Nutritional supplementation during tuberculosis treatment to improve clinical symptoms: a double-blinded placebo-controlled randomized trial†
Yang
Yang
a,
Jing
Cai
a,
Xinfang
Wang
a,
Kuan
Zhao
a,
Zhixuan
Lei
a,
Wenge
Han
b,
Xiangyu
Yin
b,
Kun
Yan
b,
Yidan
Hu
a,
Bo
Zhang
b,
Lei
Xu
a,
Xin
Guo
a,
Yanqiu
Xu
a,
Ke
Xiong
a,
Tianlin
Gao
 a,
Yan
Ma
a,
Feng
Zhong
a,
Yan
Ma
a,
Feng
Zhong
 a,
Qiuzhen
Wang
a,
Yongye
Sun
a,
Qiuzhen
Wang
a,
Yongye
Sun
 a,
Jinyu
Wang
a,
Jinyu
Wang
 *a and
Aiguo
Ma
*a and
Aiguo
Ma
 *a
*a
aInstitute of Nutrition and Health, School of Public Health, Qingdao University, 308 Ningxia Road, Qingdao, Shandong 266021, China. E-mail: magfood@qdu.edu.cn; wangjinyu@qdu.edu.cn; Tel: +86-138-0452-2696 Tel: +86-176-6760-7037
bDepartment of Respiratory and Critical Care Medicine, Weifang Respiratory Disease Hospital, Weifang, Shandong, China
First published on 21st November 2024
Abstract
Background and aims. Undernutrition coexists with tuberculosis and is associated with adverse treatment outcomes. Nutrition packages have been incorporated into tuberculosis patient care in some regions but there are little data on its effectiveness. The aim of this study is to evaluate the effect of a nutrition package on the treatment response and nutritional status of tuberculosis patients. Methods. We conducted a double-blinded placebo-controlled randomized trial in 360 pulmonary tuberculosis patients with concurrent diabetes or prediabetes. The participants were randomly assigned to receive a daily nutrition package (112 kcal, 9 g protein, and micronutrients) or a daily placebo package (112 kcal, 3 g protein, and no micronutrients) during tuberculosis treatment. The intervention lasted for six months. All participants received standard pulmonary tuberculosis treatment. The clinical symptoms, sputum smear, chest computed tomography, and nutritional status were monitored during the intervention. Results. The nutrition package improved the expectoration (intervention vs. placebo: 34.1% vs. 48.3% in week 1, 27.8% vs. 45.0% in week 2, 25.9% vs. 38.6% in week 3, 25.6% vs. 35.4% in month 1, 15.3% vs. 22.9% in month 2) and chest pain (2.3% vs. 9.0% in week 3, 3.6% vs. 8.3% in month 1, 4.3% vs. 10.0% in month 2, 1.8% vs. 6.4% in month 4). The nutrition package also increased hemoglobin, albumin, and lymphocyte counts. The nutrition package did not influence the sputum smear conversion in the whole population [hazard ratio (95% CI): 1.031 (0.685, 1.550), P = 0.885], but accelerated the conversion in patients without cavity [2.583 (1.180, 5.656), P = 0.018]. Conclusions. The nutrition package improved the clinical symptoms (e.g. chest pain and expectoration) and alleviated undernutrition (e.g. anemia and hypoproteinemia) among tuberculosis patients. The study was registered at the China Clinical Trial Registry Center (no. ChiCTR1900022294; https://www.chictr.org.cn).
1. Introduction
Tuberculosis is an infectious disease caused by Mycobacterium tuberculosis. Pulmonary tuberculosis is the primary type of disease, which accounts for 85% of the total cases.1 According to the data released by the World Health Organization (WHO), 7.5 million people worldwide developed active tuberculosis in 2022 and 1.3 million died from the disease.2Tuberculosis patients are characterized by a low BMI and deficiency of multiple nutrients including protein, Zn, and vitamins A, D, and E.3,4 Undernutrition is the top one risk factor for tuberculosis which accounts for more than 25% of tuberculosis cases and is associated with increased severity, worse treatment response, and increased mortality.2,3 The WHO recommended the integration of nutrition assessment, counselling, and supplementation in tuberculosis programs.5 However, most tuberculosis programs do not provide nutritional support or monitoring. Some countries, such as India, Indonesia, and Myanmar, provided nutritional support, including food baskets, food vouchers, or nutrition packages, to tuberculosis patients, but little data are reported on its effectiveness.6
Previous studies have shown that nutrition supplementation can improve the clinical symptoms, physical function and treatment adherence of tuberculosis and other diseases.7–15 Most of these studies focus on micronutrients, either single micronutrients (e.g. vitamin A, vitamin D, and Zn) or multiple micronutrients (a combination of vitamin A, thiamin, riboflavin, vitamin B6, vitamin B12, folic acid, niacin, vitamin C, vitamin D, vitamin E, selenium and copper).16 One randomized controlled trial indicated that macronutrient supplementation (a combination of protein, carbohydrate and fat) can improve weight gain, grip strength and quality of life among tuberculosis patients.8 Three randomized controlled trials indicated that food supplementation can improve treatment adherence and treatment responses among tuberculosis patients.9–11 Since tuberculosis patients are wasting and facing multiple nutrient deficiencies, a more comprehensive nutrient supplementation may provide a superior effect.
The National Health Commission of China noticed the nutrition needs of tuberculosis patients and funded our project to test the effects of nutritional support on the treatment response and nutritional status of tuberculosis patients. We provided nutritional support in the form of a nutrition package which was easy to dispense and use and contained both macronutrients (protein, carbohydrate, and fat) and micronutrients (iron, calcium, zinc, folate, niacin, and vitamins A, B1, B2, B6, B12, C, D, and E). The dosage of the micronutrients ranged from one-third to one full dosage of the recommended nutrient intake (RNI) for Chinese residents, except for vitamin B6 whose dosage was 25% to 43% higher than the RNI depending on the age of the patients (the tuberculosis drug interferes with the metabolism of vitamin B6).17,18
The WHO reported the co-epidemics of tuberculosis and diabetes (over 15% of tuberculosis patients were combined with diabetes) and highlighted the urgent need to target this population for tuberculosis control.19 Tuberculosis patients with concurrent diabetes or prediabetes have worse clinical manifestations (e.g. higher risks of the pulmonary cavity and severe respiratory symptoms), delayed sputum conversion, and higher risks of recurrence and death.20 Therefore, we targeted this population for nutritional supplementation.
In this work, we conducted a double-blinded placebo-controlled randomized trial to investigate the effects of nutritional supplementation on the tuberculosis treatment response [e.g. clinical symptoms, sputum smear conversion, and chest computed tomography (CT) result] and nutritional status (e.g. anemia, weight gain, and hypoproteinemia) of tuberculosis patients with concurrent diabetes or prediabetes. The intervention lasted for six months.
2. Subjects and methods
2.1 Study design and patients
We conducted this double-blinded placebo-controlled randomized trial at a hospital located in Weifang city, Shandong province, China. Weifang is an inland city with 9.4 million residents and a gross domestic product of around ten thousand dollars per capita. The enrollment period was from May 2019 to September 2021 and all patients completed follow-up in May 2022. The enrollment was suspended from January 2020 to June 2020 due to the outbreak of COVID-19.Newly diagnosed pulmonary tuberculosis patients (aged 18–80 years old) with concurrent diabetes or prediabetes were eligible. The patients were enrolled within two days after the tuberculosis diagnosis. The inclusion criteria included the following : (1) newly diagnosed pulmonary tuberculosis patients; (2) aged 18–80 years old; and (3) with concurrent diabetes or prediabetes. The exclusion criteria included the following: (1) extrapulmonary tuberculosis or drug-resistant tuberculosis; (2) with other serious diseases including HIV, liver or kidney diseases, malignant tumors, psychological illness; (3) pregnancy or lactation; (4) taking nutritional supplementation in the previous two months; and (5) soybean allergy. The pulmonary tuberculosis was diagnosed by a combination of clinical symptoms (e.g. cough, hemoptysis, weight loss, fever and night sweats), chest computed tomography and sputum smear results according to the WHO's guidelines.21 The drug-resistant tuberculosis and extrapulmonary tuberculosis were not included in this study. The patients who were not expected to survive for 6 months were not included. Diabetes and prediabetes were diagnosed according to the criteria of the International Diabetes Federation.22
The study was approved by the Medical Ethics Committee of Qingdao Center for Disease Control and Prevention (no. 201805) and conducted in accordance with the Declaration of Helsinki. The study was registered at the China Clinical Trial Registry Center (no. ChiCTR1900022294). All participants provided written informed consent.
2.2 Randomization and masking
A simple randomization was used in this study. A research investigator (YY) generated the randomization sequence using SAS software. The enrolled patients were randomly assigned (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to receive the standard tuberculosis treatment together with a nutrition or placebo package. The assignment for each participant was put into an envelope. When each patient was enrolled, the hospital staff (WH) opened the envelope to assign the patient to A or B. The nutrition and placebo packages had the same appearance and taste and were labeled as A and B, respectively. The patients, research investigators, and hospital staff did not know the assignment of A and B. The assignment was maintained by one research investigator (AM) and revealed after the end of the study.
1) to receive the standard tuberculosis treatment together with a nutrition or placebo package. The assignment for each participant was put into an envelope. When each patient was enrolled, the hospital staff (WH) opened the envelope to assign the patient to A or B. The nutrition and placebo packages had the same appearance and taste and were labeled as A and B, respectively. The patients, research investigators, and hospital staff did not know the assignment of A and B. The assignment was maintained by one research investigator (AM) and revealed after the end of the study.
2.3 Procedures
The patients received the standard tuberculosis treatment according to the Chinese National Guidelines for Tuberculosis Prevention and Control, which consists of a two-month intensive phase using isoniazid, rifampicin, pyrazinamide, and ethambutol and a four-month continuation phase using isoniazid and rifampicin.23 The patients were asked to stay in hospital for the first month during treatment and discharged after that. The patients took a nutrition or placebo package orally every day during the six-month treatment. During hospitalization, the nutrition or placebo packages were distributed daily by the hospital staff. After discharge, the patients took home the nutrition or placebo packages with medications and were asked to attend the hospital at months 2, 4 and 6 for examination.The nutrition package was a dietary supplement. The nutrition package contained 9.1 g of protein, 2.6 g of fat, 13.0 g of carbohydrate, 9.0 mg of iron, 320 mg of calcium, 8.0 mg of zinc, 350 μg (retinol equivalent) of vitamin A, 7.5 μg of vitamin D, 140 μg of folate, 2.0 μg of vitamin B12, 5.0 mg of vitamin E, 1.2 mg of vitamin B1, 1.2 mg of vitamin B2, 2.0 mg of vitamin B6, 10.0 mg of niacin, and 80.0 mg of vitamin C. The placebo contained 3.0 g of protein, 1.3 g of fat, and 22.0 g of carbohydrate. The dosage of the micronutrients was based on the RNI for Chinese residents, which ranged from one-third to one of the RNI.18 The dosage of vitamin B6 was 1.25–1.43 of the RNI (for different age groups) because its metabolism is interfered by isoniazid.17,18 The standard tuberculosis treatment usually lasted for 6 months; the nutritional intervention lasted throughout the tuberculosis treatment. The nutrition and placebo packages were produced by Tiantian'ai Biotechnology Co., Ltd (Shandong, China).
At the screening visit, the patients’ demographic information was collected including age, gender, disease history, education level, and smoking status. Fasting blood samples and sputum samples were collected before and 1, 2, 3, 4, 8, 16 and 24 weeks after the intervention. The blood samples were analyzed for fasting blood glucose, total protein, albumin, hemoglobin, and complete blood count. Hypoproteinemia was defined as an albumin content below 35 g L−1.24 Lymphocytopenia was defined as a lymphocyte count below 1 × 109 per L.25 Anemia was defined as a hemoglobin content below 130 g L−1 in men and below 120 g L−1 in women.26 Sputum smear was conducted and examined microscopically for the presence and number of acid-fast bacteria (AFB). The chest CT scan was conducted before and six months after the intervention. We used the chest CT severity score to evaluate the chest damage.27 Chest CT severity score = proportion of total lung affected (100%) + 40% if cavity present. The blood test, sputum smear, and chest CT were conducted by the hospital staff. The clinical symptoms including cough, expectoration, hemoptysis, chest pain, fatigue, night sweats, fever, and loss of appetite were surveyed by trained research investigators using a structured questionnaire before and 1, 2, 3, 4, 8, 16 and 24 weeks after the intervention. The symptom score was used to assess the symptom severity. The presence of any of the typical tuberculosis signs and symptoms (cough, expectoration, hemoptysis, chest pain, fatigue, night sweats, loss of appetite, fever and anemia) was scored as one point. The tuberculosis score ranges from 0–9.
2.4 Outcomes
The primary efficacy outcome of the study was the proportion of patients with persistent tuberculosis symptoms after two to six months of tuberculosis treatment. The clinical symptoms generally improve within the first few weeks of tuberculosis treatment and are important factors to assess the treatment response. Persistent or recurrent clinical symptoms are signs of treatment failure and require prompt investigation. The secondary outcomes were time to sputum smear conversion, chest CT severity, and the proportion of patients with malnutrition (e.g. anemia, hypoproteinemia, and lymphocytopenia) after tuberculosis treatment.2.5 Statistical analysis
According to previous studies, 66%, 72%, and 38% of tuberculosis patients had cough, expectoration, and chest pain after two months of tuberculosis treatment in the placebo group.12,28 The sample size was calculated using the following formula: with a two-sided significance of 0.05 and 1 − β = 0.8. To detect a 40% difference in the incidence of cough, expectoration, and chest pain between the intervention and placebo groups, 110, 90, and 285 participants were needed, respectively. Assuming a drop-out rate of 20%, a sample size of 356 patients was needed.
with a two-sided significance of 0.05 and 1 − β = 0.8. To detect a 40% difference in the incidence of cough, expectoration, and chest pain between the intervention and placebo groups, 110, 90, and 285 participants were needed, respectively. Assuming a drop-out rate of 20%, a sample size of 356 patients was needed.
The statistical analyses were conducted using SPSS 27.0 software and the significance was tested at the 0.05 level. The difference of numerical data between the two groups was analyzed by a t-test (if normally distributed) or a Mann–Whitney U test (if not normally distributed). A repeated measures ANOVA was also used. The difference of categorical data between the two groups was analyzed by a χ2 test or Fisher's exact test. A log-rank test was used to compare the sputum smear conversion time between the two groups. A Cox proportional hazard regression model was established for potential predictors of sputum smear conversion, including intervention allocation, age, gender, baseline sputum smear, BMI, white blood cell count, neutrophil count, anemia, diabetes, hypoproteinemia, lymphocytopenia, and cavitation. The age, gender, and baseline sputum smear were included as covariates in the multivariate Cox proportional hazard regression model. Subgroup analysis was performed for age, gender, BMI and cavitation.
3. Results
3.1 Effect of nutritional supplementation on clinical symptoms
Between May 2019 and September 2021, 360 tuberculosis patients were randomly assigned to the intervention and placebo group (Fig. 1, N = 180 in each group). Thirty-one patients had no follow-up results. A total of 329 patients were included in the final intention-to-treat analyses (176 in the intervention group and 153 in the placebo group). The demographic and baseline characteristics were comparable between the two groups except for the smoking status. The proportion of patients smoking cigarettes was slightly higher in the placebo group than that in the intervention group (Table 1).| Intervention | Placebo | P | |||
|---|---|---|---|---|---|
| N | Values | N | Values | ||
| Data are presented as n (%) or mean (standard deviation) unless otherwise stated. The difference between the two groups was analyzed by a χ2 test (category data), a t-test (normally distributed numerical data) or a Mann–Whitney U test (non-normally distributed numerical data). IQR, inter-quartile range; AFB, acid-fast bacillus; HFB, high-power field; CT, computed tomography. | |||||
| Median age, years (IQR) | 176 | 56.5 (41.0, 64.75) | 153 | 55.0 (46.5, 65.0) | 0.551 |
| Gender | 176 | 153 | 0.756 | ||
| Male | 132 (75.0%) | 117 (76.5%) | |||
| Female | 44 (25.0%) | 36 (23.5%) | |||
| BMI, kg m−2 | 176 | 21.6 (3.9) | 152 | 21.6 (3.2) | 0.867 |
| Diabetes status | 176 | 153 | 0.732 | ||
| No | 85 (48.3%) | 71 (46.4%) | |||
| Yes | 91 (51.7%) | 82 (53.6%) | |||
| Marital status | 176 | 153 | 0.375 | ||
| Single | 21 (11.9%) | 13 (8.5%) | |||
| Married | 148 (84.1%) | 129 (84.3%) | |||
| Widowed | 3 (1.7%) | 7 (4.6%) | |||
| Divorced | 4 (2.3%) | 4 (2.6%) | |||
| Educational level | 171 | 139 | 0.142 | ||
| None | 10 (5.8%) | 13 (9.4%) | |||
| Class I–IX | 97 (56.7%) | 88 (63.3%) | |||
| Class X–XII | 56 (32.7%) | 30 (21.6%) | |||
| Diploma or higher | 8 (4.7%) | 8 (5.8%) | |||
| Presently smokes cigarettes | 176 | 6 (3.4%) | 153 | 16 (10.5%) | 0.011 |
| Presently consumes alcohol | 176 | 6 (3.4%) | 153 | 8 (5.2%) | 0.415 |
| Baseline sputum smear | 125 | 117 | 0.453 | ||
| <3 AFB per HFB | 93 (74.4%) | 82 (70.1%) | |||
| ≥3 AFB per HFB | 32 (25.6%) | 35 (29.9%) | |||
| Cavitation on chest CT | 139 | 55 (39.6%) | 118 | 43 (36.4%) | 0.607 |
| Fasting blood glucose, mmol L−1 | 172 | 6.8 (2.8) | 149 | 6.9 (3.0) | 0.585 |
Before the intervention, the incidence of clinical symptoms including cough, expectoration, hemoptysis, fever, chest pain, fatigue, and loss of appetite was similar between the two groups (Fig. 3); the incidence of night sweats was higher in the intervention group than that in the placebo group (26.3% vs. 9.1%, P < 0.001). The incidence of night sweats decreased in the intervention group and was comparable to that in the placebo group after two weeks of treatment. The incidences of expectoration (intervention vs. placebo: 34.1% vs. 48.3% in week 1, 27.8% vs. 45.0% in week 2, 25.9% vs. 38.6% in week 3, 25.6% vs. 35.4% in month 1, 15.3% vs. 22.9% in month 2) and chest pain (2.3% vs. 9.0% in week 3, 3.6% vs. 8.3% in month 1, 4.3% vs. 10.0% in month 2, 1.8% vs. 6.4% in month 4, 0% vs. 2.9% in month 6) were significantly lower in the intervention group than those in the placebo group. Subgroup analyses were conducted by age, gender, BMI and cavitation (ESI Tables 1–4†). The results indicated that the alleviation effect of nutritional supplementation on tuberculosis symptoms (e.g. chest pain, expectoration, and cough) was more pronounced in males and patients with a BMI less than 18.5.
3.2 Nutritional supplementation on sputum smear conversion
Among the 329 patients, 97 patients had negative sputum smear at the baseline and 103 patients had no follow-up sputum smear results. The remaining 129 patients were included in the analysis of sputum smear conversion (69 in the intervention group, 60 in the placebo group). The medium time to sputum smear conversion was 91.0 days (95% CI: 55.0–127.0) for the intervention group and 82.0 (68.2–95.8) days for the placebo group, which did not differ significantly (Fig. 2, P = 0.884, log-rank test). Univariate Cox regression analyses showed that the severe baseline sputum smear (the presence of three or more acid-fast bacillus per high-power field) and male gender were associated with a delayed sputum smear conversion (ESI Table 5†). After adjusting for age, gender, and baseline sputum smear, the hazard ratio of treatment allocation was 0.998 (95% CI: 0.653–1.525). Subgroup analyses indicated that nutritional supplementation may be beneficial for patients with no lung cavity [ESI Table 4,† hazard ratio (95% CI): 2.583 (1.180–5.656)].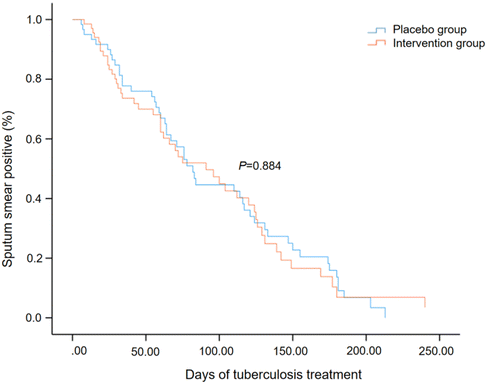 | ||
| Fig. 2 Time to sputum smear conversion between the intervention and placebo groups by a log-rank test. | ||
3.3 Nutritional supplementation on chest CT results
Lung damage was prevalent among the included tuberculosis patients (Table 2, assessed by chest CT). Before the treatment, 82.1% of patients exhibited consolidation, 26.5% of patients exhibited fibrosis, 52.5% of patients exhibited nodules, 38.1% of patients exhibited cavities, and 37.7% of patients exhibited infiltration. After six months of treatment, the prevalence of consolidation, cavity, infiltration, and chest CT severity score decreased, while the prevalence of fibrosis increased. The chest CT results between the two groups were not significantly different.| Intervention | Placebo | P | ||||
|---|---|---|---|---|---|---|
| N | Values | N | Values | |||
| Data are presented as number (%) or median (inter-quartile range). The difference between the two groups was analyzed by a χ2 test (category data) or a Mann–Whitney U test (numerical data). CT, computed tomography. | ||||||
| No. of participants with consolidation | Pre-intervention | 139 | 116 (83.5%) | 118 | 95 (80.5%) | 0.539 |
| Month 6 | 89 | 59 (66.3%) | 72 | 51 (70.8%) | 0.538 | |
| No. of participants with fibrosis | Pre-intervention | 139 | 41 (29.5%) | 118 | 27 (22.9%) | 0.231 |
| Month 6 | 89 | 36 (40.4%) | 72 | 24 (33.3%) | 0.353 | |
| No. of participants with nodules | Pre-intervention | 139 | 77 (55.4%) | 118 | 58 (49.2%) | 0.318 |
| Month 6 | 89 | 54 (60.7%) | 72 | 38 (52.8%) | 0.314 | |
| No. of participants with cavity | Pre-intervention | 139 | 55 (39.6%) | 118 | 43 (36.4%) | 0.607 |
| Month 6 | 89 | 29 (32.6%) | 72 | 28 (38.9%) | 0.406 | |
| No. of participants with infiltration | Pre-intervention | 139 | 55 (39.6%) | 118 | 42 (35.6%) | 0.512 |
| Month 6 | 89 | 18 (20.2%) | 72 | 14 (19.4%) | 0.902 | |
| Chest CT severity score | Pre-intervention | 139 | 50 (20, 90) | 118 | 40 (20, 70) | 0.544 |
| Month 6 | 89 | 20 (10, 75) | 72 | 30 (12.5, 60) | 0.752 | |
3.4 Nutritional supplementation on nutritional and immune indexes
The nutritional supplementation significantly increased the hemoglobin and albumin levels and reduced the prevalence of anemia (intervention vs. placebo: 18.9% vs. 30.2% in month 1, 11.6% vs. 18.2% in month 2, 5.5% vs. 9.6% in month 4, 5.4% vs. 12.5% in month 6; Fig. 4A) and hypoproteinemia (5.5% vs. 13.1% in month 1, 1.8% vs. 6.5% in month 2; Fig. 4B). The patients’ weight increased in response to the tuberculosis treatment; however, no significant difference existed between the two groups. The lymphocyte count was elevated in response to the tuberculosis treatment and significantly higher in the intervention group than that in the placebo group (1.6 vs. 1.5 × 109 per L in week 2, 1.7 vs. 1.6 in month 2, 1.7 vs. 1.6 in month 4; ESI Table 6†). The nutritional supplementation significantly reduced the prevalence of lymphocytopenia (9.8% vs. 24.3% in week 1, 12.7% vs. 21.1% in week 2, 7.8% vs. 20.5% in month 2, 6.3% vs. 15.6% in month 6; Fig. 4C).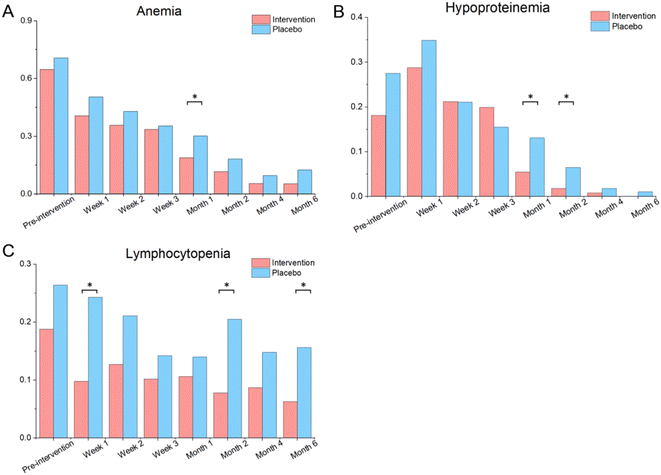 | ||
| Fig. 4 The incidence of anemia (A), hypoproteinemia (B) and lymphocytopenia (C) by treatment allocation. *P < 0.05, **P < 0.001. | ||
No patients died during the intervention. Sixty-eight adverse events were reported in the intervention group: 51 (29%) patients had nausea or vomiting, and 17 (9.7%) patients had diarrhea. Fifty-six adverse events were reported in the placebo group: 36 (23.5%) patients had nausea or vomiting, 15 (9.8%) patients had diarrhea, and 1 (0.7%) patient had rashes. The intervention was discontinued in the patient with rashes in the placebo group. The incidence of adverse events did not differ significantly between the two groups.
4. Discussion
The current double-blinded, placebo-controlled, randomized trial investigated the effects of a nutrition package, which contained both macronutrients and micronutrients, on the treatment response and nutritional status among tuberculosis patients. The intervention lasted for six months and a total of 329 tuberculosis patients were included in the final analyses. Our results indicated that the nutritional supplementation improved the clinical symptoms (e.g. chest pain, expectoration, and cough) and alleviated the undernutrition (e.g. anemia and hypoproteinemia) among tuberculosis patients. Additionally, the nutritional supplementation did not affect the sputum smear conversion in the whole population, but it did hasten the sputum smear conversion in non-cavitary tuberculosis patients.First, the current results indicated that nutritional supplementation improved the clinical symptoms (e.g. chest pain, expectoration, and cough) among tuberculosis patients. The incidence of chest pain and expectoration was 57% and 28% lower after one month of tuberculosis treatment in the intervention group than that in the placebo group. Chest pain, expectoration, and cough are typical symptoms of tuberculosis, which reflect the disease severity and influence the physical function and life quality of the tuberculosis patients. Nutritional supplementation may correct the undernutrition status of tuberculosis patients and boost the immune response to the disease. The nutrition package used in this study contained protein, carbohydrates, fat, vitamins, and minerals. Consistent with our results, three randomized controlled trials reported that vitamin D supplementation alleviated respiratory symptoms and facilitated the sputum conversion among tuberculosis patients.12,15,29 Two randomized controlled trials indicated a beneficial effect of arginine on tuberculosis symptoms.13,28 One randomized controlled trial indicated that multiple micronutrient supplementation reduced the risk of treatment failure and relapse.30 Additionally, we observed a more pronounced effect of nutritional supplementation on patients with a BMI less than 18.5, indicating a particular need for nutritional supplementation among this population.
Second, our results indicated that nutritional supplementation may facilitate sputum conversion among non-cavitary tuberculosis patients. Cavity is a border-lined, gas-filled space, which provides an immune-sheltered zone for bacterial growth.31 The cavity is poorly vascularized which limits the penetration of both drugs and nutrients. The high bacillary burden and limited penetration of nutrients in the cavity may partially explain the poor response of sputum conversion to nutritional supplementation in non-cavitary tuberculosis patients. The previous results of nutritional supplementation on sputum conversion were conflicting and this heterogeneity may be related to the cavitation.16 This needs to be investigated in future studies.
Third, our results indicated that the nutritional supplementation improved the nutritional status (e.g. reduced the prevalence of anemia and hypoproteinemia) among tuberculosis patients. The hemoglobin synthesis involves iron, folate and vitamin B12.32 The deficiency of these nutrients can lead to anemia.32 Additionally, protein deficiency is an important cause of hypoproteinemia.33 The correction of nutrition deficiency can reduce anemia and hypoproteinemia among this population. Consistently, previous clinical trials reported that food and macronutrient supplementation (a combination of protein, carbohydrate, and fat) improved the weight and physical function of tuberculosis patients8,10 and the micronutrient supplementation improved the hemoglobin and albumin levels in tuberculosis patients.34 The nutrition status is an important factor affecting the treatment outcome, tuberculosis recurrence, and long-term quality of life and mortality in tuberculosis patients.3
Fourth, our results also found that nutritional supplementation may improve the lymphocyte count among tuberculosis patients. Lymphocytes are crucial in the immune response to tuberculosis and their number and function are greatly affected by nutrients such as protein, Zn, Fe, and vitamins A, B6, C, D, and E.35 Consistent with our results, Ganmaa et al. and Martineau et al. separately reported an elevation of lymphocyte count in response to vitamin D supplementation.36,37 Additionally, Villamor et al. reported an elevation of CD3+ and CD4+ T cells in response to multiple micronutrient supplementation (a combination of retinal, vitamin B1, vitamin B2, vitamin B6, vitamin B12, vitamin C, vitamin E, folic acid, niacin, and selenium).30
During the study, 87 (26.4%) patients exhibited nausea or vomiting, 32 (9.7%) patients exhibited diarrhea, and 1 (0.3%) patient exhibited rash. No serious adverse event was observed. The incidence of adverse events did not differ significantly between the two groups. Gastrointestinal reactions and rashes are common adverse reactions of tuberculosis drugs.38 The higher incidence of adverse reactions in our study may be related to our use of structured questionnaires for the active detection of adverse reactions.
The advantages of this study include the following. First, this is one of the first randomized controlled trials which investigated the effect of a combination of macronutrients, minerals, and vitamins supplementation on tuberculosis patients. Previous clinical trials focused on the effects of micronutrient supplementation on tuberculosis patients, and only a few trials investigated the effects of macronutrients and food supplementations.16 Tuberculosis patients are typically wasting and facing multiple nutrient deficiencies. Our studies used a combination of protein, carbohydrate, fat, minerals, and vitamins and showed a beneficial effect on tuberculosis patients. Second, the current work is a strictly randomized, double-blinded, placebo-controlled trial with a sufficient sample size. Both the nutrition and placebo packages were manufactured by a third party and had the same appearance and taste. The patients and researchers did not know the treatment allocation until the end of the study. The disadvantages need to be acknowledged. First, we only followed up the tuberculosis patients for six months. A long-term follow-up may be helpful to investigate the effects of nutritional supplementation on tuberculosis recurrence and tuberculosis sequela, which warrants future studies. Second, the participants in this study were tuberculosis patients with concurrent prediabetes or diabetes. Diabetes and its medication may interfere with tuberculosis. The effect of nutritional supplementation in tuberculosis patients without prediabetes or diabetes may need further investigation. Third, the overall loss to follow-up rate of this trial is 6%. The relatively high loss to follow-up rate is partially related to the breakout of COVID-19 in this region. Fourth, the dosage of the nutrients used in this trial was based on the RNI for Chinese residents and may not be optimal for other populations.
In summary, the current double-blinded placebo-controlled randomized trial indicated a beneficial effect of a nutrition package (containing both macronutrients and micronutrients) on improving the clinical symptoms and nutritional status of tuberculosis patients during tuberculosis treatment. A routine adjunctive nutrition supplementation during tuberculosis treatment may be considered in the future.
Author contributions
AM, KX, YM, FZ, QW, YS and JW designed the study. AGM acquired the funding. JC and YY acquired the ethics permission. YY, KZ, ZL, YH, BZ, LX, XG, YX and TG conducted the trial and collected the data. WH, XY and KY performed laboratory assays and chest computed tomography. XW and JW conducted the data analysis. JW, XW and KX wrote the article. YY did the randomization. AM supervised the data collection, analysis and interpretation and reviewed the article. All authors approved the final version of the article.Data availability
Data will be made available upon request to the corresponding author with a methodologically sound proposal and a signed data access agreement.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This study was funded by the National Health Commission of China (spaq-2019–105) and the National Natural Science Foundation (818726101). The funders had no role in the design and conduct of the trial and writing of the manuscript.References
- K. Dheda, C. E. R. Barry and G. Maartens, Tuberculosis, Lancet, 2016, 387, 1211–1226 CrossRef.
- World Health Organization, Global tuberculosis report 2023, World Health Organization, Geneva, 2023 Search PubMed.
- P. Sinha, K. Lonnroth, A. Bhargava, S. K. Heysell, S. Sarkar, P. Salgame, W. Rudgard, D. Boccia, D. Van Aartsen and N. S. Hochberg, Food for thought: addressing undernutrition to end tuberculosis, Lancet Infect. Dis., 2021, 21, e318–e325 CrossRef.
- F. Xu, B. Ma, D. Wang, J. Lu, K. Xiong and J. Wang, Associating the blood vitamin A, C, D and E status with tuberculosis: a systematic review and meta-analysis of observational studies, Food Funct., 2022, 13, 4825–4838 RSC.
- World Health Organization, Nutritional care and support for patients with tuberculosis, World Health Organization, Geneva, 2013 Search PubMed.
- World Health Organization, Regional strategic plan towards ending TB in the WHO South-East Asia Region: 2021–2025, World Health Organization, Geneva, 2021 Search PubMed.
- T. Schön, D. Elias, F. Moges, E. Melese, T. Tessema, O. Stendahl, S. Britton and T. Sundqvist, Arginine as an adjuvant to chemotherapy improves clinical outcome in active tuberculosis, Eur. Respir. J., 2003, 21, 483–488 CrossRef PubMed.
- N. I. Paton, Y. K. Chua, A. Earnest and C. B. Chee, Randomized controlled trial of nutritional supplementation in patients with newly diagnosed tuberculosis and wasting, Am. J. Clin. Nutr., 2004, 80, 460–465 CrossRef CAS.
- C. Pérez-Guzmán, M. H. Vargas, F. Quiñonez, N. Bazavilvazo and A. Aguilar, A cholesterol-rich diet accelerates bacteriologic sterilization in pulmonary tuberculosis, Chest, 2005, 127, 643–651 CrossRef.
- N. Martins, P. Morris and P. M. Kelly, Food incentives to improve completion of tuberculosis treatment: randomised controlled trial in Dili, Timor-Leste, BMJ, 2009, 339, b4248 CrossRef PubMed.
- G. Jahnavi and C. H. Sudha, Randomised controlled trial of food supplements in patients with newly diagnosed tuberculosis and wasting, Singapore Med. J., 2010, 51, 957–962 CAS.
- A. P. Ralph, G. Waramori, G. J. Pontororing, E. Kenangalem, A. Wiguna, E. Tjitra, Sandjaja, D. B. Lolong, T. W. Yeo, M. D. Chatfield, R. K. Soemanto, I. Bastian, R. Lumb, G. P. Maguire, J. Eisman, R. N. Price, P. S. Morris, P. M. Kelly and N. M. Anstey, L-arginine and vitamin D adjunctive therapies in pulmonary tuberculosis: a randomised, double-blind, placebo-controlled trial, PLoS One, 2013, 8, e70032 CrossRef CAS.
- A. Farazi, O. Shafaat, M. Sofian and M. Kahbazi, Arginine adjunctive therapy in active tuberculosis, Tuberc. Res. Treat., 2015, 2015, 205016 Search PubMed.
- X. Xia, J. Zhang, X. Wang, K. Xiong, Z. Pan and J. Wang, Effects of vegetarian diets on blood lipids, blood glucose, and blood pressure: a systematic review and meta-analysis, Food Funct., 2024 10.1039/D4FO03449J.
- L. Tamara, C. B. Kartasasmita, A. Alam and D. A. Gurnida, Effects of Vitamin D supplementation on resolution of fever and cough in children with pulmonary tuberculosis: A randomized double-blind controlled trial in Indonesia, J. Global Health, 2022, 12, 04015 CrossRef.
- L. Grobler, S. Nagpal, T. D. Sudarsanam and D. Sinclair, Nutritional supplements for people being treated for active tuberculosis, Cochrane Database Syst. Rev., 2016, 6, Cd006086 Search PubMed.
- J. P. Biehl and R. W. Vilter, Effects of isoniazid on pyridoxine metabolism, J. Am. Med. Assoc., 1954, 156, 1549–1552 CrossRef CAS.
- Chinese Nutrition Society, Dietary Guidelines for Chinese Residents 2023, People's Health Publishing House, China, 2023 Search PubMed.
- J. J. Noubiap, J. R. Nansseu, U. F. Nyaga, J. R. Nkeck, F. T. Endomba, A. D. Kaze, V. N. Agbor and J. J. Bigna, Global prevalence of diabetes in active tuberculosis: a systematic review and meta-analysis of data from 2·3 million patients with tuberculosis, Lancet Global Health, 2019, 7, e448–e460 CrossRef PubMed.
- K. E. Dooley and R. E. Chaisson, Tuberculosis and diabetes mellitus: convergence of two epidemics, Lancet Infect. Dis., 2009, 9, 737–746 CrossRef PubMed.
- World Health Organization, Systematic Screening for Active Tuberculosis: Principles and Recommendations, World Health Organization, Geneva, 2013 Search PubMed.
- International Diabetes Federation, IDF Diabetes Atla, International Diabetes Federation, Belgium, 8th edn, 2017 Search PubMed.
- Chinese Center for Disease Control and Prevention, Guidelines for Tuberculosis Prevention and Control in China, People's Health Publishing House, China, 2021 Search PubMed.
- A. Gatta, A. Verardo and M. Bolognesi, Hypoalbuminemia, Intern. Emerg. Med., 2012, 7, 193–199 CrossRef.
- C. P. de Jager, P. T. van Wijk, R. B. Mathoera, J. de Jongh-Leuvenink, T. van der Poll and P. C. Wever, Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit, Crit. Care, 2010, 14, R192 CrossRef PubMed.
- World Health Organization, Haemoglobin concentration for the diagnosis of anaemia and assessment of severity, World Health Organization, Geneva, 2011 Search PubMed.
- A. P. Ralph, M. Ardian, A. Wiguna, G. P. Maguire, N. G. Becker, G. Drogumuller, M. J. Wilks, G. Waramori, E. Tjitra, Sandjaja, E. Kenagalem, G. J. Pontororing, N. M. Anstey and P. M. Kelly, A simple, valid, numerical score for grading chest X-ray severity in adult smear-positive pulmonary tuberculosis, Thorax, 2010, 65, 863–869 CrossRef PubMed.
- T. Schon, D. Elias, F. Moges, E. Melese, T. Tessema, O. Stendahl, S. Britton and T. Sundqvist, Arginine as an adjuvant to chemotherapy improves clinical outcome in active tuberculosis, Eur. Respir. J., 2003, 21, 483–488 CrossRef CAS.
- J. Wang, K. Xiong, Q. Wang, S. Zhao, Y. Liu and A. Ma, Adjunctive vitamin A and D during pulmonary tuberculosis treatment: a randomized controlled trial with a 2 × 2 factorial design, Food Funct., 2020, 11, 4762–4781 Search PubMed.
- E. Villamor, F. Mugusi, W. Urassa, R. J. Bosch, E. Saathoff, K. Matsumoto, S. N. Meydani and W. W. Fawzi, A trial of the effect of micronutrient supplementation on treatment outcome, T cell counts, morbidity, and mortality in adults with pulmonary tuberculosis, J. Infect. Dis., 2008, 197, 1499–1505 CrossRef PubMed.
- M. E. Urbanowski, A. A. Ordonez, C. A. Ruiz-Bedoya, S. K. Jain and W. R. Bishai, Cavitary tuberculosis: the gateway of disease transmission, Lancet Infect. Dis., 2020, 20, e117–e128 CrossRef CAS.
- C. D. Karakochuk, S. Y. Hess, D. Moorthy, S. Namaste, M. E. Parker, A. I. Rappaport, R. Wegmüller and O. Dary, Measurement and interpretation of hemoglobin concentration in clinical and field settings: a narrative review, Ann. N. Y. Acad. Sci., 2019, 1450, 126–146 CrossRef PubMed.
- B. R. Don and G. Kaysen, Serum albumin: relationship to inflammation and nutrition, Semin. Dial., 2004, 17, 432–437 CrossRef.
- S. Mehta, F. M. Mugusi, R. J. Bosch, S. Aboud, A. Chatterjee, J. L. Finkelstein, M. Fataki, R. Kisenge and W. W. Fawzi, A randomized trial of multivitamin supplementation in children with tuberculosis in Tanzania, Nutr. J., 2011, 10, 120 CrossRef CAS PubMed.
- P. Chandrasekaran, N. Saravanan, R. Bethunaickan and S. Tripathy, Malnutrition: Modulator of Immune Responses in Tuberculosis, Front. Immunol., 2017, 8, 1316 CrossRef.
- A. R. Martineau, P. M. Timms, G. H. Bothamley, Y. Hanifa, K. Islam, A. P. Claxton, G. E. Packe, J. C. Moore-Gillon, M. Darmalingam, R. N. Davidson, H. J. Milburn, L. V. Baker, R. D. Barker, N. J. Woodward, T. R. Venton, K. E. Barnes, C. J. Mullett, A. K. Coussens, C. M. Rutterford, C. A. Mein, G. R. Davies, R. J. Wilkinson, V. Nikolayevskyy, F. A. Drobniewski, S. M. Eldridge and C. J. Griffiths, High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial, Lancet, 2011, 377, 242–250 CrossRef CAS.
- D. Ganmaa, B. Munkhzul, W. Fawzi, D. Spiegelman, W. C. Willett, P. Bayasgalan, E. Baasansuren, B. Buyankhishig, S. Oyun-Erdene, D. A. Jolliffe, T. Xenakis, S. Bromage, B. R. Bloom and A. R. Martineau, High-Dose Vitamin D(3) during Tuberculosis Treatment in Mongolia. A Randomized Controlled Trial, Am. J. Respir. Crit. Care Med., 2017, 196, 628–637 CrossRef CAS PubMed.
- E. J. Forget and D. Menzies, Adverse reactions to first-line antituberculosis drugs, Expert Opin. Drug Saf., 2006, 5, 231–249 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo05172f |
| This journal is © The Royal Society of Chemistry 2025 |


