 Open Access Article
Open Access ArticleApple peels as an edible source of phenolic bioactive compounds with antidiabetic and antiglycation properties
Javier
Cano-Lou
 ab,
Adrián
Millán-Laleona
ab,
Adrián
Millán-Laleona
 ab,
Rares
Candrea
a,
Francisco
Les
ab,
Rares
Candrea
a,
Francisco
Les
 ab,
Ana
Pina
c,
Giovanni
Caprioli
ab,
Ana
Pina
c,
Giovanni
Caprioli
 d and
Víctor
López
d and
Víctor
López
 *ab
*ab
aDepartment of Pharmacy, Faculty of Health Sciences, Universidad San Jorge, 50830, Zaragoza, Spain. E-mail: ilopez@usj.es
bInstitute of Agri-food Research of Aragón, IA2, Zaragoza University-CITA, 50013, Zaragoza, Spain
cUnidad de Hortofruticultura, Centro de Investigación y Tecnología Agroalimentaria de Aragón (CITA), Av Montañana 930, 50059, Zaragoza, Spain
dChemistry Interdisciplinary Project (ChIP), School of Pharmacy, University of Camerino, Via Madonna delle Carceri, 62032, Camerino, Italy
First published on 10th March 2025
Abstract
Apples (Malus domestica Borkh.) are one of the most consumed fruits around the world with a high production of peels as wastes and by-products. In this work, peels from different commercial and local apple samples are explored as a source of phenolic bioactive compounds that could be directly related to the prevention of type 2 diabetes. Six different cultivars from local and commercial apple samples were processed to obtain the phenolic compounds by ultrasonication of the peels using methanol as the solvent. The phenolic content was explored using the Folin–Ciocalteu assay and the quantification of 37 individual phenolic compounds was carried out by high-performance liquid chromatography coupled with mass spectrometry (HPLC-MS/MS). Cellular viability was determined by performing the MTT assay in Caco-2 cell cultures exposed to the phenolic extracts. Subsequently, the capacity to inhibit α-glucosidase, α-amylase and pancreatic lipase enzymes, as well as antiglycation and antioxidant activities, was evaluated. These apple peel samples were considered a source of phenolic compounds with hyperoside, delphinidin 3,5-diglucoside, chlorogenic acid, phlorizin, epicatechin and procyanidin B2 as the main constituents. All samples neutralized the production of advanced glycation end-products and exhibited antiradical activities in a dose-dependent manner; four samples (Amarilla de Octubre, Manzana Helada, Verde Doncella and Pinova) inhibited α-glucosidase but only the sample known as “Amarilla de Octubre” was successful in inhibiting pancreatic α-amylase. Cytotoxicity was discarded in Caco-2 cell cultures at physiological concentrations considering these extracts as a source of phenolic compounds with antidiabetic, antiglycation and antioxidant properties.
1. Introduction
Diabetes mellitus is a chronic disease that develops when the pancreatic islet β-cells cannot produce insulin, or the body becomes resistant to insulin with a deficient action in sensitive tissues.1 This contributes to high blood glucose levels, which induce a rise in reactive oxygen species (ROS) and inflammation, culminating in long-term damage to hyperglycaemia-sensitive cells in various organs and tissues.2 The World Health Organization (WHO) classifies hyperglycaemic disorders into diabetes mellitus type 1, type 2 and gestational diabetes, with type-2 diabetes accounting for 90% of all diabetes cases, which occurs due to an excess body weight, sedentary behaviour and dietary changes.3 The International Diabetes Federation (IDF) estimates 783.2 million diabetic people by 2045, which will be directly related to inevitable factors, such as population growth, major urbanisation, and an elderly society, combined with a better diabetes treatment that increases prevalence through decreased mortality.4 Some strategies on modifiable factors such as lifestyle behaviour and a healthy diet are required to stall the rise of developing diabetes and its complication.5 Plant-based diets or herbal supplements have traditionally had beneficial effects on chronic disease and have been associated with a decrease in the chance of developing type 2 diabetes.6,7 One of the most common components in plant-derived foods is polyphenols,8 which are phenolic compounds with a survival and antioxidant function as secondary metabolites in plants.9 These phenolic compounds might help in the prevention of metabolic and non-communicable diseases such as type 2 diabetes in part due to their antiradical and anti-inflammatory properties associated with specific antidiabetic properties such as enzyme inhibitory properties, anti-glycation activity, improvement of beta cell function and insulin resistance, as well as regulation of carbohydrate metabolism, among others.10 The most frequent classification of phenolic compounds includes five main classes: phenolic acids, flavonoids, tannins, stilbenes, lignans, and others.9 The flavonoid group is frequently found in plant foods, and some of the subgroups are flavonols, flavanols, flavones, isoflavones, anthocyanins and flavanones.11 These phenolic compounds could be found in high amounts in plant residues, including food by-products and wastes.12 Compared to other food-processing sectors, the agri-food industries in relation to fruits and vegetables generate increasing amounts of waste including 25–30% of peels, which are usually discarded.13 According to the Food and Agriculture Organization of the United Nations (FAO), 1.3 billion tons per year of food get lost or wasted in the world.14 In addition, the agri-food industries and distributors in Spain produced 0.0022 kg L−1 of by-products and 0.0004 kg L−1 of wastes for each kg L−1 of end products.15 Phenolic compounds from this huge volume of plant residues could be extracted and transformed into health-promoting ingredients for the development of innovative food products such as nutraceuticals, food supplements, cosmetics or medicinal products16 and could provide a source of a great variety of phytochemicals as hypoglycaemic drugs to prevent diabetes type 2.17,18 Certain peels from fruits and vegetables are edible, but in many cases, they are also frequent residues found in the food industry. In this context, it is remarkable to delve into the added value from residues of apple-derived products which are usually processed as peeled fruits, producing a large quantity of peel as industrial waste.19 Furthermore, peels are considered as a great source of nutrients and phytochemicals with higher bioactivity than the pulp.20,21 Nevertheless, the quantitative distribution of phenolic compounds is variable depending not only on the plant type or the plant organ, but also on the genetics, maturity and climatic conditions.22 The main goal of this manuscript is to investigate whether edible peels from autochthonous and commercial apple cultivars can also be used as a natural matrix of phenolic bioactive compounds for different industrial applications.2. Materials and methods
2.1 Apple peel samples
Six different varieties of fresh apples (Pinova and Verde Doncella as commercial samples; Esperiega de Ademuz, Manzana Helada, Amarilla de Octubre and Borau 01 as local autochthonous cultivars) were collected in 2022 from Garcipollera (Huesca, Aragon) between the months of August–October. After the apples were peeled, the pulp and the peel were completely separated in different parts. The peel tissue was frozen at −80 °C and lyophilized.2.2 Extraction of phenolic compounds and determination of total phenolic content (TPC)
For this study, the extraction of phenolic compounds was achieved using methanol and the solid liquid ratio (SLR) was a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 mixture. The method used for the extraction was treatment with intense ultrasonic waves for 20 minutes at room temperature, also known as ultrasound-assisted extraction (UAE). UAE was used as an effective extraction method of bioactive compounds, such as phenolic compounds.13 The liquid extract was centrifuged at 4000g for 10 minutes, the supernatant was filtered by clarification filtration with filter paper, and the solvent was evaporated using a rotary evaporator. The end product was a semi-solid mixture of each apple peel extract. TPC was determined using the Folin–Ciocalteu assay. A volume of 100 μL of the Folin–Ciocalteu reagent 10% (Sigma-Aldrich, Barcelona, Spain) was added to each well along with 20 μL of diluted extracts in methanol. After being kept for 3 minutes in the dark at ambient temperature, the reaction solution was mixed with 80 μL of Na2CO3 (10%).
33 mixture. The method used for the extraction was treatment with intense ultrasonic waves for 20 minutes at room temperature, also known as ultrasound-assisted extraction (UAE). UAE was used as an effective extraction method of bioactive compounds, such as phenolic compounds.13 The liquid extract was centrifuged at 4000g for 10 minutes, the supernatant was filtered by clarification filtration with filter paper, and the solvent was evaporated using a rotary evaporator. The end product was a semi-solid mixture of each apple peel extract. TPC was determined using the Folin–Ciocalteu assay. A volume of 100 μL of the Folin–Ciocalteu reagent 10% (Sigma-Aldrich, Barcelona, Spain) was added to each well along with 20 μL of diluted extracts in methanol. After being kept for 3 minutes in the dark at ambient temperature, the reaction solution was mixed with 80 μL of Na2CO3 (10%).
The reaction was concluded after 30 minutes of incubation under the previous conditions, and the absorbance (760 nm) was measured on a Synergy H1 reader (BioTek® Instruments, Inc., Winooski, VT, USA). A standard curve of gallic acid was used to quantify the TPC, which was expressed as milligrams of gallic acid equivalent (GAE) per gram of the extract.
2.3 Analysis of phenolic compounds by HPLC-MS/MS
Briefly, the analytical separation of the analytes was carried out on a Synergi Polar–RP C18 column preceded by a pre-column. The solvents used as mobile phases were water (A) and methanol each with 0.1% of formic acid at a flow rate of 0.8 mL min−1 in the gradient elution mode. The volume used for injection was 2 μL, and the temperature of the oven was 30 °C.
The source of ionization used was an electrospray ionization (ESI) operating in negative and positive modes and the detector was a triple quadrupole working in the dynamic MRM (multiple reaction monitoring) mode.
2.4 Cell viability assays
Caco-2 cells (epithelial cells isolated from colon tissue) were acquired from ATCC. The cell culture was treated with DMEM medium (Sigma Aldrich), 10% foetal bovine serum (FBS) (Sigma Aldrich) and 1% penicillin–streptomycin (Sigma Aldrich) in an incubator under a 5% CO2 atmosphere and at 37 °C. For the MTT assay, firstly, Caco-2 cells were seeded in 96-well plates at 2 × 104 cells per well for 24 hours. After that period, certain cells were treated with different apple peel extracts from 62.5 to 1000 μg extract per mL in 1% FBS and DMEM, meanwhile other cells (control) were cultivated with 1% FBS and DMEM without treatment for 24 hours. Following that, 100 μL of MTT stock solution (0.4 mg mL−1 in 10% FBS) was added in each well and the plate was collocated in an incubator under a 5% CO2 atmosphere and at 37 °C, in the dark for 3 hours. Once the formazan crystals were formed, the MTT solution was removed and 100 μL of DMSO was added. Finally, the dissolution of the crystals was carried out by agitation, and a purple colour appeared. The absorbance was read at 550 nm in a Synergy H1 Hybrid Multi-Mode Reader (Biotek, Bad Friedrichshall, Germany). The viability was calculated using the following formula (eqn (1)):| Cell viability (%) = [Abssample/Abscontrol] × 100 | (1) |
2.5 Enzyme inhibitory assays of α-glucosidase, α-amylase and lipase
| Inhibition (%) = [Abscontrol − Abssample/Abscontrol] × 100 | (2) |
2.6 Antiglycation and antioxidant activities
2.7 Statistical analysis
Each assay was carried out at least three times on different days and all results were expressed as the mean ± standard error (SEM) of different assays. GraphPad Prism v.8.0 (GraphPad Software, San Diego, CA, USA) was applied to execute data analyses such as nonlinear regressions, a one-way analysis of variance (ANOVA) with Dunnett's post hoc test, Student's t-test, and Pearson correlations and statistics with a 95% confidence level and 5% of wise significance. The determination of linear correlation was performed for the total polyphenol content using the Folin–Ciocalteu assay and antidiabetic, anti-glycation or antioxidant properties.2.8 Chemical structure generation
The chemical structures of most of the phenolic compounds found in the extract samples were generated using ChemDraw v. 21.0.0 software (PerkinElmer Informatics, Waltham, Massachusetts, USA).3. Results
3.1 Phenolic content using the Folin–Ciocalteu assay and HPLC-MS/MS
According to the TPC obtained from the Folin–Ciocalteu assay, the best apple peel sample is Amarilla de Octubre (142.80 ± 26.61 mg GAE per g extract) (Table 1), revealing significant differences against the rest of the samples, either autochthonous or commercial samples. Nevertheless, the commercial sample known as Pinova (52.81 ± 13.70 mg GAE per g extract), and the autochthonous sample of Borau 01 (41.79 ± 5.82 mg GAE per g extract) showed the lowest polyphenol content, compared to the rest of the samples.| Samples | Folin–Ciocalteu (mg GAE per g extract) |
|---|---|
| Amarilla de Octubre | 142.80 ± 26.61 |
| Esperiega de Ademuz | 69.21 ± 8.98* |
| Verde Doncella | 67.43 ± 11.89* |
| Manzana Helada | 61.94 ± 11.18* |
| Pinova | 52.81 ± 13.70* |
| Borau 01 | 41.79 ± 5.82** |
Considering HPLC-MS/MS analysis, 37 individual phenolic compounds of interest in six apple peel extracts from different apple cultivars were monitored (Table 2). The phenolic groups detected in the samples belong to flavonols, flavan-3-ols, anthocyanins, dihydrochalcones and cinnamic acid compounds. Nevertheless, none of the hydroxybenzoic acid, stilbene and flavanone compounds were identified. Amarilla de Octubre, as determined by HPLC-MS/MS, is the best sample as it contains 3 times higher phenolics (24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840.98 μg g−1) than Borau 01 (8576.39 μg g−1). The highest number of different phenolic compounds analysed (16 compounds) were detected in Amarilla de Octubre, while 14 compounds were revealed in Borau 01, and 15 were detected in the other samples.
840.98 μg g−1) than Borau 01 (8576.39 μg g−1). The highest number of different phenolic compounds analysed (16 compounds) were detected in Amarilla de Octubre, while 14 compounds were revealed in Borau 01, and 15 were detected in the other samples.
| Compounds | Amarilla de Octubre | Pinova | Esperiega de Ademuz | Manzana Helada | Verde Doncella | Borau 01 |
|---|---|---|---|---|---|---|
| n.d: not detected. | ||||||
| Hydroxybenzoic acids | ||||||
| Gallic acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 3-Hydroxybenzoic acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 4-Hydroxybenzoic acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Cinnamic acids | ||||||
| Chlorogenic acid | 2105.95 | 154.30 | 910.65 | 333.76 | 20.77 | 2312.85 |
| Neochlorogenic acid | 39.05 | n.d. | 18.22 | 16.09 | n.d. | n.d. |
| Caffeic acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Vanillic acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Syringic acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| P-Coumaric acid | n.d. | 15.05 | n.d. | n.d. | n.d. | 10.94 |
| Ferulic acid | n.d. | 73.39 | n.d. | n.d. | n.d. | n.d. |
| Ellagic acid | 104.85 | 55.28 | 100.34 | 103.25 | 308.72 | n.d. |
| trans-Cinnamic acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Stilbenes | ||||||
| Resveratrol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Dihydrochalcones | ||||||
| Phloridzin | 341.08 | 157.32 | 3092.59 | 1894.48 | 62.29 | 408.87 |
| Phloretin | 0.18 | 0.89 | 0.82 | 0.46 | n.d. | 0.14 |
| Flavonols | ||||||
| Quercetin | 12.45 | 1387.82 | 6.60 | 5.53 | 10.34 | 33.07 |
| Kaempferol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Myricetin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Isorhamnetin | n.d. | 12.30 | n.d. | n.d. | n.d. | n.d. |
| Kaempferol-3-glucoside | 24.54 | 10.10 | 7.50 | 5.37 | 9.85 | 6.71 |
| Quercitrin | 925.76 | 1447.40 | 517.20 | 952.75 | 793.34 | 442.78 |
| Rutin | 302.41 | 541.97 | 74.68 | 148.06 | 666.50 | 34.83 |
| Hyperoside | 4246.61 | 6336.00 | 2766.28 | 2677.83 | 3495.94 | 1209.07 |
| Isoquercitrin | 2973.13 | 4517.45 | 1669.32 | 1943.33 | 2168.95 | 770.70 |
| Flavan-3-ols | ||||||
| Catechin | 515.34 | n.d. | 181.13 | 113.11 | 87.73 | 108.17 |
| Procyanidin A2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Procyanidin B2 | 4297.35 | n.d. | 1160.39 | 1331.09 | 471.07 | 1086.90 |
| Epicatechin | 5244.89 | n.d. | 1977.79 | 1255.01 | 296.31 | 1186.63 |
| Flavanones | ||||||
| Naringenin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Hesperidin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Anthocyanin | ||||||
| Delphinidin-3,5-diglucoside | 3707.40 | 5456.33 | 1979.19 | 1992.60 | 2611.10 | 948.11 |
| Delphinidin-3-galactoside | 15.80 | n.d. | n.d. | n.d. | n.d. | 16.60 |
| Cyanidin-3-glucoside | n.d. | 303.24 | n.d. | n.d. | n.d. | n.d. |
| Petunidin-3-glucoside | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Pelargonidin-3-glucoside | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Pelargonidin-3-rutinoside | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Malvidin-3-galactoside | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Total polyphenolic content (μg g −1 ) | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840.98 840.98 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 484.65 484.65 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 462.70 462.70 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 772.71 772.71 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 002.2 002.2 |
8576.39 |
The most abundant phenolic compounds contained in the samples were hyperoside, delphinidin 3,5-diglucoside, epicatechin, procyanidin B2, phlorizin and chlorogenic acid (Fig. 1). However, hyperoside followed by delphinidin 3,5-diglucoside were found in high quantities in all the samples. Hyperoside belongs to the flavonol compounds as a glycosylated variety of quercetin, and its levels varied from 6336.00 μg g−1 (Pinova) to 1209.07 μg g−1 (Borau 01). However, delphinidin 3,5-diglucoside is included in the anthocyanin compounds as a glycosylated form of delphinidin, and its levels varied between 5456.33 μg g−1 (Pinova) and 948.11 μg g−1 (Borau 01).
It is noteworthy that the commercial samples are a better source of flavonols and anthocyanins but have a shortage of flavan-3-ols, dihydrochalcones and cinnamic acids (Fig. 2). The phenolic fraction of commercial samples of Pinova and Verde Doncella was made up of 69.58% and 64.94% flavonols, along with 28.19% and 23.73% anthocyanins, respectively, with hyperoside, delphinidin 3,5-diglucoside and isoquercitrin being the most abundant compounds in both samples. The main differences between them were the absence of flavan-3-ol compounds in Pinova, compared to the presence of 7.77% flavan-3-ols in the Verde Doncella sample. Procyanidin B2 (471.07 μg g−1), epicatechin (296.31 μg g−1) and catechin (87.73 μg g−1) were contained in Verde Doncella.
Nevertheless, for the autochthonous samples, a lower proportion of flavonols and anthocyanins was obtained in their TPC, with respect to the commercial samples. The decreased ratio of flavonols and anthocyanins is due to a notable increase in other types of phenolic compounds such as flavon-3-ols, dihydrochalcones and cinnamic acids (Fig. 2).
The sample of Amarilla de Octubre showed the most abundant quantities of flavonols and anthocyanins among all the autochthonous samples. Although, in comparison with the commercial samples, a lower percentage of 34.16% and 14.92% was obtained in their TPC, respectively. This is attributed to the great amounts of flavon-3-ol compounds existing as epicatechin (5244.89 μg g−1) or its dimerized form called procyanidin B2 (4297.35 μg g−1).
Similarly, it is worth noting that phlorizin, the glycosylated form of phloretin, which belongs to dihydrochalcone compounds, appears as an important phenolic compound in the autochthonous extracts of Esperiega de Ademuz (3092.59 μg g−1) and Manzana Helada (1894.48 μg g−1). Apart from that, the main difference between them is the fluctuation of the amount of cinnamic acids, flavonols and anthocyanins. Esperiega de Ademuz showed higher quantities of cinnamic acids as chlorogenic acid (910.65 μg g−1). Nonetheless, Manzana Helada was made up of 44.88% flavonols, along with 15.60% anthocyanins, being the second autochthonous sample beyond Amarilla de Octubre with a rich amount of these types of phenolic compounds.
In addition, despite the low TPC of the autochthonous sample of Borau 01, chlorogenic acid from cinnamic acids, function as a significant compound (2312.85 μg g−1). Therefore, being the sample with the largest amount of this type of phenolic compound, the sample of Borau 01 is considered as a good source of that substance.
3.2 Cell viability in Caco-2 cultures
The cytotoxicity of the apple peel phenolic extracts was evaluated in a wide range of concentrations, from lower and more physiological to very high and non-physiological concentrations. The MTT assay showed that Amarilla de Octubre, Manzana Helada and Esperiega de Ademuz, induced a slight decrease in the cell viability at high concentrations compared to the commercial samples (Verde Doncella and Pinova) (Fig. 3). The three autochthonous samples with the lowest viability, and the commercial sample of Pinova at 1000 μg mL−1 showed significant differences compared to the control cells without treatment, in terms of viability. Nonetheless, the cell viability was superior by 50% at the highest analysed concentration of all samples; considering that 1000 μg mL−1 is a very high and non-physiologically relevant concentration, apple peel extracts can be considered as non-cytotoxic.3.3 Evaluation of the antidiabetic activity against α-glucosidase, α-amylase and lipase
The substance used as reference in the α-glucosidase assay was the therapeutic agent of acarbose, with a half-maximal inhibitory concentration (IC50) of 220 ± 64.10 μg mL−1. Only four extracts (Amarilla de Octubre, Manzana Helada, Verde Doncella and Pinova) were able to inhibit α-glucosidase as indicated in Fig. 4. Amarilla de Octubre showed the lowest IC50 value related to the highest activity. The most powerful sample is followed by Manzana Helada and the commercial sample of Verde Doncella, which had interesting results as well. The commercial sample of Pinova was less effective than the previous mentioned samples. Nevertheless, no activity was found in the inhibition of α-glucosidase in the autochthonous samples of Borau 01 and Esperiega de Ademuz. | ||
| Fig. 4 Antidiabetic activity against (A) α-glucosidase (Saccharomyces cerevisiae) and (B) α-amylase (porcine pancreas). | ||
The substance used as reference in the α-amylase assay was gallic acid, a phenolic compound with an IC50 value of 909 ± 15 μg mL−1. The autochthonous sample of Amarilla de Octubre was the unique sample with enough capacity to inhibit the α-amylase enzyme, showing promising antidiabetic activity.
Regarding the lipase inhibition assay, the substance used as reference in the lipase assay was the therapeutic agent of orlistat, with an IC50 value of 27.68 ± 1.53 μg mL−1. None of the extracts were able to show activities, as they did not reach a concentration of 2000 μg mL−1.
In the set of the antidiabetic assays, none of the samples were able to achieve the reference pure substances (Table 3). However, the IC50 value of autochthonous samples of Amarilla de Octubre and Manzana Helada were very similar to the IC50 of reference substances used in the α-glucosidase assays, with no significant difference between them. Commercial samples of Verde Doncella showed an interesting result as well in the α-glucosidase inhibition assay, with no significant difference compared to the acarbose.
| IC50 values (μg mL−1) in the in vitro assays | ||||
|---|---|---|---|---|
| Extracts | α-Glucosidase | α-Amylase | AGEs | DPPH |
| n.a: no activity. | ||||
| A. Octubre | 625.51 ± 52.23ns | 1796.51 ± 40.95**** | 158.69 ± 10.04ns | 23.78 ± 3.69ns |
| M. Helada | 1074 ± 77.53ns | n.a | 190.95 ± 8.14ns | 51.68 ± 5.13* |
| E. Ademuz | n.a | n.a | 383.41 ± 14.18*** | 56.09 ± 8.23* |
| Pinova | 2150 ± 926.49* | n.a | 1006.87 ± 82.32**** | 92.43 ± 10.80**** |
| V. Doncella | 1250 ± 63.51ns | n.a | 221.61 ± 14.12* | 59.98 ± 3.08* |
| Borau 01 | n.a | n.a | 378.33 ± 14.90**** | 106.45 ± 21.88**** |
| Acarbose | 220 ± 64.10 | — | — | — |
| Gallic acid | — | 909 ± 15 | — | 0.47 ± 0.07 |
| Aminoguanidine | — | — | 55.01 ± 5.63 | — |
3.4 Evaluation of the inhibition of AGEs formation, and the antioxidant activity against radical DPPH
The substance used as reference in the AGEs inhibition assay was the investigational drug of aminoguanidine, with an IC50 value of 55.01 ± 5.63 μg mL−1. All apple peel samples were able to inhibit AGEs formation. It should be emphasized that the high activity of the autochthonous sample of Amarilla de Octubre was indicated by the lowest IC50 value, followed by Manzana Helada and the commercial sample of Verde Doncella. Nevertheless, some of the autochthonous samples (Borau 01, Esperiega de Ademuz) and the commercial sample of Pinova showed the lowest activity, with the commercial sample of Pinova being the least effective.The substance used as reference in the DPPH inhibition assay was gallic acid from phenolic compounds. It revealed an outstanding profile as radical scavengers, showing an IC50 value of 0.471 ± 0.0791 μg mL−1. All apple peel samples were able to scavenge DPPH radicals. It should be noted that the high activity of the autochthonous sample of Amarilla de Octubre was indicated by the lowest IC50 value. Other autochthonous and commercial samples such as Esperiega de Ademuz, Manzana Helada and Verde Doncella had optimal and similar results in terms of inhibitory activity. Nevertheless, the autochthonous and commercial samples of Borau 01 and Pinova showed the worst results.
None of the apple peel extracts (Table 3) were able to achieve IC50 values of the reference pure substances. However, the IC50 value of autochthonous sample of Amarilla was very comparable to the IC50 value of the reference substance used in AGE and DPPH assays, with no significant difference between them. The IC50 value of the other autochthonous sample of Manzana Helada was very similar to the IC50 value of aminoguanidine used in the AGE inhibition assay, with no significant difference as well.
3.5 Determination of linear correlation of TPC using the Folin–Ciocalteu assay and antidiabetic, antiglycative or antioxidant properties
Linear correlation analysis of TPC was obtained using the Folin–Ciocalteu assay and the IC50 values of the antidiabetic, antiglycation and antioxidant assays using the Pearson correlation coefficient (Fig. 5). No correlation was found between the α-glucosidase and AGE assays and TPC, which suggests that TPC is not related to antidiabetic activity. However, regarding the antioxidant activity, a clear correlation was found between the DPPH assay and TPC.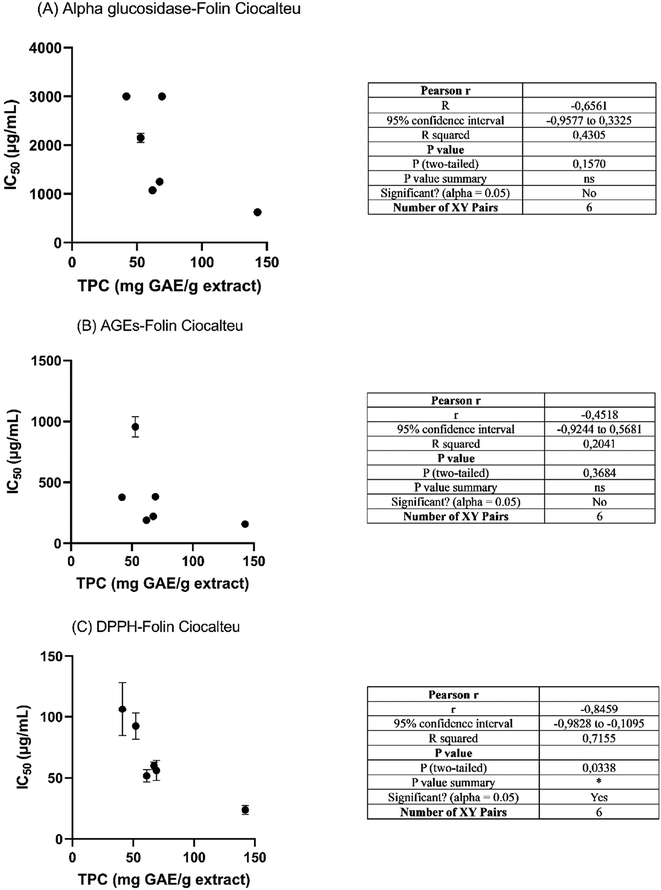 | ||
| Fig. 5 Correlation statistical analysis of TPC using the Folin–Ciocalteu assay and IC50 values for α-glucosidase (A), AGEs (B) and DPPH (C) (ns = not significant, *p < 0.05). | ||
4. Discussion
Apple peels are edible fruit parts but also a residue generated by the fruit and food industry all over the world. Considering that according to the WHO, 422 million people worldwide have diabetes, we here hypothesized whether these fruit peels could be an excellent source of phenolic compounds with bioactive properties such as antidiabetic, antiglycation or antioxidant properties and different food or health applications. The total extractable phenolic content was analysed using the Folin–Ciocalteu assay and ranges from 41 to 142 mg g−1 of apple peel extract. The concentration of TPC is greater in the apple peel than that in the apple pulp obtained in a previous research study from Aragón and Navarra apple cultivars,24 and this result is consistent with other comparative studies between the peel and the pulp of the apple.25The Folin–Ciocalteu assay method is usually used as a normalized method for analysing the TPC and uses the Folin–Ciocalteu reagent to oxidise the phenolic compounds. These phenolic compounds analysed are the main objective of this study, and the amounts of these bioactive compounds in the apple peel extracts are relevant to revalorizing these types of wastes and by-products. However, the TPC assay used is based on a redox reaction, and other compounds than polyphenols such as ascorbic acid and sugars can reduce the Folin–Ciocalteu reagent, and an overestimated result of phenolics can be produced.26 Nevertheless, the TPC can be very useful in comparing the amount of phenolic compounds between the different samples. In this assay, only Amarilla de Octubre has a significant difference among the other samples. Therefore, a complementary study of the antioxidant phytochemicals was needed, and HPLC-MS was selected as a reference method to analyse the overall phenolic compounds and 37 individual phenolic compounds of the samples. The TPC obtained by HPLC-MS was lower than that obtained by the Folin–Ciocalteu method. This finding is attributed to the fact that only 37 phenolic compounds were detected in this analysis and that many compounds such as polymerized polyphenols cannot be detected by HPLC-MS, such as proanthocyanidins (flavan-3-ol) and other oligomeric phenolics.27 Nevertheless, the individual phenolic groups found in the extracts were flavonols, flavan-3-ol, anthocyanins, dihydrochalcones and cinnamic acids, which are similar to the results of other previous studies on apple peels.19
Among all samples, the autochthonous sample of Amarilla de Octubre was the singular sample with the highest amount of TPC, contrasted by the Folin–Ciocalteu assay and with the identification of the major individual phenolic compounds by HPLC-MS. These results align with the best antidiabetic and antioxidant activities in all bioassays, showing no significant difference with all the reference substances, apart from the lipase and α-amylase assays.
In the other apple peel extracts, no significant differences were found in the TPC using the Folin–Ciocalteu assay, which indicates a low variability in the total polyphenols between different apple peel varieties. These results are in contrast with the previous studies of the TPC using the Folin–Ciocalteu assay in the apple pulp from commercial and autochthonous varieties where a significant difference was found in the TPC between them.24 Nevertheless, the ratio of the 37 individual phenolic compounds analysed by HPLC-MS showed a certain variability in the phenolic groups between the samples.
The autochthonous samples of Amarilla de Octubre followed by Manzana Helada showed the highest amount of flavonols and anthocyanins among all the autochthonous samples, combined with greater amounts of flavan-3-ols or dihydrochalcones compounds, respectively, compared to the commercial samples. These findings agree with the lowest IC50 value in the inhibition of α-glucosidase, formation of AGEs and DPPH radical scavenging, among all extracts. Both extracts showed similar results to acarbose and aminoguanidine in the antidiabetic assays, but only Amarilla de Octubre exhibited a similar activity to gallic acid in the inhibition of DPPH and was able to inhibit the α-amylase enzyme. The commercial sample of Verde Doncella showed great results as well in the antidiabetic and antioxidant bioassays with similar activity to acarbose in the inhibition of α-glucosidase, and it is attributable to the high amounts of flavonols and anthocyanins, along with a significant amount of flavan-3-ols compared to the other commercial samples. Nevertheless, none of the extracts were able to achieve the inhibition of lipase, and none of the extracts apart from Amarilla de Octubre inhibited the α-amylase enzyme.
Correlations for the TPC using the Folin–Ciocalteu assay and the α-glucosidase or advanced glycation end-product assays were not found, which could mean that other phytochemicals might be acting as bioactive compounds in these types of properties. Previous studies suggest that triterpenes found in apples, such as oleanolic and ursolic acids, may act as α-glucosidase inhibitors.28,29 Therefore, the ratio of the individual phenolic compounds and their synergism can not only influence the antidiabetic activity, but also the antioxidant activity.30 Some of these individual phenolic compounds found in high quantities in the samples were glycosylated flavonoids (hyperoside, delphinidin 3,5-diglucoside, and phlorizin), which modify the inhibitory activity of the α-amylase and α-glucosidase enzymes depending on the conjugation site and the type of sugar.31 In addition, the glycosylated chemical structure produces higher water solubility with higher bioavailability, which is a common issue of the phenolic compounds.32
5. Conclusion
Six apple peel samples from local and commercial cultivars were explored in terms of phytochemicals and can be considered a rich source of phenolic compounds (particularly flavonols, anthocyanins, flavan-3-ols, dihydrochalcones, and cinnamic acids). The samples showed antidiabetic and antioxidant properties without altering the cell viability at physiological concentrations. These bioassays indicated that the autochthonous local cultivar known as Amarilla de Octubre showed the highest phenolic content and the lowest IC50 value in the bioassays, suggesting its potential use for the development of nutraceuticals or pharmaceutical products for the prevention or treatment of type-2 diabetes complications as well as for the extraction of certain phytochemicals. The most representative individual phenolic compounds found in the apple peel samples are glycosylated flavonols such as hyperoside and isoquercitrin, flavan-3-ols such as procyanidin B2 and epicatechin, or anthocyanins such as delphinidin 3,5-diglucoside.Abbreviations
| AGEs | Advanced glycation end products |
| DNS | Dinitrosalicylic acid |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| ESI | Electrospray ionization |
| FAO | Food and Agriculture Organization of the United Nations |
| FBS | Foetal bovine serum |
| GAE | Gallic acid equivalent |
| HPLC-MS/MS | High performance liquid chromatography coupled with mass spectrometry |
| IC50 | Half-maximal inhibitory concentration |
| IDF | International Diabetes Federation |
| NPB | p-4-Nitrophenyl butyrate |
| ROS | Reactive oxygen species |
| SEM | Standard error of mean |
| SLR | Solid–liquid ratio |
| TPC | Total phenolic content |
| UAE | Ultrasound-assisted extraction |
| WHO | World Health Organization |
Data availability
The authors confirm that the data supporting the findings of this study are available within the article. Raw data and derived data supporting the findings of this study will be available from the corresponding author upon request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is part of the APPLEDIV Project (Multidisciplinary approach for exploiting genetic diversity to increase the value of autochthonous apple: valorizing local apple by-products and wastes through bioactive compounds; reference PID2022–141847OR-C33) funded by the Research State Agency in the 2022 call for Projects of Generation Knowledge oriented towards societal challenges and the previous project APPLECUT (PID2019–108081RR-C21) founded in the 2019 call of the Research State Agency. The authors thank Teva Pharma S.L.U for the research PhD grant to Javier Cano-Lou, the Government of Aragon for financial support from the Phyto-pharm group (ref. B44_23R) and Universidad San Jorge for funding Internal Project 2425013 through the Call for Internal Research Projects (Academic Year 2024–2025).References
- Y. Zheng, S. Ley and F. Hu, Global aetiology and epidemiology of type 2 diabetes mellitus and its complications, Nat. Rev. Endocrinol., 2018, 14(2), 88–98 CrossRef PubMed.
- E. Rendra, V. Riabov, D. M. Mossel, T. Sevastyanova, M. C. Harmsen and J. Kzhyshkowska, Reactive oxygen species (ROS) in macrophage activation and function in diabetes, Immunobiology, 2019, 224(2), 242–253 CrossRef CAS PubMed.
- World Health Organization, WHO [Internet]. Health topics: Diabetes [cited 2024 Oct 15]. Available from: https://www.who.int/health-topics/diabetes#tab=tab_1.
- H. Sun, P. Saeedi, S. Karuranga, M. Pinkepank, K. Ogurtsova and B. B. Duncan, et al., IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045, Diabetes Res. Clin., 2022, 183–109119 CAS.
- T. Geng, K. Zhu, Q. Lu, Z. Wan, X. Chen and L. Liu, et al., Healthy lifestyle behaviors, mediating biomarkers, and risk of microvascular complications among individuals with type 2 diabetes: A cohort study, PLoS Med., 2023, 20(1), e1004135 CrossRef PubMed.
- V. K. Sullivan, H. Kim, L. E. Caulfield, L. M. Steffen, E. Selvin and C. M. Rebholz, Plant-Based Dietary Patterns and Incident Diabetes in the Atherosclerosis Risk in Communities (ARIC) Study, Diabetes Care, 2024, 47(5), 803–809 CrossRef PubMed.
- K. A. Amirehsani, J. Hu, D. C. Wallace and T. P. McCoy, Herbal/Plant Remedies and Supplements Used by Hispanics/Latinxs for Diabetes: Source of Functional Foods?, Sci. Diabetes Self-Manage. Care., 2021, 47(1), 94–104 Search PubMed.
- M. Guasch-Ferré, J. Merino, Q. Sun, M. Fitó and J. Salas-Salvadó, Dietary Polyphenols, Mediterranean Diet, Prediabetes, and Type 2 Diabetes: A Narrative Review of the Evidence, Oxid. Med. Cell. Longevity, 2017, 2017, 6723931 CrossRef PubMed.
- C. Manach, A. Scalbert, C. Morand, C. Rémésy and L. Jiménez, Polyphenols: food sources and bioavailability, Am. J. Clin. Nutr., 2004, 79(5), 727–747 CrossRef PubMed.
- D. De Paulo Farias, F. F. de Araújo, I. A. Neri-Numa and G. M. Pastore, Antidiabetic potential of dietary polyphenols: A mechanistic review, Food Res. Int., 2021, 145, 110383 CrossRef PubMed.
- J. J. C. Serina and P. C. M. F. Castilho, Using polyphenols as a relevant therapy to diabetes and its complications, a review, Crit. Rev. Food Sci. Nutr., 2022, 62(30), 8355–8387 CrossRef PubMed.
- S. P. Sha, D. Modak, S. Sarkar, S. K. Roy, S. P. Sah and K. Ghatani, et al., Fruit waste: a current perspective for the sustainable production of pharmacological, nutraceutical, and bioactive resources, Front. Microbiol., 2023, 14, 1260071 CrossRef PubMed.
- N. P. Nirmal, A. C. Khanashyam, A. S. Mundanat, K. Shah, K. S. Babu and P. Thorakkattu, et al., Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry, Foods, 2023, 12(3), 556 CrossRef PubMed.
- J. Gustavsson, C. Cederberg, U. Sonesson, R. van Otterdijk and A. Meybeck, Global food losses and food waste - Extent, causes and prevention, En: FAO. Save Food! Congress, Düsseldorf. Rome, 2011 Search PubMed.
- Ministerio de Agricultura, Pesca y Alimentación, Informe del Desperdicio Alimentario en España-Octubre 2023, Dirección General de Alimentación, Madrid, 2023 Search PubMed.
- R. De la Peña-Armada and I. Mateos-Aparicio, Sustainable Approaches Using Green Technologies for Apple By-Product Valorisation as A New Perspective into the History of the Apple, Molecules, 2022, 27(20), 6937 CrossRef PubMed.
- B. Dinda, M. Dinda, A. Roy and S. Dinda, Dietary plant flavonoids in prevention of obesity and diabetes, Adv. Protein Chem. Struct. Biol., 2020, 120, 159–235 Search PubMed.
- C. M. Weaver, Bioactive foods and ingredients for health, Adv. Nutr., 2014, 5(3), 306S–311S CrossRef PubMed.
- J. Popiolek-Kalisz and P. Glibowski, Apple Peel Supplementation Potential in Metabolic Syndrome Prevention, Life, 2023, 13(3), 753 CrossRef PubMed.
- M. Kalinowska, A. Bielawska, H. Lewandowska-Siwkiewicz, W. Priebe and W. Lewandowski, Apples: content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties, Plant Physiol. Biochem., 2014, 84, 169–188 CrossRef CAS PubMed.
- B. Łata, A. Trampczynska and J. Paczesna, Cultivar variation in apple peel and whole fruit phenolic composition, Sci. Hortic., 2009, 121(2), 176–181 CrossRef.
- R. C. Pimpão, T. Dew, P. B. Oliveira, G. Williamson, R. B. Ferreira and C. N. Santos, Analysis of phenolic compounds in Portuguese wild and commercial berries after multienzyme hydrolysis, J. Agric. Food Chem., 2013, 61(17), 4053–4062 CrossRef PubMed.
- A. M. Mustafa, S. Angeloni, D. Abouelenein, L. Acquaticci, J. Xiao and G. Sagratini, et al., A new HPLC-MS/MS method for the simultaneous determination of 36 polyphenols in blueberry, strawberry and their commercial products and determination of antioxidant activity, Food Chem., 2022, 367, 130743 CrossRef CAS PubMed.
- A. Millán-Laleona, F. J. Bielsa, E. Aranda-Cañada, C. Gómez-Rincón, P. Errea and V. López, Antioxidant, Antidiabetic, and Anti-Obesity Properties of Apple Pulp Extracts (Malus domestica Bork): A Comparative Study of 15 Local and Commercial Cultivars from Spain, Biology, 2023, 12(7), 891 CrossRef PubMed.
- K. Wolfe, X. Wu and R. H. Liu, Antioxidant activity of apple peels, J. Agric. Food Chem., 2003, 51(3), 609–614 CrossRef PubMed.
- S. Sultana, K. Foster, T. Bates, M. L. Hossain, L. Y. Lim and K. Hammer, et al., Determination of Physicochemical Characteristics, Phytochemical Profile and Antioxidant Activity of Various Clover Honeys, Chem. Biodivers., 2024, 21(5), e202301880 CrossRef PubMed.
- O. Vidal-Casanella, J. Moreno-Merchan, M. Granados, O. Nuñez, J. Saurina and S. Sentellas, Total Polyphenol Content in Food Samples and Nutraceuticals: Antioxidant Indices versus High Performance Liquid Chromatography, Antioxidants, 2022, 11(2), 324 Search PubMed.
- H. Hussain, I. R. Green, I. Ali, I. A. Khan, Z. Ali and A. M. Al-Sadi, et al., Ursolic acid derivatives for pharmaceutical use: a patent review (2012–2016), Expert Opin. Ther. Pat., 2017, 27(9), 1061–1072 Search PubMed.
- S. T. Cargnin and S. B. Gnoatto, Ursolic acid from apple pomace and traditional plants: A valuable triterpenoid with functional properties, Food Chem., 2017, 220, 477–489 Search PubMed.
- W. J. Yeh, S. M. Hsia, W. H. Lee and C. H. Wu, Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings, J. Food Drug Anal., 2017, 25(1), 84–92 CrossRef PubMed.
- H. Cao and X. Chen, Structures required of flavonoids for inhibiting digestive enzymes, Anticancer Agents Med. Chem., 2012, 12(8), 929–939 CrossRef PubMed.
- M. Fujimori, K. Kadota, K. Kato, Y. Seto, S. Onoue and H. Sato, et al., Low hygroscopic spray-dried powders with trans-glycosylated food additives enhance the solubility and oral bioavailability of ipriflavone, Food Chem., 2016, 190, 1050–1055 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |



