Effect of proanthocyanidins on cognitive improvement in thyroxin-induced aging mice
Chong
Yuan†
ac,
Hongtao
Ren†
ac,
Kexin
Hu
ac,
Linlin
Chen
abc,
Ke
Yue
bc,
Kunmiao
He
bc,
Qiuying
Yu
 *abc,
Na
Wang
*abc and
Gaiping
Zhang
bc
*abc,
Na
Wang
*abc and
Gaiping
Zhang
bc
aZhengzhou Key Laboratory of Nutrition and Health Food, College of Food Science and Technology, Henan Agricultural University, Zhengzhou 450002, China. E-mail: yuqiuyingzf@163.com
bInternational Joint Research Center of National Animal lmmunology, College of Veterinary Medicine, Henan Agricultural University, Zhengzhou 450046, China. E-mail: na-wang@163.com
cLonghu Laboratory, Zhengzhou 450046, China
First published on 9th December 2024
Abstract
As the population ages, functional dietary supplements are increasingly used to reduce age-related diseases, especially in the field of cognitive impairment. In this study, a thyroxine (Th)-induced aging model was established, and the effect of proanthocyanidins (Pc) on cognitive impairment of aging mice was evaluated based on cognitive ability, neuroinflammation and immune level. The results showed that Pc significantly reduced AchE activity compared to the Model group, improving learning deficits and spatial memory in aged mice (P < 0.01). Further study showed that Pc could maintain the organism's redox balance, markedly increasing T-AOC, GSH, and SOD levels (P < 0.01) while reducing MPO and MDA levels (P < 0.01). Pc also improved systemic inflammation, raising the levels of the anti-inflammatory cytokine PF4 and significantly lowering pro-inflammatory factors in the blood (P < 0.01). In the DG region of the hippocampus, Pc effectively repaired nerve damage, inhibited the over-activation of microglia and astrocytes, down-regulated GFAP and IBA-1 proteins (P < 0.01), and then reduced neuroinflammation. Additionally, Pc supplementation also significantly increased the levels of WBC, Lymph, Mid, and Gran in aged mice (P < 0.01), aiding in the recovery of leukocyte counts. At the same time, the CD3+ level and CD4+/CD8+ ratio were significantly increased (P < 0.01) to maintain the dynamic balance of lymphocyte subsets in aging mice and enhance the immune capacity of aging mice. The study revealed that Pc, as a dietary supplement, can effectively alleviate cognitive impairment in the elderly population. This provides a new dietary nutrition supplement strategy for the health of the aging population.
1 Introduction
In recent decades, the number of elderly people has increased significantly, and the World Health Organization estimates that by 2050, the proportion of the world's elderly population is expected to account for 20%.1 This demographic shift is accompanied by an increase in age-related cognitive decline, manifesting in conditions such as memory loss, dementia, and Alzheimer's disease (AD).2 The growing burden of caring for individuals with cognitive impairments poses significant challenges, both for families and public health systems.3Currently, the primary clinical treatments for cognitive impairment include medications such as tacrine, donepezil, galantamine, and memantine, which function by inhibiting N-methyl-D-aspartate receptors or targeting acetylcholinesterase.4,5 However, due to the irreversibility of cognitive degradation in the aging process, these drugs cannot completely treat cognitive impairment. Moreover, prolonged use of these medications often leads to diminished efficacy and various side effects that exacerbate the metabolic burden on older adults.6 Therefore, exploring safer and more efficient long-term prevention strategies has become the focus of many researchers. Recent studies have shown that reasonable dietary combination has a significant impact on promoting human health and preventing the occurrence and development of age-related diseases.7,8 A normal diet combined with dietary supplements can play a role in cognitive protection and improvement without changing the original dietary pattern. Many research studies have found that naturally occurring compounds with immune-boosting and antioxidant properties can be used as potential nutritional supplements to mitigate cognitive impairment.9–12 For example, quercetin has been shown to enhance learning and cognitive memory by promoting NK cell maturation and reducing amyloid-β accumulation in the hippocampus of AD mice.13 Similarly, Zhang et al. found that apple polysaccharide (AP) could maintain the structural integrity of hippocampal tissue and reduce age-related cognitive dysfunction in aging mice.14
Procyanidins (Pc), a class of naturally occurring polyphenols found in plants like peanuts, grape seeds, and others, have demonstrated immune-stimulating and cognitive-enhancing effects,15 as well as potential in preventing neurodegenerative diseases.16,17 Research by Li et al. and Gao et al. suggests that Pc-rich grape seed extract can maintain AKT and ERK activity, repair synaptic structures in the hippocampus, and prevent oxidative stress related to aging in the brain.18,19 These findings are particularly relevant for the elderly with mild cognitive impairments. At present, the improvement effect of natural ingredients such as Pc on cognitive impairment is mainly focused on the development of new drugs and the study of the mechanism of drug action. However, the research on the prevention and improvement of cognitive impairment in the elderly population as a dietary supplement is still insufficient.
In this study, the potential of Pc as a dietary supplement to alleviate thyroxine (Th)-induced cognitive impairment in mice was investigated based on three aspects of cognitive ability, neuroinflammation and immune response through dietary supplementation. This provides a basis for evaluating the potential of Pc as a safe and convenient dietary supplement to prevent and alleviate the development of cognitive impairment in the elderly population.
2 Materials and methods
2.1 Experimental materials
Pc was sourced from Solepol Technology Co., Ltd (Beijing, China), which was extracted from grape seeds and the purity was greater than 95%, Th was obtained from Renhe Pharmaceuticals (China). Kits for glutathione peroxidase activity (GSH), myeloperoxidase activity (MPO), and acetylcholinesterase (AchE) activity were supplied by Solarbio Co., Ltd (Beijing, China). Superoxide dismutase (SOD), malondialdehyde (MDA), and total antioxidant capacity (T-AOC) kits were procured from Nanjing Jianjian Co., Ltd (Nanjing, China). ELISA kits for Interleukin (IL)-1β, Interleukin (IL)-6, Tumor Necrosis Factor (TNF)-α, Mononuclear Chemokine (MCP)-1, and Platelet Factor (PF)-4 were provided by NeoBioscience Technology Co., Ltd (China). Anti-Mouse CD3-APC, CD4-FITC, and CD8-PE antibodies for flow cytometry were supplied by Lianke Bio Co., Ltd (China).2.2 Experimental animals
Female BALB/c mice, aged six weeks and weighing an average of 20 ± 2 grams, were obtained from the Laboratory Animal Centre of Zhengzhou University. The mice were raised under a 12/12 h light/dark cycle with controlled humidity (50 ± 5%) and ambient temperature (25 ± 2 °C). The animals were acclimated before the experiment and provided with food and water ad libitum throughout the study.2.3 Establishment of an aging mouse model
After a one-week acclimation period, the mice were randomly divided into six groups (n = 9) and treated using a modified method based on Feng et al.:20Control group (NC): received daily gavage of distilled water for 8 weeks.
Model group (Model): administered Th at 320 mg kg−1 by gavage daily for 8 weeks.
Pc prophylaxis group (prevent): given Pc at 100 mg kg−1 by gavage daily, followed by Th at 320 mg kg−1 by gavage 8 hours later for 8 weeks.
Pc supplementation groups (low, medium, high doses: Pc-L, Pc-M, Pc-H): received Th at 320 mg kg−1 daily, followed by Pc at 50 mg kg−1, 100 mg kg−1 and 200 mg kg−1 by gavage 8 hours later for 8 weeks. The experimental flow is shown in Fig. 1A.
2.4 Behavioral experiments
2.5 Histopathological sections
At the end of the eighth week, brain tissues were collected from euthanized mice and preserved in 10% neutral formalin. Then, the tissues were washed with PBS, embedded in paraffin, and cut into sections of 5 μm thickness. Hematoxylin and eosin staining was performed, with hematoxylin staining nuclei blue-purple and eosin staining the cytoplasm pink. After dehydration, sections were examined under a microscope.2.6 Immunofluorescence staining
Paraffin-embedded brain tissue sections were washed three times with PBS and incubated with the blocking solution containing 10% goat serum (NGS) and 3% Triton X-100 for 1 h at room temperature. Sections were then incubated with primary antibodies against glial fibrillary acidic protein (GFAP) and ionized calcium-binding adapter molecule 1 (IBA-1) diluted for 12 h at 4 °C. Following three PBS rinses, sections were incubated with diluted secondary antibodies for 1 h at room temperature. After additional PBS washes, sections were stained with 4′,6-diamidino-2-phenylindole (DAPI) for 10 min to visualize nuclei. Finally, brain sections were examined and imaged using a fluorescence microscope, with consistent acquisition parameters for all images.2.7 Real-time fluorescence quantitative PCR (RT-qPCR)
Total RNA was extracted from hippocampal tissues using the Trizol method, and RNA concentration was measured with a Nanodrop. Following the manufacturer's instructions, 1 μg RNA sample was reverse transcribed into cDNA. RT-qPCR was performed using SYBR qPCR Master Mix and specific primers. The thermal cycle conditions were as follows: initial denaturation at 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s, 60 °C for 30 s, and 65 °C for 30 s. Primer sequences synthesized by Sangon Biotech Co., Ltd (Shanghai) are listed in Table 1.| Name | Forward primers (5′–3′) | Reverse primers (5′–3′) |
|---|---|---|
| IL-1β | TCGCAGCAGCACATCAACAAG | TAGCCCTCCATTCCTGAAAGC |
| IL-6 | GAGAGGAGACTTCACAGAGGATACC | TCATTTCCACGATTTCCCAGAGAAC |
| TNF-α | CACGCTCTTCTGTCTACTGAACTTC | CTTGGTGGTTTGTGAGTGTGAGG |
| MCP-1 | CTACTATTCCTGATGGCACTTCTCTTG | GAACCTCTGTCCGTGATGATCTTC |
| PF4 | TGGTCCCGAAGAAAGCGATGG | GGCTGGTGATGTGCTTAAGATGG |
| β-Actin | TGGCATTGTTACCAACTGGGAC | TCACGGTTGGCCTTAGGGTTC |
2.8 Whole blood cytometry
20 μL of fresh peripheral blood was collected from the mouse eyes using capillary glass tubes and placed in anticoagulated EP tubes. The samples were diluted with whole blood diluent, and then analyzed using a whole blood cell analyzer.2.9 Serum biochemical test
At the end of the eighth week, the mice were fasted for 4 h. 100 μL blood sample was collected from the mouse eyes using capillary glass tubes and centrifuged at 4000 rpm for 15 min at 4 °C to isolate the serum. The levels of GSH, T-AOC, SOD, MPO, MDA, and AchE in the serum were measured using a biochemical analysis kit.2.10 Enzyme-linked immunosorbent assay (ELISA)
The serum was obtained by centrifuging mouse blood at 4000 rpm for 15 min at 4 °C. The levels of PF-4, TNF-α, IL-1β, and IL-6 in the serum were measured using ELISA kits. The absorbance was detected at 450 nm with an enzyme-labeled instrument.2.11 Flow cytometry experiment
100 μL of anticoagulated blood was mixed with 5 μL of Anti-Mouse CD3-APC, CD4-FITC, and CD8-PE antibodies, respectively. After incubation for 30 min at 4 °C in the dark, 1 mL of erythrocyte lysate was added and kept away from light for 10 min. The mixture was then centrifuged at 4500 rpm for 15 min at 4 °C, and the supernatant was discarded. The remaining cell pellet was resuspended in 500 μL PBS and analyzed using a flow cytometer.2.12 Statistical analysis
All the data were expressed as mean ± SE from at least three independent experiments. Differences between groups were measured using one-way analysis of variance (ANOVA) with SPSS software. Bar charts and line charts were drawn using the Graphpad Prism software. Flow cytometry data were analyzed using CytExpert software, and the animal behavior and locomotor trajectories were analyzed with DeepLabCut. The relative fluorescence intensity of immunofluorescence images was determined using ImageJ software.3 Results
3.1 Establishment of an aging mouse model
Studies have shown that long-term excessive intake of Th could increase the metabolic rate of mice, disrupt the redox balance, and accelerate the aging process in mice.21 Compared with the NC group, after 8 weeks of continuous Th administration, mice in the Model group exhibited significantly higher oxidative stress levels, specifically T-AOC, GSH and SOD were significantly decreased (P < 0.01) and MPO and MDA levels were significantly increased (P < 0.01), indicating that the aging mouse model was successfully established.3.2 Effects of Pc on the cognitive level in aging mice
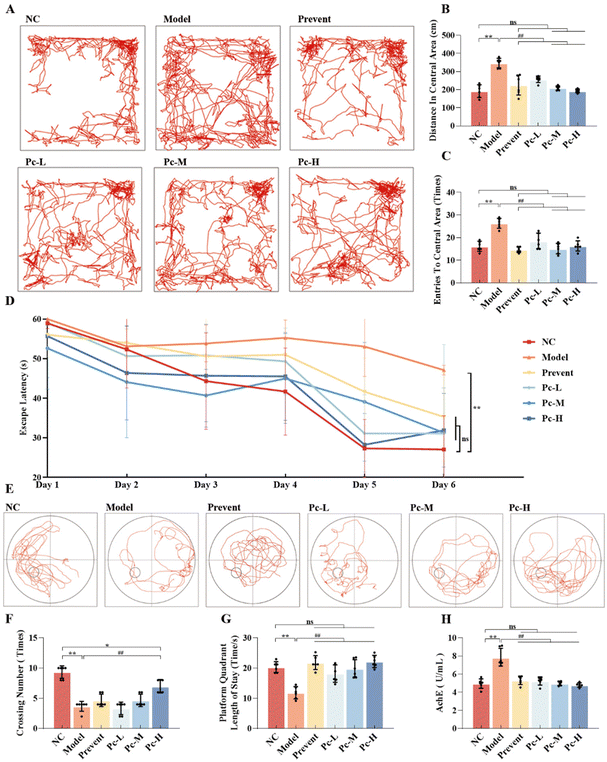
To evaluate the cognitive function of senescent mice, the Morris water maze and open field experiments were conducted. Results from the open field test revealed that compared with the NC group, the Model group had worse cognitive ability to the new environment in the open field, and the staying time and moving distance in the central area were significantly increased (P < 0.01). After Pc treatment, the behavior of the mice improved, and the movement trajectories were closer to those of healthy controls. In addition, the mice treated with Pc also showed a significant reduction in both central zone entries and distance traveled within the zone (P < 0.01). The results indicate that dietary Pc supplementation can significantly improve the spatial memory ability of aging mice in the open field (Fig. 2A–C).The Morris water maze experiment was used to further assess the effect of Pc on the learning and memory capabilities of the aging mice. As shown in Fig. 2D, mice in the Model group showed worse learning ability in the first 5 days of spatial exploration, took a longer time to find the platform and exhibited prolonged escape latency (P < 0.01). After Pc supplementation, the learning and memory abilities were significantly improved, and the escape latency was significantly reduced (P < 0.01). With the extension of the training cycle, the mice treated with Pc presented better learning ability. On the sixth day (Fig. 2E–G), the Model group demonstrated a poor performance in the localization cruise experiment, with fewer platform crossings and shorter dwell times in the target quadrant (P < 0.01). However, Pc-treated mice showed a marked increase in both dwell time and platform crossings (P < 0.01), alongside more reasonable movement patterns during exploration. The results further reveal that Pc supplementation can significantly slow down the cognitive impairment process of aging mice.
Given that acetylcholine (Ach), a neurotransmitter essential for proper brain signaling, is directly linked to cognitive function, the AchE activity was measured in aging mice. Fig. 2H shows that the activity of AchE in the serum of the Model group was significantly higher than that of the control group (P < 0.01), indicating that the neurotransmission pathway of aging mice was affected. After Pc intervention, the AchE activity was significantly down-regulated (P < 0.01), suggesting that the nerve conduction and the normal cognitive function in aging mice were repaired.
In summary, Pc supplementation could significantly enhance spatial memory and learning capacity in the cognitively challenged mice and demonstrate positive neuromodulation effects.
3.3 Effects of Pc on nerve damage in aging mice
The hippocampus, a critical brain region for cognitive function, is particularly vulnerable to age-related tissue deterioration. H&E staining of hippocampal tissues revealed that the nerve cells in the DG region of mice in the Model group were scattered, the number was reduced, and the structure was loose and incomplete, and some nerve nuclei were shrunk and stained deeply, indicating that aging caused damage to the hippocampus nerve tissue of mice, resulting in cognitive impairment. In contrast, Pc-treated groups exhibited a more intact hippocampal structure, with neatly arranged nerve cells, round nuclei, and lighter staining (Fig. 3A). The results indicate that Pc supplementation could repair nerve damage in hippocampal tissue of aging mice to a certain extent.The results of immunofluorescence staining showed that the relative fluorescence intensity of GFAP and IBA-1 proteins in the hippocampal DG region of Model group mice was significantly stronger than that in the NC group (P < 0.01), indicating that microglia and astrocytes were over-activated. After Pc intervention, the relative fluorescence intensity of GFAP and Iba-1 protein decreased significantly (P < 0.05) and remained at the normal level (Fig. 3B–D). The results demonstrate that Pc supplementation could inhibit the overactivation of microglia and astrocytes and slow down the occurrence of nerve damage.
Studies have shown that overexpression of GFAP and IBA-1 proteins is linked to neuroinflammation, which leads to cognitive impairment.22 The results showed that compared with the NC group, the expressions of pro-inflammatory factors IL-1β, IL-6, TNF-α and MCP-1 in the hippocampus of the Model group were significantly increased (P < 0.01), and the expressions of the anti-inflammatory factor PF4 were significantly decreased (P < 0.01) which indicated that the level of neuroinflammation is higher in the model group. Pc intervention significantly down-regulated the mRNA levels of pro-inflammatory (P < 0.01) and up-regulated the level of PF4 (P < 0.01), thereby reducing the neuroinflammation (Fig. 3E–I).
These results suggest that Pc supplementation can significantly improve the hippocampal tissue damage of mice with cognitive impairment, inhibit the overactivation of microglia and astrocytes, and then reduce the neuroinflammation in the brain of mice with cognitive impairment.
3.4 Effects of Pc on oxidative stress and systemic inflammation in aging mice
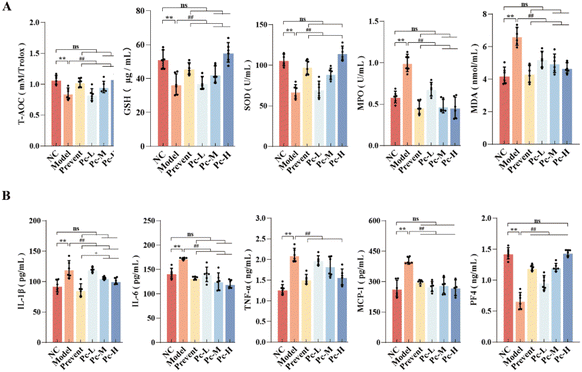
Oxidative stress is an important factor that leads to the decline of cognitive function. With age, the ability of the antioxidant system is weakened, and the accumulation of free radicals will cause neuronal damage. Fig. 4A shows that Pc supplementation could significantly increase the levels of T-AOC, GSH, and SOD in aging mice (P < 0.01) and significantly decrease the levels of MPO and MDA (P < 0.01). These findings suggest that Pc supplementation could reduce the oxidative stress and maintain the redox balance in aging mice.Aging can lead to a redox imbalance, resulting in the accumulation of metabolites in the blood and tissues, thus disrupting the immune balance of the original microenvironment, and eventually causing chronic inflammation. Inflammatory mediators can act on the brain through the blood–brain barrier, leading to cognitive decline. As shown in Fig. 4B, compared with the NC group, the serum levels of pro-inflammatory factors IL-1β, IL-6, TNF-α and MCP-1 in the Model group were significantly increased (P < 0.01), and the levels of the anti-inflammatory factor PF4 were significantly decreased, indicating a higher level of systemic chronic inflammation in the Model group. Pc supplementation could significantly reduce the levels of pro-inflammatory (P < 0.01) and increase the PF4 levels, suggesting that Pc could mitigate systemic inflammation and oxidative stress. Furthermore, preventive PC supplementation was more effective than dietary supplementation in reducing chronic inflammation.
3.5 Effect of Pc on leukocyte deficiency in aging mice
With the age increasing, the body's immune system gradually weakens, affecting the effectiveness of the immune response and increasing the risk of infection and inflammation, which can exacerbate the cognitive impairment. Immune senescence is characterized by a decrease in the number and function of immune cells and an increase in inflammatory factors that affect cognitive function. The results of routine blood tests revealed that the white blood cells (WBC), lymphocytes (Lymph), intermediate cells (Mid), and granulocytes (Gran) in the Model group were significantly decreased (P < 0.01), indicating the presence of immune aging in mice (Fig. 5A–E). Pc supplementation significantly alleviated the symptoms of leukopenia in aging mice, maintaining the normal levels of WBC, Lymph, Mid, and Gran (P < 0.01). Additionally, the preventive PC supplementation was more effective than dietary supplementation in maintaining of the Mid levels.3.6 Effect of Pc on lymphocyte subpopulations in aging mice
In contrast to the NC group, the flow cytometry analysis results revealed a significant decrease in the CD3+ levels (P < 0.01) and the CD4+/CD8+ ratios (P < 0.01) in aging mice (Fig. 6A–D). Pc intervention significantly enhanced the CD3+ level and the CD4+/CD8+ ratio in aging mice, and the results showed that Pc supplementation improved the immune ability of aging mouse lymphocytes and maintained the lymphocyte function.4 Discussion
In recent years, population aging has become an increasingly serious social problem, and cognitive impairment has a high probability of occurrence in the elderly population, which seriously affects the quality of life of the elderly population and has become a globally recognized medical and health problem.23 At present, most of the research on cognitive impairment focuses on the irreversible situation before it occurs, and attempts to reduce the risk factors of cognitive impairment from the perspective of prevention and delay of the process have gradually become a research focus. In this study, the effects of Pc on cognitive dysfunction were evaluated by intervention and prevention of aging mice with dietary supplementation of Pc. We found that Pc could enhance the anti-inflammatory ability of the body by improving the balance of immune cell subsets, such as the CD3+ level and the CD4+/CD8+ ratio, and this immune regulation provided the basis for subsequent neuroprotection. Since neuroinflammation is a key factor in aging related cognitive decline, Pc could reduce neuroinflammation, especially microglia and astrocytes, by regulating the body's immune capacity, thus promoting the repair of nerve damage. In addition, oxidative stress is closely related to immune response, and excessive oxidative damage will not only exacerbate inflammation, but also lead to neurological dysfunction. As an antioxidant, Pc could significantly improve the level of oxidative stress by enhancing the activity of antioxidant enzymes (such as T-AOC, GSH and SOD) and reducing oxidative stress markers (such as MPO and MDA), effectively protect the nervous system from oxidative damage, and further promote the recovery of cognitive function.Dietary adjustment represents a gentle intervention for the elderly and has broad prospects in the prevention and mitigation of cognitive impairment. Numerous studies have demonstrated that natural active compounds as dietary supplements can improve the development of cognitive impairment.24,25 Studies have shown that curcumin could improve cognitive impairment and alleviate neuronal metabolic dysfunction in amyloid preprotein transgenic mice via the Thrb/SIRT3 axis.26 Resveratrol (RES) has been found to involve in the regulation of neurotransmitters and oxidative stress, alleviating LPS-induced neuroinflammation, improving cognitive impairment and episodic memory, which might play a role through the PI3K-Akt pathway according to network pharmacological analysis.27 In this study, Pc supplementation could enhance the spatial memory and learning ability of cognitively impaired mice and significantly improve the course of action, residence time and crossing times of aging mice in open field and water maze tests. In addition, the study found that prophylactic Pc supplementation had better cognitive protection in mice. These results indicate that Pc has potential preventive and ameliorative effects on the development of cognitive impairment in aging individuals.
Neuroinflammation, occurring with the damage of nerve cells and tissues, plays a crucial role in the pathogenesis of cognitive impairment in the elderly. Many natural active components have been explored as dietary supplements and therapeutic agents to mitigate neuroinflammatory responses in neurodegenerative disease models.28–31 It is found that corydalis (CH), a traditional Chinese medicine in plateau areas, has potential antioxidant and anti-inflammatory effects and can be used in the treatment of neuroinflammation.32 It has been reported that natural cinnamaldehyde (CA) and its derivatives have also been suggested to improve the synaptic architecture and prevent oxidative stress in models of Alzheimer's and Parkinson's diseases.33 Additionally, studies have shown that dietary supplementation of inulin can enhance synaptic plasticity, maintain the blood–brain barrier integrity, and reduce neuroinflammation through CREB/BDNF signaling.34 Pc from grape seed has been suggested to alleviate DSS-induced colitis in mice by modulating the gut flora composition and reducing oxidative stress and inflammatory cytokines.35 Pc plays a cognition improving role by reducing the levels of neuroinflammation. Specifically speaking, the findings in this study demonstrated that Pc supplementation could repair neural tissues and reduce neuroinflammation by restoring hippocampal tissue, decreasing microglial and astrocyte hyperactivation, and down-regulating the expression of inflammatory factors IL-1β, IL-6, TNF-α, and MCP-1.
In the context of aging, an increase in reactive oxygen species leads to oxidative damage in cells and tissues, which can activate intracellular signaling pathways and promote the release of inflammatory factors. At the same time, the inflammatory mediators produced during the inflammatory process can also promote the production of reactive oxygen species, thus aggravating oxidative stress.36,37 At the same time, further oxidative stress has an impact on the structure, function and production of immune cells, leading to immune aging.38 The immune system plays a vital role in maintaining neurocognitive function.39 As individuals age, aging leads to the loss or overactivation of the innate immune system, causing chronic inflammation throughout the body that accumulates. Leukopenia, a hallmark of immune aging, is thought to contribute significantly to neurological impairment in the elderly.40 Neutrophil immune aging weakens interactions with the adaptive immune system, leading to a decline in immunity. In addition, the reduction of CD4+/CD8+ ratio is one of the important markers of T cell “immune aging”.41 Natural substances with immunomodulatory properties are increasingly being investigated for their potential functions.42 A previous study has found that zonisamycin C might reduce neuroinflammation and improve cognition in Alzheimer's disease models by activating the Ras-Raf-MEK-ERK signaling pathway, leading to increased inflammatory protein production by microglia.43 Ginsenoside (Rg1) could reduce chronic inflammation, inhibit AIM2 inflammasome activation, activate the Nrf2 signaling pathway, and improve LPS-induced cognitive impairments and neuronal iron mortality in mice.44 In this study, it was found that Pc supplementation, especially preventive supplementation, could significantly reduce the oxidative stress and inflammatory cytokine levels, increase leukocytes, lymphocytes, intermediate cells, and granulocyte counts, and restore CD3+ levels and CD4+/CD8+ ratios in lymphocyte subpopulations of aging mice. These findings suggest that Pc supplementation can decrease systemic chronic inflammation in aging mice, reverse leukopenia caused by immune aging, maintain lymphocyte subsets at a relatively normal level, and delay immune aging.
5 Conclusion
This study concludes that Pc supplementation can effectively mitigate cognitive impairments in aging mice. Pc can significantly improve the spatial memory and learning disability of aging mice, enhance the antioxidant capacity and reduce inflammatory cytokine levels, thus inhibiting the overactivation of microglia and astrocytes and reducing the level of neuroinflammation. Furthermore, Pc supplementation can help to restore the number of white blood cells, maintain the dynamic balance of lymphocyte subsets in aging mice, and enhance the immune capacity of aging mice. In addition, the results show that prophylactic Pc supplementation is more effective when the dosage is the same. These results provide a theoretical foundation for the effective prevention and intervention of the occurrence and development of cognitive disorders in the elderly population using Pc as a natural dietary supplement without changing the original dietary habits.Ethical statement
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Henan Agricultural University and approved by the Animal Ethics Committee of Henan Agricultural University (Ethics No.: HNND2024031107).Author contributions
Chong Yuan: writing – original draft, visualization, validation, and methodology. Hongtao Ren: writing – review & editing, software, and methodology. Kexin Hu: methodology, software, and data curation. Linlin Chen: methodology and investigation. Ke Yue: writing – review & editing and methodology. Kunmiao He: software and formal analysis. Qiuying Yu: methodology, data curation, writing – review & editing, formal analysis, and data curation. Na Wang: writing – review & editing, resources, data curation, methodology, and funding acquisition. Gaiping Zhang: project administration.Data availability
Data for this article are available at Science Date Bank at https://doi.org/10.57760/sciencedb.12131.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research was funded by the Innovation and Ecological Support Special Project of Henan Province (HARS-22-05-Z1) and the Key Scientific and Technological Project of Henan Province (242102320264).References
- A. Jedrusek-Golinska, D. Górecka, M. Buchowski, K. Wieczorowska-Tobis, A. Gramza-Michalowska and K. Szymandera-Buszka, Recent progress in the use of functional foods for older adults: A narrative review, Compr. Rev. Food Sci. Food Saf., 2020, 19, 835–856 CrossRef
.
- Z. Arvanitakis, R. C. Shah and D. A. Bennett, Diagnosis and Management of Dementia: Review, JAMA, J. Am. Med. Assoc., 2019, 322, 1589–1599 CrossRef
.
- L. F. Jia, Y. F. Du, L. Chu, Z. J. Zhang, F. Y. Li, D. Y. Lyu, Y. Li, Y. Li, M. Zhu, H. S. Jiao, Y. Song, Y. Q. Shi, H. Zhang, M. Gong, C. B. Wei, Y. Tang, B. Y. Fang, D. M. Guo, F. Wang, A. H. Zhou, C. B. Chu, X. M. Zuo, Y. Y. Yu, Q. Yuan, W. Wang, F. Li, S. L. Shi, H. Y. Yang, C. K. Zhou, Z. L. Liao, Y. Lv, Y. Li, M. C. Kan, H. Y. Zhao, S. Wang, S. S. Yang, H. Li, Z. L. Liu, Q. Wang, W. Qin, J. P. Jia and C. Grp, Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study, Lancet Public Health, 2020, 5, E661–E671d CrossRef
.
- B. R. Jin and H. Y. Liu, Comparative efficacy and safety of cognitive enhancers for treating vascular cognitive impairment: systematic review and Bayesian network meta-analysis, Neural Regener. Res., 2019, 14, 805–816 CrossRef CAS
.
- L. Gorecki, A. Misiachna, J. Damborsky, R. Dolezal, J. Korabecny, L. Cejkova, K. Hakenova, M. Chvojkova, J. Z. Karasova, L. Prchal, M. Novak, M. Kolcheva, S. Kortus, K. Vales, M. Horak and O. Soukup, Structure-activity relationships of dually-acting acetylcholinesterase inhibitors derived from tacrine on N-methyl-D-Aspartate receptors, Eur. J. Med. Chem., 2021, 219, 16 CrossRef
.
- Q. F. Zhou, K. Ma, H. H. Hu, X. L. Xing, X. Huang and H. Gao, Extracellular vesicles: Their functions in plant-pathogen interactions, Mol. Plant Pathol., 2022, 23, 760–771 CrossRef PubMed
.
- T. Rescigno, L. Micolucci, M. F. Tecce and A. Capasso, Bioactive Nutrients and Nutrigenomics in Age-Related Diseases, Molecules, 2017, 22, 26 CrossRef
.
- J. Luo, H. W. Si, Z. Q. Jia and D. M. Liu, Dietary Anti-Aging Polyphenols and Potential Mechanisms, Antioxidants, 2021, 10, 20 Search PubMed
.
- Q. C. Pereira, I. M. Fortunato, F. D. Oliveira, M. C. Alvarez, T. W. D. Santos and M. L. Ribeiro, Polyphenolic Compounds: Orchestrating Intestinal Microbiota Harmony during Aging, Nutrients, 2024, 16, 29 Search PubMed
.
- S. Melgar-Locatelli, M. C. Manas-Padilla, A. Castro-Zavala, P. Rivera, M. D. Razola-Díaz, F. J. Monje, C. Rodriguez-Pérez and E. Castilla-Ortega, Diet enriched with high-phenolic cocoa potentiates hippocampal brain-derived neurotrophic factor expression and neurogenesis in healthy adult micewith subtle effects on memory, Food Funct., 2024, 15, 8310–8329 RSC
.
- F. J. Li, X. N. Liu, B. Jiang, X. Y. Li, Y. Q. Wang, X. J. Chen, Y. H. Su, X. J. Wang, J. Luo, L. F. Chen, J. T. Li, Q. Lv, J. Xiao, J. Wu, J. P. Ma and P. Qin, Tea, coffee, and caffeine intake and risk of dementia and Alzheimer's disease: a systematic review and meta-analysis of cohort studies, Food Funct., 2024, 15, 8330–8344 RSC
.
- H. Ö. Osmanlıoğlu and M. Nazıroğlu, Resveratrol Modulates Diabetes-Induced Neuropathic Pain, Apoptosis, and Oxidative Neurotoxicity in Mice Through TRPV4 Channel Inhibition, Mol. Neurobiol., 2024, 61, 7269–7286 CrossRef
.
- T. T. Su, H. T. Shen, M. Y. He, S. S. Yang, X. Gong, C. Huang, L. L. Guo, H. Wang, S. Y. Feng, T. T. Mi, M. L. Zhao, Q. Liu, F. J. Huo, J. K. Zhu, J. B. Zhu, H. B. Li and H. L. Liu, Quercetin promotes the proportion and maturation of NK cells by binding to MYH9 and improves cognitive functions in aged mice, Immun. Ageing, 2024, 21, 18 CrossRef
.
- W. M. Zhang, Y. C. Zhong, Z. Y. Wang, F. R. Tang and C. H. Zheng, Apple polysaccharide improves age-matched cognitive impairment and intestinal aging through microbiota-gut-brain axis, Sci. Rep., 2024, 14, 14 CrossRef
.
- Z. P. Wang, X. L. Li, X. T. Ren, S. Q. Zhao, W. W. Chen, C. Fan, Y. L. Xu, X. J. Pi, Y. D. Zhang, T. Wang and S. Rong, Procyanidins Extracted from the Lotus Seedpod Ameliorate Cognitive Impairment through CREB-BDNF Pathway Mediated LTP in APP/PS1 Transgenic Mice, Curr. Pharm. Biotechnol., 2023, 24, 1560–1567 CAS
.
- J. Chen, Y. X. Chen, Y. F. Zheng, J. W. Zhao, H. L. Yu and J. J. Zhu, Relationship between Neuroprotective Effects and Structure of Procyanidins, Molecules, 2022, 27, 18 CrossRef PubMed
.
- Y. Zhao, C. Q. Jiang, J. Y. Lu, Y. H. Sun and Y. Y. Cui, Research progress of proanthocyanidins and anthocyanidins, Phytother. Res., 2023, 37, 2552–2577 CrossRef
.
- B. C. Li, J. Cheng, G. W. Cheng, H. L. Zhu, B. Y. Liu, Y. H. Yang, Q. Dai, W. F. Li, W. Bao and S. Rong, The effect of grape seed procyanidins extract on cognitive function in elderly people with mild cognitive impairment: A randomized, double-blind, placebo-controlled clinical trial, Heliyon, 2023, 9, 8 Search PubMed
.
- W. L. Gao, X. H. Li, X. P. Dun, X. K. Jing, K. Yang and Y. K. Li, Grape Seed Proanthocyanidin Extract Ameliorates Streptozotocin-induced Cognitive and Synaptic Plasticity Deficits by Inhibiting Oxidative Stress and Preserving AKT and ERK Activities, Curr. Med. Sci., 2020, 40, 434–443 CrossRef CAS PubMed
.
- Q. Feng, W. K. Xia, G. X. Dai, J. A. Lv, J. Yang, D. S. Liu and G. M. Zhang, The Aging Features of Thyrotoxicosis Mice: Malnutrition, Immunosenescence and Lipotoxicity, Front. Immunol., 2022, 13, 23 Search PubMed
.
- Q. Feng, G. Y. Li, W. K. Xia, G. X. Dai, J. D. Zhou, Y. Xu, D. S. Liu and G. M. Zhang, The anti-aging effects of Renshen Guben on thyrotoxicosis mice: Improving immunosenescence, hypoproteinemia, lipotoxicity, and intestinal flora, Front. Immunol., 2022, 13, 19 Search PubMed
.
- M. Santello, N. Toni and A. Volterra, Astrocyte function from information processing to cognition and cognitive impairment, Nat. Neurosci., 2019, 22, 154–166 CrossRef CAS PubMed
.
- D. O'Connor, A. M. Molloy, E. Laird, R. A. Kenny and A. M. O'Halloran, Sustaining an ageing population: the role of micronutrients in frailty and cognitive impairment, Proc. Nutr. Soc., 2023, 82, 315–328 CrossRef
.
- L. X. Chen, Y. L. Zhen, X. C. Wang, J. J. Wang and G. Q. Zhu, Neurovascular glial unit: A target of phytotherapy for cognitive impairments, Phytomedicine, 2023, 119, 19 Search PubMed
.
- L. X. Wang, R. Zhao, X. M. Li, P. Shao, J. G. Xie, X. N. Su, S. J. Xu, Y. Huang and S. B. Hu, Lactobacillus rhamnosus GG improves cognitive impairmentsin mice with sepsis, PeerJ, 2024, 12, 18 Search PubMed
.
- M. Liu, X. D. Zhang and Y. Wang, Curcumin Alleviates Aβ42-Induced Neuronal Metabolic Dysfunction via the Thrb/SIRT3 Axis and Improves Cognition in APPTG Mice, Neurochem. Res., 2021, 46, 3166–3178 CrossRef CAS
.
- G. L. Yin, C. X. Pan, H. Liu, C. Z. Dong, X. Chang, W. Zhou, S. S. Wang and Z. Y. Du, Oxyresveratrol Improves Cognitive Impairments and Episodic-like Memory through Modulating Neuroinflammation and PI3K-Akt Signaling Pathway in LPS-Induced Mice, Molecules, 2024, 29, 16 Search PubMed
.
- Z. H. Chen, X. R. Wang, S. M. Du, Q. Liu, Z. F. Xu, Y. Guo and X. W. Lin, A review on traditional Chinese medicine natural products and acupuncture intervention for Alzheimer's disease based on the neuroinflammatory, Chin. Med., 2024, 19, 33 CrossRef
.
- L. Li, S. Fan, W. Zhang, D. Li, Z. Yang, P. Zhuang, J. Han, H. Guo and Y. Zhang, Duzhong Fang Attenuates the POMC-Derived Neuroinflammation in Parkinsonian Mice, J. Inflammation Res., 2021, 14, 3261–3276 CrossRef
.
- J. M. T. Chu, A. Abulimiti, B. S. H. Wong, G. D. Zhao, S. H. Xiong, M. M. Zhao, Y. Y. Wang, Y. Chen, J. Q. Wang, Y. Zhang, R. C. C. Chang, H. Yu and G. T. C. Wong,
Sigesbeckia orientalis L. Derived Active Fraction Ameliorates Perioperative Neurocognitive Disorders Through Alleviating Hippocampal Neuroinflammation, Front. Pharmacol., 2022, 13, 14 Search PubMed
.
- J. J. Song, Y. Zhao, X. Q. Shan, Y. Y. Luo, N. Hao and L. Zhao, Active ingredients of Chinese medicine with immunomodulatory properties: NF-κB pathway and Parkinson's disease, Brain Res., 2024, 1822, 9 CrossRef
.
- J. Wang, Q. T. Liu, D. Y. Shen, J. P. Bai, Y. Hu, Q. Huang, H. J. Yu, N. N. He, X. Y. Qin and R. F. Lan, Network pharmacology analysis of the active ingredients of Corydalis hendersonii Hemsl. and their effects on eliminating neuroinflammation and improving motor functions in MPTP-intoxicated mice, J. Ethnopharmacol., 2024, 318, 16 Search PubMed
.
- M. Hajinejad, M. Ghaddaripouri, M. Dabzadeh, F. Forouzanfar and S. Sahab-Negah, Natural Cinnamaldehyde and Its Derivatives Ameliorate Neuroinflammatory Pathways in Neurodegenerative Diseases, BioMed Res. Int., 2020, 2020, 9 Search PubMed
.
- L. Wang, Z. Wang, Y. Lan, Y. Tuo, S. Ma and X. Liu, Inulin Attenuates Blood-Brain Barrier Permeability and Alleviates Behavioral Disorders by Modulating the TLR4/MyD88/NF-kappaB Pathway in Mice with Chronic Stress, J. Agric. Food Chem., 2023, 71, 13325–13337 CrossRef CAS
.
- K. L. Sheng, G. H. Zhang, M. Sun, S. M. He, X. W. Kong, J. M. Wang, F. F. Zhu, X. D. Zha and Y. Z. Wang, Grape seed proanthocyanidin extract ameliorates dextran sulfate sodium-induced colitis through intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokines and gut microbiota modulation, Food Funct., 2020, 11, 7817–7829 RSC
.
- E. S. Cannizzo, C. C. Clement, R. Sahu, C. Follo and L. Santambrogio, Oxidative stress, inflamm-aging and immunosenescence, J. Proteomics, 2011, 74, 2313–2323 CrossRef CAS
.
- T. McGarry, M. Biniecka, D. J. Veale and U. Fearon, Hypoxia, oxidative stress and inflammation, Free Radicals Biol. Med., 2018, 125, 15–24 CrossRef CAS
.
- C. Hunsche, O. Hernandez, A. Gheorghe, L. E. Díaz, A. Marcos and M. De la Fuente, Immune dysfunction and increased oxidative stress state in diet-induced obese mice are reverted by nutritional supplementation with monounsaturated and n-3 polyunsaturated fatty acids, Eur. J. Nutr., 2018, 57, 1123–1135 CrossRef CAS
.
- L. P. de Sousa, F. L. Ribeiro-Gomes, R. F. de Almeida, T. M. E. Souza, G. L. Werneck, D. O. Souza and C. T. Daniel-Ribeiro, Immune system challenge improves recognition memory and reverses malaria-induced cognitive impairment in mice, Sci. Rep., 2021, 11, 11 CrossRef
.
- W. Drew, D. V. Wilson and E. Sapey, Inflammation and neutrophil immunosenescence in health and disease: Targeted treatments to improve clinical outcomes in the elderly, Exp. Gerontol., 2018, 105, 70–77 CrossRef
.
- G. C. Muller, M. G. V. Gottlieb, B. L. Correa, I. Gomes, R. N. Moresco and M. E. Bauer, The inverted CD4:CD8 ratio is associated with gender-related changes in oxidative stress during aging, Cell. Immunol., 2015, 296, 149–154 CrossRef CAS
.
- A. Gasmi, M. Shanaida, O. Oleshchuk, Y. Semenova, P. K. Mujawdiya, Y. Ivankiv, O. Pokryshko, S. Noor, S. Piscopo, S. Adamiv and G. Bjorklund, Natural Ingredients to Improve Immunity, Pharmaceuticals, 2023, 16, 28 CrossRef
.
- X. M. Wang, Z. X. Li, R. Sun, X. L. Li, R. R. Guo, X. Y. Cui, B. X. Liu, W. J. Li, Y. Yang, X. Y. Huang, H. L. Qu, C. Liu, Z. L. Wang, Y. H. Lü and C. W. Yue, Zunyimycin C enhances immunity and improves cognitive impairment and its mechanism, Front. Cell. Infect. Microbiol., 2022, 12, 16 Search PubMed
.
- P. M. Ji, Q. F. Shi, L. L. Kong, Y. Liu, Y. Su, R. Sun, H. M. Zhou, H. Y. Xu, W. P. Li and W. Z. Li, Ginsenoside Rg1 attenuates chronic inflammation-induced renal fibrosis in mice by inhibiting AIM2 inflammasome in an Nrf2-dependent manner, J. Funct. Foods, 2024, 116, 13 Search PubMed
.
Footnote |
| † These authors have equally contributed to this work and share first authorship. |
| This journal is © The Royal Society of Chemistry 2025 |