Open Access Article
Open Access ArticleCocoa–carob blend acute intake modifies miRNAs related to insulin sensitivity in type 2 diabetic subjects: a randomised controlled nutritional trial†
Marisol
Villalva
 ab,
Esther
García-Díez
ab,
Esther
García-Díez
 b,
María del Carmen
López de las Hazas
b,
María del Carmen
López de las Hazas
 c,
Oreste
Lo Iacono
d,
José Ignacio
Vicente-Díez
e,
Sara
García-Cabrera
e,
Marta
Alonso-Bernáldez
c,
Alberto
Dávalos
c,
Oreste
Lo Iacono
d,
José Ignacio
Vicente-Díez
e,
Sara
García-Cabrera
e,
Marta
Alonso-Bernáldez
c,
Alberto
Dávalos
 cf,
María Ángeles
Martín
cf,
María Ángeles
Martín
 bg,
Sonia
Ramos
bg,
Sonia
Ramos
 bg and
Jara
Pérez-Jiménez
bg and
Jara
Pérez-Jiménez
 *bg
*bg
aInstitute of Food Science Research (CIAL), Universidad Autónoma de Madrid, CEI UAM + CSIC, Madrid, Spain
bDepartment of Metabolism and Nutrition, Institute of Food Science, Technology and Nutrition, Spanish Research Council (ICTAN-CSIC), Calle Jose Antonio Novais, 6, 28040 Madrid, Spain. E-mail: jara.perez@ictan.csic.es
cLaboratory of Epigenetics of Lipid Metabolism, Madrid Institute for Advanced Studies (IMDEA)-Food, CEI UAM + CSIC, Madrid, Spain
dServicio de Aparato Digestivo, Hospital General Universitario/Instituto de Investigación Sanitaria Gregorio Marañón, Madrid, Spain
eMonóvar Health Center, Primary Care Management, Madrid Region Health Service, Madrid, Spain
fConsorcio CIBER de la Fisiopatología de la Obesidad y Nutrición (CIBERObn), Instituto de Salud Carlos III (ISCIII), Madrid, Spain
gCIBER Diabetes and Associated Metabolic Diseases: Diabetes and Associated Metabolic Diseases Networking Biomedical Research Centre | CIBERDEM, Carlos III Health Institute (ISCIII), Madrid, Spain
First published on 20th March 2025
Abstract
Postprandial metabolic disturbances are exacerbated in type 2 diabetes (T2D). Cocoa and carob, despite showing promising effects on these alterations in preclinical studies, have not yet been jointly tested in a clinical trial. Therefore, this acute, randomised, controlled, crossover nutritional trial evaluated the postprandial effects of a cocoa–carob blend (CCB) in participants with T2D (n = 20) and overweight/obesity. The subjects followed three treatments: hypercaloric breakfast (high-sugar and high-saturated fat, 900 kcal) as the control (treatment C); the same breakfast together with 10 g of the CCB, with 5.6 g of dietary fibre and 1.6 g of total polyphenols (treatment A); and the same breakfast after consuming the CCB (10 g) the night before (treatment B). Various analyses were performed, including the determination of the clinical markers of T2D (fasting and postprandial glucose and insulin, GLP-1, and glycaemic profile), satiety evaluation, analysis of exosomal miRNA expression and ex vivo determination of inflammation modulation. No effect on glucose homeostasis (glucose, insulin, and GLP-1) was found in the study population. However, eight exosomal miRNAs were found to be significantly modified owing to CCB supplementation compared with treatment C, with three of them (miR-20A-5p, miR-23A-3p, and miR-17-5p) associated with an improvement in insulin sensitivity. Furthermore, the CCB caused a decrease in hunger feelings (0–120 min), as assessed by the visual analogue scale (VAS). Finally, treatment A caused a significant decrease in the glucose increment within 0–30 min of treatment in subjects with overweight. No significant modifications were found in the other assessed parameters. The acute intake of the CCB by subjects with T2D showed modest although significant results, which need to be validated in a long-term randomised controlled trial.
Introduction
Type 2 diabetes (T2D) is a chronic metabolic disorder with a serious and increasing impact on public health systems.1 Chronic exposure to high blood glucose levels, as occurs in T2D, is one of the main mechanisms of the damage caused to a large number of organs and tissues, generating numerous complications that are commonly associated with this disease.2 This glucotoxicity induces excess oxidative stress, inflammation and several other changes, which create a feedback loop, aggravating cardiometabolic disturbances.3 At the same time, there is a complex and powerful interaction between T2D and other closely linked pathologies, including obesity and non-alcoholic fatty liver disease (NAFLD).4 In this regard, current lifestyles are responsible for the strong connection between T2D and obesity, with 90% of people suffering from T2D exhibiting overweight or obesity, leading to the term “diabesity” for the simultaneous occurrence of both epidemic diseases.5,6Among all the metabolic alterations caused by T2D, those present in the postprandial state are particularly relevant, being considered an independent predictor of future cardiovascular events, especially when meals are high in calories, carbohydrates and/or saturated fats, even in nondiabetic subjects.7 This is owing to the increased oxidative stress and inflammation associated with this state.8,9 Moreover, it has been observed that the altered release of incretin hormones in insulin resistance situations contributes to the decreased postprandial satiety observed in subjects with obesity.10
Owing to the complications associated with T2D and the consequent increasing use of drugs during disease development, dietary approaches may help delay those alterations, as well as the incorporation of additional pharmacological treatments. Thus, polyphenols are being investigated. These are secondary plant metabolites, which, besides other biological activities, have shown a potential benefit in the prevention or management of T2D.11 These antidiabetic activities may be mediated by several mechanisms of action, such as modulation of glucose homeostasis, thereby promoting insulin signalling pathways, or their ability to decrease oxidative stress and inflammatory processes.12 Moreover, polyphenol-rich diets have shown to be able to modulate the expression of exosomal miRNAs, potentially leading to beneficial outcomes.13 However, in order to obtain the most optimal benefits from these biological activities, their metabolic transformations after ingestion and, particularly, their interaction with the gut microbiota are crucial to ultimately generate active metabolites.14 Interestingly, an important fraction of polyphenols is linked to dietary fibre, a constituent with wide evidence for its ability to reduce the risk of T2D.15,16 Indeed, dietary fibre has a relevant property in the context of diabesity, namely, its ability to modulate satiety through complementary mechanisms, such as delaying gastric emptying and regulating the levels of satiety-related hormones.17
Cocoa and carob are two vegetal materials with high polyphenol and dietary fibre contents, along with the presence of certain bioactive compounds, i.e., methylxanthines and D-pinitol, respectively.18,19 Based on their composition and previous evidence demonstrating their T2D-modulating potential,20 we developed a new functional food based on a cocoa–carob blend (CCB) that was rich in polyphenols and dietary fibre, as well as possessed proper sensory acceptance.21 Chronic supplementation with a CCB-rich diet has shown therapeutic potential against diabetic cardiomyopathy and for improving intestinal health in a T2D animal model.22,23 However, this product has not been tested in humans. Therefore, the aim of this study was to explore the potential effects of the CCB during the postprandial state (by comparing the effects of intact compounds present in the product and those from microbial-derived metabolites) in subjects with T2D through an acute randomised crossover controlled nutritional trial. A comprehensive assessment of the potential biological modifications was carried out, including the clinical markers of T2D, satiety evaluation, analysis of exosomal miRNA expression and ex vivo determination of inflammation modulation.
Methods
Supplemented product
The CCB was prepared by mixing pure cocoa powder (a kind gift from Idilia S.L., Barcelona, Spain) and carob flour (Casa Ruiz Granel Selecto S.L., Madrid, Spain) in a proportion of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 w/w, respectively. The detailed CCB composition, together with its sensory acceptance, has been described elsewhere.21 Briefly, the product had a high dietary fibre content (55.7 g per 100 g), was mostly (92%) insoluble and had a high content of polyphenols (16.7 g per 100 g). Regarding the polyphenols, they were mostly flavanols, present both as an extractable fraction, i.e., oligomers and low molecular weight polymers (2.9 g per 100 g), and a non-extractable fraction, i.e. high molecular weight polymers (9.2 g per 100 g). Detailed HPLC-MS analysis showed that the main individual flavanol constituents were (epi)gallocatechin, procyanidin trimer C1, (−)-epicatechin, procyanidin dimer B2 and (+)-catechin. Relevant amounts of a phenolic acid, i.e. vanillic acid, were also detected, while other phenolic acids or flavanols were present as minor constituents.
40 w/w, respectively. The detailed CCB composition, together with its sensory acceptance, has been described elsewhere.21 Briefly, the product had a high dietary fibre content (55.7 g per 100 g), was mostly (92%) insoluble and had a high content of polyphenols (16.7 g per 100 g). Regarding the polyphenols, they were mostly flavanols, present both as an extractable fraction, i.e., oligomers and low molecular weight polymers (2.9 g per 100 g), and a non-extractable fraction, i.e. high molecular weight polymers (9.2 g per 100 g). Detailed HPLC-MS analysis showed that the main individual flavanol constituents were (epi)gallocatechin, procyanidin trimer C1, (−)-epicatechin, procyanidin dimer B2 and (+)-catechin. Relevant amounts of a phenolic acid, i.e. vanillic acid, were also detected, while other phenolic acids or flavanols were present as minor constituents.
Study population
The study included an acute randomised crossover controlled nutritional trial approved by the Ethics Committee (internal approval 011/2020) and the Clinical Research Ethical Committee of the University Hospital Puerta de Hierro-Majadahonda (2020/01/28, further modifications were approved on 2020/11/23). The participation of primary care medical doctors in the recruitment process was approved by Research Central Commission Primary Care Direction from Madrid Region (first approval by 2020/10/21, further modifications were approved by 2020/11/12). All participants provided written informed consent prior to completing the study procedures. The study procedures were designed considering the Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects, the Council of Europe Convention on Human Rights and Biomedicine and the European Union Directive on Clinical Trials 536/2014. The trial was registered in the public repository Clinical Trials database (NCT4383639), and all the derived data are publicly available at the CSIC repository (https://hdl.handle.net/10261/367662).Subjects were enrolled by their primary care doctors (S. G.-C. and J. I. V.-D.), in Madrid city. The inclusion criteria for the trial were: T2D diagnosed patients under treatment with metformin as the only antidiabetic drug, age 40–70 years old, and body mass index > 25 kg m−2. The exclusion criteria were: body mass index > 40 kg m−2; diagnosed or receiving medication for cardiometabolic or thyroid pathologies, Hb1Ac ≥ 7%, systolic pressure ≥ 150 mmHg or diastolic pressure ≥ 100 mmHg, fasting triglycerides ≥ 350 mg dL−1, fasting total cholesterol ≥ 280 mg dL−1, pregnant or lactating, habitual intake of dietary supplements with antioxidants or dietary fibre, adherence to a vegetarian diet, allergy or intolerance to cocoa or carob, previous bariatric surgery procedure, or current participation in any other dietary intervention trial.
During the screening process, and in order to fulfill the inclusion and exclusion criteria, the subjects provided a blood analysis obtained within the last year (or specifically prescribed by their medical doctor if unavailable) with the requested parameters (glucose, Hb1Ac, and lipid profile). Since the recruitment and follow-up of the study took place between March 2021 and March 2022, with strong Covid-19 workload on the primary care services (where the medical doctors where affiliated), it was not possible to repeat the blood analysis right at baseline. Blood pressure was measured using an automated digital oscillometric device (Omron M6 Comfort from Omron Corporation, Tokyo, Japan), and the mean of two readings was taken. Anthropometric variables, such as height, body weight, and abdominal and hip circumferences were measured. Additionally, for ensuring an overview of the basal state of the volunteers, creatinine, uric acid, bilirubin, hepatic enzymes, alkaline phosphatase and basic haematological parameters were analysed, allowing subsequent determination of the Modification of Diet in Renal Disease-4 (MDR-4) values. Next, hepatic steatosis presence and progression were assessed through abdominal echography (Acuson Juniper Ultrasound, Siemens, Munich, Germany, transductor convex transductor at a frequency of 3.5–5 mHz) and FibroScan transient elastography (Echosens 530, Siemens, with M and XL probes), which can determine the degree of ultrasound attenuation due to hepatic fat.
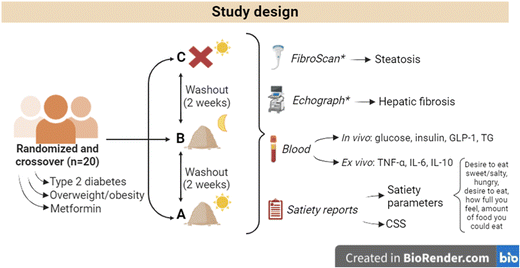
Study design
This acute randomised controlled nutritional trial included three different interventions, as shown in Fig. 1. Briefly, the interventions, separated by a 2-week wash-out period, were assigned to the subjects in a randomised order. This order was obtained with the random sequence generator https://www.random.org with no restriction applied. J. P.-J. generated the random allocation sequence and assigned the interventions. The three treatments were as follows: (A) hypercaloric breakfast with CCB (10 g) supplementation, (B) hypercaloric breakfast after consuming CCB (10 g) the night before (i.e., 10 h earlier) and (C) hypercaloric breakfast as the control intervention. For treatments A and C, when the participants arrived at the institution, they did not know which treatment they would receive; whereas in the case of treatment B they knew in advance since they received the product and had to consume it the day before the visit.A detailed description of the hypercaloric breakfast, which was rich in sugars and saturated fats with a total caloric value of 900 kcal, is provided in the ESI (Table S1†). Basically, it constituted full-cream milk, pineapple juice, honey and croissants. No appropriate placebo for replacing the CCB was found; nevertheless, the caloric value of the provided CCB dose was 21 kcal, which could be considered negligible. In treatments A and B, the CCB was dissolved in 250 mL of full-cream milk, while in treatment C the participants received the same volume of full-cream milk. Volunteers were instructed to follow their regular diet and daily activities between the three visits. However, and in order to limit the potential effect of high dietary polyphenol consumption close to the intervention, the subjects were required to refrain from consuming polyphenol-rich foods such as wine, coffee, tea, cocoa, whole bread, virgin oil olive, nuts, legumes and certain fruits and vegetables such as berries or artichoke 72 h prior to each visit. A detailed list of polyphenol-rich foods was provided to the volunteers, and, at their visits, they were asked questions in order to verify that they had followed those instructions.
Three subjects requested the replacement of lactose-free milk during breakfast. Four subjects were unable to complete the breakfast provided on the first day of intervention; therefore, on the remaining two visits the amount was adjusted accordingly to ensure full consumption of the breakfast.
Sample size calculation
The primary outcome of the trial was the postprandial increase in circulating insulin. Based on a randomised controlled trial focused on the effect of polyphenols in the regulation of postprandial glucose metabolism,24 a 20% decrease in postprandial insulin was considered the minimum value required to reach significance. Also, from the published data on the variance of this parameter24 and considering an alpha value of 0.05 and a power of 80%, the minimum sample size was established as 20 subjects, and then increased to 25 participants considering a withdrawal rate of 20%.The secondary outcomes of this study were the satiety parameters (based on validated tests) and the following blood determinations: glucose, GLP-1, triglycerides, exosomal miRNAs and several cytokines (tumour necrosis factor-α [TNF-α], interleukin-6 [IL-6] and [IL-10]) and lipopolysaccharide (LPS)-induced ex vivo levels.
Finally, in an exploratory approach, the whole group was subdivided into two subgroups based on their body mass index (BMI), or parameters related to glucose and triglyceride homeostasis, and satiety perception, (BMI ≤ 30 kg m−2 indicating overweight and BMI > 30 kg m−2 corresponding to obesity).
Sampling and biochemical analyses
In each visit, fasted subjects attended the Unit of Human Nutrition at ICTAN-CSIC between 8:00 am and 9:00 am and a catheter was placed by a qualified nurse. Then, a basal blood sample was collected, the subject fulfilled the first satiety test and then breakfast was provided. The following blood samples were collected 30, 60, 120, 180, 240 and 270 min later. Urine samples were collected at each visit during the volunteer's stay in the Unit of Human Nutrition, i.e., during 0–270 min. Thus, the samples did not correspond to the first urine of the day. This procedure was chosen because they should be related to the time elapsed from CCB intake: no CCB intake for treatment C, 0–270 min from intake for treatment A, and 10–14.5 h from intake for treatment B. Details on the sample and data collection are shown in Fig. 1. Plasma from the blood samples was obtained after centrifugation at 3000g and 4 °C for 15 min and was stored at −80 °C for further analysis. Aliquots of whole blood samples were used for ex vivo experiment, as detailed below.Fasting and postprandial blood glucose were determined by applying the enzyme electrode method, using the FreeStyle Optium Neo Meter from Abbott (Chicago, IL, USA). Plasma levels of insulin and total GLP-1 were measured using commercial ELISA kits (Merck-Millipore; Burlington, MS, USA). The areas under the curves for glucose and insulin were estimated using the trapezoidal function. Plasma triglycerides were measured using a commercial kit (Cromakit SL; Maracena, Granada, Spain).
Ex vivo study of LPS-induced cytokine production
Blood collected at 0, 120 and 240 min in a random selection of 14 subjects (due to logistic reasons related to performing these treatments at the same time as the full trial protocol was being carried out) was incubated in Petri dishes with bacterial lipopolysaccharide (LPS, Merck, Darmstadt, Germany) in a proportion 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v. Previously the LPS was dissolved in Dulbecco's modified eagle medium (DMEM, Merck, Darmstadt, Germany) to reach a final concentration of 20 μg mL−1. The whole blood together with the LPS mixture was incubated for 4 h at 37 °C, in order to stimulate inflammatory cytokine production. The supernatants were then centrifuged at 3000g and 4 °C for 15 min for plasma recovery. Then, IL-6, IL-10 and tumour necrosis TNF-α were measured by ELISA kits and their corresponding areas under the curve (AUCs) were estimated using the trapezoidal function.
4 v/v. Previously the LPS was dissolved in Dulbecco's modified eagle medium (DMEM, Merck, Darmstadt, Germany) to reach a final concentration of 20 μg mL−1. The whole blood together with the LPS mixture was incubated for 4 h at 37 °C, in order to stimulate inflammatory cytokine production. The supernatants were then centrifuged at 3000g and 4 °C for 15 min for plasma recovery. Then, IL-6, IL-10 and tumour necrosis TNF-α were measured by ELISA kits and their corresponding areas under the curve (AUCs) were estimated using the trapezoidal function.
Satiety
Satiety was evaluated by applying a validated test with several questions.25,26 The test was provided to the volunteers at different times during each intervention (0, 60, 120, 180 and 270 min) and, each time, the questions were randomly asked to avoid automatic responses. Each parameter was evaluated on a scale of 0–10 using the visual analogue scale (VAS). From this test, the values of each question were assessed, as well as the AUC for the different time intervals. Additionally, the composite satiety score (CSS) index was calculated, based on the following equation: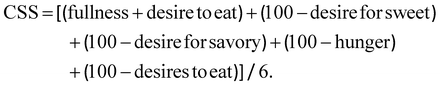 | (1) |
Analysis of exosomal miRNA expression
miRNA analysis was performed on samples from the basal time and at 2 h for the three interventions. First, the exosomes were isolated from 500 μL of plasma using the miRCURY Exosome Serum/Plasma Kit (Qiagen, Denmark), according to the manufacturer's protocol.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000–1
1000–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 1× PBS on the thawed samples. To visualize the particles, 60 s capture videos were shot using a 638 nm laser in three replicates. Particle analysis was performed using the NanoSight NTA 3.1. program.
000 and 1× PBS on the thawed samples. To visualize the particles, 60 s capture videos were shot using a 638 nm laser in three replicates. Particle analysis was performed using the NanoSight NTA 3.1. program.
Results of pathway enrichment analysis were presented in circus plots using the on-line application, available at https://circos.ca/.
Statistics
For statistical analysis, first, outliers (>1.5 IQR) and extreme values (>3.0 IQR) were identified using box-and-whisker plots, and extreme values were excluded from the results. Second, the possible effect of randomization for the group assignment (first treatment assigned) was evaluated; since no statistical differences were observed, data for all the subjects were processed together. Subsequently, normal data distribution was evaluated based on the kurtosis coefficient (between −1 and +1) and, in case data did not follow Gaussian distribution, log or square-root transformation was performed. For variables with a normal distribution, one-way analysis of variance (ANOVA) was performed, and for variables with a non-normal distribution, the equivalent non-parametric Friedman test was chosen. Statistically significant differences between the means were determined using the Tukey and Wilcoxon post-hoc tests. Independent statistical analysis was applied for the values at each time point, the absolute increments observed between baseline and the maximum level, and the AUC values for different intervals. In the ex vivo experiments, statistical analysis was also applied over time for the same treatment to establish whether significant modifications in the inflammation status were achieved.For parameters related to glucose and triglyceride homeostasis, as well as satiety perception, sub-groups based on BMI values (Group Ovw, BMI ≤ 30 kg m−2 indicating overweight and Group Obe, BMI > 30 kg m−2 corresponding to obesity) were established, since about half of the participants were distributed among both categories (n = 8 and n = 12, respectively) and the BMI is a relevant marker in the context of T2D. Nevertheless, it should be noted that this was just an exploratory analysis since there was not enough statistical power (the sample size calculation for the primary outcome was performed for the whole group). In particular, for assessing postprandial insulin (the primary outcome of the study), ANOVA was applied at each time point, since all specific parameters (values at different time points, maximum value, time to reach the maximum value, absolute increment and AUC) followed a normal distribution, or could be adjusted to it (by applying a decimal logarithm for the AUC 0–60 min and square root for 30–60 min increments).
For the analysis of miRNA expression, modifications between the baseline and 2 h plasma levels for each intervention were assessed by a non-parametrical paired t-test, while intra-group comparisons were performed by Wilcoxon test.
In all the cases, a two-tailed p < 0.05 was considered significant. All data are expressed as the mean ± standard error of the mean (SEM). All experimental determinations in the biological samples were performed at least in duplicate. All these data were analysed using the SPSS IBM Statistics 29 package for Windows.
Results
Subject characteristics
Fig. 2 presents the CONSORT diagram for the recruitment process. Recruitment took place between March 2021 and January 2022, while the patients were followed up between April 2021 and March 2022. The primary care medical doctors involved in the study spread the information among their patients and, initially, 256 subjects requested information, although 178 did not fulfill the inclusion criteria. Among the other 78 potential participants, 50 declined to participate after finding out all the details about the trial (particularly, staying at ICTAN-CSIC during three full mornings). Twenty-eight subjects consented to attend the enrolment visit when basal determinations were collected and the informed consent was signed, although 8 of them finally declined to participate. Finally, 20 subjects were enrolled in and completed the trial, after being randomly assigned to the treatment in order to fulfill the three intervention periods. As all 20 participants completed the trial, the sample size was met, considering that there was a margin of 5 for potential withdrawals. | ||
| Fig. 2 CONSORT diagram for the clinical trial on the postprandial state effect of a cocoa–carob blend in subjects with type 2 diabetes treated with metformin. | ||
The basal values for the subjects are shown in Table 1. The average age was 60.5 ± 1.7 years old and three participants were women, representing 15% of the whole group. The values for the cardiometabolic markers (blood pressure, blood glucose, HbA1c, total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides) were all within normal ranges, as were the haematological and immunological basal values. Additionally, a study of the liver by FibroScan and echography was carried out, providing full characterisation for all the participants, and showing a high prevalence (90%) of steatosis in the study population with a score exceeding 238 dB/m, although no fibrosis was detected (all participants showed values < 7.0 kPa).
| Parameter | Complete group, n = 20 | Group Ovw, n = 8 | Group Obe, n = 12 |
|---|---|---|---|
| Age (years) | 60.5 ± 1.7 | 62.0 ± 2.1 | 58.7 ± 2.1 |
| Sex | 85% male; 15% female | 75% male; 25% female | 91.7% male; 8.3% female |
| Body mass index, BMI (kg m−2) | 31.2 ± 0.7 | 27.7 ± 0.5 # | 33.4 ± 0.5 # |
| Systolic pressure (mmHg) | 132.8 ± 2.0 | 128.4 ± 2.8 | 135.8 ± 2.5 |
| Diastolic pressure (mmHg) | 79.4 ± 2.1 | 75.6 ± 3.8 | 81.9 ± 2.2 |
| Glucose (mg dL−1) | 117.9 ± 4.2 | 103.1 ± 2.0 # | 126.5 ± 1.4 # |
| HbA1c (%) | 6.3 ± 0.1 | 6.2 ± 0.2 | 6.4 ± 0.1 |
| Triglycerides (mg dL−1) | 146.5 ± 14.3 | 160.8 ± 31.5 | 137.0 ± 12.3 |
| HDL-Cholesterol (mg dL−1) | 43.8 ± 1.6 | 43.1 ± 3.3 | 44.3 ± 1.8 |
| LDL-Cholesterol (mg dL−1) | 100.8 ± 5.8 | 104.5 ± 12.2 | 98.5 ± 5.9 |
| Total Cholesterol (mg dL−1) | 172.9 ± 6.2 | 173.6 ± 14.1 | 172.3 ± 6.1 |
| Creatinine (mg dL−1) | 0.91 ± 0.04 | 0.9 ± 0.1 | 0.92 ± 0.04 |
| Modification of Diet in Renal Disease, MDRD-4 | 83.9 ± 2.9 | 81.2 ± 4.6 | 86.0 ± 3.9 |
| Chronic Kidney Disease Epidemiology Collaboration, CKD-EPI | 83.9 ± 2.9 | 81.2 ± 4.6 | 86.0 ± 3.9 |
| Uric acid (mg dL−1) | 6.7 ± 0.3 | 6.2 ± 0.4 | 6.9 ± 0.4 |
| Bilirubin (mg dL−1) | 0.7 ± 0.1 | 0.5 ± 0.1 | 0.8 ± 0.1 |
| Alanine aminotransferase, ALT (Ul L−1) | 36.9 ± 4.9 | 32.0 ± 7.0 | 40.1 ± 6.8 |
| Gamma-glutamyl transferase, GGT (Ul L−1) | 51.8 ± 8.3 | 58.3 ± 17.4 | 48.2 ± 9.2 |
| Alkaline phosphatase, ALP (Ul L−1) | 62.3 ± 3.6 | 68.0 ± 6.7 | 59.2 ± 4.2 |
| Abdominal circumference (cm) | 104.7 ± 2.5 | 94.0 ± 2.6 # | 111.8 ± 1.9 # |
| Hip circumference (cm) | 105.4 ± 1.8 | 98.5 ± 1.2 # | 109.9 ± 2.1 # |
| Body fat (%) | 29.9 ± 1.3 | 29.4 ± 2.6 | 30.4 ± 1.4 |
| Body muscle mass (kg) | 56.7 ± 2.5 | 48.5 ± 3.5 # | 62.1 ± 2.4 # |
| Visceral fat (%) | 14.6 ± 1.0 | 10.6 ± 1.4 # | 17.2 ± 0.7 # |
| Abdominal fat (%) | 32.7 ± 1.4 | 32.6 ± 3.0 | 32.8 ± 1.4 |
| Abdominal muscle mass (kg) | 31.0 ± 1.9 | 26.5 ± 4.2 | 33.6 ± 1.3 |
| Steatosis | 90% yes; 10% no | 75% yes; 25% no | 100% yes |
| Portal vein size (mm) | 10.3 ± 0.2 | 9.9 ± 0.2 # | 10.5 ± 0.2 # |
| Spleen size (cm) | 10.2 ± 0.2 | 9.8 ± 0.2 # | 10.5 ± 0.2 # |
| FibroScan stiffness (kPa) | 5.5 ± 0.4 | 5.1 ± 0.6 | 5.8 ± 0.6 |
| Controlled attenuation parameter, CAP | 302.0 ± 12.1 | 296.1 ± 20.6 | 305.9 ± 15.4 |
| Fibrosis grade | 0.9 ± 0.2 | 0.7 ± 0.2 | 1.0 ± 0.3 |
The values obtained were evaluated for the entire group (n = 20) and after the participants were classified based on their BMI (kg m−2), with 31.0 ± 0.7 kg m−2 being the mean value for the whole group. Therefore, two groups were created: Group Ovw for those participants overweight (BMI ≤ 30, n = 8) and Group Obe for those with obesity (BMI > 30, n = 12). Significant differences (p < 0.05) between the sub-groups were found for anthropometric measurements, as expected, and for basal blood glucose, in terms of the portal vein size and spleen size.
No adverse or unintended effects derived from CCB consumption were reported.
Effects of CCB supplementation on glucose and triglyceride homeostasis
The values for plasma insulin, glucose and triglycerides (mean levels over time, increases at each interval of time, maximum concentration and AUC) are provided in Tables 2, 3 and 4, respectively. Furthermore, the same data for GLP-1 values are provided in the ESI (Table S2†). All these cardiometabolic biomarkers showed a peak after breakfast, indicating activation of postprandial metabolism.| Parameter | Complete group, n = 20 | Group Ovw, n = 8 | Group Obe, n = 12 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | B | A | C | B | A | C | B | A | |
| T max: time to reach the maximum concentration. | |||||||||
| Time (min) | |||||||||
| 0 | 10.4 ± 1.1 | 12.7 ± 1.3 | 11.2 ± 1.3 | 8.6 ± 1.6 # | 11.6 ± 2.0 * | 8.8 ± 2.1 # | 11.7 ± 1.5 | 13.4 ± 1.7 | 12.8 ± 1.5 |
| 30 | 52.1 ± 6.3 | 68.5 ± 7.8 | 58.0 ± 6.5 | 48.0 ± 10.2 | 65.1 ± 13.0 | 61.1 ± 12.1 | 54.8 ± 8.4 | 70.8 ± 10.2 | 55.9 ± 7.6 |
| 60 | 87.7 ± 9.5 | 90.7 ± 11.2 | 90.0 ± 8.7 | 89.2 ± 17.3 | 85.8 ± 16.5 | 84.9 ± 7.0 | 86.8 ± 11.5 | 94.0 ± 15.6 | 93.4 ± 14.0 |
| 120 | 91.5 ± 11.3 | 110.9 ± 11.7 | 95.4 ± 11.7 | 92.3 ± 17.2 | 109.6 ± 18.7 | 89.8 ± 19.2 | 91.0 ± 15.6 | 111.8 ± 15.6 | 99.2 ± 11.4 |
| Maximum value | 103.1 ± 10.3 | 120.2 ± 12.2 | 110.6 ± 9.3 | 108.4 ± 17.2 | 113.4 ± 18.5 | 111.5 ± 14.2 | 99.5 ± 13.3 | 124.8 ± 16.7 | 109.9 ± 12.8 |
| T max (min) | 90.4 ± 7.6 | 95.2 ± 8.1 | 95.5 ± 7.1 | 91.4 ± 12.0 | 98.6 ± 11.2 | 91.4 ± 11.6 | 89.9 ± 10.2 | 92.8 ± 11.7 | 98.2 ± 9.4 |
| Increases | |||||||||
| 0–30 | 41.7 ± 5.8 | 55.8 ± 6.9 | 46.8 ± 5.9 | 80.6 ± 16.2 | 76.1 ± 15.6 | 52.3 ± 10.7 | 43.2 ± 8.0 | 57.4 ± 9.1 | 43.1 ± 7.1 |
| 0–60 | 77.3 ± 9.2 | 78.1 ± 10.7 | 78.8 ± 8.5 | 80.6 ± 16.2 | 74.2 ± 15.6 | 76.1 ± 7.6 | 75.1 ± 11.4 | 80.7 ± 15.0 | 80.6 ± 13.6 |
| 0–120 | 81.1 ± 11.0 | 97.9 ± 11.2 | 84.2 ± 9.7 | 80.9 ± 16.1 | 97.0 ± 18.0 | 80.9 ± 18.8 | 79.3 ± 15.5 | 98.4 ± 15.0 | 86.4 ± 10.9 |
| AUC (mg dL −1 min −1 ) | |||||||||
| 0–30 | 966 ± 109 | 1266 ± 144 | 1095 ± 113 | 876 ± 180 | 1204 ± 248 | 1061 ± 213 | 1027 ± 141 | 1308 ± 182 | 1117 ± 131 |
| 0–60 | 2990 ± 307 | 3170 ± 367 | 3076 ± 278 | 2948 ± 553 | 2957 ± 523 | 2856 ± 225 | 3018 ± 376 | 3311 ± 518 | 3222 ± 443 |
| 0–120 | 6241 ± 722 | 7621 ± 753 | 6553 ± 664 | 6111 ± 1116 | 7404 ± 1185 | 5989 ± 1207 | 6328 ± 985 | 7747 ± 1008 | 6929 ± 788 |
| 30–60 | 2102 ± 238 | 2404 ± 256 | 2152 ± 203 | 2015 ± 397 | 2229 ± 367 | 2234 ± 258 | 2160 ± 310 | 2520 ± 359 | 2098 ± 299 |
| 60–120 | 5499 ± 606 | 6312 ± 605 | 5746 ± 519 | 5487 ± 952 | 6180 ± 789 | 5287 ± 590 | 5507 ± 820 | 6389 ± 865 | 6052 ± 778 |
| Parameter | Complete group, n = 20 | Group Ovw, n = 8 | Group Obe, n = 12 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | B | A | C | B | A | C | B | A | |
| T max: time to reach the maximum concentration. | |||||||||
| Time (min) | |||||||||
| 0 | 116.8 ± 4.0 | 117.1 ± 4.0 | 111.9 ± 3.7 | 111.5 ± 4.0 | 109.6 ± 5.0 | 107.1 ± 48 | 120.3 ± 6.0 | 122.0 ± 5.4 | 115.0 ± 5.1 |
| 30 | 163.8 ± 5.6 | 166.5 ± 4.3 | 156.1 ± 5.1 | 164.5 ± 8.6 | 161.8 ± 5.3 | 145.8 ± 5.7 | 163.3 ± 7.6 | 169.6 ± 6.1 | 162.9 ± 7.1 |
| 60 | 161.1 ± 6.8 | 164.2 ± 6.7 | 161.7 ± 6.1 | 158.5 ± 9.6 | 155.0 ± 6.1 | 153.8 ± 11.6 | 162.8 ± 9.7 | 170.3 ± 10.3 | 167.0 ± 6.6 |
| 120 | 136.6 ± 7.2 | 144.2 ± 7.2 | 141.7 ± 7.9 | 124.9 ± 10.0 | 128.4 ± 5.1 | 132.8 ± 11.6 | 144.4 ± 9.6 | 154.7 ± 10.6 | 148.0 ± 10.6 |
| 180 | 116.9 ± 7.1 | 118.5 ± 6.4 | 117.3 ± 6.7 | 105.1 ± 8.3 | 105.3 ± 6.2 | 107.6 ± 8.0 | 124.8 ± 10.0 | 127.3 ± 9.3 | 123.8 ± 9.6 |
| 240 | 104.7 ± 5.6 | 107.1 ± 4.2 | 106.7 ± 5.7 | 96.5 ± 7.7 | 103.1 ± 6.6 | 103.0 ± 5.3 | 110.2 ± 7.7 | 109.7 ± 5.5 | 109.1 ± 9.0 |
| Maximum value | 175.4 ± 5.8 | 180.8 ± 4.1 | 170.2 ± 5.0 | 169.4 ± 7.8 | 171.0 ± 2.8 | 164.4 ± 8.6 | 179.3 ± 8.3 | 187.3 ± 6.0 | 174.1 ± 6.1 |
| T max (min) | 53.7 ± 6.1 | 54.0 ± 6.1 | 62.0 ± 7.3 | 46.5 ± 5.7 | 46.1 ± 6.0 | 49.4 ± 5.3 | 58.5 ± 9.3 | 59.3 ± 9.3 | 70.4 ± 11.2 |
| Increases | |||||||||
| 0–30 | 47.0 ± 4.7 | 49.4 ± 4.1 | 44.2 ± 3.9 | 53.0 ± 8.8 * | 52.1 ± 5.4 #, * | 38.6 ± 4.8 # | 42.9 ± 5.2 | 47.6 ± 5.9 | 47.9 ± 5.5 |
| 0–60 | 44.3 ± 7.3 | 47.1 ± 6.5 | 49.9 ± 6.4 | 47.0 ± 11.8 | 45.4 ± 9.6 | 46.6 ± 13.6 | 42.5 ± 9.7 | 48.3 ± 9.2 | 52.0 ± 6.1 |
| 0–120 | 19.8 ± 6.9 | 27.1 ± 7.0 | 29.9 ± 7.2 | 13.4 ± 11.6 | 18.8 ± 6.1 | 25.1 ± 12.1 | 24.1 ± 8.7 | 32.7 ± 10.9 | 33.0 ± 9.2 |
| 0–180 | 0.1 ± 6.4 | 1.4 ± 6.2 | 5.5 ± 6.2 | −6.4 ± 8.5 | −4.4 ± 8.0 | 0.5 ± 9.9 | 4.4 ± 9.1 | 5.3 ± 8.8 | 8.8 ± 8.1 |
| 0–240 | −12.1 ± 5.4 | −10.0 ± 4.5 | −5.2 ± 5.4 | −15.0 ± 7.2 | −6.5 ± 6.3 | −4.1 ± 5.6 | −10.2 ± 7.8 | −12.3 ± 6.3 | −5.9 ± 8.4 |
| 30–60 | −2.7 ± 6.2 | −2.3 ± 7.0 | 5.7 ± 5.9 | −6.0 ± 7.2 | −6.8 ± 10.0 | 8.0 ± 13.5 | −0.4 ± 9.4 | 0.7 ± 9.9 | 4.1 ± 4.6 |
| 60–120 | −24.5 ± 7.3 | −20.0 ± 5.4 | −20.0 ± 5.7 | −33.6 ± 9.9 | −26.6 ± 6.9 | −21.5 ± 7.0 | −18.4 ± 10.1 | −15.6 ± 7.7 | −19.0 ± 8.5 |
| 120–180 | −19.7 ± 4.2 | −25.7 ± 4.5 | −24.4 ± 6.1 | −19.8 ± 7.8 | −23.1 ± 6.0 | −24.6 ± 8.7 | −19.7 ± 5.0 | −27.4 ± 6.5 | −24.3 ± 8.7 |
| 180–240 | −12.2 ± 4.3 | −11.4 ± 5.5 | −10.7 ± 4.8 | −8.6 ± 3.9 | −2.1 ± 7.0 | −4.6 ± 7.1 | −14.6 ± 6.8 | −17.4 ± 7.5 | −14.7 ± 6.4 |
| Maximum increase | 58.6 ± 4.9 | 63.7 ± 4.0 | 58.4 ± 5.1 | 57.9 ± 6.4 | 61.4 ± 5.6 | 57.3 ± 9.9 | 59.0 ± 6.3 | 65.3 ± 5.6 | 59.1 ± 5.6 |
| AUC (mg dL −1 min −1 ) (/10) | |||||||||
| 0–30 | 436 ± 15 | 440 ± 12 | 43 ± 17 | 433 ± 20 | 416 ± 12 | 384 ± 20 | 438 ± 21 | 455 ± 18 | 457 ± 21 |
| 0–60 | 845 ± 26 | 868 ± 29 | 832 ± 25 | 819 ± 29 | 816 ± 23 | 793 ± 38 | 863 ± 39 | 903 ± 44 | 860 ± 33 |
| 0–120 | 1564 ± 74 | 15960 ± 62 | 1540 ± 62 | 1433 ± 71 | 1445 ± 50 | 1454 ± 83 | 1651 ± 109 | 1640 ± 88 | 1598 ± 87 |
| 0–180 | 2114 ± 87 | 2133 ± 80 | 2085 ± 83 | 1954 ± 89 | 1949 ± 74 | 1939 ± 80 | 2218 ± 125 | 2256 ± 112 | 2182 ± 123 |
| 0–240 | 2680 ± 101 | 2707 ± 82 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260 ± 97 260 ± 97 |
1509 ± 126 | 2570 ± 121 | 2521 ± 107 | 2793 ± 140 | 2798 ± 105 | 2695 ± 145 |
| 30–60 | 484 ± 18 | 508 ± 16 | 461 ± 15 | 473 ± 25 | 489 ± 16 | 457 ± 20 | 492 ± 26 | 521 ± 25 | 463 ± 21 |
| 60–120 | 930 ± 5 | 931 ± 43 | 931 ± 44 | 858 ± 57 | 844 ± 21 | 867 ± 68 | 979 ± 860 | 988 ± 67 | 973 ± 56 |
| 120–180 | 724 ± 31 | 776 ± 40 | 784 ± 44 | 681 ± 43 | 701 ± 31 | 707 ± 48 | 753 ± 43 | 826 ± 60 | 835 ± 64 |
| 180–240 | 679 ± 40 | 680 ± 27 | 650 ± 33 | 6160 ± 58 | 628 ± 34 | 625 ± 38 | 720 ± 52 | 715 ± 37 | 666 ± 50 |
| Glycemic profile | 2.4 ± 0.3 | 2.1 ± 0.2 | 2.6 ± 0.3 | 2.3 ± 0.5 | 2.3 ± 0.3 | 2.4 ± 0.3 | 2.5 ± 0.3 | 2.1 ± 0.3 | 2.7 ± 0.5 |
| Parameter | Complete group, n = 19 | Group Ovw, n = 7 | Group Obe, n = 12 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | B | A | C | B | A | C | B | A | |
| T max: time to reach the maximum concentration. | |||||||||
| Time (min) | |||||||||
| 0 | 99.4 ± 8.0 | 100.1 ± 7.9 | 104.9 ± 9.5 | 103.0 ± 9.7 | 103.4 ± 13.7 | 115.4 ± 15.7 | 97.3 ± 12.3 | 98.2 ± 25.8 | 98.7 ± 12.1 |
| 30 | 98.0 ± 7.7 | 92.6 ± 7.8 | 97.0 ± 9.0 | 108.3 ± 11.6 | 101.6 ± 16.6 | 102.3 ± 11.0 | 92.0 ± 14.6 | 87.3 ± 30.2 | 94.0 ± 13.0 |
| 60 | 105.9 ± 8.3 | 101.5 ± 8.8 | 112.0 ± 9.2 | 118.6 ± 11.9 | 112.3 ± 17.4 | 126.4 ± 13.3 | 98.6 ± 15.0 | 95.3 ± 25.4 | 103.6 ± 12.0 |
| 120 | 128.1 ± 8.9 | 122.2 ± 9.4 | 139.3 ± 12.3 | 141.5 ± 15.5 | 131.6 ± 18.3 | 164.2 ± 19.9 | 120.3 ± 22.0 | 116.8 ± 27.8 | 124.8 ± 14.6 |
| 180 | 138.0 ± 10.0 | 132.2 ± 11.2 | 142.7 ± 11.4 | 153.8 ± 19.2 | 148.2 ± 25.8 | 165.8 ± 22.2 | 128.8 ± 24.4 | 122.9 ± 24.2 | 129.2 ± 11.7 |
| 240 | 142.9 ± 10.9 | 133.4 ± 13.0 | 146.5 ± 14.0 | 159.7 ± 20.8 | 141.7 ± 26.5 | 170.3 ± 26.1 | 133.1 ± 29.5 | 128.6 ± 26.4 | 132.7 ± 15.5 |
| 270 | 141.5 ± 11.0 | 137.4 ± 14.5 | 137.4 ± 13.0 | 158.0 ± 20.0 | 155.2 ± 33.6 | 164.7 ± 26.2 | 131.9 ± 30.0 | 127.6 ± 24.7 | 121.4 ± 12.5 |
| Maximum value | 151.3 ± 10.8 | 163.6 ± 13.8 | 163.6 ± 13.2 | 167.0 ± 19.2 | 164.8 ± 31.6 | 189.2 ± 23.2 | 142.1 ± 29.4 | 144.9 ± 27.7 | 148.6 ± 15.0 |
| T max (min) | 224.0 ± 11.5 * | 227.7 ± 12.2 * | 182.8 ± 14.6 # | 228.1 ± 20.8 | 223.6 ± 23.2 | 176.6 ± 25.6 | 221.6 ± 14.2 * | 230.2 ± 14.7 * | 186.4 ± 18.4 # |
| Increases | |||||||||
| 0–30 | −1.3 ± 3.5 | −7.5 ± 5.0 | −7.8 ± 3.0 | 5.3 ± 6.9 | −1.8 ± 4.5 | −13.2 ± 5.8 | −5.2 ± 2.9 | −10.9 ± 5.9 | −4.7 ± 3.2 |
| 30–60 | 7.9 ± 3.1 | 8.9 ± 3.9 | 14.9 ± 3.9 | 10.2 ± 4.9 | 10.7 ± 5.6 | 24.1 ± 8.5 | 6.5 ± 6.8 | 7.9 ± 5.6 | 9.6 ± 3.1 |
| 60–120 | 22.2 ± 3.2 | 20.7 ± 3.2 | 27.4 ± 5.5 | 22.9 ± 5.2 | 19.3 ± 5.6 | 37.9 ± 9.2 | 21.8 ± 9.0 | 21.5 ± 7.1 | 21.2 ± 6.5 |
| 120–180 | 9.9 ± 3.6 | 10.0 ± 5.6 | 3.5 ± 5.5 | 12.3 ± 7.5 | 16.7 ± 12.9 | 1.5 ± 8.8 | 8.5 ± 5.9 | 6.2 ± 6.4 | 4.8 ± 7.4 |
| 180–240 | 4.9 ± 3.8 | 1.2 ± 5.6 | 3.9 ± 6.4 | 5.9 ± 5.1 | −6.5 ± 6.9 | 4.6 ± 10.4 | 4.3 ± 7.5 | 5.7 ± 10.0 | 3.4 ± 8.5 |
| 240–270 | −1.4 ± 2.6 | 4.3 ± 4.7 | −9.2 ± 5.1 | −1.8 ± 2.9 #, * | 13.4 ± 7.7 * | −5.6 ± 8.1 # | −1.2 ± 7.3 | −1.0 ± 5.2 | −11.2 ± 6.7 |
| Maximum increase | 51.9 ± 6.0 | 56.7 ± 9.5 | 58.7 ± 9.7 | 64.0 ± 12.3 | 61.4 ± 21.3 | 73.8 ± 16.4 | 44.9 ± 17.8 | 53.9 ± 13.8 | 49.9 ± 11.8 |
| AUC (mg dL −1 min −1 ) (/10) | |||||||||
| 0–30 | 305 ± 24 | 298 ± 22 | 323 ± 32 | 328 ± 32 | 314 ± 45 | 330 ± 42 | 292 ± 47 | 288 ± 92 | 319 ± 45 |
| 0–60 | 622 ± 47 | 625 ± 49 | 661 ± 56 | 669 ± 60 | 667 ± 90 | 737 ± 86 | 594 ± 81 | 601 ± 153 | 616 ± 72 |
| 0–120 | 1397 ± 100 | 1353 ± 98 | 1484 ± 126 | 1475 ± 139 | 1415 ± 185 | 1700 ± 207 | 1352 ± 199 | 1316 ± 323 | 1358 ± 154 |
| 0–180 | 2146 ± 154 | 2104 ± 157 | 2247 ± 176 | 2321 ± 251 | 2282 ± 343 | 2533 ± 319 | 2044 ± 322 | 1999 ± 440 | 2080 ± 202 |
| 0–240 | 2929 ± 217 | 2820 ± 224 | 3026 ± 258 | 3166 ± 350 | 2958 ± 464 | 3424 ± 453 | 2790 ± 493 | 2739 ± 601 | 2794 ± 306 |
| 0–270 | 3490 ± 613 | 3211 ± 268 | 3270 ± 268 | 3523 ± 385 | 3049 ± 663 | 3782 ± 493 | 3094 ± 562 | 3049 ± 663 | 2971 ± 294 |
| 30–60 | 302 ± 22 | 300 ± 24 | 303 ± 25 | 333 ± 31 | 333 ± 47 | 350 ± 36 | 284 ± 37 | 281 ± 77 | 276 ± 33 |
| 60–120 | 730 ± 59 | 668 ± 53 | 767 ± 63 | 784 ± 78 | 714 ± 108 | 879 ± 93 | 698 ± 105 | 641 ± 166 | 693 ± 81 |
| 120–180 | 773 ± 60 | 755 ± 59 | 847 ± 67 | 890 ± 113 | 853 ± 123 | 969 ± 122 | 705 ± 134 | 698 ± 148 | 776 ± 76 |
| 180–240 | 855 ± 62 | 802 ± 72 | 855 ± 80 | 945 ± 116 | 869 ± 156 | 1001 ± 140 | 802 ± 167 | 762 ± 152 | 770 ± 93 |
| 240–270 | 401 ± 35 | 390 ± 43 | 418 ± 42 | 458 ± 69 | 426 ± 90 | 513 ± 92 | 368 ± 94 | 369 ± 74 | 363 ± 33 |
Regarding the primary outcome of the study, i.e. postprandial insulin, CCB supplementation could not modify insulinaemia at the different assessed times after the breakfast provided to the participants (Table 2). Besides, insulin levels showed significant differences (p < 0.05) at 0 min when the CCB was consumed the night before the visit (intervention B), compared with the control and treatment A.
Considering glycaemia (Table 3), CCB supplementation, either at breakfast or the night before, did not significantly modify either glucose levels at different times nor the other parameters evaluated when the whole group was assessed. On the contrary, when the subjects were classified according to their BMI, a significant decrease (p < 0.05) in the glycaemia increment from 0 to 30 min was observed in those with overweight (BMI ≤ 30, n = 8) when consuming the CCB at breakfast compared with the control breakfast (Table 3). No significant differences were found between the treatments for any group in terms of GLP-1 values (Table S2†).
Regarding triglycerides (Table 4), the time to reach their maximum concentration was diminished in the complete group when the intake of CCB took place together with breakfast (intervention A) (p < 0.05), compared to treatments C (control) and B. This was also observed in the group with obesity (group Obe, BMI ≤ 30, n = 12). Moreover, in the group who were overweight (group Ovw, BMI ≤ 30, n = 8), the triglyceride increment from 240 to 270 min was significantly higher during intervention B compared to intervention A (p < 0.05), but it was not different with respect to the control treatment.
Effects of CCB supplementation on LPS-induced ex vivo cytokine production
The results for LPS-induced inflammatory cytokines for all the samples included in this assessment are shown in the ESI, Table S3.† The values at 120 min and 240 min for the three cytokines studied, i.e. TNF-α, IL-6 and IL-10, were not significantly different to those at time 0 when comparing among the same treatments. Although, the AUC values from 0–240 min were significantly higher (p < 0.05) than the AUC values from 0–120 min for the three treatments, no significant differences among the treatments were found, either in concentration or in AUC values for the three cytokines assayed.Effects of CCB supplementation on satiety
The values of the satiety parameters analysed and the CSS are detailed in Table 5. In both groups, the two CCB interventions, i.e. treatments A and B, decreased hunger feelings in the first 0–120 min following treatment (p < 0.05, question number 3), while treatment B decreased the desire to eat (p < 0.05, question number 5) within 0–60 min as measured based on AUC values when compared with the control intervention. Similarly, at 0 min, the consumption of the product the night before (intervention B) reduced the desire to eat something salty in the Ovw group (BMI ≤ 30, n = 8) (p < 0.05, question number 4). Additionally, the desire to eat (question number 5) significantly decreased (AUC 0–60 min) when the CCB was consumed the night before (treatment B) in the Obe group (BMI > 30, n = 12). No differences were found for all the other satiety variables evaluated. All the data of the satiety perception study can be found in the ESI (Fig. S1–S3†).| Questions (no.) | Complete group, n = 20 | Group Ovw, n = 8 | Group Obe, n = 12 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | B | A | C | B | A | C | B | A | |
| Question 1: How strong is your desire to eat something sweet? 2: How full do you feel? 3: How hungry do you feel? 4: How strong is your desire to eat something salty? 5: How strong is your desire to eat? 6: How much food do you think you could eat? | |||||||||
| Satiety perception t = 0 | |||||||||
| 1 | 3.8 ± 0.4 | 2.9 ± 0.4 | 3.3 ± 0.4 | 4.0 ± 0.5 | 2.9 ± 0.7 | 2.9 ± 0.6 | 3.7 ± 0.6 | 2.9 ± 0.5 | 3.6 ± 0.5 |
| 2 | 2.7 ± 0.4 | 2.6 ± 0.4 | 3.5 ± 0.5 | 3.1 ± 0.6 | 2.8 ± 0.7 | 3.1 ± 0.8 | 2.4 ± 0.5 | 2.4 ± 0.4 | 3.8 ± 0.7 |
| 3 | 4.6 ± 0.5 | 3.6 ± 0.3 | 3.9 ± 0.3 | 3.6 ± 0.7 | 3.1 ± 0.4 | 3.8 ± 0.6 | 5.2 ± 0.6 | 3.8 ± 0.4 | 4.0 ± 0.5 |
| 4 | 4.7 ± 0.5 | 2.7 ± 0.4 | 3.4 ± 0.4 | 3.3 ± 0.6 # | 1.9 ± 0.4 * | 3.3 ± 0.8 # | 4.0 ± 0.6 | 3.2 ± 0.4 | 3.4 ± 0.6 |
| 5 | 4.9 ± 0.4 | 3.9 ± 0.3 | 4.1 ± 0.3 | 4.3 ± 0.6 | 3.4 ± 0.6 | 4.0 ± 0.5 | 5.3 ± 0.5 | 4.2 ± 0.4 | 4.1 ± 0.4 |
| 6 | 5.1 ± 0.3 | 4.7 ± 0.4 | 4.9 ± 0.3 | 4.8 ± 0.4 | 4.4 ± 0.5 | 4.5 ± 0.4 | 5.2 ± 0.4 | 4.9 ± 0.5 | 5.2 ± 0.4 |
| CSS | 80.1 ± 0.2 | 80.8 ± 0.2 | 80.7 ± 0.2 | 80.5 ± 0.3 | 81.2 ± 0.3 | 80.8 ± 0.3 | 79.8 ± 0.4 | 80.6 ± 0.3 | 80.6 ± 0.4 |
| AUC (0–60 min) | |||||||||
| 1 | 177 ± 17 | 143 ± 13 | 153 ± 16 | 188 ± 17 | 135 ± 17 | 128 ± 18 | 170 ± 24 | 148 ± 18 | 170 ± 21 |
| 2 | 246 ± 25 | 239 ± 22 | 281 ± 30 | 274 ± 26 | 244 ± 31 | 255 ± 35 | 228 ± 36 | 235 ± 29 | 298 ± 42 |
| 3 | 189 ± 14 | 161 ± 9 | 168 ± 11 | 158 ± 20 | 146 ± 13 | 158 ± 11 | 210 ± 16 | 170 ± 11 | 175 ± 15 |
| 4 | 179 ± 20 | 135 ± 15 | 174 ± 15 | 165 ± 30 | 109 ± 15 | 176 ± 16 | 188 ± 24 | 153 ± 20 | 173 ± 21 |
| 5 | 210 ± 12 * | 167 ± 11 # | 176 ± 11 #, * | 184 ± 13 | 154 ± 16 | 161 ± 12 | 228 ± 14 * | 175 ± 12 # | 185 ± 14 #, * |
| 6 | 240 ± 16 | 231 ± 19 | 228 ± 17 | 221 ± 21 | 206 ± 20 | 191 ± 17 | 253 ± 20 | 248 ± 28 | 253 ± 23 |
| AUC (0–120 min) | |||||||||
| 1 | 336 ± 33 | 288 ± 37 | 312 ± 30 | 330 ± 28 | 285 ± 37 | 270 ± 33 | 340 ± 51 | 290 ± 36 | 340 ± 41 |
| 2 | 456 ± 52 | 414 ± 42 | 504 ± 51 | 488 ± 64 | 375 ± 34 | 475 ± 58 | 435 ± 71 | 440 ± 64 | 525 ± 73 |
| 3 | 402 ± 34 * | 327 ± 17 # | 348 ± 19 # | 338 ± 42 | 300 ± 21 | 323 ± 16 | 445 ± 41 | 345 ± 21 | 365 ± 29 |
| 4 | 342 ± 36 | 282 ± 28 | 339 ± 31 | 278 ± 41 | 2323 ± 25 | 345 ± 40 | 385 ± 46 | 315 ± 40 | 335 ± 41 |
| 5 | 417 ± 29 | 360 ± 23 | 369 ± 21 | 360 ± 31 | 3223 ± 36 | 354 ± 27 | 455 ± 39 | 385 ± 25 | 380 ± 27 |
| 6 | 489 ± 33 | 474 ± 40 | 486 ± 34 | 420 ± 36 | 428 ± 44 | 413 ± 31 | 535 ± 42 | 505 ± 56 | 535 ± 46 |
Effects of CCB supplementation on exosomal miRNA expression
The characteristics of the isolated exosomes obtained from NTA are provided in the ESI (Fig. S4†). No significant size or protein content changes were observed between 0 and 2 h after extract intake. Moreover, as shown in Fig. S4B,† plasma exosomes were enriched in CD63 and TSG101, whereas other non-exosomal proteins (calnexin and the endoplasmic reticulum) showed a lesser proportion. This slight contamination could be derived from using the precipitation kit as the isolation technique.Regarding the miRNA expression levels, among the 87 miRNAs analysed, 32 showed significant changes in at least one of the three interventions when comparing baseline to 2 h-later levels (data not shown). Of these 32, 9 miRNAs whose fold change varied |0.5| between both time points were selected for further analysis: miR-15b-5p, miR-17-5p, miR-20a-5p, miR-20b-5p, miR-23a-3p, miR-146a-5p, miR-369-3p, miR-223-3p, and miR-483-5p (Fig. 3). The results showed that the exosomal miR-15b-5p, miR-20a-5p, miR-20b-5p, and miR-23a-3p plasma levels were upregulated after the hypercaloric breakfast taken together with a supplementation of cocoa and carob-derived polyphenols (intervention A). At the same time, the miR-17-5p, miR-146a-5p, miR-369-3p, and miR-483-5p circulating levels were significantly downregulated when the participants consumed the polyphenols the night before having the hypercaloric breakfast (intervention B). Also, miR-17-5p was commonly modulated by both interventions with polyphenols (A and B), showing a more significant change when taken at the same time (intervention A). None of these miRNAs were significantly altered in the control intervention (treatment C), while miR-223-3p was significantly modified in all three interventions, which could reflect its susceptibility to change under dietary intervention, and for this reason, it was excluded for further analysis.
Finally, Fig. 4 shows the potential targets and pathways involved in the miRNAs modulated by the CCB. We found 96 target genes for treatment A and 83 genes for treatment B; these were involved in biological processes (BPs), molecular functions (MFs) and associated KEGG pathways. The overrepresented pathways for treatment A included the PI3K-Akt, FoxO or MAPK signalling pathways. While for treatment B, these included monocyte chemotaxis, inflammatory response and the chemokine-mediated signalling pathway.
Discussion
The increasing prevalence of T2D and the consequent diabesity6 make it necessary to address new strategies for their management, especially considering their multiple associated complications. In this context, cocoa and carob, as sources of bioactive compounds, have been proven to have potential as adjuvants in the treatment of metabolic disorders, mostly through in vitro and preclinical studies. Herein, we performed the first randomised controlled nutritional trial with the CCB, a product that combines the bioactive constituents of cocoa and carob and that was previously studied in an animal model of T2D with positive results.22,23 The trial was conducted in subjects with T2D, treated with metformin and exhibiting overweight/obesity; results were assessed either for the whole group or separately for subjects with overweight or obesity. It was found that although both situations cause metabolic alterations, they differentially affect other health outcomes. No gender stratification was possible for data processing since only 15% of the subjects were women. Nevertheless, this data coincide with the current distribution of T2D, which is more common in men.27In contrast to our hypothesis for this randomised controlled nutritional trial, supplementation with 10 g of the CCB during breakfast or the night before did not cause an overall significant variation in the primary outcome of the study, i.e. on postprandial insulin. This hypothesis was based on the existing evidence on the beneficial effects of polyphenols and dietary fibre in postprandial insulinaemia, as shown in reviews on clinical trials on the topic.28,29 Although, at the same time, the same analysis of the literature revealed other studies with null effects in the same variable, which evidences the complexity of the involved factors in nutrition studies.
Similarly to the observed results for insulin, moreover, it did not affect other parameters of glucose homeostasis (postprandial glucose and GLP-1 levels), compared to the control intervention. Nevertheless, a significant decrease in the increase in glucose from 0 to 30 min was shown in the overweight subgroup when consuming the product at the same time as breakfast (intervention A). The physiological relevance of this reduction is that postprandial glucose peaks are considered an independent risk factor for cardiovascular diseases due to different mechanisms of action; this is particularly important in the context of T2D, with a high prevalence of associated cardiovascular complications. This CCB effect may be associated with its polyphenol profile. In this sense, some randomised controlled trials have reported a reduction of glucose levels after supplementation with polyphenol-rich chocolate in young participants30 or supplements enriched in cocoa flavonoids in older subjects,31 although other studies did not find such results.32 In the case of carob, although the evidence is still limited, a trial conducted with 40 healthy volunteers aimed to evaluate the effect of consuming a drink enriched with bioactive compounds from carob. After 12 weeks of supplementation and under a normocaloric diet, the authors found that there was a decrease in glucose levels in subjects after breakfast, lunch, and dinner.33
Regarding other parameters involved in glucose homeostasis, the CCB did not significantly modify postprandial insulin levels, in contrast to a trial where the intake of cagaita (Eugenia dysenterica DC) fruit juice by dysglycaemic subjects with metabolic syndrome, together with breakfast, led to a significant decrease in plasma insulin levels compared with only breakfast.34 Interestingly, another study on blackcurrant extract did not observe modifications in postprandial sensitivity after acute intake by subjects with overweight/obesity, but a significant improvement in insulin sensitivity was observed after consuming the product for eight days.35 Therefore, a similar effect after chronic CCB supplementation could not be excluded. In our trial, no modification was found in GLP-1 levels after CCB supplementation. Other postprandial studies with acute supplementations in healthy adults with flavanol-rich products have reported both beneficial and null effects on active GLP-1.36,37 Herein, total GLP-1 was measured but the discrepant results may be mostly linked to the fact that the postprandial regulation of GLP-1 in subjects with overweight/obesity is not yet completely understood, as evidenced from the results in different cohorts.
Another significant effect associated with CCB supplementation was an improvement in the postprandial satiety as assessed by the validated VAS, an approach that, in an earlier meta-analysis, provided a consistently significant (although of low magnitude) correlation with further energy intake.38 It is likely that this effect was mostly due to the high dietary fibre content of CCB since, for instance, a previous trial with two nutraceuticals containing only polyphenols or a combination of polyphenols and dietary fibre found that the second one was more efficient for satiety regulation in subjects who were overweight/obese, by both objective and subjective determinations.39 Nevertheless, a trial where healthy subjects were supplemented with a polyphenol-rich extract from pomegranate (lacking dietary fibre) found significant improvements in satiety parameters as measured by VAS, although the mechanism of action was not assessed.40 At the same time, the obtained results for the subjective assessment of satiety perception seem to be in contradiction with the lack of effect in GLP-1 levels. In this sense, it should be mentioned that other studies based on a supplementation with polyphenol-rich or dietary fibre-rich products also reported significant effects in VAS not accompanied by modifications in GLP-1.39,41 Due to the complexity of all the signals involved in appetite regulation,42 more research is needed to ascertain how dietary polyphenols and dietary fibre may contribute to the neuroendocrine regulation of hunger.
Another relevant aspect regarding the satiety effects obtained here is the fact that the modulating effects took place not only when the product was consumed together with breakfast but also when it was consumed the night before, which highlights the contribution of the derived metabolites, resulting from dietary fibre and/or from polyphenols. In this way, a previous study on bread or porridge from rye kernel, compared with white bread, found a decrease in satiety not only 3 h after but even 7 h after intake.43 Moreover, another study focused on supplementation with several pulses compared with macaroni and cheese found that the effects on subjective appetite led to a significant decrease in energy intake in the next meal.44 Besides, a study on brown beans compared with white bread found that their consumption the night before not only produced a significant decrease in subjective hunger the next morning, but also in the circulating levels of ghrelin and in clinical markers such as postprandial glucose, postprandial insulin and IL-8.45
Regarding ex vivo analysis, cytokine release after LPS stimulation was not modified either after treatment A or B. Similar previous ex vivo studies where subjects were supplemented with bioactive-rich samples such as extra virgin olive oil (in patients undergoing coronary angiography) and a spice blend (in subjects with overweight/obesity) observed significant modifications in the final levels of IL-10 and IL-6, respectively, compared with control groups, even when the LPS treatment did not induce significant modifications over time in the control group.46,47 Since consistent evidence supports the role of polymeric flavanols as anti-inflammatory agents, the results observed here would be likely associated with an insufficient dose; indeed, the mentioned trial with a spice blend found an ex vivo anti-inflammatory effect with a dose of 6 g of the blend but not with a 2 g dose.46
This trial also aimed to evaluate the underlying molecular modifications that may occur due to CCB supplementation beyond just the clinical markers. In this context, miRNA modulation has been suggested as an additional mechanism of action of polyphenol,48 mostly after chronic intake, although a recent trial also found differences during the postprandial state.49 The results observed here indicated that circulating miRNAs transported in exosomes could be both induced and repressed in response to CCB consumption. In particular, a tendency towards an improvement in insulin sensitivity was observed. Thus, among the miRNAs significantly increased by treatment A, miR-20a-5p has been found to be downregulated in T2D and its overexpression associated with an enhancement in insulin sensitivity and hepatic glycogen synthesis, as well as a decrease in hepatic steatosis.50,51 Similarly, miR-23a-3p has been reported to be decreased in patients with T2D compared with people with normal glucose tolerance test results,52 while the CCB induced its release into the circulation. Furthermore, miR-17-5p, with increased expressions after treatments A and B compared with after treatment C, has been reported to improve insulin sensitivity and decrease steatosis.53 It should be mentioned that all three of them were significantly modified after treatment A, while only miR-17-5p was significantly modified (and to a lesser extent) after treatments A and B. Thus, the effect of CCB supplementation on miRNA expression seems to be more connected with the phenolic compounds directly observed after intake than to the metabolites derived from prolonged transformations after intake. Nevertheless, further studies are needed in order to confirm these effects, since the overexpression of miR-15b-5p detected after treatment A has been connected with a decrease in insulin sensitivity.54 Additionally, it is remarkable that miR-146a-5p, increased after treatment B, has been reported to be highly expressed in the milk-derived extracellular vesicles from pregnant rats receiving a diet enriched in resistant starch, a type of dietary fibre; thus, there may be a connection with this dietary constituent.55 The fact that these modifications in miRNA expression were not connected with changes in the markers of glucose homeostasis may be due with the fact that miRNA expression may express subtle modifications that may be present before the clinical markers are affected.56
The main strengths of this trial were that the composition of the supplemented product was fully characterized and that a robust crossover design was established, with comprehensive assessment of baseline subject characteristics, including a study of the liver status performed via echography and FibroScan (indeed, most participants exhibited NAFLD, although they were unaware, according to other evidence on the underdiagnosis of this pathology).57 Furthermore, we explored the molecular mechanism underlying clinical modifications, based on exosomal miRNA analyses, which has been scarcely assessed in previous postprandial studies. The main limitation is the acute design, based on the effect of a single serving of the product; for this reason, despite the randomised controlled design, it should be considered a pilot approach. Moreover, it was not possible to ascertain whether the observed effects were due to polyphenols, dietary fibre or both. Furthermore, future studies should include objective measurements of satiety and hunger, which would greatly complement the subjective results reported here. Some population adjustments in future studies are also recommended, including a replication study, specifically for a female population, considering the high proportion of male participants in this trial, and a replication study with enough statistical data, for validating the specific effects observed in individuals with overweight/obesity.
In summary, this acute randomised crossover controlled nutritional trial on the postprandial effects of CCB in subjects with T2D showed promising significant differences regarding postprandial hyperglycaemia in subjects who were overweight (when CCB was consumed together with a hypercaloric breakfast); satiety perception in the whole group and in subjects who were obese (when CCB was consumed together with a hypercaloric breakfast or the night before); and significant upregulations or downregulations of several miRNAs, which are mostly involved in pathways related to insulin sensitivity. At the same time, no adverse effect was reported. These results may be attributed to a combination of the mechanisms of action reported for both polyphenols and dietary fibre as bioactive constituents of CCB. A chronic intervention randomised controlled trial with this product should be guaranteed in order to confirm the observed results, as well as the potential sustained metabolic modifications.
Author contributions
M. A.-M., S. R. and J. P.-J. conceived and planned the study. S. G.-C., J. I. V.-D. and J. P.-J. identified and selected the patient cohort. M. V., E. G.-D and J. P.-J. performed the nutritional intervention and sample processing. M. V., E. G.-D, A. D., M. A.-B. and M. C. L.-H. performed analytical procedures. O. L.-I. was responsible for hepatic steatosis assessment and data processing. M. V. performed the statistical analysis. M. V. and E. G.-D. generated the figures. E. G.-D., M. V., and J. P. J. integrated the data and wrote the original manuscript. M. V., M. A.-M., S. R. and J. P. J. wrote and edited the revised version. All the authors contributed to data analysis and discussion and agreed to submit the manuscript.Data availability
The data for this article, including all the raw data used in the generation of tables and figures (also those provided as ESI†) are available at the public repository digital.csic.es of the Spanish Research Council (CSIC) at https://hdl.handle.net/10261/367662.The data for this article, including all the raw data used in the generation of tables and figures (also those provided as ESI†) are available at the public repository digital.csic.es of the Spanish Research Council (CSIC) at https://digital.csic.es/handle/10261/367662.
Conflicts of interest
J. P.-J. participated as an invited speaker at a workshop organized by EIG, joining Spanish carob companies; travel expenses were covered by EIG, but she did not receive any direct payment. J. P.-J. received research funding from ADM Wild Valencia SAU for carob analysis. M. A. M. has participated as an invited speaker in different outreach activities organized by the Fundación Caixa Forum and CSIC. M. A. M. is the author of a dissemination book on chocolate published by Editorial Catarata, Madrid, Spain. M. A. M. received research funding from the FECYT (FCT-15-9827) to carry out dissemination activities on the benefits of cocoa. The remaining authors declare no competing interest.Acknowledgements
This work was supported by the grant RTI2018-095059-B-I00, funded by MCIN/AEI/10.13039/501100011033/ and “ERDF A way of making Europe”. The study was partially funded by TED2021-130962B-C21 and financed by MICIU/AEI/10.13039/501100011033 and the European Union NextGenerationEU/PRTR. None of the funders were involved in the assessment and interpretation of the data or in manuscript preparation. E. G.-D. was the recipient of a contract from Comunidad de Madrid (PEJ-2020-AI/BIO-18529). M. V. was the recipient of a postdoctoral contract “Margarita Salas” (Plan de Recuperación, Transformación y Resiliencia) from the Spanish Ministry of Universities and Madrid Autonomous University (CA1/RSUE/2021-00588). MCLH is a recipient of of a Ramon y Cajal Grant RYC2022-037626-I, MCIU/AEI/10.13039/501100011033 and FSE+. CIBEROBN (CB22/03/00068) and CIBERDEM (CB/07/08/0013) are initiatives of the Instituto de Salud Carlos III, Spain. Ms M. Nogués Cañadas, Mr I. Escudero Abad and Ms. M. Renard are thanked for technical assistance. Ms Macarena Paredes-López, from Siemens, provided technical support during abdominal echographia and transient elastography assessments.References
- L. Guariguata, D. R. Whiting, I. Hambleton, J. Beagley, U. Linnenkamp and J. E. Shaw, Global estimates of diabetes prevalence for 2013 and projections for 2035, Diabetes Res. Clin. Prac., 2014, 103, 137–149 CrossRef CAS PubMed.
- J. M. Forbes and M. E. Cooper, Mechanisms of diabetic complications, Physiol. Rev., 2013, 93, 137–188 CrossRef CAS PubMed.
- C. P. Domingueti, L. M. S. A. Dusse, M. das Graças Carvalho, L. P. de Sousa, K. B. Gomes and A. P. Fernandes, Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications, J. Diabetes Complications, 2016, 30, 738–745 CrossRef PubMed.
- G. Targher, K. E. Corey, C. D. Byrne and M. Roden, The complex link between NAFLD and type 2 diabetes mellitus—mechanisms and treatments, Nat. Rev. Gastroenterol. Hepatol., 2021, 18, 599–612 CrossRef PubMed.
- American Society for Metabolic and Bariatric Surgery, Type 2 Diabetes and Metabolic Surgery, 2018, https://asmbs.org/resources/type-2-diabetes-and-metabolic-surgery-fact-sheet Search PubMed.
- M. I. Schmidt and B. B. Duncan, Diabesity: an inflammatory metabolic condition, Clin. Chem. Lab. Med., 2003, 41, 1120–1130 CAS.
- J. Jiang, L. Zhao and L. Lin, et al., Postprandial Blood Glucose Outweighs Fasting Blood Glucose and HbA1c in screening Coronary Heart Disease, Sci. Rep., 2017, 7, 14212 CrossRef PubMed.
- E. Wright Jr., J. L. Scism-Bacon and L. C. Glass, Oxidative stress in type 2 diabetes: the role of fasting and postprandial glycaemia, Int. J. Clin. Pract., 2006, 60, 308–314 CrossRef CAS PubMed.
- E. C. Meessen, M. V. Warmbrunn, M. Nieuwdorp and M. R. Soeters, Human postprandial nutrient metabolism and low-grade inflammation: a narrative review, Nutrients, 2019, 11, 3000 CrossRef CAS PubMed.
- C. Verdich, S. Toubro, B. Buemann, J. Lysgård Madsen, J. Juul Holst and A. Astrup, The role of postprandial releases of insulin and incretin hormones in meal-induced satiety—effect of obesity and weight reduction, Int. J. Obes., 2001, 25, 1206–1214 CrossRef CAS PubMed.
- M. Guasch-Ferré, J. Merino, Q. Sun, M. Fitó and J. Salas-Salvadó, Dietary polyphenols, Mediterranean diet, prediabetes, and type 2 diabetes: a narrative review of the evidence, Oxid. Med. Cell. Longev., 2017, 2017, 6723931 CrossRef PubMed.
- T. Nie and G. J. Cooper, Mechanisms underlying the antidiabetic activities of polyphenolic compounds: A review, Front. Pharmacol., 2021, 12, 798329 CrossRef CAS PubMed.
- D. C. Mantilla-Escalante, M. C. López de las Hazas and M. C. Crespo, et al., Mediterranean diet enriched in extra-virgin olive oil or nuts modulates circulating exosomal non-coding RNAs, Eur. J. Nutr., 2021, 60, 4279–4293 CrossRef CAS PubMed.
- A. Cortés-Martín, M. V. Selma, F. A. Tomás-Barberán, A. González-Sarrías and J. C. Espín, Where to look into the puzzle of polyphenols and health? The postbiotics and gut microbiota associated with human metabotypes, Mol. Nutr. Food Res., 2020, 64, 1900952 CrossRef PubMed.
- J. Pérez-Jiménez, M. E. Díaz-Rubio and F. Saura-Calixto, Non-extractable polyphenols, a major dietary antioxidant: Occurrence, metabolic fate and health effects, Nutr. Res. Rev., 2013, 26, 118–129 CrossRef PubMed.
- A. Reynolds, J. Mann, J. Cummings, N. Winter, E. Mete and L. Te Morenga, Carbohydrate quality and, human health: a series of systematic reviews and meta-analyses, Lancet, 2019, 393, 434–445 CrossRef CAS PubMed.
- M. Müller, E. E. Canfora and E. E. Blaak, Gastrointestinal transit time, glucose homeostasis and metabolic health: modulation by dietary fibers, Nutrients, 2018, 10, 275 CrossRef PubMed.
- R. Franco, A. Oñatibia-Astibia and E. Martínez-Pinilla, Health benefits of methylxanthines in cacao and chocolate, Nutrients, 2013, 5, 4159–4173 CAS.
- J. I. López-Sánchez, D. A. Moreno and C. García-Viguera, D-pinitol, a highly valuable product from carob pods: Health-promoting effects and metabolic pathways of this natural super-food ingredient and its derivatives, AIMS Agric. Food, 2018, 3, 41–63 Search PubMed.
- M. A. Qasem, M. I. Noordin, A. Arya, A. Alsalahi and S. N. Jayash, Evaluation of the glycemic effect of Ceratonia siliqua pods (Carob) on a streptozotocin-nicotinamide induced diabetic rat model, PeerJ, 2019, 6, e4788 CrossRef PubMed.
- E. García-Díez, H. Sánchez-Ayora, M. Blanch, S. Ramos, M. Á. Martín and J. Pérez-Jiménez, Exploring a cocoa–carob blend as a functional food with decreased bitterness: Characterization and sensory analysis, LWT–Food Sci. Technol., 2022, 165, 113708 CrossRef.
- E. García-Díez, M. E. López-Oliva and A. Caro-Vadillo, et al., Supplementation with a Cocoa–Carob blend, alone or in combination with metformin, attenuates diabetic cardiomyopathy, cardiac oxidative stress and inflammation in Zucker diabetic rats, Antioxidants, 2022, 11, 432 CrossRef PubMed.
- E. García-Díez, M. E. López-Oliva, F. Perez-Vizcaino, J. Pérez-Jiménez, S. Ramos and M. Á. Martín, Dietary Supplementation with a Cocoa–Carob Blend Modulates Gut Microbiota and Prevents Intestinal Oxidative Stress and Barrier Dysfunction in Zucker Diabetic Rats, Antioxidants, 2023, 12, 1519 CrossRef PubMed.
- R. Törrönen, E. Sarkkinen, T. Niskanen, N. Tapola, K. Kilpi and L. Niskanen, Postprandial glucose, insulin and glucagon-like peptide 1 responses to sucrose ingested with berries in healthy subjects, Br. J. Nutr., 2012, 107, 1445–1451 CrossRef PubMed.
- D. H. Dong HongLin, L. J. Sargent and Y. Chatzidiakou, et al., Orange pomace fibre increases a composite scoring of subjective ratings of hunger and fullness in healthy adults, Appetite, 2016, 107, 478–485 CrossRef PubMed.
- D. Handayani, L. D. A. Oktafiani, R. T. Abednego and S. Andarini, Effect of international and Indonesian fast foods on plasma ghrelin and glucagon like peptide-1 (GLP-1) levels, hunger and satiety scores is similar in obese adults, Pak. J. Nutr., 2017, 16, 38–44 CrossRef CAS.
- A. Kautzky-Willer, M. Leutner and J. Harreiter, Sex differences in type 2 diabetes, Diabetologia, 2023, 66, 986–1002 Search PubMed.
- D. W. Davis, J. W. Navalta, G. R. McGinnis, R. Serafica, K. Izuora and A. Basu, Effects of Acute Dietary Polyphenols and Post-Meal Physical Activity on Postprandial Metabolism in Adults with Features of the Metabolic Syndrome, Nutrients, 2020, 12(4), 1120 CAS.
- S. Tsitsou, C. Athanasaki, G. Dimitriadis and E. Papakonstantinou, Acute Effects of Dietary Fiber in Starchy Foods on Glycemic and Insulinemic Responses: A Systematic Review of Randomized Controlled Crossover Trials, Nutrients, 2023, 15(10), 2383 CAS.
- A. Leyva-Soto, R. A. Chavez-Santoscoy, L. R. Lara-Jacobo, A. V. Chavez-Santoscoy and L. N. Gonzalez-Cobian, Daily consumption of chocolate rich in flavonoids decreases cellular genotoxicity and improves biochemical parameters of lipid and glucose metabolism, Molecules, 2018, 23, 2220 Search PubMed.
- L. Munguia, et al., High flavonoid cocoa supplement ameliorates plasma oxidative stress and inflammation levels while improving mobility and quality of life in older subjects: a double-blind randomized clinical trial, J. Gerontol., Ser. A, 2019, 74, 1620–1627 CAS.
- J. Rynarzewski, L. Dicks and B. F. Zimmermann, et al., Impact of a usual serving size of flavanol-rich cocoa powder ingested with a diabetic-suitable meal on postprandial cardiometabolic parameters in type 2 diabetics—a randomized, placebo-controlled, double-blind crossover study, Nutrients, 2019, 11, 417 CAS.
- C. Bañuls, S. Rovira-Llopis and R. Falcón, et al., Chronic consumption of an inositol-enriched carob extract improves postprandial glycaemia and insulin sensitivity in healthy subjects: A randomized controlled trial, Clin. Nutr., 2016, 35, 600–607 Search PubMed.
- R. L. de Araujo, F. A. Tomas-Barberan, R. F. Dos Santos, J. A. Martinez-Blazquez and M. I. Genovese, Postprandial glucose-lowering effect of cagaita (Eugenia dysenterica DC) fruit juice in dysglycemic subjects with metabolic syndrome: An exploratory study, Food Res. Int., 2021, 142, 110209 Search PubMed.
- A. Nolan, R. Brett, J. A. Strauss, C. E. Stewart and S. O. Shepherd, Short-term, but not acute, intake of New Zealand blackcurrant extract improves insulin sensitivity and free-living postprandial glucose excursions in individuals with overweight or obesity, Eur. J. Nutr., 2021, 60, 1253–1262 CAS.
- K. Stote, A. Corkum, M. Sweeney, N. Shakerley, T. Kean and K. Gottschall-Pass, Postprandial effects of blueberry (Vaccinium angustifolium) consumption on glucose metabolism, gastrointestinal hormone response, and perceived appetite in healthy adults: a randomized, placebo-controlled crossover trial, Nutrients, 2019, 11, 202 CAS.
- Y. Kawakami, Y. Watane and M. Mazuka, et al., Effect of cacao polyphenol-rich chocolate on postprandial glycemia, insulin, and incretin secretion in healthy participants, Nutrition, 2021, 85, 111128 CAS.
- B. C. Sadoul, E. A. H. Schuring, D. J. Mela and H. P. F. Peters, The relationship between appetite scores and subsequent energy intake: an analysis based on 23 randomized controlled studies, Appetite, 2014, 83, 153–159 Search PubMed.
- M. Redondo-Puente, R. Mateos and M. A. Seguido, et al., Appetite and satiety effects of the acute and regular consumption of green coffee phenols and green coffee phenol/oat β-glucan nutraceuticals in subjects with overweight and obesity, Foods, 2021, 10, 2511 CAS.
- A. Stockton and E. A. Al-Dujaili, Effect of pomegranate extract consumption on satiety parameters in healthy volunteers: A preliminary randomized study, Foods, 2022, 11, 2639 CAS.
- G. Costabile, E. Griffo, P. Cipriano, C. Vetrani, M. Vitale, G. Mamone, A. A. Rivellese, G. Riccardi and R. Giacco, Subjective satiety and plasma PYY concentration after wholemeal pasta, Appetite, 2018, 125, 172–181 CrossRef PubMed.
- O. B. Chaudhri, V. Salem, K. G. Murphy and S. R. Bloom, Gastrointestinal satiety signals, Annu. Rev. Physiol., 2008, 70, 239–255 CrossRef CAS PubMed.
- H. Isaksson, A. Rakha, R. Andersson, H. Fredriksson, J. Olsson and P. Aman, Rye kernel breakfast increases satiety in the afternoon - an effect of food structure, Nutr. J., 2011, 10, 31 CrossRef PubMed.
- R. C. Mollard, C. L. Wong, B. L. Luhovyy and G. H. Anderson, First and second meal effects of pulses on blood glucose, appetite, and food intake at a later meal, Appl. Physiol. Nutr. Metab., 2011, 36(5), 634–642 CrossRef CAS PubMed.
- A. Nilsson, E. Johansson, L. Ekström and I. Björck, Effects of a brown beans evening meal on metabolic risk markers and appetite regulating hormones at a subsequent standardized breakfast: a randomized cross-over study, PLoS One, 2013, 8(4), e59985 CrossRef CAS PubMed.
- E. S. Oh, K. S. Petersen, P. M. Kris-Etherton and C. J. Rogers, Spices in a high-saturated-fat, high-carbohydrate meal reduce postprandial proinflammatory cytokine secretion in men with overweight or obesity: a 3-period, crossover, randomized controlled trial, J. Nutr., 2020, 150, 1600–1609 CrossRef PubMed.
- N. Khandouzi, A. Zahedmehr and J. Nasrollahzadeh, Effect of polyphenol-rich extra-virgin olive oil on lipid profile and inflammatory biomarkers in patients undergoing coronary angiography: A randomised, controlled, clinical trial, Int. J. Food Sci. Nutr., 2021, 72, 548–558 CrossRef CAS PubMed.
- D. Milenkovic, B. Jude and C. Morand, miRNA as molecular target of polyphenols underlying their biological effects, Free Radical Biol. Med., 2013, 64, 40–51 CrossRef CAS PubMed.
- R. V. Bastos, M. S. Dorna and F. Chiuso-Minicucci, et al., Acute green tea intake attenuates circulating microRNA expression induced by a high-fat, high-saturated meal in obese women: A randomized crossover study, J. Nutr. Biochem., 2023, 112, 109203 CrossRef CAS PubMed.
- D. Ye, G. Lou, T. Zhang, F. Dong and Y. Liu, MiR–17 family–mediated regulation of Pknox1 influences hepatic steatosis and insulin signaling, J. Cell. Mol. Med., 2018, 22, 6167–6175 CrossRef CAS PubMed.
- W. Fang, J. Guo and Y. Cao, et al., Micro RNA–20a–5p contributes to hepatic glycogen synthesis through targeting p63 to regulate p53 and PTEN expression, J. Cell. Mol. Med., 2016, 20, 467–1480 Search PubMed.
- Z. Yang, H. Chen and H. Si, et al., Serum miR-23a, a potential biomarker for diagnosis of pre-diabetes and type 2 diabetes, Acta Diabetol., 2014, 51, 823–831 CAS.
- Y. Jiang, L. Wei and H. Zhang, et al., MiR-17-5p promotes glucose uptake of HTR8/SVneo trophoblast cells by inhibiting TXNIP/NLRP3 inflammasome pathway, Diabetes Metab. Syndr. Obes., 2022, 15, 3361–3374 CAS.
- W. D. Li, J. R. Xia and Y. S. Lian, MiR-15b can target insulin receptor to regulate hepatic insulin signaling in mice, Anim. Cells Syst., 2019, 23, 82–89 CAS.
- D. Lu, Y. Liu and L. Kang, et al., Maternal fiber-rich diet promotes early-life intestinal development in offspring through milk-derived extracellular vesicles carrying miR-146a-5p, J. Nanobiotechnol., 2024, 22, 65 CAS.
- N. Mazgutova, I. Witvrouwen, B. Czippelova, Z. Turianikova, J. Cernanova Krohova, P. Kosutova, M. Kuricova, D. Cierny, P. Mikolka, E. Van Craenenbroeck and M. Javorka, Involvement of circulating microRNAs in the pathogenesis of atherosclerosis in young patients with obesity, Physiol. Res., 2024, 73(S3), S755–S769 CAS.
- J. M. Clark, D. R. H. Cryer, M. Morton and J. H. Shubrook, Nonalcoholic fatty liver disease from a primary care perspective, Diabetes Obes. Metab., 2023, 25(6), 1421–1433 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo04498c |
| This journal is © The Royal Society of Chemistry 2025 |