DIPEA-induced Si–H activation of siloxane for hydrosilylation polymerization via metal-free photocatalysis†
Hangcen
Xie
ab,
Rui
Xu
ab,
Bin
Huang
a,
Pingping
Lou
a,
Hua-Feng
Fei
 *ab and
Zhijie
Zhang
*a
*ab and
Zhijie
Zhang
*a
aKey Laboratory of Science and Technology on High-tech Polymer Materials, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, P. R. China.. E-mail: feihuafeng@iccas.ac.cn; zhangzj@iccas.ac.cn
bSchool of Chemical Sciences, University of Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 13th November 2024
Abstract
Although metal-free hydrosilylation of siloxanes is essential for the industrial preparation of organosilicon compounds due to its unique advantages, such as the avoidance of the use and residue of precious metals, efficient metal-free silicon hydrogenation reactions are still rare. Herein, we report a straightforward visible light-driven metal-free hydrosilylation reaction based on siloxanes and silicon vinyl groups, catalyzed by the synergistic effect of the organic photooxidation catalyst 2,4,6-tris(diphenylamino)-5-fluoroisophthalonitrile and the base N,N′-diisopropylethylamine, which undergo electron transfer and selectively induce Si–H activation during catalysis. After optimization, the silicon vinyl conversion rate in the hydrosilylation reaction exceeded 99% without using any traditional hydrogen atom transfer reagents. Mechanistic studies based on experimental data and theoretical calculations revealed that the reaction proceeds through a free radical reaction and is thermodynamically feasible. The proposed methodology efficiently affords linear polymer formation via a stepwise growth approach. Furthermore, it can crosslink commercial high-molecular-weight polyvinyl silicone oil with disiloxane, realizing the gelation of the material.
Introduction
In the hydrosilylation reaction, the Si–H bond in organosilicon compounds adds to the double or triple bonds of unsaturated organic compounds. This is an important reaction to produce various organic silicon compounds and silicone rubbers containing special functional groups.1 The hydrosilylation reaction is also the most studied and widely used type of reaction in organic silicon chemistry2 and is predominantly catalyzed by transition metal catalysts. Precious metals such as platinum, rhodium, and ruthenium (Pt, Rh, and Ru)3–6 and their complexes represent the most important examples. The two most widely used Pt catalysts are Speier's catalyst7 and Karstedt's catalyst (Fig. 1). The Pt catalysts have also been developed to improve reaction activity and recyclability by replacing ligands8–11 and changing the loading form.12,13 These catalysts can promote the hydrosilylation process of olefins under room-temperature or high-temperature conditions. The most well-known application of the hydrosilylation reaction is the production of addition-type silicone rubber.2 Addition-type silicone rubber has been extensively utilized in various fields, including electronics, aerospace, and medicine, due to its exceptional electrical insulation, biocompatibility, high- and low-temperature resistance, and chemical corrosion resistance. Pt compounds catalyze the hydrosilylation reaction under certain conditions for curing, which is currently the mainstream method for preparing addition molded silicone rubber. The most prevalent example of achieving polymer curing through hydrosilylation is long-chain polydimethylsiloxane (PDMS) terminated with divinyl groups. Solidification of PDMS occurs by adding oligomeric crosslinked chains and a small amount of Pt catalyst under certain conditions, and this type of technology is currently relatively mature. Nevertheless, due to their low abundance on Earth and their high cost, industrial applications of these precious metals are constrained.14Non-precious metals, such as iron, cobalt, copper, and nickel15–19 have been explored for many years in hydrosilylation reactions. However, preserving non-precious metal complex catalysts is usually quite challenging, and their syntheses necessitate strict adherence to specific requirements. Compared to traditional Pt catalysts, these transition metal complexes often require higher temperatures or extended reaction times to catalyze the desired reaction. In addition, the substrate application range of these catalysts is relatively narrow, and the most successful examples are limited to specific olefins and silanes. Thus, the lack of universality and versatility limits their application. Furthermore, metal residues are difficult to remove from the product, which impedes their application in biological, electronic, and optical devices. Researchers have also investigated the potential of metal-free catalysts as an alternative solution. Lewis acid catalysts, such as electron-deficient boranes,20 have been demonstrated to achieve hydrosilylation. However, their efficacy is limited to the hydrosilylation reaction of electron-rich olefins.
The use of photons to drive chemical reactions can be considered green and sustainable. Especially compared to thermochemical reactions, photochemical reactions exhibit several advantages, including mild reaction conditions, certain group selectivity, and the capacity to obtain products that are challenging to produce through conventional synthetic chemical methods. The field of photocatalytic hydrosilylation has also advanced in the past ten years. Some Pt photocatalysts, such as acetylacetone platinum21 and trimethyl (methylcyclopentadienyl)platinum(IV) [(Me–Cp)Pt(Me)3],22 have also been developed for photocatalytic hydrosilylation. Such catalysts could have future applications in producing intricate geometric-shaped silicone rubber through photolithography or using precise 3D printing. Nevertheless, the quantity of Pt complexes utilized (≥250 ppm) in photocatalytic hydrosilylation is considerably greater than that employed in thermal polymerization (5–20 ppm), which can markedly augment production costs. Besides, these Pt photocatalysts are limited in variety and are heat-sensitive, which is not conducive to transportation and long-term storage. In recent years, alternatives to Pt photocatalysts have also been researched. Tetrabutylammonium decatungstate (TBADT)23 can be used for photocatalytic activation of Si–H bonds in trisubstituted silanes and the hydrosilylation of electron-deficient olefins. Dimanganese decacarbonyl Mn2(CO)10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 has been demonstrated to undergo a visible light-induced addition reaction with phenylacetylene and dimethylphenylsilane. These catalytic processes show high yield and selectivity. Recently, Wu et al.25 employed 2,4,5,6-tetra-9H-carbazol-9-yl-1,3-benzenedicarbonitrile, 4CzIPN, as the photocatalyst, affording high selectivity and conversion rates for both electron-deficient and electron-rich olefins. On this basis, Pan et al.26 further polymerized aliphatic dienes and polysubstituted silanes using hydrosilylation reactions. However, photocatalytic hydrosilylation typically requires additives, such as hydrogen atom transfer (HAT) reagents, and these methods are only suitable for the reaction between aliphatic olefins and selected groups of silanes. Neutral eosin Y can also serve as a direct HAT photocatalyst, achieving stepwise custom functionalization of multihydrosilanes and quantitative conversion of hydrosilanes to silyl chlorides under visible-light irradiation using dichloromethane as a chlorinating agent.27,28 To date, there are very few successful reports on implementing a photocatalytic hydrosilylation reaction between siloxanes and silicone vinyl groups.
24 has been demonstrated to undergo a visible light-induced addition reaction with phenylacetylene and dimethylphenylsilane. These catalytic processes show high yield and selectivity. Recently, Wu et al.25 employed 2,4,5,6-tetra-9H-carbazol-9-yl-1,3-benzenedicarbonitrile, 4CzIPN, as the photocatalyst, affording high selectivity and conversion rates for both electron-deficient and electron-rich olefins. On this basis, Pan et al.26 further polymerized aliphatic dienes and polysubstituted silanes using hydrosilylation reactions. However, photocatalytic hydrosilylation typically requires additives, such as hydrogen atom transfer (HAT) reagents, and these methods are only suitable for the reaction between aliphatic olefins and selected groups of silanes. Neutral eosin Y can also serve as a direct HAT photocatalyst, achieving stepwise custom functionalization of multihydrosilanes and quantitative conversion of hydrosilanes to silyl chlorides under visible-light irradiation using dichloromethane as a chlorinating agent.27,28 To date, there are very few successful reports on implementing a photocatalytic hydrosilylation reaction between siloxanes and silicone vinyl groups.
Herein, we report a metal-free hydrosilylation polymerization reaction driven by visible light without using additives like HAT reagents. The possible reaction mechanism of the above reaction was investigated. The organic photocatalyst 3DPAFIPN and DIPEA jointly activated the Si–H bond of 1,1,3,3-tetramethyldisiloxane (MMH), followed by the addition of the Si–H bond to the carbon–carbon double bond, resulting in the formation of the hydrosilylation product. Finally, linear polycondensation polymers could be obtained through the gradual growth and polymerization of free radicals.
Results and discussion
We commenced our study by evaluating the light-induced hydrosilylation of 1,3-divinyl-1,1,3,3-tetramethyldisiloxane (MMVi) with MMH (5b) under blue LED irradiation (λmax = 470 nm). After carefully investigating a series of photocatalysts, solvents, and base (Tables S1–4†), the combination of a catalytic amount of the organophotoredox catalyst 3DPAFIPN (5a, 3 mol%) and DIPEA (50 mol%) in acetonitrile (MeCN) under blue LED irradiation for 24 h provided the best result (Fig. 2). Replacing the catalyst with a Pt catalyst showed comparable results. Notably, the photocatalyst 2,4,5,6-tetra-9H-carbazol-9-yl-1,3-benzenedicarbonitrile (4CzIPN, 1a), which has been commonly employed in previous studies investigating hydrosilylation reactions,29 was unable to catalyze the addition reaction. Furthermore, we attempted to utilize the relevant derivatives of 4CzIPN, such as 2,4,5,6-tetrakis(3,6-diphenyl-9H-carbazol-9-yl) isophthalonitrile (4CzIPN-Ph, 3a) and 3,4,5,6-tetrakis (3,6-di-tert-butyl-9H-carbazol-9-yl) phthalonitrile (4CzIPN-Bu, 2a). The photocatalyst 2a did not enhance the hydrosilylation reaction. While photocatalysis with 3a could deliver the corresponding polymer, the conversion rate of olefins was notably low (39%) under the used reaction conditions (Table 1). Only 2,4,6-tris(diphenylamino)-3,5-difluorobenzonitrile (3DPA2FBN, 4a), which has a redox potential similar to 3DPAFIPN (5a),30 provided appropriate outcomes. Therefore, the effect of the dosage of the photoinitiator on the hydrosilylation of MMVi with MMH was further investigated using 5a as the photocatalyst. A higher conversion was obtained using 3 mol% of 5a (based on MMVi). Among the various screened solvents, MeCN provided the best reactivity.| Entry | PC | Bis(silane) | Conv.a (%) | [Si–H] /[C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C]b C]b |
M n | Đ |
|---|---|---|---|---|---|---|
a Conversion rate of dienes based on crude 1H NMR analysis, using 1,4-dioxane as an internal standard.
b Consumption ratio of Si–H bonds and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds based on crude 1H NMR analysis. C bonds based on crude 1H NMR analysis.
|
||||||
| 1 | 1a | 5b | NR | — | — | — |
| 2 | 2a | 5b | NR | — | — | — |
| 3 | 3a | 5b | 39 | 0.45 | 953 | 1.21 |
| 4 | 4a | 5b | 87 | 0.83 | 3803 | 1.08 |
| 5 | 5a | 5b | >99 | 0.87 | 4711 | 1.22 |
| 6 | 5a | 1b | NR | — | — | — |
| 7 | 5a | 2b | 97 | 0.37 | 3703 | 1.07 |
| 8 | 5a | 3b | 70 | 0.28 | 3322 | 1.03 |
| 9 | 5a | 4b | 99 | 0.69 | 3723 | 1.07 |
| 10 | 5a | 6b | 39 | 0.51 | 795 | 1.18 |
| 11 | 5a | 7b | 55 | 0.02 | — | — |
A thorough investigation was also conducted to ascertain the optimal amount of DIPEA. The highest vinyl conversion was achieved when DIPEA was introduced at a concentration of 50 mol%. It is noteworthy that despite the relatively high quantity of base employed, it can be reused (Fig. S1†), demonstrating the sustainability potential of the proposed hydrosilylation. The photocatalyst and DIPEA may form electron donor–acceptor (EDA) complexes during photo-induced electron transfer31–33 (PIET). Furthermore, the formation of EDA complexes is concentration-dependent. When the concentration of the acceptor is too low, the distance between electron donor and acceptor in solution is considerable, restricting the charge transfer process. The EDA formation constant (KET) is maximal at a specific stoichiometric ratio for given electron donors and acceptors.34 At this point, the reaction activity is the highest. When the molar ratio exceeds the optimal stoichiometric ratio, the possibility of back-electron transfer increases. Additionally, since DIPEA is a base, it can acquire protons from DIPEA free radical cations (DIPEA˙+), resulting in the formation of protonated DIPEA,35,36 which exhibits poor electron acceptor properties and ultimately affects reaction efficiency.
No hydrosilylation product was detected in the absence of photocatalysts in control experiments. Furthermore, the reaction did not proceed successfully without DIPEA and blue light irradiation. These results demonstrate that all these components are necessary to complete the reaction successfully. Efficient conversion could also be achieved by replacing DIPEA with the appropriate amounts of triethylamine (TEA) and N,N-diisopropylmethylamine. However, it proved challenging to achieve the desired outcomes with potassium carbonate K2CO3. These results indicate that such organic bases may have a more significant role than previously anticipated in providing an alkaline environment during the reaction. These organic bases are employed extensively as reductant or electron transfer reagents in organic synthesis.37,38
Various silanes were investigated under the optimized reaction conditions (Table 1). The properties of the employed bis(silanes) play a decisive role in the conversion rate of the reaction and the consumption ratio of Si–H/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C. Diethylsilane was difficult to react to under the optimized reaction conditions, presumably due to its electron-donating ability. Replacing the alkyl group in silane with a benzene ring resulted in a notable enhancement in reaction activity.39 The conversion rates of methylphenylsilane (MePhSiH2, 2b) and diphenylsilane (Ph2SiH2, 4b) were significantly increased. These results are consistent with previously published research findings.40,41 Relevant 135 DEPT 13C NMR spectra (Fig. S27 and S28†) verified that CH2 bonds were formed during the reaction process. In addition, the observed CH peaks attributed to the benzene ring also confirmed the effective introduction of silane through the hydrosilylation reaction. Due to its unique electronic effect, the benzene ring may play a role in stabilizing free radical formation following the Si–H bond rupture. However, a significant decrease in reaction efficiency was observed when three benzene rings were connected to silicon, resulting in greater steric hindrance. Nevertheless, it should be highlighted that the conversion rate is negatively impacted when the Si–H of disilane originates from two silicon atoms within the molecule, even when these two silicon atoms are connected by a benzene ring. In siloxanes, however, this phenomenon is slightly different. Furthermore, the efficiency of the reaction was reduced by approximately 60% when the four methyl groups in 5b were replaced with their isopropyl counterparts, probably because the presence of isopropyl groups increases steric hindrance, while their electron-donating capacity is slightly higher than that of methyl groups. Ultimately, the addition reaction of Si–H on the siloxane resulted in different outcomes. As the Si–O chain in the center became longer, the activity of Si–H decreased. Especially when three silicon atoms were present in the Si–O chain and the side groups of the Si atoms in the center were replaced by two benzene rings, the overall conversion rate of the reaction was sharply reduced, and the participation of Si–H in the reaction was also markedly diminished. Differentiating between the characteristic peaks of Si atoms with two benzene rings in 29Si NMR is quite challenging. Furthermore, the limited integrated area of peaks belonging to the benzene ring in the 1H NMR also indicated the inefficiency of this reaction (Fig. S15†). Since silanes need to overcome a higher energy barrier to lose the second H atom after losing the first H atom, the presence of monosubstituted Si–H can be clearly observed in the NMR spectrum of the reaction product of 2b, which may be the reason for the lower consumption ratio of Si–H/Si–Vi in some substances.
C. Diethylsilane was difficult to react to under the optimized reaction conditions, presumably due to its electron-donating ability. Replacing the alkyl group in silane with a benzene ring resulted in a notable enhancement in reaction activity.39 The conversion rates of methylphenylsilane (MePhSiH2, 2b) and diphenylsilane (Ph2SiH2, 4b) were significantly increased. These results are consistent with previously published research findings.40,41 Relevant 135 DEPT 13C NMR spectra (Fig. S27 and S28†) verified that CH2 bonds were formed during the reaction process. In addition, the observed CH peaks attributed to the benzene ring also confirmed the effective introduction of silane through the hydrosilylation reaction. Due to its unique electronic effect, the benzene ring may play a role in stabilizing free radical formation following the Si–H bond rupture. However, a significant decrease in reaction efficiency was observed when three benzene rings were connected to silicon, resulting in greater steric hindrance. Nevertheless, it should be highlighted that the conversion rate is negatively impacted when the Si–H of disilane originates from two silicon atoms within the molecule, even when these two silicon atoms are connected by a benzene ring. In siloxanes, however, this phenomenon is slightly different. Furthermore, the efficiency of the reaction was reduced by approximately 60% when the four methyl groups in 5b were replaced with their isopropyl counterparts, probably because the presence of isopropyl groups increases steric hindrance, while their electron-donating capacity is slightly higher than that of methyl groups. Ultimately, the addition reaction of Si–H on the siloxane resulted in different outcomes. As the Si–O chain in the center became longer, the activity of Si–H decreased. Especially when three silicon atoms were present in the Si–O chain and the side groups of the Si atoms in the center were replaced by two benzene rings, the overall conversion rate of the reaction was sharply reduced, and the participation of Si–H in the reaction was also markedly diminished. Differentiating between the characteristic peaks of Si atoms with two benzene rings in 29Si NMR is quite challenging. Furthermore, the limited integrated area of peaks belonging to the benzene ring in the 1H NMR also indicated the inefficiency of this reaction (Fig. S15†). Since silanes need to overcome a higher energy barrier to lose the second H atom after losing the first H atom, the presence of monosubstituted Si–H can be clearly observed in the NMR spectrum of the reaction product of 2b, which may be the reason for the lower consumption ratio of Si–H/Si–Vi in some substances.
A distinct optical switching effect can be observed in Fig. 3b on the hydrosilylation reaction of 5b with MMVi. Monitoring the reaction process through 1H NMR demonstrated an obvious increase in the conversion rate of vinyl groups when the mixture was illuminated, while the consumption of these groups exhibited a negligible change when the blue light was extinguished. This outcome is a prominent feature of photocatalytic reactions. FT-IR spectroscopic analysis (Fig. 3a) and reaction kinetics tests (Fig. S4†) provided insight into the temporal variation of the vinyl conversion rate and product molecular weight throughout the reaction process. The data indicated that the consumption of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds and Si–H bonds enhanced gradually with time; however, the molecular weight did not rise significantly in the early stages of the reaction. Instead, a sudden increase occurred after a specific time, a characteristic feature of condensation polymerization. There was no significant difference in the polymerization effect between these two types of catalysts. The molecular weight of the final polymer was comparatively low, consistent with the molecular weight obtained by model reaction catalysis using a highly active Pt catalyst produced in our laboratory. According to the fitting results illustrated in Fig. S4c,† it was speculated that after reaching a specific molecular weight threshold, the reactivity of the vinyl group at the end of the polymer decreased. This may be the potential explanation for the low molecular weight of the product. In other words, in the later stages of the addition reaction, the occurrence of a step-growth mechanism was difficult because disproportionation termination reactions were more advantageous than extracting hydrogen from silane.41,42
C bonds and Si–H bonds enhanced gradually with time; however, the molecular weight did not rise significantly in the early stages of the reaction. Instead, a sudden increase occurred after a specific time, a characteristic feature of condensation polymerization. There was no significant difference in the polymerization effect between these two types of catalysts. The molecular weight of the final polymer was comparatively low, consistent with the molecular weight obtained by model reaction catalysis using a highly active Pt catalyst produced in our laboratory. According to the fitting results illustrated in Fig. S4c,† it was speculated that after reaching a specific molecular weight threshold, the reactivity of the vinyl group at the end of the polymer decreased. This may be the potential explanation for the low molecular weight of the product. In other words, in the later stages of the addition reaction, the occurrence of a step-growth mechanism was difficult because disproportionation termination reactions were more advantageous than extracting hydrogen from silane.41,42
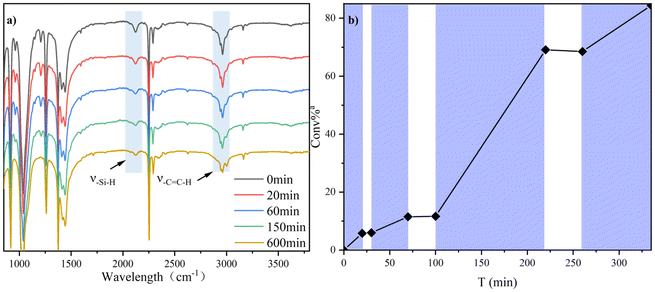 | ||
Fig. 3 (a) IR-t tests; (b) Light on–off experiments for hydrosilylation of 5b with MMVi. Consumption ratio of Si–H bonds and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds is based on 1H NMR analysis. C bonds is based on 1H NMR analysis. | ||
Mechanism
No polymers were detected when the radical scavengers TEMPO, hydroquinone, or 1,1-diphenylethylene were added to the reaction, implying a radical-based process (Scheme S3†). The consumption ratio of Si–H and Si–Vi was not equal to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 1) during the actual reaction process, indicating the presence of other free radical reactions in addition to the hydrosilylation reaction. A potential mechanism for the hydrosilylation of siloxanes and silicone vinyl groups is proposed in Fig. 4. In the presence of blue light, the photocatalyst (PC) absorbs energy and transitions to the excited state PC*. From 19F NMR37 and Stern–Vomer studies,42,43 it can be hypothesized that a single electron transfer (SET) process occurs between the photoexcited state PC* and DIPEA (Fig. S2 and S3†). The reduction of PC* by DIPEA forms the reduced photocatalyst PC˙−, while DIPEA itself becomes a nitrogen radical cation. Afterward, a HAT process occurs from the Si–H bond of the bis(silane) to the temporary nitrogen radical cation, simultaneously producing the silane radical and tertiary amine cations.38 Then, the addition of the silane radical to MMVi generates a transient radical adduct. After a series of successive SET processes with the PC˙− and proton transfer with the nitrogen radical cation, the transient radical adducts ultimately form addition products. At this juncture, PC and DIPEA are regenerated, thereby completing the catalytic cycle.
1 (Table 1) during the actual reaction process, indicating the presence of other free radical reactions in addition to the hydrosilylation reaction. A potential mechanism for the hydrosilylation of siloxanes and silicone vinyl groups is proposed in Fig. 4. In the presence of blue light, the photocatalyst (PC) absorbs energy and transitions to the excited state PC*. From 19F NMR37 and Stern–Vomer studies,42,43 it can be hypothesized that a single electron transfer (SET) process occurs between the photoexcited state PC* and DIPEA (Fig. S2 and S3†). The reduction of PC* by DIPEA forms the reduced photocatalyst PC˙−, while DIPEA itself becomes a nitrogen radical cation. Afterward, a HAT process occurs from the Si–H bond of the bis(silane) to the temporary nitrogen radical cation, simultaneously producing the silane radical and tertiary amine cations.38 Then, the addition of the silane radical to MMVi generates a transient radical adduct. After a series of successive SET processes with the PC˙− and proton transfer with the nitrogen radical cation, the transient radical adducts ultimately form addition products. At this juncture, PC and DIPEA are regenerated, thereby completing the catalytic cycle.
To verify the above mechanism, we performed density functional theory (DFT) calculations44–47 (Fig. 5 and Fig. S5†). To some extent, the results of the calculation corroborate the proposed hypothesis. Firstly, 3DPAFIPN in the excited state can readily attack DIPEA to produce the free radical B while releasing 19.60 kcal mol−1 of energy. Subsequently, the obtained free radicals further react with MMH (1) to form the intermediate 1_radical, having a very small energy barrier and releasing 28.1 kcal mol−1 of energy during the process. Then, the intermediate 1_radical undergoes an addition reaction with MMVi to obtain another intermediate, ultimately generating the target product P under an energy release of 33.0 kcal mol−1. The ΔG values of each reaction step throughout the entire process are negative (Table S5†), indicating that the above assumptions are thermodynamically feasible.
Finally, the optimized hydrosilylation reaction was performed on a gram-scale (Scheme S2†), and the conversion of the vinyl was monitored to demonstrate the efficiency of the synthetic method. The conversion rate of the vinyl group decreased during the same reaction conditions. However, the conversion rate of the vinyl group exceeded 99% when extending the light irradiation time, which may be attributed to a short penetration of light into bigger flasks and high photon loss caused by reflection and dispersion effects. In addition, due to the difficulty of reactants and active free radical groups contacting each other in solution as the volume of the reaction system increases, more energy has to be absorbed to complete the reaction, which is also a possible important reason for the observed conversion profile. Noteworthy, although the conversion rate of vinyl was still high, the consumption ratio of Si–H/Si–Vi decreased. Since it is a common practice in organic synthesis to maintain an excess of a certain reactant to increase yield effectively, replacing the current batch reactor with a continuous injection system might be a more effective method for further reaction optimization.
Cross-linking of the PVMS and Bi(siloxanes)
Crosslinked polymers exhibit a certain mechanical strength and chemical resistance, and their widespread utilization in functional materials has been the focus of many studies for a long time. Therefore, crosslinking commercial polyvinyl silicone oil with disiloxane was attempted using this metal-free radical hydrosilylation addition method (Fig. 6). A modest quantity of solvent was added to enhance the solubility of the photocatalyst in silicone oil. Initially, the silicone oil was irradiated under blue light for 24 h in the presence of the photocatalyst without adding silane. This process did not result in the formation of a polymer gel (Table S6†). No gelation was observed even if the illumination time was extended to 48 h. When the ratio of Si–H bonds to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds was equal to or greater than one, visible polymer gels were formed in the reaction mixture (Fig. 6). Other solvents, such as toluene, DCM, and others, were also unsuccessful in gel formation. However, the consumption of Si–H and C
C bonds was equal to or greater than one, visible polymer gels were formed in the reaction mixture (Fig. 6). Other solvents, such as toluene, DCM, and others, were also unsuccessful in gel formation. However, the consumption of Si–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in the reaction mixture could be observed by 1H NMR and other analytical techniques. The difficulty in achieving gelation may be attributed to the high molecular weight of polyvinyl silicone oil, which impedes the migration of generated active free radicals within the material, thereby reducing the overall activity of the reaction. Interestingly, the mixture remained a flowable liquid after exposure to blue light at a 0.5 ratio of Si–H to C
C bonds in the reaction mixture could be observed by 1H NMR and other analytical techniques. The difficulty in achieving gelation may be attributed to the high molecular weight of polyvinyl silicone oil, which impedes the migration of generated active free radicals within the material, thereby reducing the overall activity of the reaction. Interestingly, the mixture remained a flowable liquid after exposure to blue light at a 0.5 ratio of Si–H to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds. This phenomenon resulted from the high activity of the Si–H bonds, which may be consumed by the reaction with water in the atmosphere, rendering it challenging to attain the desired gel state.
C bonds. This phenomenon resulted from the high activity of the Si–H bonds, which may be consumed by the reaction with water in the atmosphere, rendering it challenging to attain the desired gel state.
Conclusions
In summary, we have developed a blue-light-driven metal-free hydrosilylation polymerization reaction for both bis(silane) and disiloxane with silicone vinyl groups using a photocatalyst and base instead of traditional HAT additives. The hydrosilylation reaction using this strategy exhibited excellent efficiency in forming linear polymers in a step-growth manner. The hydrosilylation reaction and self-polymerization of vinyl double bonds occurred simultaneously during the polymerization process of the monomers. The reaction followed a thermodynamically feasible free-radical mechanism, and a reasonable mechanism was proposed regarding the specific process and steps of the reaction based on experimental data and theoretical calculations. The findings of this study corroborate the feasibility of employing metal-free photocatalysts in hydrosilylation and provide an important research foundation and insights for efficient metal-free photocatalytic hydrosilylation reactions.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
The Analytical Instrumental Center for Physicochemical Analysis and Measurements, Chinese Academy of Sciences supported facilities used in this work.References
- R. Hofmann, M. Vlatković and F. Wiesbrock, Polymers, 2017, 9, 354 CrossRef.
- D. Wang, J. Klein and E. Mejía, Chem. – Asian J., 2017, 12, 1180–1197 CrossRef CAS.
- T. K. Meister, K. Riener, P. Gigler, J. Stohrer, W. A. Herrmann and F. E. Kühn, ACS Catal., 2016, 6, 1274–1284 CrossRef CAS.
- U. Kanbur, R. J. Witzke, J. Xu, M. S. Ferrandon, T. A. Goetjen, A. J. Kropf, F. A. Perras, C. Liu, T. D. Tilley, D. M. Kaphan and M. Delferro, ACS Catal., 2023, 13, 13383–13394 CrossRef CAS.
- L. Yang, X. Wu, W. Lu, Y. Lu and Z. Zhang, Org. Lett., 2024, 26, 2287–2291 CrossRef CAS PubMed.
- L. Li, W.-S. Huang, Z. Xu and L.-W. Xu, Sci. China: Chem., 2023, 66, 1654–1687 CrossRef CAS.
- J. L. Speier, J. A. Webster and G. H. Barnes, J. Am. Chem. Soc., 1956, 79, 974–979 CrossRef.
- J. J. Hu, F. Li and T. S. A. Hor, J. Organomet. Chem., 2009, 28, 1212–1220 CrossRef CAS.
- D. Zhang, J. Wang, S. Chen, X. Cheng, T. Li, J. Zhang and A. Zhang, Langmuir, 2012, 28, 16772–16781 CrossRef CAS PubMed.
- G. Pan, C. Hu, S. Hong, H. Li, D. Yu, C. Cui, Q. Li, N. Liang, Y. Jiang, L. Zheng, L. Jiang and Y. Liu, Nat. Commun., 2021, 12, 64 CrossRef CAS PubMed.
- Y. Inoue, T. Ishida, N. Sano, T. Ya jima, H. Sogawa and F. Sanda, Macromolecules, 2022, 55, 5711–5722 CrossRef CAS.
- N. Ishigami, H. Ago, Y. Motoyama, M. Takasaki, M. Shinagawa, K. Takahashi, T. Ikuta and M. Tsuji, Chem. Commun., 2007, 1626–1628 RSC.
- H. T. Yang, Z. P. Fang, X. Y. Fu and L. F. Tong, Catal. Commun., 2008, 9, 1092–1095 CrossRef CAS.
- X. Du and Z. Huang, ACS Catal., 2017, 7, 1227–1243 CrossRef CAS.
- A. Gorczyński, M. Zaranek, S. Witomska, A. Bocian, A. R. Stefankiewicz, M. Kubicki, V. Patroniak and P. Pawluć, Catal. Commun., 2016, 78, 71–74 CrossRef.
- I. Pappas, S. Treacy and P. J. Chirik, ACS Catal., 2016, 6, 4105–4109 CrossRef CAS.
- Y. Toya, K. Hayasaka and H. Nakazawa, Organometallics, 2017, 36, 1727–1735 CrossRef CAS.
- S. Chen, X. He, Y. Jin, Y. Lan and X. Shen, Nat. Commun., 2022, 13, 3691 CrossRef CAS.
- C. Chen, Z. Luo, H. Zeng, C. Hao, K.-F. Yang, Z. Li, G.-Q. Lai and P. Zhang, Macromolecules, 2024, 57, 8146–8153 CAS.
- D. W. Kim, S. Joung, J. G. Kim and S. Chang, Angew. Chem., Int. Ed., 2015, 54, 14805–14809 CrossRef CAS PubMed.
- T. Mayer, D. Burget, G. Mignani and J. P. Fouassier, J. Polym. Sci., Part A: Polym. Chem., 1996, 34, 3141–3146 CrossRef CAS.
- S. Marchi, M. Sangermano, P. Meier and X. Kornmann, Macromol. React. Eng., 2015, 9, 360–365 CrossRef CAS.
- H. Qrareya, D. Dondi, D. Ravelli and M. Fagnoni, ChemCatChem, 2015, 7, 3350–3357 CrossRef CAS.
- H. Liang, Y.-X. Ji, R.-H. Wang, Z.-H. Zhang and B. Zhang, Org. Lett., 2019, 21, 2750–2754 CrossRef CAS PubMed.
- R. Zhou, Y. Y. Goh, H. Liu, H. Tao, L. Li and J. Wu, Angew. Chem., Int. Ed., 2017, 56, 16621–16625 CrossRef CAS PubMed.
- Z. Huang, Z. Chen, Y. Jiang, N. Li, S. Yang, G. Wang and X. Pan, J. Am. Chem. Soc., 2021, 143, 19167–19177 CrossRef CAS PubMed.
- X. Fan, M. Zhang, Y. Gao, Q. Zhou, Y. Zhang, J. Yu, W. Xu, J. Yan, H. Liu, Z. Lei, Y. C. Ter, S. Chanmungkalakul, Y. Lum, X. Liu, G. Cui and J. Wu, Nat. Chem., 2023, 15, 666–676 CrossRef CAS PubMed.
- X. Fan, P. Xiao, Z. Jiao, T. Yang, X. Dai, W. Xu, J. D. Tan, G. Cui, H. Su, W. Fang and J. Wu, Angew. Chem., Int. Ed., 2019, 58, 12580–12584 CrossRef CAS PubMed.
- Z. Qu, T. Tian, Y. Tan, X. Ji, G.-J. Deng and H. Huang, Green Chem., 2022, 24, 7403–7409 RSC.
- E. Speckmeier, T. G. Fischer and K. Zeitler, J. Am. Chem. Soc., 2018, 140, 15353–15365 CrossRef CAS.
- A. Aguirre-Soto, C.-H. Lim, A. T. Hwang, C. B. Musgrave and J. W. Stansbury, J. Am. Chem. Soc., 2014, 136, 7418–7427 CrossRef CAS.
- I. Papadopoulos, A. Bosveli, T. Montagnon, I. Zachilas, D. Kalaitzakis and G. Vassilikogiannakis, Chem. Commun., 2024, 60, 5494–5497 RSC.
- S. Wang, X. Luo, Y. Wang, Z. Liu, Y. Yu, X. Wang, D. Ren, P. Wang, Y.-H. Chen, X. Qi, H. Yi and A. Lei, Nat. Chem., 2024, 16, 1621–1629 CrossRef CAS.
- A. K. Wortman and C. R. J. Stephenson, Chem, 2023, 9, 2390–2415 CAS.
- F. Brandl, S. Bergwinkl, C. Allacher and B. Dick, Chem. – Eur. J., 2020, 26, 7946–7954 CrossRef CAS.
- J.-Y. Hou, L. Zhang, S.-Y. He, M.-L. Ye, J. Chen, T.-L. Huang, G.-H. Lv, L. Hai, Z.-Z. Yang and Y. Wu, Org. Chem. Front., 2023, 10, 6200–6204 RSC.
- L. Chen, Q. Qu, C. K. Ran, W. Wang, W. Zhang, Y. He, L. L. Liao, J. H. Ye and D. G. Yu, Angew. Chem., Int. Ed., 2023, 62, e202217918 CrossRef CAS PubMed.
- K. R. Bajya, M. Kumar, A. Ansari and S. Selvakumar, Adv. Synth. Catal., 2023, 365, 976–982 CrossRef.
- Z. Wei, Z. Chen, F. Xue, Y. Yue, S. Wu, Y. Zhang, B. Wang, Y. Xia, W. Jin and C. Liu, Green Chem., 2024, 26, 10189–10195 RSC.
- M.-H. Kim, J. Lee, H.-S. Ham, H. C. Cha and H.-G. Woo, J. Nat. Sci., 2009, 2, 1–12 Search PubMed.
- T.-H.-Y. Quach, X. Allonas, C. Croutxé-Barghorn, D. Le Nouen and M. Sangermano, RSC Adv., 2022, 12, 8458–8465 RSC.
- M. Wu, S. Huang, H. Hou, J. Lin, M. Lin, S. Zhou, Z. Zheng, W. Sun and F. Ke, RSC Adv., 2022, 12, 14724–14728 RSC.
- Y. Meng, C. Pan, N. Liu, H. Li, Z. Liu, Y. Deng, Z. Wei, J. Xu and B. Fan, Green Chem., 2024, 26, 300–305 RSC.
- J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999–3093 CrossRef CAS.
- F. Weigend, Phys. Chem. Chem. Phys., 2006, 8, 1057–1065 RSC.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 Search PubMed.
- C. Bannwarth, E. Caldeweyher, S. Ehlert, A. Hansen, P. Pracht, J. Seibert, S. Spicher and S. Grimme, Wiley Interdiscip. Rev. Comput. Mol. Sci., 2020, 11, e1493 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The additional synthesis details and characterization. See DOI: https://doi.org/10.1039/d4gc04501g |
| This journal is © The Royal Society of Chemistry 2025 |