Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The influence of fruit juice extracts on glucose intestinal transporters and antioxidant genes in a Caco-2 and HT29-MTX co-culture cell system†
Magdalena
Köpsel
 a,
Tina
Kostka
a,
Tina
Kostka
 ab,
Miriam
Rodriguez-Werner
c and
Tuba
Esatbeyoglu
ab,
Miriam
Rodriguez-Werner
c and
Tuba
Esatbeyoglu
 *a
*a
aDepartment of Molecular Food Chemistry and Food Development, Institute of Food and One Health, Gottfried Wilhelm Leibniz University Hannover, Am Kleinen Felde 30, 30167 Hannover, Germany. E-mail: koepsel@foh.uni-hannover.de; esatbeyoglu@foh.uni-hannover.de
bDivision of Food Chemistry and Toxicology, Department of Chemistry, RPTU Kaiserslautern-Landau, Erwin-Schrödinger-Strasse 52, 67663 Kaiserslautern, Germany. E-mail: kostka@rhrk.uni-kl.de
cTentamus chelab GmbH, Carl-Zeiss-Str. 16, 30966 Hemmingen, Germany. E-mail: miriam.rodriguez-werner@tentamus.com
First published on 3rd February 2025
Abstract
In recent years, the interest of consumers in fruit juice extracts as nutraceuticals has increased. Fruits, especially red berries, contain valuable bioactive compounds such as polyphenols. Polyphenols are often associated with anti-oxidant, anti-inflammatory, anti-diabetic, anti-cancer, cardioprotective and gastroprotective properties. However, the relationship between the various effects of fruit juice extracts and their influence on the permeability of the intestinal barrier, as well as their influence on glucose transport across the intestinal membrane, is not known. Therefore, in the present study, anthocyanins and copigments were obtained from 11 fruit juice extracts by XAD7 column chromatography and characterized their health-promoting effects, as well as their influence on the intestinal membrane. Chokeberry, pomegranate and blueberry extracts showed the highest antioxidant activity, but showed incomplete regeneration of the intestinal membrane upon treatment-induced higher permeability. This may depended on the high anthocyanin level of these extracts. Treatments with gojiberry extract, elderberry extract and the copigment fraction of apple achieved the best suitable regeneration of the intestinal barrier. The transcription of epithelial glucose transporters GLUT1 und GLUT2 as well as for the oxidative stress genes catalase (CAT) and superoxide dismutase (SOD) were most effectively reduced by chokeberry extract. To sum up, fruit juice extracts possess high antioxidant potentials and can reduce the expression of antioxidant enzymes and glucose transporters in colon cells. While the glucose uptake may be reduced, the intestinal permeability is increased, which varies due to the extract composition. Therefore, fruit juice extracts need to be fractionated and characterized in more detail to identify the health-beneficial compounds.
1. Introduction
Major problems currently affecting human health are cardiovascular diseases, infections, cancer and diabetes.1 All of these diseases are associated with oxidative stress, which leads to cell-dependent apoptosis and the progression of pathogenesis. To prevent the formation of free radicals that lead to cell damage, antioxidants play an important role in human health.2 The antioxidant and beneficial effects of fruit and fruit juices has already been well described in the literature. Studies have shown that regular consumption of chokeberry juice can reduce the incidence of urinary tract infections3 as well as lowering the blood pressure and total cholesterol levels, thus potentially having cardioprotective effects.4–6 These positive effects are mainly attributed to polyphenols, known as secondary plant metabolites.7,8 Polyphenols occur in fruits, vegetables, spices and herbs.9 Moreover, polyphenols can affect the intestinal epithelium by strengthening its barrier function as an inflammatory prevention against gut microbiota.The integrity of the human intestinal barrier is critical to human health for the maintenance of physiological intestinal permeability, which modulates the transport and absorption of nutrients (e.g. sugars, vitamins, amino acids, fatty acids and other lipids) and other food-related compounds (e.g. polyphenols). The permeability is significantly affected by the diet. For instance, the Western diet is characterized by high fat and sugar consumption, which increases the permeability and may induce an inflammatory response.10 In contrast, the Mediterranean diet, rich in fruit, vegetables and fibre, has been shown to prevent increased intestinal permeability.11 Regard to these; numerous studies have been conducted in recent years to test the efficacy of dietary interventions to improve the intestinal barrier function and prevent associated diseases, with a particular focus on polyphenols.12,13 Some studies have already confirmed the positive effects of e.g. polyphenols from apples on the intestine and the intestinal epithelium.14–17 While high concentrations of glucose reduced the viability of Caco-2 cells, these effects were inhibited by fruit polyphenols.16 It was also seen that polyphenols reduced the glucose uptake by modulation of corresponding transporter proteins.16 Glucose transport is of great importance for energy metabolism. The cellular glucose uptake takes place in the cell membrane mainly through two transporters depending on the present luminal glucose concentration. At low concentrations (<30 mM), glucose is transported in the cell against a concentration gradient by an active transport mechanism in which glucose is transported together with sodium ions via the high-affinity sodium-dependent glucose transporter 1 (SGLT1).18 At higher concentrations (>30 mM), glucose is mainly transported by the facilitated transporter with lower affinity, GLUT2.18 The further transport of glucose in the blood vessel is mediated by basolateral GLUT2 transporters.18 Polyphenols have been shown to interact with certain sugar transporters, for example by competitively inhibiting SGLT1,19 or by inhibiting GLUT2.20 Therefore, polyphenols have the potential to influence glucose uptake in the intestine. The effects of some polyphenols on the sugar transporters GLUT2 and SGLT1 have already been tested in intestinal Caco-2 cells. The study of Manzano & Williamson14 showed that strawberry and apple polyphenols are potent inhibitors of GLUT2 and SGLT1 at concentrations predicted after food intake. Therefore, the consumption of strawberry and apple juices could influence glucose uptake by inhibiting glucose transports like GLUT1, GLUT2 and SGLT1, which may influence the activity of DPP4, CAT and SOD as associated enzymes.14 While there are already several studies available, analysing the effects of fruit juice extracts on intestinal permeability and uptake, the results are poorly comparable. The generation of the extracts varied greatly, while the experimental setup was different. For the first time, a broad range of fruit juice extracts will be generated under the same conditions and tested simultaneously, to compare the results. Moreover, the more realistic co-culture model of differentiated Caco-2 and HT29-MTX cells will be used.
This study aimed to investigate 11 different extracts from fruit juices (blueberry, sour cherry, elderberry, red grape, chokeberry, cranberry, pomegranate, haskapberry, goji berry, apple and plum). While the individual polyphenol content and composition was identified, their influence on the permeability of the intestinal barrier, their antioxidant properties and their efficacy with regard to glucose transport across the intestinal membrane were analyzed. The observed effects were discussed in relation to the individual polyphenol composition in order to identify the most effective and promising fruits or compounds for future health-promoting and health-maintaining applications. The enrichment of polyphenols was carried out by preparing a sugar-free XAD7-extract from different conventional fruit juices. The effects of each extract on intestinal permeability were then analyzed by in vitro tests using an established cellular transwell intestinal model with Caco-2 and HT29-MTX cells in co-culture, as well as the expression of the markers GLUT1, GLUT2, DPP4 and SGLT1, which are involved in glucose metabolism, and the oxidative stress markers CAT and SOD.
2. Materials and methods
2.1 Chemicals
Double deionized water was obtained from Elga Labwater Purelab Flex 3 (Veolia, Celle, Germany). Amberlite XAD-7, ammonium carbonate, 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS, 98%) calcium chloride dihydrate, carbazole, galacturonic acid monohydrate, potassium sodium tartrate tetrahydrate, copper(II) sulfate-5-hydrate, sodium hydrogen carbonate, sodium fluorescein and hydrochloric acid (37%) were purchased from Sigma-Aldrich (Steinheim, Germany) and Merck KGaA (Darmstadt, Germany). Potassium peroxodisulfate (K2S2O8) was obtained from Riedel-de-Haën (Seelze, Germany). Acetonitrile (HPLC grade) and acetic acid (HPLC grade) were purchased from VWR International (Leuven, Belgium). Folin–Ciocalteu reagent and dimethyl sulfoxide (p.a.) was purchased from Merck KGaA. Ethanol (denatured with methyl ethyl ketone, 99%) was purchased from Walter CMP (Kiel, Germany). Gallic acid monohydrate and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox, ≥98% purity) were purchased from Fluka (Buchs, Switzerland). Formic acid (HPLC grade), methanol (HPLC grade), ethyl acetate (≥99% p.a.) and PBS were purchased from Th. Geyer (Höxter, Germany). Capric acid (C10), acetic acid (conc.), potassium chloride (≥99% p.a.), potassium hydrogen phosphate (≥99% p.a.), sodium chloride (≥99% p.a.), sodium hydroxide (≥98% p.a.), Triton X-100 and trypan blue solution (0.4%) were purchased from Carl Roth (Karlsruhe, Germany). Dulbecco's Modified Eagle Medium (DMEM), Fetal Bovine Serum Standard (FBS), Hanks’ Balanced Salt Solution (HBSS, without phenol red, with Ca2+, Mg2+ and 0.35 g L−1 NaHCO3), MEM Non Essential Amino Acid Solution (NEAAS), penicillin–streptomycin and trypsin/EDTA were purchased from PAN Biotech (Aidenbach, Germany). Glucose Colorimetric Assay Kit was purchased from Elabscience (Texas, USA).2.2 Fruit juices samples
Pomegranate (Punica granatum), blueberry (Vaccinum myrtillus), elderberry (Sambucus nigra), sour cherry (Prunus cerasus), grape (Vitis vinifera), plum (Prunus domestica) and apple (Pyrus malus) juices were kindly provided by the company fruit press van Nahmen (Hamminkeln, Germany). Chokeberry (Aronia melanocarpa) juice was kindly provided by the company Aronia Original (Dresden, Germany). Cranberry (Vaccinium macrocarpon) and haskap (Lonicera caerulea) juice were obtained from Rabenhorst (Unkel, Germany). Goji berries (Lycium barbarum) were kindly provided by the company goji-Hof Hagenburg (Hagenburg, Germany). The goji berry juice was manually generated from berries by mini screw press.2.3 Preparation of phenolic fruit extracts from fruit juices by adsorption on an amberlite XAD7 resin
The extracts were generated according to Kostka and coworkers.21 Briefly, direct fruit juice (0.5 L) was filtered using a pleated filter and applied onto an Amberlite XAD7 column (100 × 7 cm), which had been conditioned with 2 L of ethanol and then with 2 L of water. The XAD7 column was washed with water in order to eliminate carbohydrates, proteins, organic acids, soluble fibers and minerals. Retained phenolic compounds were eluted with 1.5 L ethanol/acetic acid (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v/v). Solvents were evaporated under reduced pressure at <40 °C, and the residue was freeze-dried to abtain a XAD7 extract.
1; v/v). Solvents were evaporated under reduced pressure at <40 °C, and the residue was freeze-dried to abtain a XAD7 extract.
2.4 Quantification of polyphenols by HPLC-PDA
Selected polyphenols in the extracts were analyzed using an HPLC system (1100/1200 series, Agilent, Waldbronn, Germany). HPLC-PDA analyses were performed according to IFU method no. 71. HPLC-PDA quantification was performed on a Nucleosil 100 C18, 5 μ, 250 mm × 4.6 mm column (Macherey-Nagel, Düren, Germany) using an HPLC pump, a degasser, an autosampler, a column oven and a multiwavelength detector. HPLC measurements were done using solvent systems A (water/formic acid (9/1; v/v)) and B (water/formic acid/acetonitrile (4/1/5; v/v/v)) at a flow rate of 0.8 mL min−1 and 40 °C using the following gradient: 0 min, 12% B; 1 min, 12% B; 26 min, 30% B; 35 min, 100% B; 38 min, 100% B; 43 min, 12% B; 50 min, 12% B. The injection volume was 20 μL. The following standards were used for quantification: cyanidin-3-O-glucoside (5.0–500.0 mg L−1, LOQ (limit of quantification): 1.98 mg L−1; LOD (limit of detection): 7.19 mg L−1) at λ = 520 nm, quercetin (0.25–200.0 mg L−1, LOQ: 0.84 mg L−1; LOD: 3.09 mg L−1) at λ = 360 nm, chlorogenic acid (5-CQA, 0.50–100.0 mg L−1, LOQ: 5.60; LOD: 20.18) at λ = 320 nm, catechin (0.2–250.0 mg L−1, LOQ: 0.53 mg L−1; LOD: 1.94 mg L−1) at λ = 278 nm and rutin (0.25–200.0 mg L−1, LOQ: 0.84 mg L−1; LOD: 3.09 mg L−1) at λ = 360 nm.2.5 Fractionation by adsorptive membrane chromatography
According to Juadjur and Winterhalter22 and Kostka et al.,21,23 anthocyanins (λ = 520 nm) were separated from other non-colored phenolic compounds, so-called copigments (λ = 280 nm), on a cellulose membrane adsorber (type Sartobind S 150 mL) from Sartorius (Göttingen, Germany). Briefly, chokeberry and apple XAD7-extract (1 g) was dissolved in 1 L methanol/acetic acid (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v/v) and applied onto the cellulose membrane. Chokeberry was chosen as a representative of the red fruits due to its excellent antioxidant properties. The apple is a representative of the yellow fruits, so that differences can be made with regard to the anthocyanin content, which is relatively low in apples. First, copigments were eluted with 1 L ethanol/acetic acid (19
1; v/v) and applied onto the cellulose membrane. Chokeberry was chosen as a representative of the red fruits due to its excellent antioxidant properties. The apple is a representative of the yellow fruits, so that differences can be made with regard to the anthocyanin content, which is relatively low in apples. First, copigments were eluted with 1 L ethanol/acetic acid (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v/v). Afterwards, the retained anthocyanins were eluted with 1 L of a mixture of an aqueous NaCl (1 N) solution and ethanol (1
1; v/v). Afterwards, the retained anthocyanins were eluted with 1 L of a mixture of an aqueous NaCl (1 N) solution and ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v/v). Both fractions were concentrated in vacuo and the residue was freeze-dried. NaCl from the anthocyanin fraction was removed by Amberlite XAD-7, as described in section 2.3. The anthocyanin fraction was eluted with methanol/acetic acid (19
1; v/v). Both fractions were concentrated in vacuo and the residue was freeze-dried. NaCl from the anthocyanin fraction was removed by Amberlite XAD-7, as described in section 2.3. The anthocyanin fraction was eluted with methanol/acetic acid (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v/v), and the solvents were evaporated under reduced pressure at <40 °C and freeze-dried. To remove the polymers from the membrane, the membrane was regenerated with 1 N NaOH and the polymers were eluted with a mixture of NaCl (0.1 N) and ethanol (2
1; v/v), and the solvents were evaporated under reduced pressure at <40 °C and freeze-dried. To remove the polymers from the membrane, the membrane was regenerated with 1 N NaOH and the polymers were eluted with a mixture of NaCl (0.1 N) and ethanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8; v/v). For detailed information, see Kostka et al. and Ostberg-Potthoff et al..21,24
8; v/v). For detailed information, see Kostka et al. and Ostberg-Potthoff et al..21,24
2.6 Determantion of total phenolic content (TPC) using the Folin–Ciocalteu reagent
To evaluate the total phenolic content, the Folin–Ciocalteu reagent was used. Phenolic substances react with the Folin–Ciocalteu reagent as a redox reduction. This produces a blue-colored complex, which can be measured spectroscopically at 765 nm. The evaluation was carried out as gallic acid equivalents (GAE), which served as standards and antioxidant reference substance. Samples of XAD-7 fruit juice extracts were prepared in ethanol at a concentration of 2.5 mg mL−1 and then sonicated for 10 min and centrifuged at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min. Finally, the fruit juice extract samples were diluted 1
000g for 10 min. Finally, the fruit juice extract samples were diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v) with water. For the gallic acid stock solution (aqueous solution 1 mg mL−1), 10 mg gallic acid was weighed into a 10 mL volumetric flask and made up to the mark with H2O. The gallic acid calibration standards were freshly prepared on each test day. For each measurement, 20 μL of the prepared sample solution/calibration standard/blind value was pipetted in triplicate into the wells of a 96-microtiter plate. Water was used as blank. To start the reaction, 100 μL of Folin reagent was pipetted into each well using an integrated injector (Tecan M200 spectrophotometer, Männedorf, Switzerland) and shaken for 3 s. After an incubation time of 5 min, 100 μL of 75 g L−1 sodium carbonate solution in water was injected via another injector. After renewed shaking via the integrated shaking plate for 3 sec, the absorbance was measured at 765 nm after 60 min. The results were calculated using the gallic acid standard curve and expressed as milligram gallic acid equivalents per mg extract (mg GAE per g extract).
20 (v/v) with water. For the gallic acid stock solution (aqueous solution 1 mg mL−1), 10 mg gallic acid was weighed into a 10 mL volumetric flask and made up to the mark with H2O. The gallic acid calibration standards were freshly prepared on each test day. For each measurement, 20 μL of the prepared sample solution/calibration standard/blind value was pipetted in triplicate into the wells of a 96-microtiter plate. Water was used as blank. To start the reaction, 100 μL of Folin reagent was pipetted into each well using an integrated injector (Tecan M200 spectrophotometer, Männedorf, Switzerland) and shaken for 3 s. After an incubation time of 5 min, 100 μL of 75 g L−1 sodium carbonate solution in water was injected via another injector. After renewed shaking via the integrated shaking plate for 3 sec, the absorbance was measured at 765 nm after 60 min. The results were calculated using the gallic acid standard curve and expressed as milligram gallic acid equivalents per mg extract (mg GAE per g extract).
2.7 TEAC (trolox equivalent antioxidant capacity)-assay
Antioxidant capacities of the samples were determined by applying ABTS radical scavenging ability assays. Trolox was used as a standard using concentrations of 0.1, 0.2, 0.3, 0.4, 0.5, 0.75 and 1 mM. For ABTS radical scavenging ability, an ABTS radical stock solution was prepared by mixing ABTS (final concentration of 7 mM) with K2S2O8 (final concentration 2.5 mM) using double deionized water. The stock solution was stored at 4 °C for at least 16 h and protected from light. For ABTS working solutions, the stock solution was diluted with ethanol until an absorbance of 0.7 at 734 nm. Samples of fruit juice extracts were prepared in methanol at a concentration of 2.5 mg mL−1 and then sonicated for 10 min and centrifuged at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min. Finally, the fruit juice extract samples were diluted 1
000g for 10 min. Finally, the fruit juice extract samples were diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (v/v) with methanol, followed by preparation of concentration series of each extract (30, 45, 60, 75, 90, 105 and 135 μg mL−1). Briefly, 10 μL of the sample or Trolox standard was mixed with 200 μL of the ABTS working solution. After incubation for 6 min at room temperature, absorbance values were recorded at 734 nm using an Infinite M200 spectrophotometer (Tecan). Results were calculated as Trolox equivalents. Therefore, the concentration curve was calculated for each extract and positive control. In the next step the slope of the extracts were divided by the slope of the Trolox standard curve and multiplied with 100 to get percentual Trolox equivalents (% TE). Furthermore, the IC50 values were calculated as μg/mL for each tested compound.
2 (v/v) with methanol, followed by preparation of concentration series of each extract (30, 45, 60, 75, 90, 105 and 135 μg mL−1). Briefly, 10 μL of the sample or Trolox standard was mixed with 200 μL of the ABTS working solution. After incubation for 6 min at room temperature, absorbance values were recorded at 734 nm using an Infinite M200 spectrophotometer (Tecan). Results were calculated as Trolox equivalents. Therefore, the concentration curve was calculated for each extract and positive control. In the next step the slope of the extracts were divided by the slope of the Trolox standard curve and multiplied with 100 to get percentual Trolox equivalents (% TE). Furthermore, the IC50 values were calculated as μg/mL for each tested compound.
2.8 Cell culture
Human colon adenocarcinoma cells (Caco-2 cells) were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ; Braunschweig, Germany). Human intestinal cells (HT29-MTX cells) were obtained from the European Collection of Authenticated Cell Cultures (ECACC; Porton Down, UK). Caco-2 cells and HT29-MTX cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 4.5 g L−1 glucose and stable glutamine supplemented with 10% fetal bovine serum (FBS), 1% non-essential amino acids, 100 U mL−1 penicillin and 100 μg mL−1 streptomycin (all from Pan-Biotech, Aidenbach, Germany). Both cell lines were incubated at 37 °C in a humidified atmosphere with 5% CO2 and the medium was changed every 2–3 days. Trypsin-EDTA (0.05%) was used to passage cells once a confluency of 70–80% was reached. The passage number of cells used in experiments ranged from 10 up to 26. Caco-2 cells and HT29-MTX cells in particular are already well characterized and have been used in numerous anti-cancer studies to date, making them well suitable as a simulated intestinal barrier model for a direct comparison of the different fruit juice extracts.For the transport experiment, Caco-2 and HT29-MTX cells were co-seeded in inserts (0.4 μm pore diameter, 1 × 108 pore density, 0.3 cm2 growth area insert, Sarstedt, Nümbrecht, Germany) of 24-well plates. Two hundred microliters of growth medium with 5 × 104 cell per well density were added to the apical compartment and 800 μL of growth medium was transferred to the basolateral compartment. In detail, 3.5 × 104 cell per well of Caco-2 cells and 1.5 × 104 cell per well HT29-MTX cells were seeded into the inserts. Before transport experiments, cells differentiated to converging monolayers for 21 days post-seeding. During differentiation, monolayer quality of Caco-2 and HT29-MTX cells was screened by measuring the transepithelial electrical resistance (TEER; Millicell© ERS-2 Volt-Ohm Meter; Millipore, Bedford, MA, USA) value. Only differentiated cells exhibiting consistent TEER values were used in the transport experiment. Growth medium was replaced with fresh one every two days.
2.9 Cell viability assay: resazurin metabolism
Cell viability was assessed using the resazurin-based assay, which quantifies the metabolic conversion of resazurin to the fluorescent compound resorufin. A total of 2.5 × 103 cells (1.75 × 103 Caco-2 and 7.5 × 102 HT29-MTX cells) were seeded into 96-well plates and differentiated to converging monolayers for 14 days post-seeding. After 14 days, cells were treated with XAD7-extracts of fruit juices at varying concentrations of 50, 200 and 1000 μg mL−1. Triton-X (1% (v/v)) was used as a positive control. After 24 h of treatment, the supernatant was discarded, and the cells were incubated for 2 h with resazurin solution (10% (v/v); 100 μL per well) diluted in culture medium. The fluorescence was measured by 560 nm excitation and 690 nm emission against blank wells containing medium only. Spectrophotometric analysis were performed using an Infinite M200 spectrophotometer (Tecan). The assay was performed in three biological replicates, with three technical replicates per treatment. Cell viability was calculated using the following formula: | (1) |
The viability of the vehicle control was considered 100%. Non-cytotoxic concentrations of the test compounds were used for all experiments.
2.10 Transepithelial electrical resistance (TEER)
The electrical resistance of co-culture of Caco-2 and HT29-MTX cells seeded on inserts was measured using the Millicell® ERS-2 unit equipped with an STX1 electrode (Millipore, Bedford, MA, USA) to assess their barrier function. Cell culture inserts without cells were measured as reference. From an independent TEER value of 300 Ω cm2 (24 well transwells), the differentiated cell layer in the inserts was regarded as simulated intestinal epithelium and thus ready for experiments. Capric acid C10 (10 mM L−1) was used as a positive control leading to a decrease in TEER values by enhance the tight junction permeability.2.11 Analysis of the transport of glucose and sodium fluorescein
Even if the TEER values are a reliable and widely used marker for permeability, the results are sensitive to temperature and electrode position.25 Therefore, it is recommended measuring the paracellular transport of fluorescent molecules like sodium fluorescein as done in the recent study. Transport experiment was performed on differentiated co-cultured monolayers, prepared according to previously explained procedure.26 Briefly, the growth medium was removed from the apical and basolateral sides of the inserts. The apical and basolateral sides of the inserts were washed with PBS. XAD7 fruit juice extracts were diluted with HBSS (1000 μg mL−1) and cells were treated at 37 °C and 5% CO2 for 4 h. In addition, 10 mM glucose and 5 μM sodium fluorescein were added to each of the sample solutions. During the treatment, each insert was filled with a volume of 500 μL sample solution and each well with a volume of 1000 μL HBSS. During treatment, 200 μL of samples were obtained from both the apical and basolateral side after 2 h and 4 h. TEER value of the cells was controlled before and after 1, 2, 3, 4, 6, 8, 10, 22 and 24 h of the experiments to maintain the transepithelial electrical resistance, which would significantly drop to the level of cell-free wells if the epithelium was accidentally damaged. After 4 h-treatment, HBSS was removed and growth medium was added. Then the cells were allowed for another incubation for 24 h to detect any irreversible damage in the cell monolayer. Thus, after 24 h, TEER value of the cells was control, showing constant values of 300 Ω cm2, while values of at least 200 Ω cm2 were recommended.27 All samples were stored at −20 °C until further analysis. At the same time, at least two controls were included: one cell-free untreated and one cell-containing insert. The cell-containing insert was incubated without treatment substance only with solvent (HBSS) to serve as a negative control.2.12 Calculation of the apparent permeability coefficient (Papp)
The apparent permeability coefficient was calculated according to the following eqn (2) and (3):| dQ = dC × V | (2) |
 | (3) |
2.13 GOPOD (glucose oxidase/peroxidase) assay
Glucose concentration was measured using a D-glucose assay kit (glucose oxidase/peroxidase; GOPOD, biomol Germany) at 505 nm in a spectrophotometer. The GOPOD reagent was mixed and added to the samples from the basolateral chamber. After incubation for 15 min at 37 °C, absorbance was measured at 505 nm. The absorbance values were converted to glucose concentrations using a standard curve.2.14 Cell treatment and RNA extraction for qPCR analyses
The mRNA levels of target genes were quantified using RT-qPCR (QuantStudio3 Real-Time PCR System, ThermoFisher Scientific, Darmstadt, Germany). For this purpose, Caco-2 and HT29-MTX cells were seeded in co-culture in 6-well plates at a density of 6.0 × 104 cells per well. In detail, 4.2 × 104 cell per well of Caco-2 cells and 1.8 × 104 cell per well HT29-MTX cells were seeded differentiated to converging monolayers for 14 days post-seeding. In a first time-dependent experiment the cells were treated with chokeberry extract (1000 μg mL−1) for 1–8 h. Afterwards, extract-dependent experiments were performed by treating the cells with 1000 μg mL−1 for 4 h at 37 °C and 5% CO2. For the fruit juice extract stock solutions with a concentration of 50 mg mL−1, 10 mg XAD7-extract was dissolved in 200 μL DMSO. The treatment solutions were daily prepared. Untreated cells were included as a control. These were incubated without treatment substance only with solvent (DMEM) to serve as a negative control. The cell treatment was performed in the form of biological replicates at different passage numbers, as well as in technical triplicates contained within a biological replicate. When cells were harvested to generate cell pellets for further qPCR analysis, the culture medium was first removed and discarded. The cells in the 6-well plate were then washed with 2 mL PBS incubated with 500 μL trypsin/EDTA for 7 min at 37 °C. After the incubation period, 1.5 mL of medium was added and transferred into 2.0 mL reaction vessels. Subsequently, the cell suspension was centrifuged for 10 min at RT and 1000g. After centrifugation, the supernatant was carefully removed and discarded. Cell pellets were then washed with 1.5 mL PBS each and centrifuged again for 10 min at RT and 1000g. To prevent degradation, the cell pellets were frozen in liquid nitrogen and stored at −80 °C until further analysis.RNA extraction from the cell pellets was performed using phenol extraction. An addition of 1000 μL Trizol was added to the cell pellet. The reaction vessels were shaken by hand for 5 min at room temperature until the cell pellets were completely dissolved. Then, 200 μL of chloroform was added to the reaction vessel and shaken by hand for 15 s followed by an incubation for 3 min at room temperature. The reaction tubes were then centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g and 4 °C for 15 min. After centrifugation, 400 μL of the upper phase was transferred to a fresh 1.5 mL reaction tube and 500 μL isopropanol was added. The cell suspension was mixed by inverting several times (∼10×). This was followed by another incubation at room temperature for 10 min and subsequent centrifugation at 12
000g and 4 °C for 15 min. After centrifugation, 400 μL of the upper phase was transferred to a fresh 1.5 mL reaction tube and 500 μL isopropanol was added. The cell suspension was mixed by inverting several times (∼10×). This was followed by another incubation at room temperature for 10 min and subsequent centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g and 4 °C for 10 min. The supernatant was carefully and completely removed. Then, 1000 μL of 70% ethanol was added to the pellet and shaken for 10 min to wash the pellet. The pellet was then centrifuged at 7 500g and 4 °C for 5 min and the supernatant of the pellet was carefully and completely removed. The remaining 70% ethanol still adhering to the pellet was allowed to evaporate. Finally, the pellet was resuspended in 25 μL nuclease-free water and stored at −20 °C for further processing and analysis. The integrity and purity of RNA samples were verified using agarose gel electrophoresis (2%), and the UV absorbance ratio (A260/A280 nm) was measured via Nanodrop One C (Thermo Fisher scientific, Darmstadt, Germany). The range of the acceptable absorbance ratio was 1.85–2.00. RNA concentrations were determined using Nanodrop One C (Thermo Fisher scientific) and were adjusted to 1000 ng μL−1 for the subsequent cDNA synthesis.
000g and 4 °C for 10 min. The supernatant was carefully and completely removed. Then, 1000 μL of 70% ethanol was added to the pellet and shaken for 10 min to wash the pellet. The pellet was then centrifuged at 7 500g and 4 °C for 5 min and the supernatant of the pellet was carefully and completely removed. The remaining 70% ethanol still adhering to the pellet was allowed to evaporate. Finally, the pellet was resuspended in 25 μL nuclease-free water and stored at −20 °C for further processing and analysis. The integrity and purity of RNA samples were verified using agarose gel electrophoresis (2%), and the UV absorbance ratio (A260/A280 nm) was measured via Nanodrop One C (Thermo Fisher scientific, Darmstadt, Germany). The range of the acceptable absorbance ratio was 1.85–2.00. RNA concentrations were determined using Nanodrop One C (Thermo Fisher scientific) and were adjusted to 1000 ng μL−1 for the subsequent cDNA synthesis.
2.15 cDNA-synthesis
For cDNA synthesis, the samples were thawed on ice and diluted in a 1.5 mL reaction vessel at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (v/v) with RNAse-free water. In addition, a 1
30 (v/v) with RNAse-free water. In addition, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 (v/v) dilution of one control and one sample of each treatment were prepared from the initial dilution in order to check the linearity of the cDNA synthesis. Subsequently, 2 μL of the 1
16 (v/v) dilution of one control and one sample of each treatment were prepared from the initial dilution in order to check the linearity of the cDNA synthesis. Subsequently, 2 μL of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 diluted RNA was pipetted into a 96-well plate. Then 10 μL of Mastermix DNAse (1.2 μL DNase 1 U μL−1, 1.2 μL DNase buffer 10× and 7.6 μL RNAse-free water) digest were added to the RNA. The plate was then sealed with adhesive foil, briefly centrifuged and incubated at 37 °C for 30 min. At the end of the incubation period, the plate was incubated for a further 15 min at 70 °C to degrade DNA and then stored on ice. After complete cooling, the 96-well plate was again briefly centrifuged and 1 μL of Random Nonamers (50 pmol μL−1) was added to each well. The 96-well plate was again tightly sealed with adhesive foil, briefly centrifuged and incubated at 70 °C for 5 min. The plate was then also briefly centrifuged again and 7 μL of the master mix cDNA (0.5 μL RT M-MLV 200 U μL−1, 4.0 μL RT M-MLV buffer 5×, 2.0 μL dNTPs 10 mM and 0.5 μL RNAse-free H2O) was added. Two samples without reverse transcriptase (−RT) were also included as a control. After repeated short centrifugation, the plate was incubated at 37 °C for 60 min, followed by incubation at 70 °C for 15 min. The 96-well plate including the cDNA was then frozen at −18 °C until further analysis.
30 diluted RNA was pipetted into a 96-well plate. Then 10 μL of Mastermix DNAse (1.2 μL DNase 1 U μL−1, 1.2 μL DNase buffer 10× and 7.6 μL RNAse-free water) digest were added to the RNA. The plate was then sealed with adhesive foil, briefly centrifuged and incubated at 37 °C for 30 min. At the end of the incubation period, the plate was incubated for a further 15 min at 70 °C to degrade DNA and then stored on ice. After complete cooling, the 96-well plate was again briefly centrifuged and 1 μL of Random Nonamers (50 pmol μL−1) was added to each well. The 96-well plate was again tightly sealed with adhesive foil, briefly centrifuged and incubated at 70 °C for 5 min. The plate was then also briefly centrifuged again and 7 μL of the master mix cDNA (0.5 μL RT M-MLV 200 U μL−1, 4.0 μL RT M-MLV buffer 5×, 2.0 μL dNTPs 10 mM and 0.5 μL RNAse-free H2O) was added. Two samples without reverse transcriptase (−RT) were also included as a control. After repeated short centrifugation, the plate was incubated at 37 °C for 60 min, followed by incubation at 70 °C for 15 min. The 96-well plate including the cDNA was then frozen at −18 °C until further analysis.
2.16 Real time quantitative polymerase-chain reaction (RT-qPCR)
To perform qPCR, the cDNA samples were thawed on ice. Standards were then prepared by combining 2 μL of cDNA from each of the total samples, except for the −RT samples. These were then diluted within a serial dilution using RNAse-free water. For qPCR, the cDNA samples were diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with RNAse-free water. The master mix was then prepared. For each gene, a master mix (2.2 μL RNAse-free water, 5.0 μL SYBR Green Master Mix, 0.4 μL Primer forward 10 μM and 0.4 μL Primer reverse 10 μM) was prepared and stored on ice. On a 96 well plate, 2 μL of the 1
2 with RNAse-free water. The master mix was then prepared. For each gene, a master mix (2.2 μL RNAse-free water, 5.0 μL SYBR Green Master Mix, 0.4 μL Primer forward 10 μM and 0.4 μL Primer reverse 10 μM) was prepared and stored on ice. On a 96 well plate, 2 μL of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 diluted cDNA was mixed with 8 μL of master mix. The samples were applied in triplicate. In addition, one control per primer was added without cDNA, containing only water and the qPCR master mix to reveal, primer dimers or contaminations. The plate was tightly sealed with adhesive foil, briefly centrifuged and qPCR was performed. A thermocyler (QuantStudio3 Real-Time PCR System; Thermo Fisher scientific, Darmstadt, Germany) was used to perform 40 reaction cycles under the following conditions: 50 °C for 2 min (one time), 95 °C for 10 min, 95 °C for 15 s, 60 °C for 60 s, 95 °C for 15 s, 60 °C for 60 s and 95 °C for 1 s. RT-qPCR was performed with the designed primers and the corresponding cDNA for primer establishment. In Table S1† the primer pairs used in this study and the efficiencies in each case are shown.
2 diluted cDNA was mixed with 8 μL of master mix. The samples were applied in triplicate. In addition, one control per primer was added without cDNA, containing only water and the qPCR master mix to reveal, primer dimers or contaminations. The plate was tightly sealed with adhesive foil, briefly centrifuged and qPCR was performed. A thermocyler (QuantStudio3 Real-Time PCR System; Thermo Fisher scientific, Darmstadt, Germany) was used to perform 40 reaction cycles under the following conditions: 50 °C for 2 min (one time), 95 °C for 10 min, 95 °C for 15 s, 60 °C for 60 s, 95 °C for 15 s, 60 °C for 60 s and 95 °C for 1 s. RT-qPCR was performed with the designed primers and the corresponding cDNA for primer establishment. In Table S1† the primer pairs used in this study and the efficiencies in each case are shown.
2.17 Statistical analysis
Statistical analysis was carried out using the software Prism (version 10.1.1; GraphPad, La Jolla, CA, USA). Data were analyzed for normality of distribution by Shapiro–Wilk test, followed by a one-way ANOVA including Tukey's multiple comparison test for normally distributed data. Otherwise the non-parametric Kruskal–Wallis test with multiple comparison test was performed. Differences were considered as significant when p < 0.05, while data are shown as means ± SD (Standard Deviation) of at least three independent experiments.3 Results
3.1 Chemical characterization of fruit juice XAD7 extracts, anthocyanin and copigment fractions using HPLC-PDA
Selected phenolic compounds of the generated fruit juice extracts of apple, chokeberry, goji berry, pomegranate, haskapberry, blueberry, elderberry, cranberry, plum, sour cherry and red grape, as well as the anthocyanin/polyphenol fraction and the copigment fractions of apple and chokeberry were quantified by HPLC-PDA analyses. The quantified main components of the XAD7 fruit juice extracts are listed in Table 1. The haskapberry extract showed the highest anthocyanin content by far, determined as cyanidin-3-O-glucoside. However, the fruit juice extracts of blueberry and elderberry and the anthocyanin fraction of chokeberry also showed high levels of anthocyanins determined as cyanidin-3-O-glucoside equivalents. The extract of cranberry had the lowest content of cyanidin-3-O-glucoside, but contained the highest content of catechin of all analyzed fruit juice extracts. By comparison, the highest chlorogenic acid content, determined as 5-O-caffeoylquinic acid (5-CQA), was found in the apple extract, followed by the chokeberry juice extract. However, the apple polyphenol and copigment fractions contained only low levels of chlorogenic acid. Quercetin was present in higher amounts in cranberry and sour cherry extracts. Haskapberry and elderberry extracts had the highest rutin content. It is also noticeable that no cyanidin-3-O-glucoside and no rutin was detectable in the apple extract, as well as in the polyphenol and copigment fractions of the apple. While the apple polyphenol fraction consisted mainly of the components catechin, chlorogenic acid and quercetin, only small amounts of catechin and chlorogenic acid were detectable in the apple copigment fraction. The anthocyanin fraction of chokeberry, on the other hand, showed particularly high levels of cyanidin-3-O-glucoside.| Fruit juice extracts | Polyphenol [g per 100 g extract] | ||||
|---|---|---|---|---|---|
| Catechin | Chlorogenic acid (5-CQA) | Cyanidin-3-O-glucoside | Quercetin | Rutin | |
| LOD: limit of detection (s. 2.4) | |||||
| Apple (Pyrus malus) | 0.033 ± 0.008e | 12.2 ± 1.58abcde | <LOD | 0.032 ± 0.012defh | <LOD |
| Apple polyphenol fraction | 0.086 ± 0.068de | 0.762 ± 0.025f | <LOD | 0.019 ± 0.001eg | <LOD |
| Apple copigment fraction | 0.020 ± 0.026de | 0.178 ± 0.003g | <LOD | <LOD | <LOD |
| Chokeberry (Aronia melanocarpa) | 0.031 ± 0.006e | 6.81 ± 0.319a | 5.03 ± 0.210d | 0.076 ± 0.010bceh | 0.382 ± 0.021cd |
| Chokeberry Anthocyan fraction | 0.032 ± 0.008e | 0.326 ± 0.038g | 14.3 ± 0.087b | 0.009 ± 0.01fgi | <LOD |
| Chokeberry copigment fraction | 0.026 ± 0.009e | 6.565 ± 0.044a | 0.445 ± 0.058gh | 0.109 ± 0.011bc | 0.402 ± 0.013c |
| Gojibeery (Lycium barbarum) | 0.263 ± 0.057de | 0.244 ± 0.043g | <LOD | 0.009 ± 0.001hi | 0.322 ± 0.008d |
| Pomegranate (Punica granatum) | 0.312 ± 0.103bcde | 0.305 ± 0.020g | 0.895 ± 0.099g | 0.008 ± 0.006dgi | <LOD |
| Haskapberry (Lonicera caerulea) | 0.341 ± 0.111bcde | 3.126 ± 0.045b | 27.6 ± 0.34a | 0.051 ± 0.006cefh | 1.091 ± 0.050b |
| Blueberry (Vaccinum myrtillus) | 0.203 ± 0.006d | 2.16 ± 0.037d | 6.78 ± 0.075c | 0.097 ± 0.007b | <LOD |
| Elderberry (Sambucus nigra) | 0.033 ± 0.008e | 1.61 ± 0.046e | 6.46 ± 0.095c | 0.048 ± 0.007efh | 2.73 ± 0.076a |
| Cranberry (Vaccinium macrocarpon) | 1.47 ± 0.031a | 0.414 ± 0.034g | 0.187 ± 0.053h | 0.226 ± 0.005a | 0.089 ± 0.004f |
| Plum (Prunus domestica) | 0.543 ± 0.041c | 2.66 ± 0.057c | <LOD | 0.019 ± 0.009fgi | 0.236 ± 0.068cdef |
| Sour cherry (Prunus cerasus) | 0.033 ± 0.006e | 2.33 ± 0.027d | 1.38 ± 0.096f | 0.204 ± 0.003a | 0.169 ± 0.004e |
| Red grape (Vitis vinifera) | 0.876 ± 0.003b | 0.330 ± 0.038g | 3.67 ± 0.031e | 0.056 ± 0.004cf | 0.459 ± 0.041cd |
3.2 Radical scavenging activity effects of fruit juice extracts
3.3 Effects of fruit juice extracts on gut barrier
Fig. 2B shows the changes in TEER values when the cells were treated with the apple and chokeberry extract fractions. Here, a decrease in TEER values to 40% after 6 h after the start of treatment can be seen in relation to the initial TEER value. When cells are treated with fruit juice extract of chokeberry, as well as their anthocyanin and copigment fractions, a significant reduction in TEER values can be seen 6 hours after the start of treatment. The best regeneration in TEER values after 22 h after the start of treatment was seen in the treatment with the copigment fraction of the apple. TEER values of up to 70% were achieved in relation to the original TEER value.
As shown in Fig. 3A, cells treated with pomegranate and apple extracts showed the highest, but non-significant Papp value or the highest flow of sodium fluorescein from the apical to the basolateral compartment after 2 h of treatment. Cells treated with sour cherry and red grape extract showed the lowest Papp value and the lowest flow of sodium fluorescein. Fig. 3B shows the flow of sodium fluorescein from the apical to the basolateral compartment in relation to the treatment with the individual fractions of chokeberry and apple. A relatively high transport of sodium fluorescein compared to the control can be seen in the treatment with the fruit juice extract of apple. All other fractions showed no significant differences in the transport of sodium fluorescein in relation to XAD7-extract and also in comparison to the control.
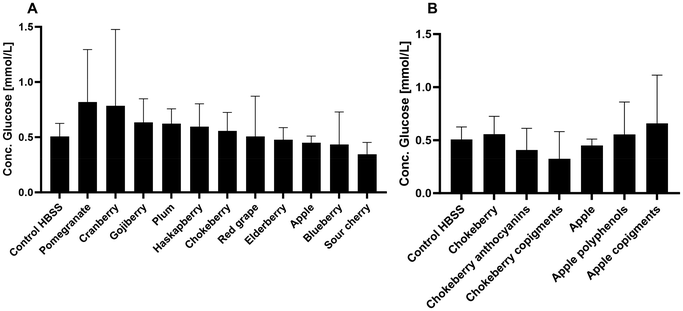
3.4 Glucose-colorimetric-assay
The glucose oxidase/peroxidase assay was used to measure the glucose content in the basolateral compartment after cell treatment with the different XAD7 fruit juice extracts and treatment with glucose to determine if any of the fruit juice extracts had an effect on the glucose transport or uptake of glucose in the cells. In Fig. 4A, the results showed that the pomegranate and cranberry extracts have a slight tendency to increase the glucose concentration in the basolateral compartment after the 4-hour period. Fig. 4A also showed that the treatment with sour cherry extract had the lowest detectable concentration of glucose in the basolateral compartment, even lower than the control treatment with HBSS. Chokeberry extract and the copigment fraction of apple showed a tendency to increase the glucose concentration in the basolateral compartment (Fig. 4B). In contrast, treatment of the cells with the anthocyanin and copigment fractions of chokeberry appeared to reduce glucose transport. Overall, however, it can be summarized that the results do not show any significant differences, but only individual tendencies between the various treatments with fruit juice extracts on the cells of the co-culture.3.5 Effects of chokeberry juice extract on the expression of the genes of GLUT1, GLUT2, SGLT1, DPP4, CAT and SOD on mRNA level in a co-culture of Caco-2 and HT29-MTX cells over different time intervals
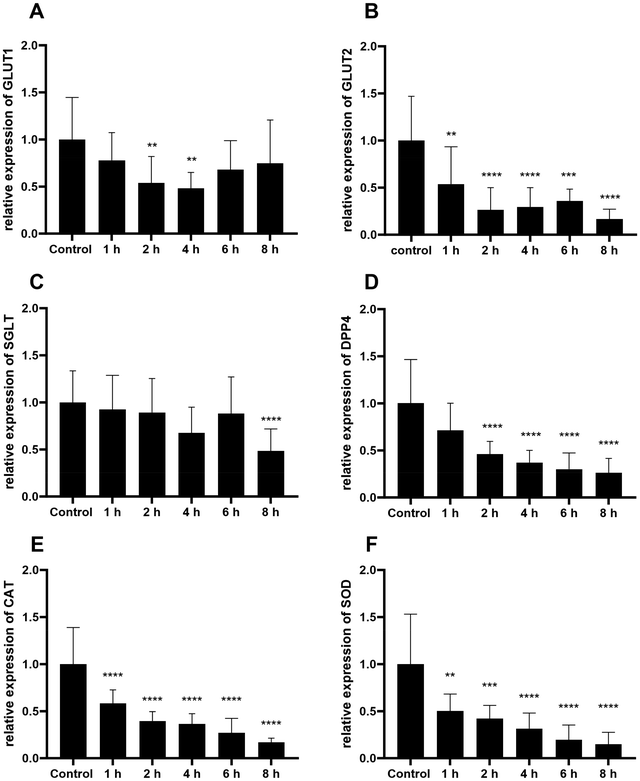
The chokeberry extract was further analyzed because of the highest content of chlorogenic acid and cyanidin-3-O-glucoside. Quantitative real-time PCR was used to determine the effects of the different XAD7-extracts of the following genes at mRNA level: GLUT1, GLUT2, DPP4, SGLT1, CAT and SOD. The GLUT1, GLUT2, SGLT1 and DPP4 genes are particularly important for glucose uptake in cells, whereas the CAT and SOD genes play a role in relation to oxidative stress. In order to determine the best possible time for cell extraction and at the simultaneous time of highest expression, a time experiment was carried out with different treatment time intervals of 1–8 h using chokeberry extract. As shown in Fig. 5A, there was a significant reduction in the expression of GLUT1 after a treatment time of 2 and 4 h in relation to the control. After a treatment time of 1, 6 and 8 h, the expression of GLUT1 was equal to that of the control. Treatment in relation to the transcription of GLUT2 resulted in a significant decrease in expression after 1 h of treatment. However, the level of expression in the time interval from 2 to 6 h did not differ marginally from each other. In the case of SGLT1, a significant decrease in expression could only be seen after 8 h of treatment. In the case of DPP4, a significant decrease was generally recognizable after 2 h of treatment, but this did not differ between 2 and 8 h treatment times.There was also a significant decrease in CAT expression after 1 h of treatment in contrast to the control. Again, there were no significant differences between the tested treatment periods. However, there was a tendency that CAT was expressed at the lowest level after 8 h of treatment. The expression of SOD showed a steady decline with increasing treatment time, with the lowest expression of SOD also occurring after 8 h treatment.
3.6 Effects of transcription of GLUT1, GLUT2, SGLT1, DPP4, CAT and SOD mRNA in co-culture of Caco-2 and HT29-MTX cells by different XAD7-fruit juice extracts
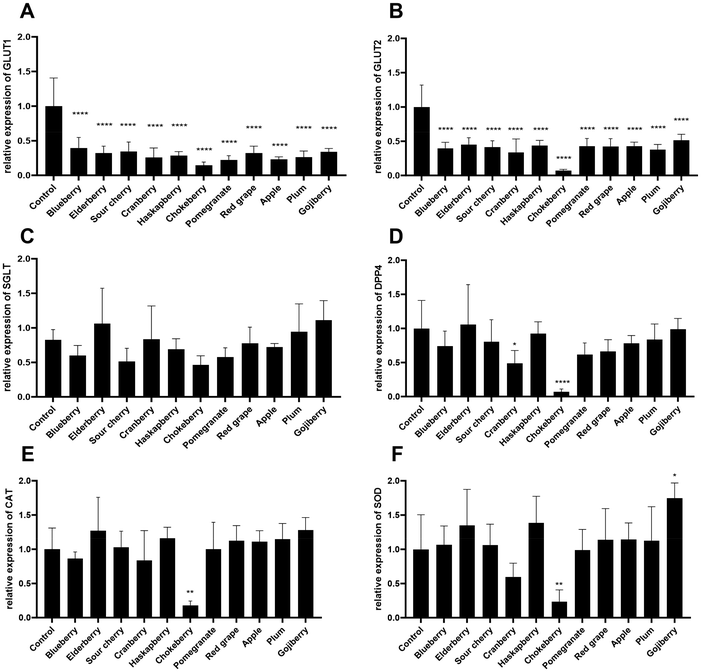
Based on our own previous studies, which showed significant effects of chokeberry extract after 4 h of treatment for most of the tested genes, all fruit juice extracts were analyzed according to this experimental setup. The influence on the transcription of the glucose transporters GLUT1, GLUT2 and SGLT1, as well as on the proteolytic enzyme DPP4 and on the antioxidant enzymes CAT and SOD was quantified (Fig. 6). After a 4-hour treatment with different XAD7 fruit juice extracts, there was a significant decrease in the expression of GLUT1 compared to the control, but there were no differences in the expression of GLUT1 between the treatment of each fruit juice extract. Likewise, a significant decrease in the expression of GLUT2 in relation to the control was recognizable in all treatments with fruit juice extracts. In particular, the treatment with chokeberry extract showed a significant reduction in the expression of GLUT2. No significant differences between the individual fruit juice extracts were recognizable in the expression of SGLT. There were also no significant changes in the expression of DPP4, with the exception of the treatment with chokeberry fruit juice extract. In the expression of CAT and SOD, with the exception of the chokeberry treatment, no significant changes in expression were recognizable either. However, it is notable that the expression of these two genes are slightly higher on average compared to the control.4. Discussion
The aim of this study was to investigate and compare the influence of 11 different fruit juice extracts on the intestinal permeability in a Caco-2 and HT29-MTX co-culture cell system. As already described in the literature, red fruits in particular contain various bioactive substances, such as polyphenols, which are associated with health-promoting effects. Especially with regard to chronic intestinal diseases and oxidative stress, it is important to investigate the effects of these fruit juice extracts in more detail.4.1 TEAC and TPC
According to Nowak,28 an high antioxidant capacity was determined for chokeberry and pomegranate juice, while the values of apple juice and nectar were significantly lower. Such results are comparable with the results of the recent publication. Furthermore, it was shown that the chokeberry extract is as effective as Trolox, a well known antioxidant, while the effects of pomegranate extract was even higher. Thus, the utilisation of pomegranate extract as natural antioxidant mixture should be taken into consideration. The high radical scavenging effect of pomegranate, chokeberry and blueberry, which was determined in this study using the TEAC assay, was confirmed by the TPC assay. A total polyphenolic content of 643 mg GAE per g extract was determined for the fruit juice extract of chokeberry and a total phenolic content of 609 mg GAE per g extract for pomegranate. Blueberry showed a significantly lower TPC compared to pomegranate, but the third highest content of all tested extracts with 506 mg GAE per g. The results for the antioxidant potential of pomegranate are comparable to those of Kostka and coworkers.21 In the literature, TPC values between 1.7 and 5.3 mg GAE per g are given for pure pomegranate juice.29–31 However, the preparation of XAD7-extract leads to an enrichment of the polyphenols present, resulting in a higher antioxidant capacity, so that the TPC value for the extract is significantly higher than for the pure juice. The determination of the TPC showed that the fruit juice extracts of chokeberry, pomegranate and blueberry had the highest content of TPC. It can therefore be assumed that chokeberry, pomegranate and blueberry have the highest antioxidant effect of the analyzed fruit juice extracts. Based on the quantification of selected polyphenol compounds using HPLC-PDA, this high antioxidant effect can be attributed to the compounds cyanidin-3-O-glucoside and catechin. Chokeberry and blueberry juice extracts in particular contain high amounts of cyanidin-3-O-glucoside. High amounts of catechin were detected in the fruit juice extracts of pomegranate and blueberry. This compound is also known to have antioxidant effects. Chokeberry has the highest content of flavonol glycosides (around 270 mg kg−1 fresh weight) compared to other berries, such as blueberry, cranberry, blackberry, blackcurrant and gojiberry, and the highest content of proanthocyanidins and anthocyanins compared to cranberry, bilberry, grape, cherry and raspberry.32 Nowak and colleagues were able to detect 40–100 g polyphenols per kg in chokeberries.33 Blueberries (30–38 g kg−1), blackcurrants (22–28 g kg−1) and cranberries (22–24 g kg−1) have also been shown to contain relatively high levels of polyphenols.28 The blueberry fruit juice extract examined here also had a very high cyanidin-3-O-glucoside content of 68 g per kg extract. In contrast, a high catechin content of 15 g per kg extract was detected in the fruit juice extract of cranberry. Analyses by Pedro et al. revealed contents of 1059 to 1094 mg GAE per 100 g in goji berries, which is also comparable to the TPC content analyzed in the current study.34A positive trend in the correlation of TPC and antioxidant capacity can be observed for pomegranate, chokeberry and blueberry in the study described here. The variety and concentration of antioxidants and their radical scavenging activity also strongly depend on the type and variety of fruit. It should be noted that all pre-harvest processes, environmental conditions, the degree of ripeness at harvest, post-harvest storage and the respective processing methods are important factors for the phytochemical profile of the fruit.35
4.2 TEER
Polyphenol-rich fruits and extracts are widely discussed as natural antioxidants suitable for application in functional food and beverages. Nevertheless, their effects on the intestinal barrier should be also considered. It is shown in the literature that the fruit-induced effects on gut permeability and TEER values varied greatly. Extracts of black tea, green tea and apples increased the cellular barrier function in Caco-2 monolayers, while chicory extracts showed no effects except of higher permeability due to compound-induced cytotoxicity and necrosis.17,36–38 Such an increase in TEER values and decrease in permeability are mainly induced by higher expression of tight junction proteins like claudin-1, claudin-4, occluding or zonula occludens 1 (ZO-1).17,39–41 An increase in permeability can be induced by delocalization of the junction anchoring protein ZO-1 from the cell membrane to the cytoplasm, documented for the anthocyanin pelargonidin in strawberries.42 ZO-1 delocalization may be responsible for the strong decrease in TEER values, reported in the recent study. After 6 h after the start of treatment (just 2 h of recovery), a significant decrease in TEER values was observed in some cases. These results correspond to other studies in the literature documenting a strong decrease in TEER values by fruit extracts e.g. lemon, cranberry, strawberry, red grape, galangal, marigold, tomato, mango, ginger and hop extracts.37,42–44 It should be mentioned that most of the studies were performed using Caco-2 monolayers, which do not form a mucus layer as a second barrier for bioactive compounds.45,46 The use of the co-culture intestinal model (Caco-2 and HT29-MTX cells) is a more physiological in vitro model for intestinal uptake, while the TEER values are still comparable between co-culture and Caco-2 monoculture studies.46After 22 hours from the start of treatment (18 hours recovery time), a partial, almost complete regeneration of intestinal permeability can be assumed. However, this regeneration of the intestinal barrier appears to be dependent on the individual fruit juice extracts. The best regeneration in relation to the initial value and in comparison to all other fruit juice extracts was observed in the treatment with gojiberry, blueberry and elderberry. In contrast, chokeberry, pomegranate, apple and cranberry showed a significant drop after 6 h of treatment, as well as incomplete regeneration of TEER values even after 24 h of treatment. From this it can be concluded that the influence of the different fruit juice extracts is not associated with their antioxidant effects, but perhaps rather to the different composition of the individual fruits and the polyphenols present in different concentrations. Lamson and colleagues were able to show that extracts from red tomatoes and red grapes effectively increased the permeability of Caco-2 monolayers, while yellow tomatoes and green grapes did not.42 Thus, especially the colour-given compounds e.g. anthocyanins may be responsible for the reduction of the barrier function. In contrast, quercetin, kaempferol and the flavines showed barrier-strengthening effects.39,47,48 To analyse such compound-specific effects the cells in the recent study were also treated with individual fractions of chokeberry and apple. Both extracts and all fractions induced a decrease in TEER values of up to 40% seen 6 h after the start of treatment compared to the initial TEER value. Despite of the different composition of the extracts, there was no significant difference between chokeberry and apple. Moreover, apple polyphenols and copigments showed similar effects, because of lacking anthocyanins. As seen in the study of Lamson and coworkers, anthocyanins possess a high potential in permeability increase.42 The anthocyanin fraction of chokeberry showed the lowest TEER values as well as the worst recovery. The effects of the whole chokeberry extract was tendentially higher to the anthocyanin fraction and verified the hypothesis of anthocyanins being mainly involved in intestinal barrier dysfunction. It was already shown that anthocyanins like pelargonidin can rearrange the actin filaments at the luminal cell surface and reduce the expression of tight junctions.42 These effects would lead to higher permeability over time. However, in relation to the cyanidin-3-O-glucoside content of the tested fruit extracts, the TEER values varied. Elderberry and blueberry extracts consisted of relatively high levels of the anthocyanin compound, while blueberry showed one of the lowest and elderberry one of the highest TEER values after treatment. In contrast to Lamson and coworkers, other studies revealed protective effects of anthocyanin-rich extracts against intestinal barrier dysfunctions by increasing the number of goblet cells and mucus production and maintaining the tight junction expression.49–51 Thus, the individual composition of the extracts is essential, while synergistic or antagonistic effects may occurred. Another important point is the occurrence of polyphenol polymeric compounds in the fruit juice extracts. Induced by different conditions like oxidation processes, small molecules and/or self-assembly, tannic acid, gallic acid and quercetin can be polymerized forming oligomers and polymers.52 These polymers could be present to a high proportion in the fruit juice extract and significantly influence the TEER results. Ci and colleagues were able to separate the monomeric, oligomeric and polymeric fraction of pear polyphenols. Interestingly, as higher was the polymerization grade, as higher were the anti-diabetic effects.53 Thus, the characterization and structure-dependent, beneficial effects should be focused in the future of polyphenol analyses.
Finally, the investigations in this study have shown that the fruit juice extracts of gojiberry and elderberry can prevent or attenuate an increase in intestinal permeability and that these extracts could be used, for example, in the treatment and prevention of chronic intestinal diseases. In contrast, extracts from chokeberry, cranberry, apple and pomegranate appear to increase intestinal permeability, which means that these extracts can be used, for example, to increase the absorption of substances across the intestinal membrane, such as medications.
4.3 Sodium fluorescein
Apple and pomegranate extract showed the highest Papp values and fluorescein flux, while the TEER was significantly reduced. Thus, the permeability as well as paracellular transport was increased by these extracts, which was shown by both methods, while the effects on the Papp values was non-significant. In general, the sodium fluorescein transport assay showed no significances for any of the fruit juice extract due to the high standard deviations. Therefore, no robust findings can be drawn from this results. Nevertheless the assay revealed first insights in some interesting points, which should be focused and analyzed in detail in future studies. Among others, it is interesting to note that most of the extracts showed constant Papp values for both time points. However, sour cherry and red grape extract first reduced the Papp compared to control after 2 h, while after 4 h the transport was increased. This could possibly be an indication that the substance may not be transported by increasing the permeability through the intestinal membrane, but that the transport could possibly also take place via other intracellular transport mechanisms. It could also be an indication that the fluorescent dye is mainly transported at the beginning of the treatment period. For instance, red grape and sour cherry extracts showed the lowest Papp values, even lower compared to the control, while the TEER value was not increased. A limited paracellular transport would indicate a lower permeability and a higher TEER result, but that was not detectable here. As mentioned above the validity of TEER measurements are restricted by outer influencing factors like temperature. Moreover, TEER depends on paracellular ion flux like sodium and chloride.39 It is known that the expression of tight junction proteins is affected by polyphenols such as quercetin and these tight junction proteins can regulate the ion flux. A higher expression of claudin-4 reduced the transport of sodium ions, resulted in higher TEER values.54 Furthermore, claudin-16 induce the formation of magnesium Mg2+ and calcium Ca2+ channels, which further influence the TEER.55 Due to these outer and cellular influencing factors the results of the fluorescein transport seem to be more reliable.However, even if the results of TEER and fluorescein transport varied, the level of anthocyanins seems to be essential. The loss of cyanidin-3-O-glucoside in apple, plum and gojiberry extracts led to high Papp values, while the TEER was reduced and the antioxidant potential was significantly lower compared to the red fruit extracts. Thus, anthocyanin-rich extracts are affective against oxidative stress, but may induce an increase of intestinal permeability. The effects of different anthocyanins should be analyzed more extensively in the future in order to investigate the structure–activity relationships in more detail.
4.4 GOPOD
Thus, the treatment with sour cherry seems to show a rather reduced transport of glucose compared to the other fruit juice extracts and also compared to the control. In case of pomegranate and cranberry extract the glucose transport was tendentially increased, which could be an effect by the high catechin contents. It can also be seen in the treatments with the individual sour cherry extract and chokeberry fractions that there is a supposedly lower glucose transport in the treatment with the catechin-free chokeberry copigment fraction compared to the control. However, this is merely a tendency, so that no significant differences can generally be assumed here. According to the literature,56–58 anthocyanins, which possibly influence the tight junction proteins and permeability, also appear to be rather insignificant for glucose transport, which could be confirmed by the investigations in this study. Extracts and fractions rich in cyanidin-3-O-glucoside or other anthocyanins showed no effects on glucose uptake and transport in these studies. In contrast to the results described here, however, some studies in the literature also show an anthocyanin-dependent regulation of glucose uptake. For instance, strawberry extracts as well as anthocyanins extracted from blueberries and acerola showed the significant reduction in glucose uptake.14,42,56,58–60 In contrast to that, Barberis and coworkers revealing an increase in glucose absorption by pomegranate and blueberry extracts.54 Finally, the impact of fruit extract on the glucose transport especially in view of antidiabetic activities is still not known for certain. It is difficult to compare all studies to each other due to complex and different composition of the extracts as well as compound-modification like digestion in the in vivo studies. Nevertheless, studies already showed that some bioactive compounds, in particular anthocyanins, phenolic acids and tannins, can influence the shape of the blood glucose curve and thus the absorption and transport of glucose. These studies have shown that these bioactive compounds can lead to an altered pattern of intestinal glucose uptake, possibly due to interactions between the compounds and enterocyte sugar transporters.14,20,614.5 qPCR
To ensure that the time of highest possible expression is used for the analysis with regard to the regulation of the individual genes, the different time intervals of the treatment were first analyzed using a time trial. These were investigated using the fruit juice extract of chokeberry as an example. It was found that for the two glucose transporters GLUT1 and GLUT2, expression was most suitable after a treatment time of 4–6 h. For SGLT1, no significant differences were seen between treatment times, with the exception of significantly lower expression of the gene after 8 h. It can therefore be assumed that SGLT is produced relatively stable at the same level by the co-culture when treated with fruit juice extract for treatment periods between 1 and 6 h a significant and time-dependent decrease in DPP4 gene expression was observed.Basically, it can be seen that cranberry and chokeberry extracts inhibited the formation of DPP4 after 4 h of treatment in contrast to the control (Fig. 6). To exert their effects, procyanidins may use various mechanisms, such as direct interaction and modulation of the activity of signalling proteins and/or prevention of oxidation.62 Procyanidins may also interact with transcription factors and enzymes.63,64 Several studies have demonstrated an antihyperglycemic effect of grape seed-derived procyanidins in insulin-resistant animals.59,60 Given the emerging role of DPP4 as a target for the regulation of glucose homeostasis, it could be hypothesized that the antihyperglycemic effect of procyanidins may also be mediated through the modulation of DPP4. There are few studies on the effects of phenolic compounds on DPP4 activity, and the published results show variable effects. González-Abuín et al.65 have already shown in studies that grape seed-derived procyanidins reduce DPP4 activity and expression. Moreover, chokeberry juice and a black bean anthocyanin fraction significantly reduced DPP4 activity, while cyanidin-3,5-diglucoside was identified as bioactive compound.66 Thus, anthocyanins seem to be essential in DPP4 inhibition. However, elderberry and blueberry extracts also consist of high levels of cyanidin-3-O-glucoside, but lack of significant DPP4 regulation.
When treated with the individual fruit juice extracts, the results showed that the expression of the glucose transporters GLUT1 and GLUT2 is significantly reduced compared to the control. GLUT1 is expressed at the same significant low level on average in all treatments with fruit juice extracts. The same applies to the expression of GLUT2, except for the treatment of the cells with chokeberry fruit juice extract. There, the expression of the gene was further reduced compared to the other extracts, so that it can be concluded that the intake of fruit juice extracts inhibits glucose uptake. According to the qPCR results the glucose uptake should be significantly reduced, but the glucose transport studies showed otherwise. No effect was detected for any other extracts. Reasons for this discrepancy could be the compound-induced higher permeability shown by the reduced TEER values. If the paracellular space was widened, glucose may reach the basolateral compartment without active transport through the cells. Furthermore, the influence on mRNA levels did not directly implicate changes on the protein biosynthesis of GLUT proteins. Nevertheless, Müller and colleagues showed that the reduction of GLUT2 expression by guava fruit extract significantly reduced the glucose uptake in mice.67 Similar results were shown using apple polyphenol extract, which reduced the blood glucose level in mice and humans by inhibition of SGLT1.15 No significant differences were observed in the expression of GLUT1, GLUT2, SGLT1 and DPP4 when the cells were treated with fruit juices. However, the extract of chokeberry should have the lowest expression of the genes studied compared to all extracts, which would be a result of an inhibitory effect.
Alzaid et al.56 observed that when Caco-2 cells were treated with a polyphenol-rich berry extract of blueberry, cranberry, elderberry, raspberry seed and strawberry, which is particularly rich in anthocyanins, a significant reduction in the expression of the GLUT2 and SGLT1 genes was observed with increasing treatment duration and that this inhibited glucose uptake. In addition, the polyphenol-rich berry extract inhibited the expression of the glucose transporter. The concentrations of berry extract used in Williamson's study (around 4 mmol L−1 – highest dose) correspond to the amounts of polyphenols found in the intestinal lumen after consuming polyphenol-rich drinks or fruits.68 There is evidence that glucose absorption across the apical membrane of enterocytes is mediated by a combination of SGLT1 and GLUT2,18 although the exact contribution of GLUT2 to lumenal glucose uptake remains controversial. Johnston, et al.61 and Kwon, et al.20 have previously shown that polyphenols regulate glucose uptake in Caco-2 cells, with polyphenols acting primarily on sodium-dependent transport. The expression of both GLUT1 and GLUT2 was significantly reduced in the present study after treatment of the cells with the fruit juice extracts, indicating possible positive effects on the intestinal glucose transport mechanism. Interestingly, data from Alzaid, et al.56 suggest that the effects of berry polyphenols on glucose transporter mRNA expression are limited to the major intestinal glucose transporters SGLT1 and GLUT2 and have no effect on the expression of the ubiquitous glucose transporter GLUT1. Despite significant reductions in GLUT2 and SGLT1 mRNA, there was no significant effect on total glucose uptake when treated with a chronic berry extract.56
Numerous in vivo and in vitro studies have also shown that phenolic compounds have a protective effect on inflammation by reducing ROS.66 While antioxidant compounds can play a direct role in radical scavenging, endogenous antioxidant enzymes can protect against oxidative stress.69 In order to achieve a direct antioxidant effect of polyphenols, a high bioavailability of the polyphenol-rich compounds in the tissue would be required so that the compounds can react directly with ROS. However, the content of dietary polyphenols in tissues is probably lower than that of endogenous antioxidant enzymes, so there is reason to suggest that the antioxidant effect is due to an indirect change in cell signalling and associated gene transcription, which controls the activity of endogenous antioxidants. The activity of endogenous antioxidants in cells is controlled by several enzymes, including SOD and CAT. SOD catalyzes the first step in the generation of hydrogen peroxide, which is subsequently converted to water and oxygen by CAT.70,71 In this study, CAT expression was decreased steadily over time when cells are treated with chokeberry extract. This steady decrease in the expression of SOD is also evident with the increase in treatment time. The results showed that the expression of the CAT and SOD genes generally decreased when the cells are treated with chokeberry extract over the tested treatment period. This could be an indication that the antioxidants contained in the chokeberry already have the ability to scavenge some of the radicals that may be present, meaning that the genes no longer need to be expressed as strongly by the cell. The individual compounds of chokeberries would have to be examined for their redox potential in future studies.
A significant decrease in the expression of the CAT and SOD genes can only be observed in the treatment with chokeberry extract compared to the control. In contrast, the SOD gene is significantly more strongly expressed when the cells are treated with gojiberry extract than in the control. One reason for this could be the low antioxidant content of the extract compared to the other fruit juice extracts examined, which was analyzed in the TEAC and TPC assays. The results obtained in this study are partially comparable with the results of Miao et al.72 Miao et al.72 also investigated the CAT and SOD expression in Caco-2 cells treated with apple polyphenols. The results of Miao et al.72 showed that apple polyphenols effectively reduced the degree of oxidative damage and increased the antioxidant capacity of the cells. In the recent study no significant effect of the apple extract, rich in chlorogenic acid, on SOD and CAT mRNA levels was measurable. This suggests that apple polyphenols activate certain protective signalling pathways in the cells that suppress the H2O2 cascade reaction. Miao et al.72 suggest that the compounds chlorogenic acid, procyanidin, epicatechin and phloridzin in apple polyphenols contribute to a strong antioxidant effect. It is generally known that the structure of the polyphenolic hydroxyl groups confers antioxidant activities to the components such as chlorogenic acid. The fruit juice extract of chokeberry used in this study also has high levels of chlorogenic acid (5-CQA) and the anthocyanin cyanidin-3-O-glucoside, so that the effects shown on the CAT and SOD genes can be attributed to these compounds. Chlorogenic acid has been shown to reduce membrane damage and lipid oxidation. Furthermore, chlorogenic acid stimulates the activity of antioxidant enzymes and alters the transcription levels of antioxidant enzymes and genes associated with phenol metabolism, thus exerting an effective antioxidant effect.72 In contrast, procyanidins showed significant ROS scavenging ability. In a similar study, it was reported that a procyanidin extract from Granny Smith apples also reduced oxidative stress in Caco-2 cells by increasing the concentration of antioxidant enzymes, e.g. SOD and CAT.73
5. Conclusion
According to our investigations, chokeberry, pomegranate and blueberry juice extracts showed the highest radical scavenging activity and total phenolic content. Due to this high antioxidant potential, chokeberry extract induced lower expression of CAT and SOD. Most extracts significantly increased the permeability of Caco-2 and HT29-MTX co-culture monolayers, while this effect seemed mainly induced by anthocyanins, which just showed a low recovery especially in case of the chokeberry anthocyanin fraction. Interestingly, in case of the glucose transport the anthocyanin content of the extracts appeared less essential. Catechin-rich extracts of pomegranate and cranberry increased slightly the glucose transport compared to control. Chokeberry reduced effectively GLUT1, GLUT2 as well as DPP4 which related to blood glucose regulation. Nevertheless, all extracts reduced GLUT1 and GLUT2 mRNA expression. SGLT1 was not significantly modified by any of the fruit extracts. In conclusion, fruit extracts have antioxidant potential and anti-diabetic effects by glucose transport regulation. However, they increase the intestinal permeability, which may induce endotoxemia and inflammatory response. These mechanisms should be analyzed in further studies in addition to the further identification of the most effective substances in fruit extracts.Author contributions
Investigation, M. K., M. R.-W. (chemical analyses), M. K. and T. K. (cell culture studies); writing – original draft preparation, M. K., T. K. and T. E.; writing – review and editing, M. K., T. K. and T. E.; visualization, M. K., T. K. and T. E.; conceptualisation, T.K. and T.E.; supervision, T. E.; project administration, T. E. All authors have read and agreed to the published version of the manuscript.Data availability
Data will be made available on request. All relevant data are within the manuscript, and all others are available by M. K. upon reasonable request.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The publication of this article was funded by the Open Access Fund of Leibniz Universität Hannover.We thank Maike Ehler and Johann Hornbacher for their excellent technical support. We thank Tentamus chelab laboratory for its excellent support in chemical analysis. We thank Obstkelterei van Nahmen (Hamminkeln, Germany), Aronia Original (Dresden, Germany) and goji-Hof Hagenburg (Hagenburg, Germany) for supplying of juices and fruits.
References
- D. S. Jones and S. H. Podolsky, The burden of disease and the changing task of medicine, N. Engl. J. Med., 2012, 366, 2333–2338 CrossRef CAS PubMed.
- G. S. P. Lima, F. Vianello, C. R. Corrêa, R. A. S. Da Campos and M. G. Borguini, Polyphenols in fruits and vegetables and its effect on human health, Food Nutr. Sci., 2014, 05, 1065–1082 CAS.
- M. Handeland, N. Grude, T. Torp and R. Slimestad, Black chokeberry juice (Aronia melanocarpa) reduces incidences of urinary tract infection among nursing home residents in the long term—a pilot study, Nutr. Res., 2014, 34, 518–525 CrossRef CAS.
- J. Hawkins, C. Hires, C. Baker, L. Keenan and M. Bush, Daily supplementation with Aronia melanocarpa (chokeberry) reduces blood pressure and cholesterol: a meta analysis of controlled clinical trials, J. Diet. Suppl., 2021, 18(5), 517–530 CrossRef CAS PubMed.
- G. Istas, E. Wood, M. Le Sayec, C. Rawlings, J. Yoon, V. Dandavate, D. Cera, S. Rampelli, A. Costabile, E. Fromentin and A. Rodriguez-Mateo, Effects of aronia berry (poly)phenols on vascular function and gut microbiota: a double-blind randomized controlled trial in adult men, Am. J. Clin. Nutr., 2019, 110, 316–329 CrossRef PubMed.
- N. Tasic, V. L. J. Jakovljevic, M. Mitrovic, B. Djindjic, D. Tasic and D. Dragisic, Citakovi, Black chokeberry aronia melanocarpa extract reduces blood pressure, glycemia and lipid profile in patients with metabolic syndrome: a prospective controlled trial, Mol. Cell. Biochem., 2021, 476, 2663–2673 CrossRef CAS PubMed.
- M. Gośliński, D. Nowak and A. Szwengie, Multidimensional comparative analysis of bioactive phenolic compounds of honeys of various origin, Antioxidants, 2021, 10(4), 530 CrossRef PubMed.
- S. Y. Wang and H. Jiao, Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen, J. Agric. Food Chem., 2000, 48, 5677–5684 CrossRef CAS PubMed.
- P. Fresco, F. Borges, C. Diniz and M. P. M. Marques, New insights on the anticancer properties of dietary polyphenols, Med. Res. Rev., 2006, 26, 747–766 CrossRef CAS PubMed.
- M. W. Rohr, C. A. Narasimhulu, T. A. Rudeski-Rohr and S. Parthasarathy, Negative effects of a high-fat diet on intestinal permeability: A review, Adv. Nutr., 2020, 11, 77–91 CrossRef PubMed.
- C. S. Guerreiro, Â Calado, J. Sousa and J. E. Fonseca, Diet, microbiota, and gut permeability-The unknown triad in rheumatoid arthritis, Front. Med., 2018, 5, 349 CrossRef PubMed.
- S. Bernardi, C. Del Bo’, M. Marino, G. Gargari, A. Cherubini, C. Andrés-Lacueva, N. Hidalgo-Liberona, G. Peron, R. González-Dominguez, P. Kroon, B. Kirkup, M. Porrini, S. Guglielmetti and P. Riso, Polyphenols and intestinal permeability: Rationale and future perspectives, J. Agric. Food Chem., 2020, 68, 1816–1829 CrossRef CAS PubMed.
- N. Hidalgo-Liberona, R. González-Domínguez, E. Vegas, P. Riso, C. Del Bo’, S. Bernardi, G. Peron, S. Guglielmetti, G. Gargari, P. A. Kroon, A. Cherubini and C. Andrés-Lacueva, Increased intestinal permeability in older subjects impacts the beneficial effects of dietary polyphenols by modulating their bioavailability, J. Agric. Food Chem., 2020, 68, 12476–12484 CrossRef CAS PubMed.
- S. Manzano and G. Williamson, Polyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal Caco-2 cells, Mol. Nutr. Food Res., 2010, 54, 1773–1780 CrossRef CAS.
- C. Schulze, A. Bangert, G. Kottra, K. E. Geillinger, B. Schwanck, H. Vollert and H. Daniel, Inhibition of the intestinal sodium-coupled glucose transporter 1 (SGLT1) by extracts and polyphenols from apple reduces postprandial blood glucose levels in mice and humans, Mol. Nutr. Food Res., 2014, 58, 1795–1808 CrossRef CAS PubMed.
- S. Sharma, P. Tripathi, J. Sharma and A. Dixit, Flavonoids modulate tight junction barrier functions in hyperglycemic human intestinal Caco-2 cells, Nutrition, 2020, 78, 110792 CrossRef CAS.
- R. A. M. Vreeburg, E. E. van Wezel, F. Ocaña–Calahorro and J. J. Mes, Apple extract induces increased epithelial resistance and claudin 4 expression in Caco-2 cells, J. Sci. Food Agric., 2012, 92, 439–444 CrossRef CAS.
- G. L. Kellett, E. Brot-Laroche, O. J. Mace and A. Leturque, Sugar absorption in the intestine: the role of GLUT2, Annu. Rev. Nutr., 2008, 28, 35–54 CrossRef CAS PubMed.
- F. Alvardo, Hypothesis for the interaction of phlorizin and phloretin with membrane carriers for sugars, Biochim. Biophys. Acta, 1967, 483–495 CrossRef.
- O. Kwon, P. Eck, S. Chen, C. P. Corpe, J.-H. Lee, M. Kruhlak and M. Levine, Inhibition of the intestinal glucose transporter GLUT2 by flavonoids, FASEB J., 2007, 21, 366–377 CrossRef CAS.
- T. Kostka, J. J. Ostberg-Potthoff, K. Briviba, S. Matsugo, P. Winterhalter and T. Esatbeyoglu, Pomegranate (Punica granatum L.) Extract and its anthocyanin and copigment fractions-free radical scavenging activity and influence on cellular oxidative stress, Foods, 2020, 9(11), 1617 CrossRef CAS PubMed.
- A. Juadjur and P. Winterhalter, Development of a novel adsorptive membrane chromatographic method for the fractionation of polyphenols from bilberry, J. Agric. Food Chem., 2012, 60, 2427–2433 CrossRef CAS.
- T. Kostka, J. J. Ostberg-Potthoff, J. Stärke, C. Guigas, S. Matsugo, V. Mirčeski, L. Stojanov, S. K. Veličkovska, P. Winterhalter and T. Esatbeyoglu, Bioactive phenolic compounds from lingonberry (Vaccinium vitis-idaea L.): Extraction, chemical characterization, fractionation and cellular antioxidant activity, Antioxidants, 2022, 11(3), 467 CrossRef CAS.
- J. J. Ostberg-Potthoff, K. Berger, E. Richling and P. Winterhalter, Activity-guided fractionation of red fruit extracts for the identification of compounds influencing glucose metabolism, Nutrients, 2019, 11(5), 1166 CrossRef CAS.
- B. Srinivasan, A. R. Kolli, M. B. Esch, H. E. Abaci, M. L. Shuler and J. J. Hickman, TEER measurement techniques for in vitro barrier model systems, J. Lab. Autom., 2015, 20, 107–126 CrossRef CAS.
- W. Ting, C. Grootaert, S. Voorspoels, G. Jacobs, J. Pitart, S. Kamiloglu, S. Possemiers, M. Heinonen, N. Kardum, M. Glibetic, G. Smagghe, K. Raes and J. van Camp, Aronia (Aronia melanocarpa) phenolics bioavailability in a combined in vitro digestion/Caco-2 cell model is structure and colon region dependent, J. Funct. Foods, 2017, 38, 128–139 CrossRef.
- K. Palm, K. Luthman, A. L. Ungell, G. Strandlund, F. Beigi, P. Lundahl and P. Artursson, Evaluation of dynamic polar molecular surface area as predictor of drug absorption: comparison with other computational and experimental predictor, J. Med. Chem., 1998, 41, 5382–5392 CrossRef CAS PubMed.
- D. Nowak, M. Gośliński and E. Wojtowicz, Comparative analysis of the antioxidant capacity of selected fruit juices and nectars: Chokeberry juice as a rich source of polyphenols, Int. J. Food Prop., 2016, 19, 1317–1324 CrossRef CAS.
- M. I. Gil, F. A. Tomás-Barberán, B. Hess-Pierce, D. M. Holcroft and A. A. Kader, Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing, J. Agric. Food Chem., 2000, 48, 4581–4589 CrossRef CAS.
- V. Kozik, K. Jarzembek, A. Jędrzejowska, A. Bąk, J. Polak, M. Bartoszek and K. Pytlakowska, et al., Investigation of antioxidant activity of pomegranate juices by means of electron paramagnetic resonance and UV-Vis spectroscopy, J. AOAC Int., 2015, 98, 866–870 CrossRef CAS PubMed.
- N. P. Seeram, M. Aviram, Y. Zhang, S. M. Henning, L. Feng, M. Dreher and D. Heber, Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the united states, J. Agric. Food Chem., 2008, 56, 1415–1422 CrossRef CAS PubMed.
- T. Esatbeyoglu, M. Rodríguez-Werner and P. Winterhalter, Fractionation and isolation of polyphenols from Aronia melanocarpa by countercurrent and membrane chromatography, Eur. Food Res. Technol., 2017, 243, 1261–1275 CrossRef CAS.
- D. Nowak, M. Gośliński and L. Kłębukowska, Antioxidant and antimicrobial properties of selected fruit juices, Plant Foods Hum. Nutr., 2022, 77, 427–435 CrossRef CAS.
- A. C. Pedro, M. L. Pérez-Rodríguez, M.-C. Sánchez-Mata, R. Z. Bisinella, C. Oliveira, C. de Soltovski, E. Schnitzler, C. D. Bet, G. M. Maciel and C. W. I. Haminiuk, Biological activities, chromatographic profile and thermal stability of organic and conventional goji berry, Food Meas., 2022, 16, 1263–1273 CrossRef.
- G. A. Manganaris, V. Goulas, A. R. Vicente and L. A. Terry, Berry antioxidants: small fruits providing large benefits, J. Sci. Food Agric., 2014, 94, 825–833 CrossRef.
- E. Azzini, G. Maiani, I. Garaguso, A. Polito, M. S. Foddai, E. Venneria, A. Durazzo, F. Intorre, L. Palomba, M. L. Rauseo, G. Lombardi-Boccia and F. Nobili, The potential health benefits of polyphenol-rich extracts from Cichorium intybus L. Studied on Caco-2 cells model, Oxid. Med. Cell. Longevity, 2016, 2016, 1594616 CrossRef.
- Y. Konishi, Modulations of food-derived substances on intestinal permeability in Caco-2 cell monolayers, Biosci., Biotechnol., Biochem., 2003, 67, 2297–2299 CrossRef CAS PubMed.
- C. B. van Buiten, J. D. Lambert and R. J. Elias, Green tea polyphenols mitigate gliadin-mediated inflammation and permeability in vitro, Mol. Nutr. Food Res., 2018, 62, e1700879 CrossRef PubMed.
- M. Amasheh, S. Schlichter, S. Amasheh, J. Mankertz, M. Zeitz, M. Fromm and J. D. Schulzke, Quercetin enhances epithelial barrier function and increases claudin-4 expression in Caco-2 cells, J. Nutr., 2008, 138, 1067–1073 CrossRef CAS PubMed.
- T. Suzuki and H. Hara, Quercetin enhances intestinal barrier function through the assembly of zonula corrected occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells, J. Nutr., 2009, 139, 965–974 CrossRef CAS.
- M. C. Valenzano, K. DiGuilio, J. Mercado, M. Teter, J. To, B. Ferraro, B. Mixson, I. Manley, V. Baker, B. A. Moore, J. Wertheimer and J. M. Mullin, Remodeling of tight junctions and enhancement of barrier integrity of the Caco-2 intestinal epithelial cell layer by micronutrients, PLoS One, 2015, 10, e0133926 CrossRef.
- N. G. Lamson, K. C. Fein, J. P. Gleeson, A. N. Newby, S. Xian, K. Cochran, N. Chaudhary, J. R. Melamed, R. L. Ball, K. Suri, V. Ahuja, A. Zhang, A. Berger, D. Kolodieznyi, B. F. Schmidt, G. L. Silva, K. A. Gloria and K. Whitehead, The strawberry-derived permeation enhancer pelargonidin enables oral protein delivery, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2207829119 CrossRef CAS.
- I. Epriliati, B. D'Arcy and M. Gidley, Nutriomic analysis of fresh and processed fruit products. 2. During in vitro simultaneous molecular passages using Caco-2 cell monolayers, J. Agric. Food Chem., 2009, 57, 3377–3388 CrossRef CAS PubMed.
- K. Hashimoto, N. Matsunaga and M. Shimizu, Effect of vegetable extracts on the transepithelial permeability of the human intestinal Caco-2 cell monolayer, Biosci., Biotechnol., Biochem., 1994, 58, 1345–1346 CrossRef.
- A. Béduneau, C. Tempesta, S. Fimbel, Y. Pellequer, V. Jannin, F. Demarne and A. Lamprecht, A tunable Caco-2/HT29-MTX co-culture model mimicking variable permeabilities of the human intestine obtained by an original seeding procedure, Eur. J. Pharm. Biopharm., 2014, 87, 290–298 CrossRef.
- P. Hoffmann, M. Burmester, M. Langeheine, R. Brehm, M. T. Empl, B. Seeger and G. Breves, Caco-2/HT29-MTX co-cultured cells as a model for studying physiological properties and toxin-induced effects on intestinal cells, PLoS One, 2021, 16, e0257824 CrossRef CAS.
- H.-Y. Park, Y. Kunitake, N. Hirasaki, M. Tanaka and T. Matsui, Theaflavins enhance intestinal barrier of Caco-2 cell monolayers through the expression of AMP-activated protein kinase-mediated Occludin, Claudin-1, and ZO-1, Biosci., Biotechnol., Biochem., 2015, 79, 130–137 CrossRef CAS PubMed.
- T. Suzuki, S. Tanabe and H. Hara, Kaempferol enhances intestinal barrier function through the cytoskeletal association and expression of tight junction proteins in Caco-2 cells, J. Nutr., 2011, 141, 87–94 CrossRef CAS.
- E. Cremonini, E. Daveri, A. Mastaloudis, A. M. Adamo, D. Mills, K. Kalanetra, S. N. Hester, S. M. Wood, C. G. Fraga and P. Oteiza, Anthocyanins protect the gastrointestinal tract from high fat diet-induced alterations in redox signaling, barrier integrity and dysbiosis, Redox Biol., 2019, 26, 101269 CrossRef CAS PubMed.
- T. Le Phuong Nguyen, F. F. Thi, J. Remenyik, J. R. Homoki, P. Gogolák, I. Bácskay, P. Fehér, Z. Ujhelyi, G. Vasvári, M. Vecsernyés and J. Váradi, Protective effect of pure sour cherry anthocyanin extract on cytokine-induced inflammatory Caco-2 monolayers, Nutrients, 2018, 10(7), 861 CrossRef PubMed.
- Y. Wang and T. Hatabu, Mulberry juice freeze-dried powder attenuates the disease severity by the maintaining of colon mucosa in mice with DSS-induced acute colitis, Biosci., Biotechnol., Biochem., 2019, 83, 914–922 CrossRef CAS.
- T. Liu, P. Pan, H. Shi, J. Feng and X.-Z. Zhang, Assembled polyphenol–based systems as advanced therapeutics, J. Polym. Sci., 2024, 62, 297–323 CrossRef CAS.
- Z. Ci, C. Jiang, Y. Cui and M. Kojima, Suppressive effect of polyphenols from Immature pear fruits on blood glucose levels, J. Food Nutr. Res., 2018, 6, 445–449 CrossRef CAS.
- A. Barberis, A. Garbetta, A. Cardinali, G. Bazzu, I. D’Antuono, G. Rocchitta, A. Fadda, V. Linsalata, G. D’Hallewin, P. A. Serra and F. Minervini, Real-time monitoring of glucose and phenols intestinal absorption through an integrated Caco-2TC7cells/biosensors telemetric device: Hypoglycemic effect of fruit phytochemicals, Biosens Bioelectron., 2017, 88, 159–166 CrossRef PubMed.
- P. J. Kausalya, S. Amasheh, D. Günzel, H. Wurps, D. Müller, M. Fromm and W. Hunziker, Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of Claudin-16, J. Clin. Invest., 2006, 116, 878–891 CrossRef CAS.
- F. Alzaid, H.-M. Cheung, V. R. Preedy and P. A. Sharp, Regulation of glucose transporter expression in human intestinal Caco-2 cells following exposure to an anthocyanin-rich berry extract, PLoS One, 2013, 8, 78932 CrossRef.
- S. Deprez, I. Mila, J. F. Huneau, D. Tome and A. Scalbert, Transport of proanthocyanidin dimer, trimer, and polymer across monolayers of human intestinal epithelial Caco-2 cells, Antioxid. Redox Signaling, 2001, 6, 957–967 CrossRef PubMed.
- P.-W. Zhang, F.-X. Chen, L. Di, W.-H. Ling and H.-H. Guo, A CONSORT-compliant, randomized, double-blind, placebo-controlled pilot trial of purified anthocyanin in patients with nonalcoholic fatty liver disease, Medicine, 2015, 94, e758 CrossRef CAS PubMed.
- R. Cermak, S. Landgraf and S. Wolffram, Quercetin glucosides inhibit glucose uptake into brush-border-membrane vesicles of porcine jejunum, Br. J. Nutr., 2004, 91, 849–855 CrossRef CAS.
- T. Hanamura, C. Mayama, H. Aoki, Y. Hirayama and M. Shimizu, Antihyperglycemic effect of polyphenols from acerola (Malpighia emarginata DC.) fruit, Biosci., Biotechnol., Biochem., 2006, 70, 1813–1820 CrossRef CAS PubMed.
- K. Johnston, P. Sharp, M. Clifford and L. Morgan, Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells, FEBS Lett., 2005, 579, 1653–1657 CrossRef CAS PubMed.
- C. G. Fraga and P. I. Oteiza, Dietary flavonoids: Role of (-)-epicatechin and related procyanidins in cell signaling, Free Radicals Biol. Med., 2011, 51, 813–823 CrossRef CAS.
- J. M. Del Bas, M. L. Ricketts, I. Baiges, H. Quesada, A. Ardevol, M. J. Salvadó, G. Pujadas, M. Blay, L. Arola, C. Bladé, D. D. Moore and J. Fernandez-Larrea, Dietary procyanidins lower triglyceride levels signaling through the nuclear receptor small heterodimer partner, Mol. Nutr. Food Res., 2008, 52, 1172–1181 CrossRef CAS PubMed.
- Y. Gu, W. J. Hurst, D. A. Stuart and J. D. Lambert, Inhibition of key digestive enzymes by cocoa extracts and procyanidins, J. Agric. Food Chem., 2011, 59, 5305–5311 CrossRef CAS PubMed.
- N. González-Abuín, N. Martínez-Micaelo, M. Blay, G. Pujadas, S. Garcia-Vallvé, M. Pinent and A. Ardévol, Grape seed-derived procyanidins decrease dipeptidyl-peptidase 4 activity and expression, J. Agric. Food Chem., 2012, 60, 9055–9061 CrossRef PubMed.
- F. A. Moura, K. Queiroz, J. C. de Andrade, F. Dos Santos, O. R. P. Araújo and M. O. F. Goulart, Antioxidant therapy for treatment of inflammatory bowel disease: Does it work?, Redox Biol., 2015, 6, 617–639 CrossRef CAS PubMed.
- U. Müller, F. Stübl, B. Schwarzinger, G. Sandner, M. Iken, M. Himmelsbach, C. Schwarzinger, N. Ollinger, V. Stadlbauer, O. Höglinger, T. Kühne, P. Lanzerstorfer and J. Weghuber, In vitro and in vivo inhibition of intestinal glucose transport by guava (Psidium guajava) extracts, Mol. Nutr. Food Res., 2018, 62, e1701012 CrossRef PubMed.
- G. Williamson, Possible effects of dietary polyphenols on sugar absorption and digestion, Mol. Nutr. Food Res., 2013, 57, 48–57 CrossRef CAS PubMed.
- Y. Du, H. Guo and H. Lou, Grape seed polyphenols protect cardiac cells from apoptosis via induction of endogenous antioxidant enzymes, J. Agric. Food Chem., 2007, 55, 1695–1701 CrossRef CAS PubMed.
- T. Finkel and N. J. Holbrook, Oxidants, oxidative stress and the biology of ageing, Nature, 2000, 408, 239–247 CrossRef CAS PubMed.
- B. Zhang, Z. Deng, Y. Tang, P. X. Chen, R. Liu, D. Dan Ramdath, Q. Liu, M. Hernandez and R. Tsao, Bioaccessibility, in vitro antioxidant and anti-inflammatory activities of phenolics in cooked green lentil (Lens culinaris), J. Funct. Foods, 2017, 32, 248–255 CrossRef CAS.
- T. Miao, G. Song and J. Yang, Protective effect of apple polyphenols on H2O2-induced oxidative stress damage in human colon adenocarcinoma Caco-2 cells, Chem. Pharm. Bull., 2023, 71, 262–268 CrossRef CAS PubMed.
- H. Wu, T. Luo, Y. M. Li, Z. P. Gao, K. Q. Zhang, J. Y. Song, J. S. Xiao and Y. P. Cao, Granny Smith apple procyanidin extract upregulates tight junction protein expression and modulates oxidative stress and inflammation in lipopolysaccharide-induced Caco-2 cells, Food Funct., 2018, 9, 3321–3329 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo03950e |
| This journal is © The Royal Society of Chemistry 2025 |