Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pregnancy-related changes in microbiome are disrupted by obesogenic diet exposure: implications for offspring microbiome development†
Kyoko
Hasebe
 a,
Michael D.
Kendig
a,
Michael D.
Kendig
 ab,
Nadeem O.
Kaakoush
ab,
Nadeem O.
Kaakoush
 a,
Aynaz
Tajaddini
a,
Sonia
Hesam-Shariati
a,
R.
Frederick Westbrook
a,
Aynaz
Tajaddini
a,
Sonia
Hesam-Shariati
a,
R.
Frederick Westbrook
 c and
Margaret J.
Morris
c and
Margaret J.
Morris
 *a
*a
aSchool of Biomedical Sciences, UNSW Sydney, NSW, Australia. E-mail: m.morris@unsw.edu.au
bSchool of Life Sciences, University of Technology Sydney, NSW, Australia
cSchool of Psychology, UNSW Sydney, NSW, Australia
First published on 25th April 2025
Abstract
Pregnancy can alter gut microbiota composition, but effects of an obesogenic diet on both mother and offspring microbiome can be obscured by confounding factors. This study examined changes in gut microbiota composition prior to pregnancy, and across gestation and lactation in rat dams fed either a high-fat, high-sugar Cafeteria (Caf) diet or Chow. Microbiome development was assessed in male and female offspring weaned onto chow. Caf diet consumption during pregnancy increased weight gain and adiposity, with increased glucose and plasma leptin and lower folate and B12 levels indicating metabolic disturbance in dams. α- and β diversity measures in Caf-fed dams showed no change in Bacteroidetes and Firmicutes abundance across pregnancy compared with Chow dams, who showed reduced Firmicutes at gestation and mid-lactation. Offspring born to Caf versus Chow dams exhibited greater adiposity and plasma leptin at weaning (3 weeks); at 14 weeks these changes were only observed in males. Maternal Caf diet induced clear differences in β diversity in weanlings but not α diversity. Caf weanlings had lower plasma folate but higher B12 levels compared to chow counterparts. Maternal folate levels were positively associated with maternal and weanling gut microbiota, specifically OTU2 Romboutsia and OTU3_Lactobacillus relative abundance. SourceTracker analysis revealed similarities in the gut microbiota of Chow weanlings and maternal gut microbiota observed during lactation, whereas the microbiota of Caf weanlings was similar to the maternal gut microbiota during gestation. Maternal Caf diet had marginal effects on gut microbiota composition in adult offspring consuming regular chow.
Introduction
During pregnancy, physiological changes to the immune and endocrine systems nourish the developing fetus and impact metabolic status of the mother. While current evidence suggests that gut microbiota composition is strongly associated with physiological changes during pregnancy,1,2 observations regarding the temporal dynamics of any changes in the maternal gut microbiome during pregnancy are inconsistent. In humans, Koren et al.3 reported changes in maternal gut microbiota diversity from the first to third trimester, specifically increases of Proteobacteria and Actinobacteria, and decreased α diversity. In contrast, DiGiulio et al.4 found that pregnancy progression was not associated with α- and β diversity in weekly samples from vagina, stool, saliva and tooth/gum. Similarly, Yang et al.5 found limited gestational age-associated variations in human gut microbiota, while noting variability associated with host factors such as age, pre-pregnancy body mass index (BMI) and disease states. Finally, Rasmussen et al.6 reported a significant increase in Shannon diversity, but no difference in Faith's Phylogenetic Diversity, from weeks 24 to 36 of pregnancy, a decline in Lactobacillus relative abundance from week 24 until birth, and no detectable change in β diversity.Fluctuations in maternal microbiota have also been observed over gestation in animal models. Changes in relative abundances of Clostridium spp., Akkermansia muciniphila, Methanobrevibacter spp., Bacteroides/Prevotella spp., and Roseburia spp. were observed in faecal samples from pregnant Sprague-Dawley rats across pre-pregnancy, gestation day 14 and lactation day 19.7 Using a porcine model, Ji et al. reported increased microbiota α diversity and differences in β diversity across gestation and lactation.8 Zhang et al.9 reported increases in two α diversity indices (Shannon and Faith's Phylogenetic Diversity) during gestation in goats compared with baseline, while differences in maternal microbiota β diversity from baseline to gestation and lactation were driven by decreased relative abundance of Anaerovoracaceae Family XIII AD3011 group at gestation. They also reported that alterations in pro- and anti- inflammatory cytokine levels in serum were accompanied by changes in maternal microbiota. The differences noted above between human and animal data regarding changes in maternal gut microbiota across pregnancy and lactation could relate to the inability to precisely control diet and other potential confounding factors in human microbiota studies during pregnancy.
Maternal diet is one of the key factors affecting maternal gut microbiota. Using a primate model, Ma et al.10 tested the effects of a maternal high fat (HFD) or control diet on the early life offspring gut microbiome. They found that maternal diet during the perinatal period, not maternal obesity per se, appeared to shape offspring gut microbial community in the first year of life. In a human study of 163 mother–child dyads whereby mothers were categorised as consuming high fat diet and control diet based on food intake at gestation, the relative abundance of Bacteroides in the gut microbiota was significantly depleted in neonates exposed to HFD during gestation.11
Micronutrients including folate and B12, play an essential role in healthy pregnancy and offspring development.12–14 Emerging evidence suggests a relationship between poor maternal folate and B12 status and offspring metabolic health outcomes.15,16 Furthermore, poor dietary choices during pregnancy affect the maternal gut microbiota community, and indirectly might affect offspring gut microbiota formation.17,18 However, little is known regarding how poor dietary patterns affect maternal folate and B12 status during pregnancy, and the nature of associations among maternal folate and B12 status, maternal gut microbiota, offspring gut microbiota and other offspring outcomes.
In the present study, we tracked changes in maternal gut microbiota at multiple times across pre-pregnancy, gestation and lactation to examine the dynamic effects of maternal nutrient-poor, highly-processed ‘cafeteria’ foods high in sugar, fat and salt (Cafeteria diet) on microbiota across these stages. Maternal metabolic state was measured at the end of lactation along with plasma folate and B12 status. We sought to determine whether maternal microbiota composition is associated with offspring microbiota at two ages, weaning (3 weeks), proximal to maternal Caf diet exposure, and at 14 weeks old, after being weaned onto chow.
Methods
Ethics statement
The experimental protocol was approved by the Animal Care and Ethics Committee of the University of New South Wales (Ethics number:19/74A) in accordance with the guidelines for the use and care of animals for scientific purposes 8th edition (National Health and Medical Research Council, Australia).Subjects
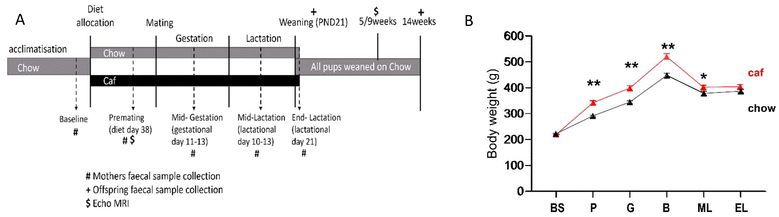
This experiment used a subset of animals from a larger study investigating the impact of maternal diet on offspring behaviour. Young adult female (approximately 7–8 weeks of age; body weight ∼200 g) and male (approximately 8–9 weeks of age; body weight ∼300 g) Sprague-Dawley rats were obtained from a commercial supplier (Animal Resource Centre, WA, Australia) and housed by sex, 4 per cage in a colony room maintained at 18–22 degrees (12 h light/dark cycle). All rats were provided with potable water. Standard chow (14 kJ g−1, 65% carbohydrate, 22% protein and 13% fat; Premium Rat Maintenance diet, Gordon's Stockfeeds, NSW, Australia) were continuously available. Following acclimatisation, female rats were randomly allocated to receive chow (Chow; n = 10) or Caf (n = 15), a diet consisting of a selection of cakes, biscuits and protein sources e.g., meat pie and dim sims. Mean body weight of female Chow group was 221 ± 2 g (SEM) while the Caf group was 220 ± 1 g (SEM). The core component of the diet used in this study and micronutrients of each item (mg g−1) are shown in ESI Table 1.† Foods varied daily, with chow and water always available, as described previously.19The experimental design is shown in Fig. 1A. After six weeks of diet, females were mated with chow fed males by co-housing two females and one male for five days. The male was then removed. Pregnancy was inferred based on weight gain and females were housed individually from approximately gestation day 16. Litters were standardised to six male and six female pups, where possible, on postnatal day (PND1). Dams and offspring were weighed every three days during lactation. From weaning (PND20) to 14 weeks of age, all pups were fed chow regardless of the maternal diet. At weaning and at 14 weeks of age, subsets of offspring (n = 1–2/sex/litter) were anesthetised by i.p. injection of ketamine (100 mg kg−1)/xylazine (15 mg kg−1) and decapitated. Retroperitoneal (RP) adipose tissue (identified as the triangular pad of fat attached to the lateral abdominal wall) was collected bilaterally.
Maternal faecal samples were collected on five occasions (Fig. 1A): at baseline (prior to introduction of Caf diet), pre-mating (diet day 38), mid-gestation (gestation day 11–13), mid-lactation (lactation day 10–13), and at the end of lactation when offspring were weaned (PND20). Offspring faecal samples were collected from the subset euthanised at weaning (PND20) and from their siblings continued on chow diet and euthanised at 14 weeks old.
Endpoint measures
When pups were weaned (PND20), dams were deeply anesthetised (ketamine/xylazine, 100/15 mg kg−1 respectively, i.p.) and body weight, girth, and nasoanal length measured. Blood was obtained by cardiac puncture, collected in Eppendorf tubes containing 10 μl EDTA and rats were immediately decapitated. Retroperitoneal (RP) fat pads were weighed. Plasma was stored at −30 °C for analysis of leptin and insulin (CrystalChem Inc., Chicago, IL, USA), triglyceride content (Roche triglyceride reagent, Sigma glycerol standard), and concentrations of maternal vitamin B12 and folate (Monobind Inc., CA, USA).Faecal DNA extraction
Fresh faeces were collected, placed into a sterile tube, immediately frozen on dry ice then stored at −80 °C. Faecal DNA extraction was performed using the PowerSoil® DNA Isolation Kit (Qiagen, Clayton, Victoria, Australia) according to the manufacturer's instructions. DNA concentration and quality were measured using a DeNovix DS-11 Spectrophotometer (DeNovix, Inc., Delaware, USA) and stored at −80 °C.16S rRNA gene amplicon sequencing and raw data analysis
Microbial community diversity was assessed by 16S rRNA gene amplicon sequencing (Illumina 2 × 250 bp MiSeq chemistry, V4 region, 515F-806R primer pair; Ramaciotti Centre for Genomics, UNSW Sydney) using a standard protocol.20 The sequence data were then analysed using Mothur (version 1.42.321), which included removal of ambiguous bases and homopolymers longer than 15 base pairs, alignment with SILVA database version 132,22 chimera checking with VSEARCH (version 2.13.3), and classification against the RDP Ribosomal Database training set (version18_03202018). Sequences were clustered into operational taxonomic units (OTU) at 97% nucleotide identity to generate an OTU count table and a taxonomic classification file. Commands were derived from MiSeq SOP23 and modified as required. Sequence data were subsampled to n = 4191 total clean reads/sample.Statistical analysis
Data were analysed using SPSS (v28, IBM). Effects of the diet on maternal body weight during pregnancy were assessed in a mixed-ANOVA with factors of maternal diet (Chow or Caf) and time (baseline, premating, mid-gestation, mid-lactation and end-lactation), applying a Greenhouse–Geisser correction where appropriate. Dams’ endpoint measures were assessed by independent samples t-tests. Endpoint measures in weanlings and at 14 weeks were assessed in two-way ANOVAs with maternal diet (Chow or Caf) and sex (male or female) as between-subjects factors. OTU tables were standardised by dividing feature read counts by total number of reads in each sample to calculate relative abundances. Standardised data were then square root transformed and sample resemblances were calculated using Bray–Curtis similarities. β diversity was assessed by Non-metric Multi-dimensional Scaling (NMDS) plots, Permutational Multivariate Analysis of Variance (PERMANOVA), and Permutational Analysis of Multivariate Dispersions (PERMDISP) with Bray–Curtis resemblance matrices. Analyses were completed using PRIMER-e v7 (Primer-e Ltd, Plymouth, UK).24 Repeated measures ANOVA was used for analyses of relative abundance in dams with significance level set p < 0.025 and the Bonferroni test was used to control for inflation of the Type 1 error rate by multiple comparisons. SourceTracker25 assessed similarities between maternal biota at multiple time points and offspring microbiota via the Galaxy web application.26,27 Figures were generated in GraphPad Prism v9 and PRIMER-e v7. Results are expressed as mean ± SEM and considered significant at p < 0.05.Results
Maternal body weight and endpoint measures
Fig. 1A shows the experimental design of this study. Analysis of maternal body weight from baseline to lactation revealed a significant time × diet interaction (F(2.86,62.84) = 17.11, p < 0.001), and main effects of time (F(2.86,62.84) = 71.07, p < 0.001) and diet (F(1,22) = 15.11, p < 0.001), indicating that the Caf diet significantly increased body weight at premating, mid-gestation, and mid-lactation periods (Fig. 1B). As shown in Table 1, gestational weight gain did not differ significantly between Chow and Caf dams, but during lactation body weight declined in the Caf group while remaining stable in the Chow group (p < 0.001, Table 1). Consequently, at the end of lactation, body weight did not differ between groups. Nonetheless, RP fat mass, levels of blood glucose and plasma leptin were higher in Caf than Chow dams, and plasma B12 and folate levels were significantly lower in Caf than Chow dams.| Chow (n = 10) | Caf (n = 15) | p value | |
|---|---|---|---|
| Data are expressed as mean ± SEM. Independent t tests were used for analyses. Mann–Whitney tests for blood glucose measures. | |||
| Premating - after five weeks of Caf diet | |||
| Body weight (g) | 291.1 ± 3.23 | 342.8 ± 7.82 | <0.0001 |
| Fat mass (% in body weight) | 11.39 ± 0.57 | 23.18 ± 1.16 | <0.0001 |
| Lean mass (g) | 238.7 ± 3.87 | 237.8 ± 3.52 | 0.87 |
| Blood glucose, fasted (mmol L−1) | 5.85 ± 0.09 | 6.38 ± 0.16 | 0.017 |
| Changes in body weight (g) | |||
| Gestational weight gain % | 46.8 ± 2.5 | 45.5 ± 1.8 | 0.315 |
| Weight change over lactation % (day20 vs. day1) | 2.2 ± 1.1 | −10.5 ± 1.5 | <0.001 |
| Endpoint measures | |||
| Body weight (g) | 386.89 ± 7.15 | 404.77 ± 8.19 | 0.138 |
| Girth (cm) | 17.5 ± 0.27 | 17.6 ± 0.29 | 0.670 |
| Naso-anal length (cm) | 22.6 ± 0.18 | 23.1 ± 0.13 | 0.023 |
| RP fat (g) | 3.12 ± 0.28 | 5.54 ± 0.26 | <0.001 |
| Blood glucose, non-fasted (mmol L−1) | 6.4 ± 0.13 | 6.9 ± 0.16 | 0.035 |
| Plasma leptin (ng ml−1) | 3.75 ± 0.29 | 5.1 ± 0.38 | 0.018 |
| Plasma insulin (ng ml−1) | 1.09 ± 0.10 | 1.42 ± 0.18 | 0.139 |
| Plasma triglyceride (nmol L−1) | 2.01 ± 0.26 | 1.79 ± 0.37 | 0.647 |
| Plasma folate (ng ml−1) | 77.36 ± 2.64 | 57.12 ± 2.7 | <0.0001 |
| Plasma B12 (pg ml−1) | 976.4 ± 71.73 | 732.2 ± 64.93 | 0.02 |
Maternal diet effects on microbiota composition across pregnancy
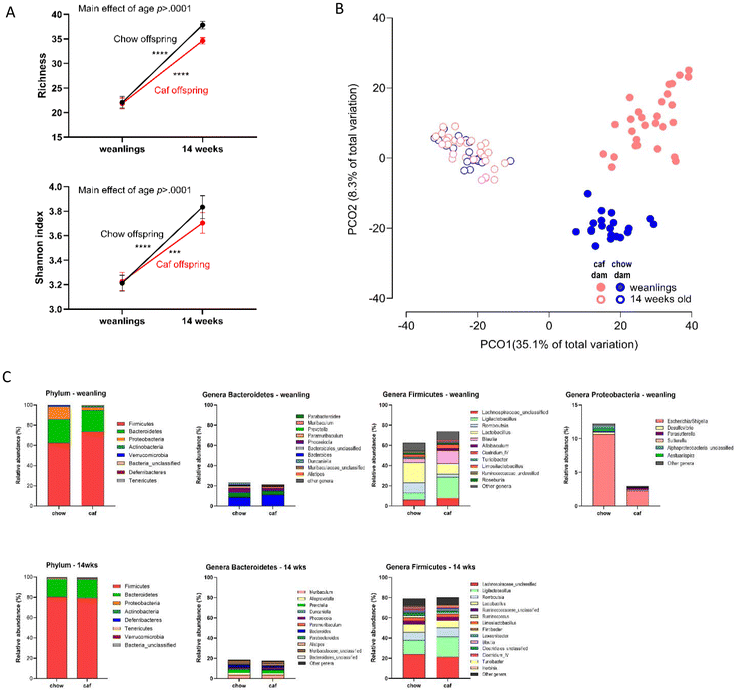
Repeated measures ANOVA indicated a significant interaction between time and Caf diet (F(4,60) = 8.50, p < 0.001) and a main effect of time (F(4,60) = 3.60, p < 0.05) on richness (Fig. 2A), which was significantly higher in Caf than Chow dams at mid- and end-lactation (p < 0.001 and p < 0.01 respectively). Similarly, a significant interaction between time and Caf diet was observed for Shannon index (F(4,60) = 3.02, p < 0.05), which was significantly higher in Caf dams at mid- and end-lactation compared with Chow dams (p < 0.05 and p < 0.01 respectively) (Fig. 2B).In a principal coordinate analysis (PCO) with Bray–Curtis resemblance (Fig. 2C), PCO1 axis represents variation on diet and PCO2 axis represents progression of pregnancy. For Chow dams, permutational MANOVA (PERMANOVA) indicated a significant main effect of time on microbiota composition (Pseudo-F = 3.51, p < 0.01). Each timepoint differed significantly (p values ranging from 0.0002 to 0.0048), except premating and gestation timepoints (p = 0.0525, ESI Table 2.1†). However, analyses of centroid distance (distance-based test for homogeneity of multivariate dispersions, PERMDISP) showed a significant difference between time points (F(4,35) = 5.87, p < 0.01), including between baseline and mid-lactation (p < 0.01) and end-lactation (p < 0.001), and between premating and end of lactation (p < 0.05, ESI Table 2.2†). Hence, differences in dispersion may contribute to the effects of PERMANOVA in Chow dams. To further investigate the dispersion effects, we conducted Analysis of Similarity (ANOSIM) to assess similarities between each timepoint (overall R statistic R = 0.55, p = 0.0001), with significant pairwise comparisons between all times (R ranging from 0.227 to 0.864; p values 0.043 to 0.0002). For Caf dams, PERMANOVA revealed significant differences in β diversity across time (Pseudo-F = 3.56, p < 0.001), with all time points significantly different from one another (ESI Table 2.1†). Unlike Chow dams, however, no differences in dispersion were observed (PERMDISP: F(4,25) = 0.51, p > 0.05, see ESI Table 2.2†).
The change in Bacteroidetes abundance across time differed between groups (F(4,60) = 5.70, p < 0.001) with significantly higher Bacteroidetes abundance in Chow than Caf dams at gestation and mid-lactation (p < 0.05 and p < 0.001 respectively). Firmicutes abundance also differed between groups over time (F(4,60) = 5.29, p < 0.01) with significantly lower Firmicutes in Chow than Caf dams at gestation and mid-lactation (p < 0.05 and p < 0.001 respectively). Within group analyses in Chow dam indicated significant differences in abundance of Bacteroidetes (F(4,24) = 3.76, p < 0.05) and Firmicutes (F(4,24) = 5.03, p < 0.01) from baseline to end lactation (Fig. 2D). Bacteroidetes abundance was significantly higher at lactation than baseline (p < 0.05) while Firmicutes abundance was significantly lower at lactation than baseline and premating (both ps < 0.05). In the case of Caf dams, repeated measure ANOVA indicated that there was no significant difference in Bacteroidetes and Firmicutes abundance levels from baseline to end lactation (Fig. 2E).
Associations between maternal plasma folate and B12 levels and maternal gut microbiota
The relationship(s) between maternal plasma measures and maternal microbiome β diversity at the end of lactation were examined using distance based linear models (DistLM). Variables included were plasma B12, folate, insulin and triglyceride, blood glucose, gestation weight gain (GWG) (%) and body weight. Maternal plasma folate (p < 0.01) and B12 (p < 0.05) were significantly associated with maternal β diversity (ESI Table 3†).Endpoint measures in male and female offspring at weaning and 14 weeks of age
Table 2 summarises endpoint measures in offspring at weaning and 14 weeks of age. Factorial ANOVA with maternal diet (Chow versus Caf) and sex (male versus female) confirmed that maternal Caf diet consumption significantly increased weanling blood glucose (F(1,44) = 15.771, p < 0.001), plasma leptin (F(1,44) = 35.059, p < 0.001), RP fat mass (F(1,44) = 46.994, p < 0.001) and plasma B12 levels (F(1,41) = 10.81, p < 0.01). On the other hand, there was a significant interaction between maternal diet and sex on weanling plasma folate level (F(1,41) = 4.58, p < 0.05), with maternal Caf diet consumption significantly lowered weanling plasma folate concentrations (F(1,41) = 41.37, p < 0.0001). It also confirmed that male weanlings had significantly greater RP fat mass than females (F(1,44) = 4.411, p < 0.05). There were no significant effects of maternal diet or sex on weanling body weight. At 14 weeks of age, maternal Caf diet was associated with greater RP fat mass (F(1,46) = 8.466, p < 0.01), and male offspring from Caf dams were significantly heavier than those from Chow dams (p < 0.05). There was a significant interaction between sex and maternal diet (F(1,46) = 7.202, p < 0.05) and a significant maternal diet effect (F(1,46) = 6.337, p < 0.05) on plasma leptin levels. Bonferroni post hoc comparisons confirmed that maternal Caf diet significantly elevated plasma leptin in male but not in female offspring (p < 0.05; Table 2). We measured average daily food (chow) consumption at two age ranges, from 3 to 9 weeks, and from 9 to 14 weeks. Male offspring from Caf dam at 9 to 14 weeks showed significantly higher food consumption compared with chow counterparts (p < 0.01, ESI Fig. 2†). There was no difference in average food consumption between Chow and Caf males at 3 to 9 weeks of age. For females, there were no differences in food consumption between Chow and Caf groups at the two age ranges (ESI Fig. 2†).| Weaning | Offspring from Chow dams | Offspring from Caf dams | Two-way ANOVA (Mat diet × sex) | ||
|---|---|---|---|---|---|
| Male (10) | Female (10) | Male (14) | Female (14) | ||
| Body weight (g) | 43.7 ± 2.1 | 42.8 ± 1.6 | 44.3 ± 1.1 | 44.5 ± 1.2 | n.s. |
| RP fat (g) | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.16 ± 0.02*** | 0.11 ± 0.01***^^ | Mat diet (p < 0.001) |
| Sex (p < 0.05) | |||||
| Blood glucose (mmol L−1) | 7.9 ± 0.18 | 7.7 ± 0.16 | 8.5 ± 0.17* | 8.2 ± 0.14** | Mat diet (p < 0.001) |
| Plasma leptin (ng ml−1) | 2.71 ± 0.33 | 2.97 ± 0.43 | 5.93 ± 0.60** | 6.36 ± 0.61** | Mat diet (p < 0.001) |
| Plasma insulin (ng ml−1) | 0.83 ± 0.06 | 1.03 ± 0.12 | 1.03 ± 0.14 | 1.3 ± 0.15 | n.s. |
| Plasma folate (ng ml−1) | 38.9 ± 3.5 | 43.3 ± 3.5 | 26.9 ± 2.1** | 19.3 ± 2.2**** | Mat diet × sex (p < 0.05) |
| Mat diet (p < 0.0001) | |||||
| Plasma B12 (pg ml−1) | 1034.4 ± 46.0 | 1017.6 ± 42.0 | 1197.2 ± 50.5* | 1150.3 ± 39.3* | Mat diet (p < 0.01) |
| 14 weeks | Male (10) | Female (10) | Male (15) | Female (15) | |
|---|---|---|---|---|---|
| All offspring were fed chow diet after weaning. Factorial ANOVA with diet (Chow and Caf) and sex (male and female) were used for analyses. Data are expressed as mean ± SEM. Plasma insulin data were analysed by nonparametric Kruskal–Wallis test. Retroperitoneal (RP) fat; post hoc dam diet effect within same sex (Chow male vs. Caf male or Chow female vs. Caf female), *p < 0.05, **p < 0.01, ***p < 0.001; post hoc sex effect, ^p < 0.05, ^^p < 0.01, ^^^p < 0.001. | |||||
| Body weight (g) | 537.7 ± 19.2 | 305.1 ± 10.2 | 582.3 ± 16.9* | 304.37 ± 8.0 | Sex (p < 0.001) |
| RP fat (g) | 6.0 ± 1.07 | 3.3 ± 0.33^ | 9.0 ± 0.83** | 4.4 ± 0.34^^^ | Mat diet (p < 0.01) |
| Sex (p < 0.001) | |||||
| Blood glucose (mmol L−1) | 10.2 ± 0.59 | 9.4 ± 0.27 | 10.7 ± 0.53 | 9.9 ± 0.75 | n.s. |
| Plasma leptin (ng ml−1) | 2.70 ± 0.30 | 3.17 ± 0.39 | 4.28 ± 0.25*** | 3.12 ± 0.27^^ | Mat diet × sex (p < 0.05) |
| Mat diet (p < 0.05) | |||||
| Plasma insulin (ng ml−1) | 0.52 ± 0.07 | 0.49 ± 0.10 | 1.16 ± 0.26 | 0.60 ± 0.08 | n.s. |
Influence of maternal microbiota on offspring microbiota
Offspring α diversity was analysed by two-way ANOVA with factors of age (weanling versus 14 weeks) and maternal diet (Chow versus Caf). Species richness and Shannon index significantly increased with age (species richness F(1,94) = 44.30, p < 0.0001; Shannon index F(1,94) = 221.0, p < 0.0001) in offspring from both Chow and Caf dams (ps < 0.001 to <0.0001; Fig. 3A). There was no significant interaction between maternal diet and time, and no main effect of maternal diet. There was no significant sex effect on α diversity indices.Fig. 3B shows PCO of weanlings and 14 weeks-old offspring; PCO1 axis showing separation by age (weaning or 14 weeks), and PCO2 axis by maternal diet in weanling, but not in 14 weeks-old offspring. All weanlings and 14 weeks offspring were fed chow after weaning. When Chow and Caf offspring β diversities were directly compared at weaning and 14 weeks of age, maternal Caf diet had a significant effect on β diversity in Caf versus Chow weanlings (PERMANOVA, Pseudo F = 10.18, p = 0.0001; PERMDISP F = 2.74, p = 0.1), and 14 weeks-old offspring (PERMANOVA, Pseudo F = 2.4679, p = 0.005; PERMDISP F = 1.23, p = 0.38). Sex did not differentiate β diversity at either age and maternal diet did not interact with sex. Descriptive representative taxa in weanlings and 14 weeks old offspring are shown in Fig. 3C. It appears that maternal Caf diet consumption suppressed proportions of Escherichia/Shigella within Phylum Proteobacteria in weanlings, and instead, promoted Firmicutes relative abundance in weanlings.
Influence of maternal plasma folate and B12 levels on offspring gut microbiota
DistLM was used to examine the relationship between maternal plasma folate and B12 levels, and offspring gut microbiota (weaning and 14 weeks). Weanling β diversity was significantly associated with maternal plasma B12 (p < 0.01), folate (p < 0.001) and insulin (p < 0.05), and blood glucose (p < 0.01) (ESI Table 3†). β diversity in 14 weeks offspring was significantly associated with maternal plasma B12 levels (p < 0.05) and insulin (p < 0.01) (ESI Table 3†).Further, distance based redundancy analysis (dbRDA) combined with correlation analysis revealed that the relative abundances of three OTUs in weanlings, OTU2_Romboutsia, OTU3_Lactobacillus, and OTU14 Blautia, were positively associated with maternal plasma folate level (ESI Fig. 1A†). To complement this finding, associations between these OTUs in dams and maternal folate levels were examined.
Maternal folate levels were significantly associated with the relative abundance of OTU2_Romboutsia and OTU3_Lactobacillus in dams whilst OTU14_Blautia abundance in dams was not associated with maternal folate levels (ESI Fig. 1B†). When the relative abundance of these OTUs in dams from baseline to end lactation were examined, there were significant diet and time interactions on the abundance of OTU2 Romboutsia (p < 0.01) and OTU3_Lactobacillus (p < 0.05), and post hoc comparisons indicated that Caf diet significantly reduced abundance of these taxa from premating to end lactation (ESI Fig. 1C†).
Similarity of maternal and offspring gut microbiota
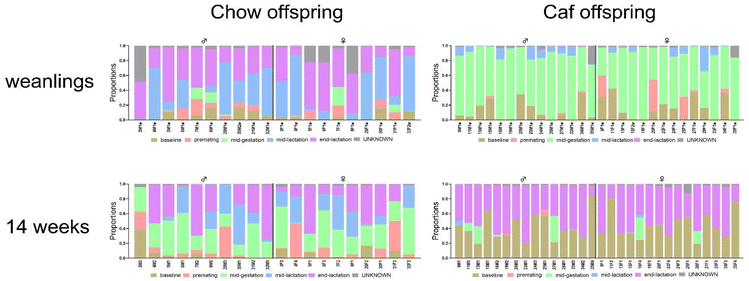
We were interested in whether the maternal gut microbiota influenced her offspring gut microbiota composition, and whether there was a relationship between maternal gut microbiota at a particular time and the offspring gut microbiota. To investigate this, we used SourceTracker analysis to assess similarities in gut microbiota composition between dams at multiple time points and their offspring at weaning and 14 weeks. SourceTracker computed maternal gut microbiota composition at baseline, premating, mid-gestation, mid- and end-lactation, and offspring gut microbiota composition at weaning and 14 weeks, and assessed contributions of maternal gut microbiota at each time point to weanling and 14 week-old offspring gut microbiota composition, respectively. Results showed that the majority of Chow weanlings gut microbiota composition were accounted for by maternal gut microbiota at mid- and end-lactation (blue and purple respectively), (Fig. 4, upper left panel). In contrast, Caf weanling microbiota composition (Fig. 4, upper right panel) was similar to that of Caf dam gut microbiota at mid-gestation (represented by light green). In adult offspring from Chow dams (Fig. 4, bottom left panel), microbiota composition was similar to a mixture of maternal gut microbiota at mid-gestation (green), mid- and end-lactation (blue and purple respectively), whereas microbiota of adult offspring (14 weeks) from Caf dams were more similar to maternal gut microbiota at the baseline and end-lactation (brown and purple).Discussion
This experiment showed that consumption of a high-fat, high-sugar, cafeteria diet altered the trajectory of gut microbiota composition of rat dams across pregnancy and lactation. The decrease in microbiota species richness and Shannon index observed in Chow dams during lactation was absent in Caf dams. Similarly, the fluctuations in two major phyla, Bacteroidetes and Firmicutes across gestation and lactation in Chow dams were absent in Caf-fed dams. Others have demonstrated changes in alpha diversity across pregnancy. Reduced alpha diversity across pregnancy has been reported in several human28–31 and animal32 studies with some exceptions.33–36 However, our study is perhaps the most comprehensive assessment of faecal microbiota changes across pre-mating, gestation and lactation, and the contrasting effects in animals consuming two very different quality diets. Our results accord with Astbury and colleagues,32 who observed that alpha diversity reduced across over gestation in dams fed chow but increased over gestation in dams fed a high-fructose diet. Our results advance this result through the use of an ecologically valid ‘western’ style diet high in both fat and sugar.Identifying the impact of diet on gut microbiota composition across human pregnancy can be obscured by numerous confounding factors such as intake of supplements, and differences in overall dietary intake. Hence, the present results might shed light on the diverse findings from studies on maternal microbiota in humans.
Although PERMANOVA indicated that both Chow and Caf maternal gut microbiota differed from baseline to lactation, PCO and PERMDISP suggested that Chow maternal gut microbiota showed more distinct changes between timepoints whereas Caf maternal gut microbiota differed less over time, possibly due to disruptive effects of Caf diet on the gut microbiota. Previous studies have reported changes in maternal gut microbiota in rats fed standard chow and high-fat/high sucrose diet,7 sows fed commercial gestational diets,8 and goats fed a standardised diet,9 which is in line with the changes in Chow dams in our study. In contrast, there were fewer changes induced by pregnancy in the dams consuming Caf diet, which may be one of the factors contributing to the diverse findings reported in human pregnancy.4–6
The present experiment found that maternal Caf diet suppressed changes in Bacteroidetes and Firmicutes abundance during pregnancy and lactation in comparison to their abundance in dams fed chow. More specifically, the changes in the two phyla observed here reflected an interaction between Caf diet and the two major phyla with the progression of pregnancy, rather than the Firmicutes/Bacteroidetes ratio, an indicator of health or disease states.37 Whether the suppression of changes in the two major phyla seen with the Caf diet influences pregnancy outcomes, offspring gut microbiota, and offspring development requires further investigation.
Maternal Caf diet consumption generated a robust obesity phenotype in dams, with a 20% difference in body weight prior to mating, persisting to parturition. Of note, Caf dams lost weight during lactation such that group weights did not differ at endpoint, though adiposity and plasma leptin remained higher in Caf dams, indicating residual metabolic impairment. Offspring born to Caf dams exhibited a mild obesity phenotype, with greater adiposity, plasma leptin and blood glucose than Chow dam offspring at weaning, and increased adiposity in adulthood, with a sex-specific increase in body weight in males, consistent with our previous work.38 Lastly, we did not find sex specific differences on α- and β-diversity on offspring gut microbiota at weaning and 14 weeks of age. Our previous study39 and an earlier work from our lab40 also found that there was no sex specific difference in offspring gut microbiota. In our previous study, we found that postnatal diet had strong effect on shaping offspring gut microbiota.39 A recent study also found similar results on mice offspring gut microbiota and showed that the postnatal environment partly linked with sex difference in gut microbiota, using a cross-fostering technique.41 Given that offspring in this study was fed the same chow diet regardless of dam's diet, it is possible that the sex difference in offspring gut microbiota is very subtle, hence we were unable to detect.
Our study showed that cafeteria diet consumption from prepregnancy to lactation significantly lowered abundance of OTU2_Romboutsia and OTU3_Lactobacillus in dams. We further identified that lower abundance of these taxa in dams were associated with lower maternal folate levels. In addition, weanlings from Caf dams showed lower abundance of OTU2_Romboutsia and OTU3_Lactobacillus, as well as lowered folate levels compared with Chow counterparts, indicating vertical transmission of these taxa from dams to weanlings. It is noteworthy that some Lactobacillus species are known to produce folic acid.42–44 Interestingly, there was no association between maternal folate level and these taxa in offspring gut microbiota at 14 weeks of age. Since folate compositions in chow and Caf diet are unknown, we are unable to comment on whether this was related to the 14 weeks offspring consuming chow diet, features of offspring gut microbiota composition, or interaction of these two factors. On the other hand, we showed that the lower maternal B12 levels in Caf dams than Chow dams were associated with gut microbiota at weaning and 14 weeks. Contrary to folate status at weaning, weanlings from Caf dams had slightly higher circulating B12 concentrations compared with chow counterparts. Both folate and B12 are essential vitamins required for maintenance of maternal health and fetal/offspring development.14,17,18 However, associations between circulating folate and B12 and intestinal gut microbiota are dynamic and complex, and may depend on host status, dietary intake and other environmental factors.45 Our study shows that the unhealthy cafeteria diet exerted detrimental effects on the status of essential vitamins in dams, with changes also evident in offspring at weaning. It is unclear to what extent the reduction observed here may have had negative consequences on offspring development, and further investigation is required to test this.
Our previous work showed that adult offspring gut microbiota were more affected by post weaning diet (chow vs. Caf), than by maternal diet.39 SourceTracker analysis in the present study revealed more nuanced maternal influences on offspring gut microbiota; while these were not marked in α and β diversity measures, different patterns of maternal gut microbiota contributions were observed in both diet groups.
Our study showed alterations of maternal gut microbiota during pregnancy to lactation under the influence of a cafeteria diet that mimics the nutrient poor, high-sugar, high-fat and highly processed diets eaten by many people, including pregnant mothers, around the world. Although the robust animal model used allowed for the effects of maternal diet on offspring biome to be identified, this may not represent a real-world scenario wherein children are likely to maintain dietary habits introduced by their parents,46–48 whereas rat offspring in our model were weaned at PND21 and fed standard chow. While the diet regimen in our rodent model has strong relevance to human dietary patterns, our focus was on the impact of diet on gut microbiota, and hence, our design minimised potential confounding factors in a laboratory setting, which is not feasible in humans. While this affords a degree of rigor not possible in clinical scenarios, it limits the applicability of our findings to humans. Moreover, in this study we were unable to assess the fibre content of each diet (Chow and Caf) along with other food additives which could interact with macronutrients and subsequently differentially influence the gut microbiota, between chow and Caf fed animals.49 Finally, our findings do not permit causal inferences to be drawn; however, we still believe the findings are valuable in demonstrating a link between maternal nutritional status and offspring microbiome composition. Further in-depth experimental work on this association is warranted.
Conclusions
This study showed that supplementing rats’ standard chow with a selection of nutrient-poor, highly-processed ‘cafeteria’ foods high in sugar, fat and salt, alters the trajectory of maternal rat gut microbiota from pre-pregnancy to lactation. While outside the scope of the present study, continued work is needed to establish causality how the maternal microbiome is altered by specific nutritive components, such as sugar,32 fat50,51 and food processing.52 Nonetheless, our study shows that maternal Caf diet not only dysregulates the normal pregnancy-associated changes in microbiota composition, but programs metabolic phenotypes and the development of the microbiome in offspring. While the gut microbiota of Caf offspring differed from that of Chow offspring at weaning, the difference was less marked at 14 weeks of age when offspring were consuming chow.Author contributions
Conception and design of research: MJM and RFW; performed the experiments: MDK, AT and SHS; analysed the data: KH and NOK; interpretation of the results of experiments: KH, MDK, NOK, RFW and MJM; prepared the figures: KH; drafted manuscript: KH, MDK and MJM. All authors read and approved the final manuscript.Data availability
The sequence data for this study have been deposited in the European Nucleotide Archive (ENA) at EMBL-EBI under accession number PRJEB81782 (https://www.ebi.ac.uk/ena/browser/view/PRJEB81782). This study is currently set on private and will be released upon acceptance. Preliminary report of these findings can be found here.53Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research was supported by project funding by the National Health and Medical Research Council of Australia to MJM and RFW [1161418].References
- B. Abu-Raya, C. Michalski, M. Sadarangani and P. M. Lavoie, Maternal Immunological Adaptation During Normal Pregnancy, Front. Immunol., 2020, 11, 575197 CrossRef CAS PubMed.
- M. Tian, Q. Li, T. Zheng, S. Yang, F. Chen, W. Guan and S. Zhang, Maternal microbe-specific modulation of the offspring microbiome and development during pregnancy and lactation, Gut Microbes, 2023, 15, 2206505 CrossRef PubMed.
- O. Koren, J. K. Goodrich, T. C. Cullender, A. Spor, K. Laitinen, H. K. Bäckhed, A. Gonzalez, J. J. Werner, L. T. Angenent, R. Knight, F. Bäckhed, E. Isolauri, S. Salminen and R. E. Ley, Host remodeling of the gut microbiome and metabolic changes during pregnancy, Cell, 2012, 150, 470–480 CrossRef CAS PubMed.
- D. B. DiGiulio, B. J. Callahan, P. J. McMurdie, E. K. Costello, D. J. Lyell, A. Robaczewska, C. L. Sun, D. S. Goltsman, R. J. Wong, G. Shaw, D. K. Stevenson, S. P. Holmes and D. A. Relman, Temporal and spatial variation of the human microbiota during pregnancy, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 11060–11065 CrossRef CAS PubMed.
- H. Yang, R. Guo, S. Li, F. Liang, C. Tian, X. Zhao, Y. Long, F. Liu, M. Jiang, Y. Zhang, J. Ma, M. Peng, S. Zhang, W. Ye, Q. Gan, F. Zeng, S. Mao, Q. Liang, X. Ma, M. Han, F. Gao, R. Yang, C. Zhang, L. Xiao, J. Qin, S. Li and C. Zhu, Systematic analysis of gut microbiota in pregnant women and its correlations with individual heterogeneity, npj Biofilms Microbiomes, 2020, 6, 32 CrossRef PubMed.
- M. A. Rasmussen, J. Thorsen, M. G. Dominguez-Bello, M. J. Blaser, M. S. Mortensen, A. D. Brejnrod, S. A. Shah, M. H. Hjelmsø, J. Lehtimäki, U. Trivedi, H. Bisgaard, S. J. Sørensen and J. Stokholm, Ecological succession in the vaginal microbiota during pregnancy and birth, ISME J., 2020, 14, 2325–2335 CrossRef CAS PubMed.
- H. A. Paul, K. H. Collins, M. R. Bomhof, H. J. Vogel and R. A. Reimer, Potential Impact of Metabolic and Gut Microbial Response to Pregnancy and Lactation in Lean and Diet-Induced Obese Rats on Offspring Obesity Risk, Mol. Nutr. Food Res., 2018, 62, 867–879 CrossRef PubMed.
- Y. J. Ji, H. Li, P. F. Xie, Z. H. Li, H. W. Li, Y. L. Yin, F. Blachier and X. F. Kong, Stages of pregnancy and weaning influence the gut microbiota diversity and function in sows, J. Appl. Microbiol., 2019, 127, 867–879 CrossRef CAS PubMed.
- K. Zhang, G. Liu, Y. Wu, T. Zhang, M. Guo, Y. Lei, X. Cao, L. Suo, D. Brugger, X. Wang, Y. Yang and Y. Chen, Gut Microbial Succession Patterns and Metabolic Profiling during Pregnancy and Lactation in a Goat Model, Microbiol. Spectrum, 2023, 11, e0295522 CrossRef PubMed.
- J. Ma, A. L. Prince, D. Bader, M. Hu, R. Ganu, K. Baquero, P. Blundell, R. Alan Harris, A. E. Frias, K. L. Grove and K. M. Aagaard, High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model, Nat. Commun., 2014, 5, 3889 CrossRef CAS PubMed.
- D. M. Chu, K. M. Antony, J. Ma, A. L. Prince, L. Showalter, M. Moller and K. M. Aagaard, The early infant gut microbiome varies in association with a maternal high-fat diet, Genome Med., 2016, 8, 77 CrossRef PubMed.
- A. M. Henderson, D. C. Tai, R. E. Aleliunas, A. M. Aljaadi, M. B. Glier, E. E. Xu, J. W. Miller, C. B. Verchere, T. J. Green and A. M. Devlin, Maternal folic acid supplementation with vitamin B(12) deficiency during pregnancy and lactation affects the metabolic health of adult female offspring but is dependent on offspring diet, FASEB J., 2018, 32, 5039–5050 CrossRef CAS PubMed.
- E. Castaño-Moreno, V. Castillo, R. Peñailillo, M. N. Llanos, R. Valenzuela and A. M. Ronco, Fatty acid and lipid metabolism in liver of pregnant mice and their offspring is influenced by unbalanced folates/vitamin B12 diets, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2020, 154, 102057 CrossRef PubMed.
- V. S. Tanwar, S. Ghosh, S. Sati, S. Ghose, L. Kaur, K. A. Kumar, K. V. Shamsudheen, A. Patowary, M. Singh, V. Jyothi, P. Kommineni, S. Sivasubbu, V. Scaria, M. Raghunath, R. Mishra, G. R. Chandak and S. Sengupta, Maternal vitamin B(12) deficiency in rats alters DNA methylation in metabolically important genes in their offspring, Mol. Cell. Biochem., 2020, 468, 83–96 CrossRef CAS PubMed.
- Z. Li, R. M. Gueant-Rodriguez, D. Quilliot, M. A. Sirveaux, D. Meyre, J. L. Gueant and L. Brunaud, Folate and vitamin B12 status is associated with insulin resistance and metabolic syndrome in morbid obesity, Clin. Nutr., 2018, 37, 1700–1706 CrossRef CAS PubMed.
- J. A. McKay, L. Xie, C. Manus, S. A. Langie, R. J. Maxwell, D. Ford and J. C. Mathers, Metabolic effects of a high-fat diet post-weaning after low maternal dietary folate during pregnancy and lactation, Mol. Nutr. Food Res., 2014, 58, 1087–1097 CrossRef CAS PubMed.
- H. M. Guetterman, S. L. Huey, R. Knight, A. M. Fox, S. Mehta and J. L. Finkelstein, Vitamin B-12 and the Gastrointestinal Microbiome: A Systematic Review, Adv. Nutr., 2022, 13, 530–558 CrossRef CAS PubMed.
- E. Rubini, N. Schenkelaars, M. Rousian, K. D. Sinclair, L. Wekema, M. M. Faas, R. P. M. Steegers-Theunissen and S. Schoenmakers, Maternal obesity during pregnancy leads to derangements in one-carbon metabolism and the gut microbiota: implications for fetal development and offspring wellbeing, Am. J. Obstet. Gynecol., 2022, 227, 392–400 CrossRef CAS PubMed.
- S. J. Leigh, M. D. Kendig and M. J. Morris, Palatable Western-style Cafeteria Diet as a Reliable Method for Modeling Diet-induced Obesity in Rodents, J. Visualized Exp., 2019, 153, e60262 Search PubMed.
- J. G. Caporaso, C. L. Lauber, W. A. Walters, D. Berg-Lyons, C. A. Lozupone, P. J. Turnbaugh, N. Fierer and R. Knight, Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(Suppl 1), 4516–4522 CrossRef CAS PubMed.
- P. D. Schloss, S. L. Westcott, T. Ryabin, J. R. Hall, M. Hartmann, E. B. Hollister, R. A. Lesniewski, B. B. Oakley, D. H. Parks, C. J. Robinson, J. W. Sahl, B. Stres, G. G. Thallinger, D. J. Van Horn and C. F. Weber, Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities, Appl. Environ. Microbiol., 2009, 75, 7537–7541 CrossRef CAS PubMed.
- C. Quast, E. Pruesse, P. Yilmaz, J. Gerken, T. Schweer, P. Yarza, J. Peplies and F. O. Glöckner, The SILVA ribosomal RNA gene database project: improved data processing and web-based tools, Nucleic Acids Res., 2013, 41, D590–D596 CrossRef CAS PubMed.
- J. J. Kozich, S. L. Westcott, N. T. Baxter, S. K. Highlander and P. D. Schloss, Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform, Appl. Environ. Microbiol., 2013, 79, 5112–5120 CrossRef CAS PubMed.
- K. R. Clarke, Non-parametric multivariate analyses of changes in community structure, Aust. J. Ecol., 1993, 18, 117–143 CrossRef.
- D. Knights, J. Kuczynski, E. S. Charlson, J. Zaneveld, M. C. Mozer, R. G. Collman, F. D. Bushman, R. Knight and S. T. Kelley, Bayesian community-wide culture-independent microbial source tracking, Nat. Methods, 2011, 8, 761–763 CrossRef CAS PubMed.
- K. Feng, Z. Zhang, W. Cai, W. Liu, M. Xu, H. Yin, A. Wang, Z. He and Y. Deng, Biodiversity and species competition regulate the resilience of microbial biofilm community, Mol. Ecol., 2017, 26, 6170–6182 CrossRef PubMed.
- V. Jalili, E. Afgan, Q. Gu, D. Clements, D. Blankenberg, J. Goecks, J. Taylor and A. Nekrutenko, The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2020 update, Nucleic Acids Res., 2020, 48, W395–W402 CrossRef CAS PubMed.
- M. D. Mesa, B. Loureiro, I. Iglesia, S. Fernandez Gonzalez, E. Llurba Olivé, O. García Algar, M. J. Solana, M. J. Cabero Perez, T. Sainz, L. Martinez, D. Escuder-Vieco, A. Parra-Llorca, M. Sánchez-Campillo, G. Rodriguez Martinez, D. Gómez Roig, M. Perez Gruz, V. Andreu-Fernández, J. Clotet, S. Sailer, I. Iglesias-Platas, J. López-Herce, R. Aras, C. Pallás-Alonso, M. S. de Pipaon, M. Vento, M. Gormaz, E. Larqué Daza, C. Calvo and F. Cabañas, The Evolving Microbiome from Pregnancy to Early Infancy: A Comprehensive Review, Nutrients, 2020, 12, 133 CrossRef PubMed.
- C. B. Miller, P. Benny, J. Riel, C. Boushey, R. Perez, V. Khadka, Y. Qin, A. K. Maunakea and M. J. Lee, Adherence to Mediterranean diet impacts gastrointestinal microbial diversity throughout pregnancy, BMC Pregnancy Childbirth, 2021, 21, 558 CrossRef PubMed.
- S. E. Rothenberg, C. L. Wagner, B. Hamidi, A. V. Alekseyenko and M. Andrea Azcarate-Peril, Longitudinal changes during pregnancy in gut microbiota and methylmercury biomarkers, and reversal of microbe-exposure correlations, Environ. Res., 2019, 172, 700–712 CrossRef CAS PubMed.
- M. Tang, N. E. Weaver, D. N. Frank, D. Ir, C. E. Robertson, J. F. Kemp, J. Westcott, K. Shankar, A. L. Garces, L. Figueroa, A. K. Tshefu, A. L. Lokangaka, S. S. Goudar, M. Somannavar, S. Aziz, S. Saleem, E. M. McClure, K. M. Hambidge, A. E. Hendricks and N. F. Krebs, Longitudinal Reduction in Diversity of Maternal Gut Microbiota During Pregnancy Is Observed in Multiple Low-Resource Settings: Results From the Women First Trial, Front. Microbiol., 2022, 13, 823757 CrossRef PubMed.
- S. Astbury, A. Song, M. Zhou, B. Nielsen, A. Hoedl, B. P. Willing, M. E. Symonds and R. C. Bell, High Fructose Intake During Pregnancy in Rats Influences the Maternal Microbiome and Gut Development in the Offspring, Front. Genet., 2018, 9, 203 CrossRef PubMed.
- A. L. Dunlop, A. K. Knight, G. A. Satten, A. J. Cutler, M. L. Wright, R. M. Mitchell, T. D. Read, J. Mulle, V. S. Hertzberg, C. C. Hill, A. K. Smith and E. J. Corwin, Stability of the vaginal, oral, and gut microbiota across pregnancy among African American women: the effect of socioeconomic status and antibiotic exposure, PeerJ, 2019, 7, e8004 CrossRef PubMed.
- R. C. Kennedy, R. R. Fling, M. S. Robeson, A. M. Saxton, R. L. Donnell, J. L. Darcy, D. A. Bemis, J. Liu, L. Zhao and J. Chen, Temporal Development of Gut Microbiota in Triclocarban Exposed Pregnant and Neonatal Rats, Sci. Rep., 2016, 6, 33430 CrossRef CAS PubMed.
- M. L. Ruebel, S. P. Gilley, C. R. Sims, Y. Zhong, D. Turner, S. V. Chintapalli, B. D. Piccolo, A. Andres and K. Shankar, Associations between Maternal Diet, Body Composition and Gut Microbial Ecology in Pregnancy, Nutrients, 2021, 13, 3295 CrossRef CAS PubMed.
- X. Yang, M. Zhang, Y. Zhang, H. Wei, Q. Guan, C. Dong, S. Deng, H. M. Tun and Y. Xia, Ecological change of the gut microbiota during pregnancy and progression to dyslipidemia, npj Biofilms Microbiomes, 2023, 9, 14 CrossRef CAS PubMed.
- F. Magne, M. Gotteland, L. Gauthier, A. Zazueta, S. Pesoa, P. Navarrete and R. Balamurugan, The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients?, Nutrients, 2020, 12, 1474 CrossRef CAS PubMed.
- A. Tajaddini, M. D. Kendig, K. V. Prates, R. F. Westbrook and M. J. Morris, Male Rat Offspring Are More Impacted by Maternal Obesity Induced by Cafeteria Diet than Females-Additive Effect of Postweaning Diet, Int. J. Mol. Sci., 2022, 23, 1442 CrossRef CAS PubMed.
- K. Hasebe, M. D. Kendig, N. O. Kaakoush, A. Tajaddini, R. F. Westbrook and M. J. Morris, The influence of maternal unhealthy diet on maturation of offspring gut microbiota in rat, Anim. Microbiome, 2022, 4, 31 CrossRef CAS PubMed.
- S. P. Bhagavata Srinivasan, M. Raipuria, H. Bahari, N. O. Kaakoush and M. J. Morris, Impacts of Diet and Exercise on Maternal Gut Microbiota Are Transferred to Offspring, Front. Endocrinol., 2018, 9, 716 CrossRef PubMed.
- L. Daoust, B. S. Choi, S. Lacroix, V. Rodrigues Vilela, T. V. Varin, S. Dudonné, G. Pilon, D. Roy, E. Levy, Y. Desjardins, B. Chassaing and A. Marette, The postnatal window is critical for the development of sex-specific metabolic and gut microbiota outcomes in offspring, Gut Microbes, 2021, 13, 2004070 CrossRef PubMed.
- P. Kanmani, R. Satish Kumar, N. Yuvaraj, K. A. Paari, V. Pattukumar and V. Arul, Probiotics and its functionally valuable products-a review, Crit. Rev. Food Sci. Nutr., 2013, 53, 641–658 CrossRef CAS PubMed.
- M. Rossi, A. Amaretti and S. Raimondi, Folate production by probiotic bacteria, Nutrients, 2011, 3, 118–134 CrossRef CAS PubMed.
- F. Saubade, Y. M. Hemery, J. P. Guyot and C. Humblot, Lactic acid fermentation as a tool for increasing the folate content of foods, Crit. Rev. Food Sci. Nutr., 2017, 57, 3894–3910 CrossRef CAS PubMed.
- D. E. Kok, W. T. Steegenga, E. J. Smid, E. G. Zoetendal, C. M. Ulrich and E. Kampman, Bacterial folate biosynthesis and colorectal cancer risk: more than just a gut feeling, Crit. Rev. Food Sci. Nutr., 2020, 60, 244–256 CrossRef CAS PubMed.
- L. H. Bogl, K. Silventoinen, A. Hebestreit, T. Intemann, G. Williams, N. Michels, D. Molnár, A. S. Page, V. Pala, S. Papoutsou, I. Pigeot, L. A. Reisch, P. Russo, T. Veidebaum, L. A. Moreno, L. Lissner and J. Kaprio, Familial Resemblance in Dietary Intakes of Children, Adolescents, and Parents: Does Dietary Quality Play a Role?, Nutrients, 2017, 9, 892 CrossRef PubMed.
- S. M. Robson, S. C. Couch, J. L. Peugh, K. Glanz, C. Zhou, J. F. Sallis and B. E. Saelens, Parent Diet Quality and Energy Intake Are Related to Child Diet Quality and Energy Intake, J. Acad. Nutr. Diet., 2016, 116, 984–990 CrossRef PubMed.
- H. Vepsäläinen, J. Nevalainen, M. Fogelholm, L. Korkalo, E. Roos, C. Ray and M. Erkkola, Like parent, like child? Dietary resemblance in families, Int. J. Behav. Nutr. Phys. Act., 2018, 15, 62 CrossRef PubMed.
- K. E. Morrison, E. Jašarević, C. D. Howard and T. L. Bale, It's the fiber, not the fat: significant effects of dietary challenge on the gut microbiome, Microbiome, 2020, 8, 15 CrossRef CAS PubMed.
- P. E. Mann, K. Huynh and G. Widmer, Maternal high fat diet and its consequence on the gut microbiome: A rat model, Gut Microbes, 2018, 9, 143–154 CrossRef CAS PubMed.
- M. L. Tellechea, M. F. Mensegue and C. J. Pirola, The Association between High Fat Diet around Gestation and Metabolic Syndrome-related Phenotypes in Rats: A Systematic Review and Meta-Analysis, Sci. Rep., 2017, 7, 5086 CrossRef PubMed.
- P. G. de Oliveira, J. M. de Sousa, D. G. F. Assunção, E. K. S. de Araujo, D. S. Bezerra, J. Dametto and K. Ribeiro, Impacts of Consumption of Ultra-Processed Foods on the Maternal-Child Health: A Systematic Review, Front. Nutr., 2022, 9, 821657 CrossRef PubMed.
- Preliminary findings were reported in K. Hasebe, M. D. Kendig, N. O. Kaakoush, A. Tajaddini, R. F. Westbrook and M. J. Morris, BioRxiv, 2024, preprint, DOI:10.1101/2024.01.21.576569.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo05674d |
| This journal is © The Royal Society of Chemistry 2025 |