Open Access Article
Open Access ArticleSustainable nutrient enhancement of edible mushrooms via boiling and gastrointestinal simulation
Si
Qin
a,
Sunantha
Ketnawa
 b and
Nattaya
Konsue
b and
Nattaya
Konsue
 *bc
*bc
aCollege of Food Science and Technology, Hunan Agricultural University, Changsha 410128, China
bFood Science and Technology Program, School of Agro-Industry, Mae Fah Luang University, Muang, Chiang Rai 57100, Thailand. E-mail: nattaya.kon@mfu.ac.th
cResearch Center of Innovative Food Packaging and Biomaterials, Mae Fah Luang University, Muang, Chiang Rai 57100, Thailand
First published on 24th September 2025
Abstract
This study investigated the impact of boiling and in vitro gastrointestinal digestion on the nutritional properties of seven commercially available mushroom species: Volvariella volvacea, Lentinus polychrous, Lentinus squarrosulus, Pleurotus ostreatus, Astraeus odoratus, Lentinula edodes, and Auricularia auricula-judae. Boiling altered the mushroom microstructure, enhancing the release of nutrients and bioactive compounds. It increased crude protein (5–35%), amino acids (3–75%), minerals (3–30%), and phenolic compounds (1–2-fold), though the effects varied by species. A. odoratus showed the highest crude protein content (37.30%), while A. auricula-judae demonstrated the highest nutrient bioaccessibility during digestion. V. volvacea exhibited the greatest amino acid content (74.50 mg g−1). Simulated digestion further improved amino acid and phenolic availability. L. edodes and A. auricula-judae exhibited the highest phenolic bioaccessibility, likely due to lower dietary fiber. Boiling increased the total phenolic and flavonoid content in A. odoratus, suggesting the presence of heat-resistant polyphenols, but led to reductions in other species due to leaching. Antioxidant activity, assessed by DPPH and FRAP assays, increased after digestion across all species. Boiling enhanced antioxidant activity in A. odoratus and A. auricula-judae, likely due to stable compounds such as β-glucans and ergothioneine. Correlation analysis identified total phenolic content as the primary contributor to antioxidant potential, while flavonoid effects varied. These findings underscore the role of mushrooms as sustainable, nutrient-rich foods. Their efficient growth on low-input substrates and improved functionality through processing support their use in plant-based diets, meat analogues, and nutritional supplements for sustainable food system innovation.
Sustainability spotlightThis investigation promotes sustainable food systems by improving the bioaccessibility and nutritional value of edible mushrooms, which are fast-growing, low-impact crops that are cultivated on agro-waste. The work promotes the development of eco-efficient, plant-based protein alternatives by illustrating enhanced protein, amino acid, and mineral content through straightforward thermal processing. By fostering resource-efficient food innovation that reduces dependence on animal-derived proteins and mitigates environmental impact, these findings directly contribute to the United Nations sustainable development goals, particularly goal 2 (zero hunger), goal 12 (responsible consumption and production), and goal 13 (climate action). This research emphasizes the significance of mushrooms in the provision of sustainable, scalable nutrition to a burgeoning global population. |
Introduction
Edible mushrooms are increasingly valued for their combined nutritional, functional, and environmental benefits. They are naturally low in fat and digestible carbohydrates, while offering high-quality protein, dietary fiber, essential amino acids, vitamins, and minerals. Moreover, mushrooms are known for their rich content of bioactive compounds including β-glucans, flavonoids, phenolics, and complex polysaccharides, which have been associated with antioxidant, immunomodulatory, anti-inflammatory, hypoglycemic, and antitumor activities.1–3 Several edible species, particularly Lentinula edodes, Pleurotus ostreatus, and Auricularia auricula-judae, are extensively documented in medicinal mushroom studies for their health-promoting effects. These include immunomodulatory, antitumor, hepatoprotective, hypoglycemic, and neuroprotective activities, which are primarily attributed to their content of polysaccharides (especially β-glucans), terpenoids, and phenolic compounds.4,5 For example, L. edodes produces lentinan, a well-characterized β-glucan with proven immunomodulatory and antitumor effects, while P. ostreatus and A. auricula-judae exhibit antioxidant, anti-inflammatory, and metabolic regulatory properties.4–6Beyond their nutritional value, mushrooms are environmentally efficient to cultivate. They require minimal land, grow rapidly, and thrive on agricultural or industrial waste substrates, reducing food system waste and resource use. Compared to animal-based proteins, mushroom production generates significantly lower greenhouse gas emissions and conserves water and energy, making it an attractive option for sustainable protein development.1,2 As global interest in reducing reliance on meat intensifies, mushrooms are gaining recognition as functional, scalable alternatives in meat analogues, nutritional supplements, and health-focused foods.
Ng and Rosman7 found that in vitro digestion, when combined with domestic cooking (boiling, steaming, microwaving, and pressure-cooking), enhanced total antioxidant activity and carbohydrate-digestive enzyme inhibitory potential in various mushrooms, including L. edodes and Pleurotus sajor-caju. Similarly, Soler-Rivas et al.8 demonstrated that while water-soluble antioxidants in Agaricus bisporus, L. edodes, and Boletus edulis were impacted by cooking, intestinal digestion increased their antioxidant capacity, and the Caco-2 cell model confirmed partial absorption and transformation of those antioxidants. More recently, Zeng et al.9 evaluated six cooking methods on L. edodes, showing that boiling and steaming reduced some nutrients, yet roasting improved amino acid bioaccessibility during simulated digestion, and cooking methods influenced antioxidant retention. While these studies offer valuable insights, comparative evaluations across multiple species, particularly those relevant to Southeast Asia, remain limited. Moreover, most existing work has focused on antioxidant metrics and enzyme inhibition; comprehensive analyses of protein quality, mineral bioaccessibility, microstructure, and other nutritional parameters across digestion remain rare.
In real-world contexts, mushrooms are consumed as food rather than medicine. Thus, understanding how cooking methods like boiling, combined with gastrointestinal digestion, affect their nutritional and functional properties is essential for optimizing their role in sustainable diets—without overstating therapeutic potential.
Boiling is one of the most widely used culinary and industrial processing methods for mushrooms. It can result in the loss of water-soluble nutrients through leaching and heat degradation, but it may also improve the release and bioaccessibility of certain nutrients and bioactives by disrupting the mushroom's cell wall and softening the matrix.3 These effects are highly species-specific and can either enhance or reduce the final nutritional quality.
One important factor that determines the nutritional impact is bioaccessibility, which is the fraction of nutrients that are liberated from the food matrix and may be absorbed after digestion.6 Evaluating only nutrient content before and after cooking does not capture the complete picture. In particular, protein quality and digestibility can vary considerably with species and processing conditions, affecting the role of mushrooms as a viable, sustainable protein source in plant-based food development.
This study examined the effects of boiling and simulated gastrointestinal digestion on seven commercially cultivated mushroom species in Thailand: Volvariella volvacea, Lentinus polychrous, Lentinus squarrosulus, Pleurotus ostreatus, Astraeus odoratus, Lentinula edodes, and Auricularia auricula-judae. Nutritional parameters (crude protein, amino acids, and minerals), bioactive compounds (total phenolic and flavonoid content), antioxidant activities (DPPH and FRAP), and microstructural changes were analyzed before and after digestion. By highlighting species-specific differences in nutrient retention and bioavailability, the findings support the strategic use of mushrooms as nutrient-rich, environmentally sustainable components in next-generation plant-based foods.
Experimental
Samples and reagents
Fresh mushrooms, including V. volvacea (paddy straw mushroom or straw mushroom), L. polychrous, L. squarrosulus, P. ostreatus (oyster mushroom), A. odoratus (barometer earthstars, hygroscopic earthstar, false earthstar), L. edodes (shiitake mushroom), and A. auricula-judae (Jew's ear, wood ear, and jelly ear) were selected for their nutritional value, widespread popularity, and well-documented health-promoting properties, as illustrated in Fig. 1. These species are commonly consumed and recognized in various regions, making them relevant for evaluating their potential functional or nutraceutical applications. The fresh mushrooms were purchased from the local market in Chiang Rai, Thailand. Pancreatin from porcine gastric mucosa (activity of 800–2500 U mg−1 of protein, Sigma P-3292), pepsin from porcine gastric mucosa (Sigma P-7012), pancreatin from porcine pancreas (4xUSP), amyloglucosidase (activity of 3260 U mL−1), and invertase (activity ≥300 U mg−1) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Amyloglucosidase (AMG) was purchased from Megazyme International Ireland Ltd. (Wicklow, Ireland). All other chemicals used were of analytical grade.Preparation of raw and boiled mushroom samples
Fresh mushrooms (200 g) were washed using tap water and patted dry using a kitchen paper towel. After that, the washed samples were packed in low-density polyethylene (LDPE) plastic zip-lock bags. The samples were categorized as “raw” for untreated specimens. For the boiled samples, 200 g of fresh mushrooms were thoroughly washed and then cooked in boiling water at 100 °C for 10 minutes, using a mushroom-to-water ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.10 Boiled samples were drained, cooled down, and pat-dried using a kitchen paper towel. After that, the boiled samples were packed in LDPE plastic zip-lock bags and labeled as ‘boiled’.
10.10 Boiled samples were drained, cooled down, and pat-dried using a kitchen paper towel. After that, the boiled samples were packed in LDPE plastic zip-lock bags and labeled as ‘boiled’.
Preparation of dried mushroom powder and freeze-dried mushroom
Mushroom samples were sliced into 1 cm pieces and dried overnight at 60 °C in a tray dryer until the moisture content was reduced to below 10 wt%. The dried mushrooms were then ground into a fine powder using a blender, sealed in airtight plastic bags, and stored at room temperature prior to analysis of free amino acid composition and mineral profile.Both raw and boiled mushroom samples were collected before and after in vitro gastrointestinal digestion and immediately frozen at −40 °C overnight. The samples were then freeze-dried using a laboratory lyophilizer (CHRIST, Osterode am Harz, Germany) set to a condenser temperature of −80 °C and a vacuum pressure of 0.1 mbar until completely dried. All freeze-dried samples were vacuum-sealed in polyethylene bags, stored at −40 °C, and labeled as “lyophilized” for subsequent protein content and microstructure analyses.
Determination of in vitro protein digestibility and bioaccessibility of nutrients
The estimation of protein contents in lyophilized samples before and after in vitro gastrointestinal digestion was performed by the Kjeldahl method.11 The percent in vitro protein digestibility was calculated according to the following formula: | (1) |
P 0 = protein content of the sample before digestion.
P 1 = protein content of the sample after digestion.
The estimation of bioaccessibility (%) of total amino acid content and total mineral content in raw and boiled mushroom samples was calculated using the following formula:12
 | (2) |
Microstructure analysis of mushrooms
Lyophilized mushroom samples were attached onto aluminum stubs using a conductive carbon adhesive tape, vacuum-dried, and coated with gold. The mushroom surface morphology was studied using a field emission scanning electron microscope or FESEM (MIRA, Tescan, Czech Republic) at a magnification of 500× and a distance of 9.0–10.0 mm using an accelerating voltage of 5 keV.Determination of bioactive compounds
DPPH radical scavenging activity. The antioxidant activity of dried samples was evaluated using the DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging assay, following the method described.13 A 50 μL sample aliquot was mixed with 1.95 mL of 60 μM DPPH solution and incubated in the dark at room temperature for 30 min. The content was mixed, transferred to a 96-well plate, and absorbance was measured at 517 nm using a microplate reader (Thermo Fisher Scientific, Multiskan GO, Finland). Trolox was used as the standard, while methanol served as the blank. The radical scavenging activity was reported as micromoles of Trolox (TE) equivalent per gram of dried sample.
Ferric reducing antioxidant power (FRAP). The ferric reducing antioxidant power (FRAP) assay was performed.13 The FRAP reagent was freshly prepared by mixing a 300 mM acetate buffer (pH 3.6), 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) in 40 mM HCl, and 20 mM FeCl3 in a 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) ratio. A 0.4 mL sample aliquot was added to 2.6 mL of the FRAP reagent and incubated at 37 °C for 30 minutes. The content was mixed, transferred to a 96-well plate, and absorbance was measured at 595 nm using a microplate reader (Thermo Fisher Scientific, Multiskan GO, Finland). Ferrous sulfate (FeSO4) was used as the standard, and distilled water served as the blank. The ferric reducing antioxidant power was expressed as micromoles of FeSO4 equivalent per gram of dried sample.
1 (v/v) ratio. A 0.4 mL sample aliquot was added to 2.6 mL of the FRAP reagent and incubated at 37 °C for 30 minutes. The content was mixed, transferred to a 96-well plate, and absorbance was measured at 595 nm using a microplate reader (Thermo Fisher Scientific, Multiskan GO, Finland). Ferrous sulfate (FeSO4) was used as the standard, and distilled water served as the blank. The ferric reducing antioxidant power was expressed as micromoles of FeSO4 equivalent per gram of dried sample.
Mineral profile. The mineral profiles of raw and boiled mushroom ethanolic extracts, as well as raw and boiled in vitro digested mushroom samples, were analyzed using an Inductively Coupled Plasma Triple-Quadrupole Mass Spectrometer (ICP-MS/MS, Agilent Technologies, 8900 Triple Quadrupole ICP-MS), following the method of López et al. (2024) with slight modifications.15 Approximately 0.3 g of lyophilized mushroom (LM) powder was accurately weighed into Teflon cylindrical tubes. To each sample, 9 mL of concentrated Suprapur® grade HNO3 (>69% purity) and 1 mL of concentrated Suprapur® grade HCl (>36% purity) were added. The digestion was carried out using a microwave digestion system (Milestone ETHOS UP, Milestone Srl, Via Fatebenefratelli, Italy) at 200 °C (1800 watt) for 15 minutes. After digestion, the samples were cooled for 20 minutes and diluted to a final volume of 50 mL with deionized water in volumetric flasks. ICP-MS/MS analysis was performed under the following operating conditions: no gas mode; hydrogen flow rate of 7.0 mL min−1; helium flow rate of 5.0 mL min−1; nebulizer gas flow of 0.80 L min−1; and RF power of 1550 W.
Amino acid composition analysis. Mushroom extracts were subjected to acid hydrolysis before analysis, following the method of Al Azad and Ping (2021).16 Specifically, 0.2 g of dried mushroom powder was hydrolyzed with 8 mL of 6N HCl at 110 °C for 24 hours. After hydrolysis, the samples were diluted to 25 mL with distilled water. The amino acid profiles of both raw and boiled mushroom ethanolic extracts, as well as raw and boiled in vitro digested mushrooms (without acid hydrolysis), were analyzed using a Liquid Chromatography Triple Quadrupole Mass Spectrometry (LC-QqQ) system equipped with a binary pump and auto sampler, coupled to a Nexera LCMS-8060 system (Shimadzu, Japan). The sampling speed was set to 5.0 μL s−1 with a sample injection volume of 1 μL. The mobile phase consisted of 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B).
In vitro gastrointestinal digestion. The in vitro gastrointestinal digestion was carried out following the protocol outlined by Tamura et al.17 Mushroom samples (both raw and cooked) were cut into small pieces and homogenized using a kitchen blender before being subjected to the assay. Approximately 30 g of blended mushroom was combined with 140 g of distilled water in a 600 mL digestion chamber equipped with a thermometer and pH meter. The chamber was placed in a water bath set to maintain the temperature at 37 °C throughout the digestion process. The pH was first adjusted to 1.20 using varying concentrations of HCl (1 M, 3 M, and 6 M) to simulate gastric conditions. The gastric phase was initiated by adding 19 mL of pepsin solution (prepared by dissolving 0.0912 g of pepsin in gastric fluid buffer), and the pH was maintained at 1.20 throughout this stage. Aliquots were collected at 30, 60, 90, and 120 minutes during gastric digestion. To terminate the gastric phase, the pH was increased to 6.80 using NaOH (1 M, 3 M, and 6 M).
The intestinal phase was then initiated by adding 23 mL of intestinal enzyme solution, which consisted of 0.184 g pancreatin from porcine pancreas, 0.0138 g invertase and 3.68 mL amyloglucosidase in intestinal fluid buffer. Samples were collected at 0, 30, 60, 90, and 120 minutes for both the gastric and intestinal stages. All samples were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×g for 5 minutes, and the supernatant aliquots were collected for further analysis.
000×g for 5 minutes, and the supernatant aliquots were collected for further analysis.
Additional mushroom samples were collected before and after in vitro gastrointestinal digestion, frozen at −40 °C overnight and subsequently freeze-dried using a laboratory lyophilizer as previously described. The freeze-dried samples were stored in an electronically controlled humidity cabinet until further analysis, including scanning electron microscopy (SEM), protein quantification, and calculation of protein digestibility.
Statistical analysis. All tests were conducted in triplicate and presented as means ± standard deviation (SD). The results were subjected to a one-way analysis of variance (ANOVA) at a 95% significance level using IBM SPSS Statistics 26. The significance of the difference in data was determined using Tukey's HSD range test. Pearson's correlation matrix analysis at confidence levels of 99% and 95% was performed.
Results and discussion
Microstructural changes of raw and boiled mushrooms before and after in vitro gastrointestinal digestion
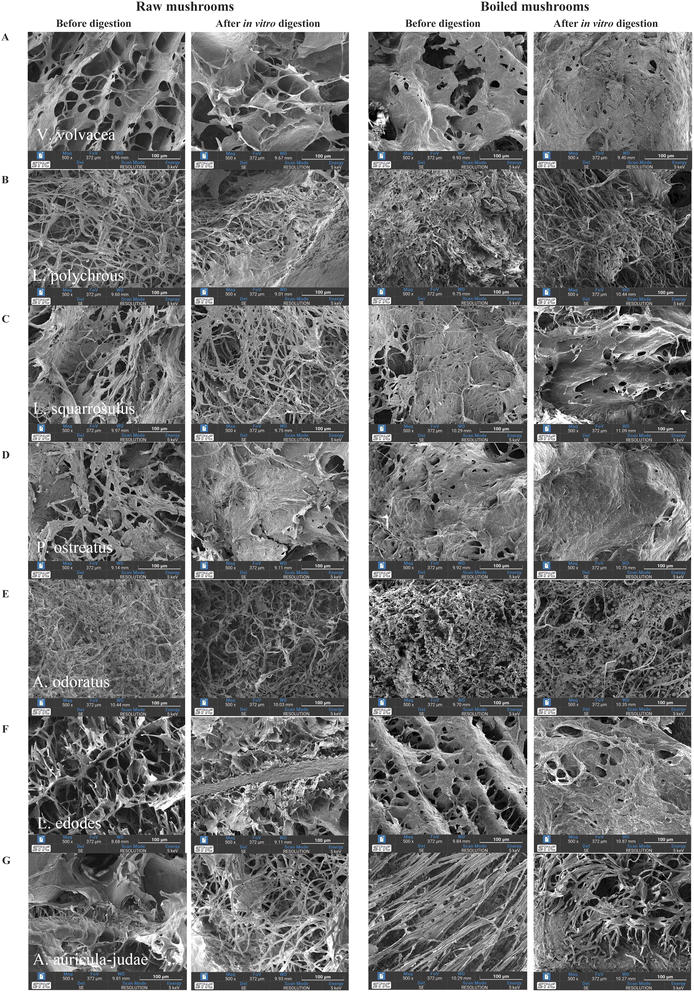
Fig. 2 illustrates SEM images (500× magnification) of the microstructures of the seven mushroom species after boiling and simulated gastrointestinal digestion. Visual inspection indicates that boiling and digestion visibly alter the structural integrity of all mushroom species, likely due to cell wall softening, polysaccharide leaching, and protein denaturation.Raw V. volvacea exhibited a relatively porous and hyphal network (Fig. 2A), which appeared disrupted after digestion. Boiling reduced structural coherence, and digestion of the boiled sample resulted in near-complete collapse of the visible microstructure. Similar trends were observed in L. polychrous and L. squarrosulus (Fig. 2B and C), where raw samples retained mycelial networks, but digestion led to deformation. In L. squarrosulus, more apparent surface voids appeared after digestion, while boiling further deteriorated the structure, likely due to polysaccharide solubilization and hyphal shrinkage. P. ostreatus showed a fibrous, porous structure in raw form (Fig. 2D). Digestion led to denser aggregation, while boiling elongated hyphae. Post-digestion of the boiled sample caused structural collapse. A. odoratus displayed compact, low-porosity morphology (Fig. 2E), which became more disrupted after digestion. Boiling produced a denser but disorganized structure, and digestion of the boiled sample caused further disintegration. In L. edodes, a sponge-like, porous matrix was evident (Fig. 2F). Digestion caused hyphal disorganization, while boiling led to partial shrinkage and coarser surface textures post-digestion. A. auricula-judae exhibited a smooth, flattened surface (Fig. 2G) with limited porosity in raw form. Digestion appeared to increase visible structural discontinuities, while boiling induced a fibrous structure that fragmented post-digestion.
While these micrographs suggest the presence of altered structural patterns such as fragmentation, hyphal disorganization, and apparent voids, we acknowledge that these are qualitative observations. Quantitative analysis (e.g., porosity measurements or image-based morphometry) was not performed, and thus claims regarding porosity enhancement are interpreted with caution.
Nonetheless, these findings align with prior studies reporting microstructural damage from boiling, such as cell wall rupture and polysaccharide solubilization.18,19 Further enzymatic degradation during digestion may contribute to hyphal fragmentation and network disassembly, consistent with previous reports.19,20 The presence of visibly altered structures—particularly in species like L. squarrosulus, A. odoratus, and A. auricula-judae—may reflect carbohydrate content and fiber swelling, although these interpretations require further validation using objective structural metrics in future work.20,21
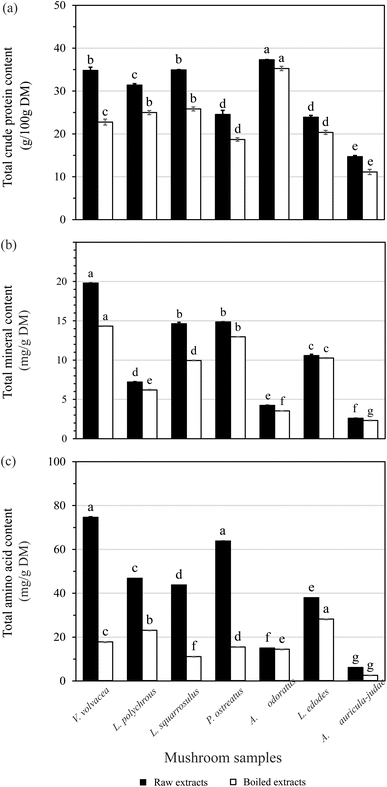
Impact of boiling
Boiling significantly reduced the crude protein content in all seven studied mushroom species, with losses ranging from 5% to 35%, as shown in Fig. 3a. The smallest reduction was observed in A. odoratus (5.44%), while the greatest was recorded in V. volvacea (34.72%). This decline may be attributed to the solubilization and leaching of nitrogenous compounds and minerals during the boiling process.27 A comparable trend was noted by Nie et al., who reported a 9.3% reduction in protein content for L. edodes which lower than the 14.89% observed in our study.18
The effect of boiling on crude protein content was relatively modest in A. odoratus and L. edodes (both <15%). In the case of A. odoratus, this could be attributed to its high total dietary fiber and glucan content (77.1 g/100 g DM and 26.1 g/100 g DM, respectively), which may help preserve protein during cooking by forming a rigid cell matrix.25 Additionally, L. edodes is known to have a high proportion (approximately 90%) of water-insoluble proteins, which are less susceptible to loss during boiling.28 Since boiling mainly affects water-soluble proteins, peptides, and amino acids, most of the protein content in L. edodes remained intact.
Boiling led to a reduction in total mineral content ranging from 3% to 30%. The smallest loss was observed in Lentinula edodes, while Lentinus squarrosulus experienced the greatest decrease. After boiling, the ranking of total mineral content (mg g−1 DM) among the species was as follows: Volvariella volvacea (14.33) > Pleurotus ostreatus (12.96) > L. edodes (10.26) > L. squarrosulus (9.96) > Lentinus polychrous (6.21) > Astraeus odoratus (3.54) > Auricularia auricula-judae (2.32). In addition to the overall reduction in mineral content, significant losses were recorded in individual macro- and trace elements, including potassium, magnesium, calcium, manganese, iron, zinc, copper, and selenium. These findings suggest that boiling promotes the leaching of minerals into the cooking water, as detailed in SI Table S5. This trend is consistent with the findings of Lee et al. (2019), who investigated the effects of boiling on L. edodes.30 Their study reported notable decreases in potassium, magnesium, and phosphorus following boiling.
The observed reduction in both protein and mineral content following boiling is primarily attributed to the diffusion of minerals into the cooking water.31 This finding aligns with the previous work of R. Puupponen-Pimiä et al.,32 who reported that mineral losses during cooking are due to leaching rather than degradation. Interestingly, while boiling reduces mineral content, it can also improve the nutritional quality of mushrooms by lowering levels of antinutritional factors. Traditional cooking methods, such as boiling, are known to reduce compounds like phytic acid, polyphenols, and certain fibers that bind minerals and limit their bioavailability.33 Therefore, although boiling causes some mineral loss, it may enhance the overall bioavailability of remaining minerals by decreasing the impact of antinutrients and breaking down structural barriers within the mushroom matrix.
The results of this study broadly align with previous findings, although reported amino acid values vary considerably depending on mushroom species, cultivation conditions, substrate composition, and analytical methodology on amino acid quantification. For instance, total amino acid contents have ranged from 4.4 mg g−1 in L. polychrous to over 100 mg g−1 in species such as P. ostreatus and L. edodes.26,34 Amino acids contribute significantly to the nutritional quality and characteristic flavor profiles of mushrooms.2 These results highlight V. volvacea and P. ostreatus as particularly rich sources of amino acids, underscoring their potential as valuable dietary protein supplements. Moreover, the species-specific differences in amino acid composition may inform their selection for use in functional food products or targeted nutritional applications.
Boiling significantly reduced total amino acid content across all species, with losses ranging from approximately 3% to 75% (Fig. 3c, Tables S3 and S4). The smallest reduction was observed in A. odoratus, while V. volvacea experienced the greatest loss. After boiling, the ranking of total amino acid content changed to: L. edodes (28.08) > L. polychrous (22.94) > V. volvacea (17.68) > P. ostreatus (15.37) > A. odoratus (14.33) > L. squarrosulus (10.99) > A. auricula-judae (2.43), as shown in Fig. 3c. The observed reduction in amino acid content is consistent with prior studies demonstrating that heat treatment can compromise the protein structure and reduce amino acid retention. For instance, it has been reported that free amino acids in L. edodes significantly (p < 0.05) decreased by 58.60% (from 5.03 to 2.09 mg g−1) following boiling.18 In comparison, our study noted a 25% reduction in total amino acids for the same species (from 37.85 to 28.08 mg g−1 DM). This decline is largely attributed to the high water solubility of free amino acids, which are readily leached into the cooking water during boiling. Consequently, the consumption of mushroom broth may help recover some of the lost amino acid content and enhance dietary intake.18
| Mushroom samples | Treatment | Estimated in vitro digestibility (%) | Estimated in vitro bioaccessibility (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Total crude protein | Total mineral content | Total amino acid content | Total phenolic content (TPC) | Total flavonoid content (TFC) | DPPH radical scavenging activity (DPPH) | Ferric reducing antioxidant power (FRAP) | ||
| a Values represent the mean and standard deviation (n = 3). Values of estimated in vitro digestibility and estimated in vitro bioaccessibility (%) were obtained from eqn (1) and (2). Small letters indicate significant differences between samples within the same treatment (p < 0.05). | ||||||||
| V. volvacea | Raw | 24.27 ± 1.46c | 25.71 ± 0.21e | 106.47 ± 5.80d | 342.72 ± 17.26b | 110.99 ± 5.14c | 129.81 ± 2.24c | 193.13 ± 10.12b |
| Boiled | 32.85 ± 1.01d | 29.03 ± 0.53cd | 350.11 ± 15.98b | 997.51 ± 51.53bc | 116.79 ± 0.20b | 133.87 ± 0.51b | 341.70 ± 6.64a | |
| L. polychrous Berk | Raw | 38.10 ± 1.44b | 58.65 ± 0.92bc | 149.51 ± 3.63d | 446.00 ± 22.26b | 62.51 ± 1.06e | 142.60 ± 0.21b | 150.37 ± 2.49bc |
| Boiled | 43.22 ± 3.01bc | 29.77 ± 2.54cd | 193.02 ± 7.58d | 1206.77 ± 56.86bc | 91.67 ± 6.48c | 141.09 ± 3.54ab | 157.20 ± 12.53c | |
| L. squarrosulus | Raw | 40.83 ± 1.17b | 38.56 ± 0.50d | 190.71 ± 13.39cd | 494.21 ± 45.91b | 72.22 ± 2.35e | 135.13 ± 3.82bc | 140.35 ± 2.35c |
| Boiled | 44.22 ± 0.52bc | 21.08 ± 1.48d | 365.78 ± 17.19b | 1189.59 ± 222.92bc | 90.51 ± 3.00c | 143.37 ± 5.25a | 127.32 ± 10.84d | |
| P. ostreatus | Raw | 37.93 ± 0.49b | 37.58 ± 0.57d | 137.73 ± 9.34d | 600.20 ± 37.92b | 133.56 ± 0.18b | 154.64 ± 3.69a | 157.04 ± 6.55bc |
| Boiled | 38.22 ± 1.84cd | 23.76 ± 0.40cd | 232.15 ± 6.18 cd | 1518.24 ± 176.11b | 66.97 ± 3.86d | 149.55 ± 0.36a | 174.54 ± 7.68 bc | |
| A. odoratus | Raw | 39.70 ± 1.87b | 62.71 ± 0.73b | 355.82 ± 29.39b | 410.80 ± 58.42b | 114.20 ± 15.95c | 128.72 ± 5.45c | 182.14 ± 4.83bc |
| Boiled | 55.31 ± 1.27a | 54.41 ± 1.45b | 264.68 ± 4.44c | 436.27 ± 32.16c | 117.57 ± 7.92b | 144.07 ± 2.77a | 200.26 ± 12.60b | |
| L. edodes | Raw | 39.07 ± 0.02b | 51.29 ± 0.20c | 314.21 ± 32.80bc | 1139.36 ± 201.62a | 169.01 ± 1.45a | 158.58 ± 6.49a | 318.03 ± 43.55a |
| Boiled | 39.87 ± 0.88c | 32.35 ± 0.50c | 130.83 ± 3.37e | 1406.20 ± 74.70bc | 71.05 ± 2.36d | 148.99 ± 3.80a | 186.12 ± 15.59bc | |
| A. auricula-judae | Raw | 50.71 ± 1.17a | 99.89 ± 6.62a | 1483.09 ± 97.88a | 1451.48 ± 378.26a | 94.18 ± 5.18d | 156.53 ± 7.04a | 160.83 ± 19.60bc |
| Boiled | 49.68 ± 2.22ab | 110.40 ± 6.63a | 1002.74 ± 19.45a | 3520.55 ± 1140.72a | 136.75 ± 15.36a | 143.52 ± 5.84a | 173.61 ± 18.70bc | |
Protein digestibility is significantly influenced by the structural complexity of proteins and the accessibility of peptide bonds for enzymatic hydrolysis. Proteins embedded within complex matrices, such as polysaccharides, are less accessible to digestive enzymes, leading to reduced digestibility.37 For instance,20L. squarrosulus exhibited a protein content of 30.84%, which decreased to 11.91% after simulated intestinal digestion, corresponding to a hydrolysis rate of 59.79%. In contrast, our study found lower digestibility in raw L. squarrosulus (40.82%), which increased to 58.78% upon boiling. This suggests that the structural matrix of the mushroom may hinder enzymatic access to proteins, thereby affecting digestibility.38
The presence of polysaccharides such as β-glucans in mushroom cell walls can hinder protein digestion by increasing gastrointestinal viscosity and forming glycoprotein complexes that resist enzymatic breakdown.38 These interactions reduce protein bioavailability. Additionally, the degree of hydrolysis and type of protease used are key factors; endopeptidases, for instance, enhance digestibility by producing smaller, absorbable peptides.39 Protease treatment, as shown in Pleurotus eryngii, improves digestibility by increasing amino acid release.38 Overall, protein digestibility in mushrooms is shaped by structural complexity and polysaccharide interactions, emphasizing the need for targeted processing strategies to improve bioavailability.
These variations highlight the complex interplay between mushroom cell wall composition, the presence of antinutritional factors, and mineral bioaccessibility. Polysaccharides, such as β-glucans and chitin, prevalent in mushroom cell walls, can form complexes with minerals, reducing their solubility and hindering enzymatic breakdown during digestion.38 Additionally, compounds like phytic acid, oxalic acid, and tannins can bind minerals, further decreasing their bioavailability.33
Processing methods, including boiling, can influence the release of minerals from these complexes. For instance, boiling may facilitate the leaching of minerals into the cooking water, as observed in some species, while others may retain minerals more effectively. Understanding these dynamics is crucial for optimizing the nutritional value of mushrooms through appropriate culinary practices. In summary, the bioaccessibility of minerals in mushrooms is influenced by their intrinsic cellular structures and the presence of antinutritional factors. Species-specific responses to processing methods underscore the need for tailored approaches to enhance the nutritional benefits of edible mushrooms.
Proteolytic enzymes during digestion convert proteins into smaller peptides and free amino acids, making them more bioavailable.19 However, raw V. volvacea and P. ostreatus extracts exhibited notable reductions in total amino acids after digestion, whereas other mushroom species showed an increase. This discrepancy may be explained by the high mineral content of V. volvacea and P. ostreatus, which likely facilitates peptide-mineral complex formation during in vitro digestion. As proteins are hydrolyzed into amino acids and peptides, some exhibit strong metal-binding properties, particularly with iron, leading to reduced free amino acid levels but improved mineral bioavailability. The nitrogen of the ε-amino group from lysine and the imidazole group from histidine play a crucial role in chelation, while proline residues contribute to peptide folding, although they do not participate directly in metal complexation.40
Notably, the percentage bioaccessibility of total amino acids during in vitro digestion was higher in boiled mushrooms than in raw mushrooms, except for A. auricula-judae. Boiling mushrooms for 10 minutes generally enhanced amino acid bioaccessibility during in vitro digestion, despite a reduction in total amino acid content. This suggests that boiling facilitates the release and absorption of amino acids by denaturing proteins and increasing protease accessibility. However, excessive boiling can lead to nutrient loss and reduced digestibility due to protein aggregation and oxidation. For instance, studies on A. bisporus and L. edodes have shown that boiling can decrease free amino acid content, but the bioaccessibility of amino acids in the digestive tract may still improve due to better protein breakdown and solubility.
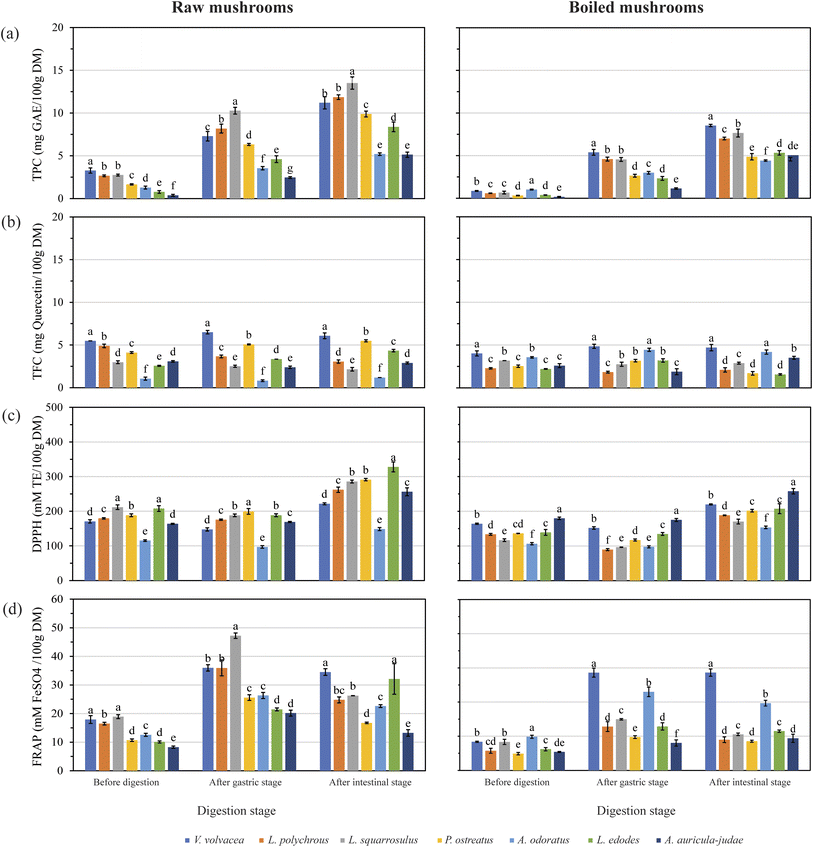
Conversely, A. odoratus exhibited the highest TPC after boiling, suggesting that its polyphenols may be more heat-resistant compared to other species. In addition, boiling may enhance the release and bioaccessibility of phenolic compounds in this species. Conversely, boiling generally reduced TPC in other mushroom species, likely due to the leaching of water-soluble phenols into the cooking water, a process that can significantly decrease antioxidant activity.42 In contrast, boiling V. volvacea resulted in a significant reduction in TPC, but in vitro digestion led to an overall increase in TPC, suggesting that digestive processes can enhance the bioaccessibility of phenolic compounds even after thermal degradation.
In this study, raw and boiled V. volvacea exhibited the highest total flavonoid content (TFC) both before and after in vitro digestion. Notably, boiling significantly increased TFC in A. odoratus, suggesting that boiling can enhance flavonoid release during digestion. Conversely, boiling led to a reduction in TFC in P. ostreatus, with the lowest TFC observed post-digestion. This variability underscores the influence of cooking methods on flavonoid stability and bioaccessibility.
Flavonoids are water-soluble and heat-sensitive, making them susceptible to degradation during cooking and digestion. In this study, boiling resulted in a significant decrease in TFC across various mushroom species, consistent with previous findings that attributed such reductions to the breakdown of mushroom tissue during heating, facilitating the release of these compounds from the food matrix.10 However, some studies suggest that certain cooking methods, such as steaming and microwaving, can enhance flavonoid content by increasing the extractability of these compounds from the cell matrix.36
The observed differences in TFC among mushroom species and cooking methods highlight the complex interplay between thermal processing and flavonoid stability. While boiling generally reduces TFC, it may also enhance the release of bound flavonoids during digestion, potentially increasing their bioaccessibility. Therefore, the impact of cooking on flavonoid content and bioaccessibility is multifaceted, depending on the specific mushroom species and cooking techniques employed.
Among the raw mushroom samples, L. squarrosulus and L. edodes showed the highest initial DPPH scavenging activity (211.66 and 207.96 mM TE/100 g DM, respectively; p < 0.05), with no significant difference between them. All raw mushrooms demonstrated increased DPPH activity following digestion. In contrast, A. odoratus exhibited the lowest initial activity (115.29 mM TE/100 g DM).
Boiling significantly enhanced DPPH activity in A. odoratus and A. auricula-judae, suggesting their antioxidant compounds—such as β-glucans, amino acids, and ergothioneine—are heat-resistant and likely extend beyond phenolics.42 Conversely, boiling reduced DPPH activity in other species, which could be due to processing depending on surface area exposure and cooking time.
FRAP values also increased after digestion across all raw mushrooms. However, A. auricula-judae, despite high DPPH activity, showed the lowest FRAP value (8.26 mM FeSO4/100 g DM). Post-digestion, the highest FRAP values were recorded in V. volvacea and L. edodes (34.5 and 32.11 mM FeSO4/100 g DM, respectively). Boiling reduced FRAP in all mushrooms; however, boiled A. odoratus initially had the highest FRAP value. During digestion, boiled V. volvacea showed a notable increase in FRAP. Flavonoid-related metal-chelation may also influence FRAP activity. This depends on the flavonoid structure, metal ion type, and pH.43 Since pH varies across the digestive tract, the stability and bioavailability of flavonoid–metal complexes may shift during digestion.
It is important to note that the TPC, TFC, DPPH, and FRAP values in this study were based on water extracts, which may not fully reflect the total antioxidant content. Some mushrooms may require alternative solvents—such as ethanol, diethyl ether, or mixed solvents—for more efficient extraction. Nevertheless, this study prioritized water-based extraction to better simulate real-life consumption conditions. Consequently, while the bioavailability of phenolic compounds may increase, the overall antioxidant capacity of the vegetable diminishes due to the loss of these compounds into the cooking medium.
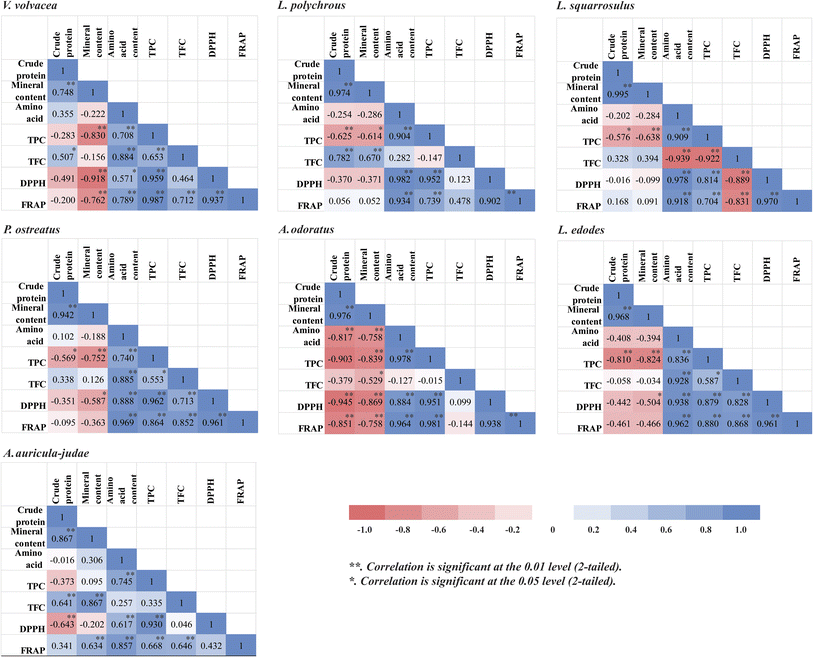
TPC showed strong correlation with DPPH (R2 = 0.814–0.962) and moderate to strong correlation with FRAP (R2 = 0.668–0.987) across species. TFC also correlated with antioxidant activity in some species, such as V. volvacea, P. ostreatus, and L. edodes. These results suggest that phenolics are major contributors to antioxidant capacity. Notably, A. auricula-judae showed no DPPH-FRAP correlation, possibly due to assay differences and solvent effects.
Interestingly, L. squarrosulus had a negative correlation between TFC and antioxidant activity, despite strong TPC-TFC correlation, indicating that non-flavonoid phenolics—like gallic acid—may dominate antioxidant effects. L. polychrous had the highest TFC, while A. odoratus showed no significant TFC-antioxidant link, likely due to low TFC (Fig. 4). Overall, these findings support TPC as the primary antioxidant contributor in mushrooms.10,44,45
Conclusions
This study demonstrated that boiling and in vitro gastrointestinal digestion significantly influenced the nutritional composition, microstructure, and bioactive properties of seven edible mushroom species. Boiling generally reduced amino acids and phenolic compounds but enhanced protein digestibility and improved the apparent bioaccessibility of several nutrients. Distinct species-specific responses were observed, underscoring the importance of choosing appropriate mushroom types and preparation methods to optimize health benefits. Microstructural analysis via SEM revealed visible disruption of fungal cell networks and the formation of structural discontinuities following processing. However, we recognize that conclusions regarding porosity enhancement are based on qualitative observations. We acknowledge the need for quantitative metrics in future work to confirm these findings. Although the study offers useful insights for functional food development, the absence of sensory and consumer acceptability data remains a limitation. Future research should include sensory evaluations to assess flavor, texture, and palatability—key factors for real-world application of mushrooms in plant-based food products. These findings collectively support the integration of edible mushrooms into sustainable, protein-rich diets and functional food formulations.Author contributions
All authors have participated in (a) the conception and design of the experiment, or the analysis and interpretation of the data; (b) drafting the article or revising it critically for important intellectual content; and (c) approval of the final version.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the SI. Supplementary information: supplementary Tables S1–S5 provide detailed compositional and digestibility data for seven mushroom species. Table S1 includes total crude protein, mineral content, amino acid profiles before and after digestion, and estimated in vitro digestibility. Table S2 presents total phenolic content, flavonoid content, antioxidant activities (DPPH, FRAP), and their in vitro bioaccessibility. Tables S3 and S4 detail essential, non-essential, and conditionally essential amino acid contents in raw, boiled, and digested extracts. Table S5 summarizes macro and trace mineral compositions in raw, boiled, and digested samples. See DOI: https://doi.org/10.1039/d5fb00346f.Acknowledgements
We are thankful for the financial support provided by the National Science, Research and Innovation Fund (NSRF), under Grant No. 662A04012 and Mae Fah Luang University.References
- A. K. Das, P. K. Nanda, P. Dandapat, S. Bandyopadhyay, P. Gullón, G. K. Sivaraman, D. J. McClements, B. Gullón and J. M. Lorenzo, Molecules, 2021, 26, 1–25 Search PubMed.
- S. Gupta, B. Summuna, M. Gupta and S. K. Annepu, in Bioactive Molecules in Food, eds. J.-M. Mérillon and K. G. Ramawat, Springer International Publishing, Cham, 2018, pp. 1–33 Search PubMed.
- I. Roncero-Ramos, M.-L. Mónica, P.-C. Margarita and C. Delgado-Andrade, Int. J. Nutr. Food Sci., 2017, 68, 287–297 CrossRef CAS PubMed.
- W. A. Elkhateeb and G. M. Daba, Int. J. Pharmaceut. Chem. Anal., 2022, 9, 1–19 CrossRef CAS.
- P. Łysakowska, A. Sobota and A. Wirkijowska, Molecules, 2023, 28, 1–15 CrossRef PubMed.
- S. A. Heleno, L. Barros, A. Martins, P. Morales, V. Fernández-Ruiz, J. Glamoclija, M. Sokovic and I. C. F. R. Ferreira, LWT–Food Sci. Technol., 2015, 63, 799–806 CrossRef CAS.
- Z. X. Ng and N. F. Rosman, J. Food Sci. Technol., 2019, 56, 865–877 CrossRef CAS PubMed.
- C. Soler-Rivas, A. C. Ramírez-Anguiano, G. Reglero and S. Santoyo, Int. J. Food Sci. Technol., 2009, 44, 2189–2197 CrossRef CAS.
- C. Zeng, H. Li, J. Li, C. Li, Z. Fang, B. Hu, C. Wang, S. Chen, X. Li, Z. Zeng and Y. Liu, Food Chem., 2024, 439, 138107 CrossRef CAS PubMed.
- L. Sun, X. Bai and Y. Zhuang, J. Food Sci. Technol., 2014, 51, 3362–3368 CrossRef CAS PubMed.
- W. Horwitz and G. W. Latimer, J. - Assoc. Off. Anal. Chem., 2012, 17, 135–138 Search PubMed.
- E. Fernández-García, I. Carvajal-Lérida and A. Pérez-Gálvez, Nutr. Res., 2009, 29, 751–760 CrossRef PubMed.
- N. Donlao, S. Wonglek, N. Bunyameen, W. N. Krom, M. Chayathatto and P. Fuggate, J. Stored Prod. Res., 2024, 105, 102241 CrossRef CAS.
- T. Aumasa, Y. Ogawa, J. Singh, W. Panpipat and N. Donlao, NFS Journal, 2023, 33, 100154 CrossRef CAS.
- A. R. López, E. Ortega-Caneda, E. Espada-Bellido, O. R. Taracena-Zepeda, M. Palma and G. Fernández-Barbero, Foods, 2024, 13, 1–13 CrossRef PubMed.
- S. Al Azad and V. C. A. Ping, Adv. Biosci. Biotechnol., 2021, 12, 286–296 CrossRef CAS.
- M. Tamura, J. Singh, L. Kaur and Y. Ogawa, Food Chem., 2016, 191, 91–97 CrossRef CAS PubMed.
- Y. Nie, Y. Mingjun, Z. Haoyu, Z. Penghui, Y. Wei and B. Li, CyTA - J. Food, 2020, 18, 543–550 CrossRef CAS.
- F. Ayimbila and S. Keawsompong, Starch Staerke, 2018, 70, 1800049 CrossRef.
- F. Ayimbila, S. Siriwong, M. Nakphaichit and S. Keawsompong, Sci. Rep., 2022, 12, 2655 CrossRef CAS PubMed.
- S. Jongaroontaprangsee, N. Chiewchan, P. Methacanon and S. Devahastin, J. Nat. Sci., 2016, 15, 141–152 Search PubMed.
- F. Ayimbila and S. Keawsompong, Curr. Nutr. Rep., 2023, 12, 290–307 CrossRef PubMed.
- M. E. Effiong, C. P. Umeokwochi, I. S. Afolabi and S. N. Chinedu, Front. Nutr., 2023, 10, 1279208 CrossRef PubMed.
- A. Srikram and S. Supapvanich, Agric. Nat. Resour., 2016, 50, 432–436 Search PubMed.
- K. Wunjuntuk, M. Ahmad, T. Techakriengkrai, R. Chunhom, E. Jaraspermsuk, A. Chaisri, R. Kiwwongngam, S. Wuttimongkolkul and S. Charoenkiatkul, J. Food Compos. Anal., 2022, 105, 104226 CrossRef CAS.
- Y. Cuptapun, D. Hengsawadi, W. Mesomya and S. Yaieiam, Agric. Nat. Resour., 2010, 44, 664–670 CAS.
- T. Reid, M. Munyanyi and T. Mduluza, Food Sci. Nutr., 2017, 5, 538–544 CrossRef CAS PubMed.
- J. Erjavec, J. Kos, M. Ravnikar, T. Dreo and J. Sabotič, Trends Biotechnol., 2012, 30, 259–273 CrossRef CAS PubMed.
- P. Kalač, in Mineral Composition and Radioactivity of Edible Mushrooms, ed. P. Kalač, Academic Press, 2019, pp. 9–24 Search PubMed.
- K. Lee, H. Lee, Y. Choi, Y. Kim, H. S. Jeong and J. Lee, Food Sci. Technol. Res., 2019, 25, 115–122 CrossRef CAS.
- N. Wang, D. W. Hatcher, R. T. Tyler, R. Toews and E. J. Gawalko, Food Res. Int., 2010, 43, 589–594 CrossRef CAS.
- R. Puupponen-Pimiä, S. T. Häkkinen, M. Aarni, T. Suortti, A.-M. Lampi, M. Eurola, V. Piironen, A. M. Nuutila and K.-M. Oksman-Caldentey, J. Sci. Food Agric., 2003, 83, 1389–1402 CrossRef.
- M. H. Ertop and M. Bektaş, Food Health, 2018, 4, 159–165 CrossRef.
- M. A. R. Mazumder, M. Sangsomboon, S. Ketnawa and S. Rawdkuen, J. Agric. Food Res., 2024, 16, 101103 CAS.
- V. Lavelli, C. Proserpio, F. Gallotti, M. Laureati and E. Pagliarini, Food Funct., 2018, 9, 1353–1372 RSC.
- X. Zhou, Q. Guan, Y. Wang, D. Lin and B. Du, Foods, 2022, 11, 1–24 Search PubMed.
- G. Goksen, G. Karabulut, M. Keklik and M. Martínez-Sanz, Food Chem., 2025, 489, 145013 CrossRef CAS PubMed.
- D. Wang, M. Zhang, J. Wan, H. Liu, Y. Wang, R. Yang, Y. Wu, D. Bao, H. Chen, G. Zou and Y. Zhao, J. Fungi, 2024, 10 Search PubMed.
- G. J. Fadimu, T. T. Le, H. Gill, A. Farahnaky, O. O. Olatunde and T. Truong, Foods, 2022, 11, 1–27 Search PubMed.
- M. E. Caetano-Silva, F. M. Netto, M. T. Bertoldo-Pacheco, A. Alegría and A. Cilla, Crit. Rev. Food Sci. Nutr., 2021, 61, 1470–1489 CrossRef CAS PubMed.
- G. Rocchetti, R. P. Gregorio, J. M. Lorenzo, F. J. Barba, P. G. Oliveira, M. A. Prieto, J. Simal-Gandara, J. I. Mosele, M. J. Motilva, M. Tomas, V. Patrone, E. Capanoglu and L. Lucini, Compr. Rev. Food Sci. Food Saf., 2022, 21, 811–842 CrossRef CAS PubMed.
- D. Morales, M. A. Bal, S. Figueredo, C. Soler-Rivas and A. Ruiz-Rodríguez, Food Bioprocess Technol., 2024, 17, 791–798 CrossRef CAS.
- K. Wojtunik-Kulesza, A. Oniszczuk, T. Oniszczuk, M. Combrzyński, D. Nowakowska and A. Matwijczuk, Nutrients, 2020, 12, 1–28 CrossRef PubMed.
- F. Yao, H. Gao, C. M. Yin, D. F. Shi and X. Z. Fan, Foods, 2023, 12, 1–15 Search PubMed.
- A. González, C. Nobre, L. S. Simões, M. Cruz, A. Loredo, R. M. Rodríguez-Jasso, J. Contreras, J. Texeira and R. Belmares, Food Chem., 2021, 346, 128884 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |