Single cobalt atom catalysis for the construction of quinazolines and quinazolinones via the aerobic dehydrocyclization of ethanol†
Xueping
Zhang
a,
Kai
Xu
a,
Yi
Zhuang
a,
Shihao
Yuan
a,
Yamei
Lin
bc and
Guo-Ping
Lu
 *a
*a
aSchool of Chemistry and Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, China. E-mail: glu@njust.edu.cn
bInternational Innovation Center for Forest Chemicals and Materials, Nanjing Forestry University, Nanjing 210037, China
cNanjing Normal University, School of Food Science and Pharmaceutical Engineering, Nanjing, 210032, China
First published on 5th November 2024
Abstract
The synthesis of N-heterocycles through the aerobic dehydrocyclization of ethanol is still significant and challenging since ethanol is the largest renewable small molecule feedstock but with high dehydrogenation activation energy. Herein, a single Co catalyst (Co1@NC-50) with oxidase-like active sites (CoN4) has been fabricated for the construction of quinazolines and quinazolinones using ethanol as the C2-synthon. The merits of this approach include the use of air as the oxidant and abundant metal recyclable catalyst, free of additives, high step and atom economy, and broad substrate scope, showing great potential for application in drug synthesis. The mechanistic insights are also gained: (1) ethanol dehydrogenation is the rate-determining step of this reaction, which is mainly implemented by ˙O2−; (2) the CoN4 site exhibits a strong ability for adsorption of O2 and ethanol, and it has the lowest ethanol dehydrogenation energy barrier than CuN4 and FeN4. To the best of our knowledge, this is the first example of single atom catalysis for the synthesis of N-heterocycles using ethanol as the C2-synthon.
Introduction
Ethanol is widely used in industry, medicine and daily life and is mainly produced through biomass fermentation.1 At present, the annual production of ethanol worldwide is about 100 million tons, making it the largest renewable small molecule feedstock.2 Most ethanol is used as fuel, and it can also be used as a C2-synthon to synthesize high-value N-heterocycles since it has both electrophilic and nucleophilic sites,3 which is undoubtedly more in line with the principles of green chemistry. The construction of N-heterocyclic compounds has always been a central topic in organic synthesis.4 As important representatives of these compounds, quinazolines and quinazolinones have been widely used in dyes,5 fluorescent materials,6 and drugs (Fig. 1).7 In view of this, it is meaningful and sustainable to develop novel strategies for the construction of quinazoline and quinazolinone skeletons using ethanol as the C2-synthon.Nevertheless, most studies have focused on the dehydrogenation coupling of aromatic alcohols (benzyl alcohols, heterocyclic methanols, aryl ethanols) for the synthesis of N-heterocycles.8 Ethanol has much higher dehydrogenation activation energy than aromatic alcohols. For example, the bond dissociation energy of the methylene C–H bond of ethanol (403 kJ mol−1) is much higher than that of benzyl alcohol (332 kJ mol−1), so the construction of N-heterocycles via ethanol as a C2-synthon is still in its infancy.
In 2015, Singh's group9 reported KOH-promoted synthesis of quinolines from ethanol and 2-aminobenzyl alcohols/2-aminobenzophenones. Several homogeneous transition metal catalysts, such as Co, Ir, Ru complexes10 and CuI11 have also been developed for the fabrication of quinazolinones through dehydrogenative coupling strategies. However, these homogeneous catalytic systems suffer from separation difficulties and the use of stoichiometric oxidants and bases. In terms of heterogeneous catalysis, several studies have disclosed the generation of benzimidazoles,12 but the reaction temperature is up to 180 °C. Two heterogeneous catalysts including oxygen lattice modified MoS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 and Ni-NiAl-LDO14 have been developed for the synthesis of methyl substituted quinolines and 1,2,3,4-tetrahydroquinolines via dehydrocyclization of ethanol (Fig. 2a). In view of the importance of quinazoline and quinazolinone frameworks and the scarcity of ethanol as a C2-synthon for the construction of these two frameworks, it is meaningful to develop an effective and abundant transition metal heterogeneous catalyst to realize this strategy.
13 and Ni-NiAl-LDO14 have been developed for the synthesis of methyl substituted quinolines and 1,2,3,4-tetrahydroquinolines via dehydrocyclization of ethanol (Fig. 2a). In view of the importance of quinazoline and quinazolinone frameworks and the scarcity of ethanol as a C2-synthon for the construction of these two frameworks, it is meaningful to develop an effective and abundant transition metal heterogeneous catalyst to realize this strategy.
Single-atom catalysts (SACs) narrow the gap between homogeneous and heterogeneous catalyses because of their 100% metal atom utilization, uniformly adjustable active sites and the characteristics of heterogeneous catalysts.15 Among them, the single metal sites anchored on the N-doped carbon structure (M–N–C) have a MNx coordination structure similar to active sites of many natural oxidases, which can effectively activate oxygen to form reactive oxygen species.16 More recently, cobalt catalysis has received widespread attention due to its inexpensiveness, easy availability and excellent redox properties.17 Co single atoms confined in N-doped carbon display excellent performance in many aerobic oxidations and their induced tandem reactions.18
Based on the above results, we have developed a Co single-atom anchored N-doped carbon catalyst (Co1@NC-50) with CoN4 sites for the coupling–annulation reaction for quinazolines and quinazolinones trigged by the aerobic oxidation of ethanol (Fig. 2b). This approach has the characteristics of high step and atom economy, a wide range of substrates, free of organic solvent and additives. To the best of our knowledge, this is the first example of single Co atom catalysis for dehydrogenation coupling of ethanol to construct N-heterocycles, providing an opportunity to study the facilitating role of Co species in the aerobic oxidation of alkyl alcohols.
Experimental
Catalyst preparation
Other precursors including CoZn@BZIF-10, CoZn@BZIF-20 and ZIF-8 were prepared by changing the amount of Co(NO3)2·6H2O (the numbers represent the molar ratios of Zn(NO3)2·6H2O and Co(NO3)2·6H2O). CoZn@BZIF-50-1 precursors were prepared without adding benzylamine.
Subsequently, the prepared precursors were calcined under a N2 atmosphere at 900 °C for 2 h to obtain an N-doped carbon-based catalyst. CoZn@BZIF-10, CoZn@BZIF-20, CoZn@BZIF-50, CoZn@BZIF-50-1 and ZIF-8 were converted into Co@NC-10, Co@NC-20, Co1@NC-50, Co@NC-50-1 and Zn@NC, respectively.
| Entry | ZIFs | M@NCa | Noteb |
|---|---|---|---|
| a NC means N-doped carbon. b 10, 20 and 50 are the molar ratios of Zn/M. | |||
| 1 | CoZn@BZIF-10 | Co@NC-10 | Zn/Co = 10 |
| 2 | CoZn@BZIF-20 | Co@NC-20 | Zn/Co = 20 |
| 3 | CoZn@BZIF-50 | Co1@NC-50 | Zn/Co = 50 |
| 4 | CoZn@BZIF-50-1 | Co@NC-50-1 | Zn/Co = 50, no use of benzylamine |
| 5 | ZIF-8 | Zn@NC | No use of Co(NO3)2·6H2O |
| 6 | FeZn@BZIF-50 | Fe@NC-50 | Zn/Fe = 50 |
| 7 | CuZn@BZIF-50 | Cu@NC-50 | Zn/Cu = 50 |
| 8 | MoZn@BZIF-50 | Mo@NC-50 | Zn/Mo = 50 |
Catalytic reaction
Typically, 0.2 mmol o-aminobenzyl alcohol, 2 mL ethanol, 1 mL NH3·H2O and 20 mg Co1@NC-50 (2.5 mol% Co) were added to a 25 mL sealed tube. The sealed tube was placed in an oil bath preheated to 100 °C, and the reaction was stirred for 6 h. After the reaction was complete, ethyl acetate was added to dilute the reaction mixture. The diluted mixture was filtered through a bed of silica gel layered over Celite, and concentrated under reduced pressure to obtain a crude product. The crude product was purified through silica gel column chromatography using petroleum ether/ethyl acetate as the eluent with the polarity gradually increasing from 20% (ethyl acetate/petroleum ether, v/v = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to 70% (ethyl acetate/petroleum ether, v/v = 7
8) to 70% (ethyl acetate/petroleum ether, v/v = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3).
3).
Catalyst characterization
An N-doped carbon supported Co single atom catalyst (Co1@NC-50) was obtained by the pyrolysis of CoZn@BZIF-50, which was derived from a one-pot self-assembly of Co(NO3)2·6H2O, Zn(NO3)2·6H2O, 2-MeIm and benzylamine (Fig. 3).19 The Co content of Co1@NC-50 is about 1.48 wt% as determined by ICP-MS (Table S1†). The scanning electron microscopy (SEM) image exhibits that CoZn@BZIF-50 is in regular dodecahedral morphology (Fig. 4a). After pyrolysis at 900 °C for 2 h, Co1@NC-50 retains the morphology of the precursor, but with partial shrinkage and shape defects (Fig. 4b). For a comparison, NC, Co@NC-10, Co@NC-20 and M@NC-50 (M = Fe, Cu and Mo) were also synthesized using a similar procedure.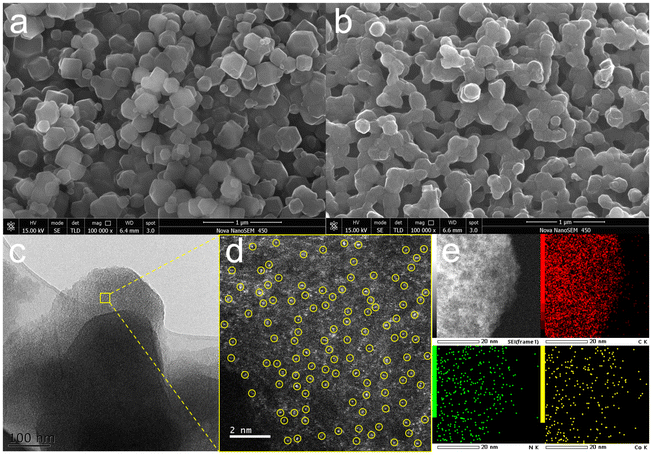 | ||
| Fig. 4 SEM images of (a) CoZn@BZIF-50 and (b) Co1@NC-50. (c) TEM image of Co1@NC-50. (d) HAADF-STEM image of Co1@NC-50. (e) The EDX elemental mapping images of C, N and Co of Co1@NC-50. | ||
The two wide peaks at 24° and 43° in the XRD pattern of Co1@NC-50 are attributed to the (002) and (101) crystal faces of graphite-carbon materials, respectively,20 and no Co characteristic diffraction peaks can be detected (Fig. S1†). There are obvious metal Co diffraction peaks at 44°, 51° and 76° (JCPDS 15-0806) in the XRD pattern of Co@NC-10 (Fig. S1†). According to the transmission electron microscopy (TEM) images, no cobalt nanoparticles are observed in Co1@NC-50 (Fig. 4c). Co@NC-10 exhibits an irregular nanotube morphology, in which obvious cobalt nanoparticles were observed (Fig. S2†).
The porous nature of Co1@NC-50 and Co@NC-10 was also studied by N2 adsorption–desorption isotherm testing. Co1@NC-50 is a type I isotherm with a BJH adsorption average pore diameter of 2.6 nm and a Brunauer–Emmett–Teller surface area (SBET) of 1115.8 m2 g−1. Co@NC-10 is a type IV isotherm with a SBET of 614.6 m2 g−1, and its pore diameter is 3.8 nm (Fig. S3 and Table S2†). It can be concluded that the cobalt content is inversely proportional to the dispersion of cobalt sites and the specific surface area of the catalyst. It is universally acknowledged that atomic dispersed metal sites and high SBET are essential to improve the metal utilization rate and catalytic activity of catalysts.21
The surface electronic structure and chemical state of Co1@NC-50 were analyzed by X-ray photoelectron spectroscopy (XPS). In the survey spectrum, elements including C, N, O, Co and Zn can be identified (Fig. S4a†). In the Co 2p spectrum of Co1@NC-50, no peaks of metallic cobalt were resolved. The binding energies of 779.9 eV and 781.5 eV are assigned to Co3+ and Co2+, respectively (Fig. S4b†), manifesting its ionic Coδ+ (2 < δ < 3).22 In the N 1s spectrum, oxidized-N (403.1 eV), graphitic-N (401.2 eV), pyrrolic-N (399.2 eV) and pyridinic-N (398.5 eV) can be detected.23 Notably, a signal of CoNx can be observed at 400.6 eV (Fig. S4c†), which verifies the existence of a Co–N coordination structure in the material.23c
The Co configurations in Co1@NC-50 is further analyzed at the atomic level by high angle annular dark-field STEM (HAADF-STEM), where a large number of bright spots marked in yellow represent a single isolated Co atom (Fig. 4d). The elements C, N and Co are uniformly distributed in Co1@NC-50 according to the EDX elemental mapping images (Fig. 4e).24
The Co site coordination structure of Co1@NC-50 was revealed at the atomic level by the Co K-edge X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS). The XANES spectrum of Co1@NC-50 exhibits a signal distinct from that of Co foil, Co2O3 and CoPc (Fig. 5a). The absorption edge or the K-edge of the sample is between Co2O3 and CoPc, indicating that the valence state of Co in the sample is between +2 and +3. In the EXAFS spectrum, Co1@NC-50 shows an obvious strong peak at 1.41 Å, which can be attributed to the Co–N scattering path, and there is no Co–Co coordination peak at 2.18 Å (Fig. 5b). These results demonstrate that Co species of Co1@NC-50 mainly exists in the form of single atoms, which is consistent with HAADF-STEM and XRD results. The EXAFS spectrum of Co1@NC-50 was analyzed using IFEFFIT fitting. The peak at 1.41 Å originates from the first-shell coordination of CoN4, and the error of the best fitting results was within a reasonable range (Fig. 5c and Table S3†).25
 | ||
| Fig. 5 (a) Co K-edge XANES spectra. (b) FT EXAFS spectra. (c) EXAFS curve fitting of Co1@NC-50 in R space. | ||
Catalytic performance
The catalytic performance of the catalyst was investigated using the annulation reaction of o-aminobenzyl alcohol, ethanol and ammonia as the model reaction (Table 2). After screening of different M@NC-50 catalysts (M = Fe, Cu, Mo), Co1@NC-50 exhibited the best catalytic activity (entries 1–4). Other M@NC-50 displayed poor catalytic capacity for this C–N bond coupling cyclization (Table S4†). Only 9% yield of 4a was observed using Co@C as the catalyst, indicating that N-doping can effectively enhance the catalytic activity of cobalt sites (entry 5).26| Entry | Catalyst | Atmosphere | Conv. (%) | Yield of 4a |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (2 mL), 3 (1 mL), catalyst (2.5 mol% of metal), air, 100 °C, 6 h. b Yields were determined by GC using mesitylene as the internal standard. c 20 mg. d 60 °C. e 80 °C. | ||||
| 1 | Fe@NC-50 | Air | 95 | 48 |
| 2 | Cu@NC-50 | Air | 95 | 30 |
| 3 | Mo@NC-50 | Air | 67 | 22 |
| 4 | Co 1 @NC-50 | Air | 97 | 97 |
| 5 | Co@C | Air | 23 | 9 |
| 6 | — | Air | 4 | — |
| 7 | NC | Air | 59 | 20 |
| 8 | Zn@NCc | Air | 64 | 24 |
| 9 | Co@NC-50-1 | Air | 76 | 30 |
| 10 | Co@NC-20 | Air | 96 | 86 |
| 11 | Co@NC-10 | Air | 95 | 48 |
| 12 | Co(NO3)2·6H2O | Air | 96 | 61 |
| 13 | Co(OAc)2·4H2O | Air | 96 | 52 |
| 14 | CoPc | Air | 84 | 61 |
| 15 | Co(acac)2 | Air | 41 | 15 |
| 16 | Co2O3 | Air | 6 | — |
| 17d | Co1@NC-50 | Air | 97 | 15 |
| 18e | Co1@NC-50 | Air | 96 | 85 |
| 19 | Co1@NC-50 | N2 | 77 | 44 |
| 20 | Co1@NC-50 | O2 | 98 | 98 |
No reaction took place in the absence of catalysts (entry 6). Poor yields of 4a were afforded in the cases of NC and Zn@NC, suggesting that Co is the main active center of this catalyst (entries 7 and 8). Compared with other Co catalysts, Co1@NC-50 provided the best results (entries 4 and 7–16). Among them, the cobalt phthalocyanine complex (CoPc) with a CoN4 coordination structure could not achieve the same catalytic activity as the Co1@NC-50 catalyst. This may be related to the large specific surface area, suitable pore structure, and unique electronegativity of Co in Co1@NC-50. Lowering the temperature is not conducive to the reaction (entries 17 and 18). The control experiments imply that molecular oxygen is necessary for the reaction (entries 19 and 20). To demonstrate the practicability of the developed synthesis methodology, compound 4a was synthesized on a gram scale using 9 mmol o-aminobenzyl alcohol (Scheme S1†), yielding a good product yield of 79%.
In order to further test the general applicability of Co1@NC-50, a series of 2-aminoaryl alcohols and aliphatic alcohols were applied in the protocol (Scheme 1). This approach is suitable for a wider range of aliphatic alcohols including methanol, ethanol, 1-propanol, 1-butanol, 1-pentanol, 1-hexanol, 1-heptanol, 1-octanol, isoheptanol and isopropanol, all of which can covert to corresponding 2-substituted quinazolines in 31–92% isolated yields (4a–4k). Both 2-aminoaryl alcohols with electron-donating (–Me, –OMe) and weak electron-withdrawing (–F, –Cl) groups afford the desired products in medium to excellent yields (4l–4o). Generally, electron-rich 2-aminoaryl alcohols are more likely to be oxidized and coupled to trigger the reaction.27 In addition, both 2-amino-3-hydroxypyridine and 1-(2-aminophenyl) ethanol could be converted to the corresponding quinazolines (4p–4r). The yield of the reaction between benzyl alcohol and o-aminobenzyl alcohol was 67% (4s).
2-Aminobenzophenone derivatives were also explored to obtain the quinazoline products 6 (Scheme 2). 2-Aminobenzophenones with weak electron-withdrawing (–F, –Cl, –Br) groups provide the corresponding quinazolines with high yields (6b–6e), while the strong electron withdrawing (–NO2) group inhibits the reaction (6f).
 | ||
| Scheme 2 Substrate range of 2-aminobenzophenone derivatives 5.a,b (a) Reaction conditions: 5 (0.2 mmol), 2 (2 mL), 3 (1 mL), Co1@NC-50 (2.5 mol% of Co, 20 mg), 100 °C, 6 h, air. (b) Isolated yields. | ||
In order to further expand the scope of this transformation, direct synthesis of quinazolinone from isatoic anhydrides was also attempted (Scheme 3). This transformation could be achieved by increasing the reaction temperature and prolonging the reaction time. A series of alkyl alcohols could react with isatoic anhydride to form corresponding coupling products (8a–8d). In addition, the electronic effect of the substituents has little effect on this reaction (8e–8j).
 | ||
| Scheme 3 Substrate range of isatoic anhydrides 7 and alkyl alcohols 2.a,b (a) Reaction conditions: 1 (0.2 mmol), 2 (2 mL), 3 (1 mL), Co1@NC-50 (2.5 mol% Co), 120 °C, 24 h, air. (b) Isolated yields. | ||
It should be noted that the synthesis of 2-methyl-substituted quinazolines and quinazolinones have significant implications in the field of medicinal chemistry (Fig. 1). As shown in Scheme 4a, a 84% yield of 6g was produced by the annulation of (2-aminophenyl)(2-chlorophenyl)methanone, ethanol and ammonia, which can be used as an intermediate for the synthesis of ER176 (positron emission tomography (PET) radioactive ligand).288a, 8e, and 8g can serve as intermediates for the synthesis of Methaqualone (sedative-hypnotic drug),29Raltitrexed (thymidylate synthase inhibitor)30 and CHEMBL5087955 (epithelial growth factor receptor),31 respectively (Scheme 4b). Furthermore, a microtubule blocker, Verubulin8ab, was synthesized by the chlorination and amination reactions using 8a as the raw material.32
Catalytic mechanism
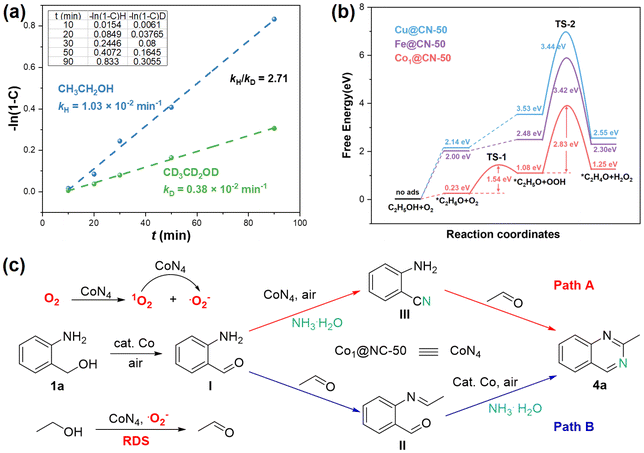
According to control experiments (Scheme S2†), compounds I–III should be the intermediates of this tandem reaction. This assumption was also verified by reaction tracking experiments (Fig. 6a). All the possible intermediates I–III can be detected by GC-MS at the initial stage of the reaction (Fig. S6†), and these compounds will gradually be consumed as the reaction continues.According to kinetic studies, it can be observed that (1) the ethanol concentration was positively correlated with the initial reaction rate (Fig. 6b); (2) O2 concentration was not related to the initial reaction rate (Fig. 6c); (3) 2-aminobenzyl alcohol and ammonia were negatively correlated with the initial reaction rate (Fig. S5†). These results indicate that the activation of ethanol may be included in the rate-determining step (RDS) of the reaction.33
To further elucidate the reaction mechanism, we conducted free radical capture (Fig. 7) and H/D kinetic isotope effect (KIE) experiments (Fig. 8a). The addition of TEMPO and t-butanol (˙OH scavenger) to the model reaction has little effects on the reaction (Fig. 7a and b). β-Carotene and p-benzoquinone (BQ) were also applied to the reaction to trap 1O2 ˙O2−, respectively.34 β-Carotene has a moderate inhibitory effect on the reaction, and only a trace amount of 4a is afforded in the presence of BQ (Fig. 7c and d). Furthermore, β-Carotene and BQ as radical scavengers were added to the reactions of III with 2, and I with 2, respectively (Fig. S7 and S8†). The inhibition of BQ on both reactions mentioned above is greater than that of β-Carotene. It can be concluded that (1) ˙O2− plays the main role in the dehydrogenation of alcohols; (2) 1O2 should generate ˙O2− through a single electron transfer process to achieve alcohol dehydrogenation;35 (3) ˙OH is not generated during the reaction process, or it has little effect on the reaction.
In view of the Electron Paramagnetic Resonance (EPR) results (Fig. S9†), Cu@NC-50 produces more 1O2 and ˙O2− than Co1@NC-50, while the catalytic activity of Co1@NC-50 was better than Cu@NC-50 (Table 2, entries 2 and 4), further confirming that the O2 activation step is not the RDS. The H/D KIE experiments were also performed to further explore the RDS of the reaction. The value of kH/kD is 2.71, which was calculated by parallel experiments using ethanol/deuterated ethanol as substrates (Fig. 8a), so the RDS may be the dehydrogenation of ethanol.15b
In addition, the energy barriers of the RDS (the aerobic dehydrogenation of ethanol) on different MN4 sites were calculated using Density Functional Theory (DFT) (Fig. 8b and S10–S12†). The order of the energy barrier is CoN4 (2.83 eV) < FeN4 (3.42 eV) < CuN4 (3.44 eV), which is consistent with the experimental results (Table 2, entries 1, 2 and 4). Based on the DFT calculation results, CoN4 has stronger adsorption for O2 and ethanol than CuN4 and FeN4, which may be the main reason for the lowest energy barrier of CoN4-catalyzed ethanol aerobic dehydrogenation.
Based on experiments and previous studies,36 two possible reaction pathways are proposed for the Co-catalyzed C–N bond coupling cyclization (Fig. 8c). Initially, the CoN4 sites activate O2 to generate 1O2 and ˙O2−, among which ˙O2− plays a major role in the oxidation of ethanol, while 1O2 generates ˙O2− through a single electron transfer process to achieve alcohol dehydrogenation.35 The Co-catalyzed aerobic oxidation of 1a to afford the corresponding aldehyde I. There are two pathways for the generation of 4a from I. Path A: 2-Aminobenzonitrile (III) is formed by the ammoxidation of I. Then, III is condensed with acetaldehyde to produce the target product 4a. Path B: Intermediate II is obtained by the condensation of acetaldehyde with I. Finally, II undergoes cyclization with ammonia to yield the product 4a.
Catalyst recyclability and stability
The recyclability and reusability of Co1@NC-50 were investigated by the model reaction. The reaction time was shortened to 3 h in order to better detect the loss of catalytic activity of the catalyst during recycling. In each catalytic cycle, the catalyst was simply separated by centrifugation, washed with ethyl acetate and reused in the next reaction after drying. Co1@NC-50 maintained high catalytic activity (62%–69%) after 6 runs (Fig. S13†). The results of ICP-MS, TEM and XRD suggest that there is almost no loss of Co content and no aggregation of Co sites in the recovered catalyst (Table S1 and Fig. S14, S15†).Conclusions
In conclusion, we have disclosed for the first time C–N coupling cyclization using ethanol as a C2-synthon catalyzed by an N-doped carbon supported cobalt single atom catalyst with oxidase-like CoN4 for the preparation of quinazolines and quinazolinones. This transformation provides several advantages including the use of air as the oxidant and abundant metal recyclability catalyst, free of additives, high step and atom economy, and a wide range of substrates (38 examples), thereby exhibiting high potential in drug synthesis. Kinetic studies suggest that the dehydrogenation of ethanol is the RDS for the reaction, and further coupling cyclization is carried out through two pathways.Co1@NC-50 can effectively activate O2 to generate 1O2 and ˙O2−, and ˙O2− plays a major role in the oxidation of ethanol. CoN4 more easily adsorbs O2 and ethanol and has a lower dehydrogenation energy barrier than other MN4 (M = Cu and Fe) sites, so Co1@NC-50 exhibits the best catalytic performance in this reaction. Furthermore, Co1@NC-50 has good stability and recyclability, which can be recycled at least six times. This chemistry not only provides a new and sustainable access for the synthesis of N-heterocycles from ethanol, but also offers mechanistic insights into the aerobic oxidation of alkyl alcohols.
Data availability
Data are available on request from the authors.The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Conflicts of interest
There are no conflicts to declare.References
- S. Mohapatra, C. Mishra, S. S. Behera and H. Thatoi, Application of pretreatment, fermentation and molecular techniques for enhancing bioethanol production from grass biomass-A review, Renewable Sustainable Energy Rev., 2017, 78, 1007–1032 CrossRef CAS
.
-
(a) C. C. Meyer, N. P. Stafford, M. J. Cheng and M. J. Krische, Ethanol: unlocking an abundant renewable C2-feedstock for catalytic enantioselective C–C coupling, Angew. Chem., 2021, 133, 10636–10640 Search PubMed
; (b) Z. L. Yuan, L. Huang, Y. S. Liu, Y. Sun, G. H. Wang, X. Li, J. A. Lercher and Z. H. Zhang, Synergy of oxygen vacancies and base sites for transfer hydrogenation of nitroarenes on ceria nanorods, Angew. Chem., Int. Ed., 2024, 63, e202317339 CrossRef CAS PubMed
.
-
(a) A. Cook and S. G. Newman, Alcohols as substrates in transition-metal-catalyzed arylation, alkylation, and related reactions, Chem. Rev., 2024, 124, 6078–6144 CrossRef CAS PubMed
; (b) K. K. Sun, H. B. Shan, G. P. Lu, C. Cai and M. Beller, Synthesis of N-heterocycles via oxidant-free dehydrocyclization of alcohols using heterogeneous catalysts, Angew. Chem., Int. Ed., 2021, 60, 25188–25202 CrossRef CAS PubMed
.
-
(a) A. Nandakumar, S. P. Midya, V. G. Landge and E. Balaraman, Transition-metal-catalyzed hydrogen-transfer annulations: access to heterocyclic scaffolds, Angew. Chem., Int. Ed., 2015, 54, 11022–11034 Search PubMed
; (b) M. Maji, D. Panja, I. Borthakur and S. Kundu, Org. Recent advances in sustainable synthesis of N-heterocycles following acceptorless dehydrogenative coupling protocol using alcohols, Chem. Front., 2021, 8, 2673–2709 Search PubMed
; (c) J. P. Michael, Quinoline, quinazoline and acridone alkaloids, Nat. Prod. Rep., 2007, 24, 223–246 RSC
.
-
(a) M. H. Alhalafi, Novel azo-dye quinazolinones as colorimetric chemosensors for detection of cobalt and ferrous ions in aqueous medium, J. Saudi Chem. Soc., 2023, 27, 101685 CrossRef CAS
; (b) S. Liu, L. L. Bu, Y. M. Zhang, J. Y. Yan, L. Li, G. R. Li, Z. B. Song and J. Huang, Subtle structural changes of dyes lead to distinctly different fluorescent behaviors in cellular context: the role of G-quadruplex DNA interaction using coumarin-quinazolinone conjugates as a case study, Anal. Chem., 2021, 93, 5267–5276 CrossRef CAS PubMed
.
-
(a) Z. M. Xing, W. H. Wu, Y. X. Miao, Y. Q. Tang, Y. K. Zhou, L. F. Zheng, Y. Fu, Z. B. Song and Y. Y. Peng, Recent advances in quinazolinones as an emerging molecular platform for luminescent materials and bioimaging, Org. Chem. Front., 2021, 8, 1867–1889 RSC
; (b) S. M. Kim, J. H. Yun, H. Hana and J. Y. Lee, A design strategy of bipolar host materials for more than 30 times extended lifetime in phosphorescent organic light-emitting diodes using benzocarbazole and quinazoline, J. Mater. Chem. C, 2017, 5, 9072–9079 Search PubMed
.
-
(a) K. S. V. Horn, W. N. Burda, R. Fleeman, L. N. Shaw and R. Manetsch, Antibacterial activity of a series of N2,N4-disubstituted quinazoline-2,4-diamines, J. Med. Chem., 2014, 57, 3075–3093 CrossRef
; (b) I. Dobrescu, E. Hammam, J. M. Dziekan, A. Claës, L. Halby, P. Preiser, Z. Bozdech, P. B. Arimondo, A. Scherf and F. Nardella, Plasmodium falciparum eukaryotic translation initiation factor 3 is stabilized by quinazoline-quinoline bisubstrate inhibitors, ACS Infect. Dis., 2023, 9(6), 1257–1266 CrossRef CAS
; (c) A. R. Martirosyan, R. R. Bata, A. B. Freeman, C. D. Clarke, R. L. Howard and J. S. Strobl, Differentiation-inducing quinolines as experimental breast cancer agents in the MCF-7 human breast cancer cell model, Biochem. Pharmacol., 2004, 68, 1729–1738 CrossRef CAS PubMed
; (d) P. J. Atkinson, S. M. Bromidge, M. S. Duxon, L. M. Gaster, M. S. Hadley, B. Hammond, C. N. Johnson, D. N. Middlemiss, S. E. North, G. W. Price, H. K. Rami, G. J. Riley, C. M. Scott, T. E. Shaw, K. R. Starr, G. Stemp, K. M. Thewlis, D. R. Thomas, M. Thompson, A. K. K. Vong and J. M. Watson, 3,4-Dihydro-2H-benzoxazinones are 5-HT1A receptor antagonists with potent 5-HT reuptake inhibitory activity, Bioorg. Med. Chem. Lett., 2005, 15, 737–741 CrossRef CAS PubMed
.
- Selected literatures on the synthesis of quinazolines and quinazolinones from aryl alcohols:
(a) T. Song, P. Ren, Z. M. Ma, J. J. Xiao and Y. Yang, Highly dispersed single-phase Ni2P nanoparticles on N,P-doped porous carbon for efficient synthesis of N-heterocycles, ACS Sustainable Chem. Eng., 2020, 8, 267–277 CrossRef CAS
; (b) K. Chakrabarti, M. Majia and S. Kundu, Cooperative iridium complex-catalyzed synthesis of quinoxalines, benzimidazoles and quinazolines in water, Green Chem., 2019, 21, 1999–2004 RSC
; (c) T. Y. Su, K. K. Sun, G. P. Lu and C. Cai, Synthesis of quinazolinones via a tandem hydrogen-transfer strategy catalyzed by N,S Co-doped carbon-anchored Co nanoparticles, ACS Sustainable Chem. Eng., 2022, 10, 3872–3881 CrossRef CAS
; (d) K. Gopalaiah, A. Sainia and A. Devi, Iron-catalyzed cascade reaction of 2-aminobenzyl alcohols with benzylamines: synthesis of quinazolines by trapping of ammonia, Org. Biomol. Chem., 2017, 15, 5781–5789 RSC
; (e) L. Y. Jiang, J. Shang, X. Y. Peng, Z. L. Li, M. Q. Zhu, Y. W. Cheng, P. F. Xie and L. H. Ren, Single atom catalysts enable quasi-homogeneous synthesis of quinazoline, Chem. Eng. J., 2023, 474, 145651 CrossRef CAS
; (f) Z. D. Tan, Z. X. Fu, J. Yang, Y. Wu, L. Cao, H. F. Jiang, J. Li and M. Zhang, Hydrogen transfer-mediated multicomponent reaction for direct synthesis of quinazolines by a naphthyridine-based iridium catalyst, iScience, 2020, 23, 101003 CrossRef CAS PubMed
; (g) F. Wang, F. Y. Zhu, E. X. Ren, Q. Zhang, G. P. Lu and Y. M. Lin, Fe–FeOx nanoparticles encapsulated in N-doped carbon material: a facile catalyst for selective synthesis of quinazolines from alcohols in water, Catal. Sci. Technol., 2022, 12, 7018–7026 RSC
; (h) M. Heidary, M. Khoobi, S. Ghasemi, Z. Habibi and M. A. Faramarzi, Synthesis of quinazolinones from alcohols via laccase-mediated tandem oxidation, Adv. Synth. Catal., 2014, 356, 1789–1794 CrossRef CAS
; (i) J. He, B. Han, C. S. Xian, Z. Hu, T. F. Fang and Z. H. Zhang, Hydrogen-bond-mediated formation of C–N or C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond during photocatalytic reductive coupling reaction over CdS nanosheets, Angew. Chem., Int. Ed., 2023, 63, e202404515 CrossRef
N bond during photocatalytic reductive coupling reaction over CdS nanosheets, Angew. Chem., Int. Ed., 2023, 63, e202404515 CrossRef .
- N. Anand, S. Koley, B. J. Ramulua and M. S. Singh, Metal-free aerobic one-pot synthesis of substituted/annulated quinolines from alcohols via indirect Friedländer annulation, Org. Biomol. Chem., 2015, 13, 9570–9574 RSC
.
-
(a) F. Li, L. Lu and P. C. Liu, Acceptorless dehydrogenative coupling of o-aminobenzamides with the activation of methanol as a C1 source for the construction of quinazolinones, Org. Lett., 2016, 18, 2580–2583 CrossRef CAS PubMed
; (b) B. C. Roy, S. A. Samim, D. Panja and S. Kundu, Tandem synthesis of quinazolinone scaffolds from 2-aminobenzonitriles using aliphatic alcohol-water system, Catal. Sci. Technol., 2019, 9, 6002–6006 RSC
; (c) S. A. Samim, B. C. Roy, S. Nayak and S. Kundu, Cobalt-catalyzed tandem transformation of 2-aminobenzonitriles to quinazolinones using hydration and dehydrogenative coupling strategy, J. Org. Chem., 2020, 85, 11359–11367 CrossRef CAS
.
- M. B. Reddy, K. Prasanth and R. Anandhan, Visible-light induced copper(I)-catalyzed oxidative cyclization of o-aminobenzamides with methanol and ethanol via HAT, Org. Biomol. Chem., 2020, 18, 9601–9605 RSC
.
-
(a) Z. H. Sun, G. Bottaria and K. Barta, Supercritical methanol as solvent and carbon source in the catalytic conversion of 1,2-diaminobenzenes and 2-nitroanilines to benzimidazoles, Green Chem., 2015, 17, 5172–5181 RSC
; (b) F. Feng, J. Ye, Z. Cheng, X. L. Xu, Q. F. Zhang, L. Ma, C. S. Lu and X. N. Li, Cu–Pd/γ-Al2O3 catalyzed the coupling of multi-step reactions: direct synthesis of benzimidazole derivatives, RSC Adv., 2016, 6, 72750–72755 RSC
; (c) S. Sharma, A. Sharma and P. Das, Supported Rhodium (Rh@PS) catalyzed benzimidazoles synthesis using ethanol/methanol as C2H3/CH source, Adv. Synth. Catal., 2019, 361, 67–72 CrossRef CAS
; (d) Z. H. Sun, G. Bottaria and K. Barta, Supercritical methanol as solvent and carbon source in the catalytic conversion of 1,2-diaminobenzenes and 2-nitroanilines to benzimidazoles, Green Chem., 2015, 17, 5172–5181 RSC
.
- C. F. Zhang, Z. Y. Gao, P. N. Ren, J. M. Lu, Z. P. Huang, K. Y. Su, S. C. Zhang, J. J. Mua and F. Wang, Oxygen-implanted MoS2 nanosheets promoting quinoline synthesis from nitroarenes and aliphatic alcohols via, an integrated oxidation transfer hydrogenation–cyclization mechanism, Green Chem., 2022, 24, 1704–1713 RSC
.
- J. Zhang, Z. An, Y. R. Zhu, X. Shu, H. Y. Song, Y. T. Jiang, W. L. Wang, X. Xiang, L. L. Xu and J. He, Ni0/Niδ+ synergistic catalysis on a nanosized Ni surface for simultaneous formation of C–C and C–N bonds, ACS Catal., 2019, 9, 11438–11446 CrossRef CAS
.
-
(a) M. B. Gawande, P. Fornasiero and R. Zbořil, Carbon-based single-atom catalysts for advanced applications, ACS Catal., 2020, 10, 2231–2259 CrossRef CAS
; (b) X. P. Zhang, G. P. Lu, K. Wang, Y. M. Lin, P. C. Wang and W. B. Yi, Lignin-derived Zn single atom/N-codoped porous carbon for α-alkylation of aromatic ketones with alcohols via borrowing hydrogen strategy, Nano Res., 2022, 15, 1874–1881 CrossRef CAS
; (c) X. X. Liu, L. Huang, Y. D. Ma, G. Q. She, P. Zhou, L. F. Zhu and Z. H. Zhang, Enable biomass-derived alcohols mediated alkylation and transfer hydrogenation, Nat. Commun., 2024, 15, 7012 CrossRef CAS PubMed
.
-
(a) Z. Li, F. N. Liu, C. X. Chen, Y. Y. Jiang, P. J. Ni, N. N. Song, Y. Hu, S. B. Xi, M. M. Liang and Y. Z. Lu, Regulating the N coordination environment of Co single-atom nanozymes for highly efficient oxidase mimics, Nano Lett., 2023, 23, 1505–1513 CrossRef CAS
; (b) W. W. Wu, L. Huang, X. Y. Zhu, J. X. Chen, D. Y. Chao, M. H. Li, S. L. Wu and S. J. Dong, Reversible inhibition of the oxidase-like activity of Fe single-atom nanozymes for drug detection, Chem. Sci., 2022, 13, 4566–4572 RSC
; (c) X. Li, S. C. Ding, Z. Y. Lyu, P. Tieu, M. Y. Wang, Z. X. Feng, X. Q. Pan, Y. Zhou, X. H. Niu, D. Du, W. L. Zhu and Y. H. Lin, Single-atomic iron doped carbon dots with both photoluminescence and oxidase-like activity, Small, 2022, 18, 2203001 CrossRef CAS PubMed
.
-
(a) R. V. Jagadeesh, K. Murugesan, A. S. Alshammari, H. Neumann, M. M. Pohl, J. Radnik and M. Beller, MOF-derived cobalt nanoparticles catalyze a general synthesis of amines, Science, 2017, 358, 326–332 CrossRef CAS
; (b) Y. H. Hu, Y. S. Zou, H. W. Yang, H. T. Ji, Y. Jin, Z. F. Zhang, Y. G. Liu and W. B. Zhang, Precise synthesis of chiral Z-allylamides by cobalt-catalyzed asymmetric sequential hydrogenations, Angew. Chem., Int. Ed., 2023, 62, e202217871 CrossRef CAS
; (c) S. Pattanaik and C. Gunanathan, Cobalt-catalyzed selective synthesis of disiloxanes and hydrodisiloxanes, ACS Catal., 2019, 9, 5552–5561 CrossRef CAS
; (d) D. Xu, R. R. Liu, J. F. Li, H. C. Zhao, J. T. Ma and Z. P. Dong, Atomically dispersed Co–N4 sites anchored on N-doped carbon for aqueous phase transfer hydrogenation between nitroarenes and saturated N-heterocycles, Appl. Catal., B, 2021, 299, 120681 CrossRef CAS
; (e) K. K. Sun, D. D. Li, G. P. Lu and C. Cai, Hydrogen auto-transfer synthesis of quinoxalines from o-nitroanilines and biomass-based diols catalyzed by MOF-derived N,P co-doped cobalt catalysts, ChemCatChem, 2021, 13, 373–381 CrossRef CAS
.
-
(a) B. Y. Li, J. F. Kou, G. Zeng, J. T. Ma and Z. P. Dong, Atomically dispersed Co on N-doped porous carbon for the highly efficient catalytic oxidation of aromatic alcohols to acids and nitriles, ACS Catal., 2023, 13, 16286–16299 CrossRef CAS
; (b) B. F. Chen, S. P. Li, S. J. Liu, M. H. Dong, B. X. Han, H. Z. Liu and L. R. Zheng, Aerobic selective oxidation of methylaromatics to benzoic acids over Co@N/Co-CNTs with high loading CoN4 species, J. Mater. Chem. A, 2019, 7, 27212–27216 RSC
; (c) J. L. Sun, H. F. Jiang, P. H. Dixneuf and M. Zhang, Multicomponent reductive coupling for selective access to functional γ-lactams by a single-atom cobalt catalyst, J. Am. Chem. Soc., 2024, 146, 11289–11298 CAS
; (d) J. L. Sun, H. F. Jiang, P. H. Dixneuf and M. Zhang, Reductive coupling of nitroarenes and HCHO for general synthesis of functional ethane-1,2-diamines by a cobalt single atom catalyst, J. Am. Chem. Soc., 2023, 145, 17329–17336 CrossRef CAS PubMed
; (e) J. L. Sun, C. G. Ci, H. F. Jiang, P. H. Dixneuf and M. Zhang, Utilizing nitroarenes and HCHO to directly construct functional N-heterocycles by supported cobalt/amino acid relay catalysis, Angew. Chem., Int. Ed., 2023, 62, e202303007 CrossRef CAS PubMed
; (f) P. Zhou, L. Jiang, F. Wang, K. J. Deng, K. L. Lv and Z. H. Zhang, High performance of a cobalt-nitrogen complex for the reduction and reductive coupling of nitro compounds into amines and their derivatives, Sci. Adv., 2017, 3, e1601945 CrossRef PubMed
.
- K. K. Sun, H. B. Shan, H. Neumann, G. P. Lu and M. Beller, Efficient iron single-atom catalysts for selective ammoxidation of alcohols to nitriles, Nat. Commun., 2022, 13, 1848 CrossRef CAS PubMed
.
- G. P. Lu, H. B. Shan, Y. M. Lin, K. Zhang, B. J. Zhou, Q. Zhong and P. C. Wang, A Fe single atom on N,S-doped carbon catalyst for performing N-alkylation of aromatic amines under solvent-free conditions, J. Mater. Chem. A, 2021, 9, 25128–25135 RSC
.
-
(a) T. Rui, G. P. Lu, X. Zhao, X. Cao and Z. Chen, The synergistic catalysis on Co nanoparticles and CoNx sites of aniline-modified ZIF derived Co@NCs for oxidative esterification of HMF, Chin. Chem. Lett., 2021, 32, 685–690 CrossRef CAS
; (b) X. Liang, N. H. Fu, S. C. Yao, Z. Li and Y. D. Li, The progress and outlook of metal single-atom-site catalysis, J. Am. Chem. Soc., 2022, 144, 18155–18174 CrossRef CAS
.
-
(a) Y. C. Guo, F. Liu, L. Feng, X. M. Wang, X. Zhang and J. S. Liang, Single Co atoms anchored on nitrogen-doped hierarchically ordered porous carbon for selective hydrogenation of quinolines and efficient oxygen reduction, Chem. Eng. J., 2022, 429, 132150 CrossRef CAS
; (b) Y. M. Lin, R. F. Nie, Y. T. Li, X. Wu, J. Q. Yu, S. H. Xie, Y. J. Shen, S. J. Mao, Y. Z. Chen, D. Lu, Z. B. Bao, Q. W. Yang, Q. L. Ren, Y. W. Yang, F. D. Liu, L. Qi, W. Y. Huang and Z. G. Zhang, Highly efficient and anti-poisoning single-atom cobalt catalyst for selective hydrogenation of nitroarenes, Nano Res., 2022, 15, 10006–10013 CrossRef CAS
; (c) Z. J. Li, Z. Y. Wang, S. B. Xi, X. X. Zhao, T. Sun, J. Li, W. Yu, H. M. Xu, T. S. Herng, X. Hai, P. Lyu, M. Zhao, S. J. Pennycook, J. Ding, H. Xiao and J. Lu, Tuning the spin density of cobalt single-atom catalysts for efficient oxygen evolution, ACS Nano, 2021, 15, 7105–7113 CrossRef CAS
.
-
(a) J. J. Gao, Y. X. Hu, Y. Wang, X. R. Lin, K. L. Hu, X. Lin, G. Q. Xie, X. J. Liu, K. M. Reddy, Q. H. Yuan and H. J. Qiu, MOF structure engineering to synthesize Co–N–C catalyst with richer accessible active sites for enhanced oxygen reduction, Small, 2021, 17, 2104684 CrossRef CAS PubMed
; (b) F. Y. Zhu, H. C. Qiu, F. Wang, X. Zhang, G. P. Lu, Y. M. Lin and H. Huang, Bio-inspired iron-based carbonic anhydrase mimic for CO2 hydration and conversion, ACS Sustainable Chem. Eng., 2023, 11, 7388–7397 CrossRef CAS
; (c) L. Yang, L. Shi, D. Wang, Y. L. Lv and D. P. Cao, Single-atom cobalt electrocatalysts for foldable solid-state Zn-air battery, Nano Energy, 2018, 50, 691–698 CrossRef CAS
.
- J. Q. Li, K. Wang, Y. J. Jiang, J. Huang and S. M. Liu, Advances of single-atomic cobalt catalysts in liquid-phase selective oxidative reactions, Small Sci., 2023, 3, 2300079 CrossRef CAS
.
-
(a) P. Q. Yin, T. Yao, Y. E. Wu, L. R. Zheng, Y. Lin, W. Liu, H. X. Ju, J. F. Zhu, X. Hong, Z. X. Deng, G. Zhou, S. Q. Wei and Y. D. Li, Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts, Angew. Chem., Int. Ed., 2016, 55, 10800–10805 CrossRef CAS
; (b) F. L. Cao, W. X. Ni, Q. S. Zhao, L. B. Wang, S. Xue, Y. P. Li, D. B. Kong, M. B. Wu and L. J. Zhi, Precisely manipulating the local coordination of cobalt single-atom catalyst boosts selective hydrogenation of nitroarenes, Appl. Catal., B, 2024, 346, 123762 CrossRef CAS
.
-
(a) M. L. Pan, J. Wang, G. D. Gao and J. W. Chew, Incorporation of single cobalt active sites onto N-doped graphene for superior conductive membranes in electrochemical filtration, J. Membr. Sci., 2020, 602, 117966 CrossRef CAS
.
-
(a) F. Wang, F. Y. Zhu, E. X. Ren, G. F. Zhu, G. P. Lu and Y. M. Lin, Recent advances in carbon-based iron catalysts for organic synthesis, Nanomaterials, 2022, 12, 3462 CrossRef CAS PubMed
; (b) Z. Li, J. H. Zhang, X. T. Jing, J. Dong, H. F. Liu, H. J. Lv, Y. N. Chi and C. W. Hu, A polyoxometalate@covalent triazine framework as a robust electrocatalyst for selective benzyl alcohol oxidation coupled with hydrogen production, J. Mater. Chem. A, 2021, 9, 6152–6159 RSC
; (c) A. Bagherzade, F. Nemati and H. T. Nahzomi, Computational and experimental evidence of Pd supported P-doped porous graphitic carbon nitride as a highly efficient and exceptionally durable photocatalyst for boosted visible-light-driven benzyl alcohol oxidation, J. Phys. Chem. Solids, 2021, 152, 109985 CrossRef CAS
.
-
(a) S. Castellano, S. Taliani, C. Milite, I. Pugliesi, E. D. Pozzo, E. Rizzetto, S. Bendinelli, B. Costa, S. Cosconati, G. Greco, E. Novellino, G. Sbardella, G. Stefancich, C. Martini and F. D. Settimo, Synthesis and biological evaluation of 4-phenylquinazoline-2-carboxamides designed as a novel class of potent ligands of the translocator protein, J. Med. Chem., 2012, 55, 4506–4510 CrossRef CAS PubMed
; (b) Z. J. Wang, W. T. Chen, C. He, G. L. Zhang and Y. P. Yu, Palladium(II)-catalyzed three-component tandem cyclization reaction for the one-pot assembly of 4-arylquinazolines, Synthesis, 2021, 53, 1356–1364 CrossRef CAS
.
-
(a) G. N. Vaidya, A. Khan, H. Verma, S. Kumar and D. Kumar, Structure ligation relationship of amino acids for the amination cross-coupling reactions, J. Org. Chem., 2019, 84, 3004–3010 CrossRef CAS PubMed
; (b) J. A. Inger, E. R. Mihan, J. U. Kolli, C. W. Lindsley and A. M. Bender, DARK classics in chemical neuroscience: methaqualone, ACS Chem. Neurosci., 2023, 14, 340–350 CrossRef CAS PubMed
.
- S. L. Cao, Y. P. Feng, Y. Y. Jiang, S. Y. Liu, G. Y. Ding and R. T. Li, Synthesis and in vitro antitumor activity of 4(3H)-quinazolinone derivatives with dithiocarbamate side chains, Bioorg. Med. Chem. Lett., 2005, 15, 1915–1917 CrossRef CAS PubMed
.
- J. Ramharter, D. Kessler, P. Ettmayer, M. H. Hofmann, T. Gerstberger, M. Gmachl, T. Wunberg, C. Kofink, M. Sanderson, H. Arnhof, G. Bader, K. Rumpel, A. Zöphel, R. Schnitzer, J. Böttcher, J. C. O'Connell, R. L. Mendes, D. Richard, N. Pototschnig, I. Weiner, W. Hela, K. Hauer, D. Haering, L. Lamarre, B. Wolkerstorfer, C. Salamon, P. Werni, S. Munico-Martinez, R. Meyer, M. D. Kennedy, N. Kraut and D. B. McConnell, One atom makes all the difference: getting a foot in the door between SOS1 and KRAS, J. Med. Chem., 2021, 64, 6569–6580 CrossRef CAS PubMed
.
- R. H. V. Nishimura, T. Santos, V. E. Murie, L. C. Furtado, L. V. Costa-Lotufo and G. C. Clososki, Efficient N-arylation of 4-chloroquinazolines en route to novel 4-anilinoquinazolines as potential anticancer agents, Beilstein J. Org. Chem., 2021, 17, 2968–2975 CrossRef CAS PubMed
.
-
(a) Y. Z. Wang, S. Y. Furukawa and N. Yan, Identification of an active NiCu catalyst for nitrile synthesis from alcohol, ACS Catal., 2019, 9, 6681–6691 CrossRef CAS
; (b) C. F. Zhang, Z. Y. Gao, P. N. Ren, J. M. Lu, Z. P. Huang, K. Y. Su, S. C. Zhang, J. J. Mu and F. Wang, Oxygen-implanted MoS2 nanosheets promoting quinoline synthesis from nitroarenes and aliphatic alcohols via an integrated oxidation transfer hydrogenation-cyclization mechanism, Green Chem., 2022, 24, 1704–1713 RSC
.
-
(a) S. Suleman, Y. Zhang, Y. Y. Qian, J. W. Zhang, Z. Y. Lin, Ö. Metin, Z. Meng and H. L. Jiang, Turning on singlet oxygen generation by outer-sphere microenvironment modulation in porphyrinic covalent organic frameworks for photocatalytic oxidation, Angew. Chem., Int. Ed., 2024, 63, e202314988 CrossRef CAS PubMed
; (b) C. S. Xian, J. He, Y. R. He, J. B. Nie, Z. Yuan, J. Sun, W. N. Martens, J. Z. Qin, H. Y. Zhu and Z. H. Zhang, High nitrile yields of aerobic ammoxidation of alcohols achieved by generating ˙O2− and Br˙ radicals over iron-modified TiO2 photocatalysts, J. Am. Chem. Soc., 2022, 144, 23321–23331 CrossRef CAS PubMed
.
- S. Kumari, S. Roy, P. Arora and S. Kundu, Visible light-mediated synthesis
of quinazolinones and benzothiadiazine-1,1-dioxides utilizing aliphatic alcohols, Org. Biomol. Chem., 2024, 22, 4172–4178 RSC
.
-
(a) Z. H. Zhang, X. N. Zhang, L. P. Mo, Y. X. Lia and F. P. Ma, Catalyst-free synthesis of quinazoline derivatives using low melting sugar-urea-salt mixture as a solvent, Green Chem., 2012, 14, 1502 RSC
; (b) S. Kumari, S. Roy, P. Arora and S. Kundu, Visible light-mediated synthesis of quinazolinones and benzothiadiazine-1,1-dioxides utilizing aliphatic alcohols, Org. Biomol. Chem., 2024, 22, 4172–4178 RSC
; (c) Z. Y. Chen, J. X. Chen, M. C. Liu, J. C. Ding, W. X. Gao, X. B. Huang and H. Y. Wu, Unexpected copper-catalyzed cascade synthesis of quinazoline derivatives, J. Org. Chem., 2013, 78, 11342–11348 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc04928d |
| This journal is © The Royal Society of Chemistry 2025 |