Substituting animal protein with black soymilk reduces advanced glycation end product level and improves gut microbiota composition in obese prediabetic individuals: a randomized crossover intervention trial†
Yu-Ho
Chang
 ab,
Pei-Ni
Lee
b,
Cheng-Hsu
Chen
cde,
Hsin-Yi
Yang
ab,
Pei-Ni
Lee
b,
Cheng-Hsu
Chen
cde,
Hsin-Yi
Yang
 f,
Chi-Hao
Wu
f,
Chi-Hao
Wu
 a,
Jia-Yau
Doong
f and
Wan-Ju
Yeh
a,
Jia-Yau
Doong
f and
Wan-Ju
Yeh
 *a
*a
aGraduate Program of Nutrition Science, National Taiwan Normal University, Taipei, Taiwan. E-mail: wandayeh@ntnu.edu.tw; Tel: +886-2-7749-6988
bDepartment of Nutrition, Taipei Hospital, Ministry of Health and Welfare, New Taipei City, Taiwan
cDepartment of Post-Baccalaureate Medicine, College of Medicine, National Chung Hsing University, Taichung, Taiwan
dDivision of Nephrology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan
eDepartment of Life Science, Tunghai University, Taichung, Taiwan
fDepartment of Nutritional Science, Fu Jen Catholic University, New Taipei City, Taiwan
First published on 26th November 2024
Abstract
Prediabetes (PreDM) and obesity increase the risk of type 2 diabetes. Individuals with these conditions often consume diets higher in animal protein than in plant protein, which are associated with elevated levels of dietary advanced glycation end products (dAGEs). Increased dAGE intake has been linked to blood glucose abnormalities, oxidative stress, and dysbiosis of the microbiota, all of which exacerbate metabolic disorders. Black soybeans, as a plant-based protein source, contain substantially lower levels of dAGEs compared with pork. This study aimed to investigate the effects of substituting animal protein with black soybeans on advanced glycation end product (AGE) levels, oxidative stress, and the gut microbiota in individuals with both PreDM and obesity. This study was a randomized crossover intervention trial conducted over 16 weeks. We recruited men and women aged 20–64 years with both prediabetes and obesity. This study had four periods: 0–4 weeks for the run-in period, 4–8 weeks and 12–16 weeks for the pork or black soymilk intervention period, and 8–12 weeks for the wash-out period. During the intervention period, the participants consumed pork or black soymilk with similar protein content as their dietary protein source. The participants maintained 3 day dietary records, and we measured anthropometric items and collected blood and fecal samples for analysis. The results showed that partially substituting pork with black soymilk as a dietary protein source for 4 weeks significantly reduced dAGE intake. The black soymilk group also exhibited significantly lower blood AGE fluorescence intensity, oxidative stress, and levels of glycative stress markers. Furthermore, black soymilk consumption significantly increased the relative abundance of short-chain fatty acid-producing genera compared with pork consumption. In conclusion, partially substituting dietary pork with black soymilk may reduce serum AGE levels, reduce oxidative and glycation stress, and increase the abundance of short-chain fatty acid-producing microbiota in individuals with both PreDM and obesity. Registration number of Clinical Trial: NCT05290519 (ClinicalTrials.gov).
1. Introduction
Being overweight or obese, while having prediabetes (PreDM), substantially increases the risk of developing type 2 diabetes (T2DM).1,2 Poor glycemic control, oxidative stress, inflammation, and gut microbiota dysbiosis accelerate this progression.2,3 Advanced glycation end products (AGEs), formed from reactions between reducing sugars and amino acids, can promote the development of PreDM.4 Individuals with PreDM have higher AGE levels relative to healthy individuals, and elevated AGE levels combined with oxidative stress create a vicious cycle that accelerates the progression to T2DM.4,5Higher animal protein intake is linked to increased insulin resistance and a higher risk of PreDM and T2DM.6–8 Animal-based proteins contain more dietary AGEs (dAGEs) than plant-based proteins like soybeans.9 Approximately 10% of dAGEs are absorbed into the bloodstream, with one-third excreted in urine within 48 hours, two-thirds remaining in tissues for about 24 hours, and 90% excreted in feces.10 Thus, long-term high AGE diets lead to AGE accumulation. Low dAGE diets significantly reduce blood malondialdehyde (MDA) levels in T2DM patients and may also impact gut microbiota diversity in renal failure patients.11,12 Therefore, dietary AGE content affects systemic AGE levels, oxidative stress, and gut microbiota composition.
Black soybeans, commonly cultivated in Asia, serve as a high-quality source of plant-based protein. Compared with yellow soybeans, black soybeans have seed coats that contain higher levels of anthocyanins.13 Studies show that soybean milk, with equivalent protein content, has lower dAGE levels compared to meat,9 and consuming black soybeans for 4 weeks significantly reduces oxidative stress in healthy adults.14In vitro research suggests that anthocyanin cyanidin-3-glucoside from black soybean coats inhibits the formation of fructosamine, indicating anti-glycation properties.15 Thus, the present study evaluates the effects of partially substituting dietary pork with black soymilk, at a constant caloric intake, on AGE levels, oxidative stress, and the gut microbiota in individuals with prediabetes and obesity.
2. Materials and methods
2.1 Participants
For this randomized crossover intervention trial, we recruited individuals with both prediabetes and obesity. The research protocol was approved by the Ethical Committee of National Taiwan Normal University (202008HM003) and registered on ClinicalTrials.gov under (NCT05290519). Informed consent was obtained from each participant before enrollment.Eligible participants were men or women aged between 20 and 64 years who consumed a non-vegetarian diet. The inclusion criteria were established in accordance with the Ministry of Health and Welfare's guidelines (Taiwan) for adult obesity: a body mass index (BMI) of between 27 and 35 kg m−2, or a waist circumference of ≥90 cm for men or ≥80 cm for women, and a fasting blood glucose concentration between 100 and 125 mg dL−1.
The exclusion criteria for the trial were having specific chronic conditions (e.g., hypertension, cardiovascular diseases, endocrine disorders, acute or chronic liver or kidney diseases, mental illnesses, or binge eating or anorexia) and being on long-term medication. We also excluded individuals who regularly consumed probiotics, prebiotic supplements, or antibiotics, engaged in weight loss activities in the month preceding the trial, or had allergies to pork or soy products.
2.2 Study design
The trial at National Taiwan Normal University had two intervention periods, with participants assigned to either a meat group or a black soymilk group. The trial spanned 16 weeks over four phases. The participants maintained 3 day 24 h dietary records every two weeks, and we conducted anthropometric assessments and collected blood and fecal samples before and after the interventions. The participants adhered to their customary diet throughout the run-in and wash-out periods. Subsequently, during intervention period 1, the participants were randomly assigned to either the meat group or the black soymilk group by a lottery conducted by the researcher. After intervention period 1, a wash-out period was imposed, after which the participants started intervention period 2 (Fig. 1). Throughout the trial period, the participants were required to maintain their customary level of physical activity.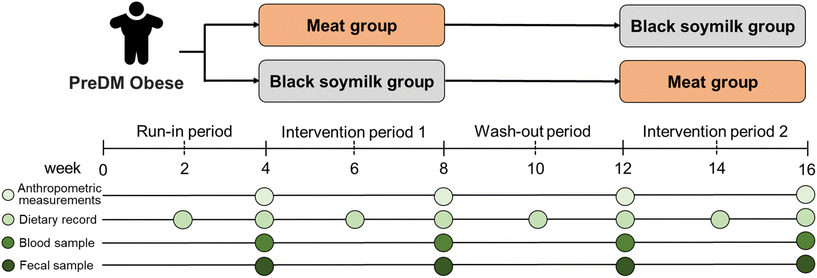 | ||
| Fig. 1 Randomized crossover study assessing effects of interventions involving meat and black soymilk consumption on individuals with prediabetes and obesity. | ||
2.3 Intervention
After reviewing the 3 day 24 h dietary records maintained by the participants during the run-in period, the researchers determined that the average daily caloric intake for all participants was 1800 kcal. Therefore, during the intervention periods, the participants were prescribed a daily caloric intake of 1800 kcal, with the proportions of daily total caloric intake allocated to carbohydrates, proteins, and fats set at 55%, 17%, and 28%, respectively.During the intervention periods, the participants were restricted to consuming protein-rich foods provided by the trial execution unit, with no allowance for additional protein-rich foods or dairy products. After the protein content in cereals, whole grains, and vegetables was accounted for, the participants could consume approximately 50 g of protein daily from protein-rich foods. The daily dietary regimen for the black soymilk group entailed consuming two bottles of no-sugar added black soymilk (Uni-Sunshine, Taiwan), each containing approximately 15.2 g of protein. Additionally, one 95 g packet of cooked low-fat pork contributed approximately 21 g of protein. Black soymilk served as a substitute for approximately 60% of the daily dietary protein intake by weight. The meat group participants each consumed three packets of cooked pork daily, which was prepared by the research team using dry-heat cooking methods, including frying, stir-frying, and grilling. Each daily serving of cooked pork comprised one 73.5 g packet of low-fat ground pork (providing 15 g of protein) and two 86 g packets of pork tenderloin (each providing 17 g of protein), which were distributed across three meals. The participants had to visit the research center weekly during the intervention period to obtain experimental samples (i.e., pork and black soymilk samples). They were required to return the sample packaging and record the time of consumption to ensure compliance with the experimental protocol.
2.4 Fluorescence intensity of AGEs in dietary and serum samples collected during the intervention periods
Analyses were conducted in accordance with the protocol outlined by Chen et al.16 Samples of cooked pork and black soymilk were freeze-dried using a freeze dryer to remove moisture, after which they were ground into powder. Approximately 0.05 g of food sample or 50 μL of serum sample was added to 250 μL of reducing borate buffer. The borate buffer and sodium borohydride were mixed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (pH 9.2) and allowed to react in the dark for 2 h. Subsequently, 400 μL of methanol (67-56-1, Genestar, Kuraray, USA), 100 μL of chloroform (67-66-3, Duksan Chemical, Seoul, Korea), and 300 μL of ddH2O were added and mixed thoroughly. Protein precipitation was performed, followed by centrifugation (Z326K, HERMLE, Germany) at 4000g and 25 °C to remove the supernatant. The pellet was hydrolyzed with 500 μL of 6 N hydrochloric acid (7647-01-0, J.T Baker, Radnor, USA) at 110 °C for 24 h. After the completion of the reaction, the samples were filtered through a 0.22 μm filter. Fluorescent AGEs were detected using a fluorescence spectrophotometer (F-7000, Hitachi, Japan). The AGE fluorescence was scanned as follows: vesperlysine A and B (Ex. 366 nm; Em. 442 nm), vesperlysine C (Ex. 345 nm; Em. 405 nm), pentosidine (Ex. 335 nm; Em. 385 nm), lysyl-pyrropyridine (Ex. 370 nm; Em. 448 nm), 2-(2-furoyl)-4(5)-(2-furanyl)-1H-imidazole (Ex. 380 nm; Em. 440 nm), argpyrimidine (Ex. 335 nm; Em. 400 nm), crossline (Ex. 379 nm; Em. 463 nm), and fluorolink (Ex. 380 nm; Em. 460 nm). Food samples were analyzed in triplicate from three independent samples, and blood samples were analyzed in duplicate.
1 ratio (pH 9.2) and allowed to react in the dark for 2 h. Subsequently, 400 μL of methanol (67-56-1, Genestar, Kuraray, USA), 100 μL of chloroform (67-66-3, Duksan Chemical, Seoul, Korea), and 300 μL of ddH2O were added and mixed thoroughly. Protein precipitation was performed, followed by centrifugation (Z326K, HERMLE, Germany) at 4000g and 25 °C to remove the supernatant. The pellet was hydrolyzed with 500 μL of 6 N hydrochloric acid (7647-01-0, J.T Baker, Radnor, USA) at 110 °C for 24 h. After the completion of the reaction, the samples were filtered through a 0.22 μm filter. Fluorescent AGEs were detected using a fluorescence spectrophotometer (F-7000, Hitachi, Japan). The AGE fluorescence was scanned as follows: vesperlysine A and B (Ex. 366 nm; Em. 442 nm), vesperlysine C (Ex. 345 nm; Em. 405 nm), pentosidine (Ex. 335 nm; Em. 385 nm), lysyl-pyrropyridine (Ex. 370 nm; Em. 448 nm), 2-(2-furoyl)-4(5)-(2-furanyl)-1H-imidazole (Ex. 380 nm; Em. 440 nm), argpyrimidine (Ex. 335 nm; Em. 400 nm), crossline (Ex. 379 nm; Em. 463 nm), and fluorolink (Ex. 380 nm; Em. 460 nm). Food samples were analyzed in triplicate from three independent samples, and blood samples were analyzed in duplicate.
2.5 Body composition
Waist circumference was measured at the midpoint between the lowest rib and the iliac crest, with both inhalation and exhalation accounted for. We used a body composition analyzer (Inbody 3.0, Biospace, Seoul, Korea) to assess body weight, skeletal muscle mass, and body fat mass. Before measurements were taken, the participants were asked to remove all metal accessories and electronic devices from their bodies. Their BMI was also calculated.2.6 Biochemical parameters
Before and after the interventions, we collected fasting blood samples from the participants. Fasting glucose levels were detected using a blood glucose meter (FreeStyle Precision Neo, Abbott Laboratories, IL, USA). The blood samples were then centrifuged at 4 °C and 1000g for 15 min (MEGAFUGE 8R, Thermo Scientific, Waltham, USA), and serum samples were collected for analysis. Fasting insulin, triglycerides (TG), cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine (Cr), blood urea nitrogen (BUN), and high-sensitivity C-reactive protein (hs-CRP) were analyzed using an autoanalyzer (AU5820, Beckman Coulter, Brea, California, USA). Homeostatic model assessment of insulin resistance (HOMA-IR) was conducted by applying the following equation: (fasting glucose [mg dL−1] × insulin [mU L−1])/405. Serum total antioxidant status (TAS) (HSTA001, Bio-Techne, Minneapolis, MN, USA), protein carbonyl (10005020, Cayman, Ann Arbor, MI, USA), soluble RAGE (sRAGE; RD191116200R, Biovendor, Brno, Czech Republic), and lipopolysaccharide-binding protein (LBP) (DY870-05, R&D, Minneapolis, MN, USA) levels were analyzed using commercial kits. Serum malondialdehyde (MDA) levels were measured using an established method.17 Adiponectin and leptin levels were measured using LEGENDplex multiplex assays (741063, BioLegend, San Diego, CA, USA) and detected using a flow cytometer (FACSCanto II, BD, NJ, USA).2.7 Dietary record and physical activity
The participants recorded a 3 day 24 h dietary record biweekly, documenting all food consumed during the days, including two weekdays and one weekend day. We used the Simplified Nutrition Calculation Table (developed by the Taiwan Dietitian Association) to compute the participants’ calorie and nutrient intake. Dietary AGE intake was calculated using the database established by Uribarri et al.;9 for foods not included in the database, estimates were made using similar categories and cooking methods. The participants were instructed to maintain their usual physical activity levels during the trial. We used the short form of the International Physical Activity Questionnaire to quantitatively assess metabolic equivalent (MET) levels, which were categorized as “low physical activity” (<600 MET-min per week), “moderate physical activity” (600–3000 MET-min per week), and “high physical activity” (>3000 MET-min per week).182.8 Gut microbiota analysis
The participants provided fresh stool samples collected in tubes containing DNA preservation solution. Stool DNA analysis was conducted using the methods from a previous study.19 DNA was isolated using NucleoSpin 96 (Macherey-Nagel, Düren, Germany), and sample disruption was performed using a Vortex-Genie 2. The V3–V4 region of bacterial 16S rDNA was amplified through polymerase chain reaction, tagged with Illumina adapters, and purified through agarose gel electrophoresis. Sequencing was conducted using the MiSeq Reagent Kit V3 (Illumina, USA) and an Illumina MiSeq sequencer. Next-generation sequencing was employed for sequence data analysis using USEARCH (version 10.0), mothur (version 1.38), and a database created by Clinical Microbiomics A/S. Sequence clustering with 97% similarity was used to generate operational taxonomic units. The Shannon diversity index was used to estimate community diversity levels. Alpha diversity indices were employed to assess the richness, evenness, and overall diversity of the microbial communities within each group.20 Beta diversity was used to compare differences in community composition between the different groups. Species were classified hierarchically using the RDP Classifier (v2.11) with a 0.8 threshold. Microbial community compositions were statistically analyzed and depicted in bar charts. Spearman coefficients were used to analyze the relationships of serum and dietary AGE level with microbial composition, and the results were visualized using a heatmap.2.9 Outcome
The primary outcome of this trial was the effect of black soymilk on serum AGE levels. The secondary outcomes were the effects of black soymilk on glycative stress-related parameters and microbiota composition.2.10 Statistical analysis
Given the lack of preliminary data and available research for estimating the expected intervention difference, the sample size was set at 15 participants per group to achieve a statistical power of 80% (type I error 20%, two-tailed t-test) for detecting a clinically meaningful difference (effect size of 0.8) in the primary outcome. Sample size calculations were performed using G*Power software (version 3.1).The results were reported in terms of the mean ± standard deviation. Normality for continuous variables was examined using the Shapiro–Wilk test. Group comparisons at baseline were performed using an independent sample t-test for normally distributed continuous variables or a Mann–Whitney U test for non-normally distributed continuous variables. Within-group differences (before and after intervention within the group) were analyzed using a paired t-test or Wilcoxon signed-rank test depending on data normality. Between-group differences (delta values between the meat group and the black soymilk group) were determined using an independent sample t-test or Mann–Whitney U test. Statistical analyses were performed using SPSS software (version 25, IBM Corporation, Armonk, NY, USA). Statistical significance was defined by a two-tailed p value of <0.05.
3. Results
3.1 Baseline characteristics and trial compliance
Sixteen participants were selected from 174 enrolled individuals and randomly assigned to either the meat group or the black soymilk group (Fig. 2). The 16 participants had an average age of 46 years and an average BMI of 29.6 kg m−2. Their weight averaged 80 kg, and their waist circumference averaged 98.8 cm for men and 92.2 cm for women. Fasting blood glucose levels averaged 111 mg dL−1, meeting the recruitment criteria (ESI Table 1†). One participant completed only one period of the experiment because of the COVID-19 pandemic.Throughout the trial, the participants’ average macronutrient intake comprised 46% carbohydrates, 17% protein, and 36% fat. The participants consumed less carbohydrates and more fat than planned, whereas their protein intake remained within the target range. Compliance was assessed through the timely submission of packaging corners, with all participants returning them punctually. The participants maintained a dietary record on a biweekly basis, exhibiting no significant disparities in caloric or macronutrient intake during the trial; this finding indicates good dietary adherence (ESI Table 2†).
3.2 Dietary records and physical activity assessments
Black soymilk and pork samples prepared using various cooking methods were assessed to determine the fluorescence intensity of various AGEs. The results indicated significantly lower AGE fluorescence intensity in black soymilk than in both raw and cooked ground pork and raw tenderloin (ESI Table 3†).The dietary assessment revealed no significant differences in calorie intake and macronutrient composition between the pork and the black soymilk intervention at baseline. Additionally, four weeks of the black soymilk intervention resulted in significant decreases in dAGE intake (p = 0.001) and dAGEs/energy (p = 0.001) compared to pre-intervention measures. Furthermore, compared to the pork intervention, the black soymilk intervention also yielded significant reductions in dAGE intake (p = 0.006) and dAGEs/energy (p = 0.001) after four weeks (Table 1).
| Meat | Black soymilk | Differences between groups | ||||||
|---|---|---|---|---|---|---|---|---|
| Before (n = 16) | After (n = 16) | Treatment P-value | Before (n = 15) | After (n = 15) | Treatment P-value | Δ mean (95% CI) | P-Value | |
| Values are presented as means ± standard deviations. A matched-pairs Wilcoxon signed-rank test or a paired t-test was performed to compare changes before and after the interventions (within-group variations). Between-group differences (delta values between the meat and black soymilk groups) were determined using an independent samples t-test or a Mann–Whitney U test. Before the intervention, an independent samples t-test or a Mann–Whitney U test was conducted (for comparisons between the meat and black soymilk groups). P < 0.05 indicates significance. Abbreviations: meat, meat group; black soymilk, black soymilk group; d, day; dAGEs, dietary advanced glycation end products; kU, kilounit; and MET, metabolic equivalent. | ||||||||
| Dietary intakes | ||||||||
| Energy (kcal d−1) | 1714 ± 570 | 1663 ± 395 | 0.623 | 1966 ± 640 | 1724 ± 260 | 0.058 | −190.3 (−446.2–85.6) | 0.169 |
| Carbohydrate (%) | 50 ± 4 | 44 ± 11 | 0.083 | 45 ± 10 | 48 ± 8 | 0.297 | 8.6 (0.9–16.4) | 0.031 |
| Protein (%) | 18 ± 5 | 18 ± 3 | 0.431 | 18 ± 5 | 19 ± 2 | 0.293 | 0.2 (−3.7–4.2) | 0.904 |
| Fat (%) | 34 ± 4 | 38 ± 9 | 0.162 | 36 ± 9 | 34 ± 5 | 0.254 | −6.1 (−12.9–0.6) | 0.073 |
| Carbohydrate (g d−1) | 214 ± 76 | 193 ± 80 | 0.085 | 222 ± 86 | 211 ± 61 | 0.363 | 10.7 (−20.3–41.7) | 0.487 |
| Protein (g d−1) | 77 ± 37 | 75 ± 11 | 0.570 | 87 ± 37 | 80 ± 16 | 0.753 | — | 0.737 |
| Fat (g d−1) | 65 ± 23 | 67 ± 7 | 0.698 | 78 ± 31 | 64 ± 9 | 0.164 | −16.2 (−34.8–2.3) | 0.083 |
| dAGE content (kU d−1) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 406 ± 7742 406 ± 7742 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 101 ± 1179 101 ± 1179 |
0.148 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 117 ± 6565 117 ± 6565 |
5911 ± 795 | 0.001 | — | 0.006 |
| dAGEs/energy (kU kcal−1) | 6 ± 4 | 8 ± 2 | 0.077 | 7 ± 2 | 4 ± 1 | 0.001 | −5.0 (−7.3 to −2.6) | 0.001 |
| Physical activity (MET-min per week) | 1045 ± 863 | 1204 ± 1164 | 0.776 | 1111 ± 1151 | 964 ± 754 | 0.510 | −305.7 (−906.5–295.0) | 0.307 |
The physical activity assessment indicated similar MET values between the meat and the black soymilk groups at pre-intervention. Subsequently, no significant differences in MET values were identified at post-intervention in both the pork and the black soymilk compared with their baseline (Table 1).
3.3 Anthropometric measurements, biochemical analyses, and serum AGE analysis
Anthropometric measurements indicated no significant differences in terms of the changes in weight, BMI, waist circumference, muscle mass, body fat percentage, and blood pressure between the meat and the black soymilk groups (ESI Table 4†). Furthermore, there were no significant differences in body weight and body composition throughout the study period (ESI Table 5†).Serum analysis showed no significant pre-intervention differences between the meat and the black soymilk groups in blood glucose, insulin, lipid profiles (TC, TG, HDL-C, and LDL-C), MDA, protein carbonyl, or liver and kidney function indicators (AST, ALT, Cr, and BUN). In the meat group, serum Cr levels increased significantly (p = 0.048) but remained within the normal range and protein carbonyl levels increased significantly (p = 0.007) at post-intervention. In the black soymilk group, serum MDA levels significantly decreased (p = 0.032) at post-intervention. Compared with 4 weeks of pork consumption, 4 weeks of black soymilk consumption significantly reduced MDA (p = 0.048) and protein carbonyl (p = 0.001) levels. Between the 4 week intervention involving pork consumption and that involving black soymilk consumption, no significant differences were identified in the serum levels of adiponectin, leptin, hs-CRP, or LBP, and the adiponectin–leptin ratio (Table 2).
| Meat | Black soymilk | Differences between groups | ||||||
|---|---|---|---|---|---|---|---|---|
| Before (n = 16) | After (n = 16) | Treatment P-value | Before (n = 15) | After (n = 15) | Treatment P-value | Δ mean (95% CI) | P-value | |
| Values are presented as means ± standard deviations. A matched-pairs Wilcoxon signed-rank test or a paired t-test was performed to compare changes before and after the interventions (within-group variations). Between-group differences (delta values between the meat and black soymilk groups) were determined using an independent samples t-test or a Mann–Whitney U test. Before the intervention, an independent samples t-test or a Mann–Whitney U test was conducted (for comparisons between the meat and black soymilk groups). P < 0.05 indicates significance. Abbreviations: meat, meat group; black soymilk, black soymilk group; HOMA-IR, homeostatic model assessment for insulin resistance; TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; Cr, creatinine; BUN, blood urea nitrogen; TAS, total antioxidant status; MDA, malondialdehyde; sRAGE, soluble receptor for advanced glycation end products; hs-CRP, high sensitivity C-reactive protein; and LBP, lipopolysaccharide-binding protein. | ||||||||
| Fasting blood glucose (mg dL−1) | 112.6 ± 8.8 | 108.2 ± 15.6 | 0.283 | 109.8 ± 7.2 | 113.5 ± 16.4 | 0.244 | 8.2 (−0.7–17.1) | 0.070 |
| Insulin (mU L−1) | 14.6 ± 10.6 | 15.8 ± 9.6 | 0.698 | 11.9 ± 6.6 | 14.8 ± 12.3 | 0.977 | — | 0.813 |
| HOMA-IR | 4.1 ± 3.2 | 4.4 ± 3.4 | 0.877 | 3.2 ± 1.8 | 4.3 ± 3.8 | 0.730 | — | 0.621 |
| TG (mg dL−1) | 146.4 ± 78.2 | 133.5 ± 53.0 | 0.489 | 150.6 ± 56.8 | 133.1 ± 55.7 | 0.160 | — | 0.418 |
| TC (mg dL−1) | 177.8 ± 29.9 | 174.6 ± 25.6 | 0.575 | 182.8 ± 35.6 | 175.1 ± 31.7 | 0.066 | −4.5 (−18.6–9.5) | 0.513 |
| HDL-C (mg dL−1) | 48.7 ± 9.9 | 50.2 ± 10.2 | 0.365 | 48.1 ± 10.8 | 50.9 ± 12.1 | 0.168 | 1.3 (−3.8–6.4) | 0.606 |
| LDL-C (mg dL−1) | 107.6 ± 27.9 | 108.6 ± 22.0 | 0.846 | 110.9 ± 29.9 | 113.3 ± 26.1 | 0.576 | 1.4 (−12.8–15.6) | 0.841 |
| AST (IU L−1) | 24.1 ± 12.7 | 20.6 ± 7.0 | 0.181 | 19.9 ± 5.3 | 20.1 ± 4.9 | 0.823 | — | 0.204 |
| ALT (IU L−1) | 32.4 ± 20.6 | 29.9 ± 23.0 | 0.320 | 28.3 ± 13.9 | 28.5 ± 13.2 | 0.972 | 2.6 (−4.6–9.8) | 0.460 |
| Cr (mg dL−1) | 0.78 ± 0.29 | 0.82 ± 0.31 | 0.048 | 0.83 ± 0.39 | 0.83 ± 0.32 | 0.909 | −0.05 (−0.12–0.03) | 0.199 |
| BUN (mg dL−1) | 14.0 ± 4.6 | 15.5 ± 5.7 | 0.244 | 15.7 ± 6.0 | 15.9 ± 6.7 | 0.755 | −1.1 (−3.7–1.5) | 0.379 |
| TAS (mM Trolox) | 1.5 ± 0.7 | 1.6 ± 0.9 | 0.640 | 1.4 ± 0.7 | 1.4 ± 1.4 | 0.572 | — | 0.527 |
| MDA (nmol mL−1) | 9.7 ± 2.9 | 10.8 ± 4.1 | 0.298 | 10.7 ± 3.3 | 9.2 ± 3.2 | 0.032 | — | 0.048 |
| Protein carbonyl (nmol mL−1) | 18.2 ± 3.2 | 20.9 ± 3.0 | 0.007 | 21.2 ± 5.6 | 17.8 ± 4.0 | 0.057 | −6.1 (−9.3 to −2.8) | 0.001 |
| sRAGE (pg mL−1) | 742.3 ± 322.1 | 662.9 ± 261.0 | 0.005 | 741.0 ± 267.4 | 686.9 ± 252.7 | 0.066 | 25.3 (−49.3–99.8) | 0.493 |
| Adiponectin (μg mL−1) | 30.8 ± 58.2 | 14.7 ± 10.0 | 0.148 | 14.6 ± 9.0 | 31.6 ± 68.4 | 0.820 | — | 0.213 |
| Leptin (ng mL−1) | 14.0 ± 5.4 | 13.6 ± 6.5 | 0.641 | 13.5 ± 6.8 | 13.5 ± 6.8 | 0.970 | 0.4 (−2.4–3.2) | 0.755 |
| Adiponectin–leptin ratio | 2.4 ± 4.1 | 1.2 ± 0.8 | 0.490 | 1.1 ± 0.7 | 1.9 ± 2.7 | 0.649 | — | 0.333 |
| hs-CRP (mg dL−1) | 0.29 ± 0.28 | 0.29 ± 0.23 | 0.796 | 0.28 ± 0.27 | 0.25 ± 0.20 | 0.691 | −0.01 (−0.09–0.06) | 0.698 |
| LBP (ng L−1) | 652.6 ± 149.0 | 667.8 ± 205.6 | 0.702 | 750.6 ± 216.7 | 699.9 ± 209.9 | 0.313 | −65.9 (−192.1–60.4) | 0.295 |
At pre-intervention, the participants exhibited no significant differences in the serum fluorescence intensities of the various types of AGEs. After the 4 week intervention involving pork consumption, significant increases in the serum fluorescence intensities of vesperlysine C (p = 0.006), pentosidine (p = 0.003), and argpyrimidine (p = 0.001) were identified. After the 4 week intervention involving black soymilk consumption, the fluorescence intensity of vesperlysine C significantly decreased (p = 0.047). Compared with the meat group, the black soymilk group exhibited significantly lower fluorescence intensities of vesperlysine C (p = 0.007), pentosidine (p = 0.009), lysyl-pyrropyridine (p = 0.024), argpyrimidine (p = 0.004), crossline (p = 0.038), and fluorolink (p = 0.038) in serum (Fig. 3). However, no significant difference in serum sRAGE level was identified (Table 2).
3.4 Gut microbiota analysis
We analyzed the gut microbiota on the basis of species richness by using the Shannon index for alpha diversity and principal component analysis for beta diversity. No significant differences were identified between the meat and black soymilk at post-intervention (Fig. 4a and b).Analysis of bacterial relative abundance at the genus level revealed that after the 4 week black soymilk intervention, the relative abundances of Butyricicoccus (p = 0.045) and Rothia (p = 0.013) significantly increased (Fig. 4d and e). Correlation results revealed that serum fluorescence AGE levels were negatively correlated with the relative abundances of Prevotella and Roseburia. By contrast, both dAGE/energy and serum AGE fluorescence intensity were positively correlated with the relative abundances of UCG-002 and Ruminococcus (Fig. 4f).
4. Discussion
In this randomized crossover trial, we recruited participants with both PreDM and obesity who did not have dyslipidemia and abnormal liver and kidney functions. The results showed that replacing approximately 60% of dietary animal protein with black soybean protein by weight for 4 weeks—without altering the total daily caloric intake—led to significant reductions in dAGE intake and serum AGE fluorescence intensity compared with a diet comprising meat as the protein source. Although body weight and composition were unaffected, oxidative and glycemic stress was significantly lower, and the abundance of short-chain fatty acid (SCFA)-producing bacteria was substantially increased. Asian dietary practices typically involve meat cooking methods that result in higher dAGEs. This study proposes that consuming two bottles of black soymilk per day is a practical approach consistent with Asian dietary habits and has not been previously studied.4.1 Effects of replacing dietary meat with black soymilk on dAGE and systemic AGE levels
In previous studies where healthy adult participants were provided foods cooked using high-temperature dry heat (high dAGEs) or steaming (low dAGEs), serum AGE levels were significantly lower in the low dAGE group than in the high dAGE group after 4–6 weeks.21,22 This indicates that decreased dAGE intake leads to a decrease in AGE accumulation. Previous studies have indicated that individuals with obesity and prediabetes prefer consuming animal-based protein foods and meals with higher levels of AGEs.8,23,24 Animal proteins contain a considerable amount of furosine, an amino acid found in cooked meat products and other processed foods that is an early-stage product of the Maillard reaction.25,26 According to the Simplified Nutrition Calculation Table (developed by the Taiwan Dietitian Association), meat with 100 grams of protein contains more lysine than soybeans. Studies also indicate that animal-based foods high in protein and fat are rich in lysine and arginine, which are potential targets for glycation.27,28Suantawee et al. reported the protective effects of anthocyanin against methylglyoxal and glucose-induced protein glycation and oxidative DNA damage.29 Therefore, consuming black soymilk reduces serum AGE fluorescence intensity, suggesting that the anthocyanins in black soybean seed coats contribute to the anti-glycation effects. sRAGE functions as a decoy receptor that reduces the binding of AGEs to RAGE, thereby mitigating oxidative stress and inflammation.30 However, higher serum sRAGE levels may also indicate overstimulation of cell surface RAGE, which, if persistent, can exacerbate pro-inflammatory processes and worsen pathological conditions. Given the inconsistent findings across different clinical studies, further research is needed to validate the use of serum sRAGE as a marker for glycative stress and disease progression.31
4.2 Effects of replacing dietary meat with black soymilk on oxidative stress, glycative stress, and inflammation
Hyperglycemia accelerates the formation of AGEs, which bind to RAGE receptors, increasing oxidative stress and inflammation.32,33 In an intervention trial, a 6 week low dAGE diet for T2DM patients reduced serum AGE fluorescence intensity and blood MDA levels compared to a normal diet,11 suggesting that reducing dAGE intake lessens AGE accumulation and mitigates oxidative stress. In an animal study, rats on a high-fat diet treated with soy isoflavone extract for 4 weeks showed decreased colonic MDA and blood protein carbonyl levels, along with increased antioxidant activities.34 Additionally, human trials with anthocyanin supplements daily for 12 weeks also reported reduced serum MDA levels.35 In this study, we found that the no-sugar-added black soymilk, which is rich in anthocyanins, showed a higher antioxidant capacity than yellow soymilk, although both had lower antioxidant activity compared to Trolox (ESI Table 6†). The results indicated that the 4 week black soymilk intervention resulted in significantly lower oxidative and glycative stress compared to the pork intervention. This finding may be related to reduced dietary AGE intake and the increased consumption of soy isoflavones or anthocyanins. Overweight and obesity can increase oxidative stress.36 A previous study indicated that participants on a low-AGE diet for 2 weeks had reduced dAGE and urinary AGE excretion without weight change.37 In the present study, substituting pork with black soymilk while maintaining the usual caloric intake did not significantly affect body weight or composition. Thus, these decreases in oxidative stress are likely to be independent of changes in body weight.Visceral adipose tissue secretes adipokines such as adiponectin and leptin, which play crucial roles in regulating glucose metabolism, insulin sensitivity, and inflammation.38 Adiponectin levels are typically lower in obesity and possess anti-inflammatory effects, while leptin levels are elevated and could promote inflammation.39–41 The adiponectin–leptin ratio serves as an indicator of adipose dysfunction and is negatively correlated with inflammation.42,43 In this study, the black soybean milk intervention for 4 weeks increased the adiponectin–leptin ratio, although hs-CRP levels did not change significantly. In contrast, the pork intervention resulted in a decrease in this ratio. These findings suggest that replacing animal protein with black soybean milk may help reduce low-grade inflammation in obese prediabetic individuals.
4.3 Effects of replacing dietary meat with black soymilk on inflammation and the gut microbiota
In individuals with obesity, an imbalance in the gut microbiota can impair barrier function and increase intestinal permeability, allowing lipopolysaccharides (LPSs) into the bloodstream, where LBP binds to LPS and activates TLR4 to trigger inflammation.44,45 Higher levels of blood LBP are associated with increased trunk fat and insulin resistance in preDM.46 Butyrate, essential for gut barrier integrity, binds to G protein-coupled receptor 43 and SLC5A8, regulating tight junction proteins and potentially reducing low-grade inflammation in obesity.47–49 Previous studies have reported that a short-term reduction in dAGE intake lowers circulating AGEs in patients undergoing peritoneal dialysis and affects their gut microbiota composition.12 Animal studies indicate that high-AGE diets decrease butyrate-producing bacteria,50,51 while feeding rats freeze-dried soymilk increases SCFA-producing bacteria,52 and soybean oligosaccharides boost Bifidobacterium and Lactobacillus.53Butyricicoccus, a butyrate-producing genus,54 is less abundant in rats with type 1 DM, and this lower abundance is correlated with inflammation and faster disease progression;55 by contrast, Rothia abundance is positively correlated with fecal butyrate levels.56 In the present study, a 4 week black soymilk intervention reduced dAGE intake and serum AGE fluorescence intensity and increased the relative abundances of Butyricicoccus and Rothia, and the serum AGE fluorescence intensity was negatively correlated with the levels of the SCFA-producing genera Prevotella and Roseburia.57,58The inflammatory marker hs-CRP is higher in individuals with T2DM than those with PreDM.59 Studies showed that patients with T2DM have a significantly higher abundance of Collinsella, which is linked to increased serum cholesterol and atherosclerosis.60 This suggests that T2DM involves greater inflammatory responses and gut microbiota dysbiosis compared to PreDM. In the present study, we noted no significant differences in serum inflammatory markers or blood LBP levels before versus after the interventions. Although the level of hs-CRP was elevated in individuals with both preDM and obesity compared with healthy individuals, it remained within the normal range.61,62 Thus, substituting pork with black soymilk for 4 weeks in individuals with both preDM and obesity can increase gut butyrate-producing bacteria, potentially mitigating tight junction injury and exacerbating inflammation.
4.4 Strengths and limitations
Previous studies on dAGE intake effects required specific cooking methods, such as steaming or consuming raw foods, to minimize dAGE formation.21,37 Although these methods ensure rigorous experimental control, they can be challenging for an individual to maintain over the long term in their everyday life. In the present study, participants replaced approximately 60% of their dietary pork protein with black soymilk while maintaining their usual caloric intake and healthy eating pattern. This approach effectively reduced dAGE intake and serum AGE fluorescence intensity. Additionally, this trial is the first to focus on individuals with both preDM and obesity, suggesting that recommending a low dAGE diet for these individuals may become more feasible in the future.The present study also has several limitations. (1) Its sample size is small. The focus on examining the effects of dAGEs from a disease prevention perspective required recruiting individuals with both preDM and obesity, and the narrow range of blood glucose levels in this group resulted in fewer eligible participants. (2) The recruitment and testing processes were affected by the severe COVID-19 pandemic, which restricted the locations for recruitment and reduced participant willingness. (3) It was challenging to fully control for the potential effects of gender and age on the study outcomes.
5. Conclusion
Replacing approximately 60% of animal protein from pork with plant-based protein from black soymilk in the daily diet of individuals with both preDM and obesity can reduce dietary AGE intake as well as systemic AGE levels, glycative stress, and oxidative stress. Additionally, this dietary change increases the relative abundance of butyrate-producing bacteria. Therefore, partially incorporating black soymilk as a protein source in the diet of omnivorous individuals with both preDM and obesity may have beneficial effects.Author contributions
Conceptualization, project administration, supervision, and project administration: Wan-Ju Yeh. Funding acquisition: Pei-Ni Lee, Hsin-Yi Yang, and Wan-Ju Yeh. Formal analysis, investigation, methodology, and visualization: Yu-Ho Chang, Cheng-Hsu Chen, and Wan-Ju Yeh. Writing – original draft: Yu-Ho Chang, Pei-Ni Lee, and Wan-Ju Yeh. Writing – review & editing: Yu-Ho Chang, Hsin-Yi Yang, Chi-Hao Wu, Jia-Yau Doong, and Wan-Ju Yeh.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare that they have no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.Acknowledgements
This work was supported by grants from Taipei Hospital, Ministry of Health and Welfare (grant number: 202203), and the Ministry of Science and Technology (grant number: 110–2320-B-003–003). The authors would like to express their gratitude to Uni-Sunshine (Taiwan) for providing the no-sugar-added black soymilk used in their research.References
- B. Ahmed, R. Sultana and M. W. Greene, Adipose tissue and insulin resistance in obese, Biomed. Pharmacother., 2021, 137, 111315, DOI:10.1016/j.biopha.2021.111315.
- S. Alvarez, R. Coffey, P. M. Mathias and A. M. Algotar, Prediabetes, 2024, Treasure Island, FL, PMID: 29083606 Search PubMed.
- D. W. Lam and D. LeRoith, Metabolic syndrome, in Endotext, 2000, South Dartmouth, MA, PMID: 25905173 Search PubMed.
- T. Jiang, Y. Zhang, F. Dai, C. Liu, H. Hu and Q. Zhang, Advanced glycation end products and diabetes and other metabolic indicators, Diabetol. Metab. Syndr., 2022, 14(1), 104, DOI:10.1186/s13098-022-00873-2.
- K. Nowotny, T. Jung, A. Hohn, D. Weber and T. Grune, Advanced glycation end products and oxidative stress in type 2 diabetes mellitus, Biomolecules, 2015, 5(1), 194–222, DOI:10.3390/biom5010194.
- Z. Chen, O. H. Franco, S. Lamballais, M. A. Ikram, J. D. Schoufour, T. Muka and T. Voortman, Associations of specific dietary protein with longitudinal insulin resistance, prediabetes and type 2 diabetes: The Rotterdam Study, Clin. Nutr., 2020, 39(1), 242–249, DOI:10.1016/j.clnu.2019.01.021.
- B. Azemati, S. Rajaram, K. Jaceldo-Siegl, J. Sabate, D. Shavlik, G. E. Fraser and E. H. Haddad, Animal-protein intake is associated with insulin resistance in Adventist Health Study 2 (AHS-2) calibration substudy participants: a cross-sectional analysis, Curr. Dev. Nutr., 2017, 1(4), e000299, DOI:10.3945/cdn.116.000299.
- E. Viguiliouk, S. E. Stewart, V. H. Jayalath, A. P. Ng, A. Mirrahimi, R. J. de Souza, A. J. Hanley, R. P. Bazinet, S. Blanco Mejia, L. A. Leiter, R. G. Josse, C. W. Kendall, D. J. Jenkins and J. L. Sievenpiper, Effect of replacing animal protein with plant protein on glycemic control in diabetes: A systematic review and meta-analysis of randomized controlled trials, Nutrients, 2015, 7(12), 9804–9824, DOI:10.3390/nu7125509.
- J. Uribarri, S. Woodruff, S. Goodman, W. Cai, X. Chen, R. Pyzik, A. Yong, G. E. Striker and H. Vlassara, Advanced glycation end products in foods and a practical guide to their reduction in the diet, J. Am. Diet. Assoc., 2010, 110(6), 911–916, DOI:10.1016/j.jada.2010.03.018.
- T. Koschinsky, C. J. He, T. Mitsuhashi, R. Bucala, C. Liu, C. Buenting, K. Heitmann and H. Vlassara, Orally absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy, Proc. Natl. Acad. Sci. U. S. A., 1997, 94(12), 6474–6479, DOI:10.1073/pnas.94.12.6474.
- C. Luévano-Contreras, M. E. Garay-Sevilla, K. Wrobel, J. M. Malacara and K. Wrobel, Dietary advanced glycation end products restriction diminishes inflammation markers and oxidative stress in patients with type 2 diabetes mellitus, J. Clin. Biochem. Nutr., 2013, 52(1), 22–26, DOI:10.3164/jcbn.12-40.
- R. Yacoub, M. Nugent, W. Cai, G. N. Nadkarni, L. D. Chaves, S. Abyad, A. M. Honan, S. A. Thomas, W. Zheng, S. A. Valiyaparambil, M. A. Bryniarski, Y. Sun, M. Buck, R. J. Genco, R. J. Quigg and J. C. He, Advanced glycation end products dietary restriction effects on bacterial gut microbiota in peritoneal dialysis patients; a randomized open label controlled trial, PLoS One, 2017, 12(9), e0184789, DOI:10.1371/journal.pone.0184789.
- Y. Yamashita, H. Sakakibara, T. Toda and H. Ashida, Insights into the potential benefits of black soybean (Glycine max L.) polyphenols in lifestyle diseases, Food Funct., 2020, 11(9), 7321–7339, 10.1039/d0fo01092h.
- Y. Yamashita, A. Nakamura, F. Nanba, S. Saito, T. Toda, J. Nakagawa and H. Ashida, Black soybean improves vascular function and blood pressure: A randomized, placebo controlled, crossover trial in humans, Nutrients, 2020, 12(9), 2755, DOI:10.3390/nu12092755.
- G. Prasanna and P. Jing, Cyanidin-3-O-glucoside functions like chemical chaperone and attenuates the glycation mediated amyloid formation in albumin, Arch. Biochem. Biophys., 2018, 643, 50–56, DOI:10.1016/j.abb.2018.02.012.
- J. Y. Chen, G. C. Yen, N. T. Tsai and J. A. Lin, Risk and benefit of natural and commercial dark brown sugars as evidenced by phenolic and Maillard reaction product contents, J. Agric. Food Chem., 2021, 69(2), 767–775, DOI:10.1021/acs.jafc.0c04795.
- D. Tsikas, Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges, Anal. Biochem., 2017, 524, 13–30, DOI:10.1016/j.ab.2016.10.021.
- P. H. Lee, D. J. Macfarlane, T. H. Lam and S. M. Stewart, Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review, Int. J. Behav. Nutr. Phys. Act., 2011, 8(1), 115, DOI:10.1186/1479–5868–8–115.
- C. Iribarren, M. K. Magnusson, L. K. Vigsnæs, I. Aziz, I. D. Amundsen, T. Šuligoj, N. Juge, P. Patel, M. Sapnara, L. Johnsen, N. Sørensen, J. Sundin, H. Törnblom, M. Simrén and L. Öhman, The effects of human milk oligosaccharides on gut microbiota, metabolite profiles and host mucosal response in patients with irritable bowel syndrome, Nutrients, 2021, 13(11), 3836, DOI:10.3390/nu13113836.
- T. Andermann, A. Antonelli, R. L. Barrett and D. Silvestro, Estimating alpha, beta, and gamma diversity through deep learning, Front. Plant Sci., 2022, 13, 839407, DOI:10.3389/fpls.2022.839407.
- I. Birlouez-Aragon, G. Saavedra, F. J. Tessier, A. Galinier, L. Ait-Ameur, F. Lacoste, C. N. Niamba, N. Alt, V. Somoza and J. M. Lecerf, A diet based on high-heat-treated foods promotes risk factors for diabetes mellitus and cardiovascular diseases, Am. J. Clin. Nutr., 2010, 91(5), 1220–1226, DOI:10.3945/ajcn.2009.28737.
- R. Semba, S. K. Dgebauer, D. J. Baer, K. Sun, R. Turner, H. A. Silber, S. Talegawkar, L. Ferrucci and J. A. Novotny, Dietary intake of advanced glycation end products did not affect endothelial function and inflammation in healthy adults in a randomized controlled trial, J. Nutr., 2014, 144(7), 1037–1042, DOI:10.3945/jn.113.189480.
- Y. Wang and M. A. Beydoun, Meat consumption is associated with obesity and central obesity among US adults, Int. J. Obes., 2009, 33(6), 621–628, DOI:10.1038/ijo.2009.45.
- N. P. Mendes, F. G. Candido, F. X. Valente, M. Peluzio, L. L. Juvanhol and R. C. G. Alfenas, Dietary advanced glycation end products, body composition, and anthropometric measures: A cross-sectional analysis in women with excess body weight, Nutr., Metab. Cardiovasc. Dis., 2024, 34(7), 1721–1730, DOI:10.1016/j.numecd.2024.03.011.
- N. Aljahdali and F. Carbonero, Impact of Maillard reaction products on nutrition and health: Current knowledge and need to understand their fate in the human digestive system, Crit. Rev. Food Sci. Nutr., 2019, 59(3), 474–487, DOI:10.1080/10408398.2017.1378865.
- J. L. Scheijen, E. Clevers, L. Engelen, P. C. Dagnelie, F. Brouns, C. D. Stehouwer and C. G. Schalkwijk, Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database, Food Chem., 2016, 190, 1145–1150, DOI:10.1016/j.foodchem.2015.06.049.
- G. Münch, D. Schicktanz, A. Behme, M. Gerlach, P. Riederer, D. Palm and R. Schinzel, Amino acid specificity of glycation and protein–AGE crosslinking reactivities determined with a dipeptide SPOT library, Nat. Biotechnol., 1999, 17, 1006–1010, DOI:10.1038/13704.
- M. Mahmoudinezhad, M. A. Farhangi, H. Kahroba and P. Dehghan, Personalized diet study of dietary advanced glycation end products (AGEs) and fatty acid desaturase 2 (FADS2) genotypes in obesity, Sci. Rep., 2021, 11(1), 19725, DOI:10.1038/s41598-021-99077-3.
- T. Suantawee, H. Cheng and S. Adisakwattana, Protective effect of cyanidin against glucose- and methylglyoxal-induced protein glycation and oxidative DNA damage, Int. J. Biol. Macromol., 2016, 93(PtA), 814–821, DOI:10.1016/j.ijbiomac.2016.09.059.
- K. Prasad and M. Mishra, AGE-RAGE stress, stressors, and antistressors in health and disease, Int. J. Angiol., 2018, 27(1), 1–12, DOI:10.1055/s-0037-1613678.
- J. D. Erusalimsky, The use of the soluble receptor for advanced glycation-end products (sRAGE) as a potential biomarker of disease risk and adverse outcomes, Redox Biol., 2021, 42, 101958, DOI:10.1016/j.redox.2021.101958.
- H. Vlassara and J. Uribarri, Advanced glycation end products (AGE) and diabetes: Cause, effect, or both?, Curr. Diabetes Rep., 2014, 14, 1–10, DOI:10.1007/s11892-013-0453-1.
- C. Sharma, A. Kaur, S. S. Thind, B. Singh and S. Raina, Advanced glycation end-products (AGEs): An emerging concern for processed food industries, J. Food Sci. Technol., 2015, 52(12), 7561–7576, DOI:10.1007/s13197–015–1851−y.
- Q. Luo, D. Cheng, C. Huang, Y. Li, C. Lao, Y. Xia, W. Liu, X. Gong, D. Hu, B. Li, X. He and Z. Chen, Improvement of colonic immune function with soy isoflavones in high-fat diet-induced obese rats, Molecules, 2019, 24(6), 1139, DOI:10.3390/molecules24061139.
- H. Zhang, Z. Xu, H. Zhao, X. Wang, J. Pang, Q. Li, Y. Yang and W. Ling, Anthocyanin supplementation improves anti-oxidative and anti-inflammatory capacity in a dose-response manner in subjects with dyslipidemia, Redox Biol., 2020, 32, 101474, DOI:10.1016/j.redox.2020.101474.
- I. Perez-Torres, V. Castrejon-Tellez, M. E. Soto, M. E. Rubio-Ruiz, L. Manzano-Pech and V. Guarner-Lans, Oxidative stress, plant natural antioxidants, and obesity, Int. J. Mol. Sci., 2021, 22(4), 1786, DOI:10.3390/ijms22041786.
- B. de Courten, M. P. de Courten, G. Soldatos, S. L. Dougherty, N. Straznicky, M. Schlaich, K. C. Sourris, V. Chand, J. L. Scheijen, B. A. Kingwell, M. E. Cooper, C. G. Schalkwijk, K. Z. Walker and J. M. Forbes, Diet low in advanced glycation end products increases insulin sensitivity in healthy overweight individuals: A double-blind, randomized, crossover trial, Am. J. Clin. Nutr., 2016, 103(6), 1426–1433, DOI:10.3945/ajcn.115.125427.
- S. Milić, D. Lulić and D. Štimac, Non-alcoholic fatty liver disease and obesity: Biochemical, metabolic and clinical presentations, World J. Gastroenterol., 2014, 20(28), 9330–9337, DOI:10.3748/wjg.v20.i28.9330.
- I. Jialal, B. Adams-Huet, F. Duong and G. Smith, Relationship between retinol-binding protein-4/adiponectin and leptin/adiponectin ratios with insulin resistance and inflammation, Metab. Syndr. Relat. Disord., 2014, 12(4), 227–230, DOI:10.1089/met.2014.0013.
- G. Biondi, N. Marrano, A. Borrelli, M. Rella, G. Palma, I. Calderoni, E. Siciliano, P. Lops, F. Giorgino and A. Natalicchio, Adipose tissue secretion pattern influences β-cell wellness in the transition from obesity to type 2 diabetes, Int. J. Mol. Sci., 2022, 23(10), 5522, DOI:10.3390/ijms23105522.
- R. V. Considine, M. K. Sinha, M. L. Heiman, A. Kriauciunas, T. W. Stephens and M. R. Nyce, et al., Serum immunoreactive-leptin concentrations in normal-weight and obese humans, N. Engl. J. Med., 1996, 334(5), 292–295, DOI:10.1056/NEJM199602013340503.
- D. Lettieri-Barbato, E. Giovannetti and K. Aquilano, Effects of dietary restriction on adipose mass and biomarkers of healthy aging in human, Aging, 2016, 8(12), 3341–3355, DOI:10.18632/aging.101122.
- G. Frühbeck, V. Catalán, A. Rodríguez and J. Gómez-Ambrosi, Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk, Adipocytez, 2018, 7(1), 57–62, DOI:10.1080/21623945.2017.1402151.
- R. Medzhitov and T. Horng, Transcriptional control of the inflammatory response, Nat. Rev. Immunol., 2009, 9(10), 692–703, DOI:10.1038/nri2634.
- A. E. Mohr, M. Crawford, P. Jasbi, S. Fessler and K. L. Sweazea, Lipopolysaccharide and the gut microbiota: Considering structural variation, FEBS Lett., 2022, 596(7), 849–875, DOI:10.1002/1873-3468.14328.
- C. M. Tilves, J. M. Zmuda, A. L. Kuipers, C. S. Nestlerode, R. W. Evans, C. H. Bunker, A. L. Patrick and I. Miljkovic, Association of lipopolysaccharide-binding protein with aging-related adiposity change and prediabetes among African ancestry men, Diabetes Care, 2016, 39(3), 385–391, DOI:10.2337/dc15–1777.
- H. M. Hamer, D. Jonkers, K. Venema, S. Vanhoutvin, F. J. Troost and R. J. Brummer, Review article: the role of butyrate on colonic function, Aliment. Pharmacol. Ther., 2008, 27(2), 104–119, DOI:10.1111/j.1365–2036.2007.03562.x.
- S. C. Chang, M. H. Shen, C. Y. Liu, C. M. Pu, J. M. Hu and C. J. Huang, A gut butyrate-producing bacterium Butyricicoccus pullicaecorum regulates short-chain fatty acid transporter and receptor to reduce the progression of 1,2-dimethylhydrazine-associated colorectal cancer, Oncol. Lett., 2020, 20(6), 327, DOI:10.3892/ol.2020.12190.
- L. K. Brahe, A. Astrup and L. H. Larsen, Is butyrate the link between diet, intestinal microbiota and obesity-related metabolic diseases?, Obes. Rev., 2013, 14(12), 950–959, DOI:10.1111/obr.12068.
- R. Mastrocola, D. Collotta, G. Gaudioso, M. Le Berre, A. S. Cento, G. Ferreira Alves, F. Chiazza, R. Verta, I. Bertocchi, F. Manig, M. Hellwig, F. Hava, C. Cifani, M. Aragno, T. Henle, L. Joshi, K. Tuohy and M. Collino, Effects of exogenous dietary advanced glycation end products on the cross-talk mechanisms linking microbiota to metabolic inflammation, Nutrients, 2020, 12(9), 2497, DOI:10.3390/nu12092497.
- J. Wang, W. Cai, J. Yu, H. Liu, S. He, L. Zhu and J. Xu, Dietary advanced glycation end products shift the gut microbiota composition and induce insulin resistance in mice, Diabetes, Metab. Syndr. Obes., 2022, 15, 427–437, DOI:10.2147/DMSO.S346411.
- S. M. Lee, H. W. Han and S. Y. Yim, Beneficial effects of soy milk and fiber on high cholesterol diet-induced alteration of gut microbiota and inflammatory gene expression in rats, Food Funct., 2015, 6(2), 492–500, 10.1039/c4fo00731j.
- Y. Ma, X. Wu, V. Giovanni and X. Meng, Effects of soybean oligosaccharides on intestinal microbial communities and immune modulation in mice, Saudi J. Biol. Sci., 2017, 24(1), 114–121, DOI:10.1016/j.sjbs.2016.09.004.
- A. Geirnaert, A. Steyaert, V. Eeckhaut, B. Debruyne, J. B. Arends, F. Van Immerseel, N. Boon and T. Van de Weile, Butyricicoccus pullicaecorum, a butyrate producer with probiotic potential, is intrinsically tolerant to stomach and small intestine conditions, Anaerobe, 2014, 30, 70–74, DOI:10.1016/j.anaerobe.2014.08.010.
- Q. Ma, Y. Li, J. Wang, P. Li, Y. Duan, H. Dai, Y. An, L. Cheng, T. Wang, C. Wang, T. Wang and B. Zhao, Investigation of gut microbiome changes in type 1 diabetic mellitus rats based on high-throughput sequencing, Biomed. Pharmacother., 2020, 124, 109873, DOI:10.1016/j.biopha.2020.109873.
- D. Li, Y. Li, W. Dai, H. Wang, C. Qiu, S. Feng, Q. Zhou, W. Wang, X. Feng, K. Yao, Y. Liu, Y. Yang, Z. Yang, X. Xu, S. Li, J. Wei and K. Zhou, Intestinal Bacteroides sp. imbalance aasociated with the occurrence of childhood undernutrition in China, Front. Microbiol., 2019, 10, 2635, DOI:10.3389/fmicb.2019.02635.
- K. Nie, K. Ma, W. Luo, Z. Shen, Z. Yang, M. Xiao, T. Tong, Y. Yang and X. Wang, Roseburia intestinalis: A beneficial gut organism from the discoveries in genus and species, Front. Cell. Infect. Microbiol., 2021, 11, 757718, DOI:10.3389/fcimb.2021.757718.
- P. Portincasa, L. Bonfrate, M. Vacca, M. De Angelis, I. Farella, E. Lanza, M. Khalil, D. Q. Wang, M. Sperandio and A. Di Ciaula, Gut microbiota and short chain fatty acids: Implications in glucose homeostasis, Int. J. Mol. Sci., 2022, 23(3), 1105, DOI:10.3390/ijms23031105.
- Z. Wang, X. H. Shen, W. M. Feng, G. F. Ye, W. Qiu and B. Li, Analysis of inflammatory mediators in prediabetes and newly diagnosed type 2 diabetes patients, J. Diabetes Res., 2016, 2016, 7965317, DOI:10.1155/2016/7965317.
- X. Zhang, D. Shen, Z. Fang, Z. Jie, X. Qiu, C. Zhang, Y. Chen and L. Ji, Human gut microbiota changes reveal the progression of glucose intolerance, PLoS One, 2013, 8(8), e71108, DOI:10.1371/journal.pone.0071108.
- A. Ghule, T. K. Kamble, D. Talwar, S. Kumar, S. Acharya, A. Wanjari, S. A. Gaidhane and S. Agrawal, Association of serum high sensitivity C-reactive protein with pre-diabetes in rural population: A two-year cross-sectional study, Cureus, 2021, 13(10), e19088, DOI:10.7759/cureus.19088.
- H. Alzamil, Elevated serum TNF-α is related to obesity in type 2 diabetes mellitus and is associated with glycemic control and insulin resistance, J. Obes., 2020, 2020, 5076858, DOI:10.1155/2020/5076858.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo04717f |
| This journal is © The Royal Society of Chemistry 2025 |



