Open Access Article
Open Access ArticleFlaxseed powder supplementation in non-alcoholic fatty liver disease: a randomized controlled clinical trial
Yanyan
Tian
a,
Yuhao
Zhou
a,
Wang
Liao
 a,
Jiayue
Xia
a,
Qiaosheng
Hu
b,
Qing
Zhao
b,
Rui
Zhang
b,
Guiju
Sun
a,
Jiayue
Xia
a,
Qiaosheng
Hu
b,
Qing
Zhao
b,
Rui
Zhang
b,
Guiju
Sun
 *a,
Ligang
Yang
*a,
Ligang
Yang
 *a and
Lihua
Li
*b
*a and
Lihua
Li
*b
aKey Laboratory of Environmental Medicine and Engineering of Ministry of Education, and Department of Nutrition and Food Hygiene, School of Public Health, Southeast University, Nanjing 210009, China. E-mail: yangligang2012@163.com; gjsun@seu.edu.cn
bLianshui People's Hospital Affiliated to Kangda College of Nanjing Medical University, Huai'an, Jiangsu 223400, China. E-mail: lihua200121@126.com
First published on 22nd January 2025
Abstract
Background: Non-alcoholic fatty liver disease (NAFLD) has become a growing public health problem worldwide, and dietary interventions have important potential in the prevention and treatment of NAFLD. Moreover, previous animal studies have shown that flaxseed has a good improvement effect in animal NAFLD models. Objectives: Assess whether flaxseed powder could improve the liver lipid content in patients with NAFLD. Methods: In this 12-week randomized controlled clinical trial, 50 patients were randomly assigned to the flaxseed group (n = 25) and the control group (n = 25). The flaxseed group received 30 g d−1 flaxseed powder orally before lunch or dinner along with health education, while the control group received only health education. The primary outcome was the intrahepatic lipid content assessed by the proton density fat fraction estimated by magnetic resonance imaging, and secondary outcomes were body composition measurements, liver function, and glucolipid metabolism. Results: Patients in the flaxseed group showed significantly lower liver fat content, body fat percentage, obesity index, visceral fat area, serum total bilirubin (TBIL), direct bilirubin (DBIL), indirect bilirubin (IBIL), aspartate aminotransferase (AST), total cholesterol (TC), and triglyceride (TG) levels after a 12-week intervention compared to pre-intervention levels, while serum apolipoprotein A1 (Apo A1) and high-density lipoprotein cholesterol (HDL-C) levels were significantly increased, with all differences being statistically significant (P < 0.05). Analysis of the gut microbiota showed that, at the phylum level, flaxseed intervention significantly increased the abundance of Bacteroides and Actinobacteria, while decreasing the ratio of Firmicutes to Bacteroidetes. At the genus level, the relative abundance of Clostridium_sensu_stricto_1, Parasutterella, Lachnospiraceae_NK4A136_group, Eubacterium_xylanophilum_group, and Bifidobacterium in the gut microbiota of the flaxseed group was significantly higher than that of the control group (P < 0.05), whereas the relative abundance of Coriobacteriaceae_UCG-002 was significantly lower than that of the control group (P < 0.05). Conclusions: Flaxseed powder intervention for 12 weeks had the effect of improving liver lipid deposition, liver function, body composition indicators, and lipid metabolism in patients with NAFLD. It also regulated the gut microbiota in NAFLD patients, increasing the abundance of beneficial bacteria while reducing harmful bacteria. This suggested that flaxseed is one of the natural and effective foods for improving NAFLD.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is one of the most common chronic liver diseases globally, affecting 38% of the population, characterized by the accumulation of fat in the liver.1 The pathologic stages of NAFLD include nonalcoholic fatty liver (NAFL), nonalcoholic steatohepatitis (NASH), followed by the development of liver fibrosis and cirrhosis, and finally leading to the development of hepatocellular carcinoma.2 Among them, about 1/5 of NAFLD patients show stage development into NASH, which is a major cause of cirrhosis and liver cancer.3 With the rapid development of the social economy over the past few decades, the prevalence of NAFLD continues to rise. A meta-analysis involving 8.5 million people showed that the global prevalence of NAFLD increased from 25.3% in 2000 to 38.0% in 2019.4 Furthermore, the increasing prevalence of obesity among young people exposes the population to NAFLD at an earlier age, and the prolonged course of the disease leads more individuals to develop liver fibrosis and cirrhosis.5 Although progress has been made in research on the treatment of NAFLD, there is currently no effective clinical drug regimen for NAFLD. Recent studies have found that dietary structure is closely related to the occurrence of NAFLD. Poor dietary habits are an important cause of NAFLD, and regulation of dietary structure, such as energy restriction and rational nutrition, could reduce metabolic syndrome.6 The Mediterranean diet, low-carbohydrate diet, and diets rich in fruits, nuts, and whole grains had a significant positive impact on improving NAFLD, providing valuable guidance and insights for the prevention and treatment of related diseases.7–12Flaxseed, as a nutritionally rich food, has a long history of cultivation in Europe and Asia. Due to its high content of ALA, lignans, and dietary fiber, it has become an important functional food ingredient.13 ALA not only helps improve blood lipids and blood pressure, but it also inhibits inflammatory reactions, relieves platelet aggregation, and helps prevent thrombus formation.14–16 n-3 PUFA could reduce intrahepatic lipid accumulation, improve liver enzyme levels, increase insulin sensitivity, and exhibit anti-inflammatory effects.17,18 Lignans are one of the most important and abundant phytochemicals in flaxseed, proven to offer a range of health benefits, including anti-cancer, antioxidant, neuroprotective, cardioprotective, and estrogenic properties.19–21 Flaxseeds contain more than 10% soluble dietary fiber; consuming sufficient dietary fiber could reduce plasma triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and plasma cholesterol in rats, alleviating atherosclerosis.22 In addition, dietary fiber supplementation could improve the gut microbiota in NAFLD patients, producing short-chain fatty acids to slow the progression of NAFLD.23,24 Given that flaxseeds are rich in bioactive substances, the American National Cancer Institute has listed them as one of the six major plants targeted for cancer research.25,26 However, the current nutritional research on flaxseed is mainly based on a single component of flaxseed, but the preparation and extraction of a single component of the process is complex and expensive and has small functional coverage. Whole flaxseed, as an organic whole, contains a wider variety of active ingredients, offers more comprehensive coverage, is easy to consume, and has a higher value for application and promotion.
In this article, we designed a randomized controlled clinical trial to evaluate the effectiveness of supplemental flaxseed powder as an adjunct intervention to a healthy diet in the treatment of NAFLD. The primary outcome was the liver fat content assessed by magnetic resonance imaging that estimated the proton density fat fraction (MRI-PDFF), while secondary outcomes included liver function, glucose–lipid metabolism, anthropometric measurements, and gut microbiota. This study aimed to explore the impact of flaxseed powder on mild to moderate NAFLD, providing new theoretical insights into the relationship between flaxseed and human health.
Methods
Participants and study design
This was a 12-week randomized controlled clinical trial (open-label), conducted from September 2022 to September 2023 at the People's Hospital of Lianshui County, Huai'an City, Jiangsu Province, China. The flaxseed was golden flaxseed purchased from Canmar Foods Ltd in Canada and contained the following per 100 g: protein – 20.0 g, fat – 48.7 g (saturated fatty acid – 3.3 g, polyunsaturated fatty acid – 40.0 g [omega-3 polyunsaturated fatty acid – 33.3 g and omega-6 polyunsaturated fatty acid – 6.7 g], monounsaturated fatty acid – 5.3 g and trans fatty acid – 0 g), carbohydrate – 20.0 g (dietary fiber – 20.0 g and sugar – 0 g), cholesterol – 0 g, sodium – 66.7 mg and lignan – 1.6 g. This trial was not blinded because it was difficult to find a placebo with the same color, smell, and characteristics. The study protocol was approved by the Ethics Approval Committee of Zhongda Hospital Affiliated to Southeast University (approval no. 2022ZDSYLL321-P01), and the study received informed consent and signed informed consent forms from all participants. The trial was registered at https://www.chictr.org.cn as ChiCTR2400091299.From October 1st to 30th, 2022, volunteers were recruited at the Outpatient and Physical Examination Center of Lianshui County People's Hospital. Based on previous studies,27–29 the largest sample size was calculated using the MRI-PDFF value as the dependent variable, with an expected difference in MRI-PDFF of 30%, assuming a two-sided α level of 0.05, a power of 80%, and a sample size of 25 patients in each group, taking into account a 10% dropout. Local residents aged between 18 and 65 diagnosed with NAFLD through previous MRI-PDFF examination (MRI-PDFF ≥ 5%)30,31 and willing to participate in the study were eligible. Exclusion criteria included: (1) patients with severe NAFLD (MRI-PDFF > 25%); (2) patients with diabetes, cardiovascular disease, digestive disorders affecting absorption, organ transplants, organ failure, malignant tumors and genetic diseases, and those using immunosuppressants; (3) those who had previously or currently suffered from viral hepatitis, drug-induced liver disease, autoimmune liver disease, and other conditions that could lead to fatty liver; (4) weight loss of >5% of their body weight in the past 6 months or currently following a weight loss diet; excessive alcohol consumption (women consuming >70 g of ethanol per week, men consuming >140 g of ethanol per week); (5) women during pregnancy, lactation, and menopause; and (6) history of flaxseed allergy, intake of nutritional supplements, probiotics, prebiotics, antibiotics, and proton pump inhibitors within 3 months, and consumption of large amounts of nuts, flaxseed, and sesame.
After completing the baseline assessment, 54 eligible NAFLD patients officially participated in the trial. Based on the patients’ age, gender, body mass index (BMI), nutrient intake, liver fat content, and other indicators, 54 eligible subjects were randomly divided into 2 groups, flaxseed group (n = 27) and control group (n = 27), by a statistical analyst who was not involved in this study using SPSS 22.0. Neither the subjects nor the researchers were aware of the random allocation sequence until the grouping was completed. The flaxseed group received 30 g d−1 flaxseed powder orally before lunch or dinner along with health education, while the control group received only health education. The content of health education included distributing oil-limiting bottles and NAFLD health education manuals to subjects, as well as providing weekly health knowledge related to NAFLD. Subjects were surveyed in weeks 0 and 12 with questionnaires (sociodemographic information, general health information, and 3-day 24 h food recall), physical examinations (height, weight, waist circumference, hip circumference, and body composition measurements), laboratory tests, and MRI-PDFF, while also collecting fecal samples to assess changes in the composition of the gut microbiota. In this study, the 3-day 24 h food recall questionnaire was collected by professional nutritionists through face-to-face interviews. The nutritionists provided three dietary record tables, and participants randomly selected two weekdays and one weekend to record all food and beverages consumed. During the questionnaire filling, the nutritionists provided daily food models to assist participants in better understanding the survey and estimating food weights more accurately. After completing the food records, the nutritionists checked the accuracy and completeness of all the recorded information. The 3-day 24 h dietary survey results were then input into the nutrition calculator developed by the Nutrition and Health Institute of the Chinese Center for Disease Control and Prevention (CDC) to calculate the participants’ daily dietary intake and analyze the nutrient content of the diet. Throughout the entire trial period, a revisit and retrieval of packaging bags was conducted every 10 days to observe any adverse reactions in the subjects and assess adherence, to ensure the quality of the trial, and flaxseed powder was distributed every 30 days.
Determination of the intrahepatic fat content by MRI-PDFF
The main outcome of this study was determined by measuring the change in the percentage of hepatic steatosis using MRI-PDFF in the participants. All examinations were conducted at the Imaging Department of the Lianshui County People's Hospital using the General Electric (GE) company's SIGNA Architect 3.0 T MRI scanner. The same MRI physician performed all MRI-PDFF examinations on the participants, utilizing an abdominal coil in combination with respiratory gating and segmental breath-holding to execute the MRI scans. Subjects should fast for 4 hours before liver MRI to minimize motion artifacts caused by excessive gastrointestinal peristalsis during the examination. Additionally, any metal objects should be removed from the body before the examination to ensure uniformity of the magnetic field. During the examination, the subject should lie supine, and the subject should hold their breath to cooperate with the examination. Three areas with fewer blood vessels, uniform liver texture, and away from the liver edge should be selected for measurement during the examination to increase the accuracy of fat quantification. Each scan should start at the end of inhalation to minimize the interference of respiratory movements. After the scan was completed, the liver fat content of three areas of the liver texture was obtained, and the maximum value was taken to calculate the overall liver fat content, quantitatively calculating the grade of fatty liver.Basic information collection and anthropometric assessment
At the beginning of the trial, the researchers collected the subjects’ socio-demographic and general health information through face-to-face interviews, including age, gender, occupation, education level, smoking history, alcohol consumption history, daily activity level, history of previous diseases, previous medication use and so on. In addition, 3-day, 24-hour dietary recall data and dietary frequency questionnaires were collected consecutively by nutrition professionals at the beginning and end of the trial, supplemented by daily food modeling to help subjects estimate food weights more accurately. The results of the dietary survey were then entered into the FeiHua Nutrition Calculator software to calculate the daily dietary intake of the subjects and to analyze the nutrient intake. The DBA-450 Body Composition Analyzer was used for body composition measurement based on the bioelectrical impedance measurement method, and a Meilen MSG005-H ultrasonic measuring instrument was used to measure the height, with participants removing shoes, socks, outerwear, hats, and other heavy clothing before measurement to ensure the accuracy of the measurement results. A uniform standard soft ruler was provided to measure the waist circumference and hip circumference, and the average of the two measurements was taken as the final value.Serum sample collection and measurement
Peripheral venous blood samples from the subjects were collected for biochemical testing before and at the end of the experiment. Subjects maintained a light diet the day before blood collection and fasted on food and water for 8 hours before blood collection. Blood samples were collected by medical personnel between 8:00–9:00 a.m. at the Health Examination Center of Lianshui County People's Hospital, and then sent to the Laboratory Department of Lianshui County People's Hospital for the measurement of blood routine, blood glucose and lipid metabolism related indexes, and liver function indexes within half an hour after the blood collection. Biochemical parameters related to glucose, liver function, renal function, inflammation, and lipid profile were measured using a Cobas 8000 automated biochemical analyzer, while fasting insulin (FINS) levels were measured using a MAGLUMI 400 PLUS fully automated chemiluminescence meter. All biochemical assessments were performed in the same laboratory using standard methods.Fecal sample collection and microbial DNA extraction and sequencing
At the baseline and week 12, fecal samples from subjects were collected for sequencing. The fecal collection process followed the International Human Microbiome Standard (IHMS) procedure for fecal collection, and the collected fecal samples were stored in a refrigerator at −80 °C until testing. A commercial DNA extraction kit (NobleRyder) was used to extract bacterial DNA from microbial samples obtained from the subjects, utilizing cetyltrimethylammonium bromide for the process. Primers 341F (5′-CCTACGGGGNGGCWGCAG-3′) and 805R (5′-GACTACHVGGGGTATCTAATCC-3′) were used to amplify the 16S rRNA gene, especially the V3–V4 region. The PCR amplification conditions were as follows: initial denaturation at 98 °C for 30 s, then denaturation at 98 °C for 10 s, annealing at 54 °C for 30 s, and extension at 72 °C for 45 s in 32 cycles. The PCR amplification conditions were as follows: initial denaturation at 98 °C for 30 s, denaturation at 98 °C for 10 s, annealing at 54 °C for 30 s, extension at 72 °C for 45 s, a total of 32 cycles, and finally extension at 72 °C for 10 min. Ultrapure water was used throughout as a negative control to exclude false positive experimental results. Then the PCR products were purified using 2% agarose gel electrophoresis. The size and number of the amplified library were assessed using an Agilent 2100 Bioanalyzer (Agilent, USA) and an Illumina library quantification kit (Kapa Biosciences, Woburn, MA, USA). The sequencing platform used was the NovaSeq PE250 platform, with the selected instrument being the NovaSeq 6000 sequencer, and the matching reagent was the NovaSeq 6000 SP Reagent Kit (500 cycles).Statistical analysis
The questionnaires were entered using the EPIDATA version 3.1 software with double entry, and the data were processed and analyzed using SPSS 22.0 (version 22.0, SPSS Inc., Chicago, IL, USA). Quantitative data were analyzed based on the Shapiro–Wilk test. Normally distributed data were described as means ± SD, and the Student's t-test was used to analyze differences between the two groups. For non-normally distributed data, it was described as the median (25th percentile, 75th percentile), and the Mann–Whitney U test was used to analyze and compare the differences between the two groups of data. Qualitative data were analyzed using the chi-square test and described in terms of composition ratios. In statistical analysis, the significance level was set at 0.05, and if P < 0.05, the difference between the two groups was considered statistically significant.The software used for gut microbial diversity analysis was Qiime 2, which could calculate both microbial alpha and beta diversity, and inter-group variability was analyzed using the Student's t-test or the Wilcoxon test. Inter-group difference analysis was conducted using either the Student's t-test or the Wilcoxon test, and the Spearman rank test was used to compute the correlation between changes in participants’ clinical outcomes and relative abundance changes in the gut microbiota. R3.5.2 (R Core Team) and GraphPad Prism 9.0 were used for chart plotting.
Results
Patient flow, tolerability, and compliance
The flowchart of the trial is shown in Fig. 1. At the beginning of the trial, 102 patients with NAFLD registered to participate. Among them, 32 patients did not meet the inclusion criteria, 4 could not be contacted, 6 refused to attend medical examinations on time, and 3 were unable to control their diet and could not take the flaxseed on time. After screening, 54 individuals enrolled in the randomization, with 27 in each of the flaxseed group and the control group. In the flaxseed group, 2 participants discontinued the study, one was lost to follow-up and one was hospitalized due to a fracture. In the control group, 2 participants also discontinued the study, 1 was transferred for work reasons to another location, and 1 was diagnosed with breast cancer requiring surgery. No adverse reactions were reported during the trial, and the participants showed good compliance. Among those who completed the final follow-up, the compliance rate for daily flaxseed intake exceeded 90%.Baseline characteristics
The baseline sociodemographic, anthropometric, daily dietary intake, physical activity level, liver fat content, liver function, lipid profile, blood glucose, and FINS data of the subjects are shown in Table 1. There were no significant differences between the flaxseed group and the control group on all indicators at the baseline.| Characteristics | Flaxseed group (n = 25) | Control group (n = 25) | p-value |
|---|---|---|---|
| Abbreviations: BMI, body mass index; WHR, waist to hip ratio; VFA, visceral fat area; MET, metabolic equivalent; MRI-PDFF, magnetic resonance imaging proton density fat fraction; TBIL, total bilirubin; DBIL, direct bilirubin; IBIL, indirect bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transpeptidase; CHE, cholinesterase; TC, total cholesterol; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Apo A1, apolipoprotein A1; Apo B, apolipoprotein B; LP(a), lipoprotein (a); GLU, glucose; FINS, fasting insulin. Values are displayed as n (%), mean ± SD, or median (IQR). | |||
| Age, year | 35.44 ± 10.85 | 36.32 ± 10.00 | 0.767 |
| Sex | 0.395 | ||
| Male, n (%) | 13 (52.0) | 10 (40.0) | |
| Female, n (%) | 12 (48.0) | 15 (60.0) | |
| Occupation | 0.658 | ||
| Professionals/technicians, n (%) | 13 (52.0) | 13 (52.0) | |
| Sales/workers/farmers, n (%) | 8 (32.0) | 6 (24.0) | |
| Other, n (%) | 4 (16.0) | 6 (24.0) | |
| Educational attainment | 0.768 | ||
| Primary school and below, n (%) | 2 (8.0) | 1 (4.0) | |
| Junior and senior high school, n (%) | 6 (24.0) | 8 (32.0) | |
| College and above, n (%) | 17 (68.0) | 16 (64.0) | |
| Anthropometry | |||
| Height, cm | 168.59 ± 7.43 | 166.33 ± 9.69 | 0.358 |
| Weight, kg | 90.5 ± 12.1 | 87.4 ± 15.2 | 0.440 |
| BMI, kg m−2 | 31.33 (29.50, 34.40) | 31.13 (28.39, 33.34) | 0.550 |
| Body fat, % | 38.02 ± 5.10 | 37.53 ± 4.73 | 0.727 |
| Obesity degree, % | 152.70 (143.90, 161.70) | 147.70 (142.10, 157.40) | 0.105 |
| WHR | 0.91 ± 0.07 | 0.94 ± 0.05 | 0.119 |
| VFA, cm2 | 199.20 ± 72.86 | 172.62 ± 60.06 | 0.166 |
| Skeletal muscle mass, kg | 30.96 ± 5.60 | 30.44 ± 5.78 | 0.748 |
| Daily dietary intake | |||
| Energy, kcal d−1 | 2157.36 ± 909.00 | 2044.08 ± 828.72 | 0.647 |
| Protein, g d−1 | 75.30 (58.60, 101.70) | 65.70 (54.10, 86.80) | 0.105 |
| Fat, g d−1 | 57.80 (31.90, 85.40) | 53.70 (35.40, 83.20) | 0.816 |
| Carbohydrates, g d−1 | 299.81 ± 142.41 | 303.68 ± 132.90 | 0.921 |
| Dietary fiber, g d−1 | 9.90 ± 5.35 | 11.01 ± 5.93 | 0.491 |
| Vitamin B1, mg d−1 | 1.10 (0.70, 1.70) | 1.22 (0.90, 1.80) | 0.547 |
| Vitamin B2, mg d−1 | 0.88 (0.50, 1.90) | 1.12 (0.50, 1.90) | 0.992 |
| Vitamin B3, mg d−1 | 12.68 ± 9.64 | 16.05 ± 11.37 | 0.263 |
| Vitamin C, mg d−1 | 66.85 (14.60, 139.40) | 49.10 (16.30, 89.90) | 0.621 |
| Vitamin E, mg d−1 | 9.98 (3.40, 19.10) | 9.83 (6.80, 17.80) | 0.869 |
| Zinc, mg d−1 | 8.60 ± 5.55 | 10.87 ± 7.75 | 0.240 |
| Selenium, mg d−1 | 40.41 (13.70, 69.20) | 41.70 (19.60, 79.20) | 0.669 |
| Physical activity, MET-min per week | 2478 (1409, 3943) | 2573 (1517, 4172) | 0.648 |
| MRI-PDFF (%) | 10.80 (9.10, 18.63) | 10.30 (7.64, 14.10) | 0.159 |
| Liver function | |||
| TBIL (μmol L−1) | 14.98 (10.60, 16.90) | 12.33 (10.20, 15.20) | 0.204 |
| DBIL (μmol L−1) | 3.94 (3.20, 5.50) | 3.42 (2.80, 4.70) | 0.09 |
| IBIL (μmol L−1) | 10.68 (7.60, 12.40) | 8.38 (6.10, 10.10) | 0.055 |
| ALT (U L−1) | 40.00 (34.00, 57.00) | 34.00 (26.00, 52.50) | 0.171 |
| AST (U L−1) | 23.00 (21.50, 28.00) | 22.00 (19.00, 27.00) | 0.173 |
| GGT (U L−1) | 35.00 (30.50, 54.00) | 30.00 (23.50, 40.00) | 0.109 |
| CHE (U L−1) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 205.48 ± 1142.70 205.48 ± 1142.70 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 680.44 ± 1103.55 680.44 ± 1103.55 |
0.105 |
| Blood lipids | |||
| TC (mmol L−1) | 5.22 ± 0.80 | 5.05 ± 0.65 | 0.422 |
| TG (mmol L−1) | 2.11 ± 0.74 | 2.69 ± 1.65 | 0.120 |
| HDL-C (mmol L−1) | 1.01 ± 0.09 | 0.98 ± 0.12 | 0.307 |
| LDL-C (mmol L−1) | 3.43 ± 0.76 | 3.21 ± 0.53 | 0.243 |
| Apo A1 (mmol L−1) | 0.94 ± 0.09 | 0.93 ± 0.09 | 0.621 |
| Apo B (mmol L−1) | 0.94 ± 0.01 | 0.91 ± 0.14 | 0.463 |
| LP(a) (mmol L−1) | 93.50 (45.50, 236.40) | 133.80 (58.30, 211.30) | 0.491 |
| Glycemic control parameters | |||
| GLU (mmol L−1) | 5.06 ± 0.56 | 5.40 ± 0.62 | 0.463 |
| FINS (pmol L−1) | 113.36 (92.70, 136.60) | 114.70 (93.50, 127.70) | 0.977 |
Dietary energy and nutrient intake, and physical activity levels of the subjects
Table 2 shows the nutritional intake and physical activity of the flaxseed group and the control group at the baseline and week 12; there were no significant differences between the two groups in terms of energy, nutrition, or physical activity.| Characteristics | Flaxseed group (n = 25) | p a | Control group (n = 25) | p b | ||
|---|---|---|---|---|---|---|
| Baseline (n = 25) | Week 12 (n = 25) | Baseline (n = 25) | Week 12 (n = 25) | |||
| Abbreviation: MET, metabolic equivalent. Values are displayed as mean ± SD, or median (IQR). a,bp values indicate differences between the control and treatment groups at the baseline and 12 weeks. | ||||||
| Energy, kcal d−1 | 2157.36 ± 909.00 | 2023 ± 845.67 | 0.878 | 2044.08 ± 828.72 | 2154.08 ± 749.67 | 0.539 |
| Protein, g d−1 | 75.30 (58.60, 101.70) | 79.20 (61.40, 98.30) | 0.763 | 65.70 (54.10, 86.80) | 61.83 (49.64, 88.27) | 0.227 |
| Fat, g d−1 | 57.80 (31.90, 85.40) | 54.20 (36.70, 89.30) | 0.698 | 53.70 (35.40, 83.20) | 59.6 (46.80, 80.60) | 0.816 |
| Carbohydrates, g d−1 | 299.81 ± 142.41 | 276.56 ± 126.89 | 0.425 | 303.68 ± 132.90 | 274.39 ± 118.60 | 0.434 |
| Dietary fiber, g d−1 | 9.90 ± 5.35 | 10.3 ± 4.89 | 0.673 | 11.01 ± 5.93 | 10.82 ± 3.67 | 0.731 |
| Vitamin B1, mg d−1 | 1.10 (0.70, 1.70) | 1.13 (0.80, 1.80) | 0.621 | 1.22 (0.90, 1.80) | 1.05 (0.80, 1.60) | 0.628 |
| Vitamin B2, mg d−1 | 0.88 (0.50, 1.90) | 0.92 (0.48, 1.93) | 0.342 | 1.12 (0.50, 1.90) | 1.09 (0.60, 1.85) | 0.870 |
| Vitamin B3, mg d−1 | 12.68 ± 9.64 | 11.99 ± 8.63 | 0.385 | 16.05 ± 11.37 | 15.62 ± 9.73 | 0.473 |
| Vitamin C, mg d−1 | 66.85 (14.60, 139.40) | 68.43 (25.29, 125.36) | 0.632 | 49.10 (16.30, 89.90) | 43.50 (14.62, 76.40) | 0.384 |
| Vitamin E, mg d−1 | 9.98 (3.40, 19.10) | 10.04 (4.21, 19.37) | 0.531 | 9.83 (6.80, 17.80) | 8.79 (5.30, 17.6.40) | 0.737 |
| Zinc, mg d−1 | 8.60 ± 5.55 | 8.40 ± 5.02 | 0.872 | 10.87 ± 7.75 | 9.93 ± 7.45 | 0.390 |
| Selenium, mg d−1 | 40.41 (13.70, 69.20) | 42.32 (14.78, 62.73) | 0.691 | 41.70 (19.60, 79.20) | 43.10 (21.50, 68.60) | 0.683 |
| Physical activity, MET-min per week | 2478 (1409, 3943) | 2583 (1672, 3856) | 0.839 | 2573 (1517, 4172) | 2450 (1493, 4078) | 0.721 |
Effects of flaxseed powder consumption on body composition
As shown in Table 3, after 12 weeks of flaxseed intervention, there was a significant reduction in body fat percent (P = 0.001), obesity degree (P = 0.008), and visceral fat area (P = 0.017) compared to the baseline. Based on the analysis results of the differences between the baseline and 12 weeks, the subjects in the flaxseed group showed a mean decrease weight loss of 2.4 kg and a 6.4% reduction in body fat percentage, in addition to a significant decrease in the visceral fat area. However, there were no differences observed between the two groups in terms of skeletal muscle mass.| Characteristics | Flaxseed group | Control group | ||||
|---|---|---|---|---|---|---|
| Baseline (n = 25) | Week 12 (n = 25) | Change | Baseline (n = 25) | Week 12 (n = 25) | Change | |
| Abbreviations: BMI, body mass index; WHR, waist to hip ratio; VFA, visceral fat area. Values are displayed as n (%), mean ± SD, or median (IQR). a indicates a statistically significant difference between the baseline and week 12 in the flaxseed group or the control group, p < 0.05; b indicates a statistically significant difference between the flaxseed group and the control group at week 12, p < 0.05; c indicates a statistically significant difference between the change from the baseline to week 12 in the flaxseed group and the control group, p < 0.05. | ||||||
| Weight, kg | 90.5 ± 12.1 | 88.1 ± 10.6 | −2.4 ± 2.3 | 87.4 ± 15.2 | 87.5 ± 14.7 | 0.1 ± 2.9c |
| BMI, kg m−2 | 31.33 (29.50, 34.40) | 30.67 (28.72, 32.62) | −0.92 (−1.36, −0.23) | 31.13 (28.39, 33.34) | 31.13 (28.39, 33.34) | 0.02 (−0.43, 0.70)c |
| Body fat, % | 38.02 ± 5.10 | 31.62 ± 3.62a | −6.40 ± 2.16 | 37.53 ± 4.73 | 36.22 ± 5.33b | −1.30 ± 1.12c |
| Obesity degree, % | 152.70 (143.90, 161.70) | 143.40 (130.10, 151.80)a | −12.80 (−15.70, −11.70) | 147.70 (142.10, 157.40) | 142.80 (134.10, 153.50) | −5.30 (−23.40, 9.80) |
| WHR | 0.91 ± 0.07 | 0.89 ± 0.04 | −0.02 ± 0.08 | 0.94 ± 0.05 | 0.93 ± 0.03b | −0.01 ± 0.06 |
| VFA, cm2 | 199.20 ± 72.86 | 156.20 ± 46.75a | −28.90 (−41.70, −21.40) | 172.62 ± 60.06 | 168.90 ± 63.73 | −11.30 (−26.10, 48.90)c |
| Skeletal muscle mass, kg | 30.96 ± 5.60 | 31.81 ± 5.76 | 0.90 (0.30, 1.40) | 30.44 ± 5.78 | 31.48 ± 6.79 | 1.10 (−0.10, 2.20) |
Effect of flaxseed powder consumption on biochemical indicators
As shown in Table 4, both the liver function indicators of the flaxseed group and the control group subjects improved, but the improvement was more pronounced in the flaxseed group. In terms of liver function indexes, serum total bilirubin (TBIL) and direct bilirubin (DBIL) in both the flaxseed group and control group decreased significantly at 12 weeks with statistical differences, while indirect bilirubin (IBIL) and aspartate aminotransferase (AST) decreased significantly only in the flaxseed group, and the control group also showed a decreasing trend, but with smaller decreases. No significant changes in alanine aminotransferase (ALT) and cholinesterase (CHE) were observed before and after the intervention in both groups. In terms of lipid profiles, there were also statistically significant differences (P < 0.05) between the two groups in TG, HDL-C, and ApoA1 after 12 weeks of intervention. Although there was no statistical difference in the baseline and 12-week LDL-C levels between the two groups, the flaxseed group showed a reduction of 0.23 mmol L−1 from the baseline, while the control group only showed a decrease of 0.05 mmol L−1. After 12 weeks of intervention with flaxseed powder, the total cholesterol (TC), TG, high-density lipoprotein cholesterol (HDL-C), and apolipoprotein A1 (Apo A1) levels of subjects in the flaxseed group improved significantly compared with the baseline. In addition, the flaxseed group showed a decrease in TG by 0.4 mmol L−1 from the baseline (P < 0.05), and an increase in HDL-C and ApoA1 by 0.10 mmol L−1 and 0.12 mmol L−1 from the baseline (P < 0.05), respectively, while the control group showed minimal changes compared to the baseline. However, no beneficial effects of flaxseed power intervention were observed in glycemic control parameters.| Characteristics | Flaxseed group | Control group | ||||
|---|---|---|---|---|---|---|
| Baseline (n = 25) | Week 12 (n = 25) | Change | Baseline (n = 25) | Week 12 (n = 25) | Change | |
| Abbreviations: TBIL, total bilirubin; DBIL, direct bilirubin; IBIL, indirect bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transpeptidase; CHE, cholinesterase; TC, total cholesterol; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Apo A1, apolipoprotein A1; Apo B, apolipoprotein B; LP(a), lipoprotein (a); GLU, glucose; FINS, fasting insulin. Values are displayed as n (%), mean ± SD, or median (IQR). a indicates a statistically significant difference between the baseline and week 12 in the flaxseed group or the control group, p < 0.05; b indicates a statistically significant difference between the flaxseed group and the control group at week 12, p < 0.05; c indicates a statistically significant difference between the change from the baseline to week 12 in the flaxseed group and the control group, p < 0.05. | ||||||
| Liver function | ||||||
| TBIL (μmol L−1) | 14.98 (10.60, 16.90) | 9.71 (7.80, 12.60)a | −4.16 (−5.30, −2.90) | 12.33 (10.20, 15.20) | 9.13 (5.80, 14.10)a | −3.21 (−4.10, −2.40)c |
| DBIL (μmol L−1) | 3.94 (3.20, 5.50) | 3.29 (2.30, 3.70)a | −1.03 (−1.80, −0.80) | 3.42 (2.80, 4.70) | 2.44 (1.90, 4.00)a | −0.80 (−1.00, −0.50)c |
| IBIL (μmol L−1) | 10.68 (7.60, 12.40) | 6.80 (5.70, 8.40)a | −3.29 ± 1.29 | 8.38 (6.10, 10.10) | 6.69 (4.00, 10.1) | −1.78 ± 2.13c |
| ALT (U L−1) | 40.00 (34.00, 57.00) | 37.00 (28.00, 49.00) | −6.00 (−12.00, −3.00) | 34.00 (26.00, 52.50) | 37.00 (21.50, 42.50) | −4.00 (−12.00, −2.00) |
| AST (U L−1) | 23.00 (21.50, 28.00) | 18.00 (16.00, 23.00)a | −6.00 (−6.00, −4.00) | 22.00 (19.00, 27.00) | 20.00 (18.00, 26.00) | −1.00 (−2.50, −1.00)c |
| GGT (U L−1) | 35.00 (30.50, 54.00) | 36.00 (22.50, 54.50) | −8.00 (−10.00, 0.50) | 30.00 (23.50, 40.00) | 24.00 (16.50, 41.50) | −6.00 (−7.00, −4.00) |
| CHE (U L−1) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 205.48 ± 1142.70 205.48 ± 1142.70 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 827.76 ± 2145.83 827.76 ± 2145.83 |
377.72 ± 1725.43 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 680.44 ± 1103.55 680.44 ± 1103.55 |
9948.28 ± 1969.47 | 732.16 ± 998.36 |
| Blood lipids | ||||||
| TC (mmol L−1) | 5.22 ± 0.80 | 4.75 ± 0.78a | −0.34 (−0.50, −0.20) | 5.05 ± 0.65 | 4.73 ± 0.70 | −0.45 (−0.50, −0.40) |
| TG (mmol L−1) | 2.11 ± 0.74 | 1.71 ± 0.63a | −0.40 ± 0.36 | 2.69 ± 1.65 | 2.65 ± 1.52b | −0.04 ± 0.60c |
| HDL-C (mmol L−1) | 1.01 ± 0.09 | 1.12 ± 0.17a | 0.10 ± 0.08 | 0.98 ± 0.12 | 0.98 ± 0.12b | 0.00 ± 0.06c |
| LDL-C (mmol L−1) | 3.43 ± 0.76 | 3.20 ± 0.98 | −0.23 ± 0.28 | 3.21 ± 0.53 | 3.16 ± 0.52 | −0.05 ± 0.14c |
| Apo A1 (mmol L−1) | 0.94 ± 0.09 | 1.06 ± 0.13a | 0.12 ± 0.04 | 0.93 ± 0.09 | 0.94 ± 0.05b | 0.01 ± 0.05c |
| Apo B (mmol L−1) | 0.94 ± 0.01 | 0.88 ± 0.26 | −0.06 ± 0.08 | 0.91 ± 0.14 | 0.90 ± 0.11 | −0.01 ± 0.15 |
| LP(a) (mmol L−1) | 93.50 (45.50, 236.40) | 48.70 (23.20, 187.30) | −22.50 (−44.00, −18.40) | 133.80 (58.30, 211.30) | 87.50 (48.00, 157.60) | −38.80 (−59.00, −14.80) |
| Glycemic control parameters | ||||||
| GLU (mmol L−1) | 5.06 ± 0.56 | 5.10 ± 0.49 | 0.03 ± 0.56 | 5.40 ± 0.62 | 5.25 ± 0.83 | −0.15 ± 1.03 |
| FINS (pmol L−1) | 113.36 (92.70, 136.60) | 106.86 (95.30, 146.40) | 7.42 (−29.70, 43.80) | 114.70 (93.50, 127.70) | 105.03 (90.70, 159.00) | 10.96 (−30.40, 51.90) |
Effect of flaxseed powder consumption on liver fat content
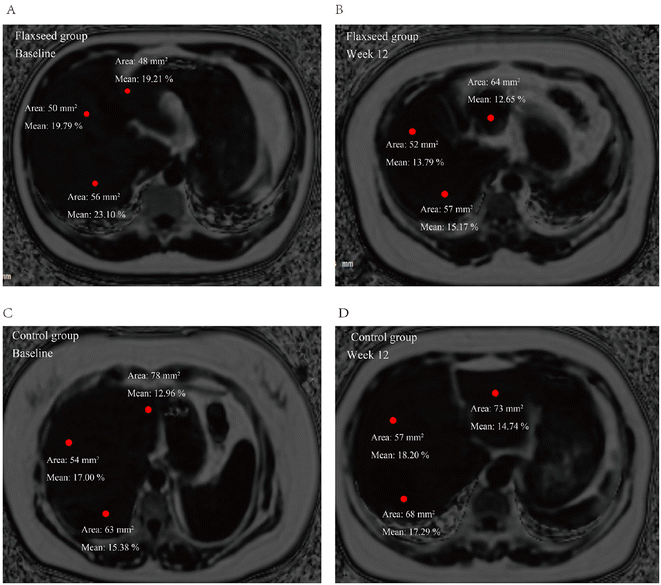
After 12 weeks of intervention, the comparison of differences in MRI-PDFF between the two groups also showed a statistically significant difference (P = 0.001) (Table 5). Furthermore, the liver fat content of the participants in the flaxseed group decreased by an average of 5.28% compared to the baseline (P = 0.006). Although the liver fat content in the control group also decreased by 1.23%, there was no statistically significant difference (P = 0.225) (Table 5). Further subgroup analysis results (Table 5) showed that flaxseed powder intervention did not find significant improvement in MRI-PDFF in 5%–10% of NAFLD subjects, although there was a downward trend, but no statistical difference. In contrast, in NAFLD participants with MRI-PDFF levels between 10% and 25%, flaxseed powder showed a positive intervention effect on the liver fat content; the liver fat content of the participants in the flaxseed group decreased by an average of 8.87% from the baseline (P = 0.006). The liver fat content of each subject during the trial is shown in Fig. 2. Fig. 3 shows MRI-PDFF images of the liver fat content in 2 subjects. Fig. 3A and B show comparisons of the liver fat content at the baseline and at 12 weeks in 1 randomly selected subject in the flaxseed group. The liver fat content of the participant in the flaxseed group (male, 37 years old) decreased from 23.10% at the baseline to 15.17% at 12 weeks. Fig. 3C and D show comparisons of the liver fat content at the baseline and 12 weeks for 1 randomly selected subject in the control group, which increased from 17.00% at the baseline to 18.20% at 12 weeks for the control group participant (male, 35 years old).| Characteristics | Flaxseed group | Control group | ||||
|---|---|---|---|---|---|---|
| Baseline | Week 12 | Change | Baseline | Week 12 | Change | |
| Abbreviation: MRI-PDFF, magnetic resonance imaging proton density fat fraction. Values are displayed as median (IQR). a indicates a statistically significant difference between the baseline and week 12 in the flaxseed group or the control group, p < 0.05; b indicates a statistically significant difference between the flaxseed group and the control group at week 12, p < 0.05; c indicates a statistically significant difference between the change from the baseline to week 12 in the flaxseed group and the control group, p < 0.05. | ||||||
| MRI-PDFF (%) | 10.80 (9.10, 18.63) (n = 25) | 8.17 (6.70, 11.22)a (n = 25) | −5.28 (−8.77, −3.30) | 10.30 (7.64, 14.10) (n = 25) | 8.85 (5.09, 15.41) (n = 25) | −1.23 (−2.31, 0.51)c |
| MRI-PDFF (5%–10%) | 9.10 (8.96, 9.20) (n = 12) | 6.65 (5.17, 11.71) (n = 12) | −2.91 (−3.80, −2.57) | 7.64 (6.08, 9.50) (n = 13) | 5.25 (9.10, 7.47) (n = 13) | −0.11 (−2.29, −0.43) |
| MRI-PDFF (10%–25%) | 18.62 (16.52, 20.20) (n = 13) | 9.66 (7.65, 10.65)a (n = 13) | −8.87 (−9.55, −7.59) | 15.71 (12.40, 19.62) (n = 12) | 13.44 (11.13, 18.45)b (n = 12) | −1.52 (−3.12, 1.06)c |
Effects of flaxseed powder consumption on the relative abundance of the gut microbiota
To explore the potential effects of flaxseed powder on the gut microbiota of NAFLD patients, 16S rRNA gene sequencing was performed on the feces of all participants. Fig. 4A showed the baseline differences in the gut microbiota distribution at the phylum level between the two groups. The results indicated that there was no statistically significant difference in the distribution of major phyla between the intervention group and the control group at the baseline. After 12 weeks of intervention, the relative abundance of Bacteroidetes and Actinobacteria in the gut microbiota of the intervention group was significantly higher than that of the control group, while the relative abundance of Firmicutes in the intervention group was significantly lower than that of the control group (P < 0.05) (Fig. 4B). Fig. 5A shows the baseline differences in the gut microbiota distribution at the genus level between the two groups. The results showed no statistically significant difference in the distribution of gut microbiota at the genus level between the two groups at the baseline. Fig. 5B shows the genera with different distributions at the genus level of the gut microbiota between the two groups at 12 weeks. After 12 weeks of flaxseed powder intervention, the relative abundance of Clostridium_sensu_stricto_1, Parasutterella, Lachnospira-ceae_NK4A136_group, Eubacterium_xylanophilum_group, and Bifidobacterium in the gut microbiota of the intervention group was significantly higher than that of the control group, while the relative abundance of Coriobacteriaceae_UCG-002 was significantly lower than that of the control group (P < 0.05) (Fig. 5B).Analysis of the correlation between the gut microbiota and glucose–lipid metabolism and liver function in patients with NAFLD
To further investigate whether the improvement of glycolipid metabolism and liver function indicators in NAFLD patients was related to changes in the gut microbiota, the Spearman method was used to perform correlation analysis on the glycolipid metabolism, liver function indicators, and gut microbiota with different abundances in NAFLD patients at 12 weeks, as shown in Fig. 6. The analysis revealed that Clostridium_sensu_stricto_1, Lachnospiraceae_N-K4A136_group, and Bifidobacterium were significantly correlated with numerous indicators of glycolipid metabolism and liver function (P < 0.05). Among them, Bifidobacterium was significantly positively correlated with serum Apo A1 and HDL-C levels, and significantly negatively correlated with AST, TBIL, DBIL, I-BIL, LDL-C, lipoprotein (a) (LP(a)), TC, and TG levels (P < 0.05). Clostridium_sensu_stricto_1 was negatively correlated with glucose (P < 0.05). Coriobacteriaceae_UCG-002 was significantly positively correlated with serum ALT, AST, DBIL, and TG levels (P < 0.05). However, the Lachnospiraceae_NK4A136_group showed a significant negative correlation with serum AST and TG levels (P < 0.05).Discussion
This randomized controlled clinical trial aimed to explore the effects of flaxseed powder intervention on improving NAFLD patients’ conditions. Results indicated that taking 30 g of flaxseed powder daily for 12 weeks could reduce liver fat content, obesity and visceral fat area, improve liver function and blood lipid profile, and increase the abundance of gut microbiota species in NAFLD patients. Previous clinical studies have shown that flaxseed powder has a significant improvement effect on NAFLD patients,32–34 consistent with our findings.Currently, liver biopsy is the gold standard for evaluating liver histopathology in patients with NAFLD. However, this invasive technique might cause complications associated with the procedure. Therefore, it is not practical to use it to quantify the dynamic changes in fatty liver.35 In this study, MRI-PDFF was used to measure the liver fat content. MRI-PDFF is the most reliable non-invasive method for detecting steatosis, and due to its feasibility and accuracy, it has been adopted as a primary outcome indicator in multiple clinical trials.36 The results of this study indicated that after 12 weeks of intervention, the liver fat content in the flaxseed intervention group showed a significant reduction compared to the baseline, while no such change was observed in the control group. Interestingly, further subgroup analysis revealed that flaxseed intervention significantly reduced the liver fat content in patients with moderate NAFLD, whereas in the mild NAFLD subgroup, there was no statistically significant difference in the liver fat content before and after the intervention. In an animal study conducted by Chao Yang et al.,33 it was found that flaxseed powder significantly improved hepatic steatosis in NAFLD mice, with effects being dose-dependent – the higher the flaxseed powder dose, the more evident the improvement. In two other randomized, controlled, clinical trials, both of which used 30 g of whole flaxseed powder for 12 weeks of intervention in patients with NAFLD, liver steatosis and fibrosis were assessed using transient elastography in all patients, and the results indicated significant improvement in both fibrosis scores and the percentage of steatosis in the flaxseed intervention group.29,37 However, both studies did not perform a stratified analysis, so it remained unclear whether flaxseed powder interventions had different effects on mild NAFLD versus moderate NAFLD. Additionally, there has been limited prior research on related populations; thus, this study also offers a new perspective for future research on using flaxseed powder to improve the condition of NAFLD patients.
In the current study, a 12-week intervention with flaxseed powder led to a significant reduction in body weight, BMI, body fat percentage, and visceral fat area in patients with NAFLD. Body composition indicators were associated with the risk and severity of NAFLD. NAFLD patients with a BMI greater than 25 kg m−2 had a higher risk of liver fibrosis compared to those with normal weight, and the reduction of body weight delayed the progression of NAFLD,38,39 and body fat percentage was highly sensitive in predicting NAFLD risk.40 Previously, flaxseed has been widely used for weight loss among individuals who are overweight or obese. Research has shown that flax lignans can help prevent obesity or aid in weight loss by regulating adiponectin levels and up-regulating the level of fat oxidation in skeletal muscles. In addition, flax lignans could reduce the synthesis levels of sterol regulatory elements necessary for TG production and increase short-chain fatty acid concentrations in the intestines, thereby enhancing satiety to promote weight loss.41 Meanwhile, the soluble fiber in flaxseed dietary fiber has also been shown to suppress appetite and delay gastric emptying by absorbing water and swelling to exert a weight loss effect.42 A three-arm randomized controlled trial conducted by Bongartz et al.43 also demonstrated that flaxseed soluble dietary fiber supplementation reduced body weight and waist–hip circumference in overweight and obese patients. Meanwhile, the high concentration of ALA contained in flaxseed also played an important role in the weight reduction effect. ALA was converted into eicosapentaenoic acid and docosahexaenoic acid when it entered the body, thus exerting a weight reduction effect.44 Finally, flaxseed dietary fiber altered the composition of the gut microbiota, thereby accelerating intestinal absorption of lignans and promoting the formation of lignan metabolite enterolactone, which was negatively correlated with weight gain.45 This also reflected the superiority of flaxseed powder supplementation compared to supplementation with individual flaxseed components.
Flaxseed is considered natural and effective in improving liver function in NAFLD patients.46 Serum bilirubin levels were used to assess the ability of hepatocytes to process bilirubin. Elevated serum TBIL levels should be divided into direct and indirect bilirubin components. Elevated DBIL (conjugated bilirubin) was indicative of hepatocyte dysfunction or cholestasis, whereas damage to hepatocytes led to a decrease in the liver's ability to process bilirubin, which could lead to an elevation of serum bilirubin levels.47 Asymptomatic elevation of liver enzymes was a possible clinical manifestation of NAFLD. Among these, ALT showed the strongest correlation with fat accumulation in the liver and was most commonly found in the cytoplasm of hepatocytes. Serum ALT has historically served as an indicator of inflammation from hepatocellular injury in diseases such as cirrhosis, hepatitis, hepatocellular carcinoma, and alcoholic liver disease.48–50 Since ALT is a sensitive indicator for recognizing NAFLD injury, it was frequently elevated when liver injury was moderate.51,52 In individuals with NAFLD, ALT was usually greater than AST and AST/ALT < 1 was typical. The cytoplasm and mitochondria of cells contained AST, and as the disease progressed, hepatocytes released more AST than ALT because the mitochondria of the cells were damaged, and the elevation of ALT and AST varied with the extent and duration of liver disease.53,54 The results of this study showed that the serum TBIL, DBIL, IBIL and AST levels of NAFLD patients in the intervention group at the end of the intervention were significantly lower than those before the intervention, and the serum TBIL and DBIL levels of the control group also improved at the end of the intervention, but the improvement was not as significant as that of the intervention group, which suggested that flaxseed has the ability to ameliorate the role of liver injury in NAFLD patients. Previous studies have reached similar conclusions. In a 12-week randomized controlled trial conducted by Yari et al.29 with 50 NAFLD patients, the intervention group consumed 30 g of flaxseed powder daily. Both the intervention and control groups also received health education. The results showed improvement in the liver fat content and liver function indicators in both groups, with the intervention group showing more significant liver function improvement. In addition, Davis et al.55 investigated the ameliorative effect of flaxseed meal on fatty liver in NAFLD model laying hens, and the results showed that defatted flaxseed meal and whole flaxseed meal could significantly reduce the serum level of AST in NAFLD laying hens, and at the same time, the whole flaxseed meal was able to reduce hepatocyte swelling, which indicated that flaxseed could effectively improve hepatocyte injury and restore liver function.
In addition, the results of this study showed that after 90 days of flaxseed powder intervention in patients with NAFLD, the levels of TG decreased and the levels of HDL-C and Apo A1 increased in the lipid indexes of the intervention population. HDL-C had the role of reverse cholesterol, and Apo A1 was an important component of HDL-C, and the increase in the levels of HDL-C and Apo A1 and the decrease in the levels of TG and LDL-C indicated the restoration of lipid homeostasis.56 This result indicated that flaxseed powder had an improving effect on lipid metabolism disorders in NAFLD patients, which was consistent with the results of previous studies. Yang et al. investigated the effect of flaxseed on high-fat diet-induced NAFLD model mice, and intervened with the addition of flaxseed at the doses of 10 g, 20 g, and 30 g of flaxseed per 100 g of feed, which showed that the effect of flaxseed on the NAFLD model mice at the doses of 10 g per 100 g and 20 g per 100 g flaxseed intervention significantly reduced serum TG and LDL-C levels in NAFLD model mice.33 In addition, a flaxseed powder intervention study conducted on 50 patients with dyslipidemia found that taking 30 g of flaxseed powder daily for 90 days effectively increased serum HDL-C levels, reduced TC levels, and lowered the risk of atherosclerosis.57 Flaxseed is rich in n-3 PUFA, and has been shown to be used to ameliorate NAFLD; it could reduce fatty acid and cholesterol biosynthesis by decreasing the expression of lipogenic genes and decreasing the number of lipid synthesizing transcription factors.58 For many years, the physiological activities of lignans, especially their antioxidant properties, has been extensively studied. The antioxidant activity of flaxseed lignans, expressed through their metabolites, could effectively lower serum cholesterol and reduce the development of atherosclerosis.59 Furthermore, a high intake of flax lignans could also reduce serum TC.41 A double-blind, randomized crossover trial conducted by Kristensen et al. examined the lipid metabolism improvement effects of flaxseed dietary fiber. The results showed that supplementing with flaxseed dietary fiber reduced serum TC levels by 12% and LDL-C levels by 15%.60 Therefore, the lipid metabolism improvement effect of flaxseed might be partially attributed to its high n-3 PUFA content, lignans, and flaxseed dietary fiber. In addition, a meta-analysis showed that whole flaxseed was more effective in improving lipid metabolism compared to a single component. This also suggested a synergistic effect between the biological functions of different components of flaxseed.61
Previous studies have shown that multiple alpha diversity indices of the gut microbiota are decreased and species richness is significantly reduced in patients with NAFLD compared to healthy populations.62 The results of this study indicated that daily consumption of 30 g of flaxseed powder significantly improved the species richness of the gut microbiota in patients with NAFLD, with notable increases in the Shannon and Simpson indices, leading to enhanced diversity of the gut microbiome. At the phylum level, the relative abundance changes of Firmicutes and Bacteroidetes were the primary differences in the gut microbiota between NAFLD patients and healthy individuals; the abundance of Firmicutes significantly increased in NAFLD patients, while the abundance of Bacteroidetes significantly decreased, resulting in a significantly higher Firmicutes/Bacteroidetes (F/B) ratio.63 The increase in the F/B ratio indicated an increase in the overall fermentation activity of the microbiota, leading to increased efficiency of energy absorption in the intestine.64 The F/B ratio was positively correlated with obesity and BMI.65 In the results of the present study, the abundance of Firmicutes in the gut microbiota of the intervention group was significantly lower than that of the control group, while the abundance of Bacteroidetes was significantly higher than that of the control group, leading to a significant decrease in the F/B ratio. Furthermore, the abundance of Firmicutes and Bacteroidetes in the gut was related to the dietary fiber content. A study on dietary fiber intake and fecal microbiota showed that individuals with low dietary fiber intake had a gut abundance of Firmicutes that was twice that of individuals with high dietary fiber intake. In contrast, Bacteroidetes was significantly enriched in the high dietary fiber intake group,66 and animal experiments had also indicated that dietary fiber improved energy homeostasis by regulating gut microbiota abundance and lowering the F/B ratio in obese mice.67 Furthermore, a study had shown that n-3 PUFA in flaxseed could reduce the F/B ratio.68 Flaxseed had a high content of dietary fiber and n-3 PUFA, so it had a better effect on the improvement of gut microbiota. At the genus level, our research found that compared to the control group, the intervention with flaxseed powder significantly improved the abundance of Clostridium sensu stricto 1, Parasutterella, Eubacterium xylanophilum group, Lachnospiraceae NK4A136 group, and Bifidobacterium in the gut microbiota of the intervention group subjects. The abundance of Clostridium sensu stricto 1 in the gut microbiota of NAFLD mice was significantly reduced and was negatively correlated with body weight, serum ALT and TG concentrations, insulin resistance, and liver weight.69Parasutterella had been shown to participate in bile acid metabolism by altering microbial metabolites, including bilirubin and bile acid derivatives, thus playing a role in maintaining bile acid homeostasis.70Lachnospiraceae_NK4A136_group is a type of probiotic associated with obesity and can produce butyrate and improve intestinal barrier function.71Bifidobacterium belongs to the Actinobacteria phylum and has the function of improving intestinal barrier function and preventing pathogen invasion, and is negatively correlated with the visceral fat content and degree of obesity.72 It had also been demonstrated that Bifidobacterium abundance in the gut microbiota of NAFLD patients was inversely related to serum TC levels.73Bifidobacterium was also sensitive to a high-fiber diet, and the supplementation of dietary fiber led to its significant enrichment.74 A multi-ethnic cross-sectional study found that the abundance of Eubacterium xylanophilum group was negatively correlated with the total body fat content.75 Animal studies had also shown that Eubacterium xylanophilum group contributed to the improvement of obesity by regulating the concentration of branched-chain amino acids in circulation.76 Additionally, our results showed that flaxseed powder intervention significantly reduced the abundance of Coriobacteriaceae_UCG-00. It has been reported that Coriobacteriaceae_UCG-002 can produce cytotoxic metabolites such as toluene and phenol, which impair intestinal barrier function.77 Meanwhile, animal experiments showed that the abundance of Coriob-acteriaceae_UCG-002 in the gut was significantly and positively correlated with body weight, blood glucose level, serum LDL-C level, serum leptin level, and hepatic TG level in NAFLD mice.78 At the same time, the Spearman correlation analysis indicated that the differences in gut microbiota mentioned above were significantly correlated with numerous indices of glucose–lipid metabolism and liver function, suggesting that the improvement of flaxseed powder in patients with NAFLD was partially attributable to changes in the abundance of gut microbiota.
However, there were some limitations to this study. Firstly, in the current experiment, only one dosage of flaxseed powder for a 12-week intervention was used, so there is no awareness of the relationship between NAFLD improvement and the dosage and duration of flaxseed intervention. Previous animal experiments had shown that different doses of flaxseed powder might have different intervention effects, and higher doses of flaxseed powder and longer intervention time might produce better effects in patients with NAFLD.32,33 Secondly, compared to liver biopsy, MRI-PDFF only provided information on changes in the liver fat content, but lacked information on inflammation, hepatocyte ballooning degeneration, and fibrosis. Multiple studies had shown that MRI-PDFF had a good correlation with histological grade changes in liver fat deposition, and MRI-PDFF could more comprehensively assess the fat content of the entire liver.79–81 Thirdly, the included population was small, and future population studies with larger sample sizes might be needed to further confirm the effect of flaxseed powder on improving NAFLD patients. Finally, further animal experiments are needed in later studies to explore the specific mechanism through which flaxseed improves NAFLD.
Conclusion
In conclusion, this randomized controlled clinical trial showed that flaxseed powder intervention for 12 weeks moderately reduced the liver fat content of NAFLD patients, while it also improved many indicators such as body composition, liver function, and lipid metabolism of NAFLD patients, and also increased the abundance of beneficial bacteria and decreased the abundance of harmful bacteria in the gut microbiota of NAFLD patients. This might be related to the rich content of ALA, n-3 PUFA, lignans, and dietary fiber in flaxseed. Dietary interventions had important potential in the prevention and treatment of NAFLD. Our study highlighted the potential of flaxseed as an emerging superfood adjunct for the treatment of NAFLD.Abbreviations
| ALA | α-Linolenic acid |
| ALT | Alanine aminotransferase |
| Apo A1 | Apolipoprotein A1 |
| Apo B | Apolipoprotein B |
| AST | Aspartate aminotransferase |
| BMI | Body mass index |
| CHE | Cholinesterase |
| DBIL | Direct bilirubin |
| FINS | Fasting insulin |
| F/B | Firmicutes/Bacteroidetes |
| GGT | Gamma-glutamyl transpeptidase |
| HDL-C | High-density lipoprotein cholesterol |
| IBIL | Indirect bilirubin |
| IHMS | International Human Microbiome Standard |
| LDL-C | Low-density lipoprotein cholesterol |
| LP(a) | Lipoprotein (a) |
| MRI-PDFF | Magnetic resonance imaging estimated proton density fat fraction |
| NAFL | Nonalcoholic fatty liver |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Nonalcoholic steatohepatitis |
| n-3 PUFA | n-3 polyunsaturated fatty acids |
| TBIL | Total bilirubin |
| TC | Total cholesterol |
| TG | Triglyceride |
Author contributions
LY, GS and LL: designed the research; YT, YZ, WL, QH, QZ and RZ: conducted the research; YT and YZ: analyzed the data; YT and LY: wrote the article; and YT, LY, GS and LL: had primary responsibility for the final content; and all authors read and approved the final manuscript.Data availability
The data described in the article, code book, and analytic code will be made available upon request.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China under Grant [82073551] and the Huai'an Natural Science Research Plan (Joint Special Project) Health Research Project [HABL202261]. We appreciate the valuable suggestions and assistance provided by the People's Hospital of Lianshui County, Huai'an City, Jiangsu Province, China, for this experiment.References
- V. W. Wong, M. Ekstedt, G. L. Wong and H. Hagström, Changing epidemiology, global trends and implications for outcomes of NAFLD, J. Hepatol., 2023, 79, 842–852 Search PubMed.
- S. L. Friedman, B. A. Neuschwander-Tetri, M. Rinella and A. J. Sanyal, Mechanisms of NAFLD development and therapeutic strategies, Nat. Med., 2018, 24, 908–922 Search PubMed.
- Z. Younossi, Q. M. Anstee, M. Marietti, T. Hardy, L. Henry, M. Eslam, J. George and E. Bugianesi, Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention, Nat. Rev. Gastroenterol. Hepatol., 2018, 15, 11–20 Search PubMed.
- Z. M. Younossi, A. B. Koenig, D. Abdelatif, Y. Fazel, L. Henry and M. Wymer, Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes, Hepatology, 2016, 64, 73–84 Search PubMed.
- C. Estes, Q. M. Anstee, M. T. Arias-Loste, H. Bantel, S. Bellentani, J. Caballeria, M. Colombo, A. Craxi, J. Crespo, C. P. Day, Y. Eguchi, A. Geier, L. A. Kondili, D. C. Kroy, J. V. Lazarus, R. Loomba, M. P. Manns, G. Marchesini, A. Nakajima, F. Negro, S. Petta, V. Ratziu, M. Romero-Gomez, A. Sanyal, J. M. Schattenberg, F. Tacke, J. Tanaka, C. Trautwein, L. Wei, S. Zeuzem and H. Razavi, Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030, J. Hepatol., 2018, 69, 896–904 Search PubMed.
- Q. Yang, L. Zhang, Q. Li, M. Gu, Q. Qu, X. Yang, Q. Yi, K. Gu, L. Kuang, M. Hao, J. Xu and H. Yang, Characterization of microbiome and metabolite analyses in patients with metabolic associated fatty liver disease and type II diabetes mellitus, BMC Microbiol., 2022, 22, 105 Search PubMed.
- M. C. Ryan, C. Itsiopoulos, T. Thodis, G. Ward, N. Trost, S. Hofferberth, K. O'Dea, P. V. Desmond, N. A. Johnson and A. M. Wilson, The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease, J. Hepatol., 2013, 59, 138–143 Search PubMed.
- Z. M. Younossi, S. Zelber-Sagi, L. Henry and L. H. Gerber, Lifestyle interventions in nonalcoholic fatty liver disease, Nat. Rev. Gastroenterol. Hepatol., 2023, 20, 708–722 Search PubMed.
- M. Romero-Gómez, S. Zelber-Sagi and M. Trenell, Treatment of NAFLD with diet, physical activity and exercise, J. Hepatol., 2017, 67, 829–846 Search PubMed.
- C. Properzi, T. A. O'Sullivan, J. L. Sherriff, H. L. Ching, G. P. Jeffrey, R. F. Buckley, J. Tibballs, G. C. MacQuillan, G. Garas and L. A. Adams, Ad Libitum Mediterranean and Low-Fat Diets Both Significantly Reduce Hepatic Steatosis: A Randomized Controlled Trial, Hepatology, 2018, 68, 1741–1754 Search PubMed.
- L. Haigh, C. Kirk, K. El Gendy, J. Gallacher, L. Errington, J. C. Mathers and Q. M. Anstee, The effectiveness and acceptability of Mediterranean diet and calorie restriction in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis, Clin. Nutr., 2022, 41, 1913–1931 Search PubMed.
- G. M. Cunha, G. Guzman, L. L. Correa De Mello, B. Trein, L. Spina, I. Bussade, J. Marques Prata, I. Sajoux and W. Countinho, Efficacy of a 2-Month Very Low-Calorie Ketogenic Diet (VLCKD) Compared to a Standard Low-Calorie Diet in Reducing Visceral and Liver Fat Accumulation in Patients With Obesity, Front. Endocrinol., 2020, 11, 607 Search PubMed.
- S. F. Wu, X. C. Wang, W. Qi and Q. B. Guo, Bioactive protein/peptides of flaxseed: A review, Trends Food Sci. Technol., 2019, 92, 184–193 Search PubMed.
- P. Nuotio, M. A. Lankinen, T. Meuronen, V. D. de Mello, T. Sallinen, K. A. Virtanen, J. Pihlajamäki, M. Laakso and U. Schwab, Dietary n-3 alpha-linolenic and n-6 linoleic acids modestly lower serum lipoprotein(a) concentration but differentially influence other atherogenic lipoprotein traits: A randomized trial, Atherosclerosis, 2024, 395, 117562 Search PubMed.
- S. Stivala, S. Gobbato, N. Bonetti, G. G. Camici, T. F. Lüscher and J. H. Beer, Dietary alpha-linolenic acid reduces platelet activation and collagen-mediated cell adhesion in sickle cell disease mice, J. Thromb. Haemostasis, 2022, 20, 375–386 Search PubMed.
- M. Takić, S. Ranković, Z. Girek, S. Pavlović, P. Jovanović, V. Jovanović and I. Šarac, Current Insights into the Effects of Dietary α-Linolenic Acid Focusing on Alterations of Polyunsaturated Fatty Acid Profiles in Metabolic Syndrome, Int. J. Mol. Sci., 2024, 25, 4909 Search PubMed.
- G. S. Masterton, J. N. Plevris and P. C. Hayes, Review article: omega-3 fatty acids - a promising novel therapy for non-alcoholic fatty liver disease, Aliment. Pharmacol. Ther., 2010, 31, 679–692 Search PubMed.
- D. G. Bouzianas, S. D. Bouziana and A. I. Hatzitolios, Potential treatment of human nonalcoholic fatty liver disease with long-chain omega-3 polyunsaturated fatty acids, Nutr. Rev., 2013, 71, 753–771 Search PubMed.
- A. Mueed, M. Ibrahim, S. Shibli, P. Madjirebaye, Z. Deng and M. Jahangir, The fate of flaxseed-lignans after oral administration: A comprehensive review on its bioavailability, pharmacokinetics, and food design strategies for optimal application, Crit. Rev. Food Sci. Nutr., 2024, 64, 4312–4330 Search PubMed.
- J. Peterson, J. Dwyer, H. Adlercreutz, A. Scalbert, P. Jacques and M. L. McCullough, Dietary lignans: physiology and potential for cardiovascular disease risk reduction, Nutr. Rev., 2010, 68, 571–603 Search PubMed.
- D. García-Mateos, R. García-Villalba, J. A. Otero, J. A. Marañón, J. C. Espín, A. I. Álvarez and G. Merino, An altered tissue distribution of flaxseed lignans and their metabolites in Abcg2 knockout mice, Food Funct., 2018, 9, 636–642 Search PubMed.
- Y. Y. Shim, B. Gui, Y. Wang and M. J. T. Reaney, Flaxseed (Linum usitatissimum L.) oil processing and selected products, Trends Food Sci. Technol., 2015, 43, 162–177 Search PubMed.
- P. Cronin, S. A. Joyce, P. W. O'Toole and E. M. O'Connor, Dietary Fibre Modulates the Gut Microbiota, Nutrients, 2021, 13, 1655 Search PubMed.
- D. Zhou and J. G. Fan, Microbial metabolites in non-alcoholic fatty liver disease, World J. Gastroenterol., 2019, 25, 2019–2028 Search PubMed.
- S. F. De Silva and J. Alcorn, Flaxseed Lignans as Important Dietary Polyphenols for Cancer Prevention and Treatment: Chemistry, Pharmacokinetics, and Molecular Targets, Pharmaceuticals, 2019, 12, 68 Search PubMed.
- A. Calado, P. M. Neves, T. Santos and P. Ravasco, The Effect of Flaxseed in Breast Cancer: A Literature Review, Front. Nutr., 2018, 5, 4 Search PubMed.
- S. Rezaei, M. R. Sasani, M. Akhlaghi and A. Kohanmoo, Flaxseed oil in the context of a weight loss programme ameliorates fatty liver grade in patients with non-alcoholic fatty liver disease: a randomised double-blind controlled trial, Br. J. Nutr., 2020, 123, 994–1002 Search PubMed.
- N. Khodadadi, A. Sadeghi, H. Poustchi, B. Abbasi, M. Nilghaz, E. Melekoglu, Z. Yari and A. Hekmatdoost, Effectiveness of flaxseed consumption and fasting mimicking diet on anthropometric measures, biochemical parameters, and hepatic features in patients with Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): a randomized controlled clinical trial, Nutr. Diabetes, 2024, 14, 93 Search PubMed.
- Z. Yari, M. Rahimlou, T. Eslamparast, N. Ebrahimi-Daryani, H. Poustchi and A. Hekmatdoost, Flaxseed supplementation in non-alcoholic fatty liver disease: a pilot randomized, open labeled, controlled study, Int. J. Food Sci. Nutr., 2016, 67, 461–469 Search PubMed.
- Chinese Society of Hepatology, Chinese Medical Association, Guidelines for the prevention and treatment of metabolic dysfunction-associated (non-alcoholic) fatty liver disease (Version 2024), Chin. J. Hepatol., 2024, 32, 418–434 Search PubMed.
- J. Jung, A. Han, E. Madamba, R. Bettencourt, R. R. Loomba, A. S. Boehringer, M. P. Andre, J. W. Erdman, Jr., W. D. O'Brien, Jr., K. J. Fowler, C. B. Sirlin and R. Loomba, Direct Comparison of Quantitative US versus Controlled Attenuation Parameter for Liver Fat Assessment Using MRI Proton Density Fat Fraction as the Reference Standard in Patients Suspected of Having NAFLD, Radiology, 2022, 304, 75–82 Search PubMed.
- C. Yang, M. Wan, D. Xu, D. Pan, H. Xia, L. Yang and G. Sun, Flaxseed Powder Attenuates Non-Alcoholic Steatohepatitis via Modulation of Gut Microbiota and Bile Acid Metabolism through Gut-Liver Axis, Int. J. Mol. Sci., 2021, 22, 10858 Search PubMed.
- C. Yang, L. Yang, Y. Yang, M. Wan, D. Xu, D. Pan and G. Sun, Effects of flaxseed powder in improving non-alcoholic fatty liver by regulating gut microbiota-bile acids metabolic pathway through FXR/TGR5 mediating, Biomed. Pharmacother., 2023, 163, 114864 Search PubMed.
- M. Parikh, B. C. Hirst, K. A. O'Hara, T. G. Maddaford, J. A. Austria, A. Stamenkovic, L. Yu, B. Kura, B. Garg, T. Netticadan, S. D. Proctor and G. N. Pierce, Beneficial Effects of Dietary Flaxseed on Non-Alcoholic Fatty Liver Disease, Nutrients, 2024, 16, 466 Search PubMed.
- A. A. Bravo, S. G. Sheth and S. Chopra, Liver biopsy, N. Engl. J. Med., 2001, 344, 495–500 Search PubMed.
- J. Gu, S. Liu, S. Du, Q. Zhang, J. Xiao, Q. Dong and Y. Xin, Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: a meta-analysis, Eur. Radiol., 2019, 29, 3564–3573 Search PubMed.
- Z. Yari, M. Cheraghpour, S. M. Alavian, M. Hedayati, H. Eini-Zinab and A. Hekmatdoost, The efficacy of flaxseed and hesperidin on non-alcoholic fatty liver disease: an open-labeled randomized controlled trial, Eur. J. Clin. Nutr., 2021, 75, 99–111 Search PubMed.
- S. Sookoian and C. J. Pirola, Systematic review with meta-analysis: the significance of histological disease severity in lean patients with nonalcoholic fatty liver disease, Aliment. Pharmacol. Ther., 2018, 47, 16–25 Search PubMed.
- L. M. Glass, R. C. Dickson, J. C. Anderson, A. A. Suriawinata, J. Putra, B. S. Berk and A. Toor, Total body weight loss of ≥ 10% is associated with improved hepatic fibrosis in patients with nonalcoholic steatohepatitis, Dig. Dis. Sci., 2015, 60, 1024–1030 Search PubMed.
- M. Ariya, F. Koohpayeh, A. Ghaemi, S. Osati, S. H. Davoodi, J. M. Razzaz, G. Javedan, E. Ehrampoush and R. Homayounfar, Assessment of the association between body composition and risk of non-alcoholic fatty liver, PLoS One, 2021, 16, e0249223 Search PubMed.
- S. Fukumitsu, K. Aida, N. Ueno, S. Ozawa, Y. Takahashi and M. Kobori, Flaxseed lignan attenuates high-fat diet-induced fat accumulation and induces adiponectin expression in mice, Br. J. Nutr., 2008, 100, 669–676 Search PubMed.
- M. Zarei, S. Adeli, S. Hosseini and E. Daneshzad, The effect of flaxseed intake on appetite reduction: A systematic review of randomized clinical trials, Phytother. Res., 2022, 36, 3792–3804 Search PubMed.
- U. Bongartz, U. Hochmann, B. Grube, R. Uebelhack, F. Alt, C. Erlenbeck, L. V. Peng, P. W. Chong and P. De Costa, Flaxseed Mucilage (IQP-LU-104) Reduces Body Weight in Overweight and Moderately Obese Individuals in a 12-week, Three-Arm, Double-Blind, Randomized, and Placebo-Controlled Clinical Study, Obes. Facts, 2022, 15, 395–404 Search PubMed.
- M. Takic, B. Pokimica, G. Petrovic-Oggiano and T. Popovic, Effects of Dietary α-Linolenic Acid Treatment and the Efficiency of Its Conversion to Eicosapentaenoic and Docosahexaenoic Acids in Obesity and Related Diseases, Molecules, 2022, 27, 4471 Search PubMed.
- Y. Hu, Y. Song, A. A. Franke, F. B. Hu, R. M. van Dam and Q. Sun, A Prospective Investigation of the Association Between Urinary Excretion of Dietary Lignan Metabolites and Weight Change in US Women, Am. J. Epidemiol., 2015, 182, 503–511 Search PubMed.
- J. Yang, M. Fernández-Galilea, L. Martínez-Fernández, P. González-Muniesa, A. Pérez-Chávez, J. A. Martínez and M. J. Moreno-Aliaga, Oxidative Stress and Non-Alcoholic Fatty Liver Disease: Effects of Omega-3 Fatty Acid Supplementation, Nutrients, 2019, 11, 872 Search PubMed.
- P. Y. Kwo, S. M. Cohen and J. K. Lim, ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries, Am. J. Gastroenterol., 2017, 112, 18–35 Search PubMed.
- M. Duan, X. Chi, H. Xiao, X. Liu and H. Zhuang, High-normal alanine aminotransferase is an indicator for liver histopathology in HBeAg-negative chronic hepatitis B, Hepatol. Int., 2021, 15, 318–327 Search PubMed.
- P. S. Sung, A different detection method reveals a new role of alanine aminotransferase as an indicator of liver fibrosis, Korean J. Intern. Med., 2020, 35, 295–297 Search PubMed.
- X. D. Wang, C. W. Pan, G. Y. Zhou, F. Gao, F. L. Wang, R. Q. Fu, E. H. Xiao, P. Li, H. Zhang and M. H. Zheng, Effect of liver steatosis on liver stiffness measurement in chronic hepatitis B patients with normal serum alanine aminotransferase levels: A multicentre cohort study, J. Viral Hepatitis, 2022, 29, 196–204 Search PubMed.
- J. F. Chen, Z. Q. Wu, H. S. Liu, S. Yan, Y. X. Wang, M. Xing, X. Q. Song and S. Y. Ding, Cumulative effects of excess high-normal alanine aminotransferase levels in relation to new-onset metabolic dysfunction-associated fatty liver disease in China, World J. Gastroenterol., 2024, 30, 1346–1357 Search PubMed.
- J. Yang, Y. Hou, Q. Zhang and Y. Wang, Normal serum alanine aminotransferase levels for screening metabolic dysfunction-associated fatty liver disease, J. Formosan Med. Assoc., 2023, 122, 1092–1093 Search PubMed.
- S. McPherson, S. F. Stewart, E. Henderson, A. D. Burt and C. P. Day, Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease, Gut, 2010, 59, 1265–1269 Search PubMed.
- Y. Zou, L. Zhong, C. Hu and G. Sheng, Association between the alanine aminotransferase/aspartate aminotransferase ratio and new-onset non-alcoholic fatty liver disease in a nonobese Chinese population: a population-based longitudinal study, Lipids Health Dis., 2020, 19, 245 Search PubMed.
- J. E. Davis, J. Cain, C. Small and D. B. Hales, Therapeutic effect of flax-based diets on fatty liver in aged laying hens, Poult. Sci., 2016, 95, 2624–2632 Search PubMed.
- L. R. Marques, T. A. Diniz, B. M. Antunes, F. E. Rossi, E. C. Caperuto, F. S. Lira and D. C. Gonçalves, Reverse Cholesterol Transport: Molecular Mechanisms and the Non-medical Approach to Enhance HDL Cholesterol, Front. Physiol., 2018, 9, 526 Search PubMed.
- S. Saxena and C. Katare, Evaluation of flaxseed formulation as a potential therapeutic agent in mitigation of dyslipidemia, Biomed. J., 2014, 37, 386–390 Search PubMed.
- G. S. de Castro and P. C. Calder, Non-alcoholic fatty liver disease and its treatment with n-3 polyunsaturated fatty acids, Clin. Nutr., 2018, 37, 37–55 Search PubMed.
- P. Lan, M. Du, Y. Teng, M. G. Banwell, H. Nie, M. J. T. Reaney and Y. Wang, Structural Modifications of a Flaxseed Lignan in Pursuit of Higher Liposolubility: Evaluation of the Antioxidant and Permeability Properties of the Resulting Derivatives, J. Agric. Food Chem., 2019, 67, 14152–14159 Search PubMed.
- M. Kristensen, M. G. Jensen, J. Aarestrup, K. E. Petersen, L. Søndergaard, M. S. Mikkelsen and A. Astrup, Flaxseed dietary fibers lower cholesterol and increase fecal fat excretion, but magnitude of effect depend on food type, Nutr. Metab., 2012, 9, 8 Search PubMed.
- C. Yang, H. Xia, M. Wan, Y. Lu, D. Xu, X. Yang, L. Yang and G. Sun, Comparisons of the effects of different flaxseed products consumption on lipid profiles, inflammatory cytokines and anthropometric indices in patients with dyslipidemia related diseases: systematic review and a dose-response meta-analysis of randomized controlled trials, Nutr. Metab., 2021, 18, 91 Search PubMed.
- X. Su, S. Chen, J. Liu, Y. Feng, E. Han, X. Hao, M. Liao, J. Cai, S. Zhang, J. Niu, S. He, S. Huang, K. Lo and F. Zeng, Composition of gut microbiota and non-alcoholic fatty liver disease: A systematic review and meta-analysis, Obes. Rev., 2024, 25, e13646 Search PubMed.
- R. Loomba, V. Seguritan, W. Li, T. Long, N. Klitgord, A. Bhatt, P. S. Dulai, C. Caussy, R. Bettencourt, S. K. Highlander, M. B. Jones, C. B. Sirlin, B. Schnabl, L. Brinkac, N. Schork, C. H. Chen, D. A. Brenner, W. Biggs, S. Yooseph, J. C. Venter and K. E. Nelson, Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease, Cell Metab., 2017, 25, 1054–1062 Search PubMed.
- P. J. Turnbaugh, R. E. Ley, M. A. Mahowald, V. Magrini, E. R. Mardis and J. I. Gordon, An obesity-associated gut microbiome with increased capacity for energy harvest, Nature, 2006, 444, 1027–1031 Search PubMed.
- F. J. Verdam, S. Fuentes, C. de Jonge, E. G. Zoetendal, R. Erbil, J. W. Greve, W. A. Buurman, W. M. de Vos and S. S. Rensen, Human intestinal microbiota composition is associated with local and systemic inflammation in obesity, Obesity, 2013, 21, E607–E615 Search PubMed.
- C. De Filippo, D. Cavalieri, M. Di Paola, M. Ramazzotti, J. B. Poullet, S. Massart, S. Collini, G. Pieraccini and P. Lionetti, Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 14691–14696 Search PubMed.
- H. Wang, T. Hong, N. Li, B. Zang and X. Wu, Soluble dietary fiber improves energy homeostasis in obese mice by remodeling the gut microbiota, Biochem. Biophys. Res. Commun., 2018, 498, 146–151 Search PubMed.
- T. Liu, H. Hougen, A. C. Vollmer and S. M. Hiebert, Gut bacteria profiles of Mus musculus at the phylum and family levels are influenced by saturation of dietary fatty acids, Anaerobe, 2012, 18, 331–337 Search PubMed.
- Y. Ni, X. Wang, Q. Wu, Y. Yao, Y. Xu, Y. Li, Q. Feng, M. Zhou and X. Gou, Qushi Huayu decoction ameliorates non-alcoholic fatty liver disease in rats by modulating gut microbiota and serum lipids, Front. Endocrinol., 2023, 14, 1272214 Search PubMed.
- T. Ju, J. Y. Kong, P. Stothard and B. P. Willing, Defining the role of Parasutterella, a previously uncharacterized member of the core gut microbiota, ISME J., 2019, 13, 1520–1534 Search PubMed.
- X. Q. He, D. Liu, H. Y. Liu, D. T. Wu, H. B. Li, X. S. Zhang and R. Y. Gan, Prevention of Ulcerative Colitis in Mice by Sweet Tea (Lithocarpus litseifolius) via the Regulation of Gut Microbiota and Butyric-Acid-Mediated Anti-Inflammatory Signaling, Nutrients, 2022, 14, 2208 Search PubMed.
- S. Takahashi, D. Anzawa, K. Takami, A. Ishizuka, T. Mawatari, K. Kamikado, H. Sugimura and T. Nishijima, Effect of Bifidobacterium animalis ssp. lactis GCL2505 on visceral fat accumulation in healthy Japanese adults: a randomized controlled trial, Biosci. Microbiota, Food Health, 2016, 35, 163–171 Search PubMed.
- X. Zhang, O. O. Coker, E. S. Chu, K. Fu, H. C. H. Lau, Y. X. Wang, A. W. H. Chan, H. Wei, X. Yang, J. J. Y. Sung and J. Yu, Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites, Gut, 2021, 70, 761–774 Search PubMed.
- L. Chen, B. Liu, L. Ren, H. Du, C. Fei, C. Qian, B. Li, R. Zhang, H. Liu, Z. Li and Z. Ma, High-fiber diet ameliorates gut microbiota, serum metabolism and emotional mood in type 2 diabetes patients, Front. Cell. Infect. Microbiol., 2023, 13, 1069954 Search PubMed.
- C. P. Lozano, L. R. Wilkens, Y. B. Shvetsov, G. Maskarinec, S. Y. Park, J. A. Shepherd, C. J. Boushey, J. R. Hebert, M. D. Wirth, T. Ernst, T. Randolph, U. Lim, J. W. Lampe, L. Le Marchand and M. A. J. Hullar, Associations of the Dietary Inflammatory Index with total adiposity and ectopic fat through the gut microbiota, LPS, and C-reactive protein in the Multiethnic Cohort-Adiposity Phenotype Study, Am. J. Clin. Nutr., 2022, 115, 1344–1356 Search PubMed.
- L. Zhang, Y. Yue, M. Shi, M. Tian, J. Ji, X. Liao, X. Hu and F. Chen, Dietary Luffa cylindrica (L.) Roem promotes branched-chain amino acid catabolism in the circulation system via gut microbiota in diet-induced obese mice, Food Chem., 2020, 320, 126648 Search PubMed.
- Z. Yu, D. Li and H. Sun, Herba Origani alleviated DSS-induced ulcerative colitis in mice through remolding gut microbiota to regulate bile acid and short-chain fatty acid metabolisms, Biomed. Pharmacother., 2023, 161, 114409 Search PubMed.
- S. Hao, L. Ming, Y. Li, H. Lv, L. Li, T. Jambal and R. Ji, Modulatory effect of camel milk on intestinal microbiota of mice with non-alcoholic fatty liver disease, Front. Nutr., 2022, 9, 1072133 Search PubMed.
- A. Tang, J. Tan, M. Sun, G. Hamilton, M. Bydder, T. Wolfson, A. C. Gamst, M. Middleton, E. M. Brunt, R. Loomba, J. E. Lavine, J. B. Schwimmer and C. B. Sirlin, Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis, Radiology, 2013, 267, 422–431 Search PubMed.
- Z. Permutt, T. A. Le, M. R. Peterson, E. Seki, D. A. Brenner, C. Sirlin and R. Loomba, Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD, Aliment. Pharmacol. Ther., 2012, 36, 22–29 Search PubMed.
- I. S. Idilman, H. Aniktar, R. Idilman, G. Kabacam, B. Savas, A. Elhan, A. Celik, K. Bahar and M. Karcaaltincaba, Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy, Radiology, 2013, 267, 767–775 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |