 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pumpkin seed flour (a cold-pressed by-product): characterization and rheological assessment in gluten-free bread premixes
Juan José
Burbano
 *a,
Manuel
Rojas
a,
Marina
Fernández Bravo
a,
Julio
Vidaurre-Ruiz
bc,
Ritva
Repo Carrasco Valencia
bc and
María Jimena
Correa
*a,
Manuel
Rojas
a,
Marina
Fernández Bravo
a,
Julio
Vidaurre-Ruiz
bc,
Ritva
Repo Carrasco Valencia
bc and
María Jimena
Correa
 *a
*a
aCentro de Investigación y Desarrollo en Ciencia y Tecnología de los Alimentos (CIDCA), Facultad de Ciencias Exactas-Universidad Nacional de La Plata, Comisión de Investigaciones Científicas de la Provincia de Buenos Aires, Consejo Nacional de Investigaciones Científicas y Técnicas, 47 y 116, C.P. 1900 La Plata, Argentina. E-mail: jburbano@exactas.unlp.edu.ar; mjcorrea@biol.unlp.edu.ar
bDepartamento de Ingeniería de Alimentos y Productos Agropecuarios, Universidad Nacional Agraria La Molina, Lima, Peru
cCentro de Investigación e Innovación en Productos Derivados de Cultivos Andinos (CIINCA), Universidad Nacional Agraria La Molina, Avenida La Molina s/n, Lima 12, Peru
First published on 24th September 2025
Abstract
Cold-pressed oil production generates high-quality oil and a nutrient-rich press cake as a by-product. Cucurbita pepo seeds (var. styrica) are typically used for this purpose. This work aims to add value to the press cake by-product by characterizing the techno-functional, physicochemical, nutritional, and microstructural properties of pumpkin seed flour (PSF), and evaluating the impact of PSF on the pasting and rheological behaviour of gluten-free model premixes (rice flour based). Nutritionally, PSF contained 41% protein. Although lipids accounted for one-third of its composition (predominantly unsaturated fatty acids), PSF showed high oxidative stability. Regarding its techno-functional properties, PSF showed good emulsion activity (55%), high thermal emulsion stability (51%) and good water, oil, and organic molecule retaining capacities. Characterization of PSF and rice flour (RF) showed that the two flours complement each other when forming composite mixtures. In addition, the suitability of PSF for breadmaking was determined by evaluating its effect on the rheology of premixes. The premixes were prepared by substituting RF with PSF at 10%, 20% and 30%. The viscoamylograph showed that regardless of the substitution level, PSF formed premixes that were less susceptible to retrogradation and had the potential to enhance oven spring during baking. At 20% and 30% substitution, PSF also improved the rheological quality of the gluten-free dough models, acting similarly to xanthan gum. This effect could be related to the ability of PSF to retain water and to form emulsions. These results showed the aptitude of PSF to be included in formulations intended for gluten-free bakery products.
Sustainability spotlightReusing and revaluing by-products contributes to the circular economy. Pumpkin seed flour (PSF) is a by-product of cold-pressed oil production. PSF is rich in protein, healthy lipids and dietary fibre, and it showed good techno-functional properties and a heterogeneous microstructure. Moreover, its incorporation improved the technological (pasting and rheological) properties of premixes for gluten-free bread. Therefore, this research aligns with the United Nations' Sustainable Development Goals, especially Goals 12 and its targets 12.2 (efficient use of natural resources), 12.3 (reduce the food waste at the consumer level) and 12.5 (reduce waste by reusing). |
1 Introduction
Obtaining seed oil can be achieved through a variety of common methods, including mechanical pressing, supercritical extraction, and solvent extraction. The chosen method directly impacts both the physicochemical properties of the oils and their final price. For this reason, cold-press mechanical extraction stands out as the optimal choice when seeking high-quality oil at a reasonable price,1 as it also yields an equally high-quality pressed cake as a by-product.The Styrian pumpkin (Cucurbita pepo subsp. pepo var. styriaca), which originated from south-eastern Austria, is distinguished from other varieties by its hull-less seeds. This characteristic makes it a prime source for oil extraction.2,3 The production of this high-quality oil results in a press cake rich in proteins and residual oil, which, through milling, is transformed into pumpkin seed flour (PSF). On the other hand, gluten-free bakery products are usually of low nutritional quality because their main components are refined starches and flours. Among them, rice flour (RF) is one of the most common due to its wide availability, hypoallergenicity, smooth flavour, and low price.4 However, RF has low protein content and lacks bioactive compounds and saturated fat, with starch being its main component.5 Furthermore, gluten-free flours like RF do not have the ability to form a network structure similar to gluten, which is essential for creating the matrix of products like bread. Consequently, many components, including hydrocolloids, various protein sources, and emulsifiers, are added to achieve products of high technological quality. In this regard, pressed-cake by-products have proven to be valuable ingredients for the development of gluten-free bakery products.6–9 Particularly, the high protein and good dietary fibre content of PSF could be optimal for increasing the nutritional quality and simultaneously helping the formation of a suitable matrix for bread. Therefore, the formulation of premixes could be the perfect vehicle for encouraging the reuse of this by-product, thereby promoting the circular economy. Thus, this work aims to add value to the pumpkin seed press cake by-product by characterizing the techno-functional, physicochemical, nutritional, and microstructural properties of pumpkin seed flour (PSF), and evaluating the impact of PSF on the pasting and rheological behaviour of gluten-free premixes.
2 Materials and methods
2.1. Materials
Pumpkin seed flour (PSF) was provided by Grupo Aceites del Desierto SRL (Córdoba, Argentina). Rice flour (RF) (Santa María, Argentina) and xanthan gum (Onza de oro, Condiment S.A., Argentina) were acquired at a local market. All ingredients were certified gluten-free, and all reagents were of analytical or chromatography grade. Table 1 shows the proximal composition of PSF and RF (100 g wet basis).| Pumpkin seed flour | Rice flour | |
|---|---|---|
| a Nutritional information provided by each manufacturer. | ||
| Calories (kcal) | 500 | 366 |
| Proteins (g) | 41 | 6 |
| Total fat (g) | 35 | 1.2 |
| Saturated fat (g) | 6.5 | 0.4 |
| Carbohydrates (g) | 5 | 80 |
| Dietary fibre (g) | 4.3 | 2.4 |
| Sodium (mg) | 10.7 | 0 |
2.2. Methods
2.2.1.1 Fatty acid profile. The PSF fatty acid profile was determined by gas chromatography, after in situ transesterification to obtain fatty acid methyl esters (FAMEs). The methodology of Park and Goins10 was followed with minor modifications: 0.2 g of PSF was weighed into a screw-cap test tube, and 1 mL 0.5 N NaOH in methanol and 200 μL of methylene chloride (CH2Cl2) were added. After injecting N2 the tube was immediately closed, and after this, the tube was heated in a water bath at 90 °C for 10 min. The tube was cooled with running water before addition of 1 mL of 14% boron trifluoride (BF3) in methanol. After injecting N2, the tube was tightly closed, and the heating continued in a water bath at 90 °C for 10 min. The tube was cooled with running water, and then, 1 mL of Milli-Q water and 500 μL of hexane (C6H14) were added. The tube was shaken vigorously in order to transfer the FAMEs to hexane. The tube was centrifuged at 3000 rpm (Rolco CM2036 Duron, Rolco Srl, Argentina) for 15 min. The top layer was collected with a (1 mL) syringe and filtered through a 0.22 μm Nylon filter directly into an amber vial. The FAMEs in hexane were analysed in an Agilent 7890A gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) with a flame ionization detector (injector temperature, 250 °C). The chromatograph was equipped with a DB-23 capillary column (30 m length, 0.25 mm ID × 0.25 μm film thickness), and a split ratio of 50
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was employed. A Supelco 37 component FAME MIX was employed as the external standard.
1 was employed. A Supelco 37 component FAME MIX was employed as the external standard.
2.2.1.2 Lipid nutritional quality. Lipid nutritional quality of PSF was evaluated with the following indices: the atherogenic index (AI) (eqn (1)), the thrombogenic index (TI) (eqn (2)) and the hypocholesterolaemic/hypercholesterolaemic fatty acid ratio (HH) (eqn (3)).11 These indices are related to the impact of fatty acid composition on the risk of developing cardiovascular disease.
 | (1) |
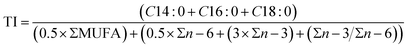 | (2) |
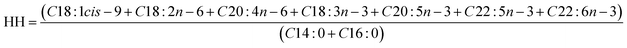 | (3) |
In addition, the iodine value of PSF was calculated according to eqn (4).12
IV = (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 × 0.899) + (C18 1 × 0.899) + (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 × 1.814) + (C18 2 × 1.814) + (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 × 2.737) 3 × 2.737) | (4) |
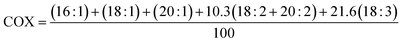 | (5) |
2.2.3.1 Moisture content. Due to its high unsaturated lipid content, the moisture content of PSF was measured in a vacuum oven (70 °C, 50–55 mbar) (Arcane, China) connected to a diaphragm pump (Vacuubrand PC 500 Series-CVC 3000, Germany) until constant weight was achieved.13 The assay was performed at least in triplicate.
2.2.3.2 Water activity (aw). a w of PSF was measured at 25 °C using an AquaLab (Decagon Devices, Inc Pullman, USA).
2.2.3.3 Colour. CIE-L*a*b* parameters of flours (PSF and RF) were evaluated using a colorimeter (Chroma Meter CR-400C, Minolta, Osaka, Japan).
2.2.3.4 pH values. A 10% (w/v) suspension of PSF was prepared with distilled water in centrifuge tubes. The suspension was allowed to rest for 30 minutes and the pH values were measured.
2.2.3.5 Particle size measurements. The measurement was performed by laser diffraction using a Mastersizer 2000E (Malvern Instruments Ltd, UK) with a Hydro 2000 MU accessory at a pump speed of 2000 rpm. PSF was suspended in distilled water (10% w/w). Refractive indices of 1.33 for water and 1.52 for PSF were used. The assay was performed with the application of ultrasonic treatment to favour the dispensability (ultrasonic displacement of 10 μm for 10 s).15 The size of PSF was evaluated in terms of the 10th (D10), 50th, (D50) and 90th (D90) percentiles and span ((D90 − D10)/D50), which were obtained from the volume-weighted distribution using the Mastersizer 2000E software (version 5.54).
2.2.5.1 Emulsion activity and stability of PSF. PSF (1.5 g) was homogenized with 50 mL of water for 30 seconds using an Ultra-Turrax homogenizer (T25 basic, IKA-Werke, Germany) at 9500 rpm. Then, 25 mL of sunflower oil was added, and the mixture was homogenized for 30 seconds. Afterwards, another 25 mL of oil was added, and the mixture was homogenized for 90 seconds. The emulsion was divided evenly into two 50 mL centrifuge tubes. The first tube was centrifuged at 3000 rpm for 5 min (CM 2036, Rolco SRL, Argentina) to determine emulsion activity. To determine the emulsion stability, the second centrifuge tube was heated in a water bath at 85 °C for 15 min. Then, the mixture was also centrifuged. The assay was conducted in triplicate. Emulsion activity and stability were calculated according to the following equations.16
| Emulsion activity (%) = vol2/vol1 × 100 | (6) |
| Emulsion stability (%) = vol3/vol1 × 100 | (7) |
2.2.5.2 Interaction of PSF with water, oil and organic molecules. The water holding capacity (WHC), oil absorption capacity (OAC) and organic molecule absorption capacity (OMAC) of PSF were determined as follows: WHC and OAC were determined using the methodology by Burbano et al.17 OMAC was determined following the OAC methodology, but after oil was added, the sample was maintained for 24 h at 20 °C before being centrifuged.
The rheological behaviour of GFD was measured using the method given by Burbano et al.19 with slight modifications. Measurements were performed at 25 °C in a hybrid rheometer (HR 20, TA Instruments, USA) with parallel-plate geometry (diameter 40 mm, with a 2 mm gap). Liquid paraffin was applied around the sample to prevent the dough from drying during testing. Stress sweeps were performed in the range between 0.01 and 30 Pa at a constant frequency of 1 Hz in order to determine the linear viscoelastic range (LVR) of each formulation. Frequency sweeps were performed within the LVR from 0.1 to 40 Hz. From the mechanical spectrum, the storage (G′), loss (G′′) and complex (G*) moduli were obtained as a function of frequency. The dynamic complex modulus (G*) was fitted to a weak gel model.
3 Results and discussion
3.1. Nutritional properties
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) linoleic acid (18
linoleic acid (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, cis n − 6) (41.6%), followed by oleic acid (C18
2, cis n − 6) (41.6%), followed by oleic acid (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, cis n − 9) (40.5%), palmitic acid (16
1, cis n − 9) (40.5%), palmitic acid (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) (12.2%), stearic acid (18
0) (12.2%), stearic acid (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) (5.11%), arachidic acid (20
0) (5.11%), arachidic acid (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) (0.31%), α-linolenic acid (18
0) (0.31%), α-linolenic acid (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) (0.28%) and eicosenoic acid (20
3) (0.28%) and eicosenoic acid (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (0.060%) These values were similar to those previously reported for cold-pressed pumpkin seed oil.22 Essential fatty acids, which cannot be synthesized by the human body and must be obtained from the diet, are involved in different physiological functions. In this regard, PSF showed considerable amounts of linoleic acid but negligible amounts of α-linolenic acid.
1) (0.060%) These values were similar to those previously reported for cold-pressed pumpkin seed oil.22 Essential fatty acids, which cannot be synthesized by the human body and must be obtained from the diet, are involved in different physiological functions. In this regard, PSF showed considerable amounts of linoleic acid but negligible amounts of α-linolenic acid.
Table 2 shows the calculated iodine value (IV) for PSF. The value was consistent with the degree of unsaturated lipids in its composition (∑UFA: 82.4%) and coincides with the previous IV determined for pumpkin seed oil, also obtained by cold pressing.23
| Nutritional indices | Oxidative stability | ||
|---|---|---|---|
| a Mean ± standard deviation (SD); HH: hypocholesterolaemic/hypercholesterolaemic fatty acid ratio; AI: atherogenic index; TI: thrombogenic index; SFA: saturated fatty acid; MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid; UFA: unsaturated fatty acid; IV: iodine value; COX: calculated oxidizability; OSI: oxidative stability index; Q10: temperature acceleration factor. | |||
| HH | 6.76 | COX | 4.75 |
| AI | 0.15 | OSI at 130 °C (hours) | 19.1 ± 0.2 |
| TI | 0.41 | OSI at 140 °C (hours) | 9.5 ± 0.1 |
| ∑SFA (%) | 17.6 | OSI at 150 °C (hours) | 4.42 ± 0.09 |
| ∑MUFA (%) | 40.5 | OSI at 160 °C (hours) | 2.45 ± 0.05 |
| ∑PUFA (%) | 41.9 | Activation energy (kJ mol−1) | 100.5 |
| ∑UFA/∑SFA | 4.7 | Q 10 | 2.0 ± 0.1 |
| IV | 113 | Shelf life at 20 °C (months) | 45 ± 3 |
In addition, the calculated lipid nutritional indices for PSF are shown in Table 2. These indices are useful for estimating the impact of food on health. The atherogenic and thrombogenic indices were very low, which could indicate that PSF lipids had an anti-atherogenic action and they do not stimulate platelet aggregation. Also, PSF could have a potential protecting role against coronary heart disease since a high HH value (6.76) was obtained.14
3.2. Oxidative stability
Lipids are the second main component of PSF, and a high percentage of them are unsaturated (high IV value). Consequently, oxidation could limit its shelf life, leading to rancidity. This chemical phenomenon modifies the flavour, aroma, and appearance of foods. Despite this, PSF exhibited high oxidative stability, as the calculated oxidizability (COX) value was 4.75, which is higher than that previously reported for another press cake by-product (almond flour: 2.82).24 In addition, from the oxidative stability indices obtained from the Rancimat assay (Table 2), a shelf life of 45 months was estimated for storage at 20 °C. This high stability could be related to the presence of remnant seed particles that protect the fatty acids from oxidation, as well as to the presence of bioactive compounds with antioxidant capacity.23,25 Although the Rancimat estimation is useful, it is recommended to perform a real-time storage assay at different temperatures to determine the actual shelf life.3.3. Physicochemical properties of the flours
Table 3 shows the physicochemical and colour parameters of PSF. PSF had a low water activity value (0.523), which indicates that it does not favour the growth of microorganisms. Consistent with this, PSF also had a low moisture content (≈5%), which is below the maximum level (≤15%) for various flours set by the European Union.26 To use PSF (Fig. 1A) as an ingredient in different baking formulations, its appearance is of utmost importance. The instrumental colour of PSF (Table 3) and rice flour (L*: 93.5 ± 0.3, a*: 0.13 ± 0.03, and b*: 6.36 ± 0.05) was determined to compare the impact of PSF on composite formulations. While PSF was characterised by a bright green colour, rice flour presented a higher L* value, reflecting its whiteness. Thus, depending on the level of PSF used, it could slightly or significantly modify the final colour of the baked product. However, in the case of gluten-free bakery products, this could be a positive attribute because these types of products are commonly white due to the starches used in their formulations. Finally, PSF presented an almost neutral pH (6.74), showing that it would not inhibit or modify the fermentation process during baking.| Parameters | Value |
|---|---|
| a Mean ± SD. (n ≥ 4). OAC: oil absorption capacity, OMAC: organic molecular absorption capacity and WHC: water holding capacity. | |
| a w – water activity | 0.523 ± 0.004 |
| Moisture content (%) | 5.1 ± 0.1 |
| pH value | 6.74 ± 0.01 |
| L* value – luminosity | 48 ± 1 |
| a* value | 1.1 ± 0.3 |
| b* value | 21.6 ± 0.8 |
| WHC (g of water per 100 g of PSF) | 114 ± 4 |
| OAC (g oil per 100 g of PSF) | 74 ± 4 |
| OMAC (g oil per 100 g of PSF) | 79 ± 4 |
| Emulsion activity (%) | 55 ± 2 |
| Emulsion stability (%) | 51 ± 1 |
3.4. Thermal properties of PSF
Protein was the main component in PSF, so the thermal transitions observed by differential scanning calorimetry (DSC) can be attributed to denaturation of that protein under controlled heating. The sample showed an endothermic signal at around 92 °C (Tonset 80 ± 1 °C, Tpeak 91.5 ± 0.4 °C and Tfinal 106.7 ± 0.4 °C) and a denaturation enthalpy (ΔH) of 7.0 ± 0.3 J per g solids. These values are within the range previously indicated for pumpkin seed protein isolates.27 Furthermore, PSF showed a high value of ΔT1/2 (11.0 ± 0.9 °C), which could be related to the protein heterogeneity in pumpkin seeds28 and/or the protective effect of some remaining fragments towards the protein bodies in PSF.3.5. Techno-functional properties of PSF
The evaluation of the techno-functional properties of the by-products allows the prediction of how they will behave when used as ingredients. Table 3 shows the results of how PSF interacted with water, oil, and organic molecules (WHC, OAC and OMAC, respectively). PSF retained good amounts of water and oil, likely related to its fibre and protein content. The absorption and retention of water and oil by flours are important in the breadmaking process because they are related to the consistency of the dough or batter during kneading and subsequent baking time. In addition, OMAC values are associated with the ability of dietary fibre to interact with carcinogenic and mutagenic substances and with bile acids, leading to a hypocholesterolemic effect.29 OMAC of PSF was 79, and values near 100 have been reported as healthy.30On the other hand, PSF showed the ability to form emulsions, which is quite important in the food industry and particularly, for the development of gluten-free products since batters are emulsion systems. At its natural pH, PSF formed a light green emulsion with a few dark green particles. The PSF emulsion activity and stability (Table 3) are related to its proteins and remnant lipids after the cold-press oil extraction. All these techno-functional properties directly influence the sensory attributes of the final product, such as the desired texture of baked goods.31
3.6. Spectrophotometric analysis
Fig. 3 shows the ATR-FTIR spectra of RF and PSF. Both flours presented a signal at 3280 cm−1, corresponding to the stretching vibration of the O–H bond, which is related to the presence of water and polyphenol molecules. The RF signal was stronger, which coincides with its higher moisture content (12.5 ± 0.2%), while the PSF signal is likely a result of the presence of both water and those bioactive molecules.23 PSF exhibited stronger signals at 2923 cm−1 and 2854 cm−1 that arise from the stretching vibrations of C–H present in aliphatic –CH2 and –CH3 of triglycerides. At 1744 cm−1, a signal appeared in PSF that is associated with the vibrations of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of triglycerides and phospholipids. The signals at 1633, 1641 and 1536 cm−1 are related to proteins; the former two are mainly assigned to C
O group of triglycerides and phospholipids. The signals at 1633, 1641 and 1536 cm−1 are related to proteins; the former two are mainly assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching (amide I) and the latter to N–H bending (60%) and C–N stretching (40%) (amide II).32,33 Finally, both flours presented signals at around 1000 cm−1 assigned to the C–O stretching of carbohydrates. As expected, this signal was much stronger on the RF spectrum at 995 cm−1.
O stretching (amide I) and the latter to N–H bending (60%) and C–N stretching (40%) (amide II).32,33 Finally, both flours presented signals at around 1000 cm−1 assigned to the C–O stretching of carbohydrates. As expected, this signal was much stronger on the RF spectrum at 995 cm−1.
3.7. Microstructural analysis
Confocal scanning laser microscopy enables the differentiation of distinct macroscopic components within the observed samples. The microstructure of PSF was compared to that of rice flour, which is widely used in gluten-free baking. PSF was dyed with a mix of Nile red, rhodamine B and Calcoflour white. Meanwhile, RF was dyed with a mix of fluorescein isothiocyanate (FITC), rhodamine B and Calcoflour white. These two mixes of fluorophores were used to colour lipids, starch, proteins and fibre. Fig. 1 shows the micrographs of PSF and RF by CSLM. In the micrographs, fluorophore channels can be selectively switched on or off to facilitate observation. As expected after milling, Fig. 1B (Nile red channel off) shows PSF protein all over the image. Moreover, some fibre fragments from the seeds (in blue)2 were observed. In this micrograph of PSF, when proteins and lipids share the same position, the fluorophore colocalization is observed as a yellow-greenish colour (image B′ shows all respective channels on). This colocalization phenomenon could explain PSF's ability to form emulsions. Also, Fig. 1C shows that starch is the major component of RF. This flour showed high heterogeneity in terms of its components, i.e. blue and red structures were visible corresponding to fibre fragments and protein bodies, respectively. In contrast to PSF, RF had a low protein content. Therefore, to facilitate observation, the FITC channel was switched off (image C′).3.8. Pasting properties of premixes
In the milling industry, the pasting profile of the premixes, as determined by RVA, provides useful information on viscosity changes during heating. Besides, a correlation has been found between RVA parameters (setback)34 and how the breads would behave during storage. Fig. 4 shows the pasting profile of pumpkin seed flour (PSF), the premixes of rice flour with increasing replacements of PSF and premix control with xanthan gum (Control-XG). All formulations presented the typical pasting profile for a baking premix, while PSF presented a differentiated profile. The viscosity of PSF slightly increased (654 ± 0.7 cP) during heating (≈90 °C) and then dropped rapidly, remaining constant (≈356 cP). The initial increase in viscosity coincides with the protein denaturation temperature, as seen by DSC. Moreover, the subsequent decrease would be related to the interaction of the denatured proteins with the lipids of PSF under the constant shear stress. Besides, PSF starch (∼3%)25 could also contribute to its profile. | ||
| Fig. 4 Pasting behaviour of premixes of rice flour (Control) with increasing replacements of pumpkin seed flour (PSF) and a premix of rice flour with 1% xanthan gum (Control-XG). | ||
Table 4 shows pasting parameters of the premixes with PSF. PSF increased the pasting temperature of the premixes (p < 0.05), which shows that when PSF is included in a dough formulation the dough setting occurs at higher temperature. Thus, dough/batter could have more time to expand in the oven (oven spring). At the same time, the peak, minimum and final viscosities progressively decreased as the substitution level increases, which could be related to a combined effect: starch dilution and water restriction due to the ability of PSF to interact with water. A lower minimum viscosity reflects a higher stability of starch granules to heating which could be related to lower water availability. In addition, lower final viscosities are indicative of a lower tendency to form gels.34 On the other hand, the RVA profile exhibited by Control-XG was comparable to the pasting profile of PSF20%. This effect is relevant since the rheological behaviour of dough is determinant of the final volume of bread.18 In terms of secondary parameters, the premixes with PSF and XG showed less physical disruption during the assay (a decrease in breakdown values, p < 0.05). Likewise, the use of PSF and XG caused a decrease in the setback values. This suggests that these premixes could be less susceptible to retrogradation, which is favourable for bakery products since retrogradation is a key factor in determining shelf life.34
| Pasting temperature (°C) | Viscosities (cP) | Secondary parameters (cP) | ||||
|---|---|---|---|---|---|---|
| Peak | Minimum | Final | Breakdown | Setback | ||
| η p | η min | η f | η p − ηmin | η f − ηmin | ||
| a Mean ± SD. Different letters in the same column indicate significant differences (p < 0.05, n ≥ 3). | ||||||
| Control | 73.5 ± 0.0a | 3978 ± 28e | 2765 ± 30d | 5437 ± 8e | 1213 ± 2e | 2672 ± 21c |
| Control-XG | 73.5 ± 0.2a | 2513 ± 24b | 2271 ± 25b | 4025 ± 6b | 242 ± 1a | 1754 ± 31a |
| PSF 10% | 83.1 ± 0.1b | 3316 ± 21d | 2632 ± 7c | 4867 ± 88d | 684 ± 13d | 2235 ± 95b |
| PSF 20% | 85.2 ± 0.5c | 2720 ± 34c | 2224 ± 33b | 4392 ± 3c | 497 ± 1c | 2169 ± 36b |
| PSF 30% | 86.0 ± 0.6c | 2110 ± 8a | 1744 ± 19a | 3610 ± 67a | 366 ± 11b | 1866 ± 86a |
3.9. Rheological properties of model gluten-free doughs
The rheological properties of the five model gluten-free dough formulations were evaluated. These are considered model dough samples because they were prepared with only RF, PSF, water, and salt, whereas real systems usually contain many other ingredients. However, these ingredients could be used as the base for a baking formulation. On this basis, the loss, storage and complex moduli were obtained as a function of frequency. In all samples (Fig. 5A), throughout the entire test range, the storage modulus (G′) was higher than the respective loss modulus (G′′), suggesting a predominance of solid character in all five formulation samples. A similar tendency was previously reported for gluten-free dough and batters.18,35 The behaviour of G′ and G′′ of the control dough showed values ranging from 517 to 1664 Pa and from 125 to 1193 Pa, respectively (data not shown in Fig. 5A). Additionally, increasing the replacement of RF with PSF led to a significant increase in the moduli values (G′, G′′ and G*).Fig. 5B shows the complex modulus of the samples, which were modelled according to the weak gel approach (R2 ≥ 0.978). However, for the control dough (RF, salt, and water), the modelling was only successful from 0.1 to 20 Hz. Therefore, GFD-Control is not shown in Fig. 5B. This behaviour of the GFD-Control sample could be related to the fact that this sample showed poor water retention and was prone to syneresis during manipulation.
In this approach (weak gel model), the dough matrix is considered a network formed by weak strands that interconnect topological points, with weak interactions responsible for its stabilization.36 From this modelling, two parameters, AF and z, were calculated (Table 5). AF is related to the strength of the interactions present in the weak gel. In this case, AF values (Pa s1/z) increased considerably with the use of PSF (p < 0.05). The control dough containing xanthan gum showed a moderate gel strength that was between to that of the GFD-PSF10% and GFD-PSF20% doughs. This indicates that replacing RF with PSF at 20% resulted in a dough with a viscoelastic behaviour similar to that achieved with xanthan gum, a hydrocolloid commonly and effectively used in gluten-free bread production. The rheological behaviour exhibited by dough with PSF was in agreement with the previously discussed techno-functional properties of PSF, particularly its capacity to interact with water and its ability to form emulsions. Finally, the coordination degree (z) of flow units in these dough formulations decreased with the use of PSF (p < 0.05). z values went from 10 to 8.3 as the substitution level of PSF increased. This parameter is related to the number of interactions in the dough matrix, which could be explained by the difference in particle size between the flours (PSF and RF). The larger PSF particles created fewer, but stronger, connection points.
| A F (Pa s1/z) | z | R 2 | |
|---|---|---|---|
| a Mean ± SD. † Since control dough was modelled from 0.1 to 20 Hz, it was not included in the one-way ANOVA. Different letters in the same column indicate significant differences (p < 0.05, n ≥ 3). AF: gel strength, z: coordination number. | |||
| GFD-control† | 792 ± 3 | 8.9 ± 0.1 | 0.966 |
| GFD-control-XG | 13702 ± 284b | 7.04 ± 0.03a | 0.996 |
| GFD-PSF10% | 4524 ± 94a | 10 ± 1c | 0.982 |
| GFD-PSF20% | 18563 ± 375c | 9.0 ± 0.4bc | 0.978 |
| GFD-PSF30% | 36672 ± 176d | 8.3 ± 0.1ab | 0.991 |
4 Conclusion
Pumpkin seed flour (PSF), obtained through a cold-pressing process, presented a high nutritional value and promising techno-functional properties. Although lipids represent one-third of its composition (with 82.4% being unsaturated fatty acids), PSF showed a high oxidative stability. Characterization through FTIR, microstructural, and particle size analyses showed that PSF and rice flour complement each other when forming composite mixtures. Furthermore, viscoamylograph results demonstrated that PSF (at 10% to 30%) was suitable for developing gluten-free premixes, as premixes with PSF exhibited improved pasting properties. Moreover, PSF enhanced the rheological quality of the gluten-free dough model systems when used at 20% and 30%, even acting as a typical hydrocolloid (xanthan gum). This effect could be related to the ability of PSF to retain water and to form emulsions. Therefore, the next step will be to focus on the effect of using PSF on the overall quality of gluten-free bread.Author contributions
JJB and MJC: conceptualization, methodology, investigation, data curation, writing – original draft, and writing – review & editing. MR, MFB and JVR: investigation. RRCV and MJC: resources, project administration and funding acquisition.Conflicts of interest
The authors declare no conflict of interest.Data availability
The data supporting this article have been included in the main document. The corresponding authors can be contacted for data sharing.Acknowledgements
Special thanks to Aceites del Desierto SRL for the donation of pumpkin seed flour. Also, the authors thank Daniela Igartúa and Dario Cabezas (particle size measurements), Enrique Portiansky (CSLM) and Fernanda Hamet (rheological measurements) for their kind assistance.References
- J. M. Roncero, M. Álvarez-Ortí, A. Pardo-Giménez, R. Gómez, A. Rabadán and J. E. Pardo, Virgin almond oil: Extraction methods and composition, Grasas Aceites, 2016, 67, e143, DOI:10.3989/gya.0993152.
- J. Murovec, K. Drašlar and B. Bohanec, Detailed analysis of Cucurbita pepo seed coat types and structures with scanning electron microscopy, Botany, 2012, 90, 1161–1169, DOI:10.1139/b2012-088.
- G. O. Fruhwirth and A. Hermetter, Seeds and oil of the Styrian oil pumpkin: Components and biological activities, Eur. J. Lipid Sci. Technol., 2007, 109, 1128–1140, DOI:10.1002/ejlt.200700105.
- M. Arslan, A. Rakha, Z. Xiaobo and M. A. Mahmood, Complimenting gluten free bakery products with dietary fiber: Opportunities and constraints, Trends Food Sci. Technol., 2019, 83, 194–202, DOI:10.1016/j.tifs.2018.11.011.
- S. Jan, C. Ghoroi and D. C. Saxena, Effect of particle size, shape and surface roughness on bulk and shear properties of rice flour, J. Cereal. Sci., 2017, 76, 215–221, DOI:10.1016/j.jcs.2017.04.015.
- L. d. L. d. O. Pineli, L. A. de Aguiar, G. T. de Oliveira, R. B. A. Botelho, M. d. D. F. P. Ibiapina and H. C. De Lima, et al., Use of Baru (Brazilian Almond) Waste from Physical Extraction of Oil to Produce Gluten Free Cakes, Plant Foods Hum. Nutr., 2015, 70, 50–55, DOI:10.1007/s11130-014-0460-7.
- J. J. Burbano, D. M. Cabezas and M. J. Correa, Gluten-free cakes with walnut flour: a technological, sensory, and microstructural approach, Int. J. Food Sci. Technol., 2022, 57, 4772–4781, DOI:10.1111/ijfs.15591.
- B. Zdybel, R. Różyło and A. Sagan, Use of a waste product from the pressing of chia seed oil in wheat and gluten-free bread processing, J. Food Process. Preserv., 2019, 43(8), e14002, DOI:10.1111/jfpp.14002.
- J. J. Burbano and M. J. Correa, Short-Term Storage of Gluten-Free Cakes with Walnut Flour, a By-Product from Walnut Cold-Pressed Oil Extraction, in A Cross-Disciplinary Exploration of STEM, ed. Y. Torres, A. M. Beltrán, M. Felix, E. Peralta and D. F. Larios Marín, Springer Nature, Switzerland, Cham, 2025, pp. 308–320, DOI:10.1007/978-3-031-99987-1_32.
- P. W. Park and R. E. Goins, In Situ Preparation of Fatty Acid Methyl Esters for Analysis of Fatty Acid Composition in Foods, J. Food Sci., 1994, 59, 1262–1266, DOI:10.1111/j.1365-2621.1994.tb14691.x.
- E. Symoniuk, N. Ksibi, M. Wroniak, M. Lefek and K. Ratusz, Oxidative Stability Analysis of Selected Oils from Unconventional Raw Materials Using Rancimat Apparatus, Appl. Sci., 2022, 12, 10355, DOI:10.3390/app122010355.
- M. L. Martínez and D. M. Maestri, Oil chemical variation in walnut (Juglans regia L.) genotypes grown in Argentina, Eur. J. Lipid Sci. Technol., 2008, 110, 1183–1189, DOI:10.1002/ejlt.200800121.
- J. J. Burbano and M. J. Correa, Composition and physicochemical characterization of walnut flour, a by-product of oil extraction, Plant Foods Hum. Nutr., 2021, 76, 233–239, DOI:10.1007/s11130-021-00898-4.
- K. Ratusz, E. Symoniuk, M. Wroniak and M. Rudzińska, Bioactive Compounds, Nutritional Quality and Oxidative Stability of Cold-Pressed Camelina (Camelina sativa L.) Oils, Appl. Sci., 2018, 8, 2606, DOI:10.3390/app8122606.
- O. Y. A. Moscoso, M. E. Lionello, L. Garófalo, J. J. Burbano, D. M. Cabezas and M. J. Correa, Effect of ultrasonic treatment on soybean okara to be used as a gluten-free bread improver, Int. J. Food Sci. Technol., 2023, 58, 3827–3837, DOI:10.1111/ijfs.16484.
- S. Jitngarmkusol, J. Hongsuwankul and K. Tananuwong, Chemical compositions, functional properties, and microstructure of defatted macadamia flours, Food Chem., 2008, 110, 23–30, DOI:10.1016/j.foodchem.2008.01.050.
- J. J. Burbano, D. M. Cabezas and M. J. Correa, Characterization and Techno-Functional Properties of High Protein Walnut Flour from an Oil by-Product, Plant Foods Hum. Nutr., 2024, 79, 810–818, DOI:10.1007/s11130-024-01219-1.
- C. Huamaní-Perales, J. Vidaurre-Ruiz, D. M. Cabezas, M. J. Correa, J. J. Burbano and J. C. Rodríguez-Soto, et al., Tarwi okara (Lupinus mutabilis) native and modified by high-intensity ultrasound as a gluten-free bread improver, Food Hydrocoll., 2025, 163, 111131, DOI:10.1016/j.foodhyd.2025.111131.
- J. J. Burbano, D. M. Cabezas and M. J. Correa, Effect of walnut flour addition on rheological, thermal and microstructural properties of a gluten free-batter, LWT–Food Sci. Technol., 2022, 154, 112819, DOI:10.1016/j.lwt.2021.112819.
- J. J. Burbano, A. D. Gara, C. Ferrero and M. J. Correa, Gluten-free bread made with non-defatted ground walnuts: microstructural, technological and nutritional properties, Int. J. Food Sci. Technol., 2025, vvae084, DOI:10.1093/ijfood/vvae084.
- AACC, Approved Methods of the American Association of Cereal Chemists, The American Association of Cereal Chemists, St. Paul, MN, USA, 2000 Search PubMed.
- S. Neđeral, M. Petrović, D. Vincek, D. Pukec, D. Škevin and K. Kraljić, et al., Variance of quality parameters and fatty acid composition in pumpkin seed oil during three crop seasons, Ind. Crop. Prod., 2014, 60, 15–21, DOI:10.1016/j.indcrop.2014.05.044.
- G. Zhang, Z. Li and M. Fu, Comparison of quality and oxidative stability of pumpkin seed (Cucurbita maxima) oil between conventional and enzymatic extraction methods, Sustainable Food Technol., 2024, 2, 1033–1040, 10.1039/D4FB00080C.
- J. J. Burbano and M. J. Correa, Almond flour a by-product of oil extraction: nutritional characterisation and impact on rheological properties of premixes for bakery products, Int. J. Food Sci. Technol., 2024, 59, 3126–3133, DOI:10.1111/ijfs.17055.
- M. Z. Amin, T. Islam, F. Mostofa, M. J. Uddin, M. M. Rahman and M. A. Satter, Comparative assessment of the physicochemical and biochemical properties of native and hybrid varieties of pumpkin seed and seed oil (Cucurbita maxima Linn.), Heliyon, 2019, 5, e02994, DOI:10.1016/j.heliyon.2019.e02994.
- CR-EEC No 1580 R. COMMISSION REGULATION (EEC) No 1580/93, https://www.legislation.gov.uk/eur/1993/1580/contents/adopted, accessed 2 Nov2021.
- D. Sert, H. Rohm and S. Struck, Ultrasound-Assisted Extraction of Protein from Pumpkin Seed Press Cake: Impact on Protein Yield and Techno-Functionality, Foods, 2022, 11, 4029 CrossRef CAS PubMed.
- M. Habib, S. Singh, N. A. Sagar, S. Ahmad, I. Qureshi and S. Jan, et al., Physicochemical and functional characterization of pumpkin seed protein isolate, Sustainable Food Technol., 2025, 3, 445–455, 10.1039/D4FB00268G.
- Y. N. Mora, J. C. Contreras, C. N. Aguilar, P. Meléndez, I. D. L. Garza and R. Rodríguez, Chemical Composition and Functional Properties from Different Sources of Dietary Fiber, Am. J. Food Nutr., 2013, 1, 27–33, DOI:10.12691/ajfn-1-3-2.
- O. A. Vázquez, G. Rosado-Rubio, L. Chel-Guerrero and D. Betancur-Ancona, Physicochemical properties of a fibrous fraction from chia (Salvia hispanica L.), LWT–Food Sci. Technol., 2009, 42, 168–173 CrossRef.
- S. Bernardi, A. L. Lupatini-Menegotto, D. L. Kalschne, É. L. Moraes Flores, P. R. S. Bittencourt and E. Colla, et al., Ultrasound: a suitable technology to improve the extraction and techno-functional properties of vegetable food proteins, Plant Foods Hum. Nutr., 2021, 76, 1–11, DOI:10.1007/s11130-021-00884-w.
- S. W. Ellepola, S. M. Choi and C. Y. Ma, Conformational study of globulin from rice (Oryza sativa) seeds by Fourier-transform infrared spectroscopy, Int. J. Biol. Macromol., 2005, 37, 12–20, DOI:10.1016/j.ijbiomac.2005.07.008.
- A. Dogan, G. Siyakus and F. Severcan, FTIR spectroscopic characterization of irradiated hazelnut (Corylus avellana L.), Food Chem., 2007, 100, 1106–1114, DOI:10.1016/j.foodchem.2005.11.017.
- S. Balet, A. Guelpa, G. Fox and M. Manley, Rapid Visco Analyser (RVA) as a Tool for Measuring Starch-Related Physiochemical Properties in Cereals: a Review, Food Anal. Methods, 2019, 12, 2344–2360, DOI:10.1007/s12161-019-01581-w.
- B. Madhavi, N. Asaithambi, A. Kumar, P. Maiti, D. Chandra Rai and R. K. Duary, Impact of chickpea aquafaba-based emulsion on the physicochemical, nutritional, rheological and structural characteristics of little millet (Panicum sumatrense Roth.) flour cake, Sustainable Food Technol., 2025, 3, 286–299, 10.1039/D4FB00293H.
- D. Gabriele, B. de Cindio and P. D’Antona, A weak gel model for foods, Rheol. Acta, 2001, 40, 120–127, DOI:10.1007/s003970000139.
| This journal is © The Royal Society of Chemistry 2025 |




