Open Access Article
Open Access ArticleStudies on the physiochemical, thermal, structural and functional attributes of vetch starch (Vicia villosa) and protein from Kashmir valley
Toiba Majeed,
Aamir Hussain Dar * and
Tariq Ahmad Ganaie
* and
Tariq Ahmad Ganaie
Department of Food Technology, Islamic University of Science and Technology, Awantipora, Kashmir, India. E-mail: daraamirft@gmail.com
First published on 3rd November 2025
Abstract
Vicia villosa (vetch), a leguminous plant and a significant source of starch and protein, is primarily used for animal nutrition, but it also has potential for human consumption. This study aimed to isolate starch and protein from vetch seeds and evaluate them based on various physicochemical, functional, structural, and thermal characteristics. The extracted protein and starch exhibited high purity with a C-type crystalline structure. The chemical composition of starch revealed moisture (7.7 ± 0.20), protein (0.09 ± 0.01), fat (0.02 ± 0.00) and ash (0.03 ± 0.01) contents, while that of protein showed moisture, fat, and ash in the range of 9.2 ± 0.3, 2.3 ± 0.26 and 2.05 ± 0.05 g/100 g. SEM revealed an oval or kidney-shaped starch structure, while a heterogenous lamellar structure with some particle aggregation in the case of vetch starch. The DSC analysis demonstrated early gelatinization with increased enthalpy (6.94 J g−1) in vetch starch, suggesting a stable crystalline region and adequate heat resistance whereas vetch protein displayed two separate denaturation peaks (86.19 °C and 227.86 °C), affirming the existence of vicilin (7S) and legumin (11S) fractions, which provide structural stability and functionality during processing. SDS-PAGE displayed polypeptide sub-units ranging between 25 kDa and 75 kDa in molecular weight with the majority of bands characteristic of vicilin and legumin. The pasting behavior of vetch starch showed an effective swelling tendency with strong gel forming capacity, as evident from the high peak, setback and final viscosities – attributes favorable for thickening, texturizing, and film production. Also, vetch protein demonstrated promising functional properties (emulsifying, gelling, and foaming), highlighting its potential applications in food, packaging and various industrial sectors.
Sustainability spotlightThe present study provides a pathway for technological valorization of the underutilized pulse variety Vicia villosa. Utilizing Vicia villosa, a natural and underutilized legume, promotes biodiversity, decreases reliance on conventional crops, and strengthens local agricultural markets. Vetch is well-known for its abundant protein content and nutritional value, making it a sustainable plant protein source. Vetch protein isolates have superior emulsifying and foaming capabilities, indicating possible applications in food formulations. Also, vetch starch has remarkable properties like a high gelatinization temperature, making it a viable source for both the food and non-food sectors. Thus, the physiochemical and functional investigation of vetch starch and protein suggests the potential for biodegradable film synthesis, providing environmentally acceptable alternatives to synthetic packaging. Overall, the research aims to promote zero-waste valorization and circular bioeconomy models by extracting starch and protein from locally available biomass, improving efficient use of resources and facilitating the transition towards green protein, collectively aimed at building a sustainable and resilient food ecosystem. |
1 Introduction
Global population was calculated to be 8.2 billion in 2024 (ref. 1) which is likely to continue growing for the next 50 to 60 years, reaching approximately 9.8 billion in 2050 and 10.3 billion by the mid-2080s.2 With this drastic increase in global population, the need for food is expected to increase by 50%, necessitating a boost in agricultural productivity.3 This spike in population, along with altering dietary preferences toward a protein-rich diet and increasing environmental concerns about conventional livestock farming, has put enormous pressure on agricultural systems to find novel sources of protein. Additionally, the increased need for sustainable, cheaper and new sources of plant-based carbohydrates led to studies on different legume varieties. Although legumes like soybeans, chickpeas, and lentils are widely employed, lesser-known and underutilized legumes provide unexplored potential for the development of nutritional and functional ingredients. Vicia villosa, which is also known as hairy vetch, is one of approximately 150 species that comprise the genus Vicia and the family Fabaceae (legume). It is a cool-season, creeping-growth legume, cultivated for green manure, pasture, silage, and grain for cattle feed under the native name “kalamatar”. The smooth, spherical, black or brown vetch seeds have a diameter between 3.5 and 5.0 mm and are categorized as an underutilized legume. Currently found throughout the world, vetch is essentially considered a multi-purpose crop because it is utilized as pasture or silage for livestock and as green manure or a cover crop in agriculture.4 However, these plants are native to tropical Africa, North and South America, East and Central Asia, and Europe. Vicia villosa seeds are considered a good source of carbohydrates, protein, fiber, and minerals like magnesium, iron, potassium, etc. and are valuable for human well-being. They also contain various phenolic compounds and flavonoids, including daidzein, quercetin, formononetin, diosmetin, kaempferol, luteolin, myricetin, petunidin, malvidin, P-hydroxy benzoic acid and gallic acids.5 It is mostly used as a source of protein for poultry and cattle, but it can also be consumed by humans. This leguminous plant produces seeds that have significantly high starch content ranging from 30–47% (DW basis).6 The seeds of V. villosa have significant amounts of crude protein, ranging from 17–25% and in some studies up to 28%.7–9 Thus, vetch is not only an important source of starch and protein for farm animals, but it also offers a potential diet for humans. Vicia villosa, as a non-conventional source of starch, has the ability to broaden the range of necessary functional qualities required to generate high-value food products and promote the use of sustainable agricultural resources.Even though legumes like soybean, chickpea, Vicia sativa and lentil have been investigated, Vicia villosa (especially from the Kashmir valley) has received less attention despite having similar or higher protein and starch content. Existing studies on vetch species have mostly concentrated on their proximate composition and value as animal feed, paying little attention to their techno-functional, structural, or thermal properties. Furthermore, integrated investigations utilizing multi-technique analyses, including differential scanning calorimetry (DSC), X-ray diffraction (XRD), rapid visco analysis (RVA), and scanning electron microscopy (SEM), are still lacking for V. villosa. Therefore, by offering a thorough assessment of the protein and starch extracted from Vicia villosa, this study advances current knowledge by systematically characterizing its physicochemical, thermal, structural, and functional properties, emphasizing the plant's potential as an underutilized, sustainable source of functional ingredients. By investigating these properties, it is possible to assess their potential applications in various food systems to highlight the importance of vetch seeds as a novel source for broader utilization beyond agricultural uses.
2 Materials and methods
2.1 Materials and chemical reagents
Vetch (Vicia villosa) seeds were procured from Srinagar local markets. Seeds were cleaned of dust and other contaminants and stored at 20 °C for future use. The study employed only analytical-grade substances.2.2 Physico-chemical properties
Protein isolate recovery was measured by weighing the extracted isolates per 100 g of vetch seed, while the protein yield of isolates was calculated using the following equation.
2.3 Pasting properties
Pasting parameters of vetch seed starch were computed by means of a Rapid-visco Analyzer (Tech Master, Pertain Instruments, Warriewood, Australia) using the procedure described by Wani et al.16 An aqueous starch suspension containing 3 g of starch (10.7%, w/w) on a 14% moisture basis was prepared in an aluminum canister (28 g capacity). Initially, the sample was held at 50 °C for 1 min, then heated to 95 °C at a rate of 12.2 °C and maintained at this temperature for 2.5 min, followed by cooling to 50 °C at a rate of 11.8 °C min−1 and again held for 2 min. During the test, the paddle rotated continuously at a speed of 160 rpm, except for the initial 10 seconds when it rotated faster at 960 rpm for proper dispersion of the sample.2.4 Differential scanning calorimetry (DSC)
Vetch starch and protein samples were subjected to differential scanning calorimetry in order to determine their onset (Tonset), peak (Tpeak), and end (Tend) temperatures as well as their enthalpy (ΔH). A Differential Scanning Calorimeter (Perkin Elmer, Model: DSC 6000) was used to monitor the DSC thermograms of the protein and starch samples. Samples of 4.1 mg (starch) and 4.9 mg (protein) were hermetically sealed and then heated in aluminum pans between 20 and 150 °C for starch and 20 to 250 °C for protein at a scanning rate of 10 °C min−1. As a reference, an empty pan was used.2.5 SDS-PAGE
The molecular weight of unmodified vetch protein isolate was determined by SDS-PAGE utilizing a BIO-RAD Mini-PROTEAN system, as described by Strauch & Lila19 with minor changes. A powdered protein sample of 0.2 mg was solubilized in 500 μL Laemmle buffer (5×) and denatured for 20 min at 85 °C. The sample was then subjected to centrifugation at rpm of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for 5 min. Afterwards the gels were run at a steady voltage of 90 V for 2 to 2.5 hours at room temperature in a premixed 12% resolving gel (Acryl-bis, Tris, SDS, APS and TEMED). Then the gels were stained using Hi-Media Coomassie Brilliant Blue Stain (G-250, MB092-25G) for 1.5 hours and finally de-stained with double distilled water overnight at a shaking speed of 12. The gels were again rinsed with double-distilled water until protein bands started appearing on the gel and imaged with a ChemiDoc MP Imaging System (Invitrogen by Thermo Fischer Scientific, Model: CL1500, Singapore). The molecular weights were estimated using a standard from 11 to 75 kDa.
000 for 5 min. Afterwards the gels were run at a steady voltage of 90 V for 2 to 2.5 hours at room temperature in a premixed 12% resolving gel (Acryl-bis, Tris, SDS, APS and TEMED). Then the gels were stained using Hi-Media Coomassie Brilliant Blue Stain (G-250, MB092-25G) for 1.5 hours and finally de-stained with double distilled water overnight at a shaking speed of 12. The gels were again rinsed with double-distilled water until protein bands started appearing on the gel and imaged with a ChemiDoc MP Imaging System (Invitrogen by Thermo Fischer Scientific, Model: CL1500, Singapore). The molecular weights were estimated using a standard from 11 to 75 kDa.
2.6 Fourier transforms infrared (FTIR) spectroscopy
The FTIR spectra of starch and protein samples were captured on a FTIR spectrophotometer (PerkinElmer) coupled to an ATR accessory at room temperature. Finely ground starch and protein powders were measured in the mid-infrared region with a resolution of 4 cm−1 and over a spectral range of 500–4000 cm−1.2.7 Scanning electron microscopy (SEM)
To interpret SEM micrographs of vetch starch and protein granules, a field emission scanning electron microscope (JSM-6480 LV, JEOL, Japan) was used following the protocol of Jafari et al.20 The dried samples were coated with gold particles using a sputter coater before examination. The pictures were taken at an acceleration voltage of 15 kV.2.8 X-ray diffraction (XRD)
The crystalline structure of vetch starch and protein samples was analyzed with an X-ray diffractometer (Model: D8 Advance DAVINCI, Bruker AXS Inc. Madison, WI, USA) according the methodology of de Oliveira Filho et al.21 The samples were scanned over a 2θ range of 5 °C to 40 °C at an average temperature of 3.2° per minute with a step value of 0.0131°.2.9 Functional properties
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 1 min in a 250 mL measuring cylinder with a high-speed homogenizer (IKA, Ultra Turrax, Model: T 18 digital, Germany). Foaming capacity was calculated as the percent increase in the volume of foam of protein dispersion upon homogenization. The foam stability was analyzed by measuring the volume of foam over time.
000 rpm for 1 min in a 250 mL measuring cylinder with a high-speed homogenizer (IKA, Ultra Turrax, Model: T 18 digital, Germany). Foaming capacity was calculated as the percent increase in the volume of foam of protein dispersion upon homogenization. The foam stability was analyzed by measuring the volume of foam over time.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 1 min. Immediately after homogenization, 50 μL of emulsion was pipetted out from the bottom of the centrifuge tube into 5 mL of 0.1% (w/v) SDS solution at 0 and 10 min. The absorbance of the resulting emulsion was recorded as initial absorbance (A0) and the absorbance after 10 min (A10) at 500 nm using a UV spectrophotometer (SP-UV 300, SPECTRUM, PerkinElmer Company, New York). The absorbances measured at (A0) and (A10) after emulsion formation were used to calculate the emulsifying activity index (EAI) and the emulsion stability index (ESI).
000 rpm for 1 min. Immediately after homogenization, 50 μL of emulsion was pipetted out from the bottom of the centrifuge tube into 5 mL of 0.1% (w/v) SDS solution at 0 and 10 min. The absorbance of the resulting emulsion was recorded as initial absorbance (A0) and the absorbance after 10 min (A10) at 500 nm using a UV spectrophotometer (SP-UV 300, SPECTRUM, PerkinElmer Company, New York). The absorbances measured at (A0) and (A10) after emulsion formation were used to calculate the emulsifying activity index (EAI) and the emulsion stability index (ESI).where DF is the dilution factor (100), c is the initial concentration of protein (g mL−1), φ is the optical path (0.01), θ is the oil volume fraction of the emulsion (0.25) and A0 and A10 are the absorbances of the emulsion at 0 and 10 min, respectively.
2.10 Statistical analysis
All studies were carried out in triplicate and the data were presented as the average of three readings. An analysis of variance (ANOVA) with a significance level of 5% was done and Duncan's test was performed for assessing differences between means using commercial data analysis software (SPSS Inc., Chicago, IL, USA). Statistical analysis was performed using OriginPro 2018 software (OriginLab Corporation, Northampton, MA, USA).3 Results and discussion
3.1 Physico-chemical properties
| Parameter | Vetch starch | Vetch protein |
|---|---|---|
| a ND: not-detected. Values expressed are mean ± standard deviation. | ||
| Moisture (g/100 g) | 7.70 ± 0.20 | 9.20 ± 0.30 |
| Protein (g/100 g) | 0.09 ± 0.01 | 85.66 ± 0.57 |
| Fat (g/100 g) | 0.02 ± 0.00 | 2.30 ± 0.26 |
| Ash (g/100 g) | 0.03 ± 0.01 | 2.05 ± 0.05 |
| Carbohydrate (g/100 g) | 92.16 ± 0.00 | ND |
The carbohydrate content of vetch starch was estimated to be 91.16 (g/100 g), respectively. Also, the apparent amylose content of vetch seed starch was 31.25 g/100 g. These values are consistent with the findings of Tarahi et al.26 for bitter vetch starch, Wani et al.27 for Bengal gram starch, and Wani et al.11 for kidney bean protein isolates.
Protein isolate recovery of vetch protein was calculated to be 13 g/100 g, whereas protein yield was estimated to be 46.4 g/100 g (Table 2). The observed data regarding the recovery of vetch protein isolates are consistent with the results reported by Stone et al.22 and Sofi et al.14 for pea protein and chickpea protein isolates.
| Parameter | Vetch starch | Vetch protein |
|---|---|---|
| a ND = not detected. Values expressed are mean ± standard deviation. | ||
| Amylose (g/100 g) | 31.25 ± 0.50 | ND |
| Yield (g/100 g) | 28.56 ± 0.23 | |
| Protein isolate recovery (g/100 g) | ND | 13 ± 1.50 |
| Protein yield (g/100 g) | ND | 50.61 ± 3.90 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Color | ||
| L* | 93.93 ± 0.15 | 83.51 ± 0.46 |
| a* | −0.14 ± 0.01 | 3.80 ± 0.25 |
| b* | 6.48 ± 0.09 | 18.20 ± 0.26 |
The swelling index (SI) ranged between 2.75 and 13.71, and 2.90 to 11.09 g g−1 for vetch starch and protein respectively. The SI of both vetch starch and protein showed an increase with the increase in temperature from 50 °C to 90 °C (Table 3). The increase in swelling power could be attributed to the breakdown of intermolecular interactions, such as hydrogen bonding, as temperature increases. The solubility index also increased with increasing temperature from 50–90 °C. The water solubility index of vetch starch ranged from 0.002 to 0.19 and from 0.03 to 0.14 for vetch protein. These results are comparable with those reported for wheat, yellow pea starch, and cowpea cultivars32–34 but little higher than that for wheat which may be due to the difference in amylose content. The swelling power is highly influenced by the amylose content; materials with a lower amylose concentration often have higher swelling power. The moderate swelling capacity of vetch starch provides significant water-binding ability and suggest its possible application in food systems that require viscosity development, thickening, or moisture retention. Additionally, because of its neutral solubility, it is advantageous for films used for packaging materials. Vetch protein, on the other hand, has lower swelling and solubility, which suggests that it has more structural stability and regulated water uptake. These qualities are beneficial for high-protein food products or composite edible films where integrity and decreased moisture sensitivity are crucial.
| Parameter | Vetch starch | Vetch protein |
|---|---|---|
| a Values expressed are mean ± standard deviation. Means in the row with different superscripts are significantly different at p ≤ 0.05. | ||
| Swelling index (g g−1) | ||
| 50 °C | 2.75 ± 0.04a | 2.90 ± 0.04a |
| 60 °C | 3.81 ± 0.14a | 4.10 ± 0.17a |
| 70 °C | 7.17 ± 0.32b | 6.96 ± 0.11b |
| 80 °C | 11.14 ± 0.17c | 10.66 ± 0.32c |
| 90 °C | 13.71 ± 0.21d | 11.09 ± 0.21c |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Solubility index (g g−1) | ||
| 50 °C | 0.002 ± 0.00p | 0.03 ± 0.025p |
| 60 °C | 0.03 ± 0.01p | 0.20 ± 0.03p |
| 70 °C | 0.11 ± 0.02q | 0.29 ± 0.02q |
| 80 °C | 0.16 ± 0.01r | 0.13 ± 0.02r |
| 90 °C | 0.19 ± 0.02 s | 0.14 ± 0.03 s |
| Parameter | Vetch starch |
|---|---|
| a Values expressed are mean ± standard deviation. Means in the row with different superscripts are significantly different at p ≤ 0.05. | |
| Syneresis (%) | |
| 0 h | 00a |
| 24 h | 36.51 ± 0.51b |
| 48 h | 40.57 ± 0.52c |
| 72 h | 43.32 ± 0.43d |
| 96 h | 45.52 ± 0.47d |
| 120 h | 48.25 ± 0.23e |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Light transmittance (%) | |
| 0 h | 5.15 ± 0.34c |
| 24 h | 3.68 ± 0.02b |
| 48 h | 1.72 ± 0.01a |
| 72 h | 1.27 ± 0.03a |
| 96 h | 0.73 ± 0.05a |
| 120 h | 0.49 ± 0.01a |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Freeze–thaw (%) | |
| 0 h | 00a |
| 24 h | 3.30 ± 0.17a |
| 48 h | 11.56 ± 0.25b |
| 72 h | 17.70 ± 0.20c |
| 96 h | 27.33 ± 0.35d |
| 120 h | 30.43 ± 0.40d |
An increase in the syneresis of starch gels was observed with an increase in storage duration at 4 °C. Vetch starch displayed the highest (48.25 ± 0.23) syneresis on the 5th day of storage, while the lowest was reported on day zero of storage. One possible explanation for this rise is the interaction between dispersed amylose and amylopectin during storage, which creates junction zones and causes water to be expelled, leading to phase separation. Also, grains with high amylose content exhibit greater syneresis because amylose molecules tend to retrograde more quickly during cooling or storage. These findings were in accordance with the findings of Sofi etal.17 for Vicia faba (broad bean) starch.
3.2 Pasting properties
The pasting behavior of starch encompasses a series of intricate processes that occur post-gelatinization, involving irreversible swelling, solubilization, crystallite melting, the release of amylose, loss of order, birefringence and gel formation. The extent of these changes is determined by starch type, concentration, temperature, solutes, and shear, resulting in viscosity development, making starch useful for both culinary and non-food applications, particularly in imparting texture. Granular swelling is thought to be the primary cause of crystallite disintegration during gelatinization process.37The pasting properties of vetch starch are given in Table 7. Peak viscosity (PV) is the tendency of starch to expand in response to a temperature increase prior to physical breakdown. Peak viscosity is expected to be affected by the degree of amylose leaching, the formation of amylose–lipid complexes, friction among swollen granules, and competition for free water between leached amylose and ungelatinized granules. A greater peak viscosity may be beneficial for their utilization as a thickening agent in food systems.10 Peak viscosity of native vetch starch was found to be 2378.66 cP.
Trough viscosity (TV) is the viscosity drop, following peak viscosity as a result of disruption of swollen granules due to increased temperature and shearing force.17 The TV of starch paste was 1698 cP, showing a decrease from peak viscosity.
Breakdown viscosity (BDV) represents the differences in peak viscosity and trough viscosity and measures the degree of disintegration in the swollen granules of starch. Vetch starch showed a BDV of 680 cP.10
Final viscosity indicates an increase in gel viscosity due to decreased movement of water molecules as the temperature drops. The final and setback viscosities are largely due to re-ordering or polymerization of leached out amylose & long linear amylopectin. FV of vetch starch was found to be 3497.66 cP.6
Setback viscosity (SBV) represents the reorganization capacity of linear and branched starch chains; thus, it measures the retrogradation potential of starch. It is the restoration of viscosity that occurs during the cooling of a hot starch slurry. The SBV of vetch starch was observed to be 1799.33 cP.35
Pasting temperature is the lowest temperature required for cooking. A lower pasting temperature suggests weaker resistance to swelling and disintegration. The pasting temperature of vetch starch was 76.80 °C. Similar results for all pasting properties have been observed by Wani et al.10 and Fu et al.6 for kidney bean and vetch starches.
3.3 Differential scanning calorimetry (DSC)
DSC is a simple and fast technique that requires no special sample preparation. It is also recognized as an environmentally friendly approach because it does not require any harmful chemicals or solvents. It has contributed significantly to the study of the thermal characteristics of polymers like carbohydrates and proteins. DSC is a thermo-analytical technology that determines the amount of heat required to raise a sample's temperature compared to a reference material at varying temperatures. The difference in heat flow is then measured as a peak. The area below the curve, which shows whether the thermal process is exothermic (creating energy) or endothermic (consuming energy), is exactly proportional to the enthalpy change.38 The DSC curve of vetch starch and protein exhibits an endothermic peak revealing the gelatinization behavior of these molecules. The DSC analysis of vetch starch exhibits a major endothermic peak at the beginning which can be attributed to the presence of higher initial moisture content. The onset (To) temperature was 54.58 °C which indicates possible pre-damage and early gelatinization of amylopectin. This also highlights the loss of birefringence and aligns with lower pasting viscosity. Owing to a greater gelatinization temperature, the peak temperature (Tp) is found to be at 68.22 °C. An early onset and a slight delayed peak temperature indicate the possible amylose complexation and the reduction in native water mobility. It may also reflect a greater concentration of amorphous regions and variations in granule size. This effect also continued, resulting in a higher gelatinization enthalpy of 6.94 J g−1. The enthalpy values are slightly lower compared to previously reported results which may be due to a greater proportion of amylose double helices relative to the overall crystallinity. The concluding temperature (Tc) was 71.06 °C which further validates the presence of double helices. The vetch protein isolate exhibits two endothermic peaks. The first denaturation (Td) or peak temperature of protein is at 86.19 °C indicating the denaturation of vicilin type protein (7S) with an enthalpy of 140.378 J g−1. This enthalpy reflects the area change upon denaturation (i.e. heat required for inducing denaturation). The second denaturation (Td) at 227.86 °C indicates relatively higher thermal stability for the legumin type sub-protein fraction (11S).39 The increased stability is also related to the purity and origin of the protein isolate. This also indicates the possible transition of a rubbery state and attainment of glass transition temperature (Tg). In this case of vetch protein isolate, it can be observed that the linearity in the confrontational mobility and its alignment in an orderly fashion would have increased crystallinity, thereby leading to higher Td and Tg. Time, coupled with temperature, alters the protein structure, starting with unfolding and denaturing, leading to a reversible glassy state. The attainment of vitrification (glassy state) in gelled systems and dehydrated products can be positively influenced by these protein isolates. For polymer processing, it is required to increase the moisture content of the protein isolate so that the melting or transition is better prevented. The enthalpy also indicates the orderly change of protein from its native to denatured condition. Here, the enthalpy was found to be 35.050 J g−1 which reflects its resistance to breaking hydrophobic bonds and causing minimal damage to its orderly structure. The exact understanding and reason can be assessed by supporting data on viscosity and XRD.3.4 SDS-PAGE
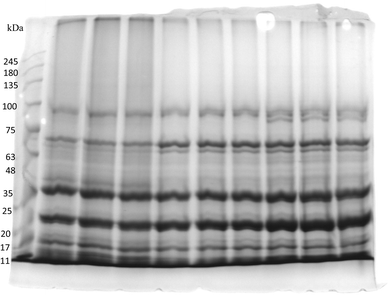
SDS-PAGE is the most preferred gel electrophoretic method for protein analysis. It works on the principle of separation of proteins based on their size and thus can be used to determine the molecular mass of proteins. SDS is an anion-based detergent that unfolds and intensely binds with proteins to produce linear polypeptide chains. The results of SDS-PAGE analysis are shown in Fig. 7. Vetch protein expresses a less complex protein profile in which polypeptide sub-units range between 25 kDa and 75 kDa in molecular weight. Overall, most of the bands and the peptides that have been identified are associated with storage proteins like vicilin and legumin. However, several other metabolic proteins were also detected as small constituents. Visual examination of the SDS-PAGE profile shows that the majority of strong bands emerge in three regions; 23, 35 and 70 kDa. The sub-units corresponding to 70 kDa belong to convicilin and the polypeptide units around 35 kDa correspond to acidic polypeptide of legumin subunits the polypeptide sub-units at 23 kDa may be assigned to the basic polypeptide of 11S legumin. The unique bands around 97 kDa may be related to aggregates resulting from the splitting and joining of disulfide bonds among acidic and basic polypeptide units. These polypeptide patterns were comparable to the observations of Chen et al.40 and Ladjal-Ettoumi et al.41 However, a clear band between 11 and 18 kDa was found which may correspond to the water-soluble metabolic protein albumins. These findings are consistent with the findings of Schumacher et al.29 for pea protein isolates.3.5 Fourier transforms infrared (FTIR) spectroscopy
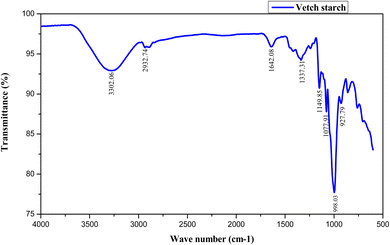
FTIR spectroscopy is a potent analytical instrument for identifying polymeric materials. It is used to quantify particular functional groups where the strength of absorbance peaks correlates with the quantity of functional groups present in the sample. The FTIR spectrum of vetch starch is illustrated in Fig. 1. The spectra represent peaks at different wavenumbers. The broad and dominant peak was found at 3302.6 cm−1 which represents –OH stretching, indicating the abundance of hydroxyl groups in starch granules. The broadness of the band represents substantial inter- and intra-molecular hydrogen bonding which plays a vital role in defining starch crystal structure, gelatinization, and retrogradation tendency.42 The second absorption band at 2932.74 cm−1 was ascribed to the stretching of the C–H bond of CH2 groups in glucose units of starch, which indicates the aliphatic nature of starch molecules. The band at 1642.08 cm−1 is associated with bending oscillations of H–O–H groups showing hydrogen bonding among the molecules, which in turn indicates bound water molecules within the amorphous regions of starch. The peak at 1337.31 cm−1 is ascribed to the twisting of –CH2 and the band at 1155.4 cm−1 depicts C–O, C–C and O–H bond stretching and folding or bending of C–O–H. A sharp band at 1077.91 cm−1 was related to the vibration of C–O–H deformation and C–C and C–O stretching. Another sharp band at 998 cm−1 represents the existence of α-(1,4)-glycosidic linkages and C–O–C stretching vibrations, validating the structural identity of amylose and amylopectin chains. The results of FTIR spectra were in agreement with the data of Tahari et al.26 for bitter vetch starch.Fig. 2 presents the FTIR spectra of vetch protein. A prominent absorption band at 3276.31 cm−1 describes the symmetrical and asymmetrical stretching vibrations of peaks of O–H in proteins, and its intensity reflects the extent of intermolecular interactions and hydration capacity of proteins. A band at 2927 cm−1 represents the stretching vibration absorbance peak of aliphatic C–H groups in proteins.43 A sharp band at 1633.52 cm−1 indicates the absorption peak produced through the stretching vibration of the amide I band (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), a highly susceptible region for examining protein secondary structures such α-helix, β-sheet, and random coil conformations, while another sharp band at 1527.82 cm−1 was associated with the bending vibration of the amide II band (N–H) in proteins. The absorption peak near 1453.96 cm−1 displayed the bending vibration from the absorption peak of –CH2–, both for proteins as well as polysaccharides, suggesting that the protein isolate may include trace amounts of carbohydrates. Furthermore, the absorption peak at 1233.32 cm−1 corresponds to N–H bending, and the band at 920.29 cm−1 usually corresponds to C–H vibrations in insoluble fibers or side chain groups. Similar results were also observed in FTIR spectra of pea protein.44 The presence of amide I, II, and III bands, together with supplementary side-chain absorptions, confirms that vetch protein predominantly displays a globular shape characterized by substantial β-sheet conformations and some related carbohydrate residues.
O), a highly susceptible region for examining protein secondary structures such α-helix, β-sheet, and random coil conformations, while another sharp band at 1527.82 cm−1 was associated with the bending vibration of the amide II band (N–H) in proteins. The absorption peak near 1453.96 cm−1 displayed the bending vibration from the absorption peak of –CH2–, both for proteins as well as polysaccharides, suggesting that the protein isolate may include trace amounts of carbohydrates. Furthermore, the absorption peak at 1233.32 cm−1 corresponds to N–H bending, and the band at 920.29 cm−1 usually corresponds to C–H vibrations in insoluble fibers or side chain groups. Similar results were also observed in FTIR spectra of pea protein.44 The presence of amide I, II, and III bands, together with supplementary side-chain absorptions, confirms that vetch protein predominantly displays a globular shape characterized by substantial β-sheet conformations and some related carbohydrate residues.
The FTIR study elucidates the structure–function relationship of vetch biopolymers. Significant intermolecular linkages that improve the starch and protein isolates' ability to bind water and expand are provided by the strong O–H stretching and broader –OH bands. Prominent amide I and II bands of vetch protein indicate organized β-sheet structures, suggesting enhanced heat stability and significant gel-forming capacity. The solubility, water retention, and overall functionality of the protein and starch extracted from vetch are all directly impacted by these molecular interactions.
SEM image analysis of vetch protein revealed a heterogeneous lamellar structure with some particle aggregation giving it a near-spherical morphology with particle size ranging from 1 to 8 μm, Fig. 4. As is evident from the figure the surface roughness varies suggesting an irregular or non-uniform protein matrix. There is a dense and well-connected network with minimal fractures which depicts its good mechanical stability. This information is in agreement with that mentioned by Zhang et al.47 and Barreras-Urbina et al.48 for common vetch protein and wheat protein.47,48 The protein is appropriate for emulsification applications because of its rough and heterogeneous surface morphology and pores, which are likely to improve water absorption, solubility, and interfacial activity. Strong molecular associations are suggested by the dense, interconnected matrix with few fractures, which may result in better mechanical stability and film-forming capabilities.49
The protein network's near-spherical aggregates may further strengthen barrier qualities and structural integrity. These characteristics are highly desirable in the context of edible film formation, where the ability to develop a cohesive, crack-free network translates into improved mechanical strength and barrier properties.
3.6 X-ray diffraction (XRD)
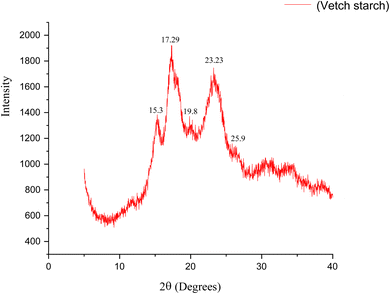
XRD is one of the potent non-destructive techniques employed to characterize crystalline materials and is useful to explain crystalline orientation, grain size and crystal defects. Based on XRD patterns, starch granule crystal polymorphs are often classified into three forms: A-type, B-type, and C-type. C-type starches are highly complex mainly due to the varying contents and distributions of A and B-type crystals. Starch extracted from various plant sources exhibits distinct X-ray diffraction spectra. A-Type crystals are commonly found in starches from cereals. B-Type is common in tuber crops and maize. However, a C-type crystalline structure is exhibited by starches from plants like green bananas, tapioca, and peas. As illustrated in Fig. 5, five peaks at 15.3°, 17.29°, 19.8°, 23.23° and 25.9° 2θ values were observed in vetch starch, indicating that this starch is a typical C-type crystal.16,46,47 The presence of significant and sharp peaks at 17.29° and 23.23° indicates a moderate crystalline structure, suggesting A-type crystallinity, which implies a more compact structure. This packing is linked to decreased water permeability and slower enzymatic digestion. On the other hand, the comparatively reduced intensity of the peak at 15.3° indicates lower crystallinity, which implies a combination of amorphous regions that are mostly caused by irregular amylopectin chains and amorphous amylose matrices.50 Also, the peak at 19.8° is noteworthy as it indicates the presence of V-type crystallinity, which occurs when amylose forms inclusion networks with lipids or small molecules. Although less significant, this characteristic peak may indicate some level of amylose–lipid interaction in vetch starch, which could affect heating and pasting properties. This semi-crystalline structure is congruent with the structural arrangement of legume starches and is directly related to their gelatinization, retrogradation, and digestible characteristics.51 Thus, the XRD data validate the C-type crystallinity of vetch starch while also providing information about its structural integrity and functional potential in food and non-food applications. The C-type crystallinity along with moderate A-type and V-type components, signifies a semi-crystalline structure that affects water permeability, gelatinization, retrogradation, and digestibility.The structural properties of vetch protein, studied by XRD, are shown in Fig. 6. The vetch protein showed two peaks: a high crystalline peak at a 2θ value of 20.0° and a small peak at 9.0°, corresponding to those described by Zhao et al.52 and Jin et al.43 The dominant peak at 20.0° is indicative of β-sheet secondary structures and the small peak around 9.0° shows the presence of α-helix or lamellar structures respectively. The complete diffraction pattern is significantly broad, indicating that the protein has minimal to moderate crystallinity. Thus, it can be suggested that vetch protein exhibits low to moderate crystallinity reflecting the amorphous nature of these proteins and supporting that vetch protein is a globular protein. Also, the protein's amorphous structure implies its versatility in molecular packing, which may affect its solubility, surface activity, and film-forming behavior. Amorphous proteins are more soluble in water and solvents, which improves their hydration and swelling properties. β-Sheets promote strong intermolecular hydrogen bonding and can form ordered aggregates to strengthen the protein network. Vetch protein's increased solubility, hydration, swelling capacity, and film-forming potential are all correlated with low to moderate crystallinity, which includes amorphous patches and significant β-sheet structures. This agrees with SEM observations of dense, linked protein matrices.
3.7 Functional properties
The ability of biopolymers like proteins and starches to imbibe water or oil for enhanced consistency in foods is known as their water/oil absorption capacity. The water and oil absorption capacities of native starch and protein are presented in Table 5. WAC is mainly due to the presence of hydrophilic groups on its molecules, causing it to swell and absorb large amounts of water. Vetch starch showed a WAC of 2.43 g g−1. The WAC of a food product is an indicator of the degree of starch gelatinization since it quantifies the starch's capacity to retain water after swelling in excess water, which is equivalent to the weight of the resulting gel. It depends on the ability of hydrophilic groups to imbibe water molecules as well as the ability of molecules to form gels.53
| Parameter | Vetch starch | Vetch protein |
|---|---|---|
| a Values expressed are mean ± standard deviation. WAC – water absorption capacity, OAC – oil absorption capacity. | ||
| WAC (g g−1) | 2.43 ± 0.15 | 4.62 ± 0.05 |
| OAC (g g−1) | 1.93 ± 0.05 | 3.23 ± 0.05 |
The OAC of starch was found to be 1.93 g g−1. The results align with those observed by Gao et al.54 for pea starch, and Sofi et al.17 for broad bean starch. Oil absorption capacity is primarily due to the physical trapping of oil by the macromolecules, like starch, protein. It serves as an indicator of how quickly proteins attach to fat in food compositions. In dietary systems where optimal oil absorption is desired, the proteins' capacity to interact with oil is significant. It enables flour to have practical applications in various food products. When using flours in food preparation, their ability to absorb oil is crucial for facilitating improvements in mouthfeel and flavor.53
The WAC of vetch protein was found to be 4.62 g g−1. The oil absorption capacity of protein is lower as compared to its water absorption capacity and was found to be 3.23 g g−1. The relatively low oil absorption capacity of vetch protein may be ascribed to the extent of denaturation of its protein structure. Comparable results were also observed by Chen et al.40 for common vetch protein and Schumacher et al.29 for different varieties of pea protein isolates.
| Parameter | pH 3 | pH 5 | pH 7 |
|---|---|---|---|
| a Values expressed are mean ± standard deviation. | |||
| Emulsion capacity (m2 g−1) | 48.99 ± 0.67 | 37.17 ± 0.50 | 85.11 ± 0.27 |
| Emulsion stability (min) | 65.60 ± 0.36 | 45.96 ± 0.19 | 97.65 ± 0.60 |
| Parameter | pH 4 | pH 7 | pH 10 |
|---|---|---|---|
| Foaming capacity (%) | 86.33 ± 0.57 | 108 ± 1 | 129.33 ± 0.57 |
| Foaming stability (min) | 50.56 ± 0.55 | 92.03 ± 0.75 | 102.96 ± 0.25 |
Foam stability is the capacity of foam to preserve its original configuration over time and prevent or retard the coalescence of gas. Foaming stability of protein isolates at different pH values was studied over a 1-hour period (Table 6). The percentage of foam volume that remains after a given time duration is known as foam stability. Vetch protein has the highest foam volume at pH 10 (102.96) and the lowest at pH 4 (50.56). Similar outcomes were also reported by Shen et al.55 and Wani et al.11
The emulsion solubility index provides a measure of the stability of a diluted emulsion over a specified time duration. Substantial differences in emulsion stability were also observed among different pH values, being highest at pH 7 (97.65 min) and lowest at pH 5 (46.96 min). Greater emulsion stability at neutral pH may be attributed to balanced electrostatic interactions between emulsifier molecules at the oil–water interface, thus preventing droplet aggregation (Table 7). Comparable results were observed by Wani et al.11 for kidney bean protein isolates.
| Pasting property | Vetch starch |
|---|---|
| a Values expressed are mean ± standard deviation [1 cP = 0.001 Pa s]. PV – peak viscosity, TV – trough viscosity, BDV – breakdown viscosity, FV – final viscosity, SB – set back, PT – pasting temperature, PkT – peak time. | |
| PV (cP) | 2378.66 ± 20.64 |
| TV (cP) | 1698 ± 18.52 |
| BDV (cP) | 680 ± 30.51 |
| FV (cP) | 3497.66 ± 36.01 |
| SB (cP) | 1799.33 ± 15.30 |
| PT (°C) | 76.80 ± 0.22 |
| PkT (min) | 5.20 ± 0.30 |
4 Conclusion
This work extensively examined the physicochemical, functional, structural, and thermal properties of starch and protein isolates produced from Vicia villosa (vetch) seeds, an underutilized legume with exceptional importance in culinary and industrial applications. The proximate composition revealed a high starch yield and a substantial amount of protein. The apparent amylose content of starch was in the range of other legume starches and the color characteristics of the isolated starch and protein show a negligible amount of any pigment or contaminant. The starch exhibited significant swelling capacity and solubility, accompanied by considerable gel clarity as evidenced by light transmission. Analysis of syneresis and freeze–thaw stability showed that vetch starch produces stable gels with minimal water segregation during storing and freezing cycles. Thermal analysis by RVA revealed that the vetch starch exhibits an intense peak viscosity (3610.33 cP) and a comparatively low pasting temperature (73.27 °C), providing robust gel forming ability and exceptional resilience to breakdown under shear and heat Table 8. Also, structural analysis by XRD showed C-type crystallinity in starch which is characteristic of legumes, while scanning electron microscopy (SEM) indicated well-organized, elliptical to polygonal starch granules with smooth surfaces. SDS-PAGE examination of the protein isolate revealed a varied molecular weight distribution consistent with legume globulin patterns. In conclusion, vetch protein and starch possess satisfactory functional, structural, and thermal properties, suggesting diverse applications. Specifically, vetch protein shows promise as an emulsifier in plant-based formulations while vetch starch offers potential as a sustainable material for biodegradable packaging. Future research is expected to focus on application-based studies, such as developing and testing vetch starch–protein composite films or edible coatings, to confirm their efficacy in practical applications and improve their commercial use.| Parameter | Value | |
|---|---|---|
| Vetch starch | Vetch protein | |
| Onset temperature (To) | 54.58 °C | 65 °C |
| Peak temperature (Tp) | 66.22 °C | 86.19 °C and 227.86 |
| Conclusion temperature (Tc) | 71.06 °C | 247 °C |
| Enthalpy change ΔH | 6.94 J g−1 | 35.050 J g−1 |
Conflicts of interest
There are no conflicts to decalre.Data availability
All data generated or analysed during this study are included in the manuscript. No new crystallographic data were generated. This study did not involve any human participants.Acknowledgements
The authors are thankful to the Anusandhan Research Foundation (ANRF), Government of India Erstwhile (SERB scheme) under the SERB-SURE Scheme vide Sanction order SUR/2022/003847, for supporting and funding the research.References
- United Nations, World Population Prospectus, Department of Economic and Social Affairs Population Division, 2024, https://population.un.org/wpp/ Search PubMed.
- United Nations, Peace dignity and equality on a healthy planet, https://www.un.org/en/global-issues/population.
- K. Pandian, M. R. A. F. Mustaffa, G. Mahalingam, A. Paramasivam, A. John Prince, M. Gajendiren, A. R. Rafiqi Mohammad and S. T. Varanasi, Synergistic conservation approaches for nurturing soil, food security and human health towards sustainable development goals, J. Hazard. Mater. Adv., 2024, 16, 100479, DOI:10.1016/j.hazadv.2024.100479.
- V. Nguyen, S. Riley, S. Nagel, I. Fisk and I. R. Searle, Common vetch: a drought tolerant, high protein neglected leguminous crop with potential as a sustainable food source, Front. Plant Sci., 2020, 11, 818, DOI:10.3389/fpls.2020.00818.
- M. J. Hassan and E. J. Kadhim, Phytochemical Investigation of some Chemical Compounds Present in Vicia villosa L. Widely Grown in Iraq, Int. J. Drug Deliv. Technol., 2021, 11(2), 548–554, DOI:10.25258/ijddt.11.2.54.
- L. Fu, L. Liu, W. Chen, Q. Wang, X. Lv, J. Wang, Z. Ji, G. Yu, Q. Liu and X. Zhang, Physicochemical and functional characteristics of starches from common vetch (Vicia sativa L.), LWT-Food Sci. Technol., 2020, 131, 109694, DOI:10.1016/j.lwt.2020.109694.
- B. S. Yancheshmeh, L. M. Marvdashti, A. Emadi, A. Abdolshahi, A. Ebrahimi and N. Shariatifar, Evaluation of physicochemical and functional properties of Vicia villosa seed protein, Food Anal. Methods, 2022, 1–16, DOI:10.1007/s12161-021-02185-z.
- M. Badzadeh, F. Zaragarzadeh and B. Esmaiepour, Chemical composition of some forage Vicia spp, Iran, J. Food Agric. Environ., 2008, 6, 178–180 Search PubMed . http://www.isfae.org/scientificjournal.php.
- H. Akdeniz, A. Koc, A. Hossain and A. El Sabagh, Nutritional values of four hairy vetch (Vicia villosa Roth) varieties grown under Mediterranean environment, Fresenius Environ. Bull., 2018, 27(8), 5385–5390 Search PubMed.
- I. A. Wani, D. S. Sogi, A. A. Wani, B. S. Gill and U. S. Shivhare, Physico-chemical properties of starches from Indian kidney bean (Phaseolus vulgaris) cultivars, Int. J. Food Sci. Technol., 2010, 45(10), 2176–2185, DOI:10.1111/j.1365-2621.2010.02379.x.
- I. A. Wani, D. S. Sogi, U. S. Shivhare and B. S. Gill, Physico-chemical and functional properties of native and hydrolyzed kidney bean (Phaseolus vulgaris L.) protein isolates, Food Res. Int., 2015, 76, 11–18, DOI:10.1016/j.foodres.2014.08.027.
- AOAC, Official Methods of Analysis, Association of Official Analytical Chemists, AOAC Inc., Arlington, 1990 Search PubMed.
- N. Sit, S. C. Deka and S. Misra, Optimization of starch isolation from taro using combination of enzymes and comparison of properties of starches isolated by enzymatic and conventional methods, J. Food Sci. Technol., 2015, 52(7), 4324–4332, DOI:10.1007/s13197-014-1462-z.
- S. A. Sofi, J. Singh, K. Muzaffar, D. Majid and B. N. Dar, Physicochemical characteristics of protein isolates from native and germinated chickpea cultivars and their noodle quality, Int. J. Gastronomy Food Sci., 2020, 22, 100258, DOI:10.1016/j.ijgfs.2020.100258.
- A. A. Wani, I. A. Wani, P. R. Hussain, A. Gani, T. A. Wani and F. A. Masoodi, Physicochemical properties of native and γ-irradiated wild arrowhead (Sagittaria sagittifolia L.) tuber starch, Int. J. Biol. Macromol., 2015, 77, 360–368, DOI:10.1016/j.ijbiomac.2015.03.012.
- I. A. Wani, M. Jabeen, H. Geelani, F. A. Masoodi, I. Saba and S. Muzaffar, Effect of gamma irradiation on physicochemical properties of Indian Horse Chestnut (Aesculus indica Colebr.) starch, Food Hydrocoll., 2014, 35, 253–263, DOI:10.1016/j.foodhyd.2013.06.002.
- B. A. Sofi, I. A. Wani, F. A. Masoodi, I. Saba and S. Muzaffar, Effect of gamma irradiation on physicochemical properties of broad bean (Vicia faba L.) starch, LWT-Food Sci. Technol., 2013, 54(1), 63–72, DOI:10.1016/j.lwt.2013.05.021.
- R. Hoover and W. S. Ratnayake, Starch characteristics of black bean, chick pea, lentil, navy bean and pinto bean cultivars grown in Canada, Food Chem., 2002, 78(4), 489–498, DOI:10.1016/S0308-8146(02)00163-2.
- R. C. Strauch and M. A. Lila, Pea protein isolate characteristics modulate functional properties of pea protein–cranberry polyphenol particles, Food Sci. Nutr., 2021, 9(7), 3740–3751, DOI:10.1002/fsn3.2335.
- M. Jafari, A. Koocheki and E. Milani, Effect of extrusion cooking on chemical structure, morphology, crystallinity and thermal properties of sorghum flour extrudates, J. Cereal Sci., 2017, 75, 324–331, DOI:10.1016/j.jcs.2017.07.011.
- J. G. de Oliveira Filho, B. R. Albiero, L. Cipriano, C. C. de Oliveira Nobre Bezerra, F. C. A. Oldoni, M. B. Egea, H. M. C. de Azeredo and M. D. Ferreira, Arrowroot starch-based films incorporated with a carnauba wax nanoemulsion, cellulose nanocrystals, and essential oils: A new functional material for food packaging applications, Cellulose, 2021, 28(10), 6499–6511, DOI:10.1007/s10570-021-03945-0.
- A. K. Stone, A. Karalash, R. T. Tyler, T. D. Warkentin and M. T. Nickerson, Functional attributes of pea protein isolates prepared using different extraction methods and cultivars, Food Res. Int., 2015, 76, 31–38, DOI:10.1016/j.foodres.2014.11.017.
- I. A. Wani, D. S. Sogi, U. S. Shivhare and B. S. Gill, Physico-chemical and functional properties of native and hydrolyzed kidney bean (Phaseolus vulgaris L.) protein isolates, Food Res. Int., 2015, 76, 11–18, DOI:10.1016/j.foodres.2014.08.027.
- K. N. Pearce and J. E. Kinsella, Emulsifying properties of proteins: evaluation of a turbidimetric technique, J. Agric. Food Chem., 1978, 26(3), 716–723, DOI:10.1021/jf60217a041.
- K. Shevkani, N. Singh, A. Kaur and J. C. Rana, Structural and functional characterization of kidney bean and field pea protein isolates: A comparative study, Food Hydrocoll., 2015, 43, 679–689, DOI:10.1016/j.foodhyd.2014.07.024.
- M. Tahari, F. Shahidi and S. Hedayati, A novel starch from bitter vetch (Vicia ervilia) seeds: a comparison of its physicochemical, structural, thermal, rheological and pasting properties with conventional starches, Int. J. Food Sci. Technol., 2022, 57(10), 6833–6842, DOI:10.1111/ijfs.16021.
- I. A. Wani, G. Farooq, N. Qadir and T. A. Wani, Physico-chemical and rheological properties of Bengal gram (Cicer arietinum L.) starch as affected by high temperature short time extrusion, Int. J. Biol. Macromol., 2019, 131, 850–857, DOI:10.1016/j.ijbiomac.2019.03.135.
- R. Mukhtar, A. Shah, N. Noor, A. Gani, I. A. Wani, B. A. Ashwar and F. A. Masoodi, γ-Irradiation of oat grain–Effect on physico-chemical, structural, thermal, and antioxidant properties of extracted starch, Int. J. Biol. Macromol., 2017, 104, 1313–1320, DOI:10.1016/j.ijbiomac.2017.05.092.
- T. Schumacher, T. Steinmacher, E. Köster, A. Wagemans, J. Weiss and M. Gibis, Physico-chemical characterization of ten commercial pea protein isolates, Food Hydrocol., 2025, 162, 110996, DOI:10.1016/j.foodhyd.2024.110996.
- Shubeena, I. A. Wani, A. Gani, P. Sharma, T. A. Wani, F. A. Masoodi, A. Hamdani and S. Muzafar, Effect of acetylation on the physico-chemical properties of Indian Horse Chestnut (Aesculus indica L.) starch, Starch/Staerke, 2015, 67(3–4), 311–318, DOI:10.1002/star.201400156.
- C. Wijaya, Q. D. Do, Y. H. Ju, S. P. Santoso, J. N. Putro, L. Laysandra, F. E. Soetaredjo and S. Ismadji, Isolation and characterization of starch from Limnophila aromatica, Heliyon, 2019, 5(5), e01622, DOI:10.1016/j.heliyon.2019.e01622.
- N. A. Bhat, I. A. Wani, A. M. Hamdani, A. Gani and F. A. Masoodi, Physicochemical properties of whole wheat flour as affected by gamma irradiation, LWT-Food Sci. Technol., 2016, 71, 175–183, DOI:10.1016/j.lwt.2016.03.024.
- S. Rai, A. Poonia and S. Pandey, The Determination of the Physicochemical, Functional & Structural Properties of Yellow Pea Starch, Int. J. Agric. Sci. Res., 2019, 9(2), 81–88 Search PubMed.
- S. Hamid, S. Muzzafar, I. A. Wani and F. A. Masoodi, Physicochemical and functional properties of two cowpea cultivars grown in temperate Indian climate, Cogent Food Agric, 2015, 1(1), 1099418, DOI:10.1080/23311932.2015.1099418.
- Y. I. Cornejo-Ramírez, O. Martínez-Cruz, C. L. Del Toro-Sánchez, F. J. Wong-Corral, J. Borboa-Flores and F. J. Cinco-Moroyoqui, The structural characteristics of starches and their functional properties, CyTA-J. Food, 2018, 16(1), 1003–1017, DOI:10.1080/19476337.2018.1518343.
- Z. Ao and J. Jane, Characterization and modeling of the a- and b-granule starches of wheat, triticale, and barley, Carbohydr. Polym., 2007, 67, 46–55, DOI:10.1016/j.carbpol.2006.04.013.
- A. Gunaratne and H. Corke, STARCH|Analysis of Quality, Encyclopedia of Grain Science, Academic Press, 2004, pp. 202–212, DOI:10.1016/B0-12-765490-9/00005-7.
- E. J. Munson, Analytical techniques in solid-state characterization, Developing Solid Oral Dosage Forms, Academic Press, 2009, pp. 61–74, DOI:10.1016/B978-0-444-53242-8.00003-5.
- C.-H. Tang, Thermal denaturation and gelation of vicilin-rich protein isolates from three Phaseolus legumes: A comparative study, LWT-Food Sci. Technol., 2008, 41(8), 1380–1388, DOI:10.1016/j.lwt.2007.08.025.
- W. Chen, Y. Wang, X. Lv, G. Yu, Q. Wang, H. Li, J. Wang, X. Zhang and Q. Liu, Physicochemical, structural and functional properties of protein isolates and major protein fractions from common vetch (Vicia sativa L.), Int. J. Biol. Macromol., 2022, 216, 487–497, DOI:10.1016/j.ijbiomac.2022.07.030.
- Y. Ladjal-Ettoumi, H. Boudries, M. Chibane and A. Romero, Pea, chickpea and lentil protein isolates: Physicochemical characterization and emulsifying properties, Food Biophys., 2016, 11, 43–51, DOI:10.1007/s11483-015-9411-6.
- S. V. Kumar, V. A. Sajeev and S. Kumar, The influence of bound water on the FTIR characteristics of starch and starch nanocrystals obtained from selected natural sources, Starch/Staerke, 2018, 1700026, DOI:10.1002/star.201700026.
- D. Jin, Y. Qu, W. Lu, L. Ge, H. Wang, J. Xue, D. Deng, Q. Zhang, K. Cheng and H. Yang, A study on the synergistic interaction of pea protein isolate and whey protein isolate for enhancing the stability and quality of krill oil emulsion, Food Hydrocoll., 2024, 157, 110426, DOI:10.1016/j.foodhyd.2024.110426.
- S. He, Z. Lei, Y. Wang, G. Li, X. Gao, W. Guo and J. Huang, Isolation of pea protein-polysaccharide natural mixtures and physicochemical properties investigation, J. Food Meas Charact., 2025, 1–14, DOI:10.1007/s11694-025-03137-5.
- X. Zhang, N. Tang, X. Jia, D. Geng and Y. Cheng, Multi-scale comparison of physicochemical properties, refined structures, and gel characteristics of a novel native wild pea starch with commercial pea and mung bean starch, Foods, 2023, 12(13), 2513, DOI:10.3390/foods12132513.
- X. Zhang, Y. Cheng, X. Jia, D. Geng, X. Bian and N. Tang, Effects of extraction methods on physicochemical and structural properties of common vetch starch, Foods, 2022, 11(18), 2920, DOI:10.3390/foods11182920.
- J. Zhang, X. Mao, J. Zhang and Q. Liu, Structural changes and functional characteristics of common vetch isolate proteins altered by different pH-shifting treatments, Int. J. Biol. Macromol., 2024, 282, 136887, DOI:10.1016/j.ijbiomac.2024.136887.
- C. G. Barreras-Urbina, F. Rodríguez-Félix, G. A. López-Ahumada, S. E. Burruel-Ibarra, J. A. Tapia-Hernández, D. D. Castro-Enríquez and E. O. Rueda-Puente, Microparticles from Wheat-Gluten Proteins Soluble in Ethanol by Nanoprecipitation: Preparation, Characterization, and Their Study as a Prolonged-Release Fertilizer, Int. J. Polym. Sci., 2018, 2018(1), 1042798, DOI:10.1155/2018/1042798.
- M. H. R. Bhuiyan, L. Liu, A. Samaranayaka and M. Ngadi, Prediction of pea composites physicochemical traits and techno-functionalities using FTIR spectroscopy, LWT-Food Sci. Technol., 2024, 208, 116667, DOI:10.1016/j.lwt.2024.116667.
- Y. Zhou, R. Hoover and Q. Liu, Relationship between α-amylase degradation and the structure and physicochemical properties of legume starches, Carbohydr. Polym., 2004, 57(3), 299–317, DOI:10.1016/j.carbpol.2004.05.010.
- D. M. Dries, S. V. Gomand, J. A. Delcour and B. Goderis, V-type crystal formation in starch by aqueous ethanol treatment: The effect of amylose degree of polymerization, Food Hydrocoll., 2016, 61, 649–661, DOI:10.1016/j.foodhyd.2016.06.026.
- X. Zhao, H. Zhu, B. Zhang, J. Chen, Q. Ao and X. Wang, XRD, SEM, and XPS analysis of soybean protein powders obtained through extraction involving reverse micelles, J. Am. Oil Chem. Soc., 2015, 92, 975–983, DOI:10.1007/s11746-015-2657-9.
- H. Twinomuhwezi, C. G. Awuchi and M. Rachael, Comparative study of the proximate composition and functional properties of composite flours of amaranth, rice, millet, and soybean, Am. J. Food Sci.Nutr., 2020, 6(1), 6–19 Search PubMed.
- L. Gao, Y. Wu, C. Wan, P. Wang, P. Yang, X. Gao, M. Eeckhout and J. Gao, Structural and physicochemical properties of pea starch affected by germination treatment, Food Hydrocoll., 2022, 124, 107303, DOI:10.1016/j.foodhyd.2021.107303.
- Y. Shen, X. Tang and Y. Li, Drying methods affect physicochemical and functional properties of quinoa protein isolate, Food Chem., 2021, 339, 127823, DOI:10.1016/j.foodchem.2020.127823.
| This journal is © The Royal Society of Chemistry 2025 |