocks from tire pyrolysis oil distillation: defossilization of the petrochemical industry†
Juan Daniel
Martínez
 *a,
Alberto
Sanchís
*a,
Alberto
Sanchís
 a,
Alberto
Veses
a,
Alberto
Veses
 a,
Andreas
Kapf
b,
José Manuel
López
a,
Andreas
Kapf
b,
José Manuel
López
 a,
María Soledad
Callén
a,
María Soledad
Callén
 a,
Tomás
García
a,
Tomás
García
 a and
Ramón
Murillo
a and
Ramón
Murillo
 a
a
aInstituto de Carboquímica (ICB-CSIC), Miguel Luesma Castán 4, 50018 Zaragoza, Spain. E-mail: jdmartinez@icb.csic.es
bPyrum Innovations AG, Dieselstraße 8, D-66763 Dillingen, Germany
First published on 3rd December 2024
Abstract
The recovery of waste-based feedstocks is an important step in the defossilization of the petrochemical industry and thus in the circular economy for petroleum-based products that have reached the end of their useful life such as end-of-life tires (ELT). This work is part of the European BLACKCYCLE project, and focuses on the distillation performance of tire pyrolysis oil (TPO) obtained from an industrial scale plant, ranked at the ninth technology readiness level (TRL-9). The influence of different reboiler temperatures and reflux ratios on the yields and characteristics of the resulting streams was investigated using a pilot scale packed distillation column under industrially relevant conditions, classified within the fifth technology readiness level (TRL-5). The distillation process was shown to be capable of continuously producing a light fraction (LF) with a very high concentration of benzene, toluene, ethylbenzene and xylenes (BTEX) suitable for high value chemicals. Similarly, a heavy fraction (HF) with a high C/H ratio, high flash point and high presence of polycyclic aromatic hydrocarbons (PAH) is obtained, making it an attractive alternative to carbon black oil. These results are quite outstanding to accomplish the recovery of waste-based value-added feedstocks in such a way that the carbon embedded in the ELT is retained in the petrochemical industry. This work is committed to the development of green, affordable and practical recycling processes to fill the gap in the production of sustainable chemical commodities, while paving the way to address one of the industry's greatest challenges: the defossilization of the petrochemical industry.
1. Introduction
The recycling of post-consumer products such as end-of-life tires (ELT) has become a major challenge worldwide, not only because they are designed to be robust and extremely durable, but also because they are very resistant to biological and chemical treatments (difficult to degrade under typical environmental conditions). In addition, they are continuously generated due to the increasing tire demand. The total amount of tires worldwide was estimated at 30.9 Mt in 2019,1 and the annual growth rate is expected to be 3–4% in the coming years.2 Although most of the ELT generated in developed countries are processed by material and energy recovery processes, significant amounts accumulate in landfills or open fields in developing countries.3 Material recovery includes the production of chips, granules and powders for various civil engineering applications, and even as a preliminary step for energy recovery, which ultimately uses the ELT as a fuel. However, current circular economy regulations aim to recover secondary raw materials that allow carbon to be retained rather than significantly degraded. Therefore, the use of ELT as fuel in combustion systems results in a poor return on value not only because the carbon is degraded to CO2, but also because only a very small amount of energy is recovered (30–35 MJ kg−1) compared to the high energy input required to produce a tire (200 MJ kg−1).1Situated below “reuse” in the waste hierarchy, recycling is expected to contribute significantly to the defossilization and circular economy of most of society's petroleum-based products. In particular, the recycling of ELT should retain the embedded tire carbon to enable a carbon-based petrochemical industry to become circular. This approach enables the transition from a linear to a circular carbon economy while addressing waste management. In addition, the recovery of carbo-chemicals is an excellent example of how the petrochemical industry can implement defossilization processes by displacing as much fossil crude oil as possible. Petrochemicals, indispensable to modern society, involve diverse carbon-intensive activities that are accompanied by negative externalities due to the high resulting carbon footprint. This is due to the greenhouse gas emissions associated not only with fossil fuel-fired heating systems, but also with the fossil feedstock itself from which the petrochemical commodity is derived. Efforts to reduce the former greenhouse gases are referred to as decarbonization, while efforts to replace carbon in chemical products through the use of recovered feedstocks or renewable sources are referred to as defossilization.4 The petrochemical industry is projected to continue to grow and recognizes the limited capacity of the earth to provide conventional fossil carbon feedstocks to meet the challenges ahead.5 Therefore, alternative carbon sources from renewable sources such as biomass and end-of-life products are expected to play an important role, as well as CO2 (from CCS/CCU: carbon capture sequestration and carbon capture utilization) when conversion technologies are fully developed and economically competitive5 (Fig. 1).
Mechanical and chemical recycling is a collection of different technologies that transform polymer waste such as ELT into various secondary raw materials including base chemicals and monomers. Both approaches are complementary, and are expected to help in bridging the gap between waste management and the petrochemical industry.5–8 Pyrolysis is a thermochemical process that falls under the general definition of chemical recycling because it changes the chemical structure of the waste.9 Pyrolysis is currently experiencing an outstanding surge and is likely the leading technology for thermochemical recycling of complex waste such ELT due to its flexibility, robustness, and more recently, favorable economics. Defined as the thermochemical breakdown of macromolecules by energization with heat and usually in the absence of oxygen,10 pyrolysis is capable to digging out the embedded value of the ELT.11 During this process, the rubber compounds (natural rubber, butyl rubber and styrene–butadiene rubber) are separated from the non-volatile components (carbon blacks, SiO2, ZnO, etc.).12,13 Thus, the pyrolysis of ELT produces tire pyrolysis gas (TPG) and tire pyrolysis oil (TPO) associated with the volatile compounds, and raw recovered carbon black (RRCB) related to the carbon blacks used in tire manufacturing. TPG has a remarkable calorific value with important concentrations of H2, and CH4, among others, and is capable of providing the energy required by pyrolysis. TPO is an interesting mixture of liquid hydrocarbon compounds, and typically contains single aromatic compounds such as benzene, toluene, ethylbenzene, and xylenes (BTEXs), as well as heavier hydrocarbon complexes.14,15 RRCB can be improved by milling to enhance reinforcing properties. In this case, and once wires and fabrics have been removed, this fraction should be referred to recovered carbon black (rCB) in accordance with ASTM standard D8178.16 Pyrolysis offers a valuable way to turn the ELT to secondary raw materials for making products, and acts as an environmentally sound alternative to others pathways of the waste hierarchy.17
The highest yields from the pyrolysis of ELT are RRCB (35–45 wt%) and TPO (30–50 wt%).12,13 The former continues to pursue a market niche from a circular economy perspective as a substitute for virgin carbon black, with active support from ASTM, as evidenced by recently published standards.16,18,19 The latter also offers interesting possibilities for use as a substitute for fossil resources in the petrochemical industry, particularly for the extraction of high value chemicals such as BTEX, as well as heavy hydrocarbon feedstocks for carbon black production. The BTEX is one of the top 15 petrochemical commodities20 and has been produced using fossil fuels.21 These monoaromatics are key platform chemicals for the production of multiple derivatives22 or in other words, drive the global consumption of various chemicals, plastics, synthetic fibers and rubber, among others.23 On the other hand, carbon black is also an interesting global commodity, listed as one of the top 50 industrial chemicals with a wide range of applications, the tire industry being the largest application.24 Carbon black is typically produced from high molecular weight hydrocarbon-based feedstocks that are rich in aromatics such as coal tar and from the bottoms of steam crackers and fluid catalytic crackers.25 Therefore, the recovery of BTEX and raw materials for carbon black production from TPO is considered a very attractive option for defossilization in the petrochemical industry, especially considering the enormous potential of many operating refineries to become “waste refineries” as suggested elsewhere.26
Among other benefits, the recovery of these feedstocks from end-of-life products minimizes dependence on non-renewables sources and provides a significant boost to the circular economy. Most chemicals and products are based on finite fossil resources and are therefore highly vulnerable to volatile oil prices, in addition to having high CO2 emissions. The petrochemical subsector is difficult to defossilize not only because of the high heat input required to drive the refinery operations, but also because the raw materials used to produce these commodities are derived from fossil resources, as discussed above. The industrial sector (8.7 Gt CO2 per y) is the second-largest CO2 emitter after the electricity sector (12.3 Gt CO2 per y), and includes the petrochemical, iron, steel, and cement subsectors, among others.27,28 Roughly speaking, the primary energy consumption of these subsectors in 2019 accounted for 1005, 800 and 250 Mtoe per y, respectively, making the petrochemical subsector the largest energy consumer, ahead of the steel and cement subsectors.27,28
This work was carried out as part of the BLACKCYCLE project and demonstrates, under relevant industrial conditions, the recovery of BTEX and raw materials for carbon black production by distillation using a packed column operating in continuous mode. The distillation plant has been designed and erected specifically to operate with TPO, and is at the fifth technological readiness level (TRL-5).11,29 An extensive experimental campaign was conducted to evaluate the effect of reboiler temperature and reflux ratio on the resulting distillation yields, namely the light fraction (LF), which contains the BTEX compounds, and the heavy fraction (HF), which is considered as a feedstock for carbon black production. The TPO used in the distillation unit was obtained from an industrial-scale pyrolysis plant based on the moving bed reactor, which aligns with the ninth technological readiness level (TRL-9).
This work is committed to the development of green, affordable and practical recycling processes to fill the gap in the production of sustainable chemical commodities. In addition, the pyrolysis process of ELT and the refinement/separation of value-added chemicals at a high level of technological maturity exemplifies one of the major challenges for the development of industrial green chemistry around end-of-life products. In this way, this work represents an opportunity to address these challenges by demonstrating not only that the industrial-scale pyrolysis process is real, but also that the production of important, marketable, high-value chemicals that can replace petroleum-based feedstocks is well on its way to industrial deployment. Moreover, as stated by García-García et al.,30 the pyrolysis of end-of-life products is fully consistent with the engineering principles of green chemistry31 in particular atom economy (principle 2) and design for energy efficiency (principle 6). With respect to the principle 2, pyrolysis allows virtually all of the atoms present in the ELT to be reused, in this case as chemical precursors, thus avoiding the generation of waste. This work focuses on TPO using an industrially relevant conditions distillation column to recover BTEX and an alternative feedstock to carbon black oil, demonstrating how crude oil could be displaced in the petrochemical industry. Regarding principle 6, pyrolysis requires energy to drive the endothermic reactions, and this energy could be obtained by using the TPG as a fuel in order to produce either hot flue gases or electricity after the use of engines/turbines. It is also worth noting that pyrolysis is closely related to the E-factor,32 which in turn is closely related to the principles of green chemistry, as this process reduces the amount of final waste generated. The results presented here, are expected to positively impact the goals of the EU Green Deal and some of the UN Sustainable Development Goals (7, 11, 12 and 13), by pursuing the production of waste-based chemical commodities through a circular economy strategy that integrates a pyrolysis technology at TRL-9 and a distillation unit at TRL-5.
2. Materials and methods
2.1. Pyrolysis
The TPO used in this work was produced by Pyrum Innovations AG, which uses a patented moving bed reactor technology to perform the pyrolysis of ELT.33,34 The plant is located in Dillingen/Saar (Germany) and is capable of processing about 5000 t per y of shredded ELT. Following its development and commissioning in 2015, the plant is currently operating continuously twenty-four hours a day, seven days a week; and is therefore aligned with the ninth technological readiness level (TRL-9). A general diagram of the whole plant is shown in Fig. 2. As it is observed, the plant consists of different systems such as feeding, pyrolysis reactor, condensation, and RRCB collection and milling. The feeding system includes shredding and separation units for both wire and fabric, and delivers rubber granules (<8 mm). Both streams are separated from the ELT by magnetic and pneumatic systems, respectively, providing suitable raw materials for other processes.The pyrolysis reactor system consists of a moving bed reactor that is electrically heated to temperatures between 500 and 750 °C. Under these conditions, Pyrum's process yields approximately 35% TPO, 45% RRCB and 20% TPG. The rubber granules are pyrolyzed from the top to the bottom by weight of the material and the residence time is determined by the dosing device installed on the reactor (at least one hour). The reactor itself is designed as a vertical, cylindrical gas-tight apparatus. The condensation unit receives the vapors generated during pyrolysis and produces both TPO and TPG. The latter is sent to a combined heat and power (CHP) unit after being filtered and purified. The electricity generated by the CHP provides the energy needed to power both the pyrolysis reactor and the RRCB mill. The RRCB is continuously extracted from the bottom part of the reactor via sluice systems, and then fed to the milling and pelletizing systems.
2.2. Distillation
The pilot scale distillation plant consists of a packed column and is capable of processing up to 20 kg h−1 of TPO, placing the plant at the fifth technological readiness level (TRL-5). Technical details are provided in other works that also demonstrate its functionality and versatility in distilling various TPOs derived from different pyrolysis technologies.11,29 The distillation column considers eight theoretical minimum equilibrium stages, resulting in a total packing height of 3.5 m. The column has an inside diameter of 11 cm and uses randomly arranged 1′′ stainless steel pall rings as packing, with openings made by folding strips of the surface into the ring (bulk density of 481 kg m−3 and surface area of 210 m2 m−3).As shown in Fig. 3, the TPO is stored in two interconnected vessels (V1 and V2) and pumped to the column using a calibrated peristaltic pump (TPO pump). A guided wave radar level meter (Levelflex FMP50) connected to V2 continuously monitors the TPO level (LT2). Although in this work the TPO is fed at the center of the column, it is also possible to feed it from two other points located at the bottom (stripping section) and top of the column (rectifying section). Each feed line is equipped with pneumatic valves that are connected to the control and acquisition system. The column is equipped with eight thermocouples (Ti) and pressure transducers (pi) that are located along the length of the distillation column, including those of the reboiler. These signals are useful for monitoring the progress of the distillation process, i.e. transient and steady state, as well as for the identification of possible malfunctions.
The reboiler at the bottom of the column provides the energy for the distillation of the TPO. The reboiler consists of a 5 kW electrical resistance array immersed in the TPO. As a result, the generated vapors travel upward across the column in constant contact with the downward TPO stream. In the condenser, the low-molecular-weight vapors leaving the distillation column are recovered as liquids and are referred to as the LF. The condenser uses tap water and consists of a shell-and-tube counterflow heat exchanger. Two containers (V3 and V4) connected to each other store the LF leaving the condenser. V3 is connected to a guided wave radar level transmitter (Levelflex FMP50, LT3) and is used as a reservoir for pumping the LF to the distillation column to evaluate the reflux influence during the distillation process. V4 is used for storage and sampling. A supply line connects V3 and V4 to a second peristaltic pump (reflux pump) and the top of the column. Finally, just below the reboiler, the higher molecular weight compounds go down the distillation column. This stream is the resultant HF, which is collected in the V5 container also equipped with a guided wave radar level meter (Levelflex FMP50, LT5).
2.3. Experimental planning
This work shows the influence of the reboiler temperature (190, 210, 230, 250 and 270 °C) and reflux ratio (up to 1.5) on both LF and HF yields and composition, and the experimental campaign performed is shown in Table S1.† Based on previous distillation experiments, this temperature range was selected to recover BTEX compounds in the LF.11,29 The reflux ratio in a packed distillation column is typically in the range of 1 to 3,35,36 and is defined as the ratio of the liquid mass flow returned to the column to the liquid mass flow removed as product. In other words, it is the ratio between the LF returned to the distillation column (ṁLF-R) and the LF collected in T3 (ṁLF). In all cases, the TPO feed rate was 20 kg h−1. There was no pre-heating of either the TPO or the reflux before it was fed to the distillation column. Temperature and pressure signals were recorded and plotted instantaneously during all tests, while LF and HF yields were determined by weighing at the end of the experiment. Each run at the distillation pilot plant takes one day and includes TPO loading, column heating, distillation, shutdown and cooling, and LF and HF discharge.2.4. Characterization
The boiling range distribution of TPO was measured by gas chromatography (GC) using the method described in the ASTM D2887 standard, and the PerkinElmer Clarus 590. The TPO and the resulting HF were characterized in terms of elemental analysis (Thermo Flash 1112, UNE-EN 15407), heating value (Parr 6400, UNE-EN 15400), and flash point (Grabner Instruments, ASTM D6460). These analytical methods were difficult to apply to the LF samples due to the high volatility of the samples and therefore the results were not repeatable. The TPO and the HF samples were also analyzed by GC coupled to a quadrupole mass spectrometer (MS) (PerkinElmer Clarus 690 – PerkinElmer Clarus SQ 8). The system was equipped with a capillary column, Elite-5 ms (5% diphenyl-95% dimethylpolysiloxane; 60 m × 0.25 mm ID, 0.250 μm df). The identified individual compounds were 99 which were grouped into a total of six families (BTEX, substituted benzenes, indanes and indenes, heterocyclic compounds, and polynuclear aromatic hydrocarbons (PAH)) based on the percentage of relative area. All injections were performed in duplicate. In addition, all samples including the LF were analyzed by gas chromatography (PerkinElmer Clarus 590 GC) to determine the concentration of benzene, toluene, xylenes (o-xylene, m-xylene, p-xylene), ethylbenzene (BTEX) and limonene using a wide-range FID detector and a 60 m DB-5 ms capillary column (0.25 mm ID and 0.25 μm df). The boiling range distribution led to the simulated distillation (SimDist) curve, and was obtained by using a programmable on-column (POC) injector, a wide-range FID detector and a 10 m Elite-2887 column (0.53 mm ID and 2.65 μm df). The analytical procedure used for GC analysis (BTEX and limonene, and SimDist) and GC/MS is detailed in previous works.11,293. Results and discussion
3.1. Tire pyrolysis oil
The characterization of the TPO produced by Pyrum Innovations AG is shown in Table 1. For comparison, the characterization of other TPOs used in the distillation column is also included: TPO-Greenval,11 and TPO-ICB.29 The former was produced in an industrial-scale pyrolysis plant in Zaragoza, Spain, capable of continuously processing approximately 5000 t per y of ELT granules. This plant is owned by Greenval Technologies and based on the single-auger technology using a license from the Spanish Council for Scientific Research (ICB-CSIC).37 The latter came from a TRL-5 pilot-scale pyrolysis plant, which is also based on the single-auger reactor (4 kg h−1) and is also located in Zaragoza. This unit is used to study different pyrolysis conditions, including not only temperature, mass flow rate and residence time, but also ELT type and size, with the goal of validating the process under industrially relevant conditions. The details of both plants are available elsewhere.38 Although both TPO-Greenval and TPO-ICB were obtained in a similar reactor configuration and both have similar basic properties (Table 1), the presence and concentration of chemical compounds is different. As will be discussed later, this is probably due to the longer residence time of volatiles in the industrial plant compared to the pilot plant. The comparison of these TPOs with that produced by Pyrum provides an interesting backdrop to demonstrate the technical feasibility of distilling this type of alternative hydrocarbon, although they have important differences.| Equipment/method | Parameter | Pyrum | Greenval | ICB |
|---|---|---|---|---|
| Thermo Flash 1112, UNE-EN 15307 | Carbon (wt%) | 88.8 | 88.0 | 89.2 |
| Hydrogen (wt%) | 9.2 | 9.8 | 9.1 | |
| Nitrogen (wt%) | 0.9 | 0.9 | 0.6 | |
| Sulfur (wt%) | 0.8 | 0.7 | 1.1 | |
| From elemental analysis | C/H | 0.80 | 0.75 | 0.82 |
| Parr 6400, UNE-EN 15400 | HCV (MJ kg−1) | 41.6 | 42.0 | 39.2 |
| Picnometry | Density @ 25 °C (g ml−1) | 0.93 | 0.92 | 0.90 |
| Crison Titromatic, ASTM E203 | Water content (ppm) | 165 | 153 | 148 |
| Grabner Instruments, ASTM D6460 | Flash point (°C) | <25 | <25 | <25 |
| Mettler Toledo T50 | pH | 7.6 | 6.4 | 6.2 |
| Simulated distillation (ASTM D2887) | IBP (°C) | 64 | 69 | 90 |
| T 50 (°C) | 204 | 243 | 271 | |
| FBP (°C) | 532 | 514 | 534 | |
| Gas chromatography (PerkinElmer Clarus 590) – FID detector and 60 m DB-5 ms capillary column (0.25 mm ID and 0.25 μm df) | Benzene (wt%) | 4.0 | 2.1 | 2.4 |
| Toluene (wt%) | 11.4 | 6.2 | 6.2 | |
| Ethyl-benzene (wt%) | 2.6 | 1.0 | 1.3 | |
| (p + m)-Xylene (wt%) | 7.9 | 5.0 | 4.1 | |
| o-Xylene + styrene (wt%) | 2.4 | 1.8 | 2.5 | |
| Total BTEX (wt%) | 28.2 | 16.2 | 16.5 | |
| Limonene (wt%) | 0.1 | 2.7 | 5.1 |
As it can be observed in Table 1, the results of the elemental (C, H, N and S contents) and calorific (HCV: higher calorific value) analyses are the expected ones, and also compare well with the results presented in various reviews on the subject.12,13 It should also be noted that the basic properties of TPO-Pyrum (density, water content, flash point and pH) are consistent with those of TPO-Greenval and TPO-ICB. In general, these properties warn of the need to take precautions during storage and use to reduce the risk of fire and corrosion. In addition, the C/H ratio (0.80) suggests a high degree of aromaticity, which is confirmed by the significant amounts of BTEX (28.2 wt%) and a practically negligible concentration of limonene. In this regard, a high concentration of BTEX is expected when both a high residence time and a high temperature profile are used during pyrolysis. It has been shown in the literature that BTEX is derived not only from the occurrence of the Diels–Alder reactions among the small chain alkenes12,13 but also from the benzene ring in the styrene–butadiene rubber chain contained in the ELT.39 Conversely, at very short residence times and low temperatures, the TPO typically contains large amounts of aliphatic compounds, including limonene, and the more secondary reactions occur, the lower their presence in the TPO.40 As observed in Table 1, the TPOs previously used in the distillation column have shown lower BTEX concentrations and more limonene, consistent with the temperature and residence time used in their production.11,29
On the other hand, the SimDist curve of the TPO used in this work (TPO-Pyrum), as well as those discussed above (TPO-ICB and TPO-Greenval), is shown in Fig. 4. As can be seen, the boiling point distribution for all curves is very broad and appears to be in the same approximate range (64–535 °C), i.e. with similar initial and final boiling points (IBP and FBP, respectively). However, when comparing TPO-Pyrum to TPO-ICB and TPO-Greenval, the temperature at which 50% of the TPO evaporates (T50) is significantly lower, likely due to a higher presence of BTEX in the former. Although a possible influence on tire composition is also expected,41 this difference is mainly related to the inherent characteristics of the pyrolysis reactor, i.e. the temperature profile along the reactor and the residence time of both solids and volatiles, as discussed above. The TPO studied here comes from a technology that uses higher temperatures and longer residence times, while the other TPOs were produced by using the auger technology under less severe conditions. Therefore, secondary reactions are expected to dominate in the production of TPO-Pyrum, which is also reflected in the lower yield compared to the others: 40 ± 2 wt% for both TPO-Greenval and TPO-ICB.
3.2. Distillation process performance
Fig. 5 and 6 depict the pressure and temperature profiles of the distillation column, respectively, including the different stages of the process: heating (startup), TPO feed, pseudo-steady state, steady state and shutdown, for the experimental run 2. All of the experiments showed the same pattern of reaching steady state conditions, which is an important achievement in TPO refining because it reveals an appropriate control strategy that is difficult to achieve in processes involving unknown hydrocarbon feedstocks such as TPO. A detailed explanation of the distillation column in terms of pressure and temperature patterns, including operating procedures can be found in an earlier paper.11The TPO feed is characterized by a sudden increase in pressure at several points in the column, which quickly stabilizes resulting in a stable profile for both pressure and temperature. However, the pressure in the reboiler continued to increase up to 160 min of operation, which is why this stage is referred to as the pseudo-steady state. During this pseudo-steady state and later during the steady state (where the pressure in the reboiler remained unchanged), the temperatures in the column were practically the same over the operating time, and decreased from bottom to top, as expected (T10 > T4). The steady-state temperature profile of the column for all experiments is shown in the ESI (Fig. S1, S2, S3, S4, and S5†).
The heat supply at the bottom of the column and its distribution along the column appear to be adequate to promote a proper internal flow circulation of the upward vapor stream and the downward liquid stream with no visible symptoms of malfunction. Neither the temperature nor the pressure signals showed any signs of distillation problems such as flooding, suggesting stable operation conditions.42 Except for the reboiler pressure (which always showed the highest value), all signals were quite stable. After 160 min of operation, the reboiler pressure stabilized around 26 mbar, indicating a steady state condition. Since the heavier TPO compounds are held at the bottom of the column during boiling, this pressure considers not only the static head, but also some friction, expansion, contraction, and phase change phenomena. Pressure is considered as the most important parameter in distillation43 as it controls hydraulic and thermodynamic phenomena that could lead to malfunctions.44 Under steady state conditions, the pressure at the top of the column (commonly referred to as the column pressure) is slightly above atmospheric (2 mbar), so the pressure drop of the column is approximately 24 mbar. According to Ray and Das,45 the pressure drop in packed distillation columns varies between 5 and 125 mm of water column per meter of packing height (in this case, between 1.5 and 43 mbar), which confirms a suitable operation and a remarkable milestone.
3.3. Yields
The LF yields for all experiments, along with the temperature at the top of the column (T4), are shown in Fig. 7. The HF yield is the remaining fraction (not shown). The relative error of these yields is less than 3% in all cases. It is also important to point out that some char particles were observed after each distillation run, mainly adhering to the resistance bars of the reboiler. Since the resulting amount was much lower than that of LF and HF, it was not considered as a product. As it can be observed in Fig. 7, the higher the temperature of the reboiler, the higher the LF yield, because the higher the fractionation severity, as expected. Light compounds tend to be enriched in the vapor stream going up the column, while the high molecular weight compounds are expected to be found in the liquid stream going down the column.The reflux ratio also has an effect on the resulting yields, with a clear tendency for the runs performed at 230 °C, 250 °C and 270 °C. In these experiments, the LF yield decreases with the reflux ratio in comparison to the experiments without reflux. The observed lower yield of LF with reflux is consistent with the theoretical results obtained by Aspen Hysys carried out in the design of the distillation column.29 This pattern is related to the temperature at the top of the column, which decreases when the reflux is used because some of the LF is returned to the column lowering the temperature of the rectifying section. The same finding was previously observed in the distillation column using a TPO with different properties.11 As shown in Fig. 7, the difference between the LF yield produced with and without reflux is greater as the reboiler temperature increases because more LF is produced and therefore more liquid is introduced to the top of the column to maintain the same reflux ratio. It is worth noting again that both TPO and reflux were fed to the distillation column at room temperature, so preheating strategies for both streams may be of interest in future work to optimize the distillation operation depending on the compound or family of compounds to be recovered.
3.4. Characterization
Reboiler temperatures above 210 °C result in lower BTEX concentrations in the LF, probably because other compounds are also pulled out of the distillation column as the temperature at the top of the column also increases (see Fig. 7) allowing more compounds to be present in the overhead product. Fig. 8 also suggests that reflux slightly increases BTEX concentrations in the LF with a pronounced effect at the highest reboiler temperature (270 °C). Increasing the reflux ratio to 1.5 at this temperature results in a greater difference in the BTEX concentration when comparing the resulting LF obtained with and without reflux. The sharpness of the rectifying section is promoted by the reflux ratio in order to enrich the concentration of light compounds in the upward vapor stream by separating the heavier compounds contained in it. This improvement in the quality of the LF is strongly dependent on the matter and energy exchange between the liquid and vapor phases, which could be seen somehow by comparing the temperature variation along the column with and without reflux. As can be seen in Fig. S2, S3, S4 and S5 (ESI†), the reflux ratio effect results in a less flat temperature profile in the middle part of the column (the temperature difference between T8 and T4 is always lower in the experiments without reflux than in those with reflux). This pattern is expected to provide better conditions to promote the fractionation process. However, it should be noted that each stage in a packed distillation column (height equivalent to a theoretical plate) has a different efficiency along the column because the volume of the vapor stream, composition, and temperature vary with height.
On the other hand, Fig. 11 shows the flash point of the HF obtained in the experimental campaign, as well as that of the starting TPO. Flash point is considered a key property in handling, transportation and storage, and for carbon black production feedstocks it is generally accepted that it should be higher than 65 °C in order to avoid potential fire hazards.25 However, this value may vary depending on the standards of the carbon black production company. When the feedstock exceeds the regulatory flash point, flammable vapors are released, creating an explosive atmosphere if exposed to an ignition source. Typical feedstocks used to produce carbon black, such as those derived from steam crackers, catalytic crackers, and coal tar distillation, have flash points of 70 °C, 130 °C, and 90 °C, respectively.46
As observed in Fig. 11, the flash point of the HF increases with the reboiler temperature and is significantly improved with respect to the value of the starting TPO. This increasing trend in flash point with reboiler temperature also indicates the low presence of low molecular weight compounds in the HF. A maximum flash point of 83 °C is achieved at both 250 °C and 270 °C reboiler temperatures. The reflux ratio does not seem to help raise the flash point, but rather to lower it, at least when high reboiler temperatures are used in the distillation column. These results are quite interesting as there is no data in the literature on the variation of the flash point of the HF derived from TPO distillation.
In this work, PAH consider molecules containing only carbon and hydrogen, and their chemical structure consists of two or more fused aromatic rings fused in various arrangements with a pair of carbon atoms shared between the rings.22 PAHs are highly desirable for carbon black production24,49 and the more suitable feedstocks for carbon black production are mainly based on three or four ring aromatics.46 The aromatic composition of a typical carbon black oil consists of 10–15% monocyclic, 50–60% bicyclic, 25–35% tricyclic, and 5–10% tetracyclic aromatics.54 Therefore, the resulting HF meets the basic requirements to be considered as a feedstock for carbon black: high C/H ratio, high flash point, and high presence of PAH, which makes it possible to claim about the possibility of recovering waste-based value-added feedstocks from the distillation of TPO.
3.5. BTEX recovery efficiency and mass balance closure
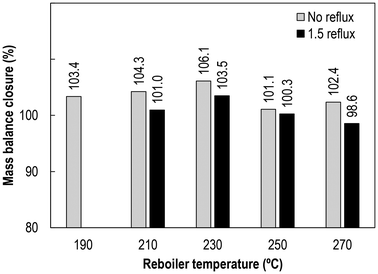
The BTEX recovery efficiency in the LF was calculated based on the amount of BTEX in the LF divided by the amount contained in the TPO, and the results for all the experimental runs are shown in Fig. 14. Contrary to the trend found for the BTEX concentration in both LF and HF, the BTEX recovery efficiency increases linearly with the reboiler temperature. The highest BTEX recovery efficiency (≈100%) is found at the highest reboiler temperature tested (270 °C). However, higher BTEX recoveries do not mean higher BTEX concentration in the LF, as discussed in section 3.4.1. Interestingly, styrene has the highest boiling point of all the BTEXs (145 °C, 1 atm), which is the same temperature found at the top of the column in the experiment conducted at the highest reboiler temperature without reflux (run 5, see Fig. 6). This observation suggests that temperature control at the top of the column plays a key role in achieving good fractionation of multi-component hydrocarbons such as TPO.The mass balance closure (MBC) regarding the BTEX distribution between the inlet (TPO) and outlet streams (LF and HF) for each experiment is shown in Fig. 15. It is observed that the MBC is quite satisfactory (98.6–106.1%), taking into consideration the complexity in the operation of these plants under industrially relevant conditions, as well as the associated error not only with the measurement of the resulting yields, but also in the determination of the BTEX concentrations by GC. Therefore, the results discussed along this paper are quite reliable regarding the characteristics of the resulting products derived from the distillation of the tire pyrolysis oil, and provide an outstanding impetus for realizing defossilization in the petrochemical industry in such a way that the carbon embedded in the ELT is properly retained and converted into value-added feedstocks.
4. Conclusions
This work shows the influence of the two main control variables in packed distillation columns for the recovery of waste-based feedstocks from tire pyrolysis oil: reboiler temperature and reflux ratio. The fact that these results were obtained under industrially relevant conditions opens the door to a strong promotion of the circular economy not only in the tire sector but also in the petrochemical industry. Considering the high BTEX concentration in the TPO used for distillation, the light fraction was found to have a very high BTEX concentration with a maximum of 85.5 wt% and 81.3% recovery efficiency at 210 °C reboiler temperature and 1.5 reflux ratio. Higher severity distillation conditions result in a highly aromatic heavy hydrocarbon fraction suitable for carbon black production. For example, at 270 °C and 1.5 reflux ratio, the C/H ratio, the flash point, and the PAH presence in the resulting heavy fraction were 0.93, 69 °C and 55.6% relative area, respectively. Both the light and the heavy fractions can be considered as value-added feedstocks for the petrochemical industry, particularly as precursor substitutes for BTEX and carbon black oil, and can meet the growing global demand for carbon-based chemicals and derivatives in a sustainable manner.Author contributions
JD Martínez: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing – review & editing. A Sanchís, A Veses, A Kapf, JM López, MS Callén, T García: methodology, investigation, formal analysis. R Murillo: project administration, resources, supervision, writing – review & editing.Data availability
All data supporting the findings of this study are included in the main text and the ESI.† All of relevant data are available from the corresponding authors upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is part of the BLACKCYCLE project (For the circular economy of tyre domain: recycling end of life tyres into secondary raw materials or tyres and other product applications) which has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement no. 869625. The authors would also like to thank the Regional Government of Aragon (DGA) for the support provided under the research groups support program and CSIC for the interdisciplinary thematic platforms SusPlast (Sustainable Plastics) and SosEcoCir (Sustainability and Circular Economy).References
- F. Valentini and A. Pegoretti, End-of-life options of tyres. A review, Adv. Ind. Eng. Polym. Res., 2022, 5(4), 203–213, DOI:10.1016/j.aiepr.2022.08.006.
- Smithers , Global tire manufacturing output to grow 3.4% year-on-year to 2024. https://www.smithers.com. Access: 15/05/2023.
- J. D. Martínez, An overview of the end-of-life tires status in some Latin American countries: proposing pyrolysis for a circular economy, Renewable Sustainable Energy Rev., 2021, 144, 111032, DOI:10.1016/j.rser.2021.111032.
- Z. J. Schiffer and K. Manthiram, Electrification and decarbonization of the chemical industry, Joule, 2017, 1(1), 10–14, DOI:10.1016/j.joule.2017.07.008.
- J.-P. Lange, Towards circular carbo-chemicals – the metamorphosis of petrochemicals, Energy Environ. Sci., 2021, 14, 4358, 10.1039/d1ee00532d.
- J.-P. Lange, Managing plastic waste-sorting, recycling, disposal, and product redesign, ACS Sustainable Chem. Eng., 2021, 9, 15722–15738, DOI:10.1021/acssuschemeng.1c05013.
- H. Li, H. A. Aguirre-Villegas, R. D. Allen, X. Bai, C. H. Benson, G. T. Beckham, S. L. Bradshaw, J. L. Brown, R. C. Brown, M. A. Sanchez, V. S. Cecon, J. B. Curley, G. W. Curtzwiler, S. Dong, S. Gaddameedi, J. E. Garcia, I. Hermans, M. S. Kim, J. Ma, L. O. Mark, M. Mavrikakis, O. O. Olafasakin, T. A. Osswald, K. G. Papanikolaou, H. Radhakrishnan, M. A. Sánchez Castillo, K. L. Sánchez-Rivera, K. N. Tumu, R. C. Van Lehn, K. L. Vorst, M. M. Wright, J. Wu, V. M. Zavala, P. Zhou and G. W. Huber, Expanding plastics recycling technologies: chemical aspects, technology status and challenges, Green Chem., 2022, 24, 8899–9002, 10.1039/D2GC02588D.
- H. Luo, H. Tyrrell, J. Bai, R. I. Muazu and X. Long, Fundamental, technical and environmental overviews of plastic chemical recycling, Green Chem., 2024, 26, 11444–11467, 10.1039/D4GC03127J.
- K. Ragaert, C. Ragot, K. M. Van Geem, S. Kersten, Y. Shiran and S. De Meester, Clarifying European terminology in plastics recycling, Curr. Opin. Green Sustainable Chem., 2023, 44, 100871, DOI:10.1016/j.cogsc.2023.100871.
- A. J. Bowles, Á. Nievas and G. D. Fowler, Consecutive recovery of recovered carbon black and limonene from waste tyres by thermal pyrolysis in a rotary kiln, Sustainable Chem. Pharm., 2023, 32, 100972, DOI:10.1016/j.scp.2023.100972.
- J. D. Martínez, A. Veses, M. S. Callén, J. M. López, T. García and R. Murillo, On fractioning the tire pyrolysis oil in a pilot-scale distillation plant under industrially relevant conditions, Energy Fuels, 2023, 37(4), 2886–2896, DOI:10.1021/acs.energyfuels.2c03850.
- J. D. Martínez, N. Puy, R. Murillo, T. García, M. V. Navarro and A. M. Mastral, Waste tyre pyrolysis - A review, Renewable Sustainable Energy Rev., 2013, 23, 179–213, DOI:10.1016/j.rser.2013.02.038.
- P. T. Williams, Pyrolysis of waste tyres: a review, Waste Manage., 2013, 33, 1714–1728, DOI:10.1016/j.wasman.2013.05.003.
- Á. Muelas, M. S. Callén, R. Murillo and J. Ballester, Production and droplet combustion characteristics of waste tire pyrolysis oil, Fuel Process. Technol., 2019, 196, 106149, DOI:10.1016/j.fuproc.2019.106149.
- F. Campuzano, A. G. Abdul Jameel, W. Zhang, A.-H. Emwas, A. F. Agudelo, J. D. Martínez and S. M. Sarathy, Fuel and chemical properties of waste tire pyrolysis oil derived from a continuous twin auger reactor, Energy Fuels, 2020, 34(10), 12688–12702, DOI:10.1021/acs.energyfuels.0c02271.
- ASTM D8178-22, Standard Terminology Relating to Recovered Carbon Black (rCB), 2022. DOI: 10.1520/D8178-22.
- F. Campuzano, J. D. Martínez, A. F. Agudelo Santamaría, S. M. Sarathy and W. L. Roberts, Pursuing the end-of-life tire circularity: an outlook toward the production of secondary raw materials from tire pyrolysis oil, Energy Fuels, 2023, 37(13), 8836–8866, DOI:10.1021/acs.energyfuels.3c00847.
- ASTM D8466-22, Standard Guide for Recovered Carbon Black—Carbon Black Test Methods for Testing rCB, 2022. DOI:10.1520/D8466-22.
- ASTM F8474-22, Standard Test Method for Recovered Carbon Black (rCB)—Compositional Analysis by Thermogravimetry (TGA), 2022. DOI:10.1520/D8474-22.
- G. Busca, Production of gasolines and monocyclic aromatic hydrocarbons: from fossil raw materials to green processes, Energies, 2021, 14(13), 4061, DOI:10.3390/en14134061.
- A. M. Niziolek, O. Onel and C. A. Floudas, Production of benzene, toluene, and xylenes from natural gas via methanol: Process synthesis and global optimization, AIChE J., 2016, 62(5), 1531–1556, DOI:10.1002/aic.15144.
- G. Lezcano, I. Hita, Y. Attada, A. Bendjeriou-Sedjerari, A.H Jawad, A. Lozano-Ballesteros, M. Sun, N. Al-Mana, M. AlAmer, E.Z Albaher and P. Castaño, Selective ring-opening of polycyclic to monocyclic aromatics: A data- and technology-oriented critical review, Prog. Energy Combust. Sci., 2023, 99, 101110, DOI:10.1016/j.pecs.2023.101110.
- International Energy Agency, The future of petrochemicals. Towards more sustainable plastics and fertilisers, 2018. DOI:10.1787/9789264307414-en. Access: 16/05/2023.
- C. O. Okoye, I. Jones, M. Zhu, Z. Zhang and D. Zhang, Manufacturing of carbon black from spent tyre pyrolysis oil - A literature review, J. Cleaner Prod., 2021, 279, 123336, DOI:10.1016/j.jclepro.2020.123336.
- H. Westenberg, H. Gromes and D. Rechenbach, Carbon black from tire-derived pyrolysis oil, Kautsch. Gummi Kunstst., 2021, 6(21), 47–51 Search PubMed.
- R. Palos, A. Gutiérrez, F. J. Vela, M. Olazar, J. M. Arandes and J. Bilbao, Waste refinery: the valorization of waste plastics and end-of-life tires in refinery units. A review, Energy Fuels, 2021, 35(5), 3529–3557, DOI:10.1021/acs.energyfuels.0c03918.
- International Energy Agency , World Energy Outlook, 2021. https://www.iea.org/reports/world-energy-outlook-2021, Access: 16/05/2023.
- International Energy Agency , Key World Energy Statistics, 2021. https://www.iea.org/reports/key-world-energy-statistics-2021, Access: 16/05/2023.
- J. D. Martínez, A. Sanchís, A. Veses, M. S. Callén, J. M. López, T. García and R. Murillo, Design and operation of a packed pilot scale distillation column for tire pyrolysis oil: Towards the recovery of value-added raw materials, Fuel, 2024, 358, 130266, DOI:10.1016/j.fuel.2023.130266.
- G. García-García, M. A. Martín-Lara, M. Calero and G. Blázquez, Environmental impact of different scenarios for the pyrolysis of contaminated mixed plastic waste, Green Chem., 2024, 26, 3853–3862, 10.1039/D3GC04396G.
- P. T. Anastas and J. C. Warner, Green chemistry: theory and practice, Oxford University Press, 2000 Search PubMed.
- R. A. Sheldon, The E factor at 30: a passion for pollution prevention, Green Chem., 2023, 25, 1704–1728, 10.1039/D2GC04747KB.
- Pyrolysis method and apparatus for carrying out the method, International Patent, WO 2010/127664A1, Inventor: Klaus-Peter Schulz, Assignee: Pyrum Innovations International S.A Search PubMed.
- Thermal reactor, International Patent, WO 2012/092924A1, Inventor: Klaus-Peter Schulz, Assignee: Pyrum Innovations International S.A Search PubMed.
- W. L. McCabe, J. C. Smith and P. Harriot, Distillation, in Unit Operations of Chemical Engineering, McGraw-Hill Inc., 7th edn, 2004, ch. 21 Search PubMed.
- J. M. Coulson and J. F. Richardson, Distillation, in Chemical Engineering Design, Butterworth-Heinemann, 2005, ch. 11 Search PubMed.
- Procedimiento y aparato de termolisis de polimeros de desecho, International Patent, WO 2012/127085A1, Inventor: Ramón Murillo Villuendas, Assignee: Consejo Superior de Investigaciones Científicas, Tecnologias Ineditas Medioambientales S L Search PubMed.
- A. Veses, J. D. Martínez, A. Sanchís, J. M. López, T. García, G. García and R. Murillo, Pyrolysis of end-of-life tires: moving from a pilot prototype to a semi-industrial plant using auger technology, Energy Fuels, 2024, 38(17), 17087–17099, DOI:10.1021/acs.energyfuels.4c02748.
- J. Li, D. Zheng, Z. Yao, S. Wang, R. Xu, S. Deng, B. Chen and J. Wang, Formation mechanism of monocyclic aromatic hydrocarbons during pyrolysis of styrene butadiene rubber in waste passenger car tires, ACS Omega, 2022, 7(47), 42890–42900, DOI:10.1021/acsomega.2c04994.
- N. M. Mkhize, P. van der Gryp, B. Danon and J. F. Görgens, Effect of temperature and heating rate on limonene production from waste tyre pyrolysis, J. Anal. Appl. Pyrolysis, 2016, 120, 314–320, DOI:10.1016/j.jaap.2016.04.019.
- A. Sanchís, A. Veses, J. D. Martínez, J. M. López, T. García and R. Murillo, The role of temperature profile during the pyrolysis of end-of-life-tyres in an industrially relevant conditions auger plant, J. Environ. Manage., 2022, 317, 115323, DOI:10.1016/j.jenvman.2022.115323.
- H. Z. Kister, Common Techniques for Distillation Troubleshooting, in Distillation Operation and Applications, ed. A. Górak and H. Schoenmakers, Elsevier Inc., 2014, ch. 2 Search PubMed.
- H. Z. Kister, Distillation Operation, McGraw Hill, 1990 Search PubMed.
- Z. Li, Y. Chen, Y. Yang, C. Liu, M. Lucquiaud and J. Jia, Flow regime transition in countercurrent packed column monitored by ECT, Chem. Eng. J., 2021, 420(Part 1), 129841, DOI:10.1016/j.cej.2021.129841.
- S. Ray and G. Das, Distillation, in Process Equipment and Plant Design. Principles and Practices, ed. S. Ray and G. Das, Elsevier Inc., 2020, ch. 11 Search PubMed.
- J. B. Donnet, R. C. Bansal and M. J. Wang. Carbon Black, Science and technology, CRC Press, 2nd edn, 1993 Search PubMed.
- M. Srivastava, I. D. Singh and H. Singh, Structural characterization of petroleum based feedstocks for carbon black production, Pet. Sci. Technol., 1999, 17(1–2), 67–80, DOI:10.1080/10916469908949707.
- S. V. Shurupov, Some factors that govern particle carbon formation during pyrolysis of hydrocarbons, Proc. Combust. Inst., 2000, 28(2), 2507–2514, DOI:10.1016/S0082-0784(00)80666-9.
- A. Javadi, S. Soltanieh, S. Sahebdelfar, D. Bastani and K. Javadi, Estimation of temperature and residence time of carbon black oil furnace industrial reactors, Proceedings of IMECE2006, IMECE2006-15671, 2006, pp. 69–77. DOI:10.1115/IMECE2006-15671.
- F. Campuzano, A. G. Abdul Jameel, W. Zhang, A.-H. Emwas, A. F. Agudelo, J. D. Martínez and S. M. Sarathy, On the distillation of waste tire pyrolysis oil: A structural characterization of the derived fractions, Fuel, 2021, 290, 120041, DOI:10.1016/j.fuel.2020.120041.
- M.-J. Wang, C. A. Gray, S. A. Reznek, K. Mahmud and Y. Kutsovsky, Kirk-Othmer Encyclopedia of chemical technology: Carbon black, 2003. DOI:10.1002/0471238961.0301180204011414.a01.pub2.
- A. Boulamanti and J. A. Moya. Energy efficiency and GHG emissions: Prospective scenarios for the chemical and petrochemical Industry, Science for policy report: EUR 28471 EN, 2017. DOI:10.2760/20486.
- Y. Abdallas Chikri and W. Wetzels, Decarbonization options for the Dutch carbon black industry. PBL Netherlands Environmental Assessment Agency, TNO, 2020. https://resolver.tno.nl/uuid:38ed9922-ce9c-4e8c-8388-e13276694a08m Search PubMed.
- European Commission, Integrated pollution prevention and control. Reference document on best available techniques for the manufacture of large volume inorganic chemicals - solids and others industry, 2007. https://eippcb.jrc.ec.europa.eu/reference/large-volume-inorganic-chemicals-solids-and-others-industry.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc05185h |
| This journal is © The Royal Society of Chemistry 2025 |